Abstract
Background
Over 250 million children under 5 years, globally, are at risk of developmental delay. Interventions during the first 2 years of life have enduring positive effects if children at risk are identified, using standardized assessments, within this window. However, identifying developmental delay during infancy is challenging and there are limited infant development assessments suitable for use in low- and middle-income (LMIC) settings. Here, we describe a new tool, the Oxford Neurodevelopment Assessment (OX-NDA), measuring cognition, language, motor, and behaviour, outcomes in 1-year-old children. We present the results of its evaluation against the Bayley Scales of Infant Development IIIrd edition (BSID-III) and its psychometric properties.
Methods
Sixteen international tools measuring infant development were analysed to inform the OX-NDA’s construction. Its agreement with the BSID-III, for cognitive, motor and language domains, was evaluated using intra-class correlations (ICCs, for absolute agreement), Bland-Altman analyses (for bias and limits of agreement), and sensitivity and specificity analyses (for accuracy) in 104 Brazilian children, aged 12 months (SD 8.4 days), recruited from the 2015 Pelotas Birth Cohort Study. Behaviour was not evaluated, as the BSID-III’s adaptive behaviour scale was not included in the cohort’s protocol. Cohen’s kappas and Cronbach’s alphas were calculated to determine the OX-NDA’s reliability and internal consistency respectively.
Results
Agreement was moderate for cognition and motor outcomes (ICCs 0.63 and 0.68, p < 0.001) and low for language outcomes (ICC 0.30, p < 0.04). Bland-Altman analysis showed little to no bias between measures across domains. The OX-NDA’s sensitivity and specificity for predicting moderate-to-severe delay on the BSID-III was 76, 73 and 43% and 75, 80 and 33% for cognition, motor and language outcomes, respectively. Inter-rater (k = 0.80-0.96) and test-rest (k = 0.85-0.94) reliability was high for all domains. Administration time was < 20 minutes.
Conclusion
The OX-NDA shows moderate agreement with the BSID-III for identifying infants at risk of cognitive and motor delay; agreement was low for language delay. It is a rapid, low-cost assessment constructed specifically for use in LMIC populations. Further work is needed to evaluate its use (i) across domains in populations beyond Brazil and (ii) to identify language delays in Brazilian children.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12887-022-03794-1.
Keywords: Neurodevelopment, Early child development, Infant development, Developmental delay, OX-NDA, 2015 Pelotas birth cohort study
Background
In 2015, Early Child Development (ECD) was, for the first time, included in the Sustainable Development Goals as indicator 4.2.1: “the proportion of children under 5 years of age who are developmentally on track in health, learning and psychosocial well-being, by sex” [1]. Nevertheless, over 250 million children, globally, are at risk of not achieving their developmental potential due to poverty and stunting alone [2]. A disadvantaged start in life limits children’s abilities to benefit from education leading to lower productivity and social consequences that affect not only present but also future generations [3–7]. Moreover, brain stimulation interventions during the first 2 years of life have been to have enduring positive effects if children at risk are identified [3], using standardized assessments, and receive such interventions, within this window. One of the key challenges in the ECD landscape, therefore, is the early and accurate identification of children at risk, using standardized methodologies, to target interventions and to enable cross-population comparisons [5, 7, 8].
Many developmental batteries assess ECD outcomes during the first year of life [9] (Additional file 1: Table S1). However, only a few can be administered reliably by non-specialists and be applied across population groups from high-, middle- and low-income settings at relatively low costs [9–12]. Between 2013 and 2018, a WHO commissioned team identified only 3 initiatives that attempted to address these technical and logistical challenges [12]: (i) the WHO Gross Motor Milestones [13, 14], (ii) the Ages and Stages Questionnaires (ASQ) [15], and (iii) the Guide for Monitoring Child Development (GMCD) [16, 17]. Two of these (the ASQ and GMCD) were caregiver-reports; and the WHO milestones, although observer-rated, focussed exclusively on measuring gross motor skills.
Previous ECD research has shown that a mixed-methodology approach, combining direct-assessments with observer- and caregiver-reports, provides advantages over either method alone. This approach has been successfully applied in several developmental tests including the Batelle Developmental Inventory (BDI) [18, 19], the Test de Aprendizaje y Desarrollo Infantil (TADI) [20] and the INTERGROWTH-21st Neurodevelopment Assessment (INTER-NDA; www.inter-nda.com) [21]. The latter is a rapid, standardized, psychometrically valid 37-item international ECD instrument measuring cognition, fine and gross motor, language and behaviour outcomes in children aged 22 to 30 months [21, 22]. It can be administered reliably by non-specialists to identify children at risk of delay, and its norms are international ECD standards constructed according to the WHO’s prescriptive approach [23].
The adoption of the INTER-NDA - for the identification of children at risk of delay and for cross-population comparisons - by the clinicians and researchers, internationally, prompted demand for a tool, similar in conceptual and technical constructs, for the comprehensive and reliable assessment of outcomes in younger children [24]. The objective of this study is (i) to describe the rationale and methodology leading to the construction of a novel infant development assessment meeting the aforementioned specifications (the Oxford Neurodevelopment Assessment; OX-NDA) and (ii) to evaluate its performance against the Bayley Scales of Infant Development III edition (BSID-III) [25]. The specific aims of the current study were to: (i) examine agreement between OX-NDA and BSID-III domain scores; (ii) evaluate the OX-NDA’s sensitivity and specificity in predicting moderate-to-severe developmental delay on the BSID-III; and (iii) determine the OX-NDA’s internal consistency and rater reliability.
Methods
Participants and procedures
Infants enrolled in the 2015 Pelotas Birth Cohort Study [26], were consecutively recruited to participate in the OX-NDA validation study. Children with known severe hearing or vision impairments, and non-singleton children, were excluded from participation.
The 2015 Pelotas Birth Cohort is a large epidemiological study of child health, growth and development in the city of Pelotas in Southern Brazil. The cohort included 4275 newborns born between 1 January and 31 December 2015. Mothers and children were assessed at birth, and at 3, 12, 24 and 48 months post birth. Details of the cohort have been previously published [26]. The cohort is registered at clinicaltrials.gov [NCT03271723].
