Abstract
Background:
Abnormal blood pressure (BP) responses to exercise can predict adverse cardiovascular outcomes, but their optimal measurement and definitions are poorly understood. We combined frequently sampled BP during cardiopulmonary exercise testing (CPET) with vascular stiffness assessment to parse cardiac and vascular components of exercise BP.
Methods:
CPET with BP measured every two minutes and resting vascular tonometry were performed in 2858 Framingham Heart Study (FHS) participants. Linear regression was used to analyze sex-specific exercise BP patterns as a function of arterial stiffness (carotid-femoral pulse wave velocity; CFPWV) and cardiac-peripheral performance (defined by peak O2 pulse).
Results:
Our sample was balanced by sex (52% women) with mean age 54±9 years and 47% with hypertension. We observed variability in CFPWV and peak O2 pulse across individuals with clinically defined exercise hypertension (peak systolic BP [SBP] in men ≥210 mm Hg; in women ≥190 mm Hg). Despite similar resting SBP and cardiometabolic profiles, individuals with higher peak O2 pulse displayed higher peak SBP (P≤0.017) alongside higher fitness levels (P<0.001), suggesting that high peak exercise SBP in the context of high peak O2 pulse may in fact be favorable. While both higher (favorable) O2 pulse and higher (adverse) arterial stiffness were associated with greater peak SBP (P<0.0001 for both), the magnitude of association of CFPWV with peak SBP was higher in women (sex-CFPWV interaction P<0.0001). In sex-specific models, exercise SBP measures accounting for workload (e.g., SBP during unloaded exercise, SBP at 75 watts, and SBP/workload slope) were directly associated with the adverse features of greater arterial stiffness and lower peak O2 pulse.
Conclusions:
Higher peak exercise SBP reflects a complex trade-off between arterial stiffness and cardiac-peripheral performance that differs by sex. Studies of BP responses to exercise accounting for vascular and cardiac physiology may illuminate mechanisms of hypertension and clarify clinical interpretation of exercise BP.
Subject terms: Epidemiology, exercise, hypertension, vascular stiffness
Graphical Abstract
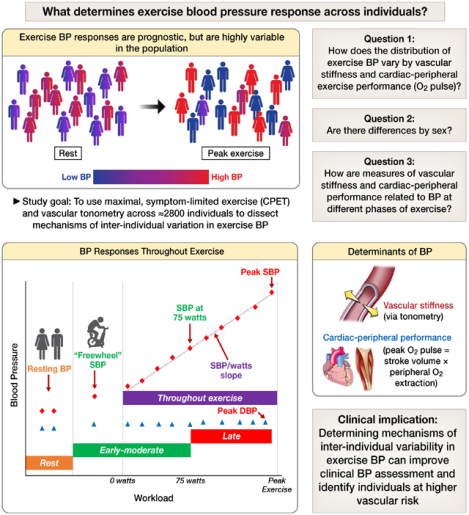
Abbreviations: CPET, cardiopulmonary exercise testing; BP, blood pressure; SBP, systolic blood pressure; DBP, diastolic blood pressure. In the lower left panel, red diamonds represent systolic blood pressures and blue diamonds represent diastolic blood pressures.
INTRODUCTION
Exercise testing unmasks abnormal physiological responses that are not apparent at rest. Over recent decades, exaggerated blood pressure (BP) responses to exercise have been studied in relation to elevated ambulatory BP1,2 and incident hypertension and cardiovascular disease (CVD)3–8. A rapid rise in BP during exercise has been reported to reflect adverse vascular function4. However, despite their established relevance for disease prediction and pathophysiology, the use of exercise BP responses in clinical settings is limited by a lack of consensus on the definition of abnormal values9,10. While clinical exercise testing recommendations advocate for the use of peak systolic BP (SBP) threshold values (≥210 mm Hg in men, ≥190 mm Hg in women)9, individuals with high fitness levels may achieve higher peak systolic BP as a consequence of greater cardiac and skeletal muscle (peripheral) performance during exercise, which would not necessarily confer higher CVD risk11,12. To overcome these limitations, prior observational studies have largely relied on submaximal BP measures3–8, but protocols used to derive submaximal BP measures lack standardization. Moreover, prior studies have not examined the physiological contributions of cardiac and vascular function to exercise BP responses in community-dwelling individuals, which are critical factors in determining the potential adverse cardiovascular consequences of abnormal exercise BP responses.
Here, we quantify BP responses during exercise in a large group of community-dwelling individuals who underwent maximum effort cardiopulmonary exercise testing (CPET) and evaluation of resting arterial stiffness (via tonometry). Combining CPET and vascular function testing allowed us to parse the relations of arterial stiffness and the cardiac-peripheral performance (represented by the peak O2 pulse, composed of cardiac stroke volume × peripheral O2 extraction) with BP changes with exercise. Given potential differences among men and women in the epidemiology, physiology, and clinical implications of BP regulation, we evaluated sex differences in exercise BP and its physiological correlates13,14. We hypothesized that a high peak exercise SBP would be observed in individuals with either higher arterial stiffness or higher cardiac-peripheral performance and that BP measures that incorporate workload would be more closely associated with adverse vascular characteristics and corresponding CVD risk factors (Graphical Abstract).
METHODS
Data sharing
Data used for the present investigation will be made available upon reasonable request. Framingham Heart Study (FHS) data are publicly available through the National Institutes of Health database of genotypes and phenotypes (https://www.ncbi.nlm.nih.gov/gap/).
Study sample
Enrollment and characteristics of the FHS Generation Three, Omni Generation Two, and New Offspring Spouse cohorts have been reported15,16. Briefly, these three cohorts were enrolled together and attended their first study visit in 2002–2005 and their second study visit in 2008–2011. At the third study visit (2016–2019), 3117 of 3521 participants (89%) consented to undergo maximum effort CPET, as described17,18. Only information from the third study visit was used for the present investigation. From individuals who performed CPET, we excluded those with missing gas exchange measures (N=13), sub-maximum volitional effort (peak respiratory exchange ratio [RER] <1.05; N=139), missing tonometry measures (N=56), or missing blood pressure measures or covariates (N=51), yielding 2858 individuals eligible for the present analysis. Institutional Review Boards at Boston University and Massachusetts General Hospital approved all study protocols. All participants provided written informed consent.
Exercise testing and BP quantification
CPET methods have been reported previously and are reproduced below for fidelity of scientific communication19. Participants were encouraged to fast overnight (including caffeinated beverages) prior to the CPET and arterial tonometry assessments and to not perform exercise prior to arrival at the study visit. CPET assessment and arterial tonometry were performed primarily during the mornings in the context of a ≈four-hour study visit19. Maximal effort CPET was performed on a cycle ergometer (Lode, Netherlands) using one of two incremental ramp protocols (15 and 25 watts/minute). Participants were assigned to a specific ramp protocol by study staff based on an estimate of the predicted peak watts after considering age, sex, weight, height, and physical activity level19. The CPET assessment was conducted in a separate room with an adjustable air conditioner unit to avoid high temperatures during exercise. The exercise protocol consisted of three minutes of unloaded (“freewheel”) exercise followed by incremental ramp exercise. We obtained breath-by-breath gas exchange measures (MedGraphics, St. Paul, MN) throughout exercise17,18. Heart rate was monitored continuously during exercise with wireless electrocardiogram (ECG) equipment (Mortara, Milwaukee, WI). Peak oxygen uptake (VO2) was assessed as the highest 30-second median during the final minute of exercise and peak O2 pulse was calculated as the peak VO2 divided by the peak heart rate17,18. The percent predicted peak VO2 was calculated using the Wasserman-Hansen equations (equations included in the Supplemental Material)20. BP was measured every two minutes during exercise using a manual sphygmomanometer. Peak BP was measured immediately after termination of loaded exercise. BP measures used for this analysis include the following: (1) resting systolic and diastolic BP while the participant was seated on the cycle immediately prior to exercise (without a specified resting period); (2) unloaded exercise (turning the pedals with no added resistance) systolic BP (SBP), measured at minute 2 of the unloaded period; (3) SBP at 75 watts (occurring at minute 3 for the 25 watts/minute and minute 5 for the 15 watts/minute ramp protocols); and, (4) the “SBP/W slope”, calculated as (peak SBP – rest SBP)/peak workload. Participants were encouraged to exercise until exhaustion and testing was only stopped early for safety criteria (including chest pain with ECG changes, complex ectopy or high-degree atrioventricular block, symptomatic fall in SBP >20 mm Hg, marked exercise hypertension [SBP >240 mm Hg, diastolic BP (DBP) >120 mm Hg], oxygen desaturation, neurologic compromise, or at the discretion of the supervising clinician). To account for sex differences in workload, we multiplied the SBP/W slope by the sex-specific 1-standard deviation change in workload. Resting hypertension was defined, using the average of two measurements taken while the participant was seated in a chair following a five-minute period of rest prior to exercise, as SBP ≥130 mm Hg, DBP ≥80 mm Hg, or use of BP lowering medications21. An exaggerated SBP response to exercise was defined as systolic BP ≥210 in men and ≥190 mm Hg in women, consistent with published recommendations3,9,22.
