Abstract
Aims:
Oxidative stress and neuronal apoptosis play crucial roles in the pathological processes of secondary injury after intracerebral hemorrhage (ICH). Aryl hydrocarbon receptor (AHR), together with its endogenous ligand kynurenine, is known to mediate free radical accumulation and neuronal excitotoxicity in central nervous systems. Herein, we investigate the pathological roles of kynurenine/AHR after ICH.
Results:
Endogenous AHR knockout alleviated reactive oxygen species accumulation and neuronal apoptosis in ipsilateral hemisphere at 48 h after ICH in mice. The ICH insult resulted in an increase of total and nucleus AHR protein levels and AHR transcriptional activity. Inhibition of AHR provided both short- and long- term neurological benefits by attenuating mitochondria-mediated oxidative stress and neuronal apoptosis after ICH in mice. RhoA-Bax signaling activated mitochondrial death pathway and participated in deleterious actions of AHR. Finally, we reported that exogenous kynurenine aggravated AHR activation and mediated the brain mentioned earlier. Male animals were used in the experiments.
Innovation:
We show for the first time that kynurenine/AHR mediates mitochondria death and free radical accumulation, at least partially via the RhoA/Bax signaling pathway. Pharmacological antagonists of AHR and kynurenine may ameliorate neurobehavioral function and improve the prognosis of patients with ICH.
Conclusion:
Kynurenine/AHR may serve as a potential therapeutic target to attenuate mitochondria-mediated oxidative stress and neuronal cells impairment in patients with ICH. Antioxid. Redox Signal. 37, 1111–1129.
Keywords: Intracerebral hemorrhage, kynurenine, apoptosis, oxidative stress, aryl hydrocarbon receptor
Introduction
Intracerebral hemorrhage (ICH) constitutes 15–20% of strokes, yet it accounts for 49% of global burden death from stroke (Joundi et al, 2021). The catastrophic cascade of secondary injury after ICH followed by perihematomal edema and hematoma expansion evolves over hours to days, providing an amenable therapeutic time window (Lim-Hing and Rincon, 2017; Murthy et al, 2015; Tschoe et al, 2020).
It is believed that oxidative stress and neuronal apoptosis plays an important role in the devastating pathological processes of secondary injury after ICH (Hu et al, 2016; Leasure et al, 2021). Therefore, aiming at finding out a therapeutic target to mitigate oxidative stress and neuronal apoptosis remains an imperative but unmet need in the uphill battle with ICH.
The aryl hydrocarbon receptor (AHR) is first discovered as a ligand-activated transcription factor located in the cytoplasm with its highly conserved PER-ARNT-SIM (PAS) domain (Rothhammer and Quintana, 2019; Vecsei et al, 2013). As demonstrated by the phenotype in AHR knockout mice (Fernandez-Salguero et al, 1997), AHR was postulated to be linked with vascular and cardiac homeostasis, immune system function, and neoplasm development in response to endogenous ligands such as kynurenine and tryptophan metabolite under normal cell physiology (Cuartero et al, 2014; Kou and Dai, 2021).
Innovation
Aryl hydrocarbon receptor (AHR) is a ligand-activated transcription factor with increasing importance in cellular physiology. However, its actions in models of acute injury by circulatory pathologies such as intracerebral hemorrhage (ICH) have not been illustrated. Mitochondria-mediated oxidative stress and neuronal apoptosis play crucial roles in the pathological processes of secondary injury after ICH. Therefore, we show for the first time that kynurenine/AHR mediates mitochondria death and free radical accumulation, at least partially via the RhoA/Bax signaling pathway. The AHR may serve as a potential therapeutic target in patients with ICH. Pharmacological antagonists of AHR and kynurenine may ameliorate neurobehavioral function and improve the prognosis of patients with ICH.
On activation, AHR shuttled into the nucleus with the formation of a complex with AHR nuclear translocator (ARNT), and it bound to the upstream of target genes promoter fragments such as Tsp-1, Cyp1a1 (Androutsopoulos et al, 2009; Dietrich, 2016; Stejskalova et al, 2011). Though the pathophysiological roles of AHR in the neurodevelopment have been extensively studied (Juricek and Coumoul, 2018), the mechanisms underlying neuroprotection remain poorly explored.
Of note, kynurenine is the endogenous ligand of AHR, metabolized from tryptophan to neuroactive compounds regulating N-methyl-D-aspartate (NMDA) receptor function and it induces free radical production (Savitz, 2020; Vecsei et al, 2013). Kynurenine/AHR is postulated to link with reactive oxygen species (ROS) production in viral myocarditis and atherosclerosis (Yi et al, 2018). The inhibition of kynurenine/AHR protects the mouse brain against excitotoxic insult in Huntington disease and aging-related disorders (Natividad et al, 2018; Rothhammer and Quintana, 2019). Therefore, it is of great interest to determine whether kynurenine/AHR is involved in the pathological process of ICH and contributes to the oxidative stress and neuronal apoptosis.