Participating children were evaluated on the OX-NDA and the BSID-III between 10 and 14 months by separate assessors, blinded to each other results. Assessments were conducted in Brazilian Portuguese in the children’s homes, in the presence of a primary caregiver over two consecutive days. In half the sample, the BSID-III was administered first; in the remaining sample, the OX-NDA was administered first. We did not evaluate children on both instruments in a single sitting to avoid children’s underperformance due to fatigue from repeated testing and because of the BSID-III’s long administration time (60-90 minutes). The reliability of the OX-NDA was determined for four non-specialist field assessors across 10 assessments.
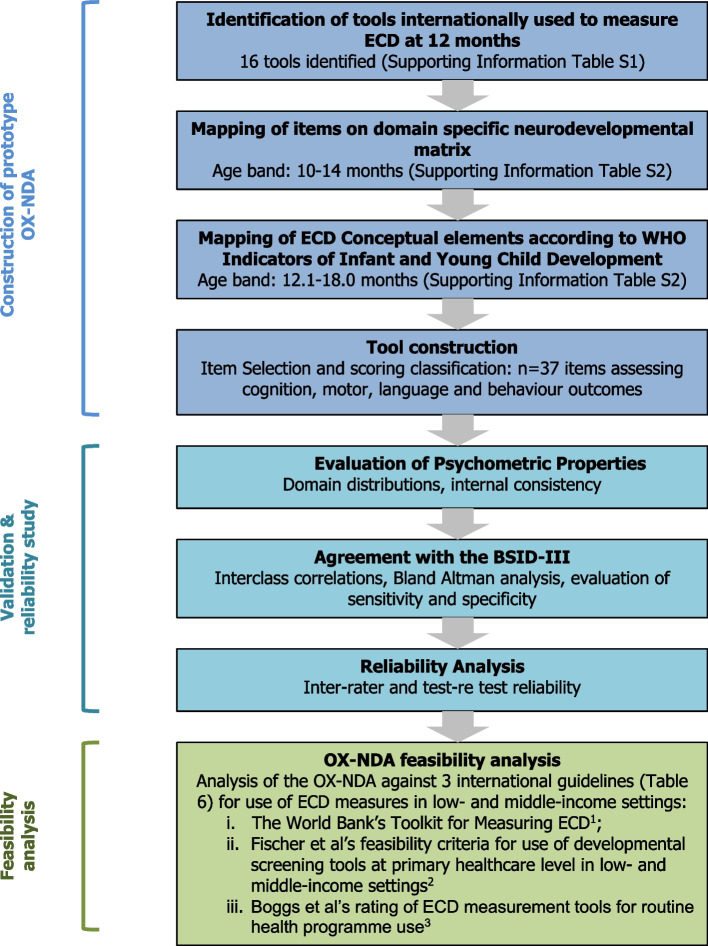
The design, development and evaluation process of the OX-NDA is summarised in Fig. 1.
Fig. 1.
Flow chart of OX-NDA development and study process
Measures
The BSID-III: The BSID-III measures cognition, language, motor, social-emotional, and adaptive behaviour outcomes in children aged 1-42 months [25, 27]. The thresholds for moderate and severe delay on its composite scores (Mean 100, SD 15) are 71-85 and ≤ 70, respectively [27]. Its latest norms were developed from 1700 US children recruited in 2004 and selected to match the 2000 United States census population of children [25]. The cost of the BSID-III kit, at the time of writing this paper, is GBP 1521.64, the cost per form per child is GBP 4.89 [27]. In this study, the adaptive behaviour subscale of the BSID-III was not included in the cohort’s protocol.
The OX-NDA: The OX-NDA is a novel multi-dimensional ECD assessment for children aged 10-14 months [24] consisting of 37 items grouped into cognition, motor, language, and positive and negative behaviour domains (Table 1). Of these items, 21 are directly assessed, 13 are concurrently observed and 3 are caregiver reported. Item scores range from 4 (highest) to 1 (lowest) with ‘unable to assess’ being scored as ‘X’: items scored ‘unable to assess’ are excluded from the calculation of mean domain scores. Its kit (Fig. 2) costs GBP 100.00 in the UK, but sourcing items locally can substantially reduce costs. There is no fee per child for use. The OX-NDA items were created in English and were translated into Brazilian Portuguese according to the WHO Mental Health Initiative translation guidelines which includes processes of translation, back translation and cultural customization [28].
Table 1.
OX-NDA Domain Classification, Scoring Formulae and Interpretation
| OX-NDA domain | Number of items contributing to domain | Range for item scores | Constituent item numbers for domain | Raw domain score estimation | Scaling formula for conversion of raw domain scores to standardized scores (range 0-100) | Interpretation of domain score |
|---|---|---|---|---|---|---|
| Cognition | 15 | 1 - 4 | 1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 13, 14, 15, 16 | Mean of constituent item scores | ((x – 1) / 3)) * 100 | Higher score reflects better performance |
| Motor | 8 | 1 - 4 | 12, 17, 18, 22, 23, 24, 25, 26 | Mean of constituent item scores | ((x – 1) / 3)) * 100 | Higher score reflects better performance |
| Language | 7 | 1 - 4 | 19, 20, 21, 27, 28, 29, 30 | Mean of constituent item scores | ((x – 1) / 3)) * 100 | Higher score reflects better performance |
| Positive behaviour | 5 | 1 - 3 | 31,32,33,34,35 | Mean of constituent item scores | ((x – 1) / 2)) * 100 | Higher score reflects better performance |
| Negative behaviour | 2 | 1 - 3 | 36,37 | Mean of constituent item scores | ((x – 1) / 2)) * 100 | Lower score reflects better performance |
Fig. 2.

OX-NDA Kit
The construction of the OX-NDA involved five stages:
A landscape analysis of widely used tools measuring child development at 12 months across populations (Additional file 1: Table S1)
An analysis mapping the conceptual basis of the constituent items within each tool onto a ‘neurodevelopment domain’ matrix (Additional file 2: Table S2).