Vascular stiffness assessment
Applanation tonometry was performed on the right brachial, femoral, and carotid arteries on supine participants after a five-minute resting period using a custom transducer and data acquisition system (NIHem Hemodynamic Workstation, Cardiovascular Engineering, Inc., Norwood, MA), as previously described23. Arterial waveforms were signal-averaged using the electrocardiographic R wave as the fiducial point. Signal-averaged brachial artery waveforms were calibrated with systolic and diastolic auscultatory BP and integrated to derive mean arterial pressure. Carotid-femoral pulse wave velocity (CFPWV) was calculated as carotid-femoral transit distance (measured as the difference in body surface measurements from the suprasternal notch to the femoral and carotid sites) divided by carotid-femoral transit time delay (measured using the foot of the carotid and femoral waveforms). The augmentation index was calculated as the difference between the first systolic inflection point and the peak waveform (i.e., the augmentation pressure) divided by the total pulse pressure and multiplied by 100. Central pressure and flow waveforms were used to perform time domain waveform separation analysis in order to obtain forward and backward pressure waveforms23. Forward wave amplitude was defined as the amplitude of the forward pressure wave. Characteristic impedance was calculated in the time domain by dividing the pressure increase by the flow increase up to 95% of peak flow23.
Covariate assessment
Prevalent CVD was defined as a history of myocardial infarction, stroke, or heart failure, or by self-report of taking medications for heart failure, angina or chest pain, atrial fibrillation/heart rhythm abnormality, stroke, peripheral arterial disease, or claudication. Diabetes was defined by fasting blood glucose ≥126 mg/dL, non-fasting blood glucose ≥200 mg/dL, or use of glucose lowering medications. Smoking was defined by self-report as current smoking (within the 1-year period preceding the study visit) vs. former or never smoking. The physical activity index was calculated based on the reported time spent performing specific activities24.
Statistical analysis
Baseline characteristics, exercise responses, and vascular stiffness measures were summarized and tabulated. Peak O2 pulse was natural log-transformed for analysis to reduce skewness. CFPWV was inverse transformed to reduce heteroscedasticity and skewness and multiplied by −1000 to convert units (ms/m) and restore directionality (i.e., expressed as −1000/CFPWV for analysis), consistent with prior work4,25. Distributions of rest/exercise BP measures and physiological correlates (CFPWV and peak O2 pulse) were examined separately in men and women. Baseline and exercise measures were compared according to categories of CFPWV and peak O2 pulse using two sample t-tests for continuous variables and chi-square tests for categorical variables.
We investigated the relations of peak SBP (dependent variable) with CFPWV and peak O2 pulse (independent variables) using linear models adjusted for age, sex, resting (pre-exercise) SBP, and hypertension medication use. Due to differences noted in the distributions of BP indices in men and women, we evaluated for effect modification by sex on the associations of CFPWV and peak O2 pulse with peak SBP using multiplicative interaction terms (i.e., sex-CFPWV and sex-peak O2 pulse interaction terms were added to separate models including covariates above). Based on evidence of effect modification and different distributions of CFPWV, peak O2 pulse, and peak SBP by sex, all subsequent models were conducted separately in men and women. Accordingly, we evaluated sex-specific models relating peak SBP to CFPWV and peak O2 pulse adjusting for age, resting SBP, and hypertension medication use. These models were used to estimate the marginal means of peak SBP across the 5th to 95th percentile values of (non-transformed) CFPWV and peak O2 pulse separately in men and women (emmeans package in R). Peak SBP estimation was performed using transformed variables (−1000/CFPWV and the natural logarithm of peak O2 pulse) and joint effects plots were shown on the original scale for CFPWV and peak O2 pulse for interpretability.
Next, we evaluated the cross-sectional associations of vascular stiffness measures (independent variables) with BP measures during exercise (dependent variables) in sex-specific linear models adjusted for age, hypertension treatment, and resting SBP. In an additional model, we also adjusted for potential confounders of body mass index (BMI), smoking, and menopause status (in women). In models with peak DBP as the dependent variable, we substituted resting DBP for resting SBP as a covariate. All independent and dependent variables were mean-centered and standardized to unit variance within each sex to facilitate comparison and interpretation. In sensitivity analyses, associations of BP responses with vascular stiffness measures were evaluated after excluding individuals with hypertension. A Bonferroni-adjusted P-value threshold of 0.01 (0.05/5 dependent or “outcome” variables) was used to determine statistical significance and control for multiple hypothesis testing. All analyses were performed using R version 4.1.3 (R project, www.rproject.org).
RESULTS
Clinical characteristics
Study participants had a mean age of 54±9 years and BMI of 28.1±5.3 kg/m2, with 52% women and 9.2% nonwhite individuals (Table 1). Peak VO2, was 21.0±5.9 ml/kg/min (98% predicted) in women and 26.1±6.8 ml/kg/min (93% predicted) in men. Nearly half (47%) of participants had resting hypertension. Resting and exercise BP measures differed substantially in men and women with men demonstrating higher values for all BP measures except for the SBP at 75 watts. Peak O2 pulse and CFPWV were also higher in men versus women (Table 1 and Figure 1).
Table 1.
Characteristics of the study sample
| Variable | Overall (N=2858) | Women (N=1493) | Men (N=1365) | P-value |
|---|---|---|---|---|
| Age, years | 54±9 | 53±9 | 54±9 | 0.13 |
| Nonwhite race, N (%) | 263 (9.2) | 137 (9.2) | 126 (9.2) | 1.00 |
| Height, in | 66.8±3.7 | 64.3±2.5 | 69.5±2.7 | <0.001 |
| Body mass index, kg/m2 | 28.1±5.3 | 27.1±5.6 | 29.1±4.7 | <0.001 |
| Hypertension medication use, N (%) | 599 (21) | 256 (17) | 343 (25) | 0.001 |
| AV nodal blocking medication use, N(%) | 230 (8.0) | 98 (6.6) | 132 (9.7) | 0.003 |
| Hypertension, N (%) | 1349 (47) | 540 (36) | 809 (59) | <0.001 |
| Prevalent cardiovascular disease, N (%) | 104 (3.6) | 37 (2.5) | 67 (4.9) | 0.85 |
| Diabetes, N (%) | 206 (7.2) | 69 (4.6) | 137 (10.0) | <0.001 |
| Current smoking, N (%) | 164 (5.7) | 84 (5.6) | 80 (5.9) | <0.001 |
| Total Cholesterol, mg/dL | 191±36 | 196±35 | 184±36 | <0.001 |
| HDL Cholesterol, mg/dL | 60±19 | 68±19 | 51±15 | <0.001 |
| Physical activity index | 34±6 | 33±4 | 35±7 | <0.001 |
| SBP at rest, mm Hg | 119±14 | 116±14 | 123±13 | <0.001 |
| SBP during freewheel exercise, mm Hg | 130±17 | 127±18 | 134±16 | <0.001 |
| SBP at 75 watts, mm Hg | 153±21 | 154±22 | 152±19 | 0.06 |
| SBP at peak, mm Hg | 181±25 | 172±23 | 191±23 | <0.001 |
| SBP/workload slope, mm Hg/watts | 0.3±0.1 | 0.4±0.2 | 0.3±0.1 | <0.001 |
| DBP at rest, mm Hg | 79±8 | 78±8 | 82±8 | <0.001 |
| DBP at peak, mm Hg | 84±10 | 82±9 | 87±10 | <0.001 |
| Resting heart rate, beats/min | 71.9±12.0 | 74.0±11.9 | 69.6 ±11.6 | <0.001 |
| Peak heart rate, beats/min | 152.1±18.7 | 152.5± 18.2 | 151.6 ±19.3 | 0.18 |
| Peak O2 pulse, ml/beat | 12.4±3.9 | 9.7±2.1 | 15.3±3.2 | <0.001 |
| Carotid-femoral pulse wave velocity, m/s | 7.9±1.9 | 7.5±1.7 | 8.3±2.0 | <0.001 |
| Forward wave amplitude, mm Hg | 48±12 | 47±12 | 48±12 | 0.02 |
| Characteristic impedance | 207 (70) | 222 (75) | 190 (60) | <0.001 |
| Augmentation index, % | 16.6±11.8 | 20.9±10.9 | 11.9±10.9 | <0.001 |
| Peak respiratory exchange ratio | 1.23±0.09 | 1.21±0.09 | 1.24±0.09 | <0.001 |
| Peak workload, watts | 173±60 | 136±35 | 214±54 | <0.001 |
| Peak VO2, ml/kg/min | 23.4±6.8 | 21.0±5.9 | 26.1±6.8 | <0.001 |
| Percent predicted peak VO2, % | 96 (20) | 98 (21) | 93 (18) | <0.001 |
Values are displayed as mean ± SD for continuous variables and N (%) for categorical variables. Abbreviations: SBP, systolic blood pressure; DBP, diastolic blood pressure; VO2, oxygen uptake. P-value reflects a comparison of men and women using two sample t-tests for continuous variables and chi-square tests for categorical variables.
