Abstract
Intracellular flow cytometry analysis of perforin production by CD8+ T cells showed levels were greatly reduced in tuberculosis (TB) patients compared to healthy controls. Reduced cytotoxic-T-lymphocyte activity was also obtained with CD8+ T cells from TB patients compared to healthy controls in The Gambia. A change in antigen recognition was noted between the two groups of donors: in addition to recognition of Ag85A and Ag85B, as seen in healthy donors, a prominent ESAT-6 response was found in TB patients.
Tuberculosis (TB) remains a major public health problem worldwide for which no effective vaccine is available (18). Estimates show that up to one-third of the world's population is infected with Mycobacterium tuberculosis, the etiological agent of human TB, yet only ca. 10% of those infected ever develop disease. TB still accounts for approximately 12 million new cases of active tuberculosis and 3 million deaths annually worldwide, making TB one of the leading causes of death due to a single infectious agent (7).
Cell-mediated immunity is the major protective immune response against intracellular bacteria such as M. tuberculosis. The interaction between gamma interferon-secreting CD4+ T cells and infected macrophages has been well characterized in humans and animal models and is known to be essential in the protective response against TB (1, 12, 17).
More recently, evidence from the murine model of TB has linked major histocompatibility class I (MHC-I) restricted CD8+ T cells with protective immunity against M. tuberculosis infection. β2-Microglobulin gene knockout mice, which lack MHC-I and functional CD8+ T cells, have been shown to be more susceptible to M. bovis BCG and M. tuberculosis infection (8, 12). CD8+ T cells are capable of producing macrophage-activating cytokines and display cytolytic activity against infected macrophages (2, 15, 21–23). However, their exact role in the protective immune response to human TB remains to be determined.
In both murine in vivo and human in vitro studies, strong CTL activity has been observed against macrophages infected or pulsed with M. bovis BCG, M. tuberculosis, purified protein derivative, culture filtrate proteins, or the secreted antigens Ag85A, Ag85B, 19-kDa, 38-kDa, and ESAT-6 (4–6, 13, 16, 21, 25–26). However, in the murine model, perforin, granzyme, and Fas pathway knockout mice show little change in at least the early course of infection with M. tuberculosis in the lung (3, 14). This suggests that cell-mediated cytotoxicity (cytotoxic-T-lymphocyte [CTL]) activity may not be the most important function of these cells in the immune response against mycobacteria. The study of immune responses to M. tuberculosis in healthy donors and in those with active disease will be essential to determine the correlates of protective immunity and to allow the design of an effective vaccine against the disease.
We present here a comparative study of the CD8+ T-cell responses in healthy BCG-vaccinated donors and in untreated pulmonary TB patients. By stimulating CD8+ T cells with either M. bovis BCG or M. tuberculosis H37Ra, we studied perforin production and CTL activity of these cells and investigated the antigen recognition patterns in these two groups. Compared to healthy donors, TB patients showed a marked reduction in perforin production, which correlated with a reduction in CTL activity. Strong recognition of Ag85A and Ag85B by CD8+ T cells was observed in both TB patients and healthy donors. In addition, we observed a relatively strong CTL response against ESAT-6 in TB patients, which was virtually absent in healthy BCG-vaccinated donors.
Ten pulmonary TB patients (10 males; mean age, 25 ± 6 years) who attended the Medical Research Council (MRC) TB Clinic, Fajara, The Gambia, and 10 healthy M. bovis BCG-vaccinated Gambian donors, with BCG scars, who had been vaccinated in childhood (10 males; mean age, 28 ± 4 years) were studied for their CD8+ CTL response to mycobacteria.
All samples were collected and tested over a 6-week period. TB patient blood samples were obtained with informed consent prior to the start of chemotherapy. Pulmonary TB was confirmed by sputum smear and chest X-ray examinations. All patients were seronegative for human immunodeficiency virus. Most patients studied had extensive pulmonary involvement: eight had pulmonary cavities; two had miliary disease.
Informed consent was obtained from all patients and control donors, and this study was approved by the MRC Scientific Coordinating Committee, The Gambia, The Gambian Government-MRC Ethics Committee, and the London School of Hygiene & Tropical Medicine Ethics Committee.
