Abstract
The rationale for using thalidomide (THD) as a treatment for nausea and vomiting during pregnancy in the late 1950s appears to have been based on its sedative or hypnotic properties. In contrast to contemporaneous studies on the anti-emetic activity of phenothiazines, we were unable to identify publications reporting preclinical or clinical evaluation of THD as an anti-emetic. Our survey of the literature revealed a clinical study in 1965 showing THD reduced vomiting in cancer chemotherapy which was substantiated by similar studies from 2000, particularly showing efficacy in the delayed phase of chemotherapy-induced nausea and vomiting. To identify the mechanism(s) potentially involved in thalidomide's anti-emetic activity we reviewed its pharmacology in the light of nausea and vomiting mechanisms and their pharmacology with a particular emphasis on chemotherapy and pregnancy. The process identified the following potential mechanisms: reduced secretion of Growth Differentiation Factor 15, suppression of inflammation/prostaglandin production, downregulation of cytotoxic drug induced upregulation of iNOS, and modulation of BK (KCa1.1) channels and GABAA/glutamate transmission at critical points in the emetic pathways (nucleus tractus solitarius, area postrema). We propose ways to investigate these hypothesized mechanisms and discuss the associated challenges (e.g., objective quantification of nausea) in addition to some of the more general aspects of developing novel drugs to treat nausea and vomiting.
Keywords: Chemotherapy, GDF15, Hyperemesis gravidarum, Nausea, Thalidomide, Vomiting
Graphical abstract
Highlights
-
•
The mechanism of the anti-emetic effect of thalidomide in pregnancy and chemotherapy is not known.
-
•
Thalidomide's anti-emetic use and pharmacology is reviewed and potential mechanisms discussed.
-
•
Thalidomide-induced reduction of GDF15, iNOS and proinflammatory gene expression are possible mechanisms.
-
•
Potential neural targets are KCa1.1, GABAA/glutamate at critical points in emetic pathways.
-
•
The challenges of testing proposed mechanisms and developing novel anti-emetics are discussed.
Abbreviations
- AMPA
α-amino-3-hydroxy = 5-methyl-4-isoxazoleproprionic acid receptor
- AP
area postrema
- AVP
arginine vasopressin
- BBB
blood brain barrier
- BK
the KCa 1.1 potassium activated calcium channel
- cAMP
cyclic adenosine mono-phosphate
- CB1
cannabinoid receptor1
- CINV
chemotherapy-induced nausea and vomiting
- Cmax
maximum concentration achieved
- COVID-19
Coronavirus disease 2019
- COX
cyclooxygenase enzyme
- CRBN
cereblon
- CRR
complete response rate (see text for definition in relation to specific parameters)
- CRL4
cullin-RING ligase 4
- D
dopamine receptor
- EC
enterochromaffin cells
- FOLFOX
5-fluorouracil, leucovorin, and oxaliplatin
- GABA
gamma-aminobutyric acid
- GDF15
growth differentiation factor 15
- GDNF
glial derived neurotrophic factor
- GFRAL
GDNF family receptor alpha like proto-oncogene tyrosine protein kinase receptor
- H
histamine receptor
- 5-HT3 RA
5-hydroxytryptamine 3 receptor antagonist
- iNOS
inducible nitric oxide synthase
- IL
Interleukin
- KCa
1.1 potassium activated calcium channel
- M
muscarinic acetylcholine receptor
- mRNA
messenger ribonucleic acid
- MPTP
1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine
- NF-κB
nuclear factor-κB
- NLRP3
NOD-like receptor, pyrin domain containing-3
- NMDA
N-methyl -D-aspartate receptor
- nNOS
neuronal nitric oxide synthase
- NTS
nucleus tract solitarius
- PDE4
phosphodiesterase 4 enzyme
- PONV
post-operative nausea and vomiting
- PTZ
pentylenetetrazole
- REM
rapid eye movement (sleep)
- RYR
ryanodine receptor
- SARS-CoV-2
Severe Acute Respiratory Syndrome coronavirus 2
- SP
substance P
- tmax
time at which maximum concentration was achieved
- T3
thyroxine
- T4
triiodothyronine
- THD
thalidomide
- TRPV1
transient receptor potential channel V1
- TGF-β
transforming growth factor β
- TNFα
tumour necrosis factor-α
1. Introduction
The teratogenic effects of thalidomide (THD) administration during early pregnancy in humans are unfortunately well known, particularly in giving rise to limb deformation (Lenz, 1962; McBride, 1961). The molecular mechanism responsible is now known to be due to THD binding to cereblon, inhibiting CRL4CRBN E3 ubiquitin ligase activity (Ito et al., 2010; Chang and Stewart, 2011; Peach et al., 2020; Yamamoto et al., 2022). THD was used clinically as a “non-toxic” sedative, beginning in late 1956 and continuing until its withdrawal in 1961 (Franks et al., 2004; Rehman et al., 2011). In particular, THD was used to clinically manage women experiencing nausea and vomiting during the first trimester of pregnancy, more popularly called “morning sickness”, although it can occur at any time of day (Whitehead et al., 1992; Flaxman and Sherman, 2000; Liu et al., 2022). Surprisingly, THDs ability to directly alleviate “morning sickness” does not appear to have been based on preclinical studies or controlled clinical trials. In addition, no mechanism was proposed at the time for this action of THD, beyond a hypothesis that reduced nausea and vomiting would result from a decreased anxiety effect (sedation) following drug treatment.
In the last 5 years there has been an increasing body of evidence showing efficacy of THD against anti-cancer chemotherapy-induced nausea and vomiting (CINV) (Zhang et al., 2017; Alhifany et al., 2020; Wang et al., 2020; Xie et al., 2022). Due to this resurgence of interest, we reviewed the historical literature on THD's use to control nausea and vomiting, together with current knowledge of the pharmacology of THD. Our analysis identified varying levels of evidence that THD has actions against nausea and vomiting in three different clinical settings but that the mechanism of this activity remains unknown.
Describing drugs by their therapeutic orientation (e.g., anti-emetic, anti-hypertensive, parasympatholytic, diuretic) rather than by activity at their predominant molecular target has recently been challenged (Seifert and Alexander, 2022). In the area of drugs with potential ‘anti-emetic’ actions this terminology does not differentiate between clinically important differences in drug action against nausea and vomiting so in this review, where possible, the use of the term ‘anti-emetic’ has been avoided except when used historically (Sanger and Andrews, 2022).
We discuss the most likely pharmacological mechanisms of action(s) of THD against nausea and vomiting, suggest how they can be tested and discuss the implications for the treatment of nausea in particular, often a poorly-met clinical need in many patient groups (Sanger and Andrews, 2018).
2. What is thalidomide?
Thalidomide (α-(N-phthalimido)glutarimide; C13H10N2O4) (Fig. 1) is a glutamic acid derivative and an analog of glutethimide (Sneader, 2005). The reported date of synthesis by the pharmaceutical company Chemie Grünenthal is inconsistent, with Theoret (1962) stating 1953 but others giving 1954 (Stephens and Brynner, 2001; Rehman et al., 2011). THD is a racemic mixture of two optical isomers, S-and R–enantiomers which rapidly interconvert in physiological solutions (https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=732) and undergo spontaneous hydrolysis at physiological pH (Schumacher et al., 1965a). Enantiomeric specificity of effects has been identified for THD's teratogenic (S-, Vargesson, 2015) and sedative activity (R+: Höglund et al., 1998; Reist et al., 1998; Eriksson et al., 2001), and ability to reduce tumour necrosis factor α (TNFα) release from mononuclear blood cells (S-, Wnendt et al., 1996).
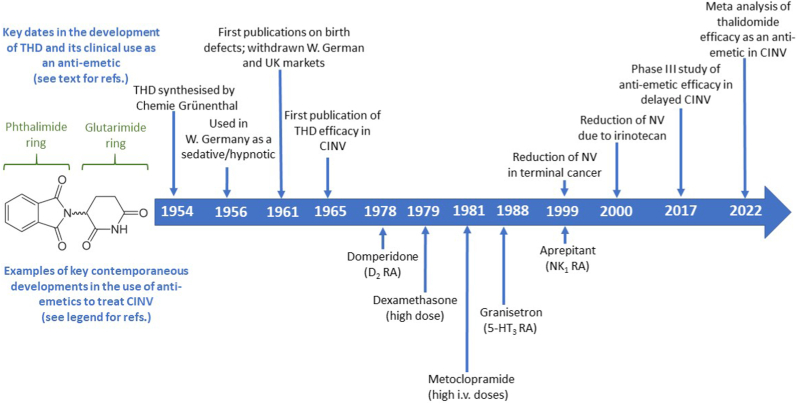
Fig. 1.
Timeline showing the key dates in the development of thalidomide and its clinical use as an ‘anti-emetic’ (upper row; see text for references and details) and examples of key contemporaneous developments in the use of ‘anti-emetics’ to treat anti-cancer chemotherapy –induced nausea and vomiting (CINV). Sources are: Domperidone, Huys, 1978; Dexamethasone, Baker et al., 1979; Metoclopramide (high dose), Gralla et al. (1981); Granisetron, Cassidy et al., 1988; Aprepitant, Navari et al., 1999 (see References for details).
One complication in understanding the pharmacology of THD is that it is degraded into multiple metabolites, with at least 12 identified in the rat (Fabro et al., 1965; Schumacher et al., 1965b). Absorption of THD occurs within the digestive tract with plasma tmax values of 2–4 h following oral dosing in humans (Eriksson et al., 2001) and a Cmax of ∼2 μg/ml (∼10 μM) following a dose of 200 mg (Teo et al., 1999; Bai et al., 2013). THD is blood-brain barrier (BBB) permeant, with a brain/plasma ratio of 0.89 in rodents (Huang et al., 2005). It should be noted that the BBB is relatively permeable in areas implicated in emetic mechanisms (area postrema [AP] and the immediate subjacent part of the nucleus tractus solitarius [NTS]; Stern et al., 2011), so these brain regions may be exposed to the more polar metabolites present in plasma.
Since the identification of the anti-angiogenic and immunomodulatory effects of THD there has been continuing interest in developing analogues with increased therapeutic potency and reduced toxicity (Peach et al., 2020); for example, lenalidomide is widely used in anti-cancer chemotherapy (Sherbet, 2015). Research into “thalidomide-like” drugs has also been undertaken to identify compounds lacking cereblon binding due to the glutarimide ring (Fig. 1) but which preserve the immunomodulatory activity, such as N-Adamantyl Phthalimidine (Hsueh et al., 2021). We do not discuss THD analogues or THD-like drugs further as they have not been tested directly for anti-emetic effects.
3. Thalidomide's sedative and hypnotic activity
The sedative activity of THD was first identified through a 50% reduction in spontaneous motor activity in mice, but without loss of the righting reflex, and without overt symptoms of central nervous system depression or toxicity in doses up to 4 g/kg (Kunz, 1956). These observations were subsequently confirmed and extended to show that, in mice, THD increased barbiturate-induced sleeping time and potentiated the catatonia produced by chlorpromazine or reserpine (Osterloh, 1959; Somers, 1960). THD-mediated sedation was later shown in dogs (Kuhn and Van Maanen, 1961), rats (Fabro et al., 1965), rabbits (Kimura, 1973) and cats (Kaitin, 1985) but not in monkeys (Kuhn and Van Maanen, 1961). In humans, early reports indicated sedation and improved sleep at the dose of 25–200 mg three times daily (Jung, 1956; Salter et al., 1959; Lasagna, 1960). These findings are supported by more recent clinical trials (e.g., a double-blind study by Höglund et al., 1998) including a small study in healthy males using polysomnography, showing an increase in both rapid-eye movement (REM) and stage 3–4 sleep at a dose of 100mg/night (Kanbayashi et al., 1999). The dose of THD used in the sleep studies is comparable to that used in recent clinical trials of anti-emetic efficacy (100–200 mg; see below). It is notable that in the Discussion of their 1965 publication Fabro et al. (1965, p.359) commented “Man, however, appears to be a species uniquely sensitive to the sedative and embryotoxic effects of the drug, since the dose used clinically is relatively low (100 to 200 mg)”.
Importantly, THD was shown to have a wide safety margin in dogs and this was supported by subsequent clinical findings of recovery following ingestion of 16–160 times the sedative dose (see Mann, 1984, for detailed analysis of original publications in German). This safety margin was interpreted as an indication that THD was “safe” to use; at the time only limited preclinical safety tests were undertaken and were not mandatory either in Germany or the United Kingdom (see Ferner and Aronson, 2022 for history of medicines regulation in the UK), the two initial markets for THD. Thus, THD was brought into wide-spread use, since in humans it was orally active, did not cause gastric irritation or vomiting, had no serious dependence issues (Mann, 1984), and appeared to induce deep, natural sleep without a “morning hang-over” (a point made in a letter to the Lancet in 1960 reporting 4 cases of peripheral neurological effects of long term THD treatment; Florence, 1960). It was marketed initially in Germany (January 1957) as a day-time sedative (Contergan™, 25–50 mg, given twice or four times daily) to reduce stress and anxiety and as a night-time hypnotic to facilitate sleeping (Contergan forte™, 50–100 mg) (Daemmrich, 2015).
4. Origins of thalidomide's use as an anti-emetic in pregnancy
Academic accounts of the history of THD focus on its use as a drug with sedative or hypnotic (“tranquilizer”) properties (e.g., Mann, 1984; Stephens and Brynner, 2001; Botting, 2016; Appelbe, 2005; Sneader, 2005). Employing sedatives for the treatment of women in pregnancy was not unusual in the 1950s so it is perhaps unsurprising that THD was used in this role, and according to Daemmrich (2015) “Taking thalidomide to treat nausea during pregnancy appears to have spread among physicians and pregnant women in West Germany during the first half of 1959”.
As far as we can ascertain there are no published (peer review) clinical studies on the efficacy of THD in treating either ‘morning sickness’ or the intense nausea and vomiting occurring during later stages of pregnancy (hyperemesis gravidarum). However, the UK Sunday Times newsapaper investigation into THD initially published in 1979 provides some important insights regarding anti-emetic effects. The quotations and page number references in the following sections are from the 1980 paperback edition of the book “Suffer the Children: The story of Thalidomide” (Sunday Times Insight Team, 1980).
Smith, Kline and French were offered the American licence for THD by Chemie Grünenthal (Sunday Times Insight Team, 1980, p.31) and so investigated its properties in 1956 reporting that “thalidomide had no capacity to block a conditioned-escape reflex(suggesting it had no tranquillizing potential), no antiemetic activity (our underline), no anti-histamine activity, and only slight to moderate anti-spasmodic activity” (Sunday Times Insight Team, 1980, p.32-33). There are no details of the antiemetic test, but it is likely to have used the dog and investigated the effect of THD against systemic apomorphine or intragastric copper sulphate-induced vomiting as this was the model commonly in use at the time (Borison and Wang, 1953). In 1960 in Australia, the New South Wales representative of Distillers Company Biochemicals (Australia) Ltd. provided samples of Distaval (THD) to the pharmacy of the Women's Hospital, Sydney via Dr J. Newlinds. One of the clinicians at this hospital, Dr W. McBride, subsequently treated as an emergency admission a pregnant woman unresponsive to anti-emetic therapy who had been vomiting for several days and in danger of miscarrying; after two doses (100 mg each) of THD the vomiting stopped, and the patient gave birth to a healthy child in 1961. The Sunday Times Insight Team (1980, p.16) reported “McBride was very impressed and began to prescribe Distaval for pregnant women who complained of morning sickness, nervousness, or inability to sleep. The drug appeared both effective and without side effects”. It is interesting to note that it was Dr McBride who was amongst the first clinicians to recognise and publish the link between thalidomide administration in early pregnancy and congenital abnormalities (McBride, 1961, but see also Lenz, 1962). A further coincidence is that Dr Newlinds (see above) was not particularly interested in “another sedative”, as they were undertaking a trial of thiethylperazine (Torecan™; a phenothiazine), then marketed by Sandoz as an anti-nauseant and therefore of potential utility in treating morning sickness (see Sunday Times Insight Team, 1980, p.15). The results of the trial on the effects of thiethylperazine on nausea and vomiting in pregnancy were published in 1964 (Newlinds, 1964).
