Abstract
The genes encoding the glycosyltransferases responsible for the addition of the five sugars in the α-oligosaccharide (α-OS) moiety of lipooligosaccharide (LOS) have been identified. Disruption of these glycosyltransferase genes singly or in combination results in corresponding truncations in LOS. In the present work we show that sequential deletion of the terminal four sugar residues of gonococcal α-OS had no discernible effect on the invasion of human conjunctival, endometrial, and cervical cell lines. However, deletion of the proximal glucose, which resulted in the complete deletion of α-OS, significantly impaired invasion of the gonococci into all three cell lines. The effect of deleting α-OS on invasion was independent of and additive to the known invasion-promoting factor OpaA. These data suggest that the proximal glucose residue of the α-OS chain of LOS is required for efficient invasion of gonococci into host mucosa.
Neisseria gonorrhoeae (the gonococcus [GC]), a gram-negative bacterium that naturally infects only humans, is the causative agent of gonorrhea. Gonorrhea is one of the more prevalent sexually transmitted diseases, with more than 600,000 cases reported every year in the United States (82). Currently, no gonococcal vaccine is available, mainly due to the high frequency of phase and antigenic variation of GC surface structures.
Early in vitro studies of GC invasion were done with fallopian tube explants obtained from patients undergoing surgical sterilization or hysterectomy. In this model it was shown that GC are internalized by nonciliated cells and sequestered within membrane-bound vacuoles which transcytose, fuse with the cells' basolateral membranes, and release the bacteria into the submucosa (22, 26, 39–41, 80), where they can initiate an inflammatory reaction and invade the bloodstream to spread systemically.
Most of the recent in vitro studies of GC adherence and invasion have been done with cell lines. Internalization by epithelial cells requires viable GC (52) and occurs by a process termed parasite-directed endocytosis (38). This mechanism is reminiscent of classical phagocytosis (which is microfilament dependent and thus inhibited by the cytochalasins), but differs in that it occurs in ordinarily nonphagocytic cells and appears to be initiated by bacterial rather than host cell factors. There is evidence from studies using cell lines as well as direct examination of urethral discharge that intracellular GC reside both within vacuoles and free in the cytoplasm (43, 64, 81).
Three GC surface structures of particular relevance to the initiation of GC infection include pili, opacity (Opa) proteins, and lipooligosaccharide (LOS). Pili are filamentous outer membrane appendages that cause an initial attachment of GC to epithelial and endothelial cells (55, 56, 69, 78) via human membrane cofactor protein CD46 (30). GC recovered from the urethras of infected male human volunteers form piliated colonies exclusively, indicating a role in the initiation of the infection in vivo (73). Furthermore, extensive antigenic variation of pilus components occurs by a variety of mechanisms (17, 27) throughout the course of both natural and experimental infections (63, 73), allowing immune evasion and tissue tropism. Efficient invasion of some human epithelial cells by GC occurs in the nonpiliated (P−) rather than the pillated (P+) phase (23, 35).
Opacity (Opa) proteins are a family of GC outer membrane proteins that mediate tight adherence to and invasion of host cells (3, 5, 14, 32, 65). There are 11 different complete Opa genes in GC strain MS11, with antigenic variation being determined by which Opa proteins happen to be turned on or off in a particular GC (6). This phase variation of individual Opa proteins occurs at a frequency of ∼10−3 and is mainly controlled at the translational level by a recA-independent slipped-strand mispairing mechanism (4, 6, 37, 44, 68). It has been shown that OpaA mediates efficient GC strain MS11 invasion of Chang conjunctival, HEC-1-B endometrial, and ME-180 cervical human epithelial cell lines (32, 35). Adherence of OpaA+ GC and their internalization by epithelial cells is mediated through the binding of OpaA to epithelial heparan sulfate proteoglycans (9, 20, 77).
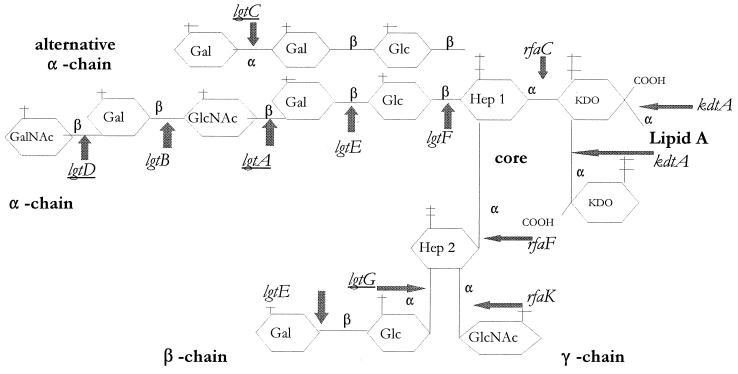
Neisseria gonorrhoeae expresses (LOS), which lacks the O-antigen sugar repeats present in the lipopolysaccharides (LPS) of enteric bacteria. GC LOS consists of lipidA, which is embedded in the outer membrane and attached to two 2-keto-3-deoxy-mannooctulosonic acid (KDO) molecules. Attached to the first KDO residue are two heptoses carrying the α-, β-, and γ-oligosaccharide chains (Fig. 1); this is in contrast with enteric LPS, which contains a single oligosaccharide chain. Schneider et al. challenged human volunteers with GC strain MS11 var A, which predominantly expresses a lactosyl group as its α-oligosaccharide (α-OS) (31), and found that the majority of the variants recovered after the onset of dysuria and discharge expressed higher-molecular-weight LOS molecules (60). An isolate with the full-length α-OS was named variant C (var C) (60) and is the parental variant we chose for this study. LOS varies independently of other GC surface structures (including Opa proteins and pili) at a frequency of ∼10−3 in the absence of selective pressures (1, 61).
FIG. 1.
Generic structure of GC LOS. The underlined genes can phase vary (on/off) due to the presence of poly(G) or poly(C) tracts that potentially result in slipped-strand errors during replication (2, 7, 12, 19, 24, 85). The alternative α-chain is expressed when lgtA is off and lgtC is on (19). Note that a deletion of rfaF leads to the absence of the α-chain as well as the absence of Hep2 with its β- and γ-chains (18, 46, 47, 62). Futhermore, an rfaK mutant contains Hep2 but lacks α-, β-, and γ-chains (28).
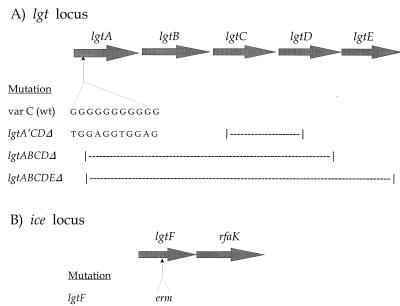
The genes encoding the glycosyltransferases responsible for the stepwise addition of each sugar to gonococcal α-OS have been identified and are labeled in Fig. 1. The lgtA to lgtE genes are located in a single cluster (Fig. 2A) that is not adjacent to other LOS synthesis genes. Four of the genes (lgtA, -B, -D, and -E) encode the glycosyltransferases that add the four terminal sugars of α-OS, whereas lgtC encodes the α-galactosyltransferase responsible for the alternative α-chain (19). lgtA (lsi-2) was also independently identified by Danaher et al. (12). lgtA, -C, and -D can phase vary due to the presence of poly(G) tracts within the gene sequences; these nucleotide repeats can cause slipped-strand errors in replication, resulting in translational frameshifts (7, 12, 19, 24, 85). lgtF encodes the glycosyltransferase that adds the proximal Glc of α-OS to Hep1; it is located immediately upstream of rfaK, which adds the γ-chain (Fig. 1 and 2B). The two genes are cotranscribed, and this operon has been named ice, for inner core extension (29). lgtF was previously identified in the meningococcus (MC) (29), and we subsequently cloned the GC homolog (this study).
FIG. 2.
Mutations in the lgt genes. The poly(G) tract in lgtA was altered by replacing the guanines with thymine or adenine in the third position of the codons in the reading frame; this locks lgtA into an on position by abolishing its ability to phase vary (19, 85). The lgtF gene was disrupted by the insertion of a modified erythromycin resistance cassette (erm); the construct was nonpolar (i.e., did not affect the downstream rfaK gene), as it was devoid of the erm transcriptional terminator (see text). In this figure, the extents of the deletions are marked by broken lines, and insertions are indicated by up arrows.
