Abstract
Abscesses are a classic host response to infection by many pathogenic bacteria. The immunopathogenesis of this tissue response to infection has not been fully elucidated. Previous studies have suggested that T cells are involved in the pathologic process, but the role of these cells remains unclear. To delineate the mechanism by which T cells mediate abscess formation associated with intra-abdominal sepsis, the role of T-cell activation and the contribution of antigen-presenting cells via CD28-B7 costimulation were investigated. T cells activated in vitro by zwitterionic bacterial polysaccharides (Zps) known to induce abscess formation required CD28-B7 costimulation and, when adoptively transferred to the peritoneal cavity of naïve rats, promoted abscess formation. Blockade of T-cell activation via the CD28-B7 pathway in animals with CTLA4Ig prevented abscess formation following challenge with different bacterial pathogens, including Staphylococcus aureus, Bacteroides fragilis, and a combination of Enterococcus faecium and Bacteroides distasonis. In contrast, these animals had an increased abscess rate following in vivo T-cell activation via CD28 signaling. Abscess formation in vivo and T-cell activation in vitro required costimulation by B7-2 but not B7-1. These results demonstrate that abscess formation by pathogenic bacteria is under the control of a common effector mechanism that requires T-cell activation via the CD28–B7-2 pathway.
Abscess formation is a distinct pathological response to certain bacterial pathogens. In clinical situations, the development of abscesses associated with intra-abdominal sepsis causes chronic illness and can be fatal in infected patients. Bacteroides fragilis is the most commonly isolated anaerobic bacterium isolated from these cases (23). Studies with rodent models have shown that the ability of B. fragilis to cause these infections is mainly attributable to the presence of a unique capsular polysaccharide (CP) on this organism (22). Intraperitoneal implantation of a monomicrobial culture of B. fragilis or its purified capsular polysaccharide, PS A, in conjunction with sterile cecal contents promotes abscess formation (37). Abscess induction by PS A is dependent on the presence of positively and negatively charged groups associated with its repeating unit structure. Structurally distinct polymers that possess this zwitterionic charge motif, such as PS B from B. fragilis or the Streptococcus pneumoniae type 1 CP can also induce abscesses in this manner (37). The presence of positively charged groups on bacterial polysaccharides is rare, and those polysaccharides lacking the zwitterionic charge motif do not possess this activity.
Attempts to define the immunologic events leading to the development of abscesses by B. fragilis have been carried out with athymic or T-cell-depleted animals and suggest that T cells may be required for the induction of this host response (21, 28, 30). However, the mechanism by which these cells mediate this process is not known. Recently, we have demonstrated that zwitterionic bacterial polysaccharides (Zps) such as PS A and the S. pneumoniae type 1 CP activate human and rat CD4+ T cells in vitro, while bacterial polysaccharides lacking this charge motif did not have this activity (4, 15, 35). The activity was specific to these carbohydrates and not due to contaminating protein or lipopolysaccharide. T-cell activation in this system required the presence of class II-bearing antigen-presenting cells (APCs) and could be blocked by major histocompatibility complex class II-specific antibody (35). The strict cell-mediated control of the biologic properties associated with Zps conflicts with the current dogma regarding the immune response to this class of macromolecules and suggested that they have a direct effect on T cells in vivo.
Recent studies have shown that the interaction of CD28 on T cells with its ligands B7-1 and B7-2 on APCs is a major T-cell costimulatory pathway that controls the T-cell response to a variety of antigens (2, 29, 34). Ligation of CD28 with its counterreceptor B7 promotes cell cycle progression and increases interleukin-2 (IL-2) production by regulating IL-2 mRNA at the level of transcription and translation (9). The fusion protein CTLA4Ig contains the extracellular domain of CTLA4 (a homologue of CD28) fused to the human immunoglobulin G1 (IgG1) heavy chain (17) and functions to prevent engagement of the CD28-B7 axis. Blocking costimulatory signaling by anti-B7 monoclonal antibodies or the fusion protein CTLA4Ig, which binds with high affinity to B7-1 and B7-2, has been demonstrated to be very effective in inhibiting the immune responses in a variety of autoimmune and transplant models (5, 25, 29). In these studies, CTLA4Ig had a pronounced effect in reducing the levels of T-cell- and monocyte-derived cytokines and chemokines thought to play a key role in the disease process. Recently a clinical trial with CTLA4Ig in patients with psoriasis has been conducted with encouraging results (1).
In the present study, we investigated the relationship of T-cell activation by an unusual class of bacterial polysaccharides to the ability of certain bacteria to induce intra-abdominal abscess formation in animals. The results demonstrate that T-cell activation by Zps is mediated by the CD28-B7 pathway and that signaling via this interaction modulates intra-abdominal abscess formation by different bacterial pathogens.
MATERIALS AND METHODS
Bacterial strains and polysaccharide preparations.
B. fragilis NCTC 9343, Bacteroides distasonis 8503, and Enterococcus faecium 838970 were obtained from the Channing Laboratory stock culture collection. Staphylococcus aureus PS 80 was a kind gift from Jean Lee, Channing Laboratory. PS A from B. fragilis NCTC 9343 was prepared as previously described (38). PS A was isolated by hot phenol-water extraction, gel filtration chromatography, and isoelectric focusing. The S. pneumoniae type 1 CP was obtained from the American Type Culture Collection (Manassas, Va.) and treated with 2 M NaOH for 1 h at 80°C to remove the contaminating cell wall polysaccharide, C substance. Following purification by gel filtration chromatography, the polysaccharides were subjected to isoelectric focusing, dialyzed, lyophilized, and stored in 3 M NaCl to prevent aggregation. Polysaccharides were prepared in sterile, pyrogen-free saline for administration to animals.
Animal model for intra-abdominal sepsis and bacterial strains.