In this study, we explored the role of AHR in experimental ICH by using a collagenase injection model in vivo. Our data showed that the inhibition of kynurenine/AHR activation could ameliorate neurological deficits as well as alleviate mitochondria-mediated oxidative stress and neuronal apoptosis through the RhoA/Bax signaling pathway after the induction of ICH in mice (The proposed schematic is shown in Fig. 1).
FIG. 1.
Graphic schematic pathway. The effects of Kynurenine/AHR on mitochondria-mediated oxidative stress and neuronal apoptosis and underlying mechanisms after ICH in mice. The cell type in representative figure is neuron. AHR, aryl hydrocarbon receptor; ICH, intracerebral hemorrhage; Trp, tryptophan; TDO, tryptophan 2,3-dioxygenase; IDO, indoleamine-2,3-dioxygenase; KYN, kynurenine; ARNT, aryl hydrocarbon receptor nuclear translocator; TMF, trimethoxyflavone.
Results
Animal use and mortality
A total of 377 mice were used in our in vivo experiments, of which 309 underwent ICH surgery, 56 underwent sham surgery, and 12 underwent naive underwent intraventricular injection. The mortality rate of the ICH mice in this study was 3.7% (14/377). The detailed mortality in each group is listed in Supplementary Table S1.
AHR exacerbated mitochondria-mediated oxidative stress and apoptosis after ICH in mice
We first examined whether AHR participates in oxidative stress and apoptosis after ICH. The AHR CRISPR knockout mice, in which the expression level of AHR after ICH was lower than in the ICH group, were also neuroprotective, showing improved neurobehavioral deficits (Modified Garcia Test and Forelimb Placement Test) 48 h after ICH (n = 6–10; p < 0.05; Fig. 2A–C). In vivo genome editing efficacy was confirmed by AHR expression level in the ipsilateral hemisphere between Control CRISPR and AHR CRISPR Knockout group in naive mice (n = 6; p < 0.05; Supplementary Fig. S1).
FIG. 2.
AHR exacerbated neurobehavioral function and oxidative stress after ICH in mice. (A, B) Neurobehavioral testing using modified Garcia test and forelimb placing test in different groups 48 h after ICH. (C) Representative Western blot bands of AHR, 4-HNE, HO-1, and Bcl-2/Bax proteins in different groups. (D) Quantitative analysis of AHR, 4-HNE, HO-1, and Bcl-2/Bax proteins. **p < 0.01; *p < 0.05. The error bars represent mean ± SD. Kruskal–Wallis test (Wilcoxon signed-rank test) followed by post hoc Tukey method. n = 6–10/each group. CRISPR, clustered regularly interspaced short palindromic repeat; KO, knockout.
There was no statistically significant difference between the ICH group and the ICH + Control CRISPR group in protein levels of AHR and downstream proteins, as well as neurobehavior scores (n = 6–10; both p > 0.999; Fig. 2A–D), which excluded any off-target effects caused by the CRISPR system. The protein levels of cellular damage marker, 4-Hydroxynonena (4-HNE) (damage elicited by oxidized lipids etc.), was significantly increased in the ICH group and ICH + Control CRISPR, compared with Sham group 48 h after ICH (n = 6; both p < 0.05; Fig. 2C, D). The AHR CRISPR Knockout significantly decreased the ICH-induced upregulation of 4-HNE (n = 6; p < 0.05; Fig. 2C, D).
Also, AHR CRISPR Knockout further reversed the oxidative stress and apoptosis by upregulation of heme oxygenase 1 (HO-1) and Bcl-2/Bax protein levels compared with ICH and ICH +Control CRISPR group (n = 6; both p < 0.05; Fig. 2C, D).
Consistently, TUNEL-positive neurons were significantly increased at 48 h after ICH combined with an increased number of cleaved caspases 3-positive cells, indicating a loss in viability that is likely through an increase in apoptosis (n = 4; p < 0.05, compared with Sham group; Fig. 3A, B). Notably, the loss of neuron viability was further alleviated with AHR CRISPR Knockout after ICH (n = 4; p < 0.05, compared to ICH+Control CRISPR group; Fig. 3A, B).
FIG. 3.
In Vivo AHR CRISPR Knockdown ameliorated neuronal apoptosis after ICH in mice. (A) Representative microphotographs to show immunohistochemistry staining of cleaved caspase-3 cells (cleaved caspase-3 positive cells, dark brown, left column), and immunohistochemistry staining of neuron (NeuN, red) with TUNEL-positive cells (dark brown, right column) 48 h after ICH, scale bar = 50 μm. The brain slice on the left of top panel indicates the location of staining quantification (small black box). (B) Quantitative analysis of cleaved caspase-3 positive cells and TUNEL-positive neurons 48 h after ICH. **p < 0.01. The error bars represent mean ± SD. Kruskal–Wallis test (Wilcoxon signed-rank test) followed by post hoc Tukey method. n = 4/each group. NeuN, neuron; TUNEL, terminal deoxynucleotidyl transferase dUTP.
