Abstract
This study aimed to analyze the associations of nonalcoholic fatty liver disease (NAFLD) with dental parameters, while controlling for socio-demographics, health-related habits, and each of the metabolic syndrome (MetS) components, consequences, and related conditions among a nationally representative sample of young and middle-aged adults. To that end, we analyzed data from the dental, oral, medical epidemiological (DOME) cross-sectional records-based study that combined comprehensive socio-demographic, medical, and dental databases of a nationally representative sample of military personnel. Included were 132,529 subjects aged 18–50 who attended military dental clinics for one year. The prevalence of NAFLD in the study population was 0.7% (938/132,529). The following parameters maintained a statistically positive association with NAFLD in the multivariate analysis (from highest to lowest OR): male sex (OR = 3.91 (2.29–6.66)), hyperlipidemia (OR = 3.69 (2.75–4.95)), diabetes Type 2 (OR = 3.14 (2.21–4.46)), hypertension (OR = 1.67 (1.30–2.14)), periodontitis (OR = 1.42 (1.06–1.89)), body mass index (BMI) (OR = 1.15 (1.13–1.18)), and age (OR = 1.08 (1.06–1.09)). The multivariate analysis established a profile of the “patient vulnerable to NAFLD”, including older age, male sex, and other MetS components, including diabetes type 2, hypertension, hyperlipidemia, BMI, and periodontitis. This profile aligns with the current new definition of metabolic dysfunction-associated fatty liver disease (MAFLD). We also analyzed the associations of the sum of the standard dental unit (SDU) scores of planned (SDU-P) and delivered (SDU-D) dental procedures per patient with NAFLD using receiver operating characteristic (ROC) analysis. The SDU-P (planned) score exhibited excellent discrimination for NAFLD (area under the curve (AUC) = 0.718 (0.703–0.734)). Overall, the results confirmed the hypothesis of this research, i.e., that NAFLD is associated with dental morbidity, particularly with periodontitis.
Keywords: fatty liver, nonalcoholic fatty liver disease (NAFLD), metabolic syndrome, metabolic dysfunction-associated fatty liver disease (MAFLD), diabetes type 2, hyperlipidemia, hypertension, obesity, periodontitis, dental caries, decayed teeth, electronic medical record, electronic dental record
1. Introduction
Non-alcoholic fatty liver disease (NAFLD) is the most common chronic liver disease worldwide, representing the hepatic manifestation of metabolic syndrome (MetS) [1]. Given this current understanding, in 2020, the Asian Pacific Association for the Study of the Liver (APASL), including a panel of international experts from 22 countries, redefined NAFLD as metabolic dysfunction-associated fatty liver disease (MAFLD) [2]. NAFLD affects about a quarter of the global adult population, ranging from 5–42%, depending on the diagnostic criteria [1,2,3], and poses a major health and economic burden to all societies, yet it has no approved pharmacotherapy [1,2]. The criteria for MAFLD are based on evidence of hepatic steatosis, in addition to one of the following three criteria: overweight/obesity, presence of type 2 diabetes mellitus, or evidence of metabolic dysregulation [2]. While NAFLD is diagnosed per exclusion, MAFLD applies positive diagnostic criteria and does not exclude alcohol intake or other liver diseases [2]. However, given its complex pathophysiology, the APASL states that it is unlikely that a single diagnostic test will become available, as was the case for MetS, which has multiple definitions [4].
NAFLD also shares some risk factors with dental conditions, including periodontitis and dental caries. For example, the consumption of simple sugars (glucose and fructose) is a factor leading to NAFLD and dental caries, and several hypotheses link NAFLD and periodontitis, through periodontal pathogens, inflammatory mediators, and oxidative stress [5]. Periodontal disease is characterized by the inflammatory destruction of the tooth-supporting tissues, including the cement, periodontal ligament, and alveolar bone. Periodontitis is the sixth most common human disease, with a global prevalence of almost 50% of the adult population. The severe form affects 9.8% (796 million people) globally [6,7]. Periodontitis is the leading cause of tooth loss in the adult population worldwide, with major negative consequences on masticatory function, thereby affecting their nutrition, esthetics, and overall quality of life [8]. Dental caries is also a prevalent disease, with untreated caries of the permanent dentition considered the most prevalent condition worldwide, with a global prevalence of 35% for all ages combined [9]. Dental caries is a diet-dependent, transmissible microbiologically mediated disease that also follows an infectious and chronic disease model [10]. Due to its predominance, dental caries is considered the most important oral disease and is of medical, social, and economic importance [11].
Associations between dental problems and MetS have been previously investigated, including by our group [12,13,14,15,16,17]. Over the last few decades, more than 60 different systemic conditions have been investigated in relation to periodontitis [18]. A significant body of evidence supports independent associations between severe periodontitis, including diabetes, cardiovascular disease, chronic obstructive pulmonary disease, and chronic kidney disease, and even with all-cause and cardiovascular mortality in several populations [19]. In particular, a “bidirectional” relationship has been ascertained for the associations between periodontitis and diabetes, leading to the designation of periodontitis as the “sixth complication of diabetes mellitus”[20]. The associations between dental status and NAFLD have been less studied, compared to the associations with diabetes and cardiovascular diseases. The available literature demonstrates conflicting results, with some reporting that NAFLD is positively associated with periodontitis and tooth loss [5,21,22,23,24], while other studies failed to replicate the previously reported associations between periodontitis and NAFLD [25,26].
These conflicting results could be attributed to the limitations of the published studies, such as heterogeneity in the definitions of dental and systemic diseases and the presence of possible confounders that were not always considered. For example, there are well-known common risk factors for multiple chronic diseases, including increased age, socioeconomic status, smoking, sugar consumption, and obesity [27]. Considering these limitations, there is a need for large-scale epidemiological studies on the association between NAFLD and dental morbidities that employ a rigorous protocol regarding dental and medical disease definitions and consider the existence of many possible confounders. It is also important to study the associations between NAFLD and dental morbidities in the context of the MetS cluster, and periodontitis should be studied in the context of other dental and oral conditions that might exist concomitantly in an individual, i.e., within the context of “dental cluster”, as we previously described [15]. Moreover, there is a need to study the association between NAFLD and the dental burden, which is a function of the number of both the planned and the delivered dental procedures, which, to the best of our knowledge, has not been studied yet. Particularly, it is important to assess the associations between dental status and NAFLD among young and middle-aged adults, a less studied population in this context.
To address the gap in the literature, the primary objective of this study was to test the hypothesis that poor dental status, as reflected by the presence of periodontitis and by a higher prevalence of dental treatment needs, will be the predictor of the research outcome of NAFLD diagnosis. Specific objectives were to:
-
A.
Measure the prevalence of NAFLD and study the associations of NAFLD, with the prevalence of the following planned and delivered dental procedures: (1) fillings, (2) endodontic treatments, (3) post-treatment, (4) crowns, (5) extractions, (6) periodontal disease, and (7) missing teeth, as well as with the medical and dental attendance patterns. This specific goal will enable us to address the “dental cluster”.
-
B.
To address possible confounders, we aim to further explore the associations of NAFLD with dental status in a multivariate model controlling for (1) socio-demographic parameters, (2) health-related risk habits, and (3) each of the MetS components and consequences and related conditions, including diabetes type 2, hypertension, hyperlipidemia, impaired glucose tolerance (IGT), obesity, cardiovascular disease, S/P stroke, S/P transient ischemic attack (TIA), obstructive sleep apnea (OSA), and (5) auxiliary test results, including the blood tests used in the assessment of MetS related conditions. This specific goal will enable us to address the “MetS cluster”.
2. Methods
2.1. Data Source
The current study used data from the “The Dental, Oral, Medical Epidemiological (DOME)” study [14,15,17,28,29,30,31]. The DOME study is based on a structured records-based repository that combines comprehensive socio-demographic, medical, and dental databases of a nationally representative sample of military personnel from the Israel Defense Forces (IDF) [30]. The protocol and methods of data collection of the DOME study were previously detailed in-depth [30].
Ethical approval. Approval for the study was obtained from the Medical Corps Institutional Review Board (IRB number: IDF-1281-2013). Considering the retrospective study design, including anonymous records analyses, an exemption from written informed consent was given by the IRB.
Inclusion criteria: The socio-demographic, medical, and dental records of all IDF military personnel, aged 18 years and older of both sexes, who visited military dental clinics of the IDF between 1 January 2015 and 1 January 2016, for which there are records in the socio-demographic medical dental patient record (DPR).
Exclusion criteria: Subjects with a lack of data in these databases.
The research consisted of 132,529 records of patients who fulfilled the eligibility criteria.