An animal model for intra-abdominal sepsis was utilized for these studies with some modification (36). Briefly, male Wistar rats (180 to 200 g; Charles River Laboratories, Wilmington, Mass.) were anesthetized with a single intraperitoneal injection of 0.15 ml of pentobarbital sodium (Nembutal, 50 mg/ml; Abbott Laboratories, North Chicago, Ill.). An anterior midline incision (0.5 cm) was made through the abdominal wall, and 0.5 ml of inoculum was inserted directly into the peritoneal cavity. Inocula for these experiments contained B. fragilis NCTC 9343 (108 CFU/rat), a mixture of B. distasonis (5 × 107 CFU/rat) and E. faecium (5 × 107 CFU/rat), S. aureus PS 80 (107 CFU/rat), or 20 μg of PS A mixed with rat sterile cecal contents (SCC) as described previously (36). The dose of S. aureus employed in these studies did not induce mortality in animals (data not shown). SCC are used as an adjuvant for abscess formation and do not induce the formation of abscesses when implanted alone into the peritonea of rats. The incisions were closed with silk sutures, and animals were returned to their cages. Six days later, surviving animals were necropsied and examined for intra-abdominal abscesses by two observers blind to the identity of each group. The presence of one or more abscesses in an animal as defined previously (36) was scored as a positive result.
CD28-B7 studies.
Human CTLA4Ig, Y100FIg, and the control L6Ig were from Bristol-Myers Squibb (Princeton, N.J.) (13). Human CTLA4Ig binds specifically to human or rat B7-1 and B7-2, while Y100FIg binds to human or rat B7-1 only. Binding to B7-2 is undetectable (13). For all in vivo experiments, CTLA4Ig and Y100FIg were administered at doses that have been shown to inhibit CD28-B7 interactions (26). The dose of B7-2-specific antibody used in these experiments was given according to the manufacturer's recommendation (see below). Fusion L6Ig was used as a control Ig fusion protein. This molecule has the same Ig heavy chain fused to an irrelevant protein. Rat CD28-, B7-1-, and B7-2-specific monoclonal antibodies (IgG1) were obtained from Pharmingen (San Diego, Calif.). An irrelevant IgG1 antibody was used as a control for these antibodies. A monoclonal antibody specific for CD28 (500 μg) that is known to positively signal for T-cell proliferation and IL-2 secretion was selected for use (32). For animal experiments, all fusion proteins and monoclonal antibodies were administered at the time (T) of challenge (T = 0) unless otherwise designated. For in vitro T-cell proliferation experiments, all fusion proteins were used at a concentration of 50 μg/ml, while the monoclonal antibody specific for rat CD28 was used at a concentration of 1 μg/ml.
T-cell proliferation assay.
For human CD4+ T-cell proliferation assays, cells were obtained from leukopacs (discarded white cells from anonymous platelet donors) as previously described (6, 12). Mononuclear cells were separated by Ficoll-Hypaque sedimentation to eliminate red blood cells and polymorphonuclear leukocytes (PMNs). The mononuclear layer, which consisted of T cells, B cells, and mononuclear cells, was depleted of B cells and monocytes by passage over a nylon wool column. Nylon-passed cells, which were greater than 98% CD3 positive (as determined by fluorescence-activated cell sorter [FACS] analysis), were used as responder cells or further depleted with antibodies to CD8 (OKT8) followed by negative selection with magnetic beads as described previously (6, 12). A portion of these cells was saved prior to placement on nylon wool and were used as autologous feeder cells following irradiation with 6.4 kilorads with a cesium source for 4.8 min. Responder cells (5 × 104 cells/well) were added to 2.5 × 105 irradiated feeder cells and cultured in U-bottom 96-well plates (Corning-Costar Corp., Cambridge, Mass.) with RPMI 1640 and 5% fetal calf serum. S. pneumoniae type 1 CP was added to wells at a concentration of 20 μg/ml. At 6 days postculture, cells were pulsed with 1 μCi of [3H]thymidine/well 6 h prior to harvest in order to measure cell proliferation. Cells were washed extensively and harvested, and the amount of radioactive uptake was counted by liquid scintillation. Data were expressed as the average of triplicate wells ± the standard deviation of counts per minute represented. For all proliferation experiments, data represent typical results from at least five different experiments. For blocking experiments, fusion proteins were added at a concentration of 50 μg/ml. For in vitro experiments with the monoclonal antibody specific for rat CD28, a proliferation assay was employed as previously described using rat CD4+ T cells (4). Splenic T cells were purified by passage over nylon wool columns, depleted of CD8+ T cells, and cocultured with an equal number of irradiated splenocytes (1.5 kilorads). Cells were cultured with S. pneumoniae type 1 CP (20 μg/ml) alone or in the presence of the CD28-specific antibody (1 μg/ml) or an isotype-matched murine antibody (IgG1) for 4 days.
T-cell transfer and abscess induction.
T-cell transfer studies in which rat CD4+ T cells were stimulated with the S. pneumoniae type 1 CP (20 μg/ml) alone or in the presence of CTLA4Ig or L6Ig were performed. Rat T cells were cultured in vitro with irradiated APCs as described above in the presence of S. pneumoniae type 1 CP alone or with the appropriate fusion protein (50 μg/ml). Cultures were harvested after 4 days, and T cells were isolated by repeated nylon wool passage. FACS analysis showed that respective cell populations were >95% pure. The enriched T-cell population was implanted (106 cells/animal) into the peritoneal cavities of animals through a 0.5-cm-long midline incision. Shortly thereafter, 0.5 ml of SCC was placed into the peritoneal cavities of animals as described above.
Statistical evaluation.
