Abstract
Background: The remnant-like particle cholesterol (RLP-C) has been demonstrated to be associated with residual cardiovascular risk. The meta-analysis aimed to evaluate the impact of baseline RLP-C on the incidence of major cardiovascular adverse events (MACEs) in patients with coronary artery disease (CAD). Methods: A systematic literature search was performed in PubMed and Embase electronic databases from the inception of the databases through 1 October 2022. Studies evaluating the association between baseline RLP-C and the risk of MACEs in patients with CAD were included. Hazard ratios (HRs) with 95% confidence intervals (CIs) were pooled by a random-effect method (RLP-C analyzed as a categorical variable) and a fixed-effects model (RLP-C analyzed as a continuous variable). Results: Ten studies including 18,053 subjects were finally included in this meta-analysis. In our pooled analysis, compared to CAD patients with the lowest RLP-C category, the CAD patients with the highest RLP-C category had a significantly higher risk of future MACEs during follow-up (HR 1.79, 95% CI, 1.42–2.26, I2 = 60.31%, p < 0.01), which was consistent with outcomes of meta-analysis with the RLP-C analyzed as a continuous variable (HR 1.40, 95% CI, 1.28–1.53, I2 = 38.20%, p < 0.01). The sensitivity analysis confirmed the robustness of the results, and no significant publication bias was identified. Conclusion: The present meta-analysis suggests that the RLP-C was associated with an increased risk of long-term MACEs in patients with CAD at baseline. It is necessary to conduct randomized controlled trials to explore whether reducing the RLP-C level is conducive to reducing residual cardiovascular risk, even coronary plaque regression.
Keywords: remnant-like particle cholesterol, coronary artery disease, residual cardiovascular risk
1. Introduction
Coronary artery disease (CAD) is the most common cause of death all over the world, resulting in a huge economic and medical burden [1,2]. The rise of cholesterol is one of the widely accepted mechanisms that cause atherosclerosis, and the oxidized low-density lipoprotein (LDL) particles play an important role [3,4]. Therefore, lipid lowering therapy focusing on lowering low-density lipoprotein cholesterol (LDL-C) has been widely applied to the primary and secondary prevention of CAD [5]. With the application of statins, ezetimibe, and proprotein convertase subtilisin/kexin type 9 (PCSK9) inhibitors, the LDL-C level and the risk of adverse cardiovascular events of CAD patients have been significantly decreased [6,7,8]. However, there is also a considerable residual cardiovascular risk that may be driven by non-high density lipoprotein cholesterol (non-HDL-C), lipoprotein (a), and remnant-like particle cholesterol (RLP-C) [9,10,11].
In recent years, triglyceride-rich lipoproteins (TRLs) including chylomicron remnant, intermediate-density lipoprotein (IDL), and very low-density lipoprotein (VLDL), have been regarded as potential cardiovascular risk factors besides LDL-C, especially for patients with metabolic disorders [12,13]. Most studies have suggested that the RLP-C, as the cholesterol content of a subset of the TRLs, was significantly associated with the increasing risk of CAD and major cardiovascular adverse events (MACEs) [14,15,16]. However, a few studies indicated that there was no significant association between RLP-C and cardiovascular events [17,18]. In view of the conflicting data, we carried out a systematic review and meta-analysis to explore the association between RLP-C and the risk of MACEs in patients with CAD, and to obtain a quantitative estimate of the risk.
2. Method
2.1. Literature Retrieval Strategy
We searched published studies in PubMed and Embase electronic databases from the inception of these databases to 1 October 2022, using the following retrieval methods described in Item S1 in Supplementary Materials. Only full-length articles were screened, while conference abstracts would be excluded. There was no language limitation.
2.2. Inclusion and Exclusion Criteria
Inclusion criteria: (1) cohort, or nest case-control study evaluating the association between RLP-C and the risk of MACEs in CAD patients, (2) RLP-C was measured at baseline, (3) the endpoints of studies included MACEs, (4) studies reported hazard ratio (HR), relative risk (RR), or Odds ratio (OR) with 95% confidence intervals (CI) after adjustment of confounding factors, (5) the median follow-up time was over six months.
Exclusion criteria: (1) not all participants were diagnosed with CAD at baseline, (2) cross-sectional studies, and (3) conference abstract without full text. The screening of eligible studies was conducted by Yang and Wang, and disagreements were judged by a third reviewer (Y.-D.T.)
RLP-C was defined as the cholesterol content of a subset of TRLs, i.e., chylomicron remnants, VLDL, and IDL in the non-fasting state, and VLDL and IDL in the fasting state; calculated by the formula: TC minus HDL-C minus LDL-C. Non-HDL-C was calculated by the formula: TC minus HDL-C. The definition of MACEs was in accordance with the criteria of the original research. The MACEs were typically defined as a composite endpoint including cardiac death, myocardial infarction, stroke and repeat revascularization. When multiple studies deriving from the same data source were appropriate, we incorporated only the one with the maximum sample size. Moreover, if both the outcomes of calculated RLP-C and measured RLP-C were available, we used the outcomes of calculated RLP-C. We used the Newcastle-Ottawa quality assessment scale to evaluate the quality of cohort and nest case-control studies [19], where 7–9 points was assumed high quality, 5–6 points was assumed moderate quality, and 0–4 points was assumed low quality.
2.3. Data Extraction
Two reviewers (Yang and Wang) extracted detailed data from included studies. The extracted data included the last name of the first author, year of publication, country of origin, study design, sample size, subject characteristics (age, sex, type of CAD and mean LDL-C), follow-up time, outcomes reported, and confounding factors adjusted in the multivariate regression analyses.