Disruption of the α-OS glycosyltransferase genes singly or in combination results in the stable expression of truncated LOS, as evidenced by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), immunochemical, and structural analyses (19, 29, 79; this study). Data presented herein specifically address the effect of these stable truncations in the α-OS of LOS on the interaction of N. gonorrhoeae strain MS11 with Chang conjunctival, HEC-1-B endometrial, and ME-180 cervical cells. These cell lines represent tissues that are natural sites for infection by GC (75). Adherence and invasion of the MS11 LOS mutants was measured by the gentamicin selection assay (15, 35). We show that the proximal glucose but not the terminal four sugars of α-OS is necessary for efficient GC invasion into these cell lines.
(This study was presented in part at the 11th International Pathogenic Neisseria Conference, Nice, France, 1 to 6 November 1998.)
MATERIALS AND METHODS
Reagents and supplies.
Reagents and chemicals were obtained from Sigma Chemical Co. (St. Louis, Mo.) or Fisher Scientific (Springfield, N.J.). Restriction enzymes and prestained molecular weight markers were purchased from New England Biolabs (Beverly, Mass.). Tissue culture plates were purchased from Falcon (Franklin Lakes, N.J.). Tissue culture medium was purchased from Gibco-BRL (Grand Island, N.Y.), and fetal bovine serum (FBS) was purchased from Hyclone Laboratories, Inc. (Logan, Utah).
Bacterial strains.
The parental wild-type (wt) strain used was N. gonorrhoeae MS11 var C, a recent human-passaged strain that expresses full-length LOS (60). GC strain MS11 expressing wt or truncated forms of LOS were either stored at −70°C in Greave's solution (5% monosodium glutamate and 5% bovine serum albumin in sterile distilled water) or passed daily on GC agar supplemented with 1% IsoVitaLex (70) and then incubated at 37°C in 6% CO2. Escherichia coli strain HB101 was stored at −70°C in Greave's solution or passed on Luria agar and then incubated at 37°C in 6% CO2. The GC were monitored for piliation by viewing colony morphology under a dissecting microscope (72) and for expression of the invasion-promoting OpaA by colony morphology (35, 70, 71), SDS-PAGE, and Western blotting.
Protein electrophoresis and immunoblotting.
GC were grown overnight on GC agar plates; opaque colonies were analyzed to verify OpaA expression, and transparent colonies were analyzed to verify lack of detectable Opa protein expression. The colonies were suspended in Laemmli buffer (33) and heated at 100°C for 5 min; the lysates were then subjected to SDS-PAGE. The Opa proteins were also detected by SDS-PAGE and immunoblotting (74) using rabbit polyclonal anti-OpaA antiserum (3) as the primary antibody, with alkaline phosphatase-conjugated goat anti-rabbit immunoglobulin (Sigma) as the secondary antibody. The blot was developed with 5-bromo-4-chloro-3-indolylphosphate and p-nitro blue tetrazolium chloride in a Tris-MgCl2 buffer. GC subcultured and stored in frozen aliquots for further study expressed either OpaA or no detectable Opa proteins and were either piliated or nonpiliated.
Electrophoretic analysis of LOS.
GC, either swabbed from GC agar plates or pelleted from culture in proteose peptone liquid medium (19), were suspended in sample buffer, heated at 100°C for 5 min, and incubated with proteinase K (∼500 μg/ml) for 1 h at 55°C. LOS was analyzed by tricine-SDS-PAGE and silver staining (34).
Construction of pLGTA′CDΔ.
In order to prevent undesired phase variation, the poly(G) tract in lgtA (Fig. 2A) was altered by oligonucleotide-mediated mutagenesis, using the method of Zoller et al. (86) as modified by Sambrook et al. (57). The two primers were the M13 reverse primer and the mutagenic primer C GAA TTG GCA AAG TCT GGA GGT GGA GAA TAT ATT GCG CGC, which was synthesized by the Rockefeller University Technology Center. The template was a cloned copy of the wt lgtA. As indicated by the underlined segment of the mutagenic primer sequence, 11 G's [equivalent to the poly(G) tract in GC strain MS11 lgtA (85)] were replaced with thymine or adenine in the third position of the codons in the reading frame; thus, the gene's poly(G) tract was eliminated, in effect locking lgtA into an on position by abolishing its ability to phase vary without affecting the amino acid sequence. E. coli cells containing a plasmid with the mutated sequence were identified by colony hybridization with the mutagenic primer labeled with 32P (57). The mutated lgtA gene was verified by DNA sequencing and renamed lgtA′. The deletion of lgtC and lgtD (Fig. 2A) was accomplished by digesting a plasmid containing the complete lgt locus with Tth111I and religating to create pLGTACDΔ; this mutation removed a DNA segment consisting of 635 nucleotides from the 3′ end of lgtC plus 462 nucleotides from the 5′ end of lgtD. Subsequently, the mutated lgtA′ was substituted for the wild-type lgtA in pLGTACDΔ by subcloning to yield pLGTA′CDΔ.
Construction of pNGLGTF::erm.
An Neisseria menigitidis lgtF amplicon was generated by a PCR protocol using MC strain BNCV genomic DNA as the template and MC lgtF primers CK23 and CK24 (29). The CK23-CK24 lgtF amplicon was labeled with enhanced chemiluminescence (Amersham-Pharmacia) and used to probe a GC strain 15253-λZapII phage library by plaque hybridization (2). This screen yielded a phagemid clone, pGCLGTF, containing 626 bp of the ∼750-bp lgtF gene as well as a ∼6-kb upstream sequence. In order to disrupt lgtF, pGCLGTF was digested with BsiWI at 493 nucleotides into the lgtF gene fragment and filled with Klenow enzyme; a 789-bp erythromycin (erm) resistance cassette (19) was then inserted into this site (Fig. 2B). The resultant plasmid, named pGCLGTF::erm, was further modified in four sequential steps. First, it was reduced in size from 9,902 to 6,927 bp by digestion with EcoRV, which removed ∼3 kb of the DNA sequence upstream of lgtF. Second, the erm gene was reversed by cutting out the cassette with BsiWI, religating the fragments, and selecting a clone with the erm gene inverted to the same orientation as lgtF. Third, the erm transcriptional terminator and the downstream, incomplete 133-bp lgtF 3′ sequence were removed by digestion with Ppu10I and SpeI. Fourth, a 1.2-kb amplicon (generated from GC strain FA1090 genomic DNA using lgtF and rfaK primers) consisting of the complete 265-bp 3′ end of lgtF plus the entire rfaK gene was subcloned into the Ppu10I and SpeI sites to obtain the recombination substrate vector pNGLGTF::erm.
The modified erm cassette of pNGLGTF::erm contains the sequence up to bp 2196 (Ppu10I site) of the original ermC′ cassette of vector pIM13 (GenBank accession no. M13761) (42); hence, the pNGLGTF::erm construct was nonpolar, as it was devoid of the transcriptional terminator. Furthermore, the presence of glucosamine (GlcNAc is added by rfaK to make the γ-chain) was determined by carbohydrate analysis of MS11 lgtF LOS by high-pH anion-exchange chromatography (2, 16), confirming the nonpolar nature of the mutation (data not shown).
Generation of MS11 LOS mutants.
Plasmids carrying the lgtABCDΔ, lgtABCDEΔ, and lgtF mutations (Fig. 2), which conferred erythromycin resistance, were purified from E. coli XL1-Blue MRF′ by alkaline lysis, verified by restriction analysis, and used to transform MS11 var C (OpaA+ P+) by means of the spot dilution method (13). In brief, the GC colonies (16 to 18 h old) grown on GC agar were suspended and diluted in proteose peptone liquid medium to ∼104 CFU/ml. Fresh GC agar was spread with 200 μl of the final dilutions and then spotted with 20 μl of plasmid preparations (∼1 mg/ml). Plates were incubated at 37°C in 6% CO2 for 30 h to allow transformation, recombination, expression of phenotypic markers, and colonial growth. Colonies were picked from the plasmid-containing areas and then passed thrice on GC agar containing erythromycin (2 μg/ml) for identification of transformants. Plasmid pLGTA′CDΔ was purified from E. coli XL1-Blue MRF′ and used to transform the erythromycin-resistant MS11 lgtABCDEΔ (OpaA+ P+) by means of the spot dilution technique. Colonies were picked from the plasmid-containing areas and passed twice on GC agar; transformants were chosen by screening for sensitivity to erythromycin (2 μg/ml). The MS11 LOS mutants were analyzed for LOS and opacity phenotypes.
Comparison of growth rates.
