Abstract
Numerous studies have reported that long‐term musical training can affect brain functionality and induce structural alterations in the brain. Singing is a form of vocal musical expression with an unparalleled capacity for communicating emotion; however, there has been relatively little research on neuroplasticity at the network level in vocalists (i.e., noninstrumental musicians). Our objective in this study was to elucidate changes in the neural network architecture following long‐term training in the musical arts. We employed a framework based on graph theory to depict the connectivity and efficiency of structural networks in the brain, based on diffusion‐weighted images obtained from 35 vocalists, 27 pianists, and 33 nonmusicians. Our results revealed that musical training (both voice and piano) could enhance connectivity among emotion‐related regions of the brain, such as the amygdala. We also discovered that voice training reshaped the architecture of experience‐dependent networks, such as those involved in vocal motor control, sensory feedback, and language processing. It appears that vocal‐related changes in areas such as the insula, paracentral lobule, supramarginal gyrus, and putamen are associated with functional segregation, multisensory integration, and enhanced network interconnectivity. These results suggest that long‐term musical training can strengthen or prune white matter connectivity networks in an experience‐dependent manner.
Keywords: brain asymmetry, emotion, graph theory, language, structural connectivity, vocal training
Musical training (both voice and piano) could enhance connectivity among emotion‐related regions of the brain, such as the amygdala. Voice training reshaped the architecture of experience‐dependent networks, such as those involved in vocal motor control, sensory feedback, and language processing. Long‐term musical training can strengthen or prune white matter connectivity networks in an experience‐dependent manner.
1. INTRODUCTION
Intensive musical training has been widely studied, due to its ability to induce structural neuroplasticity in brain regions associated with perception, motor control, and cognitive functions (Foster & Zatorre, 2010; Groussard et al., 2014; Habibi et al., 2018; Hyde et al., 2009; Miendlarzewska & Trost, 2013; Schmithorst & Wilke, 2002; Shenker et al., 2021; Wang et al., 2019). Emerging neuroimaging evidence has revealed a strong relationship between structural connectivity and long‐term training in various instrumental musicians (Dhakal et al., 2021; Elmer et al., 2016; Jancke et al., 2012; Li et al., 2014). Most previous studies reported local changes in the architecture of white matter, such as microstructural changes in the fiber tract organization in various regions of the brain. However, little is known about how the topological properties of brain networks in noninstrumental musicians are changed after long‐term training. In this work, we adopted graph theory measurements to characterize the architectures of structural brain networks in musicians after long‐term vocal training (Leipold et al., 2021; Li et al., 2014).
The neuroplasticity patterns presented by musicians differ according to the specifics of their musical training (Barrett et al., 2013). Among the variety of musical training, singing is the most important form of musical expression, as it is an innate ability (Knight, 1999; Nettl, 2015) and universal human activity (Lomax, 2017). Singing is also distinct in that practitioners use their bodies as an instrument (Zarate, 2013). One previous brain imaging study reported that long‐term vocal training enhances structural connectivity between widespread brain regions related to sound perception, sound production, and kinesthetic motor control (Halwani et al., 2011). They also reported increased leftward asymmetry in white matter tracts related to language learning (e.g., arcuate fasciculus) in singers. However, there has been relatively little research on structural network architectures altered by vocal training.
The means by which musicians convey emotion in music also vary, depending on the specifics of musical training. For example, instrumental musicians exploit a set of skills to translate their finger touches and body movements into emotional intentions. Singers manipulate acoustic parameters (e.g., tempo, pitch, and phonation) in the singing voice for emotion expression (Juslin & Laukka, 2003; Scherer, 2003). Professional singers express anger through the use of vibrato, sadness by the absence of vibrato at a slow tempo with low voice intensity, and fear by the presence of steep spectral slope (Jansens et al., 1997). One recent study using multiple discriminant analysis demonstrated robust vocal signatures in the interpretation and expression of emotional content by professional opera singers (Scherer et al., 2017). The authors reported that the acoustic parameters of anger and joy can both be characterized by high levels of loudness, vocal dynamics, and perturbation variation. Singers might utilize a motor system that is intrinsically linked to innate emotional expression, and then develop volitional control over emotional vocalization in the context of speech motor control. In the current study, we hypothesized that the intensive musical practice that involves the interpretation of emotions could strengthen the connectivity between brain regions related to emotional expression and motor control. We also speculated that this connectivity would differ between professional singers and instrumental musicians.
Graph theory has been widely used to characterize the architecture of networks in human brains (Bassett & Bullmore, 2006; Loui et al., 2011; Rubinov & Sporns, 2010; Sporns, 2014, 2016). Graph theory metrics used to measure the organization of networks can be divided into local (regional) and global. Regional metrics included strength centrality (SC), betweenness centrality (BC), and local efficiency (LE). Strength centrality reflects the correlation between one region and the others. Betweenness centrality measures the importance of nodes to overall network integrity (Brandes, 2001; Kintali, 2008), which has been defined as the fraction of all shortest paths passing through a given node in the network (Rubinov & Sporns, 2010). Local efficiency measures the fault tolerance of a system, as an indication of communication efficiency between the direct neighbors of a node (Latora & Marchiori, 2001). Global metrics included density, global efficiency, mean local efficiency, mean clustering coefficient, characteristic path length, mean betweenness centrality, and transitivity (Rubinov & Sporns, 2010). Global efficiency measures network integration, indicating the ability to transmit information across the whole brain (Achard & Bullmore, 2007; Estrada & Hatano, 2008). Mean local efficiency measures the mean communication efficiency of the whole brain. These metrics describe the importance of brain regions, the efficiency of information transfer among brain regions, and the integration and segregation of specific brain networks.
In this study, we adopted the graph theory framework and diffusion‐weighted images to identify changes in structural networks in the brain after long‐term musical training. We recruited vocalists, instrumental musicians, and nonmusicians to elucidate the effects of long‐term vocal training on the architecture of white matter networks. Note that most vocalists received long‐term piano training before maturation of the voice during adolescence (Gackle, 2019; Williams & Harrison, 2019). Unlike vocalists, pianists are required to replay long motor sequences rapidly using both hands, which relies on the precise retrieval of motor memory functions (Heun et al., 2004; Landau & D'Esposito, 2006). A functional activation study showed that pianists had greater rightward asymmetry activity in several regions related to motor sequence learning (Landau & D'Esposito, 2006). In contrast, singers exhibited left lateralization in the white matter tracts associated with language learning (Halwani et al., 2011). Here, we hypothesized that long‐term vocal training would strengthen connectivity and network efficiency among brain regions related to auditory‐motor control, sensory feedback, and emotion expression. We also speculated that vocalists would exhibit leftward asymmetry in language‐related areas, as a result of intensive singing exercises.
2. MATERIALS AND METHODS
2.1. Participants
We initially recruited 100 right‐handed participants (age range: 20–40); however, 5 participants were excluded due to signal loss in their diffusion‐weighted images. This resulted in 95 participants overall, which included 35 vocalists (V; male = 5), 27 pianists (P; male = 6), and 33 nonmusician control participants (C; male = 6). The musicians were college students or graduates who majored in vocal or piano performance. Control participants were nonmusicians who were mainly college students majoring in liberal arts. In the vocalist group, the mean vocal training was 7.68 ± 3.1 years, involving 13.4 ± 7.1 training hours per week. In the pianist group, the mean piano training was 16.22 ± 2.2 years, involving 21.3 ± 7.5 training hours per week.