A second analysis mapping these items onto the WHO Indicators of Infant and Young Child Development (IYCD) ECD elements for 6.1-18 month age bands [12] (Additional file 2: Table S2).
Selection, by a panel of regional and international experts in ECD measurement, of the most appropriate ECD elements (n = 41) for inclusion in the prototype OX-NDA and construction of a 5-point performance reporting scale for each item (Table 1), similar to that of the INTER-NDA, to objectively measure a child’s level of achievement for the respective item (Additional file 3: Table S3).
Initial piloting and assessment of internal consistency (Table 3) resulted in excluding 4 items. The OX-NDA format for further psychometric testing and validation against the BSID-III therefore consisted of 37 items (Additional file 3: Table S3).
Table 3.
Internal consistencies of OX-NDA domain scores
| OX-NDA Domain | Prototype OX-NDA | Final OX-NDA | ||
|---|---|---|---|---|
| N (items) | Cronbach’s alphas | N (items) | Cronbach’s alphas | |
| Cognition | 15 | 0.71 | 15 | 0.71 |
| Motor | 8 | 0.70 | 8 | 0.70 |
| Language | 8 | 0.30 | 7 | 0.40 |
| Executive Function | 3 | 0.42 | 0 | – |
| Positive behaviour | 5 | 0.72 | 5 | 0.72 |
| Negative behaviour | 2 | 0.22 | 2 | 0.22 |
OX-NDA The Oxford Neurodevelopment Assessment
Sample size and statistical analysis
Statistical analysis was conducted in Stata 15 software (Stata Corp., College Station, TX, USA). Accounting for attrition, a sample size of 104 was estimated to detected associations at a confidence level of 95% and significance level of < 0.05. Raw mean OX-NDA domain scores were calculated and converted to standardised scaled scores (Mean 50, SD 25, range 0 to 100; Table 1), and distributions explored. The following analyses were carried out:
Internal consistency: was determined for OX-NDA domain scores using Cronbach’s alphas. Values ≥0.7 were considered “good” [29, 30].
Agreement between the OX-NDA and BSID-III: was evaluated using four statistical methods, as recommended by Lee [31] and Bland and Altman [32]:
-
i.
Continuous correlations (Pearson) to determine whether children who scored high on the OX-NDA also scored high on the BSID-III;
-
ii.
Single measure intra-class correlation coefficients (ICCs) for absolute agreement between each domain, using a two-way mixed effects model (to quantify the strength of the association between OX-NDA and BSID-III scores);
-
iii.
Bias and limits of agreement statistics; and
-
iv.
Bland-Altman plots to identify whether the OX-NDA scores differed systematically across different levels of the BSID-III.
-
3.
Sensitivity and Specificity Analyses: OX-NDA scores were compared between children scoring ≥85 (to assess OX-NDA sensitivity) and < 85 on BSID-III composites (to assess specificity). Cut-offs with highest sensitivity and specificity for each OX-NDA domain were determined using Receiver Operating Characteristics (ROC) analyses. Positive and negative predictive values and positive and negative likelihood ratios for each OX-NDA cut-off threshold were calculated.
-
4.
Inter-rater and Test-re test Reliability: was determined using Cohen’s kappa coefficients [33].
Ethics
Our study was approved by the Research Ethics Committee of the Medical School (No 1.400.585) and the School of Physical Education at the Federal University of Pelotas, Brazil (CAAE registration number: 26746414.5.0000.5313). Mothers provided written informed consent on behalf of their children.
Results
Sample characteristics
Of the cohort’s 4018 children followed-up at 12 months, a sub-sample of 104 was randomly selected to participate in the OX-NDA study. Other than age at weaning, there were no significant differences in socio-demographic and health characteristics between the OX-NDA sample and the cohort (Table 2). Children were assessed at a mean age of 367.6 (SD 8.4) days, 55 (53%) were male, and 84 (80.7%) were born at or near term. Mean birth weight was 3.2 (SD 0.5) kg. At the time of the assessment, children weighed 10.1 (SD 1.2) kg (corresponding to the 75th-85th centiles for girls and 50th-75th centiles for boys on the age-specific WHO child growth standards), and measured 75.1 (SD 3.1) cm in length (corresponding to the 50th-75th WHO centiles for girls and 25th-50th centiles for boys). The mean OX-NDA assessment time was 20.0 (SD 5.0) minutes.
Table 2.
