Abstract
Comparison of the phenotypic expression of Mycoplasma gallisepticum strain R low (passage 15) to that of strain R high (passage 164) revealed that three proteins, i.e., the cytadhesin molecule GapA, a 116-kDa protein (p116), and a 45-kDa protein (p45), are missing in strain R high. Sequence analysis confirmed that the insertion of an adenine 105 bp downstream of the gapA translational start codon resulted in premature termination of translation in R high. A second adenine insertion had also occurred at position 907. Restoration of expression of wild-type gapA in R high (clone designated GT5) allowed us to evaluate the extent to which the diminished cytadherence capacity could be attributed to GapA alone. The results indicated that GT5 attached to the same limited extent as the parental R high, from which it was derived. The cytadherence capability of the parental R high was not restored solely by gapA complementation alone, indicating that either p116 or p45 or both may play a role in the overall cytadherence process. The gene encoding p116 was found to be immediately downstream of gapA in the same operon and was designated crmA. This gene exhibited striking homology to genes encoding molecules with cytadhesin-related functions in both Mycoplasma pneumoniae and Mycoplasma genitalium. Transcriptional analysis revealed that crmA is not transcribed in R high. We are currently constructing a shuttle vector containing both the wild-type gapA and crmA for transformation into R high to assess the role of CrmA in the cytadherence process.
Consistent with the interactions of typical noninvasive pathogenic bacteria and their host cells, Mycoplasma gallisepticum must first establish a specific and firm attachment to its target cell through a process known as cytadhesion in order to avoid rapid clearance by innate host defense mechanisms. This is a prerequisite for the initiation of the processes that result in host cell alterations and pathogenesis. Recent studies on the molecular mechanisms of M. gallisepticum cytadherence have revealed a complex multifactorial cytadhesion process involving the coordinate action of the primary cytadhesin molecule, GapA, while implicating additional proteins as potential cytadherence-related molecules in a manner reminiscent of the complex cytadherence mechanism of the human respiratory pathogen Mycoplasma pneumoniae. M. pneumoniae cytadherence has been demonstrated to involve the coordinate action of the primary cytadhesin molecule, P1 (11, 12, 21), in concert with an array of high-molecular-weight accessory proteins (7, 8). Prolonged colonization and survival of M. gallisepticum in the host requires maximal utilization of cytadhesin molecules in association with the cytadherence-related molecules. This ultimately confers a selective advantage for that strain of pathogenic M. gallisepticum over those with diminished cytadherence capacity. The aim of this study was to characterize the molecular basis for the diminished cytadherence capacity as well as to investigate the biochemical and molecular properties of the molecules observed to be involved in the phenotypic alterations in the avirulent R high strain.
MATERIALS AND METHODS
Organisms and culture conditions.
M. gallisepticum strains R low (passage 15) and R high (passage 164) (gifts from Sharon Levisohn, Department of Food Animal and Equine Medicine, College of Veterinary Medicine and Poultry Diseases, North Carolina State University, Raleigh) were cultured in Frey's medium (3) at 37°C until mid-logarithmic phase was achieved. Escherichia coli INVαF′ (Invitrogen, Carlsbad, Calif.) and E. coli XL1-Blue competent cells were grown in Luria broth (10 g of tryptone, 5 g of yeast extract, and 5 g of NaCl per liter) and on Luria plates at 37°C.
SDS-PAGE and amino acid analysis.
Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) was performed according to the protocol described by Laemmli (15). Triton X-114 (TX-114) phase partitioning was performed according to the method of Bordier (1). Proteins that were identified as differentially expressed were excised and electroeluted from a polyacrylamide gel using a Bio-Rad electroelution unit. The eluted proteins were run into SDS–10% polyacrylamide gels and then subjected to in-gel digestion with Lys-C protease, followed by sequence analysis according to the procedure of Edman and Begg (2).
Immunoblot analysis.
