Abstract
The mechanism of killing of obligately intracellular Rickettsia conorii within human target cells, mainly endothelium and, to a lesser extent, macrophages and hepatocytes, has not been determined. It has been a controversial issue as to whether or not human cells produce nitric oxide. AKN-1 cells (human hepatocytes) stimulated by gamma interferon, tumor necrosis factor alpha, interleukin 1β, and RANTES (regulated by activation, normal T-cell-expressed and -secreted chemokine) killed intracellular rickettsiae by a nitric oxide-dependent mechanism. Human umbilical vein endothelial cells (HUVECs), when stimulated with the same concentrations of cytokines and RANTES, differed in their capacity to kill rickettsiae by a nitric oxide-dependent mechanism and in the quantity of nitric oxide synthesized. Hydrogen peroxide-dependent intracellular killing of R. conorii was demonstrated in HUVECs, THP-1 cells (human macrophages), and human peripheral blood monocytes activated with the cytokines. Rickettsial killing in the human macrophage cell line was also mediated by a limitation of the availability of tryptophan in association with the expression of the tryptophan-degrading enzyme indoleamine-2,3-dioxygenase. The rates of survival of all of the cell types investigated under the conditions of activation and infection in these experiments indicated that death of the host cells was not the explanation for the control of rickettsial infection. This finding represents the first demonstration that activated human hepatocytes and, in some cases, endothelium can kill intracellular pathogens via nitric oxide and that RANTES plays a role in immunity to rickettsiae. Human cells are capable of controlling rickettsial infections intracellularly, the most relevant location in these infections, by one or a combination of three mechanisms involving nitric oxide synthesis, hydrogen peroxide production, and tryptophan degradation.
Among spotted fever group (SFG) rickettsiae, six species are closely related genetically and cause similar clinical and pathologic manifestations with overlapping spectra of severity. The order of decreasing overall severity is Rickettsia rickettsii, R. conorii, and R. sibirica. At the less severe end of the spectrum, R. japonica, R. africae, and R. honei have not been documented to cause a fatal outcome. R. akari and R. australis are SFG rickettsiae substantially more distantly related to this cluster of six SFG rickettsial pathogens (30, 31, 40). The cell wall of each of these organisms contains the major, immunodominant antigens: rickettsial outer membrane proteins A and B and nonendotoxic lipopolysaccharide (45). R. conorii and R. rickettsii are typical SFG rickettsiae, small, obligately intracellular, gram-negative bacteria that attach to a host cell receptor via one or more adhesins, including rickettsial outer membrane protein A (20). The rickettsiae induce internalization by phagocytosis in association with phospholipase A2 activity, apparently of rickettsial origin, and escape from the phagosome into the cytosol by an unidentified mechanism (39, 41, 51, 54). In the cytosol, R. conorii stimulates the polymerization of host cell F actin, which propels the bacterium through the cytoplasm and across the cell membrane into the adjacent cell or extracellular space (14, 42).
Boutonneuse fever caused by R. conorii is a classic SFG rickettsiosis transmitted to humans by tick bite inoculation of rickettsiae into the skin. Proliferation of rickettsiae in the inoculation site endothelial network results in focal dermal and epidermal necrosis, an eschar or tache noire (49). Rickettsiae spread via lymphatic vessels to the regional lymph nodes, resulting in lymphadenopathy, and via the bloodstream to the lungs, brain, liver, kidneys, heart, spleen, and skin (47). The target cells that have been identified for humans are mainly endothelium and, to a lesser extent, macrophages (47). A mouse model of R. conorii infection mimics the visceral pathologic lesions and distribution of rickettsiae in the tissues of human boutonneuse fever and Rocky Mountain spotted fever (51). In the mouse model, R. conorii is observed not only in endothelial cells and macrophages but also in hepatocytes. The murine hepatic lesions resemble those observed in human R. conorii infection (52).
In mouse and guinea pig models of R. conorii infection, rickettsiae are killed within the cytoplasm of the target cells of infection, namely, endothelium, macrophages, and hepatocytes (51, 53). In infected mice and in mouse endothelial cell cultures, intracellular rickettsicidal activity is mediated by the stimulation of inducible synthesis of nitric oxide by the combination of tumor necrosis factor alpha (TNF-α) and gamma interferon (IFN-γ) (8, 50). This study was undertaken to evaluate the ability of target cells of R. conorii in boutonneuse fever—human endothelial cells, macrophages, and hepatocytes—to kill rickettsiae residing in the cytosol upon cytokine and chemokine activation. Because cytokines alone do not trigger nitric oxide production, the report that RANTES regulated by activation, normal T-cell-expressed and -secreted chemokine) could induce nitric oxide production by human macrophages led us to investigate whether this chemokine could play a role in the induction of nitric oxide synthesis by human endothelial cells and hepatocytes (44). The roles of three potential antirickettsial mechanisms were evaluated: (i) nitric oxide derived via inducible nitric oxide synthase (iNOS), (ii) production of the reactive oxygen species hydrogen peroxide, and (iii) tryptophan degradation via indoleamine-2,3-dioxygenase (IDO).
MATERIALS AND METHODS
Rickettsia.
R. conorii (Malish 7 strain), an isolate that was obtained from a patient in South Africa and that was subsequently passaged an unknown number of times in the yolk sac of embryonated chicken eggs, was obtained from the American Type Culture Collection (ATCC; Manassas, Va.; catalog no. VR-613). In our laboratory, the organism was plaque purified and had the following passage history: 39 passages in yolk sac of embryonated chicken eggs and 4 passages in Vero cells.
The organism retains virulence in humans, as demonstrated by the development of inoculation site lesions, fever, and systemic symptoms in two experimentally infected volunteers who had given Institutional Review Board-approved informed consent. The stock, aliquots of a 10% suspension of infected yolk sac containing 4 × 106 PFU per ml, was stored at −70°C.
Cell cultures.
Pools of human umbilical vein endothelial cells (HUVECs) were purchased from Clonetics (San Diego, Calif.). Other HUVEC cultures were established from individual or pooled human umbilical cords as described previously (46). The growth medium was EGM-2 (Clonetics). The cells were subcultured when the monolayer became confluent, two or three times per week. In this study, the cells were used between passages 3 and 7.