As explained in previous publications, a remarkable opportunity exists in Israel for research on dental-systemic associations by utilization of the comprehensive information restored in the military databases and captured by the DOME repository. The Israeli military population is large and constitutes a credible data source for epidemiological studies among young and middle-aged adults [14,15,17,28,29,30,31,32]. This is partially due to conscription in Israel for all Jewish, Druze, or Circassian citizens aged 18 and older [30]. Of importance is the fact that the service in the IDF includes individuals with a complex medical background, excluding subjects who are unfit for service, due to severe health reasons (physical or mental), and even for military exemption recipients, there is an option to apply for volunteering [33,34]. Dental care services are included in the comprehensive medical care basket and are provided to IDF military personnel free of charge [30,35]. In the context of this study, in general, the military population does not include heavy consumers of alcohol, since it is forbidden.
2.2. Data Collection
A full description of the data collection of the DOME study had been published previously [30]. Briefly, the DOME structured repository captures 3 military electronic databases: Dental Patient Record (DPR), medical (i.e., computerized patient record (CPR)), and socio-demographic computerized systems that store personal socio-demographic profiles and dental and medical records of all military personnel [30].
2.3. Study Variables
We analyzed the associations of NAFLD as dependent, with various independent, variables. Definitions of the variables available in the DOME repository have been detailed in the DOME protocol and methods paper previously [30], and will be described briefly.
Definitions of Socio-Demographic Parameters.Age (years), sex (men/women), duration of service (months), education (high school and below/technical college/academic), locality of residence (urban Jewish, urban non-Jewish, rural), socio-economic status (SES), as retrieved from the Israeli Ministry of the Interior records (low (1st–4th deciles)/medium (5th–7th deciles)/high (8th–10th deciles)), and birth country (western Europe, eastern Europe, Asia, Ethiopia, Africa, North America, South America, Israel) [30].
Definitions of Health-Related Habits. Based on self-reporting: current smoker (yes/no), teeth brushing at least once a day (yes/no), consumption of cariogenic diet (snacks and/or sweets consumption between or instead of meals (yes/no)), consumption of sweetened beverages (sweet drinks consumption above one cup a day (yes/no)) [30].
Definitions of Medical and Dental Attendance Patterns.
Measurement health care utilization during the study period included: appointments with general physicians, periodontal specialists, and oral medicine specialists, the total number of dental appointments, scaling and root planning appointments, and non-attendance to scheduled dental appointments [30].
Definitions of Medical Diagnoses and Auxiliary Test Results. The medical diagnoses of MetS components, consequences, and associated morbidities as well as the auxiliary test results were retrieved from the CPR as described previously [14,15,30] and included:
Medical Diagnoses: Diagnoses on the CPR are based on the ICD-9-CM:
-
1.
The dependent variable—nonalcoholic fatty liver disease (NAFLD), equivalent to 2015 ICD-9-CM Diagnosis Code 571.8: other chronic nonalcoholic liver disease.
-
2.
Other systemic conditions related to MetS were included as independent variables and were also based on ICD-9-CM diagnoses, including diabetes mellitus, impaired glucose tolerance (IGT), hyperlipidemia, hypertension, cardiovascular disease, obesity (BMI > 30 kg/m2), obstructive sleep apnea (OSA), S/P (status post) transient ischemic attack (TIA), and S/P stroke [14,15,30].
Auxiliary Test Results: The auxiliary test results retrieved from the CPR included blood tests used in the assessment of MetS components, as described previously weight (in kilograms), body mass index-BMI (weight/height2 (kg/m2)), C reactive protein-CRP (mg/L), glycated hemoglobin-HbA1c (%), fasting glucose (mg/dL), cholesterol (mg/dL), high-density lipoprotein-HDL (mg/dL), low-density lipoprotein-LDL (mg/dL), triglycerides (mg/dL), very low-density lipoprotein-VLDL (mg/dL), and non-HDL cholesterol (mg/dL) [14,15,30].
Definitions of Dental Parameters. Details on the standardized uniform codes employed in the DPR for each dental procedure and diagnosis can be found in previous publications [15,17,30]. Briefly, dental codes in the DPR are equivalent to the nomenclature used by the American Dental Association’s (ADA) current dental terminology (CDT) [36]. The dental procedures were drawn from the DPR as planned (i.e., treatment plan) and delivered (i.e., actual treatments that were performed) [15,17,30]. In addition to procedures, the DPR data include records of the presence of periodontal disease and a count of missing teeth for any reason (excluding wisdom teeth) [30]. The definition of periodontitis was detailed in the DOME protocol paper and was based on the American Academy of Periodontology from 2010 to 2018 (current data was collected in 2016). Furthermore, even before 2018, routinely collected data included age, smoking habits, and metabolic morbidity, including diabetes, and for those with diabetes, the glycated hemoglobin (HbA1c) was also measured. Moreover, due to possible pseudo pockets, radiographic bone loss was considered mandatory to establish a diagnosis of periodontitis radiographic bone loss, which is defined as the distance between the crestal margin and the cementoenamel junction, which is greater than 2 mm in more than one tooth, with no visible cause, such as faulty restoration, overhang, etc [30].
Standard Dental Unit (SDU). To employ the concept of “clustering” in dentistry, a standardized scoring system that quantifies dental needs and actual performance and their burden is needed. As we detailed previously, for several decades, the IDF dental corps routinely employs a standardized scoring system for dental procedures, termed the standard dental unit (SDU) [15]. The SDU score of each dental procedure represents the time and complexity of the executed procedure. For example, the SDU score of the procedure of “dental filling” (amalgam or composite) equals 1 SDU, “endodontic treatment of one root canal” equals 1.25 SDU, while the “crown” procedure equals 4 SDU. From the DOME repository, we computed for each patient the sum of planned dental procedures (SDU-P), as well as the sum of the actual treatments that were performed de facto (SDU-D). The definitions and comparisons of the SDU scores and CDT-related coding can be found elsewhere [15]. In this study, we also analyzed the associations of NAFLD with the SDU-P and SDU-D using ROC analysis (see below—statistical methods).
2.4. Statistical Methods
Statistical analyses were performed using SPSS software version 27.0 (IBM, Chicago, IL, USA). Descriptive statistics included the presentation of continuous variables as means and standard deviations (SD) and categorical variables as frequencies and percentages.
Univariate analysis. Performed between NAFLD as a dependent variable and the independent variables using Pearson’s chi-square test or likelihood ratio test (for categorical parameters) and with a non-paired t-test for independent samples (for continuous variables). Odds ratios (OR) were calculated with linear regression for continuous variables, with binary logistic regression for categorical variables.
Multicollinearity analyses. Following the univariate analyses, we performed multicollinearity tests using linear regression to examine the collinearity of the independent variables. In a case where two or more variables were highly collinear, only one of them was included in the model, and it was decided by the context which of the variables will be included in the analysis. The variance inflation factors (VIFs), which are 1/tolerance, are calculated using linear regression analysis. While VIF < 10 is usually considered indicative of collinearity, in weaker models, VIF > 2.5 may be a cause for concern; therefore, the current study used VIF < 2.5 as a cutoff.
Multivariate analysis. Following univariate analysis and collinearity statistics, a multivariate binary logistic regression analysis was performed for NAFLD as the dependent variable with statistically significant independent variables in the univariate analysis, which were not highly collinear.
Receiver operating characteristic (ROC) analysis: ROC analyses were performed on the SDU-P and SDU-D as predictors of NAFLD, and the area under the curve (AUC) was calculated to assess their discriminative ability. Cut-offs for AUC discrimination were as follows: (a) AUC ≤ 0.5: no discrimination, i.e., randomly; (b) 0.6 ≥ AUC > 0.5: poor discrimination, (c) 0.7 ≥ AUC > 0.6: acceptable discrimination, (d) 0.8 ≥ AUC > 0.7: excellent discrimination, and (e) AUC > 0.9: outstanding discrimination [37].
3. Results
3.1. The Associations between NAFLD and Socio-Demographic Parameters
The research consisted of 132,529 records of patients who fulfilled the eligibility criteria. There were 938 patients with NAFLD diagnosis; therefore, the prevalence of NAFLD was 0.7% (938/132,529). Table 1 presents a comparison of the socio-demographic characteristics between patients diagnosed with NAFLD and those without NAFLD. Compared to those without NAFLD, those with NAFLD had statistically significant positive associations, with the following parameters:
-
(1)
Male sex (OR (95% CI) men vs. women: 6.29 (4.69–8.47)).
-
(2)
Education: technical vs. high school education (OR = 26.73 (22.44–31.83)) and academic vs. high school education (OR = 17.17 (14.48–20.36)).
-
(3)
SES: low vs. high SES (OR= 1.19 (0.86–1.65)) and medium vs. high SES (OR = 1.25 (1.09–1.43)).
-
(4)
Birth country: being an immigrant from the following birth countries, compared to native Israelis: Africa (OR = 4.77 (2.60–8.73)), Asia (3.79 (2.18–6.61)), western Europe (OR = 1.49 (1.22–1.83)), eastern Europe (OR = 1.19 (0.70–2.02)).
-
(5)
Older age (OR = 1.19 (1.18–1.20)).
-
(6)
More time in service (OR = 1.14 (1.13–1.15)).
Table 1.