Western blots of R low, R high, and GT5 SDS-PAGE-separated proteins were transferred to BA-S85 nitrocellulose (Schleicher & Schuell, Inc., Keene, N.H.) according to the protocol described by Towbin et al. (22). For colony immunoblots, nitrocellulose membranes (82-mm diameter and 0.45-μm pore size) were placed onto M. gallisepticum colonies grown on agar plates as described by Goh et al. (6). All membranes were blocked with 5% bovine serum albumin in phosphate-buffered saline (PBS) at 4°C overnight and washed three times with PBS-Tween. The membranes were reacted with rabbit anti-GapA serum at a dilution of 1:8,000, incubated for 1 h at 37°C with gentle rocking, and washed three times with PBS-Tween. Peroxidase-conjugated goat anti-rabbit immunoglobulin G (heavy plus light chains) (Sigma, St. Louis, Mo.) was added at a final concentration of 1:7,500, and the membranes were incubated at room temperature for 1 h with gentle rocking. The membranes were washed three times with PBS, and the reaction products were visualized by the addition of 4-chloro-1-naphthol in the presence of hydrogen peroxide.
Nucleic acid extractions.
R low (passage 15), R high (passage 164), and GT5 genomic DNAs were extracted according to the method of Hempstead (9), and total RNAs were extracted from 30 ml of mid-logarithmic-phase culture according to the hot-phenol extraction protocol described by Sambrook et al. (19) The concentrations and purities were determined spectrophotometrically using absorbance measurements at 260 nm and ratios of absorbance measurements at 260 and 280 nm, respectively (19). Plasmid DNAs were extracted using either the Qiagen Plasmid Midi kit protocol as described by the manufacturer (Qiagen, Inc., Santa Clarita, Calif.) or miniplasmid preparations according to the protocol described by Holmes and Quigley (10).
PCR and RT-PCR.
PCRs were performed in a total volume of 50 μl containing 50 ng of template; a 250 mM concentration each of dATP, dTTP, dGTP, and dCTP; 1.5 mM MgCl2; 400 ng of each primer (synthesized by the University of Connecticut Biotechnology Center); and 2.5 U of AmpliTaq (Applied Biosystem/Perkin Elmer, Norwalk, Conn.) The targeted regions were amplified under the following conditions: 25 cycles of 94°C for 1 min, 5°C below the melting temperature of the primer for 1 min, and 72°C for 2 min, followed by 1 cycle at 72°C for 10 min.
The GeneAmp RNA PCR kit (Perkin-Elmer Roche, Branchburg, N.J.) was used to generate R low and R high cDNAs. Reverse transcriptase PCRs (RT-PCRs) were performed in a total volume of 20 μl containing 5 mM MgCl2; a 1mM concentration each of dATP, dCTP, dTTP, and dGTP; 1 U of RNase inhibitor (1 U/ml); of 2.5 U of murine leukemia virus reverse transcriptase (2.5 U/ml); 2.5 mM random hexamers and 1 μg template. A Perkin-Elmer model 480 thermal cycler was used, and the reactions proceeded under the following conditions: one cycle of 42°C for 15 min, 99°C for 5 min, and 5°C for 5 min. Subsequent PCR amplifications of the respective cDNAs were performed as described above. The primer pairs utilized are described in Table 1.
TABLE 1.
Primer pairs used to amplify gapA and crmA from M. gallisepticum
| Gene or procedure | Amplicon | Size (bp) | Primers | Positiona |
|---|---|---|---|---|
| gapA | A | 1,008 | 5′ AGA CCA AAC TTC CCT AAC 3′ (forward) | 1–1008 |
| 5′ CCT CCT CCA GCA GCA CTA 3′ (reverse) | ||||
| B | 462 | 5′ GCC GGA TTG ATT TGT ATG 3′ (forward) | 644–1105 | |
| 5′ CAG AAG TAG AAG CAG TAG GA 3′ (reverse) | ||||
| C | 725 | 5′ TGA TAA TCC TAA TGC TGT 3′ (forward) | 1447–2171 | |
| 5′ ACT TGT TTT GTG TTT CC 3′ (reverse) | ||||
| B′/C | 1,511 | 5′ GCC GGA TTG ATT TGT ATG 3′ (forward) | 661–2171 | |
| 5′ ACT TGT TTT GTG TTT CC 3′(reverse) | ||||
| D | 1,135 | 5′ ATT AGT AAG CCA GCT GGT 3′ (forward) | 2137–3471 | |
| 5′ CTT ACG GTT TTG AGA CAT TG 3′ (reverse) | ||||
| E | 1,050 | 5′ TAA TGT AAT CGG TCA AGG TGC 3′ (forward) | 3042–4091 | |
| 5′ CCA GCA AAA TCA TCA CTT AG 3′ (reverse) | ||||
| crmA | F | 1,507 | 5′ CTA AGT GAT GAT TTT GCT GG 3′ (forward) | 4071–5577 |
| 5′ ARR TAR TTD CCC ATD GCD GG 3′ (reverse) | ||||
| G | 850 | 5′ CAA GAG TAG GTA CTG AAA CC 3′ (forward) | 5510–6359 | |
| 5′ SWY TGR TTW GTW ACR AA 3′ (reverse) | ||||
| H | 2,024 | 5′ GTG GCT AGA AAC TTC GTT ACA 3′ (forward) | 6331–8354 | |
| 5′ CKR TTY TCR TCN GTY CRT T 3′ (reverse) | ||||
| RT-PCR | I | 462 | 5′ GCC GGA TTG ATT TGT ATG 3′ (forward) | 644–1105 |
| 5′ CAG AAG TAG AAG CAG TAG GA 3′ (reverse) | ||||
| J | 350 | 5′ TAA GAA GAC TCC ACA AAT GCT 3′ (forward) | 3579–3928 | |
| 5′ TAG CAT CTA GCG TTC TTG CTT G 3′ (reverse) |
Positions refer to locations in gapA or crmA.
