ABSTRACT
Unsaturated fatty acids might be involved in the prevention of and improvement in mental disorders, but the evidence on these associations has not been comprehensively assessed. This umbrella review aimed to appraise the credibility of published evidence evaluating the associations between unsaturated fatty acids and mental disorders. In this umbrella review, systematic reviews and meta-analyses of studies comparing unsaturated fatty acids (including supplementation, dietary intake, and blood concentrations) in participants with mental disorders with healthy individuals were included. We reanalyzed summary estimates, between-study heterogeneity, predictive intervals, publication bias, small-study effects, and excess significance bias for each meta-analysis. Ninety-five meta-analyses from 29 systematic reviews were included, encompassing 43 studies on supplementation interventions, 32 studies on dietary factors, and 20 studies on blood biomarkers. Suggestive evidence was only observed for dietary intake, in which higher intake of fish was associated with reduced risk of depression (RR: 0.78; 95% CI: 0.69, 0.89) and Alzheimer disease (RR: 0.74; 95% CI: 0.63, 0.87), and higher intake of total PUFAs might be associated with a lower risk of mild cognitive impairment (RR: 0.71; 95% CI: 0.61, 0.84). Evidence showed that PUFA supplementation was favorable but had weak credibility in anxiety, depression, attention-deficit/hyperactivity disorder (ADHD), autism spectrum disorder (ASD), dementia, mild cognitive impairment, Huntington's disease, and schizophrenia (P-random effects <0.001–0.040). There was also weak evidence on the effect of decreased circulating n–3 (ɷ-3) PUFAs among patients on risk of ADHD, ASD, bipolar disorder, and schizophrenia (P-random effects <10−6–0.037). Our results suggest that higher levels of unsaturated fatty acids may relieve symptoms or reduce the risk of various mental disorders; however, the strength of the associations and credibility of the evidence were generally weak. Future high-quality research is needed to identify whether PUFA interventions should be prioritized to alleviate mental disorders.
Keywords: mental disorders, unsaturated fatty acids, n–3 PUFA, umbrella review, meta-analysis
Statement of Significance: The credibility of published evidence on the association between unsaturated fatty acids and mental disorders remains controversial. Our findings suggest the overall credibility of evidence is low, in which suggestive/weak evidence indicates the protective effect of high consumption of unsaturated fatty acids or fish and weak evidence indicates the broad differences in circulating unsaturated fatty acids and the potential value of omega-3 polyunsaturated fatty acid supplementation interventions for various mental disorders.
Introduction
Currently, mental disorders remain among the top 10 leading causes of disease burden worldwide (1). The Global Burden of Diseases Study (GBD) 2019 showed that the proportion of global disability-adjusted life-years (DALYs) attributed to mental disorders increased from 3.1% to 4.9% between 1990 and 2019 (1). In 2020, only 52% of the WHO's 194 member states met the target related to mental health promotion and prevention programs, which was considerably below the 80% target (2). The global conflict between the increasing burden of mental disorders and the insufficient investment in mental health highlights the growing need to evaluate effective prevention and management strategies for mental disorders (1–3).
Unsaturated fatty acids, as one of the most important dietary nutrients, might be associated with neurodevelopment and brain function, as well as behavior and mental health (4, 5). Characterized by the number and position of double carbon bonds, unsaturated fatty acids include MUFAs, n–3 PUFAs [including ɑ-linolenic acid (ALA; 18:3n−3), EPA, and DHA], and n–6 PUFAs [including linoleic acid (LA; 18:2n–6) and arachidonic acid (AA; 20:4n−6)]. In early life, obtaining adequate DHA and AA from the mother is essential for the myelination and proper neurodevelopment of the fetus (6, 7). In addition, n–3 PUFAs and n–6 PUFAs are highly enriched in brain tissue (8) and participate in numerous biological processes in the brain (e.g., metabolism, neurotransmission, synaptogenesis, and inflammation) (4, 5, 9, 10). Various mental disorders such as Alzheimer disease (AD), dementia, attention-deficit/hyperactivity disorder (ADHD), autism spectrum disorder (ASD), mood disorders, and schizophrenia have been suggested to be associated with altered levels and functions related to unsaturated fatty acids in the brain (8, 11–15).
To date, a large number of meta‐analyses have been conducted to assess the role of unsaturated fatty acids in mental disorders from multiple perspectives, but the available evidence remains controversial. The assessment of various kinds of bias (e.g., publication bias, reporting bias, residual confounding bias, and researcher allegiance) in these meta-analyses was often insufficient (16), which might result in overestimated efficacy or false significance (17, 18). Moreover, the appraisal of the evidence has not been formally determined across different mental disorders. To overcome these limitations, we conducted an umbrella review of the relevant meta-analyses, which have increasingly consolidated the highest level of evidence on this topic (19). We aimed to systemically assess the role of unsaturated fatty acids as alternative or complementary (adjunctive) interventions, dietary factors, or peripheral biomarkers for various mental disorders, and generate hierarchies of evidence.
Methods
Literature search strategy and eligibility criteria
We conducted an umbrella review to systematically review and evaluate all available systematic reviews and meta-analyses on the topic of unsaturated fatty acids in mental disorders. PubMed, Embase, PsycINFO, and the Cochrane Database of Systematic Reviews were searched for papers published between database inception and 20 April 2022, and no language restriction was applied. The complete search strategy is provided in the Supplemental Methods. In accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines (20), 2 investigators (XG and XS) independently screened titles, abstracts, and full texts to identify potentially relevant systematic reviews (Figure 1). We also manually searched the reference lists from relevant studies to reduce missing records in database searches. In case of discrepancies, a third investigator (FZ) was involved, and consensus was reached by discussion.
FIGURE 1.
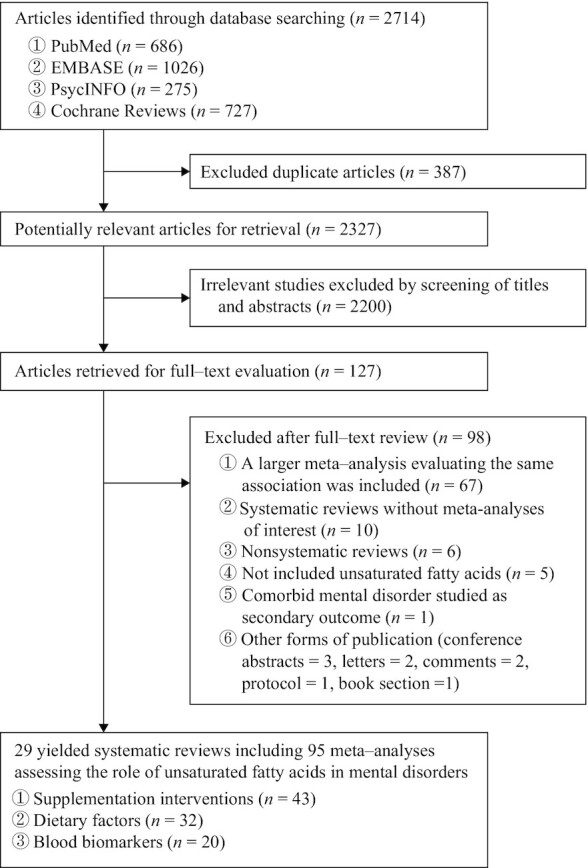
Study selection profile.
We included systematic reviews and meta-analyses that evaluated the role of unsaturated fatty acids as interventions, risk factors, or biomarkers for all types of mental disorders and evaluations of unsaturated fatty acids, including but not limited to nutritional supplements, dietary intake, or blood concentrations. For eligible systematic reviews, mental disorders should be assessed using structured psychiatric diagnostic interviews or validated or commonly used rating scales. Systematic reviews without study-level effect sizes and 95% CIs were excluded. When 2 or more systematic reviews existed for the same association or comparison, we included the most recent systematic review with the largest number of individual studies providing study-level estimates, in agreement with umbrella review methodology (21). For systematic reviews that did not report sufficient data for reanalysis, we contacted the corresponding authors to obtain the necessary data.
Data extraction and quality assessment
From each included systematic review, we extracted information on the first author, publication year, number of included studies, outcomes, reported unsaturated fatty acids, and summary meta-analytic estimates. The following information was extracted from each individual study: publication year, study design (i.e., cohort design, case–control design, or clinical trial design), population (i.e., children, adolescents, middle-aged adults, or older adults), sample size, reported unsaturated fatty acids, outcomes and corresponding assessment criteria, and maximally adjusted study-specific estimates [i.e., mean difference (MD), standardized mean difference (SMD; including Hedges’ g and Cohen's d), OR, or RR] with 95% CIs.
The methodological quality of the included systematic reviews was critically appraised using AMSTAR 2 (A Measurement Tool to Assess Systematic Reviews), a 16-item rating scale with good interrater reliability and usability (22). AMSTAR 2 is not intended to generate an overall score; instead, it rates the confidence of systematic reviews into 4 broad categories (high, moderate, low, and critically low) based on review design, literature screening, data extraction, and individual study quality assessment (22).
Data analysis
We followed the analytic approach that was developed and reproduced in previous umbrella reviews (23–26). The pooled effect size, 95% CI, and P value of each meta-analysis were re-estimated in their original form under random-effects models using the DerSimonian and Laird method (27). The statistical significance P-value threshold of pooled effect estimates was set at <0.05, and additional P-value thresholds were set at <10−3 and <10−6 to assess the credibility of evidence (28, 29). For between-study heterogeneity, we performed Cochran's Q tests (30) (P < 0.10 indicated the existence of heterogeneity) and calculated the I2 statistic (31) (I2 ≥ 50% represented high inconsistency).
To enable comparison of the effects of various interventions on the same outcome, we further re-estimated unstandardized MDs as SMDs by the method of Cohen and converted all SMDs into equivalent ORs based on Hasselblad and Hedges’ method (32–34). Subsequently, we estimated the 95% prediction intervals, which specify the uncertainty as to whether the effect will persist in a future study examining the same research question (35, 36). Prediction intervals excluding the null value (i.e., 1 in the case of RRs or ORs) infer that the effect would be expected in a new study (35, 36). Egger's regression asymmetry test was used to identify potential publication bias (37). The presence of small-study effects was established at an Egger's P < 0.10, with the estimate of the largest component study (the study with the smallest SE) being more conservative than the summary estimate based on random-effects models (23–26).
We evaluated the excess significance to examine whether the observed number of studies (O) with nominally statistically significant results (P < 0.05) in each meta-analysis was larger than their expected number (E) (38). For each meta-analysis, the expected number of significant studies was estimated from the sum of the statistical power estimates for each individual study (26), using an algorithm from a noncentral t distribution and the effect size of the largest study in each meta-analysis as the plausible power for the tested association (39). For each meta-analysis, the significance threshold of the excess significance bias was set at P < 0.10. Excess significance for single meta-analysis was established at P < 0.10 (1-sided P < 0.05 with O > E, as previously proposed). All statistical analyses were performed using Stata version 14.0 (StataCorp). The P values were all 2-tailed.
Determining the credibility of evidence
In accordance with previous umbrella reviews (23–26, 40), the following criteria were used to determine the level of evidence: 1) P < 10−6 based on random-effects meta-analysis, 2) >1000 participants, 3) P < 0.05 of the largest study, 4) between-study heterogeneity with I² < 50%, 5) no evidence of small-study effects, 6) 95% prediction interval that excluded the null value, and 7) no excess significance bias. Based on the results of statistical analyses, we categorized the credibility of each evidence as class I (convincing evidence that met all criteria), class II (highly suggestive evidence that met 1 to 3 of the criteria), class III (suggestive evidence criteria that required only a P < 0.001 by random-effects and >1000 participants), class IV (weak evidence that required only a P < 0.05 under random-effects), and no significant evidence (P ≥ 0.05 under random-effects).
Results
A total of 2714 records were identified through a systematic database search. After duplicate removal and the inspection of titles and abstracts, 127 full-text articles were screened for eligibility. Ultimately, 29 systematic reviews involving 96 meta-analyses met the umbrella review inclusion criteria and were included for reanalysis (Figure 1) (11–15, 41–64). Details of the excluded reviews with the reasons for exclusion are provided in the Supplemental Results. From the included systematic reviews, we extracted information on the role of the assessed unsaturated fatty acids and mental disorders of interest (Table 1). Among these systematic reviews, 43 meta-analyses assessed the efficacy of unsaturated fatty acid supplementation interventions, 32 meta-analyses assessed the effect of unsaturated fatty acid intake, and 20 meta-analyses assessed differences in peripheral unsaturated fatty acid concentrations between healthy controls and patients with mental disorders.