Figure 1. Distributions of rest and exercise blood pressure measures and their physiological correlates in men and women.

The probability density functions are plotted for men and women separately for resting systolic blood pressure (SBP), peak exercise SBP, natural log (peak O2 pulse), transformed carotid-femoral pulse wave velocity (−1000/CFPWV). Dotted lines represent sex-specific mean values.
Distributions and relations of physiological correlates of peak SBP
The distribution of peak SBP across resting SBP measures demonstrated that ≈25% of men and women had peak SBP above clinical thresholds for elevated exercise SBP; however, these individuals exhibited varied levels of resting SBP and did not necessarily demonstrate lower peak workloads or peak O2 pulse (Figure 2). To further explore the relations of peak O2 pulse and CFPWV with peak SBP, we examined individual characteristics according to categories defined by low versus high values for peak O2 pulse and CFPWV (Table S1). Despite higher BMI and similar resting SBP and cardiometabolic risk profiles, individuals with lower CFPWV (below sex-specific median) but higher peak O2 pulse (above sex-specific median) displayed higher mean peak SBP (P<0.001 for both men and women) and higher peak fitness levels (peak VO2; P<0.001 for men and women) than individuals with lower CFPWV and lower peak O2 pulse. Similarly, individuals with higher CFPWV and higher peak O2 pulse demonstrated higher peak SBP than individuals with higher CFPWV and lower peak O2 pulse (P<0.001 in women, P=0.017 in men) despite similar resting SBP (P>0.05 in women and men). Notably, the workloads were markedly higher (P<0.001) in individuals with higher O2 pulse, suggesting that the higher SBP observed in these individuals was consistent with greater external work performed. Collectively, these findings support the notion that a higher peak SBP may not solely reflect adverse vascular function and can be observed in the setting of either higher achieved workload and cardiac-peripheral performance (O2 pulse) or higher arterial stiffness (CFPWV).
Figure 2. The distribution of peak systolic blood pressure across ranges of resting systolic blood pressure.
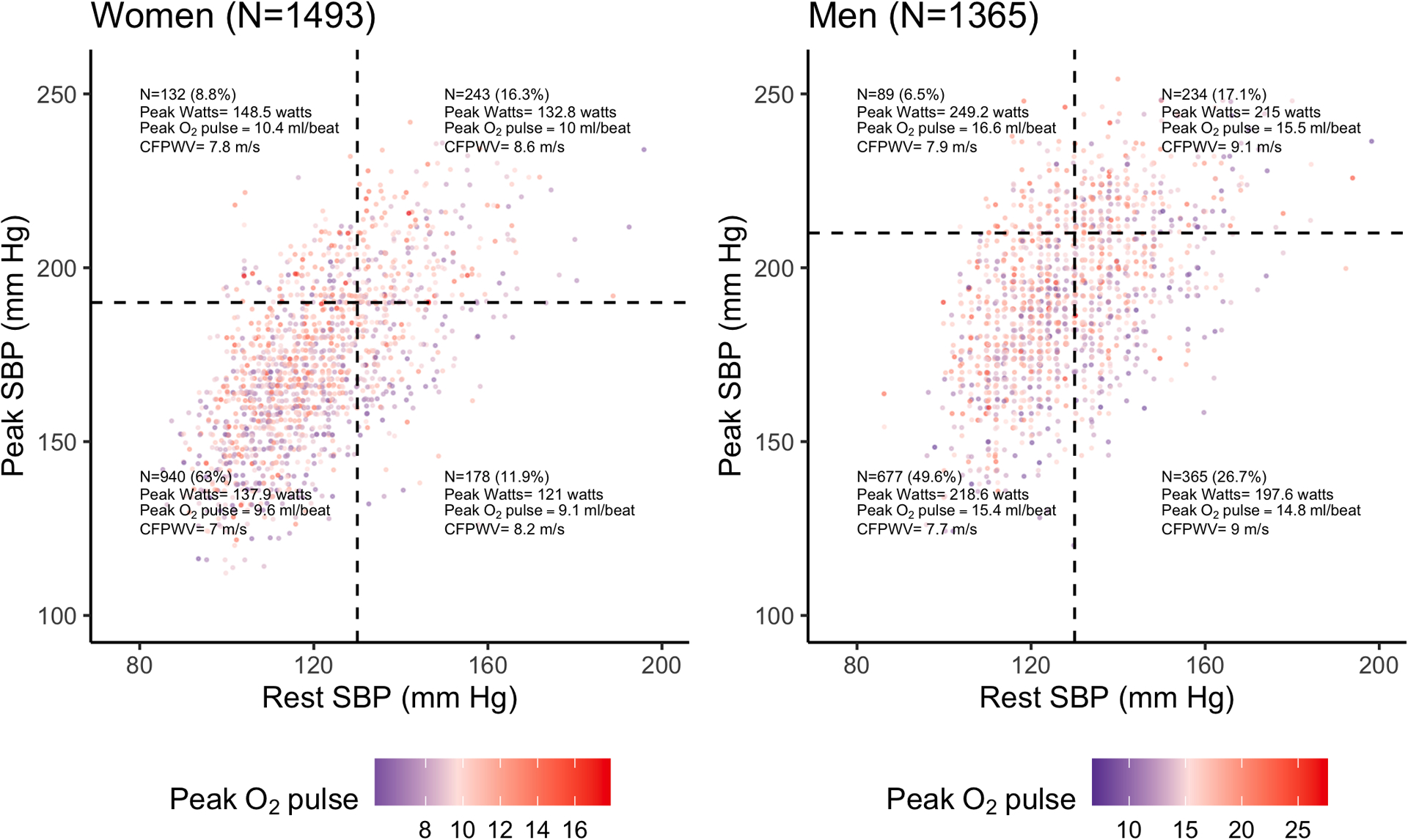
Rest and peak exercise systolic blood pressure (SBP) were plotted for each participant separately in men and women. Points are colorized by the peak O2 pulse achieved. Dotted lines were placed at 130 mm Hg to denote elevated rest SBP and 210 mm Hg for men and 190 mm Hg for women to denote elevated peak SBP. The N and mean workload (“peak watts”), peak O2 pulse, and carotid-femoral pulse wave velocity are displayed for each quadrant.
Joint relations of arterial stiffness and peak O2 pulse with peak SBP
We next examined joint relations of resting CFPWV and peak O2 pulse (independent variables) with peak SBP (dependent variable) using multivariable modeling. A linear model that included CFPWV, peak O2 pulse, age, sex, resting SBP, and hypertension treatment explained 47% of the variability in peak exercise SBP, and CFPWV and peak O2 pulse were both directly related to peak SBP (Table S2). Effect modification by sex on the relation of CFPWV with peak SBP was observed (P<0.0001). By contrast, the sex interaction with peak O2 pulse was not significant (P=0.61). Given the different distributions of CFPWV, peak O2 pulse, and peak SBP in men and women and the significant interaction observed by sex, we then elected to construct all subsequent models separately in men and women (Table S2). Similar relations of higher peak O2 pulse with greater peak SBP were observed in women and men, consistent with lack of a significant sex interaction. However, the magnitude of association of peak SBP with CFPWV was higher in women (75% higher regression coefficient in women; Table S2 and Figure 3A). We specified model-estimated marginal means for peak SBP across CFPWV and peak O2 pulse to visualize their relations with peak SBP (Figure 3B). A higher peak SBP was observed with higher CFPWV and with higher peak O2 pulse. Adjusted for resting SBP, at the same peak O2 pulse, a given increment in CFPWV was associated with a higher increment in peak SBP in women versus men.