Cloning and transformation.
PCR products were cloned into the PCRII vector of the TA cloning kit according to the protocol of the manufacturer (Invitrogen). The vectors containing the correct inserts were transformed into E. coli INVαF′ (Invitrogen) competent cells according to the manufacturer's protocol. White colonies were selected and the inserts were sequenced, as described below. Plasmid, pISM2062, containing the modified transposon Tn4001mod (14), was used as the vector to insert wild-type gapA into a GapA− clonal isolate of R high. The R high culture used for transformations was reconfirmed to be negative for gapA expression by immunoblotting immediately prior to use. A 4,112-bp fragment containing the gapA gene was amplified from M. gallisepticum strain R low using forward (5′ GGGGGATCCAGACCAAACTTCCCTAAC 3′) and reverse (5′ GGGGGATCCAGCAAAATCATCACTTAG 3′) primers. Tn4001mod contains a unique BamHI site at the end of the IS256L arm (7, 14). The fragment containing the gapA gene was cloned into the BamHI site of the Tn400lmod. Recombinant clones were selected with the insert oriented so that gapA was transcribed from an outward-reading promoter in IS256L. R high was transformed by a modification of the method of King and Dybvig (13). Briefly, organisms from 1 ml of overnight culture were harvested and washed in ST buffer (500 mM sucrose, 10 mM Tris [pH 6.5]). Washed cells were suspended in 500 μl of 100 mM CaCl2 and incubated on ice for 30 min. Yeast RNA (20 μg) and the Tn4001-gapA vector DNA (10 μg) were added along with 4 ml of 40% polyethylene glycol, and the suspension was incubated for 2 min at room temperature. The suspension was diluted with 20 ml of ST buffer, and the cells were harvested by centrifugation at 10,000 × g for 15 min. The cell pellet was suspended in 2 ml of Frey's medium (3) and incubated at 37°C for 3 h. After incubation, 50 μg of gentamicin ml−1 was added, and the broth cultures were incubated at 37°C overnight, plated on solid medium containing 15 μg of gentamicin ml−1, and incubated further at 37°C. Single colonies were picked, propagated, analyzed by immunoblotting, using anti-GapA serum, for the expression of gapA and by Southern hybridization (20) of HindIII-digested genomic DNA using both 32P-labeled gapA as a probe, and then reprobed with 32P-labeled Tn4001 DNA. The Southern hybridization conditions were as follows. Probes were incubated with the blot (42°C with 45% [vol/vol] formamide) for 16 h. Filters were washed twice with 2× SSC (300 mM NaCl, 30 mM sodium citrate, pH 7.0)–0.1% SDS and O.2× SSC–0.1% SDS for 3 min at room temperature, followed by two additional washes at 50°C in 0.16× SSC–0.1% SDS for 15 min. The filters were dried and exposed to film (Fuji Rx film; Fisher Scientific, Pittsburgh, Pa.) using intensifying screens. Those clones which were positive for GapA by immunoblotting and possessed the complemented wild-type copy of the gapA were analyzed further. The restriction fragment containing the Tn4001-gapA insert was excised and cloned into pBluescript SK11(+) (Stratagene), which had been previously digested with HindIII and treated with calf intestinal alkaline phosphatase, at a 2:1 insert DNA-to-vector ratio. The ligation mixture was transformed into E. coli XL1-Blue competent cells (19) and selected for on Luria broth plates containing 100 μg of ampicillin ml−1 and 25 μg of X-Gal (5-bromo-4-chloro-3-indolyl-β-d-galacto-pyranoside) ml−1. The DNA sequence was determined as described below (using oligonucleotide primers synthesized from the ends of the insertion sequence element and primer walking).