TABLE 1.
Characteristics and quality assessments of eligible meta-analyses evaluating the associations between unsaturated fatty acids and mental disorders1
| Study, year (ref) | Population | Mental disorders | Outcomes | Outcome assessments | No. of studies (participants, n) | Study design | Intervention/comparison | Effect metrics | AMSTAR 2 rating2 |
|---|---|---|---|---|---|---|---|---|---|
| Unsaturated fatty acids supplementation | |||||||||
| Xu et al., 2022 (64) | Adults | Schizophrenia | Symptoms | Positive and Negative Syndrome Scale | 6 (317) | Randomized controlled studies | EPA/DHA/EPA+DHA vs. placebo | MD | ●●○○Low |
| Appleton et al., 2021 (56) | Adults | Depression | Depressive symptoms, adverse events, quality of life | Beck Depression Inventory, Montgomery Asberg Depression Rating Scale, Hamilton Depression Rating Scale, and others | 34 (1924) | Randomized controlled studies | n–3 fatty acids supplementation vs. placebo | SMD, OR | ●●●●High |
| de Andrade Wobido et al., 2021 (57) | Children | Autism spectrum disorder | Symptoms | Aberrant Behavior Checklist, Social Responsiveness Scale | 13 (372) | Clinical trial, community trial | n–3 and n–6 fatty acids supplementation vs. placebo | SMD | ●●●●High |
| Goh et al., 2021 (58) | Adults | Schizophrenia | Symptoms | Positive and Negative Syndrome Scale and General Psychopathology Scale | 14 (950) | Randomized controlled studies | n–3 fatty acids supplementation vs. placebo or non-supplementation | SMD | ●●●●High |
| Händel et al., 2021 (59) | Children | Attention-deficit/hyperactivity disorder | Symptoms, behavioral difficulties, quality of life | Multiple psychopathology scales assessed the parent-reported core symptoms, teacher-reported core symptoms, parent-reported behavioral difficulties, teacher-reported behavioral difficulties, quality of life (including diarrhea, gastrointestinal discomfort, and nausea) | 31 (1775) | Randomized controlled studies | n–3 and n–6 fatty acids supplementation vs. placebo and/or regular diet | SMD | ●●●●High |
| Suradom et al., 2021 (60) | Pregnant and postpartum women | Depression | Prevention and treatment of depression severity | Edinburgh Postnatal Depression Scale, Postpartum Depression Screening Scale,Center for Epidemiological Studies–Depression Scale | 11 (3181) | Randomized controlled studies | n–3 and n–6 fatty acids supplementation vs. placebo | SMD | ●●○○Low |
| Xu et al., 2021 (61) | Elderly | Mild cognitive impairment | Symptoms | Mini-Mental State Examination | 3 (96) | Randomized controlled studies | Unsaturated fatty acids vs. antioxidant or non-supplementation | MD | ●○○○Critically low |
| Araya-Quintanilla et al., 2020 (52) | The elderly | Alzheimer disease | Symptoms | Narcissistic Personality Inventor, Mini-Mental State Examination, Alzheimer's Disease Assessment Scale–Cognitive section | 6 (758) | Randomized controlled studies | n–3 fatty acids supplementation vs. placebo | SMD | ●○○○Critically low |
| Luo et al., 2020 (53) | Adults | Depression | Depressive symptoms | Hamilton Depression Rating Scale or others | 10 (910) | Randomized controlled studies | n–3 and n–6 fatty acids supplementation (≥2000 mg/d and <2000 mg/d) vs. placebo | SMD | ●○○○Critically low |
| Mocking et al., 2020 (54) | Pregnant and postpartum women | Perinatal and postpartum depression | Depressive symptoms | Edinburgh Postnatal Depression Scale; Hamilton Depression Rating Scale; Montgomery-Asberg Depression Rating Scale, or others | 18 (4052) | Randomized controlled studies | n–3 and n–6 fatty acids supplementation vs. placebo or regular diet | SMD | ●●●●High |
| Zhang et al., 2020 (55) | The elderly | Mild cognition decline | Cognition | Mini-Mental State Examination | 7 (434) | Randomized controlled studies | n–3 and n–6 fatty acids supplementation vs. placebo | WMD | ●○○○Critically low |
| Devoe et al., 2019 (48) | Youth (age between 13 and 33) | Psychosis | Symptoms | Scale for the Assessment of Positive Symptoms, Brief Psychiatric Rating Scale, the Positive and Negative Syndrome Scale, the Scale of Prodromal Symptoms, and the Comprehensive Assessment of At-Risk Mental States | 3 (347) | Randomized controlled studies | n–3 fatty acids supplementation vs. placebo | SMD | ●●○○Low |
| Morsy et al., 2019 (49) | Adults | Huntington's disease | Total motor score; total motor score-4 | Unified Huntington disease rating scale (UHDRS) or other evaluations | 4 (782) | Randomized controlled studies | Ethyl-EPA vs. placebo | MD | ●●●○Medium |
| Zhang et al., 2019 (51) | Children | Depression | Depressive symptoms | The Children's Depression Rating Scale (CDRS), revised CDRS, Beck Depression Inventory, and Children's Depression Inventory | 4 (153) | Randomized controlled studies | n–3 fatty acids supplementation vs. placebo | SMD | ●○○○Critically low |
| Su et al., 2018 (47) | Adults | Anxiety | Symptoms | Clinician-administered post-traumatic stress disorder scale, Child Behavior Checklist anxiety subscale, children's Yale-Brown obsessive-compulsive scale, depression, anxiety, and stress scales, generalized anxiety disorder questionnaire, Hospital Anxiety and Depression Scale anxiety subscale, Hamilton anxiety rating scale, impact of event scale–revised, and Yale-Brown obsessive-compulsive scale | 19 (1203) | Clinical trials | n–3 fatty acids supplementation vs. placebo or education | Hedges' g | ●●●●High |
| Rosenblat et al., 2016 (45) | Adults | Bipolar depression | Symptoms | Bipolar Depression Rating Scale, Clinical Global Impressions Scale, Clinical Global Impressions Scale–Improvement–Bipolar, Clinical Global Impressions Scale–Improvement–Depression, Clinical Global Impressions Scale–Improvement–Mania, Clinical Global Impressions Scale–Severity–Bipolar, Clinical Global Impressions Scale–Severity–Depression, Clinical Global Impressions Scale–Severity–Mania, Hamilton Depression Rating Scale, Inventory of Depressive Symptomatology–Clinical Rating Scale, Montgomery–Asberg Depression Rating Scale, Quality of Life Enjoyment and Satisfaction Scale, Social and Occupational Functioning Assessment Scale, and Young Mania Rating Scale | 4 (140) | Randomized controlled studies | n–3 fatty acids supplementation vs. placebo | SMD | ●●○○Low |
| Tan et al., 2016 (46) | Children | Specific learning disorders | Adverse effects (gastrointestinal disturbances) | — | 2 (116) | Randomized controlled studies, quasi-RCTs | PUFA vs. placebo | RR | ●●●●High |
| Cooper et al., 2015 (42) | Children | Attention-deficit/hyperactivity disorder | Symptoms and brain functions | Wechsler Intelligence Scale for Children, test of variables of attention, digit span backwards (recalling a string of numbers backwards), immediate or delayed word recall, Wide Range Achievement Test, or others | 24 (8658) | Randomized controlled studies | n–3 fatty acids supplementation vs. placebo | SMD | ●○○○Critically low |
| Yang et al., 2015 (43) | Women | Depression | Depressive symptoms | Comprehensive evaluation (including Montgomery–Asberg Depression Rating Scale, Hamilton Depression Rating Scale, Clinical Global Impression, Beck Depression Inventory, or Geriatric Depression Scale) | 8 (267) | Randomized controlled studies | DHA and EPA vs. placebo | SMD | ●●●●High |
| Dietary unsaturated fatty acids intake | |||||||||
| Kosti et al., 2022 (63) | The elderly | Dementia,Alzheimer disease | Risk of dementia/Alzheimer disease | Cambridge Mental Disorders of the Elderly Examination, Clinical Dementia Rating Diagnostic and Statistical Manual of Mental Disorders-III-Revised, Geriatric Mental State Schedule, Mini-Mental State Examination Wechsler Memory Scale Revised | 9 (440,572) | Cohort | Highest intake of fish vs. reference group | RR | ●●●●High |
| Zhu et al., 2021 (62) | The elderly | Alzheimer disease | Risk of Alzheimer disease | NR | 14 (54,177) | Cohort | Highest intake of n–3 fatty acids vs. reference group | RR | ●●○○Low |
| Qu et al., 2019 (50) | Adults | Parkinson disease | Risk of Parkinson disease | NR | 9 (778,571) | Cohort, case-control | Highest intake of n–3 and n–6 fatty acids vs. reference group | RR | ●○○○Critically low |
| Grosso et al., 2016 (44) | Adults | Depression | Risk of depression | Center for Epidemiologic Studies–Depression, Composite International Diagnostic Interview Short Form, Depression, Anxiety and Stress Scales, Geriatric Depression Scale, Edinburgh Post-partum Depression Scale, and Munich-Composite International Diagnostic Interview | 16:15 (255,076) | Cohort, case-control | Highest intake of n–3 fatty acids and fish vs. reference group | RR | ●○○○Critically low |
| Zhang et al., 2016 (15) | The elderly | Alzheimer disease; cognitive decline; dementia; mild cognitive impairment; Parkinson disease | Risk of cognitive impairment | NR | 21 (181,580) | Cohort | Highest intake of n–3 fatty acids and fish vs. reference group | RR | ●●●●High |
| Tsai et al., 2014 (41) | Adults | Suicide | Suicide mortality | — | 3 (205,357) | Cohort | Highest intake of n–3 or n–6 fatty acids vs. reference group | RR | ●○○○Critically low |
| Circulating unsaturated fatty acids | |||||||||
| Mazahery et al., 2017 (11) | Children | Autism spectrum disorder | Circulating n–3, n–6 fatty acids and ratios between n–3/n–6 fatty acids | — | 15 (1193) | Case-control | Autism spectrum disorder vs. typically developing control | SMD | ●●●●High |
| McNamara et al., 2016 (12) | Youth | Bipolar disorder | Circulating n–3 and n–6 fatty acids | — | 6 (265) | Case-control | Bipolar disorder vs. typically developing control | SMD | ●○○○Critically low |
| Zhang et al., 2016 (15) | The elderly | Alzheimer disease; dementia; cognitive decline | Risk of cognitive impairment | — | 21 (181,580) | Cohort | A 1% increment of blood DHA concentrations | RR | ●●●●High |
| Hawkey et al., 2014 (13) | Children | Attention-deficit/hyperactivity disorder | Circulating n–3 fatty acids | — | 9 (586) | Case-control | Attention-deficit/hyperactivity disorder vs. typically developing control | Hedges' g | ●●●○Medium |
| van der Kemp et al., 2012 (14) | The elderly | Schizophrenia | Circulating n–3 and n–6 fatty acids | — | 14 (873) | Cohort, case-control | Schizophrenia vs. typically developing control | Cohen's d | ●○○○Critically low |
AMSTAR 2, A Measurement Tool to Assess Systematic Reviews; MD, mean difference; NR, not reported; RCT, randomized controlled trial; ref, reference; SMD, standardized mean difference; WMD, weighted mean difference.
AMSTAR 2 used 16 items to assess methodological quality of systematic reviews on the basis of the validity of review design, literature screening, data extraction, and individual study quality assessment. Details of the quality assessment for eligible reviews were provided in Supplemental Table 1.
Of the 29 systematic reviews identified, 11 had high-quality ratings according to the AMSTAR 2 scoring system (11, 15, 43, 46, 47, 54, 56–59, 63), 2 had moderate quality ratings (13, 49), and 16 received a low or critically low-quality rating (Table 1) (12, 14, 41, 42, 44, 45, 48, 50–53, 55, 60–62, 64). AMSTAR 2 detected that, in 8 reviews, the methods were not established prior to the conduct of the review and 10 reviews did not provide the list of excluded studies with justification of the exclusions (details are reported in Supplemental Table 1).