AHR expression is increased in the post-hemorrhagic brain
We next examined the temporal expression and cellular localization of AHR after ICH. Western blot analysis showed low expression of AHR in Sham mice (n = 6; p < 0.05; Fig. 4A). After ICH, AHR levels increased in the ipsilateral hemisphere, in which AHR was upregulated and peaked at 48 h, lasting to 72 h. The ARNT functioned as the chaperone protein of AHR and formed an AHR/ARNT heterodimer, leading to a shuttle inside the nucleus. Consistently, similar trends were found in Western blot of ARNT expression after ICH in the ipsilateral hemisphere of the mice (n = 6; p < 0.05; Supplementary Fig. S2).
FIG. 4.
Intracerebral hemorrhage induces AHR overexpression and transcriptional activity in the ipsilateral hemisphere in mice. (A) Temporal expression of AHR with representative Western blot bands and quantitative analysis of AHR at 6, 24, 48, 72 h and 7 days in the ICH group. n = 6/per group. (B) Double immunofluorescence staining for AHR (green) with neurons (NeuN, red), microglia (Iba-1, red), and astrocytes (GFAP, red) around the hematoma at 48 h in different groups after ICH. The brain slice on the right of top panel indicates the location of staining quantification (small black box). Scale bar = 50 μm, n = 4 for each group. (C, D) Representative Western blot bands and quantitative analysis of nuclear/cytoplasm AHR ratio at 24, 48, and 72 h in the ICH group. n = 4/per group. (E) Representative Western blot bands and quantitative analysis of CYP1A1 at 6, 24, 48, 72 h and 7 days in the ICH group. n = 6/per group. The error bars represent mean ± SD. *p < 0.05. **p < 0.01. Kruskal–Wallis test (Wilcoxon signed-rank test), Tukey's post hoc test. GFAP, glial fibrillary acidic protein; IBA-1, ionized calcium binding adaptor molecule-1.
To determine the cell type responsible for the increased AHR after ICH, double immunofluorescence staining 48 h after ICH was used (n = 4; Fig. 4B and Supplementary Fig. S3). Mice in Sham group showed some diffuse AHR immunoreactivity in neurons in the ipsilateral hemisphere, whereas ICH insult increased AHR expression mainly in neurons (colocalization of AHR and NeuN) and astrocytes (colocalization of AHR and glial fibrillary acidic protein, GFAP) around hematoma at 48 h. Rare immunoreactivity was observed in microglial (colocalization of AHR and IBA-1).
Exposure to ICH induced AHR nuclear translocation in mouse brain
Because the activity of AHR as a transcription factor was regulated by shuttling from cytoplasm to nucleus, AHR subcellular location was accessed by Western blot in nuclear/cytoplasmic fraction. The result showed a two-fold increase in AHR nuclear/cytoplasmic ratio at 48 h after ICH in brains of ICH-exposed animals compared with the sham group (n = 4; p < 0.05; Fig. 4C, D). Also, we performed Western blot to confirm the induction and the temporal changes of the AHR target gene Cyp1a1 after ICH compared with animals (n = 6; p < 0.05; Fig. 4E). These data suggested that in vivo ICH induced AHR nuclear translocation and transcriptional activity.
6,2,4-trimethoxyflavone improved neurobehavioral deficits in an AHR-dependent manner
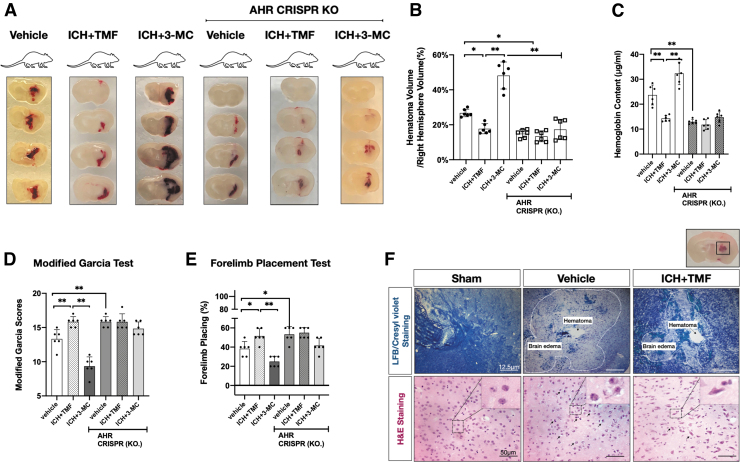
We used the pharmacological approach to inhibit or to activate AHR in both wildtype and AHR CRISPR KO mice. Mice subjected with ICH were treated with AHR antagonist 6,2,4-trimethoxyflavone (TMF) (5 mg/kg, i.p.) or AHR agonist 3-Methylcholanthrene (3-MC) (10 mg/kg, i.p.). The administration of TMF significantly decreased the hematoma determined either by hematoma volume or by hemoglobin assay at 48 h (n = 6; p < 0.05; Fig. 5A–C). Mice treated with TMF also presented a higher Modified Garcia score and Forelimb placement score (n = 6; p < 0.05; Fig. 5D, E).
FIG. 5.