DNA sequencing.
Sequencing was accomplished by primer walking and was performed at the Keck Foundation Biotechnology Resource Laboratory at the Yale University School of Medicine in New Haven, Conn., and at the University of Connecticut Macromolecular Characterization Facility of the Biotechnology Center. DNA sequencing reactions were performed using a Taq DyeDeoxy Terminator Cycle Sequencing Kit according to the protocol of the manufacturer (Perkin-Elmer) and analyzed on an Applied Biosystems 373A Stretch DNA sequencer. DNAMAN (Lynnon BioSoft, Quebec, Canada) was used for the alignments of the amino acids. The stem-loop structures were determined using DNAMAN and MacDNASIS (Hitachi Software Engineering America, San Bruno, Calif.).
Pulse-chase analysis.
Pulse-chase analysis of R low and R high was performed by the method of Popham et al. (18) at both 37 and 4°C. The cells were then subjected to TX-114 phase partitioning, and the proteins were electrophoresed in a 10% polyacrylamide gel. They were transferred to BA-S85 nitrocellulose (Schleicher & Schuell, Inc.) and then dried and exposed to X-ray film (Fuji Rx film; Fisher Scientific) at −70°C for 48 h. Following autoradiographic analysis, the membranes were immunoblotted with anti-GapA serum as described above.
Cytadherence assay.
MRC-5 cell culture cytadherence assessments were performed as previously described (4, 5) using tritium-labeled M. gallisepticum R low, R high, and GT5.
Nucleotide sequence accession number.
The GenBank accession number for the sequence reported in this paper is AF214004.
RESULTS
Comparative analysis of R low and R high.
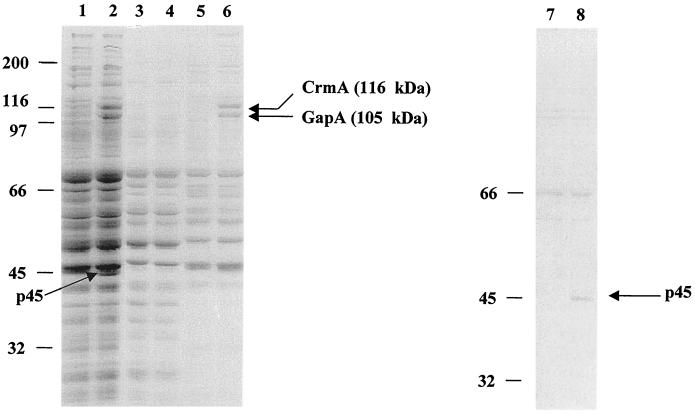
The analysis of both R low and R high following TX-114 phase partitioning and SDS-PAGE is shown in Fig. 1. GapA (lane 6) is missing from R high, as are two additional proteins; one in the insoluble phase with a molecular mass of 116 kDa (lane 6) and the other in the detergent phase with a molecular mass of 45 kDa (lane 8). These proteins are designated p116 and p45, respectively. The three proteins were isolated from R low and subjected to amino acid analysis after in-gel digestion with Lys-C protease to generate internal amino acid data (2). A peak from the suspected GapA protein was sequenced, resulting in 10 amino acids. This sequence was found to be 100% identical to our published GapA sequence (GenBank accession no. U44804). Western blot analysis (22) utilizing anti-GapA serum (Fig. 2A) resulted in a positive reaction in R low but not in R high. These data verified the identity of this protein (which is absent from R high) as GapA. Additionally, they indicated that the lack of expression is not a consequence of size variation or stable truncation of GapA, since no size variant was recognized. Southern blot analysis (20) was performed on both R low and R high to determine if the gapA gene was present in R high. The results indicated that the gene is present in both. RT-PCR amplification of gapA indicated that the gapA gene is also transcribed in both strains (Fig. 2C).