Unsaturated fatty acid supplementation interventions for mental disorders
A total of 43 meta-analyses assessed the efficacy of unsaturated fatty acid supplementation on improving mental disorders, including AD, mild cognitive impairment, anxiety, bipolar disorder (BP), depression, perinatal depression, postpartum depression, ADHD, ASD, specific learning disorders, Huntington's disease, schizophrenia, and psychosis. However, only 12 reanalyses reported a nominally statistically significant summary effect using random-effects models (P < 0.05), and only one 95% prediction interval excluded the null value (Table 2). Significant heterogeneity (I2 > 50%) was observed in all statistically significant comparisons, with the exception of the meta-analysis on the efficacy of an n–3 PUFA or n–6 PUFA supplementation intervention for ASD, Huntington's disease, and schizophrenia (Table 2). The risk of small-study effects bias was observed in 2 comparisons, whereas excess of significance bias was detected in 8 comparisons. However, 17 comparisons consisted of less than 5 individual studies, in which case the power of the test was reduced.
TABLE 2.
Quantitative synthesis and evidence grading for meta‐analyses of unsaturated fatty acids supplementation interventions for participants with mental disorders1
| Study, year (ref) | Mental disorders | Outcomes | Unsaturated fatty acids supplementation | No. of studies (participants, n) | Original effect metrics | Random-effects summary estimate (95% CI) | Random-effects P | I 2, % | Converted as equivalent OR (95% CI) | 95% Prediction interval | Egger's test> P | Largest study estimate (95% CI) | Significant studies | Grading | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| O/E | P | ||||||||||||||
| Xu et al., 2022 (64) | Schizophrenia | Positive and Negative Syndrome Scale total scores | EPA/DHA/EPA+DHA | 6 (317) | MD | −3.274 (−5.449, −1.098) | 0.003 | 22.2 | 0.326 (0.061, 1.751) | (0.001, 144.492) | 0.22 | 1.11 (0.49, 2.52) | 1/0.33 | 0.29 | Not significant |
| Appleton et al., 2021 (56) | Depression | Depressive symptoms | Total n–3 PUFAs | 33 (1848) | SMD | −0.401 (−0.642, −0.160) | 0.001 | 80.7 | 0.484 (0.313, 0.748) | (0.052, 4.482) | 0.05 | 0.83 (0.59, 1.18) | 10/2.16 | <10−3 | Weak |
| Quality of life | Total n–3 PUFAs | 12 (476) | SMD | −0.376 (−0.816, 0.063) | 0.093 | 78.9 | 0.506 (0.228, 1.121) | (0.029, 8.799) | 0.26 | 1.63 (0.72, 3.68) | 5/1.52 | 0.01 | Not significant | ||
| Adverse events | Total n–3 PUFAs | 24 (1503) | OR | 1.267 (0.977, 1.644) | 0.075 | 5.7 | — | (0.840, 1.912) | 0.48 | 1.26 (0.83, 1.91) | 2/1.70 | 0.69 | Not significant | ||
| de Andrade Wobido et al., 2021 (57) | Autism spectrum disorder | Aberrant Behavior Checklist | Total n–3 and n–6 PUFAs | 5 (183) | SMD | −0.183 (−0.341, −0.025) | 0.023 | 0.0 | 0.718 (0.540, 0.955) | (0.531, 0.970) | 0.80 | 0.79 (0.31, 2.03) | 1/1.46 | — | Weak |
| Social Responsiveness Scale | Total n–3 and n–6 PUFAs | 5 (238) | SMD | −0.059 (−0.239, 0.122) | 0.524 | 46.2 | 0.953 (0.691, 1.314) | (0.337, 2.694) | <0.01 | 1.88 (1.36, 4.83) | 1/4.11 | — | Not significant | ||
| Goh et al., 2021 (58) | Schizophrenia | Positive and Negative Syndrome Scale total scores | Total n–3 PUFAs | 9 (443) | SMD | −0.295 (−0.527, −0.064) | 0.012 | 28.1 | 0.586 (0.386, 0.891) | (0.230, 1.497) | 0.81 | 1.18 (0.55, 2.52) | 3/0.55 | 0.01 | Weak |
| Positive and Negative Syndrome Scale– Positive scale | Total n–3 PUFAs | 6 (266) | SMD | −0.105 (−0.349, 0.139) | 0.400 | 0.0 | 0.827 (0.531, 1.287) | (0.442, 1.547) | 0.86 | 0.82 (0.38, 1.75) | 0/0.38 | — | Not significant | ||
| Positive and Negative Syndrome Scale–Negative scale | Total n–3 PUFAs | 3 (195) | SMD | 1.193 (−0.654, 3.039) | 0.205 | 96.7 | 8.661 (0.306, 245.036) | (<0.001, >1000) | 0.08 | 0.67 (0.31, 1.46) | 1/0.42 | 0.37 | Not significant | ||
| Positive and Negative Syndrome Scale–General Psychopathology scale | Total n–3 PUFAs | 2 (145) | SMD | −0.388 (−1.103, 0.327) | 0.287 | 77.7 | 0.495 (0.136, 1.806) | — | — | 0.93 (0.44, 2.03) | 1/0.10 | 0.10 | Not significant | ||
| Händel et al., 2021 (59)2 | Attention-deficit/hyperactivity disorder | Parent-reported core symptoms | Total n–3 and n–6 PUFAs | 24 (1754) | SMD | −0.167 (−0.318, −0.015) | 0.031 | 60.7 | 0.739 (0.562, 0.972) | (0.245, 2.228) | 0.04 | 1.54 (1.36, 3.24) | 8/1.04 | 0.05 | Weak |
| Teacher-reported core symptoms | Total n–3 and n–6 PUFAs | 10 (640) | SMD | −0.062 (−0.310, 0.186) | 0.626 | 55.0 | 0.894 (0.571, 1.401) | (0.241, 3.324) | 0.26 | 1.14 (0.59, 2.22) | 2/0.58 | 0.11 | Not significant | ||
| Parent-reported behavioral difficulties | Total n–3 and n–6 PUFAs | 7 (682) | SMD | −0.014 (−0.169, 0.141) | 0.859 | 0.0 | 0.975 (0.736, 1.291) | (0.675, 1.409) | 0.73 | 0.74 (0.41, 1.31) | 0/0.89 | — | Not significant | ||
| Teacher-reported behavioral difficulties | Total n–3 and n–6 PUFAs | 5 (377) | SMD | −0.041 (−0.346, 0.264) | 0.791 | 49.1 | 0.928 (0.534, 1.612) | (0.178, 4.824) | 0.38 | 0.96 (0.49, 1.88) | 1/0.25 | 0.23 | Not significant | ||
| Quality of life | Total n–3 and n–6 PUFAs | 2 (191) | SMD | 0.014 (−0.288, 0.316) | 0.929 | 0.0 | 1.025 (0.593, 1.771) | — | — | 0.98 (0.52, 1.88) | 0/0.10 | — | Not significant | ||
| Suradom et al., 2021 (60) | Depression | Prevention of depression severity | Total n–3 and n–6 PUFAs | 10 (1027) | SMD | −0.033 (−0.201, 0.134) | 0.697 | 23.2 | 0.942 (0.695, 1.275) | (0.493, 1.797) | 0.45 | 1.20 (0.71, 2.03) | 1/0.78 | 0.56 | Not significant |
| Treatment of depression severity | Total n-3 and n-6 PUFAs | 4 (209) | SMD | −0.138 (−0.543, 0.268) | 0.506 | 30.7 | 0.780 (0.374, 1.623) | (0.070, 8.640) | 0.31 | 1.54 (0.56, 4.18) | 0/0.55 | — | Not significant | ||
| Xu et al., 2021 (61) | Mild cognitive impairment | Mini-Mental State Examination | Unsaturated fatty acids | 3 (96) | MD | 0.658 (−0.008, 1.325) | 0.053 | 59.8 | 3.293 (0.986, 10.995) | (<0.001, >1000) | 0.44 | 1.10 (0.33, 3.64) | 2/0.16 | 0.01 | Not significant |
| Araya-Quintanilla et al., 2020 (52) | Alzheimer disease | Narcissistic Personality Inventor | Total n–3 PUFAs | 2 (543) | SMD | −0.342 (−1.077, 0.393) | 0.362 | 93.7 | 0.539 (0.142, 2.037) | — | — | 0.28 (0.19, 0.41) | 1/1.99 | — | Not significant |
| Mini-Mental State Examination | Total n–3 PUFAs | 3 (283) | SMD | 0.582 (−0.434, 1.597) | 0.262 | 0.0 | 2.865 (0.456, 18.016) | (<0.001, >1000) | 0.79 | 2.06 (0.19, 22.09) | 0/1.34 | — | Not significant | ||
| Alzheimer's Disease Assessment Scale– Cognitive section | Total n–3 PUFAs | 3 (239) | SMD | 1.096 (−1.031, 3.224) | 0.312 | 96.9 | 7.275 (0.155, 342.043) | (<0.001, >1000) | 0.11 | 216.09 (99.23, 470.60) | 1/2.99 | — | Not significant | ||
| Luo et al., 2020 (53)2 | Depression | Depressive symptoms | Total n–3 and n–6 PUFAs (≥2000 mg/d) | 4 (160) | SMD | −0.941 (−1.581, −0.301) | 0.004 | 66.9 | 0.182 (0.057, 0.580) | (0.001, 22.190) | 0.05 | 0.54 (0.24, 1.18) | 3/0.73 | 0.02 | Weak |
| Depressive symptoms | Total n-3 and n-6 PUFAs (<2000 mg/d) | 7 (985) | SMD | −0.630 (−1.186, −0.075) | 0.026 | 90.8 | 0.319 (0.117, 0.874) | (0.010, 9.976) | 0.13 | 0.82 (0.58, 1.16) | 2/0.68 | 0.14 | Weak | ||
| Mocking et al., 2020 (54) | Perinatal depression | Depressive symptoms | Total n–3 and n–6 PUFAs | 14 (3781) | SMD | −0.072 (−0.188, 0.045) | 0.227 | 19.0 | 0.879 (0.712, 1.084) | (0.571, 1.352) | 0.95 | 0.83 (0.67, 1.04) | 1/1.71 | 0.68 | Not significant |
| Postpartum depression | Depressive symptoms | Total n–3 and n–6 PUFAs | 4 (423) | SMD | −0.656 (−1.690, 0.378) | 0.213 | 92.7 | 0.305 (0.047, 1.982) | (<0.001, >1000) | 0.10 | 0.03 (0.01, 0.06) | 2/3.99 | — | Not significant | |
| Zhang et al., 2020 (55) | Mild cognitive impairment | Mini-Mental State Examination | Total n–3 PUFAs | 7 (434) | WMD | 0.852 (0.039, 1.665) | 0.040 | 52.2 | 1.646 (1.009, 2.685) | (0.424, 6.395) | 0.55 | 0.98 (0.59, 1.62) | 1/0.35 | 0.30 | Weak |
| Devoe et al., 2019 (48) | Psychosis | Attenuated psychotic symptoms | Total n–3 PUFAs | 3 (347) | SMD | −0.309 (−0.878, 0.261) | 0.288 | 81.1 | 0.572 (0.204, 1.604) | (<0.001, >1000) | 0.74 | 0.91 (0.57, 1.46) | 1/0.17 | 0.16 | Not significant |
| Morsy et al., 2019 (49) | Huntington disease | Total motor score | Ethyl-EPA | 2 (285) | MD | −2.720 (−4.763, −0.677) | 0.009 | 0.0 | 0.071 (0.001, 3.786) | — | — | 0.52 (0.35, 0.79 | 2/1.42 | 1.00 | Not significant |
| Total motor score-4 | Ethyl-EPA | 2 (285) | MD | −2.225 (−3.843, −0.607) | 0.007 | 9.7 | 0.025 (0.008, 0.080) | — | — | 0.01 (0.01, 0.03) | 2/1.99 | 1.00 | Weak | ||
| Zhang et al., 2019 (51) | Depression in children | Depressive symptoms | Total n–3 PUFAs | 4 (153) | SMD | −0.119 (−0.533, 0.296) | 0.575 | 30.5 | 0.807 (0.381, 1.709) | (0.069, 9.412) | 0.03 | 1.22 (0.43, 3.49) | 1/0.25 | 0.23 | Not significant |
| Su et al., 2018 (47)2 | Anxiety | Symptoms | Total n–3 PUFAs | 19 (1203) | Hedges' g | −0.374 (−0.666, −0.081) | 0.012 | 89.9 | 0.508 (0.300, 0.863) | (0.049, 5.300) | 0.10 | 1.29 (0.88, 1.87) | 6/2.11 | 0.01 | Weak |
| Rosenblat et al., 2016 (45) | Bipolar depression | Depressive symptoms | Total n–3 PUFAs | 4 (140) | SMD | −0.364 (−0.735, 0.007) | 0.054 | 8.3 | 0.517 (0.265, 1.012) | (0.092, 2.895) | 0.79 | 0.48 (0.20, 1.14) | 0/0.83 | — | Not significant |
| Tan et al., 2016 (46) | Specific learning disorders | Adverse effects | PUFAs | 2 (116) | RR | 1.402 (0.237, 8.281) | 0.710 | 0.0 | 1.402 (0.237, 8.281) | — | — | 2.05 (0.19, 21.7) | 0/0.63 | — | Not significant |
