Abstract
Genome-wide association studies (GWAS) have identified the PICALM (Phosphatidylinositol binding clathrin-assembly protein) gene as the most significant genetic susceptibility locus after APOE and BIN1. PICALM is a clathrin-adaptor protein that plays a critical role in clathrin-mediated endocytosis and autophagy. Since the effects of genetic variants of PICALM as AD-susceptibility loci have been confirmed by independent genetic studies in several distinct cohorts, there has been a number of in vitro and in vivo studies attempting to elucidate the underlying mechanism by which PICALM modulates AD risk. While differential modulation of APP processing and Aβ transcytosis by PICALM has been reported, significant effects of PICALM modulation of tau pathology progression have also been evidenced in Alzheimer’s disease models. In this review, we summarize the current knowledge about PICALM, its physiological functions, genetic variants, post-translational modifications and relevance to AD pathogenesis.
Keywords: PICALM, Alzheimer’s disease, GWAS, amyloid β, neurofibrillary tangles, microglia
1. Physiopathology of Alzheimer’s Disease and Genetic Risk Factors
Alzheimer’s disease (AD) is the most common cause of dementia, manifesting itself in cognitive deficits. AD is characterized by two neuropathological hallmarks: extracellular deposition of fibrils made up of amyloid β (Aβ) peptides as senile plaques [1] and intracellular accumulation of hyperphosphorylated tau as neurofibrillary tangles (NFTs) [2] (for review, see [3]).
Aβ is produced by successive cleavages of β-amyloid precursor protein (APP), encoded by APP gene located in chromosome 21, by β-secretase (BACE1) and γ-secretase [4]. APP is cleaved by BACE1 at its N terminal domain to release soluble APP-β and the membrane-bound APP C-terminal fragment (β-CTF or C99). The γ-secretase is a complex of four protein subunits consisting of presenilin (PSEN), Nicastrin, presenilin enhancer (PEN) and APH1 (anterior pharynx-defective 1). Sequential cleavages of APP by BACE1 and γ-secretase lead to production of Aβ peptides of 38-, 40- and 42-amino acids in length. Aβ peptides are principally produced in endosomes and are released from neurons in a synaptic activity-dependent manner [5]. Aβ peptides adopt aggregation-prone conformations that are resistant to proteolysis and form oligomers, protofibrils and fibrils. Due to hydrophobicity at its C-terminus, Aβ42 has greater aggregation properties [6].
Tau is a microtubule-associated protein and has a role in stabilizing neuronal microtubules. Tau is encoded by MAPT (microtubule-associated protein tau) gene located on chromosome 17. Tau is natively soluble and unfolded, but tau can be aggregated into oligomers and fibrils in the presence of pathologically misfolded tau or polyanions [7]. In AD, tau aggregates form paired helical filaments (PHF) or straight filaments [8]. Compelling evidence suggests that tau pathology, defined by the accumulation of hyperphosphorylated aggregated forms of tau, is strongly associated with neurodegeneration and cognitive impairment in AD [6,9]. Tau pathology propagates between neuroanatomically connected areas throughout the brain during the progression of AD. The stereotypical aspect of tau pathology progression forms the basis of neuropathological Braak staging of tau pathology [10]. The stage of tau pathology better correlates with cognitive decline than Aβ load in AD patients [9]. Numerous studies have suggested that both synthetic and AD brain-derived tau fibrils can propagate in a prion-like manner by recruiting normal unfolded tau into pathological tau aggregates [11]. Tau aggregation can also be found in neurons and glia of other human neurological disorders, so-called tauopathies, such as frontotemporal lobar degeneration (FTLD) with tau pathology, progressive supranuclear palsy (PSP), Pick disease, corticobasal degeneration (CBD), etc. [12].
AD can be generally classified into two subgroups according to the age of onset. AD cases occurring earlier than age 65 are termed early-onset AD (EOAD), constituting less than 5% of all cases. Early-onset AD includes familial AD (FAD) inherited in an autosomal-dominant manner and can present at a very early age of onset and with a more rapid rate of disease progression, constituting 1–2% of AD cases. A number of FAD mutations have been identified in the genes of APP, Presenilin1 (PSEN1) and Presenilin2 (PSEN2) (γ-secretase component) [13].
Since age is the most important risk factor for AD [14], the great majority of cases, estimated at more than 95%, occur after age 65, constituting late-onset AD (LOAD). There was little knowledge of genetic risk factors for LOAD except for Apolipoprotein (APOE) risk allele APOE4 till the breakthrough made by genome-wide association studies (GWAS) [15,16]. To date, GWAS have identified about 75 single nucleotide polymorphisms (SNPs), including APOE4, BIN1, PICALM, CLU, CR1, ABCA7, TREM2, MS4A and APH1B, with a significant association for LOAD susceptibility [17]. These LOAD-susceptibility genes are functionally implicated in multiple cellular processes such as synaptic function, lipid metabolism, inflammation, endocytosis, cytoskeletal transports, and tau and amyloid pathways in AD pathologies [18,19,20]. Nevertheless, the mechanisms by which the GWAS hit genes modulate the AD risk remain largely elusive and have been under active scrutiny in the field of functional genomics in the post GWAS era. The vast majority of these variants are located in the noncoding region [21], and such variants are supposed to exert phenotypic effects via the perturbation of transcriptional gene promoters and enhancers [22].
Among them, several independent GWAS have identified SNPs significantly associated with LOAD in the PICALM gene (see Section 3.1) coding phosphatidylinositol binding clathrin-assembly protein PICALM (also known as CALM that stands for clathrin assembly lymphoid myeloid leukemia). Numerous GWAS have suggested that PICALM is one of the most significant susceptibility genes for LOAD after APOE4 and BIN1 [15,17,19,23,24,25,26,27]. Since the discovery of the genetic implication of PICALM in LOAD, many studies have been conducted to decipher the roles of PICALM in AD pathogenesis. In light of these findings, we describe in this review the protein structure and known post-translational modifications of PICALM. We summarize the emerging link between PICALM and pathogenic proteins found in AD brains to better understand the mechanisms by which PICALM contributes to AD pathogenesis via Aβ-dependent and Aβ-independent pathways.
2. Biology of PICALM
2.1. Tissue Expression, Cellular Functions and Protein Interaction of PICALM
The human PICALM gene is located on chromosome 11 [28]. There are 24 transcripts as splice variants, 15 of them coding protein [28], whose sizes vary from 134 to 652 amino acids [29], and the molecular weights of the major isoforms are estimated at 65–75 kDa due to alternative splicing of exon 13 [30]. PICALM is a ubiquitous protein and is expressed in the brain, muscle, kidney, urinary bladder, connective tissues, bone marrow, lymphoid tissues and in female tissues, including breast and placenta, https://www.proteinatlas.org/ENSG00000073921-PICALM/tissue (accessed on 1 June 2022). [31]. In the human brain, PICALM is abundantly expressed in the microglia, oligodendrocytes, endothelial cells, neurons, vascular mural and choroid plexus cells (for further information on the localization of PICALM in the AD brain, see 3.3.1) [30,32,33,34,35,36]. In the mouse brain, PICALM mRNA is highly expressed in the hippocampus [31], in particular in the dentate gyrus granule cells, as detected by a spatial transcriptomic study [37,38].
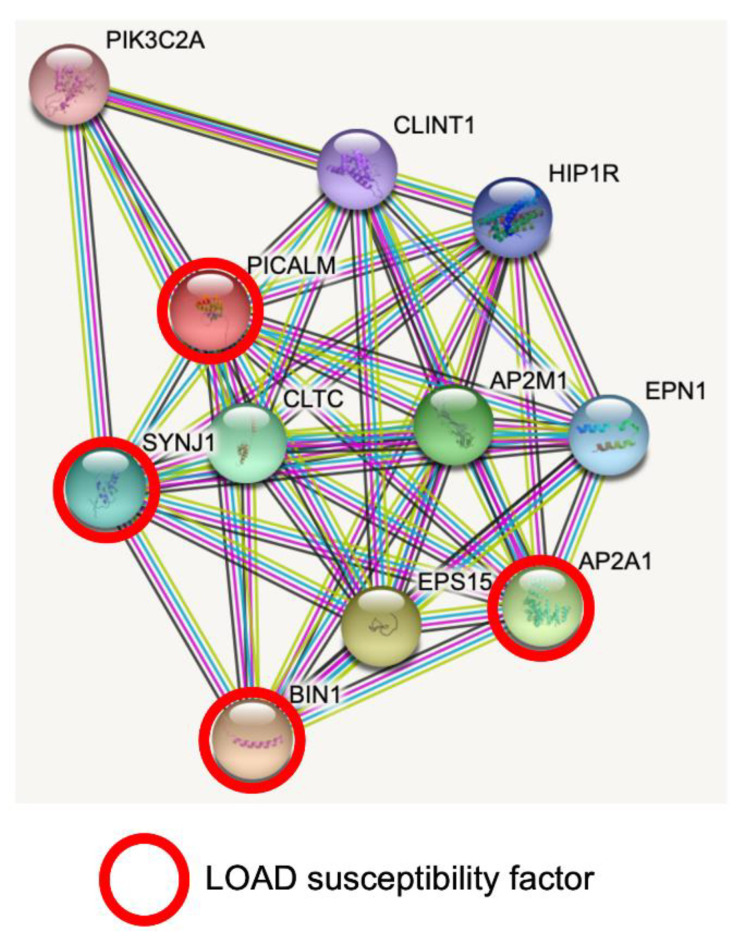
STRING database analysis revealed predicted protein–protein interactions of PICALM [39,40]. PICALM interacts with key proteins for clathrin-mediated endocytosis, intracellular trafficking and signaling (Figure 1). Interestingly, PICALM tightly interacts with other proteins encoded by GWAS hit genes for LOAD, such as BIN1, CD2AP, EPHA1, AP2A1 and AP2A2 [41]. PICALM is almost perfectly colocalized with the AP2 adaptor and AP2 α-adaptin subunits (encoded by AP2A1 and AP2A2) [42,43]. Interestingly, gene variants, splicing defects and altered expression of AP2A1 and AP2A2 have been recently shown to have a significant association with LOAD susceptibility [41]. Mounting evidence suggests PICALM has multiple roles in cellular functions and homeostasis as a clathrin adaptor for clathrin-mediated endocytosis, erythroid maturation, transferrin uptake, lipid homeostasis, autophagy, neuronal polarity, neuritic prolongation and synaptic vesicle turnover [44].
Figure 1.
PICALM interacts directly with several GWAS hits identified in LOAD. Predicted protein–protein interactions by STRING database analysis (https://string-db.org (accessed on 1 June 2022)) show several key proteins for clathrin-mediated endocytosis and intracellular trafficking as PICALM interactors [40]. AP2A1 and BIN1 have been identified as GWAS hits [41]. SNPs of SYNJ1 have significant associations with disease onset of AD [45]. CLTC: Clathrin heavy chain 1. SYNJ1: Synaptojanin1. PIK3C2A: Phosphatidylinositol 4-phosphate 3-kinase C2 domain-containing subunit α. CLINT1: Clathrin interactor 1. HIP1R: Huntingtin-interacting protein 1-related protein. AP2M1: AP2 complex subunit mu. EPN1: Epsin-1. AP2A1: AP2 complex subunit α-1. EPS15: Epidermal growth factor receptor substrate 15. BIN1: Myc box-dependent-interacting protein 1.
2.1.1. PICALM and Clathrin-Mediated Endocytosis
Clathrin-mediated endocytosis, also called receptor-mediated endocytosis, is a process leading to the internalization of plasma membrane-associated cargo molecules and subsequent trafficking through intracellular vesicle compartments. PICALM is necessary for clathrin assembly at the plasma membrane by binding to AP2 and clathrin (Figure 2A). PICALM interacts simultaneously with AP1 and clathrin at the trans-Golgi network in HeLa cells [43]. Several independent studies have reported that the depletion of PICALM leads to an increase in the size of clathrin-coated vesicles (CCVs) and to an increase in heterogeneity in the CCVs shape in cultured cells of rat hippocampus [46] and of HeLa cells [43,47]. CRISPR/Cas9-mediated PICALM disruption also leads to an increase in the number of early endosomes in HeLa cells [48]. A similar observation was obtained by the depletion of uncoordinated protein-11 (unc-11), the ortholog of PICALM in neurons of C. elegans [49]. Another study suggests that PICALM is required for efficient clathrin coat maturation in fibroblasts [50].
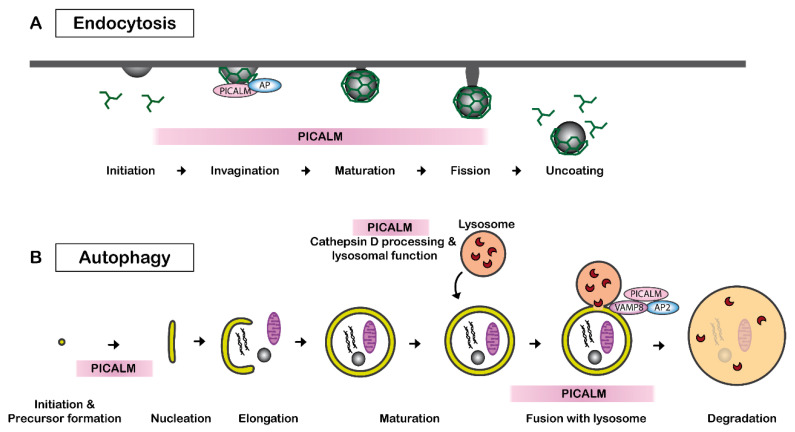
Figure 2.
PICALM regulates clathrin-mediated endocytosis and autophagy. (A) PICALM is involved in clathrin-mediated endocytosis (CME) as a clathrin adaptor by facilitating the formation of clathrin-coated vesicles (CCVs). The interaction between PICALM, adaptor proteins (AP) and clathrin is critical to maintaining the form and sizes of CCVs. PICALM interacts with AP2 at the plasma membrane and AP 1 in the trans-Golgi network to form CCVs. (B) PICALM is also involved in multiple steps of autophagy process. PICALM is involved in the autophagy precursor formation at the autophagosome-lysosome fusion [51], maturation of Cathepsin D and lysosomal function [48].
2.1.2. PICALM, Hematopoiesis, Transferrin-Uptake and Cholesterol Homeostasis
Chromosomal translocations of PICALM have been observed in patients with acute lymphoblastic leukemia and acute myeloid leukemia [52]. In mice, nonsense point mutations of PICALM cause abnormal iron distribution, growth retardation, shortened life span and hematopoietic abnormalities [53].
PICALM is a critical protein, and homozygous PICALM knockout mice are dwarfed and die within 1 month after birth due to a deficiency in erythroid maturation and transferrin uptake [54]. There are controversies in the literature on the effects of PICALM on transferrin uptake. While some studies suggest that PICALM overexpression or down-expression did not alter transferrin uptake in HeLa cells or HEK293 cells [55,56], others observed a significant effect on transferrin uptake in the liver, bone marrow and erythroid cells from PICALM-deficient mice [50,54]. It is thus speculated that the deletion of PICALM may be partially compensated by other endocytic proteins in certain types of cells. Indeed, PICALM is dispensable in myeloid and B-lymphoid development [50].
Additionally, PICALM also modulates cellular cholesterol homeostasis, as PICALM expression influences the expression of genes encoding proteins involved in cholesterol biosynthesis and lipoprotein uptake [57]. Knockdown of PICALM leads to up to a 50% increase in cholesterol biosynthesis genes in in HEK293, murine embryonic fibroblasts (MEF) and Cath-a-differentiated (CAD) neuronal cells [57].