Sample Characteristics
| Children participating in the OX-NDA evaluation study (n = 104) Mean (SD) or number (%) |
Children in the Pelotas 2015 Birth Cohort not participating in the OX-NDA evaluation study (n = 3914) Mean (SD) or number (%) |
p value | |
|---|---|---|---|
| Socio-demographic, prenatal and perinatal characteristics | |||
| Maternal age at recruitment, years | 27.1 (6.6) | 27.5 (6.2) | 0.51 |
| Monthly family income | 3585.6 (5667.7) | 3010.9 (4377.3) | 0.55 |
| Duration of mother’s formal education, years | 10.1 (4.0) | 10.7 (4.0) | 0.09 |
| Maternal employment status | 49 (47.1%) | 1878 (47.9%) | 0.06 |
| Maternal infections (including HIV, rubella, syphilis, hepatitis B, CMV, toxoplasmosis, tuberculosis and malaria) | 10.0 (11.6%) | 348 (9.6%) | 0.49 |
| Maternal substance abuse (including alcohol) and smoking | 20 (19.2%) | 582 (16.1%) | 0.18 |
| Maternal prenatal anxiety and depression/mental stress | 15 (14.4%) | 402 (11.1%) | 0.26 |
| Maternal preeclampsia and eclampsia | 10 (9.6%) | 227 (6.3%) | 0.10 |
| Perinatal and Neonatal characteristics | |||
| Gestational age at delivery, weeks | 38.7 (2.0) | 38.4 (2.4) | 0.34 |
| Birth weight, kg | 3.2 (0.5) | 3.2 (0.5) | 0.39 |
| Birth length, cm | 48.3 (2.6) | 40.2 (3.2) | 0.64 |
| Head circumference at birth, cm | 33.9 (1.5) | 33.9 (2.9) | 0.94 |
| Apgar score at 5 min | 9.5 (0.6) | 9.7 (4.3) | 0.13 |
| Boys | 55 (53.0%) | 1848 (51.1%) | 0.72 |
| Postnatal characteristics | |||
| Only child | 51 (49.0%) | 1905 (48.7%) | 0.95 |
| Mean age of 12-month / OX-NDA assessment (days) | 367.6 (8.4) | – | – |
| Significant morbidity during the first year of life1 | 4 (3.8%) | 153 (5.1%) | 0.73 |
| Weight at 1 year, kg | 10.1 (1.2) | 9.9 (1.4) | 0.28 |
| Length at 1 year, cm | 75.1 (3.1) | 75.0 (3.0) | 0.63 |
| Immunized for age | 90 (100%) | 2955 (99.7%) | 0.60 |
| Age at which infant was weaned, months | 5.7 (3.4) | 4.1 (3.8) | *0.001 |
| BSID-III assessment conducted before OX-NDA | 52 (50%) | – | – |
| Duration between OX-NDA and BSID-III assessments at 1 year, day | 1 (0) | – | – |
*p < 0.05
OX-NDA The Oxford Neurodevelopment Assessment
1Signficant morbidity during the first year of life is defined as any life threatening or life altering condition, requiring prolonged hospitalization and/or treatment, such as epilepsy, metabolic disorders, endocrinological disorders, haematological disorders including haemophilia, oncological diagnosis, any conditions requiring surgery, congenital cardiac conditions, prolonged ventilation included home-based ventilatory support and/or neurological conditions requiring prolonged treatment and/or surveillance
Internal consistency
For the final OX-NDA (consisting of 37 items), Cronbach’s alpha values (Table 3) were satisfactory (≥0.70) for the cognition, motor and positive behaviour; acceptable (0.40) for language, and weak (< 0.4) for negative behaviour [30].
Concurrent validity between the OX-NDA and BSID-III
Strong positive correlations were observed between cognition and motor scores of the BSID-III and the OX-NDA (Table 3, Pearson’s r = 0.50-0.52, p < 0.001). The ICCs for the BSID-III and OX-NDA domains showed similarly strong associations for cognition and motor outcomes (ICCs 0.63-0.68, p < 0.001), and low associations for language outcomes (ICC 0.30, p = 0.04).
The Bland-Altman analysis (Table 4) indicated no, or very low, bias in the subscales, suggesting little to acceptable difference between OX-NDA and BSID-III scores [32]. The Bland-Altman plots (Additional file 4: Fig. S4) and the linear regression analyses (Table 4) of difference scores (BSID-III minus OX-NDA) revealed positive associations between the measures.
Table 4.
Evaluation of agreement between OX-NDA and BSID-III
| OX-NDA Domain (n = 104) |
BSID-III composite scores (n = 104) |
Correlation analysis | Bland Altman Analysis | Linear regression of difference scores1 | Comparison of OX-NDA domain scores between children scoring ≥85 and < 85 on the BSID-III | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Pearson’s r (p) | ICC (95% CI, p) | Bias | Lower limit of agreement | Upper limit of agreement | r | Children scoring < 85 on the BSID-III Mean (SD) |
Children scoring ≥85 on the BSID-III Mean (SD) |
t (p) | ||
| Cognition | Cognition | 0.50** (< 0.001) | 0.66** (0.50-0.77, < 0.001) | 0.10 | −67.88 | −23.88 | 0.16** | 50.42 (10.66) | 65.82 (9.77) | 3.08** (< 0.001) |
| Motor | Motor | 0.52** (< 0.001) | 0.68** (0.53-0.78, < 0.001) | 0.36 | −42.33 | 0.84 | 0.27** | 60.31 (14.28) | 80.68 (9.54) | 6.09** (< 0.001) |
| Language | Language | 0.18 (0.07) | 0.30* (− 0.04 – 0.52, 0.041) | 0.08 | −77.42 | −24.28 | 0.28** | 53.12 (11.26) | 61.72 (9.49) | 1.54 (0.13) |
OX-NDA The Oxford Neurodevelopment Assessment; BSID-III: The Bayley Scales of Infant Development III edition; Pearson’s r: Pearson’s correlation coefficients; ICC: interclass correlation coefficients; CI: Confidence intervals; t: independent sample t test
**p < 0.001, *p < 0.05
1Difference scores were calculated as BSID-III minus OX-NDA scores
Sensitivity and specificity analysis
Fewer than 10% of the study’s children obtained BSID-III composite scores < 85, no children scored < 70. Children scoring low (< 85) on the BSID-III composites also scored low on the OX-NDA across all domains (Table 4). The sensitivity and specificity of the OX-NDA cognition and motor scores in predicting moderate to severe delay on the BSID-III (composite scores < 85) was strong (Additional file 5: Table S5) at cut-offs of ≤60 (sensitivity 76%, specificity 75%), ≤73 (sensitivity 73%, specificity 80%), and ≤ 71 (sensitivity 70%, specificity 25%), respectively. This was low for the OX-NDA language score at a cut-off of ≤60 (sensitivity 43%, specificity 33%) [34, 35].
Reliability analysis
The inter-rater and test-retest reliability was high (Cohen’s k = 0.80-0.96, 95% CI: 0.78-0.97 and Cohen’s k = 0.85-0.94, 95% CI: 0.80-0.95) across all OX-NDA domains (Table 5).
Table 5.