AKN-1 cells, a human hepatocyte cell line established from wedge sections of normal liver, were a gift from Andreas K. Nussler (University of Ulm, Ulm, Germany) (24). The growth medium was an equal mixture of Ham F-12 and Williams Medium E (both from Gibco) supplemented with 22.7 mM sodium bicarbonate, 0.8 mg of fatty acid-free albumin per ml, 65 μM ethanolamine, 7.18 μM linoleic acid, 7 mM glucose, 0.4 mM sodium pyruvate, 0.1 mM ascorbic acid, 15 mM HEPES, 5 μg of insulin per ml, 5 μg of transferrin per ml, 5 μg of selenious acid per ml, and 10−7 M dexamethasone. The cells were subcultured twice weekly.
THP-1 cells, a human monocytic cell line derived from the peripheral blood of a patient with acute monocytic leukemia, were purchased from ATCC (catalog no. TIB 202). The cells were grown in Dulbecco's minimal essential medium containing 10% bovine calf serum (BCS) and were subcultured two or three times per week.
Human monocytes were prepared from the peripheral blood of four healthy donors. The white blood cells were separated from 15 to 30 ml of blood by density gradient centrifugation in Ficoll-Paque (Amersham Pharmacia Biotech, Uppsala, Sweden). The monocytes were isolated by using C14 magnetic microbeads (Miltesivi Biotech, Auburn, Calif.) according to the manufacturer's instructions. The monocytes were adjusted to concentrations of 0.5 × 106 to 1.0 × 106/ml according to the yield from each donor.
Reagents.
Human recombinant TNF-α was purchased from Boehringer Mannheim Biochemicals (Cambridge, Mass.). RANTES and NG-monomethyl-l-arginine (NGMMLA) were obtained from R&D Systems, Inc. (Minneapolis, Minn.), and Calbiochem-Novabiochem Corp. (La Jolla, Calif.), respectively. Human recombinant IFN-γ and interleukin 1β (IL-1β) were purchased from Genzyme (Cambridge, Mass.), and catalase and l-tryptophan were purchased from Sigma Chemical Company (St. Louis, Mo.).
Experimental design.
The monolayers of HUVECs, AKN-1, THP-1, or human peripheral blood monocyte cells cultivated in 24-well plates were treated with RANTES at 500 ng/ml and either 2,000 U of human recombinant TNF-α per ml, 1,000 U of recombinant human IFN-γ per ml, and 100 U of recombinant human IL-1β per ml or one-half or one-fourth these doses overnight at 37°C in an atmosphere containing 5% CO2. Then, the treated cells were infected with rickettsiae to evaluate the antirickettsial activity associated with nitric oxide synthesis. Similar studies were performed without RANTES to evaluate the antirickettsial effects associated with the production of hydrogen peroxide and with the limitation of the availability of tryptophan by IDO activity. In preliminary studies, the effect of adding RANTES was evaluated and shown not to influence the results of hydrogen peroxide production or IDO activity in AKN-1 and THP-1 cells. NGMMLA (1 mM), catalase (100 μM), and l-tryptophan (80 μg/ml) was added to each cell type for the determination of the effects of nitric oxide synthesis, hydrogen peroxide production, and tryptophan limitation, respectively.
Renografin (Bracco Diagnostic Inc., Princeton, N.Y.)-purified R. conorii was inoculated at multiplicities of infection of 0.3 or 0.6 into HUVECs and 0.12 into AKN-1 cells, THP-1 cells, and human peripheral blood monocytes. The plates were centrifuged at 770 × g for 10 min to increase the attachment of the rickettsiae to the cells. The infected cells were incubated at 37°C for 2 to 3 days. The cells were scraped from the wells and centrifuged at 440 × g for 10 min. The supernatant was saved for assay of the nitrite level. The pellets were suspended in 3 ml of Eagle's minimum essential medium (EMEM; Gibco) containing 1% BCS and then sonicated for 40 s (Braun-Sonic 2000 Ultrasonicator; Ultrasonic Power Corp., Freeport, Ill.) at a power of 28 W to release the intracellular rickettsiae. After centrifugation at 440 × g for 10 min, serial 10-fold dilutions were made of the supernatants containing the released rickettsiae; 0.2 ml of each dilution was added in duplicate to Vero cell monolayers in 24-well plates for plaque assay of the infectious rickettsial content (51). After 2 h of incubation at 37°C, 0.5% agarose in EMEM containing 1% BCS and cycloheximide (final concentration, 1 μg/ml) was added. Three days later, the plaques were counted under an inverted microscope.
Nitrite level.
One hundred microliters of supernatant from each well was divided into aliqouts, which were placed in 96-well U-bottom plates. One hundred microliters of Griess reagent was added per well (13). After incubation at room temperature for 15 to 20 min, the optical densities were measured at 540 nm with an enzyme-linked immunosorbent assay reader (Bio-TEK Instruments, Inc., Winooski, Vt.).
RT-PCR.
RNA was extracted with UltraspecRNA (BiotecX, Houston, Tex.) from cells that had been infected with rickettsiae and treated with cytokines and with or without RANTES or that were uninfected and uninduced. Reverse transcription (RT)-PCR was performed according to the instructions of the manufacturer of the 5′ rapid amplification of cDNA ends (RACE) system (Gibco). The human iNOS primers were 5′CTG TCC TTG GAA ATT TCT GTT3′ and 5′TGG CCA GAT GTT CCT CTA TT3′. The PCR program was 95°C for 30 s, 57°C for 30 s, and 72°C for 75 s for 35 cycles, followed by a final extension at 72°C for 7 min. The iNOS PCR product was 488 bp long.
The human IDO primers were as follows: forward primer, 5′TTC TCC GGC CAC CTG TTT TCA TAG T3′, and reverse primer, 5′TAG TCT GCT CCT CTG GTG CCC CCT C3′ (328-bp product). Amplification of DNA by PCR was performed as follows: 94°C for 30 s, 55°C for 30 s, and 72°C for 1 min for 35 cycles plus extension at 72°C for 7 min. Primers for the glyceraldehyde-3-phosphate dehydrogenase (G3PDH) housekeeping gene (450-bp product) were obtained from Clontech Laboratory, Inc. (Palo Alto, Calif.). The PCR products were detected after separation by electrophoresis in a 1.5% agarose gel and staining with ethidium bromide.
RESULTS
Human cells were observed to use different pathways singly or in combination to control the growth and survival of intracellular rickettsiae. In R. conorii infection, three mechanisms were verified as having a role in the intracellular killing of rickettsiae by one or more of the host cell types.
Effect of nitric oxide production on rickettsial survival.
