Abstract
Cpp1p is a putative mitogen-activated protein (MAP) kinase phosphatase that suppresses Candida albicans hyphal formation at 25°C through its probable substrate, the Cek1p filamentation MAP kinase. Here we report that expression of the serum-induced genes SAP4-6 and HYR1 increased several fold in hyphal forms of a cpp1/cpp1 null mutant, while the rate and extent of hyphal development up to 5 h were normal. Therefore, we provide evidence that Cpp1p represses hyphal gene expression by acting through a Cek1p-independent mechanism. SAP4-6 and HYR1 transcripts were undetectable in a null mutant of another key regulator of filamentation, Efg1p; thus, Efg1p and Cpp1p oppose each other during the expression of these genes in hyphal forms.
Deletion of the mitogen-activated protein (MAP) kinase phosphatase Cpp1p of the human opportunistic pathogen Candida albicans was shown to derepress hyphal formation under noninducing conditions (2), and virulence of a cpp1/cpp1 mutant strain was reduced in murine models of candidiasis (2, 5). Hyphal development of C. albicans is controlled by at least two regulatory cascades, both of which involve phosphorylation of downstream components. There is a predominant pathway that functions in response to a variety of stimuli, including serum, which involves the kinase Tpk2p and the basic helix-loop-helix transcription factor Efg1p (for enhanced filamentous growth) (10, 14, 17). A second pathway that operates via a nutritionally regulated MAP kinase cascade (Cst20p-Hst7p-Cek1p) phosphorylates and activates the transcription factor Cph1p (6, 7, 9). The development of a yeast cell to a multicellular mycelium that consists of hyphal forms is an important step during pathogenesis, because nonfilamentous strains of C. albicans are avirulent (8, 10).
It was suggested that Cpp1p acts to block the yeast-to-hyphal transition under noninducing conditions through inactivation of the MAP kinase Cek1p (2), because previous studies showed that the morphological phenotypes of the cpp1/cpp1 mutation during late stages of hyphal development (4 days) were reversed by additional deletion of the CEK1 gene (2). In this study, we show that under inducing conditions at 37°C, Cpp1p is a potent suppressor of serum-induced hyphal gene expression. Surprisingly, this activity does not require the MAP kinase Cek1p.
Cpp1p is a suppressor of Cek1p-independent hyphal gene expression.
The downstream target genes of Cpp1p-dependent signal transduction were investigated. We determined the mRNA expression level of the type 4, 5, and 6 isogenes (SAP4-6) of the secreted aspartyl proteinase (a putative virulence factor [12]) and the HYR1 gene (coding for a nonessential cell wall component [1]) during hyphal formation. The C. albicans strains used for this study are listed in Table 1. For the induction of hyphal development, cells were grown for 48 to 72 h in YPD (1% yeast exract, 2% Bactopeptone, 2% glucose) and diluted into CLICKS RPMI-1640 medium supplemented with 10% selected fetal calf serum to a final density of 20 × 106 cells/ml. Total RNA was extracted from yeast and hyphal forms, and Northern blots were carried out as published previously (16). For detection of the type 4, 5, and 6 isogenes of secreted aspartic proteinase SAP4-6 mRNA, we labeled (by random priming) BglII restriction fragments derived from the cloned coding regions of SAP4-6. For the detection of HYR1 mRNA, we used a cloned HYR1 PCR product spanning the coding region as a template for probe synthesis. ACT1 hybridization was used to control for equal sample loading as described previously (3). Plasmids for probe synthesis for the detection of EFG1 and ACT1 mRNA were kindly provided by J. Ernst, Düsseldorf, Germany. Residual radioactivity was stripped from the membranes prior to hybridization with a different probe (16). Autoradiograms were quantitated with a Fuji BAS3000 phosphorimager and accessory software.
TABLE 1.
C. albicans strains used in this study
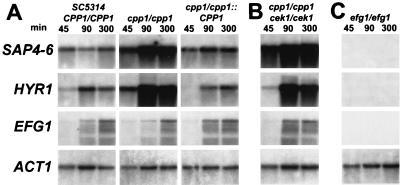
As shown in Fig. 1A, the C. albicans wild-type strain SC5314 and the cpp1/cpp1::CPP1 control strain (integrated chromosomal copy of CPP1) were induced to similar levels of SAP4-6 and HYR1 expression after initiation of hyphal formation. However, hypha-forming cells of the cpp1/cpp1 mutant expressed approximately 10-fold more SAP4-6 and HYR1 mRNA (Fig. 1A). Because the cpp1/cpp1 phenotype of derepressed filamentous growth under noninducing conditions at room temperature was reverted by deletion of the MAP kinase gene CEK1 (2), we measured the expression of SAP4-6 and HYR1 in the cpp1/cpp1 cek1/cek1 double mutant under inducing conditions as detailed above and compared it to that of the cpp1/cpp1 single mutant and the isogenic control strains. The overexpression of SAP4-6 and HYR1 observed in the cpp1/cpp1 mutant also occurred in the cpp1/cpp1 cek1/cek1 double mutant (Fig. 1B). Hence, derepressed SAP4-6 and HYR1 gene expression in the cpp1/cpp1 mutant does not require functional Cek1p MAP kinase activity.