Figure 3. Joint associations of peak O2 pulse and carotid-femoral pulse wave velocity with peak systolic blood pressure.
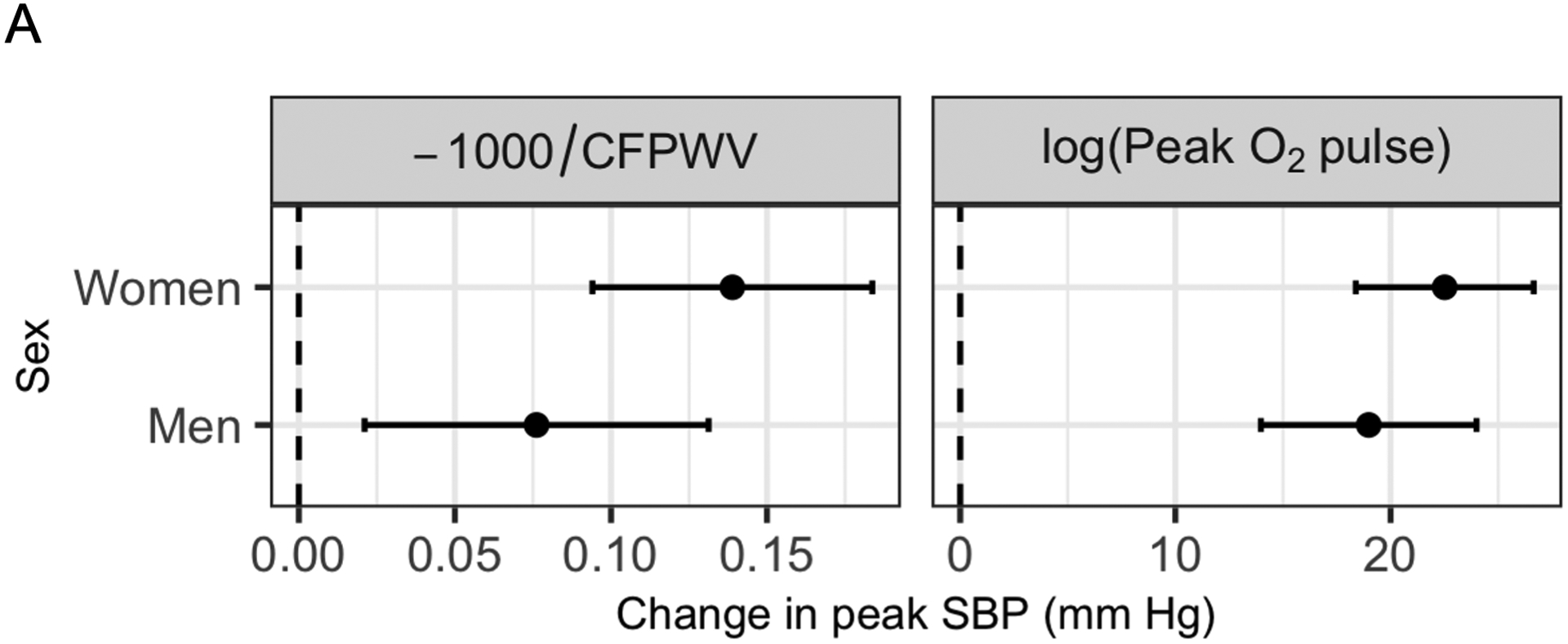
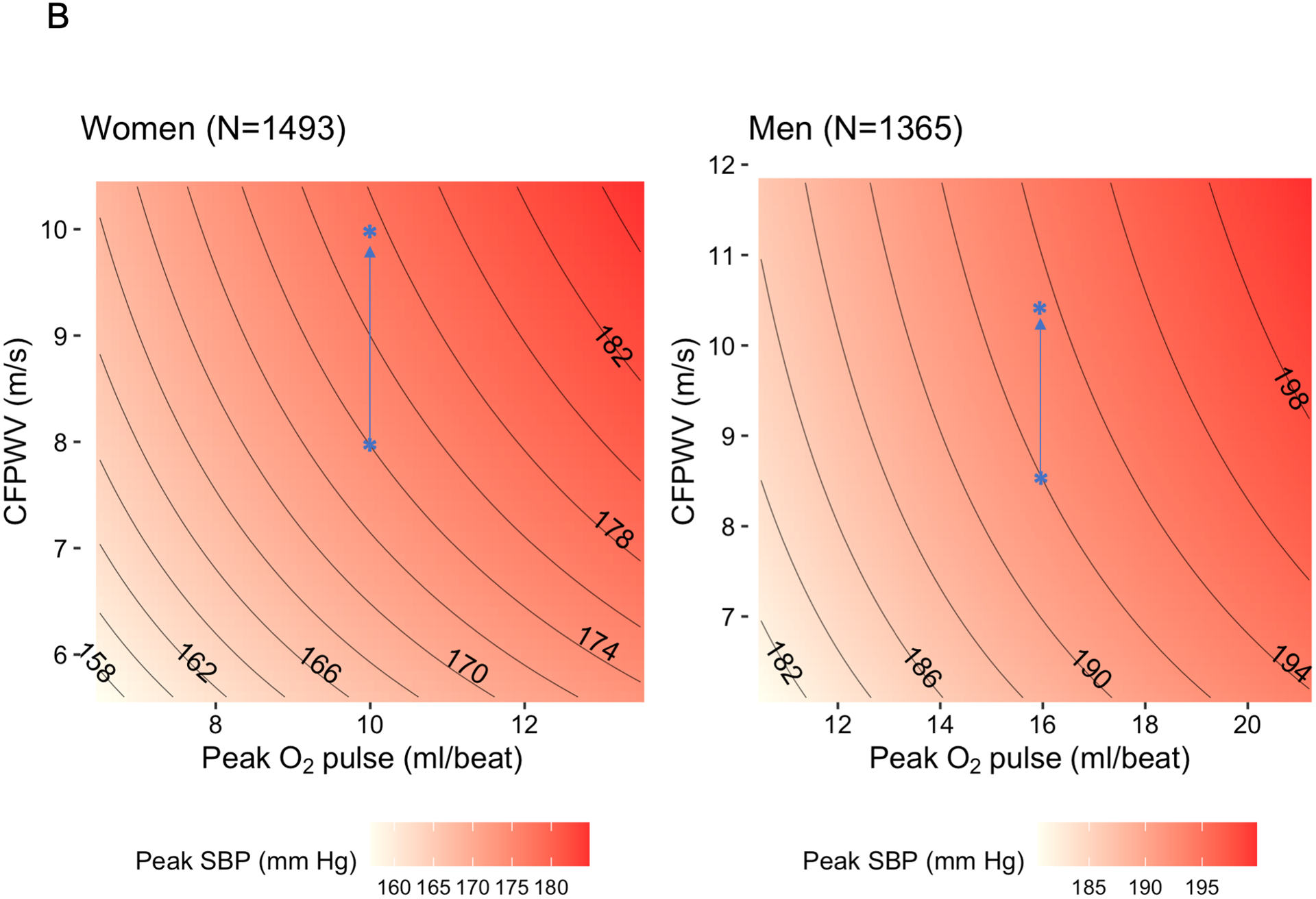
In panel (A), the estimated beta coefficients and 95% confidence intervals are displayed for carotid-femoral pulse wave velocity (CFPWV, transformed) and for peak O2 pulse (natural log transformed) separately in men and women. Estimated regression coefficients were calculated in sex-stratified linear models with peak systolic blood pressure (SBP) as the dependent variable and transformed CFPWV, log(peak O2 pulse), age, hypertension treatment, and resting SBP as independent variables. Panel (B) displays estimated marginal means for peak SBP (using the models described above) as a function of CFPWV and peak O2 pulse (5th-95th percentiles of non-transformed values) using a joint effects plot. These plots demonstrate a “trade-off” between CFPWV and peak O2 pulse in relation to peak SBP. The magnitude of effect of CFPWV on peak SBP is higher in women than men. Overlaid in blue are two illustrative examples. For a woman with CFPWV of 8 m/s and peak O2 pulse of 10 ml/beat, the predicted peak SBP is 174 mm Hg. An increase in CFPWV of 2 m/s (with no change in peak O2 pulse) would correspond to a ≈3.5 mm Hg higher predicted peak SBP. By contrast, in a man with a CFPWV of 8.5 m/s and a peak O2 pulse of 16 ml/beat, the predicted peak SBP is 192 mm Hg. A 2 m/s increase in CFPWV (with no change in peak O2 pulse) would result in a ≈1.7 mm Hg increase in predicted peak SBP.
