ABSTRACT
Previous studies have shown beneficial effects of coenzyme Q10 (CoQ10) supplementation on blood pressure (BP). However, the optimal intake of CoQ10 for BP regulation in patients with cardiometabolic disorders is unknown, and its effect on circulating CoQ10 is also unclear. We aimed to assess the dose-response relation between CoQ10 and BP, and quantify the effect of CoQ10 supplementation on the concentration of circulating CoQ10 by synthesizing available evidence from randomized controlled trials (RCTs). A comprehensive literature search was performed in 3 databases (PubMed/MEDLINE, Embase, and Cochrane Library) to 21 March, 2022. A novel 1-stage restricted cubic spline regression model was used to evaluate the nonlinear dose-response relation between CoQ10 and BP. Twenty-six studies comprising 1831 subjects were included in our meta-analysis. CoQ10 supplementation significantly reduced systolic blood pressure (SBP) (−4.77 mmHg, 95% CI: −6.57, −2.97) in patients with cardiometabolic diseases; this reduction was accompanied by a 1.62 (95% CI: 1.26, 1.97) μg/mL elevation of circulating CoQ10 compared with the control group. Subgroup analyses revealed that the effects of reducing SBP were more pronounced in patients with diabetes and dyslipidemia and in studies with longer durations (>12 wk). Importantly, a U-shaped dose-response relation was observed between CoQ10 supplementation and SBP level, with an approximate dose of 100–200 mg/d largely reducing SBP (χ2 = 10.84, Pnonlinearity = 0.004). The quality of evidence was rated as moderate, low, and very low for SBP, diastolic blood pressure (DBP), and circulating CoQ10 according to the Grading of Recommendations, Assessment, Development, and Evaluation approach (GRADE), respectively. The current finding demonstrated that the clinically beneficial effects of CoQ10 supplementation may be attributed to the reduction in SBP, and 100–200 mg/d of CoQ10 supplementation may achieve the greatest benefit on SBP in patients with cardiometabolic diseases. This study was registered on PROSPERO as CRD42021252933.
Keywords: CoQ10 supplementation, cardiometabolic disorders, blood pressure, dose-response meta-analysis, GRADE
Statement of Significance: This systematic review and meta-analysis provided a recommended dose of 100–200 mg/d to achieve the greatest benefit in the regulation of blood pressure in patients with cardiometabolic diseases.
Introduction
Cardiometabolic disorders, such as CVDs, type 2 diabetes, and metabolic syndrome (MetS), are the leading causes of death globally (1). As one of the modifiable risks for cardiometabolic disorders, high blood pressure (BP) is associated with the strongest evidence for causation and it has a high prevalence of exposure affecting 31.1% of adults (1.39 billion) worldwide (2–4). Therefore, it is of great significance for patients with cardiometabolic disorders to reduce morbidity and mortality by reducing high BP (5). Numerous clinical medications are applied for the treatment of high BP. However, long-term use of medications exerts harmful effects, including renal or cardiac dysfunction, cough, and depression (6). Therefore, supplementary nutritional strategies have been taken into account as a common approach for the control and/or early prevention of hypertension (6, 7).
Coenzyme Q10 (CoQ10), also known as ubiquinone in nature, is a lipid-soluble molecule derived mainly from endogenous synthesis. CoQ10 plays an essential role in the electron-transport chain of mitochondrial oxidative phosphorylation (8). CoQ10 has also received attention for its essential role in energy metabolism and antioxidant protection (9). However, there is evidence that CoQ10 deficiency has been implicated in patients with cardiometabolic disorders (10). As a nutritional supplement, a number of small and short-term randomized controlled trials (RCTs) have suggested that CoQ10 supplementation has beneficial effects on BP (11–13), although the optimal intake was unclear. A previous meta-analysis with an insufficient sample size of 50 participants of 2 RCTs demonstrated that CoQ10 did not have any effects on either systolic blood pressure (SBP) or diastolic blood pressure (DBP) (14). Another meta-analysis of RCTs published in recent years reviewed 17 trials with a total of 684 participants and showed that CoQ10 supplementation significantly decreased SBP in people with metabolic diseases (15). Similarly, the previous meta-analysis lacked an analysis of the optimal intake of CoQ10 supplementation for the daily recommendation. In addition, the efficacy of CoQ10 supplementation on circulating CoQ10 warrants further evaluation. Furthermore, limited information on evidence quality and evidence certainty also warrants further evaluation to ascertain the potential clinical translatability of CoQ10 supplementation. Therefore, considering all the above-mentioned points, a comprehensive update of previous systematic reviews and meta-analyses is needed.
To provide healthy food choices for daily recommendation in patients with cardiometabolic disorders, we examined the efficacy of CoQ10 supplementation for BP and circulating CoQ10 via a systematic review and meta-analysis of available RCTs. Furthermore, we assessed a novel dose-response relation to determine the effective dose of CoQ10 supplementation on attenuating BP (16) and evaluated the certainty of the evidence using the Grading of Recommendations Assessment, Development, and Evaluation (GRADE) approach.
Methods
This systematic review was performed according to PRISMA and has been registered on PROSPERO as CRD42021252933 (17).
Literature search
We conducted a systematic search of online databases (PubMed/MEDLINE, Embase, and Cochrane Library) through to 21 March, 2022, for reports of RCTs that had tested the effect of CoQ10 on BP. We used “Coenzyme Q10” and “blood pressure” as key terms. Detailed search strategies are reported in Supplemental Table 1. We also checked the reference lists of articles, related reviews, and meta-analyses to avoid omitting publications. Title and abstract screening and subsequent full-text evaluation were performed in duplicate by 2 authors (DZ and YL). A third author (ML) helped to resolve differences. The process of study selection is shown in Supplemental Figure 1.
Eligibility criteria
Studies with the following conditions were included in this meta-analysis: 1) Had accessible full articles published in English, 2) were RCTs with parallel or crossover designs, and 3) performed on individuals aged ≥18 y with cardiometabolic disorders, including CVDs, diabetes, MetS, dyslipidemia, hypertension, and obesity (these patients are regularly prescribed medications or no medication), 4) reported one of the following measures: SBP or DBP, and 5) had an appropriate controlled design (if CoQ10 was administered as an adjunct to another drug/supplement, the control group had to receive the same drug/supplement).
Exclusion criteria
Studies with one of the following conditions were excluded: 1) animal experiments, 2) studies that did not provide sufficient information for the outcomes of interest, 3) the duration of intervention was <2 wk, and 4) nonoriginal research (reviews, editorials, or commentaries), abstracts, unpublished studies, and duplicated studies.
Data extraction
Data extraction was independently carried out by 2 investigators (YL and DZ), and any discrepancy was resolved by the third investigator. We extracted the following data from the included studies: First author name, publication year, country, age and sex of participants, health status, sample size, study design (crossover or parallel), duration, intervention type or dosage of CoQ10 intervention, medication contamination, whether the study received industry funding and whether the detection of circulating CoQ10, SBP, or DBP showed differences between intervention and control groups, along with the SD at the end of the intervention periods.
Quality assessment
The Cochrane risk-of-bias tool was used to evaluate bias in studies, including domains of random sequence generation, allocation concealment, blinding of participants and researchers, blinding of outcome assessment, incomplete outcome data, and selective reporting. Other biases, including comparisons of baseline, were also assessed. Consequently, terms including “Low,” “High,” or “Unclear” were used to classify each domain of study bias (18). Two reviewers (DZ and SD) independently evaluated biases. Disagreements on the risk-of-bias ratings were resolved through discussion and adjudication by a third investigator (ZL).
Data synthesis and analysis
To evaluate the overall effects of CoQ10 supplementation on BP, we compared the mean changes in SBP or DBP between the CoQ10 treatment group and placebo groups after intervention by calculating weighted mean differences (WMDs) and 95% CIs using a random-effects meta-analysis model (19). We also estimated WMDs for circulating CoQ10 concentrations to assess the effectiveness of CoQ10 supplementation on CoQ10 status.
When mean changes were not reported (e.g., only the mean BP values at baseline and again at postintervention were noted in the study), the following formula was used to derive such changes: Mean change = final postintervention BP value – baseline value for the same; and subsequently, changes in SDs were calculated using the following formula:
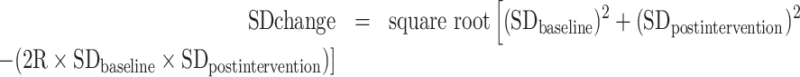 |
(1) |
The best correlation coefficient (R) for each parameter was estimated from studies in which mean ± SD changes were reported. Additionally, the standard error of the mean (SEM) was transformed to SD using the following formula: SD = SEM × √n (n = number of participants in each group).
Heterogeneity was evaluated through the Cochran (Q) and I-squared tests (I2). The percentages of I2 at ∼25%, 50%, and 75% indicate low, medium, and high heterogeneity, respectively. Given the existing heterogeneity between studies, when I2 exceeded 50% or P <0.05, the random-effect model was used; otherwise, the fixed-effect model was applied. To discern the potential sources of heterogeneity, we carried out stratified analyses based on health status, study design, duration of intervention, the dosage of CoQ10, and type of control. Several exploratory subgroup analyses of moderators (i.e., mean age, baseline BP and CoQ10, medication and supplementation contamination, and whether the study received industry funding) were also conducted to explore study protocols. In a sensitivity analysis, we excluded trials at high risk-of-bias to access the effects of CoQ10 supplementation on BP. Finally, a 1-stage restricted cubic spline regression model analysis was performed to assess the possible dose-response relation of CoQ10 supplementation and BP (16, 20). When 10 or more trial comparisons were available, publication bias was investigated by inspection of contour-enhanced funnel plots (21) and Begg's test (at P <0.05) (22). If publication bias was suspected, the Duval and Tweedie trim-and-fill method was utilized to adjust for any omissions and potential bias (23).