2.1.3. The Roles of PICALM in Neuronal Polarity and Synaptic Vesicle Sorting
PICALM and its neuronal homolog assembly protein 180 (AP180) have quite interesting functions in neuronal cells. Endocytosis is reduced in PICALM- or AP180-deficient neurons [58]. Interestingly, PICALM and AP180 have critical roles in establishing the polarity and neuritic growth in neuronal cells. While treatments with siRNA targeting PICALM lead to dendritic dystrophy, treatments with siRNA targeting AP180 abolish axonal extension in rat primary neuronal culture. In contrast, neurons overexpressing AP180 or PICALM generate multiple axons [58].
PICALM is expressed in neurons and is present in synapses [36]. PICALM regulates membrane fusion by binding to R-SNAREs (soluble NSF attachment protein receptors) such as VAMP2, VAMP3 and VAMP8 [59,60]. Furthermore, PICALM regulates the internalization of VAMP2 (Synaptobrevin 2), the most abundant synaptic vesicle protein and a major R-SNARE component [56]. Since the AP180 N-terminal homology (ANTH) domain (see chapter 2.2) of PICALM interacts with VAMP2, both PICALM and AP180 regulate endocytic sorting of synaptic vesicles at active zones for neurotransmission [59]. PICALM and related ANTH-domain-containing proteins modulate the surface levels of calcium-permeable AMPA-type glutamate receptors (AMPARs) that mediate fast excitatory neurotransmission and excitotoxicity [61]. PICALM is thereby supposed to play roles in synaptic plasticity and learning by modulating both long-term potentiation (LTP) and long-term depression (LTD) [62].
2.1.4. PICALM and Autophagy
PICALM plays a key role in multiple steps in autophagy (Figure 2B). PICALM regulates the endocytosis of VAMP2, VAMP3 and VAMP8, which are essential for the autophagy process [60]. PICALM knockdown leads to a reduced fusion of autophagosome precursor vesicles, which is a VAMP2- and VAMP3-dependent process, and thus decreases autophagosome biogenesis. PICALM knockdown also influences the process of autophagosome-lysosome fusion, which is a VAMP8-dependent process [63]. Therefore, PICALM reduction results in a general reduction in autophagic flux in HeLa, HEK, CAD and MEF cells [51]. Additionally, PICALM is also implicated in the maturation of Cathepsin D, which is a critical lysosomal component [48]. Disruption of exon1 of PICALM leads to deficits in the maturation of Cathepsin D and autophagy in HeLa cells [48].
2.2. Protein Structure of PICALM
PICALM is constituted of an N-terminal ANTH-domain binding phosphatidyl inositol 4,5 bisphosphate (PtdIns(4,5)P2) and a C-terminal clathrin adaptor domain (Figure 3A) [42,64].
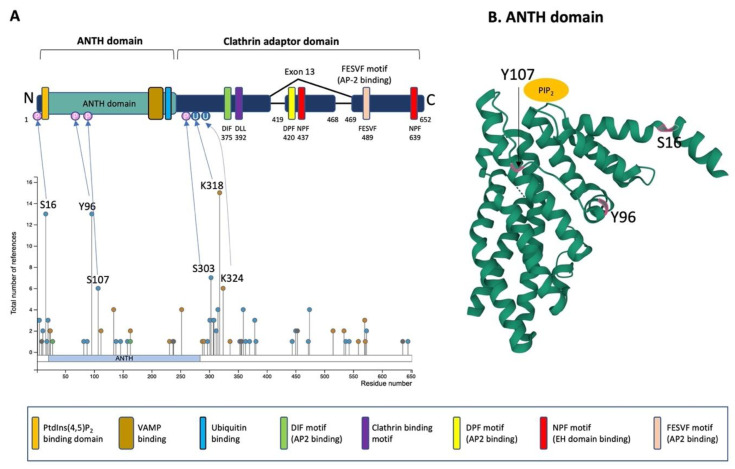
Figure 3.
Schematic illustration of PICALM structure and post-translational modifications. (A) Schematic structure of PICALM. PICALM is constituted of two distinct domains: the ANTH (AP180 N-terminal homology) domain and clathrin adaptor domain. ANTH domain contains a PtdIns(4,5)P2 binding domain and vesicle-associated membrane protein (VAMP) binding domain. Clathrin adaptor domain contains several motifs that are critical to interact with endocytic protein, including DIF, DLL Clathrin binding, DPF, NPF and FESVF motifs. DIF motif binds to AP2. DLL motif is conserved and has weak affinity to Clathrin DPF (Aspartic acid-Proline-Phenylalanine) motifs. NPF (Asparagine-Proline-Phenylalanine) motif binds to AP2 and EH domains (Eps15 Homology domain). Four phosphorylation sites (S16, Y96, S107 and S303) and two ubiquitination sites (K318 and K324) have been described in more than 5 references and validated by mass-spectrometry and thus are highlighted [65]. Altogether, the reported PTMs of PICALM are 41 phosphorylation (S2, S5, T11, S16, T18, S20, S23, T82, Y88, Y96, S107, Y138, T146, T158, Y237, S297, T301, S303, S307, S308, T312, S315, S353, T355, T356, S359, T363, T370, T379, S381, S444, S450, S453, T472, S474, K515, K534, S537, S543, T573 and S645), 15 ubiquitination (K24, K112, K134, K163, K231, K252, K288, K291, K318, K324, K336, K515, K559, K570 and K571), 1 acetylation (K28), 1 mono-methylation (R9), 1 di-methylation (R636) and 1 sumoylation (K238). (B) Computational modeling of the structure of PICALM N-terminal ANTH domain is shown (https://www.rcsb.org/structure/3ZYM (accessed on 1 June 2022)) [66]. PICALM ANTH domain is constituted of 11 α-helices. Three known phosphorylation sites (S16, Y96 and S107) in ANTH domain are highlighted in pink.
The ANTH domain is a membrane-binding domain with specificity for phosphatidylinositol 4,5-bisphosphate (PtdIns(4,5)P2) and acts as a universal adaptor for nucleation of clathrin coats. In silico modeling of N-terminal ANTH domain suggests a structure consisting of 11 α-helices [60] (Figure 3B). The PtdIns(4,5)P2 binding residues (Kx9Kx(K/R)(H/Y) are present on several helices. The PtdIns(4,5)P2 binding sites contain evolutionally conserved basic amino acid residues (K28, K38, K40 and K41, numbering according to the PICALM isoform of 652 amino acids) forming a positively charged patch on the surface to interact with phosphates of PtdIns(4,5)P2 [64]. The ANTH domain also contains the binding region for R-SNARE proteins, and PICALM can bind simultaneously PtdIns(4,5)P2 and VAMP8 [60]. PICALM interacts with R-SNARE proteins via its hydrophobic amino acid residues such as L219, F240, M244, I272 and L251 [60]. A recent study has shown that the ANTH domain directly binds to ubiquitin, and the ANTH domain can act as an adaptor for ubiquitinated protein [67].
In the C-terminal clathrin adaptor domain of PICALM contains critical motifs that allow PICALM to interact with clathrin and other endocytic machinery proteins. The clathrin binding motif is DLLDLQ (392–397 in the isoform of 652 amino acids). AP2-binding motifs are DIF (D375, I376 and F377), DPF (D420, P421 and F422) and FESVF (F489, E490, S491, V492 and F493). The asparagine-proline-phenylalanine (NPF) motif binds to Eps15 homology (EH) domains found in proteins associated with endocytosis and vesicle trafficking. Since exon 13 contains one DPF (AP2 binding) and one NPF (EH domain binding) motif, the splice variant without exon 13 is supposed to have reduced interaction with AP2 and other protein partners owning EH-domains [43].
2.3. Post-Translational Modifications of PICALM
PICALM undergoes several post-translational modifications (PTM), such as phosphorylation, methylation, ubiquitination, sumoylation and acetylation [65,66,68,69,70,71,72,73,74]. These post-translational modifications have been identified on numerous amino acid residues of PICALM: 41 phosphorylation, 15 ubiquitination, 1 acetylation, 1 mono-methylation, 1 di-methylation and 1 sumoylation sites have been described so far. Certain factors are known to induce some of the post-translational modifications on PICALM, such as phosphorylation by Aβ treatment in primary neuronal culture [75], by BI2536, a selective inhibitor of protein kinase Polo-Like Kinase 1 (PLK1), inducing phosphorylation (Y138, T146 and S537) [76], by ischemia (S303 and S315) [77] and by nocodazole, an inhibitor of microtubule polymerization (S474 and S645) [78]. The most well-characterized PTM of PICALM are the four phosphorylation sites (S16, Y96, S107 and S303) and two ubiquitination sites (K318 and K324) that have been validated by mass-spectrometry and have been described for more than 5 times [65]. While the phosphorylation on the ANTH domain may influence the interactions of PICALM with PtdIns(4,5)P2 or R-SNARE proteins, the other phosphorylation residues are located in the C-terminal clathrin adaptor domain exhibiting pivotal functions in protein–protein interaction of PICALM with clathrin and other endocytic machinery proteins. Therefore, it is speculated that phosphorylation of PICALM should play a role in regulating interaction with PtdIns(4,5)P2 and other protein binding partners. For instance, the R-SNARE binding domain contains several sites that can undergo PTM, such as one phosphorylation (Y237), two ubiquitination (K231 and K252) and one sumoylation (K238), and thus, these PTM may regulate the interaction between PICALM and R-SNARE.
PICALM is a substrate of calpain and caspase [33,79,80]. Nevertheless, it remains largely unknown if the phosphorylation and ubiquitination of PICALM affect its protein stability or degradation.
Given that PICALM contains more than 130 putative phosphorylation sites, PICALM phosphorylation may be dysregulated in AD brains not only in terms of its global phosphorylation level but also in the pattern of site-specific phosphorylation. Indeed, Aβ exposure increases PICALM phosphorylation in primary rat neuronal cortical culture [75] though phosphorylation sites and the involved kinases remain to be identified. While it is speculated that phosphorylation may change the interaction of PICALM with its binding partners such as R-SNAREs, PtdIns(4,5)P2 or clathrin, it remains elusive which kinases are involved in PICALM phosphorylation, whether PICALM phosphorylation is misregulated during the progression of AD, whether PICALM phosphorylation has any impact on its protein stability/degradation or its subcellular localization and whether phosphorylation of PICALM may cause a loss of function and/or a gain of toxicity.
3. PICALM and Alzheimer’s Disease Pathology
3.1. PICALM Gene and AD
3.1.1. Gene Location and Genetic Variants of PICALM
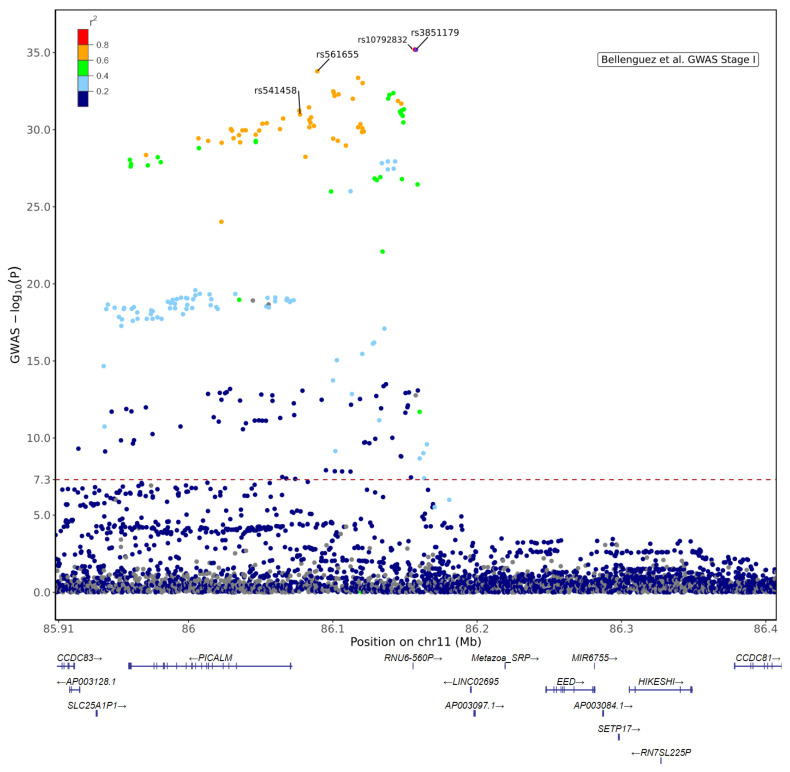
In humans, the PICALM gene is located on chromosome 11q14 (Figure 4). Since several independent GWAS have reported the involvement of PICALM SNPs in LOAD susceptibility, many other studies have replicated and confirmed the association of PICALM genetic variants with LOAD in diverse cohorts (see review [44]). There have been several LOAD-associated SNPs identified within or near the PICALM gene so far (Table S1). A recent large GWAS (n = 788,989) suggests that the most significant SNP associated with LOAD susceptibility is rs3851179, located in the intergenic regions between PICALM and EED [17].
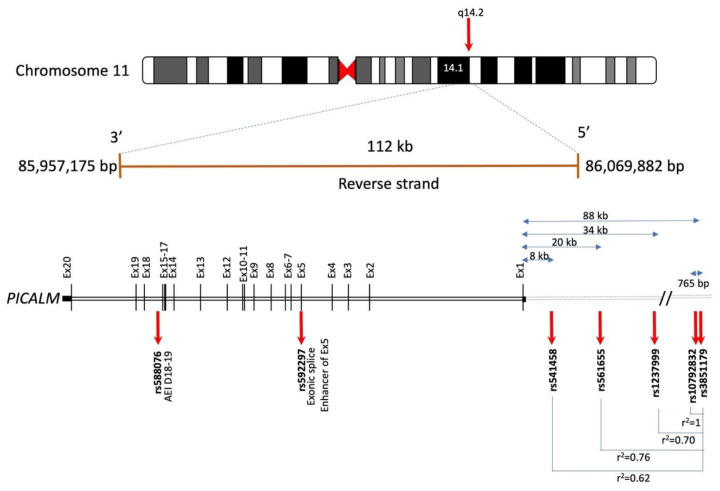
Figure 4.
Schematic illustration of PICALM genomic location and some of the LOAD-associated SNPs based on the information available in Gene database (https://genome.ucsc.edu/ (accessed on 1 June)) and e!Ensembl (http://www.ensembl.org/ (accessed on 1 June 2022) (accession number ENSG00000073921, GRCh38:CM000673.2). Human PICALM gene spans 112 kb on chromosome 11q14 and encodes 20 exons. The most significant LOAD-associated SNP is rs3851179, located approximately 88 kb upstream of PICALM in the 5′ intergenic region [17]. rs10792832 is 765 bp from rs3851179 and in full linkage disequilibrium (LD) with rs3851179 (r2 = 1). rs1237999, located 34 kb upstream of PICALM, is localized in a CCCTC binding factor in a regulatory region [81]. rs561655 is the lead SNP of PICALM locus in [82], located 20 kb upstream of PICALM gene, a putative transcription factor binding site [15] and in LD with rs3851179 (r2 = 0.76). rs541458, located in 8 kb upstream of PICALM gene, is also genome-wide significant [15] and is in LD with rs3851179 (r2 = 0.62). rs592297 is located in exon 5 and is an exonic splice enhancer [29]. rs588076 is located in the intron 17 of PICALM and is associated with the allelic expression imbalance (AEI) in PICALM isoform lacking exons 18 and 19 (D18-19) [83].
rs3851179T is a protective allele reported to reduce AD risk by 9–29% among various cohorts [81]. rs3851179 is located in a region 88 kb upstream of the PICALM gene (Figure 4) and is likely involved in transcription factor binding [84].
rs3851179 is in linkage disequilibrium (LD) with other genome-wide significant SNPs associated with LOAD, such as rs10792832 [24], rs1237999 [81], rs561655 [82] and rs541458 [15] (r2 = 1, r2 = 0.7, r2= 0.762 and r2 = 0.622, respectively) (Figure 5; Table S1).