The Associations between NAFLD and Socio-Demographic Parameters.
| Parameter | Variable | Without NAFLD (N, %) |
NAFLD (N, %) |
p Value | OR and 95% CI # |
|---|---|---|---|---|---|
| NAFLD diagnosis (No/Yes) | 131,591 (99.3) | 938 (0.7) | |||
| Sex | Men | 98,575 (99.1) | 891 (0.9) | <0.001 ^ | 6.29 (4.69–8.47) |
| Women | 33,016 (99.9) | 47 (0.1) | 1 | ||
| Education (Y) | High school | 111,908 (99.8) | 204 (0.2) | <0.001 * | 1 |
| Technicians | 7081 (95.4) | 345 (4.6) | 26.73 (22.44–31.83) | ||
| Academic | 12,427 (97.0) | 389 (3.0) | 17.17 (14.48–20.36) | ||
| Locality of residence | Urban Jewish | 112,635 (99.3) | 833 (0.7) | <0.001 * | 0.26 (0.16–0.43) |
| Urban Non-Jewish | 17,835 (99.5) | 83 (0.5) | 0.16 (0.09–0.28) | ||
| Rural | 567 (97.3) | 16 (2.7) | 1 | ||
| Socio-economic status (SES) | Low | 5677 (99.3) | 42 (0.7) | 0.005 * | 1.19 (0.86–1.65) |
| Medium | 68,093 (99.2) | 526 (0.8) | 1.25 (1.09–1.43) | ||
| High | 56,359 (99.4) | 348 (0.6) | 1 | ||
| Birth country | Western Europe | 10,463 (99.0) | 108 (1.0) | <0.001 * | 1.49 (1.22–1.83) |
| Eastern Europe | 1701 (99.2) | 14 (0.8) | 1.19 (0.70–2.02) | ||
| Asia | 496 (97.4) | 13 (2.6) | 3.79 (2.18–6.61) | ||
| Ethiopia | 2180 (99.8) | 5 (0.2) | 0.33 (0.13–0.80) | ||
| Africa | 334 (96.8) | 11 (3.2) | 4.77 (2.60–8.73) | ||
| North America | 2854 (99.8) | 5 (0.2) | 0.25 (0.10–0.61) | ||
| South America | 952 (99.5) | 5 (0.5) | 0.76 (0.31–1.83) | ||
| Israel | 112,582 (99.3) | 777 (0.7) | 1 | ||
| Parameter | NAFLD | Mean ± SD | p value ** | OR (95% CI) ## | |
| Age | No | 21.78 ± 5.88 | <0.001 | 1.19 (1.18–1.20) | |
| Yes | 36.81 ± 7.67 | ||||
| Time in service | No | 3.02 ± 6.13 | <0.001 | 1.14 (1.13–1.15) | |
| Yes | 18.46 ± 9.17 | ||||
^ Pearson Chi-Square; * likelihood ratio, ** non-paired t-test, # binary logistic regression; ## generalized linear models.
A statistically significant negative association between NAFLD and socio-demographic parameters was seen for:
-
(1)
The locality of residence: urban Jewish vs. rural locality (OR = 0.26 (0.16–0.43)) and urban non-Jewish vs. rural locality (OR = 0.16 (0.09–0.28)).
-
(2)
Birth country: being an immigrant from the following birth countries compared to native Israelis: Ethiopia (OR = 0.33 (0.13–0.80)), North America (OR = 0.25 (0.10–0.61)), and South America (OR = 0.76 (0.31–1.83)).
3.2. The Associations between NAFLD and MetS Components, Consequences, and Related Conditions
Table 2 presents a comparison of MetS-related conditions between patients diagnosed with NAFLD and those without NAFLD diagnosis. Those with NAFLD had a statistically significant positive association with the following MetS-related conditions (from the highest to lowest OR): obesity (OR = 45.45 (38.46–52.63)), hyperlipidemia (OR = 38.46 (33.33–45.45)), IGT (OR = 34.95 (22.47–54.37)), Diabetes Type 2 (OR = 33.33 (27.02–41.66)), obstructive sleep apnea (OR = 16.13 (11.76–22.22)), stroke (OR = 15.63 (8.62–27.78)), hypertension (OR = 13.51 (11.76–15.62)), transient ischemic attack (OR = 11.49 (5.92–22.22)), and cardiovascular disease (OR = 7.81 (6.62–9.17)).
Table 2.
The Associations between NAFLD and Metabolic Syndrome (MetS) related conditions.
| Parameter | Variable | No NAFLD | NAFLD | p Value ^ | OR and 95% CI # |
|---|---|---|---|---|---|
| Hypertension | No | 128,473 (99.5) | 693 (0.5) | <0.001 | 1 |
| Yes | 3118 (92.7) | 245 (7.3) | 13.51 (11.76–15.62) | ||
| Hyperlipidemia | No | 124,527 (99.8) | 276 (0.2) | <0.001 | 1 |
| Yes | 7064 (91.4) | 662 (8.6) | 38.46 (33.33–45.45) | ||
| Impaired glucosetolerance (IGT) | No | 131,488 (99.3) | 913 (0.7) | <0.001 | 1 |
| Yes | 103 (80.5) | 25 (19.5) | 34.95 (22.47–54.37) | ||
| Diabetes type 2 | No | 131,321 (99.3) | 863 (0.7) | <0.001 | 1 |
| Yes | 270 (78.3) | 75 (21.7) | 33.33 (27.02–41.66) | ||
| Obesity | No | 124,826 (99.8) | 255 (0.2) | <0.001 | 1 |
| Yes | 6765 (90.8) | 683 (9.2) | 45.45 (38.46–52.63) | ||
| Cardiovascular disease | No | 128,161 (99.4) | 770 (0.6) | <0.001 | 1 |
| Yes | 3430 (95.3) | 168 (4.7) | 7.81 (6.62–9.17) | ||
| Obstructive sleep apnea (OSA) | No | 131,308 (99.3) | 903 (0.7) | <0.001 | 1 |
| Yes | 283 (89.0) | 35 (11.0) | 16.13 (11.76–22.22) | ||
| Stroke | No | 131,509 (99.3) | 928 (0.7) | <0.001 | 1 |
| Yes | 82 (89.1) | 10 (10.9) | 15.63 (8.62–27.78) | ||
| Transient ischemic attack (TIA) | No | 131,500 (99.3) | 930 (0.7) | <0.001 | 1 |
| Yes | 91 (91.9) | 8 (8.1) | 11.49 (5.92–22.22) |
^ Pearson Chi-Square; # binary logistic regression.
3.3. The Associations of NAFLD with Auxiliary Test Results
Table 3 presents the associations of NAFLD with auxiliary test results, including blood tests used in the assessment of MetS-related conditions. Compared to those without NAFLD, those with NAFLD exhibited weak positive associations with all auxiliary test results, apart from HDL, which had a negative association with NAFLD. OR were close to 1, except for BMI (OR = 1.231 (1.217–1.245)) and HbA1c (OR = 1.277 (1.143–1.427)) (Table 3).
Table 3.
The Associations of NAFLD with Auxiliary Test results.
| Parameter | Without NAFLD | NAFLD | p Value * | OR and 95% CI ## | ||
|---|---|---|---|---|---|---|
| N | Mean ± SD | N | Mean ± SD | |||
| Weight (kilograms) | 65,810 | 73.04 ± 32.44 | 807 | 93.95 ± 17.22 | <0.001 | 1.005 (1.004–1.005) |
| Body mass index (BMI) | 65,589 | 24.20 ± 4.24 | 805 | 30.28 ± 4.64 | <0.001 | 1.231 (1.217–1.245) |
| C reactive protein (CRP) (mg/L) | 29,878 | 3.74 ± 10.12 | 540 | 5.54 ± 12.83 | <0.001 | 1.010 (1.005–1.015) |
| Glycated hemoglobin (HbA1c) (%) | 1727 | 5.35 ± 0.92 | 216 | 5.78 ± 1.18 | <0.001 | 1.277 (1.143–1.427) |
| Fasting glucose (mg/dL) | 2421 | 86.95 ± 11.65 | 106 | 91.00 ± 16.79 | 0.016 | 1.020 (1.008–1.032) |
| Cholesterol (mg/dL) | 27,280 | 175.43 ± 35.48 | 900 | 187.79 ± 37.96 | <0.001 | 1.009 (1.007–1.011) |
| High-density lipoprotein (HDL) (mg/dL) | 27,273 | 48.49 ± 11.79 | 900 | 41.81 ± 8.83 | <0.001 | 0.938 (0.931–0.945) |
| Low-density lipoprotein (LDL) (mg/dL) | 19,359 | 108.05 ± 29.92 | 854 | 114.95 ± 31.85 | <0.001 | 1.007 (1.005–1.009) |
| LDL cholesterol calculated (mg/dL) | 16,788 | 108.10 ± 30.26 | 670 | 115.18 ± 33.10 | <0.001 | 1.007 (1.005–1.009) |
| Triglycerides (mg/dL) | 27,283 | 102.75 ± 62.00 | 900 | 156.49 ± 95.03 | <0.001 | 1.007 (1.006–1.008) |
| Very low-density lipoprotein (VLDL) (mg/dL) | 27,234 | 20.30 ± 10.93 | 897 | 29.90 ± 14.67 | <0.001 | 1.051 (1.047–1.055) |
| Non-HDL cholesterol (mg/dL) | 16,035 | 128.76 ± 34.82 | 787 | 144.69 ± 35.39 | <0.001 | 1.012 (1.010–1.014) |
* non-paired t-test, ## generalized linear models.