Statistical analyses were conducted using STATA software, version 16.0 (Stata Corp), and R software, version 4.1.1 (http://www.r-project.org/). A P value of <0.05 was considered to represent statistical significance.
Certainty assessment
GRADEpro GDT software (24) was used to assess the certainty of evidence according to the GRADE guidelines (gradeworkinggroup.org) for primary outcomes based on areas of study design, risk of bias, inconsistency, indirectness, imprecision, and other considerations, such as publication bias, effect size, and potential confounding (25, 26).
Results
Flow and characteristics of the included studies
We screened 1425 titles or abstracts. The review flow diagram is shown in Supplemental Figure 1. Of these, 26 trials (11–13, 27–50) reported the effect of CoQ10 on SBP comprising 29 arms and DBP comprising 26 arms, and the main characteristics of the included studies are shown in Table 1. These publications were dated between 1997 and 2021 and were conducted in Denmark (4 trials), Australia (4 trials), Finland (1 trial), Iran (4 trials), Iraq (2 trials), Singapore (1 trial), Mexico (1 trial), New Zealand (1 trial), India (1 trial), Japan (1 trial), China (2 trials), Slovakia (1 trial), America (1 trial), Korea (1 trial), and Europe (1 trial). Twenty-three studies were designed as parallel studies, with the remaining 3 as crossover designs. The study duration ranged from 1 to 24 mo with sample sizes between 24 and 420 participants. Participants’ ages ranged from 28 to 68 y. Studies included RCTs across health conditions, including diabetes, MetS, CVD, dyslipidemia, hypertension, and obesity. Although the majority of investigations enrolled both sexes, only 2 studies exclusively utilized female participants. Among them, 16 studies also measured circulating CoQ10.
TABLE 1.
Study characteristics of the 26 trials included in the analysis1
| Study | Country | Sex (male/female) | Age, y, mean ± SD QG/PG | Health status | Sample size (intervention/control) | Design/duration (mo) | Intervention type | Intervention | Treatment | Received industry funding? | Detection circulating CoQ10? | Outcome | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CoQ10 intake, mg/d | Control | Hypoglycemic2 | Hypotension3 | Hypolipidemic4 | |||||||||||
| Andersen et al. 1997 (27) | Denmark | QG:10/7 | QG:33.5 ± 2.0 | Insulin-dependent diabetes mellitus | 34 (17/17) | P/3 mo | Capsules | 100 | Placebo | Insulin 100.0 | NA | NA | Yes | No | SBP, DBP |
| PG:9/8 | PG:35.3 ± 2.4 | ||||||||||||||
| Chew et al. 2008 I (28) | Australia | QG:13/3 | QG:61.3 ± 4.1 | T2D5 | 36 (16/20) | P/6 mo | Capsules | 200 | Placebo | QG: 56.2 | QG:50.0 | QG:31.2 | Yes | Yes | SBP, DBP |
| PG:14/6 | PG:62.4 ± 8.8 | PG:75.0 | PG:65.0 | PG:25.0 | |||||||||||
| Chew et al. 2008 II (28) | Australia | F + CoQ10:13/6 | F + CoQ10:63.0 ± 9.4 | T2D5 | 38 (19/19) | P/6 mo | Capsules | 200 | F + placebo | QG:73.7 | QG:36.8 | QG:47.4 | Yes | Yes | SBP, DBP |
| F:13/6 | F:64.8 ± 7.3 | PG:75.0 | PG:65.0 | PG:25.0 | |||||||||||
| Eriksson et al. 1999 (29) | Finland | NA | QG:65 ± 5 | T2D | 23 (12/11) | P/6 mo | Capsules | 100 | Placebo | OHA or diet | NA | NA | Yes | Yes | SBP, DBP |
| PG:64 ± 7 | |||||||||||||||
| Gholami et al. 2019 (30) | Iran | 0/70 | QG:52.97 ± 1.04 | T2D5 | 70 (35/35) | P/3 mo | Capsules | 100 | Placebo | QG:48.6 | QG:100.0 | QG:100.0 | No | Yes | SBP, DBP |
| PG:53.68 ± 1.14 | PG:57.1 | PG:100.0 | PG:100.0 | ||||||||||||
| Hamilton et al. 2009 (31) | Australia | NA | 68 ± 6 | T2D5 | 46 (23/23) | C/3 mo | Capsules | 200 | Placebo | 83.0 | 52.0 | 100.0 | Yes | Yes | SBP, DBP |
| Henriksen et al. 1999 (32) | Denmark | QG:10/7 | QG:35.5 ± 8.2 | T1DM5 | 34 (17/17) | P/3 mo | Capsules | 100 | Placebo | NA | NA | NA | Yes | Yes | SBP, DBP |
| PG:9/8 | PG:35.3 ± 10.0 | ||||||||||||||
| Lim et al. 2008 (33) | Singapore | QG:17/23 | QG:54 ± 9 | T2D5 | 80 (40/40) | P/3 mo | Capsules | 200 | Placebo | QG:51.0 | QG:20.0 | QG:58.0 | Yes | No | SBP, DBP |
| PG:22/18 | PG:53 ± 9 | PG:51.0 | PG:23.0 | PG:58.0 | |||||||||||
| Rodriguez-Carrizalez et al. 2016 (34) | Mexico | QG:11/9 | QG:28.2 ± 3.7 | T2D | 40 (20/20) | P/6 mo | NA | 400 | Placebo | NA | NA | NA | No | No | SBP, DBP |
| PG:9/11 | G:29.3 ± 0.8 | ||||||||||||||
| Zarei et al. 2018 (12) | Iran | 0/68 | QG:53.1 ± 6.23 | T2D5 | 68 (34/34) | P/3 mo | Capsules | 100 | Placebo | QG:55.9 | QG:100.0 | QG:100.0 | No | Yes | SBP, DBP |
| PG:53.35 ± 6.56 | PG:66.7 | PG:100.0 | PG:100.0 | ||||||||||||
| Young et al. 2012 (35) | New Zealand | 30 (15/15) | 64 ± 3.9 | MetS5 | 60 (30/30) | C/3 mo | Capsules | 200 | Placebo | NA | 21.0 | NA | No | No | SBP, DBP |
| Hodgson et al. 2002 I (13) | Australia | F + CoQ10:14/5 | F + CoQ10:51.7 ± 6.97 | MetS5 | 37 (19/18) | P/3 mo | Capsules | 200 | F | NA | NA | QG:100.0 | No | Yes | SBP, DBP |
| F:14/4 | F:53.6 ± 10.18 | PG:100.0 | |||||||||||||
| Hodgson et al. 2002 II (13) | Australia | QG:17/2 | QG:52.3 ± 6.1 | MetS5 | 37 (19/18) | P/3 mo | Capsules | 200 | Placebo | NA | NA | NA | No | Yes | SBP, DBP |
| PG:13/5 | PG:55.2 ± 9.76 | ||||||||||||||
| Singh et al. 2018 (36) | India | QG:23/4 | QG:48.5 ± 9.5 | CVD | 55 (27/28) | P/6 mo | Capsules | 120 | B vitamins | NA | F | NA | Yes | No | SBP, DBP |
| PG:22/6 | PG:48.7 ± 9.3 | ||||||||||||||