2.4. Statistical Analysis
STATA version 17.0 was used to perform statistical analysis. The association of baseline RLP-C with the risk of future MACEs was evaluated by calculating the pooled HR and 95% CI. We extracted the HR and 95% CI of the future MACEs in subjects with the highest RLP-C group compared with those with the lowest RLP-C group, when the studies analyzed RLP-C as a categorical variable. When the studies analyzed RLP-C as a continuous variable, we extracted the HR and 95% CI of future MACEs per 1-SD/1-log unit increment of the RLP-C according to the reporting of the original studies. We used the Galbraith plots, the I2 statistic and Cochran’s Q test to assess the between-study heterogeneity. When the p value of the Q test was <0.1 and I2 was >50%, we used the random-effects model to calculate the pooled HR and 95% CI, otherwise, we used the fixed-effects model to calculate them. The significance of the pooled HR was assessed by the Z test (p < 0.05 was regarded as statistically significant). Subgroup analysis was performed to explore the potential source of heterogeneity. Moreover, sensitivity analysis (excluding one study at a time) was carried out to assess the robustness of the outcomes. Moreover, we would conduct funnel plots to evaluate potential publication bias.
3. Results
3.1. Characteristics of Included Studies
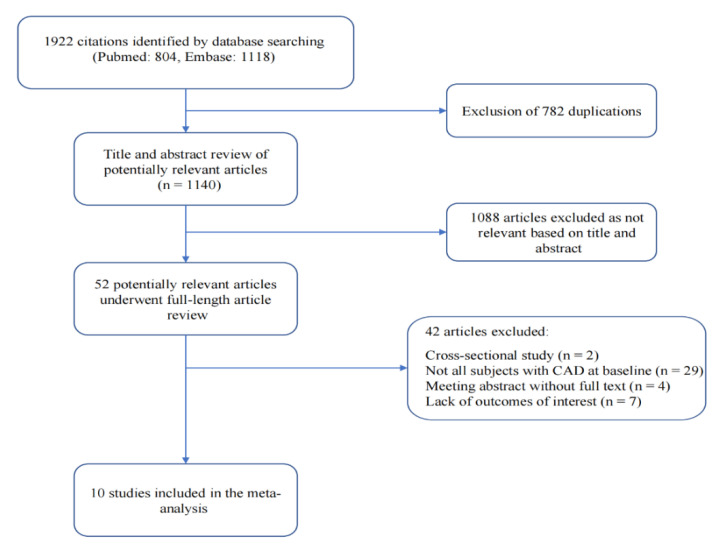
The present meta-analysis finally included 10 studies comprising 18,053 subjects according to our retrieving methods and screen criteria (Figure 1). These studies included participants from two countries (four from Japan, six from China). Most European and American studies were excluded because they included the general population rather than the population with coronary heart disease at baseline. Half of the studies were prospective cohort studies, and half of the studies were retrospective cohort studies. Four studies included subjects with acute coronary syndrome (ACS), four studies included patients with stable CAD, and two studies included patients with CAD. The average age of the included patients ranged from 55 to 70 years old, and the proportion of female patients varied from 9 to 43%. The median follow-up time ranged from 12 to 54.9 months. All the studies scored more than 7 points based on the Newcastle–Ottawa quality assessment scale, suggesting good study quality (Table 1).
Figure 1.
Flow chart of meta-analysis for exclusion/inclusion of individual articles.
Table 1.
Main characteristics of the studies included in this meta-analysis of the association between the RLP-C and MACEs.
| Country | Study Design | Subjects | Sample Size | Sex Female | Age (Years) | RLP-C Analysis | Mean LDL-C (mmol/L) | Follow-Up Time (Months) | Outcome Reported | Confounder Adjustment | Quality Assessment (Newcastle–Ottawa Scale) | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Shao et al. 2022 [15] | China | Retrospective cohort study | Patients with ACS undergoing PCI | 1716 | 23.3% | 60 ± 10 | RLP-C > 75th vs. RLP-C ≤ 75th | 2.44 ± 0.80 | 30.9 | MACE (354) | Age, sex, BMI, current smoking, hypertension, diabetes, past MI, past PCI, CKD, statins on admission, discharged drugs, complete revascularization, STEMI, hs-CRP, GRACE risk score, left main or multivessel disease. | Selection: 4 Comparability: 2 Outcome: 2 |
| Nguyen et al. 2014 [20] | Japan | Prospective cohort study | Patients with ACS undergoing PCI | 190 | 27.9% | 70.2 (63.0 − 79.0) | RLP-C ≥ 5.4 mg/dL vs. RLP-C < 5.4 mg/dL | 2.57 ± 0.80 | 30 | MACE (42), Cardiac death (2), MI (10), Ischemia-driven revascularization (25), Stroke (30) | Age, sex, smoking, BMI, DM, HTN, Multivessel CAD, hs-CRP, HbA1c, TG, HDL-C, and LDL-C. | Selection: 4 Comparability: 2 Outcome: 2 |
| Nakamura et al. 2016 [21] | Japan | Prospective cohort study | Patients with stable CAD | 560 | 43.0% | 64 ± 9 | Continuous | 2.31 (1.92–2.51) | 33 | MACE (40), Cardiac death (13), MI (2), Ischemia-driven revascularization (17), Stroke (8) | Multivessel CAD, CRP, eGFR, BNP, non-HDL-C, and ApoA-I. | Selection: 4 Comparability: 1 Outcome: 2 |