Inter-rater and test-re test reliability of the OX-NDA
| OX-NDA domain | Kappa | 95% CIs |
|---|---|---|
| Inter-rater reliability | ||
| Cognition | 0.87 | 0.84-0.90 |
| Motor | 0.96 | 0.95-0.97 |
| Language | 0.84 | 0.80-0.88 |
| Positive Behaviour | 0.82 | 0.79-0.86 |
| Negative Behaviour | 0.80 | 0.78-0.85 |
| Test-re test reliability | ||
| Cognition | 0.94 | 0.93-0.95 |
| Motor | 0.92 | 0.89-0.94 |
| Language | 0.89 | 0.85-0.93 |
| Positive Behaviour | 0.86 | 0.83-0.90 |
| Negative Behaviour | 0.85 | 0.80-0.87 |
OX-NDA The Oxford Neurodevelopment Assessment; CI: Confidence intervals
VI. Feasibility Analysis for Use Across Populations and Settings.
The OX-NDA was assessed against three international guidelines for measuring ECD in low- and middle-income settings (Table 6): the World Bank’s Toolkit for Measuring ECD [10]; Fischer et al’s feasibility criteria for use of developmental screening tools at primary healthcare level in low- and middle-income settings [11] and Boggs’ et al’s rating of ECD measurement tools for routine health programme use [9]. The OX-NDA met all but one World Bank and Fischer criteria, and scored 19 (of a maximum total of 24) on Boggs et al’s rating.
Table 6.
Assessment of the OX-NDA against feasibility criteria for use of an early child developmental assessment in a low- and middle-income setting
| Feasibility Criteria | Does OX-NDA fulfil the criteria? | Additional details |
|---|---|---|
| World Bank Toolkit for Examining ECD1 | ||
| Psychometrically adequate, valid and reliable | Yes | ICCs 0·63 and 0·68 (p < 0·001) between BSID-III and OX-NDA for cognition, and total score domains; and motor composite; ICC 0.30 (p < 0.04) for language composite. Internal consistency satisfactory. Sensitivity in predicting BSID-III composite scores < 85 (moderate delay) was 76, 73, and 43% for the OX-NDA cognition, motor and language domains at cut-off scores of <=60, 73, and 60 respectively. Specificity in predicting BSID-III composite scores < 85 (moderate delay) was 75, 80, and 33% for the OX-NDA cognition, motor, and language domains at cut-off scores of <=60, 73, and 60 respectively. Inter-rater reliability and test-rest reliability was k = 0.80-0.96, 95% CI: 0.78-0.97 and k = 0.85-0.94, 95% CI: 0.80-0.95 across all domains. |
| Balanced in terms of number of items at the lower end to avoid children with low scores | Yes | Age range of items extend to 6 months |
| Enjoyable for children to take (e.g. interactive, colourful materials) | Yes | |
| Relatively easy to adapt to various cultures | Yes | Adapted via cultural customisation session during training and currently in use in Brazil, India, and Grenada |
| Easy to use in low-resource settings, e.g. not requiring much material | Yes | Cost of kit GBP 100.0; no fee per use; manuals and forms available upon request, mobile phone/tablet based OX-NDA E-form available. |
| Not too difficult to obtain or too expensive | Yes | See above |
| Able to be used in a wide age range | No | Moderately narrow age range (10 to 14 months) |
| Fischer et al’s feasibility criteria for use of developmental screening tools at primary healthcare level in low-middle income settings2 | ||
| Results understood by health workers | Yes | Cut-offs for moderate-to-severe delay |
| Reliable | Yes | High, inter-rater reliability and test-rest reliability of k = 0.80-0.96, 95% CI: 0.78-0.97 and k = 0.85-0.94, 95% CI: 0.80-0.95 across all subscales. |
| Valid | Yes | See above |
| Acceptable to caregivers | Yes | |
| Provides information that is relevant to primary care providers | Yes | Cut-offs |
| Information that can be used for referrals of early intervention | Yes | Cut-offs |
| Information that is useful for anticipatory guidance | Unknown | |
| Results understood by caregivers | Yes | |
| Staff members have the expertise to answer questions | Yes | Session on maternal questions and responses included in training package. |
| Access to application | Yes | Manuals, paper forms and E-form available upon request. |
| Training involved | Yes | Time taken to train assessors in the OX-NDA: 1 day for ≤3 assessors, 2 days for 3-5 assessors, 3 days for 5-10 assessors |
| How long it takes to administer the tool | Yes | 15-25 minutes |
| Cover multiple areas of child development | Yes | Cognition, language, motor skills, and behaviour (positive and negative) |
| Cost of the tool | Yes | Cost of kit GBP100.0; no fee per use; mobile phone/tablet based OX-NDA E-form available. |
| Minimal adaptation needed | Yes | Sessions on cultural customisation and translation included in training |
| Educational level of staff members | Yes | Primary education; non-specialist personnel |
| How many staff members to administer the tool | Yes | 1 |
| Local norms available | Yes | Cut-offs based on Brazilian sample. Research to develop international norms on-going. |
| Space | Yes | Minimal space for storage of kit and forms. Mobile phone/tablet based OX-NDA E-form available. Home-based assessments possible. |
| Boggs et al’s rating of early child development outcome measurement tools for routine health programme use3 | ||
| Validity | Rating: 2 | Validity somewhat below widely accepted threshold (0.5 to 0.7) against another performance-based tool e.g. BSID-III |
| Reliability | Rating: 3 | High, inter-rater reliability and test-rest reliability of k = 0.80-0.96, 95% CI: 0.78-0.97 and k = 0.85-0.94, 95% CI: 0.80-0.95 across all subscales. |
| Cultural Adaptability | Rating: 3 | Easy modification of items, materials and procedures |
| Accessibility | Rating: 2 |
Tool administration, scoring and interpretation, all available online, but some intellectual property or other restrictions. Minimal cost to tool <US$ 10 per child App (mobile phone/tablet based OX-NDA E-form) available |
| Training | Rating: 2 | Moderate (> 1 hour to < 1 day), requires standardization and training on direct assessment of children’s abilities, no certification requirement. |
| Administration time | Rating: 2 | < 15 to 20 minutes, minimum to moderate scoring. |
| Geographical uptake | Rating: 3 | Used in at least three continents (Asia, Europe, South America) |
| Clinical relevance and utility | Rating: 2 |
Sensitivity in predicting BSID-III composite scores < 85 (moderate delay) was 76, 73, and 43% for the OX-NDA cognition, motor and language domains at cut-off scores of <=60, 73 and 60 respectively. Specificity in predicting BSID-III composite scores < 85 (moderate delay) was 75, 80, and 33% for the OX-NDA cognition, motor and language domains at cut-off scores of <=60, 73, and 60 respectively. Further research to develop international norms, and contextually appropriate referral pathways underway. |
ECD Early child development
OX-NDA The Oxford Neurodevelopment Assessment
BSID-III The Bayley Scales of Infant Development III edition
ICC interclass correlation coefficients
CI Confidence intervals
k Cohen’s kappa coefficient
1Fernald LCH, Kariger P, Engle P, et al. Examining Early Child Development in Low-Income Countries: A Toolkit for the Assessment of Children in the First 5 Years of Life. Washington DC: The World Bank, 2009
2Fischer VJ, Morris J, Martines J. Developmental screening tools: feasibility of use at primary healthcare level in low-and middle-income settings. Journal of health, population, and nutrition 2014;32(2):314
3Boggs D, Milner KM, Chandna J, et al. Rating early child development outcome measurement tools for routine health programme use. Archives of disease in childhood 2019;104 (Suppl 1):S22-S33
Discussion
The OX-NDA is a multi-dimensional, mixed methodology instrument for measuring cognitive, motor and behaviour outcomes in children aged 10 to 14 months: its psychometric validity was low for the language domain. The rationale for its construction was to provide a comprehensive neurodevelopmental assessment that can be administered reliably and rapidly to young children by non-specialists in low-resource settings at relatively low costs. The preliminary evidence obtained from this study supports the OX-NDA’s use as a valid and reliable measure of early cognitive, motor and behaviour outcomes in Brazilian infants.