Recombinant vaccinia viruses (rVV) expressing the mycobacterial proteins ESAT-6, Ag85A, and Ag85B were constructed as previously described (10). rVV were cultivated by growth in the thymidine kinase-disrupted osteosarcoma cell line 143 (TK−143). Viruses were harvested and purified by sucrose cushion density centrifugation prior to aliquoting at −70°C. On the day of use aliquots were thawed and sonicated before use in assays.
Stocks of M. bovis BCG (Glaxo-Evans strain) and M. tuberculosis H37Ra were generated by culture of bacilli in complete Middlebrook 7H9 medium containing glycerol and Middlebrook ADC enrichment (Difco, Detroit, Mich.). Aliquots of bacteria were frozen and stored at −70°C prior to use.
Peripheral blood mononuclear cells (PBMC) were isolated from heparanized venous blood by density gradient centrifugation over Lymphoprep (Nycomed Pharma, Oslo, Norway). Freshly isolated PBMC were washed twice in RPMI 1640 and resuspended in culture medium. Two million PBMC were cultured per well in 24-well tissue culture plates (Nunclon, Roskilde, Denmark) with either no antigen, live M. bovis BCG (1 bacillus/macrophage), or live M. tuberculosis H37Ra (1 bacillus/macrophage). The culture medium consisted of RPMI 1640 supplemented with 10% autologus plasma, 2 mM l-glutamine (Gibco-BRL, Paisley, United Kingdom), and 50 μg of ampicillin (Sigma) per ml. After 7 days of culture at 37°C in 5% CO2, the cells were harvested and tested.
Purified CD8+ T cells were obtained by positive enrichment using the magnetic cell sorting (MACS) system (Miltenyi Biotec, Bergisch-Gladbach, Germany). Briefly, 7-day-cultured PBMC were labeled with anti-CD8 microbeads (20 μl/107 cells) in incubation buffer (phosphate-buffered saline [PBS]–0.5% bovine serum albumin [BSA]–2 mM EDTA; 80 μl/107 cells) for 15 min at 4°C. After one washing step, the cells were resuspended in degassed incubation buffer, and enrichment was performed using LS+ columns and the MidiMACS magnet according to the manufacturers' instructions. The resulting CD8+ T-cell population was ≥95% pure, as determined by flow cytometry for the surface markers αβ T-cell receptor, CD3, and CD8 and showed <5% contamination with CD4+ T cells and CD56+ natural killer cells.
Intracellular perforin production by CD8+ T cells was assessed by two-color flow cytometry. Perforin secretion was blocked by incubation with 3 μg of brefeldin A (Sigma) per ml for 16 h at 37°C. Cells were washed twice in cold PBS and stained for surface CD8 with fluorescein isothiocyanate-conjugated antibody (Becton Dickinson, Oxford, United Kingdom) at 4°C for 30 min in incubation buffer (PBS–0.5% BSA–0.1% sodium azide); phycoerythrin (PE)-conjugated CD25 antibody was used to stain cells for surface CD25 expression (Becton Dickinson). Cells were washed twice in cold incubation buffer before fixation in 4% paraformaldehyde at 4°C overnight. Fixation was followed by incubation for 30 min in FACS Cytofix/Cytoperm solution (PharMingen, San Diego, Calif.). Cells were washed in 1× PermWash solution (PharMingen). Staining for intracellular perforin was performed in 1× PermWash with containing PE-conjugated monoclonal antibody (MAb) against perforin (PharMingen) for 30 min at 4°C. Cells were then washed in PBS and analyzed using CellQuest software on a FACSCalibur apparatus (Becton Dickinson), and 30,000 to 50,000 events were acquired for each sample.