| Cao et al. 2020 [22] | China | Retrospective cohort study | Patients with CAD | 4431 | 28.9% | 58.32 ± 12.29 | Continuous | 2.44 ± 0.89 | 61.2 | MACE (541), Cardiac death (75), UAP requiring hospitalization (132), MI (44), Repeat revascularization (181), Stroke (109) |
Age, sex, smoking, BMI, DM, HTN, Family history of CAD, Baseline statin, TC, TG, HDL-C, non-HDL-C, Apo B, and LDL-C. | Selection: 4 Comparability: 2 Outcome: 2 |
| Hong et al. 2017 [17] | China | Retrospective Cohort study | Patients with stable CAD and diabetes mellitus | 328 | 36.2% | 59.2 ± 9.4 | Continuous | 2.50 ± 1.00 | 12 | MACE (47), Cardiac death (3), UAP requiring hospitalization (5), MI (8), Repeat revascularization (32) | Age, sex, smoking, BMI, HTN, Family history of CAD, Gensini scores, Lp (a), HbA1c, hs-CRP, Fibrinogen, Neutrophil count and LDL-C. | Selection: 4 Comparability: 2 Outcome: 1 |
| Zhao et al. 2020 [14] | China | Retrospective cohort study | Patients with NSTE-ACS undergoing PCI | 2419 | 28.2% | 60.08 ± 8.97 | RLP-C > 50th vs. RLP-C ≤ 50th, Continuous | 2.50 ± 0.88 | 36 | MACE (454), all-cause death (21), MI (117), ischemia-driven revascularization (316) | Age, BMI, heart rate, SBP, DM, prior MI, prior PCI, prior CABG, prior stroke, TG, TC, HDL-C, hs-CRP, eGFR, FBG, HbA1c, LVEF, principal diagnosis, discharged drugs, Left main disease, muti-vessel disease, CTO disease, diffuse disease, bifurcation disease, and number of stents. | Selection: 4 Comparability: 2 Outcome: 1 |
| Gao et al. 2022 [23] | China | Prospective cohort study | Patients with MINOCA | 1179 | 36.5% | 55.70 ± 11.8 | RLP-C > 50th vs. RLP-C ≤ 50th, Continuous | 2.29 ± 0.76 | 41.7 | MACE (168), All-cause death (18), UAP or HF requiring hospitalization (119), MI (41), Repeat revascularization (46), Stroke (109) |
Age, sex, BMI, MI type, HTN, DM, and dyslipidemia. | Selection: 4 Comparability: 2 Outcome: 2 |
| Kugiyama et al. 1999 [24] | Japan | Prospective cohort study | Patients with CAD | 135 | 34.0% | 65.00 ± 9.70 | T3 vs. T1-T2 | NA | 26.8 | MACE (45) | Age, sex, smoking, HTN, DM, hypercholesterolemia, hypertriglyceridemia, low levels of HDL cholesterol, stenosis of left main coronary artery, and the number of diseased coronary arteries. | Selection: 4 Comparability: 2 Outcome: 1 |
| Fujihara et al. 2019 [25] | Japan | Prospective cohort study | Patients with stable CAD | 256 | 9.0% | 67.0 (60.0 –74.0) | Continuous | 1.60 (1.45–1.73) | 38 | MACE (33), Cardiac death (2), HF requiring hospitalization (9), MI (1), ischemia-driven revascularization (13), Stroke (30), PAD requiring endovascular treatment (1), aortic aneurysms requiring surgical treatment (3) | Smoking, TG, Lp (a), HbA1c, and ApoB. | Selection: 4 Comparability: 1 Outcome: 2 |
| Liu et al. 2020 [26] | China | Retrospective cohort study | Patients with stable CAD | 6839 | 27.6% | 58.10 ± 10.70 | RLP-C > 50th vs. RLP-C ≤ 50th, Continuous | 2.44 ± 0.92 | 54.9 | MACE (462), Cardiac death (197), MI (94), Stroke (171) | Age, sex, smoking status, prior MI, HTN, DM, LVEF, TG, LDL-C, HDL-C creatinine, statin use and types at admission, and statintypes on discharge. |
Selection: 4 Comparability: 2 Outcome: 2 |
Abbreviation: RLP-C, remnant-like particle cholesterol; MACEs, major cardiovascular adverse events; CAD, coronary artery disease, ACS, acute coronary syndrome; PCI, percutaneous coronary intervention; BMI, body mass index; MI, myocardial infarction; STEMI, ST-segment elevated myocardial infarction; CKD, chronic kidney disease; hs-CRP, high-sensitivity C-reactive protein; GRACE, Global Registry of Acute Coronary events; DM, diabetes mellitus; HTN, hypertension; TC, total cholesterol; TG, triglyceride; HDL-C, low-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; eGFR, estimated glomerular filtration rate; Apo A-I, BNP, B-type natriuretic peptide; apolipoprotein A-I; Apo B, apolipoprotein B; Lp (a), lipoprotein a; CABG, coronary artery bypass graft; FBG, fasting blood glucose; LVEF, left ventricular ejection fraction; CTO, chronic total occlusion; UA, uric acid; ALT, alanine aminotransferase; Cre, creatinine; CT, computed tomography; DM, diabetes mellitus; HTN, hypertension; CVD, cardiovascular disease; TC, total.cholesterol.