3.4. The Associations of NAFLD with Health-Related Habits and Medical and Dental Attendance Patterns
Table 4 presents a comparison of the health-related habits and medical and dental attendance patterns between patients with NAFLD and those without NAFLD.
Table 4.
The Associations of NAFLD with Health-Related Habits and Attendance Patterns.
| Parameter | Variable | Without NAFLD | NAFLD | p Value ^ | OR and 95% CI # |
|---|---|---|---|---|---|
| Smoking | No | 125,060 (99.5) | 585 (0.5) | <0.001 | 1 |
| Yes | 6531 (94.9) | 353 (5.1) | 11.55 (10.10–13.2) | ||
| Teeth brushing once a day or more | No | 17,557 (98.5) | 263 (1.5) | <0.001 | 1 |
| Yes | 39,381 (99.3) | 295 (0.7) | 0.50 (0.42–0.59) | ||
| Consumption of a cariogenic diet | No | 34,106 (98.8) | 415 (1.2) | <0.001 | 1 |
| Yes | 22,832 (99.4) | 143 (0.6) | 0.52 (0.43–0.62) | ||
| Consumption of sweetened beverages | No | 32,601 (98.8) | 408 (1.2) | <0.001 | 1 |
| Yes | 24,337 (99.4) | 150 (0.6) | 0.49 (0.41–0.59) | ||
| Parameter | Variable | Without NAFLD | NAFLD | p Value ** | OR and 95% CI ## |
| Total number of appointments with a general physician | 14.19 ± 11.96 | 17.85 ± 15.01 | <0.001 | 1.016 (1.016–1.024) | |
| The number of times where scaling was performed | 0.63 ± 0.86 | 1.07 ± 1.22 | <0.001 | 1.41 (1.35–1.47) | |
| The number of times where root planning was performed | 0.06 ± 0.57 | 0.47 ± 1.56 | <0.001 | 1.35 (1.30–1.40) | |
| The number of examinations by an oral medicine specialist | 0.01 ± 0.17 | 0.07 ± 0.43 | <0.001 | 1.59 (1.38–1.82) | |
| The number of examinations by a periodontal specialist | 0.03 ± 0.27 | 0.21 ± 0.68 | <0.001 | 1.84 (1.70–1.99) | |
| Total number of dental appointments | 5.81 ± 10.14 | 15.26 ± 18.16 | <0.001 | 1.03 (1.02–1.03) | |
| Non-attendance to scheduled dental appointments | 0.98 ± 2.646 | 2.17 ± 4.218 | <0.001 | 1.06 (1.05–1.08) | |
^ Pearson Chi-Square; ** non-paired t-test, # binary logistic regression; ## generalized linear models.
NAFLD and Health-Related Habits. Compared to those without NAFLD, those with NAFLD had a statistically significant positive association with smoking (OR = 11.55 (10.10–13.2)). NAFLD diagnosis had a statistically significant negative association with teeth brushing once a day or more (OR = 0.50 (0.42–0.59)), consumption of a cariogenic diet (OR = 0.52 (0.43–0.62)), and the consumption of sweetened beverages (OR = 0.49 (0.41–0.59)) (Table 4).
NAFLD and Attendance patterns to medical and dental services. Compared to those without NAFLD diagnosis, those with NAFLD had statistically significant more: appointments with a general physician (OR = 1.016 (1.016–1.024)), scaling (OR = 1.41 (1.35–1.47)), root planning (OR = 1.35 (1.30–1.40)), appointments with oral medicine specialists (OR = 1.59 (1.38–1.82)) and with periodontal specialists (OR = 1.84 (1.70–1.99)), more total dental appointments (OR 1.030 (1.027–1.033)), and were more likely to not attend to scheduled dental appointments (OR = 1.068 (1.056–1.081)) (Table 4).
3.5. The Associations of NAFLD with Dental and Oral Status
Table 5 presents the associations between NAFLD and dental and oral status represented by diagnoses, as well as by dental procedures requirements and actual performance among the study population. Compared to those without NAFLD diagnosis, those with NAFLD had a statistically significant positive association with periodontitis diagnosis (OR = 2.41 (1.97–2.96)), presence of an oral soft tissue lesion in an oral examination (OR = 7.25 (5.81–9.09)), and all dental procedures requirements and actual performance, except for the number of teeth that required one surface amalgam filling (OR = 0.82 (0.76–0.88)), the number of teeth where one surface amalgam filling was performed (OR = 0.94 (0.87–1.03)), the number of teeth that required two amalgam fillings on two surfaces (0.79 (0.71–0.88)), and the total number of teeth that required fillings (OR = 0.97 (0.94–1.00)) (Table 5).
Table 5.
The Associations between NAFLD and Dental Status.z.
| Parameter | Variable | Without NAFLD | NAFLD | p Value ^ | OR and 95% CI # |
|---|---|---|---|---|---|
| Periodontitis | No | 51,424 (99.1) | 442 (0.9) | <0.001 | 1 |
| Yes | 5514 (97.9) | 116 (2.1) | 2.41 (1.97–2.96) | ||
| Presence of an oral soft tissue lesion in an oral examination | No | 130,013 (99.3) | 859 (0.7) | <0.001 | 1 |
| Yes | 1578 (95.2) | 79 (4.8) | 7.25 (5.81–9.09) | ||
| Parameter | Without NAFLD | NAFLD | p Value ** | OR and 95% CI ## | |
| Mean ± SD | Mean ± SD | ||||
| The number of teeth that required one surface amalgam filling | 0.61 ± 1.21 | 0.39 ± 0.88 | <0.001 | 0.82 (0.76–0.88) | |
| The number of teeth where one surface amalgam filling was performed | 0.29 ± 0.78 | 0.26 ± 0.70 | <0.009 | 0.94 (0.87–1.03) | |
| The number of teeth that required two amalgam fillings on two surfaces | 2.03 ± 1.46 | 1.67 ± 1.12 | <0.001 | 0.79 (0.71–0.88) | |
| The number of teeth where two surface amalgam fillings were performed | 0.32 ± 0.84 | 0.47 ± 0.86 | <0.001 | 1.17 (1.10–1.24) | |
| The number of teeth that required three and more amalgam fillings on surfaces | 0.11 ± 0.45 | 0.14 ± 0.47 | <0.035 | 1.14 (1.01–1.28) | |
| The number of teeth where three and more surface amalgam fillings were performed | 0.07 ± 0.32 | 0.13 ± 0.40 | <0.001 | 1.44 (1.27–1.64) | |
| The number of teeth that required four or more surfaces amalgam fillings | 0.01 ± 0.11 | 0.02 ± 0.15 | <0.025 | 1.72 (1.22–2.43) | |
| The number of teeth where four or more surfaces amalgam fillings were performed | 0.03 ± 0.18 | 0.07 ± 0.31 | <0.001 | 1.91 (1.58–2.31) | |
| The number of teeth that required resin-based composite fillings on one to four surfaces, anterior | 0.24 ± 0.79 | 0.30 ± 0.93 | <0.057 | 1.08 (1.01–1.15) | |
| The number of teeth where resin-based composite fillings were performed on one to four surfaces, anterior | 0.25 ± 0.86 | 0.45 ± 2.14 | <0.005 | 1.11 (1.07–1.15) | |
| Total number of teeth that required fillings | 1.55 ± 2.41 | 1.42 ± 2.13 | <0.048 | 0.97 (0.94–1.00) | |
| Total number of teeth where fillings were performed | 1.00 ± 1.96 | 1.49 ± 2.81 | <0.001 | 1.08 (1.06–1.10) | |
| Total number of teeth that required endodontic treatment | 0.08 ± 0.38 | 0.13 ± 0.41 | 0.001 | 1.21 (1.09–1.34) | |
| Total number of teeth where endodontic treatment was performed | 0.07 ± 0.33 | 0.18 ± 0.48 | <0.001 | 1.53 (1.39–1.69) | |
| The number of teeth that required prefabricated (direct, post and core) | 0.10 ± 0.42 | 0.15 ± 0.43 | 0.002 | 1.20 (1.08–1.34) | |
| The number of teeth on which prefabricated (direct, post and core) was performed | 0.10 ± 0.39 | 0.25 ± 0.61 | <0.001 | 1.44 (1.341. 55) | |
| The number of teeth that required crowns | 0.15 ± 0.57 | 0.26 ± 0.70 | <0.001 | 1.22 (1.14–1.30) | |
| The number of teeth where a crown was performed | 0.05 ± 0.53 | 0.34 ± 1.40 | <0.001 | 1.21 (1.16–1.25) | |
| The number of teeth that required extractions | 0.14 ± 0.51 | 0.22 ± 0.71 | 0.001 | 1.23 (1.13–1.34) | |
| Total number of teeth where extractions were performed | 0.10 ± 0.42 | 0.23 ± 0.79 | <0.001 | 1.27 (1.19–1.36) | |
| Missing teeth | 0.57 ± 1.28 | 1.30 ± 1.67 | <0.001 | 1.15 (1.12–1.18) | |
^ Pearson Chi-Square; ** non-paired t-test, # binary logistic regression; ## generalized linear models.