2.2. MRI data acquisition
All MRI images were acquired using a 3‐Tesla scanner (MAGNETOM Trio, A Tim System; Siemens Medical Solution, Erlangen, Germany) with a 32‐channel head coil. Diffusion spectrum images (DSIs) were obtained using spin‐echo diffusion echo‐planar imaging sequence (EPI) with the following parameters: repetition time (TR) = 9700 ms, echo time (TE) = 136 ms, 56 axial slices, field of view (FOV) = 200 × 200 mm2, matrix size = 80 × 80, and slice thickness = 2.5 mm. The diffusion acquisition scheme comprised 102 diffusion‐encoding directions (Chen et al., 2015). A diffusion spectrum imaging (DSI) dataset consisted of 101 DWIs with variable b‐values (b max = 4000 s/mm2; 16 distinct b‐values in the present study) and a b 0 volume image (Hsu et al., 2015). The acquisition scheme was modified from the DSI203 scheme (Kuo et al., 2008). The volume image in the DSI dataset corresponded to 102 isotopic grid points in q‐space (Wedeen et al., 2005). High resolution T1‐weighted images were acquired using three‐dimensional magnetization‐prepared rapid gradient echo (3D‐MPRAGE) sequence with the following parameters: TR = 2530 ms, TE = 3.03 ms, 192 axial slices, flip angle = 7°, FOV = 224 × 256 mm2, matrix size = 224 × 256, and slice thickness = 1 mm.
2.3. Image processing and analysis
To ensure image quality, diffusion‐weighted images were inspected visually to verify that they were unaffected by significant signal dropout due to head motion, which is a common practice for DWI pretreatment (Chen et al., 2015). For the datasets with signal loss, the images that could not be recovered by subsequent image processing were discarded (Chen et al., 2015). Hence, each image in our data was visually scrutinized, and datasets with more than three axial slices of signal loss were discarded. DWIs also underwent denoising using MRtrix3 (http://www.mrtrix.org/) to improve the signal‐to‐noise ratio and reduce scanning artifacts (Veraart et al., 2016). The brain area was extracted from T1‐weighted images and DWIs using BET package in FSL (http://fsl.fmrib.ox.ac.uk). Both the T1‐weighted and DWIs were normalized to the standard Montreal Neurological Institute (MNI) space by the following steps: (1) The b 0 images were co‐registered to individual T1‐weighted images; (2) The T1‐weighted image was normalized to the standard MNI space; (3) The deformation field from step (1) and (2) was applied to DWI data in the standard MNI space. For the measurement of network characteristics, the Automated Anatomical Labeling (AAL) atlas (Tzourio‐Mazoyer et al., 2002) was applied to individual DWIs using the same inverse deformation field.
Reconstruction of the orientation distribution function (ODF) and whole‐brain fiber tracking were performed using DSI Studio with optimized parameter settings (Yeh et al., 2013). Generalized fractional anisotropy (GFA) was computed for each voxel. A maximum of 1,000,000 streamlines were reconstructed for each individual using deterministic tractography and the standard protocol with the following parameters: step size (1.1 mm), fiber length (range: 20–500 mm), quantitative anisotropy threshold (0.01–0.10), turning angle threshold (60°), and smoothing (20%). Redundant streamlines at a Hausdorff distance of less than 1 mm were then removed (Fernandez‐Miranda et al., 2015; Yeh et al., 2010, 2013, 2018).
2.4. Measurement of structural network characteristics
Brain networks were analyzed using graph‐based information related to structural connectivity. This involved defining 90 nodes () involving whole‐brain structural connectivity using the AAL atlas without cerebellar regions. The weight of the edge between each pair of nodes was estimated by calculating the total number of end‐to‐end streamlines. Connectivity matrix measurements were calculated using the Brain Connectivity Toolbox (BCT) as indices of the topological properties of the networks (Rubinov & Sporns, 2010).
We constructed anatomical brain networks for each subject and calculated the properties using weighted networks. The number of streamlines was converted to edge weights, , for subsequent analysis using the following equation:
where is the connectivity matrix of the kth participant and is the number of streamlines between node i and node j in .
The degree of an individual node (indicating the importance of the node in the network) represents the number of edges connected to other nodes in this network (Rubinov & Sporns, 2010). Density was defined as the mean degree of the entire network.
The effects of musical training on structural connectivity were detected by computing the following local (regional) and global network metrics. The regional metrics included strength centrality, betweenness centrality, and local efficiency. Strength centrality of node , , was defined as the summation of all neighboring edge weights, as follows:
| (1) |
Betweenness centrality measures the importance of nodes to overall network integrity (Brandes, 2001; Kintali, 2008), which has been defined as the fraction of all shortest paths in the network passing through a given node (Rubinov & Sporns, 2010), written as follows:
where is the number of shortest paths between node and node passing through node , and is the shortest path number between node and node .
Local efficiency of node , , was defined as follows:
where is the length of the shortest path between node and node containing only neighbors of node . This metric measures the level of system fault tolerance, as an indication of communication efficiency between the first neighbors of node , when node is removed (Latora & Marchiori, 2001).
The global metrics included density, global efficiency, mean local efficiency, mean clustering coefficient, characteristic path length, mean betweenness centrality, and transitivity (for more details, see Rubinov & Sporns, 2010).
Global efficiency, , was the average of (Latora & Marchiori, 2001):
where was the average of the value inversely proportional to , the shortest distance between node and node , . Global efficiency is a measurement of network integration, indicating the ability to transmit information across the whole brain (Achard & Bullmore, 2007; Estrada & Hatano, 2008).
Mean local efficiency of the whole brain, , was defined as follows:
The above‐mentioned global network metrics were also calculated separately in the right and left hemispheres. We utilized the local (regional) lateralization measure with the strength asymmetry index to compare connectivity strength between the two hemispheres in each region of the brain (Catani et al., 2007):
where , respectively, represent the strength centrality of the left and right hemispheres in brain region j, as defined in Equation (1).
2.5. Statistical analysis
Statistical analyses were performed using SPSS 21 (IBM Corp., Armonk, NY; Version 21) and MATLAB (Mathworks, Natick, MA; version 9). In the demographic characteristics, two‐sample t‐tests were used to compare continuous variables and chi‐square tests were used to compare nominal variables. The difference of connectivity matrices among the three groups was tested with network‐based statistic (NBS; Zalesky et al., 2010) for the local changes before graph‐theoretical measurement. NBS controls for the family‐wise error rate (in a weak sense) when mass‐univariate testing is performed on all the connections in the graph. Differences in the number of streamlines and network metrics among the three groups were assessed with one‐way Kruskal‐Wallis tests (p < .05) followed by post hoc independent sample permutation tests (iterations = 10,000 iterations) (p < .05, corrected) using the t max method (Blair & Karniski, 1993), which adjusts the p‐values of each variable for multiple comparisons (i.e., the number of nodes [n = 90]) and strongly controls the family wise error‐rate (FWE) (Blair & Karniski, 1993; Westfall & Young, 1993). Results were visualized with BrainNet Viewer (Xia et al., 2013). The Spearman correlation coefficient was used to estimate the association between accumulated vocal training duration and the network metrics, with the level of significance set at α = 0.05.
3. RESULTS
3.1. Demographic characteristics
The pianists (P) in this study were significantly younger than the vocalists (V) and nonmusicians (C) (P, 21.7 ± 1.4 years; V, 23.0 ± 2.3 years; C, 23.0 ± 1.7 years; p < .05; Table 1). There were no significant differences among the three groups in terms of gender or handedness. In terms of vocal training history, vocalists had undergone training significantly longer than pianists (V, 7.68 ± 3.1 years; P, 0.67 ± 1.4; p < .01). The duration of piano training was not different between musician groups (V, 15.0 ± 4.2 years; P, 16.22 ± 2.1 years).
TABLE 1.