Across cognitive and motor domains, the OX-NDA demonstrated good concurrent validity with the BSID-III, satisfactory psychometric properties and high levels of inter-rater and test-retest reliability. Importantly, its sensitivity and specificity in predicting moderate-to-severe cognitive and motor delay in 1-year-olds was satisfactory. The OX-NDA’s concurrent validity with the BSID-III for the language domain was low. It is possible that this may reflect the observation that language, as a construct, is highly influenced by culture and it was, as such, not possible for us to ascertain from our dataset whether it is the OX-NDA or the BSID-III that have limited function in this context.
Across domains, the OX-NDA’s reliability, when administered by non-specialists, was high and its administration time was shorter and cost per child was lower than comparable measures. Previous studies have shown that ECD assessments for older ages and constructed using similar approaches, such as the INTER-NDA [22] and TADI [20], have yielded similar results.
Our study was limited in that all children were Brazilian, recruited from the city of Pelotas, and that the comparison of the OX-NDA’s behaviour domain with the BSID-III was not ascertained. Its agreement with the BSID-III language composite was limited and further work is needed to ascertain why this was the case and how its performance in the language domain may be improved. Moreover, its ability to predict severe delay could not be determined as no children in our study scored < 70 on the BSID-III composite scores. Additionally, the OX-NDA’s age range is narrow (10 to 14 months). Finally, item selection was guided by the expert panel, initial piloting and assessment of internal consistency: we did not conduct a factorial analysis for item selection which limited our ability to scrutinise relations between observed and latent variables.
Nevertheless, the study’s strengths lie in the detailed validation of the OX-NDA, using multiple statistical techniques, in a population-based sample from a low-and-middle-income setting against the BSID-III [25], a well-established measure of ECD, considered, by some, to be a gold-standard ECD assessment. Our study design controlled for child fatigue and contamination of results during sequential developmental testing sessions.
The OX-NDA offers several practical and conceptual advantages over other infant developmental assessments (Table 6, Additional file 1: Table S1). First, it reliably measures multiple domains of infant development (cognition, motor, and behaviour). For each domain, outcomes are reported on a 5-point scale characterising the child’s performance across a spectrum and offering a level of granularity beyond that provided by many infant development batteries. Second, it was designed to be free from cultural biases and is based upon objective reporting (rather than subjective judgement) of the child’s performance. Its rigorous standardization protocol ensures children are assessed uniformly and reliably. Moreover, it can be administered reliably by non-specialist assessors in a short assessment time and at relatively low costs. Third, because it is a mixed-methodology assessment, it applies the advantages of direct assessment as well as caregiver- and observer-reports. It assesses cognitive processing by presenting children with new tasks and captures previously demonstrated abilities while being minimally affected by reporter and recall biases.
Nevertheless, although the OX-NDA fulfilled most requirements for a population-level ECD measure when assessed against the World Bank’s [10], Fischer et al’s [11] and Boggs’ et al’s criteria [9] (Table 6); the findings of this study may not be generalizable to culturally and linguistically disparate LMICs and further research is needed to evaluate its adaptability and applicability in screening for infant development delay in populations beyond Brazil. Additionally, further work is needed to examine (and improve) its performance in the language domain. Currently, such studies are on-going in the West Indies, Indonesia, India, and Senegal.
Identifying children at risk of delay during early childhood and comparing outcomes across populations are essential prerequisites for achieving indicator 4.2.1 of the Sustainable Development Goals. The OX-NDA measure presented here contributes an important component to the care of young children: a unique, standardized developmental tool that can be applied by non-specialists in low-resource settings to measure neurodevelopmental outcomes in infants reliably, rapidly and at relatively low costs and to identify those children at risk of delays.
Conclusions
The evidence presented here shows the OX-NDA to be a psychometrically sound measurement tool for the assessment of cognitive, motor and behaviour outcomes in Brazilian infants. Its mixed-methodology, multidimensional approach; relatively low costs; and reliability when administered by non-specialists make it an attractive candidate ECD measure for research and clinical efforts aimed at identifying infants at risk of delay in low-resource settings. Its ability to identify language delay in Brazilian infants was low and further work is needed to examine (and improve) its performance in the language domain. Research efforts, focusing on its adaptation and application in LMIC settings beyond Brazil, are on-going.
Supplementary Information
Acknowledgements
We are grateful to the mothers and children who have contributed to the study. We gratefully acknowledge the contribution of Hua-Fang (Lily) Liao, Executive Director of the Chinese Association of Early Intervention Professional for Children with Developmental Delays for providing information on the Comprehensive Developmental Inventory for Infants and Toddlers.