Comparison of groups with regard to abscess formation was made by chi-square analysis. Comparison of in vitro T-cell proliferation data was made by the unpaired t test. Statistical analysis was performed by commercially available software (InStat; GraphPad Software, Inc., San Diego, Calif.).
RESULTS
Effect of CTLA4Ig treatment on abscess induction following bacterial challenge.
We first investigated the effect of CD28-B7 blockade on abscess formation induced by challenge with different bacterial inocula. Animals were treated with CTLA4Ig and challenged with B. fragilis and SCC. Significantly fewer animals receiving this treatment developed abscesses than in the saline-treated control group (Table 1; 27% compared with 73%, respectively; P = 0.002).
TABLE 1.
Effect of CTLA4Ig treatment on intra-abdominal abscess formation by different bacterial pathogens
| Treatmenta | Challenge (intraperitoneal) speciesb | No. of animals positive for abscesses/total (%) | Pc |
|---|---|---|---|
| Saline | B. fragilis | 22/30 (73) | |
| Saline | B. distasonis + E. faecium | 28/34 (82) | |
| Saline | S. aureus | 10/10 (100) | |
| CTLA4Ig | B. fragilis | 6/22 (27) | 0.002 |
| CTLA4Ig | B. distasonis + E. faecium | 9/32 (28) | <0.0001 |
| CTLA4Ig | S. aureus | 2/10 (20) | 0.001 |
| L6Ig | B. fragilis | 7/10 (70) |
The fusion protein (500 μg/ml) was administered via the intracardiac route.
The challenge dose is reported in Materials and Methods.
Compared to the respective saline control.
Similar studies were performed using a heterologous challenge inoculum consisting of the synergistic combination of B. distasonis and E. faecium. Treatment with CTLA4Ig reduced abscess formation from 82% in the saline-treated control group to 28% (Table 1; P < 0.0001). Finally, treatment of animals with CTLA4Ig reduced the incidence of abscesses following challenge with S. aureus. All animals treated with saline and challenged with this organism had abscesses, while CTLA4Ig-treated animals had a 20% abscess rate (P = 0.001).
Delayed administration of CTLA4Ig.
To determine the effect of delayed CTLA4Ig administration, treatment was delayed by 24 or 48 h relative to challenge with B. fragilis. Animals given treatment at the time of challenge (as described above) had a 22% abscess rate compared with 80% for the saline-treated control group (P < 0.03; data not shown). Delay of treatment by 24 h resulted in an increase in abscess formation (60%; not statistically significant), while administration of CTLA4Ig 48 h following challenge yielded an abscess rate of 88%, which was comparable to that for the saline-treated control group.
Effect of CTLA4Ig treatment on abscess induction by PS A and S. pneumoniae type 1 CP.
Animals were treated with 500 μg of CTLA4Ig via the intracardiac route and immediately challenged with 20 μg of PS A or the S. pneumoniae type 1 CP mixed with SCC. The results are shown in Table 2. Treatment with CTLA4Ig reduced abscess formation by PS A from 71% in the saline-treated control group to 23% (P = 0.004). CTLA4Ig-treated animals challenged with the S. pneumoniae type 1 CP had an abscess rate of 23% compared to 88% in the saline-treated control group (P = 0.0002). Administration of the fusion protein control (L6Ig) did not have an effect (abscess rate = 80% in animals challenged with PS A).
TABLE 2.
Effect of CTLA4Ig treatment on intra-abdominal abscess formation by PS A
| Treatmenta | Challenge (intraperitoneal) proteinb | No. of animals positive for abscesses/total (%) | Pc |
|---|---|---|---|
| Saline | PS A | 12/17 (71) | |
| CTLA4Ig | PS A | 5/22 (23) | 0.004 |
| Saline | S. pneumoniae type 1 CP | 14/16 (88) | |
| CTLA4Ig | S. pneumoniae type 1 CP | 3/15 (20) | 0.0002 |
| L6Ig | PS A | 8/10 (80) | NSd |
The fusion protein (500 μg/ml) was administered via the intracardiac route.
Animals challenged with 20 μg/rat.
Compared to the saline control.
NS, not significant.
Role of B7-1 and B7-2 in abscess induction.
The contribution of B7-1 and B7-2 to abscess formation was investigated. The results are shown in Table 3. Treatment of animals with Y100FIg, a fusion protein that binds to and blocks B7-1 but not B7-2, did not reduce the incidence of abscesses following challenge with B. fragilis (abscess rate = 90%). However, administration of a B7-2-specific monoclonal antibody completely prevented abscess formation (P = 0.0001 compared to the saline-treated control group). Treatment with the fusion protein control (L6Ig) or an isotype-matched control did not have this effect.
TABLE 3.
Effect of blockade of B7-1 or B7-2 on intra-abdominal abscess formation by B. fragilisb
| Treatmenta | No. of animals positive for abscesses/total (%) | Pc |
|---|---|---|
| Saline | 17/20 (85) | |
| Y100FIg (binds to B7-1) | 9/10 (90) | NSd |
| L6Ig | 7/10 (70) | NS |
| Anti-B7-2 | 0/7 (0) | 0.0001 |
| IgG control | 5/5 (100) | NS |
Fusion protein (500 μg/ml) was administered via the intracardiac route.
Animals were challenged with B. fragilis at 108 CFU/rat.
Compared to the saline control.
NS, not significant.
Potentiation of abscess formation by CD28 signaling.
The role of CD28 in abscess formation was determined. A monoclonal antibody specific for CD28 that has been shown to provide a positive signal for T-cell activation in the absence of B7 ligation (32) was administered to animals at the time of challenge with B. fragilis and SCC. The CD28-specific antibody (500 μg) was administered via the intracardiac route. A suboptimal dose of B. fragilis (5 × 107 CFU/animal) that induces abscesses in approximately 50% of animals was employed. Results are shown in Table 4. Animals receiving saline and challenged with B. fragilis had a 42% abscess rate, while 83% of animals receiving the antibody specific for CD28 had abscesses. The administration of the monoclonal antibody significantly increased the number of animals with abscesses compared to the corresponding number for the saline control group (P < 0.02).