3.6. Receiver Operating Characteristic (ROC) Analyses of Planned and Delivered Standard Dental Units (SDU-P and SDU-D) as Predictors of NAFLD
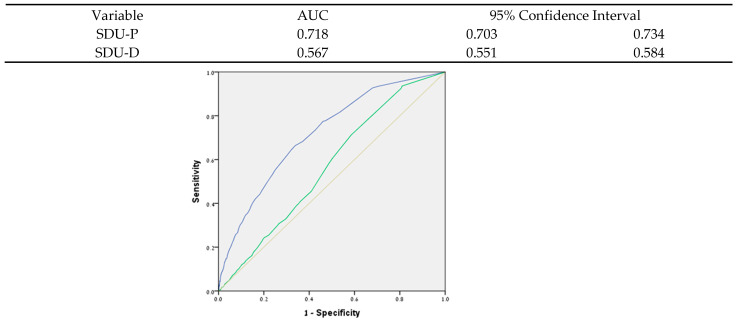
Receiver operating characteristic (ROC) analyses were performed on the SDU-P and SDU-D as predictors of NAFLD. ROC curves were plotted, and the area under the curve (AUC) was calculated. Figure 1 presents ROC analyses and AUC calculations for NAFLD with SDU-P and SDU-D. As can be seen in Figure 1, the SDU-P exhibited excellent discrimination (0.8 ≥ AUC > 0.7) for NAFLD (AUC = 0.718 (0.703–0.734)), while the SDU-D exhibited poor discrimination (0.6 ≥ AUC > 0.5) for NAFLD (AUC = 0.567 (0.551–0.584)) (Figure 1). Periodontitis diagnosis had a statistically significant positive association with SDU-P (p < 0.001), but not with SDU-D (p = 0.392) (non-paired t-test, data not in a table).
Figure 1.
Receiver operating characteristic (ROC) analyses of planned and delivered Standard Dental Units (SDU-P and SDU-D) as predictors of NAFLD. AUC: Area Under the Curve SDU-P: blue line, SDU-D: green line, reference line-orange.
3.7. Multivariate Analysis of NAFLD Diagnosis as a Dependent Variable
Collinearity statistics. Before executing the multivariate analysis of NAFLD as a dependent variable, we ran a linear regression analysis to examine the collinearity between the independent variables. As can be seen in Table 6, collinearity was ruled out (VIF < 2.5).
Table 6.
Multivariate Logistic Regression Analysis and Collinearity Statistics for NAFLD diagnosis as the dependent variable with statistically significant independent parameters.
| Parameter | Multivariate Logistic Regression Analysis | Linear Regression Analysis Collinearity Statistics |
|||||
|---|---|---|---|---|---|---|---|
| B | Std. Error | p Value | OR (95% CI) | ||||
| Tolerance | VIF | ||||||
| (Intercept) | 7.19 | 0.98 | <0.001 | 0.001 (0.000–0.005) | |||
| Age | 0.07 | 0.008 | <0.001 | 1.08 (1.06–1.09) | 0.448 | 2.234 | |
| Sex: men vs. women | 1.36 | 0.27 | <0.001 | 3.91 (2.29–6.66) | 0.934 | 1.071 | |
| Locality of residence (reference: rural) | urban Jewish | −0.24 | 0.56 | 0.667 | 0.78 (0.26–2.35) | 0.998 | 1.012 |
| Urban non-Jewish | −0.69 | 0.58 | 0.233 | 0.49 (0.16–1.56) | 0.979 | 1.021 | |
| Socioeconomic status (SES) (reference: low SES) | High | 0.12 | 0.25 | 0.619 | 1.13 (0.68–1.87) | 0.948 | 1.055 |
| Medium | 0.32 | 0.24 | 0.183 | 1.39 (0.85–2.25) | 0.944 | 1.059 | |
| Birth country (reference native Israelis) | Western Europe | 0.19 | 0.16 | 0.254 | 1.21 (0.87–1.671 | 0.983 | 1.017 |
| Eastern Europe | −0.11 | 0.60 | 0.856 | 0.89 (0.27–2.93) | 0.979 | 1.022 | |
| Asia | −0.45 | 0.56 | 0.417 | 0.63 (0.21–1.90) | 0.995 | 1.005 | |
| Ethiopia | 0.07 | 0.60 | 0.897 | 1.08 (0.33–3.54) | 0.984 | 1.017 | |
| Africa | 0.42 | 0.48 | 0.382 | 1.52 (0.59–3.94) | 0.995 | 1.005 | |
| North America | 0.08 | 0.60 | 0.883 | 1.09 (0.33–3.55) | 0.987 | 1.013 | |
| South America | 0.02 | 0.61 | 0.969 | 1.02 (0.31–3.39) | 0.997 | 1.003 | |
| Smoking | 0.15 | 0.11 | 0.183 | 1.16 (0.93–1.45) | 0.749 | 1.334 | |
| Teeth brushing once a day or more | 0.05 | 0.13 | 0.696 | 1.05 (0.81–1.36) | 0.694 | 1.441 | |
| Consumption of a cariogenic diet | 0.03 | 0.16 | 0.830 | 1.03 (0.75–1.43) | 0.587 | 1.702 | |
| Sweetened beverages | −0.23 | 0.16 | 0.145 | 0.79 (0.57–1.08) | 0.576 | 1.736 | |
| Diabetes type 2 | 1.14 | 0.18 | <0.001 | 3.14 (2.21–4.46) | 0.917 | 1.090 | |
| Impaired glucose tolerance (IGT) | 0.51 | 0.42 | 0.224 | 1.67 (0.73–3.85) | 0.963 | 1.038 | |
| Hypertension | 0.51 | 0.12 | <0.001 | 1.67 (1.30–2.14) | 0.901 | 1.109 | |
| Hyperlipidemia | 1.31 | 0.15 | <0.001 | 3.69 (2.75–4.95) | 0.546 | 1.832 | |
| BMI | 0.14 | 0.01 | <0.001 | 1.15 (1.13–1.18) | 0.840 | 1.190 | |
| Cardiovascular disease | 0.02 | 0.14 | 0.901 | 1.02 (0.76–1.36) | 0.920 | 1.086 | |
| Obstructive sleep apnea (OSA) | −0.39 | 0.30 | 0.185 | 0.67 (0.37–1.21) | 0.972 | 1.029 | |
| Periodontitis | 0.35 | 0.14 | 0.017 | 1.42 (1.06–1.89) | 0.959 | 1.043 | |
| Missing teeth | −0.02 | 0.03 | 0.513 | 0.97 (0.91–1.04) | 0.768 | 1.302 | |
| Total number of teeth that required endodontic treatment | −0.04 | 0.11 | 0.740 | 0.96 (0.76–1.21) | 0.810 | 1.235 | |
| The number of teeth that required regular extraction | −0.11 | 0.07 | 0.149 | 0.89 (0.77–1.04) | 0.949 | 1.053 | |
| The number of teeth that required a crown | −0.006 | 0.05 | 0.924 | 0.99 (0.88–1.11) | 0.822 | 1.216 | |
| Presence of oral soft tissue disease | 0.03 | 0.19 | 0.873 | 1.03 (0.71–1.50) | 0.854 | 1.123 | |
Statistically significant values are in bold. VIF: variance inflation factor.
Multivariate Analysis. Following the collinearity analysis, we performed a multivariate binary logistic regression analysis for NAFLD diagnosis as the dependent variable (Table 6). The independent variables were entered into the analysis simultaneously. The following parameters maintained a statistically positive association with NAFLD in the multivariate analysis presented in Table 6 (descending order from highest to lowest OR): male sex (OR = 3.91 (2.29–6.66)), hyperlipidemia (OR = 3.69 (2.75–4.95)), diabetes Type 2 (OR = 3.14 (2.21–4.46)), hypertension (OR = 1.67 (1.30–2.14)), periodontitis (OR = 1.42 (1.06–1.89)), BMI (OR = 1.15 (1.13–1.18)), and age (OR = 1.08 (1.06–1.09)) (Table 6).