Figure 5.
Regional plot for PICALM locus based on Stage I results from [17] (Accession No GCST90027158, https://www.ebi.ac.uk/gwas/Study (accessed on 1 July 2022)). The plot shows the lead variant in Stage I + II meta-analysis, rs3851179 and +/− 250 kb extended coordinates for n = 3988 variants in chr11:85,907,598–86,407,598 (GRCh38). LD reference data derived from 1000 Genomes Project phased biallelic SNV and INDEL autosomal genetic variants called de novo GRCh38 (for selected n = 404 unrelated Non-Finnish European (NFE) sample) [85]. The lead variant rs3851179 is located in the intergenic region between PICALM and EED in which there are other non-coding genes (RNU6-560P, LINC02695 and Metazoa SRP) and a contigs (AP003097). rs10792832 (red) is in almost full LD with rs3851179. Other genome-wide significant SNPs, such as rs561655 [82,86] and rs541458 [15,87], are also in high LD with rs3851179. The red dashed line indicates the genome-wide significance threshold (p = 5 × 10−8).
There are no LOAD-associated coding variants of PICALM [29]. Two rare nonsynonymous coding variants in PICALM (rs147556602 (p.P495A) and rs117411388 (p.H458R)) have been reported, although their association with AD remains unclear [88]. Computational analyses suggest that rs561655 and rs573167, located in the intergenic region, have significant deleterious effects based on combined annotation-dependent depletion (CADD) scores (CADD 17.79 for rs561655 and 15.63 for rs573167) [89].
Furthermore, two PICALM SNPs, not genome-wide significant, are known to be involved in splicing regulation (Figure 4). rs592297, located in exon 5, is an exonic splice enhancer and is associated with PICALM lacking exons 2–4 [30]. rs588076, located in PICALM intron 17, is associated with allelic expression imbalance (AEI) of the PICALM isoform lacking exon 18-19 [83].
3.1.2. PICALM and Quantitative Trait Loci (QTL)
Analyses on expression quantitative trait loci (eQTL) suggest that there may be a cell-type specific effect of the PICALM variant, notably in microglia. Several recent PICALM eQTL analyses have consistently suggested that there is a significant colocalization between AD GWAS signals and PICALM eQTL in microglia [90,91,92,93]. PICALM rs10792832 (in almost full LD with rs3851179) is located in an open chromatin region (OCR), and the non-protective variant was associated with both lower OCR signal and gene expression by regulating chromatin accessibility [91].
Microglia may not be the only cell type affected by PICALM variants. The protective rs3851179T allele is linked to increased PICALM expression in the brain [81,94], especially implicated in PICALM expression in endothelial cells [30,34]. The rs3851179T allele is associated with increased PICALM mRNA and protein expression in induced pluripotent stem cells (iPSC) differentiated into endothelium compared to isogenic cells bearing non-protective rs3851179C [34]. The non-protective rs3851179C allele is associated with a significant reduction in PICALM isoform 2 mRNA expression in the frontal and in temporal cortex [94].
Alternative splicing of PICALM is significantly changed in AD brains in relation to LOAD-susceptibility variants [41]. Differential excision of introns in the PICALM gene was observed in the dorsolateral prefrontal cortex (DLPFC) of AD brains in correlation with the GWAS signal [41]. Raj and colleagues observed such alteration of alternative splicing in iPSC-derived neurons overexpressing tau, suggesting that accumulation of tau drives abnormal alternative splicing of key AD genes, including PICALM.
It has to be noted that the nearest coding gene of rs3851179 is EED [17]. A colocalization of the EED function association has been reported [95]. It remains elusive if EED is also involved in AD pathogenesis.
These data support the hypothesis that PICALM is the risk gene in the locus and that the potential effect for AD risk is, at least partially, via modulation of microglial PICALM expression.
3.1.3. PICALM Variants and Phenotypes Relevant to AD
Studies have shown significant associations of some of the SNPs in the PICALM locus with phenotypes relevant to AD, such as the age of onset, cognitive functions, information processing speed, spontaneous brain activity, hippocampal atrophy and Aβ or tau levels in cerebrospinal fluid (CSF) (Table 1). Some SNPs are not genome-wide significant to LOAD (rs510566, rs592297, rs17148741) but showed statistically significant association with hippocampal atrophy or Aβ and tau levels in cerebrospinal fluid (CSF) [81,96].
Table 1.
Association between PICALM SNPs and AD-related phenotypes. The location of SNPs is based on GRCh38. Linkage disequilibrium to rs3851179 is denoted as r2. Effect size shows beta coefficients. SE: standard error. NA: non-available. Hip: hippocampus. EC: entorhinal cortex.
| SNP | Location | GWAS p | r2 | Phenotype | Sample Size | Effect Size (SE) | p-Value | Reference |
|---|---|---|---|---|---|---|---|---|
| rs510566 | 85966796 | 5.669 × 10−05 | 0.042 | Risk G allele is associated with lower CSF Aβ42, higher pTau and pTau/Aβ42 ratio | AD: n = 114, MCI: n = 395, Ctrl: n = 203 | NA | p = 0.048, p = 0.0006, p = 0.0006 | [81] |
| rs510566 | 85966796 | 5.669 × 10−05 | 0.042 | Risk G allele is associated with hippocampal atrophy | AD: n = 141, MCI: n = 461, Ctrl: n = 235 | NA | p < 0.0001 | [81] |
| rs592297 | 86014894 | NA | NA | C allele was associated with faster atrophy rate of hippocampus | AD: n = 141, MCI: n = 461, Ctrl: n = 235 | NA | p < 0.0045 | [81] |
| rs592297 | 86014894 | NA | NA | C allele was associated with slower atrophy rate of posterior cingulate in MCI | AD n = 39, MCI n = 422, Ctrl n = 257 | NA | p < 0.05 | [97] |
| rs17148741 | 86054749 | 0.07777 | 0.017 | C allele is associated with hippocampal volume | n = 1400 | NA | p = 9.4 × 10−5 | [96] |
| rs541458 | 86077309 | 1.032 × 10−31 | 0.622 | Risk T allele is associated with decreased CSF Aβ | n = 412 | NA | p = 0.002 | [98] |
| rs541458 | 86077309 | 1.032 × 10−31 | 0.622 | Differential modulation of spontaneous brain activity (C vs. TT) | MCI n = 35, Ctrl n = 26 | NA | p < 0.05 | [99] |
| rs541458 | 86077309 | 1.032 × 10−31 | 0.622 | Risk T allele is associated with an earlier age at midpoint of cognitive decline | n = 1831 | NA | NA | [100] |
| rs1237999 | 86103988 | 5.091 × 10−33 | 0.696 | A allele is associated with lower CSF Aβ42, higher Tau, pTau, pTau/Aβ42 ratio | AD: n = 114, MCI: n = 395, Ctrl: n = 203 | NA | p = 0.042, p = 0.030, p = 0.011, p = 0.045 | [81] |
| rs543293 | 86109035 | 1.07 × 10−29 | 0.637 | A allele is associated with atrophy rate of posterior cingulate in MCI | AD n = 39, MCI n = 422, Ctrl n = 257 | NA | p < 0.05 | [97] |
| rs10501610 | 86133444 | 1.186 × 10−12 | 0.082 | T allele is associated with slower rise of CSF pTau and pTau/Aβ42 ratio | AD: n = 114, MCI: n = 395, Ctrl: n = 203 | NA | p = 0.0001 | [81] |
| rs2888903 | 86143134 | 1.14 × 10−28 | 0.396 | Major allele is associated with earlier age of onset of AD in individuals with down syndrome | n = 67 | 3.31 | p = 0.011 | [101] |
| rs7110631 | 86145145 | 1.367 × 10−32 | 0.740 | Risk G allele is associated with an age-dependent cognitive decline | n = 1564 | NA | p = 0.03 | [102] |
| rs7941541 | 86147496 | 2.052 × 10−32 | 0.740 | Major allele is associated with earlier age of onset of AD in individuals with down syndrome | n = 67 | Beta: 3.92 | p = 0.016 | [101] |
| rs10751134 | 86147845 | 1.618 × 10−27 | 0.483 | Major allele is associated with earlier age of onset of AD in individuals with down syndrome | n = 67 | Beta: 2.78 | p = 0.040 | [101] |
| rs3851179 | 86157598 | 6.496 × 10−36 | 1 | Protective T allele is associated with increased volume of hippocampus and entorhinal cortex | AD n = 168, MCI n = 357, Ctrl n= 215 | Beta: Hip: 0.061 (0.029), EC: 0.066 (0.021) |
Hip p = 0.04, EC n = 0.01 | [103] |
| rs3851179 | 86157598 | 6.496 × 10−36 | 1 | Protective T allele is associated with slower atrophy rate of hippocampus | AD n = 205, MCI n = 639, Ctrl n = 339 | NA | p < 0.05 | [104] |
| rs3851179 | 86157598 | 6.496 × 10−36 | 1 | Non-protective C allele is associated with earlier age of onset of AD | n = 2569 | NA | p = 0.0086 (1 side) | [105] |
| rs3851179 | 86157598 | 6.496 × 10−36 | 1 | Protective T allele is associated with better cognitive function in the oldest old | n = 1369 | Beta: 0.60 | p = 0.024 | [106] |
| rs3851179 | 86157598 | 6.496 × 10−36 | 1 | Protective T allele is associated with faster information processing speed | n = 87 | NA | p < 0.05 | [107] |
| rs3851179 | 86157598 | 6.496 × 10−36 | 1 | Protective T allele is associated with larger entorhinal cortical thickness | AD n = 245, MCI n = 434, Ctrl n = 284 | Beta: 0.043 | p = 0.034 | [108] |
PICALM rs541458T risk allele is associated with decreased Aβ in CSF [98]. A potential association of rs541458 was reported with the spontaneous brain activity of amnestic MCI [99] and with the cognitive performance of non-demented Chinese in an age-dependent manner [109].
Xu et al., reported that rs1237999, located 34 kb upstream of PICALM, is localized in a CCCTC binding factor in a regulatory region (Figure 4). rs1237999 in LD with rs3851179 and is associated with the levels of Aβ, tau and the ratio of phosphotau/Aβ in CSF [81].
Interestingly, rs10501610 is not in LD with rs3851179 (r2 = 0.082), but genome-wide significant (p = 1.186 × 10−12) and is associated with CSF biomarkers [81]. rs2888903, rs7941541 and rs10751134 are associated with the age of onset or cognitive function [101]. rs7110631 in LD (r2 = 0.740) is associated with age-dependent cognitive decline [102].
There is a significant association of PICALM rs3851179T protective allele with the change rate of adjusted hippocampal volume [103,104] and entorhinal cortex thickness [103,108] measured by magnetic resonance imaging (MRI). rs3851179T is associated with better cognitive functions in the oldest old [106], metabolic connectivity [110], and default mode network function in mild cognitive impairment (MCI) [111]. Non-protective rs3851179C allele is associated with the decline in the information processing speed [112] and earlier onset of AD [105].
It has to be noted that some studies have a rather small sample size (Table 1) and may be underpowered. Further replication of these studies in larger cohorts is warranted. Although correlation does not imply causation, these reports support the hypothesis that PICALM variants are associated with phenotypes relevant to AD.
3.2. PICALM and Aβ Pathology
3.2.1. PICALM and APP Processing
Given that the generation of Aβ peptides from APP and its accumulation are pivotal events in AD pathogenesis, researchers have sought to identify the role of PICALM on Aβ pathology. Multiple lines of evidence suggest that PICALM regulates APP processing [35,113,114,115] and Aβ transcytosis [34].
Tian et al., have reported a rather protective role of PICALM in APP metabolism. PICALM forms a complex with APP-CTF together with AP2 and microtubule-associated protein 1 light chain 3 (LC3), an autophagy marker [115]. The interaction of APP-CTFs, PICALM, AP2 and LC3 results in transporting APP-CTFs into the autophagosome in which APP-CTFs are degraded by autophagy. The interaction between APP-CTFs and PICALM consequently leads to a reduction of Aβ production [114]. This critical role of PICALM in autophagy is strengthened by another study showing that loss of exon 1 of PICALM in HeLa cells leads to an augmentation of lysosomal enzymes in the endosome-enriched fraction and accumulation of autophagy substrates such as APP-CTFs [48].
PICALM regulates APP processing via γ-secretase internalization due to a direct interaction between the PICALM ANTH domain and Nicastrin, one of the components of the γ-secretase complex [116]. Intriguingly, PICALM itself modulates APP-CTF production via controlling BACE1 expression [94].
In addition, PICALM has been reported to be a regulator of PICALM/clathrin-dependent internalization of Aβ bound to the low-density lipoprotein-receptor-related protein-1 (LRP1), a major Aβ receptor, leading to Aβ transcytosis and clearance [34].
As there are multiple implications of PICALM in the regulation of Aβ pathology (autophagy, APP processing, transcytosis), it is not surprising that there are some contradictory observations in transgenic models of amyloid pathology with reduced expression of PICALM. One study has shown a significant increase in amyloid load in PICALM+/− crossed with APPsw/0 and observed a 3.5-4-fold increase in Aβ load in the 9-month-old APPsw/0PICALM+/− mouse brain [34]. On the other hand, Kanatsu et al., observed a significant reduction of Aβ load in 12-month-old A7/PICALM+/− mouse brains. Further, Xiao et al., have reported that PICALM is colocalized with APP and PICALM expression level has a significant impact on Aβ level in APP/PS1 mice injected with adeno-associated virus (AAV)-mediated modulation of PICALM levels. While PICALM overexpression increases APP internalization and Aβ production, PICALM knockdown decreases amyloid load [35]. Furthermore, drosophila PICALM orthologue LAP overexpression did not alter the Aβ42 level in a drosophila model overexpressing Aβ42 [117]. The discrepancy of the observation on Aβ production may be related to the different transgenes with distinct mutations and the differences in amyloid doses in the models analyzed. These contradictory data imply that the effect of PICALM reduction can differ depending on the progression of Aβ pathology. Further studies are necessary to determine the function of PICALM in APP processing by using a model close to the physiological conditions of human LOAD to achieve clinical relevance.
3.2.2. PICALM and Aβ-Mediated Neurotoxicity via Glutaminergic Neurotransmission
In the yeast model, PICALM orthologue was identified as a protective modifier of Aβ toxicity [118]. Overexpression of YAP1802, the yeast orthologue of PICALM, partially rescues Aβ-induced toxicity without affecting the secretory pathway. Treusch et al., confirmed the rescuing effect of PICALM in C. elegans and rat cortical neuronal culture: overexpression of unc-11, the C. elegans orthologue of PICALM, rescues Aβ-induced glutaminergic neuron loss in C. elegans and lentiviral PICALM overexpression diminished Aβ-induced neuron loss in primary culture of rat cortical neurons. On the contrary, D’Angelo and colleagues observed that mammalian PICALM enhanced Aβ-induced toxicity in a yeast model overexpressing Aβ tagged with GFP [119]. These inconsistent observations are partly due to the heterogeneity of the Aβ species and the distinct expression level of Aβ. In addition, Aβ overexpression in these bio-engineered yeast models was driven by transgene encoding Aβ, and thus, the nature of overexpressed Aβ should differ from the Aβ peptides derived from successive cleavages of APP in a physiological/pathophysiological condition in human brains.