At around the same time (1959), Chemie Grünenthal licenced THD to Richardson-Merrell in the USA (Sunday Times Insight Team, 1980, p.95-96). Amongst a long list of possible therapeutic indications, THD was proposed as a treatment for anxiety and apprehension associated with a number of conditions including nausea and vomiting (Sunday Times Insight Team, 1980, p.95). It is also of relevance that Dr R. Pogge, the medical director of Richardson-Merrell, asked Dr R.O. Nulsen, an obstetrician and gynaecologist to “try out a new drug that might well take the place of barbiturates” (Sunday Times Insight Team, 1980, p.112-113). These studies began in 1959 and then in 1960 Dr Pogge asked Dr Nulsen “if he would try thalidomide in the next six to eight patients who were suffering from nausea in pregnancy” (Sunday Times Insight Team, 1980, p.116). We are unable to locate a publication on the outcome of this study.
From these reports, two important points emerge: i) using a drug to manage the anxiety associated with nausea and vomiting in early pregnancy, was not considered unreasonable; ii) although THD may initially have been prescribed as a sedative or hypnotic there is evidence to suggest that by 1960 there was interest in its potential to specifically treat nausea or vomiting during pregnancy. This view is supported by McBride's original Letter to the Lancet in 1961 reporting initial observations about THD and birth defects in which he comments, women “were given the drug thalidomide (‘Distaval’) during pregnancy as an anti-emetic (our underlining) or as a sedative” (McBride, 1961). Apart from this quotation and the comment by McBride quoted above (Sunday Times Insight Team, 1980, p.16) we have been unable to find publications reporting the efficacy of THD against either nausea or vomiting at any stage of pregnancy; however, the above quotations provide some very limited indications of the perception of this possibility.
The apparent absence of pivotal publications reporting data on anti-emetic efficacy in pregnancy has not hindered the widespread acceptance of THDs ‘anti-emetic’ activity particularly in ‘morning sickness’, as exemplified by the selected publications in Table 1. Research publications and reviews describing the claim of anti-emetic activity of THD in pregnancy often do not give references for these statements (see Table 1). This lack of key validation of THDs anti-emetic action prior to human use is unusual, as there were contemporaneous preclinical studies of other ‘anti-emetic’ drugs using established carnivore models (e.g., chlorpromazine, Boyd et al., 1954; Brand et al., 1954) and human clinical trials (e.g., hyoscine, Hill and Guest, 1945; dimenhydrinate, Bignall and Crofton, 1949: Gay and Carliner, 1949), including a single-blinded study of dimenhydrinate in early pregnancy (Carliner et al., 1949).
Table 1.
Selected example publications in the last 20 years commenting on the anti-emetic effects of thalidomide (THD). in pregnancy Bold text is our highlighting of key phrases. The publications illustrate either the lack of a reference to studies reporting data on anti-emetic (nausea or vomiting) efficacy in pregnancy or when references are given, they do not refer directly to studies reporting data on the anti-emetic (nausea or vomiting) efficacy of THD in pregnancy. See text for further details and discussion.
| Quotation about anti-emetic effects of thalidomide in pregnancy | Publication (ranked by year of publication) | Citation given to support statement | Comment |
|---|---|---|---|
| Thalidomide was first developed in the 1950s by the German pharmaceutical company Grünenthal as a sedative or anti-emetic for morning sickness in pregnant women. | Yamamoto et al. (2022), p. 6236 | No citation | Comment is specific for morning sickness and includes both sedative and anti-emetic actions. |
| The unintended teratogenic effect of thalidomide (THD) prescribed to treat morning sickness in pregnant women is a historic tragedy. | Xie et al. (2022), p.2 | No citation | Comment is specific for morning sickness and “prescription” implies that its treatment was the intent of the prescriber. Note that THD was available over the counter. |
| Thalidomide is a synthetic glutamic derivative, it was approved for pregnancy-associated morning sickness in 1957, although a few years later was withdrawn because of teratogenicity. | Was et al. (2022), p.9 | No citation | Comment is specific for morning sickness and the use of the word “approval” implies some form of regulatory process. |
| Thalidomide was first developed to treat morning sickness and sold over the counter to pregnant women in Germany in the 1950s, with recommended doses in the range of aspirin treatments (300–500 mg). | Schein (2021), p.16 | Schein, 2020 | Comment implies data/trials to support development. Schein, 2020, cites Rehman et al., 2011, use of THD in morning sickness |
| Thalidomide was marketed as a safe and effective sedative beginning in 1957 and was later found to be effective at treating morning sickness. | Vargesson and Stephens (2021), p.1455 | No citation | Comment indicates primary use was sedation and efficacy specifically against morning sickness was discovered subsequently. |
| Thalidomide (THD) is a derivative of glutarnic [sic]acid, which was initially used as a sedative to treat emesis in pregnancy …. | Wang et al. (2020), p. 4561 | No citation | Implies that the sedative and anti-emetic actions are linked. |
| It was very popular at the time, being distributed in at least 46 countries worldwide as an effective drug in relieving morning sickness. | Vargesson (2019), p.88 | No citation | Statement implies efficacy in morning sickness. |
| Thalidomide was developed in 1957 by the German pharmaceutical compnay Chemie Grünenthal as a sedative used by pregnant women to ameliorate morning sickness. | Gemechu et al. (2018), p.11802 | No citations | Comment is specific for morning sickness. |
| In 1957, this drug was released into the market as an over-the -counter drug of a non-addictive/non-barbiturate sedative as well as an anti-emetic. Thalidomide ameliorated “morning sickness” in pregnant women and was tragically believed to be harmless. | Yashiro et al. (2018), p. 2250. | No citation | Amelioration implies efficacy against morning sickness. |
| Thalidomide was introduced in the 1950s as a safe antiepileptic drug. In 1957, it was commercialized as a safe sedative and was widely used as an antiemetic (Randall, 1990; Perri and Hsu, 2003) | Islas-Espinoza et al. (2018), p. 671 | Randall (1990); Perri and Hsu (2003) | Statement does not confine anti-emetic efficacy only to pregnancy. |
| Randall, 1990, is a brief history of THD. Perri and Hsu (2003), reviews the history of THD and its use in dermatology-it cites Stirling, 1988 [3] as the source for the statement “Pregnant women frequently treated their nausea of pregnancy with thalidomide.[3] | |||
| Thalidomide (THD) was able to significantly ameliorate nausea and vomiting in pregnancy, but was withdrawn in Europe as a result of teratogenicity in the late 1950s. | Zhang et al. (2017), p. 3559 | No citation | Amelioration implies efficacy against both nausea and vomiting and “in pregnancy” implies efficacy at any stage of pregnancy. |
| Thalidomide was first introduced in the late 1950s as a sedative for pregnant women to prevent morning sickness [28]. | Shi and Chen (2017), p. 3 | Citation to Ito and Handa, 2016 | Comment links sedation and anti-emetic efficacy. Reference [28] is Ito and Handa, 2016, Cereblon and its downstream substrates as molecular targets of immunotherapy drugs. |
| Thalidomide was released in the late 1950's as a nonaddictive, nonbarbiturate sedative by the German pharmaceutical company, Chemie-Grunenthal (Fig. 1). Thalidomide was very effective and quickly discovered to also be an effective anti-emetic and used to treat morning sickness in pregnant women. | Vargesson (2015), p. 140. | No citation | Statement implies efficacy in morning sickness. |
| Thalidomide is a sedative that was first introduced in the late 1950s for treatment of morning sickness and insomnia [1,2]. | Han et al. (2014), p. 361 | Citation given to Reid et al. (2012), and Badros (2012) | Reid et al. (2012) is a Cochrane review of the utility of thalidomide in the management of cancer cachexia and Badros, 2012 is an editorial about the use of lenalidomide in myeloma. |
| Initially marketed as Contergan, thalidomide was prescribed as a nonbarbiturate hypnotic sedative able to produce deep sleep without hangover or risk of dependency … … … Soon available world-wide, the drug became popular for its anti-emetic effect in pregnant women suffering with morning sickness. | Rehman et al. (2011), p. 291. | No citation | Statement implies efficacy in morning sickness. |
| Thalidomide, a derivative of glutamic acid, was introduced in Europe in 1954 as a sedative/hypnotic agent and was used to ameliorate nausea in pregnancy. | Liu et al. (2009), p. 692. | No citation | Comment is specific to nausea and statement implies it is efficacious at any stage of pregnancy. |
| It was initially marketed as a sedative, with its rapid speed of onset, lack of hangover effect, and apparent safety after overdose making it an alternative to barbiturates. In addition it was a powerful antiemetic, and was widely taken by pregnant women for the treatment of morning sickness. | Gordon and Goggin (2003), p. 127 | No citation | Statement implies efficacy in morning sickness. |
| Early studies done in 1953 established the anxiolytic, hypnotic, antiemetic, and adjuvant analgesic properties of thalidomide (44, 45) | Mujagic et al. (2002), p. 275 | Citations to Fabro et al. (1965) and Smithells (1966). | Fabro et al. (1965) describes the metabolism of thalidomide, its biological effects and those of some metabolites but no studies of antiemetic activity; Smithells (1966) describes mobility and mental health rehabilitation aspects of children affected by thalidomide. |
5. Recent evidence for efficacy of thalidomide as an anti-emetic in chemotherapy
More recent studies have provided evidence for efficacy of THD in treatment of nausea and vomiting. A preclinical study published in 2014 investigated the potential anti-emetic use of THD in a rat model of kaolin consumption (pica) induced by the cancer chemotherapeutic agent cisplatin (Han et al., 2014). Since rodents are unable to vomit (Sanger et al., 2011; Horn et al., 2013), increased consumption of kaolin following administration of an agent which would induce vomiting in an emetic species is used to indicate activation of pathways which may result in nausea and leaned aversions (see Stern et al., 2011). THD was given by gavage (10 mg/kg) simultaneously with cisplatin (10 mg/kg, intraperitoneally) (Han et al., 2014). THD was without effect in the first 24 h, considered to equate to the acute phase of chemotherapy-induced nausea and vomiting (CINV) but kaolin consumption was reduced in the THD group 72 h after cisplatin administration, the period believed to represent the delayed phase of high-dose cisplatin-induced nausea and vomiting in humans (Sanger and Andrews, 2018). The 5-hydroxytryptamine3 receptor antagonist (5-HT3RA) granisetron was used as a positive control, representing a widely used class of anti-emetic drug in the control of CINV, which had also demonstrated efficacy in cisplatin-induced pica in rodents (Liu et al., 2005; Malik et al., 2007). The Han et al. (2014) study did not include a group in which THD was given alone nor did it contain any data on food intake (although kaolin intake was expressed as % of total food intake) or locomotor behaviours which would have provided insights into the sedative effects of THD. This study also attempted to investigate THDs mechanism of action reporting that THD given to cisplatin-treated animals reduced the levels of substance P (SP) immunoreactivity in both the stomach (∼38% decrease) and the medulla (∼35% decrease) when measured at 33 h post cisplatin administration but was without effect on levels of gastric and medullary NK1 receptor immunoreactivity following cisplatin. However, although potentially important, these results should be treated with caution until replicated because of the lack of a THD alone group, behavioural data and the lack of key details about the methodology used for immunohistochemistry. Assuming that the data on SP are replicated, they suggest that modulation of synthesis or destruction of transmitters implicated in emesis should not be overlooked in searching for THDs anti-emetic mechanism (see below).
The ability of THD to inhibit nausea and vomiting in CINV has recently been investigated in several clinical trials (Fig. 1). The earliest trial we identified was a publication in Italian in 1965. The paper entitled [Use of the imide of N-phthalylglutamic acid (thalidomide) in the symptomatic therapy of vomiting of many patients with malignant neoplasms or caused by administration of mechlorethamine HCl] (Traldi et al., 1965) reports a reduction in the intensity of vomiting but does not comment on any effects against nausea. As far as we can ascertain, this paper was the first to propose a mechanism for the ‘anti-emetic’ effect of THD, hypothesising a blockade of visceral afferent impulses to the brainstem or interruption of the vomiting reflex arc in the brainstem; the authors considered the second hypothesis more likely in view of THDs sedative properties. We return to these potential mechanisms below.
As part of a study of the effects of THD on cachexia in patients with terminal cancer Bruera et al. (1999) showed a significant reduction in nausea and an increase in appetite (see also Reid et al., 2012). The next clinical study reporting ‘anti-emetic’ effects of THD was in 2000 (Govindarajan et al., 2000). These authors conducted a pilot study (9 patients) and showed that when compared with historical data, the co-administration of THD (400 mg/day, administered at bedtime) appeared to prevent or greatly reduce the late onset diarrhoea, nausea and vomiting associated with use of irinotecan for treatment of metastatic colorectal cancer.
Several studies were subsequently conducted in China. For example, Liu et al. (2009) used a crossover design in patents receiving a modified FOLFOX7 chemotherapy regime (primarily for gastric and colorectal tumours) to investigate the effects of addition of THD (150 mg twice daily) to a combination of ramosetron (a 5-HT3RA) and dexamethasone on nausea and vomiting on days 2–5 following chemotherapy (delayed phase) using a crossover design between therapy cycles one and two. A significant increase in the complete response rate (CRR) in the THD group for nausea occurred on days 2–4 (∼24–34% increase in CRR, defined as no nausea) and for vomiting on days 2 and 3 (∼20% increase in CRR outcome measure for vomiting defined as no emetic episodes [vomiting and/or retching in defined time epochs] and no rescue medication). In the same study, the complete response rate for anorexia (defined as normal food intake) was higher in the THD treatment group on days 2–5. Interestingly, the frequency of sedation/dizziness was higher at 42% in the THD group compared to 9.6% without thalidomide; no other side effects differed significantly. This data supports early studies on the sedating effects of THD. In another, more recent study Zhang et al. (2017) used a randomized, multi-centre, double-blind, placebo-controlled design trial in patients receiving highly emetogenic chemotherapy to investigate the effect of addition of THD (100 mg twice daily) on days 1–5 to a palonosetron/dexamethasone regime. Both nausea and vomiting CRR were significantly increased (i.e. a reduction in both) in the THD group on days 2–5, with a 15.2% increase for vomiting CRR (no emetic episodes [vomiting and/or retching in defined time epochs] and no rescue medication) and 14% for nausea CRR (score of zero on a 4 point Likert scale). Sedation was increased in the THD group (12.6% vs. 5.6%) and insomnia reduced (7.6% vs. 15.9%). This study prompted some correspondence on the advantages and disadvantages of the addition of THD (Chong and Chan, 2018; Zhang et al., 2018). Overall, these studies show that THD can reduce nausea and vomiting, particularly in the delayed phase of CINV.