4. Discussion
The present study research analyzed the associations of NAFLD with dental parameters, while controlling for socio-demographic parameters, health-related habits, and each of the MetS components, consequences, and related conditions among a nationally representative sample of 132,529 young and middle-aged adults. The study demonstrated a statistically significant association between NAFLD and periodontitis, even following the multivariate analysis (Table 6). Moreover, the ROC analysis demonstrated that the SDU-P (planned) exhibited excellent discrimination for NAFLD (Figure 1). Periodontitis diagnosis also had a statistically significant positive association with SDU-P, but not with SDU-D. The SDU score considers the time and complexity of the procedure and represents the procedural burden. It can be concluded that SDU-P, but not SDU-D, is a better predictor of NAFLD and periodontitis. In other words, NAFLD and periodontal morbidity are associated with a higher dental treatment needs burden, rather than with dental treatments performed de facto, which is more dependent on external influencing factors, such as patient compliance, availability of treatment, treatment experience, and specialty of the treating dental professional. Overall, the results confirmed the hypothesis of this research, i.e., that NAFLD is associated with dental morbidity, particularly with periodontitis. The study established a profile of the “patient vulnerable to NAFLD”, which, following multivariate analysis, maintained the well-known risk factors for NAFLD, including older age, male sex, and other MetS components, including diabetes type 2, hypertension, hyperlipidemia, BMI, and periodontitis. This profile is in line with the current new definition of metabolic dysfunction-associated fatty liver disease (MAFLD).
4.1. Socio-Demographic Parameters and NAFLD
In line with the literature, our findings demonstrate that older age and male sex retained a statistically significant positive association with NAFLD, even following multivariate analysis. Age is associated with a higher prevalence of NAFLD, and disease progression seems to be faster in older patients, with the male gender appearing as a risk factor [38]. In women, it seems that estrogen may play a protective effect against NAFLD [39]. Previous studies also demonstrated a statistically significant association between lower SES [40] and lower education, with a higher NAFLD prevalence. However, our univariate results should be interpreted with the utmost caution, since SES, locality of residence, and birth countries lost statistical significance with NAFLD following multivariate analysis, and education did not enter the multivariate analysis, due to its collinearity with age.
4.2. Health-Related Habits and NAFLD
Previous studies have shown that NAFLD is positively associated with passive and heavy active smoking, and active smoking and BMI have been shown to have a synergistic effect on prevalent NAFLD [41]. Consumption of simple sugars (glucose and fructose) is a factor attributing to both NAFLD development and dental caries. Chronic fructose exposure can lead to inflammation, liver fat accumulation, NAFLD, and metabolic syndrome [42]. Interestingly, none of the health-related habits retained a statistically significant association with NAFLD following multivariate analysis, reflecting the importance of other parameters, such as socio-demographics, MetS components, and periodontitis, which are all associated with lifestyle habits that serve as confounders, mediators, or common cause.
4.3. MetS Components, Consequences, and Related Conditions and NAFLD
Following multivariate analysis, NAFLD maintained a statistically significant positive association with diabetes type 2, hypertension, hyperlipidemia, and BMI in line with the current new definition of metabolic dysfunction-associated fatty liver disease (MAFLD). Indeed, an intimate association exists between MAFLD and type 2 diabetes with over 70% of patients with type 2 diabetes having MAFLD; therefore, type 2 diabetes is now considered as one of the three criteria for defining MAFLD, along with overweight and obesity [2]. Excess body weight has a strong pathological link to MAFLD and is a critical determinant of adverse clinical outcomes [2]. Obesity can be classified as metabolically healthy obesity (MHO) and metabolically unhealthy obesity [43], and therefore, the presence of both excess weight and metabolic dysfunction have independent effects on the risk of MAFLD [2], as were considered in the current study. Other metabolic risk abnormalities included in the new MAFLD diagnosis were also considered in the present study, including plasma triglycerides, HDL, prediabetes, fasting glucose, HbA1c, and CRP (Table 3).
4.4. Dental Status and NAFLD
The study demonstrated a statistically significant association between NAFLD and periodontitis, independent of socioeconomic variables, health-related habits, and MetS morbidity. Moreover, the SDU-P (planned), which represents the dental procedural burden, exhibited excellent discrimination for NAFLD. To the best of our knowledge, this study is the first to perform ROC analysis to illustrate the diagnostic ability of the dental procedural burden in NAFLD diagnosis.
NAFLD shares some risk factors with periodontitis and dental caries, such as smoking and the consumption of simple sugars (glucose and fructose) [42]. In agreement, a recent cross-sectional study has shown that untreated caries, adults with <20 teeth, and/or people with moderate-severe periodontitis were more likely to have NAFLD [5]. The number of missing teeth has been also previously shown to be positively associated with a higher prevalence rate of NAFLD in males with over 6 teeth missing, but not in females [44,45]. In the present study, the parameter of missing teeth was positively associated with NAFLD in the univariate analysis, but lost its statistical significance in the multivariate analysis. This could reflect the fact that some of the tooth loss could be attributed to periodontitis, which retained a statistically significant positive association with NAFLD.
Another population-based cohort investigation concluded that a history of periodontitis might be a risk factor for NAFLD [46,47]. Individuals with hepatic disease showed a higher prevalence of periodontal disease, worse oral hygiene, and periodontal health status, compared to healthy patients [48]. In concordance, our results also demonstrated more scaling, root planning sessions, and more appointments with periodontists.
Several hypotheses link NAFLD and periodontitis, through periodontal pathogens, inflammatory mediators, and oxidative stress [49]. In a systematic review, a significant positive association was shown between NAFLD and clinical microbial periodontal parameters and between NAFLD and self-reported categories of tooth loss in males, but not females [50]. Although occurring in distant parts of the body, NAFLD and periodontitis pathologies share some etiological factors regarding their onset and portray an unbalanced inflammatory response in a susceptible host. Some of the proposed effects of P. gingivalis on hepatic cells include inhibiting glycogenesis and triggering a cascade that increases the transcription of pro-inflammatory genes, such as TNF-α and IL-1β. Therefore, the progression of both diseases can be aggravated by insulin resistance and the increased systemic pro-inflammatory profile found in diabetic and obese individuals [26,51,52]. The clinical relevance of the results is that clinicians and health authorities should be aware of the high periodontal morbidity in NAFLD patients and refer them to evaluation by dentists. Therapy requires customized treatment plans to address the specific characteristics of each patient and focus on high-risk populations.
5. Strength and Limitations
The main strengths of the current research are its large sample size (132,529 individuals), as well as the strict protocol and uniform sociodemographic, medical, and dental codes used. The records-based design is not influenced by patient recall bias, excluding health-related habits. Furthermore, the large sample size enabled us to capture the consequences of MetS, such as TIA and stroke, which are relatively uncommon among young to middle-aged adults. Moreover, routine evaluation of dental pathologies was based on both clinical and radiological examinations. This enhances the diagnosis of hidden occlusal or interproximal caries.
The limitations of the current research include the cross-sectional design, which cannot address causality and can only suggest the associations and correlations between variables. While the research considered and analyzed important factors and covariates, considering the depth of the issues discussed, other parameters were not considered, such as the severity of periodontitis, genetics and epigenetics parameters, childhood and in utero exposures, and history of health-related habits. While multiple ethnicities among a nationwide sample were included in the study, the study population captures the military personnel in Israel, which may limit the generalizability of the results. There is a need for future multicenter international longitudinal prospective studies that will include both genetic and more epidemiological data in other settings and populations to discover the underlying mechanisms underlying the observed associations found in this study.
6. Conclusions
The conclusions of this study are:
The results confirmed the hypothesis of this research, i.e., that NAFLD is associated with dental morbidity, particularly with periodontitis.
The multivariate analysis established a profile of the “patient vulnerable to NAFLD”, including older age, male sex, and other MetS components, including diabetes type 2, hypertension, hyperlipidemia, BMI, and periodontitis.
This profile aligns with the current new definition of metabolic dysfunction-associated fatty liver disease (MAFLD).
A collaborative effort is needed from the dental and general medical authorities by sharing information regarding dental and systemic morbidities.
The study also highlights the need to adopt a holistic risk management approach that considers both dental and systemic conditions.
Acknowledgments
The authors declare the self-funding of the research.
Abbreviations
| BMI | Body mass index |
| MPR | Medical patient record |
| CRP | C-reactive protein |
| CVD | Cardiovascular disease |
| DPR | Dental patient record |
| HbA1c | Glycated hemoglobin |
| HDL | High-density lipoprotein |
| IDF | Israeli Defense Force |
| LDL | Low-density lipoprotein |
| MetS NAFLD |
Metabolic syndrome Non-alcoholic fatty liver disease |
| OSA | Obstructive sleep apnea |
| SES | Socio-economic status |
| TIA | Transient ischemic attack |
| VLDL | Very low-density lipoprotein |
Author Contributions
Conceptualization, D.R., A.W. and. G.A.; methodology, D.R., A.W. and. G.A.; software, D.Z. and G.A.; validation, D.Z. and G.A.; formal analysis, G.A.; investigation, D.R., A.W. and. G.A.; resources, D.Z. and G.A.; data curation, D.Z.; writing—original draft preparation, D.R. and G.A.; writing—review and editing, A.W. and D.Z.; visualization, D.R., A.W. and. G.A.; supervision, G.A.; project administration, G.A.; funding acquisition: G.A. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the Israeli Medical Corps Institutional Review Board protocol code: IDF-1281-2013.