Authors’ contributions
MF conceptualised and designed the study, designed the data collection instruments, conducted data analysis, drafted the initial manuscript, and reviewed and revised the manuscript. IS conceptualised and designed the study, designed the data collection instruments, coordinated and supervised data collection and critically reviewed the manuscript for important intellectual content. AM conducted data analysis, and critically reviewed the manuscript for important intellectual content. SC, GINC, EA, AB, MFS, LTR and LA coordinated and supervised data collection, and, together with DM, critically reviewed the manuscript for important intellectual content. CH, IS, MFS and DB conceptualised and designed the 2015 Pelotas birth cohort study, coordinated and supervised data collection and critically reviewed the manuscript for important intellectual content. All authors approved the final manuscript as submitted and agree to be accountable for all aspects of the work.
Funding
The Wellcome Trust (grant number 095582/Z/11/Z) supports the 2015 Pelotas Birth Cohort. This study was funded by the Brazilian National Council for Scientific and Technological Development (CNPq) (Grant N° 401732/2015-0) and Bill & Melinda Gates Foundation (B&MGF) (Grant N° OPP 1142172). IS Santos, A Matijasevich and PC Hallal receive support from the Brazilian National Council for Scientific and Technological Development (CNPq). M Fernandes receives support from the Medical Research Council in the UK. None of the funding bodies had a role in the design of this study, data analysis, collection, interpretation or writing of the manuscript.
Availability of data and materials
The data that support the findings of this study are available from the DOVE research centre (contact@doveresearch.org) but restrictions apply to the availability of these data, which were used under license for the current study, and so are not publicly available. Data are however available from the authors/corresponding author upon reasonable request and with permission of the DOVE research centre (contact@doveresearch.org).
Declarations
Ethics approval and consent to participate
Our study was approved by the Research Ethics Committee of the Medical School (No 1.400.585) and the School of Physical Education at the Federal University of Pelotas, Brazil (CAAE registration number: 26746414.5.0000.5313) and complies with the principles of the Declaration of Helsinki. Mothers provided written informed consent on behalf of their children.
Consent for publication
Not applicable.
Competing interests
None to declare.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.United Nations. Transforming our world: the 2030 Agenda for Sustainable Development. p. 2015.
- 2.Janevic T, Petrovic O, Bjelic I, Kubera A. Risk factors for childhood malnutrition in Roma settlements in Serbia. BMC Public Health. 2010;10(1):509. doi: 10.1186/1471-2458-10-509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Daelmans B, Darmstadt GL, Lombardi J, Black MM, Britto PR, Lye S, et al. Early childhood development: the foundation of sustainable development. Lancet. 2017;389(10064):9–11. doi: 10.1016/S0140-6736(16)31659-2. [DOI] [PubMed] [Google Scholar]
- 4.Dua T, Tomlinson M, Tablante E, Britto P, Yousfzai A, Daelmans B, et al. Global research priorities to accelerate early child development in the sustainable development era. Lancet Glob Health. 2016;4(12):e887–e8e9. doi: 10.1016/S2214-109X(16)30218-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Engle PL, Black MM, Behrman JR, Cabral de Mello M, Gertler PJ, Kapiriri L, et al. Strategies to avoid the loss of developmental potential in more than 200 million children in the developing world. Lancet. 2007;369(9557):229–242. doi: 10.1016/S0140-6736(07)60112-3. [DOI] [PubMed] [Google Scholar]
- 6.Grantham-McGregor S, Cheung YB, Cueto S, Glewwe P, Richter L, Strupp B. Developmental potential in the first 5 years for children in developing countries. Lancet. 2007;369(9555):60–70. doi: 10.1016/S0140-6736(07)60032-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Richter LM, Daelmans B, Lombardi J, Heymann J, Boo FL, Behrman JR, et al. Investing in the foundation of sustainable development: pathways to scale up for early childhood development. Lancet. 2017;389(10064):103–118. doi: 10.1016/S0140-6736(16)31698-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Walker SP, Wachs TD, Meeks Gardner J, Lozoff B, Wasserman GA, Pollitt E, et al. Child development: risk factors for adverse outcomes in developing countries. Lancet. 2007;369(9556):145–157. doi: 10.1016/S0140-6736(07)60076-2. [DOI] [PubMed] [Google Scholar]
- 9.Boggs D, Milner KM, Chandna J, Black M, Cavallera V, Dua T, et al. Rating early child development outcome measurement tools for routine health programme use. Arch Dis Child. 2019;104(Suppl 1):S22–S33. doi: 10.1136/archdischild-2018-315431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fernald LCH, Kariger P, Engle P, Raikes A. Examining early child development in low-income countries: a toolkit for the assessment of children in the first five years of life. Washington DC: The World Bank; 2009. [Google Scholar]
- 11.Fischer VJ, Morris J, Martines J. Developmental screening tools: feasibility of use at primary healthcare level in low-and middle-income settings. J Health Popul Nutr. 2014;32(2):314. [PMC free article] [PubMed] [Google Scholar]
- 12.Lancaster GA, McCray G, Kariger P, Dua T, Titman A, Chandna J, et al. Creation of the WHO indicators of infant and young child development (IYCD): metadata synthesis across 10 countries. BMJ Glob Health. 2018;3(5):e000747. doi: 10.1136/bmjgh-2018-000747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.WHO Multicentre Growth Reference Study Group. de Onis M. WHO motor development study: windows of achievement for six gross motor development milestones. Acta Paediatr. 2006;S450:86–95. doi: 10.1111/j.1651-2227.2006.tb02379.x. [DOI] [PubMed] [Google Scholar]