FIG. 1.
SDS-PAGE of M. gallisepticum strains R low and R high. Lanes 1 and 2, total protein profiles of R high (lane 1) and R low (lane 2). Lanes 3 to 8, detergent, aqueous, or insoluble phase obtained following TX-114 phase partitioning, as follows: lane 3, R high, aqueous; lane 4) R low, aqueous; 5, R high, insoluble; 6, R low, insoluble; 7, R high, detergent; 8, R low, detergent. The arrows point to proteins present in R low but missing in R high. The molecular mass markers are represented in kilodaltons.
FIG. 2.
Analysis of the gapA operon. (A) Western blot of R low and R high reacted with anti-GapA serum. The molecular mass markers are represented in kilodaltons. (B) Adenine insertion sites at positions 105 and 907 in gapA of R high generating TAA termination codons. (C)RT-PCR analysis utilizing primer pairs I and J in R high and R low. (D) Stem-loop structures found downstream of the TAA stop codon at positions 120 and 326.
The results of pulse-chase experiments at both 37 and 4°C indicated that gapA was expressed in R low but was not expressed in R high. Identical pulse-chase experiments followed by immunoblot analysis using anti-GapA serum confirmed that gapA is expressed in R low but not in R high, and no lower-molecular-mass products were detected (data not shown).
Comparative analysis of the DNA sequences of gapA from R low and R high revealed the insertion of an adenine 105 bp downstream of the translational start site of gapA in R high, resulting in a premature translational stop signal (TAA) (Fig. 2B). Insertion of a second adenine was also found at base 907, resulting in a second TAA codon.
MRC-5 cell culture attachment tests comparing the cytadherence capabilities of R low and R high indicated that R high attached with only 24% of the efficiency of R low (data not shown).
Complementation of R high with wild-type gapA.
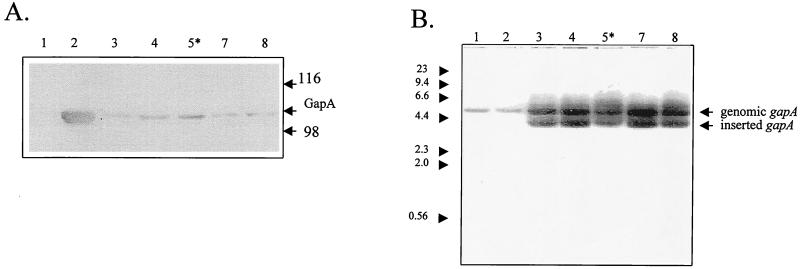
Figure 3A shows the results of anti-GapA immunoblot analysis of R high after transformation with pISM2062 containing the modified transposon Tn4001-gapA (pUCGgapA). All clones expressed gapA but not to wild-type levels. As shown in Fig. 3B, Southern hybridization of HindIII-digested genomic DNAs from these clones probed with 32P-labeled gapA resulted in hybridization with both the genomic, mutant gapA and the inserted copy of the wild-type gapA in each of the clones. The clone in lane 5 (designated GT5) was selected for further analysis.
FIG. 3.
Immunoblot and Southern hybridization analyses of M. gallisepticum R high clones transformed with Tn4001-gapA. (A) Anti-GapA immunoblot analysis. Lanes: 1, R high; 2, R low; 3 to 8, R high clones transformed with Tn4001-gapA. The molecular mass markers are represented in kilodaltons. The asterisk indicates clone GT5. (B) Southern blot analysis of HindIII-digested total genomic DNAs probed with 32P-labeled gapA. Lanes: 1, R high; 2, R low; 3 to 8, R high clones transformed with Tn4001-gapA. Molecular size markers are in kilobase pairs.
Colony immunoblotting of GT5 reacted with anti-GapA serum (data not shown) indicated that GapA was presented on the surface.
The results of cytadherence analysis indicated that GT5 attached to the same limited extent as the parental R high, from which it was derived (data not shown). The cytadherence capability of the parental R high was not restored solely by the insertion and expression of gapA alone.
The RT-PCR results using primers designed from the sequence of a suspected open reading frame (ORF) 5′ of the insertion site showed no amplification product. A BLAST search of the DNA sequence as well as negative RT-PCR results indicated that Tn4001-gapA did not insert into a transcribed ORF in GT5.
