Abstract
(1) Background: Practice guidelines define drug-eluting stents (DES) as the standard of care in coronary percutaneous coronary intervention (PCI), including in acute coronary syndrome (ACS). This is based on comparisons with bare-metal stents (BMS). However, non-drug-eluting titanium-nitride-oxide-coated stents (TiNOS) have not been taken into account. The objective of this study is to determine whether TiNOS can be used as an alternative to DES in ACS. (2) Methods: A prospective systematic literature review (SLR), conducted according to the PRISMA guidelines, was performed, wherein multiple literature databases from 2018 and 2022 were searched. Prospective, randomised, controlled trials comparing outcomes after PCI with TiNOS vs. DES in any coronary artery disease (CAD) were searched. Clinical outcomes were meta-analytic pooled risk ratios (RR) of device-oriented Major Adverse Cardiac Events (MACE) and their components. The analysis stratified outcomes reported with ACS-only vs. ACS jointly with chronic coronary syndrome (CCS). (3) Results: Five RCTs were eligible, comprising 1855 patients with TiNOS vs. 1363 with DES at a 1-year follow-up. Three enrolled patients presented with ACS only and two with ACS or CCS. The latter accounted for most of the patients. The one-year pooled RRs in those three RCTs were as follows: MACE 0.93 [0.72, 1.20], recurrent myocardial infarction (MI) 0.48 [0.31, 0.73], cardiac death (CD) 0.66 [0.33, 1.31], clinically driven target lesion revascularization (TLR) 1.55 [1.10, 2.19], and stent thrombosis (ST) 0.35 [0.20, 0.64]. Those results were robust to a sensitivity analysis. The evidence certainty was high in MACE and moderate or low in the other endpoints. (4) Conclusions: TiNOS are a non-inferior and safe alternative to DES in patients with ACS.
Keywords: meta-analysis, acute coronary syndrome, percutaneous coronary intervention, drug-eluting stents, non-drug-eluting titanium-nitride-oxide coated stents (TiNOS), Major Adverse Cardiac Events (MACE), recurrent myocardial infarction, stent thrombosis
1. Introduction
Coronary Artery Disease (CAD) is classified into two broad groups: Acute Coronary Syndrome (ACS) and chronic coronary syndromes (CCS) [1]. CCS have a variety of presentations but are without acute symptoms.
Patients with ACS have ongoing acute myocardial ischemia that can cause various symptoms ranging from cardiac arrest to electrical or haemodynamic instability and cardiac mechanical disorders. The leading ACS symptom is chest discomfort or pain. ACS with acute chest pain and persistent (>20 min) ST-segment elevation on an electrocardiogram (ECG) often reflects an acute total or subtotal coronary occlusion. Most patients with this type of ACS develop ST-segment elevation myocardial infarction (STEMI) [2]. Patients with acute chest discomfort and no persistent ST-segment elevation (NSTE-ACS) often develop non-ST-segment elevation myocardial infarction (NSTEMI), but some of them do not develop myocardial damage and are classified as having unstable angina (UA). The published evidence has established percutaneous coronary interventions (PCI) with drug-eluting stents (DES) as the standard of care in CAD, including the different presentations of ACS [2,3,4,5,6,7,8,9,10]. The purpose of using DES is to mitigate post-stenting restenosis [11,12,13]. Titanium-nitride-oxide-coated coronary stents (TiNOS) have a pharmacologically inactive, non-absorbable coating. Preclinical studies have shown less neointimal hyperplasia with TiNOS than with bare-metal stents (BMS) [14,15,16]. Several randomised, controlled clinical trials (RCTs) have compared clinical outcomes of TiNOS vs. DES in patients with ACS. Pooling the results to summarise them and enable an overall interpretation is now required. This study is the first systematic literature review (SLR) on this topic. Its objective is to determine if TiNOS can be used as an alternative to DES in ACS.
2. Materials and Methods
2.1. Foundations
This SLR was designed and conducted according to methods described in the Cochrane Handbook with the use of “Grading of Recommendations Assessment, Development and Evaluation” (GRADE) [17,18,19,20,21,22]. The protocol was registered in PROSPERO (CRD4201809062) before initiation. It is reported according to “Preferred Reporting Items for Systematic Reviews and Meta-Analyses” (PRISMA) [23].
2.2. Research Question Specification
The research question was specified using the PICOS framework and Academic Research Consortium (ARC-2) definitions [24,25]. Patients presented ACS. The intervention was PCI using TiNOS. The comparator was PCI using any DES. Outcomes constituted the device-oriented Major Adverse Cardiac Events (MACE), a composite of three items: Cardiac death (CD), recurrent myocardial infarction (MI), and clinically driven target lesion revascularisation (TLR). CD or MI was summarised jointly to reflect the safety component of MACE with a single indicator. In addition, Probable or definite ST was analysed as a stent-related serious adverse event (SAE) that may result in CD or MI. All-cause mortality (TD) was also analysed. All measures of outcome were assessed at one- and five-year follow-ups. The study methods were parallel-arm prospective RCTs.
2.3. Data Sources
Pubmed, Embase, the Cochrane Library, and Web of Science (WoS) electronic databases were queried on 8 March 2018 and 27 August 2022. The search terms were: ((bioactive OR (Titanium AND nitride AND oxide) OR TiNO OR TNO OR BAS) AND stent) AND (DES OR (drug AND eluting AND stent)) AND (RCT OR ((randomised OR randomised) AND controlled AND trial)). No exclusion filter was applied related to language, country, year, or any other aspect. The search string as interpreted by the databases’ search engines is reported in Appendix A. The websites of AHA, TCT, ESC, EuroPCR, and clinicaltrials.gov were also searched for unpublished studies meeting the question’s specifications.
The downloaded record files were imported, pooled, and sifted in EndNote X8 (Clarivate Analytics, Philadelphia, PA, USA). One reference only was selected when duplicates were identified. When different references concerned the same study, their information was pooled using the citation of the most recent one. Full articles were reviewed for all non-duplicate references.
2.4. Study Selection, Risk of Bias Analysis, Data Extraction, Certainty of Evidence Grading
Two reviewers (FD and LL) independently performed the following steps: (1) exhaustive reference screening, (2) reference classification according to the inclusion and exclusion criteria, (3) extraction of study methods, (4) of patient baseline data, (5) of treatment data, and results of each eligible RCT, (6) individual RCT risk of bias rating, and (7) assessment of the certainty of the evidence for each outcome variable according to GRADE [19,20,21,22]. The screened studies were included if they met the following criteria: provided first-hand clinical evidence with prospective inclusion; included patients with CAD treated with coronary PCI; involved implantation of either TiNOS or DES after the random allocation of the stent type; provided target outcomes reported at 1-year and/or 5-year follow-up; provided the outcomes reported as the number of patients who were included along with the number or proportion of them who presented an event of interest.
RCTs were included if IRB/ethics committee’s approval and patients’ informed consent were confirmed, the evidence was first-hand with prospective inclusion, patients were treated for CAD treated with coronary PCI, stents were either TiNOS or DES after the random allocation, target outcomes were reported at 1-year and/or 5-year follow-up as the number of patients included, and events were reported with the number or proportion of included patients. ACS refers to patients presenting ST-elevated myocardial infarction (STEMI), non-ST-elevated myocardial infarction (NSTEMI), or unstable angina pectoris at baseline as defined in ARC-2 [25]. Data extraction was stratified according to patients’ clinical presentation, i.e., ACS vs. CCS, whenever feasible. Differences were adjudicated by a third reviewer (NM). One reviewer (PK) adjudicated multiple endpoint definitions across the RCTs. The results were recorded in Review Manager software (RevMan version 5.4.1, The Nordic Cochrane Centre, Copenhagen, Denmark), and the risk of bias of individual RCTs was rated according to the criteria proposed by the Cochrane Collaboration and was implemented via that software package with operator’s blinding as an additional separate item [26].
2.5. Meta-Analysis
The two treatment arms were compared concerning each endpoint using the risk ratio (RR) defined as ((n patients with an event in TiNOS)/(n patients in TiNOS))/((n patients with an event in DES)/(n patients in DES)). RR > 1 reflected a higher frequency of events in the TiNOS arm than in the DES arm and vice versa. Outcomes were analysed on an intention-to-treat (ITT) basis, with the number of patients presenting an event counted in the randomised arm as the numerator and the sample size of the corresponding arm as the denominator. Each RR was reported with its 95% confidence interval (CI []).
Publication bias was suspected if the funnel plot of study RRs was asymmetrical and/or if Harbord’s regression test for binary variables was significant (i.e., p < 0.05) [27,28,29,30]. The set of pooled study RRs was considered homogeneous if Cochran’s Q-test was not significant (given the χ² distribution’s degrees of freedom df = k − 1 where k is the number of study RRs) and if the I² was low to moderate (i.e., I² = (Q − df)/Q × 100% ≤ 25%) [27,28,30,31,32,33,34]. Due to clinical heterogeneity concerning clinical indications and stent generations, pooled RRs were calculated using the M-H method with a random-effects model.
Pooled analyses were stratified according to patient enrollment, i.e., RCTs with ACS-only vs. RCTs with ACS and CCS without separate outcome reporting. Each pooled measure of outcomes was presented in a Forest plot. One-year and five-year outcomes were analysed separately.
Sensitivity analysis was performed by iteratively recalculating the pooled RR after removing one eligible RCT.
The certainty of the pooled evidence was rated in GRADEpro GDT software 2022 online (https://gradepro.org) [35]. Additional analyses were performed in STATA (version 16, StataCorp LP, College Station, TX, USA) using the metan and metaprop packages.
3. Results
3.1. Study Identification and Selection
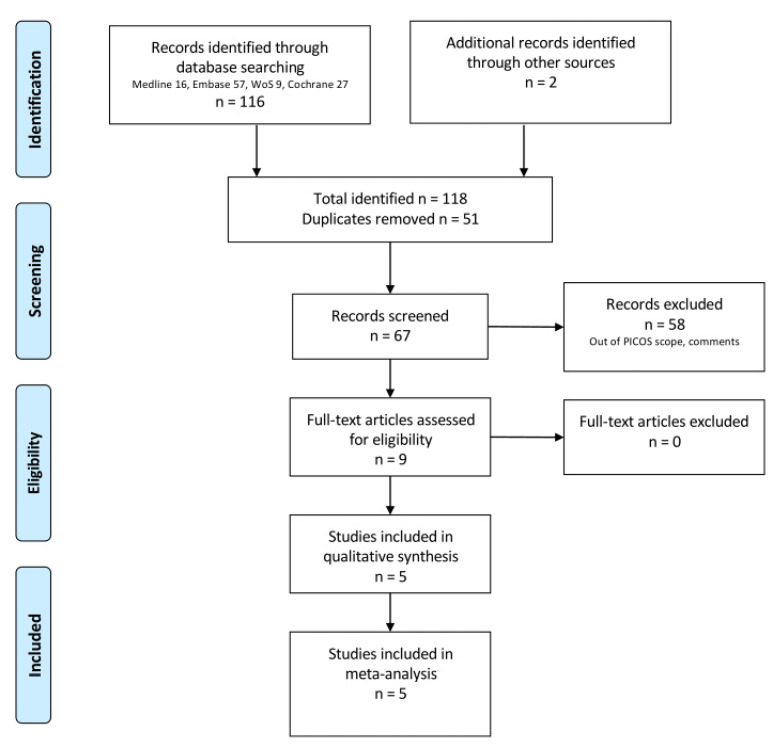
The studies’ identification, screening, and selection are described in the PRISMA flowchart (Figure 1). One hundred and eighteen references were identified and nine publications with first-hand data about five RCTs were eligible for inclusion in the meta-analysis.
Figure 1.
PRISMA flow chart.
3.2. Eligible Study Characteristics
Five RCTs were eligible, as their baseline characteristics complied with the PICOS specification (Table 1).
Table 1.
Eligible studies’ baseline characteristics.
| Study | Age and Prior Events | Clinical Presentation (Included and Pooled) | Procedural Data and Medication | Attrition, Cross-Overs | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Stent | TiNOS | DES | TiNOS N incl, % pooled |
DES N incl, % pooled |
TiNOS | DES | ||||
| TITAX-AMI DES = PES [36,37] |
Patients n age prior MI prior PCI prior CABG |
214 64 ± 11 15% 10% 7% |
211 64 ± 11 9% 5% 6% |
NSTEMI STEMI UA |
131, 61% 83, 39% 0, 0% |
114, 54% 97, 46% 0, 0% |
Stents/culprit lesion n TSL (mm) post-dilation procedural success DAPT 12 m |
1.1 ± 0.3 18.5 ± 6.4 42% 99.5% 31% |
1.1 ± 0.4 19.2 ± 7.2 35% 98.1% 65% |
LFU 1 y TiNOS: 0 DES: 0 5 y TiNOS 3 DES: 7 |
| TIDE DES = ZES [38,39] |
Patients n age prior MI prior PCI prior CABG |
152 65.9 ± 9.0 27.6% 25.7% 7.9% |
150 63.4 ± 10.5 28.7% 21.3% 25.3% 2.7% |
Stable angina NSTEMI UA |
57.9% 32.9% 9.2% |
47.3% 42.0% 10.7% |
Stents/culprit lesion n TSL (mm) device success DAPT 12 m |
1.28 ± 0.55 19.3 ± 11.1 93.0% |
1.17 ± 0.45 19.6 ± 10.0 94.6% |
LFU 1 y TiNOS: 0 DES: 2 5 y: N.R. |
| TITANIC-XV DES = EES [40] |
Patients n age prior MI prior PCI prior CABG |
83 66.5 ± 8.8 10.8% 8.4% 2.4% |
90 64.5 ± 10.1 15.6% 11.1% 2.2% |
NSTEMI other CAD Diabetics: all |
69.9% 30.1% |
60.0% 40.0% |
Stents/culprit lesion n TSL (mm) stent failure DAPT 12 m |
1.1 ± 0.3 18.72 ± 8.20 NR. |
1.1 ± 0.3 21.63 ± 9.65 NR. |
NR. |
| BASE-ACS DES = EES [41,42] |
Patients n age prior MI prior PCI prior CABG |
417 63 ± 12 13.4% 9.6% 4.8% |
410 63 ± 12 9.8% 10.5% 4.1% |
NSTEMI STEMI UA |
206, 49.4% 162, 38.8% 49, 11.8% |
187, 45.6% 159, 38.8% 64, 15.6% |
Stents/culprit lesion n TSL (mm) post-dilation stent failure DAPT: Aspirin: N.R. Clopidogrel: N.R. |
1.15 ± 0.38 20.8 ± 9.4 42.2% 0.0% |
1.14 ± 0.36 20.6 ± 8.2 43.9% 1.0% |
LFU 1 y TiNOS: 3 DES: 3 5 y TiNOS 29 DES: 28 |
| TIDES-ACS DES = EES [43,44] |
Patients n age prior MI prior PCI prior CABG |
989 62.7 ± 10.7.6% 7.0% 0.6% |
502 62.6 ± 10.5 9.0% 6.6% 1.2% |
NSTEMI STEMI |
46.3% 44.9% |
45.0% 47.6% |
Stents/culprit lesion n TSL (mm) post-dilation stent failure DAPT 12 m |
1.13 ± 0.38 20.5 ± 7.8 33.0% 0.3% 80.3% |
1.14 ± 0.37 20.6 ± 7.2 38.0% 1.0% 86.0% |
LFU 1 y TiNOS: 7 DES: 4 |
UA = unstable angina pectoris; Drugs: EES = everolimus elution; ZES = zotarolimus elution; PES = paclitaxel elution; 12 m = 12 months; 1 y = 1 year.