TABLE 4.
Effect of anti-CD28 treatment on intra-abdominal abscess formation by B. fragilisa
| Treatment | No. of animals positive for abscesses/total (%) | P |
|---|---|---|
| Saline | 8/19 (42) | |
| Anti-CD28b | 15/18 (83) | <0.02 |
A dose of B. fragilis (5 × 107 CFU/animal) was used to induce abscesses in approximately 50% of animals.
A dose of 500 μg/ml was administered via the intracardiac route.
In vitro modulation of polysaccharide-induced T-cell activation by CD28-B7 costimulation.
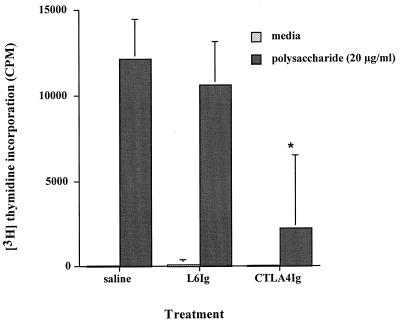
The effect of CD28-B7 blockade with CTLA4Ig on human CD4+ T-cell proliferation in vitro by a PS A polysaccharide mimetic, S. pneumoniae type 1 CP, was determined. This polysaccharide has a charge motif similar to that of PS A and exhibits the same biologic properties. Both PS A and the S. pneumoniae type 1 CP induce abscess formation in animals (37) and stimulate human T-cell activation in vitro (35). In vitro CD4+ T-cell assays in which the impact of CTLA4Ig or L6Ig on T-cell activation by the S. pneumoniae type 1 CP was assessed were performed. Results are shown in Fig. 1. The addition of 50 μg of CTLA4Ig/ml significantly reduced T-cell activation by S. pneumoniae type 1 CP (P < 0.006 compared with the saline control), while similar treatment with the control Ig did not have an effect. Further in vitro experiments demonstrated that addition of a monoclonal antibody specific for B7-2 inhibited T-cell activation, while addition of Y100FIg or a monoclonal antibody specific for B7-1 did not (data not shown).
FIG. 1.
Effect of CTLA4Ig on polysaccharide-mediated CD4+ T-cell proliferation in vitro. Addition of CTLA4Ig (50 μg/ml) to human T cells and irradiated APCs cultured with the S. pneumoniae type 1 CP (20 μg/ml) for 6 days resulted in a significant decrease in activity (P < 0.006 compared with the saline control [∗]). Addition of L6Ig at a similar concentration did not have this effect. Error bars indicate standard deviations.
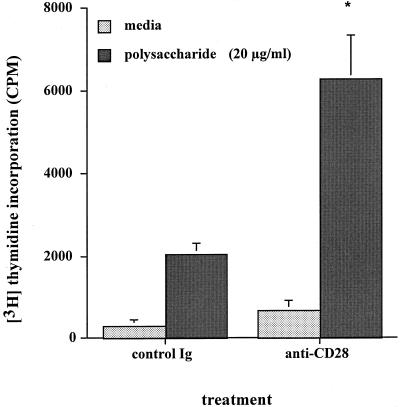
Similar rat CD4+ T-cell proliferation experiments using the monoclonal antibody specific for CD28 were performed. Addition of this antibody (1 μg/ml) to rat T-cell cultures resulted in enhanced proliferation of the T-cell response to S. pneumoniae type 1 CP compared to an isotype-matched Ig control (Fig. 2). The increase in the proliferative response to the S. pneumoniae type 1 CP in the presence of the CD28-specific antibody was approximately three times greater than the response in the presence of control antibody (P < 0.002).
FIG. 2.
Effect of CD28 signaling antibody on polysaccharide-mediated CD4+ T-cell proliferation in vitro. Rat T cells and irradiated APCs were cultured with S. pneumoniae type 1 CP (20 μg/ml) for 4 days. Addition of this antibody (1 μg/ml) to cultures resulted in enhanced proliferation of the cellular response compared to that for an isotype-matched Ig control. The increase in the proliferative response was approximately three times greater than the response in the presence of the control antibody (P < 0.002 [∗]). Error bars indicate standard deviation.
Effect of in vitro CD28-B7 blockade on abscess induction.
The effect of polysaccharide-induced T-cell activation on abscess formation in the animal model of intra-abdominal sepsis was evaluated. In these studies, naïve rat CD4+ T cells were stimulated in vitro for 4 days with S. pneumoniae type 1 CP and transferred to rats through a midline abdominal incision into the peritoneal cavity. Shortly following implantation of stimulated T cells, 0.5 ml of SCC was added to the peritoneal cavity and the incision was closed. Results are shown in Table 5. All animals implanted with T cells cultured with S. pneumoniae type 1 CP formed abscesses compared with animals given T cells cultured with medium alone (14% abscess rate; P < 0.0014). The transfer of S. pneumoniae type 1 CP-stimulated T cells cultured in vitro in the presence of CTLA4Ig did not induce abscess formation (0% abscess rate; P < 0.0002). Culture of these cells with the fusion protein control, L6Ig, had little effect on their ability to induce abscesses (abscess rate = 75%). None of the animals challenged with the SCC alone formed abscesses (data not shown).
TABLE 5.