Autologous monocytes were isolated by their adherence to plastic. PBMC were resuspended in RPMI 1640 alone. Aliquots (200 μl) of the cell suspension were added to each well of a 96-well round-bottomed tissue culture plate (Nunclon). Cells were incubated at 37°C in 5% CO2 for 1 to 2 h. Nonadherent cells were then removed, leaving only adherent monocytes which were cultured antigen free in culture medium containing 10% autologous plasma for 6 days to give monocyte-derived macrophages. On day 6 the monocyte-derived macrophages were infected with M. bovis BCG (multiplicity of infection [MOI] 5:1); M. tuberculosis (MOI 5:1); rVV expressing mycobacterial protein Ag85A, Ag85B, or ESAT-6 (MOI 10:1); a control rVV expressing no recombinant protein; or no antigen. Simultaneously, cells were loaded with 2 μCi of Na251Cr per well and incubated overnight at 37°C in 5% CO2. Target cells were then washed three times with warm RPMI and either treated with 5 μg of anti-HLA-A, -B, and -C MAb w6/32 or immunoglobulin G2a isotype control MAb (Sigma) for 1 h or resuspended in culture medium alone before being used in the cytotoxicity assay. Purified CD8+ T cells in a volume of 100 μl were then added to the washed target cells to give an effector/target (E/T) ratio of 30:1 (found to be optimal in previous experiments) and incubated at 37°C in 5% CO2 for 6 h. Supernatants (25 μl) were harvested and mixed with 125 μl of OptiPhase SuperMix scintillant (Wallac Scintilation Products, Fisher Chemicals, Loughborough, United Kingdom) before being counted on a 1450 MicroBeta TriLux liquid scintillation counter (Wallac, Turku, Finland). Spontaneous release was measured in wells containing target cells alone and was found to be <10% whether the target cells were uninfected or infected. Maximum release was calculated by lysing uninfected target cells with 5% sodium dodecyl sulfate (Sigma). The percent specific lysis was calculated as follows: [(cpmsupernatant − cpmspontaneous)/(cpmmaximum − cpmspontaneous)] × 100%.
To determine the cytolytic potential of the CD8+ T cells, intracellular staining for perforin production was performed. PBMC from healthy BCG-vaccinated donors and pulmonary TB patients were cultured as described above in the presence of no antigen, M. bovis BCG, or M. tuberculosis H37Ra. After 7 days, the cells were harvested, stained for the intracellular production of perforin, and analyzed by flow cytometry (FACS).
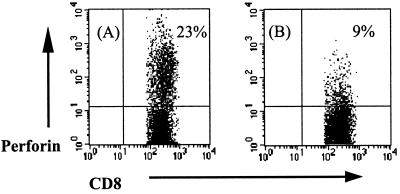
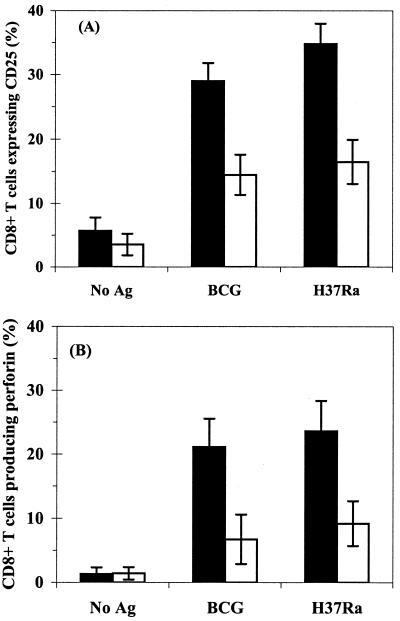
As shown in Fig. 1 and 2, stimulated CD8+ T cells from healthy donors upregulate CD25 expression (30% expression) and perforin production in response to mycobacteria (21% positive when stimulated with M. bovis BCG and 23% positive when stimulated with M. tuberculosis H37Ra). CD8+ T cells from pulmonary TB patients showed less activation with regards to CD25 expression; this correlated with reduced perforin production, with only 6 to 9% of cells staining positive for the cytolytic granule component, a reduction of >55% compared to the staining seen in CD8+ T cells from healthy donors (P < 0.005).
FIG. 1.
Flow cytometric detection of intracellular perforin by CD8+ T cells. PBMC from a healthy BCG vaccinee (A) and a pulmonary TB patient (B) were stimulated for 7 days in the presence of live M. tuberculosis. For the final 16 h of stimulation, brefeldin A was added to the cultures. Intracellular perforin staining was performed before FACS analysis. Cells were progressively gated by forward- and side-scatter for lymphocytes, and analysis was performed on the CD8+ high-staining lymphocytes. Results shown are for one BCG vaccinee and one TB patient who are representative of the 10 donors in each group tested.