3.2. Meta-Analysis Results
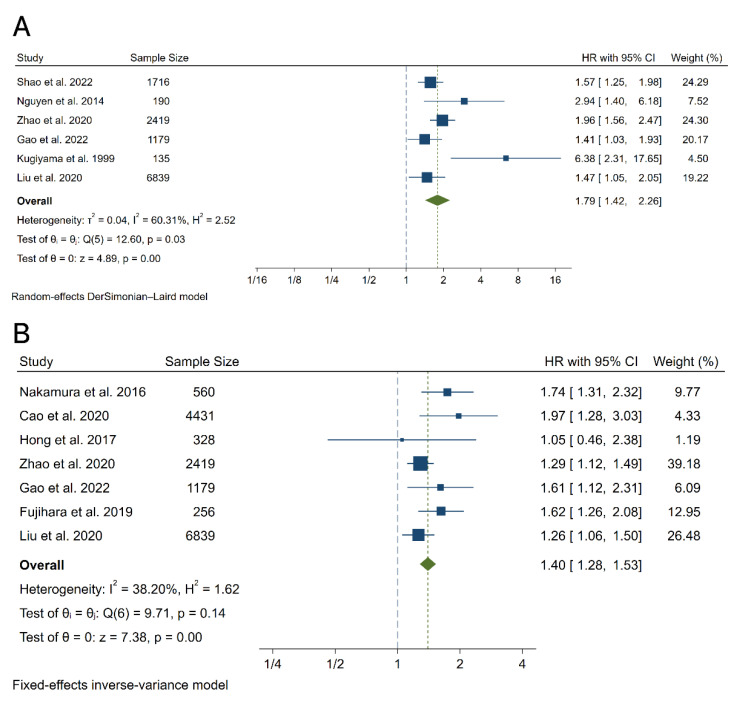
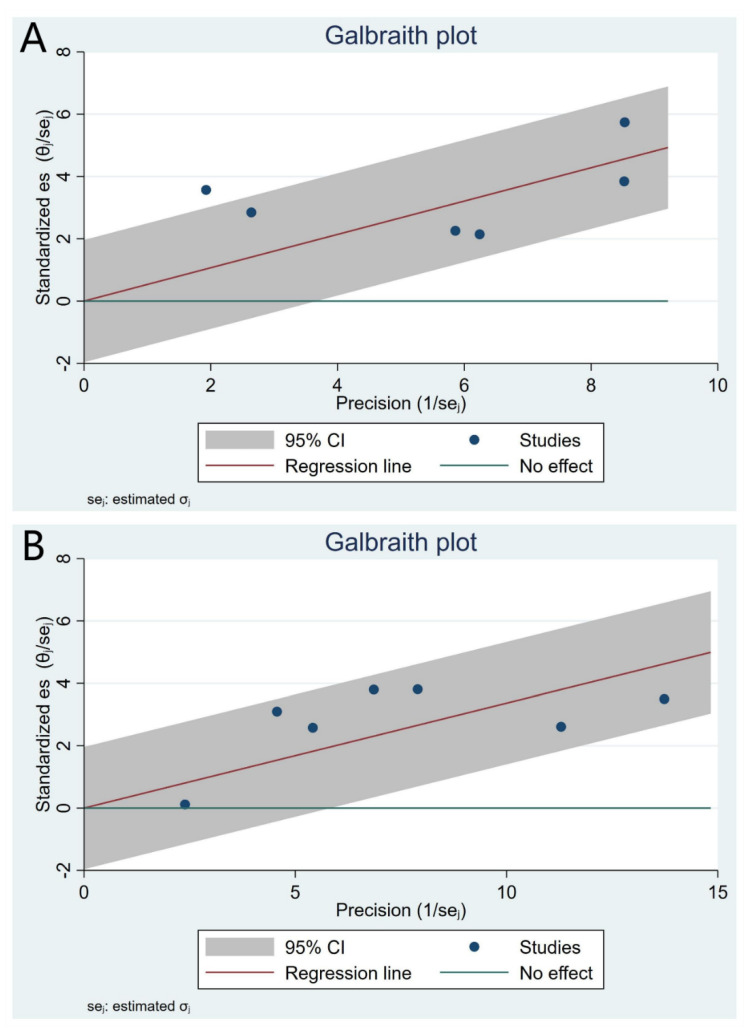
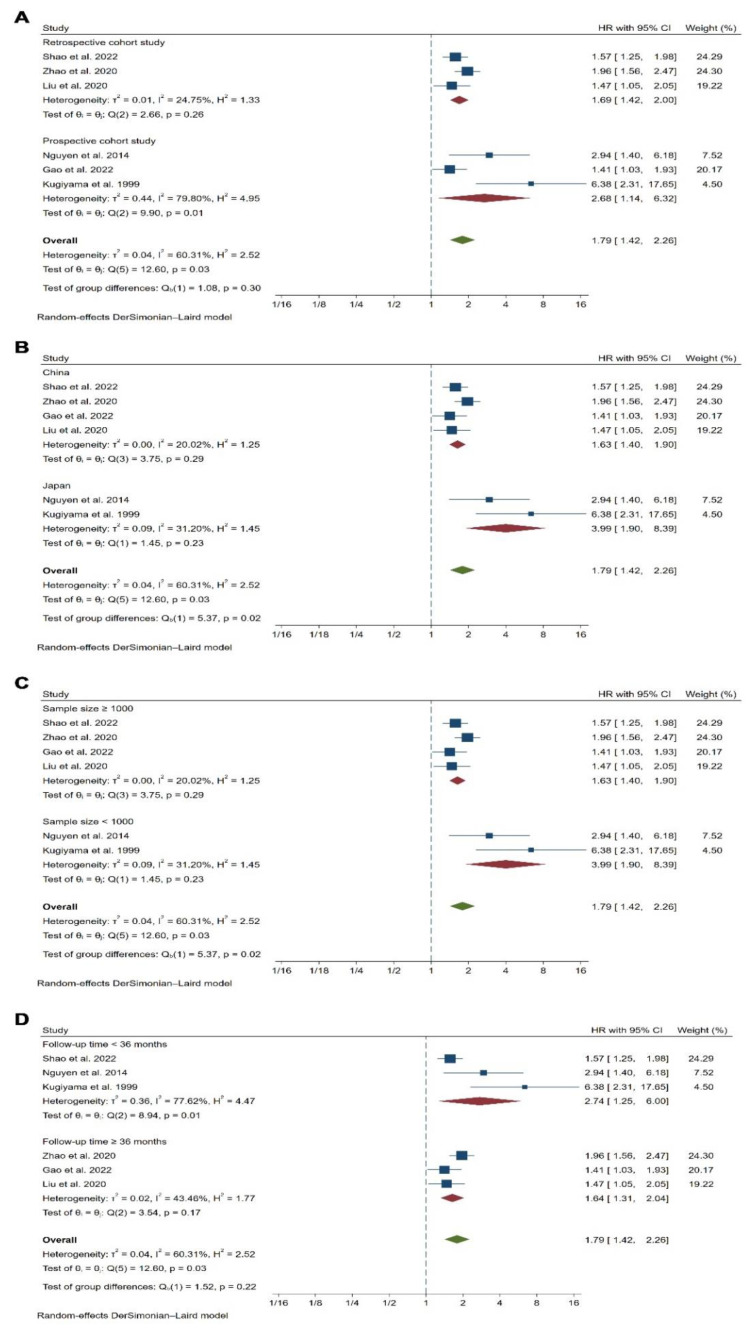
In our pooled analysis, compared to CAD patients with the lowest RLP-C category, the CAD patients with the highest RLP-C category had a significantly higher risk of future MACEs during follow-up (HR 1.79, 95% CI, 1.42–2.26, p < 0.01) with moderate heterogeneity between studies (I2 = 60.31%, Q = 12.60, p = 0.03) (Figure 2A and Figure 3A), which was consistent with outcomes of meta-analysis with the RLP-C analyzed as a continuous variable (HR 1.40, 95% CI, 1.28–1.53, p < 0.01, I2 = 38.20%, Q = 9.71, p = 0.14) (Figure 2B and Figure 3B). To explore the potential source of heterogeneity of the meta-analysis outcomes with the RLP-C analyzed as a categorical variable, we conducted subgroup analysis according to study design, country of origin, sample size, and follow-up time. The results showed that the sample size and the country of origin might contribute to the heterogeneity (Figure 4). Moreover, we conducted sensitivity analysis by excluding one study at a time, and the result indicated that no specific study affected the primary outcomes significantly (Supplementary Figure S1). The funnel plots were almost symmetric visually, indicating a possible low risk of publication bias (Supplementary Figure S2). Moreover, the Begg’s test and the Egger’s test were unable to be conducted because the available studies were not more than ten.
Figure 2.
Forest plots for the meta-analysis of the association between the RLP-C and the risk of MACEs. (A) Meta-analysis with the RLP-C analyzed as a categorical variable. (B) Meta-analysis with the RLP-C analyzed as a continuous variable. References involved in the study: [14,15,17,20,22,23,24,25,26].
Figure 3.
Galbraith plots for heterogeneity test of the meta-analysis. (A) The RLP-C analyzed as a categorical variable. (B) The RLP-C analyzed as a continuous variable.
Figure 4.
Subgroup analyses for the association between the RLP-C analyzed as a categorical variable and the risk of MACEs. (A) Subgroup analysis according to the study design. (B) Subgroup analysis according to the country of origin. (C) Subgroup analysis according to the sample size. (D) Subgroup analysis according to the follow-up time. References involved in the study: [14,15,20, 22,24,26].
4. Discussion