Demographic characteristics among the three groups
| Vocalists (N = 35) | Pianists (N = 27) | Nonmusicians (N = 33) | |
|---|---|---|---|
| Age (years) | 23.0 (2.3)* | 21.7 (1.4) ## | 23.0 (1.7) |
| Gender (F/M) | 30/5 | 21/6 | 27/6 |
| Education (years) | 16.5 (1.6) | 15.7 (1.0) | 16.1 (1.1) |
| Vocal training duration (years) | 7.68 (3.1)** | 0.67 (1.4) | ‐ |
| Piano training duration (years) | 15.0 (4.2) | 16.22 (2.1) | ‐ |
| Right‐handness | 74.3 (28.3) | 83.3 (26.0) | 80.1 (18.6) |
Note: Values are presented as mean (SD). *p < .05, ** p < .01, vocalist vs. pianists; ## p < .01, pianist vs. nonmusicians; two‐sample t‐test.
Abbreviations: F, females; M, males; SD, standard deviation.
3.2. Local structural connectivity
The results of NBS analysis showed no significant differences in the connectivity matrices among the three groups.
3.3. Global network metrics
There were no significant differences in the summation of whole‐brain streamlines or network metrics among the three groups (Figure S1, Table S1, and Figure 1a).
FIGURE 1.
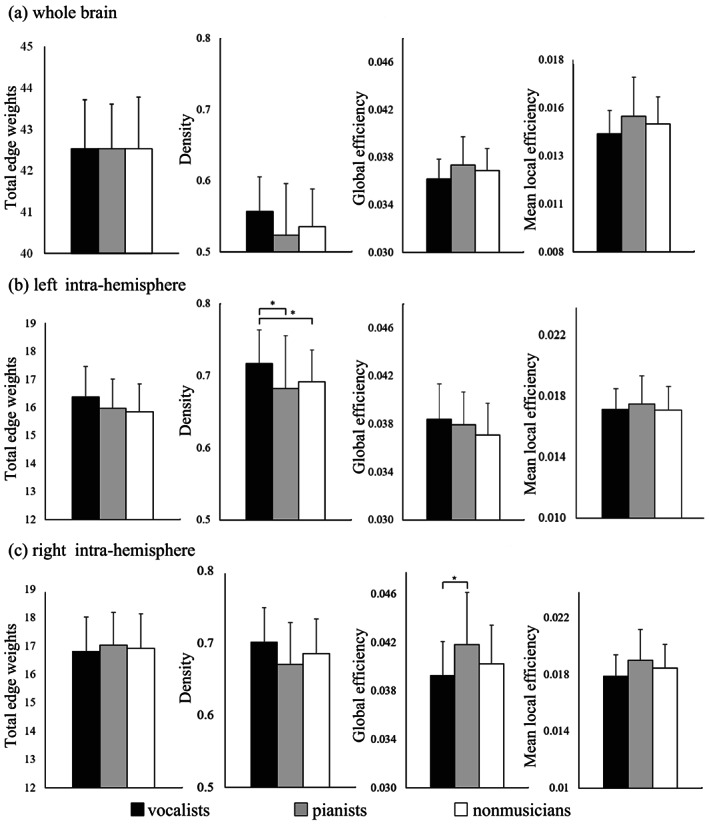
Network measurement in the whole brain (a), left intra‐hemisphere (b), and right intra‐hemisphere (c). Kruskal‐Wallis tests revealed significant group differences in terms of density in the left hemisphere (H(2) = 8.116, p = .017) and global efficiency in the right hemisphere (H(2) = 6.686, p = .035). (*FWE‐corrected p < .05; post hoc permutation test using 10,000 iterations)
Compared to nonmusicians, vocalists exhibited significantly higher network density in structural networks in the left hemisphere (Figure 1b and Table S2). Compared to pianists, vocalists exhibited significantly higher density in the left hemisphere and lower global efficiency in the right hemisphere (Figure 1b,c, and Table S2).
3.4. Local (regional) metrics
3.4.1. Strength centrality
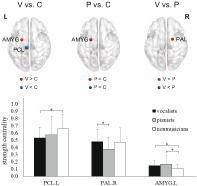
SC in the left amygdala was significantly higher in both musician groups (vocalists and pianists) than in the nonmusician group (Figure 2 and Table S3). Comparing vocalists versus nonmusicians, vocalists exhibited significantly higher SC in the left amygdala and significantly lower SC in the left paracentral lobule (PCL). Comparing pianists versus nonmusicians, pianists exhibited significantly higher SC in the left amygdala. Comparing vocalists versus pianists, pianists exhibited significantly lower SC in the right pallidum. The upper panel in Figure S2 presents a map of mean whole‐brain SC values from the entire study cohort.
FIGURE 2.
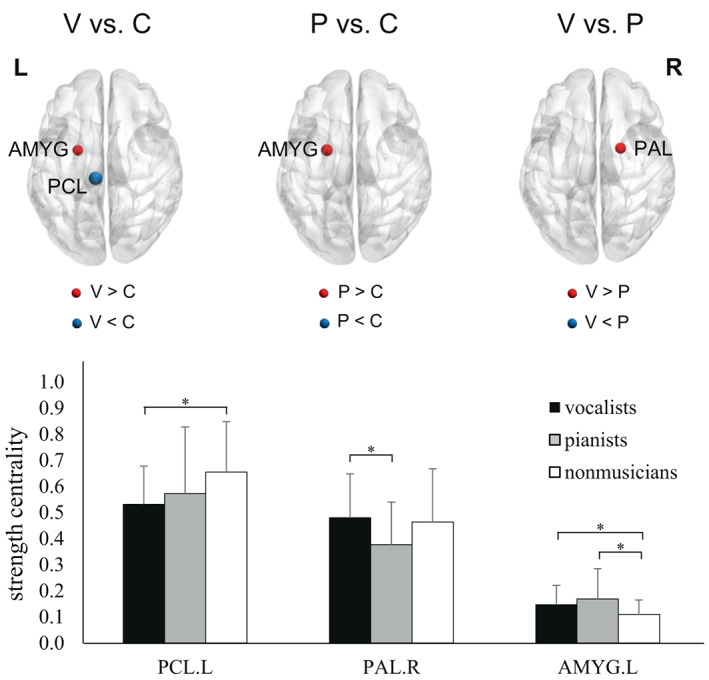
Comparison of strength centrality among the three groups. Note that the size of the sphere indicates the degree of statistical significance between the results from the two groups. The Kruskal‐Wallis tests revealed significant group differences in the left paracentral lobule (PCL; H(2) = 10.135, p = .006), right pallidum (PAL; H(2) = 6.23, p = .044), and left amygdala (AMYG; H(2) = 6.699, p = .035). P, pianists; V, vocalists; C, nonmusicians; L, left; R, right (*FWE‐corrected p < .05; post hoc permutation test using 10,000 iterations)
3.4.2. Betweenness centrality
Comparing vocalists versus nonmusicians, vocalists exhibited significantly lower BC in the left middle temporal gyrus (MTG) (Figure S3). There were no significant differences between pianists and nonmusicians. Comparing vocalists versus pianists, vocalists exhibited significantly lower BC in the left MTG while pianists exhibited significantly higher BC in the left inferior temporal gyrus (ITG), right precuneus, and cuneus. The lower panel in Figure S2 presents a map of mean whole‐brain BC values from the entire study cohort.
3.4.3. Local efficiency
Comparing vocalists versus nonmusicians, vocalists exhibited significantly lower LE in the left supramarginal gyrus (SMG), PCL, right middle frontal gyrus (MFG), and insula (Figure 3 and Table S4). There were no significant differences between pianists and nonmusicians. Comparing vocalists versus pianists, vocalists exhibited significantly lower LE in the left SMG and right MFG whereas pianists exhibited significantly higher LE in the right SMG.
FIGURE 3.