FIG. 2.
CD8+ T-cell activation and perforin production in response to M. bovis BCG and M. tuberculosis H37Ra. PBMC from 10 healthy BCG-vaccinated donors (solid bars) and 10 pulmonary TB patients (open bars) were incubated with M. bovis BCG, M. tuberculosis H37Ra, or medium alone (No Ag) for 7 days. CD25 expression (A) and perforin production (B) by CD8+ T cells were measured by two-color flow cytometry. The results are presented as the mean percentage ± the standard deviation of CD8+ T cells staining positive for the marker.
To determine the antigen specificity of CD8+ T cells, highly purified CD8+ T cells were used in a CTL assay. PBMC from healthy BCG-vaccinated donors and pulmonary TB patients were cultured in the presence of either M. bovis BCG or M. tuberculosis H37Ra. After 7 days CD8+ T cells were purified by positive selection with anti-CD8-coated magnetic beads. The cytolytic activity of purified CD8+ T cells was tested against autologous monocyte-derived macrophages labeled with 51Cr and infected with M. bovis BCG; M. tuberculosis H37Ra; rVV expressing Ag85A, Ag85B, or ESAT-6; control rVV; or no antigen. No nonspecific effector activity was found as a result of the purification procedure, as demonstrated by the lack of lysis observed against uninfected and control rVV-infected macrophages; in addition, no significant lysis was observed against rVV ESAT-6-infected macrophages by BCG-stimulated effectors.
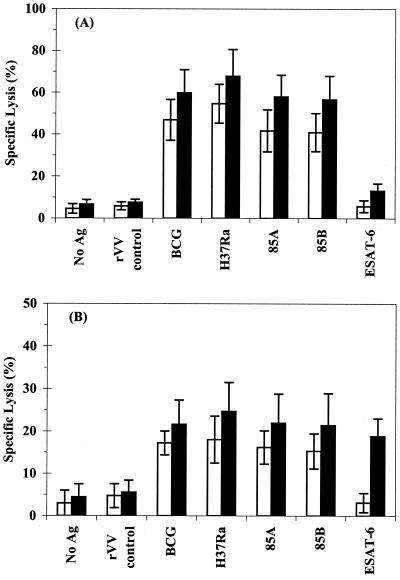
Strong CTL activity was observed by CD8+ T cells from healthy donors (mean lysis of targets infected with either M. bovis BCG or M. tuberculosis of 40 to 70%), with recognition of Ag85A and Ag85B but no significant recognition of ESAT-6 (Fig. 3A). Greatly reduced CTL activity was observed by CD8+ T cells from TB patients, with maximum levels of lysis ranging from 20 to 30% (P < 0.005). Despite the reduction in CTL activity, a clear change in antigen recognition was seen in TB patients (Fig. 3B). In addition to Ag85A and Ag85B, relatively strong recognition of ESAT-6 (∼20%) was also observed.
FIG. 3.
Antigen recognition by CD8+ T cells from BCG-vaccinated donors and TB patients. PBMC from 10 healthy donors (A) and 10 TB patients (B) were incubated in the presence of either M. bovis BCG (open bars) or M. tuberculosis H37Ra (solid bars) for 7 days. CD8+ T cells were purified by MACS. Autologus monocyte-derived macrophages were infected overnight with either M. bovis BCG, M. tuberculosis H37Ra, or rVV-expressing mycobacterial antigens. Effector cells were incubated with autologous, antigen-pulsed macrophages for 6 h at a 30:1 E/T ratio. CTL activity was measured as reflected by specific 51Cr release. The results are expressed as the mean percent specific lysis ± the standard deviation.