| Playford et al. 2003 I (37) | Australia | F + CoQ10:14/6 | F + CoQ10:52.7 ± 8.05 | MetS5 | 40 (20/20) | P/3 mo | Capsules | 200 | F | OHA 27.5 | NA | NA | No | Yes | SBP |
| F:14/6 | F:53.5 ± 9.84 | ||||||||||||||
| Playford et al. 2003 II (37) | Australia | QG:18/2 | QG:52.7 ± 6.26 | MetS5 | 40 (20/20) | P/3 mo | Capsules | 200 | Placebo | OHA 27.5 | NA | NA | No | Yes | SBP |
| PG:15/5 | PG:54.7 ± 9.39 | ||||||||||||||
| Kamikawa et al. 1985 (38) | Japan | 10/2 | 55.5 ± 6.36 | CVD | 24 (12/12) | C/1 mo | NA | 150 | Placebo | NA | NA | NA | No | No | SBP |
| Dai et al. 2011 (39) | China | QG:27/1 | QG:67.7 ± 9.4 | CVD5 | 56 (28/28) | P/2 mo | Capsules | 300 | Placebo | NA | QG:89.3 | QG:96.4 | Yes | Yes | SBP, DBP |
| PG:25/3 | PG:70.1 ± 9.8 | PG:96.4 | PG:92.9 | ||||||||||||
| Mortensen et al. 2014 (40) | Denmark | QG:154/48 | QG:62.3 ± 12 | Chronic heart failure5 | 420 (202/218) | P/24 mo | Capsules | 300 | Placebo | QG:21.8 | QG:88.1 | QG:36.6 | No | Yes | SBP, DBP |
| PG:151/67 | PG:62.3 ± 11 | PG:23.4 | PG:89.4 | PG:35.3 | |||||||||||
| Kuhlman et al. 2019 (41) | Denmark | QG:14/4 | QG:62 ± 1 | Patient in primary prevention with simvastatin | 35 (18/17) | P/2 mo | Capsules | 400 | Placebo | NA | NA | QG:100.0 | Yes | No | SBP, DBP |
| PG:8/9 | PG:64 ± 2 | PG:100.0 | |||||||||||||
| Mohseni et al. 2014 (42) | Iran | 39/13 | QG:60 ± 4 | Hyperlipidemic5 | 52 (26/26) | P/3 mo | Capsules | 200 | Placebo | NA | QG:100.0 | QG:100.0 | No | No | SBP, DBP |
| PG:61 ± 3.5 | PG:100.0 | PG:100.0 | |||||||||||||
| Toth et al. 2017 (45) | Slovakia | QG:17/18 | QG:58.4 ± 13.8 | Dyslipidemia | 70 (35/35) | P/3 mo | NA | 200 | ω-3 PUFA | NA | NA | QG:100.0 | No | No | SBP, DBP |
| PG:18/17 | PG:61.96 ± 12.2 | PG:100.0 | |||||||||||||
| Zhang et al. 2018 (11) | China | QG:14/37 | QG:51.78 ± 8.92 | Dyslipidemia5 | 101 (51/50) | P/6 mo | Capsules | 120 | Placebo | QG:0.0 | QG:0.0 | QG:0.0 | No | No | SBP, DBP |
| PG:18/32 | PG:50.02 ± 10.91 | PG:0.0 | PG:0.0 | PG:0.0 | |||||||||||
| Burke et al. 2001 (44) | America | QG:21/18 | QG:69.7 ± 4.1 | Isolated systolic hypertension5 | 71 (39/32) | P/6 mo | Hydrosoluble Q-Gel | 120 | Vitamin E | NA | NA | QG:35.9 | No | Yes | SBP, DBP |
| PG:18/14 | PG:67.3 ± 3.4 | PG:31.3 | |||||||||||||
| Lee et al. 2011 (43) | Korea | QG:11/15 | QG:42.7 ± 11.3 | Obesity5 | 36 (17/19) | P/3 mo | NA | 200 | Placebo | NA | NA | QG:0.0 | No | Yes | SBP, DBP |
| PG:10/15 | PG:42.5 ± 11.2 | PG:0.0 | |||||||||||||
| Sedeh et al. 2018 (46) | Iran | 39/29 | 18–60 | T2D | 68 (34/34) | P/18 mo | Capsules | 100 | Placebo | NA | NA | NA | No | No | SBP, DBP |
| Yasser et al. 2021 (47) | Iraq | QG:14/14 | QG:59.2 ± 5.6 | Dyslipidemia | 52 (28/24) | P/3 mo | NA | 200 | Atorvastatin | NA | NA | QG:100.0 | No | Yes | SBP, DBP |
| PG:13/11 | PG:58.1 ± 7.1 | PG:100.0 | |||||||||||||
| Mortensen et al. 2019 (48) | European | QG:90/28 | QG:65.7 ± 10.0 | CVD | 85 (40/45) | P/24 mo | NA | 300 | Standard heart failure therapy | QG:25.0 | QG:92.0 | QG:57.0 | Yes | Yes | SBP, DBP |
| PG:87/36 | PG:64.0 ± 12.0 | PG:26.0 | PG:91.0 | PG:56.0 | |||||||||||
| Dawood et al. 2021 (49) | Iraq | 30/20 | 30–65 | Prehypertensive | 50 (25/25) | P/3 mo | NA | 200 | Placebo | NA | NA | NA | No | No | SBP, DBP |
C, crossover; CoQ10, coenzyme Q10; DBP, diastolic blood pressure; F, fenofibrate; MetS, metabolic syndrome; NA, not available; OHA, oral hypoglycemic agent; P, parallel; PG, placebo group; QG, CoQ10 group; SBP, systolic blood pressure; T1DM, type 1 diabetes mellitus; T2D, type 2 diabetes.
Proportion of participants on hypoglycemic medications or other measurement.
Proportion of participants on hypotension medications.
Proportion of participants on hypolipidemic medications.
Participants were excluded due to supplementation contamination, such as CoQ10, vitamins, antioxidants etc., dietary supplementation.
Effect of CoQ10 supplementation on SBP
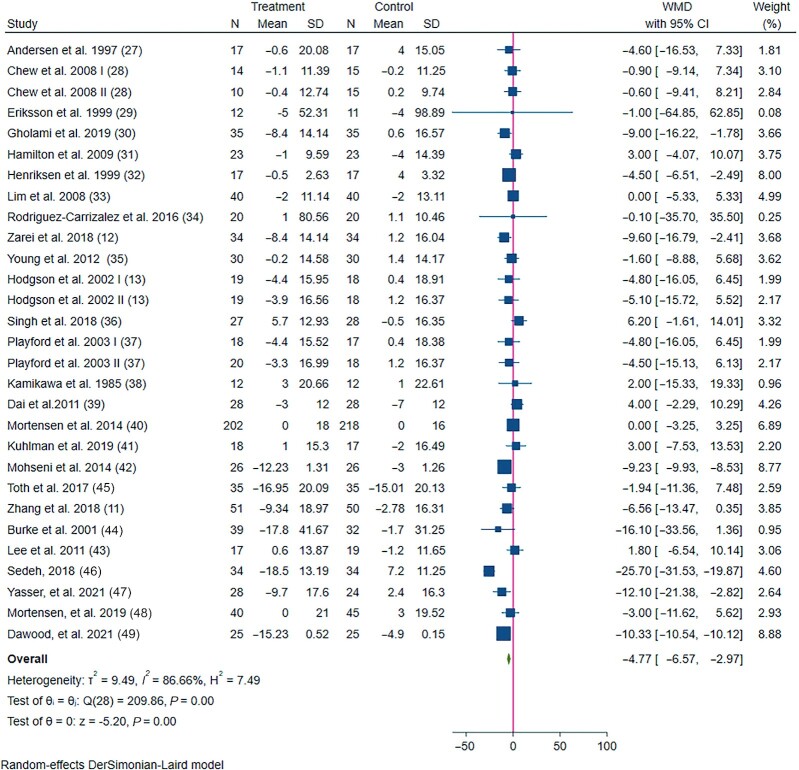
In total, 26 eligible studies (27) with 29 treatment arms including a total of 1831 participants, examined the effect of CoQ10 supplementation intake on SBP. Combining their findings, we found that SBP was significantly reduced by −4.77 mmHg (95% CI: −6.57, −2.97) compared to the control group, with significant between-study heterogeneity (Figure 1). The subgroup analysis conducted on participants' health status revealed a significant reduction in SBP in patients with diabetes and dyslipidemia, but not in CVD and MetS subjects. In addition, subgroup analysis with no medication or supplementation contamination, parallel study design, duration ≥12 wk, CoQ10 dose <200 mg/d, CoQ10 as a single supplementation, and the study not receiving industry funding showed a greater reduction in the levels of SBP (Table 2).
FIGURE 1.
Forest plot detailing WMD and 95% CIs for the effect of CoQ10 supplementation on systolic blood pressure in patients with cardiometabolic disorders. The green diamond at the bottom of each chart is the amount of overall effect size estimates in the random-effects meta-analysis. The size of each blue box reflects the relative weight apportioned to the study in the meta-analysis. The horizontal line across each blue box reflects the 95% CIs of the study. CoQ10, coenzyme Q10; WMD, weighted mean difference.
TABLE 2.