TMF improved neurological deficits and alleviated secondary injury after ICH in an AHR-dependent manner. (A–C) Photos of mice brain coronal sections of 1 mm thickness in different groups at 48 h after ICH and statistical analysis of hematoma volume and hemoglobin assay. Kruskal–Wallis test (Wilcoxon signed-rank test), Tukey's post hoc test. The error bars represent mean ± SD. *p < 0.05. **p < 0.01. n = 6/each group. (D–E) Neurobehavioral testing using modified Garcia test and forelimb placing test in different groups. n = 6/per group. Kruskal–Wallis test (Wilcoxon signed-rank test), Tukey's post hoc test. (F) Photos of mice brain coronal sections; micrographs indicate representative immunostaining with LFB/Cresyl violet staining (Scale bar = 12.5 μm) and H&E staining (Scale bar = 50 μm) from each group. n = 6 for each group. The brain slice on the right of top panel indicates the location of staining quantification (small black box). LFB, luxol fast blue; H&E, hematoxylin eosin.
Further, AHR activation with selective AHR agonist 3-MC dramatically increased hematoma accompanied with worse neurobehavioral performances (n = 6; p < 0.05; Fig. 5A–E). Finally, AHR CRISPR KO mice were treated with either TMF or 3-MC to demonstrate their AHR-dependent effects. Although mice treated with TMF, or 3-MC caused a reduction or an increase in hematoma, these effects were lost in AHR CRISPR KO group (n = 6; Fig. 5A–E), demonstrating that the neuroprotective or harmful action of TMF and 3-MC was AHR dependent.
TMF treatment alleviated brain edema and long-term neurological deficits after ICH
To observe typical histopathological characteristics of brain edema after ICH, Luxol Fast Blue (LFB) counterstained with Cresyl Violet was performed to differentiate hematoma with the area of edema around hematoma (Fig. 5F, upper panel). In addition, hematoxylin and eosin staining showed widened lacunar spaces surrounding vessels and swollen cell bodies in the region surrounding the hemorrhage in ICH mice (Fig. 5F, lower panel).
Treatment with TMF improved these characteristics after ICH in mice also measured with brain water content (n = 12; p < 0.05; Fig. 5F and Supplementary Fig. S4). Also, the rotarod test was used to examine sensorimotor and balance function. The TMF improved long-term sensorimotor and balance function, represented by longer falling latency, at 2–3 weeks after ICH (n = 15; p < 0.05, compared with Vehicle group, Fig. 6A and Supplementary Fig. S5).
FIG. 6.
Inhibition of AHR by TMF treatment improved the long-term outcomes at 28 days after ICH. (A) Rotarod tests of 10 rpm at every week. n = 15 for each group. The error bars represent mean ± SD. Two-way ANOVA-Tukey. *p < 0.05. **p < 0.01. (B, C) Escape latency and swim distance of water maze test at 28 days, n = 15 for each group. The error bars represent mean ± SD. Two-way ANOVA-Tukey. *p < 0.05. **p < 0.01. (D, E) Representative heat map of probe test showed that TMF-treated ICH mice spent more time in the probe quadrant and quantification of the probe quadrant duration in the probe trial. n = 15 for each group. The error bars represent mean ± SD. Kruskal–Wallis test (Wilcoxon signed-rank test) -Tukey. *p < 0.05. **p < 0.01. (F) Representative microphotographs of Nissl staining within the hippocampal CA1, CA3, and DG regions showed the damaged neurons (less layer of neurons, morphology of atrophic cell body, and concentrated nucleus). Brain slice on the left top panel indicates the representative microphotograph of Nissl staining location of CA1, CA3, and DG regions within the left hippocampus. Scale bar = 100 μm. (G) Quantitative analysis for Nissl-positive neurons in the ipsilateral mice brain after ICH. n = 6 for each group. The error bars represent mean ± SD. Two-way ANOVA-Tukey. *p < 0.05. **p < 0.01. CA, cornu ammonis; DG, dentate gyrus.
Morris water maze test was conducted at days 23 to 28 after ICH to evaluate the spatial memory and learning ability. As shown in Figure 6B–D, the escape latency and path length were significantly longer in the Vehicle group compared with the Sham group (n = 15; p < 0.05). However, TMF-treated ICH mice showed shorter escape latency and swimming distance to the platform on the 1st to the 5th day of Morris water maze test (n = 15; p < 0.05; Fig. 6B–D). In the probe quadrant trials on the 6th day of Morris water maze test, the Vehicle mice spent a notably less time in the target quadrant compared with the sham group and TMF significantly improved the spatial memory and learning ability (n = 15; p < 0.05; Fig. 6E).
The results of Nissl staining showed the neuron injury in the Vehicle group with the morphology of the atrophic cell body and concentrated nucleus compared with the sham group, whereas TMF treatment attenuated neuronal injury in cornu ammonis (CA)1, CA3, and dentate gyrus (DG) region of the ipsilateral hippocampus compared with the Vehicle group at 28 days after ICH (n = 6; p < 0.05; Fig. 6F, G).
AHR regulates Ras homolog family member-a mitochondria death pathway after ICH: effect of AHR pharmacological modulation
The neuronal expression of AHR in our in vivo model after ICH suggested that AHR exerted main functions in neurons (Fig. 4B). Ras homolog family member A (Rho A) is one of the most important transducers of signals from G-protein-coupled receptors and the extracellular matrix involving cell survival or apoptosis, which was believed to be Bcl-2-sensitive. A previous study suggested that AHR increased promoter activities for the AHR binding sites in the promoter region of RhoA. Therefore, we explored Bcl-2 and Bax expression after ICH or AHR pharmacological inhibition.