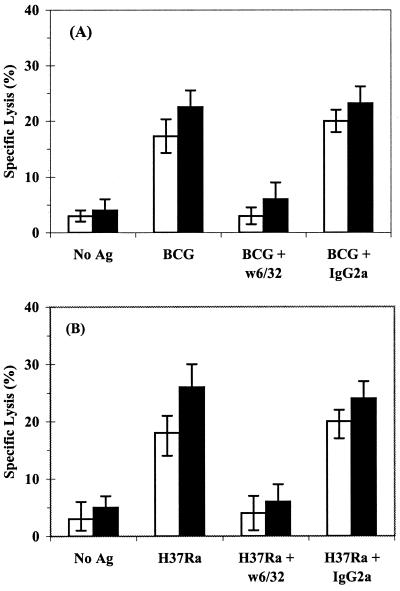
Blocking experiments were performed to confirm that the CD8+ CTL response was MHC-I restricted. Classical CD8+ T-cell CTL activity was observed with CD8+ T cells from TB patients, since pretreatment of infected macrophages with anti-HLA-A, -B, and -C MAb (w6/32) reduced the levels of lysis of both M. bovis BCG- and M. tuberculosis-infected macrophages by more than 75% (Fig. 4), whereas an isotype control had no significant effect.
FIG. 4.
MHC-I restriction of M. bovis BCG- and M. tuberculosis-specific CD8+ T cells from TB patients. 51Cr-labeled macrophages were infected with either M. bovis BCG (A) or M. tuberculosis H37Ra (B) and preincubated for 1 h with w6/32 (anti-HLA-A, -B, or -C) or immunoglobulin G2a MAb as an isotype control; uninfected macrophages were also included as target cells (No Ag). CD8+ T cells prestimulated with either M. bovis BCG (open bars) or M. tuberculosis H37Ra (solid bars) for 7 days were purified by MACS and added at a 30:1 E/T ratio for the cytotoxicity assay. The data represent the mean specific lysis ± the standard deviation of 10 TB patients.
Earlier studies have demonstrated the presence of human MHC-I-restricted CD8+ T cells specific for M. bovis BCG (21, 23) and M. tuberculosis (2, 15). Furthermore, these CD8+ T cells can be found both in the peripheral blood and in the alveolar tissue spaces of healthy BCG-vaccinated and tuberculin skin test-positive persons (2, 15, 21–23). The current study was designed to compare the CTL function and antigen specificity of CD8+ T cells found in the peripheral blood of healthy BCG-vaccinated donors with those of untreated pulmonary TB patients.
CD8+ T cells from both healthy controls and TB patients were readily activated by M. bovis BCG and M. tuberculosis bacilli. These activated CD8+ T cells were shown to possess cytolytic granules containing perforin. The number of perforin-producing CD8+ T cells and the levels of perforin produced by CD8+ T cells were both found to be greatly reduced in TB patients compared to healthy donors.
Highly purified CD8+ T-cell populations from both groups of donors exhibited CTL activity against both live bacilli and rVV-expressing mycobacterial secreted proteins. CD8+ T cells from pulmonary TB patients showed reduced CTL activity, and this corresponded with decreased perforin staining of activated CD8+ T cells. Healthy controls demonstrated strong recognition of whole M. bovis BCG and M. tuberculosis bacilli. In addition, in accordance with previous findings, Ag85A and Ag85B appear to be major antigenic targets for CD8+ T cells from healthy BCG-vaccinated donors (21). A strong CD8+ T-cell response to Ag85A and Ag85B was also seen in TB patients. Recognition of ESAT-6 was observed in TB patients only, with all 10 patients having a significant ESAT-6 response. A weak ESAT-6 response was observed in some healthy donors; this might be expected in an area where TB is endemic such as The Gambia; however, compared to the Ag85 response, the recognition was minimal. When the lysis of M. bovis BCG- and M. tuberculosis-infected target cells was taken as maximal reactivity, then the lysis in response to ESAT-6 was almost as strong as that to the live mycobacteria. The ESAT-6 response was minimal or absent in the M. bovis BCG-stimulated effectors of both study groups, thus supporting previous data demonstrating the loss of ESAT-6 from vaccine strains of M. bovis BCG (11). It was, however, interesting that in a country such as The Gambia, where TB is endemic, the healthy donors did not show a stronger response to ESAT-6. These data support the use of ESAT-6 as a marker of M. tuberculosis infection. ESAT-6 recognition by CD4+ T cells has been shown to be increased in TB patients compared to healthy controls (19, 24). A CD8+ T-cell epitope from ESAT-6 has also been identified in TB patients (13).