Nonpiliated wt and LOS mutant GC grown for ∼18 h on GC agar plates were suspended in proteose peptone broth to an optical density (OD) of 0.3 at 540 nm. Suspensions were diluted 1:10 into fresh proteose peptone broth supplemented with 1% IsoVitaLex and grown at 37°C in a shaker incubator. The OD540 of each culture was measured at 1-h intervals.
Cell culture.
Chang conjunctival cells (ATCCCCL 20.2), HEC-1-B endometrial cells (ATCC HTB 113), and ME-180 cervical cells (ATCC HTB 33) were maintained in RPMI 1640 medium plus 1 mM sodium pyruvate and 5% FBS in tissue culture flasks incubated at 37°C in 6% CO2.
Adherence and invasion assays.
The gentamicin survival assay was used to measure adherence and invasion (15, 35). We confirmed the susceptibility of our MS11 LOS mutants to gentamicin by their lack of growth on GC agar containing gentamicin (100 μg/ml). For infection experiments, monolayers were grown in RPMI 1640 plus 1 mM sodium pyruvate and 5% FBS in 24-well tissue culture plates. Confluent epithelial cell monolayers, obtained after 24 h of incubation of ∼2 × 105 epithelial cells per well at 37°C in 6% CO2, were determined to contain ∼4 × 105 epithelial cells per monolayer. Immediately before use, the monolayers were rinsed once with RPMI 1640 medium with no FBS.
Wt and LOS mutant GC (nonpiliated and expressing either OpaA or no detectable Opa protein) were grown for ∼18 h on GC agarose plates (76) at 37°C in 6% CO2 and then suspended in proteose peptone broth to an OD540 of 0.3 (∼2 × 108 CFU/ml). One hundred microliters (∼2 × 107 CFU) of the appropriate suspension was added to 1 ml of FBS-free RPMI medium (76) per Chang conjunctival cell monolayer in 24-well tissue culture plates and incubated for 5 h at 37°C in 6% CO2. For the cytochalasin D inhibition experiments, 1 ml of fresh RPMI medium containing either 10 μg of cytochalasin D (10 μl of a 1-mg/ml solution in dimethyl sulfoxide [DMSO]) per ml or 10 μl of DMSO (as a control) was added to appropriate monolayers, which were then incubated for 30 min prior to infection.
After the inoculation and a 5-h incubation, the monolayers were washed three times in phosphate-buffered saline (PBS) to remove non-cell-associated bacteria. In some assays, equal-volume samples of the different mutants were taken and plated from the cell culture medium just prior to removing non-cell-associated bacteria and verified to yield similar numbers of colonies. To measure total cell association (a combination of adherent and invasive bacteria), infected monolayers were lysed with 1 ml of saponin (1% in PBS) for 15 min at 37°C. To measure invasion, infected monolayers were incubated further with gentamicin (100 μg/ml) in RPMI for 1 h (a step which kills extracellular bacteria, while internalized bacteria survive), then washed and lysed. Appropriate dilutions were made and plated on GC agar for CFU counts; in some assays, dilutions were also plated on agar containing gentamicin (100 μg/ml) to verify susceptibility. Monolayers were inoculated in triplicate, and each experiment was performed at least three times.
Because the consolidated data from repeated experiments done at different times did not follow a Gaussian distribution, we used a generalized Friedman-type statistical procedure which ranks all the data points within each experiment and then compares the average sum of ranks between groups in a set of experiments; this nonparametric test has been modified to handle unbalanced designs, tied values, and values that are off scale or missing at random (83, 84).
RESULTS
Disruption of the lgt genes in GC strain MS11 leads to expression of truncated LOS.
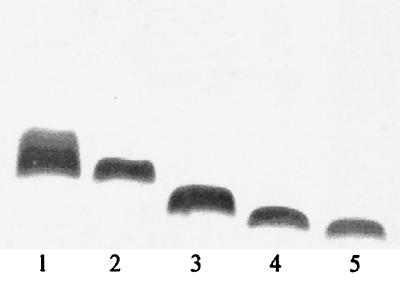
N. gonorrhoeae strain MS11 var C is a recent human-passaged strain that expresses full-length LOS (60). Our repertoire of LOS mutants (Table 1) was created by transformation-recombination of MS11 var C (OpaA+ P+) with plasmids carrying the lgtA′CDΔ, lgtABCDΔ, lgtABCDEΔ, or lgtF mutation (Fig. 2). Sequential deletion of the LOS glycosyltransferase genes was predicted to result in the expression of progressively shorter LOS chains. Accordingly, as shown in Fig. 3, the LOS bands migrated by SDS-PAGE in a manner consistent with their relative sizes, with the band corresponding to MS11 var C (wt) migrating the shortest distance and the LOS band corresponding to MS11 lgtF (completely lacking α-OS) migrating the longest distance. This also provided evidence that none of the mutants underwent spontaneous slippage in the poly(C) tract of lgtG to an in-frame position, which would have resulted in expression of the β-chain (see Fig. 1). Furthermore, the truncations of LOS had no discernible effect on the growth rate of GC in liquid medium (data not shown).
TABLE 1.
MS11 LOS mutant designation and phenotypes
| LOS mutant | Designationa | α-OS phenotypeb | Reference |
|---|---|---|---|
| MS11 var C (wt) | α-OS5 | GalNAcβ1→3Galβ1→4GlcNAcβ1→3Galβ1→4Glcβ1→4R | 60 |
| MS11 lgtA′CDΔ: | α-OS4 | Galβ1→4GlcNAcβ1→3Galβ1→4Glcβ1→4R | This study |
| MS11 lgtABCDΔ | α-OS2 | Galβ1→4Glcβ1→4R | Δ5 mutation (19) |
| MS11 lgtABCDEΔ | α-OS1 | Glcβ1→4R | Δ4 mutation (19) |
| MS11 lgtF | α-OS0 | R | 29; this study |
The subscripts in the LOS designations denote the number of sugars present in the α-OS.
R in the phenotypes refers to the remaining LOS structure, including the inner core and lipid A (see Fig. 1).
FIG. 3.
MS11 mutant α-OS phenotypes. Silver-stained tricine-SDS-PAGE gel scanned using Adobe Photoshop 4.0 and Microtek ScanMaker III. Note that var C expresses several forms of α-OS (represented by the band pattern in lane 1), including the tetrasaccharide (represented by the predominant band) and full-length pentasaccharide chains (60). This is possibly due to factors such as phase variation in lgtD (see Fig. 1), incomplete glycosyltransferase activity, or the elaboration of α-OS repeats (25). Lanes: 1, MS11 var C; 2, MS11 lgtA′CDΔ; 3, MS11 lgtABCDΔ; 4, MS11 lgtABCDEΔ; 5, MS11 lgtF.
GC strain MS11 LOS mutants are unaltered in expression of OpaA.
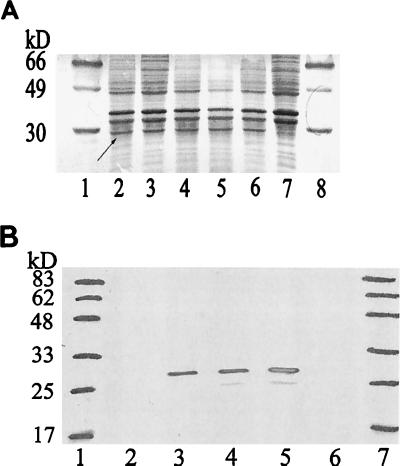
Wt and LOS mutant GC (Table 1) used for study were nonpiliated (because pili are known to interfere with GC invasion in vitro) and expressed either OpaA or no detectable Opa proteins (35). Expression of the ∼30-kDa OpaA was similar by SDS-PAGE analysis in GC expressing wt or mutant LOS (Fig. 4A, lanes 2 to 6). The identity of OpaA was further confirmed for the lgtF mutant by Western blotting with polyclonal anti-OpaA antiserum (Fig. 4B), which recognized OpaA in extracts from E. coli expressing recombinant OpaA, MS11 var C (OpaA+), and MS11 lgtF (OpaA+) (lanes 3 to 5) but not in extracts from E. coli carrying a plasmid control (lane 2) and MS11 var C (Opa−) (lane 6).
FIG. 4.