The present meta-analysis supplied a systematic review of published articles and a quantitative estimation of the association between the RLP-C and the risk of MACEs in patients with CAD. The results suggested that the CAD patients with the higher baseline RLP-C level had a significantly higher risk of future MACEs during follow-up regardless of whether the RLP-C was regarded as a category variable or continuous variable, with low risk of publication bias. Several studies were used twice because they reported results regarding RLP-C as categorical variable and continuous variable, respectively. The outcomes of subgroup analysis indicated that the moderate heterogeneity might result from different sample size and country of origin. Moreover, the result of sensitivity analysis suggested the stability of our primary outcomes. In view of the results above, the RLP-C may be the next therapeutic target for patients with a considerable cardiovascular residual risk.
To our knowledge, the present study is the first meta-analysis estimating the association between the baseline RLP-C and future MACEs in patients diagnosed with CAD at baseline. As we all know, the LDL-C-lowering therapy is the primary treatment for patients with CAD. Nevertheless, some patients are still under considerable cardiovascular residual risk after effective LDL-C-lowering therapy. In that case, the TRLs may play an important role in the cardiovascular residual risk, especially for patients with metabolic disorders, such as diabetes mellitus, metabolic syndrome, and insulin resistance [13,27]. In recent years, the RLP-C, as the cholesterol content of a subset of the TRLs, has been emphasized in the primary prevention and secondary prevention of CAD. Quispe et al. [28] included 17,532 ASCVD-free subjects at baseline from three prospective cohorts. The result of pooled analysis showed that log RLP-C correlated with increasing ASCVD risk (HR, 1.65, 95% CI, 1.45–1.89) after median follow-up of 18.7 years. Shao et al. [15] consecutively included 1716 ACS patients undergoing percutaneous coronary intervention. They defined the primary endpoint as MACEs, including all-cause mortality, non-fatal stroke, non-fatal myocardial infarction, and unplanned repeat revascularization, and the median follow-up time was 2.5 years. The outcomes suggested that baseline RLP-C > 75th was associated with the higher risk of long-term MACEs (HR, 1.572, 95%CI, 1.25–1.98, p < 0.001). Fujihara and his colleagues [25] consecutively enrolled 247 individuals with stable CAD and LDL-C levels lower than 70 mg/dL on statin treatment. During a mean follow-up time of 3.2 years, 33 MACEs occurred. The outcomes of multivariate Cox regression analysis indicated that RLP-C was associated with increasing risk of adverse cardiovascular events in patients with stable CAD (HR1.62, 95% CI, 1.26–2.07, p < 0.01).