FIG. 1.
Northern blot analysis of SAP4-6, HYR1, EFG1, and ACT1 gene expression of the C. albicans wild-type strain SC5314 (CPP1/CPP1) and the cpp1/cpp1 and cpp1/cpp1::CPP1 (A), cpp1/cpp1 cek1/cek1 (B), and efg1/efg1 (C) isogenic mutant strains. The genotype and the duration (minutes) of serum-induced hyphal formation prior to RNA extraction are delineated at the top of each column, respectively. The origins of probes used for hybridization are given on the left-hand side (SAP4-6, HYR1, EFG1, and ACT1).
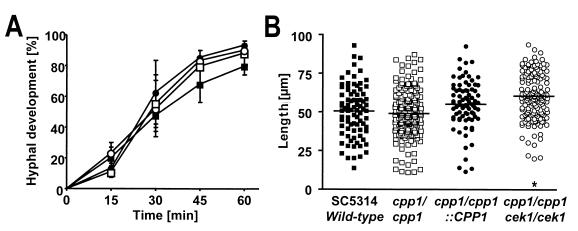
Since hyphal formation at room temperature was derepressed in the cpp1/cpp1 mutant (2), the increase in hyphal gene expression might simply reflect derepressed hyphal formation that occurred at 37°C as well. Therefore, serum-induced hyphal development was quantitated by using a Zeiss axiophot microscope equipped with a video device. As illustrated in Fig. 2A, we observed similar percentages of germ tubes emerging from blastoconidia of the SC5314 wild-type strain, the cpp1/cpp1::CPP1 control strain, and the cpp1/cpp1 and cpp1/cpp1 cek1/cek1 mutant strains (79.8% ± 5.8%, 93.4% ± 2.6%, 90.3% ± 3.9%, and 90.2% ± 3.1% at 60 min; mean percentage of germ tube-positive blastoconidia ± standard error of three independent experiments with at least 200 cells/time point). The length of the filaments at 4 h was measured on calibrated video images with MetaView software (Universal Imaging Corporation, West Chester, Pa.). The SC5314 wild-type strain, the cpp1/cpp1::CPP1 control strain, and the cpp1/cpp1 mutant produced filaments of similar length (51.38 ± 1.8, 55.8 ± 1.7, and 49.9 ± 1.2 μm, respectively), whereas the filaments of the cpp1/cpp1 cek1/cek1 double mutant strain extended slightly further to 60.6 ± 1.2 μm (Fig. 2B; mean hyphal length ± standard error of three independent experiments with n > 80 hyphal forms; P < 0.05 for cpp1/cpp1 cek1/cek1 versus all other samples). Chlamydospore formation (15) on cornmeal agar was not affected by deletion of CPP1 (data not shown). We concluded that the rate and extent of hyphal development of C. albicans at 37°C in vitro are not controlled by Cpp1p. Therefore, the derepressed hyphal expression of SAP4-6 and HYR1 of the cpp1/cpp1 and the cpp1/cpp1 cek1/cek1 mutants is not the result of hyperfilamentation of these mutants at 37°C. In fact, this result suggests that Cpp1p acts on a separate signal transduction pathway that specifically regulates expression of SAP4-6 and HYR1, but not the extent of hyphal formation at 37°C.
FIG. 2.
Percentage of germ tube-positive blastoconidia after 15, 30, 45, and 60 min (A) and extent of filaments (micrometers) after 4 h of serum-induced hyphal formation (B). ■, SC5314 wild-type (CPP1/CPP1); □, cpp1/cpp1; ●, cpp1/cpp1::CPP1; ○, cpp1/cpp1 cek1/cek1. (A) Illustrated values are the mean percentage of germ tube-positive blastoconidia ± standard error of three independent experiments with at least 200 cells/time point (B) Each scatter represents cumulative data of hyphal length from three independent experiments with n > 80 hyphal forms each. Horizontal bars represent mean hyphal length. ∗, P < 0.05.