OpaA expression by MS11 LOS mutants. (A) Coomassie brilliant blue-stained SDS-PAGE gel scanned using Adobe Photoshop 4.0 and Microtek ScanMaker III. Lanes: 1, size markers; 2, MS11 var C (OpaA+); 3, MS11 lgtA′CDΔ (OpaA+); 4, MS11 lgtABCDΔ (OpaA+); 5, MS11 lgtABCDEΔ (OpaA+); 6, MS11 lgtF (OpaA+); 7, MS11 var C (Opa−); 8, size markers. Note: the arrow (lane 1) points to the 30-kDa OpaA band; this is absent from Opa−GC (lane 7). (B) Western blotting with polyclonal anti-OpaA rabbit antiserum, blot scanned using Adobe Photoshop 4.0 and Microtek ScanMaker III. Lanes: 1, size markers; 2, E. coli(pGEM-3Z) expressing no Opa proteins; 3, E. coli(pEXA) expressing OpaA; 4, MS11 var C (OpaA+); 5, MS11 lgtF (OpaA+); 6, MS11 var C (OpaA−); 7, size markers. Note: the recombinant vector pEXA consists of a β-lactamase–OpaA fusion in vector pGEM-3Z (3).
Only complete truncation of MS11 α-OS impairs GC invasion into human epithelial cell lines.
Cell association and invasion of the MS11 LOS mutants (Table 1) into Chang conjunctival, HEC-1-B endometrial, and ME-180 cervical cells were measured by the gentamicin survival assay (15, 35). Gentamicin is an aminoglycoside which does not readily permeate eukaryotic cell membranes; thus, internalized bacteria are shielded from the bactericidal effects of this antibiotic (15). Total cell association is a measure of the combination of adherent and invasive bacteria. In our hands, the proportion of gentamicin-resistant organisms was only ∼1% of the measurement of cell association; thus, total cell association provided a good estimate of adherence, and we hereafter use the terms interchangeably.
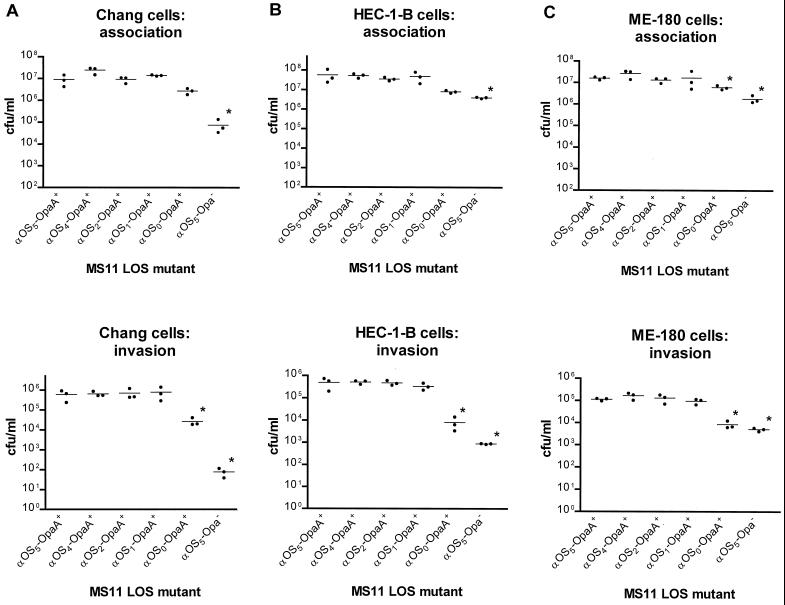
We found adherence and invasion of MS11 lgtA′CDΔ, lgtABCDΔ, and lgtABCDEΔ (Table 1) into Chang, HEC-1-B, and ME-180 cell lines to be comparable to those of the wt (Fig. 5, P > 0.1). In contrast, MS11 lgtF (Table 1) was significantly less invasive (Fig. 5, P ≤ 0.05) in these cell lines. This mutant was also consistently but only slightly less adherent than wt, with statistical significance being reached in ME-180 cells (Fig. 5C, P < 0.05) but not in Chang (Fig. 5A, P > 0.1) or HEC-1-B (Fig. 5B, P > 0.1) cells. As expected, in the Chang (Fig. 5A), HEC-1-B (Fig. 5B), and ME-180 (Fig. 5C) cell lines, the negative control MS11 var C (Opa−) was significantly less adherent (P < 0.05) and invasive (P < 0.001) than the positive control MS11 var C (OpaA+). These results suggest that the presence of the proximal glucose of the α-chain (added by the glycosyltransferase LgtF) is indispensable for efficient invasion into all three cell lines and has a significantly greater effect on invasion than on adherence in Chang and HEC-1-B cells.
FIG. 5.
GC adherence to and invasion of human epithelial cells. Chang conjunctival (A), HEC-1-B endometrial (B), and ME-180 cervical (C) cells were infected with nonpiliated N. gonorrhoeae strain MS11 wt and LOS mutants (Table 1), all variants expressing either OpaA or no detectable Opa protein. After a 5-h incubation, cell-associated (predominantly adherent) GC were determined by lysis of the epithelial cells and plating of the released bacteria for CFU counts. Invasive GC were determined by gentamicin treatment of the infected monolayers to kill extracellular bacteria, lysis of the epithelial cells, and plating of the surviving intracellular bacteria. Monolayers were infected in triplicate, and the assays were done at least three times. One representative experiment is shown for each cell type tested. The graphs are plotted as triplicate values (dots) with means (horizontal lines). Data were analyzed by a modified Friedman statistical procedure (83, 84). Asterisks mark values reaching statistical significance compared to MS11 var C (OpaA+).
α-OS glucose and OpaA have an additive effect on the invasion of Chang conjunctival and ME-180 cells.
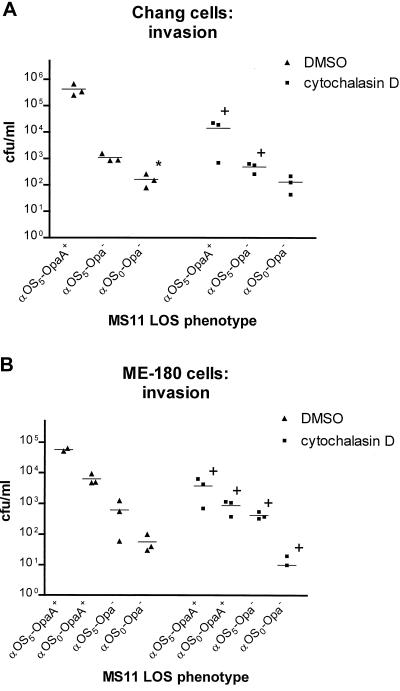
A relevant question that we sought to address is whether Opa and LOS (α-chain) act sequentially in a single pathway or via independent parallel pathways. To this end, we determined whether the lgtF mutation in an Opa− background would cause levels of invasion even lower than with MS11 var C (Opa−) (Table 1) in Chang conjunctival and ME-180 cervical cells. A sequential, OpaA-dominant pathway would be expected to result in no further reduction in invasion by MS11 lgtF (Opa−) (Table 1). In contrast, we found that (in the absence of cytochalasin D), MS11 lgtF (Opa−) was less invasive than MS11 var C (Opa−) in Chang cells (Fig. 6A, P < 0.05) and also in ME-180 cells, though this latter P value did not quite reach significance (Fig. 6B, P = 0.09). In ME-180 cells, MS11 lgtF (Opa−) was also less invasive than MS11 lgtF (OpaA+) (Fig. 6B, P < 0.05). These data argue for two independent pathways rather than one sequential pathway, since the absence of both OpaA and the LOS α-chain (proximal glucose) caused a greater reduction in invasion than did either property alone.
FIG. 6.
Additive effect of α-OS glucose and OpaA on MS11 invasion. Gentamicin protection assays were done using tissue culture medium containing either cytochalasin D (10 μg/ml) in DMSO or an equal volume of DMSO without cytochalasin D as a control (see text). Monolayers were infected in triplicate, and each experiment was done at least three times. Shown is one representative experiment each for Chang conjunctival (A) and ME-180 (B) cells. The graphs are plotted as triplicate values (dots) with means (horizontal lines). Data were analyzed by a modified Friedman statistical procedure (83, 84). The data in the left portion of each graph illustrate the additive effect of the proximal glucose and OpaA, in that MS11 lgtF (Opa−) was consistently less invasive than either MS11 lgtF (OpaA+) in ME-180 cells or MS11 var C (Opa−) in both Chang and ME-180 cells. The asterisk marks the value for invasion by MS11 lgtF (Opa−) that reached statistical significance compared to MS11 var C (Opa−). As expected, the invasion of OpaA+ GC was consistently inhibited by cytochalasin D; this is shown in the right portion of each graph. The plus signs denote significant inhibition of invasion by cytochalasin D.