Two recent systematic reviews of the CINV studies with THD define its ability to inhibit nausea and vomiting effects. The first looked at 14 randomized control trials including 1744 patients receiving mostly a platinum-based chemotherapy with a 5-HT3RA with or without dexamethasone (Wang et al., 2020). The analysis showed that THD enhanced the complete response rate of nausea and vomiting in the delayed and overall phases; the efficacy of the 100 and 200 mg/day doses appeared similar. A further systematic review and meta-analysis included 34 studies published from 2009 to 2018, involving a total of 3168 patients receiving highly ‘emetogenic’ chemotherapy (Xie et al., 2022). In terms of complete responder rate, THD plus a 5-HT3RA with or without dexamethasone was significantly higher than 5-HT3RA with or without dexamethasone in the acute phase (74.4% vs. 67.4%), delayed phase (70.6% vs 50.4%), and overall phase (68.4% vs 53.4%). In terms of the no nausea rate, the THD treatment group was also significantly higher than the control group in the acute phase (61.7% vs. 55.5%), delayed phase (50.5% vs. 30.0%), and overall phase (44.6% vs. 29.9%). These analyses show that although THD has some efficacy in the acute phase of CINV the efficacy is greater in the delayed phase.
6. What is the mechanism of the ‘anti-emetic’ activity of thalidomide?
The above clinical studies in CINV, terminal cancer and the historical reports of THD efficacy in pregnancy provide a basis for attempting to identify the mechanism(s) underlying its effects against nausea and vomiting. Additionally, a recent study reporting the use of THD to treat symptoms of COVID-19 commented on the possible therapeutic benefit of its anti-emetic effect (Li et al., 2021). Nausea, vomiting and diarrhoea are symptoms of SARS-coV-2 infection with the mechanisms implicated in emesis proposed to be similar to those involved in CINV (see Andrews et al., 2020 for review).
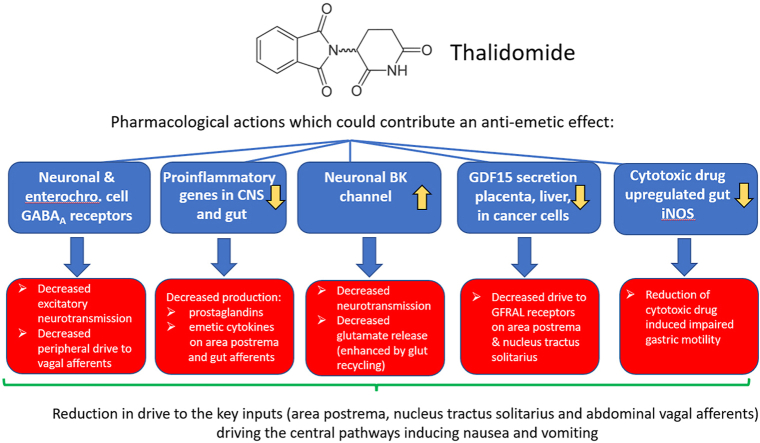
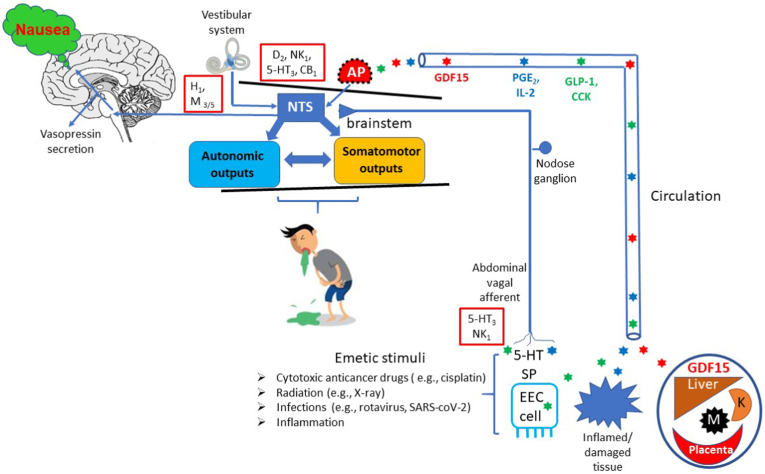
To identify how THD could exert an effect against nausea and/or vomiting we examined the known pharmacology of THD for links to the mechanisms of nausea and vomiting, particularly in relation to CINV and pregnancy. Fig. 2 summarises the key pathways implicated in induction of nausea and vomiting together with their pharmacology to provide a background to the following discussion. Several of the proposed mechanisms are speculative but are included to provide testable hypotheses.
Fig. 2.
A summary of the main pathways implicated in induction of nausea and vomiting and the neurotransmitters/signalling molecules (see Stern et al., 2011; Sanger and Andrews, 2018; Zhong et al., 2021 for details and references). The main inputs (vestibular system, area postrema [AP] and abdominal vagal afferents) inducing nausea and vomiting converge on the nucleus tractus solitarius (NTS). The AP is outside the blood brain barrier and can be activated by a range of circulating substance. For example: Growth Differentiation Factor 15 (GDF15) from liver, macrophages (M), kidney (K) and placenta; Prostaglandin E2 (PGE2) and Interleukin-2 (IL-2) from inflamed/damaged tissue; Glucagon –like peptide-1 (GLP-1) and cholecystokinin (CCK) from Enteroendocrine Cells (EEC). Substances released from the EEC cells, particularly 5-hydroxytryptmaine (5-HT) and substance P (SP) can also act locally to activate the vagal afferents which can also be sensitised by inflammatory mediators. Red boxes indicate key receptors implicated in emesis in the vestibular system, dorsal vagal complex (NTS + AP) and abdominal vagal afferent activation. Cartoon from Sanger and Andrews (2018). (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
6.1. Sedative activity
Sedation is a consistently reported effect in clinical studies of THD (see Millrine and Kishimoto, 2017), including when evaluated for efficacy against CINV (Zhang et al., 2017). It may be speculated that a general reduction in arousal level may reduce perception of nausea (involving thalamo-cortical arousal) but it is less likely to affect vomiting as this can be evoked even under general anaesthesia (Andrews et al., 1990). However, other drugs with sedative/hypnotic activity such as propofol, midazolam and olanzepine also have ‘anti-emetic’ activity in humans (Borgeat et al., 1992; Tarhan et al., 2007; Davis and Sanger, 2020) so could this be the case for THD? Propofol acts on multiple ligand-gated ion channels, including the GABAA, glycine, nicotinic and weakly, 5-HT3 receptors (Trapani et al., 2000). Positive modulation of the inhibitory function of GABAA receptors by propofol (Barann et al., 2000; Trapani et al., 2000) has been demonstrated in the NTS (McDougall et al., 2008) and the AP (Cechetto et al., 2001) and could contribute to a direct anti-emetic effect in post-operative nausea and vomiting and CINV even when propofol is administered at sub-hypnotic doses (e.g., Ewalenko et al., 1996; Gan et al., 1997; Kim et al., 2000). The sedating benzodiazepine medazoline may also be efficacious against post-operative nausea and vomiting (PONV) at sub-hypnotic doses, and like propofol modulates neuronal activity by binding to the GABAA-benzodiazepine receptor complex (Tarhan et al., 2007). Finally, the antipsychotic drug olanzapine, which also causes sedation can inhibit vomiting (Davis and Sanger, 2020 for review). This drug also antagonises activity at multiple receptors (D1, D2, D3, D4, 5-HT2A, 5-HT2C, 5-HT3, 5-HT6, α1 adrenergic, H1 and m1, m2, m3, m4 receptors) many of which may be involved in the mechanisms of vomiting although antagonism of the H1 receptor is most likely responsible for the sedative actions of olanzapine within the cerebral cortex (Davis and Sanger, 2020).
With regards to THD, binding studies (see below) at a range of different receptor and enzyme targets do not suggest an ability to interact with any of the anti-emetic mechanisms suggested for propofol, medazoline or olanzapine (Kanbayashi et al., 1996). Interestingly, in silico studies suggest that THD can dock with the benzodiazepine pocket of the GABAA receptor (Asadollahi et al., 2019). These findings contrast with an inability of THD to bind to this area (Kanbayashi et al., 1996; see below) but do indicate that further studies are needed to look for an ability of THD to modulate GABAA function (see also 6.2, iii).
Neurophysiological and molecular studies of the sedative/hypnotic actions of THD show that it does not appear to involve the cereblon-mediated ubiquitin/proteasome pathway (Hirose et al., 2020). However, a THD-induced depression of cortical excitatory transmission, partially mediated pre-synaptically, was observed in the absence of an effect on inhibitory transmission (ibid). Earlier animal studies differentiated THD from pentobarbital in several ways: i) THD increased slow wave sleep and rapid eye movement sleep at doses which did not induce ataxia; ii) THD did not reduce pre-optic area activity; iii) THD enhanced the sleep-inducing effect of basal forebrain stimulation (Frederickson et al., 1977; Kaitin, 1985). THD has an acute, rapid onset sedative/hypnotic effect with a different profile from barbiturates. The sedative/hypnotic effects in both human and animal studies suggest a central action involving suppression of excitatory neurotransmission which if it occurred in critical parts of pathways receiving stimuli inducing nausea or vomiting (e.g., NTS, AP) could also directly affect induction of nausea and/or vomiting (rather than indirectly through reduced arousal).
Finally, it should be noted that the impairment of cognitive function by THD (administered 3 h before testing) involves a cereblon mediated modulation of the large-conductance calcium-activated potassium channel (BK channel, KCa 1.1) in the hippocampus (Choi et al., 2018a, Choi et al., 2018b). This observation further shows the potential for an acute central effect of THD which may be of relevance to its ‘anti-emetic action’ (see below).
6.2. Interaction with neurotransmitter receptors and functions
Receptor binding studies with canine brain tissue found no meaningful affinity of THD (up to 10−4 M) for adrenergic (α1, α2), dopamine (D2), and muscarinic (M2) receptors (Ki > 100 mM for the racemate) (Kanbayashi et al., 1996). In a screen (at 10−5 M) THD did not have significant binding to receptors for the following substances: adrenaline (α1, α2, ß); dopamine (D1, D2); histamine (H1, H2); acetylcholine (m1, m2, m3); 5-hydroxytryptamine (5-HT1, 5-HT1A, 5-HT1C, 5-HT2, 5-HT3); benzodiazepine; gamma amino butyric acid (GABAA, GABAB); glycine (strychnine-insensitive/sensitive); glutamate (AMPA); kainate (NMDA); MK-801; phencyclidine; adenosine (A1, A2); opiate; angiotensin II; arginine-vasopressin (AVP1 or V1A); atrial natriuretic factor; cholecystokinin; epidermal growth factor; substance P; substance K; neuropeptide Y; neurotensin; somatostatin; vasoactive intestinal polypeptide. THD was also without effect on adenosine, choline, dopamine, noradrenaline, 5-HT and GABA uptake or glutamate transport. Also negative in the screening study were: calcium channels type N, T, L; chloride channel (TBOB); adenylate cyclase; inositol triphosphate; protein kinase C; acetylcholinesterase; mono-amine oxidase A/B; nitric oxide synthase; choline acetyl transferase; glutamic acid decarboxylase (Kanbayashi et al., 1996). This list is taken from the publication but unfortunately important detail is lacking (e.g., type of NOS) which introduces some limitations when using these data to discuss THDs pharmacological mechanisms. The binding study should be repeated and in addition, include each isomer analysed separately, products of hydrolysis and hepatic metabolism.
The lack of THD binding to any of the receptors currently known to be capable of reducing nausea and/or vomiting by either an antagonist (H1, M3/5. D2, 5-HT3, NK1) or an agonist (cannabinoid, opiate, GABAB) suggests that the anti-emetic action is not due to action at one of these receptors (Sanger and Andrews, 2018). This is particularly relevant to the studies with CINV treated clinically by a combination of 5-HT3 and NK1 receptor antagonism together with dexamethasone (Sanger and Andrews, 2018) and the nausea and vomiting in early pregnancy where 5-HT3 RA have some efficacy (Matthews et al., 2015) as the above discussion provides some evidence for efficacy of THD in both settings. However, before dismissing an interference of THD with neurotransmitter function the binding screen should not only be replicated, but expanded as some receptors implicated in nausea and vomiting pathways are not included (e.g., TRPV1, CB1; Sanger and Andrews, 2018).
Although the above discussion argues against an effect on many receptors affecting brain neurotransmission there is a body of preclinical evidence demonstrating potential relevance in modulation of neuronal function which we briefly review.
-
i)
BK channel (KCa1.1). The large-conductance calcium-activated potassium channel (BK channel, KCa1.1) is bound and regulated by cereblon. Cereblon binds and is regulated by a CRL4CBM4 E3 ubiquitin ligase, which is in turn inhibited by THD, thus THD regulates channel activity (Kim and Oh, 2016; Choi et al., 2018a). The possibility of modulation of neuronal function by THD is consistent with the wide distribution of cereblon mRNA in the mouse and human brain in the same areas as the Kca1.1 channel (Xin et al., 2008; Aizawa et al., 2011) raising the possibility of modulation of neuronal function. By binding to cereblon, THD inhibits CRL4CBM4 E3 ubiquitin ligase activity, one of the substrates of which is the BK channel (Choi et al., 2018a). Electrophysiological studies of mouse hippocampus showed that THD (given 3 h earlier) induced BK channel hyperactivity leading to reduced presynaptic glutamate release (Choi et al., 2018b). Glutamate is an excitatory transmitter in the NTS so THD could reduce transmission through this critical part of the emetic pathway with the effect potentially amplified as it co-transmits with SP, a key neurotransmitter involved in the brainstem emetic pathways (Sanger and Andrews, 2018). The BK channel is also present in the cell bodies of vagal afferent neurones in the nodose ganglion (Li et al., 2011) so raising the possibility of THD modulation of vagal afferent activity as proposed by Traldi et al., in 1965 (Traldi et al., 1965) assuming that these cells express cereblon.
-
ii)
Glutamatergic transmission. Another substrate of CRL4CBM4 E3 ubiquitin ligase is glutamine synthase, the enzyme responsible fo glutamate recycling, catalyzing the conversion of glutamate to glutamine in astrocytes, that is converted back to glutamate in neurones. Glutamine synthetase has been demonstrated in the area postrema in the glial cells surrounding the fenestrated capillaries (D'Amelio et al., 1987). THD acting to inhibit this pathway would be expected to lead to reduced recycling of glutamate thus affecting transmission.
In an open label study of patients with refractory epilepsy THD reduced the number of seizures/month over a year (Palencia et al., 2010). Thalidomide has anticonvulsant properties (2.5–100 mg/kg) assessed using amygdaloid kindling in conscious rats (Palencia et al., 2011), with clonic seizures induced by pentylenetetrazole (PTZ) where it was similarly potent to valproic acid (Palencia et al., 2007), and in lithium-pilocarpine-induced status epilepticus in mice (Amanlou et al., 2021). THD is neuroprotective (mouse striatum) against MPTP-induced excitotoxicity (Palencia et al., 2015) and protective against ischemia/reperfusion injury (rat) (Palencia et al., 2015). Modulation of the N-methyl-D-apartic acid (NMDA) receptor/nitric oxide (nNOS) pathway is implicated in the neuromodulatory and neuroprotective effects of THD in the PTZ-seizure model (Payandemehr et al., 2014; Pourshadi et al., 2020). Studies of the anti-depressant effects of THD in mice implicate an action on the NO/cyclic GMP pathway (Rostamian et al., 2019).
Taken together the above preclinical studies of seizures indicate that THD can modulate the NMDA/NO pathways in the brain. As glutamate is an excitatory neurotransmitter in the NTS and other parts of the brainstem implicated in ‘emesis’ via the NMDA receptor, an action similar to the anti-epileptic effect of THD described above would contribute to an ‘anti-emetic’ action. This is particularly relevant to chemotherapy where cisplatin has been shown to upregulate both NMDA and AMPA receptor subunit expression in the dorsal vagal complex (including the NTS) of mice (Holland et al., 2014) and antagonism of NMDA receptors reduces acute cisplatin-induced vomiting in the ferret (Lehmann and Karrberg, 1996). Reduction of transmission at critical integrative sites within the brainstem (e.g., NTS) is one of the main mechanisms by which the clinically used anti-emetic NK1 receptor antagonists operate (Andrews and Rudd, 2015).