The studies reported 1-year follow-up data for 1855 patients in the TiNOS arm vs. 1363 in the DES arm. The quantities of available patients at 5-year follow-up were 783 vs. 773.
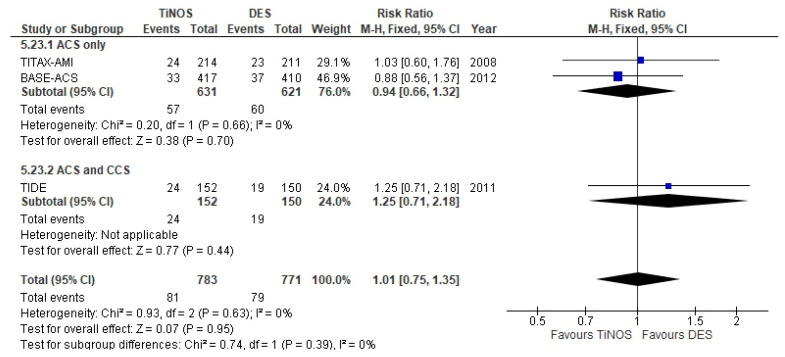
Three RCTs reported outcomes in patients with ACS only (TITAX-AMI, BASE-ACS, and TIDES-ACS). The two others reported outcomes in patients presenting ACS or CCS without stratification: TIDE enrolled 143 patients with ACS (47%) and TITANIC-XV enrolled 112 (64.7%).
3.3. Publication Bias
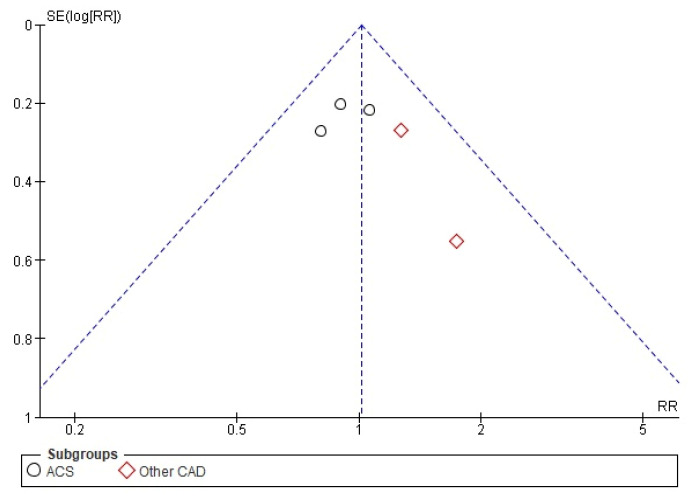
The funnel plot and the Harbord test (p = 0.263) did not detect a risk of publication bias regarding the RR of MACE in all CAD cases at 1-year follow-up (Figure 2).
Figure 2.
Publication bias of MACE RR in all RCTs at 1-year follow-up: funnel plot.
Similar conclusions were found for the other six endpoints at the 1-year follow-up in all CAD cases.
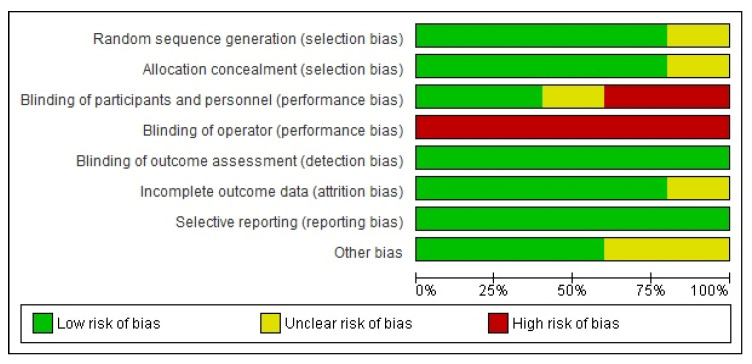
3.4. Individual Study Bias
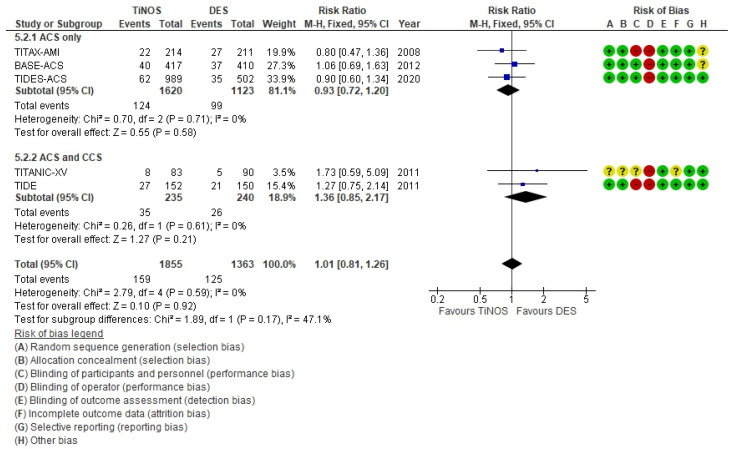
The compiled risk of bias across the studies (Figure 3) shows an overall risk of bias that is generally less than 75%, except for operator blinding. The individual RCT risk of bias is reported with the pooled 1-year MACE RR (Figure 4).
Figure 3.
Individual RCT risk of bias across studies.
Figure 4.
MACE—1 year.
There were some differences in the definitions of MACE and MI between studies, but they were applied similarly in both treatment arms.
3.5. Pooled Outcome Risk Ratios
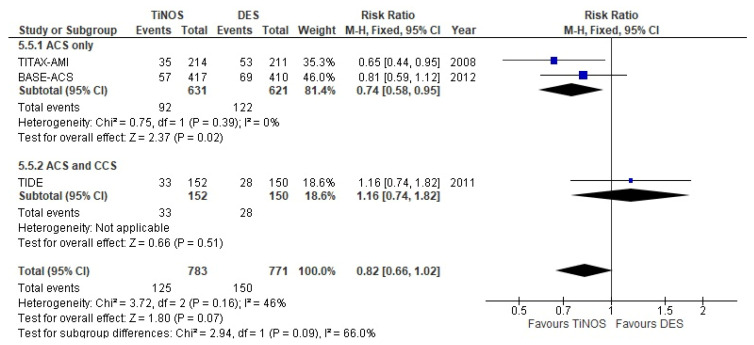
The stratified, pooled RRs of the primary endpoint, 1-year MACE (i.e., ACS-only vs. ACS and CCS, and total), show no significant risk difference between TiNOS and DES and low individual study risk bias except for operator blinding (Figure 4). The ACES only vs. ACS and CCS subgroups display no significant heterogeneity according to the Q-test (p = 0.17), which is in line with the overlapping CIs. The 47.1% I² between subgroups quantifies the visual difference.
The pooled 5-year MACE RR shows a significantly lower pooled RR favouring TiNOS in the ACS-only subgroup. Heterogeneity between the two strata is larger than at one 1-year (I² = 66%), but the confidence intervals overlap with a non-significant Q-test (p = 0.09) (Figure 5).
Figure 5.
MACE—5 years.
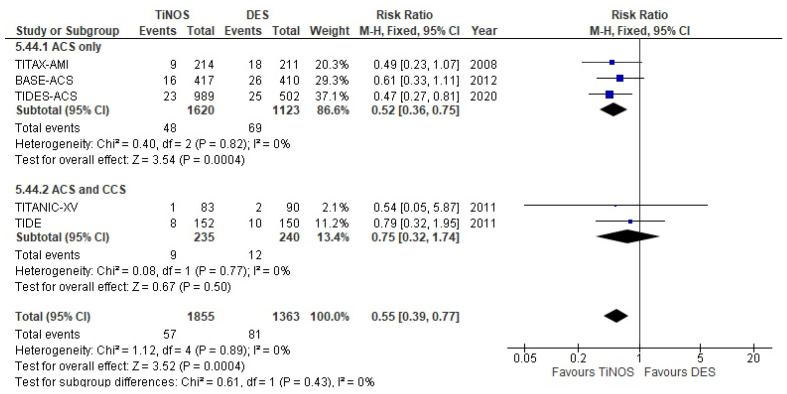
The pooled RRs of recurrent non-fatal MI or CD at 1-year and 5-year follow-ups are significant and favour TiNOS in the ACS-only subgroup (Figure 6 and Figure 7). The RRs are driven by the differences in the incidence of non-fatal MI.
Figure 6.
CD or MI—1 year.
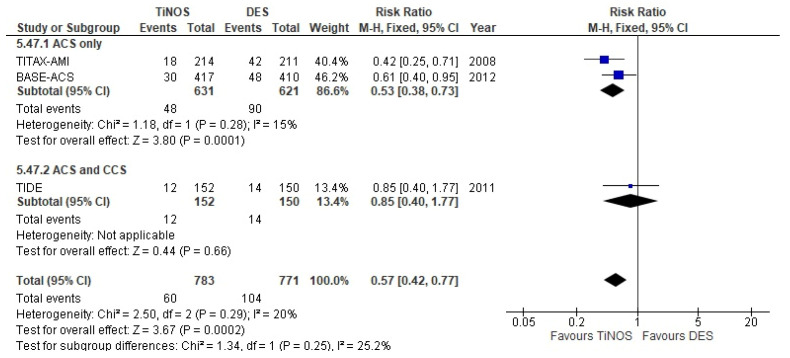
Figure 7.
CD or MI—5 years.
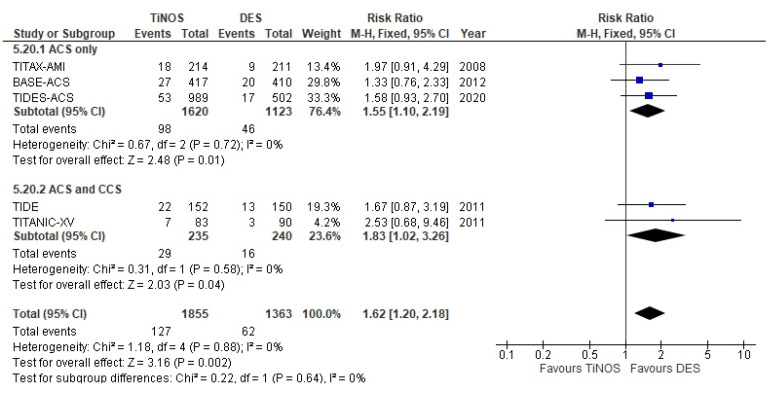
The pooled RR of TLR at a 1-year follow-up is significant, with more frequent TLRs with TiNOS than DES in ACS-only vs. ACS and CCS (Figure 8). However, the RR at 5-year follow-up reduces to non-significance as the rate of TLRs in the DES arm catches up with the rate in the TiNOS arm (Figure 9).
Figure 8.
TLR—1 year.
Figure 9.
TLR—5 years.
The pooled RRs of CD, non-fatal MI, probable or definite ST, TD, and definite ST are reported in the sensitivity analysis (Table 2). The 1-year and 5-year RRs of non-fatal MI and probable or definite ST are significant and favour TiNOS in the ACS-only subgroup.
Table 2.
Sensitivity analysis of primary and secondary endpoints.