Cytochalasin D inhibits invasion of MS11 var C (OpaA+) and MS11 lgtF (OpaA+) into Chang and ME-180 cells.
GC invasion is dependent upon actin polymerization, which is inhibited by cytochalasin D (5, 64). As expected, cytochalasin D significantly inhibited invasion of MS11 var C (OpaA+) (Table 1) in Chang conjunctival cells (Fig. 6A, P < 0.001) and ME-180 cells (Fig. 6B, P < 0.001). MS11 lgtF (OpaA+) (Table 1) was likewise significantly inhibited by cytochalasin D in ME-180 cells (Fig. 6B, P = 0.001). These results confirmed that optimum OpaA-mediated invasion by both MS11 var C and MS11 lgtF is dependent upon actin polymerization.
Invasion by MS11 var C (Opa−) (Table 1) was significantly inhibited by cytochalasin D in both Chang conjunctival cells (Fig. 6A, P < 0.05) and ME-180 cells (Fig. 6B, P < 0.01). In contrast, cytochalasin D significantly inhibited the invasion of MS11 lgtF (Opa−) (Table 1) into ME-180 cells (Fig. 6B, P < 0.01) but not into Chang conjunctival cells (Fig. 6A, P > 0.5). It is possible that the very small numbers of bacteria recovered from the MS11 lgtF (Opa−) invasion experiments included adherent GC that were gentamicin tolerant due to being sequestered within folds of the eukaryotic membrane and shielded from the effects of the antibiotic; alternatively, the recovery could represent invasion through another pathway not inhibited by cytochalasin D.
DISCUSSION
Our aim was to determine the effect of GC α-OS phenotype on GC interaction with Chang conjunctival, HEC-1-B endometrial, and ME-180 cervical cells, three human cell lines relevant to GC pathogenesis (75). We found that sequential deletion of the terminal four sugar residues of the α-OS in N. gonorrhoeae strain MS11 had no discernible effect on the bacterium's adherence to or invasion of these cell lines. In contrast, the effect of the nonpolar lgtF mutation (complete deletion of the α-chain but no discernible effect on expression of the γ-chain) was quite marked, with a slight reduction in adherence but a much greater (10- to 100-fold) reduction in invasion into all three cell types. These results suggest that the proximal glucose of the LOS α-chain plays an important role in the establishment of the mucosal infection.
We found a 5- to 10-fold lower uptake of wt GC into ME-180 cells compared to Chang conjunctival cells; this contrasts with the results of Kupsch et al. (32), who demonstrated a higher uptake of the bacteria into ME-180 cells than into Chang conjunctival cells. The reason for our contrasting results is unclear but could be due to different culture conditions—for example, growth of the GC on agarose plates and the use of serum-free medium for the invasion assays, both of which have been demonstrated to increase the rate of invasion into Chang conjunctival cells (76).
It is likely that GC do utilize more than one eukaryotic uptake pathway. For example, our data and the results of Grassmé et al. (21) confirmed the role of actin polymerization in invasion by demonstrating that cytochalasin D causes a ≥90% inhibition of GC internalization by Chang conjunctival cells. Actin polymerization (as measured by inhibition of invasion by cytochalasin D) is also required for optimum GC entry into HeLa cervical cells (5), HEC-1-B endometrial cells (8; this study), and ME-180 cervical cells (21; this study). However, it has also been found that inhibitors of microtubule assembly moderately decrease GC invasion into Chang conjunctival cells (21) and baby hamster kidney cells (52). This suggests a major requirement for a functional actin-based microskeleton, with some contribution from the microtubular network.
The cloning of the GC lgtA to lgtF genes allowed the construction of defined and stable α-OS mutants. Previously, other kinds of mutants expressing truncated forms of LOS were studied. For example, Robertson et al. cloned the GC galE gene, which encodes the metabolic enzyme UDP-galactose-4-epimerase (53). Because GC are unable to take up exogenous galactose, the galE mutant cannot incorporate galactose into its LOS and expresses an α-OS which consists of one sugar, the proximal glucose. Interestingly, this MS11 galE mutant of Robertson et al. has a structure similar to that of our MS11 lgtABCDEΔ (Table 1) and likewise exhibits wt levels of adhesion to and invasion of epithelial cells (53; this study). In contrast, a disruption of the GC lsi-1/rfaF gene leads to the production of LOS that is truncated beyond α-OS and into the inner-core region (18, 46, 47, 62) (Fig. 1), and Schwan et al. showed that this markedly reduces the invasion of Chang conjunctival cells by the OpaA+ and nonpiliated lsi-1 mutants (62). It is noteworthy that such a mutation in LOS could be linked to a clear defect in interaction with epithelial cells despite GC expression of an invasion-promoting Opa protein. Indeed, our study is the first in which a series of defined and stable LOS mutants were generated, the effect of truncations in α-OS on invasion were systematically tested, and the proximal glucose (added by the product of lgtF) was pinpointed as the specific residue in LOS that is likely to play a critical role in the initiation of GC infection of the host mucosa.
It is improbable that the lgtF mutation causes a nonspecific disruption in the GC membrane that would affect invasion. In E. coli and Salmonella spp., the minimal LPS structure required for viability consists of lipid A linked to two KDO residues (reviewed in reference 51); however, there is evidence of membrane perturbation in some enteric bacteria when there is an inability to synthesize or incorporate heptoses into this structure (45, 58). In contrast, a meningococcal lpxA mutant (which is defective in the first committed step in lipid A biosynthesis and lacks detectable LOS) is viable and exhibits normal cell envelope ultrastructure but a reduced growth rate (67). Thus, the absence of the LOS α-chain alone would not be expected to adversely affect GC membrane integrity; indeed, we found that the lgtF mutation had no effect on GC growth rate in liquid culture (data not shown).
E. coli cells expressing recombinant OpaA (rOpaA) adhere to (10, 21) but do not invade (21) Chang conjunctival cells, indicating that OpaA alone is insufficient to mediate invasion into this cell type. Kupsch et al. found that E. coli (rOpaA+) showed poor adherence to and invasion of HEC-1-B and ME-180 cells (32). These results indicate that some bacterial factor(s) other than Opa may also be involved in the internalization of GC by human epithelial cells. Indeed, Song et al. have recently demonstrated that GC expressing both pili and full-length α-OS can efficiently invade human epithelial cells in the absence of detectable Opa proteins (66). Accordingly, we have here presented evidence that the proximal glucose of α-OS is a component essential to the efficient invasion of GC into three physiologically relevant human cell lines: Chang conjunctival, HEC-1-B endometrial, and ME-180 cervical cell monolayers. We also showed data suggesting that GC OpaA and α-OS glucose operate via independent pathways.
Among the many questions that now need to be addressed are the following. First, what is the epithelial receptor for GC LOS? Many possibilities exist. It is known that GC LOS structures mimic an array of human epithelial glycosphingolipids (reviewed in reference 36). Also, researchers have reported the binding of GC LOS to a 70-kDa eukaryotic protein found on HepG2 cells (50). Additionally, Pier et al. showed that cystic fibrosis transmembrane conductance regulator is a receptor for Pseudomonas aeruginosa LPS that mediates the internalization of the bacteria by lung epithelial cells (48, 49). It is intriguing that a pseudomonal algC mutant, which resembles our MS11 lgtF mutant in lacking glucose in the core of its LPS (11, 54) (Table 1), is significantly less invasive than P. aeruginosa possessing a complete outer oligosaccharide core (49).
Another relevant question is whether the MS11 lgtF mutant is attenuated in vivo. Results of our in vitro experiments showed that MS11 lgtABCDΔ (Table 1) was comparable to MS11 var C in adherence and invasion (Fig. 5). In contrast, Schneider et al. found that MS11 var A, which, like MS11 lgtABCDΔ, expresses a lactosyl-LOS (31) (Table 1), was not as infectious as MS11 var C in clinical trials (59). Furthermore, when symptoms of gonorrhea ensued subsequent to inoculation of volunteers with MS11 var A, var C organisms were recovered in higher proportions than the expected (10−3) frequency of variation (59, 60). It would thus be interesting to compare the infectiousness of our repertoire of defined and stable MS11 LOS mutants in clinical trials. Such studies would further clarify the specific nature of the contribution of GC LOS to the establishment of the GC infection.