| Cooper et al., 2015 (42)2 | Attention-deficit/hyperactivity disorder | Attention (omission errors) | Total n–3 PUFAs | 6 (387) | SMD | −0.129 (−0.327, 0.069) | 0.200 | 0.0 | 0.792 (0.554, 1.132) | (0.477, 1.314) | 0.82 | 0.76 (0.43, 1.3) | 0/0.54 | — | Not significant |
| IQ | Total n–3 PUFAs | 5 (706) | SMD | 0.139 (−0.07, 0.351) | 0.200 | 23.3 | 1.286 (0.876, 1.888) | (0.513, 3.221) | 0.96 | 1.00 (0.55, 1.82) | 1/0.25 | 0.23 | Not significant | ||
| Inhibition | Total n–3 PUFAs | 12 (951) | SMD | −0.037 (−0.217, 0.143) | 0.687 | 38.9 | 0.935 (0.675, 1.295) | (0.396, 2.208) | 0.49 | 0.74 (0.43, 1.27) | 1/1.36 | — | Not significant | ||
| Mean reaction time | Total n–3 PUFAs | 11 (1107) | SMD | −0.001 (−0.118, 0.117) | 0.997 | 12.8 | 0.999 (0.808, 1.236) | (0.684, 1.460) | 0.86 | 1.29 (0.89, 1.85) | 1/1.16 | — | Not significant | ||
| Reaction time variability | Total n–3 PUFAs | 2 (114) | SMD | 0.290 (−0.709, 1.289) | 0.569 | 82.1 | 1.691 (0.277, 10.319) | — | — | 0.68 (0.25, 1.92) | 1/0.24 | 0.22 | Not significant | ||
| Reading | Total n–3 PUFAs | 8 (1433) | SMD | 0.014 (−0.062, 0.090) | 0.722 | 0.0 | 1.025 (0.894, 1.176) | (0.864, 1.218) | 0.22 | 1.04 (0.79, 1.34) | 0/0.42 | — | Not significant | ||
| Short-term memory | Total n–3 PUFAs | 14 (2188) | SMD | 0.067 (−0.013, 0.147) | 0.101 | 28.5 | 1.129 (0.976, 1.306) | (0.793, 1.608) | 0.41 | 1.00 (0.72, 1.39) | 2/0.70 | 0.15 | Not significant | ||
| Spelling | Total n–3 PUFAs | 6 (974) | SMD | 0.031 (−0.090, 0.153) | 0.614 | 5.1 | 1.058 (0.849, 1.319) | (0.738, 1.517) | 0.90 | 0.95 (0.66, 1.34) | 0/0.32 | — | Not significant | ||
| Working memory | Total n–3 PUFAs | 8 (1410) | SMD | 0.089 (−0.007, 0.185) | 0.068 | 3.6 | 1.175 (0.988, 1.397) | (0.917, 1.505) | 0.02 | 0.96 (0.74, 1.27) | 1/0.42 | 0.35 | Not significant | ||
| Yang et al., 2015 (43)2 | Depression in women | Depressive symptoms | EPA+DHA | 8 (267) | SMD | −0.648 (−1.120, −0.175) | 0.007 | 78.4 | 0.310 (0.132, 0.728) | (0.018, 5.344) | 0.09 | 0.53 (0.24, 1.20) | 6/1.70 | <0.01 | Weak |
All summary estimates were recalculated based on a random-effects model using the method of DerSimonian and Laird. The 95% prediction interval and Egger's test were not evaluated if available studies were <3. For excess of significance, the P value was not evaluated if the observed number of studies was smaller than expected. E, expected number of studies with positive finding; MD, mean difference; O, observed number of studies with positive finding; ref, reference; SMD, standardized mean difference; WMD, weighted mean difference.
The direction of comparison was normalized to supplementation group versus non-supplementation group.
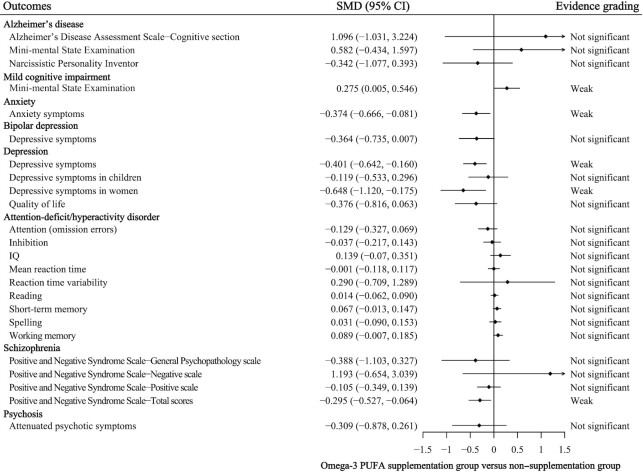
None of the 43 meta-analyses had convincing, highly suggestive, or suggestive strength of evidence according to the quantitative umbrella review criteria. In addition, the strength of the evidence was weak for 10 meta-analyses (Table 2). Weak evidence suggested that n–3 PUFA and n–6 PUFA supplementation could significantly reduce depressive symptoms in patients with depression (SMD: –0.941 to –0.401; equivalent OR: 0.182 to 0.484; P-random effects: 0.001–0.026). Weak evidence also suggested that n–3 PUFA and n–6 PUFA supplementation could significantly reduce Aberrant Behavior Checklist total scores in patients with ASD (SMD: –0.183; equivalent OR: 0.718; P-random effects: 0.023), and reduce parent-reported core symptoms in patients with ADHD (SMD: –0.167; equivalent OR: 0.739; P-random effects: 0.031). For n–3 PUFA supplementation intervention (Figure 2), there was weak evidence for its efficacy in reducing Positive and Negative Syndrome Scale total scores in patients with schizophrenia (SMD: –0.295; equivalent OR: 0.586; P-random effects: 0.012), efficacy in reducing motor scores in patients with Huntington's disease (MD: –2.225; equivalent OR: 0.025; P-random effects: 0.007), efficacy in reducing symptoms of patients with anxiety (Hedges’ g: –0.374; equivalent OR: 0.508; P-random effects: 0.012), and efficacy in improving Mini-Mental State Examination scores in patients with mild cognitive impairment (WMD: 0.852; equivalent OR: 1.646; P-random effects: 0.040).
FIGURE 2.
Efficacy of n–3 unsaturated fatty acid supplementation interventions for mental disorders with evidence grading. SMD, standardized mean difference.
Dietary unsaturated fatty acid intake and the risk of mental disorders
A total of 32 meta-analyses assessing the dietary unsaturated fatty acid intake and the risk of mental disorders, such as AD, dementia, mild cognitive impairment, Parkinson disease, depression, and suicide, were recalculated. Only 5 meta-analyses reported a marginally statistically significant summary effect using random-effects models (P < 0.05), and all 95% prediction intervals of the meta-analyses included the null value, which indicated no associations (Table 3). Significant heterogeneity (I2 > 50%) was observed in associations of fish consumption with risk of depression and intake of EPA and DHA with risk of depression (Table 3). The risk of small-study effects bias was only observed in 2 associations, whereas excess of significance bias was detected in 2 associations. However, most associations (26/32) consisted of fewer than 5 individual studies, in which case the power of the test was reduced.
TABLE 3.
Quantitative synthesis and evidence grading for meta‐analyses evaluating the associations between unsaturated fatty acids intake and risk of mental disorders1
| Study, year (ref) | Mental disorders | Composition | No. of studies (participants, n) | Effect metrics | Random-effects summary estimate (95% CI) | Random-effects P | I 2, % | 95% Prediction interval | Egger's test P | Largest study estimate (95% CI) | Significant studies | Grading | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| O/E | P | ||||||||||||
| Kosti et al., 2022 (63) | Dementia | Higher intake of fish | 9 (45,643) | RR | 0.798 (0.688, 0.925) | 0.003 | 0.0 | (0.563, 1.129) | 0.08 | 0.84 (0.71, 1.00) | 4/3.50 | 0.74 | Weak |
| Alzheimer disease | Higher intake of fish | 8 (444,022) | RR | 0.737 (0.626, 0.867) | <10−3 | 0.0 | (0.479, 1.134) | 0.71 | 0.69 (0.60, 0.80) | 5/6.49 | — | Suggestive | |
| Zhu et al., 2021 (62) | Alzheimer disease | Higher intake of total n–3 PUFAs | 4 (11,977) | RR | 0.883 (0.662, 1.177) | 0.396 | 60.7 | (0.370, 2.11) | 0.12 | 1.07 (0.91, 1.25) | 1/0.41 | 0.35 | Not significant |
| Higher intake of total n–6 PUFAs | 2 (3427) | RR | 0.826 (0.549, 1.241) | 0.358 | 55.9 | — | — | 0.76 (0.55, 1.06) | 0/1.69 | — | Not significant | ||
| Higher intake of PUFAs | 3 (8084) | RR | 0.905 (0.741, 1.105) | 0.326 | 0.0 | (0.584, 1.403) | 0.68 | 1.09 (0.79, 1.50) | 0/0.43 | — | Not significant | ||
| Higher intake of MUFA | 4 (8899) | RR | 1.154 (0.800, 1.664) | 0.442 | 64.50 | (0.380, 3.507) | 0.78 | 0.91 (0.77, 1.07) | 1/0.56 | 0.45 | Not significant | ||
| Higher intake of DHA | 3 (3892) | RR | 0.778 (0.477, 1.267) | 0.313 | 76.20 | (0.003, 185.619) | 0.67 | 0.73 (0.57, 0.95) | 2/1.66 | 1.00 | Not significant | ||
| Higher intake of EPA | 3 (3892) | RR | 0.888 (0.658, 1.198) | 0.436 | 45.08 | (0.046, 16.999) | 0.15 | 0.74 (0.57, 0.95) | 1/1.58 | — | Not significant | ||
| Qu et al., 2019 (50) | Parkinson disease | Higher intake of total n–3 PUFAs | 3 (300,321) | RR | 0.763 (0.487, 1.194) | 0.236 | 62.2 | (0.006, 104.092) | 0.50 | 0.93 (0.76, 1.14) | 1/0.39 | 0.34 | Not significant |
| Higher intake of total n–6 PUFAs | 3 (300,321) | RR | 0.990 (0.667, 1.468) | 0.959 | 55.9 | (0.015, 66.907) | 0.29 | 1.23 (1.02, 1.49) | 1/1.29 | — | Not significant | ||
| Higher intake of PUFAs | 4 (326,686) | RR | 0.864 (0.592, 1.261) | 0.449 | 74.0 | (0.173, 4.320) | 0.18 | 1.23 (1.02, 1.49) | 2/1.57 | 0.65 | Not significant | ||
| Higher intake of MUFA | 5 (327,054) | RR | 1.033 (0.865, 1.235) | 0.719 | 0.0 | (0.774, 1.380) | 0.24 | 1.13 (0.94, 1.55) | 0/1.00 | — | Not significant | ||
| Higher intake of ALA | 3 (300,513) | RR | 0.726 (0.480, 1.099) | 0.130 | 64.6 | (0.008, 70.002) | 0.22 | 0.93 (0.77, 1.13) | 1/0.39 | 0.34 | Not significant | ||
| Higher intake of LA | 3 (300,513) | RR | 0.930 (0.614, 1.409) | 0.732 | 65.6 | (0.009, 94.012) | 0.05 | 1.23 (1.02, 1.49) | 1/1.33 | — | Not significant | ||
| Higher intake of AA | 2 (299,985) | RR | 1.426 (0.753, 2.700) | 0.277 | 80.1 | — | — | 1.08 (0.90, 1.30) | 1/0.36 | 0.32 | Not significant | ||
| Higher ratio of n–3 to n–6 PUFAs | 2 (299,985) | RR | 0.890 (0.754, 1.051) | 0.170 | 0.0 | — | — | 0.87 (0.73, 1.04) | 0/0.82 | — | Not significant | ||
| Grosso et al., 2016 (44) | Depression | Higher intake of total n–3 PUFAs | 8 (25,923) | RR | 0.873 (0.722, 1.055) | 0.160 | 30.9 | (0.569, 1.339) | 0.86 | 0.74 (0.58, 0.95) | 2/4.57 | — | Not significant |
| Higher intake of EPA and DHA | 7 (77,143) | RR | 0.782 (0.667, 0.918) | 0.003 | 50.4 | (0.508, 1.206) | 0.63 | 0.65 (0.53, 0.80) | 3/6.35 | — | Weak | ||
| Higher intake of fish | 21 (200,422) | RR | 0.780 (0.688, 0.885) | <10−3 | 61.4 | (0.489, 1.244) | 0.90 | 0.76 (0.64, 0.91) | 8/15.08 | — | Suggestive | ||
| Zhang et al., 2016 (15) | Alzheimer disease | Higher intake of PUFAs | 2 (6844) | RR | 0.872 (0.334, 2.278) | 0.779 | 27.9 | — | — | 1.07 (0.82, 1.39) | 0/0.15 | — | Not significant |
| Higher intake of DHA | 3 (6476) | RR | 0.546 (0.242, 1.233) | 0.145 | 90.53 | (<0.001, >1000) | 0.11 | 1.10 (0.93, 1.31) | 2/0.31 | 0.03 | Not significant | ||