| M-H Fixed Effects RR and 95% CI after the Removal of: | ||||||
|---|---|---|---|---|---|---|
| Endpoint | None | TITAX-AMI | TIDE | TITANIC-XV | BASE-ACS | TIDES-ACS |
| Total (all RCTs)—1-year follow-up | ||||||
| MACE | 1.01 [0.81, 1.26] | 1.06 [0.83, 1.36] | 0.96 [0.75, 1.23] | 0.98 [0.78, 1.24] | 0.99 [0.76, 1.29] | 1.07 [0.82, 1.40] |
| CD or MI | 0.55 [0.39, 0.77] | 0.57 [0.39, 0.82] | 0.52 [0.36, 0.74] | 0.55 [0.39, 0.77] | 0.53 [0.36, 0.79] | 0.60 [0.40, 0.91] |
| Non-fatal MI | 0.52 [0.36, 0.76] | 0.51 [0.33, 0.78] | 0.48 [0.31, 0.73] | 0.52 [0.35, 0.76] | 0.60 [0.38, 0.93] | 0.51 [0.32, 0.82] |
| CD | 0.66 [0.33, 1.31] | 0.77 [0.37, 1.61] | 0.66 [0.33, 1.31] | 0.66 [0.33, 1.31] | 0.30 [0.11, 0.81] | 1.11 [0.43, 2.85] |
| Clinically driven TLR | 1.62 [1.20, 2.18] | 1.56 [1.13, 2.15] | 1.60 [1.15, 2.24] | 1.58 [1.16, 2.14] | 1.74 [1.22, 2.47] | 1.63 [1.14, 2.33] |
| Probable or definite ST | 0.39 [0.22, 0.69] | 0.46 [0.25, 0.84] | 0.35 [0.20, 0.64] | 0.39 [0.22, 0.69] | 0.36 [0.18, 0.72] | 0.38 [0.16, 0.87] |
| Definite ST | 0.51 [0.26, 0.99] | 0.49 [0.25, 0.97] | 0.46 [0.23, 0.92] | 0.51 [0.26, 0.99] | 0.62 [0.29, 1.36] | 0.52 [0.18, 1.44] |
| TD | 0.78 [0.48, 1.27] | 0.77 [0.46, 1.32] | 0.78 [0.47, 1.27] | 0.78 [0.48, 1.27] | 0.49 [0.26, 0.95] b | 1.22 [0.65, 2.28] |
| ACS-only RCTs—1-year follow-up | ||||||
| MACE | 0.93 [0.72, 1.20] | 0.97 [0.73, 1.30] | NA. | NA. | 0.86 [0.63, 1.19] | 0.95 [0.68, 1.33] |
| CD or MI | 0.52 [0.36, 0.75] | 0.53 [0.35, 0.80] | NA. | NA. | 0.48 [0.30, 0.75] | 0.56 [0.35, 0.90] |
| Non-fatal MI | 0.48 [0.31, 0.73] | 0.45 [0.27, 0.74] | NA. | NA. | 0.55 [0.33, 0.92] | 0.44 [0.25, 0.77] |
| CD | 0.66 [0.33, 1.31] | 0.77 [0.37, 1.61] | NA. | NA. | 0.30 [0.11, 0.81] | 1.11 [0.43, 2.85] |
| Clinically driven TLR | 1.55 [1.10, 2.19] | 1.46 [0.99, 2.15] | NA. | NA. | 1.69 [1.09, 2.63] | 1.53 [0.97, 2.40] |
| Probable or definite ST | 0.35 [0.20, 0.64] | 0.42 [0.22, 0.78] | NA. | NA. | 0.32 [0.15, 0.65] | 0.31 [0.12, 0.77] |
| Definite ST | 0.46 [0.23, 0.92] | 0.43 [0.21, 0.89] | NA. | NA. | 0.54 [0.24, 1.24] | 0.39 [0.12, 1.25] |
| TD | 0.78 [0.47, 1.27] | 0.77 [0.45, 1.32] | NA. | NA. | 0.47 [0.24, 0.93] b | 1.23 [0.64, 2.35] |
| Total (all RCTs)—5-year follow-up—Interim Results | ||||||
| MACE | 0.82 [0.66, 1.02] | 0.91 [0.70, 1.19] | 0.74 [0.58, 0.95] b | NA. | 0.83 [0.62, 1.10] | Expected |
| CD or MI | 0.57 [0.42, 0.77] | 0.67 [0.46, 0.97] | 0.53 [0.38, 0.73] | NA. | 0.53 [0.35, 0.80] | Expected |
| Non-fatal MI | 0.56 [0.39, 0.80] | 0.59 [0.37, 0.92] | 0.54 [0.37, 0.80] | NA. | 0.57 [0.35, 0.93] | Expected |
| CD | 0.68 [0.39, 1.19] | 0.93 [0.47, 1.82] | 0.59 [0.31, 1.11] | NA. | 0.55 [0.25, 1.24] | Expected |
| Clinically driven TLR | 1.01 [0.75, 1.35] | 1.00 [0.71, 1.42] | 0.94 [0.66, 1.32] | NA. | 1.13 [0.77, 1.66] | Expected |
| Probable or definite ST | 0.30 [0.14, 0.61] | 0.45 [0.19, 1.05] | 0.25 [0.12, 0.55] | NA. | 0.22 [0.07, 0.70] | Expected |
| Definite ST | 0.25 [0.11, 0.55] | 0.37 [0.14, 0.99] | 0.20 [0.09, 0.49] | NA. | 0.22 [0.07, 0.70] | Expected |
| TD | 1.03 [0.74, 1.45] | 1.14 [0.75, 1.73] | 0.95 [0.65, 1.37] | NA. | 1.05 [0.65, 1.69] | Expected |
| ACS-only RCTs—5-year follow-up—Interim Results | ||||||
| MACE a | 0.74 [0.58, 0.95] | 0.81 [0.59, 1.12] c | NA. | NA. | 0.65 [0.44, 0.95] c | Expected |
| CD or MI | 0.53 [0.38, 0.73] | 0.61 [0.40, 0.95] | NA. | NA. | 0.42 [0.25, 0.71] | Expected |
| Non-fatal MI | 0.54 [0.37, 0.80] | 0.56 [0.33, 0.94] c | NA. | NA. | 0.53 [0.30, 0.94] c | Expected |
| CD | 0.59 [0.31, 1.11] | 0.83 [0.38, 1.84] c | NA. | NA. | 0.33 [0.11, 1.00] c | Expected |
| Clinically driven TLR | 0.94 [0.66, 1.32] | 0.88 [0.56, 1.37] c | NA. | NA. | 1.03 [0.60, 1.76] c | Expected |
| Probable or definite ST a | 0.25 [0.12, 0.55] | 0.37 [0.15, 0.93] c | NA. | NA. | 0.13 [0.03, 0.57] c | Expected |
| Definite ST a | 0.20 [0.09, 0.49] | 0.28 [0.09, 0.85] c | NA. | NA. | 0.13 [0.03, 0.57] c | Expected |
| TD | 0.95 [0.65, 1.37] | 1.02 [0.63, 1.65] c | NA. | NA. | 0.85 [0.48, 1.53] c | Expected |
a sensitivity analysis in ACS at 5-year follow-up results in the RR and confidence intervals of individual RCTs; b borderline shift with 3 or fewer RCTs contributing to the pooled estimate; c results based on a single-trial; NA—Not applicable.
The Forest plots of additional endpoints are in Supplementary Materials: Figure S1: Recurrent non-fatal MI—1 year; Figure S2: Recurrent non-fatal MI—5 years; Figure S3: CD—1 year; Figure S4: CD—5 years; Figure S5: Probable or definite ST—1 year; Figure S6: Probable or definite ST—5 years.
3.6. Sensitivity Analysis—Additional Endpoints
The sensitivity analysis (Table 2) in the ACS-only group at the 1-year follow-up shows:
-
-
The robustness of the non-significant MACE’s pooled RR;
-
-
The robustness of the significant recurrent non-fatal MI’s pooled RR with less frequent events in TiNOS than in DES;
-
-
The robustness of the significant probable or definite ST’s pooled RR with less frequent events in TiNOS than in DES;
-
-
The robustness of the significant TLR’s pooled RR with more frequent events in TiNOS than in DES;
-
-
The robustness of CD’s pooled RR varied depending on the excluded study.
The total pooled RRs at the 1-year follow-up yielded similar results to ACS-only.
The sensitivity analysis in the ACS-only group at the 5-year follow-up also yielded similar results to ACS at the 1-year follow-up; however, the TLR RR became non-significant.
Sensitivity analysis in the total pooled RRs at the 5-year follow-up yielded similar results to ACS at the 5-year follow-up except for probable or definite ST, which lost robustness upon TITAX-AMI’s removal. The RR of definite ST remained robust.
3.7. GRADE: Certainty of the Evidence
Given the potential bias caused by pooling ACS and CCS, and considering that the 5-year outcomes of TIDES-ACS were not published when writing this article, the GRADE analysis focused on the 1-year outcomes in the ACS-only group (Table 3).
Table 3.
GRADE Summary of findings—TiNOS vs. DES in ACS at 1-year follow-up.
| Outcome | Risk of Bias | Inconsistency | Indirectness | Imprecision | Publication Bias |
Overall Certainty of Evidence |
|---|---|---|---|---|---|---|
| Device-oriented MACE | not serious a | not serious b | not serious c | not serious d | none | ⨁⨁⨁⨁ HIGH |
| CD or MI | not serious a | not serious b | not serious e | serious f | none | ⨁⨁⨁◯ MODERATE |
| Clinically driven TLR | not serious a | not serious b | not serious g | very serious h | none | ⨁⨁◯◯ LOW |
| Non-fatal MI | not serious a | not serious b | not serious i | serious j | none | ⨁⨁⨁◯ MODERATE |
| CD | not serious a | very serious k | not serious l | very serious m | none | ⨁◯◯◯ VERY LOW |
| Probable or definite ST | not serious a | not serious b | not serious n | very serious o | none | ⨁⨁◯◯ LOW |
| TD | not serious a | serious k,p | not serious q | very serious r | none | ⨁◯◯◯ VERY LOW |
Explanations: GRADE Working Group grades of evidence: High certainty—very confident that the true effect lies close to that of the estimate. Moderate certainty—moderately confident in the effect estimate; the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty—confidence in the effect estimate is limited; the true effect may be substantially different from the estimate. Very low certainty—very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate.
The certainty level of the evidence of MACE, the primary outcome measure, is high. However, the certainty levels of the secondary outcome measures are moderate, low, or very low.
The detailed explanations supporting each GRADE item rating are reported in Appendix B.
4. Discussion
Practice guidelines establish the use of DES as the standard of care in PCI to treat ACS [2,9,10]. However, the 2017 Cochrane review comparing the outcomes with DES vs. BMS in ACS concluded that the “evidence in this review was of low to very low quality, and the true result may depart substantially from the results presented in this review” [45]. Therefore, the comparison of the clinical outcomes of TiNOS vs. DES in ACS is relevant to determining whether TiNOS could be an alternative to DES in that group of clinical indications.
The first hypothesis of this SLR is based on preclinical demonstrations that TiNOS are associated with less neointimal hyperplasia than BMS [14,15,16]. The second hypothesis is that patients presenting with ACS have a higher risk of SAE than patients with CCS. Given that some RCTs included both patients with ACS and CCS, the SLR analysed all RCTs that compared DES to TiNOS.
The pooled RRs of MACE, CD or recurrent non-fatal MI, clinically-driven TLR, ST, and TD at the 1-year follow-up, did not significantly differ between all-RCTs and ACS-only RCTs. The latter represented 85% of the patients, and the ACS-only pooled results drove the all-RCT results. Therefore, the internal and external validation of this meta-analysis focuses on the ACS-only subgroup.
All the results were robust to the sensitivity analysis, so no single trial significantly modified them. This confirmed that excluding any ACS-only trial due to the generation of the platform, the eluted drug (e.g., paclitaxel), or a specific patient risk factor (e.g., diabetes) had no impact on the pooled results.
The results at the 5-year follow-up were consistent with those at the 1-year follow-up, although probable ST presented reduced robustness while definite ST remained robust.
No risk of publication bias was identified, and the overall risk of bias in the individual RCTs was not serious except for non-blinding operators.
At the 1-year follow-up in the ACS-only trials, TiNOS and DES displayed a non-significantly different MACE rate, and the quality of evidence was high. TiNOS displayed a significantly lower rate of CD or recurrent MI than DES, and the quality of evidence was moderate due to lower precision caused by the limited number of events observed altogether. TiNOS and DES displayed a non-significantly different TLR. TiNOS displayed lower mortality and ST rates, and the quality of evidence was low or very low with fewer observed events.
Two previously published meta-analyses comparing DES vs. BMS in ACS were identified to attempt the external validation of the DES arm of this meta-analysis [45,46]. The 2017 Cochrane review included 25 RCTs, of which most focused on STEMI, with different time horizons and RRs that were reported at the maximum follow-up. Therefore, a comparison with the 1-year and 5-year outcomes of this meta-analysis was not interpretable. The 2022 individual patient data meta-analysis (IPDM) includes 14 RCTs with a total of 22,319 patients with 34.5% of the patients treated for CCS and 65.5% for ACS. The outcomes are reported at 1-year and 5-year follow-ups. The type of ACS reported in the source publications (Table 4) shows 36.2% STEMI, 33.6% NSTEMI, and 33.6% unstable angina compared with the 42% STEMI, 47% NSTEMI, and 34% unstable angina reported in the publications of the three ACS-only RCTs of this meta-analysis. The IPDM pooled data concerned 14,628 ACS cases with 7739 DES vs. 6889 BMS (details in the IPDM Supplemental File). The IPDM and this meta-analysis show relatively similar pooled rates of CD or MI in DES at the 1-year follow-up (ratio: 0.89) and 5-year follow-up (ratio: 1.35) (Table 5). This comparability supports the validity of that endpoint in this meta-analysis. However, the pooled incidence rate of the definite ST in the DES group of this meta-analysis is 3 times higher at the 1-year follow-up than in the IPDM and 5.48 times higher at the 5-year follow-up. These ratios could have resulted from differences in the methods, the patients’ baseline risk, the types of DES used, and the relatively small number of observations in this meta-analysis. Moreover, the IPDM does not report TLR or MACE rates, so IPDM does not provide an external validation basis for those three endpoints.
Table 4.
Outcome comparison of this meta-analysis with Piccolo et al. 2022 IPDM in ACS.
| Study | N Total | N (%) with ACS |
|---|---|---|
| SPIRIT I [47] | 56 | Total: 9 (16.1%) unstable angina: 9 |
| ENDEAVOR II [48] | 1197 | Total: 359 (30%) unstable angina: 359 |
| PAINT [49] | 491 | Total: 90 (18.3%) unstable angina: 90 |
| BASKET PROVE [50] | 2314 | Total: 1492 (64.5%) unstable angina: 754 STEMI: 738 |
| CORACTO [51] | 91 | not reported |
| ISAR-CABG [52] | 610 | Total: 239 (39.2%) unstable angina: 239 |
| PRODIGY [53] | 2003 | Total: 1465 (73.1%) unstable angina: 367 STEMI: 450 NSTEMI: 648 |
| INSPIRON [54] | 57 | Total: 13 (22.8%) unstable angina: 13 |
| XIMA [55] | 740 | not reported |
| BASKET PROVE II [56] | 2291 | Total: 1446 (63.1%) unstable angina: 787 STEMI: 659 |
| LEADERS-FREE [57] | 2442 | Total: 1029 (42.1%) unstable angina: 370 NSTEMI: 554 STEMI: 105 |
| ZEUS [58] | 1606 | Total: 1016 (63.3%) unstable angina: 270 NSTEMI: 441 STEMI: 305 |
| NORSTENT [59] | 9013 | Total: 6319 (70.1%) unstable angina: 1105 NSTEMI: 2842 STEMI: 2372 |
| SENIOR [60] | 1200 | Total: 544 (45.3%) unstable angina: 109 NSTEMI: 308 STEMI: 127 |
Table 5.