Subgroup analysis of included randomized controlled trials in meta-analysis of the effect of CoQ10 supplementation on systolic and diastolic blood pressure
| Group | No. of trials | WMD (95% CI) 1 | Pdifference | I 2 , % | Pheterogeneity 2 | P for between subgroup heterogeneity3 | |
|---|---|---|---|---|---|---|---|
| Subgroup analyses of CoQ10 supplementation on SBP | |||||||
| Overall | 29 | −4.77 (−6.57, −2.97) | <0.001 | 86.66 | <0.001 | — | |
| Mean age, y | <50 | 5 | −0.71 (−6.00, 4.58) | 0.792 | 52.89 | 0.075 | 0.110 |
| ≥50 | 22 | −3.49 (−6.32, −0.66) | 0.016 | 75.91 | <0.001 | ||
| Health status | Diabetes | 10 | −5.80 (−11.11, −0.49) | 0.032 | 83.72 | <0.001 | <0.001 |
| Dyslipidemia | 5 | −6.71 (−10.90, −2.52) | 0.002 | 52.02 | 0.080 | ||
| MetS | 5 | −3.65 (−8.02, 0.72) | 0.102 | 0.00 | 0.975 | ||
| CVD | 5 | 1.11 (−1.45, 3.66) | 0.397 | 0.00 | 0.437 | ||
| Baseline SBP, mmHg | <130 | 12 | −3.24 (−6.84, 0.36) | 0.078 | 50.32 | 0.023 | <0.001 |
| ≥130 | 17 | −5.44 (−9.59, −1.28) | 0.010 | 81.21 | <0.001 | ||
| Medication contamination4 | No | 6 | −10.21 (−15.55, −4.88) | <0.001 | 91.93 | <0.001 | 0.020 |
| Yes | 23 | −2.89 (−5.76, −0.01) | 0.049 | 78.87 | <0.001 | ||
| Study design | Parallel | 26 | −5.32 (−7.16, −3.49) | <0.001 | 86.82 | <0.001 | 0.020 |
| Crossover | 3 | 0.86 (−4.00, 5.73) | 0.728 | 0.00 | 0.668 | ||
| Duration, wk | <12 | 3 | 3.58 (−1.57, 8.74) | 0.173 | 0.00 | 0.970 | <0.001 |
| ≥12 | 26 | −5.48 (−7.29, −3.68) | <0.001 | 86.33 | <0.001 | ||
| CoQ10 dosage | <200 mg/d | 10 | −7.73 (−13.89, −1.56) | 0.014 | 84.75 | <0.001 | 0.020 |
| ≥200 and <300 mg/d | 15 | −4.60 (−6.59, −2.61) | <0.001 | 86.46 | <0.001 | ||
| ≥300 mg/d | 4 | 1.81 (−2.73, 6.34) | 0.435 | 0.00 | 0.0632 | ||
| Supplementation contamination5 | No | 17 | −3.13 (−5.878, −0.39) | 0.025 | 82.08 | <0.001 | 0.290 |
| Yes | 12 | −6.53 (−12.18, −0.89) | 0.023 | 81.58 | <0.001 | ||
| CoQ10 as a single supplementation | No | 7 | −2.60 (−6.21, 1.01) | 0.158 | 48.17 | 0.072 | 0.500 |
| Yes | 22 | −5.19 (−7.12, −3.27) | <0.001 | 88.45 | <0.001 | ||
| Received industry funding? | No | 19 | −7.61 (−9.47, −5.75) | <0.001 | 82.09 | <0.001 | <0.001 |
| Yes | 10 | −2.26 (−3.86, −0.65) | 0.006 | 47.80 | 0.045 | ||
| Subgroup analyses of CoQ10 supplementation on DBP | |||||||
| Overall | 26 | −1.67 (−4.30, 0.96) | 0.210 | 99.09 | <0.001 | — | |
| Mean age, y | <50 | 5 | −2.67 (−3.88, −1.46) | <0.001 | 26.04 | 0.248 | 0.820 |
| ≥50 | 19 | −1.84 (−4.19, 0.51) | 0.124 | 99.30 | <0.001 | ||
| Health status | Diabetes | 11 | −1.23 (−4.10, 1.64) | 0.402 | 92.19 | <0.001 | 0.010 |
| Dyslipidemia | 5 | −3.22 (−13.82, 7.39) | 0.552 | 97.75 | <0.001 | ||
| MetS | 3 | −2.72 (−5.72, 0.27) | 0.075 | 0.00 | 0.963 | ||
| CVD | 4 | −0.32 (−3.56, 2.92) | 0.848 | 64.93 | 0.036 | ||
| Baseline DBP, mmHg | <80 | 14 | −1.75 (−3.27, −0.23) | 0.024 | 54.69 | 0.007 | 0.910 |
| ≥80 | 12 | −1.50 (−5.66, 2.66) | 0.480 | 99.59 | <0.001 | ||
| Medication contamination4 | No | 5 | −1.04 (−4.21, 2.13) | 0.520 | 90.82 | <0.001 | 0.760 |
| Yes | 21 | −1.88 (−6.21, 2.45) | 0.395 | 99.23 | <0.001 | ||
| Study design | Parallel | 24 | −1.87 (−4.63, 0.89) | 0.184 | 99.15 | <0.001 | 0.370 |
| Crossover | 2 | 0.78 (−4.26, 5.82) | 0.762 | 70.54 | 0.065 | ||
| Duration, wk | <12 | 2 | 4.00 (0.52, 7.48) | 0.024 | 0.00 | 1.000 | 0.010 |
| ≥12 | 24 | −2.16 (−4.90, 0.59) | 0.123 | 99.16 | <0.001 | ||
| CoQ10 dosage | <200 mg/d | 9 | −2.14 (−4.91, 0.64) | 0.132 | 90.30 | <0.001 | 0.630 |
| ≥200 and <300 mg/d | 13 | −2.08 (−7.04, 2.88) | 0.411 | 99.41 | <0.001 | ||
| ≥300 mg/d | 4 | 0.67 (−4.44, 5.78) | 0.797 | 63.89 | <0.001 | ||
| Supplementation contamination5 | No | 12 | −2.62 (−6.14, 0.91) | 0.146 | 85.57 | <0.001 | 0.600 |
| Yes | 14 | −0.99 (−5.97, 3.98) | 0.695 | 99.49 | <0.001 | ||
| CoQ10 as a single supplementation | No | 6 | −5.24 (−10.40, −0.09) | 0.046 | 80.66 | <0.001 | 0.130 |
| Yes | 20 | −0.62 (−3.62, 2.38) | 0.684 | 99.30 | <0.001 | ||
| Received industry funding? | No | 16 | −2.87 (−6.28, 0.54) | 0.099 | 99.44 | <0.001 | 0.120 |
| Yes | 10 | 0.38 (−1.95, 2.70) | 0.750 | 67.62 | 0.001 | ||
DerSimonian-Laird random effect model was used to calculate the effect size and Pdifference when the percentages of I2 >50% or Pheterogeneity; otherwise, inverse-variance fixed-effects model was used.
Cochrane Q test was used to detect the heterogeneity between studies.
Cochrane Q test was used to detect subgroup heterogeneity.
Medication contamination represented taking antidiabetic, antihypertensive or hypolipidemic drugs before entering or during the current study.
Supplementation contamination represented the consumption of CoQ10, vitamins, and antioxidants supplementation before entering or during the current study.
CoQ10, coenzyme Q10; DBP, diastolic blood pressure; MetS, metabolic syndrome; SBP, systolic blood pressure; WMD, weighted mean difference.
Effects of CoQ10 supplementation on DBP
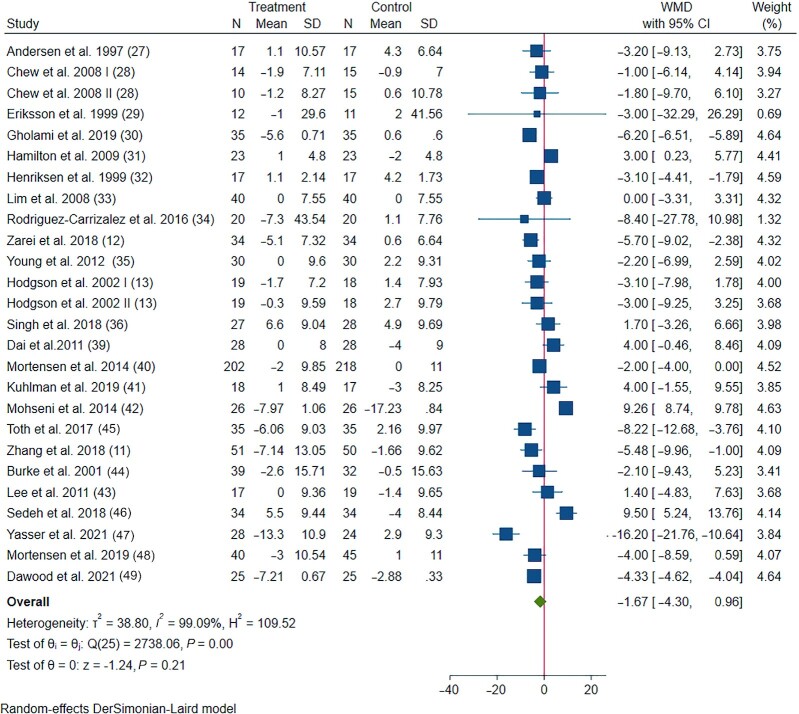
The impact of CoQ10 supplementation on DBP was assessed in 24 trials with 26 treatment arms including 1734 participants. The pooled results showed no significant reduction in DBP following CoQ10 supplementation (−1.67 mmHg, 95% CI: −4.30, 0.96), with considerable between-study heterogeneity (Figure 2). Subgroup analysis revealed that health conditions, study design, duration, CoQ10 dosage, whether CoQ10 was a single supplement, and whether the studies received industry funding explained this heterogeneity (Table 2).
FIGURE 2.
Forest plot detailing WMD and 95% CIs for the effect of CoQ10 supplementation on diastolic blood pressure in patients with cardiometabolic disorders. The green diamond at the bottom of each chart is the amount of overall effect size estimates in the random-effects meta-analysis. The size of each blue box reflects the relative weight apportioned to the study in the meta-analysis. The horizontal line across each blue box reflects the 95% CIs of the study. CoQ10, coenzyme Q10; WMD, weighted mean difference.
Effect of CoQ10 supplementation on circulating CoQ10
The impact of CoQ10 supplementation on circulating CoQ10 was assessed in 16 trials with 19 treatment arms including 983 participants. Compared with the placebo groups, circulating CoQ10 concentrations were elevated by 1.44 μg/mL (95% CI: 1.21, 1.66) (Supplemental Figure 2). These effects were generally consistent across different subgroups (Supplemental Table 2).
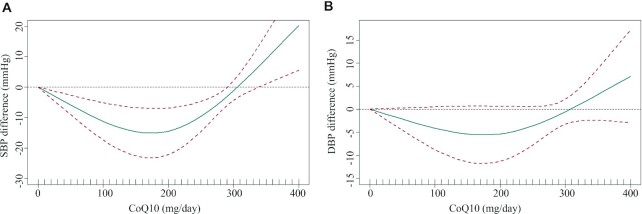
Dose-response association between CoQ10 supplementation and BP
In the dose-response meta-analysis, a U-shaped relation between CoQ10 intervention dose and the level of SBP was observed (Figure 3). Specifically, a CoQ10 dose of 100–200 mg/d had better efficacy in decreasing SBP, but not DBP (χ2 = 14.62, Pnonlinearity = 0.004; χ2 = 3.08, Pnonlinearity = 0.214, respectively).
FIGURE 3.
Dose-response effect of CoQ10 supplementation on (A) systolic blood pressure and (B) diastolic blood pressure. The average curve (green solid line) with 95% confidence limits (red dotted lines) was estimated with a 1-stage random-effects restricted cubic spline model, using 0 mg/d as a reference. CoQ10, coenzyme Q10; DBP, diastolic blood pressure; SBP, systolic blood pressure.