The AHR antagonist TMF increased ipsilateral hemisphere Bcl-2 protein levels accompanied with decreased Bax protein levels 48 h after ICH (n = 6; p < 0.05; Fig. 7A, B). After ICH, Bcl-2/Bax ratio decreased, showing a negative correlation with lesion size (n = 6, Spearman r = −0.7972, p = 0.03; Fig. 7C).
FIG. 7.
TMF modulated Mitochondria Death Pathway after ICH through AHR/Ras Homolog Family Member-A. (A, B) Representative Western blot bands and densitometric quantification of the apoptosis markers, Bcl-2 and Bax, at 48 h after ICH. Kruskal–Wallis test (Wilcoxon signed-rank test), Tukey's post hoc test. The error bars represent mean ± SD. *p < 0.05. **p < 0.01. n = 6 for each group. (C) A correlation analysis of Bcl-2/Bax ratio and hematoma volume and the Spearman correlation coefficient. n = 6/per group, Spearman r = −0.7972, p = 0.03. (D–F) Representative Western blot bands and densitometric quantification of the oxidative stress markers, HO-1, 4-HNE, and cleaved caspase-3, at 48 h after ICH. Kruskal–Wallis test (Wilcoxon signed-rank test), Tukey's post hoc test. The error bars represent mean ± SD. *p < 0.05. **p < 0.01. n = 6 for each group. (G) Representative microphotographs for FJC and TUNEL staining in the ipsilateral mice brain at 48 h after ICH. Upper panel: Immunofluorescence staining for FJC-positive cells (green) with nuclear (DAPI, blue), scale bar = 100 μm. Lower panel: Immunofluorescence staining for neuron (NeuN, red) with TUNEL-positive cells (green), scale bar = 100 μm. (H) Representative microphotographs for MitoSOX and 8-OHdG staining in the ipsilateral mice brain at 48 h after ICH. Upper panel: Immunofluorescence staining for MitoSox-positive cells (red) with nuclear (DAPI, blue), scale bar = 50 μm. Lower panel: Immunofluorescence staining for 8-OHdG-positive cells (green) with nuclear (DAPI, blue), scale bar = 50 μm. Brain slice on the right top panel indicates the location of staining quantification (small black box). (I) Representative co-immunoprecipitation Western blot bands of RhoA and AHR at 48 h after ICH. DAPI, 4′,6-diamidino2-phenylindole. FJC, fluoro-jade c; MitoSox, mitochondrial superoxide; IP, input; IB, immunoblots.
Because RhoA signaling led to activation of the apoptotic cascade, we therefore reasoned that AHR could modulate proapoptotic proteins after ICH. Indeed, we found that the AHR antagonist TMF reduced oxidative stress protein 4-HNE and proapoptotic protein cleaved caspase-3, whereas it increased HO-1 protein expression (n = 6; p < 0.05; Fig. 7D–F) with increased catabolizing ability of HO-1 (n = 4; p < 0.05; Supplementary Fig. S6). Fluoro-jade c (FJC) staining was used for neuronal degeneration analysis, and TUNEL staining was performed for the neuronal viability evaluation.
The results showed that there were significantly less FJC or TUNEL positive neurons in ICH+TMF (n = 6, p < 0.05, compared with Vehicle group, Fig. 7G and Supplementary Fig. S7A, B). Further, the 8-OHdG and MitoSox staining were evaluated for the oxidative stress injury in ipsilateral peri-hematoma brain tissue at 48 h after ICH. There were significantly more numbers of MitoSox-, and 8-OHdG-positive cells in Vehicle group than Sham group at 48 h after ICH. The oxidative injury was significantly reduced by TMF treatment (n = 4; p < 0.05; compared with Vehicle group, Fig. 7H and Supplementary Fig. S8).
Finally, AHR coimmunoprecipitation after in vivo ICH (n = 4; p < 0.05; Fig. 7I and Supplementary Fig. S9) showed an increased interaction of AHR with RhoA, suggesting that the AHR activation effect on RhoA signaling could be due, at least in part, to a direct interaction with the AHR/RhoA complex. To further explore the potential downstream molecular pathway, AHR agonist, 3-MC was used in a pair of Bax-inhibiting peptide V5, which was used to target downstream Bax.
Both Bax and cell-damage biomarker HO-1 increased at 48 h after ICH when treated with 3-MC (n = 6, p < 0.05; Supplementary Fig. S10A-E), whereas Bax-inhibiting peptide V5 showed some effects against 3-MC (n = 4, p < 0.05; Supplementary Fig. S11A, B). In contrast, TMF significantly decreased the expression of AHR, ARNT, and Bax (n = 6, p < 0.05; Supplementary Fig. S12A–D) whereas HO-1 and Bcl-2 protein expression increased (n = 6, p < 0.05; Supplementary Fig. S12E, F). The ARNT CRISPR activation significantly reversed the aforementioned protein expression (n = 6, p < 0.05; Supplementary Fig. S12A–F).