Informed Consent Statement
Patient consent was waived due to the anonymous retrospective analysis of electronic records (records-based study).
Data Availability Statement
Data sharing not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research received no external funding.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Younossi Z., Anstee Q.M., Marietti M., Hardy T., Henry L., Eslam M., George J., Bugianesi E. Global burden of NAFLD and NASH: Trends, predictions, risk factors and prevention. Nat. Rev. Gastroenterol. Hepatol. 2018;15:11–20. doi: 10.1038/nrgastro.2017.109. [DOI] [PubMed] [Google Scholar]
- 2.Eslam M., Newsome P.N., Sarin S.K., Anstee Q.M., Targher G., Romero-Gomez M., Zelber-Sagi S., Wai-Sun Wong V., Dufour J.F., Schattenberg J.M., et al. A new definition for metabolic dysfunction-associated fatty liver disease: An international expert consensus statement. J. Hepatol. 2020;73:202–209. doi: 10.1016/j.jhep.2020.03.039. [DOI] [PubMed] [Google Scholar]
- 3.Williams C.D., Stengel J., Asike M.I., Torres D.M., Shaw J., Contreras M., Landt C.L., Harrison S.A. Prevalence of nonalcoholic fatty liver disease and nonalcoholic steatohepatitis among a largely middle-aged population utilizing ultrasound and liver biopsy: A prospective study. Gastroenterology. 2011;140:124–131. doi: 10.1053/j.gastro.2010.09.038. [DOI] [PubMed] [Google Scholar]
- 4.Alberti K.G., Eckel R.H., Grundy S.M., Zimmet P.Z., Cleeman J.I., Donato K.A., Fruchart J.C., James W.P., Loria C.M., Smith S.C., Jr. Harmonizing the metabolic syndrome: A joint interim statement of the international diabetes federation task force on epidemiology and prevention; national heart, lung, and blood institute; american heart association; world heart federation; international atherosclerosis society; and international association for the study of obesity. Circulation. 2009;120:1640–1645. doi: 10.1161/CIRCULATIONAHA.109.192644. [DOI] [PubMed] [Google Scholar]
- 5.Weintraub J.A., Lopez Mitnik G., Dye B.A. Oral diseases associated with nonalcoholic fatty liver disease in the united states. J. Dent. Res. 2019;98:1219–1226. doi: 10.1177/0022034519866442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kassebaum N.J., Bernabe E., Dahiya M., Bhandari B., Murray C.J., Marcenes W. Global burden of severe periodontitis in 1990-2010: A systematic review and meta-regression. J. Dent. Res. 2014;93:1045–1053. doi: 10.1177/0022034514552491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bernabe E., Marcenes W., Hernandez C.R., Bailey J., Abreu L.G., Alipour V., Amini S., Arabloo J., Arefi Z., Arora A., et al. Global, regional, and national levels and trends in burden of oral conditions from 1990 to 2017: A systematic analysis for the global burden of disease 2017 Study. J. Dent. Res. 2020;99:362–373. doi: 10.1177/0022034520908533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tonetti M.S., Jepsen S., Jin L., Otomo-Corgel J. Impact of the global burden of periodontal diseases on health, nutrition and wellbeing of mankind: A call for global action. J. Clin. Periodontol. 2017;44:456–462. doi: 10.1111/jcpe.12732. [DOI] [PubMed] [Google Scholar]
- 9.Marcenes W., Kassebaum N.J., Bernabe E., Flaxman A., Naghavi M., Lopez A., Murray C.J. Global burden of oral conditions in 1990–2010: A systematic analysis. J. Dent. Res. 2013;92:592–597. doi: 10.1177/0022034513490168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cappelli D.P., Mobley C.C. Prevention in Clinical Oral Health Care. Mosby Elsevier St.; Louis, MO, USA: 2007. [Google Scholar]
- 11.Marthaler T.M. Changes in dental caries 1953–2003. Caries Res. 2004;38:173–181. doi: 10.1159/000077752. [DOI] [PubMed] [Google Scholar]
- 12.Abdalla-Aslan R., Findler M., Levin L., Zini A., Shay B., Twig G., Almoznino G. Where periodontitis meets metabolic syndrome-The role of common health-related risk factors. J. Oral. Rehabil. 2019;46:647–656. doi: 10.1111/joor.12798. [DOI] [PubMed] [Google Scholar]
- 13.Abdalla-Aslan R., Findler M., Zini A., Almoznino G. Caries experience, periodontal status, and metabolic morbidity in patients with psychiatric disorders. Quintessence Int. 2021;52:516–526. doi: 10.3290/j.qi.b1044091. [DOI] [PubMed] [Google Scholar]
- 14.Almoznino G., Kessler Baruch O., Kedem R., Protter N.E., Shay B., Yavnai N., Zur D., Mijiritsky E., Abramovitz I. SOS Teeth: First priority teeth with advanced caries and its associations with metabolic syndrome among a national representative sample of young and middle-aged adults. J. Clin. Med. 2020;9:3170. doi: 10.3390/jcm9103170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Abramovitz I., Zini A., Pribluda P., Kedem R., Zur D., Protter N.E., Almoznino G. “Dental Cluster” versus “metabolic cluster”: Analyzing the Associations of planned and delivered dental procedures with metabolic syndrome, utilizing data from the dental, oral, medical epidemiological (DOME) cross-sectional record-based nationwide study. Biology. 2021;10:608. doi: 10.3390/biology10070608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Almoznino G., Gal N., Levin L., Mijiritsky E., Weinberg G., Lev R., Zini A., Touger-Decker R., Chebath-Taub D., Shay B. Diet practices, body mass index, and oral health-related quality of life in adults with periodontitis- A case-control study. Int. J. Environ. Res. Public Healthy. 2020;17:2340. doi: 10.3390/ijerph17072340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Almoznino G., Zini A., Kedem R., Protter N.E., Zur D., Abramovitz I. Hypertension and its associations with dental status: Data from the dental, oral, medical epidemiological (DOME) nationwide records-based study. J. Clin. Med. 2021;10:176. doi: 10.3390/jcm10020176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Baima G., Romandini M., Citterio F., Romano F., Aimetti M. Periodontitis and accelerated biological aging: A geroscience approach. J. Dent. Res. 2021;101:125–132. doi: 10.1177/00220345211037977. [DOI] [PubMed] [Google Scholar]
- 19.Sanz M., Marco Del Castillo A., Jepsen S., Gonzalez-Juanatey J.R., D’Aiuto F., Bouchard P., Chapple I., Dietrich T., Gotsman I., Graziani F., et al. Periodontitis and cardiovascular diseases: Consensus report. J. Clin. Periodontol. 2020;47:268–288. doi: 10.1111/jcpe.13189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Loe H. Periodontal disease. The sixth complication of diabetes mellitus. Diabetes Care. 1993;16:329–334. doi: 10.2337/diacare.16.1.329. [DOI] [PubMed] [Google Scholar]
- 21.Kim J.Y., Lee G.N., Song H.C., Park Y.M., Ahn Y.B., Han K., Ko S.H. Association between fatty liver index and periodontitis: The Korea national health and nutrition examination survey. Sci. Rep. 2020;10:3805. doi: 10.1038/s41598-020-60797-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yao C., Lan D., Li X., Wang Y., Qi S., Liu Y. Porphyromonas gingivalis is a risk factor for the development of nonalcoholic fatty liver disease via ferroptosis. Microbes Infect. 2022:105040. doi: 10.1016/j.micinf.2022.105040. [DOI] [PubMed] [Google Scholar]
- 23.Ahmad A., Furuta M., Shinagawa T., Takeuchi K., Takeshita T., Shimazaki Y., Yamashita Y. Association of periodontal status with liver abnormalities and metabolic syndrome. J. Oral. Sci. 2015;57:335–343. doi: 10.2334/josnusd.57.335. [DOI] [PubMed] [Google Scholar]
- 24.Kuraji R., Sekino S., Kapila Y., Numabe Y. Periodontal disease-related nonalcoholic fatty liver disease and nonalcoholic steatohepatitis: An emerging concept of oral-liver axis. Periodontol. 2000. 2021;87:204–240. doi: 10.1111/prd.12387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Akinkugbe A.A., Barritt A.S., Cai J., Offenbacher S., Thyagarajan B., Khambaty T., Singer R., Kallwitz E., Heiss G., Slade G.D. Periodontitis and prevalence of elevated aminotransferases in the Hispanic Community Health Study/Study of Latinos. J. Periodontol. 2018;89:949–958. doi: 10.1002/JPER.17-0579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wijarnpreecha K., Panjawatanan P., Cheungpasitporn W., Lukens F.J., Harnois D.M., Pungpapong S., Ungprasert P. The association between periodontitis and nonalcoholic fatty liver disease: A systematic review and meta-analysis. J. Gastrointestin. Liver Dis. 2020;29:211–217. doi: 10.15403/jgld-841. [DOI] [PubMed] [Google Scholar]