-
iii)
GABAergic transmission. In the section discussing the sedative action of THD, modulation of brain GABAA receptor function emerged as a potential mechanism for this action. GABA-ergic transmission would be an attractive target as GABA is present in the AP and NTS (Leslie, 1985; Schwartz et al., 1986; Newton and Maley, 1987) inhibiting neuronal activity in both locations (Strominger et al., 2001; Bailey et al., 2008). As GABAA receptor activation can also reduce 5-HT release from gut enterochromaffin cells (EC) (Racke et al., 1996), studies will need to be conducted to look for an ability of THD to modulate the EC-vagal afferent pathways implicated in induction of emesis (e.g., in CINV and in emesis driven by other stimuli, such as SARS-CoV-2, radiation, and food toxins; see Andrews et al., 2020 for review).
From these studies a diversity of extracellular receptors and intracellular mechanisms have now been implicated in emetic mechanisms (see Zhong et al., 2021, for review). Thus, detailed analysis of the interaction of THD and selected metabolites with these targets should be undertaken to enable better definition of the molecular basis of THDs anti-emetic actions.
6.3. Modulation of digestive tract motility
Nausea is associated with dysrhythmia of the gastric myoelectric activity, antral motor quiescence and relaxation of the corpus. Although vomiting is not due to contraction of gastric smooth muscle, it is preceded by a giant retrograde contraction of the small intestine which progresses into the stomach (Stern et al., 2011). Thus, actions of THD on the digestive tract smooth muscle may be of relevance.
In guinea-pig isolated ileum, THD had a mild and short-lived spasmolytic action (33.3 μg/ml), reducing the spasmodic contractions to acetylcholine and histamine (Somers, 1960). Similarly, thalidomide 100 μM has been reported to inhibit contractions of prostate smooth muscle (Tamalunas et al., 2020). For each study, the mechanism of the inhibitory activity was not explored, but muscle relaxation by PDE4 inhibition seems unlikely as, the IC50 for thalidomide against PDE4 was >500 μM (Muller et al., 1998). This conclusion is further supported by the demonstration that THD (100 μM) did not elevate cAMP in human peripheral blood mononuclear cells in contrast to apremilast which showed binding to PDE4 whereas THD did not (Schafer et al., 2014). Further, thalidomide had no meaningful ability to bind to N-, T-, and L-type calcium channels (Kanbayashi et al., 1996).
The mechanism of THDs mild spasmolytic action remains unknown but its role in inhibition of vomiting seems unlikely. Such activity may be related to an ability of THD to cause constipation, a common dose-related side-effect on acute administration of the drug (Tseng et al., 1996; Stirling, 2000; Ghobrial & Rajkumar, 2003), where 80%–90% of patients can develop mild constipation. In some, severe symptoms can occur, leading to obstruction and toxic megacolon. Such severe constipation usually occurs in patients receiving high doses of thalidomide, especially those who lead a sedentary lifestyle and are more prone to develop constipation (Ghobrial & Rajkumar, 2003). In mouse, 500 mg/kg THD given orally 30 min before a charcoal meal, reduced the rate of transport through the stomach and intestines by 15%, but without reaching statistical significance (Somers, 1960). The mechanisms leading to constipation are unclear although in addition to the ability to relax smooth muscle, others have hypothesized that as with other neurotoxic agents, such as vincristine, THD may adversely affect autonomic nerve endings in the gut (Ghobrial & Rajkumar, 2003) resulting in a secondary effect on digestive tract motility.
Although an acute effect of THD on digestive tract motility is unlikely to contribute to inhibition of vomiting, THD may have a role in alleviating the reduced gastric motility in patients receiving anti-cancer chemotherapy and hence reducing nausea. In rats, the highly emetogenic agent cisplatin markedly inhibits gastric emptying (Malik et al., 2007). Molecular studies of the proximal stomach in rats analysed two days after cisplatin showed a large (3204%) increase in iNOS expression which was blocked by dexamethasone (Gale et al., 2005 and unpublished study data). And nNOS expression changes in rat proximal stomach and colon have been reported following oxaliplatin administration (Was et al., 2022 for review). THD has been shown to have weak (23 ± 8% reduction at 1 mM) NOS-inhibitory activity in vitro but the authors noted that metabolites or decomposition products might be more potent (Shimazawa et al., 2004). As TNFα is an inducer of iNOS the action of THD on TNFα could also contribute to reducing iNOS (see above, Moreira et al., 1993; Kim et al., 2004). Thus, THD could act in vivo to prevent cisplatin-induced changes in TNFα and iNOS which would otherwise lead to a depression of gastric motility, reducing gastric emptying and food intake, and induction of nausea.
6.4. Modulation of thyroid function
One of the earliest published human studies of THD (100–200 mg) reported a mild but consistent anti-thyroid effect, reducing the uptake of iodine (Murdoch and Campbell, 1958). We have not been able to find more recent publications confirming this action, but include it for completeness because of the links between thyroid function and pregnancy. Thyroid function is elevated in pregnancy and impaired uptake of iodine by the thyroid could impact thyroxine (T3) and triiodothyronine (T4) synthesis. This is relevant as increased thyroid hormone production has been implicated in both pregnancy sickness and hyperemesis gravidarum in 12/15 studies recently reviewed by Liu et al. (2022) although a causal link is not proven. Thyroid hormones are also potentially linked to nausea and vomiting during pregnancy via the ryanodine receptor2. The gene encoding the ryanodine receptor2, (RYR2) is expressed in the brainstem (Giannini et al., 1995) and is implicated in the cellular mechanism of calcium mobilisation leading to SP release and vomiting (Zhong et al., 2016). RYR2 can be overexpressed (heart tissue) by thyroid hormone administration (Jiang et al., 2000). Additionally, genetic analysis has identified a link between hyperemesis gravidarum and RYR2 variants (Fejzo et al., 2017). Interestingly, the RYR2 stress-induced calcium channel has also been implicated in Cyclical Vomiting Syndrome (Lee et al., 2015).
In hyperthyroid patients, plasma levels of Growth Differentiation Factor 15 (GDF15, see below for further discussion) are significantly increased and in mice thyroid hormones can upregulate GDF15 expression (Zhao et al., 2018). As reviewed below GDF15 has been implicated in both CINV and nausea and vomiting during pregnancy. A re-assessment of THDs effect on thyroid function would appear warranted.
6.5. Anti-inflammatory activity
THD can reduce TNFα production by enhancing degradation of TNFα mRNA (Moreira et al., 1993; Kim et al., 2004), and reduce the release of other cytokines such as IL-1β, IL-6 and IL-10 (e.g., Franks et al., 2004; Shannon et al., 2007; Deng et al., 2021). Early in vitro studies have associated the suppression of TNFα with the S-enantiomer (Wnendt et al., 1996). Further, an inflammation driven peripheral nerve hyperalgesia was blocked by THD, an effect associated with reduced TNFα synthesis which itself can trigger a cascade of pro-inflammatory cytokines resulting ultimately in the release of PGE2 (de Magalhães et al., 2020). Finally, THD inhibits cyclooxygenase (COX) COX1 and COX2 activity (with a potency comparable to aspirin; Noguchi et al., 2002).
These findings illustrate a potential peripheral action of THD which could be applicable to the abdominal vagal afferents implicated in driving nausea and vomiting. This is important because in patients receiving highly emetogenic chemotherapy, dexamethasone has been shown to reduce nausea and vomiting, particularly in the delayed phase; such activity has been used to argue that release of inflammatory mediators contributes to driving the ‘emetic’ response (Andrews and Rudd, 2015). This release is proposed to be either due to chemotherapy-induced inflammatory damage of the intestinal epithelium, releasing cytokines to act on the area postrema (e.g., TNFα, IL-1 ß activated rat area postrema neurons and astrocytes; Wuchert et al., 2009), or sensitize/drive abdominal vagal afferents (Sanger and Twycross, 1996; Andrews and Rudd, 2015), directly and/or indirectly via the release of neuroactive agents from gut enteroendocrine cells by locally produced cytokines (e.g., IL-1 ß, Kidd et al., 2009). In rat stomach the anti-cancer agent cisplatin upregulated expression of genes associated with inflammatory damage including the NLRP3 inflammasome (Gale et al., 2005; Li et al., 2020; Meng et al., 2021) and COX2 (Obara et al., 2018; see below). The role of THD in blocking the induction of pro-inflammatory gene expression caused by cisplatin provides a mechanism which could contribute to its ‘anti-emetic’ action in CINV and is supported by a recent study of expression downregulation by THD of a number of genes involved in the inflammatory response in lungs exposed to SARS-coV-2 (e.g., NF-κB, TNFα, NOS3; Sundaresan et al., 2020). Although we focus on inflammation and CINV (especially delayed phase) it is also considered to play an important role in the emetic effects of radiation (Young, 1986) making it potentially amenable to the anti-inflammatory effects of THD.
Prostaglandins (such as PGE2, PGF2) can induce vomiting (Ganesan and Karim, 1974; Smith and Mason, 1974; Kan et al., 2002) probably acting within the brainstem, (Kan et al., 2002). An action of THD on TNFα and subsequent PGE2 release could contribute to inhibition of vomiting together with COX inhibition. In pregnancy, an association has been reported between plasma PGE2 levels and nausea and vomiting (Gadsby et al., 2000). Additionally, serum TNFα is elevated in women with hyperemesis gravidarum but it is unclear if it this is a cause or a consequence (see Liu et al., 2022 for review).
6.6. Inhibition of the functions of Growth Differentiation Factor 15
A recent body of evidence has implicated GDF15 in both CINV and hyperemesis gravidarum making it a potential target for THD (e.g., Petry et al., 2018; Breen et al., 2020). In chemotherapy-damaged cancer cells THD (100 μg/ml) blocked the increase in expression and release of GDF15 protein (Dong et al., 2018). As this study demonstrates a potential interaction between THD and GDF15 we briefly review the evidence for involvement of GDF15 in nausea and vomiting to encourage further investigation.
GDF15, a divergent member of the TGF-β superfamily, acting on the glial-derived neurotrophic factor (GDNF)-family receptor α-like (GFRAL) receptor (Emmerson et al., 2017). Recently, a genome-wide association study linked GDF15 and more specifically, a single nucleotide polymorphism/coding variant of GDF15 with hyperemesis gravidarum (Fejzo et al., 2018a, b; Fejzo et al., 2019; Fejzo et al., 2022). In pregnancy, GDF15 is highly expressed in the placenta, circulating levels rise rapidly in maternal blood during the first trimester of pregnancy, remaining elevated until delivery (Moore et al., 2000) with blood GDF15 levels increased in women reporting vomiting in the second trimester, compared with women reporting no pregnancy nausea or vomiting (Petry et al., 2018). These studies implicate GDF15 in the induction of vomiting particularly in the later stages of pregnancy and in hyperemesis gravidarum, but nausea is a more common problem in early pregnancy (Whitehead et al., 1992). However, by analogy with other ‘emetics’ we hypothesise that a lower concentration of GDF15 will be required to induce nausea compared to vomiting but this requires direct investigation. The released GDF15 is proposed to act at the GFRAL receptors within the area postrema (AP: implicated in detection of circulating endogenous and exogenous agents inducing nausea and vomiting; see Fig. 2) and nucleus tractus solitarius (NTS: outputs from here project to “higher” brain regions to induce nausea and to the ventral brainstem to induce the mechanical events of vomiting). Activation of the AP and NTS by GDF15 suppresses food intake in mice and in non-human primates (Mullican et al., 2017). More recently, it has been suggested that this anorexia is a consequence of the induction of nausea and vomiting (Borner et al., 2020).
In animals and humans, GDF15 is also released into the blood circulation during administration of chemotherapeutic agents (Altena et al., 2015; Hsu et al., 2017; Borner et al., 2020), and in animals GDF15 induces acute vomiting, as well as long-term anorexia and body weight loss (Borner et al., 2020). GDF15 neutralization alleviated platinum-based chemotherapy-induced vomiting, anorexia, and weight loss in mice and/or nonhuman primates (Breen et al., 2020). Interestingly, at least one source of the GDF15 may be the stomach. Thus, in rats treated with cisplatin a reduced food consumption, increased kaolin consumption, and increased weight of the stomach contents was associated with a dramatic change in expression of genes in the stomach including GDF15 with a two-fold increase in the non-glandular stomach and a five-fold increase in the glandular stomach (Gale et al., 2005 and unpublished data).
7. Conclusions
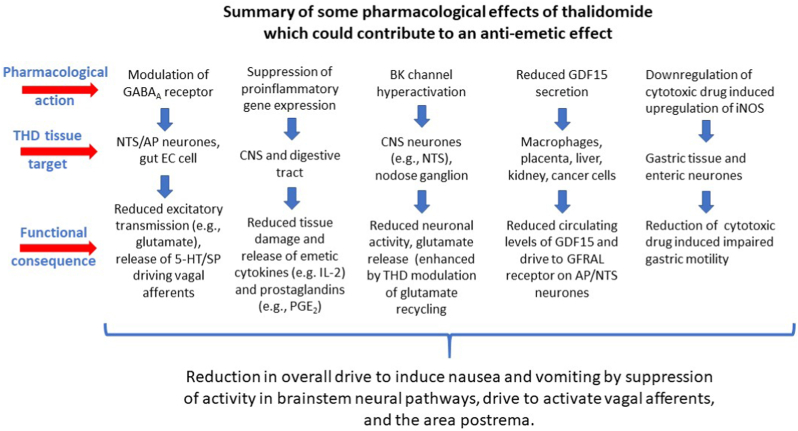
The use of THD to treat nausea and vomiting in pregnancy was based on an assumption that its sedative and hypnotic properties would indirectly reduce these symptoms rather than on data obtained from testing for such activity in animal models or clinical studies. An ‘anti-emetic’ effect of THD in CINV was first demonstrated >50 years ago and has been confirmed several times in the last ∼20 years. However, there is a lack of comparable robust data for efficacy against nausea and/or vomiting in pregnancy (Table 1) and COVID-19 (Li et al., 2021). Although understanding of the molecular actions of THD has expanded (particularly the immunomodulatory action) its ability to inhibit vomiting has not been explored in animal models (e.g., ferret, house musk shrew, least shrew) to investigate if such activity extends beyond pregnancy and CINV (i.e., is it effective against motion sickness, vomiting induced by apomorphine acting on the area postrema or gastric irritants acting via abdominal vagal afferents?). Although we identified a number of potential mechanisms which could contribute to the ability of THD to inhibit nausea and vomiting, because THD has multiple pharmacological actions it would be unwise to assume that the mechanism is the same in all circumstances. There is insufficient data on the pharmacology of THD to identify a single mechanism but the most likely mechanisms, which are not mutually exclusive, are summarised in Fig. 3. These now require direct investigation (see below) so that we may finally understand the clinical, rather than teratogenic effect for which THD is equally widely known.
Fig. 3.
A summary of some of the pharmacological effects of thalidomide which could contribute to its effects on nausea and vomiting by actions on neurotransmission in the central pathways for nausea and vomiting or by modulation of the stimuli driving emesis at peripheral sites. See text for detailed discussion and references.
8. Outlook
Pursuing the mechanisms underlying the immunomodulatory action and the teratogenic effects has given insights into the pharmacology of THD and has led to the development of THD analogues and THD-like drugs with a range of clinical applications (e.g., treatment of cancer, autoimmune disorders; Millrine and Kishimoto, 2017). However, the same has not happened for its ‘anti-emetic’ actions which have been assumed, but with the exception of the CINV studies have not been demonstrated in controlled clinical trials. Based on analysis of the limited data on THDs ability to reduce nausea and vomiting, combined with current knowledge of its pharmacology, additional studies of the pharmacology are now warranted; the outcomes may identify novel approaches to the design of drugs which inhibit nausea and/or vomiting. The studies may focus on:
-
1.