CrmA analysis.
Three peaks (8 to 10 amino acids each) from p45 and from p116 were also sequenced. One of the three amino acid peaks (FVIGGVPS) obtained from p116 matched with the translated product of our partial sequence of ORF 3 immediately downstream of gapA. Anchor PCR amplification employing a specific primer within the downstream sequence flanking gapA and the degenerate primer, designed from the amino acid sequence (VLTYPVMGGYLT) from a second peak of p116, resulted in an 1.5-kbp amplicon. A second PCR amplification using primer pair G (Table 1) resulted in an 850-bp fragment. The remainder of the gene was obtained using primer pair H (Table 1), resulting in a 2-kb amplified product. The entire ORF was sequenced and found to be a 3.2-kb gene encoding a 116-kDa protein (designated CrmA [for cytadherence-related molecule A]). Comparison of crmA sequences from R high and R low showed them to be identical. RT-PCR analysis of the crmA gene indicated that it is not transcribed in R high (Fig. 2C). Sequence comparisons revealed 41% overall amino acid identity with M. pneumoniae ORF 6 and MgpC of Mycoplasma genitalium. Hydrophobicity profiles and protein sequence analysis showed a striking homology among the last 250 amino acids of the C termini of these three proteins. These regions (Fig. 4) appear to be divided into two domains. The first domain (A) represents a surface-exposed region and is shared among cytadhesin-related molecules M. gallisepticum CrmA, M. pneumoniae ORF 6, and M. genitalium MgpC, with an overall 55% amino acid identity. The second domain (B) represents the transmembrane region and intracytoplasmic tail and is common not only among these three proteins (overall amino acid identity, 63%) but also among other mycoplasma adhesins (M. gallisepticum GapA, M. pneumoniae P1, M. genitalium MgPa [MYCGE-ADP1], and M. pirum P1-like adhesin). The overall amino acid identity among all seven of these proteins in this domain is 49%.
FIG. 4.
Amino acid domains shared among mycoplasma cytadhesins and cytadhesin-related molecules. Domain A reveals 55% shared amino acid identity among the cytadhesin-related molecules M. gallisepticum CrmA, M. pneumoniae MgpC (ORF 6), and M. genitalium MgpC. Domain B reveals 63% shared amino acid identity among the cytadhesin-related molecules stated above and 51% identity overall, including the cytadhesins M. gallisepticum GapA, M. pneumoniae P1 (ADP1), M. genitalium MgPa (ADP1), and the P1-like adhesin of M. pirum.
DISCUSSION
The high-passage R strain (R high) is a laboratory attenuated strain that resulted from multiple successive passages of the virulent R low in medium. It has previously been demonstrated (17) that R low is pathogenic for chickens, colonizing the trachea in in vivo assays and producing air sac and tracheal lesions. In contrast, R high required 4 orders of magnitude more organisms to colonize air sacs and tracheas yet produced no detectable lesions. The attenuation(s) which occurred diminished the organism's ability to adhere and to colonize in vivo. The molecular and biochemical nature of the attenuation(s) has not been evaluated until now. Our investigations have focused on the elucidation of the molecular basis for the diminished cytadherence and pathogenesis of R high. R high does not express the genes encoding three proteins, i.e., the cytadhesin molecule GapA, p116, and p45, which are expressed in R low. TX-114 phase partitioning and immunoblot analysis indicated that gapA was expressed in R low but was not expressed in R high. Pulse-chase experiments followed by immunoblot analysis using anti-GapA serum confirmed that gapA is expressed in R low but not in R high. RT-PCR results showed that gapA is transcribed in both strains. Comparative DNA sequence analysis of gapA from R low and from R high revealed that gapA is not expressed in R high due to the insertion of a single adenine at position 105, creating a stop codon and thereby resulting in a premature termination of translation. If a truncated protein was expressed, it would therefore measure approximately 4 kDa. No protein of this molecular mass was detected, indicating that a truncated protein (if expressed) was unstable and therefore immediately degraded.