Relations of arterial stiffness and peak O2 pulse with exercise BP measures at different phases of exercise
Given the higher peak workload we observed in individuals with higher peak O2 pulse (Table S1), we next sought to index SBP measures during exercise to workload and examine their relation to arterial stiffness (Figure 4). We defined the “freewheel” SBP (during unloaded [0 watt] exercise), SBP at 75 watts, and SBP-to-workload slope (SBP/W, as defined in Methods). With these measures, the opposing directionality of association became evident: peak O2 pulse was negatively associated with each workload-indexed BP measure, and CFPWV was positively associated. Highest effect estimates were observed for the association of CFPWV with the SBP/W slope. We observed minimal relations between augmentation index and exercise BP measures. On the other hand, characteristic impedance (reflecting the pulsatile load) was directly related to SBP measures that accounted for workload (SBP at 75 watts, SBP/W slope). Forward wave amplitude was related to exercise SBP responses at all stages of exercise, including peak SBP. These relations were largely consistent with additional covariate adjustment (Table S3), or when restricted to a subsample of individuals free of hypertension (Table S4).
Figure 4. Associations of blood pressure responses during exercise with different measures of vascular stiffness.
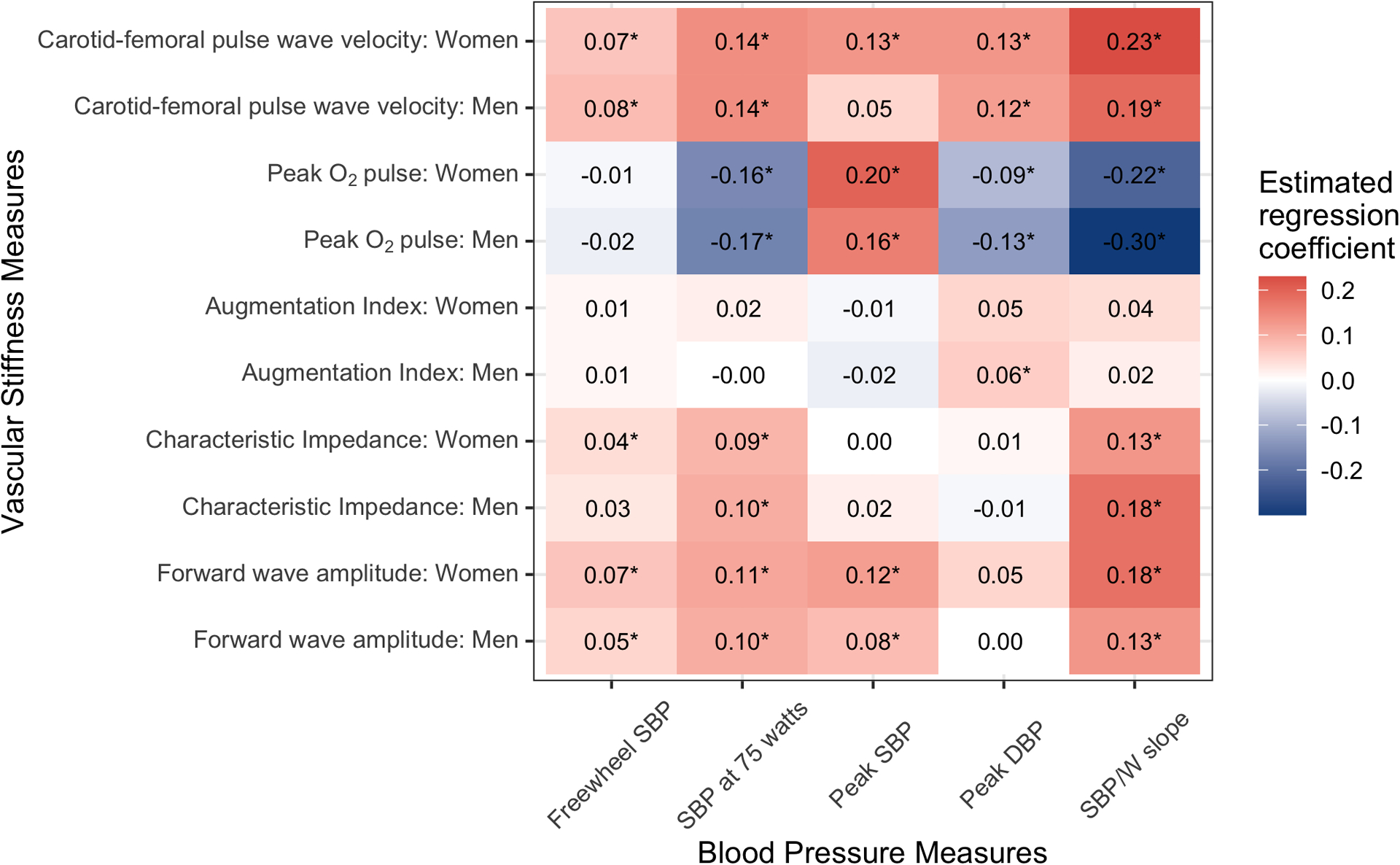
All variables shown were mean-centered and standardized for regression. Beta coefficients represent the change in BP measures (dependent variables) for a 1-standard deviation higher value of the vascular stiffness measures or peak O2 pulse. Models were adjusted for age, hypertension treatment, and resting SBP and separate models were constructed for men and women. In models with peak DBP as the dependent variable, we substituted resting DBP for resting SBP as a covariate. A Bonferroni-adjusted P-value threshold of 0.01 was used to determine statistical significance and values below this threshold are marked with an “*”.
DISCUSSION
We assessed BP responses to exercise in a large FHS sample in conjunction with measures of cardiac-peripheral performance and vascular function to characterize physiological and clinical correlates of exercise BP. Our primary result was that arterial stiffness and peak O2 pulse (reflecting cardiac and skeletal muscle performance during exercise) were jointly associated with peak exercise SBP. In effect, individuals could “achieve” a high peak SBP via mechanisms of greater cardiac stroke volume and O2 extraction in peripheral tissues (higher peak O2 pulse; a physiologically “positive” state) or via higher arterial stiffness (a physiologically “deleterious” state). Importantly, relations of arterial stiffness with peak SBP differed by sex. While the associations of peak O2 pulse with peak SBP were similar in women and men, CFPWV had a higher strength of association (as evident by the magnitude of the regression coefficient) with peak SBP in women. These observations argue against the use of a single peak SBP cut-point that does not account for stroke volume or workload to identify a hypertensive responses to exercise. Accordingly, SBP responses that incorporate workload (e.g., SBP at 75 watts, SBP/workload slope) were more closely related to adverse vascular function metrics (versus peak SBP). Collectively, these findings suggest that the magnitude of BP elevation during exercise must be interpreted in the context of its physiological contributors to clarify its clinical relevance as a reflection of adverse vascular function.
The BP response to exercise is determined in part by dynamic responses of the cardiac, peripheral (e.g., redistribution of blood flow to exercising skeletal muscle), and vascular systems26. With optimal coordination and function, the increased metabolic demands of exercise are met by an increase in cardiac output, reduction in peripheral vascular resistance, and increase in skeletal muscle O2 uptake to support increased aerobic respiration. These responses are expected to result in a rise in SBP and a minimal change in DBP26. Prior clinical and epidemiological studies in individuals without overt CVD have found that exaggerated BP responses to exercise are associated with incident hypertension7,27,28 and atherosclerotic vascular events5,6,29–32. Together with the observation that higher BP response to submaximal exercise is related to higher vascular stiffness and adverse endothelial function4, the foregoing findings may suggest that higher SBP during exercise is a reflection of adverse vascular function and attendant vascular risk. According to this reasoning, selected peak SBP thresholds are used by clinical guidelines to define a “hypertensive response to exercise” that serves as a universal marker of CVD risk9.