Kynurenine plays a deleterious role in ICH
We first explored the temporal generation of endogenous kynurenine after ICH. Brain kynurenine increased as early as at 6 h and remained elevated at 48 h after ICH. This increase was associated with an initial reduction in serum kynurenine levels at 6 h after ICH (n = 6, p < 0.05; Fig. 8A and Supplementary Fig. S13A). Also, serum kynurenine/tryptophan ratio was significantly higher in the ICH group at 48 h compared with the sham group, suggesting that the generation of endogenous kynurenine was consistent with increased activity of peripheral Indoleamine 2,3-dioxygenase (IDO) (n = 4, p < 0.05; Supplementary Fig. S14). The neuroprotection achieved by AHR inhibition after ICH in vivo implies a deleterious role of kynurenine through AHR activation.
FIG. 8.
Deleterious AHR-dependent effect of kynurenine in ICH. (A) Temporal generation of endogenous kynurenine after ICH. n = 6/per group, Kruskal–Wallis test (Wilcoxon signed-rank test), Tukey's post hoc test. The error bars represent mean ± SD. *p < 0.05. **p < 0.01. (B, C) Neurobehavioral testing using modified Garcia test and forelimb placing test in different groups. n = 6/per group, Kruskal–Wallis test (Wilcoxon signed-rank test), Tukey's post hoc test. The error bars represent mean ± SD. *p < 0.05. **p < 0.01. (D–F) Representative microphotographs and quantitative analysis for FJC, TUNEL staining in the ipsilateral mice brain in different groups at 48 h after ICH. Brain slice on the right top panel indicates the location of staining quantification (small black box). Upper panel: Immunofluorescence staining for FJC-positive cells (green) with nuclear (DAPI, blue), scale bar = 100 μm. Lower panel: Immunofluorescence staining for neuron (NeuN, red) with TUNEL-positive cells (green), scale bar = 100 μm. Kruskal–Wallis test (Wilcoxon signed-rank test), Tukey's test. n = 6 for each group. The error bars represent mean ± SD. *p < 0.05. **p < 0.01. (G, H) Representative Western blot bands and quantitative analysis of total AHR and nuclear/cytoplasm AHR ratio at 48 h in different ICH group. n = 4–6/per group. Kruskal–Wallis test (Wilcoxon signed-rank test), Tukey's test. The error bars represent mean ± SD. *p < 0.05. **p < 0.01. (I, J) Representative microphotographs and quantitative analysis for 8-OHdG staining in the ipsilateral mice brain in different groups at 48 h after ICH. Eight-OHdG-positive cells (green) with nuclear (DAPI, blue), scale bar = 50 μm. One-way ANOVA, Tukey's test. n = 6 for each group. The error bars represent mean ± SD. *p < 0.05. **p < 0.01.
Next, we measured the impact of exogenous kynurenine. Intraperitoneal administration of kynurenine (10 mg/kg) 1 h after ICH did not induce significant brain herniation (n = 4, p < 0.05; Supplementary Fig. S15A), yet it exacerbated a modified Garcia test and forelimb placement test concomitantly with upregulated total AHR expression and a two-fold increase of nucleus/cytoplasmic ratio (n = 6; p < 0.05; Fig. 8A–C, G, H).
Moreover, kynurenine-induced worsened neurobehavioral function and AHR protein levels were AHR dependent, and they were absent after coadministration of the AHR antagonist TMF (n = 6; p < 0.05; Fig. 8A–C, G, H). Likewise, there were significantly more numbers of FJC-, TUNEL-, and 8-OHdG-positive cells in the ICH+KYN group than the Vehicle group at 48 h after ICH. These neuronal damages and oxidative injury were significantly reduced by TMF treatment (n = 6; p < 0.05; compared with ICH+KYN group, Fig. 8D–F, I, J).
Besides, exogenous kynurenine elicited increased 3-nitro tyrosine deposition in the perihematomal region after ICH (Supplementary Fig. S16A) and upregulated inducible nitric oxide synthase (iNOS) protein levels compared with the Sham group (n = 4; p < 0.05; Supplementary Fig. S16B–C).
Discussion
Oxidative stress and neuronal apoptosis after ICH generate an ongoing series of pathophysiological changes from intracellular metabolic abnormalities to clinical complications (Duan et al, 2016; Xie et al, 2020). It is critical for the future development of therapies to reduce secondary brain tissue damage in ICH patients (Sonni et al, 2014). This article aimed at investigating the changes in the cerebral activation of kynurenine/AHR and its link with oxidative stress and neuronal apoptosis. Here, we reported that ICH induced AHR overexpression primarily in neurons, which participated in secondary brain injury concomitantly with the upregulation of RhoA/Bax-mediated oxidative stress and neuronal apoptosis.
We also reported that kynurenine may account for the direct activation of AHR and substantial oxidative stress, with neuronal cell loss in this setting. To the best of our knowledge, these data are the first to demonstrate a pathophysiological role of kynurenine/AHR in the setting of ICH, revealing a potential therapeutic target from the bench to bedside.