- 14.Wijnhoven TM, de Onis M, Onyango AW, Wang T, Bjoerneboe G-EA, Bhandari N, et al. Assessment of gross motor development in the WHO multicentre growth reference study. Food Nutr Bull. 2004;25(1_suppl1):S37–S45. doi: 10.1177/15648265040251S106. [DOI] [PubMed] [Google Scholar]
- 15.Small JW, Hix-Small H, Vargas-Baron E, Marks KP. Comparative use of the ages and stages questionnaires in low-and middle-income countries. Dev Med Child Neurol. 2019;61(4):431–443. doi: 10.1111/dmcn.13938. [DOI] [PubMed] [Google Scholar]
- 16.Ertem IO, Krishnamurthy V, Mulaudzi MC, Sguassero Y, Balta H, Gulumser O, et al. Similarities and differences in child development from birth to age 3 years by sex and across four countries: a cross-sectional, observational study. The lancet. Glob Health. 2018;6(3):e279–ee91. doi: 10.1016/S2214-109X(18)30003-2. [DOI] [PubMed] [Google Scholar]
- 17.Ozturk Ertem I, Krishnamurthy V, Mulaudzi MC, Sguassero Y, Bilik B, Srinivasan R, et al. Validation of the international guide for monitoring child development demonstrates good sensitivity and specificity in four diverse countries. Acta Paediatr. 2019;108(6):1074–1086. doi: 10.1111/apa.14661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Berls AT, McEwen IR. Battelle developmental inventory. Phys Ther. 1999;79(8):776–783. doi: 10.1093/ptj/79.8.776. [DOI] [PubMed] [Google Scholar]
- 19.Guidubaldi J, Perry JD. Concurrent and predictive validity of the Battelle development inventory at the first grade level. Educ Psychol Meas. 1984;44(4):977–985. doi: 10.1177/0013164484444021. [DOI] [Google Scholar]
- 20.López Vanegas NC, Peñafiel Aguirre TE. Adaptación y validación del test de Aprendizaje y desarrollo infantil “TADI” en el GAD de Calderón. Quito: UCE; 2020. [Google Scholar]
- 21.Fernandes M, Stein A, Newton CRJ, Ismail LC, Kihara M, Wulff K, et al. The INTERGROWTH-21st Project Neurodevelopment Package: A novel method for the multi-dimensional assessment of neurodevelopment in pre-school age children. PLoS One. 2014;9(11):e113360. doi: 10.1371/journal.pone.0113360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Murray E, Fernandes M, Newton CR, Abubakar A, Kennedy SH, Villar J, et al. Evaluation of the INTERGROWTH-21st neurodevelopment assessment (INTER-NDA) in 2 year-old children. PLoS One. 2018;13(2):e0193406. doi: 10.1371/journal.pone.0193406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fernandes M, José Villar AS, Urias ES, Garza C, Victora CG, Barros FC, et al. INTERGROWTH-21st Project international INTER-NDA standards for child development at 2 years of age: an international prospective population-based study. BMJ open. 2020;10(6):e035258. [DOI] [PMC free article] [PubMed]
- 24.Santos IS, Bassani DG, Matijasevich A, Halal CS, Del-Ponte B, da Cruz SH, et al. Infant sleep hygiene counseling (sleep trial): protocol of a randomized controlled trial. BMC Psychiatry. 2016;16(1):307. doi: 10.1186/s12888-016-1016-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bayley N. Bayley scales of infant and toddler development, third edition. San Antonio: Pearson Education Inc.; 2006. [Google Scholar]
- 26.Hallal PC, Bertoldi AD, Domingues MR, da Silveira MF, Demarco FF, da Silva ICM, et al. Cohort profile: the 2015 Pelotas (Brazil) birth cohort study. Int J Epidemiol. 2018;47(4):1048–104h. doi: 10.1093/ije/dyx219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Limited PE. Bayley Scales of Infant and Toddler Development, Third Edition (Bayley-III) 2020. Available from: https://www.pearsonclinical.co.uk/Psychology/ChildCognitionNeuropsychologyandLanguage/ChildGeneralAbilities/BayleyScalesofInfantandToddlerDevelopmentThirdEdition(Bayley-III)/BayleyScalesofInfantandToddlerDevelopmentThirdEdition(Bayley-III).aspx. Accessed 1 Mar 2022.
- 28.Harkness J, Pennell B, Villar A, Gebler N, Aguilar-Gaxiola S, Bilgen I. Translation procedures and translation assessment in the world mental health survey initiative. The WHO World Mental Health Surveys: Global Perspectives on the Epidemiology of Mental Disorders. 2008. pp. 91–113. [Google Scholar]
- 29.Tavakol M, Dennick R. Making sense of Cronbach's alpha. Int J Med Educ. 2011;2:53. doi: 10.5116/ijme.4dfb.8dfd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bland JM, Altman DG. Statistics notes: Cronbach's alpha. BMJ. 1997;314:572. doi: 10.1136/bmj.314.7080.572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lee J, Koh D, Ong C. Statistical evaluation of agreement between two methods for measuring a quantitative variable. Comput Biol Med. 1989;19(1):61–70. doi: 10.1016/0010-4825(89)90036-X. [DOI] [PubMed] [Google Scholar]
- 32.Martin Bland J, Altman D. Statistical Methods for Assessing Agreement Between Two Methods of Clincal Measurement. Lancet. 1986;327(8476):307–310. doi: 10.1016/S0140-6736(86)90837-8. [DOI] [PubMed] [Google Scholar]
- 33.Cohen J. A coefficient of agreement for nominal scales. Educ Psychol Meas. 1960;20:37–46. doi: 10.1177/001316446002000104. [DOI] [Google Scholar]
- 34.Heaton RK, Grant I, Matthews CG, Fastenau PS, Adams KM. Heaton, Grant, and Matthews' comprehensive norms: an overzealous attempt. J Clin Exp Neuropsychol. 1996;18(3):444–448. doi: 10.1080/01688639608409000. [DOI] [Google Scholar]
- 35.Heaton RK, Marcotte TD. Clinical neuropsychological tests and assessment techniques. 2000. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from the DOVE research centre (contact@doveresearch.org) but restrictions apply to the availability of these data, which were used under license for the current study, and so are not publicly available. Data are however available from the authors/corresponding author upon reasonable request and with permission of the DOVE research centre (contact@doveresearch.org).