It is noteworthy that Aβ is not co-immunoprecipitated with PICALM in human brains, and PICALM immunoreactivity is never observed in the center of amyloid plaques [32,33,34,35]. Thus, PICALM may presumably play roles in Aβ-induced toxicity in an indirect manner. One of the candidate intermediates between PICALM and Aβ is N-methyl-d-aspartate receptor (NMDAR). Glutaminergic neurons are the most vulnerable subtype of neurons in AD, and soluble Aβ enhances glutamate release, leading to excessive activation [120,121]. Whilst NMDAR is critical for synaptic plasticity and neuronal survival, excessive NMDAR activation leads to excitotoxicity and cell death [122]. A recent study in the drosophila model of AD has suggested that overexpression of LAP, the drosophila PICALM orthologue, rescues Aβ42-induced toxicity without affecting Aβ42 level [117].
3.3. PICALM and Tau
3.3.1. PICALM Expression and Localization in AD Brains
Different lines of evidence suggest that PICALM expression is dysregulated during the progression of AD. Under a physiological condition in the central nervous system (CNS), PICALM is ubiquitously expressed in various cell types such as endothelial, neuronal and microglial cells [30,32,33,34,35,36,123]. In AD postmortem brains, however, PICALM may undergo an alteration of expression levels and protein localizations in distinct cell types.
During the progression of AD, PICALM mRNA expression levels are increased in the brain [32,124] and in the blood [125] of AD patients. In contrast, the PICALM protein level in the total bulk tissue is decreased in AD brains [33]. Another study has shown that PICALM protein level is decreased in the isolated microvessel fraction of AD brains but is not significantly altered in the microvessel-depleted fraction of AD brains [34]. In line with this discordant observation, Tasaki et al., have recently reported that there is a general divergence between the levels of transcripts (mRNA) and protein expression in postmortem AD brain tissue. By mass-spectrometry, they observed a significant reduction of PICALM protein in a correlation with 14.3% faster cognitive decline [124].
Most of the analyses made on PICALM expression were conducted using human postmortem tissues of the affected brain regions that contain different cell types. Single-cell transcriptomic studies have shown that groups of GWAS hit genes, including PICALM, are generally upregulated in microglial cells in correlation with neuronal AD pathology [126]. While PICALM immunoreactivity in microglial cells is increased in LOAD brains [33], PICALM immunolabelling is decreased in endothelial cells [30], and PICALM protein level assessed by Western blot is decreased in the microvessel fraction of postmortem AD brain tissues [34]. Neuronal PICALM labeling is increased in AD brains [33,34] and is associated with NFTs (Figure 6A–C) [33]. PICALM and hyperphosphorylated tau are co-immunoprecipitated in AD brain lysate [33]. Since pathological tau in NFTs is ubiquitinated [127], and the ANTH domain of PICALM has an affinity to ubiquitin [67], the interaction between PICALM and tau needs to be further analyzed in non-pathological and pathological conditions to determine the role of ubiquitin to form tau–PICALM complex. Other studies have shown immunoreactivities of PICALM interactors such as PtdIns(4,5)P2 [123,128] and AP2A1 [129] in association with phosphotau in NFTs and dystrophic neurites. Of note, PICALM is not the only LOAD GWAS hit protein detected in NFTs: some other proteins encoded by GWAS hits genes are also linked with tau pathologies such as APOE4 [130], CLU [131], BIN1 [132], TPK2B [133] and AP2A1 [129].
Figure 6.
PICALM immunolabelling is increased in LOAD and FAD in correlation with the development of tau pathology. Representative immunolabelling of anti-PICALM antibody (Sigma #HPA019053) in the Cornu Ammonis regions of the hippocampus of control (A,D), LOAD (B,E) and FAD with PSEN1 mutations at R35E and E120D (C,F). In non-demented control brain, a perinuclear immunolabelling of PICALM was detected in neurons (black arrows) (A). A PICALM immunoreactivity was detected in NFTs in neuronal perikarya (blue arrows) in the affected brain areas of LOAD (B) and FAD (C). PICALM-immunoreactive microglial cells were faintly detected in control brains (arrowheads) (D), and the number and the intensity of PICALM-stained microglia were increased in LOAD (D) (arrowheads). In FAD (F) brains, the increase in PICALM immunostained microglial cells was less prominent than in LOAD. More detailed data can be found in our article [33]. Control (Ctrl), Late-onset Alzheimer’s disease (LOAD), Familial Alzheimer’s disease (FAD). Scale bar 20 µm.
3.3.2. PICALM in Primary Tauopathy Brains
To our knowledge, there is no report showing alteration of PICALM mRNA expression levels in primary tauopathy brains; however, PICALM protein localization is altered in primary tauopathies with no amyloid pathology [134]. In postmortem human tissues, PICALM immunoreactivity is associated with pathological tau in the AD brain [33] and in other tauopathies such as PSP and Pick disease [134]. In NFTs, PtdIns(4,5)P2 and PICALM are colocalized [123,128]. PICALM protein level is decreased in the affected brain areas of various tau-related neurodegenerative diseases, including AD, FTLD-tau-MAPT, corticobasal degeneration (CBD) and Lewy body dementia (LBD) diffuse type with tau pathology [33,134,135]. A significant negative correlation was observed between PICALM protein level and hyperphosphorylated tau or autophagy initiation marker Beclin1 in the postmortem human frontal cortex of these neurodegenerative diseases [134]. Such reduction of PICALM seems related to the fact that PICALM is a substrate of both calpain and caspase-3 [33,79,80] that are activated in the brains of AD and other tauopathies [136,137,138,139,140].
3.3.3. PICALM and Tau in Cellular and Animal Models
PICALM modulates tau degradation via both autophagosome formation and autolysosomal fusion in several experimental models, such as cultured cells, drosophila and zebrafish [51]. Knockdown of PICALM caused accelerated accumulation of phosphotau in HeLa cells transiently transfected with DsRed-Tau 4R (four repeats) by impairing autophagy. Nonetheless, they observed in the same study that PICALM overexpression drove tau-mediated neurodegeneration in zebrafish [51]. Thus, they observed that both knockdown and overexpression of PICALM accelerated neurodegeneration in the presence of tau pathology.
Studies in PICALM+/− mice suggest that 50% of PICALM reduction did not trigger a clear motor phenotype or any detectable tau pathology for at least up to 12 months [54,135]. On the contrary, PICALM heteroinsufficiency aggravates tau pathology in a tau transgenic mouse model. By crossing Tg30 overexpressing human double mutant tau (P301S and G272V) transgenic mice developing an age-dependent tau pathology [141] with PICALM+/− mice [54], we generated a novel transgenic mouse line Tg30xPICALM+/− [135]. Tg30xPICALM+/− exhibited an aggravation of motor deficits and tau pathology, suggesting that a reduction of PICALM in the presence of tau pathology may induce deficits in the autophagy pathway [135]. It should be emphasized that this model develops a tau pathology at least partially in a cell-autonomous manner due to a tau transgene bearing double FTLD-related mutations [141]. Since MAPT gene mutations are not linked to AD, further studies are required to decipher the role of PICALM in the development and propagation of non-mutant tau.
3.3.4. PICALM and Tau Pathology Propagation
In AD brains, tau pathology develops in a stereotypical manner across neuroanatomically connected networks [142]. Given that tau pathology propagates, at least partially, via clathrin-mediated endocytosis (CME) [143], a clathrin adaptor such as PICALM may be involved in clathrin-mediated tau internalization.
In addition to the prominent role of LRP1 in Aβ clearance [144], recent studies suggest the involvement of LRP1 in regulating tau seeding and internalization [145]. LRP1 is expressed in neuronal, glial and endothelial cells [146]. The affinity of the tau microtubule-binding domain to LRP1 is high under physiological conditions, while hyperphosphorylation of tau leads to a reduction of its binding affinity to LRP1 [147]. Therefore, their interaction may be weaker in AD brains. Since PICALM directly binds to LRP1 to regulate the internalization of LRP1 [34], it can be speculated that PICALM modulates pathological tau spreading via LRP1-mediated pathological tau internalization.
PICALM also modulates cholesterol biogenesis and lipoprotein uptake (see Section 2.1.2). Cholesterol is implicated in amyloid pathology [148] and is detected in amyloid plaques [149]. Since cholesterol depletion promotes tau uptake and aggregation [150], PICALM-mediated misregulation of cholesterol homeostasis may be related to both amyloid and tau pathology progression.
PICALM is involved in iron uptake via transferrin (see Section 2.1.2). Iron in Fe3+ (redox-inert state) is detected in NFTs of AD [151] and is implicated in tangle formation [152] and in APP processing [153]. Given PICALM is misregulated in AD brains, the PICALM-mediated iron uptake may be involved in AD pathogenesis.
A study using the HEK tau biosensor cell model [154] has suggested no effect of deletion of GWAS hit genes, including PICALM, on the FRET-based signal of tau-tau interaction [155]. Since a lipofectamine-based method was used for PHF internalization, Kolay et al., focused on the effect of a GWAS hit gene depletion on FRET-based tau-tau interaction but not on the effect on the “physiological” uptake of PHF. It remains elusive if PICALM overexpression modulates PHF uptake or tau-tau interaction. Further studies are necessary to assess the role of PICALM in modulating the prion-like tau pathology propagation in in vitro and in vivo models.
3.4. PICALM and Glial Cells
Evidence suggests that PICALM immunoreactivity in microglial cells is increased in the postmortem hippocampus of LOAD compared to non-demented controls. Such a striking increase was not evident in human FAD brains (Figure 6D–F) [33]. These observations are in line with recent studies showing the potential involvement of glial cells in AD pathogenesis [156]. Indeed, single cell transcriptome from postmortem AD brains suggests that a group of genes, including PICALM, APOE, TREM2, MEF2C and the MHC class II genes HLA-DRB1 and HLA-DRB5 are upregulated in microglial cells in correlation with AD pathology [126].
APOE4 carriers exhibit endocytic defects before significant amyloid deposition [157]. PICALM is described as a modifier of APOE4-induced endocytic defects in human iPSC-derived astrocytes and in yeast. PICALM and its yeast orthologue YAP1802 overexpression rescued APOE4-induced endosomal abnormalities in human iPSC-derived astrocytes and yeast, respectively [158]. Nonetheless, PICALM immunoreactivity is hardly detected in astrocytes in postmortem human brain tissues [33], and thus, the implication of PICALM in astrocytic endocytosis might be an adjunct contributor to AD pathogenesis.
Elevated levels of reactive oxygen species (ROS) induce neuronal lipid synthesis that leads to the sequestration of glial lipid droplets (LD) to delay neurotoxicity [159]. PICALM modulates this neuroprotective process of neuron-to-glia lipid transport. PICALM orthologue LAP knockdown in drosophila glia leads to a significantly reduced LD formation. PICALM knockdown in rat astrocytes leads to reduced uptake of neuron-derived fatty acids [159]. PICALM and other proteins encoded by LOAD GWAS hits (APOE, BIN1, CD2AP, AP2A2 and RIN3) are thus involved in LD formation and/or neuron-glial lipid transfer pathway, and knockdown of PICALM leads to blunted lipid transfer.
4. Discussion
4.1. Difficulties in Interpreting Expression Levels of PICALM in AD Brains
Cautions need to be taken to interpret the data about PICALM expression in human autopsy tissues. The results of PICALM expression are often based on the bulk tissue of human postmortem brains [32,33,124]. Since the cell type composition of brain tissue undergoes alterations during the progression of neurodegenerative disease, such bulk data may confound the interpretation of differences in gene expression between AD patients and controls.
In general, there is a widespread heterogeneity between mRNA and protein levels in postmortem human control and AD brains, including PICALM [124]. For instance, increased PICALM transcripts [32] and decreased PICALM protein level [33,34] was observed in AD brains. Due to such an obvious contradiction of PICALM mRNA and protein levels in AD, mRNA data cannot be simply used to predict the protein expression of PICALM. The first possible hypothesis of such divergence is a cellular autoregulatory mechanism with a negative feedback loop [160]. The second possible hypothesis is microRNA (miRNA)-related gene expression regulations. Post-transcriptional repression of mRNA by miRNA has been well-studied in mammalian cells [161]. Studies suggest an important regulatory role of miR-155 in neuroinflammatory conditions, including AD [162]. Interestingly, several independent studies suggest the upregulation of miR-155 in various brain regions of AD patients compared to controls as well as in the brain of transgenic mouse models of Aβ and tau pathologies [163,164]. Indeed, it has been shown that PICALM is regulated by miR-155 in mouse brains [165]. Although there is no obvious effect of miRNA-155 knockout on the expression level of PICALM transcripts in the mouse brains of these models [166], the effect of miRNA-155 overexpression on PICALM (transcript and protein) expression in human AD brains remains elusive. While further study is necessary to better understand the potential regulatory mechanisms of PICALM expression, miR-155 may provide a key pathway to untangle the divergence of mRNA and protein levels of PICALM. It would be intriguing to find correlation between the PICALM level and miR-155 in further studies. In addition, post-transcriptional regulatory effects of other non-coding RNAs, such as long non-coding RNA and circular RNA on PICALM, need to be investigated in AD models. The last but not least hypothesis to explain the divergence of mRNA and protein levels is that PICALM may undergo abnormally accelerated proteolysis in AD brains. Several lines of evidence suggest that PICALM is cleaved by both calpain and caspase [33,79,80], which are activated in AD brains [138]. Moreover, post-translational modifications of PICALM might affect its degradation, stabilization, protein–protein interaction or subcellular localization, yet such effects remain largely elusive. Further studies are thus necessary to decipher the potential abnormalities of PICALM post-translational modifications using 2D Western blot analyses and mass spectrometry from control and AD brain samples to search for AD-specific post-translational modifications of PICALM and their implications in pathology.
4.2. Challenges in Deciphering the Effect of PICALM Variants on Its Expression
Several independent studies have recently reported a significant association of the LOAD-susceptibility PICALM risk variants (rs10792832 and rs3851179 that are in full LD) on the decreased expression in microglial cells [90,91]. It should be highly informative to create AD models in which microglial PICALM expression is modulated in order to unravel the effect of reduced or increased PICALM expression in microglia.
Additionally, in iPSC-derived endothelial cells, the protective rs3851179T allele is associated with increased mRNA and protein expression of PICALM [34] and in bulk tissues when analyzed relative to cell-specific mRNA markers for microvessels and neurons [30]. Given that the effect of rs3851179T is rather modest when normalized to astrocyte marker GFAP (glial fibrillary acidic protein), it becomes more remarkable when normalized to endothelial cell markers or neuronal markers [30]. Therefore, it is possible that PICALM variants may have distinct effects on its expression in different cell types.
To decipher the inconsistencies in mRNA and protein levels of PICALM observed in postmortem human AD brain samples [124], the PICALM levels in each cell type (e.g., microglia, neuron, astrocytes, oligodendrocytes, etc.) should be analyzed in large scale diverse cohorts by high throughput single cell sequencing and further validated by RT-qPCR, Western blotting, mass-spectrometry (in distinct cell-type fractions) and by in situ hybridization and/or immunohistochemistry (in sections) of postmortem human AD brain samples [167]. As shown by Zhao et al., on the functional effect of rs3851179 on PICALM expression in iPSC-derived endothelial cells, it should be interesting to differentiate iPSC isogenic for rs3851179T/C into various cell types to examine the expression level of PICALM in distinct cell types. More work is necessary to increase the number of samples from different cohorts to improve the power of statistical analyses and pursue proteomic analyses considering heterogeneous cell populations in the brain.