ACKNOWLEDGMENTS
We thank David Stephens and Charlene Kahler for kindly providing prepublication sequence information about the MC ice locus. The invaluable editorial assistance of John McKinney, the technical expertise of Clara Eastby and James Parker, the laboratory help of Milton Brown, and the secretarial support of Jane Berger-Hassett are much appreciated. We also gratefully acknowledge Knut Wittkowski for statistical analyses, Alice Erwin for useful suggestions, and Tie Chen for supplying E. coli HB101(pGEM3Z), E. coli HB101(pEXA), N. gonorrhoeae MS11 var C, and the polyclonal anti-OpaA antiserum.
This work was funded by PHS grants AI10615 and AI26558. S.Y.M. was supported by a GE Foundation Academic Fellowship and NIH training grants GM15317 and GM07739.
REFERENCES
- 1.Apicella M A, Shero M, Jarvis G A, Griffiss J M, Mandrell R E, Schneider H. Phenotypic variation in epitope expression of the Neisseria gonorrhoeae lipooligosaccharide. Infect Immun. 1987;55:1755–1761. doi: 10.1128/iai.55.8.1755-1761.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Banerjee A, Wang R, Uljon S N, Rice P A, Gotschlich E C, Stein D C. Identification of the gene (lgtG) encoding the lipooligosaccharide β chain synthesizing glucosyl transferase from Neisseria gonorrhoeae. Proc Natl Acad Sci USA. 1998;95:10872–10877. doi: 10.1073/pnas.95.18.10872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Belland R J, Chen T, Swanson J, Fischer S H. Human neutrophil response to recombinant neisserial Opa proteins. Mol Microbiol. 1992;6:1729–1737. doi: 10.1111/j.1365-2958.1992.tb01345.x. [DOI] [PubMed] [Google Scholar]
- 4.Belland R J, Morrison S G, van der Ley P, Swanson J. Expression and phase variation of gonococcal P.II genes in Escherichia coli involves ribosomal frameshifting and slipped-strand mispairing. Mol Microbiol. 1989;3:777–786. doi: 10.1111/j.1365-2958.1989.tb00226.x. [DOI] [PubMed] [Google Scholar]
- 5.Bessen D, Gotschlich E C. Interactions of gonococci with HeLa cells: attachment, detachment, replication, penetration, and role of protein II. Infect Immun. 1986;54:154–160. doi: 10.1128/iai.54.1.154-160.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bhat K S, Gibbs C P, Barrera O, Morrison S G, Jahnig F, Stern A, Kupsch E, Meyer T F, Swanson J. The opacity proteins of Neisseria gonorrhoeae strain MS11 are encoded by a family of 11 complete genes. Mol Microbiol. 1991;5:1889–1901. doi: 10.1111/j.1365-2958.1991.tb00813.x. [DOI] [PubMed] [Google Scholar]
- 7.Burch C L, Danaher R J, Stein D C. Antigenic variation in Neisseria gonorrhoeae: production of multiple lipooligosaccharides. J Bacteriol. 1997;179:982–986. doi: 10.1128/jb.179.3.982-986.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen J C R, Bavoil P, Clark V L. Enhancement of the invasive ability of Neisseria gonorrhoeae by contact with Hec1B, an adenocarcinoma endometrial cell line. Mol Microbiol. 1991;5:1531–1538. doi: 10.1111/j.1365-2958.1991.tb00800.x. [DOI] [PubMed] [Google Scholar]
- 9.Chen T, Belland R J, Wilson J, Swanson J. Adherence of pilus− Opa+ gonococci to epithelial cells in vitro involves heparan sulfate. J Exp Med. 1995;182:511–517. doi: 10.1084/jem.182.2.511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen T, Gotschlich E C. CGM1a antigen of neutrophils, a receptor of gonococcal opacity proteins. Proc Natl Acad Sci USA. 1996;93:14851–14856. doi: 10.1073/pnas.93.25.14851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Coyne M J, Jr, Russell K S, Coyle C L, Goldberg J B. The Pseudomonas aeruginosa algC gene encodes phosphoglucomutase, required for the synthesis of a complete lipopolysaccharide core. J Bacteriol. 1997;176:3500–3507. doi: 10.1128/jb.176.12.3500-3507.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Danaher R J, Levin J C, Arking D, Burch C L, Sandlin R C, Stein D C. Genetic basis of Neisseria gonorrhoeae lipooligosaccharide antigenic variation. J Bacteriol. 1995;177:7275–7279. doi: 10.1128/jb.177.24.7275-7279.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Drazek E S, Stein D C, Deal C D. A mutation in the Neisseria gonorrhoeae rfaD homolog results in altered lipooligosaccharide expression. J Bacteriol. 1995;177:2321–2327. doi: 10.1128/jb.177.9.2321-2327.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Elkins C, Rest R F. Monoclonal antibodies to outer membrane protein PII block interactions of Neisseria gonorrhoeae with human neutrophils. Infect Immun. 1990;58:1078–1084. doi: 10.1128/iai.58.4.1078-1084.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Elsinghorst E A. Measurement of invasion by gentamicin resistance. Methods Enzymol. 1994;236:405–420. doi: 10.1016/0076-6879(94)36030-8. [DOI] [PubMed] [Google Scholar]
- 16.Erwin A L, Haynes P A, Rice P A, Gotschlich E C. Conservation of the lipopolysaccharide synthesis locus lgt among strains of Neisseria gonorrhoeae: requirement for lgtE in synthesis of the 2C7 epitope and of the β chain of strain 15253. J Exp Med. 1996;184:1233–1241. doi: 10.1084/jem.184.4.1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gibbs C P, Reimann B, Schultz E, Kaufmann A, Haas R, Meyer T F. Reassortment of pilin genes in Neisseria gonorrhoeae occurs by two distinct mechanisms. Nature. 1989;338:651–652. doi: 10.1038/338651a0. [DOI] [PubMed] [Google Scholar]
- 18.Gibson B W, Melaugh W, Phillips N J, Apicella M A, Campagnari A A, Griffiss J M. Investigation of the structural heterogeneity of lipooligosaccharides from pathogenic Haemophilus and Neisseria species and of R-type lipopolysaccharides from Salmonella typhimurium by electrospray mass spectrometry. J Bacteriol. 1993;175:2702–2712. doi: 10.1128/jb.175.9.2702-2712.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gotschlich E C. Genetic locus for the biosynthesis of the variable portion of Neisseria gonorrhoeae lipooligosaccharide. J Exp Med. 1994;180:2181–2190. doi: 10.1084/jem.180.6.2181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Grant C C R, Bos M P, Belland R J. Proteoglycan receptor binding by Neisseria gonorrhoeae MS11 is determined by the HV-1 region of OpaA. Mol Microbiol. 1999;32:233–242. doi: 10.1046/j.1365-2958.1999.01293.x. [DOI] [PubMed] [Google Scholar]
- 21.Grassmé H U C, Ireland R M, van Putten J P M. Gonococcal opacity protein promotes bacterial entry-associated rearrangements of the epithelial cell actin cytoskeleton. Infect Immun. 1996;64:1621–1630. doi: 10.1128/iai.64.5.1621-1630.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gregg C R, Melly M A, Hellerqvist C G, Coniglio J G, McGee Z A. Toxic activity of purified lipopolysaccharide of Neisseria gonorrhoeae for human fallopian tube mucosa. J Infect Dis. 1981;143:432–438. doi: 10.1093/infdis/143.3.432. [DOI] [PubMed] [Google Scholar]
- 23.Ilver D, Källström H, Normark S, Jonsson A B. Transcellular passage of Neisseria gonorrhoeae involves pilus phase variation. Infect Immun. 1998;66:469–473. doi: 10.1128/iai.66.2.469-473.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jennings M P, Hood D W, Peak I R A, Virji M, Moxon E R. Molecular analysis of a locus for the biosynthesis and phase-variable expression of the lacto-N-neotetraose terminal lipopolysaccharide structure in Neisseria meningitidis. Mol Microbiol. 1995;18:729–740. doi: 10.1111/j.1365-2958.1995.mmi_18040729.x. [DOI] [PubMed] [Google Scholar]
- 25.John C M, Schneider H, Griffiss J M. Neisseria gonorrhoeae that infect men have lippooligosaccharides with terminal N-acetyllactosamine repeats. J Biol Chem. 1999;274:1017–1025. doi: 10.1074/jbc.274.2.1017. [DOI] [PubMed] [Google Scholar]