The CD8+ T-cell response to M. bovis BCG and M. tuberculosis in healthy donors has previously been demonstrated to be MHC-I restricted (2, 15, 21–23). However, the CD8+ CTL response from TB patients has not been studied in great detail. A CD1-restricted CD8+ T-cell response has been reported by others in leprosy and TB patients (9, 20). In order to investigate the restriction of the CD8+ effector T cells, M. bovis BCG- and M. tuberculosis H37Ra-infected macrophages were pretreated with an anti-MHC-I blocking antibody. A marked reduction in the levels of lysis was seen against mycobacterium-infected macrophages treated with anti-HLA-A, -B, and -C blocking MAbs compared to untreated or isotype control antibody-treated macrophages, with the levels of lysis seen in MHC-I-blocked macrophages reduced by >75%. Previous studies using BCG-activated CD8+ CTL have shown that proteosome and Golgi inhibitors reduced CTL activity to zero (21). These data imply that the majority of CD8+ T cells against mycobacteria are restricted by MHC-I. Although a minor CD1 or nonclassical MHC-I component may be present, the absence of added interleukin-4 and granulocyte-macrophage colony-stimulating factor in the system used here makes it unlikely that sufficient CD1 expression would be present to induce efficient CD1 presentation. Other studies performed in the United Kingdom have failed to show inhibition of cytotoxicity when antibodies to CD1 were used (21).
In addition to comparisons between the two groups of patients, the study design permitted a comparison of the CD8+ T-cell response to M. bovis BCG and M. tuberculosis H37Ra. The results show that M. tuberculosis is a better stimulator of CD8+ CTL activity than is M. bovis BCG in healthy BCG-vaccinated donors and, to a lesser degree, in TB patients. This observation could be due to the ability of M. tuberculosis antigens to gain access to an alternative MHC-I presentation pathway, which may allow the presentation of more immunogenic epitopes, whereas M. bovis BCG antigens might be processed and presented via classical pathways only.
The reduced CTL activity observed in CD8+ T cells from TB patients may, however, be explained in another way. Since PBMC are the only available source of cells for this study, it could be argued that all of the mycobacterium-reactive T cells may have migrated from the blood into the site of disease, i.e., the lung.
In summary, CD8+ T cells from healthy BCG-vaccinated donors and pulmonary TB patients recognize and respond to antigens secreted by mycobacteria. The CD8+ T cells are capable of cytolytic granule formation and perforin production. CTL activity was seen against whole bacilli and particular secreted antigens. However, the characteristics of the immune responses in the two groups of donors were quite different. CD8+ T-cell activation and CTL activity were all greatly reduced in TB patients. CD8+ T cells from healthy controls exhibit strong recognition of Ag85A and Ag85B, but those from TB patients also recognize ESAT-6, supporting its role as a diagnostic tool. This study therefore suggests that CTL activity mediated by CD8+ T cells may be an important component of the protective immune response against mycobacterial infection.
Acknowledgments
We thank Ousman Jobe and Abdulrahman Hammond for supplying stocks of M. tuberculosis H37Ra. We also thank Boi Sawaneh, Musa Jawo, and Abdoulie Tunkara for their help in recruiting TB patients for the study and Ebou Sarr for recruiting healthy controls.
This work was supported by a grant from the European Commission (IC 18*CT 970236) and by an MRC-GlaxoWellcome Industrial Collaborative Studentship to S.M.S. M.R.K. is supported by a GlaxoWellcome Link Fellowship.