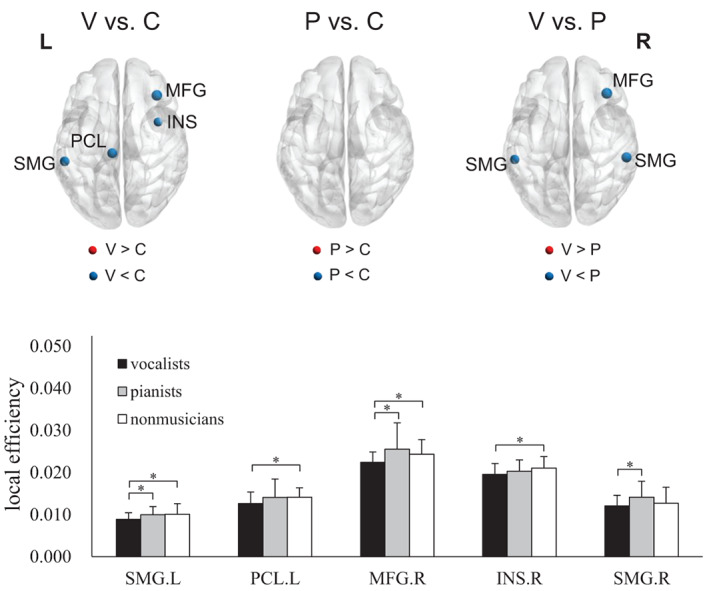
Comparison of local efficiency among the three groups. Note that the size of the sphere indicates the degree of statistical significance between the results from the two groups. Kruskal‐Wallis tests exhibited significant group differences in the left supramarginal gyrus (SMG; H(2) = 6.596, p = .037), left paracentral lobule (PCL; H(2) = 7.309, p = .026), right middle frontal gyrus (MFG; H(2) = 6.565, p = .038), right insula (INS; H(2) = 6.144, p = .046), and right supramarginal gyrus (H(2) = 7.873, p = .020). P, pianists; V, vocalists; C, nonmusicians; L, left; R, right (*FWE‐corrected p < .05; post hoc permutation test using 10,000 iterations)
3.4.4. Strength asymmetry
We observed significant differences between vocalists and pianists in terms of strength asymmetry (SA) scores in the putamen (Figure 4 and Table S5).
FIGURE 4.
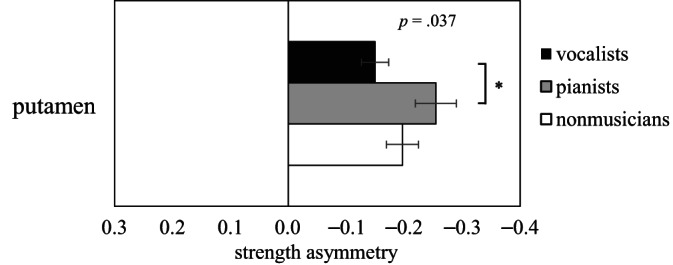
Comparison of regional strength asymmetry (L − R)/(L + R) among the three groups (H(2) = 6.59, p = .037 for one‐way Kruskal‐Wallis test; *FWE‐corrected p < .05; post hoc permutation test using 10,000 iterations)
3.5. Network metrics‐behavior associations
Significant correlations were observed between the duration of vocal training and the network metrics (Table 2). In terms of global network metrics, we observed a significant positive correlation between the duration of vocal training and network density in the left hemisphere. Significantly negative correlations were observed between the duration of vocal training and the local efficiency of local metrics in the left SMG and right MFG.
TABLE 2.
Correlation between the duration of vocal training and network metrics
| Network metrics | Region | Correlation coefficient | p‐value |
|---|---|---|---|
| Global metric | |||
| Density | Left hemisphere | 0.287 | .005 |
| Local metric | |||
| Local efficiency | Left supramarginal gyrus | −0.247 | .016 |
| Right middle frontal gyrus | −0.209 | .042 | |
4. DISCUSSION
In the current study, graph theory was used to explore the organization of white matter following long‐term vocal training. Our results revealed that long‐term vocal training modified the organization of structural networks in both global and local (nodal) facets. Compared with nonmusicians, vocalists exhibited higher density in the left hemisphere (global network metrics). Compared with pianists, vocalists exhibited higher density in the left hemispheres, but lower global efficiency in the right hemisphere. Compared with nonmusicians, both vocalists and pianists showed enhanced strength centrality in emotion‐related regions of the brain (local network metrics). These findings provided neuroimaging evidence of the piano‐ and vocal‐related effects of long‐term musical training on brain networks related to emotion processing, singing performance, and language functions.
4.1. Changes in global network organization
4.1.1. Vocalists versus nonmusicians
Compared to nonmusicians, vocalists presented higher network density in structural networks in the left hemisphere. Prior research in vocalists reported changes in the microstructure of white matter related to language and semantic memory (Ashtari et al., 2007; Halwani et al., 2011). Of note, singing requires proficiency in language processing, which is lateralized to the left hemisphere, particularly in the right‐handed (Knecht et al., 2000). Moreover, previous studies found that long‐term musical training significantly enhances not only verbal function but also visual–spatial and mathematical performance (Schlaug et al., 2005). Our results of higher network density in vocalists are indications of enhanced interconnectivity between brain regions in the left hemisphere. Our results also revealed an association between the duration of vocal training and network density in the left hemisphere, suggesting that the characteristics of long‐term vocal training may play a role in shaping the architecture of structural networks in the left hemisphere.
4.1.2. Vocalists versus pianists
Different types of musical training may differ in the way that they shape cognitive and sensory functions, in accordance with the specific demands imposed by practice (Pantev et al., 2001; Slater et al., 2017). One recent study reported that the ability to discriminate between auditory acoustic features varies according to the type of musical training (Slater et al., 2017). Compared with pianists, vocalists exhibited higher density in the left hemisphere and lower global efficiency in the right hemisphere. In comparison to piano performance, singing requires more language processing, which may cause the enhancement of network density in the left hemisphere, as stated above. Our findings in which vocalists have higher density in the left hemisphere than nonmusician and pianists indicate that the topological organization of brain networks could be shaped in a vocal‐specific manner. Unlike singers, pianists exhibited higher global efficiency only in the right hemisphere. Thus, it appears that in terms of brain networks, the main difference between pianists and singers may lie in the motor control of the muscle groups used for performing. Piano playing requires precise control of finger muscles, whereas singing requires fine motor control of phonatory muscles (e.g., vocalization, respiration, swallowing, and airway protection). The fact that pianists use left‐hand motor control in the right hemisphere far more frequently than do singers may explain the higher global efficiency in the right hemisphere (Bangert & Schlaug, 2006). Note that most vocalists underwent training in piano prior to their vocal training; however, they did not exhibit rightward asymmetry. We therefore speculate that this asymmetry may undergo a reversal after they take up vocal training. Thus, it is reasonable to expect that long‐term piano training could enhance the transfer of information within the right hemisphere. Taken together, our findings indicate that the specific characteristics of musical training (e.g., noninstrumental vs. instrumental) result in different topological characteristics in the subnetwork organization. This is a clear indication of neuroplastic network organization following long‐term training in specific musical disciplines.
4.2. Changes in local network metrics
Different types of musical training differ in the way they affect changes in the organization of local networks related to specific musical skills. For example, vocalists exhibit low local efficiency in the vocal motor control network and left asymmetry in the linguistic network. Furthermore, the rostral SMG contributes significantly to singing performance in trained singers (Kort et al., 2016). Our comparison of vocalists and pianists in terms of local efficiency suggests that professional training reshapes the network organization of the SMG and MFG. Researchers have reported a correlation between SMG activation and speech production (Bilodeau‐Mercure et al., 2015; Tremblay & Deschamps, 2016), and changes in the SMG volume can be predicted based on the age at which formal vocal training began (Kort et al., 2016). Taken together, it appears that vocalists and pianists differ in terms of the structure and function of SMG networks. It also appears that the effects of musical training could conceivably be investigated using network metrics.