Reassessing and extending the receptor binding profile of THD, its enantiomers and the major metabolites (with functional studies to validate, as appropriate for the target).
-
2.
Investigating the efficacy of THD against GDF15-dependent mechanisms.
-
3.
Determination of the spectrum of effects of THD in animal models exhibiting vomiting induced by different stimuli; there are no animal models of hyperemesis gravidarum or pregnancy sickness, and animal models of nausea remain contentious.
-
4.
Identification of non-teratogenic THD-like compounds or other molecules showing similar activity to THD in vitro and in vivo.
Clearly, the mechanism(s) by which THD inhibits nausea and vomiting differs from all currently available drugs with activity. Identification of the mechanism may therefore offer new insights into treatment of disorders in which current therapy is suboptimal or without clear efficacy (e.g., gastroparesis, chronic unexplained nausea and vomiting; Carlin et al., 2021). However, we do not yet understand the ability of THD to inhibit nausea as well as vomiting; in general, the former appears to be less well treated by existing ‘anti-emetic’ drugs (Sanger and Andrews, 2018). Measuring nausea is a challenge (animal models are contentious; Sanger and Andrews, 2018) and human studies will be required. To provide a robust evidence base for further development, it is important that assessment of nausea is not limited to self-reporting (usually a Visual Analog Scale or Likert-scale) but should include different biomarkers linked to nausea (e.g., plasma vasopressin concentration, heart rate variability, regional skin conductance and blood flow changes, and electrogastrogram) (Muth et al., 1996; Stern et al., 2011; Li et al., 2022).
Finally, the THD tragedy caused an overwhelmingly beneficial change in the way that drugs are tested prior to marketing but it also has rightly led to a very cautious approach to drug treatment of nausea and vomiting throughout pregnancy. Additionally, a recent review of the ethical issues regarding therapeutic use and research in pregnant women noted that an additional effect of the thalidomide tragedy was “to exclude all women of reproductive potential from pharmaceutical research” (Weld et al., 2021, p.7); approaches to the conduct of pharmaceutical research in pregnancy are discussed. However, nausea and vomiting is experienced during pregnancy by ∼70% of women worldwide although with large reporting variations (35%–91%) (Einarson et al., 2013) and is the most prevalent medical condition during pregnancy (Gadsby et al., 2019; Liu et al., 2022). The incidence of the maternal and foetal potentially life-threatening hyperemesis gravidarum ranges 0.3–3.6% with an average of 1.1% globally (Einarson et al., 2013). Apart from the impact on the individual, studies in the US and UK have shown that nausea and vomiting of pregnancy places a considerable impact on health service resources (Piwko et al., 2013; Gadsby et al., 2019). Although several conventional drugs have been used to treat the nausea and vomiting of pregnancy and risk benefit assessments undertaken (Mazzotta and Magee, 2000), controlled trails are lacking. A recent publication describes the protocol for a study of ondansetron and mirtazapine in hyperemesis gravidarum with patients offered metoclopramide as rescue medication (Ostenfeld et al., 2020). Whilst the trial will give a clear answer regarding efficacy, the rationale for using these drugs is their efficacy in other clinical settings (e.g., CINV, PONV, gastroparesis; see Sanger and Andrews, 2018 for review) rather than an understanding of the mechanisms in pregnancy. The data derived from understanding the mechanism(s) by which THD inhibits nausea and vomiting (and the identification of non-teratogenic compounds with the same activity), may provide a novel insight into treatment of the nausea and vomiting of pregnancy-the starting point for this review.
Ethic approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Funding
This review did not receive any specific grants for this study from funding agencies in the public, commercial, or non-profit sectors.
CRediT authorship contribution statement
Paul L.R. Andrews: Writing – original draft, Writing – review & editing. Robin S.B. Williams: Writing – review & editing. Gareth J. Sanger: Writing – review & editing.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
We thank: Prof. E.M. Tansey for drawing our attention to a number of chapters and books reviewing the history of thalidomide; Prof. J. A. Rudd for comments on an early draft of the manuscript. The responsibility for the content of the review is solely that of the authors.
Data availability
No data was used for the research described in the article.
References
- Aizawa M., Abe Y., Ito T., Handa H., Nawa H. mRNA distribution of the thalidomide binding protein cereblon in adult mouse brain. Neurosci. Res. 2011;69:343–347. doi: 10.1016/j.neures.2010.12.019. [DOI] [PubMed] [Google Scholar]
- Alhifany A.A., McBride A., Almutairi A.R., Cheema E., Shahbar A., Alatawi Y., Alharbi A.S., Babiker H., MacDonald K., Aapro M., Abraham I. Efficacy of olanzapine, neurokinin-1 receptor antagonists, and thalidomide in combination with palonosetron plus dexamethasone in preventing highly emetogenic chemotherapy-induced nausea and vomiting: a Bayesian network meta-analysis. Support. Care Cancer. 2020;28:1031–1039. doi: 10.1007/s00520-019-05210-4. [DOI] [PubMed] [Google Scholar]
- Altena R., Fehrmann R.S.N., Boer H., de Vries E.G.E., Meijer C., Gietema J.A. Growth Differentiation Factor 15 (GDF-15) plasma levels increase during bleomycin- and cisplatin-based treatment of testicular cancer patients and relate to endothelial damage. PLoS One. 2015 doi: 10.1371/journal.pone.0115372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews P.L.R., Davis C.J., Bingham S., Davidson H.I.M., Hawthorn J., Maskell L. The abdominal visceral innervation and the emetic reflex: pathways, pharmacology and plasticity. Can. J. Physiol. Pharmacol. 1990;68:325–345. doi: 10.1139/y90-047. [DOI] [PubMed] [Google Scholar]
- Amanlou A., Eslami F., Shayan M., Mortazavi P., Dehpour A.R. Anticonvulsive evaluation and histopathological survey of thalidomide synthetic analogs on lithium -pilocarpine-induced status epilepticus in rats. Res. Pharmaceut. 2021;16:586–595. doi: 10.4103/1735-5362.327505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews P.L.R., Cai W., Rudd J.A., Sanger G.J. COVID-19, nausea and vomiting. J. Gastroenterol. Hepatol. 2020;36:646–656. doi: 10.1111/jgh.15261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews P.L.R., Rudd J.A. In: Management of Chemotherapy-Induced Nausea and Vomiting: New Agents and New Uses of Current Agent. Navari R.M., editor. Springer International Publishing; 2015. The physiology and pharmacology of nausea and vomiting induced by anti-cancer chemotherapy in humans. P.5-44. [Google Scholar]
- Appelbe G.E. In: Making Medicines. A Brief History of Pharmacy and Pharmaceuticals. Anderson S., editor. Pharmaceutical Press; London, Chicago: 2005. From arsenic to thalidomide: a brief history of medicine safety; pp. 243–260. (Chapter 13) [Google Scholar]
- Asadollahi A., Asadi M., Hosseini F.S., Ekhtiari Z., Biglar M., Amanlou M. Synthesis, molecular docking, and antiepileptic activity of novel phthalimide derivatives bearing amino acid conjugated anilines. Res. Pharmaceutical Sci. 2019;14:534–543. doi: 10.4103/1735-5362.272562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badros A.Z. Lenalidomide in myeloma- a high maintenance friend. New. Engl. J. Med. 2012;366:1836–1838. doi: 10.1056/NEJMe1202819. [DOI] [PubMed] [Google Scholar]
- Bai N., Cui X.-Y., Wang J., Sun C.-G., Mei H.-K., Liang B.-B., Cai Y., Song X.-J., Gu J.-K., Wang R. Determination of thalidomide concentration in human plasma by liquid chromatography-tandem mass spectrometry. Exp. Ther. Med. 2013;5:626–630. doi: 10.3892/etm.2012.847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey T.W., Appleyard S.A., Jin Y.-H., Andresen M.C. Organization and properties of GABAergic neurones in the solitary tract nucleus (NTS) J. Neurophysiol. 2008;99:1712–1722. doi: 10.1152/jn.00038.2008. [DOI] [PubMed] [Google Scholar]
- Baker J.J., Lokey J.L., Price N.A., Winokur S.H., Bowen J., Taylor A. Nabilone as an anti-emetic. New Engl. J. Med. 1979;301:728. doi: 10.1056/NEJM197909273011318. [DOI] [PubMed] [Google Scholar]
- Barann M., Dilger J.P., Bönisch H., Göthert M., Dybek A., Urban B.W. Inhibition of 5-HT3 receptors by propofol: equilibrium and kinetic measurements. Neuropharmacology. 2000;39:1064–1074. doi: 10.1016/s0028-3908(99)00205-1. [DOI] [PubMed] [Google Scholar]
- Bignall J.R., Crofton J. Antihistamine drugs in treatment of nausea and vomiting due to streptomycin. Br. Med. J. 1949;1:13–14. doi: 10.1136/bmj.1.4591.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borgeat A., Wilder-Smith O.H.G., Saiah M., Rifat K. Subhypnotic doses of propofol possess direct antiemetic properties. Anesth. Analg. 1992;74:539–541. doi: 10.1213/00000539-199204000-00013. [DOI] [PubMed] [Google Scholar]
- Borison H.L., Wang S.C. Physiology and pharmacology of vomiting. Pharmacol. Rev. 1953;5:193–230. [PubMed] [Google Scholar]
- Borner T., Shaulson E.D., Ghidewon M.Y., Barnett A.B., Horn C.C., Doyle R.P., Grill H.J., Hayes M.R., De Jonghe B.C. GDF15 induces anorexia through nausea and emesis. Cell Metabol. 2020;31:351–362. doi: 10.1016/j.cmet.2019.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botting H.J. In: Animals and Medicine. The Contribution of Animal Experiments to the Control of Disease. Botting Regina., editor. Open Book Publishers; Cambridge, UK: 2016. The history of thalidomide. Chapter 18, p.183-198. [Google Scholar]
- Boyd E.M., Boyd C.E., Cassell W.A. The antiemetic action of chlorpromazine hydrochloride. Can. Med. Assoc. J. 1954;70:276–280. [PMC free article] [PubMed] [Google Scholar]
- Brand E.D., Harris T.D., Borison H.L., Goodman L.S. The antiemetic activity of 10-(7-dimethylaminopropyl)-2-chlorophenpthiazine (chlorpromazine) in dog and cat. J. Pharmacol. Exp. Therapeut. 1954;110:86–92. [PubMed] [Google Scholar]
- Breen D.M., Kim H., Bennett D., Calle R.A., Collins S., Esquejo R.M., He T., Joaquim S., Joyce A., Lambert M., Lin L., Pettersen B., Qiao S., Rossulek M., Weber G., Wu Z., Zhang B.B., Birnbaum M.J. GDF-15 neutralization alleviates platinum-based chemotherapy-induced emesis, anorexia, and weight loss in mice and nonhuman primates. Cell Metabol. 2020;32:938–950. doi: 10.1016/j.cmet.2020.10.023. [DOI] [PubMed] [Google Scholar]
- Bruera E., Neumann C.M., Pituskin E., Calder K., Ball K., Hanson J. Thalidomide in patients with cachexia due to terminal cancer: preliminary report. Ann. Oncol. 1999;10:857–859. doi: 10.1023/a:1008329821941. [DOI] [PubMed] [Google Scholar]
- Carlin J.L., Lieberman V.R., Dahal A., Keefe M.S., Xiao C., Birznieks G., Abell T.L., Lembo A., Parkman H., Polymeropoulos M.H. Efficacy and safety of tradipitant in patients with diabetic and idiopathic gastroparesis in a randomized, placebo- controlled trial. Gastroenterology. 2021;160:76–87e4. doi: 10.1053/j.gastro.2020.07.029. [DOI] [PubMed] [Google Scholar]
- Carliner P.E., Radman H.M., Gay L.N. Treatment of nausea and vomiting of pregnancy with Dramamine-preliminary report. Science. 1949;110:215–216. doi: 10.1126/science.110.2852.215. [DOI] [PubMed] [Google Scholar]
- Cassidy J., Raina V., Lewis C., Adams L., Soukop M., Raperort W.G., Zussman B.D., Rankin E.M., Kaye S.B. Pharmacokinetics and anti-emetic activity of BRL43694, a new selective 5HT-3 antagonist. Br. J. Cancer. 1988;58:651–653. doi: 10.1038/bjc.1988.278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cechetto D.F., Diab T., Gibson C.J., Gelb A.W. The effects of propofol in the area postrema of rats. Anesth. Analg. 2001;92:934–942. doi: 10.1097/00000539-200104000-00027. [DOI] [PubMed] [Google Scholar]
- Chang X-b, Stewart A.K. What is the functional role of the thalidomide binding protein cereblon? Int. J. Biochem. Mol. Biol. 2011;2:287–294. [PMC free article] [PubMed] [Google Scholar]
- Choi T.-Y., Lee S.-H., Kim S.-J., Jo Y., Park C.-S., Choi S.-Y. BK channel blocker paxilline attenuates thalidomide-caused synaptic and cognitive dysfunctions in mice. Sci. Rep. 2018;8 doi: 10.1038/s41598-018-36367-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi T.-Y., Lee S.-H., Kim Y.-J., Bae J.R., Lee K.M., Jo Y., Km S.-J., Lee A.-R., Choi S., Choi L.-M., Bang S., Song M.-R., Chung J., Lee K.J., Kim S.H., Park C.S., Choi S.-Y. Cereblon maintains synaptic and cognitive function by regulating BK channel. J. Neurosci. 2018;38:3571–3583. doi: 10.1523/JNEUROSCI.2081-17.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chong M.F., Chan A. Thalidomide for delayed chemotherapy-induced nausea and vomiting: where is its place in therapy? J. Clin. Oncol. 2018;36:827–828. doi: 10.1200/JCO.2017.76.4316. [DOI] [PubMed] [Google Scholar]
- Daemmrich A. Remind me again, what is thalidomide and how did it cause so much harm? Conversation. 2015 http://theconversation.com [Google Scholar]
- D'Amelio F.E., Mehler W.R., Gibbs M.A., Eng L.F., Wu J.Y. Immunocytochemical localization of glutamic acid decarboxylase (GAD) and glutamine synthetase (GS) in the area postrema of the cat. Light and electron microscopy. Brain Res. 1987;410:232–244. doi: 10.1016/0006-8993(87)90320-9. [DOI] [PubMed] [Google Scholar]