Five different pooled samples and one single-donor-derived sample of R. conorii-infected endothelial cells (HUVECs) stimulated with cytokines (TNF-α, IFN-γ, and IL-1β) and a chemokine (RANTES) have been tested. One pool of HUVECs produced a high level of nitric oxide (measured as a nitrite level of 31.1 ± 1.1 μM) [value is mean ± standard deviation]). This response was dose dependent and required all of the stimulating proteins. When the cytokine concentrations were reduced from 2,000 U of TNF-α per ml, 1,000 U of IFN-γ per ml, and 100 U of IL-1β per ml to half these doses, the nitrite level decreased to 13.2 ± 1.4 μM (Fig. 1). This nitric oxide production was inhibited by the addition to the cultures of the arginine analogue NGMMLA, an inhibitor of iNOS. The HUVECs were very sensitive to the effects of high-dose cytokines and RANTES, with substantial endothelial cell death.
FIG. 1.
Concentration of nitric oxide produced by R. conorii-infected HUVECs stimulated by the cytokines TNF-α at 2,000 U/ml, IFN-γ at 1,000 U/ml, and IL-1β at 100 U/ml (▪) or TNF-α at 1,000 U/ml, IFN-γ at 500 U/ml, and IL-1β at 50 U/ml (░⃞). The cultures of cells treated with cytokines, RANTES, and NGMMLA are indicated below the pairs of bars representing the higher and lower doses of cytokines. □, cells infected with R. conorii and receiving no treatment. Nitric oxide was measured as the concentration of nitrite and expressed as the mean and standard deviation.
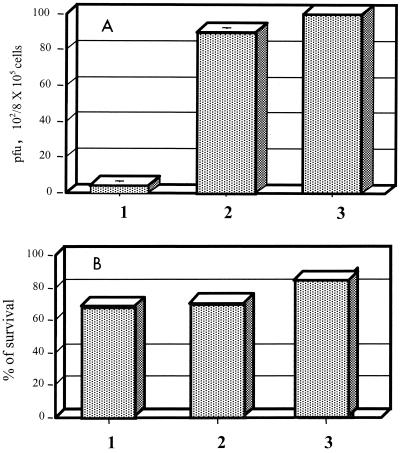
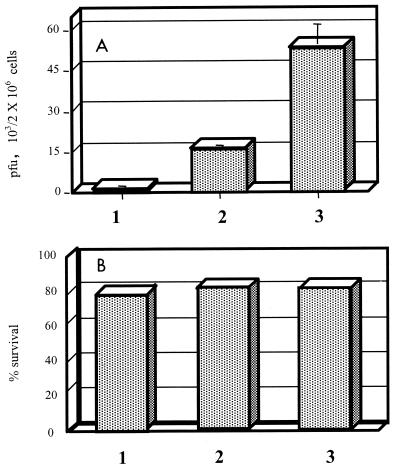
To differentiate whether the decrease in rickettsial survival was due to the direct effect of nitric oxide or was indirect, owing to death of the HUVECs associated with the production of nitric oxide, serial experiments were performed. A dose and a combination of chemokine and cytokines that resulted in nitric oxide production without reduced survival of the endothelial cells were sought. After senescence of the pooled HUVECs, a fresh HUVEC culture was established from a single umbilical cord. When these cells were treated with 500 U of TNF-α per ml, 250 U of IFN-γ per ml, 25 U of IL-1β per ml, and 500 ng of RANTES per ml, a low level of nitric oxide (3 μM nitrite) was induced. NGMMLA at a concentration of 1 mM inhibited 57% of the nitric oxide production by these cells (P < 0.05). These cytokine- and chemokine-treated HUVECs showed strong antirickettsial activity that was abrogated by the iNOS inhibitor NGMMLA, confirming the importance of the effect of nitric oxide under the conditions of these experiments (Fig. 2A). The survival of these HUVECs was not affected adversely by cytokine and chemokine activation and nitric oxide production (Fig. 2B). These results indicate that the nitric oxide produced by HUVECs acted as an intracellular rickettsicidal mechanism that was not caused by destruction of the host cells. The other four pools of HUVECs did not produce detectable (less than 1 μM) nitric oxide when induced by cytokines and RANTES.
FIG. 2.
Cellular content of infectious rickettsiae measured as PFU (A) and survival of R. conorii-infected HUVECs determined by evaluation of 100 cells by trypan blue dye exclusion (B) after treatment with TNF-α (500 U/ml), IFN-γ (250 U/ml), IL-1β (25 U/ml), and RANTES (500 ng/ml), with or without NGMMLA. Bars: 1, R. conorii-infected cells treated with cytokines and RANTES; 2, R. conorii-infected, cytokine- and chemokine-treated cells exposed to NGMMLA; 3, untreated R. conorii-infected cells. The mean and standard deviation for three repeated experiments show the features of the nitric oxide-dependent intracellular rickettsial killing mechanism.
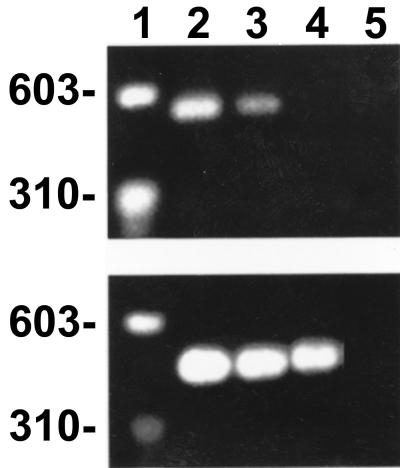
The human hepatocyte cell line AKN-1 produced nitric oxide when it was induced by TNF-α, IFN-γ, IL-1β, and RANTES (Fig. 3A). The inducible nitric oxide production by AKN-1 cells was a significant rickettsicidal mechanism and did not adversely affect the survival of the hepatocytes (Fig. 3B and C). RT-PCR using iNOS primers revealed that the AKN-1 human hepatocytes had expressed detectable mRNA of iNOS 12 h after induction by the cytokines and chemokine (Fig. 4). Infected, uninduced cells did not express mRNA of iNOS (data not shown).
FIG. 3.
Nitric oxide production measured as the concentration of nitrite (A), cellular content of infectious rickettsiae measured as PFU (B), and survival of R. conorii-infected human hepatocytes (AKN-1 cells) determined by trypan blue dye exclusion (C) after treatment with TNF-α (2,000 U/ml), IFN-γ (1,000 U/ml), IL-1β (100 U/ml), and RANTES (500 ng/ml), with or without NGMMLA (1 mM). Bars: 1, R. conorii-infected hepatocytes treated with cytokines and RANTES; 2, R. conorii-infected hepatocytes treated with cytokines, RANTES, and NGMMLA; 3, untreated R. conorii-infected hepatocytes; 4, uninfected hepatocytes exposed to medium only. The mean and standard deviation for three repeated experiments show the features of the nitric oxide-dependent intracellular rickettsial killing mechanism. Cytokine- and chemokine-activated, infected hepatocytes produced more nitric oxide than similar cultures treated with NGMMLA (P < 0.001) and than untreated, R. conorii-infected hepatocytes (P = 0.019). Cytokine- and chemokine-activated, infected hepatocytes contained fewer R. conorii than similar cultures treated with NGMMLA (P = 0.003) and than untreated, R. conorii-infected hepatocytes (P = 0.004).