By incorporating a broad range of exercise BP, arterial stiffness, and fitness levels, the current study indicates that underlying heterogeneity in the physiologic determinants of elevated exercise SBP may complicate this interpretation. We observed that SBP values above the single cut-point clinical thresholds endorsed by exercise testing guidelines9 could be achieved by individuals with lower arterial stiffness in the setting of greater cardiac-peripheral performance. Moreover, across categories of arterial stiffness, a higher peak SBP was observed in individuals with higher peak O2 pulse and favorable metabolic responses to exercise despite similar cardiometabolic profiles. These observations suggest that higher peak SBP in the context of greater cardiac-peripheral performance may largely be a consequence of augmentation in stroke volume to accommodate higher workloads. By contrast, when workload was accounted for, higher values of exercise BP measures (e.g., SBP at 0 watts of resistance, SBP at 75 watts, and SBP/watts slope) were more closely related to measures of arterial stiffness in men and women and were consequently associated with lower cardiac-peripheral performance (peak O2 pulse). The opposing direction of association for cardiac peripheral performance (peak O2 pulse) with peak SBP and SBP measures incorporating workload reflect the ability of workload-indexed SBP measures to account for the expected augmentation in cardiac stroke volume. Use of SBP measures incorporating workload therefore restore directional concordance of the relations of exercise BP measures and cardiac-peripheral performance/arterial stiffness in support of the traditional notion that a higher SBP response to exercise is adverse.
Importantly, of the exercise BP measures assessed, the SBP/watts slope exhibited the highest effect sizes in relation to higher arterial stiffness and lower O2 pulse across sex and hypertension status. Although submaximal BP measures, such as SBP at 75 watts, might be easier to obtain, inter-individual variation in the response to a given workload may complicate interpretation. For example, we observed higher SBP measures in men versus women for all exercise BP responses except SBP at 75 watts, as this workload usually represents a higher proportion of the peak workload in women versus men.
We related exercise BP responses to four different physiological measures of arterial stiffness in this study. Directions of association and effect sizes were similar for the relations of exercise BP responses with higher (adverse) CFPWV (the most widely used noninvasive correlate of arterial stiffness), forward wave amplitude (which reflects proximal arterial geometry and stiffness) and characteristic impedance (integrating pulsatile and non-pulsatile arterial load)33. However, the augmentation index, which assesses the relative contribution of reflected waves to pulse pressure, was not associated with exercise BP measures in our study. While traditionally considered a measure of higher arterial stiffness, augmentation index has complex determinants throughout the life-course and often demonstrates divergent associations with CFPWV34.
The higher effect size in women versus men for arterial stiffness in regression with peak SBP is consistent with accumulating evidence that greater arterial stiffness may partially account for a higher prevalence of heart failure with preserved ejection fraction (HFpEF) in women14. Higher peripheral vascular resistance and an exaggerated rise in BP that is not accompanied by increases in stroke volume (referred to as “ventricular-vascular uncoupling”) are commonly observed in HFpEF and may limit cardiac output augmentation and reinforce impaired exertional tolerance35–38. Women with HFpEF display higher vascular stiffness than men, a finding that is associated with a steeper rise in left ventricular heart filling pressures with exercise14. Additionally, sex-based differences in BP regulation occur before the onset of CVD: women without overt CVD experience a steeper increase in SBP after midlife than men13, have higher proximal aortic stiffness39, and demonstrate a rise in exercise peak SBP throughout the life-course, whereas peak SBP in men often plateaus in the fifth decade3. Our findings provide physiologic insights into these clinical observations and suggest that consideration of the mechanisms underlying high peak SBP may prove useful for refining HFpEF risk assessment, especially in women.
Notably, exercise on a cycle ergometer, which was performed by all participants in our study, has been previously demonstrated to lead to higher excursions in SBP and lower peak VO2 values when compared with other forms of exercise (e.g., treadmill)40. We therefore would caution against drawing conclusions about specific threshold values for use with other exercise modalities from this work. On the other hand, mean peak SBP values observed in our study sample (172 mm Hg in women, 191 mm Hg in men) were comparable to those reported for similar age groups in a large multi-center consortium of individuals undergoing treadmill exercise tests (174 mm Hg in women, 192 mm Hg in men)3. In addition, while the exact value of peak SBP and peak VO2 may differ based on exercise modality, the correlations are high for physiological measures obtained through different exercise modalities (e.g., weight-bearing vs. non weight-bearing exercise)41. As a result, the relative associations (obtained through linear regression) would be expected to be similar across exercise modalities, underscoring the relevance of our approach of defining relations of exercise measures with vascular stiffness rather than focusing on specific threshold values.
There are several findings from our study with potential clinical implications. First, we demonstrated that combining exercise BP measures with CPET may provide important contextual information regarding exercise performance. By ensuring that the peak SBP coincides with gas exchange measures of maximal volitional effort (i.e., peak RER >1.05), CPET enables uniformity in identifying the true peak SBP. Additionally, information regarding cardiorespiratory fitness, peak workload achieved, and cardiac-peripheral exercise performance can be obtained via CPET and used to complement the exercise measures themselves. Second, ≈25% of community-dwelling individuals in our study exhibited peak SBP values above existing clinical thresholds, but some of these individuals achieved high peak SBP due primarily to greater cardiac-peripheral performance. These findings in relation to cross-sectional physiological measures are therefore in concert with other studies showing that high peak SBP may not be a good predictor of adverse outcomes at the population level42, raising the prospect of using CPET (or imaging-based measures of stroke volume/cardiac performance) to clarify the physiological determinants and relevance of a high peak exercise SBP. Third, we observed that BP measures that account for workload (e.g., BP at a fixed workload, or the SBP/W slope) were directly related with higher arterial stiffness and lower cardiac-peripheral performance. Despite recent reports that the SBP/W slope may be a superior measure of adverse exercise BP responses in a clinical referral sample11,43, its clinical use has thus far been limited.
This study provides a comprehensive assessment of two of the main contributors to peak SBP in the community and characterizes the relations of exercise BP responses during exercise with arterial stiffness measures. Nevertheless, several limitations warrant consideration. BP measurements made during exercise (especially diastolic BP) can have limited precision and may exhibit circadian/daily variability44,45. In particular, noninvasive assessment of peak BP involves measurement immediately after termination of loaded exercise and thus there may be variability in the interval between the true peak BP and BP measures across participants. However, this measurement variability would be expected to bias towards the null hypothesis of no association. In addition, SBP has also been shown to be higher with cycling than with other forms of exercise (e.g., treadmill)40 and our findings should be validated using other exercise modalities, including weightbearing exercise. While we relied on periodic noninvasive BP assessment during exercise in this study, continuous BP monitoring throughout exercise may be used to uncover distinct exercise BP responses, especially in individuals with overt CVD. Despite inclusion of the FHS minority Omni 2 cohort, our sample consisted of mostly White individuals of European descent; generalizability to other populations is therefore unknown. Our findings, therefore, should be validated in larger and more diverse sample sizes and would ideally include various exercise modalities and protocols. Our modeling approach included stratification by sex, but an alternative strategy in which sex interaction terms are included for model estimation across the entire dataset could also be employed. In addition, our arterial stiffness assessment took place at rest. While recent work has demonstrated that different trajectories of arterial stiffness with exercise may also be relevant to CVD and HFpEF risk46, noninvasive assessment of changes in arterial stiffness with exercise is complex and may vary across different vascular beds47. Finally, our sample had a relatively high prevalence of individuals with hypertension. While we performed sensitivity analyses excluding individuals with hypertension, it is possible that the high prevalence influenced our findings.
In conclusion, our findings complicate the clinical interpretation of peak exercise SBP: both high (adverse) arterial stiffness and high (beneficial) cardiac-peripheral performance were associated with peak exercise SBP. Benchmarking SBP response to workload “restored” an adverse association of higher arterial stiffness and lower cardiac performance with SBP. Arterial stiffness appeared to have a higher effect on peak SBP responses in women. Future investigations of exercise BP responses that are sensitive to sex-based differences, workload, and vascular and cardiac physiology may illuminate mechanisms of hypertension and clinical interpretation of exercise BP.
Supplementary Material
HIGHLIGHTS.
While abnormal blood pressure responses to exercise have been linked to poorer cardiovascular outcomes and adverse arterial function, their definitions and determinants are incompletely defined.
We sought to parse the relations of cardiac and vascular function to different measures of exercise blood pressure using maximal effort cardiopulmonary exercise tests and resting arterial tonometry.
In 2858 Framingham Heart Study participants, an elevated peak exercise systolic blood pressure was observed in individuals with either higher (beneficial) cardiac-peripheral performance (as assessed by the peak O2 pulse) or higher (adverse) arterial stiffness, with a higher magnitude of association for arterial stiffness with peak systolic blood pressure in women versus men.