4.3. Potential Role of PICALM in Cellular Homeostasis
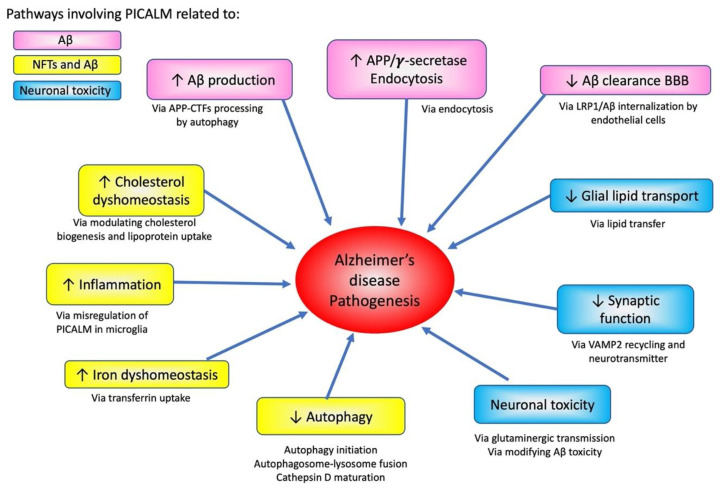
PICALM is involved in various pathological processes, such as Aβ production and transcytosis, as well as tau pathology progression (Figure 7). Given that PICALM plays versatile roles in iron homeostasis, glutaminergic neurotransmission [117], endocytosis, and autophagy, PICALM may as well be implicated in the “cellular phase” of AD proposed by Bart De Strooper and Eric Karran [168]. In this hypothesis, they propose that the accumulation of amyloid and tau pathologies at the biochemical phase is rather tolerated by neuronal cells. However, the clinical signs manifest only when the cellular homeostatic mechanisms fail along with dysfunction of the neurovascular unit, abnormal neuronal network activity and dyshomeostasis of astrocytes and microglial cells. PICALM and other endocytic proteins encoded by AD susceptibility genes may be implicated in such steps that lead to dysfunction and breakdown of finely tuned cellular physiological functions. PICALM may be a small piece of complex endocytic machinery that may be vulnerable to neurodegeneration [169].
Figure 7.
Effects of PICALM on AD pathogenesis pathways and neurodegeneration. PICALM is involved in multiple pathways of AD pathogenesis. PICALM regulates pathways related to APP processing and Aβ-related neurodegeneration (shown in pink). PICALM plays critical role related to both tau and Aβ pathologies, such as cholesterol dyshomeostasis, inflammation, iron dyshomeostasis and autophagy (yellow). PICALM is also crucial for neuronal toxicity via glial lipid transport, synaptic functions, glutaminergic transmission and modifying Aβ-toxicity (blue).
5. Conclusions
Multiple lines of evidence suggest that human PICALM polymorphism modulates AD pathogenesis. PICALM is involved not only in Aβ production and clearance but also in tau-mediated neurodegeneration and in endocytic homeostasis. The exact role of the polymorphism on PICALM expression may vary in different cell types and contribute to multiple pathways in AD pathological mechanisms. Further studies are necessary to decipher the AD-specific post-translational modifications and the functional roles of LOAD-related susceptibility alleles to provide mechanistic insights into the pathological dysregulation of PICALM.
Acknowledgments
We would like to sincerely thank Charles Duyckaerts, Marie-Claude Potier and Pierre Dourlen and Kristel Sleegers for the discussion and collaboration.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/cells11243994/s1, Table S1: Summary statistics for SNPs reaching genome-wide significance. Position, GWAS p-value, linkage disequilibrium (LD). LD reference data derived from 1000 Genomes Project (https://www.nature.com/articles/nature15393 (accessed on 1 June 2022)) phased biallelic SNV and INDEL autosomal genetic variants called de novo GRCh38 (for selected n = 404 unrelated Non-Finnish European (NFE) samples) [85]. PLINK-formatted files were obtained via (http://ftp.1000genomes.ebi.ac.uk/vol1/ftp/data_collections/1000_genomes_project/release/20190312_biallelic_SNV_and_INDEL/ (accessed on 1 June 2022)), processed, annotated quality controlled (HWE p < 1 × 10−6 excluded) and analyzed.
Author Contributions
All the coauthors participated in constructing the concept and writing the manuscript. K.A. and J.-P.B. contributed conception and design of this article. All authors contributed to the manuscript revision. All authors have read and agreed to the published version of the manuscript.
Conflicts of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Funding Statement
This study was supported by grants from the Belgian Fonds de la Recherche Scientifique Médicale to J.-P.B. (2019/0027), the Fund Aline (King Baudouin Foundation) to J.-P.B. (14001), the Foundation for Alzheimer Research (FRA/SAO) to K.L. (2021/0028) and the Génicot Fund of ULB to J.-P.B. and K.L.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Wong C.W., Quaranta V., Glenner G.G. Neuritic Plaques and Cerebrovascular Amyloid in Alzheimer Disease Are Antigenically Related. Proc. Natl. Acad. Sci. USA. 1985;82:8729–8732. doi: 10.1073/pnas.82.24.8729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brion J.P., Couck A.M., Passareiro E., Flament-Durand J. Neurofibrillary Tangles of Alzheimer’s Disease: An Immunohistochemical Study. J. Submicrosc. Cytol. 1985;17:89–96. [PubMed] [Google Scholar]
- 3.Duyckaerts C., Delatour B., Potier M.C. Classification and Basic Pathology of Alzheimer Disease. Acta Neuropathol. 2009;118:5–36. doi: 10.1007/s00401-009-0532-1. [DOI] [PubMed] [Google Scholar]
- 4.Voytyuk I., De Strooper B., Chavez-Gutierrez L. Modulation of Gamma- and Beta-Secretases as Early Prevention against Alzheimer’s Disease. Biol. Psychiatry. 2018;83:320–327. doi: 10.1016/j.biopsych.2017.08.001. [DOI] [PubMed] [Google Scholar]
- 5.Kamenetz F., Tomita T., Hsieh H., Seabrook G., Borchelt D., Iwatsubo T., Sisodia S., Malinow R. App Processing and Synaptic Function. Neuron. 2003;37:925–937. doi: 10.1016/S0896-6273(03)00124-7. [DOI] [PubMed] [Google Scholar]
- 6.Long J.M., Holtzman D.M. Holtzman. Alzheimer Disease: An Update on Pathobiology and Treatment Strategies. Cell. 2019;179:312–339. doi: 10.1016/j.cell.2019.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang Y., Mandelkow E. Tau in Physiology and Pathology. Nat. Rev. Neurosci. 2016;17:22–35. doi: 10.1038/nrn.2015.1. [DOI] [PubMed] [Google Scholar]
- 8.Goedert M. Tau Filaments in Neurodegenerative Diseases. FEBS Lett. 2018;592:2383–2391. doi: 10.1002/1873-3468.13108. [DOI] [PubMed] [Google Scholar]
- 9.Nelson P.T., Alafuzoff I., Bigio E.H., Bouras C., Braak H., Cairns N.J., Castellani R.J., Crain B.J., Davies P., del Tredici K., et al. Correlation of Alzheimer Disease Neuropathologic Changes with Cognitive Status: A Review of the Literature. J. Neuropathol. Exp. Neurol. 2012;71:362–381. doi: 10.1097/NEN.0b013e31825018f7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Braak H., Braak E. Neuropathological Stageing of Alzheimer-Related Changes. Acta Neuropathol. 1991;82:239–259. doi: 10.1007/BF00308809. [DOI] [PubMed] [Google Scholar]
- 11.Clavaguera F., Hench J., Goedert M., Tolnay M. Prion-Like Transmission and Spreading of Tau Pathology. Neuropathol. Appl. Neurobiol. 2014;41:47–58. doi: 10.1111/nan.12197. [DOI] [PubMed] [Google Scholar]
- 12.Buee L., Bussiere T., Buee-Scherrer V., Delacourte A., Hof P.R. Tau Protein Isoforms, Phosphorylation and Role in Neurodegenerative Disorders. Brain Res. Brain Res. Rev. 2000;33:95–130. doi: 10.1016/S0165-0173(00)00019-9. [DOI] [PubMed] [Google Scholar]
- 13.Tanzi R.E. The Genetics of Alzheimer Disease. Cold Spring Harb. Perspect. Med. 2012;2:a006296. doi: 10.1101/cshperspect.a006296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Guerreiro R., Bras J. The Age Factor in Alzheimer’s Disease. Genome Med. 2015;7:106. doi: 10.1186/s13073-015-0232-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Harold D., Abraham R., Hollingworth P., Sims R., Gerrish A., Hamshere M.L., Pahwa J.S., Moskvina V., Dowzell K., Williams A., et al. Genome-Wide Association Study Identifies Variants at Clu and Picalm Associated with Alzheimer’s Disease. Nat. Genet. 2009;41:1088–1093. doi: 10.1038/ng.440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lambert J.C., Heath S., Even G., Campion D., Sleegers K., Hiltunen M., Combarros O., Zelenika D., Bullido M.J., Tavernier B., et al. Genome-Wide Association Study Identifies Variants at Clu and Cr1 Associated with Alzheimer’s Disease. Nat. Genet. 2009;41:1094–1099. doi: 10.1038/ng.439. [DOI] [PubMed] [Google Scholar]
- 17.Bellenguez C., Kucukali F., Jansen I.E., Kleineidam L., Moreno-Grau S., Amin N., Naj A.C., Campos-Martin R., Grenier-Boley B., Andrade V., et al. New Insights into the Genetic Etiology of Alzheimer’s Disease and Related Dementias. Nat. Genet. 2022;54:412–436. doi: 10.1038/s41588-022-01024-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Karch C.M., Goate A.M. Alzheimer’s Disease Risk Genes and Mechanisms of Disease Pathogenesis. Biol. Psychiatry. 2015;77:43–51. doi: 10.1016/j.biopsych.2014.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dourlen P., Kilinc D., Malmanche N., Chapuis J., Lambert J.C. The New Genetic Landscape of Alzheimer’s Disease: From Amyloid Cascade to Genetically Driven Synaptic Failure Hypothesis? Acta Neuropathol. 2019;138:221–236. doi: 10.1007/s00401-019-02004-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Van Acker Z.P., Bretou M., Annaert W. Endo-Lysosomal Dysregulations and Late-Onset Alzheimer’s Disease: Impact of Genetic Risk Factors. Mol. Neurodegener. 2019;14:20. doi: 10.1186/s13024-019-0323-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Maurano M.T., Humbert R., Rynes E., Thurman R.E., Haugen E., Wang H., Reynolds A.P., Sandstrom R., Qu H., Brody J., et al. Systematic Localization of Common Disease-Associated Variation in Regulatory DNA. Science. 2012;337:1190–1195. doi: 10.1126/science.1222794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gallagher M.D., Chen-Plotkin A.S. The Post-Gwas Era: From Association to Function. Am. J. Hum. Genet. 2018;102:717–730. doi: 10.1016/j.ajhg.2018.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Seshadri S., Fitzpatrick A.L., Ikram M.A., DeStefano A.L., Gudnason V., Boada M., Bis J.C., Smith A.V., Carassquillo M.M., Lambert J.C., et al. Genome-Wide Analysis of Genetic Loci Associated with Alzheimer Disease. JAMA. 2010;303:1832–1840. doi: 10.1001/jama.2010.574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lambert J.C., Ibrahim-Verbaas C.A., Harold D., Naj A.C., Sims R., Bellenguez C., DeStafano A.L., Bis J.C., Beecham G.W., Grenier-Boley B., et al. Meta-Analysis of 74,046 Individuals Identifies 11 New Susceptibility Loci for Alzheimer’s Disease. Nat. Genet. 2013;45:1452–1458. doi: 10.1038/ng.2802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhu B., Li L.X., Zhang L., Yang S., Tian Y., Guo S.S., Zhang W., Zhao Z.G. Correlation of Picalm Polymorphism Rs3851179 with Alzheimer’s Disease among Caucasian and Chinese Populations: A Meta-Analysis and Systematic Review. Metab. Brain Dis. 2018;33:1849–1857. doi: 10.1007/s11011-018-0291-6. [DOI] [PubMed] [Google Scholar]
- 26.Kunkle B.W., Grenier-Boley B., Sims R., Bis J.C., Damotte V., Naj A.C., Boland A., Vronskaya M., van der Lee S.J., Amlie-Wolf A., et al. Genetic Meta-Analysis of Diagnosed Alzheimer’s Disease Identifies New Risk Loci and Implicates Abeta, Tau, Immunity and Lipid Processing. Nat. Genet. 2019;51:414–430. doi: 10.1038/s41588-019-0358-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jansen I.E., Savage J.E., Watanabe K., Bryois J., Williams D.M., Steinberg S., Sealock J., Karlsson I.K., Hagg S., Athanasiu L., et al. Genome-Wide Meta-Analysis Identifies New Loci and Functional Pathways Influencing Alzheimer’s Disease Risk. Nat. Genet. 2019;51:404–413. doi: 10.1038/s41588-018-0311-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ensembl. Nov, 2021. [(accessed on 1 June 2022)]. Available online: http://www.ensembl.org/ENSG00000073921.
- 29.Schnetz-Boutaud N.C., Hoffman J., Coe J.E., Murdock D.G., Pericak-Vance M.A., Haines J.L. Identification and Confirmation of an Exonic Splicing Enhancer Variation in Exon 5 of the Alzheimer Disease Associated Picalm Gene. Ann. Hum. Genet. 2012;76:448–453. doi: 10.1111/j.1469-1809.2012.00727.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Parikh I., Fardo D.W., Estus S. Genetics of Picalm Expression and Alzheimer’s Disease. PLoS ONE. 2014;9:e91242. doi: 10.1371/journal.pone.0091242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.The Human Protein Atlas. [(accessed on 1 June 2022)]. Available online: https://www.proteinatlas.org/ENSG00000073921-PICALM/tissue.