| Dementia | Higher intake of PUFAs | 2 (6844) | RR | 0.870 (0.355, 2.131) | 0.760 | 25.09 | — | — | 1.04 (0.83, 1.29) | 0/0.12 | — | Not significant | |
| Higher intake of DHA | 2 (5661) | RR | 0.804 (0.511, 1.263) | 0.344 | 90.81 | — | — | 1.00 (0.89, 1.14) | 1/0.10 | 0.10 | Not significant | ||
| Mild cognitive impairment | Higher intake of PUFAs | 3 (3386) | RR | 0.714 (0.607, 0.841) | <10−3 | 0.0 | (0.248, 2.056) | 0.70 | 0.72 (0.61, 0.85) | 1/1.05 | — | Suggestive | |
| Parkinson disease | Higher intake of DHA | 4 (141,551) | RR | 1.001 (0.974, 1.029) | 0.931 | 0.0 | (0.943, 1.063) | 0.51 | 1.01 (0.97, 1.04) | 0/0.20 | — | Not significant | |
| Tsai et al., 2014 (41) | Suicide | Higher intake of total n–3 PUFAs | 3 (205,357) | RR | 1.463 (0.950, 2.251) | 0.084 | 10.1 | (0.057, 37.437) | 0.97 | 1.47 (0.88, 2.44) | 0/0.55 | — | Not significant |
| Higher intake of total n–6 PUFAs | 3 (205,357) | RR | 0.887 (0.562, 1.401) | 0.607 | 0.0 | (0.0459, 17.149) | 0.44 | 0.82 (0.45, 1.51) | 0/0.71 | — | Not significant | ||
| Higher intake of EPA and DHA | 3 (205,357) | RR | 1.241 (0.681, 2.263) | 0.481 | 58.2 | (0.002, 786.970) | 0.21 | 0.80 (0.49, 1.28) | 0/0.29 | — | Not significant | ||
| Higher intake of ALA | 3 (205,357) | RR | 1.068 (0.734, 1.556) | 0.730 | 0.0 | (0.093, 12.222) | 0.01 | 1.27 (0.78, 2.06) | 0/0.30 | — | Not significant | ||
| Higher intake of LA | 3 (205,357) | RR | 0.664 (0.418, 1.056) | 0.084 | 0.0 | (0.033, 13.410) | 0.12 | 0.54 (0.29, 1.02) | 0/2.35 | — | Not significant | ||
| Higher intake of AA | 3 (205,357) | RR | 1.190 (0.824, 1.718) | 0.354 | 0.0 | (0.110, 12.895) | 0.27 | 1.09 (0.67, 1.77) | 0/0.21 | — | Not significant | ||
| Higher intake of fish | 3 (205,357) | RR | 0.818 (0.283, 2.364) | 0.711 | 73.7 | (<0.001, >1000) | 0.71 | 0.55 (0.31, 0.96) | 0/2.39 | — | Not significant | ||
All summary estimates were recalculated based on a random-effects model using the method of DerSimonian and Laird. The 95% prediction interval and Egger's test were not evaluated if available studies were <3. For excess of significance, the P value was not evaluated if the observed number of studies was smaller than expected. AA, arachidonic acid; ALA, α-linolenic acid; E, expected number of studies with positive finding; LA, linoleic acid; MD, mean difference; O, observed number of studies with positive finding; ref, reference; SMD, standardized mean difference.
Three meta-analyses had suggestive strength of the associations according to the quantitative umbrella review criteria, and the strength of the evidence was weak for 2 meta-analyses (Table 3). Suggestive evidence indicated that a higher intake of fish could reduce the risk of depression (pooled RR: 0.780; P-random effects: <0.001) and AD (pooled RR: 0.737; P-random effects < 0.001), and a higher intake of dietary PUFAs was associated with a lower risk of mild cognitive impairment (pooled RR: 0.714; P-random effects < 0.001). Moreover, weak evidence showed that a higher intake of dietary EPA and DHA was associated with a lower risk of depression (pooled RR: 0.782; P-random effects: 0.003), and a higher intake of fish might reduce the risk of dementia (pooled RR: 0.798; P-random effects: 0.003).
Unsaturated fatty acids as biomarkers for mental disorders
A total of 20 meta-analyses assessed the difference in circulating unsaturated fatty acids between healthy controls and patients with mental disorders, including AD, dementia, mild cognitive impairment, ADHD, ASD, BP, and schizophrenia. Eight meta-analyses reported a nominally statistically significant summary effect using random-effects models (P < 0.05), and 3 meta-analyses had 95% prediction intervals excluding the null value (Table 4). Significant heterogeneity (I2 > 50%) was observed in 5 statistically significant comparisons, with the exception of the comparison of circulating DHA between patients with BP and controls, the comparison of circulating total n–3 PUFAs between patients with ADHD and controls, and the comparison of circulating docosapentaenoic acid (DPA; 22:5n−3) between patients with schizophrenia and controls (Table 4). The risk of small-study effects bias was observed in 1 comparison, whereas excess of significance bias was detected in 6 comparisons (Table 4). Among these comparisons, 6 meta-analyses consisted of fewer than 5 individual studies, in which case the power of the test was reduced.
TABLE 4.
Quantitative synthesis and evidence grading for meta‐analyses comparing circulating unsaturated fatty acids between participants with and without mental disorders1
| Study, year (ref) | Mental disorders | Outcome | No. of studies (participants, n) | Original effect metrics | Random-effects summary estimate (95% CI) | Random-effects P | I 2, % | Converted as equivalent OR (95% CI) | 95% Prediction interval | Egger's test P | Largest study estimate (95% CI) | Significant studies | Grading | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| O/E | P | |||||||||||||
| Mazahery et al., 2017 (11) | Autism spectrum disorder | Circulating n–3 PUFAs | 5 (564) | SMD | −0.164 (−0.536, 0.209) | 0.389 | 73.0 | 0.744 (0.379, 1.459) | (0.073, 7.573) | 0.24 | 0.51 (0.32, 0.82) | 3/2.19 | 0.66 | Not significant |
| Circulating n–6 PUFAs | 5 (564) | SMD | 0.569 (−0.186, 1.323) | 0.139 | 93.2 | 2.799 (0.715, 10.968) | (0.015, 518.050) | 0.09 | 0.59 (0.37, 0.95) | 3/1.54 | 0.17 | Not significant | ||
| Circulating DHA | 14 (1291) | SMD | −1.607 (−2.482, −0.732) | <10−3 | 97.3 | 0.055 (0.011, 0.266) | (0.000, 43.982) | 0.11 | 0.45 (0.28, 0.72) | 11/6.78 | 0.03 | Weak | ||
| Circulating EPA | 11 (951) | SMD | −0.437 (−0.901, 0.027) | 0.065 | 90.2 | 0.453 (0.196, 1.050) | (0.019, 10.672) | 0.82 | 1.00 (0.62, 1.60) | 3/0.55 | 0.02 | Not significant | ||
| Circulating AA | 13 (1211) | SMD | −0.826 (−1.481, −0.172) | 0.013 | 95.5 | 0.224 (0.068, 0.733) | (0.002, 26.555) | 0.82 | 0.45 (0.28, 0.72) | 8/6.29 | 0.41 | Weak | ||
| The ratio of AA to EPA in blood | 9 (525) | SMD | 0.662 (−0.109, 1.433) | 0.093 | 93.4 | 3.311 (0.820, 13.367) | (0.023, 470.693) | 0.97 | 5.79 (3.18, 10.33) | 5/6.32 | — | Not significant | ||
| The ratio of AA to DHA in blood | 5 (336) | SMD | −0.080 (−2.223, 2.063) | 0.942 | 98.1 | 0.865 (0.018, 41.855) | (<0.001, >1000) | 0.05 | 127.84 (58.70, 278.41) | 4/4.99 | — | Not significant | ||
| McNamara et al., 2016 (12) | Bipolar disorder | Circulating DHA | 6 (265) | SMD | −0.960 (−1.243, −0.676) | <10−6 | 0.0 | 0.176 (0.105, 0.294) | (0.085, 0.364) | 0.87 | 0.17 (0.07, 0.44) | 4/5.22 | — | Weak |
| Circulating EPA | 6 (265) | SMD | −0.455 (−0.882, −0.027) | 0.037 | 56.9 | 0.438 (0.202, 0.951) | (0.045, 4.317) | 0.27 | 0.44 (0.20, 1.22) | 2/1.96 | 1.00 | Weak | ||
| Circulating LA | 4 (199) | SMD | −0.623 (−1.663, 0.418) | 0.241 | 90.3 | 0.324 (0.049, 2.129) | (<0.001, >1000) | 0.28 | 0.52 (0.19, 1.27) | 1/0.94 | 1.00 | Not significant | ||
| Circulating AA | 6 (265) | SMD | −0.186 (−0.844, 0.471) | 0.579 | 80.4 | 0.716 (0.217, 2.361) | (0.013, 40.473) | 0.69 | 0.95 (0.38, 2.43) | 3/0.31 | <0.01 | Not significant | ||
| Zhang et al., 2016 (15) | Alzheimer disease | Circulating DHA (a 1% increment of blood DHA concentrations) | 3 (1828) | RR | 0.785 (0.561, 1.098) | 0.157 | 62.75 | — | (0.020, 31.045) | 0.16 | 0.96 (0.88, 1.04) | 1/0.16 | 0.15 | Not significant |
| Dementia | Circulating DHA (a 1% increment of blood DHA concentrations) | 5 (3099) | RR | 0.939 (0.864, 1.020) | 0.136 | 57.37 | — | (0.744, 1.185) | <0.01 | 0.99 (0.96, 1.03) | 2/0.25 | 0.02 | Not significant | |
| Mild cognitive impairment | Circulating DHA (a 1% increment of blood DHA concentrations) | 2 (2497) | RR | 0.846 (0.465, 1.540) | 0.585 | 85.84 | — | — | — | 1.11 (0.97, 1.26) | 1/0.16 | 0.15 | Not significant | |
| Hawkey et al., 2014 (13)2 | Attention-deficit/hyperactivity disorder | Circulating n–3 PUFAs | 9 (586) | Hedges' g | −0.409 (−0.563, −0.255) | <10−6 | 0.0 | 0.492 (0.367, 0.659) | (0.346, 0.700) | 0.25 | 0.59 (0.41, 1.16) | 2/1.91 | 1.00 | Weak |
| van der Kemp et al., 2012 (14) | Schizophrenia | Circulating DHA | 6 (194) | Cohen's d | −0.871 (−1.290, −0.452) | <10−3 | 68.1 | 0.207 (0.097, 0.441) | (0.019, 2.269) | 0.45 | 0.485 (0.214, 1.103) | 4/1.67 | 0.06 | Weak |
| Circulating AA | 6 (194) | Cohen's d | −0.767 (−1.390, −0.144) | 0.016 | 85.7 | 0.249 (0.081, 0.771) | (0.005, 12.730) | 0.34 | 0.40 (0.17, 0.91) | 4/1.96 | 0.09 | Weak | ||
| Circulating LA | 4 (113) | Cohen's d | −0.042 (−0.327, 0.244) | 0.774 | 0.0 | 0.927 (0.553, 1.554) | (0.298, 2.883) | 0.57 | 0.88 (0.39, 2.02) | 0/0.21 | — | Not significant | ||
| Circulating DPA | 4 (113) | Cohen's d | −0.924 (−1.225, −0.624) | <10−6 | 0.0 | 0.188 (0.109, 0.323) | (0.057, 0.619) | 0.79 | 0.18 (0.08, 0.43) | 3/0.04 | 0.63 | Weak | ||
| Circulating DTA | 3 (78) | Cohen's d | −0.113 (−0.478, 0.252) | 0.544 | 12.8 | 0.815 (0.421, 1.577) | (0.005, 129.249) | 0.32 | 0.51 (0.17, 1.06) | 0/0.27 | — | Not significant | ||
All summary estimates were recalculated based on a random-effects model using the method of DerSimonian and Laird. The 95% prediction interval and Egger's test were not evaluated if available studies were <3. For excess of significance, the P value was not evaluated if the observed number of studies was smaller than expected. AA, arachidonic acid; DPA, docosapentaenoic acid; DTA, docosatetraenoic acid; E, expected number of studies with positive finding; LA, linoleic acid; MD, mean difference; O, observed number of studies with positive finding; ref, reference; SMD, standardized mean difference.