Exercise blood pressure measures incorporating workload (such as the slope of the change in systolic blood pressure during exercise/peak workload) restored directional consistency with higher values corresponding to greater arterial stiffness and poorer cardiac-peripheral performance.
Cardiac and vascular physiological measures help to clarify the complex determinants of peak systolic blood pressure and indicate that exercise blood pressure measures incorporating workload may be preferable for clinical assessment.
Sources of Funding:
The Framingham Heart Study is supported by the National Heart, Lung, and Blood Institute Contracts N01-HC-25195, HHSN268201500001I, and 75N92019D00031. MN was supported by NIH grants K23-HL138260 and R01-HL156975 and by a Career Investment Award from the Department of Medicine, Boston University School of Medicine. This work was supported by R01-HL136685 (VM and RVS), R01-HL131029 (Dr. Vasan and GDL), AHA grant 15GPSGC24800006 (GDL), and R01-HL126136 (Dr. Vasan and GFM). Dr. Vasan was supported in part by the Evans Medical Foundation and the Jay and Louis Coffman Endowment from the Department of Medicine, Boston University School of Medicine.
Disclosures:
Dr. Nayor has received speaking honoraria from Cytokinetics. Dr. Shah is supported in part by grants from the National Institutes of Health and the American Heart Association. In the past 12 months, Dr. Shah has served as a consultant for Myokardia (ongoing) and Best Doctors (ongoing), receives research funding from Amgen (concluded), had minor stock holdings in Gilead, and has current stock holdings in Pfizer. Dr. Shah is a co-inventor on a patent for ex-RNAs signatures of cardiac remodeling. Dr. Shah’s spouse works for UpToDate (Wolters Kluwer). Dr. Murthy owns stock in Amgen, General Electric and Cardinal Health. He has received speaking honoraria from, serves as a scientific advisor for, and owns stock options in Ionetix. He has received research funding and speaking honoraria from Siemens Medical Imaging. He has served as a scientific advisor for Curium and has received expert witness fees from Jubilant Draximage. He has received a speaking honorarium from 2Quart Medical. He has received non-financial research support from INVIA Medical Imaging Solutions. Dr. Lewis has research funding from the National Institutes of Health and the American Heart Association as well as Amgen, Cytokinetics, Applied Therapeutics, AstraZeneca, Sonivie in relation to projects and clinical trials investigating exercise capacity that are distinct from this work. He has served as a scientific advisor for Pfizer, Merck, Boehringer-Ingelheim, Novartis, American Regent, Relypsa, Cyclerion, Cytokinetics, and Amgen and receives royalties from UpToDate for scientific content authorship related to exercise physiology. Dr. Mitchell is owner of Cardiovascular Engineering, Inc., a company that designs and manufactures devices that measure vascular stiffness. The company uses these devices in clinical trials that evaluate the effects of diseases and interventions on vascular stiffness. He also serves as a consultant to and receives grants and honoraria from Novartis, Merck, Bayer, Servier, Philips and deCODE genetics.
NON-STANDARD ABBREVIATIONS AND ACRONYMS
- BP
blood pressure
- CVD
cardiovascular disease
- SBP
systolic blood pressure
- CPET
cardiopulmonary exercise test
- FHS
Framingham Heart Study
- RER
respiratory exchange ratio
- ECG
electrocardiogram
- VO2
oxygen uptake
- DBP
diastolic blood pressure
- CFPWV
carotid-femoral pulse wave velocity
- BMI
body mass index
Footnotes
Supplemental Material
REFERENCES
- 1.Lim PO, Donnan PT, MacDonald TM. Blood pressure determinants of left ventricular wall thickness and mass index in hypertension: comparing office, ambulatory and exercise blood pressures. J Hum Hypertens. 2001;15:627–633. [DOI] [PubMed] [Google Scholar]
- 2.Lim PO, Donnan PT, MacDonald TM. How well do office and exercise blood pressures predict sustained hypertension? A Dundee Step Test Study. J Hum Hypertens. 2000;14:429–433. [DOI] [PubMed] [Google Scholar]
- 3.Sabbahi A, Arena R, Kaminsky LA, Myers J, Phillips SA. Peak Blood Pressure Responses During Maximum Cardiopulmonary Exercise Testing: Reference Standards From FRIEND (Fitness Registry and the Importance of Exercise: A National Database). Hypertension. 2018;71:229–236. [DOI] [PubMed] [Google Scholar]
- 4.Thanassoulis G, Lyass A, Benjamin EJ, Larson MG, Vita JA, Levy D, Hamburg NM, Widlansky ME, O’Donnell CJ, Mitchell GF, et al. Relations of exercise blood pressure response to cardiovascular risk factors and vascular function in the Framingham Heart Study. Circulation. 2012;125:2836–2843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lewis GD, Gona P, Larson MG, Plehn JF, Benjamin EJ, O’Donnell CJ, Levy D, Vasan RS, Wang TJ. Exercise blood pressure and the risk of incident cardiovascular disease (from the Framingham Heart Study). Am J Cardiol. 2008;101:1614–1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shah R, Murthy VL, Colangelo LA, Reis JP, Carr JJ, Sidney S, Siddique J, Lewis CE, Lima JAC, Lewis GD, et al. Submaximal Blood Pressure Responses to Exercise in Young Adulthood and Long-Term Cardiovascular Health. J Am Coll Cardiol. 2017;70:1941–1943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Singh JP, Larson MG, Manolio TA, O’Donnell CJ, Lauer M, Evans JC, Levy D. Blood pressure response during treadmill testing as a risk factor for new-onset hypertension. The Framingham heart study. Circulation. 1999;99:1831–1836. [DOI] [PubMed] [Google Scholar]
- 8.Gottdiener JS, Brown J, Zoltick J, Fletcher RD. Left ventricular hypertrophy in men with normal blood pressure: relation to exaggerated blood pressure response to exercise. Ann Intern Med. 1990;112:161–166. [DOI] [PubMed] [Google Scholar]
- 9.Fletcher GF, Ades PA, Kligfield P, Arena R, Balady GJ, Bittner VA, Coke LA, Fleg JL, Forman DE, Gerber TC, et al. Exercise standards for testing and training: a scientific statement from the American Heart Association. Circulation. 2013;128:873–934. [DOI] [PubMed] [Google Scholar]
- 10.Williams B, Mancia G, Spiering W, Agabiti Rosei E, Azizi M, Burnier M, Clement DL, Coca A, de Simone G, Dominiczak A, et al. 2018 ESC/ESH Guidelines for the management of arterial hypertension. Eur Heart J. 2018;39:3021–3104. [DOI] [PubMed] [Google Scholar]
- 11.Hedman K, Cauwenberghs N, Christle JW, Kuznetsova T, Haddad F, Myers J. Workload-indexed blood pressure response is superior to peak systolic blood pressure in predicting all-cause mortality. Eur J Prev Cardiol. 2020;27:978–987. [DOI] [PubMed] [Google Scholar]
- 12.Pressler A, Jahnig A, Halle M, Haller B. Blood pressure response to maximal dynamic exercise testing in an athletic population. J Hypertens. 2018;36:1803–1809. [DOI] [PubMed] [Google Scholar]
- 13.Ji H, Kim A, Ebinger JE, Niiranen TJ, Claggett BL, Bairey Merz CN, Cheng S. Sex Differences in Blood Pressure Trajectories Over the Life Course. JAMA Cardiol. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lau ES, Panah LG, Zern EK, Liu EE, Farrell R, Schoenike MW, Namasivayam M, Churchill TW, Curreri L, Malhotra R, et al. Arterial Stiffness and Vascular Load in HFpEF: Differences Among Women and Men. J Card Fail. 2022;28:202–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Splansky GL, Corey D, Yang Q, Atwood LD, Cupples LA, Benjamin EJ, D’Agostino RB Sr., Fox CS, Larson MG, Murabito JM, et al. The Third Generation Cohort of the National Heart, Lung, and Blood Institute’s Framingham Heart Study: design, recruitment, and initial examination. Am J Epidemiol. 2007;165:1328–1335. [DOI] [PubMed] [Google Scholar]