- 32.Baig S., Joseph S.A., Tayler H., Abraham R., Owen M.J., Williams J., Kehoe P.G., Love S. Distribution and Expression of Picalm in Alzheimer Disease. J. Neuropathol. Exp. Neurol. 2010;69:1071–1077. doi: 10.1097/NEN.0b013e3181f52e01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ando K., Brion J.P., Stygelbout V., Suain V., Authelet M., Dedecker R., Chanut A., Lacor P., Lavaur J., Sazdovitch V., et al. Clathrin Adaptor Calm/Picalm Is Associated with Neurofibrillary Tangles and Is Cleaved in Alzheimer’s Brains. Acta Neuropathol. 2013;125:861–878. doi: 10.1007/s00401-013-1111-z. [DOI] [PubMed] [Google Scholar]
- 34.Zhao Z., Sagare A.P., Ma Q., Halliday M.R., Kong P., Kisler K., Winkler E.A., Ramanathan A., Kanekiyo T., Bu G., et al. Central Role for Picalm in Amyloid-Beta Blood-Brain Barrier Transcytosis and Clearance. Nat. Neurosci. 2015;18:978–987. doi: 10.1038/nn.4025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Xiao Q., Gil S.C., Yan P., Wang Y., Han S., Gonzales E., Perez R., Cirrito J.R., Lee J.M. Role of Phosphatidylinositol Clathrin Assembly Lymphoid-Myeloid Leukemia (Picalm) in Intracellular Amyloid Precursor Protein (App) Processing and Amyloid Plaque Pathogenesis. J. Biol. Chem. 2012;287:21279–21289. doi: 10.1074/jbc.M111.338376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Petralia R.S., Yao P.J. Ap180 and Calm in the Developing Hippocampus: Expression at the Nascent Synapse and Localization to Trafficking Organelles. J. Comp. Neurol. 2007;504:314–327. doi: 10.1002/cne.21454. [DOI] [PubMed] [Google Scholar]
- 37.Chen W.T., Lu A., Craessaerts K., Pavie B., Frigerio C.S., Corthout N., Qian X., Lalakova J., Kuhnemund M., Voytyuk I., et al. Spatial Transcriptomics and in Situ Sequencing to Study Alzheimer’s Disease. Cell. 2020;182 doi: 10.1016/j.cell.2020.06.038. [DOI] [PubMed] [Google Scholar]
- 38.Chen W.T., Lu A., Craessaerts K., Pavie B., Sala Frigerio C., Corthout N., Qian X., Lalakova J., Kuhnemund M., Voytyuk I., et al. Alzmap. [(accessed on 29 April 2018)]. Available online: https://alzmap.org/
- 39.String. [(accessed on 1 June 2022)]. Available online: https://string-db.org/
- 40.Szklarczyk D., Gable A.L., Lyon D., Junge A., Wyder S., Huerta-Cepas J., Simonovic M., Doncheva N.T., Morris J.H., Bork P., et al. String V11: Protein-Protein Association Networks with Increased Coverage, Supporting Functional Discovery in Genome-Wide Experimental Datasets. Nucleic Acids Res. 2019;47:D607–D613. doi: 10.1093/nar/gky1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Raj T., Li Y.I., Wong G., Humphrey J., Wang M., Ramdhani S., Wang Y.C., Ng B., Gupta I., Haroutunian V., et al. Integrative Transcriptome Analyses of the Aging Brain Implicate Altered Splicing in Alzheimer’s Disease Susceptibility. Nat. Genet. 2018;50:1584–1592. doi: 10.1038/s41588-018-0238-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tebar F., Bohlander S.K., Sorkin A. Clathrin Assembly Lymphoid Myeloid Leukemia (Calm) Protein: Localization in Endocytic-Coated Pits, Interactions with Clathrin, and the Impact of Overexpression on Clathrin-Mediated Traffic. Mol. Biol. Cell. 1999;10:2687–2702. doi: 10.1091/mbc.10.8.2687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Meyerholz A., Hinrichsen L., Groos S., Esk P.C., Brandes G., Ungewickell E.J. Effect of Clathrin Assembly Lymphoid Myeloid Leukemia Protein Depletion on Clathrin Coat Formation. Traffic. 2005;6:1225–1234. doi: 10.1111/j.1600-0854.2005.00355.x. [DOI] [PubMed] [Google Scholar]
- 44.Xu W., Tan L., Yu J.T. The Role of Picalm in Alzheimer’s Disease. Mol. Neurobiol. 2015;52:399–413. doi: 10.1007/s12035-014-8878-3. [DOI] [PubMed] [Google Scholar]
- 45.Miranda A.M., Herman M., Cheng R., Nahmani E., Barrett G., Micevska E., Fontaine G., Potier M.C., Head E., Schmitt F.A., et al. Excess Synaptojanin 1 Contributes to Place Cell Dysfunction and Memory Deficits in the Aging Hippocampus in Three Types of Alzheimer’s Disease. Cell Rep. 2018;23:2967–2975. doi: 10.1016/j.celrep.2018.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Petralia R.S., Wang Y.X., Indig F.E., Bushlin I., Wu F., Mattson M.P., Yao P.J. Reduction of Ap180 and Calm Produces Defects in Synaptic Vesicle Size and Density. Neuromol. Med. 2013;15:49–60. doi: 10.1007/s12017-012-8194-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Miller S.E., Mathiasen S., Bright N.A., Pierre F., Kelly B.T., Kladt N., Schauss A., Merrifield C.J., Stamou D., Honing S., et al. Calm Regulates Clathrin-Coated Vesicle Size and Maturation by Directly Sensing and Driving Membrane Curvature. Dev. Cell. 2015;33:163–175. doi: 10.1016/j.devcel.2015.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hattersley K.J., Carosi J.M., Hein L.K., Bensalem J., Sargeant T.J. Picalm Regulates Cathepsin D Processing and Lysosomal Function. Biochem. Biophys. Res. Commun. 2021;570:103–109. doi: 10.1016/j.bbrc.2021.07.024. [DOI] [PubMed] [Google Scholar]
- 49.Nonet M.L., Holgado A.M., Brewer F., Serpe C.J., Norbeck B.A., Holleran J., Wei L., Hartwieg E., Jorgensen E.M., Alfonso A. Unc-11, a Caenorhabditis Elegans Ap180 Homologue, Regulates the Size and Protein Composition of Synaptic Vesicles. Mol. Biol. Cell. 1999;10:2343–2360. doi: 10.1091/mbc.10.7.2343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ishikawa Y., Maeda M., Pasham M., Aguet F., Tacheva-Grigorova S.K., Masuda T., Yi H., Lee S.U., Xu J., Teruya-Feldstein J., et al. Role of the Clathrin Adaptor Picalm in Normal Hematopoiesis and Polycythemia Vera Pathophysiology. Haematologica. 2015;100:439–451. doi: 10.3324/haematol.2014.119537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Moreau K., Fleming A., Imarisio S., Ramirez A.L., Mercer J.L., Jimenez-Sanchez M., Bento C.F., Puri C., Zavodszky E., Siddiqi F., et al. Picalm Modulates Autophagy Activity and Tau Accumulation. Nat. Commun. 2014;5:4998. doi: 10.1038/ncomms5998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Dreyling M.H., Martinez-Climent J.A., Zheng M., Mao J., Rowley J.D., Bohlander S.K. The T(10;11)(P13;Q14) in the U937 Cell Line Results in the Fusion of the Af10 Gene and Calm, Encoding a New Member of the Ap-3 Clathrin Assembly Protein Family. Proc. Natl. Acad. Sci. USA. 1996;93:4804–4809. doi: 10.1073/pnas.93.10.4804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Klebig M.L., Wall M.D., Potter M.D., Rowe E.L., Carpenter D.A., Rinchik E.M. Mutations in the Clathrin-Assembly Gene Picalm Are Responsible for the Hematopoietic and Iron Metabolism Abnormalities in Fit1 Mice. Proc. Natl. Acad. Sci. USA. 2003;100:8360–8365. doi: 10.1073/pnas.1432634100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Suzuki M., Tanaka H., Tanimura A., Tanabe K., Oe N., Rai S., Kon S., Fukumoto M., Takei K., Abe T., et al. The Clathrin Assembly Protein Picalm Is Required for Erythroid Maturation and Transferrin Internalization in Mice. PLoS ONE. 2012;7:e31854. doi: 10.1371/journal.pone.0031854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Huang F., Khvorova A., Marshall W., Sorkin A. Analysis of Clathrin-Mediated Endocytosis of Epidermal Growth Factor Receptor by Rna Interference. J. Biol. Chem. 2004;279:16657–16661. doi: 10.1074/jbc.C400046200. [DOI] [PubMed] [Google Scholar]
- 56.Harel A., Wu F., Mattson M.P., Morris C.M., Yao P.J. Evidence for Calm in Directing Vamp2 Trafficking. Traffic. 2008;9:417–429. doi: 10.1111/j.1600-0854.2007.00694.x. [DOI] [PubMed] [Google Scholar]
- 57.Mercer J.L., Argus J.P., Crabtree D.M., Keenan M.M., Wilks M.Q., Chi J.T., Bensinger S.J., Lavau C.P., Wechsler D.S. Modulation of Picalm Levels Perturbs Cellular Cholesterol Homeostasis. PLoS ONE. 2015;10:e0129776. doi: 10.1371/journal.pone.0129776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bushlin I., Petralia R.S., Wu F., Harel A., Mughal M.R., Mattson M.P., Yao P.J. Clathrin Assembly Protein Ap180 and Calm Differentially Control Axogenesis and Dendrite Outgrowth in Embryonic Hippocampal Neurons. J. Neurosci. 2008;28:10257–10271. doi: 10.1523/JNEUROSCI.2471-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Koo S.J., Markovic S., Puchkov D., Mahrenholz C.C., Beceren-Braun F., Maritzen T., Dernedde J., Volkmer R., Oschkinat H., Haucke V. Snare Motif-Mediated Sorting of Synaptobrevin by the Endocytic Adaptors Clathrin Assembly Lymphoid Myeloid Leukemia (Calm) and Ap180 at Synapses. Proc. Natl. Acad. Sci. USA. 2012;108:13540–13545. doi: 10.1073/pnas.1107067108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Miller S.E., Sahlender D.A., Graham S.C., Honing S., Robinson M.S., Peden A.A., Owen D.J. The Molecular Basis for the Endocytosis of Small R-Snares by the Clathrin Adaptor Calm. Cell. 2011;147:1118–1131. doi: 10.1016/j.cell.2011.10.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Guo C., Ma Y.Y. Calcium Permeable-Ampa Receptors and Excitotoxicity in Neurological Disorders. Front. Neural Circuits. 2021;15:711564. doi: 10.3389/fncir.2021.711564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Azarnia Tehran D., Kochlamazashvili G., Pampaloni N.P., Sposini S., Shergill J.K., Lehmann M., Pashkova N., Schmidt C., Lowe D., Napieczynska H., et al. Selective Endocytosis of Ca2+-Permeable Ampars by the Alzheimer’s Disease Risk Factor Calm Bidirectionally Controls Synaptic Plasticity. Sci. Adv. 2022;8:eabl5032. doi: 10.1126/sciadv.abl5032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Menzies F.M., Fleming A., Rubinsztein D.C. Compromised Autophagy and Neurodegenerative Diseases. Nat. Rev. Neurosci. 2015;16:345–357. doi: 10.1038/nrn3961. [DOI] [PubMed] [Google Scholar]
- 64.Ford M.G., Pearse B.M., Higgins M.K., Vallis Y., Owen D.J., Gibson A., Hopkins C.R., Evans P.R., McMahon H.T. Simultaneous Binding of Ptdins(4,5)P2 and Clathrin by Ap180 in the Nucleation of Clathrin Lattices on Membranes. Science. 2001;291:1051–1055. doi: 10.1126/science.291.5506.1051. [DOI] [PubMed] [Google Scholar]
- 65.Phoshositeplus. [(accessed on 1 June 2022)]. Available online: https://www.phosphosite.org/
- 66.Hornbeck P.V., Kornhauser J.M., Tkachev S., Zhang B., Skrzypek E., Murray B., Latham V., Sullivan M. Phosphositeplus: A Comprehensive Resource for Investigating the Structure and Function of Experimentally Determined Post-Translational Modifications in Man and Mouse. Nucleic Acids Res. 2012;40:D261–D270. doi: 10.1093/nar/gkr1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Pashkova N., Gakhar L., Yu L., Schnicker N.J., Minard A.Y., Winistorfer S., Johnson I.E., Piper R.C. Anth Domains within Calm, Hip1r, and Sla2 Recognize Ubiquitin Internalization Signals. eLife. 2021;10:e72583. doi: 10.7554/eLife.72583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Larsen S.C., Sylvestersen K.B., Mund A., Lyon D., Mullari M., Madsen M.V., Daniel J.A., Jensen L.J., Nielsen M.L. Proteome-Wide Analysis of Arginine Monomethylation Reveals Widespread Occurrence in Human Cells. Sci. Signal. 2016;9:rs9. doi: 10.1126/scisignal.aaf7329. [DOI] [PubMed] [Google Scholar]
- 69.Mertins P., Mani D.R., Ruggles K.V., Gillette M.A., Clauser K.R., Wang P., Wang X., Qiao J.W., Cao S., Petralia F., et al. Proteogenomics Connects Somatic Mutations to Signalling in Breast Cancer. Nature. 2016;534:55–62. doi: 10.1038/nature18003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Schweppe D.K., Rigas J.R., Gerber S.A. Quantitative Phosphoproteomic Profiling of Human Non-Small Cell Lung Cancer Tumors. J. Proteom. 2013;91:286–296. doi: 10.1016/j.jprot.2013.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Sharma K., D’Souza R.C., Tyanova S., Schaab C., Wisniewski J.R., Cox J., Mann M. Ultradeep Human Phosphoproteome Reveals a Distinct Regulatory Nature of Tyr and Ser/Thr-Based Signaling. Cell Rep. 2014;8:1583–1594. doi: 10.1016/j.celrep.2014.07.036. [DOI] [PubMed] [Google Scholar]
- 72.Shiromizu T., Adachi J., Watanabe S., Murakami T., Kuga T., Muraoka S., Tomonaga T. Identification of Missing Proteins in the Nextprot Database and Unregistered Phosphopeptides in the Phosphositeplus Database as Part of the Chromosome-Centric Human Proteome Project. J. Proteome Res. 2013;12:2414–2421. doi: 10.1021/pr300825v. [DOI] [PubMed] [Google Scholar]
- 73.Zhou H., di Palma S., Preisinger C., Peng M., Polat A.N., Heck A.J., Mohammed S. Toward a Comprehensive Characterization of a Human Cancer Cell Phosphoproteome. J. Proteome Res. 2013;12:260–271. doi: 10.1021/pr300630k. [DOI] [PubMed] [Google Scholar]
- 74.Mertins P., Qiao J.W., Patel J., Udeshi N.D., Clauser K.R., Mani D.R., Burgess M.W., Gillette M.A., Jaffe J.D., Carr S.A. Integrated Proteomic Analysis of Post-Translational Modifications by Serial Enrichment. Nat. Methods. 2013;10:634–637. doi: 10.1038/nmeth.2518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Henriques A.G., Muller T., Oliveira J.M., Cova M., da Cruz E.S.C.B., da Cruz E.S.O.A. Altered Protein Phosphorylation as a Resource for Potential Ad Biomarkers. Sci. Rep. 2016;6:30319. doi: 10.1038/srep30319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kettenbach A.N., Schweppe D.K., Faherty B.K., Pechenick D., Pletnev A.A., Gerber S.A. Quantitative Phosphoproteomics Identifies Substrates and Functional Modules of Aurora and Polo-Like Kinase Activities in Mitotic Cells. Sci. Signal. 2011;4:rs5. doi: 10.1126/scisignal.2001497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Mertins P., Yang F., Liu T., Mani D.R., Petyuk V.A., Gillette M.A., Clauser K.R., Qiao J.W., Gritsenko M.A., Moore R.J., et al. Ischemia in Tumors Induces Early and Sustained Phosphorylation Changes in Stress Kinase Pathways but Does Not Affect Global Protein Levels. Mol. Cell Proteom. 2014;13:1690–1704. doi: 10.1074/mcp.M113.036392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Olsen J.V., Vermeulen M., Santamaria A., Kumar C., Miller M.L., Jensen L.J., Gnad F., Cox J., Jensen T.S., Nigg E.A., et al. Quantitative Phosphoproteomics Reveals Widespread Full Phosphorylation Site Occupancy During Mitosis. Sci. Signal. 2010;3:ra3. doi: 10.1126/scisignal.2000475. [DOI] [PubMed] [Google Scholar]