FIG. 4.
Expression of mRNA of iNOS by human cells induced with cytokines and RANTES, as determined by RT-PCR yielding the expected 488-bp product. (Top panel) Lane 1, markers; lane 2, R. conorii-infected, induced AKN-1 hepatocytes; lane 3, uninfected, induced AKN-1 hepatocytes; lane 4, infected, uninduced AKN-1 hepatocytes; lane 5, water control. (Bottom panel) RT-PCR products of mRNA extracts of G3PDH.
Nitric oxide production was not the rickettsicidal mechanism of the human monocytic cell line THP-1 or the monocytes from human peripheral blood. The combination of cytokines and RANTES did not stimulate nitric oxide production in these cells. The quantities of rickettsiae in the treated cells were not influenced by the addition of the nitric oxide synthesis inhibitor NGMMLA, another indication that nitric oxide was not an antirickettsial mechanism in THP-1 cells or human peripheral blood monocytes under these conditions (data not shown).
Role of the production of hydrogen peroxide by activated endothelial cells and macrophages in the intracellular killing of R. conorii.
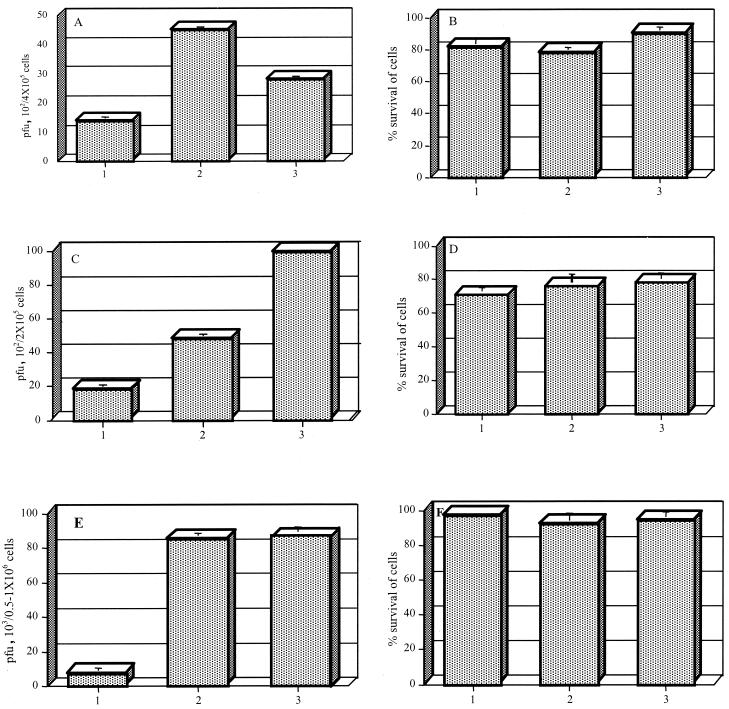
Hydrogen peroxide-dependent intracellular killing of R. conorii was demonstrated in HUVECs and THP-1 cells stimulated with cytokines only. Human peripheral blood monocytes were stimulated with both cytokines and RANTES. In three repeated experiments, catalase (100 μM) was added to inhibit the effects of hydrogen peroxide; the quantities of viable R. conorii increased 3.8-fold in HUVECs, 2-fold in THP-1 cells, and 10-fold in human peripheral blood monocytes (Fig. 5). In the AKN-1 hepatocyte cell line, hydrogen peroxide did not appear to function as an intracellular rickettsicidal mechanism because there was no change in the content of infectious rickettsiae in cells after the addition of catalase.
FIG. 5.
Infectious rickettsial content measured as PFU (A) and percent survival (B) for HUVECs infected with R. conorii; infectious rickettsial content measured as PFU (C) and percent survival (D) for THP-1 cells infected with R. conorii; and infectious content measured as PFU (E) and percent survival (F) for human peripheral blood monocytes infected with R. conorii. HUVECs were either treated with TNF-α (500 U/ml), IFN-γ (250 U/ml), and IL-1β (25 U/ml) or not treated. THP-1 cells were either treated with TNF-α (2,000 U/ml), IFN-γ (1,000 U/ml), and IL-1β (100 U/ml) or not treated. Human peripheral blood monocytes were either treated with cytokines and RANTES or not treated. Catalase was added to infected, cytokine-treated HUVECs, THP-1 cells, and human peripheral blood monocytes to determine the antirickettsial role of H2O2. Bars: 1, R. conorii-infected cells treated with cytokines; 2, R. conorii-infected cells treated with cytokines and catalase; 3, untreated R. conorii-infected cells. The results are expressed as the mean and standard deviation for three repeated experiments. Cytokine-activated, R. conorii-infected HUVECs contained fewer R. conorii than similarly activated, infected cells treated with catalase (P = 0.047) and than untreated, infected cells (P < 0.001). Cytokine-activated, R. conorii-infected THP-1 macrophages contained fewer R. conorii than similarly activated, infected cells treated with catalase (P = 0.002) and than untreated, infected cells (P = 0.006). Cytokine- and RANTES-activated, R. conorii-infected human peripheral blood monocytes contained fewer R. conorii than similarly activated, infected cells treated with catalase (P < 0.001) and than untreated, infected cells (P < 0.001).
Role of l-tryptophan depletion in the intracellular killing of R. conorii.
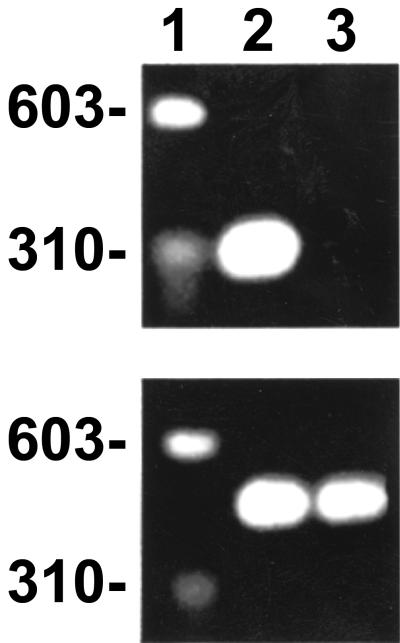
mRNA for IDO was present in THP-1 cells activated by cytokines (Fig. 6). This enzyme degrades l-tryptophan into N-formylkynurenine and kynurenine and depletes intracellular l-tryptophan pools, resulting in tryptophan starvation of intracellular parasites. Rickettsial growth was directly influenced by the l-tryptophan limitation in THP-1 cells (Fig. 7). In these cells, inhibition of rickettsial survival by activation with TNF-α, IFN-γ, and IL-1β was partially reversed by supplementation of the medium with 80 μg of l-tryptophan per ml.