In vitro assays comparing the cytadherence capabilities of R low and R high indicated that R high attached with only 24% of the efficiency of R low. The in vitro cell culture assay eliminates the innate host defense mechanisms, such as coughing and ciliary motility, which are further impediments to successful colonization by M. gallisepticum. Therefore, a greater number of organisms may adhere in the in vitro assay that might otherwise be swept away following exposure in the host animal. This likely accounts for the discrepancy noted between the number of R high organisms required for colonization in the host and the number that adhere in the cell culture assay. Restoration of expression of wild-type gapA in R high (GT5) allowed us to evaluate the extent to which the diminished cytadherence capacity could be attributed to GapA alone. Colony immunoblotting of clone GT5 reacted with anti-GapA serum indicated that GapA was exposed on the surface. It was essential for subsequent cytadherence analysis to be certain that GapA was exposed on the surface of the organism, thus allowing for proper interaction with its corresponding host cell receptor. The results of the in vitro cytadherence analysis indicated that clone GT5 attached to the same limited extent as the parental R high, from which it was derived. The cytadherence capability of the parental R high was not restored solely by the insertion and expression of gapA alone. The Tn4001-gapA insertion site in clone GT5 was then analyzed to determine whether or not the Tn4001-gapA inserted into a transcribed ORF which might encode a protein of yet-unknown function that might affect cytadherence. The sequence and RT-PCR analyses indicated that Tn4001-gapA did not insert into a transcribed ORF in clone GT5. These data suggested that one or both of the other proteins, p116 and/or p45, which are missing from R high, may play a role either in the presentation and localization of GapA or in some other cytadherence-related molecule functional capacity.
Amino acid sequence data from the peptide fragments from p116 allowed us to design degenerate oligonucleotide primers. These were then used in PCRs to obtain a partial DNA sequence and ultimately to identify the entire gene encoding p116. This gene, designated crmA, was determined to be immediately downstream of gapA (previously referred to as ORF 3) in the operon (6). The DNA sequence of crmA is identical in both R low and R high, while RT-PCR analysis demonstrated that crmA is transcribed in R low but not in R high. Lack of crmA transcription in R high is likely a consequence of the premature termination of translation of gapA upstream and of the stem-loop structures found 15 bp (ΔG −5.3 kcal) and 221 bp (ΔG −9.9 kcal) downstream of the TAA codon (Fig. 2D). The high degree of amino acid sequence identity exhibited by CrmA to ORF 6 of M. pneumoniae and to MgpC of M. genitalium suggests a functional conservation among molecules associated with and essential for effective cytadherence in these pathogenic mycoplasmas. ORF 6 of M. pneumoniae encodes a 130-kDa protein which is cleaved into a 90- and 40-kDa proteins which have been shown to be cytadherence-associated membrane proteins involved in tip structure formation (16).
The gene encoding the 45-kDa protein is not a part of the gapA operon and has not been fully characterized at this time, but it is a subject of our continuing investigation. We are currently constructing a Tn4001-gapA/crmA vector (pUCGgapA/crmA) for the purpose of transformation into R high to assess the degree of restoration of cytadherence capability. This will also provide valuable insights into the interaction of CrmA and GapA and the overall cytadherence process.
ACKNOWLEDGMENTS
This research was supported in part by USDA Agricultural Experiment Station grant CONSOO640 (to S.J.G.).
We thank Timothy Williams for critical review of the manuscript.