4.2.1. Enhanced strength centrality in the emotion‐processing networks of musicians
Musical training (instrumental and noninstrumental) involves learning how to evaluate and comprehend the emotions behind music and lyrics, which may promote interactions between brain regions related to the processing of emotion‐related information (Fruhholz et al., 2014). The intensive practice required to achieve proficiency would help strengthen and reorganize connections among the relevant neural networks. The amygdala, a core region of the limbic network, plays a key role in the processing of affective information obtained from external stimuli (Wiethoff et al., 2009) and the formation of experience‐dependent emotional memory (Vaquero et al., 2016). Our discovery of enhanced strength centrality in the left amygdala of vocalists and pianists indicates a high degree of interconnectivity between the left amygdala and other brain regions. We speculate that frequent practice in extracting the emotional value from music sculpts the local neuronal organization of limbic structures and emotion processing. This suggests that musical training, at least piano training, has effects on experience‐dependent neuroplasticity in amygdala‐centered networks, which is a generic function while performing musical arts.
4.2.2. Lower local efficiency in vocal motor control network of vocalists
The networks involved in vocal motor control and the processing of sensory feedback are crucial to maintaining precise control over the muscles used for singing (Kleber et al., 2007; Zarate, 2013). The right anterior insula, a key node in the “singing network”, regulates the integration of saliency signals and somatosensory signals during vocal production, and could therefore be modulated by vocal training (Kleber et al., 2017). The SMA, another core node in the singing network, integrates motor, visuospatial, semantic, and socio‐emotional information required for ongoing music performance (Tanaka & Kirino, 2017), which is essential for singing. In the current study, vocalists presented significantly lower local efficiency in the right insula and weak‐significantly lower efficiency in the left SMA (p = .083). A decrease in local efficiency such as this may indicate a systematic pruning of task‐irrelevant connections to strengthen functional segregation while performing cognitively demanding tasks under limited resources (Cohen & D'Esposito, 2016; Sporns, 2013). Microstructural and morphological changes in white matter occurred during professional training and brain development. Decreased local efficiency may be associated with the pruning of small white matter fibers and the enhancement of major tracts during development (Huang et al., 2015). Our discovery of lower local efficiency in the insula and SMA of vocalists suggests that specific musical training (i.e., vocal training) may reshape structural connectivity and the organization of experience‐dependent networks (i.e., the singing network) by promoting functional segregation and multisensory integration for the performance of vocal‐related skills.
Singing heavily relies on the control of breathing, regulation of airflow, and maintenance of posture. The muscles of the pelvic floor play an essential role in generating intra‐abdominal pressure, supporting breathing, maintaining the optimal posture of singing, and modulating subglottal pressure; thus, all of which affect phonation and singing (Emerich Gordon & Reed, 2020). Previous reports have shown that the paracentral lobule is associated with the muscle control of toes, feet, and pelvic floor (Asavasopon et al., 2014; Patra et al., 2021). In our study, we found lower local efficiency and strength centrality in the left paracentral lobule in vocalists compared with nonmusicians. Lower local efficiency denotes lower connections among neighboring brain regions, and lower strength centrality represents a lower degree of interconnectivity between the left paracentral lobule and all other brain regions. Taken together, these findings suggest that the paracentral lobule might lose connections with neighboring brain regions, implying that the control of pelvic floor muscles might be centralized in the paracentral lobule without synergistic work with other brain regions.
The regulation of vocal pitch is another crucial skill in singing, particularly when demonstrating examples of skillful phonation and conveying affective information in experienced singers (Zarate & Zatorre, 2008). Vocal pitch control is conducted in three stages: detecting prediction error, coordinating motor changes, and detecting sensory consequences of that action (Kort et al., 2016). Multiple brain regions, including the left auditory cortex, right inferior parietal cortex, and inferior/middle frontal gyrus, are involved in the auditory error detection and auditory feedback control of pitch production (Kort et al., 2016). In line with previous research, the local efficiency of vocalists in this study was lower than that of pianists in multiple brain regions, including the right MFG, SMG, and left Heschl gyrus (lower but not statistically significant; p = .065). Lower local efficiency is an indication of network pruning to promote functional segregation. One previous animal study reported that the learning of novel motor experiences can mediate the structural adaptation of selective neural populations associated with a specific function (Wang et al., 2011). Thus, our findings suggest that the intensive practice of singing skills may increase resource usage in brain regions related to vocal pitch control and shape the architecture of circuits involved in the regulation of vocal pitch by strengthening functional segregation for error detection and feedback control.
4.2.3. Hemispheric asymmetry changes—leftward asymmetry of strength centrality in language network of vocalists
Singers may develop expertise in linguistic expression due to not only musical training but also language training. The repertoire of nearly all singers includes lyrics in a foreign language, the learning of which may promote the development of language skills via cross‐domain transfer effects (Larrouy‐Maestri et al., 2013). Previous evidence has shown that musical training can have positive effects on foreign language processing (Kolinsky et al., 2009) and linguistic tonal processing (Marie et al., 2011). This suggests that the linguistic expression of singers may benefit from vocal training and language courses. The left thalamus has been reported to be involved in the motor‐sensory processing of human vocalization for music and language (Brown et al., 2006). Furthermore, the left putamen is related to the articulation and motor planning of multilingual speech (Abutalebi et al., 2013). Our discovery that vocalists present leftward asymmetry in strength centrality in the subcortical regions of the brain (putamen and thalamus, though not statistically significant in the thalamus; p = .054) indicates that intensive practice of multilingual pronunciation may enhance the efficiency of information transfer among related brain regions. This also suggests that multilingual pronunciation exercises may help to develop subcortical brain networks involved in monitoring articulatory processes.
4.3. Limitations
The present study has some limitations that should be considered. First, our nodal connectivity analysis focused on how vocal experiences sculpt the connectivity strength and efficiency of information transfer. We did not exploit all graph theory measurements in our derivation of nodal measurements. Second, we were unable to determine whether changes in the organization of structural networks in musicians were experience‐dependent or genetic‐dependent. Longitudinal research would be required to clarify the causal relationship between long‐term musical training and structural changes. Third, gender was an uncontrolled factor that could potentially bias our results, due to the ecology of female‐dominant art student population in Taiwan. Further studies taking these factors into account will be required.
5. CONCLUSIONS
To the best of our knowledge, this is the first study to assert that the sustained practice of musical skills can strengthen or prune white matter connectivity networks in an experience‐dependent manner. Our use of graph theory analysis made it possible to measure changes in network metrics and thereby reveal instances of neuroplasticity in global and local architectures following extended musical training (vocal and piano). Overall, our results suggest that both types of musical training strengthen connections among emotion‐related regions of the brain. We also discovered that training specific music‐related skills may reshape the structural organization of experience‐dependent networks. Vocal training appears to alter the networks related to vocal motor control, sensory feedback, and language processing by promoting functional segregation, facilitating multisensory integration, and enhancing network interconnectivity. This work provides a comprehensive assessment of brain plasticity following long‐term and complex musical learning. Our findings provide evidence on the underlying neural mechanisms of musical training and substantial insights into the neuroscientific perspective on art education and aesthetics.