Sensitivity analysis
To explore the impact of a high bias risk study on the overall effect size, we omitted 5 studies that contained high bias from the analysis (30, 31, 36, 45, 48). We found no change in the overall effect sizes of SBP and DBP. However, we found a significant reduction in DBP in younger patients (age <50 y) or patients with lower baseline DBP (baseline DBP <80 mmHg) (Supplemental Table 3). Additionally, the dose-response analysis yielded similar results to those generated using the entire dataset (χ2 = 16.46, Pnonlinearity <0.001 for SBP; χ2 = 1.61, Pnonlinearity = 0.446 for DBP, respectively) (Supplemental Figure 3). In addition, a sensitivity analysis found a similar dose-response relation between CoQ10 supplementation and SBP and DBP (χ2 = 14.64, Pnonlinearity <0.001 for SBP; χ2 = 3.68, Pnonlinearity = 1.59 DBP, respectively) in the subgroup where CoQ10 was a single supplement (28, 36, 37, 44, 45, 48, 49) (Supplemental Figure 4).
Publication bias
Visual inspection of the contour-enhanced funnel plot indicated no evidence of asymmetry in the effects of CoQ10 supplementation on BP (Supplemental Figure 5A, B). These observations were approved by the use of Begg's regression tests for SBP and DBP (PBegg's >0.05). The inverted funnel of circulating CoQ10 was asymmetric on the left and right sides (PBegg's <0.001) (Supplemental Figure 5C). Trim-and-fill estimates found that 5 imputed studies fell in the dark gray region of P >0.1, indicating that there may be unpublished documents that are not statistically significant, which may lead to publication bias. Hence, it is plausible that publication bias is the cause of the observed asymmetry in this funnel plot. However, sensitivity analysis found that 5 imputed studies did not change the overall result of CoQ10 supplementation on circulating CoQ10 (Supplemental Figure 6).
Quality assessment and grading of evidence
The quality assessment outcomes of the included studies are detailed in Supplemental Figure 7. The GRADE protocol was used to assess the certainty of the evidence (Table 3). Accordingly, studies investigating the effect of CoQ10 supplementation on SBP and DBP were regarded as moderate and low quality, respectively, due to the moderate heterogeneity between studies and relatively small sample size. The evidence for circulating CoQ10 was graded as very low due to inconsistencies or evidence of publication bias.
TABLE 3.
GRADE profile of CoQ10 supplementation for systolic blood pressure, diastolic blood pressure, and circulating CoQ10
| Quality assessment | No. of patients | Effect | Quality | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| No. of studies | Design | Risk of bias | Inconsistency | Indirectness | Imprecision | Other considerations | CoQ10 | Control | WMD (95% CI) | |
| Systolic blood pressure (follow-up 1–24 mo; better indicated by higher values) | ||||||||||
| 29 | Randomized trials | No serious risk of bias | Serious1 | No serious indirectness | No serious imprecision | None | 910 | 921 | −4.77 (−6.57, −2.97) | ⊕⊕⊕Ο MODERATE |
| Diastolic blood pressure (follow-up 1–24 mo; better indicated by higher values) | ||||||||||
| 26 | Randomized trials | No serious risk of bias | Very serious2 | No serious indirectness | No serious imprecision | None | 860 | 874 | −1.67 (−4.30, 0.96) | ⊕⊕ΟΟ LOW |
| Circulating CoQ10 (follow-up 2–24 mo; better indicated by higher values) | ||||||||||
| 19 | Randomized trials | No serious risk of bias | Very serious3 | No serious indirectness | No serious imprecision | Reporting bias4 | 481 | 503 | 1.44 (1.21, 1.66) | ⊕ΟΟΟ VERY LOW |
The test for heterogeneity is significant, and the I2 is high, 86.66%.
The test for heterogeneity is significant, and the I2 is high, 99.09%.
The test for heterogeneity is significant, and the I2 is high, 99.53%.
The Begg's test for publication bias is significant (P <0.001).
GRADE Working Group grades of evidence.
High quality: Further research is very unlikely to change our confidence in the estimate of effect.
Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate.
Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate.
Very low quality: We are very uncertain about the estimate.
CoQ10, coenzyme Q10; GRADE, Grading of Recommendations Assessment, Development, and Evaluation; WMD, weighted mean difference.
Discussion
In the present systematic review and dose-response meta-analysis, we summarized available records from 26 RCTs that examined the effects of CoQ10 supplementation on BP in patients with cardiometabolic disorders. Accordingly, our findings revealed that CoQ10 supplementation can significantly reduce SBP accompanied by the elevation of circulating CoQ10 concentrations. Subgroup analyses portrayed a greater reduction in SBP in patients with diabetes and dyslipidemia, or a longer intervention duration (>12 wk). Moreover, we found a novel U-shaped dose-response relation between the intake of CoQ10 supplementation and SBP.
Our present results, which are based largely on small and short-term RCTs, showed that CoQ10 supplementation significantly decreased SBP accompanied by elevated circulating CoQ10 concentrations in patients with cardiometabolic disorders, and the mean decrease in SBP was −4.77 mmHg. Although the overall reduction in SBP was modest in this study from an individual perspective, of note, even minor reductions in BP can yield significant reductions in CVD risk at the population level (50). A recent meta-analysis reported that a SBP reduction of 5 mmHg by pharmacotherapy reduced CVD events by 10% (52). These findings were also supported by a prior systematic review (15) of 17 RCTs involving 684 participants that found a reduction in SBP [standardized mean difference (SMD): −0.30, 95% CI: −0.52, −0.08] but not DBP (SMD: −0.08; 95% CI: −0.46, 0.29) with CoQ10 in patients with metabolic diseases. Given the moderate level of evidence certainty for SBP findings, the clinically beneficial effects of CoQ10 supplementation in people with cardiometabolic disorders may be attributed to the reduction in SBP.
Subgroup analysis showed that CoQ10 supplementation was more effective in reducing SBP in patients with dyslipidemia (−6.71 mmHg) than in all participants (−4.77 mmHg) in our study. Indeed, possible reasons may be the decrease in endogenous CoQ10 content in patients with dyslipidemia who were treated with cholesterol-lowering drugs (52–54), and circulating CoQ10 significantly increased after CoQ10 supplementation. It has been proposed that patients with lower circulating CoQ10 concentrations may have a better antihypertensive response to CoQ10 supplementation (55). In addition, previous studies have shown that the concentration of circulating CoQ10 in type 2 diabetes is lower (0.40–1.91 μmol/L CoQ10 in serum) (53, 56). Interestingly, our subgroup analysis also found that CoQ10 was more effective in reducing SBP when taking CoQ10 in patients with diabetes (−5.80 mmHg). This is consistent with a prior systematic review that found a significant effect of CoQ10 supplementation on reducing SBP in a subgroup analysis of patients with type 2 diabetes (15). Therefore, CoQ10 supplementation may achieve more health benefits in patients with dyslipidemia or diabetes.
Furthermore, subgroup analysis also indicated that interventions with longer durations demonstrated larger reductions in SBP, as compared to those with shorter durations. This might be partially due to the time that is needed to reach the threshold of lowering SBP. Concordant with the findings of the subgroup analysis on SBP in this study, several clinical trials have shown the beneficial effects of CoQ10 with long-duration administration. CoQ10 supplementation for 2 y improved heart function and reduced cardiovascular-related mortality by 43% in heart failure patients, of note, 10% of whom had type 2 diabetes (40). Similarly, in the KISEL-10 study, supplementation with 200 mg/d CoQ10 and 200 μg/d selenium for 5 y reduced cardiovascular mortality by 53% in elderly subjects, 20% of whom had diabetes (57). A longer duration of CoQ10 consumption also had antioxidant (58) and anti-inflammation (59) properties, compared with a short duration. Given that CoQ10 is easy to obtain and can be used as an attractive option for long-term use, this will achieve a greater effect on the SBP of patients with cardiometabolic disorders. Meanwhile, a larger effect of lowering SBP was experienced in patients with cardiometabolic disorders who did not take medications, and there was still a significant effect in patients currently undergoing pharmacological therapy. This may be attributed to medication contamination that may partly mask the benefits of CoQ10 supplementation on SBP. In addition, SBP-lowering effects are also valid in subgroups of both administrated supplementation and not. These results suggested that CoQ10 as a single or add-on supplementation may be beneficial for attenuating BP and reducing risk factors in patients with cardiometabolic disorders.
Importantly, by using a new “1-stage” model that allows for the inclusion of trials with only 2 levels of exposure, as is the case for most RCTs (16, 20), we conducted a dose-response meta-analysis to investigate the threshold of CoQ10 supplementation on the level of BP and showed a “U” shape relation between CoQ10 supplementation and BP. The previous meta-analysis only used the traditional forest plot to examine the overall effect size without examining its dose-response relation (60). Tabrizi et al. (15) attempted to assess the relation between CoQ10 and BP in clinical studies, but they were unable to fully characterize it because they only used forest plots, thus being unable to smoothly shape the relation between CoQ10 intake and BP over the entire range of exposure. In addition, the disappearance of the SBP control effect of high dose CoQ10 may be due to a decrease in the intestinal absorption and utilization process (61). CoQ10 is a lipophilic compound, and absorption occurs in the gastrointestinal tract via a complex active transport process. A nonlinear or zero-order absorption process has been found, suggesting that CoQ10 plasma concentration decreases as the dosage is increased (62). However, it should be noted that only 4 studies in this review used doses >400 mg/d. Thus, the relative lack of RCTs with higher CoQ10 doses limited the power to make a conclusion in the higher range of CoQ10 supplementation. Although a previous study indicated that CoQ10 appears to be well tolerated in dosages of ≤1200 mg/d in adults with long-term use (63), it seems that 100–200 mg/d is sufficient to beneficially attenuate SBP in patients with cardiometabolic disorders according to our present dose-response meta-analysis.