Outcome comparison this meta-analysis with Piccolo et al. 2022 IPDM in ACS.
| Piccolo et al. 2022 | Ratios between the Two Meta-Analyses | This SLR | |||
|---|---|---|---|---|---|
| Outcome and follow-up | DES | BMS | DES | TiNOS | |
| CD or MI 1-year | 535 | 636 | 69 | 48 | |
| 7739 | 6889 | 1123 | 1620 | ||
| 0.0691 | 0.0923 | 0.0614 | 0.0296 | ||
| DES IPDM/DES here | 0.89 | ||||
| CD or MI 5-year | 831 | 892 | 90 | 48 | |
| 7739 | 6889 | 621 | 631 | ||
| 0.1074 | 0.1295 | 0.1449 | 0.0761 | ||
| DES IPDM/DES here | 1.35 | ||||
| Definite ST 1-year | 46 | 74 | 20 | 14 | |
| 7739 | 6889 | 1123 | 1620 | ||
| 0.0059 | 0.0107 | 0.0178 | 0.0086 | ||
| DES IPDM/DES here | 3.00 | ||||
| DES IPDM/TiNOS here | 1.45 | ||||
| Definite ST 5-year | 66 | 91 | 29 | 6 | |
| 7739 | 6889 | 621 | 631 | ||
| 0.0085 | 0.0132 | 0.0467 | 0.0095 | ||
| 0.0085 | 0.0132 | DES IPDM/TiNOS here | 5.48 | ||
| DES IPDM/TiNOS here | 1.11 | ||||
Overall, the robustness to the sensitivity analysis, the low level of heterogeneity between the three ACS-only RCTs, and their low risk of bias support the internal validity of this meta-analysis of TiNOS vs. DES. The similarity in the CD or MI rates between the DES arms of the IPDM and this meta-analysis support the external validity of that endpoint. One can infer from the meta-analyses that DES presents a lower risk of CD or MI than BMS and TiNOS has a lower risk than DES.
This meta-analysis shows with high certainty of evidence, according to GRADE, that TiNOS is non-inferior to DES in ACS at the 1-year follow-up. TiNOS displays a significantly lower risk of recurrent non-fatal myocardial infarctions and probable or definite ST but a significantly higher risk of TLR. The certainty of evidence concerning ST and TLR is low due to the limited number of observations, thus the limited precision in the GRADE criteria. At any rate, the risk of TLR with TiNOS can be reduced when using short stent lengths (≤28 mm) and/or large stent diameters (>3.0 mm) [42,44].
The results at the 5-year follow-up are consistent with the 1-year results but will require the publication of TIDES-ACS final data to be confirmed.
5. Conclusions
This systematic review shows that titanium-nitride-oxide-coated stents are non-inferior to drug-eluting stents when applied to acute coronary syndrome at the one-year follow-up in terms of device-oriented major adverse cardiac events and present a lower risk of recurrent non-fatal myocardial infarction. The interim five-year results are consistent with the one-year results and are robust. Therefore, titanium-nitride-oxide-coated stents are a safe alternative to drug-eluting stents in acute coronary syndrome.
Note: Ineligible records are listed with the references [61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77,78,79,80,81,82,83,84,85,86,87,88,89,90,91,92,93,94,95,96,97,98,99,100,101,102,103,104,105,106,107,108,109,110,111,112,113,114,115,116,117,118].
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/biomedicines10123159/s1 Figure S1: Recurrent non-fatal MI—1 year; Figure S2: Recurrent non-fatal MI—5 years; Figure S3: CD—1 year; Figure S4: CD—5 years; Figure S5: Probable or definite ST—1 year; Figure S6: Probable or definite ST—5 years; List of ineligible records.
Appendix A. Detailed Search Strings in Each Database
Pubmed search string: ((bioactive OR (Titanium AND nitride AND oxide) OR TiNO OR TNO OR BAS) AND stent) AND (DES OR (drug AND eluting AND stent)) AND (RCT OR ((randomised OR randomised) AND controlled AND trial))
(“bioactivate”[All Fields] OR “bioactivated”[All Fields] OR “bioactivates”[All Fields] OR “bioactivating”[All Fields] OR “bioactivation”[All Fields] OR “bioactivations”[All Fields] OR “bioactive”[All Fields] OR “bioactives”[All Fields] OR “bioactivities”[All Fields] OR “bioactivity”[All Fields] OR ((“titanium”[MeSH Terms] OR “titanium”[All Fields] OR “titaniums”[All Fields]) AND (“nitridated”[All Fields] OR “nitridation”[All Fields] OR “nitride”[All Fields] OR “nitrided”[All Fields] OR “nitrides”[All Fields] OR “nitriding”[All Fields] OR “nitridized”[All Fields]) AND (“oxidability”[All Fields] OR “oxidable”[All Fields] OR “oxidant s”[All Fields] OR “oxidants”[Pharmacological Action] OR “oxidants”[MeSH Terms] OR “oxidants”[All Fields] OR “oxidant”[All Fields] OR “oxidate”[All Fields] OR “oxidated”[All Fields] OR “oxidates”[All Fields] OR “oxidating”[All Fields] OR “oxidation”[All Fields] OR “oxidations”[All Fields] OR “oxidative”[All Fields] OR “oxidatively”[All Fields] OR “oxidatives”[All Fields] OR “oxide s”[All Fields] OR “oxides”[MeSH Terms] OR “oxides”[All Fields] OR “oxide”[All Fields] OR “oxidic”[All Fields] OR “oxiding”[All Fields] OR “oxidisability”[All Fields] OR “oxidisable”[All Fields] OR “oxidisation”[All Fields] OR “oxidise”[All Fields] OR “oxidised”[All Fields] OR “oxidiser”[All Fields] OR “oxidisers”[All Fields] OR “oxidises”[All Fields] OR “oxidising”[All Fields] OR “oxidization”[All Fields] OR “oxidize”[All Fields] OR “oxidized”[All Fields] OR “oxidizer”[All Fields] OR “oxidizers”[All Fields] OR “oxidizes”[All Fields] OR “oxidizing”[All Fields])) OR “TiNO”[All Fields] OR “TNO”[All Fields] OR “BAS”[All Fields]) AND (“stent s”[All Fields] OR “stentings”[All Fields] OR “stents”[MeSH Terms] OR “stents”[All Fields] OR “stent”[All Fields] OR “stented”[All Fields] OR “stenting”[All Fields]) AND (“DES”[All Fields] OR (“drug”[All Fields] AND (“elutable”[All Fields] OR “elutant”[All Fields] OR “elute”[All Fields] OR “eluted”[All Fields] OR “elutent”[All Fields] OR “eluter”[All Fields] OR “eluters”[All Fields] OR “elutes”[All Fields] OR “eluting”[All Fields] OR “elution”[All Fields] OR “elutions”[All Fields]) AND (“stent s”[All Fields] OR “stentings”[All Fields] OR “stents”[MeSH Terms] OR “stents”[All Fields] OR “stent”[All Fields] OR “stented”[All Fields] OR “stenting”[All Fields]))) AND (“RCT”[All Fields] OR ((“random allocation”[MeSH Terms] OR (“random”[All Fields] AND “allocation”[All Fields]) OR “random allocation”[All Fields] OR “random”[All Fields] OR “randomization”[All Fields] OR “randomized”[All Fields] OR “randomisation”[All Fields] OR “randomisations”[All Fields] OR “randomise”[All Fields] OR “randomised”[All Fields] OR “randomising”[All Fields] OR “randomizations”[All Fields] OR “randomize”[All Fields] OR “randomizes”[All Fields] OR “randomizing”[All Fields] OR “randomness”[All Fields] OR “randoms”[All Fields] OR (“random allocation”[MeSH Terms] OR (“random”[All Fields] AND “allocation”[All Fields]) OR “random allocation”[All Fields] OR “random”[All Fields] OR “randomization”[All Fields] OR “randomized”[All Fields] OR “randomisation”[All Fields] OR “randomisations”[All Fields] OR “randomise”[All Fields] OR “randomised”[All Fields] OR “randomising”[All Fields] OR “randomizations”[All Fields] OR “randomize”[All Fields] OR “randomizes”[All Fields] OR “randomizing”[All Fields] OR “randomness”[All Fields] OR “randoms”[All Fields])) AND “controlled”[All Fields] AND (“clinical trials as topic”[MeSH Terms] OR (“clinical”[All Fields] AND “trials”[All Fields] AND “topic”[All Fields]) OR “clinical trials as topic”[All Fields] OR “trial”[All Fields] OR “trial s”[All Fields] OR “trialed”[All Fields] OR “trialing”[All Fields] OR “trials”[All Fields])))
Translations
bioactive: “bioactivate”[All Fields] OR “bioactivated”[All Fields] OR “bioactivates”[All Fields] OR “bioactivating”[All Fields] OR “bioactivation”[All Fields] OR “bioactivations”[All Fields] OR “bioactive”[All Fields] OR “bioactives”[All Fields] OR “bioactivities”[All Fields] OR “bioactivity”[All Fields]
Titanium: “titanium”[MeSH Terms] OR “titanium”[All Fields] OR “titanium’s”[All Fields] OR “titaniums”[All Fields]
nitride: “nitridated”[All Fields] OR “nitridation”[All Fields] OR “nitride”[All Fields] OR “nitrided”[All Fields] OR “nitrides”[All Fields] OR “nitriding”[All Fields] OR “nitridized”[All Fields]
oxide: “oxidability”[All Fields] OR “oxidable”[All Fields] OR “oxidant’s”[All Fields] OR “oxidants”[Pharmacological Action] OR “oxidants”[MeSH Terms] OR “oxidants”[All Fields] OR “oxidant”[All Fields] OR “oxidate”[All Fields] OR “oxidated”[All Fields] OR “oxidates”[All Fields] OR “oxidating”[All Fields] OR “oxidation”[All Fields] OR “oxidations”[All Fields] OR “oxidative”[All Fields] OR “oxidatively”[All Fields] OR “oxidatives”[All Fields] OR “oxide’s”[All Fields] OR “oxides”[MeSH Terms] OR “oxides”[All Fields] OR “oxide”[All Fields] OR “oxidic”[All Fields] OR “oxiding”[All Fields] OR “oxidisability”[All Fields] OR “oxidisable”[All Fields] OR “oxidisation”[All Fields] OR “oxidise”[All Fields] OR “oxidised”[All Fields] OR “oxidiser”[All Fields] OR “oxidisers”[All Fields] OR “oxidises”[All Fields] OR “oxidising”[All Fields] OR “oxidization”[All Fields] OR “oxidize”[All Fields] OR “oxidized”[All Fields] OR “oxidizer”[All Fields] OR “oxidizers”[All Fields] OR “oxidizes”[All Fields] OR “oxidizing”[All Fields]
stent: “stent’s”[All Fields] OR “stentings”[All Fields] OR “stents”[MeSH Terms] OR “stents”[All Fields] OR “stent”[All Fields] OR “stented”[All Fields] OR “stenting”[All Fields]
eluting: “elutable”[All Fields] OR “elutant”[All Fields] OR “elute”[All Fields] OR “eluted”[All Fields] OR “elutent”[All Fields] OR “eluter”[All Fields] OR “eluters”[All Fields] OR “elutes”[All Fields] OR “eluting”[All Fields] OR “elution”[All Fields] OR “elutions”[All Fields]
stent: “stent’s”[All Fields] OR “stentings”[All Fields] OR “stents”[MeSH Terms] OR “stents”[All Fields] OR “stent”[All Fields] OR “stented”[All Fields] OR “stenting”[All Fields]
randomized: “random allocation”[MeSH Terms] OR (“random”[All Fields] AND “allocation”[All Fields]) OR “random allocation”[All Fields] OR “random”[All Fields] OR “randomization”[All Fields] OR “randomized”[All Fields] OR “randomisation”[All Fields] OR “randomisations”[All Fields] OR “randomise”[All Fields] OR “randomised”[All Fields] OR “randomising”[All Fields] OR “randomizations”[All Fields] OR “randomize”[All Fields] OR “randomizes”[All Fields] OR “randomizing”[All Fields] OR “randomness”[All Fields] OR “randoms”[All Fields]
randomised: “random allocation”[MeSH Terms] OR (“random”[All Fields] AND “allocation”[All Fields]) OR “random allocation”[All Fields] OR “random”[All Fields] OR “randomization”[All Fields] OR “randomized”[All Fields] OR “randomisation”[All Fields] OR “randomisations”[All Fields] OR “randomise”[All Fields] OR “randomised”[All Fields] OR “randomising”[All Fields] OR “randomizations”[All Fields] OR “randomize”[All Fields] OR “randomizes”[All Fields] OR “randomizing”[All Fields] OR “randomness”[All Fields] OR “randoms”[All Fields]
trial: “clinical trials as topic”[MeSH Terms] OR (“clinical”[All Fields] AND “trials”[All Fields] AND “topic”[All Fields]) OR “clinical trials as topic”[All Fields] OR “trial”[All Fields] OR “trial’s”[All Fields] OR “trialed”[All Fields] OR “trialing”[All Fields] OR “trials”[All Fields]
Cochrane database search string: ((bioactive OR (Titanium AND nitride AND oxide) OR TiNO OR TNO OR BAS) AND stent) AND (DES OR (drug AND eluting AND stent)) AND (RCT OR ((randomised OR randomised) AND controlled AND trial)).
Web of Science search string: (((bioactive OR (Titanium AND nitride AND oxide) OR TiNO OR TNO OR BAS) AND stent) AND (DES OR (drug AND eluting AND stent)) AND (RCT OR ((randomised OR randomised) AND controlled AND trial)))
Embase search string: (((bioactive OR (Titanium AND nitride AND oxide) OR TiNO OR TNO OR BAS) AND stent) AND (DES OR (drug AND eluting AND stent)) AND (RCT OR ((randomised OR randomised) AND controlled AND trial)))/br.
Appendix B. Detailed Explanations of Each GRADE Item Rating
-
a
The risk of bias common to all RCTs was the operator’s knowledge of the type of stent. However, given the absence of significant differences in the baseline and procedural data, this risk seems to have had no effect. Other potential risks of bias appeared occasionally in some RCTs, but the sensitivity analysis shows that bias related to individual RCTs has little influence on the pooled outcomes. The study with the highest risk of bias has the smallest relative weight and does not report outcomes in ACS or at the 5-year follow-up, so the impact of the potential risk of that trial is limited.
-
b
Heterogeneity within this subgroup was low given the overlap of all the confidence intervals, the Cochran Q-test with p > 0.05, and an I² < 40%.
-
c
The three ACS RCTs report modified device-oriented MACE. Assuming half of the non-fatal MIs were in non-target vessels (i.e., 45 cases), the proportionality would lead to 27 fewer cases with TiNOS and 18 with DES. If these numbers were removed from the count of MACE, the RR would be 0.98 [0.73, 1.30], which does not change the non-inferiority conclusion.