The potential mechanism of the correlation between elevated RLP-C and the risk of MACEs in patients with CAD may include the following aspects. First, the RLP-C can invade the arterial intima and can be absorbed directly by macrophages, promoting the formation of foam cells and accelerating the progression of atherosclerosis, while the LDL can only be taken up by macrophages after being oxidative modified [27,29]. Second, the RLP-C is closely related to low-grade inflammation, with a 1-mmol/L increasing remnant cholesterol associated causally with a 28% increasing C-reactive protein [30]. Third, previous studies suggested the RLP-C was associated with endothelial dysfunction by impairing acetylcholine-induced vasodilatation and flow-mediated endothelium-dependent dilatation [31]. Fourth, the RLP-C promote the expression of plasminogen activator inhibitor-1 and induce platelet aggregation, increasing the risk of thrombus [32,33]. The atherogenic, pro-inflammatory and thrombogenic role of the RLP-C may be the underlying mechanism of the correlation between the RLP-C and cardiovascular events.
Under the condition that LDL-C lowering therapy was widely used, the inflammation and other lipid indicators, including non-HDL-C, TG, LP (a), RLP-C and Apo B, may play important roles in the residual cardiovascular risk. The cholesterol component of TRLs is regarded as the causal culprit instead of TG, because Chylomicron is unable to enter the endarterium and TG can be disintegrated by most cells [34]. Moreover, Quispe et al. [28] demonstrated that the RLP-C was associated with residual cardiovascular risk beyond Apo B, LDL-C, and non-HDL-C. Therefore, the residual inflammation risk and residual lipid (RLP-C and LP (a)) risk should be emphasized. It is the time to carried out randomized controlled trials to explore whether reducing the RLP-C level is conducive to reducing residual cardiovascular risk, even coronary plaque regression.
The study has some limitations. First, all studies included in the meta-analysis were from Asia, making it difficult to extrapolate the results to other races. Second, the type of CAD, the definitions of MACEs, follow-up time, and confounding factors adjusted were different across the studies, which may lead to potential heterogeneity. Third, as one of the sources of residual cardiovascular risk, LP(a) was adjusted as a covariate in only two studies, which may obscure the true effect of RLP-C. Fourth, the available studies were limited, and the sample sizes of studies varied greatly. The Begg’s test and the Egger’s test were unable to be conducted because the available studies were not more than ten. The results of subgroup analysis indicated that the sample size might contribute to the moderate heterogeneity. Finally, it remains unclear whether the association between the RLP-C and the risk of MACEs is linear or non-linear.
5. Conclusions
The present meta-analysis suggests that the RLP-C was associated with an increased risk of long-term MACEs in patients with CAD at baseline. It is necessary to conduct randomized controlled trials to explore whether reducing the RLP-C level is conducive to reducing residual cardiovascular risk, even coronary plaque regression.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/jcdd9120452/s1, Item S1: Search Strategy; Supplementary Figure S1. Sensitivity analysis for leave one study out. A The RLP-C analyzed as a categorical variable. B The RLP-C analyzed as a continuous variable; Supplementary Figure S2. Funnel plots for publication bias test of the meta-analysis. A The RLP-C analyzed as a categorical variable. B The RLP-C analyzed as a continuous variable.
Author Contributions
J.Y. and Y.W. conceived the study aims and design, contributed to the systematic review and data extraction, performed the analysis, interpreted the results, and drafted the manuscript. Z.X., Y.M. and C.S. contributed to the data extraction, interpretation of results, W.W. and Y.-D.T. contributed to the study design, and the revision of the manuscript. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All data and materials used in this research are freely available in electronic databases (PubMed: Embase). References have been provided.
Conflicts of Interest
The authors declare no conflict of interest.
Ethics Declarations
Ethics approval and consent to participate. Ethical approval was not applicable for this systematic review and meta-analysis.
Funding Statement
Yi-Da Tang provided financial support in the form of (National Key R&D Program of China (2020YFC2004705), Research Unit of Medical Science Research Management/Basic and Clinical Research of Metabolic Cardiovascular Diseases from Chinese Academy of Medical Sciences (2021RU003), National Natural Science Foundation of China (81825003, 91957123), Beijing Nova Program from Beijing Municipal Science & Technology Commission (Z201100006820002), and Science and Technology Project of Xicheng District Finance (XCSTS-SD2021-01)) funding. The sponsor had no role in the design or conduct of this research.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Sacco R.L., Roth G.A., Reddy K.S., Arnett D.K., Bonita R., Gaziano T.A., Heidenreich P.A., Huffman M.D., Mayosi B.M., Mendis S., et al. The Heart of 25 by 25: Achieving the Goal of Reducing Global and Regional Premature Deaths From Cardiovascular Diseases and Stroke: A Modeling Study From the American Heart Association and World Heart Federation. Glob. Heart. 2016;11:251–264. doi: 10.1016/j.gheart.2016.04.002. [DOI] [PubMed] [Google Scholar]