FUNDING INFORMATION
This work was financially supported by the Ministry of Science and Technology of Taiwan (NSC 102‐2420‐H‐075‐001‐MY3, NSC 102‐2420‐H‐010‐004‐MY3, NSC 102‐2420‐H‐010‐005‐MY3, MOST 106‐2420‐H‐010‐006‐MY2, MOST 106‐2420‐H‐010‐005, MOST 106‐2420‐H‐009‐001, MOST 110‐2314‐B‐A49A‐529), the Aim for the Top University Plan of the Ministry of Education for National Yang Ming Chiao Tung University, and Brain Research Center, National Yang Ming Chiao Tung University from The Featured Areas Research Center Program within the framework of the Higher Education Sprout Project by the Ministry of Education (MOE) in Taiwan. The funders had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
Supporting information
FIGURE S1 No significant difference was found in the number of whole‐brain streamlines among the three groups (H(2) = 1.08, p = .583 for one‐way Kruskal‐Wallis test)
FIGURE S2 Illustration of strength centrality and betweenness centrality. Note that the size of the spheres represents the mean score of the three groups. L, left hemisphere; R, right hemisphere
FIGURE S3 Comparison of betweenness centrality among the three groups. Note that the size of the sphere indicates the degree of statistical significance between the results from the two groups. The Kruskal‐Wallis tests revealed significant group differences in the left middle temporal gyrus (MTG; H(2) = 6.553, p = .038), left inferior temporal gyrus (ITG; H(2) = 9.273, p = .010), right precuneus (PCUN; H(2) = 7.816, p = .020), right cuneus (CUN; H(2) = 6.788, p = .034). P, pianists; V, vocalists; C, nonmusicians; L, left; R, right (*FWE‐corrected p < .05, post hoc permutation test using 10,000 iterations)
TABLE S1 Summary of network measurement in the study cohort
TABLE S2. Network measurements of each hemisphere in the study cohort
TABLE S3. Comparison of strength centrality in the study cohort
TABLE S4. Comparison of local efficiency in the study cohort
TABLE S5. Comparison of strength asymmetry in the study cohort
ACKNOWLEDGMENTS
The authors appreciate Taipei National University of the Arts supported the recruitment of experiment subjects.
Cheng, L.‐K. , Chiu, Y.‐H. , Lin, Y.‐C. , Li, W.‐C. , Hong, T.‐Y. , Yang, C.‐J. , Shih, C.‐H. , Yeh, T.‐C. , Tseng, W.‐Y.I. , Yu, H.‐Y. , Hsieh, J.‐C. , & Chen, L.‐F. (2023). Long‐term musical training induces white matter plasticity in emotion and language networks. Human Brain Mapping, 44(1), 5–17. 10.1002/hbm.26054
Funding information Ministry of Science and Technology, Taiwan, Grant/Award Numbers: MOST 106‐2420‐H‐009‐001, MOST 106‐2420‐H‐010‐005, MOST 106‐2420‐H‐010‐006‐MY2, MOST 110‐2314‐B‐A49A‐529, NSC 102‐2420‐H‐010‐004‐MY3, NSC 102‐2420‐H‐010‐005‐MY3, NSC 102‐2420‐H‐075‐001‐MY3; The Aim for the Top University Plan of the Ministry of Education for National Yang Ming Chiao Tung University; Brain Research Center, National Yang Ming Chiao Tung University from The Featured Areas Research Center Program within the framework of the Higher Education Sprout Project by the Ministry of Education in Taiwan
Contributor Information
Jen‐Chuen Hsieh, Email: jchsiehibru@nycu.edu.tw.
Li‐Fen Chen, Email: lfchen@nycu.edu.tw.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- Abutalebi, J. , Della Rosa, P. A. , Gonzaga, A. K. , Keim, R. , Costa, A. , & Perani, D. (2013). The role of the left putamen in multilingual language production. Brain and Language, 125, 307–315. [DOI] [PubMed] [Google Scholar]
- Achard, S. , & Bullmore, E. (2007). Efficiency and cost of economical brain functional networks. PLoS Computational Biology, 3, e17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asavasopon, S. , Rana, M. , Kirages, D. J. , Yani, M. S. , Fisher, B. E. , Hwang, D. H. , Lohman, E. B. , Berk, L. S. , & Kutch, J. J. (2014). Cortical activation associated with muscle synergies of the human male pelvic floor. The Journal of Neuroscience, 34, 13811–13818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashtari, M. , Cervellione, K. L. , Hasan, K. M. , Wu, J. , McIlree, C. , Kester, H. , Ardekani, B. A. , Roofeh, D. , Szeszko, P. R. , & Kumra, S. (2007). White matter development during late adolescence in healthy males: A cross‐sectional diffusion tensor imaging study. NeuroImage, 35, 501–510. [DOI] [PubMed] [Google Scholar]
- Bangert, M. , & Schlaug, G. (2006). Specialization of the specialized in features of external human brain morphology. The European Journal of Neuroscience, 24, 1832–1834. [DOI] [PubMed] [Google Scholar]
- Barrett, K. , Ashley, R. , Strait, D. , & Kraus, N. (2013). Art and science: How musical training shapes the brain. Frontiers in Psychology, 4, 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassett, D. S. , & Bullmore, E. (2006). Small‐world brain networks. The Neuroscientist, 12, 512–523. [DOI] [PubMed] [Google Scholar]
- Bilodeau‐Mercure, M. , Lortie, C. L. , Sato, M. , Guitton, M. J. , & Tremblay, P. (2015). The neurobiology of speech perception decline in aging. Brain Structure & Function, 220, 979–997. [DOI] [PubMed] [Google Scholar]
- Blair, R. C. , & Karniski, W. (1993). An alternative method for significance testing of waveform difference potentials. Psychophysiology, 30, 518–524. [DOI] [PubMed] [Google Scholar]
- Brandes, U. (2001). A faster algorithm for betweenness centrality. Journal of Mathematical Sociology, 25, 163–177. [Google Scholar]
- Brown, S. , Martinez, M. J. , & Parsons, L. M. (2006). Music and language side by side in the brain: A pet study of the generation of melodies and sentences. The European Journal of Neuroscience, 23, 2791–2803. [DOI] [PubMed] [Google Scholar]
- Catani, M. , Allin, M. P. , Husain, M. , Pugliese, L. , Mesulam, M. M. , Murray, R. M. , & Jones, D. K. (2007). Symmetries in human brain language pathways correlate with verbal recall. Proceedings of the National Academy of Sciences of the United States of America, 104, 17163–17168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, Y. J. , Lo, Y. C. , Hsu, Y. C. , Fan, C. C. , Hwang, T. J. , Liu, C. M. , Chien, Y. L. , Hsieh, M. H. , Liu, C. C. , Hwu, H. G. , & Tseng, W. Y. (2015). Automatic whole brain tract‐based analysis using predefined tracts in a diffusion spectrum imaging template and an accurate registration strategy. Human Brain Mapping, 36, 3441–3458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen, J. R. , & D'Esposito, M. (2016). The segregation and integration of distinct brain networks and their relationship to cognition. The Journal of Neuroscience, 36, 12083–12094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhakal, K. , Norgaard, M. , & Dhamala, M. (2021). Enhanced white matter fiber tracts in advanced jazz improvisers. Brain Sciences, 11, 506–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elmer, S. , Hänggi, J. , & Jäncke, L. (2016). Interhemispheric transcallosal connectivity between the left and right planum temporale predicts musicianship, performance in temporal speech processing, and functional specialization. Brain Structure & Function, 221, 331–344. [DOI] [PubMed] [Google Scholar]
- Emerich Gordon, K. , & Reed, O. (2020). The role of the pelvic floor in respiration: A multidisciplinary literature review. Journal of Voice, 34, 243–249. [DOI] [PubMed] [Google Scholar]
- Estrada, E. , & Hatano, N. (2008). Communicability in complex networks. Physical Review E, 77, 036111. [DOI] [PubMed] [Google Scholar]