-
d
The 95% CI of the RR of MACE included 1.0 with a lower bound slightly <0.75 in favour of TiNOS and a length slightly >0.5. This result is robust to the sensitivity analysis. A hypothetical single RCT to demonstrate the non-inferiority of TiNOS compared to DES and incorporating 1:1 randomization, a 2-sided test, α = 2.5%, power = 90%, and a reference rate of events in the three trial DES arms 99/1123 = 0.088 assuming a non-inferiority margin delta = 0.035 based on a targeted 30% RRR, would require a total sample size of n = 2760 patients, which is 1.01 times the pooled sample size. Considering that the total number of events observed is 124 + 99 = 223, reaching the GRADE rule of thumb of 300 events overall requires n = 2760 × 300/223 = 3713 patients to meet the OIS criterion. However, with the current sample size, a minimum of 20 events (9% of observed events) should be redistributed from the DES arm to the TiNOS arm for the CI to reach non-significance (RR 1.42 [1.06, 1.91]).
-
e
Publications usually do not indicate whether the MI is related to the target vessel or not, but this review assumes that recurrent MIs related to non-target vessels at a 1-year follow-up should be in equal proportion in the two treatment arms.
-
f
The 95% CI of the RR of ST is significantly <1.0 in favour of TiNOS with an upper-bound = 0.75 and a length < 0.5. This result is robust to sensitivity analysis. A hypothetical single RCT, 1:1 randomization, 2-sided test, α = 5%, power = 80%, and a reference rate of events in the 3 trial DES arms 69/1123 = 0.061 assuming delta = 0.018 based on a targeted 30% RRR requires a total sample size of n = 4788 patients, which is 1.75 times the pooled sample size. Considering the total number of events observed is 48 + 69 = 117, reaching the GRADE rule of thumb of 300 events overall requires n = 4788 × 300/117 = 12,277 patients to meet the OIS criterion. With the current sample size, a minimum of 9 events (7.7% of observed events) should be redistributed from the DES arm to the TiNOS arm for the CI to reach non-significance (RR 0.71 [0.49, 1.02]). This result is imprecise and fragile.
-
g
TLR defined as clinically driven confirmed in all three ACS trials
-
h
The 95% CI of the RR of TLR is significantly >1.0 in favour of DES with a lower bound < 1.25 and a length > 1. This result is not robust to sensitivity analysis. A hypothetical single RCT, 1:1 randomization, 2-sided test, α = 5%, power = 80%, and a reference rate of events in the 3 trial DES arms 46/1123 = 0.041 assuming delta = 0.012 based on a targeted 30% RRR requires a total sample size of n = 7308 patients, which is 2.66 times the pooled sample size. Considering the total number of events observed is 98 + 46 = 144, reaching the GRADE rule of thumb of 300 events overall requires n = 7308 × 300/144 = 15,225 patients to meet the OIS criterion. With the current sample size, a minimum of three events (2.1% of observed events) should be redistributed from the TiNOS arm to the DES arm for the CI to reach non-significance (RR 1.40 [1.00, 1.97]). This result is imprecise and fragile.
-
i
Recurrent MI is described as non-fatal in the three RCTs and the PK confirms that those included non-fatal MIs in non-target vessels. One can reasonably assume the index stent does not affect those events. Ninety non-fatal MIs were reported. Assuming half of them are related to non-target vessels (i.e., 45 cases), the proportionality with the sample size would lead to 27 fewer cases with TiNOS and 18 with DES, which would result in an RR of 0.19 [0.09, 0.42]. The inclusion of non-target MIs thus results in a dilution that is favourable to DES.
-
j
The 95% CI of the RR of non-fatal MI is significantly <1.0 in favour of TiNOS with an upper bound < 0.75 and a length < 0.5. This result is robust to sensitivity analysis. A hypothetical single RCT, 1:1 randomization, 2-sided test, α = 5%, power = 80%, and a reference rate of events in the 3 trial DES arms 55/1123 = 0.049 assuming delta = 0.015 based on a targeted 30% RRR requires a total sample size of n = 6070 patients, which is 2.21 times the pooled sample size. Considering the total number of events observed is 35 + 55 = 90, reaching the GRADE rule of thumb of 300 events overall requires n = 6070 × 300/90 = 20,233 patients to meet the OIS criterion. With the current sample size, a minimum of eight events (8.9% of observed events) should be redistributed from the DES arm to the TiNOS arm for the CI to reach non-significance (RR 0.68 [0.44, 1.05]). This result is imprecise and fragile.
-
k
Heterogeneity within that subgroup was high given the absence of overlap of confidence intervals, the significant Cochran Q-test with p = 0.005, and an I² ≥ 80%.
-
l
The definition of CD was assumed to be similar across the three ACS RCTs. This uniform reporting method across the three trials was confirmed by PK.
-
m
The 95% CI of the RR of CD is non-significant with a length > 0.5. Sensitivity analysis supports no association. A hypothetical single RCT, 1:1 randomization, 2-sided test, α = 5%, power = 80%, and a reference rate of events in the 3 trial DES arms 16/1123 = 0.014 assuming delta = 0.004 based on a targeted 30% RRR requires a total sample size of n = 21,484 patients, which is 7.83 times the pooled sample size. Considering the total number of events observed is 14 + 16 = 30, reaching the GRADE rule of thumb of 300 events overall requires n = 21,484 × 300/30 = 214,840 patients to meet the OIS criterion. With the current sample size, a minimum of three events (10% of observed events) should be redistributed from the TiNOS arm to the DES arm for the CI to reach significance (RR 0.45 [0.23 0.90]). This result is imprecise and fragile.
-
n
ARC are criteria used in all studies. Probable or definite stent thrombosis occur at 1-y FU.
-
o
The 95% CI of the RR of ST is significantly <1.0 in favour of TiNOS with an upper bound > 0.75 and a length > 0.5. This result is robust to sensitivity analysis. A hypothetical single RCT, 1:1 randomization, 2-sided test, α = 5%, power = 80%, and a reference rate of events in the 3 trial DES arms 20/1123 = 0.018 assuming delta = 0.005 based on a targeted 30% RRR requires a total sample size of n = 17,136 patients, which is 6.25 times the pooled sample size. Considering the total number of events observed is 14 + 20 = 34, reaching the GRADE rule of thumb of 300 events overall requires n = 17,136 × 300/34 = 151,200 patients to meet the OIS criterion. With the current sample size, a minimum of event event (2.9% of observed events) should be redistributed from the DES arm to the TiNOS arm for the CI to reach non-significance (RR 0.52 [0.26, 1.03]). This result is imprecise and fragile.
-
p
Heterogeneity within that subgroup was moderate given the overlap of the confidence intervals, but the Cochran Q-test was significant with p = 0.05 and I² ≥ 60%.
-
q
The definition of TD was assumed to be similar across the RCTs so the risk indirectness was rated as not serious. The fact that the sensitivity analysis showed that removing BASE-ACS from the pooled analysis led to a significant confidence interval in favour of TiNOS did not appear to be related to differences in TD definition.
-
r
The 95% CI of the RR of TD is non-significant with a length > 0.5. Sensitivity analysis supports no association. A hypothetical single RCT, 1:1 randomization, 2-sided test, α = 5%, power = 80%, and a reference rate of events in the 3 trial DES arms 29/1123 = 0.026 assuming delta = 0.008 based on a targeted 30% RRR requires a total sample size of n = 11,740 patients, which is 4.28 times the pooled sample size. Considering the total number of events observed is 29 + 29 = 58, reaching the GRADE rule of thumb of 300 events overall requires n = 11,740 × 300/58 = 60,724 patients to meet the OIS criterion. With the current sample size, a minimum of four events (6.9% of observed events) should be redistributed from the TiNOS arm to the DES arm for the CI to reach significance (RR 0.60 [0.37 0.97]). This result is imprecise and fragile.
Author Contributions
F.C.D. and L.L.: Main reviewers and meta-analysts. N.M.: Third reviewer and adjudicated disagreements. P.C.: Advised on protocol and analysis. P.P.K.: Provided additional information about the methods of the individual trials. All authors participated in finalising the manuscript. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest. No funder was involved in this academic research other than Bordeaux University.
Funding Statement
This research received no external funding.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Knuuti J., Wijns W., Saraste A., Capodanno D., Barbato E., Funck-Brentano C., Prescott E., Storey R.F., Deaton C., Cuisset T., et al. 2019 ESC Guidelines for the diagnosis and management of chronic coronary syndromes. Eur. Heart J. 2020;41:407–477. doi: 10.1093/eurheartj/ehz425. [DOI] [PubMed] [Google Scholar]
- 2.Collet J.P., Thiele H., Barbato E., Barthelemy O., Bauersachs J., Bhatt D.L., Dendale P., Dorobantu M., Edvardsen T., Folliguet T., et al. 2020 ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation. Eur. Heart J. 2021;42:1289–1367. doi: 10.1093/eurheartj/ehaa575. [DOI] [PubMed] [Google Scholar]
- 3.Levine G.N., Bates E.R., Blankenship J.C., Bailey S.R., Bittl J.A., Cercek B., Chambers C.E., Ellis S.G., Guyton R.A., Hollenberg S.M., et al. 2011 ACCF/AHA/SCAI Guideline for Percutaneous Coronary Intervention: A report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines and the Society for Cardiovascular Angiography and Interventions. Circulation. 2011;124:e574–e651. doi: 10.1161/CIR.0b013e31823ba622. [DOI] [PubMed] [Google Scholar]
- 4.O’Gara P.T., Kushner F.G., Ascheim D.D., Casey D.E., Jr., Chung M.K., de Lemos J.A., Ettinger S.M., Fang J.C., Fesmire F.M., Franklin B.A., et al. 2013 ACCF/AHA guideline for the management of ST-elevation myocardial infarction: A report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation. 2013;127:e362–e425. doi: 10.1161/CIR.0b013e3182742c84. [DOI] [PubMed] [Google Scholar]
- 5.Task Force M., Montalescot G., Sechtem U., Achenbach S., Andreotti F., Arden C., Budaj A., Bugiardini R., Crea F., Cuisset T., et al. 2013 ESC guidelines on the management of stable coronary artery disease: The Task Force on the management of stable coronary artery disease of the European Society of Cardiology. Eur. Heart J. 2013;34:2949–3003. doi: 10.1093/eurheartj/eht296. [DOI] [PubMed] [Google Scholar]
- 6.Amsterdam E.A., Wenger N.K., Brindis R.G., Casey D.E., Jr., Ganiats T.G., Holmes D.R., Jr., Jaffe A.S., Jneid H., Kelly R.F., Kontos M.C., et al. 2014 AHA/ACC guideline for the management of patients with non-ST-elevation acute coronary syndromes: Executive summary: A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;130:2354–2394. doi: 10.1161/CIR.0000000000000133. [DOI] [PubMed] [Google Scholar]
- 7.Roffi M., Patrono C., Collet J.P., Mueller C., Valgimigli M., Andreotti F., Bax J.J., Borger M.A., Brotons C., Chew D.P., et al. 2015 ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation: Task Force for the Management of Acute Coronary Syndromes in Patients Presenting without Persistent ST-Segment Elevation of the European Society of Cardiology (ESC) Eur. Heart J. 2016;37:267–315. doi: 10.1093/eurheartj/ehv320. [DOI] [PubMed] [Google Scholar]
- 8.Ibanez B., James S., Agewall S., Antunes M.J., Bucciarelli-Ducci C., Bueno H., Caforio A.L.P., Crea F., Goudevenos J.A., Halvorsen S., et al. 2017 ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation. Rev. Esp. Cardiol. 2017;70:1082. doi: 10.1016/j.rec.2017.11.010. [DOI] [PubMed] [Google Scholar]
- 9.Ibanez B., James S., Agewall S., Antunes M.J., Bucciarelli-Ducci C., Bueno H., Caforio A.L.P., Crea F., Goudevenos J.A., Halvorsen S., et al. 2017 ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation: The Task Force for the management of acute myocardial infarction in patients presenting with ST-segment elevation of the European Society of Cardiology (ESC) Eur. Heart J. 2018;39:119–177. doi: 10.1093/eurheartj/ehx393. [DOI] [PubMed] [Google Scholar]
- 10.Writing Committee M., Lawton J.S., Tamis-Holland J.E., Bangalore S., Bates E.R., Beckie T.M., Bischoff J.M., Bittl J.A., Cohen M.G., DiMaio J.M., et al. 2021 ACC/AHA/SCAI Guideline for Coronary Artery Revascularization: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. J. Am. Coll. Cardiol. 2022;79:e21–e129. doi: 10.1016/j.jacc.2021.09.006. [DOI] [PubMed] [Google Scholar]
- 11.Patterson C., Mapera S., Li H.H., Madamanchi N., Hilliard E., Lineberger R., Herrmann R., Charles P. Comparative effects of paclitaxel and rapamycin on smooth muscle migration and survival: Role of AKT-dependent signaling. Arter. Thromb. Vasc. Biol. 2006;26:1473–1480. doi: 10.1161/01.ATV.0000223866.42883.3b. [DOI] [PubMed] [Google Scholar]
- 12.Wessely R., Schomig A., Kastrati A. Sirolimus and Paclitaxel on polymer-based drug-eluting stents: Similar but different. J. Am. Coll. Cardiol. 2006;47:708–714. doi: 10.1016/j.jacc.2005.09.047. [DOI] [PubMed] [Google Scholar]
- 13.Yazdani S., Sheehy A., Pacetti S., Rittlemeyer B., Kolodgie F., Virmani R. Stent Coating Integrity of Durable and Biodegradable Coated Drug Eluting Stents. J. Interv. Cardiol. 2016;29:483–490. doi: 10.1111/joic.12303. [DOI] [PubMed] [Google Scholar]
- 14.Windecker S., Mayer I., De Pasquale G., Maier W., Dirsch O., De Groot P., Wu Y., Noll G., Leskosek B., Meier B., et al. Stent coating with titanium-nitride-oxide for reduction of neointimal hyperplasia. Circulation. 2001;104:928–933. doi: 10.1161/hc3401.093146. [DOI] [PubMed] [Google Scholar]
- 15.Ignarro L.J., Buga G.M., Wei L.H., Bauer P.M., Wu G., del Soldato P. Role of the arginine-nitric oxide pathway in the regulation of vascular smooth muscle cell proliferation. Proc. Natl. Acad. Sci. USA. 2001;98:4202–4208. doi: 10.1073/pnas.071054698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Windecker S., Simon R., Lins M., Klauss V., Eberli F., Roffi M., Pedrazzini G., Moccetti T., Wenaweser P., Togni M., et al. Randomized comparison of a titanium-nitride-oxide-coated stent with a stainless steel stent for coronary revascularization: The TiNOX trial. Circulation. 2005;111:2617–2622. doi: 10.1161/CIRCULATIONAHA.104.486647. [DOI] [PubMed] [Google Scholar]
- 17.Higgins J.P.T., Green S., editors. Cochrane Handbook for Systematic Reviews of Interventions. [(accessed on 8 March 2018)]. Version 5.1.0 Updated March 2011. Available online: https://handbook-5-1.cochrane.org.