- 79.Kim J.A., Kim H.L. Cleavage of Purified Neuronal Clathrin Assembly Protein (Calm) by Caspase 3 and Calpain. Exp. Mol. Med. 2001;33:245–250. doi: 10.1038/emm.2001.40. [DOI] [PubMed] [Google Scholar]
- 80.Rudinskiy N., Grishchuk Y., Vaslin A., Puyal J., Delacourte A., Hirling H., Clarke P.G., Luthi-Carter R. Calpain Hydrolysis of Alpha- and Beta2-Adaptins Decreases Clathrin-Dependent Endocytosis and May Promote Neurodegeneration. J. Biol. Chem. 2009;284:12447–12458. doi: 10.1074/jbc.M804740200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Xu W., Tan C.C., Cao X.P., Tan L., Initiative Alzheimer’s Disease Neuroimaging Association of Alzheimer’s Disease Risk Variants on the Picalm Gene with Picalm Expression, Core Biomarkers, and Feature Neurodegeneration. Aging. 2020;12:21202–21219. doi: 10.18632/aging.103814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Wightman D.P., Jansen I.E., Savage J.E., Shadrin A.A., Bahrami S., Holland D., Rongve A., Borte S., Winsvold B.S., Drange O.K., et al. A Genome-Wide Association Study with 1,126,563 Individuals Identifies New Risk Loci for Alzheimer’s Disease. Nat. Genet. 2021;53:1276–1282. doi: 10.1038/s41588-021-00921-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Parikh I., Medway C., Younkin S., Fardo D.W., Estus S. An Intronic Picalm Polymorphism, Rs588076, Is Associated with Allelic Expression of a Picalm Isoform. Mol. Neurodegener. 2014;9:32. doi: 10.1186/1750-1326-9-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Lutz M.W., Chiba-Falek O. Bioinformatics Pipeline to Guide Late-Onset Alzheimer’s Disease (Load) Post-Gwas Studies: Prioritizing Transcription Regulatory Variants within Load-Associated Regions. Alzheimers Dement. 2022;8:e12244. doi: 10.1002/trc2.12244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Lowy-Gallego E., Fairley S., Zheng-Bradley X., Ruffier M., Clarke L., Flicek P., Consortium Genomes Project Variant Calling on the Grch38 Assembly with the Data from Phase Three of the 1000 Genomes Project. Wellcome Open Res. 2019;4:50. doi: 10.12688/wellcomeopenres.15126.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Naj A.C., Jun G., Beecham G.W., Wang L.S., Vardarajan B.N., Buros J., Gallins P.J., Buxbaum J.D., Jarvik G.P., Crane P.K., et al. Common Variants at Ms4a4/Ms4a6e, Cd2ap, Cd33 and Epha1 Are Associated with Late-Onset Alzheimer’s Disease. Nat. Genet. 2011;43:436–441. doi: 10.1038/ng.801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kamboh M.I., Minster R.L., Demirci F.Y., Ganguli M., Dekosky S.T., Lopez O.L., Barmada M.M. Association of Clu and Picalm Variants with Alzheimer’s Disease. Neurobiol. Aging. 2012;33:518–521. doi: 10.1016/j.neurobiolaging.2010.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Vardarajan B.N., Ghani M., Kahn A., Sheikh S., Sato C., Barral S., Lee J.H., Cheng R., Reitz C., Lantigua R., et al. Rare Coding Mutations Identified by Sequencing of Alzheimer Disease Genome-Wide Association Studies Loci. Ann. Neurol. 2015;78:487–498. doi: 10.1002/ana.24466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Moreno-Grau S., de Rojas I., Hernandez I., Quintela I., Montrreal L., Alegret M., Hernandez-Olasagarre B., Madrid L., Gonzalez-Perez A., Maronas O., et al. Genome-Wide Association Analysis of Dementia and Its Clinical Endophenotypes Reveal Novel Loci Associated with Alzheimer’s Disease and Three Causality Networks: The Gr@Ace Project. Alzheimers Dement. 2019;15:1333–1347. doi: 10.1016/j.jalz.2019.06.4950. [DOI] [PubMed] [Google Scholar]
- 90.Bryois J., Calini D., Macnair W., Foo L., Urich E., Ortmann W., Iglesias V.A., Selvaraj S., Nutma E., Marzin M., et al. Cell-Type-Specific Cis-Eqtls in Eight Human Brain Cell Types Identify Novel Risk Genes for Psychiatric and Neurological Disorders. Nat. Neurosci. 2022;25:1104–1112. doi: 10.1038/s41593-022-01128-z. [DOI] [PubMed] [Google Scholar]
- 91.Kosoy R., Fullard J.F., Zeng B., Bendl J., Dong P., Rahman S., Kleopoulos S.P., Shao Z., Girdhar K., Humphrey J., et al. Genetics of the Human Microglia Regulome Refines Alzheimer’s Disease Risk Loci. Nat. Genet. 2022;54:1145–1154. doi: 10.1038/s41588-022-01149-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Young A.M.H., Kumasaka N., Calvert F., Hammond T.R., Knights A., Panousis N., Park J.S., Schwartzentruber J., Liu J., Kundu K., et al. A Map of Transcriptional Heterogeneity and Regulatory Variation in Human Microglia. Nat. Genet. 2021;53:861–868. doi: 10.1038/s41588-021-00875-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Lopes K.P., Snijders G.J.L., Humphrey J., Allan A., Sneeboer M.A.M., Navarro E., Schilder B.M., Vialle R.A., Parks M., Missall R., et al. Genetic Analysis of the Human Microglial Transcriptome across Brain Regions, Aging and Disease Pathologies. Nat. Genet. 2022;54:4–17. doi: 10.1038/s41588-021-00976-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Thomas R.S., Henson A., Gerrish A., Jones L., Williams J., Kidd E.J. Decreasing the Expression of Picalm Reduces Endocytosis and the Activity of Beta-Secretase: Implications for Alzheimer’s Disease. BMC Neurosci. 2016;17:50. doi: 10.1186/s12868-016-0288-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Gockley J., Montgomery K.S., Poehlman W.L., Wiley J.C., Liu Y., Gerasimov E., Greenwood A.K., Sieberts S.K., Wingo A.P., Wingo T.S., et al. Multi-Tissue Neocortical Transcriptome-Wide Association Study Implicates 8 Genes across 6 Genomic Loci in Alzheimer’s Disease. Genome Med. 2021;13:76. doi: 10.1186/s13073-021-00890-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Melville S.A., Buros J., Parrado A.R., Vardarajan B., Logue M.W., Shen L., Risacher S.L., Kim S., Jun G., DeCarli C., et al. Multiple Loci Influencing Hippocampal Degeneration Identified by Genome Scan. Ann. Neurol. 2012;72:65–75. doi: 10.1002/ana.23644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Xu W., Wang H.F., Tan L., Tan M.S., Tan C.C., Zhu X.C., Miao D., Yu W.J., Jiang T., Tan L., et al. The Impact of Picalm Genetic Variations on Reserve Capacity of Posterior Cingulate in Ad Continuum. Sci. Rep. 2016;6:24480. doi: 10.1038/srep24480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Schjeide B.M., Schnack C., Lambert J.C., Lill C.M., Kirchheiner J., Tumani H., Otto M., Tanzi R.E., Lehrach H., Amouyel P., et al. The Role of Clusterin, Complement Receptor 1, and Phosphatidylinositol Binding Clathrin Assembly Protein in Alzheimer Disease Risk and Cerebrospinal Fluid Biomarker Levels. Arch. Gen. Psychiatry. 2011;68:207–213. doi: 10.1001/archgenpsychiatry.2010.196. [DOI] [PubMed] [Google Scholar]
- 99.Zhuang L., Liu X., Shi Y., Liu X., Luo B. Genetic Variants of Picalm Rs541458 Modulate Brain Spontaneous Activity in Older Adults with Amnestic Mild Cognitive Impairment. Front. Neurol. 2019;10:494. doi: 10.3389/fneur.2019.00494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Sweet R.A., Seltman H., Emanuel J.E., Lopez O.L., Becker J.T., Bis J.C., Weamer E.A., DeMichele-Sweet M.A., Kuller L.H. Effect of Alzheimer’s Disease Risk Genes on Trajectories of Cognitive Function in the Cardiovascular Health Study. Am. J. Psychiatry. 2012;169:954–962. doi: 10.1176/appi.ajp.2012.11121815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Jones E.L., Mok K., Hanney M., Harold D., Sims R., Williams J., Ballard C. Evidence That Picalm Affects Age at Onset of Alzheimer’s Dementia in Down Syndrome. Neurobiol. Aging. 2013;34 doi: 10.1016/j.neurobiolaging.2013.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Chibnik L.B., Shulman J.M., Leurgans S.E., Schneider J.A., Wilson R.S., Tran D., Aubin C., Buchman A.S., Heward C.B., Myers A.J., et al. Cr1 Is Associated with Amyloid Plaque Burden and Age-Related Cognitive Decline. Ann. Neurol. 2011;69:560–569. doi: 10.1002/ana.22277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Biffi A., Anderson C.D., Desikan R.S., Sabuncu M., Cortellini L., Schmansky N., Salat D., Rosand J., Initiative Alzheimer’s Disease Neuroimaging Genetic Variation and Neuroimaging Measures in Alzheimer Disease. Arch. Neurol. 2010;67:677–685. doi: 10.1001/archneurol.2010.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Tan M.S., Yang Y.X., Xu W., Wang H.F., Tan L., Zuo C.T., Dong Q., Tan L., Suckling J., Yu J.T., et al. Associations of Alzheimer’s Disease Risk Variants with Gene Expression, Amyloidosis, Tauopathy, and Neurodegeneration. Alzheimers Res. Ther. 2021;13:15. doi: 10.1186/s13195-020-00755-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Thambisetty M., An Y., Tanaka T. Alzheimer’s Disease Risk Genes and the Age-at-Onset Phenotype. Neurobiol. Aging. 2013;34 doi: 10.1016/j.neurobiolaging.2013.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Mengel-From J., Christensen K., McGue M., Christiansen L. Genetic Variations in the Clu and Picalm Genes Are Associated with Cognitive Function in the Oldest Old. Neurobiol. Aging. 2011;32 doi: 10.1016/j.neurobiolaging.2010.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Ponomareva N.V., Andreeva T.V., Protasova M.A., Filippova Y.V., Kolesnikova E.P., Fokin V.F., Illarioshkin S.N., Rogaev E.I. Genetic Association between Alzheimer’s Disease Risk Variant of the Picalm Gene and Auditory Event-Related Potentials in Aging. Biochemistry. 2018;83:1075–1082. doi: 10.1134/S0006297918090092. [DOI] [PubMed] [Google Scholar]
- 108.Furney S.J., Simmons A., Breen G., Pedroso I., Lunnon K., Proitsi P., Hodges A., Powell J., Wahlund L.O., Kloszewska I., et al. Genome-Wide Association with Mri Atrophy Measures as a Quantitative Trait Locus for Alzheimer’s Disease. Mol. Psychiatry. 2011;16:1130–1138. doi: 10.1038/mp.2010.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Liu Z., Dai X., Zhang J., Li X., Chen Y., Ma C., Chen K., Peng D., Zhang Z. The Interactive Effects of Age and Picalm Rs541458 Polymorphism on Cognitive Performance, Brain Structure, and Function in Non-Demented Elderly. Mol. Neurobiol. 2018;55:1271–1283. doi: 10.1007/s12035-016-0358-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Chang Y.T., Huang C.W., Huang S.H., Hsu S.W., Chang W.N., Lee J.J., Chang C.C. Genetic Interaction Is Associated with Lower Metabolic Connectivity and Memory Impairment in Clinically Mild Alzheimer’s Disease. Genes Brain Behav. 2019;18:e12490. doi: 10.1111/gbb.12490. [DOI] [PubMed] [Google Scholar]
- 111.Sun D.M., Chen H.F., Zuo Q.L., Su F., Bai F., Liu C.F. Effect of Picalm Rs3851179 Polymorphism on the Default Mode Network Function in Mild Cognitive Impairment. Behav Brain Res. 2017;331:225–232. doi: 10.1016/j.bbr.2017.05.043. [DOI] [PubMed] [Google Scholar]
- 112.Ponomareva N.V., Andreeva T.V., Protasova M.S., Shagam L.I., Malina D.D., Goltsov A.Y., Fokin V.F., Illarioshkin S.N., Rogaev E.I. Quantitative Eeg During Normal Aging: Association with the Alzheimer’s Disease Genetic Risk Variant in Picalm Gene. Neurobiol. Aging. 2017;51 doi: 10.1016/j.neurobiolaging.2016.12.010. [DOI] [PubMed] [Google Scholar]
- 113.Kanatsu K., Morohashi Y., Suzuki M., Kuroda H., Watanabe T., Tomita T., Iwatsubo T. Decreased Calm Expression Reduces Abeta42 to Total Abeta Ratio through Clathrin-Mediated Endocytosis of Gamma-Secretase. Nat. Commun. 2014;5:3386. doi: 10.1038/ncomms4386. [DOI] [PubMed] [Google Scholar]
- 114.Tian Y., Chang J.C., Fan E.Y., Flajolet M., Greengard P. Adaptor Complex Ap2/Picalm, through Interaction with Lc3, Targets Alzheimer’s App-Ctf for Terminal Degradation Via Autophagy. Proc. Natl. Acad. Sci. USA. 2013;110:17071–17076. doi: 10.1073/pnas.1315110110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Tian Y., Chang J.C., Greengard P., Flajolet M. The Convergence of Endosomal and Autophagosomal Pathways: Implications for App-Ctf Degradation. Autophagy. 2014;10:694–696. doi: 10.4161/auto.27802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Kanatsu K., Hori Y., Takatori S., Watanabe T., Iwatsubo T., Tomita T. Partial Loss of Calm Function Reduces Abeta42 Production and Amyloid Deposition in Vivo. Hum. Mol. Genet. 2016;25:3988–3997. doi: 10.1093/hmg/ddw239. [DOI] [PubMed] [Google Scholar]
- 117.Yu Y., Niccoli T., Ren Z., Woodling N.S., Aleyakpo B., Szabadkai G., Partridge L. Picalm Rescues Glutamatergic Neurotransmission, Behavioural Function and Survival in a Drosophila Model of Abeta42 Toxicity. Hum. Mol. Genet. 2020;29:2420–2434. doi: 10.1093/hmg/ddaa125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Treusch S., Hamamichi S., Goodman J.L., Matlack K.E., Chung C.Y., Baru V., Shulman J.M., Parrado A., Bevis B.J., Valastyan J.S., et al. Functional Links between Abeta Toxicity, Endocytic Trafficking, and Alzheimer’s Disease Risk Factors in Yeast. Science. 2011;334:1241–1245. doi: 10.1126/science.1213210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.D’Angelo F., Vignaud H., di Martino J., Salin B., Devin A., Cullin C., Marchal C. A Yeast Model for Amyloid-Beta Aggregation Exemplifies the Role of Membrane Trafficking and Picalm in Cytotoxicity. Dis. Model. Mech. 2013;6:206–216. doi: 10.1242/dmm.010108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Li S., Hong S., Shepardson N.E., Walsh D.M., Shankar G.M., Selkoe D. Soluble Oligomers of Amyloid Beta Protein Facilitate Hippocampal Long-Term Depression by Disrupting Neuronal Glutamate Uptake. Neuron. 2009;62:788–801. doi: 10.1016/j.neuron.2009.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Talantova M., Sanz-Blasco S., Zhang X., Xia P., Akhtar M.W., Okamoto S., Dziewczapolski G., Nakamura T., Cao G., Pratt A.E., et al. Abeta Induces Astrocytic Glutamate Release, Extrasynaptic Nmda Receptor Activation, and Synaptic Loss. Proc. Natl. Acad. Sci. USA. 2013;110:E2518–E2527. doi: 10.1073/pnas.1306832110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Wang R., Reddy P.H. Role of Glutamate and Nmda Receptors in Alzheimer’s Disease. J. Alzheimers Dis. 2017;57:1041–1048. doi: 10.3233/JAD-160763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Nishikawa T., Takahashi T., Nakamori M., Hosomi N., Maruyama H., Miyazaki Y., Izumi Y., Matsumoto M. The Identification of Raft-Derived Tau-Associated Vesicles That Are Incorporated into Immature Tangles and Paired Helical Filaments. Neuropathol. Appl. Neurobiol. 2016;42:639–653. doi: 10.1111/nan.12288. [DOI] [PubMed] [Google Scholar]