REFERENCES
- 1.Andersen P, Andersen A B, Sorensen A L, Nagai S. Recall of long-lived immunity to Mycobacterium tuberculosis infection in mice. J Immunol. 1995;154:3359–3372. [PubMed] [Google Scholar]
- 2.Canaday D H, Ziebold C, Noss E H, Chervenak K A, Harding C V, Boom W H. Activation of human CD8+ αβ TCR+ cells by Mycobacterium tuberculosis via an alternate class I MHC antigen-processing pathway. J Immunol. 1999;162:327–379. [PubMed] [Google Scholar]
- 3.Cooper A M, D'Souza C, Frank A A, Orme I M. The course of Mycobacterium tuberculosis infection in the lungs of mice lacking expression of either perforin- or granzyme-mediated cytolytic mechanisms. Infect Immun. 1997;65:1317–1320. doi: 10.1128/iai.65.4.1317-1320.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Denis O, Lozes E, Huygen K. Induction of cytotoxic-T-cell responses against culture filtrate antigens in Mycobacterium bovis Bacillus Calmette-Guerin-infected mice. Infect Immun. 1997;65:676–684. doi: 10.1128/iai.65.2.676-684.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Denis O, Tanghe A, Palfliet K, Jurion F, van den Berg T-P, Vanonckelen A, Ooms J, Saman E, Ulmer J B, Content J, Huygen K. Vaccination with plasmid DNA encoding mycobacterial antigen 85A stimulates a CD4+ and CD8+ T-cell epitopic repertoire broader than that stimulated by Mycobacterium tuberculosis H37Rv infection. Infect Immun. 1998;66:1527–1533. doi: 10.1128/iai.66.4.1527-1533.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Denis O, Huygen K. Characterization of the culture filtrate-specific cytotoxic T lymphocyte response induced by bacillus Calmette-Guerin vaccination in H-2b mice. Int Immunol. 1999;11:209–216. doi: 10.1093/intimm/11.2.209. [DOI] [PubMed] [Google Scholar]
- 7.Fine P E, Rodrigues L C. Modern vaccines: mycobacterial disease. Lancet. 1990;335:1016–1020. doi: 10.1016/0140-6736(90)91074-k. [DOI] [PubMed] [Google Scholar]
- 8.Flynn J-A L, Goldstein M M, Triebold K J, Koller B, Bloom B R. Major histocompatibility complex class I-restricted T cells are required for resistance to Mycobacterium tuberculosis infection. Proc Natl Acad Sci USA. 1992;89:12013–12017. doi: 10.1073/pnas.89.24.12013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gong J, Stenger S, Zack J A, Jones B E, Bristol G C, Modlin R L, Morrissey P J, Barnes P F. Isolation of Mycobacterium-reactive CD1-restricted T cells from patients with human immunodeficiency virus infection. J Clin Investig. 1998;101:383–389. doi: 10.1172/JCI318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Harboe M, Malin A S, Dockrell H M, Winker H G, Ulvund G, Holm A, Jorgensen M C, Andersen P. B-cell epitopes and quantification of the ESAT-6 protein of Mycobacterium tuberculosis. Infect Immun. 1998;66:717–723. doi: 10.1128/iai.66.2.717-723.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Harboe M, Oettinger T, Winker H G, Rosenkrands I, Andersen P. Evidence for occurrence of the ESAT-6 protein in Mycobacterium tuberculosis and virulent Mycobacterium bovis and for its absence in Mycobacterium bovis BCG. Infect Immun. 1996;64:16–22. doi: 10.1128/iai.64.1.16-22.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ladel C H, Daugelat S, Kaufmann S H E. Immune response to Mycobacterium bovis bacille Calmette Guerin infection in major histocompatibility complex class I- and II-deficient knock-out mice: contribution of CD4 and CD8 T cells to acquired resistance. Eur J Immunol. 1995;25:377–384. doi: 10.1002/eji.1830250211. [DOI] [PubMed] [Google Scholar]