In the past few decades, AHR has long been regarded the “first-line senser” modulating downstream CYP450s enzymes' expression to metabolize xenobiotics and environmental contaminants (Hale et al, 2017). In the central nervous system, the potential deleterious role of AHR in regards to inducing oxidative stress and neuronal apoptosis had been extensively studied (Brinkmann et al, 2019; Cuartero et al, 2014; Lin et al, 2018; Wojtowicz et al, 2019). Atrazine exposure, for instance, caused neurological disorders and cerebral injury by activating AHR, followed by initiating apoptosis and producing mitochondrial dysfunction in quail cerebrum (Lin et al, 2018). In the transient middle cerebral artery occlusion (MCAO) model, cerebral ischemia leads to the activation of AHR and rats administered with TMF post-ischemia attenuate cerebral ischemia-reperfusion injury accompanied with decreased severity of apoptosis (Cuartero et al, 2014).
Consistently, our study suggested that temporal changes of AHR overexpression after ICH, particularly in the peri-hematoma zone, peaked at 48 h from initial insult. We chose the TMF, AHR antagonist as the pharmacological agent for it had neither partial agonist activity nor species dependence at the dose of 5 mg/kg i.p. (Chen et al, 2019; Cuartero et al, 2014; Nath et al, 1992; Ren et al, 2021); on administration, TMF quickly approached maximal concentration, ranging from 0.55 to 0.88 μg/mL within 1 to 2 h after administration, and then were gradually excreted with half-lives of 3–6 h (Mekjaruskul et al, 2012).
In terms of the therapeutic window, we set up the intervention interval 2 h after ICH based on the temporal trends and implications of so-called Times From Symptom Onset to Hospital Arrival in the Get With The Guidelines–Stroke Program (Tong et al, 2012). It is believed that the earlier a stroke patient arrives at the hospital with initial interventions, the better survival or prognosis the patient would get. Currently, the majority of the recommended early therapy window is around 2–4.5 h after symptom onset in ischemic stroke, with symptoms such as paralysis, numbness, or even just mild dizziness.
On the other hand, patients suffering from hemorrhagic stroke usually had more severe symptoms, such as severe headache, vomiting, and loss of consciousness. In this case, patients with ICH would have early presentation (Pulvers and Watson, 2017). Therefore, we decided to treat the animals 2 h (early presentation time) after hemorrhagic stroke. We further reported AHR nuclear translocation induced by ICH concomitantly with upregulation of ARNT.
In neonatal hypoxic-ischemic encephalopathy animal models, similarly, the inhibition of AHR attenuated perinatal asphyxia accompanied by reduced expression of ARNT and ameliorating apoptosis (Rzemieniec et al, 2020). In this context, ARNT functioned as the chaperone protein assisting AHR shuttling into the nucleus (Lindsey and Papoutsakis, 2012). Taken together, our study suggested that ICH evoked extensive neuronal apoptosis and ROS accumulation whereas pharmacological AHR antagonist mitigated aforementioned neuronal injury and oxidative stress.
However, the regulatory roles of AHR in brain pathogenesis are sometimes controversial, which, in part, may be dependent on the different pharmacological properties of AHR ligands, varies in affinity, partial agonism/antagonist properties etc.(Szelest et al, 2021). Among the putative candidates for stroke therapeutic targets, kynurenine received attention since the 1990s, with a renewed interest recently on the wave of ROS-centric perspective of central nervous system diseases (Vecsei et al, 2013).
Derived from the kynurenine pathway of essential amino acid tryptophan, kynurenine is the key endogenous AHR ligand in neurons. Under physiological conditions, kynurenine is metabolized by either tryptophan 2,3-dioxygenase (TDO) or IDO in the liver and central nervous systems (Larkin et al, 2016). In rodent models of Huntington disease, the overall increase in kynurenine pathway activity is concomitant with the degree of excitotoxicity or oxidative stress that might underlie striatal neuron dysfunction and degeneration (Stone and Darlington, 2013).
Moreover, human studies performed in ischemic stroke patients, tryptophan oxidation along the kynurenine pathway contribute to the modulation of oxidative stress partly via the glutamate receptor agonist quinolinic acid and redox-active compounds such as 3-hydroxyanthranilic acid (Colpo et al, 2019). It is reported that activated kynurenine pathway is initiated immediately after stroke and is correlated with infarct volume as well as oxidative stress induced brain damage (Darlington et al, 2007).
Brain kynurenine levels increased as early as 3 h after stroke and remained elevated 24 h afterward, which was consistent with our findings (Cuartero et al, 2014). Likewise, we now reported that local brain kynurenine concentration increased as early as 6 h after ICH and peaked at around 48 h after ICH, coinciding with nuclear/cytoplasm AHR expression. Serum kynurenine, on the other hand, decreased soon after ICH, with increased peripheral activity of IDO suggesting that the surge increase of kynurenine in brain is taken up partly, if not all, from the blood. In the meantime, differential contribution of hepatocytes and fibroblasts with kynurenine-synthesizing capacity (Marszalek-Grabska et al, 2021) together with local kynurenine synthesis in the brain by TDO (Cuartero et al, 2014) should also be taken into consideration while identifying the source of kynurenine.