- 124.Tasaki S., Xu J., Avey D.R., Johnson L., Petyuk V.A., Dawe R.J., Bennett D.A., Wang Y., Gaiteri C. Inferring Protein Expression Changes from Mrna in Alzheimer’s Dementia Using Deep Neural Networks. Nat. Commun. 2022;13:655. doi: 10.1038/s41467-022-28280-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Kumon H., Yoshino Y., Funahashi Y., Mori H., Ueno M., Ozaki Y., Yamazaki K., Ochi S., Mori T., Iga J.I., et al. Picalm Mrna Expression in the Blood of Patients with Neurodegenerative Diseases and Geriatric Depression. J. Alzheimers Dis. 2021;79:1055–1062. doi: 10.3233/JAD-201046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Mathys H., Davila-Velderrain J., Peng Z., Gao F., Mohammadi S., Young J.Z., Menon M., He L., Abdurrob F., Jiang X., et al. Single-Cell Transcriptomic Analysis of Alzheimer’s Disease. Nature. 2019;570:332–337. doi: 10.1038/s41586-019-1195-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Mori H., Kondo J., Ihara Y. Ubiquitin Is a Component of Paired Helical Filaments in Alzheimer’s Disease. Science. 1987;235:1641–1644. doi: 10.1126/science.3029875. [DOI] [PubMed] [Google Scholar]
- 128.Nishikawa T., Takahashi T., Nakamori M., Yamazaki Y., Kurashige T., Nagano Y., Nishida Y., Izumi Y., Matsumoto M. Phosphatidylinositol-4,5-Bisphosphate Is Enriched in Granulovacuolar Degeneration Bodies and Neurofibrillary Tangles. Neuropathol. Appl. Neurobiol. 2014;40:489–501. doi: 10.1111/nan.12056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Srinivasan S., Gal J., Bachstetter A., Nelson P.T. Alpha Adaptins Show Isoform-Specific Association with Neurofibrillary Tangles in Alzheimer’s Disease. Neuropathol. Appl. Neurobiol. 2022;48:e12776. doi: 10.1111/nan.12776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Huang Y., Liu X.Q., Wyss-Coray T., Brecht W.J., Sanan D.A., Mahley R.W. Apolipoprotein E Fragments Present in Alzheimer’s Disease Brains Induce Neurofibrillary Tangle-Like Intracellular Inclusions in Neurons. Proc. Natl. Acad. Sci. USA. 2001;98:8838–8843. doi: 10.1073/pnas.151254698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Zhou Y., Hayashi I., Wong J., Tugusheva K., Renger J.J., Zerbinatti C. Intracellular Clusterin Interacts with Brain Isoforms of the Bridging Integrator 1 and with the Microtubule-Associated Protein Tau in Alzheimer’s Disease. PLoS ONE. 2014;9:e103187. doi: 10.1371/journal.pone.0103187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Chapuis J., Hansmannel F., Gistelinck M., Mounier A., van Cauwenberghe C., Kolen K.V., Geller F., Sottejeau Y., Harold D., Dourlen P., et al. Increased Expression of Bin1 Mediates Alzheimer Genetic Risk by Modulating Tau Pathology. Mol. Psychiatry. 2013;18:1225–1234. doi: 10.1038/mp.2013.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Dourlen P., Fernandez-Gomez F.J., Dupont C., Grenier-Boley B., Bellenguez C., Obriot H., Caillierez R., Sottejeau Y., Chapuis J., Bretteville A., et al. Functional Screening of Alzheimer Risk Loci Identifies Ptk2b as an in Vivo Modulator and Early Marker of Tau Pathology. Mol. Psychiatry. 2017;22:874–883. doi: 10.1038/mp.2016.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Ando K., Tomimura K., Sazdovitch V., Suain V., Yilmaz Z., Authelet M., Ndjim M., Vergara C., Belkouch M., Potier M.C., et al. Level of Picalm, a Key Component of Clathrin-Mediated Endocytosis, Is Correlated with Levels of Phosphotau and Autophagy-Related Proteins and Is Associated with Tau Inclusions in Ad, Psp and Pick Disease. Neurobiol. Dis. 2016;94:32–43. doi: 10.1016/j.nbd.2016.05.017. [DOI] [PubMed] [Google Scholar]
- 135.Ando K., de Decker R., Vergara C., Yilmaz Z., Mansour S., Suain V., Sleegers K., de Fisenne M.A., Houben S., Potier M.C., et al. Picalm Reduction Exacerbates Tau Pathology in a Murine Tauopathy Model. Acta Neuropathol. 2020;139:773–789. doi: 10.1007/s00401-020-02125-x. [DOI] [PubMed] [Google Scholar]
- 136.Adamec E., Mohan P., Vonsattel J.P., Nixon R.A. Calpain Activation in Neurodegenerative Diseases: Confocal Immunofluorescence Study with Antibodies Specifically Recognizing the Active Form of Calpain 2. Acta Neuropathol. 2002;104:92–104. doi: 10.1007/s00401-002-0528-6. [DOI] [PubMed] [Google Scholar]
- 137.Grynspan F., Griffin W.R., Cataldo A., Katayama S., Nixon R.A. Active Site-Directed Antibodies Identify Calpain Ii as an Early-Appearing and Pervasive Component of Neurofibrillary Pathology in Alzheimer’s Disease. Brain Res. 1997;763:145–158. doi: 10.1016/S0006-8993(97)00384-3. [DOI] [PubMed] [Google Scholar]
- 138.Saito K., Elce J.S., Hamos J.E., Nixon R.A. Widespread Activation of Calcium-Activated Neutral Proteinase (Calpain) in the Brain in Alzheimer Disease: A Potential Molecular Basis for Neuronal Degeneration. Proc. Natl. Acad. Sci. USA. 1993;90:2628–2632. doi: 10.1073/pnas.90.7.2628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Su J.H., Zhao M., Anderson A.J., Srinivasan A., Cotman C.W. Activated Caspase-3 Expression in Alzheimer’s and Aged Control Brain: Correlation with Alzheimer Pathology. Brain Res. 2001;898:350–357. doi: 10.1016/S0006-8993(01)02018-2. [DOI] [PubMed] [Google Scholar]
- 140.Tsuji T., Shimohama S., Kimura J., Shimizu K. M-Calpain (Calcium-Activated Neutral Proteinase) in Alzheimer’s Disease Brains. Neurosci. Lett. 1998;248:109–112. doi: 10.1016/S0304-3940(98)00348-6. [DOI] [PubMed] [Google Scholar]
- 141.Leroy K., Bretteville A., Schindowski K., Gilissen E., Authelet M., de Decker R., Yilmaz Z., Buee L., Brion J.P. Early Axonopathy Preceding Neurofibrillary Tangles in Mutant Tau Transgenic Mice. Am J Pathol. 2007;171:976–992. doi: 10.2353/ajpath.2007.070345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Braak E., Braak H., Mandelkow E.M. A Sequence of Cytoskeleton Changes Related to the Formation of Neurofibrillary Tangles and Neuropil Threads. Acta Neuropathol. 1994;87:554–567. doi: 10.1007/BF00293315. [DOI] [PubMed] [Google Scholar]
- 143.Wu J.W., Herman M., Liu L., Simoes S., Acker C.M., Figueroa H., Steinberg J.I., Margittai M., Kayed R., Zurzolo C., et al. Small Misfolded Tau Species Are Internalized Via Bulk Endocytosis and Anterogradely and Retrogradely Transported in Neurons. J. Biol. Chem. 2013;288:1856–1870. doi: 10.1074/jbc.M112.394528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Kanekiyo T., Cirrito J.R., Liu C.C., Shinohara M., Li J., Schuler D.R., Shinohara M., Holtzman D.M., Bu G. Neuronal Clearance of Amyloid-Beta by Endocytic Receptor Lrp1. J. Neurosci. 2013;33:19276–19283. doi: 10.1523/JNEUROSCI.3487-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Rauch J.N., Luna G., Guzman E., Audouard M., Challis C., Sibih Y.E., Leshuk C., Hernandez I., Wegmann S., Hyman B.T., et al. Lrp1 Is a Master Regulator of Tau Uptake and Spread. Nature. 2020;580:381–385. doi: 10.1038/s41586-020-2156-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Auderset L., Cullen C.L., Young K.M. Low Density Lipoprotein-Receptor Related Protein 1 Is Differentially Expressed by Neuronal and Glial Populations in the Developing and Mature Mouse Central Nervous System. PLoS ONE. 2016;11:e0155878. doi: 10.1371/journal.pone.0155878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Cooper J.M., Lathuiliere A., Migliorini M., Arai A.L., Wani M.M., Dujardin S., Muratoglu S.C., Hyman B.T., Strickland D.K. Regulation of Tau Internalization, Degradation, and Seeding by Lrp1 Reveals Multiple Pathways for Tau Catabolism. J. Biol. Chem. 2021;296:100715. doi: 10.1016/j.jbc.2021.100715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Di Paolo G., Kim T.W. Linking Lipids to Alzheimer’s Disease: Cholesterol and Beyond. Nat. Rev. Neurosci. 2011;12:284–296. doi: 10.1038/nrn3012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Panchal M., Loeper J., Cossec J.C., Perruchini C., Lazar A., Pompon D., Duyckaerts C. Enrichment of Cholesterol in Microdissected Alzheimer’s Disease Senile Plaques as Assessed by Mass Spectrometry. J. Lipid Res. 2010;51:598–605. doi: 10.1194/jlr.M001859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Tuck B.J., Miller L.V.C., Katsinelos T., Smith A.E., Wilson E.L., Keeling S., Cheng S., Vaysburd M.J., Knox C., Tredgett L., et al. Cholesterol Determines the Cytosolic Entry and Seeded Aggregation of Tau. Cell Rep. 2022;39:110776. doi: 10.1016/j.celrep.2022.110776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Good P.F., Perl D.P., Bierer L.M., Schmeidler J. Selective Accumulation of Aluminum and Iron in the Neurofibrillary Tangles of Alzheimer’s Disease: A Laser Microprobe (Lamma) Study. Ann. Neurol. 1992;31:286–292. doi: 10.1002/ana.410310310. [DOI] [PubMed] [Google Scholar]
- 152.Rao S.S., Adlard P.A. Untangling Tau and Iron: Exploring the Interaction between Iron and Tau in Neurodegeneration. Front. Mol. Neurosci. 2018;11:276. doi: 10.3389/fnmol.2018.00276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Chen Y.T., Chen W.Y., Huang X.T., Xu Y.C., Zhang H.Y. Iron Dysregulates App Processing Accompanying with Sappalpha Cellular Retention and Beta-Secretase Inhibition in Rat Cortical Neurons. Acta Pharmacol. Sin. 2018;39:177–183. doi: 10.1038/aps.2017.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Holmes B.B., Furman J.L., Mahan T.E., Yamasaki T.R., Mirbaha H., Eades W.C., Belaygorod L., Cairns N.J., Holtzman D.M., Diamond M.I. Proteopathic Tau Seeding Predicts Tauopathy in Vivo. Proc. Natl. Acad. Sci. USA. 2014;111:E4376–E4385. doi: 10.1073/pnas.1411649111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Kolay S., Diamond M.I. Alzheimer’s Disease Risk Modifier Genes Do Not Affect Tau Aggregate Uptake, Seeding or Maintenance in Cell Models. FEBS Open Bio. 2020;10:1912–1920. doi: 10.1002/2211-5463.12928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Leng F., Edison P. Neuroinflammation and Microglial Activation in Alzheimer Disease: Where Do We Go from Here? Nat. Rev. Neurol. 2021;17:157–172. doi: 10.1038/s41582-020-00435-y. [DOI] [PubMed] [Google Scholar]
- 157.Cataldo A.M., Peterhoff C.M., Troncoso J.C., Gomez-Isla T., Hyman B.T., Nixon R.A. Endocytic Pathway Abnormalities Precede Amyloid Beta Deposition in Sporadic Alzheimer’s Disease and Down Syndrome: Differential Effects of Apoe Genotype and Presenilin Mutations. Am. J. Pathol. 2000;157:277–286. doi: 10.1016/S0002-9440(10)64538-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Narayan P., Sienski G., Bonner J.M., Lin Y.T., Seo J., Baru V., Haque A., Milo B., Akay L.A., Graziosi A., et al. Picalm Rescues Endocytic Defects Caused by the Alzheimer’s Disease Risk Factor Apoe4. Cell Rep. 2020;33:108224. doi: 10.1016/j.celrep.2020.108224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Moulton M.J., Barish S., Ralhan I., Chang J., Goodman L.D., Harland J.G., Marcogliese P.C., Johansson J.O., Ioannou M.S., Bellen H.J. Neuronal Ros-Induced Glial Lipid Droplet Formation Is Altered by Loss of Alzheimer’s Disease-Associated Genes. Proc. Natl. Acad. Sci. USA. 2021;118:e2112095118. doi: 10.1073/pnas.2112095118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Pachter J.S., Yen T.J., Cleveland D.W. Autoregulation of Tubulin Expression Is Achieved through Specific Degradation of Polysomal Tubulin Mrnas. Cell. 1987;51:283–292. doi: 10.1016/0092-8674(87)90155-3. [DOI] [PubMed] [Google Scholar]
- 161.Bartel D.P. Metazoan Micrornas. Cell. 2018;173:20–51. doi: 10.1016/j.cell.2018.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Zingale V.D., Gugliandolo A., Mazzon E. Mir-155: An Important Regulator of Neuroinflammation. Int. J. Mol. Sci. 2021;23:90. doi: 10.3390/ijms23010090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Sierksma A., Lu A., Salta E., Eynden E.V., Callaerts-Vegh Z., D’Hooge R., Blum D., Buee L., Fiers M., de Strooper B. Deregulation of Neuronal Mirnas Induced by Amyloid-Beta or Tau Pathology. Mol. Neurodegener. 2018;13:54. doi: 10.1186/s13024-018-0285-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Lukiw W.J., Alexandrov P.N. Regulation of Complement Factor H (Cfh) by Multiple Mirnas in Alzheimer’s Disease (Ad) Brain. Mol. Neurobiol. 2012;46:11–19. doi: 10.1007/s12035-012-8234-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Wu J., Gu J., Shen L., Fang D., Zou X., Cao Y., Wang S., Mao L. Exosomal Microrna-155 Inhibits Enterovirus A71 Infection by Targeting Picalm. Int. J. Biol. Sci. 2019;15:2925–2935. doi: 10.7150/ijbs.36388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Readhead B., Haure-Mirande J.V., Mastroeni D., Audrain M., Fanutza T., Kim S.H., Blitzer R.D., Gandy S., Dudley J.T., Ehrlich M.E. Mir155 Regulation of Behavior, Neuropathology, and Cortical Transcriptomics in Alzheimer’s Disease. Acta Neuropathol. 2020;140:295–315. doi: 10.1007/s00401-020-02185-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Schroeter C.B., Herrmann A.M., Bock S., Vogelsang A., Eichler S., Albrecht P., Meuth S.G., Ruck T. One Brain-All Cells: A Comprehensive Protocol to Isolate All Principal Cns-Resident Cell Types from Brain and Spinal Cord of Adult Healthy and Eae Mice. Cells. 2021;10:651. doi: 10.3390/cells10030651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.De Strooper B., Karran E. The Cellular Phase of Alzheimer’s Disease. Cell. 2016;164:603–615. doi: 10.1016/j.cell.2015.12.056. [DOI] [PubMed] [Google Scholar]
- 169.Ando K., Erneux C., Homa M., Houben S., de Fisenne M.A., Brion J.P., Leroy K. Dysregulation of Phosphoinositide 5-Phosphatases and Phosphoinositides in Alzheimer’s Disease. Front. Neurosci. 2021;15:614855. doi: 10.3389/fnins.2021.614855. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.