The direction of comparison was normalized to mental disorder group versus control group.
None of the 20 meta-analyses had convincing, highly suggestive, or suggestive strength of efficacy according to the quantitative umbrella review criteria. In addition, the credibility of the evidence was weak for 8 meta-analyses, which suggested differences in circulating unsaturated fatty acids among ADHD, ASD, BP, and schizophrenia patients and healthy controls (P-random effects <10−6–0.037).
Discussion
This umbrella review included 29 systematic reviews of studies assessing the role of unsaturated fatty acids in numerous mental disorders. Overall, the available evidence has mainly focused on long-chain PUFAs (n–3 PUFAs and n–6 PUFAs), and the effects vary among mental disorders. Current evidence suggests that n–3 PUFA supplementation intervention might have potential value, but the strength of the efficacy and credibility of the evidence were weak overall. Higher intake of unsaturated fatty acids might have protective effects on a limited number of mental disorders (i.e., depression and mild cognitive impairment), and the effect of unsaturated fatty acid intake on many mental disorders has not been evaluated. In addition, we observed broad differences in circulating unsaturated fatty acids between patients with mental disorders and controls. The between-study heterogeneity, limited study populations, prediction intervals including the null value, and risk of excess significance bias were the main factors reducing the overall confidence in the evidence.
Although linking nutrition evidence with practice remains challenging (7, 64–67), the findings of this umbrella review might have clinical practice implications. The availability of a substantial body of experimental evidence generated in unsaturated fatty acid supplementation is a major finding that should inform the establishment of guidelines for the prevention of mental disorders. Mental disorders require complex interdisciplinary treatment grounded in the use of antipsychotic medications (68). However, medical treatment is usually accompanied by a large number of adverse effects, resulting in decreased treatment compliance (69). This has contributed to the development of alternative and complementary (adjunctive) treatments. An ever-growing body of evidence has evaluated the effect of dietary and supplemental unsaturated fatty acids (especially PUFAs) on various mental illnesses, but on the basis of umbrella review criteria none of these effect sizes have reached the maximum in terms of the strength of evidence credibility ratings. Suggestive evidence has shown a protective effect of fish consumption on depression (44) and dietary PUFA intake on mild cognitive impairment (15). Although the credibility in the estimate for efficacy was not optimal, weak evidence supported the efficacy of unsaturated fatty acid supplementation for mental disorders, which demonstrated the need for future high-quality randomized controlled trials evaluating the effects of unsaturated fatty acid supplementation interventions on different mental disorders.
n–6 and n–3 PUFAs have opposite effects on inflammatory modulation (4). n–6 PUFAs, particularly AA, are important precursors of eicosanoids (including prostaglandins, thromboxanes, and leukotrienes), which regulate the inflammatory process in immune cells as inflammatory mediators (4, 8). However, EPA and DHA act as competitive inhibitors of n–6 PUFAs causing a reduction in the synthesis of proinflammatory mediators (70). On the basis of evidence grading, the mental health–related favorable effect of unsaturated fatty acid supplementation was mainly limited to n–3 PUFAs, EPA, and DHA. Moreover, n–3 and n–6 PUFAs are implicated in gene expression and regulate several genes involved in lipid metabolism and inflammatory signaling through nuclear receptors (including farnesoid X receptors, liver X receptors, NF-κB, peroxisome proliferator activated receptors, retinoid X receptors, and sterol regulatory element binding protein 1c) (71). However, n–3 PUFAs show a greater potency in modifying nuclear receptor gene expression than n–6 PUFAs (71). n–3 PUFAs downregulate inflammatory genes and lipid synthesis and stimulate fatty acid degradation (71).
PUFAs are highly enriched in the brain (8) and make up approximately 35% of the lipids in brain (72). Adequate brain DHA and ARA are essential for normal cellular processes, such as transmembrane potential, neurotransmission, and the function of ion channels (10, 73). Altered brain fatty acid composition, metabolism, and fatty acid–derived signaling systems have been associated with mental disorders (8, 74, 75). Weak evidence suggested a broad range of circulating unsaturated fatty acid shortfalls in mental disorders (including ASD, ADHD, BP, and schizophrenia), which indicated the disturbance of fatty acid metabolism and a potential decrease in the absorption of unsaturated fatty acids (11–14). Therefore, unsaturated fatty acid supplementation and the intake of an unsaturated fatty acid–enriched diet have potential value in reversing fatty acid–related imbalances in the brain and might have favorable effects on mental health conditions (4, 5, 8, 64).
The main limitations of this umbrella review include those of the included systematic reviews and, in turn, the limitations of the original studies. The most frequently reported review shortcomings, detected by AMSTAR 2, were the absence of the list of excluded studies and the justification for exclusion studies, a thorough discussion of between-study heterogeneity, and adequate investigation and discussion of publication bias. According to the umbrella review criteria, publication bias and excess significance bias cannot be excluded for some comparisons. We were also unable to quantify the differences in the dose of unsaturated fatty acids among the included meta-analyses. These limitations decreased the strength of the associations and credibility of the evidence. Based on the analytic approach of an umbrella review, analyzing numerous outcomes could increase the risk of making a type I error, and Egger's tests might lack the statistical power to detect bias when few studies are included in the meta-analysis.
Additional limitations were related to the umbrella review methodology because this approach is based on the statistical reanalysis of meta-analyses. By definition, umbrella reviews include only systematic reviews that applied a quantitative approach to data presentation, whereas systematic reviews providing qualitative descriptions of the included studies, without applying meta-analytic techniques, are excluded. However, the absence of a meta-analytical approach is typically motivated by the scarcity of sufficient and homogeneous experimental evidence, which therefore does not reach the minimum clinical and methodological requirements needed for meta-analysis. Another limitation is that we did not analyze whether the efficacy of unsaturated fatty acid supplementation interventions was moderated by the composition and dose of unsaturated fatty acids, the length of follow-up, or by other social, dietary, or lifestyle-related variables (76, 77). The analysis of these variables was not feasible due to the nature of an umbrella review. To avoid data overlapping with exaggerated test power of the actual sample, we excluded a large amount of duplicate or updated studies assessing the same association. Different meta-analyses could have differences in selection criteria and analytical approaches, which might introduce bias to the evaluation of evidence, but we only extracted the main results (i.e., the specific association between unsaturated fatty acids and mental disorders); hence, the latest study with the largest number of individual studies typically provides the most accurate estimate of the true effect size. Finally, based on the aim of this review, we did not include studies evaluating the effects of unsaturated fatty acids on the general population—for example, studies on the neurodevelopment of typically developing children (78–80).
In view of the variability in the strength of the associations and credibility of the evidence, action is required to support further research efforts for different mental disorders. Several initiatives have aimed to improve the capacity of mental health research centers to conduct high-quality nutrition studies. In terms of populations, this review suggests a need for further studies involving children and adolescents, especially in children with mood disorders. A focus on mental health along a continuum from mild psychological distress to a severely disabling condition, as suggested by the Lancet Commission on global mental health and sustainable development (81), seems to be an appropriate and feasible approach, although we note a scarcity of evidence for specific diagnostic conditions, such as sleep disorders. In terms of interventions, considering the effective dose of unsaturated fatty acids, future research efforts should be directed to ascertain which combination and dose is more feasible and effective. In terms of outcomes, the assessment of the long-term effectiveness of n–3 PUFA supplementation would be relevant, including functional and quality-of-life measures, because they were seldom considered by the studies included in this umbrella review.
Given the pressing need for evidence-based answers for people with mental health conditions, and in view of the data on the effect of unsaturated fatty acid supplementation on mental disorders, we recommend that more high-quality studies be conducted to critically evaluate the potential of unsaturated fatty acid supplementation interventions in the prevention and control of mental disorders.
Supplementary Material
Acknowledgements
The authors’ responsibilities were as follows—FZ and XG: designed the study; XG, XS, and FZ: performed the literature search and screening and extracted the data; XS, XH, CC, and SZ: conducted the data analyses; XG and XS: created the figures and tables and drafted the manuscript; XG and XS: contributed equally to the manuscript as joint first authors, whereas FZ and YY are the corresponding authors who take responsibility for the integrity of the data and the accuracy of the data analysis; FA and YY: had the final responsibility for the decision to submit for publication; and all authors: had full access to all of the study data, participated in the interpretation of results, critically revised the manuscript, and read and approved the final manuscript.
Notes
Supported by grants from National Natural Science Foundation of China (81602853 and 81801492) and the Medical Research Fund of Guangdong Province (A2020582).
Author disclosures: The authors report no conflicts of interest. The funders of this study had no role in the study design, data collection, data analysis, data interpretation, or writing of the report.
Supplemental Methods, Supplemental Results, and Supplemental Table 1 are available from the “Supplementary data” link in the online posting of the article and from the same link in the online table of contents at https://academic.oup.com/advances/.
XG and XS contributed equally to this work and should be considered as co-first authors.
Abbreviations used: AA, arachidonic acid; AD, Alzheimer disease; ADHD, attention-deficit/hyperactivity disorder; ALA, ɑ-linolenic acid; AMSTAR 2, A Measurement Tool to Assess Systematic Reviews; ASD, autism spectrum disorder; BP, bipolar disorder; LA, linoleic acid; MD, mean difference; SMD, standardized mean difference; WMD, weighted mean difference.
Contributor Information
Xuping Gao, Department of Public Health and Preventive Medicine, School of Medicine, Jinan University, Guangdong, China; Department of Child and Adolescent Psychiatry, Peking University Sixth Hospital (Institute of Mental Health), National Clinical Research Center for Mental Disorders and NHC Key Laboratory of Mental Health (Peking University Sixth Hospital), Beijing, China.
Xin Su, Department of Public Health and Preventive Medicine, School of Medicine, Jinan University, Guangdong, China.
Xue Han, Department of Public Health and Preventive Medicine, School of Medicine, Jinan University, Guangdong, China.
Huiyan Wen, Department of Public Health and Preventive Medicine, School of Medicine, Jinan University, Guangdong, China.
Chen Cheng, Department of Public Health and Preventive Medicine, School of Medicine, Jinan University, Guangdong, China.
Shiwen Zhang, Department of Public Health and Preventive Medicine, School of Medicine, Jinan University, Guangdong, China.
Wanlin Li, Department of Public Health and Preventive Medicine, School of Medicine, Jinan University, Guangdong, China.
Jun Cai, Department of Public Health and Preventive Medicine, School of Medicine, Jinan University, Guangdong, China.