Efg1p regulates SAP4-6 and HYR1 gene expression.
Efg1p is a crucial transcription factor for both hyphal formation (10, 17) and hyphal gene expression (13) of C. albicans. Therefore, we hybridized mRNA of the cpp1/cpp1 mutant grown under inducing conditions with an EFG1 probe in order to examine whether the expression of this transcription factor gene is controlled by Cpp1p. The pattern of EFG1 mRNA expression was the same for wild-type and cpp1/cpp1 mutant cells (Fig. 1A), which argues against transcriptional regulation of EFG1 by a Cpp1p-dependent mechanism. However, the efg1/efg1 mutant (10) did not express any detectable amount of SAP4-6 or HYR1 mRNA up to 4 h under inducing conditions (Fig. 1C). These results show that Efg1p is required for SAP4-6 and HYR1 expression. In the host, part of the virulence defect of the efg1/efg1 mutant (10) may be attributed to the loss of expression of the SAP4-6 genes. Since these targets of Efg1p signals are derepressed in the cpp1/cpp1 and cpp1/cpp1 cek1/cek1 mutants, we conclude that Cpp1p interacts with the signaling pathway that acts through Efg1p.
The hyperexpression of the SAP4-6 isogenes and HYR1 in the double cpp1/cpp1 cek1/cek1 mutant (Fig. 1), independent from the percentage and extent of hyphal formation (Fig. 2), indicates a minor role of Cek1p for the activation of these genes in and the generation of hyphal forms at 37°C. Inhibition of the SAP4-6 isogenes and HYR1—mediated by a yet-to-be-identified Cpp1p target in its unphosphorylated form—is removed in cpp1/cpp1 null mutants. The absence of the phosphatase would permit inappropriate release of an inactive sequestered transcription factor, because the regulatory component now is phosphorylated. Thus similar to the Saccharomyces cerevisiae MAP kinase-specific phosphatase Msg5p (11), Cpp1p seems to have more than one substrate. The observation that Efg1p and Cpp1p act in opposition to each other on SAP4-6 and HYR1 expression suggests that both regulatory proteins affect a common signal transduction pathway. The C. albicans Efg1p transcription factor contains a single potential site for protein kinase A phosphorylation at T206 (sequence IRPRVT206TT), which was reported previously to be essential in hyphal development and chlamydospore formation (15). Accordingly, Efg1p function during hyphal formation is presumably regulated by the balanced activity of kinase(s) and phosphatase(s), part of which may be the result of Cpp1p-dependent negative signals.
In summary, the MAP kinase phosphatase Cpp1p of C. albicans regulates morphological changes and transcription of hyphal genes by two mechanisms that are either MAP kinase Cek1p dependent (cell morphology at room temperature) or independent (gene transcription at 37°C), respectively. Thus, Cpp1p emerges as an important negative regulator of Cek1p-dependent morphological development and Efg1p-dependent gene expression in C. albicans.
Acknowledgments
We are indebted to J. Ernst, G. R. Fink, B. Hube, E. Leberer, M. Monod, and J. Morschhäuser for providing C. albicans strains and mutants and for critical discussion of the manuscript. We appreciate the technical assistance of A. Loichinger.
K.S. was supported by the Deutsche Forschungsgemeinschaft (grant Schr 450/3-1).