REFERENCES
- 1.Bordier C. Phase separation of integral membrane proteins in Triton-X 114 solution. J Biol Chem. 1981;256:1604–1607. [PubMed] [Google Scholar]
- 2.Edman P, Begg G. A protein sequenator. Automated equipment for sequence determination. Eur J Biochem. 1967;1:80–91. doi: 10.1007/978-3-662-25813-2_14. [DOI] [PubMed] [Google Scholar]
- 3.Frey M L, Hanson R P, Anderson D R. A medium for the isolation of avian mycoplasmas. Am J Vet Res. 1968;29:2163–2171. [PubMed] [Google Scholar]
- 4.Geary S J, Gabridge M G. Characterization of human lung fibroblast receptor site for Mycoplasma pneumoniae. Isr J Med Sci. 1987;23:462–468. [PubMed] [Google Scholar]
- 5.Geary S J, Gabridge M G, Intres R, Draper D L, Gladd M F. Identification of mycoplasma binding proteins utilizing a 100 kilodalton lung fibroblast receptor. J Receptor Res. 1989;9:465–478. doi: 10.3109/10799898909066071. [DOI] [PubMed] [Google Scholar]
- 6.Goh M S, Gorton T S, Forsyth M H, Troy K E, Geary S J. Molecular and biochemical analysis of a 105 kDa Mycoplasma gallisepticum cytadhesin (GapA) Microbiology. 1998;144:2941–2950. doi: 10.1099/00221287-144-11-2971. [DOI] [PubMed] [Google Scholar]
- 7.Hahn T W, Krebes K A, Krause D C. Expression in Mycoplasma pneumoniae of the recombinant gene encoding the cytadherence-associated protein HMW1 and identification of HMW4 as a product. Mol Microbiol. 1996;19:1085–1093. doi: 10.1046/j.1365-2958.1996.455985.x. [DOI] [PubMed] [Google Scholar]
- 8.Hahn T-W, Willby M J, Krause D C. HMW1 is required for cytadhesin P1 trafficking to the attachment organelle in Mycoplasma pneumoniae. J Bacteriol. 1998;180:1270–1276. doi: 10.1128/jb.180.5.1270-1276.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hempstead P G. An improved method for the rapid isolation of chromosomal DNA from Mycoplasma spp. Can J Microbiol. 1990;36:59–61. doi: 10.1139/m90-011. [DOI] [PubMed] [Google Scholar]
- 10.Holmes D S, Quigley M. A rapid boiling method for the preparation of bacterial plasmids. Anal Biochem. 1981;114:193–197. doi: 10.1016/0003-2697(81)90473-5. [DOI] [PubMed] [Google Scholar]
- 11.Inamine J M, Denny T P, Loechel S, Schaper U, Huang C-H, Bott K F, Hu P-C. Nucleotide sequence of the P1 attachment protein gene of Mycoplasma pneumoniae. Gene. 1988;64:217–229. doi: 10.1016/0378-1119(88)90337-x. [DOI] [PubMed] [Google Scholar]
- 12.Inamine J M, Loechel S, Hu P-C. Analysis of the nucleotide sequence of the P1 operon of Mycoplasma pneumoniae. Gene. 1988;73:175–183. doi: 10.1016/0378-1119(88)90323-x. [DOI] [PubMed] [Google Scholar]
- 13.King K W, Dybvig K. Plasmid transformation of Mycoplasma mycoides subspecies mycoides is promoted by high concentrations of polyethylene glycol. Plasmid. 1991;26:108–115. doi: 10.1016/0147-619x(91)90050-7. [DOI] [PubMed] [Google Scholar]
- 14.Knudtson K L, Minion F C. Constuction of Tn4001lac derivatives to be used as promoter probe vectors in mycoplasmas. Gene. 1993;137:217–222. doi: 10.1016/0378-1119(93)90009-r. [DOI] [PubMed] [Google Scholar]
- 15.Laemmli U K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 16.Layh-Schmitt G, Harkenthatl M. The 40-and 90-kDa membrane proteins (ORF 6 gene product) of Mycoplasma pneumoniae are responsible for the tip structure formation and P1 (adhesin) association with the Triton shell. FEMS Microbiol Lett. 1999;174:143–149. doi: 10.1111/j.1574-6968.1999.tb13561.x. [DOI] [PubMed] [Google Scholar]
- 17.Levisohn S, Dykstra M J, Lin M Y, Kleven S H. Comparison of in-vivo and in-vitro methods for pathogenicity evaluation for Mycoplasma gallisepticum in respiratory infection. Avian Pathol. 1986;15:233–246. doi: 10.1080/03079458608436284. [DOI] [PubMed] [Google Scholar]
- 18.Popham P L, Hahn T W, Krebes K A, Krause D C. Loss of HMW1 and HMW3 in noncytadhering mutants of Mycoplasma pneumoniae occurrs post-translationally. Proc Natl Acad Sci USA. 1997;94:13979–13984. doi: 10.1073/pnas.94.25.13979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1989. [Google Scholar]
- 20.Southern E. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975;98:503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- 21.Su C J, Chavoya A, Baseman J B. Regions of Mycoplasma pneumoniae cytadhesin P1 structural gene exist as multiple copies. Infect Immun. 1988;56:3157–3161. doi: 10.1128/iai.56.12.3157-3161.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Towbin H, Staehelin T, Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci USA. 1979;76:4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]