- 13.Lalvani A, Brookes R, Wilkinson R J, Malin A S, Pathan A A, Andersen P, Dockrell H, Pasvol G, Hill A V S. Human cytolytic and interferon γ-secreting CD8+ T lymphocytes specific for Mycobacterium tuberculosis. Proc Natl Acad Sci USA. 1998;95:270–275. doi: 10.1073/pnas.95.1.270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Laochumroonvorapong P, Wang J, Liu C-C, Ye W, Moreira A L, Elkon K B, Freedman V H, Kaplan G. Perforin, a cytotoxic molecule which mediates cell necrosis, is not required for the early control of mycobacterial infection in mice. Infect Immun. 1997;65:127–132. doi: 10.1128/iai.65.1.127-132.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lewinsohn D M, Alderson M R, Briden A L, Riddell S R, Reed S G, Grabstein K H. Characterization of human CD8+ T cells reactive with Mycobacterium tuberculosis-infected antigen-presenting cells. J Exp Med. 1998;187:1633–1640. doi: 10.1084/jem.187.10.1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mohagheghpour N, Gammon D, Kawamura L M, van Vollenhoven A, Benike C J, Engleman E G. CTL response to Mycobacterium tuberculosis: identification of an immunogenic epitope in the 19-kDa lipoprotein. J Immunol. 1998;161:2400–2406. [PubMed] [Google Scholar]
- 17.Orme I M, Andersen P, Boom W H. T cell responses to Mycobacterium tuberculosis. J Infect Dis. 1993;167:1481. doi: 10.1093/infdis/167.6.1481. [DOI] [PubMed] [Google Scholar]
- 18.Raviglione M C, Snider D E J, Kochi A. Global epidemiology of tuberculosis: morbidity and mortality of a worldwide epidemic. JAMA. 1995;273:220–226. [PubMed] [Google Scholar]
- 19.Ravn P, Demissie A, Eguale T, Wondwosson H, Lein D, Amoudy H A, Mustafa A S, Jensen A K, Holm A, Rosenkrands I, Oftung F, Olobo J, von Reyn F, Andersen P. Human T cell responses to the ESAT-6 antigen from Mycobacterium tuberculosis. J Infect Dis. 1999;179:637–645. doi: 10.1086/314640. [DOI] [PubMed] [Google Scholar]
- 20.Rosat J-P, Grant E P, Beckman E M, Dascher C C, Sieling P A, Frederique D, Modlin R L, Porcelli S A, Furlong S T, Brenner M B. CD1-restricted microbial lipid antigen-specific recognition found in the CD8+ αβ T cell pool. J Immunol. 1999;162:366–371. [PubMed] [Google Scholar]
- 21.Smith S M, Malin A S, Lukey P T, Atkinson S E, Content J, Huygen K, Dockrell H M. Characterization of human Mycobacterium bovis Bacille Calmette-Guerin-reactive CD8+ T cells. Infect Immun. 1999;67:5223–5230. doi: 10.1128/iai.67.10.5223-5230.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tan J S, Canaday D H, Boom W H, Balaji K N, Schwander S K, Rich E A. Human alveolar T lymphocyte responses to Mycobacterium tuberculosis antigens: role for CD4+ and CD8+ cytotoxic T cells and relative resistance of alveolar macrophages to lysis. J Immunol. 1997;159:290–297. [PubMed] [Google Scholar]
- 23.Turner J, Dockrell H M. Stimulation of peripheral blood mononuclear cells with live Mycobacterium bovis BCG activates cytolytic CD8+ T cells in vitro. Immunology. 1996;87:339–342. doi: 10.1046/j.1365-2567.1996.512590.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ulrichs T, Munk M E, Mollenkopf H, Behr-Perst S, Colangeli R, Gennaro M L, Kaufmann S H E. Differential T cell responses to Mycobacterium tuberculosis ESAT-6 in tuberculosis patients and healthy donors. Eur J Immunol. 1998;28:3949–3958. doi: 10.1002/(SICI)1521-4141(199812)28:12<3949::AID-IMMU3949>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 25.Zhu X, Venkataprasad N, Ivanyi J, Vordermeier H M. Vaccination with recombinant vaccinia virus protects mice against Mycobacterium tuberculosis infection. Immunology. 1997;92:6–9. doi: 10.1046/j.1365-2567.1997.00358.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhu X, Stauss H J, Ivanyi J, Vordermeier H M. Specificity of CD8+ T cells from subunit-vaccinated and infected H-2b mice recognising the 38-kDa antigen of Mycobacterium tuberculosis. Int Immunol. 1997;9:1669–1676. doi: 10.1093/intimm/9.11.1669. [DOI] [PubMed] [Google Scholar]