- Davis M.P., Sanger G.J. The benefits of olanzapine in palliating symptoms. Curr. Treat. Options Oncol. 2020;22:5. doi: 10.1007/s11864-020-00804-1. [DOI] [PubMed] [Google Scholar]
- De Magalhães S.F., Manzo L.P., deFaria F.M., de Oliverira-Fusaro M.C., Nishijima C.M., Veira W.F., Bonet I.J.M., Dos Santos G.G., Tambeli C.H., Parada C.A. Inflammatory pain in peripheral tissue depends on the activation of the TNF-α type 1 receptor in the primary afferent neurone. Eur. J. Neurosci. 2020;53:376–389. doi: 10.1111/ejn.14985. [DOI] [PubMed] [Google Scholar]
- Deng M.-Y., Ahmad K.A., Han Q.-Q., Wang Z.-Y., Shoaib R.M., Li X.-Y., Wang Y.-X. Thalidomide alleviates neuropathic pain through microglial IL-10/β-endorphin signaling pathway. Biochem. Pharmacol. 2021;192 doi: 10.1016/j.bcp.2021.114727. [DOI] [PubMed] [Google Scholar]
- Dong G., Zheng Q-D., Ma M., Wu S-F., Zhang R., Yao R-R., Dong Y-Y., Ma H., Gao D-M., Ye S-L., Cui J-F., Ren Z-G., Chen R-X. Angiogenesis enhanced by treatment damage to hepatocellular carcinoma through release of GDF15. Cancer Med. 2018;7:820–830. doi: 10.1002/cam4.1330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Einarson T.R., Piwko C., Koren G. Quantifying the global rates of nausea and vomiting of pregnancy: a meta -analysis. J. Population Ther. Clin. Pharmacol. 2013;20:e171–e183. [PubMed] [Google Scholar]
- Emmerson P.J., Wang F., Du Y., Liu Q., Pickard R.T., Gonciarz M.D., Coskun T., Hamang M.J., Sindelar D.K., Ballman K.K., Foltz L.A., Muppidi A., Alsina-Fernandez J., Barnard G.C., Tang J.X., Liu X., Mao X., Siegel R., Sloan J.H., Mitchell P.J., Zhang B.B., Gimeno R.E., Shan B., Wu X. The metabolic effects of GDF15 are mediated by the orphan receptor GFRAL. Nat. Med. 2017;23:1215–1219. doi: 10.1038/nm.4393. [DOI] [PubMed] [Google Scholar]
- Eriksson T., Björkman S., Höglund P. Clinical pharmacology of thalidomide. Eur. J. Clin. Pharmacol. 2001;57:365–376. doi: 10.1007/s002280100320. [DOI] [PubMed] [Google Scholar]
- Ewalenko P., Janny S., Dejonckheere M., Andry G., Wyns C. Antiemetic effect of subhypnotic doses of propofol after thyroidectomy. Br. J. Anaesth. 1996;77:463–467. doi: 10.1093/bja/77.4.463. [DOI] [PubMed] [Google Scholar]
- Fabro S., Schumacher H., Smith R.L., Stagg R.B.L., Williams R.T. The metabolism of thalidomide: some biological effects of thalidomide and its metabolites. Br. J. Pharmacol. 1965;25:352–362. doi: 10.1111/j.1476-5381.1965.tb02055.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fejzo M.S., Myhre R., Colodro-Conde L., MacGibbon K.W., Sinsheimer J.S., Prasad M.V., Reddy P.S., Pajukanta P., Nyholt D.R., Wright M.J., Martin N.G., Engel S.M., Medlnad S.E., Magnus P., Mullin P.M. Genetic analysis of hyperemesis gravidarum reveals association with intracellular calcium release channel (RYR2) Mol. Cell. Endocrinol. 2017;439:308–316. doi: 10.1016/j.mce.2016.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fejzo M.S., Sazonova O.V., Sathirapongsasuti J.F., Hallgrímsdóttir I.B., 23andMe Research Team. Vacic V., MacGibbon K.W., Schoenberg F.P., Mancuso N., Slamon D.J., Mullin P.M. Placenta and appetite genes GDF15 and IGFBP7 are associated with hyperemesis gravidarum. Nat. Commun. 2018;9 doi: 10.1038/s41467-018-03258-0. Article number: 1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fejzo M.S., Arzy D., Tian R., MacGibbon K.W., Mullin P.M. Evidence GDF15 plays a role in familial and recurrent hyperemesis gravidarum. Geburtsh. Frauenheilkd. 2018;78:866–870. doi: 10.1055/a-0661-0287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fejzo M.S., Fasching P.A., Schneider M.O., Schwitulla J., Beckmann M.W., Schwenke E., MacGibbon K.W., Mullin P.M. Analysis of GDF15 and IGFBP7 in hyperemesis gravidarum support causality. Geburtshilfe Frauenheilkd. 2019;79:382–388. doi: 10.1055/a-0830-1346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fejzo M.S., MacGibbon K.W., First O., Quan C., Mullin P.M. Whole -exome sequencing uncovers new variants in GDF15 associated with hyperemesis gravidarum. Br. J. Obstet. Gynaecol. 2022 doi: 10.1111/1471-0528.17129. (Online ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferner R.E., Aronson J.K. Medicines legislation and regulation in the United Kingdom 1500-2020. Br.J. Clin Pharamcol. 2022;2022:1–13. doi: 10.1111/bcp.15497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flaxman S.M., Sherman P.W. Morning sickness: a mechanism for protecting mother and embryo. Q. Rev. Biol. 2000;75:113–148. doi: 10.1086/393377. [DOI] [PubMed] [Google Scholar]
- Florence A.L. Is thalidomide to blame? Br. Med. J. 1960;2:1954. [Google Scholar]
- Franks M.E., Macpherson G.R., Figg W.D. Thalidomide. Lancet. 2004;363:1802–1811. doi: 10.1016/S0140-6736(04)16308-3. [DOI] [PubMed] [Google Scholar]
- Frederickson R.C., Slater I.H., Dusenberry W.E., Hewes C.R., Jones G.T., Moore R.A. A comparison of thalidomide and pentobarbital- new methods for identifying hypnotic drugs. J. Pharmacol. Exp. Therapeut. 1977;203:240–251. [PubMed] [Google Scholar]
- Gadsby R., Rawson V., Dziadulewicz E., Rousseau B., Collings H. Nausea and vomiting of pregnancy and resource implications: the NVP impact study. Br. J. Gen. Pract. 2019;69:e217–e223. doi: 10.3399/bjgp18X700745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gadsby R., Barnie-Adshead A., Grammatoppoulos D., Gadsby P. Nausea and vomiting in pregnancy: an association between symptoms and maternal prostaglandin E2. Gynecol. Obstet. Invest. 2000;50:149–152. doi: 10.1159/000010314. [DOI] [PubMed] [Google Scholar]
- Gale D., Blakemore S., Cook T., Kidwai S., Moore I., Moore G.B.T., Moore S.E., Sanger G.J., Liu Y.-L., Malik N., Andrews P.L.R. Modulation of gene expression in the glandular and non-glandular regions of the rat stomach after treatment with the anti-cancer drug cisplatin. Gastroenterology. 2005;128(Suppl. 2) A-545. [Google Scholar]
- Gan T.J., Glass P.S., Howell S.T., Canada A.T., Grant A.P., Ginsberg B. Determination of plasma concentrations of propofol associated with 50% reduction in postoperative nausea. Anesthesiology. 1997;87:779–784. doi: 10.1097/00000542-199710000-00010. [DOI] [PubMed] [Google Scholar]
- Ganesan P.A., Karim S.M.M. Acute toxicity of prostaglandins E2, F2α and 15(S) 15 methyl prostaglandin E2 methyl esters in the baboon. Prostaglandins. 1974;7:215–221. doi: 10.1016/0090-6980(74)90005-7. [DOI] [PubMed] [Google Scholar]
- Gay L.N., Carliner P.E. The prevention and treatment of motion sickness.1. Seasickness. Science. 1949;109:359. doi: 10.1126/science.109.2832.359. [DOI] [PubMed] [Google Scholar]
- Gemechu Y., Milrine D., Hashimoto S., Prakash J., Sanchenkova K., Metwally H., Gyanu P., Kang S., Kishimoto T. Humanized cereblon mice revealed two distinct therapeutic pathways of immunomodulatory drugs. Proc. Natl. Acad. Sci. USA. 2018;115:11802–11807. doi: 10.1073/pnas.1814446115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghobrial I.M., Rajkumar S.V. Management of thalidomide toxicity. Support Oncol. 2003;1:194–205. [PMC free article] [PubMed] [Google Scholar]
- Giannini G., Conti A., Mammarella S., Scrobogna M., Sorrentino V. The ryanodine receptor/calcium channel genes are widely and differentially expressed in murine brain and peripheral tissues. J. Cell Biol. 1995;128:893–904. doi: 10.1083/jcb.128.5.893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon J.N., Goggin P.M. Thalidomide and its derivatives: emerging from the wilderness. Postgrad. Med. 2003;79:127–132. doi: 10.1136/pmj.79.929.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Govindarajan R., Heaton K.M., Broadwater R., Zeitlin A., Lang N.P., Hauer-Jensen M. Effect of thalidomide on gastrointestinal toxic effects of irinotecan. Lancet. 2000;356:566e567. doi: 10.1016/s0140-6736(00)02586-1. [DOI] [PubMed] [Google Scholar]
- Gralla J., Itri L.M., Pisko S.E., Squillante A.E., Kelsen D.P., Braun D.W., Jr., Bordin L.A., Braun T.J., Young C.W. Antiemetic efficacy of high-dose metoclopramide: randomized trials with placebo and prochlorperazine in patients with chemotherapy-induced nausea and vomiting. N. Engl. J. Med. 1981;305:905–909. doi: 10.1056/NEJM198110153051601. [DOI] [PubMed] [Google Scholar]
- Han Z-x., Xu J., Wang H-m., Ma J., Sun X., Du X-p. Antiemetic role of thalidomide in a rat model of cisplatin-induced emesis. Cell Biochem. Biophys. 2014;70:361–365. doi: 10.1007/s12013-014-9921-8. [DOI] [PubMed] [Google Scholar]
- Hill I.G.W., Guest A.I. Prevention of sea-sickness in assault craft. A report of experiments under tropical conditions. Br. Med. J. 1945;2:6–11. doi: 10.1136/bmj.2.4409.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirose Y., Kitazono T., Sezaki M., Abe M., Sakimura K., Funato H., Handa H., Vogt K.E., Yanagisawa M. Hypnotic effect of thalidomide is independent of teratogenic ubiquitin/proteasome pathway. Proc. Natl. Acad. Sci. USA. 2020;117:23106–23112. doi: 10.1073/pnas.1917701117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Höglund P., Eriksson T., Björkman S.A. Double blind study of the sedative effects of thalidomide enantiomers in humans. J. Pharamakokinet. Biopharm. 1998;26:363–383. doi: 10.1023/a:1021008016719. [DOI] [PubMed] [Google Scholar]
- Holland R.A., Leonard J.J., Kensey N.A., Hannikainen P.A., De Jongh B.C. Cisplatin induces neuronal activation and increase central AMPA and NMDA receptor subunit gene expression in mice. Physiol. Behav. 2014;136:79–85. doi: 10.1016/j.physbeh.2014.02.038. [DOI] [PubMed] [Google Scholar]
- Horn C.C., Kimball B.A., Wang H., Kaus J., Dienel S., Nagy A., Gathright G.R., Yates B.J., Andrews P.L.R. Why can't rodents vomit? A comparative behavioral, anatomical, and physiological study. PLoS One. 2013;8 doi: 10.1371/journal.pone.0060537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu J.-Y., Crawley S., Hsu J.-Y., Crawley S., Chen M., Ayupova D.A., Lindhout D.A., Higbee J., Kutach A., Joo W., Gao Z., Fu D., To C., Mondal K., Li B., Kekatpure A., Wang M., Laird T., Horner G., Chan J., McEntee M., Lopez M., Lakshminarasimhan D., White A., Wang S.-P., Yao J., Yie J., Matern H., Solloway M., Haldankar R., Parsons T., Tang J., Shen W.D., Chen Y.A., Tian H., Allan B.B. Non-homeostatic body weight regulation through a brainstem-restricted receptor for GDF15. Nature. 2017;550:55–259. doi: 10.1038/nature24042. [DOI] [PubMed] [Google Scholar]
- Hsueh S.C., Luo W., Tweedie D., Kim D.S., Kim Y.K., Hwang I., Gil J.-E., Han B.-S., Chiang Y.-H., Selman W., Hoffer B.J., Greig N.H. N-Adamantyl phthalimidine: a new thalidomide-like drug that lacks cereblon binding and mitigates neuronal and synaptic loss, neuroinflammation, and behavioural deficits in traumatic brain injury and LPS challenge. ACS Pharmacol. Transl. Sci. 2021;4:980–1000. doi: 10.1021/acsptsci.1c00042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y.-J., Liao J.-F., Tsai T.-H. Concurrent determination of thalidomide in rat blood, brain and bile using multiple microdialysis coupled to liquid chromatography. Biomed. Chromatogr. 2005;19:488–493. doi: 10.1002/bmc.466. [DOI] [PubMed] [Google Scholar]
- Huys J. Cytostatic-associated vomiting effectively inhibited by domperidone (R33812) Cancer Chemother. Pharmacol. 1978;1(215):1. doi: 10.1007/BF00257152. [DOI] [PubMed] [Google Scholar]
- Islas-Espinoza A.M., Campos-Rodriguez C., Ramiers-San-Juan E. Thalidomide protects against acute pentylenetetrazole and pilocarpine -induced seizures in mice. J. Toxicol. Sci. 2018;43:671–684. doi: 10.2131/jts.43.671. [DOI] [PubMed] [Google Scholar]
- Ito T., Ando H., Suzuki T., Ogura T., Hotta K., Imamura Y., Yamaguchi Y., Handa H. Identification of a primary target of thalidomide teratogenicity. Science. 2010;327:1345–1350. doi: 10.1126/science.1177319. [DOI] [PubMed] [Google Scholar]
- Ito T., Handa H. Cereblon and its downstream substrates as molecular targets of immunomodulatory drugs. Int. J. Hematol. 2016;104:293–299. doi: 10.1007/s12185-016-2073-4. [DOI] [PubMed] [Google Scholar]
- Jiang M., Xu A., Tokmakejian S., Narayanan N. Thyroid hormone -induced overexpression of functional ryanodine receptors in the rabbit heart. Am. J. Physiol. Heart Circ. Physiol. 2000;278:H1429–H1438. doi: 10.1152/ajpheart.2000.278.5.H1429. [DOI] [PubMed] [Google Scholar]
- Jung H. Klinische Erfahrungen mit einem neuen Sedativum. Arzneim.-Forsch. 1956;6:430. [PubMed] [Google Scholar]
- Kaitin K.I. Effects of thalidomide and pentobarbital on neuronal activity in the preoptic area during sleep and wakefulness in the cat. Psychopharmacol. 1985;85:47–50. doi: 10.1007/BF00427320. [DOI] [PubMed] [Google Scholar]
- Kan K.K.W., Jones R.L., Ngan M.-P., Rudd J.A. Actions of prostanoids to induce emesis and defecation in the ferret. Eur. J. Pharmacol. 2002;453:299–308. doi: 10.1016/s0014-2999(02)02424-x. [DOI] [PubMed] [Google Scholar]
- Kanbayashi T., Nishino S., Tafti M., Hishikawa Y., Dement W.C., Mignot E. Thalidomide, a hypnotic with immune modulating properties, increases cataplexy in canine narcolepsy. Neuroreport. 1996;7:1881–1886. doi: 10.1097/00001756-199608120-00002. [DOI] [PubMed] [Google Scholar]
- Kanbayashi T., Shimizu T., Takahashi Y., Kitajima T., Takahashi K., Saito Y., Hishikawa Y. Thalidomide increases both REM and stage 3-4 sleep in human adults: a preliminary study. Sleep. 1999;22:113–115. doi: 10.1093/sleep/22.1.113. [DOI] [PubMed] [Google Scholar]