Several mechanisms have been proposed to explain the hypotensive effects of CoQ10. One of the most important mechanisms may be related to its antioxidant properties (10). Although oxidative stress reduces NO availability resulting in vasoconstriction that leads to elevated BP, CoQ10 supplementation may enhance antioxidant capacity and improve NO bioavailability exerting a direct beneficial effect on the endothelium (64, 65). A meta-analysis of 5 RCTs including 194 patients found that CoQ10 improved endothelial function, as assessed by flow-mediated dilatation (66). Another mechanism involves CoQ10 exerting an angiotensin effect upon sodium retention and decreasing the concentration of aldosterone, thus inhibiting BP levels (67). Alternatively, CoQ10 may boost the production of prostacyclin, a potent vasodilator, and enhance the sensitivity of arterial smooth muscles to prostacyclin (68). Further mechanisms that may contribute to the reduction of BP are the hypoglycemic and hypolipidemic effects of CoQ10 supplementation, as elevated blood glucose and lipid concentrations are well known to depress endothelial function via a cascade of pathophysiological events that result in the development and progression of cardiometabolic disorders. Moreover, the effects of CoQ10 on BP are also related to its anti-inflammatory abilities. Animal models have shown that CoQ10 supplementation can balance pro- and anti-inflammatory cytokines (69).
A previous study showed that a number of foods provide an exogenous source of CoQ10, but the CoQ10 content varies in different types of foods. Specifically, CoQ10 concentrations of various meats are higher than those of plant-derived foods such as grains, fruits, and vegetables. The highest CoQ10 concentration was found in beef heart (over 100 mg/kg), where the CoQ10 intake reached 100 mg/d when ≤ ∼1 kg per day of beef heart was consumed. Although beef heart is the most abundant source of CoQ10, it is not used as a daily food in some parts of the world. Other daily CoQ10-rich food products, such as sardines (5.1–64.3 mg/kg), beef (16.1–36.5 mg/kg), poultry meat (14–21 mg/kg), or peanuts (26.7 mg/kg), could substantially increase the total amount of CoQ10 (70). However, the extent to which dietary consumption of CoQ10 correlates to tissue CoQ10 concentrations is unclear. A study showed that CoQ10 intake in the daily diet is relatively low, and the average dietary intake of CoQ10 is only 3–6 mg/d (70); therefore, CoQ10 intake from the daily diet might be far from 100 mg/d. The human body can biosynthesize CoQ10, but its deficiency has also been observed in various pathological conditions, such as cardiometabolic disorders (10); thus, the intake of food with adequate CoQ10 is necessary. Additional supplementation may be necessary to improve circulating CoQ10 concentrations and attenuate SBP in patients with cardiometabolic disorders.
Strengths and limitations
This present study has several strengths. First, our study clarified the association between CoQ10 supplementation and BP in patients with cardiometabolic disorders via a systematic review and meta-analysis of updated studies. Moreover, we first conducted a novel 1-stage restricted cubic spline regression model based on 2-arm comparisons of the CoQ10 clinical trial to determine the dose-response effect of CoQ10 on BP in patients with cardiometabolic disorders and quantify its effect on the concentration of circulating CoQ10. However, several limitations merit consideration. First, most studies in this review involved the simultaneous use of diabetes treatment, and some studies permitted the use of hypotensive drugs and lipid-lowering drugs. Therefore, we cannot draw any clear conclusions regarding the efficacy of CoQ10 as a stand-alone therapeutic agent for cardiometabolic disorder management. Second, due to fewer clinical trials investigating the relation between CoQ10 supplementation and BP in patients with diabetes and dyslipidemia, we were unable to conduct restricted cubic spline regression to depict its dose response. Third, although publication bias was found for the effect on circulating CoQ10, trim-and-fill analyses showed the result is robust to additional imputed trial comparisons. Finally, the studies included were predominantly short term (<6 mo), had a relatively small participant number (n <100), and lacked relatively higher doses (doses >300 mg/d) and most of the studies lacked justification of sample size or did not report either the detailed method of blinding or the evaluation of the successfulness of blinding. Therefore, the current evaluation of this meta-analysis via the GRADE approach is only medium to very low quality. Further larger sample sizes and longer supplementation period studies with strict designs are required to confirm the beneficial effects of CoQ10 supplementation on BP in patients with cardiometabolic disorders.
Conclusions
Our findings suggest that CoQ10 supplementation may be potentially effective for clinically reducing BP in patients with cardiometabolic disorders, and these effects are more pronounced in people with diabetes or dyslipidemia. Moreover, taking 100–200 mg/d CoQ10 supplementation is recommended for attenuating SBP in patients with cardiometabolic disorders. To further determine the exact recommended intake of CoQ10 for lowering BP, we are also designing a clinical trial with different doses of CoQ10 intervention and are preparing to carry out this study in the near future.
Supplementary Material
Acknowledgements
We would like to acknowledge the assistance of Yimin Zhao in formulating a retrieval strategy.
The authors’ responsibilities were as follows—YY: designed the study; ZT: drafted the protocol and modified the final manuscript; DZ, ZL, and ML: screened and selected the trials; YL and DZ: extracted the data; YL, SD, and DZ: analyzed the data; DZ: drafted the manuscript; and all authors: read and approved the final manuscript.
Notes
This work was supported by grants from the Key Program of the National Natural Science Foundation of China (No. 82030098); the National Natural Science Foundation of China (No. 81872617); Chinese Nutrition Society Research Fund for Dietary Reference Intake; National innovation and entrepreneurship undergraduate training program (No. 202210558159 and 202210558161). The funders had no role in the study design, data collection, analysis, interpretation of data, preparation of the manuscript, or decision to submit it for publication.
Author disclosures: The authors report no conflicts of interest.
Supplemental Figures 1–7 and Supplemental Tables 1–3 are available from the “Supplementary data” link in the online posting of the article and from the same link in the online table of contents at https://academic.oup.com/advances/.
Abbreviations used: BP, blood pressure; CoQ10, coenzyme Q10; DBP, diastolic blood pressure; GRADE, Grading of Recommendations Assessment, Development, and Evaluation; MetS, metabolic syndrome; RCT, randomized controlled trial; SBP, systolic blood pressure; SMD, standardized mean difference; WMD, weighted mean difference.
Contributor Information
Dan Zhao, School of Public Health (Shenzhen), Shenzhen Campus of Sun Yat-sen University, Sun Yat-sen University, Shenzhen, Guangdong Province, PR China; Guangdong Provincial Key Laboratory of Food, Nutrition, and Health, Sun Yat-sen University, Guangzhou, Guangdong Province, PR China; Guangdong Engineering Technology Center of Nutrition Transformation, Sun Yat-sen University, Guangzhou, Guangdong Province, PR China.
Ying Liang, School of Public Health (Shenzhen), Shenzhen Campus of Sun Yat-sen University, Sun Yat-sen University, Shenzhen, Guangdong Province, PR China; Guangdong Provincial Key Laboratory of Food, Nutrition, and Health, Sun Yat-sen University, Guangzhou, Guangdong Province, PR China; Guangdong Engineering Technology Center of Nutrition Transformation, Sun Yat-sen University, Guangzhou, Guangdong Province, PR China.
Suming Dai, School of Public Health (Shenzhen), Shenzhen Campus of Sun Yat-sen University, Sun Yat-sen University, Shenzhen, Guangdong Province, PR China; Guangdong Provincial Key Laboratory of Food, Nutrition, and Health, Sun Yat-sen University, Guangzhou, Guangdong Province, PR China; Guangdong Engineering Technology Center of Nutrition Transformation, Sun Yat-sen University, Guangzhou, Guangdong Province, PR China.
Shanshan Hou, School of Public Health (Shenzhen), Shenzhen Campus of Sun Yat-sen University, Sun Yat-sen University, Shenzhen, Guangdong Province, PR China; Guangdong Provincial Key Laboratory of Food, Nutrition, and Health, Sun Yat-sen University, Guangzhou, Guangdong Province, PR China; Guangdong Engineering Technology Center of Nutrition Transformation, Sun Yat-sen University, Guangzhou, Guangdong Province, PR China.
Zhihao Liu, School of Public Health (Shenzhen), Shenzhen Campus of Sun Yat-sen University, Sun Yat-sen University, Shenzhen, Guangdong Province, PR China; Guangdong Provincial Key Laboratory of Food, Nutrition, and Health, Sun Yat-sen University, Guangzhou, Guangdong Province, PR China; Guangdong Engineering Technology Center of Nutrition Transformation, Sun Yat-sen University, Guangzhou, Guangdong Province, PR China.
Meitong Liu, School of Public Health (Shenzhen), Shenzhen Campus of Sun Yat-sen University, Sun Yat-sen University, Shenzhen, Guangdong Province, PR China; Guangdong Provincial Key Laboratory of Food, Nutrition, and Health, Sun Yat-sen University, Guangzhou, Guangdong Province, PR China; Guangdong Engineering Technology Center of Nutrition Transformation, Sun Yat-sen University, Guangzhou, Guangdong Province, PR China.
Xiaoxi Dong, School of Public Health (Shenzhen), Shenzhen Campus of Sun Yat-sen University, Sun Yat-sen University, Shenzhen, Guangdong Province, PR China.
Yiqiang Zhan, School of Public Health (Shenzhen), Shenzhen Campus of Sun Yat-sen University, Sun Yat-sen University, Shenzhen, Guangdong Province, PR China; Guangdong Provincial Key Laboratory of Food, Nutrition, and Health, Sun Yat-sen University, Guangzhou, Guangdong Province, PR China.
Zezhong Tian, School of Public Health (Shenzhen), Shenzhen Campus of Sun Yat-sen University, Sun Yat-sen University, Shenzhen, Guangdong Province, PR China; Guangdong Provincial Key Laboratory of Food, Nutrition, and Health, Sun Yat-sen University, Guangzhou, Guangdong Province, PR China; Guangdong Engineering Technology Center of Nutrition Transformation, Sun Yat-sen University, Guangzhou, Guangdong Province, PR China.