CD28-B7 blockade of polysaccharide-induced CD4+ T-cell activation prevents abscess formationa
| T-cell stimulus | Fusion protein | No. of animals positive for abscesses/ total (%) | P |
|---|---|---|---|
| Medium | 1/7 (14) | ||
| S. pneumoniae type 1 CP | 8/8 (100) | 0.0014b | |
| S. pneumoniae type 1 CP | CTLA4Ig | 0/8 (0) | 0.0002c |
| S. pneumoniae type 1 CP | L6Ig | 6/8 (75) | Not significantc |
T cells and APCs were cocultured in vitro with polysaccharide (20 μg/ml) and fusion protein (50 μg/ml) for 4 days prior to harvest. T cells were enriched by nylon wool passage, depleted of CD8+ T cells by magnetic bead depletion, and administered to rats via the intraperitoneal route. SCC were administered to animals shortly thereafter. Animals challenged with T cells alone or SCC only did not form abscesses.
Compared with medium-stimulated T cells.
Compared with S. pneumoniae type 1 CP-stimulated T cells.
DISCUSSION
Previous reports have suggested that T cells are involved in the pathogenesis of abscess formation; however, the underlying mechanism by which these cells contribute to this process remains unclear (21, 28, 30). Recently, we have shown that abscess-inducing polysaccharides from B. fragilis can stimulate rat and human CD4+ T-cell activation in vitro (4, 15, 35). These studies have shown conclusively that T-cell activation by these and other bacterial polysaccharides that have both positive and negative charges is specific to the Zps tested and is not due to contaminating proteins or lipopolysaccharide (35). This activity required the presence of class II-bearing APCs. Based on these findings, we hypothesized that T-cell activation by Zps, possibly mediated by costimulatory signals provided by APCs, was a critical step in the pathogenesis of intra-abdominal abscess formation.
In order to investigate this question, we utilized fusion proteins and antibodies that specifically inhibit T-cell activation through the blockade of the CD28-B7 costimulatory pathway. CTLA4Ig, a fusion protein that has been demonstrated in a number of recent studies (25, 29) to effectively block CD28-B7 interactions, was used to treat animals just prior to challenge with different abscess-inducing inocula. Treatment with CTLA4Ig in this manner significantly reduced the incidence of abscesses following challenge with polysaccharides that induce abscesses (PS A and the S. pneumoniae type 1 CP), B. fragilis, S. aureus, or a combination of B. distasonis and E. faecium.
It has been shown that the capsular polysaccharides of B. fragilis are responsible for experimental abscess induction by this organism (37). However, it was surprising that CTLA4Ig treatment prevented abscess formation by S. aureus or the B. distasonis-E. faecium inoculum. These organisms are associated with clinical cases of intra-abdominal abscesses (3), and preliminary data suggest that the capsular polysaccharides from S. aureus type 5 and 8 strains also induce intra-abdominal abscesses in the rat model (A. O. Tzianabos et al., unpublished data). While it is known that E. faecium possesses surface polysaccharides (14) and that B. distasonis does not, it is unclear how these organisms synergize to produce abscesses. Demonstration that CTLA4Ig treatment of animals prevented abscess formation by abscess-inducing polysaccharides or these distinct pathogenic bacteria indicated that surface polysaccharides and possibly other factors associated with these organisms mediate abscess formation through a common mechanism that involves T-cell activation via the CD28-B7 pathway. These data also indicate that T-cell costimulation is one of the necessary signals for T-cell activation by these zwitterionic polysaccharides.
The finding that the B7-2-specific antibody prevented abscess formation while the B7-1-specific fusion protein did not is intriguing. This result corroborated our finding that T-cell activation by Zps is mediated by B7-2 but not B7-1 and supports our contention that the development of this host response is completely dependent on CD28–B7-2 interactions. It has been demonstrated that B7-2 expression following antigenic stimulation occurs within 6 h and rises to higher levels while maximal B7-1 expression occurs between 18 and 24 h (2, 11, 16). This correlates with our finding that delaying the administration of CTLA4Ig by 24 or 48 h following bacterial challenge resulted in a distinct loss of protective efficacy and indicates that the process leading to the development of abscesses is initiated shortly following bacterial challenge. Recent studies have demonstrated differential requirements for B7-1 and B7-2 in mediating T-cell-dependent host responses. The antibody response to a group C meningococcal capsular polysaccharide-porin conjugate vaccine is inhibited by antibodies to B7-2 but not B7-1 (19). In another report, blockade of B7-1 with Y100FIg did not affect antibody production, cytotoxic precursors, or clearance of influenza virus but did influence lung effector function in mice. These results suggested that B7-1 is important for some immune responses to the virus in the lung but not in others (18).
The role of CD28 on T cells in the development of abscesses was demonstrated. In these experiments, animals treated with a CD28-specific monoclonal antibody (which is known to provide a positive costimulatory signal leading to T-cell proliferation and IL-2 secretion [32]) had a significantly higher abscess rate than those receiving saline. These results confirmed that the CD28-B7 costimulatory pathway is definitively involved in the regulation of abscess formation. Furthermore, because CD28 is present exclusively on T cells, direct evidence for its involvement was confirmed. The finding that CTLA4Ig and the CD28-specific signaling antibody could downregulate or upregulate abscess formation, respectively, showed that T-cell activation is a critical step leading to this host response.
Due to the limited availability of PS A, the S. pneumoniae type 1 CP was used for T-cell proliferation assays in the present studies. Each of these polymers induces experimental abscess formation and activates human and rat CD4+ T cells in vitro (4, 35). The T-cell response from inbred rats to Zps is typically lower than that of human T cells but is more consistent (4). Zps-mediated T-cell activation requires the presence of APCs and can be inhibited in vitro by the addition of major histocompatibility complex class II-specific monoclonal antibodies (35). In the present study, blockade of the CD28-B7 pathway by CTLA4Ig inhibited T-cell activation by the S. pneumoniae type 1 CP, while antibody-mediated signaling of this molecule resulted in a threefold increase in T-cell proliferation.