- Kidd M., Gustaffson B.I., Drozdov I., Modlin I.M. IL1β- and LPS-induced serotonin secretion is increased in EC cells derived from Crohn's disease. Neuro Gastroenterol. Motil. 2009;21:439–450. doi: 10.1111/j.1365-2982.2008.01210.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H., Oh K.H. Protein network interacting with BK channels. Int. Rev. Neurobiol. 2016;128:127–161. doi: 10.1016/bs.irn.2016.03.003. [DOI] [PubMed] [Google Scholar]
- Kim S.I., Han T.H., Kil H.Y., Lee J.S., Kim S.C. Prevention of postoperative nausea and vomiting by continuous infusion of subhypnotic propofol in female patients receiving intravenous patient-controlled analgesia. Br. J. Anaesth. 2000;85:898–900. doi: 10.1093/bja/85.6.898. [DOI] [PubMed] [Google Scholar]
- Kim Y.S., Kim J.S., Jung H.C., Song I.S. The effects of thalidomide on the stimulation of NF-kappaB activity and TNF-alpha production by lipopolysaccharide in a human colonic epithelial cell line. Mol. Cell. 2004;17:210–216. [PubMed] [Google Scholar]
- Kimura K. Electrographic studies of the actions of hypnotic agents. 1. Effect of various hypnotic agents on the sleep and wakefulness cycle in the rabbit. Nihon Yakurigaku Zasshi. 1973;69:599–619. [PubMed] [Google Scholar]
- Kuhn W.L., Van Maanen E.F. Central nervous system effects of thalidomide. J. Pharmacol. Exp. Therapeut. 1961;134:60–68. [PubMed] [Google Scholar]
- Kunz W. N-Phthalyl glutaminsäure-imid. Arnseimittelforschung. 1956;6:426–430. [PubMed] [Google Scholar]
- Lasagna L. Thalidomide—a new nonbarbiturate sleep-inducing drug. J. Clin. Epidemiol. 1960;11:627–631. doi: 10.1016/0021-9681(60)90061-8. [DOI] [PubMed] [Google Scholar]
- Lee J., Wong S.A., Li B.U., Boles R.G. NextGen nuclear DNA sequencing in cyclic vomiting syndrome reveals a significant association with the stress-induced calcium channel (RYR2) Neuro Gastroenterol. Motil. 2015;27:990–996. doi: 10.1111/nmo.12575. [DOI] [PubMed] [Google Scholar]
- Lehmann A., Karrberg L. Effects of N-methyl-D-aspartate receptor antagonists on cisplatin-induced emesis in the ferret. Neuropharmacology. 1996;35:475–481. doi: 10.1016/0028-3908(96)00008-1. [DOI] [PubMed] [Google Scholar]
- Lenz W. Thalidomide and congenital abnormalities. Lancet. 1962;1:45. doi: 10.1016/s0140-6736(62)91943-8. [DOI] [PubMed] [Google Scholar]
- Leslie R.A. Neuroactive substances in the dorsal vagal complex of the medulla oblongata: nucleus of the tractus solitarius, area postrema, and dorsal motor vagal nucleus of the vagus. Neurochem. Int. 1985;7:191–211. doi: 10.1016/0197-0186(85)90106-8. [DOI] [PubMed] [Google Scholar]
- Li B-Y, Glazebrook P., Kunze D.L., Schild J.H. KCa1.1 channel contributes to cell excitability in unmyelinated but not myelinated rat vagal afferents. Am. J. Cell Physiol. 2011;300:C1393–C1403. doi: 10.1152/ajpcell.00278.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y., Shi K., Qi F., You Z., Chen C., Pan J., Wu G., Chen Y.J., Chen Y., Zhou T., Li Xia J. Thalidomide combined with shot-term low -dose glucocorticoid therapy for the treatment of severe COVID-19: a case -series study. Int. J. Infect. Dis. 2021;103:507–513. doi: 10.1016/j.ijid.2020.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu C., Zhao G., Qiao D., Wag L., He Y., Zhao M., Fan Y., Jiang E. Emerging progress in nausea and vomiting of pregnancy and hyperemesis gravidarum: challenges and opportunities. Front. Med. 2022;8 doi: 10.3389/fmed.2021.809270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y-q., Yang Y-h., Zhang G-l., Meng Q., Feng X-d., Cheng Q-q., Nie K. RNA-Seq reveals inflammatory mechanisms of Xiao-Ban-Xia-Tang decoction to ameliorate cisplatin-induced emesis in a rat pica model. Biomed. Pharmacother. 2020;131 doi: 10.1016/j.biopha.2020.110699. [DOI] [PubMed] [Google Scholar]
- Li C-c., Zhang Z-r., Liu Y-h., Zhang T., Zhang X-t., Wang H., Wang X-c. Multi-dimensional and objective assessment of motion sickness susceptibility based on machine learning. Front. Neurol. 2022;13 doi: 10.3389/fneur.2022.824670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y., Zhang J., Teng Y., Zhang L., Yu P., Jin B., Zhao M., Shi J., Liu S., Song N., Li Z. Thalidomide improves prevention of chemotherapy-induced gastrointestinal side effects following a modified FOLFOX7 regimen: results of a prospective randomized crossover study. Tumori. 2009;95:691–696. doi: 10.1177/030089160909500609. [DOI] [PubMed] [Google Scholar]
- Liu Y.L., Malik N., Sanger G.J., Friedman M.I., Andrews P.L.R. Pica - a model of nausea? Species differences in response to cisplatin. Physiol. Behav. 2005;85:271–277. doi: 10.1016/j.physbeh.2005.04.009. [DOI] [PubMed] [Google Scholar]
- Malik N.M., Liu Y.-L., Cole N., Sanger G.J., Andrews P.L.R. Differential effects of dexamethasone, ondansetron and a tachykinin NK1 receptor antagonist (GR205171) on cisplatin-induced changes in behaviour, food intake, pica and gastric function in rats. Eur. J. Pharmacol. 2007;555:164–173. doi: 10.1016/j.ejphar.2006.10.043. [DOI] [PubMed] [Google Scholar]
- Mann R.D. MTP Press Limited; 1984. Modern Drug Use. An Enquiry on Historical Principles. (Chapter 8), Aspects of Modern Drug Use. [Google Scholar]
- Matthews A., Haas D.M., O'Mathúna D.P., Dowswell T. Interventions for nausea and vomiting in early pregnancy. Cochrane Database Syst. Rev. 2015;9:CD007575. doi: 10.1002/14651858.CD007575.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazzotta P., Magee L.A. A risk-benefit assessment of pharmacological and nonpharmacological treatments for nausea and vomiting of pregnancy. Drugs. 2000;59:781–800. doi: 10.2165/00003495-200059040-00005. [DOI] [PubMed] [Google Scholar]
- Meng Q., Bi P., Zhang G., Li Y., Chen S., Nie K. Forsythiae Fructus aqueous extract attenuates cisplatin-induced kaolin consumption (pica) by inhibiting NLRP3 inflammasome activation in rats. Biosci. Biotoech. Biochem. 2021;85:2054–2064. doi: 10.1093/bbb/zbab126. [DOI] [PubMed] [Google Scholar]
- McBride W.G. Thalidomide and congenital abnormalities. Lancet. 1961;2:1358. [Google Scholar]
- McDougall S.J., Bailey T.W., Mendelowitz D., Andresen M.C. Propofol enhances both tonic and phasic inhibitory currents in second -order neurons of the solitary tract nucleus (NTS) Neuropharmacology. 2008;54:552–563. doi: 10.1016/j.neuropharm.2007.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreira A.L., Sampaio E.P., Zmuidzinas A., Frindt P., Smith K.A., Kaplan G. Thalidomide exerts its inhibitory action on tumor necrosis factor alpha by enhancing mRNA degradation. J. Exp. Med. 1993;177:1675–1680. doi: 10.1084/jem.177.6.1675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millrine D., Kishimoto T. A brighter side to thalidomide: its potential use in immunological disorders. Trends Mol. Med. 2017;23:348–361. doi: 10.1016/j.molmed.2017.02.006. [DOI] [PubMed] [Google Scholar]
- Moore A.G., Brown D.A., Fairlie W.D., Bauskin A.R., Brown P.K., Munier M.L.C., Russell P.K., Salamonsen L.A., Wallace E.M., Breit S.N. The transforming growth factor-ß superfamily cytokine macrophage inhibitory cytokine-1 is present in high concentrations in the serum of pregnant women. J. Clin. Endocrinol. Metab. 2000;85:4781–4788. doi: 10.1210/jcem.85.12.7007. [DOI] [PubMed] [Google Scholar]
- Mujagic H., Chabner B.A., Mujagic Z. Mechanisms of action and potential therapeutic uses of thalidomide. Croat. Med. J. 2002;43:274–285. [PubMed] [Google Scholar]
- Muller G.W., Shire M.G., Wong L.M., Corral L.G., Patterson R.T., Chen Y., Stirling D.I. Thalidomide analogs and PDE4 inhibition. Bioorg. Med. Chem. Lett. 1998;8:2669–2674. doi: 10.1016/s0960-894x(98)00475-2. [DOI] [PubMed] [Google Scholar]
- Mullican S.E., Lin-Schmidt X., Chin C.-N., Chavez J.A., Furman J.L., Armstrong A.A., Beck S.C., South V.J., Dinh T.Q., Cash-Mason T.D., Cavanaugh C.R., Nelson S., Huang C., Hunter M.J., Rangwala S.M. GFRAL is the receptor for GDF15 and the ligand promotes weight loss in mice and nonhuman primates. Nat. Med. (N. Y., NY, U. S.) 2017;23:1150–1157. doi: 10.1038/nm.4392. [DOI] [PubMed] [Google Scholar]
- Murdoch J. McC., Campbell G.D. Antithyroid activity of N-phythalyl glutamic acid imide (K17) Br. Med. J. January. 1958;11:84–85. doi: 10.1136/bmj.1.5062.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muth E.R., Stern R.M., Thayer J.F., Koch K.L. Assessment of the multiple dimensions of nausea: the Nausea Profile (NP) J. Psychosom. Res. 1996;40:511–520. doi: 10.1016/0022-3999(95)00638-9. [DOI] [PubMed] [Google Scholar]
- Navari R.M., Reinhardt R.R., Gralla R.J., Kris M.G., Hesketh P.J., Khojasteh A., Kindler H., Grote T.H., Pendergrass K., Grunberg S.M., Carrides A.D., Gertz B.J. Reduction of cisplatin -induced emesis by a selective neurokinin -1 receptor antagonist. L-754,030 Antiemetic trials group. N. Engl. J. Med. 1999;340:190–195. doi: 10.1056/NEJM199901213400304. [DOI] [PubMed] [Google Scholar]
- Newton B.W., Maley B.E. A comparison of GABA- and GAD-like immunoreactivity within the area postrema of the rat and cat. J. Comp. Neurol. 1987;255:208–216. doi: 10.1002/cne.902550205. [DOI] [PubMed] [Google Scholar]
- Newlinds J.S. Nausea and vomiting in pregnancy. Med. J. Aust. 1964;1:234–236. [PubMed] [Google Scholar]
- Noguchi T., Shimazawa R., Nagasawa K., Hashimoto Y. Thalidomide and its analogues as cyclooxygenase inhibitors. Bioorg. Med. Chem. Lett. 2002;12:1043–1046. doi: 10.1016/s0960-894x(02)00084-7. [DOI] [PubMed] [Google Scholar]
- Obara Y., Machida T., Takano Y., Shiga S., Suzuki A., Hamaue N., Iizuka K., Hirafuji M. Cisplatin increases the number of enterochromaffin cells containing substance P in rat intestine. Naunyn Schmiedebergs Arch. Pharmacol. 2018;391:847–858. doi: 10.1007/s00210-018-1493-5. [DOI] [PubMed] [Google Scholar]
- Ostenfeld A., Petersen T.S., Futtrup T.B., Andersen J.T., Jensemn A.K., Westergaard H.B., Pedersen L.H., Løkkegard E.C.L. Validating the effect of ondansetron and mirtazapine in treating hyperemesis gravidarum (VOMIT): protocol for a randomised placebo-controlled trial. BMJ Open. 2020;10 doi: 10.1136/bmjopen-2019-034712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osterloh G. [On the effect of N-phthalyl-glutamic acid imide on other drugs] Arzneimittelforschung. 1959;9:745–747. [PubMed] [Google Scholar]
- Palencia G., Calderon A., Sotelo J. Thalidomide inhibits pentylenetetrazole-induced seizures. J. Neurol. Sci. 2007;258:128–131. doi: 10.1016/j.jns.2007.03.010. [DOI] [PubMed] [Google Scholar]
- Palencia G., Garcia E., Osorio-Rico L., Rejo-Solis C., Escamilla-Ramirez A., Sotelo J. Neuroprotective effect of thalidomide on MPTP-induced toxicity. Neurotoxicity. 2015;47:82–87. doi: 10.1016/j.neuro.2015.02.004. [DOI] [PubMed] [Google Scholar]
- Palencia G., Martinez-Juarez I.E., Calderon A., Artigas C., Sotelo J. Thalidomide for treatment of refractory epilepsy. Epilepsy Res. 2010;92:253–257. doi: 10.1016/j.eplepsyres.2010.10.003. [DOI] [PubMed] [Google Scholar]
- Palencia G., Rubio C., Custodio-Ramirez V., Paz C., Sotelo J. Strong anticonvulsant effects of thalidomide on amygdaloid kindling. Epilepsy Res. 2011;95:263–269. doi: 10.1016/j.eplepsyres.2011.04.008. [DOI] [PubMed] [Google Scholar]
- Payandemehr B., Rahimian R., Gooshe M., Bahremand A., Gholizadeh R., Berjiani S., Ahmadi-Dastgerdi M., Sarreshte-Dari A., Dianti V. Nitric oxide mediates the anticonvulsant effects of thalidomide on pentylenetertazole -induced clonic seizures in mice. Epilepsy Behav. 2014;34:99–104. doi: 10.1016/j.yebeh.2014.03.020. [DOI] [PubMed] [Google Scholar]
- Peach M.L., Beedie S.L., Chau C.H., Collins M.K., Markolovic S., Luo W., Tweedie D., Steinbach C., Greig N.H., Gütschow M., Vargesson N., Nicklaus M.C., Figg W.D. Antiangiogenic activity and in silico cereblon binding analysis of novel thalidomide analogs. Molecules. 2020;25:5683. doi: 10.3390/molecules25235683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perri A., Hsu S.A. Review of thalidomide's history and current dermatological applications. Dermatol. Online J. 2003;9:5. [PubMed] [Google Scholar]
- Petry C.J., Ong K.K., Burling K.A., Barker P., Goodburn S.F., Perry J.R.B., Acerini C.L., Hughes I.A., Painter R.C., Afink G.B., Dunger D.B., O'Rahilly S. Associations of vomiting and antiemetic use in pregnancy with levels of circulating GDF15 early in the second trimester: a nested case-control study. Wellcome Open Research. 2018;3:123. doi: 10.12688/wellcomeopenres.14818.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piwko C., Koren G., Babashov V., Vicente C., Einarson T.R. Economic burden of nausea and vomiting of pregnancy in the USA. J. Popul. Ther. Clin. Pharmacol. 2013;20:e149–e160. [PubMed] [Google Scholar]
- Pourshadi N., Rahimi N., Ghasemi M., Faghir-Ghanesfat H., Sharifzadeh M., Dehpour A.R. Anticonvulsant effects of thalidomide on pentlenetetrazole-induced seizure in mice: a role for opioidergic and nitrergic transmissions. Epilepsy Res. 2020;164 doi: 10.1016/j.eplepsyres.2020.106362. [DOI] [PubMed] [Google Scholar]
- Racke K., Reimann A., Schwörer Kilbinger H. Regulation of 5-HT release from enterochromaffin cells. Behav. Brain Res. 1996;73:83–87. doi: 10.1016/0166-4328(96)00075-7. [DOI] [PubMed] [Google Scholar]
- Randall T. Thalidomide has 37-year history. JAMA. 1990;263:1474. doi: 10.1001/jama.1990.03440110028006. [DOI] [PubMed] [Google Scholar]