Lu Zheng, Department of Public Health and Preventive Medicine, School of Medicine, Jinan University, Guangdong, China.
Junrong Ma, Department of Public Health and Preventive Medicine, School of Medicine, Jinan University, Guangdong, China.
Minqi Liao, Institute of Epidemiology, Helmholtz Zentrum München-German Research Center for Environmental Health, Neuherberg, Germany.
Wanze Ni, Department of Public Health and Preventive Medicine, School of Medicine, Jinan University, Guangdong, China.
Tao Liu, Department of Public Health and Preventive Medicine, School of Medicine, Jinan University, Guangdong, China.
Dan Liu, Department of Public Health and Preventive Medicine, School of Medicine, Jinan University, Guangdong, China.
Wenjun Ma, Department of Public Health and Preventive Medicine, School of Medicine, Jinan University, Guangdong, China.
Shasha Han, Department of Neonatology and Pediatrics, The First Affiliated Hospital, Jinan University, Guangzhou, China.
Sui Zhu, Department of Public Health and Preventive Medicine, School of Medicine, Jinan University, Guangdong, China.
Yanbin Ye, Department of Clinical Nutrition, The First Affiliated Hospital, Jinan University, Guangzhou, China.
Fang-fang Zeng, Department of Public Health and Preventive Medicine, School of Medicine, Jinan University, Guangdong, China.
Data Availability
All data included in this umbrella review were extracted from publicly available systematic reviews.
References
- 1. Ferrari AJ, Santomauro DF, Herrera AMM, Shadid J, Ashbaugh C, Erskine HEet al. Global, regional, and national burden of 12 mental disorders in 204 countries and territories, 1990-2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet Psychiatry. 2022;9(2):137–50.. 10.1016/s2215-0366(21)00395-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Department of Mental Health and Substance Use . Mental health atlas 2020. Geneva (Switzerland): WHO; 2021. [Google Scholar]
- 3. Leichsenring F, Steinert C, Rabung S, Ioannidis JPA. The efficacy of psychotherapies and pharmacotherapies for mental disorders in adults: an umbrella review and meta-analytic evaluation of recent meta-analyses. World Psychiatry. 2022;21(1):133–45.. 10.1002/wps.20941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Melo HM, Santos LE, Ferreira ST. Diet-derived fatty acids, brain inflammation, and mental health. Front Neurosci. 2019;13:265. 10.3389/fnins.2019.00265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Custers EEM, Kiliaan AJ. Dietary lipids from body to brain. Prog Lipid Res. 2022;85:101144. 10.1016/j.plipres.2021.101144. [DOI] [PubMed] [Google Scholar]
- 6. Hadley KB, Ryan AS, Forsyth S, Gautier S, Salem N Jr.. The essentiality of arachidonic acid in infant development. Nutrients. 2016;8(4):216. 10.3390/nu8040216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Heath RJ, Klevebro S, Wood TR. Maternal and neonatal polyunsaturated fatty acid intake and risk of neurodevelopmental impairment in premature infants. Int J Mol Sci. 2022;23(2):700. 10.3390/ijms23020700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Mallick R, Basak S, Duttaroy AK. Fatty acids and evolving roles of their proteins in neurological, cardiovascular disorders and cancers. Prog Lipid Res. 2021;83:101116. 10.1016/j.plipres.2021.101116. [DOI] [PubMed] [Google Scholar]
- 9. Schmitz G, Ecker J. The opposing effects of n-3 and n-6 fatty acids. Prog Lipid Res. 2008;47(2):147–55.. 10.1016/j.plipres.2007.12.004. [DOI] [PubMed] [Google Scholar]
- 10. Hashimoto M, Hossain S, Shimada T, Shido O. Docosahexaenoic acid-induced protective effect against impaired learning in amyloid beta-infused rats is associated with increased synaptosomal membrane fluidity. Clin Exp Pharmacol Physiol. 2006;33(10):934–9.. 10.1111/j.1440-1681.2006.04467.x. [DOI] [PubMed] [Google Scholar]
- 11. Mazahery H, Stonehouse W, Delshad M, Kruger MC, Conlon CA, Beck KLet al. Relationship between long chain n-3 polyunsaturated fatty acids and autism spectrum disorder: systematic review and meta-analysis of case-control and randomised controlled trials. Nutrients. 2017;9(2):155. 10.3390/nu9020155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. McNamara RK, Welge JA. Meta-analysis of erythrocyte polyunsaturated fatty acid biostatus in bipolar disorder. Bipolar Disord. 2016;18(3):300–6.. 10.1111/bdi.12386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hawkey E, Nigg JT. Omega-3 fatty acid and ADHD: blood level analysis and meta-analytic extension of supplementation trials. Clin Psychol Rev. 2014;34(6):496–505.. 10.1016/j.cpr.2014.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. van der Kemp WJ, Klomp DW, Kahn RS, Luijten PR, Hulshoff Pol HE. A meta-analysis of the polyunsaturated fatty acid composition of erythrocyte membranes in schizophrenia. Schizophr Res. 2012;141(2-3):153–61.. 10.1016/j.schres.2012.08.014. [DOI] [PubMed] [Google Scholar]
- 15. Zhang Y, Chen J, Qiu J, Li Y, Wang J, Jiao J. Intakes of fish and polyunsaturated fatty acids and mild-to-severe cognitive impairment risks: a dose-response meta-analysis of 21 cohort studies. Am J Clin Nutr. 2015;103(2):330–40.. 10.3945/ajcn.115.124081. [DOI] [PubMed] [Google Scholar]
- 16. Kim JY, Son MJ, Son CY, Radua J, Eisenhut M, Gressier Fet al. Environmental risk factors and biomarkers for autism spectrum disorder: an umbrella review of the evidence. Lancet Psychiatry. 2019;6(7):590–600.. 10.1016/s2215-0366(19)30181-6. [DOI] [PubMed] [Google Scholar]
- 17. Jüni P, Altman DG, Egger M. Systematic reviews in health care: assessing the quality of controlled clinical trials. BMJ. 2001;323(7303):42–6.. 10.1136/bmj.323.7303.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Boffetta P, McLaughlin JK, La Vecchia C, Tarone RE, Lipworth L, Blot WJ. False-positive results in cancer epidemiology: a plea for epistemological modesty. JNCI J Natl Cancer Inst. 2008;100(14):988–95.. 10.1093/jnci/djn191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Papatheodorou S. Umbrella reviews: what they are and why we need them. Eur J Epidemiol. 2019;34(6):543–6.. 10.1007/s10654-019-00505-6. [DOI] [PubMed] [Google Scholar]
- 20. Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: the PRISMA statement. BMJ. 2009;339:b2535. 10.1136/bmj.b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ioannidis JP. Integration of evidence from multiple meta-analyses: a primer on umbrella reviews, treatment networks and multiple treatments meta-analyses. Can Med Assoc J. 2009;181(8):488–93.. 10.1503/cmaj.081086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Shea BJ, Reeves BC, Wells G, Thuku M, Hamel C, Moran Jet al. AMSTAR 2: a critical appraisal tool for systematic reviews that include randomised or non-randomised studies of healthcare interventions, or both. BMJ. 2017;358:j4008. 10.1136/bmj.j4008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Belbasis L, Bellou V, Evangelou E, Ioannidis JP, Tzoulaki I. Environmental risk factors and multiple sclerosis: an umbrella review of systematic reviews and meta-analyses. Lancet Neurol. 2015;14(3):263–73.. 10.1016/s1474-4422(14)70267-4. [DOI] [PubMed] [Google Scholar]
- 24. Solmi M, Köhler CA, Stubbs B, Koyanagi A, Bortolato B, Monaco Fet al. Environmental risk factors and nonpharmacological and nonsurgical interventions for obesity: an umbrella review of meta-analyses of cohort studies and randomized controlled trials. Eur J Clin Invest. 2018;48(12):e12982. 10.1111/eci.12982. [DOI] [PubMed] [Google Scholar]
- 25. Kim JY, Son MJ, Son CY, Radua J, Eisenhut M, Gressier Fet al. Environmental risk factors and biomarkers for autism spectrum disorder: an umbrella review of the evidence. Lancet Psychiatry. 2019;6(7):590–600.. 10.1016/s2215-0366(19)30181-6. [DOI] [PubMed] [Google Scholar]
- 26. Barbui C, Purgato M, Abdulmalik J, Acarturk C, Eaton J, Gastaldon Cet al. Efficacy of psychosocial interventions for mental health outcomes in low-income and middle-income countries: an umbrella review. Lancet Psychiatry. 2020;7(2):162–72.. 10.1016/s2215-0366(19)30511-5. [DOI] [PubMed] [Google Scholar]
- 27. Field AP, Gillett R. How to do a meta-analysis. Br J Math Stat Psychol. 2010;63(3):665–94.. . [DOI] [PubMed] [Google Scholar]
- 28. Sterne JA, Davey Smith G. Sifting the evidence—what's wrong with significance tests?. BMJ. 2001;322(7280):226–31.. 10.1136/bmj.322.7280.226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ioannidis JP, Tarone R, McLaughlin JK. The false-positive to false-negative ratio in epidemiologic studies. Epidemiology. 2011;22(4):450–6.. 10.1097/EDE.0b013e31821b506e. [DOI] [PubMed] [Google Scholar]
- 30. Cochran WG. The combination of estimates from different experiments. Biometrics. 1954;10:(1):101–29. [Google Scholar]
- 31. Ioannidis JPA, Patsopoulos NA, Evangelou E. Uncertainty in heterogeneity estimates in meta-analyses. BMJ. 2007;335:(7626):914–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Hasselblad V, Hedges LV. Meta-analysis of screening and diagnostic tests. Psychol Bull. 1995;117(1):167–78.. 10.1037/0033-2909.117.1.167. [DOI] [PubMed] [Google Scholar]
- 33. Chinn S. A simple method for converting an odds ratio to effect size for use in meta-analysis. Stat Med. 2000;19(22):3127–31.. . [DOI] [PubMed] [Google Scholar]
- 34. da Costa BR, Rutjes AW, Johnston BC, Reichenbach S, Nüesch E, Tonia Tet al. Methods to convert continuous outcomes into odds ratios of treatment response and numbers needed to treat: meta-epidemiological study. Int J Epidemiol. 2012;41(5):1445–59.. 10.1093/ije/dys124. [DOI] [PubMed] [Google Scholar]
- 35. Higgins JP, Thompson SG, Spiegelhalter DJ. A re-evaluation of random-effects meta-analysis. J R Stat Soc A Stat Soc. 2009;172(1):137–59.. 10.1111/j.1467-985X.2008.00552.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Riley RD, Higgins JP, Deeks JJ. Interpretation of random effects meta-analyses. BMJ. 2011;342:d549. 10.1136/bmj.d549. [DOI] [PubMed] [Google Scholar]
- 37. Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315(7109):629–34.. 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Ioannidis JP, Trikalinos TA. An exploratory test for an excess of significant findings. Clin Trials. 2007;4(3):245–53.. 10.1177/1740774507079441. [DOI] [PubMed] [Google Scholar]
- 39. Lubin JH, Gail MH. On power and sample size for studying features of the relative odds of disease. Am J Epidemiol. 1990;131(3):552–66.. 10.1093/oxfordjournals.aje.a115530. [DOI] [PubMed] [Google Scholar]
- 40. Kim JH, Kim JY, Lee J, Jeong GH, Lee E, Lee Set al. Environmental risk factors, protective factors, and peripheral biomarkers for ADHD: an umbrella review. Lancet Psychiatry. 2020;7(11):955–70.. 10.1016/s2215-0366(20)30312-6. [DOI] [PubMed] [Google Scholar]
- 41. Tsai AC, Lucas M, Okereke OI, O'Reilly ÉJ, Mirzaei F, Kawachi Iet al. Suicide mortality in relation to dietary intake of n-3 and n-6 polyunsaturated fatty acids and fish: equivocal findings from 3 large US cohort studies. Am J Epidemiol. 2014;179(12):1458–66.. 10.1093/aje/kwu086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Cooper RE, Tye C, Kuntsi J, Vassos E, Asherson P. Omega-3 polyunsaturated fatty acid supplementation and cognition: a systematic review and meta-analysis. J Psychopharmacol. 2015;29(7):753–63.. 10.1177/0269881115587958. [DOI] [PubMed] [Google Scholar]