FIG. 6.
Expression of mRNA of IDO by human cells induced by cytokines, as determined by RT-PCR yielding the expected 328-bp product. (Top panel) Lane 1, markers; lane 2, R. conorii-infected, induced THP-1 macrophages; lane 3, uninfected, uninduced THP-1 macrophages. (Bottom panel) RT-PCR products of mRNA extracts of G3PDH.
FIG. 7.
Rickettsicidal effect of tryptophan limitation in human macrophages (THP-1 cells) treated with TNF-α (2,000 U/ml), IFN-γ (1,000 U/ml), and IL-1 β (100 U/ml), as demonstrated by supplementation with 80 μg of l-tryptophan per ml. Bars: 1, rickettsia-infected, cytokine-treated cells; 2, rickettsia-infected, cytokine-treated cells supplemented with l-tryptophan; 3, untreated, rickettsia-infected cells. (A) Infectious rickettsial content (PFU). (B) Percent survival of cells. The results represent the mean and standard deviation for three repeated experiments.
In HUVECs, AKN-1 cells, and human peripheral blood monocytes, l-tryptophan starvation was not a major cause of rickettsicidal activity. The addition of l-tryptophan to cytokine- and chemokine-treated HUVECs, AKN-1 cells, and human peripheral blood monocytes did not result in increased quantities of viable R. conorii (data not shown).
DISCUSSION
A high proportion of humans recover from Rocky Mountain spotted fever (75 to 80% in the preantibiotic era) and boutonneuse fever (95 to 99% in the contemporary period). The antimicrobial agents currently used are bacteriostatic rather than bactericidal, indicating that they merely slow bacterial growth as the host defenses, including the immune system, eliminate the rickettsiae. Survivors are immune to reinfection.
Immunity to rickettsiae involves complex interactions of CD4 and CD8 T lymphocytes, macrophages, natural killer cells, B lymphocytes, antibodies, cytokines, and chemokines (6, 8, 9, 11, 17, 18, 19, 22, 28, 29, 48, 50, 55). Animal models have provided substantial information regarding some of these immune mechanisms against Rickettsia species, indicating that cellular mechanisms are of paramount importance, particularly T lymphocytes and the cytokines TNF-α and IFN-γ (8, 9, 17, 22, 50). In animals studied over the course of infection, the quantities of morphologically intact rickettsiae increase over a period of days during the incubation period and the peak of the illness (51, 53). Then, the intracellular quantities of rickettsiae decrease, and finally they are no longer detectable by immunohistochemical analysis or cultivation. The disappearance of the rickettsiae is associated with the appearance of fragments of rickettsial antigens that are dispersed as pieces smaller than the morphologically intact organisms and as large aggregates in the host cell cytoplasm. Autophagy of rickettsiae, morphologically evident death of rickettsiae, and phagolysosomal aggregation of dead rickettsiae have been observed in vivo and in cytokine-activated murine endothelial cells (8, 50, 51). Thus, the principal antirickettsial immune mechanism in mice appears to be intracellular killing within the cytoplasm of the target cell by a cytokine-dependent, nitric oxide-dependent mechanism(s) (8, 10, 43, 50).
The data are less extensive for human infection. At sites of infection, where the control of rickettsial growth occurs, human tissues are characterized by perivascular infiltration mainly by macrophages and lymphocytes, particularly CD4 and CD8 T lymphocytes (15, 49). The role of iNOS in human defenses against infectious diseases has been controversial. Nicholson et al. detected iNOS antigen and mRNA in human alveolar macrophages from patients with active tuberculosis (23), and Rich et al. showed individual variation in the production of nitric oxide by macrophages from healthy human subjects after stimulation of the cells by infection with Mycobacterium tuberculosis (27). Monocytes from these subjects constitutively expressed iNOS mRNA, the levels of which did not increase, and produced little or no nitric oxide when stimulated with M. tuberculosis. Stimulated alveolar macrophages from different subjects showed low (mean, 4 μM nitrite), moderate (mean, 56 μM nitrite), or high (mean, 502 μM nitrite) levels of production of nitric oxide, which was diminished by iNOS inhibitors. Stimulated alveolar macrophages expressed larger amounts of iNOS mRNA and protein. Mycobacterial growth was not significantly increased by the iNOS inhibitors but was greater in low-nitric-oxide producers than in moderate-nitric-oxide producers. Also, primary human monocytes activated by RANTES, monocyte inflammatory protein 1α (MIP-1α), or MIP-1β killed intracellular Trypanosoma cruzi via the iNOS-dependent production of nitric oxide (44). Furthermore, human epithelial cell lines activated by human IFN-γ and TNF-α and bacterial lipopolysaccharide controlled the survival of intracellular Chlamydia trachomatis by a combination of mechanisms, including iNOS activity, tryptophan degradation, and iron sequestration (16).
Indeed, human cells have been demonstrated to kill or control the growth of intracellular organisms by several mechanisms. Pfefferkorn reported in 1984 that human fibroblasts activated by IFN-γ killed Toxoplasma gondii by induction of host cell degradation of tryptophan to kynurenine and formylkynurenine (25). This mechanism was subsequently extended to other cell types, particularly human monocytes or macrophages and epithelial cells, and to other organisms, e.g., C. trachomatis, C. psittaci, and Legionella pneumophila (3, 4, 12, 26, 36). The microbicidal activity was shown to be mediated by induction of the catabolic enzyme IDO and to be stimulated under certain conditions by TNF-α, sometimes in synergy with IFN-γ or IFN-β (35, 37). For the human laryngeal carcinoma epithelial cell line HEp-2, of course not a natural target of rickettsial infection, Manor and Sarov demonstrated in 1990 the principle that human cells activated synergistically by TNF-α and IFN-γ can restrict the survival of intracellular R. conorii by a tryptophan-limiting mechanism (21).