Yan Yang, School of Public Health (Shenzhen), Shenzhen Campus of Sun Yat-sen University, Sun Yat-sen University, Shenzhen, Guangdong Province, PR China; Guangdong Provincial Key Laboratory of Food, Nutrition, and Health, Sun Yat-sen University, Guangzhou, Guangdong Province, PR China; Guangdong Engineering Technology Center of Nutrition Transformation, Sun Yat-sen University, Guangzhou, Guangdong Province, PR China; China-Dietary Reference Intakes Expert Committee, Beijing, PR China.
References
- 1. Li Y, Wang DD, Ley SH, Howard AG, He Y, Lu Yet al. Potential impact of time trend of life-style factors on cardiovascular disease burden in China. J Am Coll Cardiol. 2016;68(8):818–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Lewington S, Clarke R, Qizilbash N, Peto R, Collins R, Prospective Studies Collaboration . Age-specific relevance of usual blood pressure to vascular mortality: A meta-analysis of individual data for one million adults in 61 prospective studies. Lancet. 2002;360(9349):1903–13. [DOI] [PubMed] [Google Scholar]
- 3. Fuchs FD, Whelton PK. High blood pressure and cardiovascular disease. Hypertension. 2020;75(2):285–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Mills KT, Stefanescu A, He J. The global epidemiology of hypertension. Nat Rev Nephrol. 2020;16(4):223–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Antza C, Doundoulakis I, Stabouli S, Kotsis V. Comparison among recommendations for the management of arterial hypertension issued by last US, Canadian, British and European Guidelines. High Blood Press Cardiovasc Prev. 2018;25(1):9–16. [DOI] [PubMed] [Google Scholar]
- 6. Palmer SC, Mavridis D, Navarese E, Craig JC, Tonelli M, Salanti Get al. Comparative efficacy and safety of blood pressure-lowering agents in adults with diabetes and kidney disease: A network meta-analysis. Lancet North Am Ed. 2015;385(9982):2047–56. [DOI] [PubMed] [Google Scholar]
- 7. Cameron AC, Lang NN, Touyz RM. Drug treatment of hypertension: Focus on vascular health. Drugs. 2016;76(16):1529–50. [DOI] [PubMed] [Google Scholar]
- 8. Overvad K, Diamant B, Holm L, Holmer G, Mortensen SA, Stender S. Coenzyme Q10 in health and disease. Eur J Clin Nutr. 1999;53(10):764–70. [DOI] [PubMed] [Google Scholar]
- 9. Rabanal-Ruiz Y, Llanos-González E, Alcain FJ. The use of coenzyme Q10 in cardiovascular diseases. Antioxidants. (Basel)2021;10(5):755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Flowers N, Hartley L, Todkill D, Stranges S, Rees K. Co-enzyme Q10 supplementation for the primary prevention of cardiovascular disease. Cochrane Database Syst Rev. 2014(12):CD010405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Zhang P, Yang C, Guo H, Wang J, Lin S, Li Het al. Treatment of coenzyme Q10 for 24 weeks improves lipid and glycemic profile in dyslipidemic individuals. Journal of Clinical Lipidology. 2018;12(2):417–27.e5. [DOI] [PubMed] [Google Scholar]
- 12. Zarei P, Rezvanfar MR, Ansarihadipour H, Delavar M, Abdollahi M, Khosrowbeygi A. Effects of coenzyme Q(10) supplementation on the serum levels of amylase, adenosine deaminase, catalase, and total antioxidant capacity in women with type 2 diabetes mellitus: A randomized, double-blind placebo-controlled trial. J Res Med Sci. 2018;23:91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hodgson JM, Watts GF, Playford DA, Burke V, Croft KD. Coenzyme Q10 improves blood pressure and glycaemic control: A controlled trial in subjects with type 2 diabetes. Eur J Clin Nutr. 2002;56(11):1137–42. [DOI] [PubMed] [Google Scholar]
- 14. Ho MJ, Li ECK, Wright JM. Blood pressure lowering efficacy of coenzyme Q10 for primary hypertension. Cochrane Database Syst Rev. 2016;3(3):CD007435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Tabrizi R, Akbari M, Sharifi N, Lankarani KB, Moosazadeh M, Kolahdooz Fet al. The effects of coenzyme Q10 supplementation on blood pressures among patients with metabolic diseases: A systematic review and meta-analysis of randomized controlled trials. High Blood Press Cardiovasc Prev. 2018;25(1):41–50. [DOI] [PubMed] [Google Scholar]
- 16. Crippa A, Discacciati A, Bottai M, Spiegelman D, Orsini N. One-stage dose-response meta-analysis for aggregated data. Stat Methods Med Res. 2019;28(5):1579–96. [DOI] [PubMed] [Google Scholar]
- 17. Shamseer L, Moher D, Clarke M, Ghersi D, Liberati A, Petticrew Met al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015: elaboration and explanation. BMJ. 2015;350:g7647. [DOI] [PubMed] [Google Scholar]
- 18. Higgins JP, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman ADet al. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7(3):177–88. [DOI] [PubMed] [Google Scholar]
- 20. Vinceti M, Filippini T, Malavolti M, Naska A, Kasdagli M, Torres Det al. Dose-response relationships in health risk assessment of nutritional and toxicological factors in foods: Development and application of novel biostatistical methods. EFSA Supporting Publications. 2020;17(7):1899E. [Google Scholar]
- 21. Peters JL, Sutton AJ, Jones DR, Abrams KR, Rushton L. Contour-enhanced meta-analysis funnel plots help distinguish publication bias from other causes of asymmetry. J Clin Epidemiol. 2008;61(10):991–6. [DOI] [PubMed] [Google Scholar]
- 22. Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315(7109):629–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Duval S, Tweedie R. Trim and fill: A simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics. 2000;56(2):455–63. [DOI] [PubMed] [Google Scholar]
- 24. McMaster University . GRADEpro Guideline Development Tool. Hamilton, Ontario, Canada: Evidence Prime Inc.; 2015. [Google Scholar]
- 25. Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck-Ytter Y, Alonso-Coello Pet al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ. 2008;336(7650):924–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Balshem H, Helfand M, Schünemann HJ, Oxman AD, Kunz R, Brozek Jet al. GRADE guidelines: 3. Rating the quality of evidence. J Clin Epidemiol. 2011;64(4):401–6. [DOI] [PubMed] [Google Scholar]
- 27. Andersen CB, Henriksen JE, Hother-Nielsen O, Vaag A, Mortensen SA, Beck-Nielsen H. The effect of coenzyme Q10 on blood glucose and insulin requirement in patients with insulin dependent diabetes mellitus. Mol Aspects Med. 1997;18(Suppl):307–9. [DOI] [PubMed] [Google Scholar]
- 28. Chew GT, Watts GF, Davis TM, Stuckey BG, Beilin LJ, Thompson PLet al. Hemodynamic effects of fenofibrate and coenzyme Q10 in type 2 diabetic subjects with left ventricular diastolic dysfunction. Diabetes Care. 2008;31(8):1502–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Eriksson JG, Forsén TJ, Mortensen SA, Rohde M. The effect of coenzyme Q10 administration on metabolic control in patients with type 2 diabetes mellitus. Biofactors. 1999;9(2–4):315–18. [DOI] [PubMed] [Google Scholar]
- 30. Gholami M, Rezvanfar MR, Delavar M, Abdollahi M, Khosrowbeygi A. Effects of coenzyme Q10 supplementation on serum values of gamma-glutamyl transferase, pseudocholinesterase, bilirubin, ferritin, and high-sensitivity C-reactive protein in women with type 2 diabetes. Exp Clin Endocrinol Diabetes. 2019;127(5):311–19. [DOI] [PubMed] [Google Scholar]
- 31. Hamilton SJ, Chew GT, Watts GF. Coenzyme Q10 improves endothelial dysfunction in statin-treated type 2 diabetic patients. Diabetes Care. 2009;32(5):810–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Henriksen JE, Andersen CB, Hother-Nielsen O, Vaag A, Mortensen SA, Beck-Nielsen H. Impact of ubiquinone (coenzyme Q10) treatment on glycaemic control, insulin requirement and well-being in patients with type 1 diabetes mellitus. Diabet Med. 1999;16(4):312–18. [DOI] [PubMed] [Google Scholar]
- 33. Lim SC, Lekshminarayanan R, Goh SK, Ong YY, Subramaniam T, Sum CFet al. The effect of coenzyme Q10 on microcirculatory endothelial function of subjects with type 2 diabetes mellitus. Atherosclerosis. 2008;196(2):966–9. [DOI] [PubMed] [Google Scholar]
- 34. Rodríguez-Carrizalez AD, Castellanos-González JA, Martínez-Romero EC, Miller-Arrevillaga G, Pacheco-Moisés FP, Román-Pintos LMet al. The effect of ubiquinone and combined antioxidant therapy on oxidative stress markers in non-proliferative diabetic retinopathy: A phase IIa, randomized, double-blind, and placebo-controlled study. Redox Report. 2016;21(4):155–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Young JM, Florkowski CM, Molyneux SL, McEwan RG, Frampton CM, Nicholls MGet al. A randomized, double-blind, placebo-controlled crossover study of coenzyme Q10 therapy in hypertensive patients with the metabolic syndrome. Am J Hypertens. 2012;25(2):261–70. [DOI] [PubMed] [Google Scholar]