The mechanism by which Zps activate CD4+ T cells is not known, and currently it is unclear whether they behave as superantigens, mitogens, or conventional antigens. Since studies have shown that engagement of different costimulatory pathways leads to markedly different T-cell responses (7, 20, 31, 42), it is important to ascertain whether a requirement for costimulation exists and determine the type(s) of costimulatory pathways that is involved in Zps-mediated T-cell activation. In the present study, the biologic role of T-cell activation via CD28–B7-2 by Zps was determined in T-cell transfer experiments in which rat CD4+ T cells were stimulated in vitro with the S. pneumoniae type 1 CP and implanted into the peritoneal cavities of animals along with SCC. T cells activated by the polysaccharide induced abscess formation, while coculture of these cells with CTLA4Ig resulted in the prevention of their ability to transfer abscess formation to animals.
It is important to note that activated T cells transferred without the addition of SCC did not form abscesses in these experiments. The role of SCC in the animal model may be explained by the fact that intraperitoneal administration of this material alone elicits tumor necrosis factor alpha (TNF-α) and IL-β (A. Tzianabos, unpublished results). Previously, we have shown that the release of TNF-α from resident peritoneal macrophages upregulates intracellular adhesion molecule 1 on mesothelial cells lining the abdominal cavity and leads to increased adherence of infiltrating PMNs (8). Therefore, it is possible that administration of SCC along with bacteria or activated T cells serves as a necessary adjuvant for abscess formation by upregulating the proinflammatory response. This scenario is similar to the role of Freund's adjuvant in the induction of experimental autoimmune encephalomyelitis (EAE) by myelin basic protein (33). However, unlike what is found for Freund's adjuvant in EAE, the use of SCC in the peritoneal cavity results in a course of events that closely resembles the one (i.e., spillage of colonic contents into the peritoneal cavity) that leads to abscess formation in human disease.
Recently, we have shown that CD4+ T cells activated by Zps in vitro or in vivo prevent abscess formation when these cells are administered (without adjuvants) via the intracardiac route 24 h prior to intraperitoneal challenge with B. fragilis (35). This process is mediated by the production of IL-2 by these cells (39). At the present time it is unclear why CD4+ T cells activated by Zps can both induce and prevent abscesses. We hypothesize that factors such as the route of administration of T cells (intracardiac versus intraperitoneal) and administration with SCC are responsible for these paradoxical outcomes. Our preliminary data suggest that, in addition to the production of IL-2, T cells stimulated by Zps produce chemokines that could act in concert with proinflammatory cytokines elicited by SCC in the peritoneal cavity to recruit PMNs to this site. Infiltrating PMNs could then readily bind to the activated peritoneal mesothelium to form a suitable nidus for abscess formation.
While the role of T-cell costimulation has been studied extensively in autoimmune diseases and models of transplantation, more recent studies have focused on its role in modulating the immune response to infectious diseases. This work has shown that CD28-B7 interactions have a prominent role in governing the host immune response to parasitic and bacterial infections (10, 24, 27, 40, 41, 43). The goal of the present work was to determine the specific role of T cells in intra-abdominal abscess formation. Using reagents that have been developed to study T-cell costimulation, we demonstrate that abscess-inducing Zps promote this host response via activation of CD4+ T cells requiring a “second signal” provided by APCs. This signal is initiated by CD28–B7-2 interactions and regulates abscess formation by both gram-positive and gram-negative pathogens. Further study to elucidate how this host response is controlled at the molecular level is under way.
ACKNOWLEDGMENTS
We thank Ronald Cisneros, Mary Delaney, Mathew Lawlor, Brian Hyett, and Trina Tabacco for technical assistance.
This work was supported in part by the National Institutes of Health, National Institute of Allergy and Infectious Diseases (grants AI 39576, AI 34073, and AI 34965).
REFERENCES
- 1.Abrams J R, Lebwohl M G, Guzzo C A, Jegasothy B V, Goldfarb M T, Goffe B S, Menter A, Lowe N J, Krueger G, Brown M J, Weiner R S, Birkhofer M J, Warner G L, Berry K K, Linsley P S, Krueger J G, Ochs H D, Kelley S L, Kang S. CTLA4Ig-mediated blockade of T-cell costimulation in patients with psoriasis vulgaris. J Clin Investig. 1999;103:1243–1252. doi: 10.1172/JCI5857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bluestone J A. New perspectives of CD28–B7-mediated T cell costimulation. Immunity. 1995;2:555–559. doi: 10.1016/1074-7613(95)90000-4. [DOI] [PubMed] [Google Scholar]
- 3.Brook I, Frazier E H. Microbiology of subphrenic abscesses: a 14-year experience. Am Surg. 1999;65:1049–1053. [PubMed] [Google Scholar]