- 18.Schünemann H., Brożek J., Guyatt G., Oxman A., editors. GRADE Handbook for Grading Quality of Evidence and Strength of Recommendations. 2013. [(accessed on 8 March 2018)]. Updated October 2013. The GRADE Working Group. Available online: Guidelinedevelopment.org/handbook.
- 19.Guyatt G., Oxman A., Kunz R., Woodcocke J., Brozeka J., Helfand M. GRADE guidelines: 8. Rating the quality of evidence–indirectness. J. Clin. Epidemiol. 2011. [(accessed on 8 March 2018)]. Available online: http://www.gradeworkinggroup.org/publications/JCE_series.ht. [DOI] [PubMed]
- 20.Guyatt G.H., Oxman A.D., Kunz R., Brozek J., Alonso-Coello P., Rind D., Devereaux P.J., Montori V.M., Freyschuss B., Vist G., et al. GRADE guidelines 6. Rating the quality of evidence—imprecision. J. Clin. Epidemiol. 2011;64:1283–1293. doi: 10.1016/j.jclinepi.2011.01.012. [DOI] [PubMed] [Google Scholar]
- 21.Guyatt G.H., Oxman A.D., Montori V., Vist G., Kunz R., Brozek J., Alonso-Coello P., Djulbegovic B., Atkins D., Falck-Ytter Y., et al. GRADE guidelines: 5. Rating the quality of evidence—publication bias. J. Clin. Epidemiol. 2011;64:1277–1282. doi: 10.1016/j.jclinepi.2011.01.011. [DOI] [PubMed] [Google Scholar]
- 22.Guyatt G.H., Oxman A.D., Vist G.E., Kunz R., Falck-Ytter Y., Alonso-Coello P., Schunemann H.J., Group G.W. GRADE: An emerging consensus on rating quality of evidence and strength of recommendations. BMJ. 2008;336:924–926. doi: 10.1136/bmj.39489.470347.AD. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liberati A., Altman D.G., Tetzlaff J., Mulrow C., Gotzsche P.C., Ioannidis J.P., Clarke M., Devereaux P.J., Kleijnen J., Moher D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: Explanation and elaboration. BMJ. 2009;339:b2700. doi: 10.1136/bmj.b2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Huang X., Lin J., Demner-Fushman D. Evaluation of PICO as a knowledge representation for clinical questions. AMIA Annu. Symp. Proc. 2006;2006:359–363. [PMC free article] [PubMed] [Google Scholar]
- 25.Garcia-Garcia H.M., McFadden E.P., Farb A., Mehran R., Stone G.W., Spertus J., Onuma Y., Morel M.A., van Es G.A., Zuckerman B., et al. Standardized End Point Definitions for Coronary Intervention Trials: The Academic Research Consortium-2 Consensus Document. Eur. Heart J. 2018;39:2192–2207. doi: 10.1093/eurheartj/ehy223. [DOI] [PubMed] [Google Scholar]
- 26.Savovic J., Weeks L., Sterne J.A., Turner L., Altman D.G., Moher D., Higgins J.P. Evaluation of the Cochrane Collaboration’s tool for assessing the risk of bias in randomized trials: Focus groups, online survey, proposed recommendations and their implementation. Syst. Rev. 2014;3:37. doi: 10.1186/2046-4053-3-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Egger M., Smith G.D. Bias in location and selection of studies. BMJ. 1998;316:61–66. doi: 10.1136/bmj.316.7124.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Egger M., Davey Smith G., Schneider M., Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sterne J.A.C., Egger M., Davey Smith G. Investigating and dealing with publication and other biases. In: Egger M., Davey Smith G., Altman D.G., editors. Systematic Reviews in Health Care: Meta-Analysis in Context. BMJ Publishing Group; London, UK: 2001. pp. 189–208. [Google Scholar]
- 30.Harbord R.M., Egger M., Sterne J.A. A modified test for small-study effects in meta-analyses of controlled trials with binary endpoints. Stat. Med. 2006;25:3443–3457. doi: 10.1002/sim.2380. [DOI] [PubMed] [Google Scholar]
- 31.Gavaghan D.J., Moore A.R., McQuay H.J. An evaluation of homogeneity tests in meta-analyses in pain using simulations of individual patient data. Pain. 2000;85:415–424. doi: 10.1016/S0304-3959(99)00302-4. [DOI] [PubMed] [Google Scholar]
- 32.Higgins J.P., Thompson S.G. Quantifying heterogeneity in a meta-analysis. Stat. Med. 2002;21:1539–1558. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- 33.Higgins J.P., Thompson S.G., Deeks J.J., Altman D.G. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Seeger P., Gabrielsson A. Applicability of the Cochran Q test and the F test for statistical analysis of dichotomous data for dependent samples. Psychol Bull. 1968;69:269–277. doi: 10.1037/h0025667. [DOI] [PubMed] [Google Scholar]
- 35.GRADEpro GDT: GRADEpro Guideline Development Tool [Software] 2015. [(accessed on 8 September 2022)]. McMaster University. (Developed by Evidence Prime, Inc.) Available online: https://gdt.gradepro.org.
- 36.Karjalainen P., Ylitalo A., Niemelä M., Kervinen K., Mäkikallio T., Pietili M., Sia J., Tuomainen P., Nyman K., Airaksinen K. Titanium-nitride-oxide coated stents versus paclitaxel-eluting stents in acute myocardial infarction: A 12-months follow-up report from the TITAX AMI trial. EuroInterv. J. EuroPCR Collab. Work. Group Interv. Cardiol. Eur. Soc. Cardiol. 2008;4:234–241. doi: 10.4244/EIJV4I2A42. [DOI] [PubMed] [Google Scholar]
- 37.Tuomainen P.O., Ylitalo A., Niemelä M., Kervinen K., Pietilä M., Sia J., Nyman K., Nammas W., Airaksinen K.E., Karjalainen P.P. Five-year clinical outcome of titanium-nitride-oxide-coated bioactive stents versus paclitaxel-eluting stents in patients with acute myocardial infarction: Long-term follow-up from the TITAX AMI trial. Int. J. Cardiol. 2013;168:1214–1219. doi: 10.1016/j.ijcard.2012.11.060. [DOI] [PubMed] [Google Scholar]
- 38.Pilgrim T., Räber L., Limacher A., Löffel L., Wenaweser P., Cook S., Stauffer J.C., Togni M., Vogel R., Garachemani A., et al. Comparison of titanium-nitride-oxide-coated stents with zotarolimus-eluting stents for coronary revascularization a randomized controlled trial. JACC. Cardiovasc. Interv. 2011;4:672–682. doi: 10.1016/j.jcin.2011.02.017. [DOI] [PubMed] [Google Scholar]
- 39.Pilgrim T., Räber L., Limacher A., Wenaweser P., Cook S., Stauffer J.C., Garachemani A., Moschovitis A., Meier B., Jüni P., et al. Five-year results of a randomised comparison of titanium-nitride-oxide-coated stents with zotarolimus-eluting stents for coronary revascularisation. EuroInterv. J. EuroPCR Collab. Work. Group Interv. Cardiol. Eur. Soc. Cardiol. 2015;10:1284–1287. doi: 10.4244/EIJY15M01_04. [DOI] [PubMed] [Google Scholar]
- 40.López-Mínguez J.R., Nogales-Asensio J.M., Doncel-Vecino L.J., Merchán-Herrera A., Pomar-Domingo F., Martínez-Romero P., Fernández-Díaz J.A., Valdesuso-Aguilar R., Moreu-Burgos J., Díaz-Fernández J. A randomized study to compare bioactive titanium stents and everolimus-eluting stents in diabetic patients (TITANIC XV): 1-year results. Rev. Esp. Cardiol. (Engl. Ed.) 2014;67:522–530. doi: 10.1016/j.recesp.2013.10.023. [DOI] [PubMed] [Google Scholar]
- 41.Karjalainen P., Niemelä M., Airaksinen J., Rivero-Crespo F., Romppanen H., Sia J., Lalmand J., De Bruyne B., DeBelder A., Carlier M., et al. A prospective randomised comparison of titanium-nitride-oxide-coated bioactive stents with everolimus-eluting stents in acute coronary syndrome: The BASE-ACS trial. EuroInterv. J. EuroPCR Collab. Work. Group Interv. Cardiol. Eur. Soc. Cardiol. 2012;8:306–315. doi: 10.4244/EIJV8I3A49. [DOI] [PubMed] [Google Scholar]
- 42.Karjalainen P., Nammas W., Ylitalo A., de Bruyne B., Lalmand J., de Belder A., Rivero-Crespo F., Kervinen K., Airaksinen J. Long-term clinical outcome of titanium-nitride-oxide-coated stents versus everolimus-eluting stents in acute coronary syndrome: Final report of the BASE ACS trial. Int. J. Cardiol. 2016;222:275–280. doi: 10.1016/j.ijcard.2016.07.267. [DOI] [PubMed] [Google Scholar]
- 43.Colkesen E., Eefting F., Rensing B., Suttorp M., Ten Berg J., Karjalainen P., Van Der Heyden J. TIDES-ACS Trial: Comparison of titanium-nitride-oxide coated bio-active-stent to the drug (everolimus)-eluting stent in acute coronary syndrome. Study design and objectives. Minerva Cardioangiol. 2015;63:21–29. [PubMed] [Google Scholar]
- 44.Tonino P., Pijls N., Collet C., Nammas W., Van der Heyden J., Romppanen H., Kervinen K., Airaksinen J., Sia J., Lalmand J., et al. Titanium-Nitride-Oxide-Coated versus Everolimus-Eluting Stents in Acute Coronary Syndrome: The Randomized TIDES-ACS Trial. JACC. Cardiovasc. Interv. 2020;13:1697–1705. doi: 10.1016/j.jcin.2020.04.021. [DOI] [PubMed] [Google Scholar]
- 45.Feinberg J., Nielsen E., Greenhalgh J., Hounsome J., Sethi N., Safi S., Gluud C., Jakobsen J. Drug-eluting stents versus bare-metal stents for acute coronary syndrome. Cochrane Database Syst. Rev. 2017;2021:CD012481. doi: 10.1002/14651858.CD012481.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Piccolo R., Bonaa K., Efthimiou O., Varenne O., Baldo A., Urban P., Kaiser C., de Belder A., Lemos P., Wilsgaard T., et al. Individual Patient Data Meta-analysis of Drug-eluting versus Bare-metal Stents for Percutaneous Coronary Intervention in Chronic versus Acute Coronary Syndromes. Am. J. Cardiol. 2022;182:8–16. doi: 10.1016/j.amjcard.2022.07.035. [DOI] [PubMed] [Google Scholar]
- 47.Tsuchida K., Piek J.J., Neumann F.J., van der Giessen W.J., Wiemer M., Zeiher A.M., Grube E., Haase J., Thuesen L., Hamm C.W., et al. One-year results of a durable polymer everolimus-eluting stent in de novo coronary narrowings (The SPIRIT FIRST Trial) EuroInterv. J. EuroPCR Collab. Work. Group Interv. Cardiol. Eur. Soc. Cardiol. 2005;1:266–272. [PubMed] [Google Scholar]
- 48.Fajadet J., Wijns W., Laarman G.J., Kuck K.H., Ormiston J., Munzel T., Popma J.J., Fitzgerald P.J., Bonan R., Kuntz R.E., et al. Randomized, double-blind, multicenter study of the Endeavor zotarolimus-eluting phosphorylcholine-encapsulated stent for treatment of native coronary artery lesions: Clinical and angiographic results of the ENDEAVOR II trial. Circulation. 2006;114:798–806. doi: 10.1161/CIRCULATIONAHA.105.591206. [DOI] [PubMed] [Google Scholar]
- 49.Lemos P.A., Moulin B., Perin M.A., Oliveira L.A., Arruda J.A., Lima V.C., Lima A.A., Caramori P.R., Medeiros C.R., Barbosa M.R., et al. Late clinical outcomes after implantation of drug-eluting stents coated with biodegradable polymers: 3-year follow-up of the PAINT randomised trial. EuroInterv. J. EuroPCR Collab. Work. Group Interv. Cardiol. Eur. Soc. Cardiol. 2012;8:117–119. doi: 10.4244/EIJV8I1A18. [DOI] [PubMed] [Google Scholar]
- 50.Kaiser C., Galatius S., Erne P., Eberli F., Alber H., Rickli H., Pedrazzini G., Hornig B., Bertel O., Bonetti P., et al. Drug-eluting versus bare-metal stents in large coronary arteries. N. Engl. J. Med. 2010;363:2310–2319. doi: 10.1056/NEJMoa1009406. [DOI] [PubMed] [Google Scholar]
- 51.Reifart N., Hauptmann K.E., Rabe A., Enayat D., Giokoglu K. Short and long term comparison (24 months) of an alternative sirolimus-coated stent with bioabsorbable polymer and a bare metal stent of similar design in chronic coronary occlusions: The CORACTO trial. EuroInterv. J. EuroPCR Collab. Work. Group Interv. Cardiol. Eur. Soc. Cardiol. 2010;6:356–360. doi: 10.4244/EIJV6I3A59. [DOI] [PubMed] [Google Scholar]
- 52.Mehilli J., Pache J., Abdel-Wahab M., Schulz S., Byrne R.A., Tiroch K., Hausleiter J., Seyfarth M., Ott I., Ibrahim T., et al. Drug-eluting versus bare-metal stents in saphenous vein graft lesions (ISAR-CABG): A randomised controlled superiority trial. Lancet. 2011;378:1071–1078. doi: 10.1016/S0140-6736(11)61255-5. [DOI] [PubMed] [Google Scholar]