- 26.Johnson A P, Taylor-Robinson D, McGee Z A. Species specificity of attachment and damage to oviduct mucosa by Neisseria gonorrhoeae. Infect Immun. 1977;18:833–839. doi: 10.1128/iai.18.3.833-839.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jonsson A B, Nyberg G, Normark S. Phase variation of gonococcal pili by frameshift mutation in pilC, a novel gene for pilus assembly. EMBO J. 1991;10:477–488. doi: 10.1002/j.1460-2075.1991.tb07970.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kahler C M, Carlson R W, Rahman M M, Martin L E, Stephens D S. Inner core biosynthesis of lipooligosaccharide (LOS) in Neisseria meningitidis serogroup B: identification and role in LOS assembly of the alpha 1,2 N-acetylglucosamine transferase (RfaK) J Bacteriol. 1996;178:1265–1273. doi: 10.1128/jb.178.5.1265-1273.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kahler C M, Carlson R W, Rahman M M, Martin L E, Stephens D S. Two glycosyltransferase genes, lgtF and rfaK, constitute the lipooligosaccharide ice (inner core extension) biosynthesis operon of Neisseria meningitidis. J Bacteriol. 1996;178:6677–6684. doi: 10.1128/jb.178.23.6677-6684.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kallstrom H, Liszewski M K, Atkinson J P, Jonsson A B. Membrane cofactor protein (MCP or CD46) is a cellular pilus receptor for pathogenic Neisseria. Mol Microbiol. 1997;25:639–647. doi: 10.1046/j.1365-2958.1997.4841857.x. [DOI] [PubMed] [Google Scholar]
- 31.Kerwood D E, Schneider H, Yamasaki R. Structural analysis of lipooligosaccharide produced by Neisseria gonorrhoeae, strain MS11mk (variant A): a precursor for a gonococcal lipooligosaccharide associated with virulence. Biochemistry. 1992;31:12760–12768. doi: 10.1021/bi00166a008. [DOI] [PubMed] [Google Scholar]
- 32.Kupsch E, Knepper B, Kuroki T, Heuer I, Meyer T F. Variable opacity (Opa) outer membrane proteins account for the cell tropisms displayed by Neisseria gonorrhoeae for human leukocytes and epithelial cells. EMBO J. 1993;12:641–650. doi: 10.1002/j.1460-2075.1993.tb05697.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Laemmli U K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 34.Lesse A J, Campagnari A A, Bittner W E, Apicella M A. Increased resolution of lipopolysaccharides and lipooligosaccharides utilizing tricine-sodium dodecyl sulfate-polyacrylamide gel electrophoresis, J. Immunol Methods. 1990;126:109–117. doi: 10.1016/0022-1759(90)90018-q. [DOI] [PubMed] [Google Scholar]
- 35.Makino S, van Putten J P M, Meyer T F. Phase variation of the opacity outer membrane protein controls invasion by Neisseria gonorrhoeae into human epithelial cells. EMBO J. 1991;10:1307–1315. doi: 10.1002/j.1460-2075.1991.tb07649.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mandrell R E, Apicella M A. Lipo-oligosaccharides (LOS) of mucosal pathogens: molecular mimicry and host-modification of LOS. Immunobiology. 1993;187:382–402. doi: 10.1016/S0171-2985(11)80352-9. [DOI] [PubMed] [Google Scholar]
- 37.Mayer L W. Rates of in vitro changes of gonococcal colony opacity phenotypes. Infect Immun. 1982;37:481–485. doi: 10.1128/iai.37.2.481-485.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.McGee Z A, Gorby G L, Wyrick P B, Hodinka R, Hoffman L H. Parasite-directed endocytosis. Rev Infect Dis. 1988;10(Suppl. 2):S311–S316. doi: 10.1093/cid/10.supplement_2.s311. [DOI] [PubMed] [Google Scholar]
- 39.McGee Z A, Johnson A P, Taylor-Robinson D. Pathogenic mechanisms of Neisseria gonorrhoeae: observations on damage to human fallopian tubes in organ culture by gonococci of colony type 1 or type 4. J Infect Dis. 1981;143:413–422. doi: 10.1093/infdis/143.3.413. [DOI] [PubMed] [Google Scholar]
- 40.McGee Z A, Melly M A, Gregg C R, Horn R G, Taylor-Robinson D, Johnson A P, McCutchan J A. Virulence factors of gonococci: studies using human fallopian tube organ cultures. In: Brooks G F, Gotschlich E C, Holmes K K, Sawyer W D, Young F E, editors. Immunobiology of Neisseria gonorrhoeae. Washington, D.C.: American Society for Microbiology; 1978. pp. 258–262. [Google Scholar]
- 41.Melly M A, Gregg C R, McGee Z A. Studies of toxicity of Neisseria gonorrhoeae for human fallopian tube mucosa. J Infect Dis. 1981;143:423–431. doi: 10.1093/infdis/143.3.423. [DOI] [PubMed] [Google Scholar]
- 42.Monod M, Denoya C, Dubnau D. Sequence and properties of pIM13, a macrolide-lincosamide-streptogramin B resistance plasmid from Bacillus subtilis. J Bacteriol. 1986;167:138–147. doi: 10.1128/jb.167.1.138-147.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mosleh I M, Boxberger H J, Sessler M J, Meyer T F. Experimental infection of native human ureteral tissue with Neisseria gonorrhoeae: adhesion, invasion, intracellular fate, exocytosis, and passage through a stratified epithelium. Infect Immun. 1997;65:3391–3398. doi: 10.1128/iai.65.8.3391-3398.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Murphy G L, Connell T D, Barritt D S, Koomey M, Cannon J G. Phase variation of gonococcal protein II: regulation of gene expression by slipped-strand mispairing of a repetitive DNA sequence. Cell. 1989;56:539–547. doi: 10.1016/0092-8674(89)90577-1. [DOI] [PubMed] [Google Scholar]
- 45.Parker C T, Kloser A W, Schnaitman C A, Stein M A, Gottesman S, Gibson B W. Role of the rfaG and rfaP genes in determining the lipopolysacharide core structure and cell surface properties of Escherichia coli K-12. J Bacteriol. 1992;174:2525–2538. doi: 10.1128/jb.174.8.2525-2538.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Petricoin E F, III, Danaher R J, Stein D C. Analysis of the lsi region involved in lipooligosaccharide biosynthesis in Neisseria gonorrhoeae. J Bacteriol. 1991;173:7896–7902. doi: 10.1128/jb.173.24.7896-7902.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Petricoin E F, III, Stein D C. Molecular analysis of lipooligosaccharide biosynthesis in Neisseria gonorrhoeae. Infect Immun. 1989;57:2847–2852. doi: 10.1128/iai.57.9.2847-2852.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pier G B, Grout M, Zaidi T S. Cystic fibrosis transmembrane conductance regulator is an epithelial cell receptor for clearance of Pseudomonas aeruginosa from the lung. Proc Natl Acad Sci USA. 1997;94:12088–12093. doi: 10.1073/pnas.94.22.12088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pier G B, Grout M, Zaidi T S, Olsen J C, Johnson L G, Yankaskas J R, Goldberg J B. Role of mutant CFTR in hypersusceptibility of cystic fibrosis patients to lung infections. S. 1996;271:64–67. doi: 10.1126/science.271.5245.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Porat N, Apicella M A, Blake M S. A lipooligosaccharide-binding site on HepG2 cells similar to the gonococcal opacity-associated surface protein Opa. Infect Immun. 1995;63:2164–2172. doi: 10.1128/iai.63.6.2164-2172.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Raetz C R. Bacterial lipopolysaccharides: a remarkable family of bioactive macroamphiphiles. In: Neidhardt F C, Curtiss R I, Ingraham J L, Lin E C C, Low K B, editors. Escherichia coli and Salmonella:cellular and molecular biology. Washington, D.C.: ASM Press; 1999. pp. 1035–1063. [Google Scholar]
- 52.Richardson W P, Sadoff J C. Induced engulfment of Neisseria gonorrhoeae by tissue culture cells. Infect Immun. 1988;56:2512–2514. doi: 10.1128/iai.56.9.2512-2514.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Robertson B D, Frosch M, van Putten J P M. The role of galE in the biosynthesis and function of gonococcal lipopolysaccharide. Mol Microbiol. 1993;8:891–901. doi: 10.1111/j.1365-2958.1993.tb01635.x. [DOI] [PubMed] [Google Scholar]
- 54.Rowe P S N, Meadow P M. Structure of the core oligosaccharide from the lipopolysaccharide of Pseudomonas aeruginosa PAC1R and its defective mutants. Eur J Biochem. 1983;132:329–337. doi: 10.1111/j.1432-1033.1983.tb07366.x. [DOI] [PubMed] [Google Scholar]