- 27.Sheiham A., Watt R.G. The common risk factor approach: A rational basis for promoting oral health. Community Dent. Oral. Epidemiol. 2000;28:399–406. doi: 10.1034/j.1600-0528.2000.028006399.x. [DOI] [PubMed] [Google Scholar]
- 28.Abramovitz I., Zini A., Atzmoni M., Kedem R., Zur D., Protter N.E., Almoznino G. Cognitive performance and its associations with dental caries: Results from the dental, oral, medical epidemiological (DOME) records-based nationwide study. Biology. 2021;10:178. doi: 10.3390/biology10030178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Almoznino G., Abramovitz I., Kessler Baruch O., Kedem R., Protter N.E., Levine J., Bader T., Yavnai N., Zur D., Mijiritsky E., et al. SOS Teeth: Age and sex differences in the prevalence of first priority teeth among a national representative sample of young and middle-aged adults. Int. J Environ. Res. Public. Healthy. 2020;17:4847. doi: 10.3390/ijerph17134847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Almoznino G., Kedem R., Turgeman R., Bader T., Yavnai N., Zur D., Shay B. The Dental, Oral, Medical Epidemiological (DOME) Study: Protocol and study methods. Methods Inf. Med. 2020;59:119–130. doi: 10.1055/s-0040-1718582. [DOI] [PubMed] [Google Scholar]
- 31.Abramovitz I., Zini A., Kessler Baruch O., Kedem R., Protter N.E., Shay B., Yavnai N., Zur D., Mijiritsky E., Almoznino G. SOS teeth with advanced caries and sociodemographic indicators, health-related habits and dental attendance patterns: Data from the Dental, Oral, Medical Epidemiological (DOME) nationwide records-based study. BMC Oral. Healthy. 2021;21:389. doi: 10.1186/s12903-021-01751-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Twig G., Yaniv G., Levine H., Leiba A., Goldberger N., Derazne E., Ben-Ami Shor D., Tzur D., Afek A., Shamiss A., et al. Body-Mass index in 2.3 million adolescents and cardiovascular death in adulthood. N. Engl. J. Med. 2016;374:2430–2440. doi: 10.1056/NEJMoa1503840. [DOI] [PubMed] [Google Scholar]
- 33.IDF (Israel Defense Forces) Personal Data- The Medical Profile. [(accessed on 1 November 2022)]. Available online: https://www.mitgaisim.idf.il/%D7%9B%D7%AA%D7%91%D7%95%D7%AA/english/tzav-rishon/the-medical-profile/#/
- 34.IDF Volunteering in the IDF for Military Exemption Recipients. [(accessed on 1 November 2022)]. Available online: https://www.kolzchut.org.il/en/Volunteering_in_the_IDF_for_Military_Exemption_Recipients.
- 35.Engelchin-Nissan E., Catan G., Oz N., Arieli E., Brief I., Ben Moshe R., Shmueli A. Utilization of Health Services by IDF Soldiers and Civilian Population at an Israeli HMO. Healthy Econ. Outcome Res. Open Access S1. 2017;103:2–6. [Google Scholar]
- 36.ADA (American Dental Association) ADA Guide to Dental Procedures Reported with Area of the Oral Cavity or Tooth Anatomy (or Both) [(accessed on 30 October 2022)]. Version 1. Available online: https://www.ada.org/-/media/project/ada-organization/ada/ada-org/files/publications/cdt/appendix-3_areaoftheoralcavityandtoothanatomybycdtcode_2023jan.pdf.
- 37.Yang S., Berdine G. The receiver operating characteristic (ROC) curve. Southwest Respir. Crit. Care Chron. 2017;5:34–36. doi: 10.12746/swrccc.v5i19.391. [DOI] [Google Scholar]
- 38.Dietrich P., Hellerbrand C. Non-alcoholic fatty liver disease, obesity and the metabolic syndrome. Best Pract. Res. Clin. Gastroenterol. 2014;28:637–653. doi: 10.1016/j.bpg.2014.07.008. [DOI] [PubMed] [Google Scholar]
- 39.Gutierrez-Grobe Y., Ponciano-Rodriguez G., Ramos M.H., Uribe M., Mendez-Sanchez N. Prevalence of non alcoholic fatty liver disease in premenopausal, posmenopausal and polycystic ovary syndrome women. The role of estrogens. Ann. Hepatol. 2010;9:402–409. doi: 10.1016/S1665-2681(19)31616-3. [DOI] [PubMed] [Google Scholar]
- 40.Vilar-Gomez E., Nephew L.D., Vuppalanchi R., Gawrieh S., Mladenovic A., Pike F., Samala N., Chalasani N. High-quality diet, physical activity, and college education are associated with low risk of NAFLD among the US population. Hepatology. 2022;75:1491–1506. doi: 10.1002/hep.32207. [DOI] [PubMed] [Google Scholar]
- 41.Liu Y., Dai M., Bi Y., Xu M., Xu Y., Li M., Wang T., Huang F., Xu B., Zhang J., et al. Active smoking, passive smoking, and risk of nonalcoholic fatty liver disease (NAFLD): A population-based study in China. J. Epidemiol. 2013;23:115–121. doi: 10.2188/jea.JE20120067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yki-Jarvinen H. Non-alcoholic fatty liver disease as a cause and a consequence of metabolic syndrome. Lancet Diabetes Endocrinol. 2014;2:901–910. doi: 10.1016/S2213-8587(14)70032-4. [DOI] [PubMed] [Google Scholar]
- 43.Munoz-Garach A., Cornejo-Pareja I., Tinahones F.J. Does Metabolically Healthy Obesity Exist? Nutrients. 2016;8:320. doi: 10.3390/nu8060320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Qiao F., Fu K., Zhang Q., Liu L., Meng G., Wu H., Xia Y., Bao X., Gu Y., Shi H., et al. The association between missing teeth and non-alcoholic fatty liver disease in adults. J. Clin. Periodontol. 2018;45:941–951. doi: 10.1111/jcpe.12929. [DOI] [PubMed] [Google Scholar]
- 45.Fu K.Y., Qiao F., Meng G., Zhang Q., Liu L., Song K., Niu K.J. Association between tooth missing and non-alcoholic fatty liver disease. Zhonghua Liu Xing Bing Xue Za Zhi. 2020;41:716–721. doi: 10.3760/cma.j.cn112338-20190621-00457. [DOI] [PubMed] [Google Scholar]
- 46.Akinkugbe A.A., Slade G.D., Barritt A.S., Cole S.R., Offenbacher S., Petersmann A., Kocher T., Lerch M.M., Mayerle J., Volzke H., et al. Periodontitis and non-alcoholic fatty liver disease, a population-based cohort investigation in the study of health in pomerania. J. Clin. Periodontol. 2017;44:1077–1087. doi: 10.1111/jcpe.12800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yoneda M., Naka S., Nakano K., Wada K., Endo H., Mawatari H., Imajo K., Nomura R., Hokamura K., Ono M., et al. Involvement of a periodontal pathogen, Porphyromonas gingivalis on the pathogenesis of non-alcoholic fatty liver disease. BMC Gastroenterol. 2012;12:16. doi: 10.1186/1471-230X-12-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Duseja A., Chahal G.S., Jain A., Mehta M., Ranjan A., Grover V. Association between nonalcoholic fatty liver disease and inflammatory periodontal disease: A casecontrol study. J. Indian Soc. Periodontol. 2021;25:47–54. doi: 10.4103/jisp.jisp_45_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Han P., Sun D., Yang J. Interaction between periodontitis and liver diseases. Biomed. Rep. 2016;5:267–276. doi: 10.3892/br.2016.718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Alakhali M.S., Al-Maweri S.A., Al-Shamiri H.M., Al-Haddad K., Halboub E. The potential association between periodontitis and non-alcoholic fatty liver disease: A systematic review. Clin. Oral. Investig. 2018;22:2965–2974. doi: 10.1007/s00784-018-2726-1. [DOI] [PubMed] [Google Scholar]
- 51.Albuquerque-Souza E., Sahingur S.E. Periodontitis, chronic liver diseases, and the emerging oral-gut-liver axis. Periodontol. 2000. 2022;89:125–141. doi: 10.1111/prd.12427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ishikawa M., Yoshida K., Okamura H., Ochiai K., Takamura H., Fujiwara N., Ozaki K. Oral porphyromonas gingivalis translocates to the liver and regulates hepatic glycogen synthesis through the Akt/GSK-3beta signaling pathway. Biochim. Biophys Acta. 2013;1832:2035–2043. doi: 10.1016/j.bbadis.2013.07.012. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing not applicable.