- 4.Brubaker J O, Li Q, Tzianabos A O, Kasper D L, Finberg R W. Mitogenic activity of purified capsular polysaccharide A from Bacteroides fragilis: differential stimulatory effect on mouse and rat lymphocytes in vitro. J Immunol. 1999;162:2235–2242. [PubMed] [Google Scholar]
- 5.Dong V M, Womer K L, Sayegh M H. Transplantation tolerance: the concept and its applicability. Pediatr Transplant. 1999;3:181–192. doi: 10.1034/j.1399-3046.1999.00042.x. [DOI] [PubMed] [Google Scholar]
- 6.Finberg R W, White W, Nicholson W A. Decay-accelerating factor expression on either effector or target cells inhibits cytotoxicity by human natural killer cells. J Immunol. 1992;149:2055–2060. [PubMed] [Google Scholar]
- 7.Fischer H, Gjorloff A, Hedlund G, Hedman H, Lundgren E, Kalland T, Sjogren H O, Dohlsten M. Stimulation of human naive and memory T helper cells with bacterial superantigen. Naive CD4+45RA+ T cells require a costimulatory signal mediated through the LFA-1/ICAM-1 pathway. J Immunol. 1992;148:1993–1998. [PubMed] [Google Scholar]
- 8.Gibson F C, III, Onderdonk A B, Kasper D L, Tzianabos A O. Cellular mechanism of intraabdominal abscess formation by Bacteroides fragilis. J Immunol. 1998;160:5000–5006. [PubMed] [Google Scholar]
- 9.Gimmi C D, Freeman G J, Gribben J G, Gray G, Nadler L M. Human T-cell clonal anergy is induced by antigen presentation in the absence of B7 costimulation. Proc Natl Acad Sci USA. 1993;90:6586–6590. doi: 10.1073/pnas.90.14.6586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gupta S, Vohra H, Saha B, Nain C K, Ganguly N K. Macrophage-T cell interaction in murine salmonellosis: selective down-regulation of ICAM-1 and B7 molecules in infected macrophages and its probable role in cell-mediated immunity. Eur J Immunol. 1996;26:563–570. doi: 10.1002/eji.1830260310. [DOI] [PubMed] [Google Scholar]
- 11.Hancock W W, Sayegh M H, Zheng X G, Peach R, Linsley P S, Turka L A. Costimulatory function and expression of CD40 ligand, CD80, and CD86 in vascularized murine cardiac allograft rejection. Proc Natl Acad Sci USA. 1996;93:13967–13972. doi: 10.1073/pnas.93.24.13967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Haregewoin A, Soman G, Hom R C, Finberg R W. Human gamma delta+ T cells respond to mycobacterial heat-shock protein. Nature. 1989;340:309–312. doi: 10.1038/340309a0. [DOI] [PubMed] [Google Scholar]
- 13.Harris N, Peach R, Naemura J, Linsley P S, Le Gros G, Ronchese F. CD80 costimulation is essential for the induction of airway eosinophilia. J Exp Med. 1997;185:177–182. doi: 10.1084/jem.185.1.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Huebner J, Wang Y, Krueger W A, Madoff L C, Martirosian G, Boisot S, Goldmann D A, Kasper D L, Tzianabos A O, Pier G B. Isolation and chemical characterization of a capsular polysaccharide antigen shared by clinical isolates of Enterococcus faecalis and vancomycin-resistant Enterococcus faecium. Infect Immun. 1999;67:1213–1219. doi: 10.1128/iai.67.3.1213-1219.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kalka-Moll W M, Tzianabos A O, Wang Y, Carey V J, Finberg R W, Onderdonk A B, Kasper D L. Effect of molecular size on the ability of zwitterionic polysaccharides to stimulate cellular immunity. J Immunol. 2000;164:719–724. doi: 10.4049/jimmunol.164.2.719. [DOI] [PubMed] [Google Scholar]
- 16.Lenschow D J, Walunas T L, Bluestone J A. CD28/B7 system of T cell costimulation. Annu Rev Immunol. 1996;14:233–258. doi: 10.1146/annurev.immunol.14.1.233. [DOI] [PubMed] [Google Scholar]
- 17.Linsley P S, Brady W, Urnes M, Grosmaire L S, Damle N K, Ledbetter J A. CTLA-4 is a second receptor for the B cell activation antigen B7. J Exp Med. 1991;174:561–569. doi: 10.1084/jem.174.3.561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lumsden J M, Roberts J M, Harris N L, Peach R J, Ronchese F. Differential requirement for CD80 and CD80/CD86-dependent costimulation in the lung immune response to an influenza virus infection. J Immunol. 2000;164:79–85. doi: 10.4049/jimmunol.164.1.79. [DOI] [PubMed] [Google Scholar]
- 19.Mackinnon F G, Ho Y, Blake M S, Michon F, Chandraker A, Sayegh M H, Wetzler L M. The role of B/T costimulatory signals in the immunopotentiating activity of neisserial porin. J Infect Dis. 1999;180:755–761. doi: 10.1086/314966. [DOI] [PubMed] [Google Scholar]
- 20.Ni H T, Deeths M J, Li W, Mueller D L, Mescher M F. Signaling pathways activated by leukocyte function-associated Ag-1-dependent costimulation. J Immunol. 1999;162:5183–5189. [PubMed] [Google Scholar]
- 21.Nulsen N F, Finlay-Jones J J, MacDonald R J. T-lymphocyte involvement in abscess formation in nonimmune mice. Infect Immun. 1986;52:633–636. doi: 10.1128/iai.52.2.633-636.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Onderdonk A B, Kasper D L, Cisneros R L, Bartlett J G. The capsular polysaccharide of Bacteroides fragilis as a virulence factor: comparison of the pathogenic potential of encapsulated and unencapsulated strains. J Infect Dis. 1977;136:82–89. doi: 10.1093/infdis/136.1.82. [DOI] [PubMed] [Google Scholar]
- 23.Polk B J, Kasper D L. Bacteroides fragilis subspecies in clinical isolates. Ann Intern Med. 1977;86:567–571. doi: 10.7326/0003-4819-86-5-569. [DOI] [PubMed] [Google Scholar]