- 55.Rudel T, Boxberger H, Meyer T F. Pilus biogenesis and epithelial cell adherence of Neisseria gonorrhoeae pilC double knock-out mutants. Mol Microbiol. 1995;17:1057–1071. doi: 10.1111/j.1365-2958.1995.mmi_17061057.x. [DOI] [PubMed] [Google Scholar]
- 56.Rudel T, van Putten J P M, Gibbs C P, Haas R, Meyer T F. Interaction of two variable proteins (PilE and PilC) required for pilus-mediated adherence of Neisseria gonorrhoeae to human epithelial cells. Mol Microbiol. 1992;6:3439–3450. doi: 10.1111/j.1365-2958.1992.tb02211.x. [DOI] [PubMed] [Google Scholar]
- 57.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. pp. 15.1–15.113. [Google Scholar]
- 58.Schnaitman C A, Klena J D. Genetics of lipopolysaccharide biosynthesis in enteric bacteria. Microbiol Rev. 1993;57:655–682. doi: 10.1128/mr.57.3.655-682.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Schneider H, Cross A S, Kuschner R A, Taylor D N, Sadoff J C, Boslego J W, Deal C D. Experimental human gonococcal urethritis: 250 Neisseria gonorrhoeae MS11mkC are infective. J Infect Dis. 1995;172:180–185. doi: 10.1093/infdis/172.1.180. [DOI] [PubMed] [Google Scholar]
- 60.Schneider H, Griffiss J M, Boslego J W, Hitchcock P J, Zahos K M, Apicella M A. Expression of paragloboside-like lipooligosaccharides may be a necessary component of gonococcal pathogenesis in men. J Exp Med. 1991;174:1601–1605. doi: 10.1084/jem.174.6.1601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Schneider H, Hammack C A, Apicella M A, Griffiss J M. Instability of expression of lipooligosaccharides and their epitopes in Neisseria gonorrhoeae. Infect Immun. 1988;56:942–946. doi: 10.1128/iai.56.4.942-946.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Schwan E T, Robertson B D, Brade H, van Putten J P M. Gonococcal rfaF mutants express Rd2 chemotype LPS and do not enter epithelial host cells. Mol Microbiol. 1995;15:267–275. doi: 10.1111/j.1365-2958.1995.tb02241.x. [DOI] [PubMed] [Google Scholar]
- 63.Seifert H S, Wright C J, Jerse A E, Cohen M S, Cannon J G. Multiple gonococcal pilin antigenic variants are produced during experimental human infections. J Clin Investig. 1994;93:2744–2749. doi: 10.1172/JCI117290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Shaw J H, Falkow S. Model for invasion of human tissue culture cells by Neisseria gonorrhoeae. Infect Immun. 1988;56:1625–1632. doi: 10.1128/iai.56.6.1625-1632.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Simon D, Rest R F. Escherichia coli expressing a Neisseria gonorrhoeae opacity-associated outer membrane protein invade human cervical and endometrial epithelial cell lines. Proc Natl Acad Sci USA. 1992;89:5512–5516. doi: 10.1073/pnas.89.12.5512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Song W, Ma L, Chen R, Stein D C. Role of lipooligosaccharide in Opa-independent invasion of Neisseria gonorrhoeae into human epithelial cells. J Exp Med. 2000;191:949–960. doi: 10.1084/jem.191.6.949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Steeghs L, den Hartog R, den Boer A, Zomer B, Roholl P, van der Ley P. Meningitis bacterium is viable without endotoxin. Nature. 1998;392:449–450. doi: 10.1038/33046. [DOI] [PubMed] [Google Scholar]
- 68.Stern A, Brown M, Nickel P, Meyer T F. Opacity genes in Neisseria gonorrhoeae: control of phase and antigenic variation. Cell. 1986;47:61–71. doi: 10.1016/0092-8674(86)90366-1. [DOI] [PubMed] [Google Scholar]
- 69.Swanson J. Studies on gonococcus infection. IV. Pili: their role in attachment of gonococci to tissue culture cells. J Exp Med. 1973;137:571–589. doi: 10.1084/jem.137.3.571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Swanson J. Studies on gonococcus infection. XII. Colony color and opacity variants of gonococci. Infect Immun. 1978;19:320–331. doi: 10.1128/iai.19.1.320-331.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Swanson J, Hill S A, Fischer S H. Growth on different solid media markedly affects the properties and behaviors of Opa+ gonococci. In: Conde-Glez C J, Morse S A, Rice P A, Sparling P F, Calderón E, editors. Pathobiology and immunology of Neisseriaceae. Cuernavaca, Mexico: Instituto Nacional de Salud Pública; 1994. pp. 771–776. [Google Scholar]
- 72.Swanson J, Kraus S J, Gotschlich E C. Studies on gonococcus infection. I. Pili and zones of adhesion: their relation to gonococcal growth patterns, J. Exp Med. 1971;134:886–906. doi: 10.1084/jem.134.4.886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Swanson J, Robbins K, Barrera O, Corwin D, Boslego J W, Ciak J, Blake M S, Koomey M. Gonococcal pilin variants in experimental gonorrhea. J Exp Med. 1987;165:1344–1357. doi: 10.1084/jem.165.5.1344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Towbin H, Staehlin T, Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci USA. 1979;76:4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.van Dyck E, Meheus A Z, Piot P. Laboratory diagnosis of sexually transmitted diseases. Geneva, Switzerland: World Health Organization; 1999. Gonorrhoea; pp. 1–21. [Google Scholar]
- 76.van Putten J P M, Hayes S F, Duensing T D. Natural proteoglycan receptor analogs determine the dynamics of Opa adhesin-mediated gonococcal infection of Chang epithelial cells. Infect Immun. 1997;65:5028–5034. doi: 10.1128/iai.65.12.5028-5034.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.van Putten J P M, Paul S M. Binding of syndecan-like cell surface proteoglycan receptors is required for Neisseria gonorrhoeae entry into human mucosal cells. EMBO J. 1995;14:2144–2154. doi: 10.1002/j.1460-2075.1995.tb07208.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Virji M, Kayhty H, Ferguson D J P, Alexandrescu C, Heckels J E, Moxon E R. The role of pili in the interactions of pathogenic Neisseria with cultured human endothelial cells. Mol Microbiol. 1991;5:1831–1841. doi: 10.1111/j.1365-2958.1991.tb00807.x. [DOI] [PubMed] [Google Scholar]
- 79.Wakarchuk W W, Martin A, Jennings M P, Moxon E R, Richards J C. Functional relationships of the genetic locus encoding the glycosyltransferase enzymes involved in expression of the lacto-N-neotetraose terminal lipopolysaccharide structure in Neisseria meningitidis. J Biol Chem. 1996;271:19166–19173. doi: 10.1074/jbc.271.32.19166. [DOI] [PubMed] [Google Scholar]
- 80.Ward M E, Watt P J, Robertson J N. The human fallopian tube: a laboratory model for gonococcal infection. J Infect Dis. 1974;129:650–659. doi: 10.1093/infdis/129.6.650. [DOI] [PubMed] [Google Scholar]
- 81.Weel J F L, Hopman C T P, van Putten J P M. Stable expression of lipooligosaccharide antigens during attachment, internalization, and intracellular processing of Neisseria gonorrhoeae in infected epithelial cells. Infect Immun. 1989;57:3395–3402. doi: 10.1128/iai.57.11.3395-3402.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Weisfuse I B. Gonorrhoea control and antimicrobial resistance. Lancet. 1998;351:928–928. doi: 10.1016/S0140-6736(05)60601-0. [DOI] [PubMed] [Google Scholar]
- 83.Wittkowski K M. Friedman-type statistics and consistent multiple comparisons for unbalanced designs with missing data. J Am Stat Assoc. 1988;83:1163–1169. [Google Scholar]
- 84.Wittkowski K M. An extension to Wittkowski. J Am Stat Assoc. 1992;87:258. [Google Scholar]
- 85.Yang Q-L, Gotschlich E C. Variation of gonococcal lipooligosaccharide structure is due to alterations in poly-G tracts in lgt genes encoding glycosyl transferases. J Exp Med. 1996;183:323–327. doi: 10.1084/jem.183.1.323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Zoller M J, Smith M. Oligonucleotide-directed mutagenesis: a simple method using two oligonucleotide primers and a single-stranded DNA template. Methods Enzymol. 1987;154:329–350. doi: 10.1016/0076-6879(87)54083-6. [DOI] [PubMed] [Google Scholar]