REFERENCES
- 1.Bailey D A, Feldmann P J F, Bovey M, Gow N A R, Brown A J P. The Candida albicans HYR1 gene, which is activated in response to hyphal development, belongs to a gene family encoding yeast cell wall proteins. J Bacteriol. 1996;178:5353–5360. doi: 10.1128/jb.178.18.5353-5360.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Csank C, Constantin M, Meloche S, Schröppel K, Röllinghoff M, Dignard D, Thomas D Y, Whiteway M. Derepressed hyphal growth and reduced virulence in a VH1 family related protein phosphatase mutant of Candida albicans. Mol Biol Cell. 1997;8:2539–2547. doi: 10.1091/mbc.8.12.2539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Delbrück S, Ernst J F. Morphogenesis-independent regulation of actin transcript levels in the pathogenic yeast Candida albicans. Mol Microbiol. 1993;10:859–866. doi: 10.1111/j.1365-2958.1993.tb00956.x. [DOI] [PubMed] [Google Scholar]
- 4.Gillum A M, Tsay E Y, Kirsch D R. Isolation of the Candida albicans gene for orotidine-5′-phosphate decarboxylase by complementation of S. cerevisiae ura3 and E. coli pyrF mutations. Mol Gen Genet. 1984;198:179–182. doi: 10.1007/BF00328721. [DOI] [PubMed] [Google Scholar]
- 5.Guhad F A, Csank C, Jensen H E, Thomas D Y, Whiteway M, Hau J. Reduced pathogenicity of a Candida albicans MAP kinase phosphatase (CPP1) mutant in the murine mastitis model. APMIS. 1998;106:1049–1055. doi: 10.1111/j.1699-0463.1998.tb00257.x. [DOI] [PubMed] [Google Scholar]
- 6.Köhler J R, Fink G R. Candida albicans strains heterozygous and homozygous for mutations in mitogen-activated protein kinase signaling components have defects in hyphal development. Proc Natl Acad Sci USA. 1996;93:13223–13228. doi: 10.1073/pnas.93.23.13223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Leberer E, Harcus D, Broadbent I D, Clark K L, Dignard D, Ziegelbauer K, Schmidt A, Gow N A, Brown A J, Thomas D Y. Signal transduction through homologs of the ste20p and ste7p protein kinases can trigger hyphal formation in the pathogenic fungus Candida albicans. Proc Natl Acad Sci USA. 1996;93:13217–13222. doi: 10.1073/pnas.93.23.13217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Leberer E, Ziegelbauer K, Schmidt A, Harcus D, Dignard D, Ash J, Johnson L, Thomas D Y. Virulence and hyphal formation of Candida albicans require the Ste20p-like protein kinase CaCla4p. Curr Biol. 1997;7:539–546. doi: 10.1016/s0960-9822(06)00252-1. [DOI] [PubMed] [Google Scholar]
- 9.Liu H, Köhler J R, Fink G R. Suppression of hyphal formation in Candida albicans by mutation of a STE12 homolog. Science. 1994;266:1723–1726. doi: 10.1126/science.7992058. [DOI] [PubMed] [Google Scholar]
- 10.Lo H J, Köhler J R, DiDomenico B, Loebenberg D, Cacciapuoti A, Fink G R. Nonfilamentous C. albicans mutants are avirulent. Cell. 1997;90:939–949. doi: 10.1016/s0092-8674(00)80358-x. [DOI] [PubMed] [Google Scholar]
- 11.Navarro-García F, Sánchez M, Pla J, Nombela C. Functional characterization of the MKC1 gene of Candida albicans, which encodes a mitogen-activated protein kinase homolog related to cell integrity. Mol Cell Biol. 1995;15:2197–2206. doi: 10.1128/mcb.15.4.2197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sanglard D, Hube B, Monod M, Odds F C, Gow N A R. A triple deletion of the secreted aspartyl proteinase genes SAP4, SAP5, and SAP6 of Candida albicans causes attenuated virulence. Infect Immun. 1997;65:3539–3546. doi: 10.1128/iai.65.9.3539-3546.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sharkey L L, McNemar M D, Saporito-Irwin S M, Sypherd P S, Fonzi W A. HWP1 functions in the morphological development of Candida albicans downstream of EFG1, TUP1, and RBF1. J Bacteriol. 1999;181:5273–5279. doi: 10.1128/jb.181.17.5273-5279.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sonneborn A, Bockmuhl D P, Gerads M, Kurpanek K, Sanglard D, Ernst J F. Protein kinase A encoded by TPK2 regulates dimorphism of Candida albicans. Mol Microbiol. 2000;35:386–396. doi: 10.1046/j.1365-2958.2000.01705.x. [DOI] [PubMed] [Google Scholar]
- 15.Sonneborn A, Bockmühl D P, Ernst J F. Chlamydospore formation in Candida albicans requires the Efg1p morphogenetic regulator. Infect Immun. 1999;67:5514–5517. doi: 10.1128/iai.67.10.5514-5517.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Srikantha T, Morrow B, Schröppel K, Soll D R. The frequency of integrative transformation at phase-specific genes of Candida albicans correlates with their transcriptional state. Mol Gen Genet. 1995;246:342–352. doi: 10.1007/BF00288607. [DOI] [PubMed] [Google Scholar]
- 17.Stoldt V R, Sonneborn A, Leuker C E, Ernst J F. Efg1p, an essential regulator of morphogenesis of the human pathogen Candida albicans, is a member of a conserved class of bHLH proteins regulating morphogenetic processes in fungi. EMBO J. 1997;16:1982–1991. doi: 10.1093/emboj/16.8.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]