- 2.Duggan J.P., Peters A.S., Trachiotis G.D., Antevil J.L. Epidemiology of Coronary Artery Disease. Surg. Clin. North Am. 2022;102:499–516. doi: 10.1016/j.suc.2022.01.007. [DOI] [PubMed] [Google Scholar]
- 3.Libby P., Buring J.E., Badimon L., Hansson G.K., Deanfield J., Bittencourt M.S., Tokgözoğlu L., Lewis E.F. Atherosclerosis. Nat. Rev. Dis. Primers. 2019;5:56. doi: 10.1038/s41572-019-0106-z. [DOI] [PubMed] [Google Scholar]
- 4.Bentzon J.F., Otsuka F., Virmani R., Falk E. Mechanisms of plaque formation and rupture. Circ. Res. 2014;114:1852–1866. doi: 10.1161/CIRCRESAHA.114.302721. [DOI] [PubMed] [Google Scholar]
- 5.Mach F., Baigent C., Catapano A.L., Koskinas K.C., Casula M., Badimon L., Chapman M.J., de Backer G.G., Delgado V., Ference B.A., et al. 2019 ESC/EAS guidelines for the management of dyslipidaemias: Lipid modification to reduce cardiovascular risk: The Task Force for the management of dyslipidaemias of the European Society of Cardiology (ESC) and European Atherosclerosis Society (EAS) Atherosclerosis. 2019;290:140–205. doi: 10.1016/j.atherosclerosis.2019.08.014. [DOI] [PubMed] [Google Scholar]
- 6.Navarese E.P., Robinson J.G., Kowalewski M., Kolodziejczak M., Andreotti F., Bliden K., Tantry U., Kubica J., Raggi P., Gurbel P.A. Association between Baseline LDL-C Level and Total and Cardiovascular Mortality After LDL-C Lowering: A Systematic Review and Meta-analysis. JAMA. 2018;319:1566–1579. doi: 10.1001/jama.2018.2525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fitzgerald G., Kiernan T. PCSK9 inhibitors and LDL reduction: Pharmacology, clinical implications, and future perspectives. Expert Rev. Cardiovasc. Ther. 2018;16:567–578. doi: 10.1080/14779072.2018.1497975. [DOI] [PubMed] [Google Scholar]
- 8.Grundy S.M., Stone N.J., Bailey A.L., Beam C., Birtcher K.K., Blumenthal R.S., Braun L.T., de Ferranti S., Faiella-Tommasino J., Forman D.E., et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA Guideline on the Management of Blood Cholesterol: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J. Am. Coll. Cardiol. 2019;73:e285–e350. doi: 10.1016/j.jacc.2018.11.003. [DOI] [PubMed] [Google Scholar]
- 9.Gudbjartsson D.F., Thorgeirsson G., Sulem P., Helgadottir A., Gylfason A., Saemundsdottir J., Bjornsson E., Norddahl G.L., Jonasdottir A., Jonasdottir A., et al. Lipoprotein(a) Concentration and Risks of Cardiovascular Disease and Diabetes. J. Am. Coll. Cardiol. 2019;74:2982–2994. doi: 10.1016/j.jacc.2019.10.019. [DOI] [PubMed] [Google Scholar]
- 10.Carr S.S., Hooper A.J., Sullivan D.R., Burnett J.R. Non-HDL-cholesterol and apolipoprotein B compared with LDL-cholesterol in atherosclerotic cardiovascular disease risk assessment. Pathology. 2019;51:148–154. doi: 10.1016/j.pathol.2018.11.006. [DOI] [PubMed] [Google Scholar]
- 11.Varbo A., Benn M., Nordestgaard B.G. Remnant cholesterol as a cause of ischemic heart disease: Evidence, definition, measurement, atherogenicity, high risk patients, and present and future treatment. Pharmacol. Ther. 2014;141:358–367. doi: 10.1016/j.pharmthera.2013.11.008. [DOI] [PubMed] [Google Scholar]
- 12.Ginsberg H.N., Packard C.J., Chapman M.J., Borén J., Aguilar-Salinas C.A., Averna M., Ference B.A., Gaudet D., Hegele R.A., Kersten S., et al. Triglyceride-rich lipoproteins and their remnants: Metabolic insights, role in atherosclerotic cardiovascular disease, and emerging therapeutic strategies-a consensus statement from the European Atherosclerosis Society. Eur. Heart J. 2021;42:4791–4806. doi: 10.1093/eurheartj/ehab551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chait A., Ginsberg H.N., Vaisar T., Heinecke J.W., Goldberg I.J., Bornfeldt K.E. Remnants of the Triglyceride-Rich Lipoproteins, Diabetes, and Cardiovascular Disease. Diabetes. 2020;69:508–516. doi: 10.2337/dbi19-0007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhao Q., Zhang T.Y., Cheng Y.J., Ma Y., Xu Y.K., Yang J.Q., Zhou Y.J. Prognostic impact of estimated remnant-like particle cholesterol in patients with differing glycometabolic status: An observational cohort study from China. Lipids Health Dis. 2020;19:179. doi: 10.1186/s12944-020-01355-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shao Q., Yang Z., Wang Y., Li Q., Han K., Liang J., Shen H., Liu X., Zhou Y., Ma X., et al. Elevated Remnant Cholesterol is Associated with Adverse Cardiovascular Outcomes in Patients with Acute Coronary Syndrome. J. Atheroscler. Thromb. 2022;29:63397. doi: 10.5551/jat.63397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Castañer O., Pintó X., Subirana I., Amor A.J., Ros E., Hernáez Á., Martínez-González M., Corella D., Salas-Salvadó J., Estruch R., et al. Remnant Cholesterol, Not LDL Cholesterol, Is Associated with Incident Cardiovascular Disease. J. Am. Coll. Cardiol. 2020;76:2712–2724. doi: 10.1016/j.jacc.2020.10.008. [DOI] [PubMed] [Google Scholar]