- Fernandez‐Miranda, J. C. , Wang, Y. , Pathak, S. , Stefaneau, L. , Verstynen, T. , & Yeh, F. C. (2015). Asymmetry, connectivity, and segmentation of the arcuate fascicle in the human brain. Brain Structure & Function, 220, 1665–1680. [DOI] [PubMed] [Google Scholar]
- Foster, N. E. , & Zatorre, R. J. (2010). Cortical structure predicts success in performing musical transformation judgments. NeuroImage, 53, 26–36. [DOI] [PubMed] [Google Scholar]
- Fruhholz, S. , Trost, W. , & Grandjean, D. (2014). The role of the medial temporal limbic system in processing emotions in voice and music. Progress in Neurobiology, 123, 1–17. [DOI] [PubMed] [Google Scholar]
- Gackle, L. (2019). Adolescent girls' singing development. In The Oxford handbook of singing (pp. 551–570). Oxford University Press. [Google Scholar]
- Groussard, M. , Viader, F. , Landeau, B. , Desgranges, B. , Eustache, F. , & Platel, H. (2014). The effects of musical practice on structural plasticity: The dynamics of grey matter changes. Brain and Cognition, 90, 174–180. [DOI] [PubMed] [Google Scholar]
- Habibi, A. , Damasio, A. , Ilari, B. , Veiga, R. , Joshi, A. A. , Leahy, R. M. , Haldar, J. P. , Varadarajan, D. , Bhushan, C. , & Damasio, H. (2018). Childhood music training induces change in micro and macroscopic brain structure: Results from a longitudinal study. Cerebral Cortex, 28, 4336–4347. [DOI] [PubMed] [Google Scholar]
- Halwani, G. F. , Loui, P. , Ruber, T. , & Schlaug, G. (2011). Effects of practice and experience on the arcuate fasciculus: Comparing singers, instrumentalists, and non‐musicians. Frontiers in Psychology, 2, 156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heun, R. , Freymann, N. , Granath, D. O. , Stracke, C. P. , Jessen, F. , Barkow, K. , & Reul, J. (2004). Differences of cerebral activation between superior and inferior learners during motor sequence encoding and retrieval. Psychiatry Research, 132, 19–32. [DOI] [PubMed] [Google Scholar]
- Hsu, Y. C. , Lo, Y. C. , Chen, Y. J. , Wedeen, V. J. , & Isaac Tseng, W. Y. (2015). NTU‐DSI‐122: A diffusion spectrum imaging template with high anatomical matching to the ICBM‐152 space. Human Brain Mapping, 36, 3528–3541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang, H. , Shu, N. , Mishra, V. , Jeon, T. , Chalak, L. , Wang, Z. J. , Rollins, N. , Gong, G. , Cheng, H. , Peng, Y. , Dong, Q. , & He, Y. (2015). Development of human brain structural networks through infancy and childhood. Cerebral Cortex, 25, 1389–1404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyde, K. L. , Lerch, J. , Norton, A. , Forgeard, M. , Winner, E. , Evans, A. C. , & Schlaug, G. (2009). Musical training shapes structural brain development. The Journal of Neuroscience, 29, 3019–3025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jancke, L. , Langer, N. , & Hanggi, J. (2012). Diminished whole‐brain but enhanced peri‐sylvian connectivity in absolute pitch musicians. Journal of Cognitive Neuroscience, 24, 1447–1461. [DOI] [PubMed] [Google Scholar]
- Jansens, S. , Bloothooft, G. , & de Krom, G. (1997). Perception and acoustics of emotions in singing. Proceedings of the 5th Eurospeech, Rhodes, IV, 2155–2158.
- Juslin, P. N. , & Laukka, P. (2003). Communication of emotions in vocal expression and music performance: Different channels, same code? Psychological Bulletin, 129, 770–814. [DOI] [PubMed] [Google Scholar]
- Kintali, S. (2008). Betweenness centrality: Algorithms and lower bounds. arXiv preprint arXiv:0809.1906.
- Kleber, B. , Birbaumer, N. , Veit, R. , Trevorrow, T. , & Lotze, M. (2007). Overt and imagined singing of an italian aria. NeuroImage, 36, 889–900. [DOI] [PubMed] [Google Scholar]
- Kleber, B. , Friberg, A. , Zeitouni, A. , & Zatorre, R. (2017). Experience‐dependent modulation of right anterior insula and sensorimotor regions as a function of noise‐masked auditory feedback in singers and nonsingers. NeuroImage, 147, 97–110. [DOI] [PubMed] [Google Scholar]
- Knecht, S. , Deppe, M. , Drager, B. , Bobe, L. , Lohmann, H. , Ringelstein, E. , & Henningsen, H. (2000). Language lateralization in healthy right‐handers. Brain, 123(Pt 1), 74–81. [DOI] [PubMed] [Google Scholar]
- Knight, S. (1999). Exploring a cultural myth: What adult non‐singers may reveal about the nature of singing. In Roberts B. R. A. (Ed.), The phenomenon of singing (pp. 144–154). Memorial University of Newfoundland. [Google Scholar]
- Kolinsky, R. , Cuvelier, H. , Goetry, V. , Peretz, I. , & Morais, J. (2009). Music training facilitates lexical stress processing. Music Perception, 26, 235–246. [Google Scholar]
- Kort, N. S. , Cuesta, P. , Houde, J. F. , & Nagarajan, S. S. (2016). Bihemispheric network dynamics coordinating vocal feedback control. Human Brain Mapping, 37, 1474–1485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo, L. W. , Chen, J. H. , Wedeen, V. J. , & Tseng, W. Y. (2008). Optimization of diffusion spectrum imaging and q‐ball imaging on clinical MRI system. NeuroImage, 41, 7–18. [DOI] [PubMed] [Google Scholar]
- Landau, S. M. , & D'Esposito, M. (2006). Sequence learning in pianists and nonpianists: An fMRI study of motor expertise. Cognitive, Affective, & Behavioral Neuroscience, 6, 246–259. [DOI] [PubMed] [Google Scholar]
- Larrouy‐Maestri, P. , Leybaert, J. , & Kolinsky, R. (2013). The benefit of musical and linguistic expertise on language acquisition in sung material. Musicae Scientiae, 17, 217–228. [Google Scholar]
- Latora, V. , & Marchiori, M. (2001). Efficient behavior of small‐world networks. Physical Review Letters, 87, 198701. [DOI] [PubMed] [Google Scholar]
- Leipold, S. , Klein, C. , & Jäncke, L. (2021). Musical expertise shapes functional and structural brain networks independent of absolute pitch ability. The Journal of Neuroscience, 41, 2496–2511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, J. , Luo, C. , Peng, Y. , Xie, Q. , Gong, J. , Dong, L. , Lai, Y. , Li, H. , & Yao, D. (2014). Probabilistic diffusion tractography reveals improvement of structural network in musicians. PLoS One, 9, e105508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lomax, A. (2017). Folk song style and culture. Taylor & Francis Group. [Google Scholar]
- Loui, P. , Li, H. C. , Hohmann, A. , & Schlaug, G. (2011). Enhanced cortical connectivity in absolute pitch musicians: A model for local hyperconnectivity. Journal of Cognitive Neuroscience, 23, 1015–1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marie, C. , Delogu, F. , Lampis, G. , Belardinelli, M. O. , & Besson, M. (2011). Influence of musical expertise on segmental and tonal processing in mandarin chinese. Journal of Cognitive Neuroscience, 23, 2701–2715. [DOI] [PubMed] [Google Scholar]
- Miendlarzewska, E. A. , & Trost, W. J. (2013). How musical training affects cognitive development: Rhythm, reward and other modulating variables. Frontiers in Neuroscience, 7, 279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nettl, B. (2015). The study of ethnomusicology: Thirty‐three discussions. University of Illinois Press. [Google Scholar]
- Pantev, C. , Engelien, A. , Candia, V. , & Elbert, T. (2001). Representational cortex in musicians. Plastic alterations in response to musical practice. Annals of the New York Academy of Sciences, 930, 300–314. [PubMed] [Google Scholar]
- Patra, A. , Kaur, H. , Chaudhary, P. , Asghar, A. , & Singal, A. (2021). Morphology and morphometry of human paracentral lobule: An anatomical study with its application in neurosurgery. Asian Journal of Neurosurgery, 16, 349–354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubinov, M. , & Sporns, O. (2010). Complex network measures of brain connectivity: Uses and interpretations. NeuroImage, 52, 1059–1069. [DOI] [PubMed] [Google Scholar]