The long-standing goal of understanding the mechanisms by which rickettsiae are eliminated from infected humans is substantially advanced by the present report. The ability of infected human target cells activated by cytokines, in some situations in association with the chemokine RANTES, to inhibit the survival of intracellular R. conorii was mediated by nitric oxide-dependent, H2O2-dependent, and IDO-dependent mechanisms. These data confirm the conclusion from experimental animal and mouse cell culture studies that cytokine-activated iNOS-dependent rickettsicidal activity is consistently an important mechanism in human immunity to rickettsiae only in hepatocytes activated by cytokines and RANTES (8, 43, 50). Although human endothelial cells from some individuals were shown to kill rickettsiae via a nitric oxide-dependent mechanism, the production of nitric oxide by endothelium exposed to cytokines and RANTES was not observed to occur for all donors. The precise culture conditions or genetic factors responsible for these inconsistent results have not been identified. Indeed, the most critical data, namely, whether or not human endothelial cells infected with rickettsiae synthesize nitric oxide in vivo, remain to be determined. Furthermore, these studies extend the role of cytokine-activated H2O2-mediated bactericidal activity to the control of the obligately intracellular bacterium R. conorii in its main target cells, human endothelium and macrophages. Moreover, a role has been documented for IDO-mediated limitation of the availability of intracellular tryptophan as an antirickettsial mechanism in human macrophages. To the best of our knowledge, this work represents the first demonstration that activated human endothelium and hepatocytes can kill intracellular rickettsiae, or indeed any microbial agent, via nitric oxide and that human macrophages can kill intracellular rickettsiae via IDO-mediated tryptophan starvation. It is the first demonstration that the chemokine RANTES plays a role in immunity to rickettsiae and that it activates the microbicidal function of human endothelium and hepatocytes.
Nitric oxide is associated with apoptosis of affected cells (2). Moreover, the interaction between the best-documented pathogenic mechanism of cell injury by rickettsiae, namely, the stimulation of endothelial cell production of reactive oxygen species by rickettsial infection, and rickettsicidal mechanisms is worthy of consideration (7, 34, 38). The induction of superoxide radical production might suggest a synergistic noxious effect via reaction with nitric oxide to produce the highly reactive radical peroxynitrite. However, the balanced production of superoxide and nitric oxide results in less apoptosis than the equivalent unbalanced production of either of these radicals (1, 32, 33). The scientific literature on the regulation of apoptosis fits some recent observations for rickettsia-infected endothelial cells, including activation of NF-κB by intracellular rickettsiae involving a signal transduction pathway leading to inhibition of apoptosis (5). Although apoptotic loss of endothelium could be considered a pathologic event, it favors the host more than does the survival or necrosis of an infected endothelial cell. Survival of an infected endothelial cell would permit further growth of the rickettsiae, and necrosis would release rickettsiae, allowing their spread to establish foci of infection in other cells. In contrast, rickettsia-containing apoptotic bodies would be phagocytosed rapidly and digested intracellularly. However, the rates of survival of all of the cell types investigated under the conditions of activation and infection in these experiments indicated that death of the host cells, whether by apoptosis or necrosis, was not the explanation for the control of rickettsial infection. Intracellular R. conorii organisms were killed by nitric oxide-dependent, IDO-dependent, and H2O2-dependent antirickettsial mechanisms within viable host cells.
It will be important in the future to design investigations of human subjects that will determine whether or not these mechanisms are active in vivo at the sites of infection and what the sources of cytokines and chemokines are. The most attractive hypothesis would be that perivascular infiltrations of CD4 and CD8 T lymphocytes, macrophages, and natural killer cells, the infected endothelium itself, and marginated cellular elements of blood secrete the cytokines and chemokines that activate infected endothelial cells, macrophages, and hepatocytes by paracrine and autocrine stimulation to kill intracellular rickettsiae.
ACKNOWLEDGMENTS
We thank Josie Ramirez-Kim and Kelly Cassity for expert secretarial assistance in the preparation of the manuscript, Thomas Bednarek and Gui-Min He for preparation of the figures, Gustavo Valbuena for establishment of the single-umbilical-cord HUVEC culture, and Patricia Crocquet-Valdes for contributions to the technical design.
This work was supported by a grant (AI21242) from the National Institute of Allergy and Infectious Diseases.
REFERENCES
- 1.Brüne B, Götz C, Messmer U K, Sandau K, Hirvonen M R, Lapetina E G. Superoxide formation and macrophage resistance to nitric oxide-mediated apoptosis. J Biol Chem. 1997;272:7253–7258. doi: 10.1074/jbc.272.11.7253. [DOI] [PubMed] [Google Scholar]
- 2.Brune B, von Knethen A, Sandau K B. Nitric oxide and its role in apoptosis. Eur J Pharmacol. 1998;351:261–272. doi: 10.1016/s0014-2999(98)00274-x. [DOI] [PubMed] [Google Scholar]
- 3.Byrne G I, Lehmann L K, Landry G J. Induction of tryptophan catabolism is the mechanism for gamma-interferon-mediated inhibition of intracellular Chlamydia psittacireplication in T24 cells. Infect Immun. 1986;53:347–351. doi: 10.1128/iai.53.2.347-351.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Carlin J M, Borden E C, Byrne G I. Interferon-induced indoleamine 2,3-dioxygenase activity inhibits Chlamydia psittacireplication in human macrophages. J Interferon Res. 1989;9:329–337. doi: 10.1089/jir.1989.9.329. [DOI] [PubMed] [Google Scholar]