- 53.Valgimigli M., Tebaldi M., Borghesi M., Vranckx P., Campo G., Tumscitz C., Cangiano E., Minarelli M., Scalone A., Cavazza C., et al. Two-year outcomes after first- or second-generation drug-eluting or bare-metal stent implantation in all-comer patients undergoing percutaneous coronary intervention: A pre-specified analysis from the PRODIGY study (PROlonging Dual Antiplatelet Treatment After Grading stent-induced Intimal hyperplasia studY) JACC. Cardiovasc. Interv. 2014;7:20–28. doi: 10.1016/j.jcin.2013.09.008. [DOI] [PubMed] [Google Scholar]
- 54.Ribeiro E.E., Campos C.M., Ribeiro H.B., Lopes A.C., Esper R.B., Meirelles G.X., Perin M.A., Abizaid A., Lemos P.A. First-in-man randomised comparison of a novel sirolimus-eluting stent with abluminal biodegradable polymer and thin-strut cobalt-chromium alloy: INSPIRON-I trial. EuroInterv. J. EuroPCR Collab. Work. Group Interv. Cardiol. Eur. Soc. Cardiol. 2014;9:1380–1384. doi: 10.4244/EIJV9I12A234. [DOI] [PubMed] [Google Scholar]
- 55.de Belder A., de la Torre Hernandez J.M., Lopez-Palop R., O’Kane P., Hernandez Hernandez F., Strange J., Gimeno F., Cotton J., Diaz Fernandez J.F., Carrillo Saez P., et al. A prospective randomized trial of everolimus-eluting stents versus bare-metal stents in octogenarians: The XIMA Trial (Xience or Vision Stents for the Management of Angina in the Elderly) J. Am. Coll Cardiol. 2014;63:1371–1375. doi: 10.1016/j.jacc.2013.10.053. [DOI] [PubMed] [Google Scholar]
- 56.Kaiser C., Galatius S., Jeger R., Gilgen N., Skov Jensen J., Naber C., Alber H., Wanitschek M., Eberli F., Kurz D.J., et al. Long-term efficacy and safety of biodegradable-polymer biolimus-eluting stents: Main results of the Basel Stent Kosten-Effektivitats Trial-PROspective Validation Examination II (BASKET-PROVE II), a randomized, controlled noninferiority 2-year outcome trial. Circulation. 2015;131:74–81. doi: 10.1161/CIRCULATIONAHA.114.013520. [DOI] [PubMed] [Google Scholar]
- 57.Urban P., Meredith I.T., Abizaid A., Pocock S.J., Carrie D., Naber C., Lipiecki J., Richardt G., Iniguez A., Brunel P., et al. Polymer-free Drug-Coated Coronary Stents in Patients at High Bleeding Risk. N. Engl. J. Med. 2015;373:2038–2047. doi: 10.1056/NEJMoa1503943. [DOI] [PubMed] [Google Scholar]
- 58.Valgimigli M., Patialiakas A., Thury A., McFadden E., Colangelo S., Campo G., Tebaldi M., Ungi I., Tondi S., Roffi M., et al. Zotarolimus-eluting versus bare-metal stents in uncertain drug-eluting stent candidates. J. Am. Coll Cardiol. 2015;65:805–815. doi: 10.1016/j.jacc.2014.11.053. [DOI] [PubMed] [Google Scholar]
- 59.Bonaa K.H., Mannsverk J., Wiseth R., Aaberge L., Myreng Y., Nygard O., Nilsen D.W., Klow N.E., Uchto M., Trovik T., et al. Drug-Eluting or Bare-Metal Stents for Coronary Artery Disease. N. Engl. J. Med. 2016;375:1242–1252. doi: 10.1056/NEJMoa1607991. [DOI] [PubMed] [Google Scholar]
- 60.Varenne O., Cook S., Sideris G., Kedev S., Cuisset T., Carrie D., Hovasse T., Garot P., El Mahmoud R., Spaulding C., et al. Drug-eluting stents in elderly patients with coronary artery disease (SENIOR): A randomised single-blind trial. Lancet. 2018;391:41–50. doi: 10.1016/S0140-6736(17)32713-7. [DOI] [PubMed] [Google Scholar]
- 61.Sia J., Nammas W., Collet C., De Bruyne B., Karjalainen P. Comparative study of neointimal coverage between titanium-nitric oxide-coated and everolimus-eluting stents in acute coronary syndromes ORIGINAL (NON-ENGLISH) TITLE Estudio comparativo de la cobertura neointimal entre los stents con recubrimiento de titanio-óxido nítrico y los liberadores de everolimus en el sindrome coronario agudo. Rev. Esp. Cardiol. (Engl. Ed.) 2022 doi: 10.1016/j.recesp.2022.05.011. [DOI] [PubMed] [Google Scholar]
- 62.Grube E., Buellesfeld L. BioMatrix Biolimus A9-eluting coronary stent: A next-generation drug-eluting stent for coronary artery disease. Expert Rev. Med. Devices. 2006;3:731–741. doi: 10.1586/17434440.3.6.731. [DOI] [PubMed] [Google Scholar]
- 63.Wu P., Grainger D.W. Drug/device combinations for local drug therapies and infection prophylaxis. Biomaterials. 2006;27:2450–2467. doi: 10.1016/j.biomaterials.2005.11.031. [DOI] [PubMed] [Google Scholar]
- 64.Coolong A., Kuntz R.E. Understanding the drug-eluting stent trials. Am. J. Cardiol. 2007;100:17k–24k. doi: 10.1016/j.amjcard.2007.06.004. [DOI] [PubMed] [Google Scholar]
- 65.Konorza T.F.M. Prospective, multi-center randomized trial to compare the implantation of a titanium-nitride-oxide coated stent with a paclitaxel stent in patients with acute myocardial infarction ORIGINAL (NON-ENGLISH) TITLE Prospektive, randomisierte, multizentrische studie zum vergleich der ergebnisse eines titanium-nitritoxid-beschichteten stents mit einem paclitaxel freisetzenden stent bei patienten mit akutem myokardinfarkt. HERZ. 2007;32:513. doi: 10.1007/s00059-007-3036-6. [DOI] [Google Scholar]
- 66.van der Hoeven B., Liem S., Jukema J., Suraphakdee N., Putter H., Dijkstra J., Atsma D., Bootsma M., Zeppenfeld K., Oemrawsingh P., et al. Sirolimus-Eluting Stents versus Bare-Metal Stents in Patients With. J. Am. Coll. Cardiol. 2008;51:618–626. doi: 10.1016/j.jacc.2007.09.056. [DOI] [PubMed] [Google Scholar]
- 67.van der Hoeven B., Liem S., Dijkstra J., Bergheanu S., Putter H., Antoni M., Atsma D., Bootsma M., Zeppenfeld K., Jukema J., et al. Stent Malapposition After Sirolimus-Eluting and Bare-Metal Stent Implantation in Patients with ST-Segment Elevation Myocardial Infarction. Acute and 9-Month Intravascular Ultrasound Results of the MISSION! Intervention Study. JACC Cardiovasc. Interv. 2008;1:192–201. doi: 10.1016/j.jcin.2008.02.003. [DOI] [PubMed] [Google Scholar]
- 68.Atary J., Bergheanu S., Van Der Hoeven B., Atsma D., Bootsma M., Van Der Kley F., Zeppenfeld K., Jukema J., Schalij M. Impact of sirolimus-eluting stent implantation compared to bare-metal stent implantation for acute myocardial infarction on coronary plaque composition at nine months follow-up: A Virtual Histology intravascular ultrasound analysis. Results from the Leiden MISSION! Intervention study. EuroInterv. J. EuroPCR Collab. Work. Group Interv. Cardiol. Eur. Soc. Cardiol. 2009;5:565–572. doi: 10.4244/eijv5i5a92. [DOI] [PubMed] [Google Scholar]
- 69.de Luca G., Valgimigli M., Spaulding C., Menichelli M., Brunner-La Rocca H., van der Hoeven B., Di Lorenzo E., de la Llera L., Pasceri V., Pittl U., et al. Short and long-term benefits of sirolimus-eluting stent in ST-segment elevation myocardial infarction: A meta-analysis of randomized trials. J. Thromb. Thrombolysis. 2009;28:200–210. doi: 10.1007/s11239-009-0305-7. [DOI] [PubMed] [Google Scholar]
- 70.Karjalainen P., Ylitalo A., Niemelä M., Kervinen K., Mäkikallio T., Pietilä M., Sia J., Tuomainen P., Nyman K., Airaksinen K. Two-year follow-up after percutaneous coronary intervention with titanium-nitride-oxide-coated stents versus paclitaxel-eluting stents in acute myocardial infarction. Ann. Med. 2009;41:599–607. doi: 10.1080/07853890903111018. [DOI] [PubMed] [Google Scholar]
- 71.Sant’Anna F., Batista L., Brito M., Menezes S., Ventura F., Buczynski L., Barrozo C. Randomized comparison of percutaneous coronary intervention with titanium-nitride-oxide-coated stents versus stainless steel stents in patients with coronary artery disease: RIO trial ORIGINAL (NON-ENGLISH) TITLE Estudo randomizado e comparativo da intervenção coronária percutânea com stents recobertos por titânio- óxido nítrico ou de aço inoxidável em pacientes com doença arterial coronária: Estudo RIO. Rev. Bras. Cardiol. Invasiva. 2009;17:69–75. [Google Scholar]
- 72.Atary J.Z., van der Hoeven B.L., Liem S.S., Jukema J.W., van der Bom J.G., Atsma D.E., Bootsma M., Zeppenfeld K., van der Wall E.E., Schalij M.J. Three-Year Outcome of Sirolimus-Eluting versus Bare-Metal Stents for the Treatment of ST-Segment Elevation Myocardial Infarction (from the MISSION! Intervention Study) Am. J. Cardiol. 2010;106:4–12. doi: 10.1016/j.amjcard.2010.02.005. [DOI] [PubMed] [Google Scholar]
- 73.Dibra A., Tiroch K., Schulz S., Kelbæk H., Spaulding C., Laarman G., Valgimigli M., Di Lorenzo E., Kaiser C., Tierala I., et al. Drug-eluting stents in acute myocardial infarction: Updated meta-analysis of randomized trials. Clin. Res. Cardiol. 2010;99:345–357. doi: 10.1007/s00392-010-0133-y. [DOI] [PubMed] [Google Scholar]
- 74.Konorza T.F.M. Randomized comparison of titanium-nitride-oxide coated stents with zotarolimus-eluting stents for coronary revascularisation ORIGINAL (NON-ENGLISH) TITLE Prospektive randomisierte studie zum vergleich des titan-nitrid- beschichteten stents mit dem zo-tarolimus-freisetzenden stent zur koronarrevaskularisation. HERZ. 2010;35:364. doi: 10.1007/s00059-010-3359-6. [DOI] [Google Scholar]
- 75.Morice M., Van Hout B., Berland J., Bessou J., Carrié D., Dawkins K., Mohr F., Serruys P. Economic evaluation of the French subset of the SYNTAX trial at 1 year: Drug-eluting stents compared with bypass surgery for patients with 3-vessel and/or left main coronary artery disease. Arch. Cardiovasc. Dis. Suppl. 2010;2:20. doi: 10.1016/S1878-6480(10)70061-6. [DOI] [Google Scholar]
- 76.Moschovitis A., Simon R., Seidenstucker A., Klauss V., Baylacher M., Luscher T.F., Moccetti T., Windecker S., Meier B., Hess O.M. Randomised comparison of titanium-nitride-oxide coated stents with bare metal stents: Five year follow-up of the TiNOX trial. EuroInterv. J. EuroPCR Collab. Work. Group Interv. Cardiol. Eur. Soc. Cardiol. 2010;6:63–68. doi: 10.4244/EIJV6I1A10. [DOI] [PubMed] [Google Scholar]
- 77.Karjalainen P., Nammas W. Bioactive stents for percutaneous coronary intervention: A new forerunner on the track. Interv. Cardiol. 2011;3:527–529. doi: 10.2217/ica.11.61. [DOI] [Google Scholar]
- 78.Boden H., van der Hoeven B.L., Liem S.S., Atary J.Z., Cannegieter S.C., Atsma D.E., Bootsma M., Jukema J.W., Zeppenfeld K., Oemrawsingh P.V., et al. Five-year clinical follow-up from the MISSION! Intervention Study: Sirolimus-eluting stent versus bare metal stent implantation in patients with ST-segment elevation myocardial infarction, a randomised controlled trial. EuroInterv. J. EuroPCR Collab. Work. Group Interv. Cardiol. Eur. Soc. Cardiol. 2012;7:1021–1029. doi: 10.4244/EIJV7I9A164. [DOI] [PubMed] [Google Scholar]
- 79.De Luca G., Dirksen M., Spaulding C., Kelbæk H., Schalij M., Thuesen L., Van Der Hoeven B., Vink M., Kaiser C., Musto C., et al. Drug-eluting vs. bare-metal stents in primary angioplasty: A pooled patient-level meta-analysis of randomized trials. Arch. Intern. Med. 2012;172:611–621. doi: 10.1001/archinternmed.2012.758. [DOI] [PubMed] [Google Scholar]
- 80.Lehtinen T., Airaksinen K.E., Ylitalo A., Karjalainen P.P. Stent strut coverage of titanium-nitride-oxide coated stent compared to paclitaxel-eluting stent in acute myocardial infarction: TITAX-OCT study. Int. J. Cardiovasc. Imaging. 2012;28:1859–1866. doi: 10.1007/s10554-012-0032-6. [DOI] [PubMed] [Google Scholar]
- 81.Nct The Titan versus Everolimus Intracoronary Stent (Xience V) in Diabetic Patients. [(accessed on 27 August 2022)];2012 Available online: https://clinicaltrials.gov/show/NCT01510509.