- Scherer, K. R. (2003). Vocal communication of emotion: A review of research paradigms. Speech Communication, 40, 227–256. [Google Scholar]
- Scherer, K. R. , Sundberg, J. , Fantini, B. , Trznadel, S. , & Eyben, F. (2017). The expression of emotion in the singing voice: Acoustic patterns in vocal performance. The Journal of the Acoustical Society of America, 142, 1805–1815. [DOI] [PubMed] [Google Scholar]
- Schlaug, G. , Norton, A. , Overy, K. , & Winner, E. (2005). Effects of music training on the child's brain and cognitive development. Annals of the New York Academy of Sciences, 1060, 219–230. [DOI] [PubMed] [Google Scholar]
- Schmithorst, V. J. , & Wilke, M. (2002). Differences in white matter architecture between musicians and non‐musicians: A diffusion tensor imaging study. Neuroscience Letters, 321, 57–60. [DOI] [PubMed] [Google Scholar]
- Shenker, J. J. , Steele, C. J. , Chakravarty, M. M. , Zatorre, R. J. , & Penhune, V. B. (2021). Early musical training shapes cortico‐cerebellar structural covariation. Brain Structure & Function, 227, 407–419. [DOI] [PubMed] [Google Scholar]
- Slater, J. , Azem, A. , Nicol, T. , Swedenborg, B. , & Kraus, N. (2017). Variations on the theme of musical expertise: Cognitive and sensory processing in percussionists, vocalists and non‐musicians. The European Journal of Neuroscience, 45, 952–963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sporns, O. (2013). Network attributes for segregation and integration in the human brain. Current Opinion in Neurobiology, 23, 162–171. [DOI] [PubMed] [Google Scholar]
- Sporns, O. (2014). Contributions and challenges for network models in cognitive neuroscience. Nature Neuroscience, 17, 652–660. [DOI] [PubMed] [Google Scholar]
- Sporns, O. (2016). Networks of the brain. MIT Press. [Google Scholar]
- Tanaka, S. , & Kirino, E. (2017). Dynamic reconfiguration of the supplementary motor area network during imagined music performance. Frontiers in Human Neuroscience, 11, 606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tremblay, P. , & Deschamps, I. (2016). Structural brain aging and speech production: A surface‐based brain morphometry study. Brain Structure & Function, 221, 3275–3299. [DOI] [PubMed] [Google Scholar]
- Tzourio‐Mazoyer, N. , Landeau, B. , Papathanassiou, D. , Crivello, F. , Etard, O. , Delcroix, N. , Mazoyer, B. , & Joliot, M. (2002). Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single‐subject brain. NeuroImage, 15, 273–289. [DOI] [PubMed] [Google Scholar]
- Vaquero, L. , Hartmann, K. , Ripolles, P. , Rojo, N. , Sierpowska, J. , Francois, C. , Camara, E. , van Vugt, F. T. , Mohammadi, B. , Samii, A. , Munte, T. F. , Rodriguez‐Fornells, A. , & Altenmuller, E. (2016). Structural neuroplasticity in expert pianists depends on the age of musical training onset. NeuroImage, 126, 106–119. [DOI] [PubMed] [Google Scholar]
- Veraart, J. , Fieremans, E. , & Novikov, D. S. (2016). Diffusion MRI noise mapping using random matrix theory. Magnetic Resonance in Medicine, 76, 1582–1593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, L. , Conner, J. M. , Rickert, J. , & Tuszynski, M. H. (2011). Structural plasticity within highly specific neuronal populations identifies a unique parcellation of motor learning in the adult brain. Proceedings of the National Academy of Sciences of the United States of America, 108, 2545–2550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, W. , Wei, L. , Chen, N. , Jones, J. A. , Gong, G. , & Liu, H. (2019). Decreased gray‐matter volume in insular cortex as a correlate of singers' enhanced sensorimotor control of vocal production. Frontiers in Neuroscience, 13, 815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wedeen, V. J. , Hagmann, P. , Tseng, W. Y. I. , Reese, T. G. , & Weisskoff, R. M. (2005). Mapping complex tissue architecture with diffusion spectrum magnetic resonance imaging. Magnetic Resonance in Medicine, 54, 1377–1386. [DOI] [PubMed] [Google Scholar]
- Westfall, P. H. , & Young, S. S. (1993). Resampling‐based multiple testing: Examples and methods for p‐value adjustment. John Wiley & Sons. [Google Scholar]
- Wiethoff, S. , Wildgruber, D. , Grodd, W. , & Ethofer, T. (2009). Response and habituation of the amygdala during processing of emotional prosody. Neuroreport, 20, 1356–1360. [DOI] [PubMed] [Google Scholar]
- Williams, J. , & Harrison, S. (2019). Boys' singing voice change in adolescence. In Welch G. F., Howard D. M., & Nix J. (Eds.), The Oxford handbook of singing (pp. 533–550). Oxford University Press. [Google Scholar]
- Xia, M. , Wang, J. , & He, Y. (2013). Brainnet viewer: A network visualization tool for human brain connectomics. PLoS One, 8, e68910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeh, F.‐C. , Panesar, S. , Fernandes, D. , Meola, A. , Yoshino, M. , Fernandez‐Miranda, J. C. , Vettel, J. M. , & Verstynen, T. (2018). Population‐averaged atlas of the macroscale human structural connectome and its network topology. NeuroImage, 178, 57–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeh, F. C. , Verstynen, T. D. , Wang, Y. , Fernandez‐Miranda, J. C. , & Tseng, W. Y. (2013). Deterministic diffusion fiber tracking improved by quantitative anisotropy. PLoS One, 8, e80713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeh, F. C. , Wedeen, V. J. , & Tseng, W. Y. (2010). Generalized q‐sampling imaging. IEEE Transactions on Medical Imaging, 29, 1626–1635. [DOI] [PubMed] [Google Scholar]
- Zalesky, A. , Fornito, A. , & Bullmore, E. T. (2010). Network‐based statistic: Identifying differences in brain networks. NeuroImage, 53, 1197–1207. [DOI] [PubMed] [Google Scholar]
- Zarate, J. M. (2013). The neural control of singing. Frontiers in Human Neuroscience, 7, 237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarate, J. M. , & Zatorre, R. J. (2008). Experience‐dependent neural substrates involved in vocal pitch regulation during singing. NeuroImage, 40, 1871–1887. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
FIGURE S1 No significant difference was found in the number of whole‐brain streamlines among the three groups (H(2) = 1.08, p = .583 for one‐way Kruskal‐Wallis test)
FIGURE S2 Illustration of strength centrality and betweenness centrality. Note that the size of the spheres represents the mean score of the three groups. L, left hemisphere; R, right hemisphere
FIGURE S3 Comparison of betweenness centrality among the three groups. Note that the size of the sphere indicates the degree of statistical significance between the results from the two groups. The Kruskal‐Wallis tests revealed significant group differences in the left middle temporal gyrus (MTG; H(2) = 6.553, p = .038), left inferior temporal gyrus (ITG; H(2) = 9.273, p = .010), right precuneus (PCUN; H(2) = 7.816, p = .020), right cuneus (CUN; H(2) = 6.788, p = .034). P, pianists; V, vocalists; C, nonmusicians; L, left; R, right (*FWE‐corrected p < .05, post hoc permutation test using 10,000 iterations)
TABLE S1 Summary of network measurement in the study cohort
TABLE S2. Network measurements of each hemisphere in the study cohort
TABLE S3. Comparison of strength centrality in the study cohort
TABLE S4. Comparison of local efficiency in the study cohort
TABLE S5. Comparison of strength asymmetry in the study cohort
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