- 24.Rathore A, Sacristan C, Ricklan D E, Flores V P, Stadecker M J. In situ analysis of B7–2 costimulatory, major histocompatibility complex class II, and adhesion molecule expression in schistosomal egg granulomas. Am J Pathol. 1996;149:187–194. [PMC free article] [PubMed] [Google Scholar]
- 25.Reiser H, Stadecker M J. Costimulatory B7 molecules in the pathogenesis of infectious and autoimmune diseases. N Engl J Med. 1996;335:1369–1377. doi: 10.1056/NEJM199610313351807. [DOI] [PubMed] [Google Scholar]
- 26.Reynolds J, Tam F W, Chandraker A, Smith J, Karkar A M, Cross J, Peach R, Sayegh M H, Pusey C D. CD28–B7 blockade prevents the development of experimental autoimmune glomerulonephritis. J Clin Investig. 2000;105:643–651. doi: 10.1172/JCI6710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Saha B, Jaklic B, Harlan D M, Gray G S, June C H, Abe R. Toxic shock syndrome toxin-1-induced death is prevented by CTLA4Ig. J Immunol. 1996;157:3869–3875. [PubMed] [Google Scholar]
- 28.Sawyer R G, Adams R B, May A K, Rosenlof L K, Pruett T L. CD4+ T cells mediate preexposure-induced increases in murine intraabdominal abscess formation. Clin Immunol Immunopathol. 1995;77:82–88. doi: 10.1016/0090-1229(95)90140-x. [DOI] [PubMed] [Google Scholar]
- 29.Sayegh M H, Turka L A. The role of T-cell costimulatory activation pathways in transplant rejection. N Engl J Med. 1998;338:1813–1821. doi: 10.1056/NEJM199806183382506. [DOI] [PubMed] [Google Scholar]
- 30.Shapiro M E, Kasper D L, Zaleznik D F, Spriggs S, Onderdonk A B, Finberg R W. Cellular control of abscess formation: role of T cells in the regulation of abscesses formed in response to Bacteroides fragilis. J Immunol. 1986;137:341–346. [PubMed] [Google Scholar]
- 31.Shinde S, Wu Y, Guo Y, Niu Q, Xu J, Grewal I S, Flavell R, Liu Y. CD40L is important for induction of, but not response to, costimulatory activity. ICAM-1 as the second costimulatory molecule rapidly up-regulated by CD40L. J Immunol. 1996;157:2764–2768. [PubMed] [Google Scholar]
- 32.Tacke M, Clark G J, Dallman M J, Hunig T. Cellular distribution and costimulatory function of rat CD28. Regulated expression during thymocyte maturation and induction of cyclosporin A sensitivity of costimulated T cell responses by phorbol ester. J Immunol. 1995;154:5121–5127. [PubMed] [Google Scholar]
- 33.Teitelbaum D, Arnon R, Sela M. Immunomodulation of experimental autoimmune encephalomyelitis by oral administration of copolymer. Proc Natl Acad Sci USA. 1999;96:3842–3847. doi: 10.1073/pnas.96.7.3842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Thompson C B. Distinct roles for the costimulatory ligands B7–1 and B7–2 in T helper cell differentiation. Cell. 1995;81:979–982. doi: 10.1016/s0092-8674(05)80001-7. [DOI] [PubMed] [Google Scholar]
- 35.Tzianabos A O, Finberg R W, Wang Y, Chan M, Onderdonk A B, Jennings H J, Kasper D L. T cells activated by zwitterionic molecules prevent abscesses induced by pathogenic bacteria. J Biol Chem. 2000;275:6733–6740. doi: 10.1074/jbc.275.10.6733. [DOI] [PubMed] [Google Scholar]
- 36.Tzianabos A O, Kasper D L, Cisneros R L, Smith R S, Onderdonk A B. Polysaccharide-mediated protection against abscess formation in experimental intra-abdominal sepsis. J Clin Investig. 1995;96:2727–2731. doi: 10.1172/JCI118340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tzianabos A O, Onderdonk A B, Rosner B, Cisneros R L, Kasper D L. Structural features of polysaccharides that induce intra-abdominal abscesses. Science. 1993;262:416–419. doi: 10.1126/science.8211161. [DOI] [PubMed] [Google Scholar]
- 38.Tzianabos A O, Pantosti A, Baumann H, Brisson J R, Jennings H J, Kasper D L. The capsular polysaccharide of Bacteroides fragilis comprises two ionically linked polysaccharides. J Biol Chem. 1992;267:18230–18235. [PubMed] [Google Scholar]
- 39.Tzianabos A O, Russell P R, Onderdonk A B, Gibson III F C, Cywes C, Chan M, Finberg R W, Kasper D L. IL-2 mediates protection against abscess formation in an experimental model of sepsis. J Immunol. 1999;163:893–897. [PubMed] [Google Scholar]
- 40.Walley K R, Lukacs N W, Standiford T J, Strieter R M, Kunkel S L. Elevated levels of macrophage inflammatory protein 2 in severe murine peritonitis increase neutrophil recruitment and mortality. Infect Immun. 1997;65:3847–3851. doi: 10.1128/iai.65.9.3847-3851.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang R, Fang Q, Zhang L, Radvany L, Sharma A, Noben-Trauth N, Mills G B, Shi Y. CD28 ligation prevents bacterial toxin-induced septic shock in mice by inducing IL-10 expression. J Immunol. 1997;158:2856–2861. [PubMed] [Google Scholar]
- 42.Windsor A, Walsh C, Mullen P, Cook D, Fisher B, Blocher C, Leeper-Woodford S, Sugerman H, Fowler A. Tumor necrosis factor-a blockade prevents neutrophil CD18 receptor upregulation and attenuates acute lung injury in porcine sepsis without inhibition of neutrophil oxygen radical generation. J Clin Investig. 1993;91:1459–1468. doi: 10.1172/JCI116351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ye G, Barrera C, Fan X, Gourley W K, Crowe S E, Ernst P B, Reyes V E. Expression of B7–1 and B7–2 costimulatory molecules by human gastric epithelial cells: potential role in CD4+ T cell activation during Helicobacter pylori infection. J Clin Investig. 1997;99:1628–1636. doi: 10.1172/JCI119325. [DOI] [PMC free article] [PubMed] [Google Scholar]