- 82.Tuomainen P., Ylitalo A., Niemelä M., Kervinen K., Pietilä M., Sia J., Nyman K., Nammas W., Airaksinen K., Karjalainen P. Gender-based analysis of the 3-year outcome of bioactive stents versus paclitaxel-eluting stents in patients with acute myocardial infarction: An insight from the TITAX-AMI trial. J. Invasive Cardiol. 2012;24:104–108. [PubMed] [Google Scholar]
- 83.De Luca G., Dirksen M., Spaulding C., Kelbk H., Schalij M., Thuesen L., Van der Hoeven B., Vink M.A., Kaiser C., Musto C., et al. Impact of Diabetes on Long-Term Outcome After Primary Angioplasty. Diabetes Care. 2013;36:1020–1025. doi: 10.2337/dc12-1507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.De Luca G., Dirksen M., Spaulding C., Kelbæk H., Schalij M., Thuesen L., van der Hoeven B., Vink M., Kaiser C., Musto C., et al. Time course, predictors and clinical implications of stent thrombosis following primary angioplasty: Insights from the DESERT cooperation. Thromb. Haemost. 2013;110:826–833. doi: 10.1160/th13-02-0092. [DOI] [PubMed] [Google Scholar]
- 85.De Luca G., Dirksen M., Spaulding C., Kelbæk H., Schalij M., Thuesen L., van der Hoeven B., Vink M., Kaiser C., Musto C., et al. Meta-analysis comparing efficacy and safety of first generation drug-eluting stents to bare-metal stents in patients with diabetes mellitus undergoing primary percutaneous coronary intervention. Am. J. Cardiol. 2013;111:1295–1304. doi: 10.1016/j.amjcard.2013.01.281. [DOI] [PubMed] [Google Scholar]
- 86.Karjalainen P. Neointimal coverage and vasodilator response to titanium-nitride-oxide- coated bioactive stents and everolimus-eluting stents in patients with acute coronary syndrome: Insights from the BASE-ACS trial. Int. J. Cardiovasc. Imaging. 2013;29:1693–1703. doi: 10.1007/s10554-013-0285-8. [DOI] [PubMed] [Google Scholar]
- 87.Romppanen H., Nammas W., Kervinen K., Mikkelsson J., Pietilä M., Lalmand J., Rivero-Crespo F., Pentikäinen M., Tedjokusumo P., Karjalainen P.P. Outcome of ST-elevation myocardial infarction versus non-ST-elevation acute coronary syndrome treated with titanium-nitride-oxide-coated versus everolimus-eluting stents: Insights from the BASE-ACS trial. Minerva Cardioangiol. 2013;61:201–209. [PubMed] [Google Scholar]
- 88.Velders M.A., Boden H., van der Hoeven B.L., Liem S.S., Atary J.Z., van der Wall E.E., Jukema J.W., Schalij M.J. Long-term outcome of second-generation everolimus-eluting stents and Endeavor zotarolimus-eluting stents in a prospective registry of ST-elevation myocardial infarction patients. EuroInterv. J. EuroPCR Collab. Work. Group Interv. Cardiol. Eur. Soc. Cardiol. 2013;8:1199–1206. doi: 10.4244/EIJV8I10A184. [DOI] [PubMed] [Google Scholar]
- 89.De Luca G., Dirksen M., Spaulding C., Kelbæk H., Schalij M., Thuesen L., van der Hoeven B., Vink M., Kaiser C., Musto C., et al. Drug-eluting stents in patients with anterior STEMI undergoing primary angioplasty: A substudy of the DESERT cooperation. Clin. Res. Cardiol. 2014;103:685–699. doi: 10.1007/s00392-014-0702-6. [DOI] [PubMed] [Google Scholar]
- 90.Huang Y., Ng H., Ng X., Subbu V. Drug-eluting biostable and erodible stents. J. Control. Release. 2014;193:188–201. doi: 10.1016/j.jconrel.2014.05.011. [DOI] [PubMed] [Google Scholar]
- 91.Karjalainen P., Nammas W., Airaksinen J. Optimal stent design: Past, present and future. Interv. Cardiol. 2014;6:29–44. doi: 10.2217/ica.13.84. [DOI] [Google Scholar]
- 92.Ribamar C., Almeida B., Costa R., Chamié D., Abizaid A., Perin M., Staico R., Feres F., Siqueira D., Veloso M., et al. Comparison of drug-eluting stents with durable or bioabsorbable polymer: Intracoronary ultrasound results of the BIOACTIVE trial ORIGINAL (NON-ENGLISH) TITLE Comparação de stents farmacológicos com polímero durável ou biorreabsorvível: Resultados do ultrassom intracoronário do estudo BIOACTIVE. Rev. Bras. Cardiol. Invasiva. 2014;22:3. [Google Scholar]
- 93.Tuomainen P., Sia J., Nammas W., Niemelä M., Airaksinen J., Biancari F., Karjalainen P. Pooled analysis of two randomized trials comparing titanium-nitride-oxide-coated stent versus drug-eluting stent in STEMI. Rev. Esp. Cardiol. (Engl. Ed.) 2014;67:531–537. doi: 10.1016/j.recesp.2014.01.025. [DOI] [PubMed] [Google Scholar]
- 94.Bosiers M., Deloose K., Callaert J., Verbist J., Hendriks J., Lauwers P., Schroë H., Lansink W., Scheinert D., Schmidt A., et al. Superiority of stent-grafts for in-stent restenosis in the superficial femoral artery: Twelve-month results from a multicenter randomized trial. J. Endovasc. Ther. 2015;22:1–10. doi: 10.1177/1526602814564385. [DOI] [PubMed] [Google Scholar]
- 95.Chamié D., Almeida B.O., Grandi F., Filho E.M., Costa J.R., Costa R., Staico R., Siqueira D., Feres F., Tanajura L.F., et al. Vascular response after implantation of biolimus A9-eluting stent with bioabsorbable polymer and everolimus-eluting stents with durable polymer. Results of the optical coherence tomography analysis of the BIOACTIVE randomized trial ORIGINAL (NON-ENGLISH) TITLE Resposta vascular após implante de stents liberadores de biolimus A9 com polímero bioabsorvível e stents liberadores de everolimus com polímero durável. Resultados da análise de tomografia de coerência óptica do estudo randomizado BIOACTIVE. Rev. Bras. Cardiol. Invasiva. 2015;23:28–37. doi: 10.1016/j.rbciev.2015.02.001. [DOI] [Google Scholar]
- 96.Sia J., Nammas W., Niemelä M., Airaksinen J.K.E., Lalmand J., Laine M., Tedjokusumo P., Nyman K., Biancari F., Karjalainen P.P. Gender-based analysis of randomized comparison of bioactive versus everolimus-eluting stents in acute coronary syndrome. J. Cardiovasc. Med. 2015;16:197–203. doi: 10.2459/JCM.0000000000000086. [DOI] [PubMed] [Google Scholar]
- 97.Das M. Current status on drug-eluting devices in dialysis access. CardioVascular Interv. Radiol. 2016;39:S58–S59. doi: 10.1007/s00270-016-1405-3. [DOI] [Google Scholar]
- 98.Karjalainen P.P., Niemelä M., Pietilä M., Sia J., de Belder A., Rivero-Crespo F., de Bruyne B., Nammas W. 4-Year outcome of bioactive stents versus everolimus-eluting stents in acute coronary syndrome. Scand. Cardiovasc. J. 2016;50:218–223. doi: 10.1080/14017431.2016.1177198. [DOI] [PubMed] [Google Scholar]
- 99.Varho V., Kiviniemi T.O., Nammas W., Sia J., Romppanen H., Pietilä M., Airaksinen J.K., Mikkelsson J., Tuomainen P., Perälä A., et al. Early vascular healing after titanium-nitride-oxide-coated stent versus platinum-chromium everolimus-eluting stent implantation in patients with acute coronary syndrome. Int. J. Cardiovasc. Imaging. 2016;32:1031–1039. doi: 10.1007/s10554-016-0871-7. [DOI] [PubMed] [Google Scholar]
- 100.Disclosed N. ESC 2017: Hexacath’s Titanium-Nitride-Oxide Coated Stent Matches Boston Scientific’s Synergy for Acute Coronary Syndrome. 2017. [(accessed on 27 August 2022)]. Available online: https://cardiovascularnews.com/esc-2017-hexacaths-titanium-nitride-oxide-coated-stent-matches-boston-scientifics-synergy-acute-coronary-syndrome/
- 101.Disclosed N. Study Results: Titaniam-Nitride-Oxide (TiNO) Coated Stent is Non-Inferior to Bioabsorbable Polymer Everolimus-eluting Stent (EES), for Patients Presenting with Acute Coronary Syndrome (ACS) 2017. [(accessed on 27 August 2022)]. Available online: https://www.emjreviews.com/cardiology/news/study-results-titanium-nitride-oxide-tino-coated-stent-is-non-inferior-to-bioabsorbable-polymer-everolimus-eluting-stent-ees-for-patients-presenting-with-acute-coronary-syndrome-acs/
- 102.Karjalainen P., Paana T., Ylitalo A., Sia J., Nammas W. Optical coherence tomography follow-up 18 months after titanium-nitride-oxide-coated versus everolimus-eluting stent implantation in patients with acute coronary syndrome. Acta Radiol. 2017;58:1077–1084. doi: 10.1177/0284185116683573. [DOI] [PubMed] [Google Scholar]
- 103.Karjalainen P.P., Nammas W. Titanium-nitride-oxide-coated coronary stents: Insights from the available evidence. Ann. Med. 2017;49:299–309. doi: 10.1080/07853890.2016.1244353. [DOI] [PubMed] [Google Scholar]
- 104.Karjalainen P.P., Niemelä M., Laine M., Airaksinen J.K.E., Ylitalo A., Nammas W. Usefulness of Post-coronary Dilation to Prevent Recurrent Myocardial Infarction in Patients Treated With Percutaneous Coronary Intervention for Acute Coronary Syndrome (from the BASE ACS Trial) Am. J. Cardiol. 2017;119:345–350. doi: 10.1016/j.amjcard.2016.09.057. [DOI] [PubMed] [Google Scholar]
- 105.Nammas W., Airaksinen J.K.E., Romppanen H., Sia J., de Belder A., Karjalainen P.P. Impact of Preexisting Vascular Disease on the Outcome of Patients With Acute Coronary Syndrome: Insights From the Comparison of Bioactive Stent to the Everolimus-Eluting Stent in Acute Coronary Syndrome Trial. Angiology. 2017;68:513–518. doi: 10.1177/0003319716664266. [DOI] [PubMed] [Google Scholar]
- 106.Nammas W., de Belder A., Niemelä M., Sia J., Romppanen H., Laine M., Karjalainen P. Long-term clinical outcome of elderly patients with acute coronary syndrome treated with early percutaneous coronary intervention: Insights from the BASE ACS randomized controlled trial: Bioactive versus everolimus-eluting stents in elderly patients. Eur. J. Intern. Med. 2017;37:43–48. doi: 10.1016/j.ejim.2016.07.027. [DOI] [PubMed] [Google Scholar]
- 107.Daoud F., Letinier L., Moore N., Coste P., Karjalainen P. Efficacy and safety of percutaneous coronary interventions using titanium-nitride-oxide coated bioactive stents versus drug-eluting stents in coronary artery disease. A systematic literature review and meta-analysis. Circulation. 2018;138((Suppl. 1)):A15005. [Google Scholar]
- 108.de Winter R.J., Katagiri Y., Asano T., Milewski K.P., Lurz P., Buszman P., Jessurun G.A.J., Koch K.T., Troquay R.P.T., Hamer B.J.B., et al. A sirolimus-eluting bioabsorbable polymer-coated stent (MiStent) versus an everolimus-eluting durable polymer stent (Xience) after percutaneous coronary intervention (DESSOLVE III): A randomised, single-blind, multicentre, non-inferiority, phase 3 trial. Lancet. 2018;391:431–440. doi: 10.1016/S0140-6736(17)33103-3. [DOI] [PubMed] [Google Scholar]
- 109.Hsu C., Kwan G., Singh D., Rophael J., Anthony C., van Driel M. Angioplasty versus stenting for infrapopliteal arterial lesions in chronic limb-threatening ischaemia. Cochrane Database Syst. Rev. 2018 doi: 10.1002/14651858.CD009195.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Hernandez J.M.D., Moreno R., Gonzalo N., Rivera R., Linares J.A., Fernandez G.V., Menchero A.G., del Blanco B.G., Hernandez F., Gonzalez T.B., et al. The Pt-Cr everolimus-eluting stent with bioabsorbable polymer in the treatment of patients with acute coronary syndromes. Results from the SYNERGY ACS registry. Cardiovasc. Revasc. Med. 2019;20:705–710. doi: 10.1016/j.carrev.2018.10.019. [DOI] [PubMed] [Google Scholar]
- 111.Kayssi A., Al-Jundi W., Papia G., Kucey D., Forbes T., Rajan D., Neville R., Dueck A. Drug-eluting balloon angioplasty versus uncoated balloon angioplasty for the treatment of in-stent restenosis of the femoropopliteal arteries. Cochrane Database Syst. Rev. 2019 doi: 10.1002/14651858.CD012510.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Kuroda K., Otake H., Shinohara M., Kuroda M., Tsuda S., Toba T., Nagano Y., Toh R., Ishida T., Shinke T., et al. Effect of rosuvastatin and eicosapentaenoic acid on neoatherosclerosis: The LINK-IT Trial. EuroInterv. J. EuroPCR Collab. Work. Group Interv. Cardiol. Eur. Soc. Cardiol. 2019;15:E1099–E1106. doi: 10.4244/EIJ-D-18-01073. [DOI] [PubMed] [Google Scholar]
- 113.Nct Rationale and Design of the Web basEd soCial Media tecHnology to Improvement in Adherence to Dual anTiplatelet Therapy Following Drug-Eluting Stent Implantation(WECHAT) [(accessed on 27 August 2022)];2019 doi: 10.1136/bmjopen-2019-033017. Available online: https://clinicaltrials.gov/show/NCT04119726. [DOI] [PMC free article] [PubMed]
- 114.Kuno T., Takahashi M., Hamaya R. The Randomized TIDES-ACS Trial. JACC: Cardiovasc. Interv. 2020;13:2444. doi: 10.1016/j.jcin.2020.08.004. [DOI] [PubMed] [Google Scholar]
- 115.Lemkes J.S., Janssens G.N., Van Der Hoeven N.W., Jewbali L.S.D., Dubois E.A., Meuwissen M.M., Rijpstra T.A., Bosker H.A., Blans M.J., Bleeker G.B., et al. Coronary Angiography after Cardiac Arrest without ST Segment Elevation: One-Year Outcomes of the COACT Randomized Clinical Trial. JAMA Cardiol. 2020;5:1358–1365. doi: 10.1001/jamacardio.2020.3670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Sun G., Lei L., Liu L., Liu J., He Y., Guo Z., Dai X., He L., Chen S., Liang Y., et al. Rationale and design of the Web-basEd soCial media tecHnology to improvement in Adherence to dual anTiplatelet Therapy following Drug-Eluting Stent Implantation (WECHAT): Protocol for a randomised controlled study. BMJ Open. 2020;10:e033017. doi: 10.1136/bmjopen-2019-033017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Wardle B., Ambler G., Radwan R., Hinchliffe R., Twine C. Atherectomy for peripheral arterial disease. Cochrane Database Syst. Rev. 2020 doi: 10.1002/14651858.CD006680.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Gómez-Lara J., Oyarzabal L., Brugaletta S., Salvatella N., Romaguera R., Roura G., Fuentes L., Pérez Fuentes P., Ortega-Paz L., Ferreiro J.L., et al. Coronary endothelial and microvascular function distal to polymer-free and endothelial cell-capturing drug-eluting stents. The randomized FUNCOMBO trial. Rev. Esp. Cardiol. (Engl. Ed.) 2021;74:1013–1022. doi: 10.1016/j.rec.2021.01.007. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Not applicable.