- 5.Clifton D R, Goss R A, Sahni S K, van Antwerp D, Baggs R B, Marder V J, Silverman D J, Sporn L A. NF-κB-dependent inhibition of apoptosis is essential for host cell survival during Rickettsia rickettsiiinfection. Proc Natl Acad Sci USA. 1998;95:4646–4651. doi: 10.1073/pnas.95.8.4646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Crist A E, Jr, Wisseman C L, Jr, Murphy J R. Characteristics of lymphoid cells that adoptively transfer immunity to Rickettsia mooseriinfection in mice. Infect Immun. 1984;44:55–60. doi: 10.1128/iai.44.1.55-60.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Eremeeva M E, Silverman D J. Effects of the antioxidant α-lipoic acid on human umbilical vein endothelial cells infected with Rickettsia rickettsii. Infect Immun. 1998;66:2290–2299. doi: 10.1128/iai.66.5.2290-2299.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Feng H-M, Popov V L, Walker D H. Depletion of gamma interferon and tumor necrosis factor alpha in mice with Rickettsia conorii-infected endothelium: impairment of rickettsicidal nitric oxide production resulting in fatal, overwhelming rickettsial disease. Infect Immun. 1994;62:1952–1960. doi: 10.1128/iai.62.5.1952-1960.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Feng H-M, Popov V L, Yuoh G, Walker D H. Role of T-lymphocyte subsets in immunity to spotted fever group rickettsiae. J Immunol. 1997;158:5314–5320. [PubMed] [Google Scholar]
- 10.Feng H-M, Walker D H. Interferon-γ and tumor necrosis factor-α exert their antirickettsial effect via induction of synthesis of nitric oxide. Am J Pathol. 1993;143:1016–1023. [PMC free article] [PubMed] [Google Scholar]
- 11.Gambrill M R, Wisseman C L., Jr Mechanisms of immunity in typhus infections. III. Influence of human immune serum and complement on the fate of Rickettsia mooseriwithin human macrophages. Infect Immun. 1973;8:631–640. doi: 10.1128/iai.8.4.631-640.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gebran S J, Yamamoto Y, Newton C, Klein T W, Friedman H. Inhibition of Legionella pneumophilagrowth by gamma interferon in permissive A/J mouse macrophages: role of reactive oxygen species, nitric oxide, tryptophan, and iron(III) Infect Immun. 1994;62:3197–3205. doi: 10.1128/iai.62.8.3197-3205.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Granger D L, Hibbs J J B, Broadnax L M. Urinary nitrate excretion in relation to murine macrophage activation: influence of dietary l-arginine and oral NG-monomethyl-l-arginine. J Immunol. 1991;146:1294–1302. [PubMed] [Google Scholar]
- 14.Heinzen R A, Hayes S F, Peacock M G, Hackstadt T. Directional actin polymerization associated with spotted fever group rickettsia infection of Vero cells. Infect Immun. 1993;61:1926–1935. doi: 10.1128/iai.61.5.1926-1935.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Herrero-Herrero J I, Walker D H, Ruiz-Beltran R. Immunohistochemical evaluation of the cellular immune response to Rickettsia conorii in taches noires. J Infect Dis. 1987;155:802–805. doi: 10.1093/infdis/155.4.802. [DOI] [PubMed] [Google Scholar]
- 16.Igietseme J U, Ananaba G A, Candal D H, Lyn D, Black C M. Immune control of chlamydial growth in the human epithelial cell line RT4 involves multiple mechanisms that include nitric oxide induction, tryptophan catabolism and iron deprivation. Microbiol Immunol. 1998;42:617–625. doi: 10.1111/j.1348-0421.1998.tb02332.x. [DOI] [PubMed] [Google Scholar]
- 17.Kenyon R H, Pedersen C E., Jr Immune responses to Rickettsia akariinfection in congenitally athymic nude mice. Infect Immun. 1980;28:310–313. doi: 10.1128/iai.28.2.310-313.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kokorin I N, Kabanova E A, Shirokova E M. Role of macrophages in infection with Rickettsia conorii. Acta Virol. 1980;24:137–143. [PubMed] [Google Scholar]
- 19.Kokorin I N, Kabanova E A, Shirokova E M, Abrosimova E G, Rybkina N N, Pushkareva V I. Role of T lymphocytes in Rickettsia conoriiinfection. Acta Virol. 1982;26:91–97. [PubMed] [Google Scholar]
- 20.Li H, Walker D H. rOmpA is a critical protein for the adhesion of Rickettsia rickettsiito host cells. Microb Pathog. 1998;24:289–298. doi: 10.1006/mpat.1997.0197. [DOI] [PubMed] [Google Scholar]
- 21.Manor E, Sarov I. Inhibition of Rickettsia conoriigrowth by recombinant tumor necrosis factor alpha: enhancement of inhibition of gamma interferon. Infect Immun. 1990;58:1886–1889. doi: 10.1128/iai.58.6.1886-1890.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Montenegro N R, Walker D H, Hegarty B C. Infection of genetically immunodeficient mice with Rickettsia conorii. Acta Virol. 1984;28:508–514. [PubMed] [Google Scholar]
- 23.Nicholson S, Bonecini-Almeida M G, Lapa e Silva J R, Nathan C, Xie Q, Mumford R, Weidner J R, Calaycay J, Geng J, Boechat N, Linhares C, Rom W, Ho J L. Inducible nitric oxide synthase in pulmonary alveolar macrophages from patients with tuberculosis. J Exp Med. 1996;183:2293–2302. doi: 10.1084/jem.183.5.2293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nussler A K, Silvio M D, Billiar T R, Hoffman R A, Geller D A, Selby R, Madariaga J, Simmons R L. Stimulation of the nitric oxide synthase pathway in human hepatocytes by cytokines and endotoxin. J Exp Med. 1992;176:261–264. doi: 10.1084/jem.176.1.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pfefferkorn E R. Interferon γ blocks the growth of Toxoplasma gondiiin human fibroblasts by inducing the host cells to degrade tryptophan. Proc Natl Acad Sci USA. 1984;81:908–912. doi: 10.1073/pnas.81.3.908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rapoza P A, Tahija S G, Carlin J P, Miller S L, Padilla M L, Byrne G I. Effect of interferon on a primary conjunctival epithelial cell model of trachoma. Investig Ophthalmol Vis Sci. 1991;32:2919–2923. [PubMed] [Google Scholar]
- 27.Rich E A, Torres M, Sada E, Finegan C K, Hamilton B D, Toossi Z. Mycobacterium tuberculosis(MTB)-stimulated production of nitric oxide by human alveolar macrophages and relationship of nitric oxide production to growth inhibition of MTB. Tuber Lung Dis. 1997;78:247–255. doi: 10.1016/s0962-8479(97)90005-8. [DOI] [PubMed] [Google Scholar]
- 28.Rollwagen F M. Role of natural killer cells in the early clearance of Rickettsia typhi in mice. In: Eisenstein T K, Bullock W E, Hanna N, editors. Host defenses and immunomodulation to intracellular pathogens. New York, N.Y: Plenum Press; 1988. pp. 163–168. [DOI] [PubMed] [Google Scholar]