- Rehman W., Arfons L.M., Lazarus H.M. The rise, fall and subsequent triumph of thalidomide: lessons learned in drug development. Ther. Adv. Hematol. 2011;2:291–308. doi: 10.1177/2040620711413165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid J., Mills M., Cantwell M., Cardwell C.R., Murray L.J., Donnelly M. Thalidomide for managing cancer cachexia. Cochrane Database Syst. Rev. 2012;4:CD008664. doi: 10.1002/14651858.CD008664.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reist M., Carrupt P.-A., Francotte E., Testa B. Chiral inversion and hydrolysis of thalidomide: mechanisms and catalysis by bases and serum albumin, and chiral stability of teratogenic metabolites. Chem. Res. Toxicol. 1998;11:1521–1528. doi: 10.1021/tx9801817. [DOI] [PubMed] [Google Scholar]
- Rostamian A., Gharedaghi A., Norouzi-Javidan A., Dehpour A.R. Involvement of the nitric oxide pathways in the anti-depressant -like effects of thalidomide in mice. Phyiol. Behav. 2019;208 doi: 10.1016/j.physbeh.2019.112572. [DOI] [PubMed] [Google Scholar]
- Salter C.E., Lodge-Patch I.C., Hare E.H. Thalidomide: clinical trial of a new hypnotic drug. J. Clin. Exp. Psychopathol. Q. Rev. Psychiatr. Neurol. 1959;20:243–246. [PubMed] [Google Scholar]
- Sanger G.J., Holbrook J.D., Andrews P.L.R. The translational value of rodent gastrointestinal functions: a cautionary tale. Trends Pharmacol. Sci. 2011;32:402–409. doi: 10.1016/j.tips.2011.03.009. [DOI] [PubMed] [Google Scholar]
- Sanger G.J., Andrews P.L.R. A history of drug discovery for treatment of nausea and vomiting and the implications for future research. Front. Pharmacol. 2018;9:913. doi: 10.3389/fphar.2018.00913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger G.J., Andrews P.L.R. A proposal for rational drug class terminology: a gastrointestinal perspective. Br. J. Pharmacol. 2022 doi: 10.1111/bph.15946. August 2022. [DOI] [PubMed] [Google Scholar]
- Sanger G.J., Twycross R. Making sense of emesis, pruritus, 5-HT and 5-HT3 receptor antagonists. Prog. Palliat. Care. 1996;4:7–8. [Google Scholar]
- Schafer P.H., Parton A., Capone L., Cedzik D., Brady H., Evans J.F., Man H.-W., Muller G.W., Stirling D.I., Chopra R. Apremilast is a selective PDE4 inhibitor with regulatory effects on innate immunity. Cell. Signal. 2014;26:2016–2029. doi: 10.1016/j.cellsig.2014.05.014. [DOI] [PubMed] [Google Scholar]
- Schein C.H. Repurposing approved drugs on the pathway to novel therapies. Med. Res. Rev. 2020;40:586–605. doi: 10.1002/med.21627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schein C.H. Repurposing approved drugs for cancer therapy. Br. Med. Bull. 2021;137:13–27. doi: 10.1093/bmb/ldaa045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schumacher H., Smith R.L., Williams R.T. The metabolism of thalidomide: the spontaneous hydrolysis of thalidomide in solution. Br. J. Pharmacol. 1965;25:324–337. doi: 10.1111/j.1476-5381.1965.tb02053.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schumacher H., Smith R.L., Williams R.T. The metabolism of thalidomide.: the fate of thalidomide and some of its hydrolysis products in various species. Br. J. Pharmacol. 1965;25:338–351. doi: 10.1111/j.1476-5381.1965.tb02054.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz J.-C., Agid Y., Bouthenet M.-L., Javoy-Agid F., Llorens-Cortes C., Martres M.-P., Polard H., Sales N., Taquest H. In: Nausea and Vomiting: Mechanisms and Treatment. Davis C.J., Lake-Bakaar G.V., Grahame-Smith D.G., editors. Springer-Verlag; Berlin, Germany: 1986. Neurochemical investigations into the human area postrema. p18-30. [Google Scholar]
- Seifert R., Alexander S. Perspective article: a proposal for rational drug class terminology. Br. J. Pharmacol. 2022;179:4311–4314. doi: 10.1111/bph.15916. [DOI] [PubMed] [Google Scholar]
- Shannon E., Noveck R., Sandoval F., Kamath B., Kearney M. Thalidomide suppressed interleukin-6 but not tumor necrosis factor-alpha in volunteers with experimental endotoxemia. Transl. Res. 2007;150:275–280. doi: 10.1016/j.trsl.2007.05.003. [DOI] [PubMed] [Google Scholar]
- Sherbet G.V. Therapeutic potential of thalidomide and its analogues in the treatment of cancer. Anticancer Res. 2015;35:5767–5772. [PubMed] [Google Scholar]
- Shi Q., Chen L. Cereblon: a protein crucial to the multiple functions of immunomodulatory drugs as well as cell metabolism and disease generation. J. Immunol. Res. 2017;9130608 doi: 10.1155/2017/9130608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimazawa R., Sano H., Tanatani A., Miyachi H., Hashimoto Y. Thalidomide as a nitric oxide synthase inhibitor and its structural development. Chem. Pharm. Bull. 2004;52:498–499. doi: 10.1248/cpb.52.498. [DOI] [PubMed] [Google Scholar]
- Smith E.R., Mason M.M. Toxicology of the prostaglandins. Prostaglandins. 1974;7:247–268. doi: 10.1016/0090-6980(74)90008-2. [DOI] [PubMed] [Google Scholar]
- Smithells R.W. The thalidomide syndrome comprehensive care. Clin. Pediatr. 1966;5:255–258. doi: 10.1177/000992286600500417. [DOI] [PubMed] [Google Scholar]
- Sneader W. John Wiley & Sons Ltd.; West Sussex, UK: 2005. Drug Discovery. A History. Chapter 26; pp. 355–374. The first synthetic drugs and their analogues. [Google Scholar]
- Somers G.F. Pharmacological properties of thalidomide (alpha-phthalimido glutarimide), a new sedative hypnotic drug. Br. J. Pharmacol. Chemother. 1960;15:111–116. doi: 10.1111/j.1476-5381.1960.tb01217.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephens T., Brynner R. Perseus Publishing; Cambridge, Massachusetts, USA: 2001. Dark Remedy. The Impact of Thalidomide and its Revival as a Vital Medicine; p. 216. [Google Scholar]
- Stirling D.I. Thalidomide and its impact in dermatology. Semin. Cutan. Med. Surg. 1988;17:231–242. doi: 10.1016/s1085-5629(98)80019-9. [DOI] [PubMed] [Google Scholar]
- Stirling D.I. Pharmacology of thalidomide. Semin. Hematol. 2000;37(3):5–14. [Google Scholar]
- Stern R.M., Koch K.L., Andrews P.L.R. Oxford University Press; New York, USA: 2011. Nausea: Mechanisms and Management; p. 462. [Google Scholar]
- Strominger N.L., Hori N., Carpenter D.O., Tan Y., Folger W.H. Effects of acetylcholine and GABA on neurons in the area postrema of Suncus murinus brainstem slices. Neurosci. Lett. 2001;309:77–80. doi: 10.1016/s0304-3940(01)02049-3. [DOI] [PubMed] [Google Scholar]
- Sundaresan L., Giri S., Singh H., Chatterjee S. Repurposing of thalidomide and its derivatives for the treatment of SARS-coV-2 infections: hints on molecular action. Br J. Clin. Phrmacol. 2020;87:3835–3850. doi: 10.1111/bcp.14792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sunday Times Insight Team . Futura Publications Ltd.; London, UK: 1980. Suffer the Children-The Story of Thalidomide; p. 396. [Google Scholar]
- Tamalunas A., Sauckel C., Ciotkowska A., Gratzke C., Stief C.G., Hennenberg M. Inhibition of human prostate smooth muscle contraction by thalidomide: a novel remedy in LUTS? Eur. Urol. Open. Sci. 2020;19(Suppl. 2):e989. doi: 10.1002/pros.24114. [DOI] [PubMed] [Google Scholar]
- Tarhan Ö., Canbay Ö., Çelebi N., Uzun Ş., Şahin A., Coşkun F., Aypar Ü. Subhypnotic doses of midazolam prevent nausea and vomiting during spinal anesthesia for cesarean section. Minerva Anestesiol. 2007;73:629–633. [PubMed] [Google Scholar]
- Theoret J.J. Chronology of the thalidomide story. Can. Med. Assoc. J. 1962;87:981–982. [PMC free article] [PubMed] [Google Scholar]
- Teo S.K., Colburn W.A., Thomas S.D. Single-dose oral pharmacokinetics of three formulations of thalidomide in healthy male volunteers. J. Clin. Pharmacol. 1999;39 [PubMed] [Google Scholar]
- Traldi A., Vaccari G.L., Davoli G. L’impiego dell’imide dell’acido N-ftalilglutammico (Talidomide) nella terapia sintomatica del vomito di molti pazienti affetti di neoplasia maligne o causato dalla somministrazione di cloridato di mecloretamina. Cancro. 1965;18:336–341. [PubMed] [Google Scholar]
- Trapani G., Altomare C., Liso G., Sanna E., Biggio G. Propofol in anesthesia. Mechanism of action, structure-activity relationships, and drug delivery. Curr. Med. Chem. 2000;7:249–271. doi: 10.2174/0929867003375335. [DOI] [PubMed] [Google Scholar]
- Tseng S., Pak G., Washenik K., Pomeranz M.K., Shupack J.L. Rediscovering thalidomide: a review of its mechanism of action, side effects, and potential uses. J. Am. Acad. Dermatol. 1996;35:969–979. doi: 10.1016/s0190-9622(96)90122-x. [DOI] [PubMed] [Google Scholar]
- Vargesson N. Thalidomide-induced teratogenesis: history and mechanisms. Birth Defects Res. (Part C) 2015;105:140–156. doi: 10.1002/bdrc.21096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vargesson N. The teratogenic effects of thalidomide on limbs. J. Hand Surg. 2019;44:88–95. doi: 10.1177/1753193418805249. [DOI] [PubMed] [Google Scholar]
- Vargesson N., Stephens T. Thalidomide: history, withdrawal, renaissance, and safety concerns. Expet Opin. Drug Saf. 2021;20:1455–1457. doi: 10.1080/14740338.2021.1991307. [DOI] [PubMed] [Google Scholar]
- Wang N., Xu P., Liu Y., Zhao P., Ruan J., Zheng Y., Jin J., Wang S., Yao J., Xiang D., Zhang D., Li N., Kang H., Dai Z. Efficacy and safety of thalidomide for chemotherapy-induced nausea and vomiting. J. Cancer. 2020;11:4560–4570. doi: 10.7150/jca.45678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Was H., Borkowska A., Bagues A., Tu L., Liu J.Y.H., Lu Z., Rudd J.A., Nurgali K., Abalo R. Mechanisms of chemotherapy-induced neurotoxicity. Front. Pharmacol. 2022;13 doi: 10.3389/fphar.2022.750507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weld E.D., Bailey T.C., Waitt C. Ethical issues in therapeutic use and research in pregnant and breastfeeding women. Br. J. Clin. Pharmacol. 2021;88:7–21. doi: 10.1111/bcp.14914. [DOI] [PubMed] [Google Scholar]
- Whitehead S.A., Andrews P.L.R., Chamberlain G.V.P. Characterisation of nausea and vomiting in early pregnancy: a survey of 1,000 women. J. Obstet. Gynaecol. 1992;12:364–369. [Google Scholar]
- Wnendt S., Finkam M., Winter W., Ossig J., Raabe G., Zwingenberger K. Enatioselective inhibition of THF-alpha release by thalidomide and thalidomide-analogues. Chirality. 1996;8:390–396. doi: 10.1002/(SICI)1520-636X(1996)8:5<390::AID-CHIR6>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- Wuchert F., Ott D., Rafalzik S., Roth J., Gerstberger R. Tumour necrosis factor-α, interleukin 1β and nitric oxide induce calcium transients in distinct populations of cells cultured from the rat area postrema. J. Neuroimmunol. 2009;206:44–51. doi: 10.1016/j.jneuroim.2008.10.010. [DOI] [PubMed] [Google Scholar]
- Xie J., Zhang C., Li S., Dai R., Sullivan M.A., Deng B., Xu Q., Wang J., Shi C., Zhang Y. Efficacy and safety of thalidomide as a pre-medication of chemotherapy-induced nausea and vomiting (CINV) following highly emetogenic chemotherapy (HEC): a systematic review and meta-analysis. Front. Oncol. 2022;11 doi: 10.3389/fonc.2021.818839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xin W., Xiaohua N., Pelin C., Xin C., Yaqiong S., Qihan W. Primary function analysis if human mental retardation related gene CRBN. Mol. Biol. Rep. 2008;35:251–256. doi: 10.1007/s11033-007-9077-3. [DOI] [PubMed] [Google Scholar]
- Yamamoto J., Ito T., Yamaguchi Y., Handa H. Discovery of CRBN as a target of thalidomide: a breakthrough for progress in the development of protein degraders. Chem. Soc. Rev. 2022;51:6234–6250. doi: 10.1039/d2cs00116k. [DOI] [PubMed] [Google Scholar]
- Yashiro K., Miyagawa S., Sawa Y. A lesson from the thalidomide tragedy. Circ. J. 2018;82:2250–2252. doi: 10.1253/circj.CJ-18-0775. [DOI] [PubMed] [Google Scholar]
- Young R.W. In: Nausea and Vomiting: Mechanisms and Treatment. Davis C.J., Lake-Bakaar G.V., Grahame-Smith D.G., editors. Springer-Verlag; Berlin, Germany: 1986. Mechanisms and treatment of radiation-induced nausea d vomiting. p94-109. [Google Scholar]
- Zhang L., Qu X., Teng Y., Shi J., Yu P., Sun T., Wang J., Zhu Z., Zhang X., Zhao M., Liu J., Jin B., Luo Y., Teng Z., Dong Y., Wen F., An Y., Yuan C., Chen T., Zhou L., Chen Y., Zhang J., Wang Z., Qu J., Jin F., Zhang J., Jin X., Xie X., Wang J., Man L., Fu L., Liu Y. Efficacy of thalidomide in preventing delayed nausea and vomiting induced by highly emetogenic chemotherapy: a randomized, multicentre, double-blind, placebo -controlled phase III trial (CLOG1302 study) J. Clin. Oncol. 2017;35:1–8. doi: 10.1200/JCO.2017.72.2538. [DOI] [PubMed] [Google Scholar]
- Zhang L., Qu X., Liu Y. Reply to M.F. Chong et al. J. Clin. Oncol. 2018;36:828–829. doi: 10.1200/JCO.2017.76.5784. [DOI] [PubMed] [Google Scholar]
- Zhao J., Li M., Chen Y., Zhang S., Ying H., Song Z. Elevated serum growth differentiation factor 15 levels in hyperthyroid patients. Front. Endocrinol. 2018;9:793. doi: 10.3389/fendo.2018.00793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong W., Shahbaz O., Teskey G., Beever A., Kachour N., Venketaraman V., Darmani N.A. Mechanisms of nausea and vomiting: current knowledge and recent advances in intracellular emetic signalling systems. Int. J. Mol. Sci. 2021;22:5797. doi: 10.3390/ijms22115797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong W., Chebolu S., Darmani N.A. Thapasigarin -induced activation of Ca(2+) -CaMKII-ERK in brainstem contributes to substance P release and induction of emesis in the least shrew. Neuropharmacology. 2016;103:195–210. doi: 10.1016/j.neuropharm.2015.11.023. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No data was used for the research described in the article.