- 36. Singh RB, Fedacko J, Mojto V, Pella D. Coenzyme Q10 modulates remodeling possibly by decreasing angiotensin-converting enzyme in patients with acute coronary syndrome. Antioxidants (Basel). 2018;7(8):99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Playford DA, Watts GF, Croft KD, Burke V. Combined effect of coenzyme Q10 and fenofibrate on forearm microcirculatory function in type 2 diabetes. Atherosclerosis. 2003;168(1):169–79. [DOI] [PubMed] [Google Scholar]
- 38. Kamikawa T, Kobayashi A, Yamashita T, Hayashi H, Yamazaki N. Effects of coenzyme Q10 on exercise tolerance in chronic stable angina pectoris. Am J Cardiol. 1985;56(4):247–51. [DOI] [PubMed] [Google Scholar]
- 39. Dai YL, Luk TH, Yiu KH, Wang M, Yip PM, Lee SWet al. Reversal of mitochondrial dysfunction by coenzyme Q10 supplement improves endothelial function in patients with ischaemic left ventricular systolic dysfunction: A randomized controlled trial. Atherosclerosis. 2011;216(2):395–401. [DOI] [PubMed] [Google Scholar]
- 40. Mortensen SA, Rosenfeldt F, Kumar A, Dolliner P, Filipiak KJ, Pella Det al. The effect of coenzyme Q10 on morbidity and mortality in chronic heart failure: Results from Q-SYMBIO: A randomized double-blind trial. JACC: Heart Failure. 2014;2(6):641–9. [DOI] [PubMed] [Google Scholar]
- 41. Kuhlman AB, Morville T, Dohlmann TL, Hansen M, Kelly B, Helge JWet al. Coenzyme Q10 does not improve peripheral insulin sensitivity in statin-treated men and women: The LIFESTAT study. Applied Physiology, Nutrition, and Metabolism. 2019;44(5):485–92. [DOI] [PubMed] [Google Scholar]
- 42. Mohseni M, Vafa MR, Hajimiresmail SJ, Zarrati M, Rahimi Forushani A, Bitarafan Vet al. Effects of coenzyme q10 supplementation on serum lipoproteins, plasma fibrinogen, and blood pressure in patients with hyperlipidemia and myocardial infarction. Iranian Red Crescent Medical Journal. 2014;16(10):e16433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Lee YJ, Cho WJ, Kim JK, Lee DC. Effects of coenzyme Q10 on arterial stiffness, metabolic parameters, and fatigue in obese subjects: A double-blind randomized controlled study. J Med Food. 2011;14(4):386–90. [DOI] [PubMed] [Google Scholar]
- 44. Burke BE, Neuenschwander R, Olson RD. Randomized, double-blind, placebo-controlled trial of coenzyme Q10 in isolated systolic hypertension. South Med J. 2001;94(11):1112–17. [DOI] [PubMed] [Google Scholar]
- 45. Tóth Š, Šajty M, Pekárová T, Mughees A, Štefanič P, Katz Met al. Addition of omega-3 fatty acid and coenzyme Q10 to statin therapy in patients with combined dyslipidemia. J Basic Clin Physiol Pharmacol. 2017;28(4):327–36. [DOI] [PubMed] [Google Scholar]
- 46. Sedeh BS, Sadeghi S, Mohaghegh P, Begi AK, Mohamadi R, Sabzevari Set al. Prophylactic effects of Q10 capsule on proteinuria in diabetic patients. Revista Latinoamericana de Hipertensión. 2018;13(3):291–6. [Google Scholar]
- 47. Yasser AN, Abdulridha MK, Shafek MA. Assessment of some clinical and biochemical parameters after combining coenzyme Q10 to statin in dyslipidemic patients. International Journal of Drug Delivery Technology. 2021;11(3):904–11. [Google Scholar]
- 48. Mortensen AL, Rosenfeldt F, Filipiak KJ. Effect of coenzyme Q10 in Europeans with chronic heart failure: A sub-group analysis of the Q-SYMBIO randomized double-blind trial. Cardiol J. 2019;26(2):147–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Dawood MH, Al-Yassiry MM. Effects of coenzyme Q10 administration on systolic and diastolic blood pressure in pre-hypertensive patients. Sys Rev Pharm. 2021;12(7):373–8. [Google Scholar]
- 50. Hardy ST, Loehr LR, Butler KR, Chakladar S, Chang PP, Folsom ARet al. Reducing the blood pressure-related burden of cardiovascular disease: Impact of achievable improvements in blood pressure prevention and control. J Am Heart Assoc. 2015;4(10):e002276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Rahimi K, Bidel Z, Nazarzadeh M, Copland E, Canoy D, Ramakrishnan Ret al. Pharmacological blood pressure lowering for primary and secondary prevention of cardiovascular disease across different levels of blood pressure: an individual participant-level data meta-analysis. Lancet North Am Ed. 2021;397(10285):1625–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Raizner AE, Quiñones MA. Coenzyme Q(10) for patients with cardiovascular disease: JACC focus seminar. J Am Coll Cardiol. 2021;77(5):609–19. [DOI] [PubMed] [Google Scholar]
- 53. Banach M, Serban C, Ursoniu S, Rysz J, Muntner P, Toth PPet al. Statin therapy and plasma coenzyme Q10 concentrations–A systematic review and meta-analysis of placebo-controlled trials. Pharmacol Res. 2015;99:329–36. [DOI] [PubMed] [Google Scholar]
- 54. Qu H, Meng YY, Chai H, Liang F, Zhang JY, Gao ZYet al. The effect of statin treatment on circulating coenzyme Q10 concentrations: An updated meta-analysis of randomized controlled trials. Eur J Med Res. 2018;23(1):57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Houston M. Nutrition and nutraceutical supplements for the treatment of hypertension: Part III. The Journal of Clinical Hypertension. 2013;15(12):931–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. El-ghoroury EA, Raslan HM, Badawy EA, El-Saaid GS, Agybi MH, Siam Iet al. Malondialdehyde and coenzyme Q10 in platelets and serum in type 2 diabetes mellitus: Correlation with glycemic control. Blood Coagul Fibrinolysis. 2009;20(4):248–51. [DOI] [PubMed] [Google Scholar]
- 57. Alehagen U, Johansson P, Björnstedt M, Rosén A, Dahlström U. Cardiovascular mortality and N-terminal-proBNP reduced after combined selenium and coenzyme Q10 supplementation: A 5-year prospective randomized double-blind placebo-controlled trial among elderly Swedish citizens. Int J Cardiol. 2013;167(5):1860–6. [DOI] [PubMed] [Google Scholar]
- 58. Akbari A, Mobini GR, Agah S, Morvaridzadeh M, Omidi A, Potter Eet al. Coenzyme Q10 supplementation and oxidative stress parameters: A systematic review and meta-analysis of clinical trials. Eur J Clin Pharmacol. 2020;76(11):1483–99. [DOI] [PubMed] [Google Scholar]
- 59. Fan L, Feng Y, Chen GC, Qin LQ, Fu CL, Chen LH. Effects of coenzyme Q10 supplementation on inflammatory markers: A systematic review and meta-analysis of randomized controlled trials. Pharmacol Res. 2017;119:128–36. [DOI] [PubMed] [Google Scholar]
- 60. Fotino AD, Thompson-Paul AM, Bazzano LA. Effect of coenzyme Q₁₀ supplementation on heart failure: A meta-analysis. Am J Clin Nutr. 2013;97(2):268–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Miles MV. The uptake and distribution of coenzyme Q10. Mitochondrion. 2007;7:Suppl:S72–7. [DOI] [PubMed] [Google Scholar]
- 62. Hosoe K, Kitano M, Kishida H, Kubo H, Fujii K, Kitahara M. Study on safety and bioavailability of ubiquinol (Kaneka QH) after single and 4-week multiple oral administration to healthy volunteers. Regul Toxicol Pharm. 2007;47(1):19–28. [DOI] [PubMed] [Google Scholar]
- 63. Hathcock JN, Shao A. Risk assessment for coenzyme Q10 (ubiquinone). Regul Toxicol Pharm. 2006;45(3):282–8. [DOI] [PubMed] [Google Scholar]
- 64. Watts GF, Playford DA, Croft KD, Ward NC, Mori TA, Burke V. Coenzyme Q(10) improves endothelial dysfunction of the brachial artery in type II diabetes mellitus. Diabetologia. 2002;45(3):420–6. [DOI] [PubMed] [Google Scholar]
- 65. Tsai KL, Huang YH, Kao CL, Yang DM, Lee HC, Chou HYet al. A novel mechanism of coenzyme Q10 protects against human endothelial cells from oxidative stress-induced injury by modulating NO-related pathways. J Nutr Biochem. 2012;23(5):458–68. [DOI] [PubMed] [Google Scholar]
- 66. Gao L, Mao Q, Cao J, Wang Y, Zhou X, Fan L. Effects of coenzyme Q10 on vascular endothelial function in humans: A meta-analysis of randomized controlled trials. Atherosclerosis. 2012;221(2):311–16. [DOI] [PubMed] [Google Scholar]
- 67. Langsjoen P, Langsjoen P, Willis R, Folkers K. Treatment of essential hypertension with coenzyme Q10. Mol Aspects Med. 1994;15:Suppl:s265–72. [DOI] [PubMed] [Google Scholar]
- 68. Lönnrot K, Pörsti I, Alho H, Wu X, Hervonen A, Tolvanen JP. Control of arterial tone after long-term coenzyme Q10 supplementation in senescent rats. Br J Pharmacol. 1998;124(7):1500–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Gao HL, Yu XJ, Qi J, Yi QY, Jing WH, Sun WYet al. Oral CoQ10 attenuates high salt-induced hypertension by restoring neurotransmitters and cytokines in the hypothalamic paraventricular nucleus. Sci Rep. 2016;6(1):30301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Pravst I, Zmitek K, Zmitek J. Coenzyme Q10 contents in foods and fortification strategies. Crit Rev Food Sci Nutr. 2010;50(4):269–80. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.