- 29.Rollwagen F M, Dasch G A, Jerrells T R. Mechanisms of immunity to rickettsial infection: characterization of a cytotoxic effector cell. J Immunol. 1986;136:1418–1421. [PubMed] [Google Scholar]
- 30.Roux V, Raoult D. Phylogenetic analysis of the genus Rickettsiaby 16S rDNA sequencing. Res Microbiol. 1995;146:385–396. doi: 10.1016/0923-2508(96)80284-1. [DOI] [PubMed] [Google Scholar]
- 31.Roux V, Rydkina E, Eremeeva M, Raoult D. Citrate synthase gene comparison, a new tool for phylogenetic analysis, and its application for the rickettsiae. Int J Syst Bacteriol. 1997;47:252–261. doi: 10.1099/00207713-47-2-252. [DOI] [PubMed] [Google Scholar]
- 32.Sandau K, Pfeilschifter J, Brüne B. The balance between nitric oxide and superoxide determines apoptotic and necrotic cell death of rat mesangial cells. J Immunol. 1997;158:4938–4946. [PubMed] [Google Scholar]
- 33.Sandau K, Pfeilschifter J, Brüne B. Nitric oxide and superoxide induced p53 and Bax accumulation during mesangial cell apoptosis. Kidney Int. 1997;52:378–386. doi: 10.1038/ki.1997.344. [DOI] [PubMed] [Google Scholar]
- 34.Santucci L A, Gutierrez P L, Silverman D J. Rickettsia rickettsiiinduces superoxide radical and superoxide dismutase in human endothelial cells. Infect Immun. 1992;60:5113–5118. doi: 10.1128/iai.60.12.5113-5118.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schmitz J L, Carlin J M, Borden E C, Byrne G I. Beta interferon inhibits Toxoplasma gondiigrowth in human monocyte-derived macrophages. Infect Immun. 1989;57:3254–3256. doi: 10.1128/iai.57.10.3254-3256.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shemer Y, Kol R, Sarov I. Tryptophan reversal of recombinant human gamma-interferon inhibition of Chlamydia trachomatisgrowth. Curr Microbiol. 1987;16:9–13. [Google Scholar]
- 37.Shemer-Avni Y, Wallach D, Sarov I. Reversion of the antichlamydial effect of tumor necrosis factor by tryptophan and antibodies to beta interferon. Infect Immun. 1989;57:3484–3490. doi: 10.1128/iai.57.11.3484-3490.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Silverman D J. Oxidative cell injury and spotted fever group rickettsiae. In: Anderson B E, editor. Rickettsial infection and immunity. New York, N.Y: Plenum Press; 1997. pp. 79–98. [Google Scholar]
- 39.Silverman D J, Santucci L A, Meyers N, Sekeyova Z. Penetration of host cells by Rickettsia rickettsiiappears to be mediated by a phospholipase of rickettsial origin. Infect Immun. 1992;60:2733–2740. doi: 10.1128/iai.60.7.2733-2740.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Stothard D R, Fuerst P A. Evolutionary analysis of the spotted fever and typhus groups of Rickettsiausing 16S rRNA gene sequences. Syst Appl Microbiol. 1995;18:52–61. [Google Scholar]
- 41.Teysseire N, Boudier J A, Raoult D. Rickettsia conoriientry into Vero cells. Infect Immun. 1995;63:366–374. doi: 10.1128/iai.63.1.366-374.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Teysseire N, Chiche-Portiche C, Raoult D. Intracellular movements of Rickettsia conorii and R. typhibased on actin polymerization. Res Microbiol. 1992;143:821–829. doi: 10.1016/0923-2508(92)90069-z. [DOI] [PubMed] [Google Scholar]
- 43.Turco J, Winkler H H. Role of nitric oxide synthase pathway in inhibition of growth of interferon-sensitive and interferon-resistant Rickettsia prowazekiistrains in L929 cells treated with tumor necrosis factor alpha and gamma interferon. Infect Immun. 1993;61:4317–4325. doi: 10.1128/iai.61.10.4317-4325.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Villalta F, Zhang Y, Bibb K E, Kappes J C, Lima M F. The cysteine-cysteine family of chemokines RANTES, MIP-1α, and MIP-1β induce trypanocidal activity in human macrophages via nitric oxide. Infect Immun. 1998;66:4690–4695. doi: 10.1128/iai.66.10.4690-4695.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Vishwanath S. Antigenic relationships among the rickettsiae of the spotted fever and typhus groups. FEMS Microbiol Lett. 1991;81:341–344. doi: 10.1016/0378-1097(91)90238-6. [DOI] [PubMed] [Google Scholar]
- 46.Walker D H, Firth W T, Edgell C-J S. Human endothelial cell culture plaques induced by Rickettsia rickettsii. Infect Immun. 1982;37:301–306. doi: 10.1128/iai.37.1.301-306.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Walker D H, Gear J H S. Correlation of the distribution of Rickettsia conorii, microscopic lesions, and clinical features in South African tick bite fever. Am J Trop Med Hyg. 1985;34:361–371. doi: 10.4269/ajtmh.1985.34.361. [DOI] [PubMed] [Google Scholar]
- 48.Walker D H, Henderson F W. Effect of immunosuppression on Rickettsia rickettsiiinfection in guinea pigs. Infect Immun. 1978;20:221–227. doi: 10.1128/iai.20.1.221-227.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Walker D H, Occhino C, Tringali G R, Di Rosa S, Mansueto S. Pathogenesis of rickettsial eschars: the tache noire of boutonneuse fever. Hum Pathol. 1988;19:1449–1454. doi: 10.1016/s0046-8177(88)80238-7. [DOI] [PubMed] [Google Scholar]
- 50.Walker D H, Popov V L, Crocquet-Valdes P A, Welsh C J R, Feng H-M. Cytokine-induced, nitric oxide-dependent, intracellular antirickettsial activity of mouse endothelial cells. Lab Investig. 1997;76:129–138. [PubMed] [Google Scholar]
- 51.Walker D H, Popov V L, Wen J, Feng H-M. Rickettsia conoriiinfection of C3H/HeN mice. A model of endothelial-target rickettsiosis. Lab Investig. 1994;70:358–368. [PubMed] [Google Scholar]
- 52.Walker D H, Staiti A, Mansueto S, Tringali G. Frequent occurrence of hepatic lesions in boutonneuse fever. Acta Trop. 1986;43:175–181. [PubMed] [Google Scholar]
- 53.Walker D H, Watkins N G, Dumler J S, Vishwanath S. Experimental skin lesion (tache noire) in guinea pigs after intradermal inoculation of Rickettsia conorii. Immunol Infect Dis. 1992;2:51–59. [Google Scholar]
- 54.Walker T S. Rickettsial interactions with human endothelial cells in vitro: adherence and entry. Infect Immun. 1984;44:205–210. doi: 10.1128/iai.44.2.205-210.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wisseman C L, Jr, Waddell A. Interferonlike factors from antigen- and mitogen-stimulated human leukocytes with antirickettsial and cytolytic actions on Rickettsia prowazekii. Infected human endothelial cells, fibroblasts, and macrophages. J Exp Med. 1983;157:1780–1793. doi: 10.1084/jem.157.6.1780. [DOI] [PMC free article] [PubMed] [Google Scholar]