- 43. Yang JR, Han D, Qiao ZX, Tian X, Qi D, Qiu XH. Combined application of eicosapentaenoic acid and docosahexaenoic acid on depression in women: a meta-analysis of double-blind randomized controlled trials. Neuropsychiatr Dis Treatment. 2015;11:2055–61.. 10.2147/NDT.S86581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Grosso G, Micek A, Marventano S, Castellano S, Mistretta A, Pajak Aet al. Dietary n-3 PUFA, fish consumption and depression: a systematic review and meta-analysis of observational studies. J Affect Disord. 2016;205:269–81.. 10.1016/j.jad.2016.08.011. [DOI] [PubMed] [Google Scholar]
- 45. Rosenblat JD, Kakar R, Berk M, Kessing LV, Vinberg M, Baune BTet al. Anti-inflammatory agents in the treatment of bipolar depression: a systematic review and meta-analysis. Bipolar Disord. 2016;18(2):89–101.. 10.1111/bdi.12373. [DOI] [PubMed] [Google Scholar]
- 46. Tan ML, Ho JJ, Teh KH. Polyunsaturated fatty acids (PUFAs) for children with specific learning disorders. Cochrane Database Syst Rev. 2016;9:CD009398. 10.1002/14651858.CD009398.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Su KP, Tseng PT, Lin PY, Okubo R, Chen TY, Chen YWet al. Association of use of omega-3 polyunsaturated fatty acids with changes in severity of anxiety symptoms: a systematic review and meta-analysis. JAMA Network Open. 2018;1(5):e182327. 10.1001/jamanetworkopen.2018.2327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Devoe DJ, Farris MS, Townes P, Addington J. Attenuated psychotic symptom interventions in youth at risk of psychosis: a systematic review and meta-analysis. Early Interv Psychiatry. 2019;13(1):3–17.. 10.1111/eip.12677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Morsy S, Khalil SM, Doheim MF, Kamel MG, El-Basiony DSM, Ahmed Hassan HIet al. Efficacy of ethyl-EPA as a treatment for Huntington disease: a systematic review and meta-analysis. Acta Neuropsychiatrica. 2019;31(04):175–85.. 10.1017/neu.2019.11. [DOI] [PubMed] [Google Scholar]
- 50. Qu Y, Chen X, Xu MM, Sun Q. Relationship between high dietary fat intake and Parkinson's disease risk: a meta-analysis. Neural Regeneration Res. 2019;14(12):2156–63.. 10.4103/1673-5374.262599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Zhang L, Liu H, Kuang L, Meng H, Zhou X. Omega-3 fatty acids for the treatment of depressive disorders in children and adolescents: a meta-analysis of randomized placebo-controlled trials. Child Adolesc Psychiatry Mental Health. 2019;13(1):36. 10.1186/s13034-019-0296-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Araya-Quintanilla F, Gutiérrez-Espinoza H, Sánchez-Montoya U, Muñoz-Yañez MJ, Baeza-Vergara A, Petersen-Yanjarí Met al. Effectiveness of omega-3 fatty acid supplementation in patients with Alzheimer disease: a systematic review and meta-analysis. Neurologia. 2020;35(2):105–14. [DOI] [PubMed] [Google Scholar]
- 53. Luo XD, Feng JS, Yang Z, Huang QT, Lin JD, Yang Bet al. High-dose omega-3 polyunsaturated fatty acid supplementation might be more superior than low-dose for major depressive disorder in early therapy period: a network meta-analysis. BMC Psychiatry. 2020;20(1):248. 10.1186/s12888-020-02656-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Mocking RJT, Steijn K, Roos C, Assies J, Bergink V, Ruhé HGet al. Omega-3 fatty acid supplementation for perinatal depression: a meta-analysis. J Clin Psychiatry. 2020;81(5):19r13106. 10.4088/JCP.19r13106. [DOI] [PubMed] [Google Scholar]
- 55. Zhang X, Han H, Ge X, Liu L, Wang T, Yu H. Effect of n-3 long-chain polyunsaturated fatty acids on mild cognitive impairment: a meta-analysis of randomized clinical trials. Eur J Clin Nutr. 2020;74(4):548–54.. 10.1038/s41430-019-0544-4. [DOI] [PubMed] [Google Scholar]
- 56. Appleton KM, Voyias PD, Sallis HM, Dawson S, Ness AR, Churchill Ret al. Omega-3 fatty acids for depression in adults. Cochrane Database Syst Rev. 2021;11:CD004692. 10.1002/14651858.CD004692.pub5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. de Andrade Wobido K, de Sá, Barreto da Cunha M, Miranda SS, da Mota Santana J, da Silva DCGet al. Non-specific effect of omega-3 fatty acid supplementation on autistic spectrum disorder: systematic review and meta-analysis. Nutr Neurosci. 2021:1–13.. 10.1080/1028415x.2021.1913950. [DOI] [PubMed] [Google Scholar]
- 58. Goh KK, Chen CY, Chen CH, Lu ML. Effects of omega-3 polyunsaturated fatty acids supplements on psychopathology and metabolic parameters in schizophrenia: a meta-analysis of randomized controlled trials. J Psychopharmacol. 2021;35(3):221–35.. 10.1177/0269881120981392. [DOI] [PubMed] [Google Scholar]
- 59. Händel MN, Rohde JF, Rimestad ML, Bandak E, Birkefoss K, Tendal Bet al. Efficacy and safety of polyunsaturated fatty acids supplementation in the treatment of attention deficit hyperactivity disorder (ADHD) in children and adolescents: a systematic review and meta-analysis of clinical trials. Nutrients. 2021;13(4):1226. 10.3390/nu13041226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Suradom C, Suttajit S, Oon-Arom A, Maneeton B, Srisurapanont M. Omega-3 polyunsaturated fatty acid (n-3 PUFA) supplementation for prevention and treatment of perinatal depression: a systematic review and meta-analysis of randomized-controlled trials. Nord J Psychiatry. 2021;75(4):239–46.. 10.1080/08039488.2020.1843710. [DOI] [PubMed] [Google Scholar]
- 61. Xu Z, Sun W, Zhang D, Chung VCH, Sit RWS, Wong SYS. Comparative effectiveness of interventions for global cognition in patients with mild cognitive impairment: a systematic review and network meta-analysis of randomized controlled trials. Front Aging Neurosci. 2021;13:653340. 10.3389/fnagi.2021.653340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Zhu RZ, Chen MQ, Zhang ZW, Wu TY, Zhao WH. Dietary fatty acids and risk for Alzheimer's disease, dementia, and mild cognitive impairment: a prospective cohort meta-analysis. Nutrition. 2021;90:111355. 10.1016/j.nut.2021.111355. [DOI] [PubMed] [Google Scholar]
- 63. Kosti RI, Kasdagli MI, Kyrozis A, Orsini N, Lagiou P, Taiganidou Fet al. Fish intake, n-3 fatty acid body status, and risk of cognitive decline: a systematic review and a dose-response meta-analysis of observational and experimental studies. Nutr Rev. 2022;80(6):1445–58.. 10.1093/nutrit/nuab078. [DOI] [PubMed] [Google Scholar]
- 64. Xu X, Shao G, Zhang X, Hu Y, Huang J, Su Yet al. The efficacy of nutritional supplements for the adjunctive treatment of schizophrenia in adults: a systematic review and network meta-analysis. Psychiatry Res. 2022;311:114500. 10.1016/j.psychres.2022.114500. [DOI] [PubMed] [Google Scholar]
- 65. Wood AHR, Chappell HF, Zulyniak MA. Dietary and supplemental long-chain omega-3 fatty acids as moderators of cognitive impairment and Alzheimer's disease. Eur J Nutr. 2022;61(2):589–604.. 10.1007/s00394-021-02655-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Bozzatello P, Blua C, Rocca P, Bellino S. Mental health in childhood and adolescence: the role of polyunsaturated fatty acids. Biomedicines. 2021;9(8):850. 10.3390/biomedicines9080850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Appleton KM, Voyias PD, Sallis HM, Dawson S, Ness AR, Churchill Ret al. Omega-3 fatty acids for depression in adults. Cochrane Database Syst Rev. 2021;11:CD004692. 10.1002/14651858.CD004692.pub5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Carrascal-Laso L, Franco-Martin MA, Marcos-Vadillo E, Ramos-Gallego I, Garcia-Berrocal B, Mayor-Toranzo Eet al. Economic impact of the application of a precision medicine model (5SPM) on psychotic patients. Pharmacogenomics Personalized Med. 2021;14:1015–25.. 10.2147/PGPM.S320816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Lally J, MacCabe JH. Antipsychotic medication in schizophrenia: a review. Br Med Bull. 2015;114(1):169–79.. 10.1093/bmb/ldv017. [DOI] [PubMed] [Google Scholar]
- 70. Ergas D, Eilat E, Mendlovic S, Sthoeger ZM. n-3 fatty acids and the immune system in autoimmunity. Isr Med Assoc J. 2002;4(1):34–8. [PubMed] [Google Scholar]
- 71. Schmitz G, Ecker J. The opposing effects of n-3 and n-6 fatty acids. Prog Lipid Res. 2008;47(2):147–55.. 10.1016/j.plipres.2007.12.004. [DOI] [PubMed] [Google Scholar]
- 72. Yehuda S, Rabinovitz S, Mostofsky DI. Essential fatty acids are mediators of brain biochemistry and cognitive functions. J Neurosci Res. 1999;56(6):565–70.. . [DOI] [PubMed] [Google Scholar]
- 73. Luchtman DW, Song C. Cognitive enhancement by omega-3 fatty acids from child-hood to old age: findings from animal and clinical studies. Neuropharmacology. 2013;64:550–65.. 10.1016/j.neuropharm.2012.07.019. [DOI] [PubMed] [Google Scholar]
- 74. Bazinet RP, Layé S. Polyunsaturated fatty acids and their metabolites in brain function and disease. Nat Rev Neurosci. 2014;15(12):771–85.. 10.1038/nrn3820. [DOI] [PubMed] [Google Scholar]
- 75. Shamim A, Mahmood T, Ahsan F, Kumar A, Bagga P. Lipids: an insight into the neurodegenerative disorders. Clin Nutr Exp. 2018;20:1–19.. 10.1016/j.yclnex.2018.05.001. [DOI] [Google Scholar]
- 76. Dominguez LJ, Veronese N, Vernuccio L, Catanese G, Inzerillo F, Salemi Get al. Nutrition, physical activity, and other lifestyle factors in the prevention of cognitive decline and dementia. Nutrients. 2021;13(11):4080. 10.3390/nu13114080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. D'Cruz MM, Chaturvedi SK. Sociodemographic and cultural determinants of mood disorders. Curr Opin Psychiatry. 2022;35(1):38–44.. 10.1097/yco.0000000000000766. [DOI] [PubMed] [Google Scholar]
- 78. Delgado-Noguera MF, Calvache JA, Bonfill Cosp X, Kotanidou EP, Galli-Tsinopoulou A. Supplementation with long chain polyunsaturated fatty acids (LCPUFA) to breastfeeding mothers for improving child growth and development. Cochrane Database Syst Rev. 2015;7:CD007901. 10.1002/14651858.CD007901.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Shulkin M, Pimpin L, Bellinger D, Kranz S, Fawzi W, Duggan Cet al. n-3 fatty acid supplementation in mothers, preterm infants, and term infants and childhood psychomotor and visual development: a systematic review and meta-analysis. J Nutr. 2018;148(3):409–18.. 10.1093/jn/nxx031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Leutgeb V, Köchel A, Lang L, Koch J, Schienle A. F(r)ische fürs gehirn: eine pilotstudie zur wirkung von omega-3-fettsäuren auf kognitive, emotionale und soziale verhaltensparameter bei kindergartenkindern [Effects of omega-3 fatty acids on cognitive, emotional, and social behavioral parameters in kindergarten children: a pilot study]. Kindheit Und Entwicklung: Zeitschrift Für Klinische Kinderpsychologie. 2015;24(2):86–93.. 10.1026/0942-5403/a000164. [DOI] [Google Scholar]
- 81. Patel V, Saxena S, Lund C, Thornicroft G, Baingana F, Bolton Pet al. The Lancet Commission on global mental health and sustainable development. Lancet North Am Ed. 2018;392(10157):1553–98.. 10.1016/s0140-6736(18)31612-x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data included in this umbrella review were extracted from publicly available systematic reviews.