- 16.Abraham TM, Massaro JM, Hoffmann U, Yanovski JA, Fox CS. Metabolic characterization of adults with binge eating in the general population: The framingham heart study. Obesity. 2014;22:2441–2449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nayor M, Xanthakis V, Tanguay M, Blodgett JB, Shah RV, Schoenike M, Sbarbaro J, Farrell R, Malhotra R, Houstis NE, et al. Clinical and Hemodynamic Associations and Prognostic Implications of Ventilatory Efficiency in Patients With Preserved Left Ventricular Systolic Function. Circ Heart Fail. 2020;13:e006729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nayor M, Shah RV, Miller PE, Blodgett JB, Tanguay M, Pico AR, Murthy VL, Malhotra R, Houstis NE, Deik A, et al. Metabolic Architecture of Acute Exercise Response in Middle-Aged Adults in the Community. Circulation. 2020;142:1905–1924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nayor M, Shah RV, Tanguay M, Blodgett JB, Chernofsky A, Miller PE, Xanthakis V, Malhotra R, Houstis NE, Velagaleti RS, et al. Feasibility, Methodology, and Interpretation of Broad-Scale Assessment of Cardiorespiratory Fitness in a Large Community-Based Sample. Am J Cardiol. 2021;157:56–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hansen JE, Sue DY, Wasserman K. Predicted values for clinical exercise testing. Am Rev Respir Dis. 1984;129:S49–55. [DOI] [PubMed] [Google Scholar]
- 21.Whelton PK, Carey RM, Aronow WS, Casey DE Jr., Collins KJ, Dennison Himmelfarb C, DePalma SM, Gidding S, Jamerson KA, Jones DW, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2018;71:e127–e248. [DOI] [PubMed] [Google Scholar]
- 22.Daida H, Allison TG, Squires RW, Miller TD, Gau GT. Peak exercise blood pressure stratified by age and gender in apparently healthy subjects. Mayo Clinic Proc 1996;71:445–452. [DOI] [PubMed] [Google Scholar]
- 23.Mitchell GF, Wang N, Palmisano JN, Larson MG, Hamburg NM, Vita JA, Levy D, Benjamin EJ, Vasan RS. Hemodynamic correlates of blood pressure across the adult age spectrum: noninvasive evaluation in the Framingham Heart Study. Circulation. 2010;122:1379–1386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kannel WB, Sorlie P. Some health benefits of physical activity. The Framingham Study. Arch Intern Med. 1979;139:857–861. [PubMed] [Google Scholar]
- 25.Kaess BM, Rong J, Larson MG, Hamburg NM, Vita JA, Cheng S, Aragam J, Levy D, Benjamin EJ, Vasan RS, et al. Relations of Central Hemodynamics and Aortic Stiffness with Left Ventricular Structure and Function: The Framingham Heart Study. J Am Heart Assoc. 2016;5:e002693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Le VV, Mitiku T, Sungar G, Myers J, Froelicher V. The blood pressure response to dynamic exercise testing: a systematic review. Prog Cardiovasc Dis. 2008;51:135–160. [DOI] [PubMed] [Google Scholar]
- 27.Miyai N, Arita M, Miyashita K, Morioka I, Shiraishi T, Nishio I. Blood pressure response to heart rate during exercise test and risk of future hypertension. Hypertension. 2002;39:761–766. [DOI] [PubMed] [Google Scholar]
- 28.Matthews CE, Pate RR, Jackson KL, Ward DS, Macera CA, Kohl HW, Blair SN. Exaggerated blood pressure response to dynamic exercise and risk of future hypertension. J Clin Epidemiol. 1998;51:29–35. [DOI] [PubMed] [Google Scholar]
- 29.Mundal R, Kjeldsen SE, Sandvik L, Erikssen G, Thaulow E, Erikssen J. Exercise blood pressure predicts cardiovascular mortality in middle-aged men. Hypertension. 1994;24:56–62. [DOI] [PubMed] [Google Scholar]
- 30.Wilson MF, Sung BH, Pincomb GA, Lovallo WR. Exaggerated pressure response to exercise in men at risk for systemic hypertension. Am J Cardiol. 1990;66:731–736. [DOI] [PubMed] [Google Scholar]
- 31.Schultz MG, Otahal P, Cleland VJ, Blizzard L, Marwick TH, Sharman JE. Exercise-induced hypertension, cardiovascular events, and mortality in patients undergoing exercise stress testing: a systematic review and meta-analysis. Am J Hypertens. 2013;26:357–366. [DOI] [PubMed] [Google Scholar]
- 32.Kurl S, Laukkanen JA, Rauramaa R, Lakka TA, Sivenius J, Salonen JT. Systolic blood pressure response to exercise stress test and risk of stroke. Stroke. 2001;32:2036–2041. [DOI] [PubMed] [Google Scholar]
- 33.Cooper LL, Rong J, Benjamin EJ, Larson MG, Levy D, Vita JA, Hamburg NM, Vasan RS, Mitchell GF. Components of hemodynamic load and cardiovascular events: the Framingham Heart Study. Circulation. 2015;131:354–361; discussion 361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Torjesen AA, Wang N, Larson MG, Hamburg NM, Vita JA, Levy D, Benjamin EJ, Vasan RS, Mitchell GF. Forward and backward wave morphology and central pressure augmentation in men and women in the Framingham Heart Study. Hypertension. 2014;64:259–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kaye DM, Wolsk E, Nanayakkara S, Mariani J, Hassager C, Gustafsson F, Moller JE, Sunagawa K, Burkhoff D. Comprehensive Physiological Modeling Provides Novel Insights Into Heart Failure With Preserved Ejection Fraction Physiology. J Am Heart Assoc. 2021;10:e021584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tartiere-Kesri L, Tartiere JM, Logeart D, Beauvais F, Cohen Solal A. Increased proximal arterial stiffness and cardiac response with moderate exercise in patients with heart failure and preserved ejection fraction. J Am Coll Cardiol. 2012;59:455–461. [DOI] [PubMed] [Google Scholar]
- 37.Kato S, Onishi K, Yamanaka T, Takamura T, Dohi K, Yamada N, Wada H, Nobori T, Ito M. Exaggerated hypertensive response to exercise in patients with diastolic heart failure. Hypertens Res. 2008;31:679–684. [DOI] [PubMed] [Google Scholar]
- 38.Sahlen A, Abdula G, Norman M, Manouras A, Brodin LA, Lund LH, Shahgaldi K, Winter R. Arterial vasodilatory and ventricular diastolic reserves determine the stroke volume response to exercise in elderly female hypertensive patients. Am J Physiol Heart Circ Physiol. 2011;301:H2433–2441. [DOI] [PubMed] [Google Scholar]
- 39.Coutinho T, Borlaug BA, Pellikka PA, Turner ST, Kullo IJ. Sex differences in arterial stiffness and ventricular-arterial interactions. J Am Coll Cardiol. 2013;61:96–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kim YJ, Chun H, Kim CH. Exaggerated response of systolic blood pressure to cycle ergometer. Ann Rehabil Med. 2013;37:364–372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pollock ML, Foster C, Schmidt D, Hellman C, Linnerud AC, Ward A. Comparative analysis of physiologic responses to three different maximal graded exercise test protocols in healthy women. Am Heart J. 1982;103:363–373. [DOI] [PubMed] [Google Scholar]
- 42.Hedman K, Kaminsky LA, Sabbahi A, Arena R, Myers J. Low but not high exercise systolic blood pressure is associated with long-term all-cause mortality. BMJ Open Sport Exerc Med. 2021;7:e001106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hedman K, Lindow T, Elmberg V, Brudin L, Ekstrom M. Age- and gender-specific upper limits and reference equations for workload-indexed systolic blood pressure response during bicycle ergometry. Eur J Prev Cardiol. 2020:2047487320909667. [DOI] [PubMed] [Google Scholar]
- 44.Sharman JE, LaGerche A. Exercise blood pressure: clinical relevance and correct measurement. J Hum Hypertens. 2015;29:351–358. [DOI] [PubMed] [Google Scholar]
- 45.Jones H, Pritchard C, George K, Edwards B, Atkinson G. The acute post-exercise response of blood pressure varies with time of day. Eur J Appl Physiol. 2008;104:481–489. [DOI] [PubMed] [Google Scholar]
- 46.Zern EK, Ho JE, Panah LG, Lau ES, Liu E, Farrell R, Sbarbaro JA, Schoenike MW, Pappagianopoulos PP, Namasivayam M, et al. Exercise Intolerance in Heart Failure With Preserved Ejection Fraction: Arterial Stiffness and Aabnormal Left Ventricular Hemodynamic Responses During Exercise. J Card Fail 2021;27:625–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mutter AF, Cooke AB, Saleh O, Gomez YH, Daskalopoulou SS. A systematic review on the effect of acute aerobic exercise on arterial stiffness reveals a differential response in the upper and lower arterial segments. Hypertens Res. 2017;40:146–172. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