- 17.Hong L.F., Yan X.N., Lu Z.H., Fan Y., Ye F., Wu Q., Luo S.H., Yang B., Li J.J. Predictive value of non-fasting remnant cholesterol for short-term outcome of diabetics with new-onset stable coronary artery disease. Lipids Health Dis. 2017;16:7. doi: 10.1186/s12944-017-0410-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Martin S.S., Faridi K.F., Joshi P.H., Blaha M.J., Kulkarni K.R., Khokhar A.A., Maddox T.M., Havranek E.P., Toth P.P., Tang F., et al. Remnant Lipoprotein Cholesterol and Mortality After Acute Myocardial Infarction: Further Evidence for a Hypercholesterolemia Paradox From the TRIUMPH Registry. Clin. Cardiol. 2015;38:660–667. doi: 10.1002/clc.22470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur. J. Epidemiol. 2010;25:603–605. doi: 10.1007/s10654-010-9491-z. [DOI] [PubMed] [Google Scholar]
- 20.Nguyen S.V., Nakamura T., Kugiyama K. High remnant lipoprotein predicts recurrent cardiovascular events on statin treatment after acute coronary syndrome. Circ. J. 2014;78:2492–2500. doi: 10.1253/circj.CJ-14-0380. [DOI] [PubMed] [Google Scholar]
- 21.Nakamura T., Obata J.E., Hirano M., Kitta Y., Fujioka D., Saito Y., Kawabata K., Watanabe K., Watanabe Y., Mishina H., et al. Predictive value of remnant lipoprotein for cardiovascular events in patients with coronary artery disease after achievement of LDL-cholesterol goals. Atherosclerosis. 2011;218:163–167. doi: 10.1016/j.atherosclerosis.2011.04.040. [DOI] [PubMed] [Google Scholar]
- 22.Cao Y.X., Zhang H.W., Jin J.L., Liu H.H., Zhang Y., Gao Y., Guo Y.L., Wu N.Q., Hua Q., Li Y.F. The longitudinal association of remnant cholesterol with cardiovascular outcomes in patients with diabetes and pre-diabetes. Cardiovasc. Diabetol. 2020;19:104. doi: 10.1186/s12933-020-01076-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gao S., Xu H., Ma W., Yuan J., Yu M. Remnant Cholesterol Predicts Risk of Cardiovascular Events in Patients with Myocardial Infarction with Nonobstructive Coronary Arteries. J. Am. Heart Assoc. 2022;11:e024366. doi: 10.1161/JAHA.121.024366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kugiyama K., Doi H., Takazoe K., Kawano H., Soejima H., Mizuno Y., Tsunoda R., Sakamoto T., Nakano T., Nakajima K., et al. Remnant lipoprotein levels in fasting serum predict coronary events in patients with coronary artery disease. Circulation. 1999;99:2858–2860. doi: 10.1161/01.CIR.99.22.2858. [DOI] [PubMed] [Google Scholar]
- 25.Fujihara Y., Nakamura T., Horikoshi T., Obata J.E., Fujioka D., Watanabe Y., Watanabe K., Kugiyama K. Remnant Lipoproteins Are Residual Risk Factor for Future Cardiovascular Events in Patients with Stable Coronary Artery Disease and On-Statin Low-Density Lipoprotein Cholesterol Levels <70 mg/dL. Circ. J. 2019;83:1302–1308. doi: 10.1253/circj.CJ-19-0047. [DOI] [PubMed] [Google Scholar]
- 26.Liu H.H., Guo Y.L., Zhu C.G., Wu N.Q., Gao Y., Xu R.X., Dong Q., Qian J., Dou K.F., Li J.J., et al. Synergistic effect of the commonest residual risk factors, remnant cholesterol, lipoprotein(a), and inflammation, on prognosis of statin-treated patients with chronic coronary syndrome. J. Transl. Med. 2022;20:243. doi: 10.1186/s12967-022-03448-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nordestgaard B.G. Triglyceride-Rich Lipoproteins and Atherosclerotic Cardiovascular Disease: New Insights From Epidemiology, Genetics, and Biology. Circ. Res. 2016;118:547–563. doi: 10.1161/CIRCRESAHA.115.306249. [DOI] [PubMed] [Google Scholar]
- 28.Quisp R., Martin S.S., Michos E.D., Lamba I., Blumenthal R.S., Saeed A., Lima J., Puri R., Nomura S., Tsai M., et al. Remnant cholesterol predicts cardiovascular disease beyond LDL and ApoB: A primary prevention study. Eur. Heart J. 2021;42:4324–4332. doi: 10.1093/eurheartj/ehab432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Batt K.V., Avella M., Moore E.H., Jackson B., Suckling K.E., Botham K.M. Differential effects of low-density lipoprotein and chylomicron remnants on lipid accumulation in human macrophages. Exp. Biol. Med. 2004;229:528–537. doi: 10.1177/153537020422900611. [DOI] [PubMed] [Google Scholar]
- 30.Varbo A., Benn M., Tybjærg-Hansen A., Nordestgaard B.G. Elevated remnant cholesterol causes both low-grade inflammation and ischemic heart disease, whereas elevated low-density lipoprotein cholesterol causes ischemic heart disease without inflammation. Circulation. 2013;128:1298–1309. doi: 10.1161/CIRCULATIONAHA.113.003008. [DOI] [PubMed] [Google Scholar]
- 31.Zheng X.Y., Liu L. Remnant-like lipoprotein particles impair endothelial function: Direct and indirect effects on nitric oxide synthase. J. Lipid Res. 2007;48:1673–1680. doi: 10.1194/jlr.R700001-JLR200. [DOI] [PubMed] [Google Scholar]
- 32.Sawka A.M., Singh R.J., Hiddinga H.J., McConnell J.P., Eberhardt N.L., Caplice N.M., O’Brien T. Remnant lipoproteins induce endothelial plasminogen activator inhibitor-1. Biochem. Biophys. Res. Commun. 2001;285:15–19. doi: 10.1006/bbrc.2001.5117. [DOI] [PubMed] [Google Scholar]
- 33.Saniabadi A.R., Umemura K., Shimoyama M., Adachi M., Nakano M., Nakashima M. Aggregation of human blood platelets by remnant like lipoprotein particles of plasma chylomicrons and very low density lipoproteins. Thromb. Haemost. 1997;77:996–1001. doi: 10.1055/s-0038-1656092. [DOI] [PubMed] [Google Scholar]
- 34.Nordestgaard B.G., Varbo A. Triglycerides and cardiovascular disease. Lancet. 2014;384:626–635. doi: 10.1016/S0140-6736(14)61177-6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data and materials used in this research are freely available in electronic databases (PubMed: Embase). References have been provided.