Of note, IDO catalyzed 1 rate-limiting step of tryptophan metabolism and emerges as an important regulator of cardiac remodeling as well as in the development of atherosclerosis-related inflammation (Niinisalo et al, 2008; Pedersen et al, 2011; Pertovaara et al, 2007). The IDO exerted its deleterious role on cardiac outcome through kynurenine production, as revealed by lower capillary number and increased infarct size and interstitial fibrosis through the induction of cardiomyocyte apoptosis (Melhem et al, 2021; Metghalchi et al, 2015; Pedersen et al, 2015). In line with our present findings, it is of great interest for further investigation of IDO-kynurenine in cerebrovascular dysfunction.
In a previous MCAO mice model, kynurenine 10mg/kg i.p. administration could significantly aggravate the infarct volume; in the meantime, such a dose did not lead to increased mortality (Cuartero et al, 2014). Besides, the result of a first-in-human clinical phase I trial showed that kynurenine in a broad range of doses from 50 μg/kg up to 5 mg/kg was found to be safe and well tolerated (Al-Karagholi et al, 2021). In this regard, mice were treated with kynurenine 10 mg/kg i.p. to explore its potential role after ICH.
Kynurenine was also considered as a novel endothelium-derived vasodilator through activating the cGMP and cAMP-dependent pathway, which may potentially dilate cerebral resistance vessels and the microvascular endothelium, leading to severe brain edema (Changsirivathanathamrong et al, 2011; Wang et al, 2010). Although trending to increased ICP was observed, we found no significant elevation after kynurenine use. Further experiments are warranted for the exploration of the impact of kynurenine on the cerebrovascular system.
Mice subjected to exogenous kynurenine showed hampered neurobehavioral performance at 48 h compared with the ICH group and were partially alleviated when treated with TMF. The same trends could be found in histopathological changes in terms of the degree of loss of cellular viability and ROS-related damage accumulation. Taken together, the early increase profiles of kynurenine in our study strongly suggest a detrimental role of the ligand in the hemorrhagic pathophysiological process, though some studies reported the versatile effects of supplementary kynurenine with decreased infarction lesion in ischemic stroke models (Colpo et al, 2019). In this regard, further studies are warranted to establish the role of kynurenine in stroke considering the complexity of animal models and administration route.
We further explored the potential signaling pathway. Indeed, at the molecular level, AHR regulated an increasingly large array of physiologically relevant genes by traditional transcription-dependent mechanisms. A previous study reported that cAMP response element (CRE)–binding protein (CREB) was one of the most important neuronal transcription factors known by its implication in the expression of survival and antiapoptotic genes such as brain-derived neurotrophic factor (BDNF) on binding to CRE and AHR might impair CREB-mediated neuronal BDNF gene expression (Cuartero et al, 2014). Besides, several other studies have demonstrated that AHR inhibition reduces neuronal apoptosis and neurotoxicity concomitant with an increase in Bax (Huang et al, 2018; Kajta et al, 2019; Kajta et al, 2009; Matikainen et al, 2001).
In this context, RhoA is a critical transcription factor related to mitochondria death and apoptosis injury because its so-called activity-regulated activation of mitochondria death or by activating apoptotic death signals is Bax-sensitive (Del Re et al, 2007). Indeed, our results show that the AHR antagonist TMF decreased Bax levels and reduced MitoSox+ cells, 4-HNE protein levels (biomarkers for damage elicited by oxidized lipids), and proapoptotic protein cleaved caspase-3 after ICH. 3-MC was used in a pair of Bax-inhibiting peptide V5 to elucidate the RhoA downstream signaling pathway.
These data strongly suggest that RhoA/Bax signaling participates in the detrimental role of AHR after ICH. Because in vivo coimmunoprecipitation experiments of AHR further demonstrate its direct interaction with RhoA, our results support that hemorrhagic stroke increases AHR recruitment to RhoA/Bax complexes, playing a deleterious role with regard to mitochondria death and apoptosis. However, we could not rule out other potential mechanisms' interaction between AHR/RhoA. Vav3, for example, an activator of Rho/Rac GTPases, is also an AHR transcriptional target in embryonic fibroblasts in charge of a limited subset of the developmental and physiological functions controlled by this transcriptional factor (Sauzeau et al, 2011).
Notably, AHR-deficient mice develop respiratory and cardiovascular phenotypes that resemble those of Vav3−/− mice, including hypertension, tachypnea, and sympathetic excitation (Mulero-Navarro and Fernandez-Salguero, 2016). Taken together, our results suggested that AHR mediates partially, if not all, mitochondria-mediated oxidative stress and neuronal apoptosis through the RhoA/Bax signaling pathway after ICH in mice.
Limitations
There were several limitations. First, we only used male nice. Given the possibilities that there might be different therapeutic effects in the female mouse group, further studies are warranted considering gender. Second, AHR is also expressed in microglia and astrocyte cells, involving the modulation of NMDA toxicity in many neuroinflammatory mechanisms or nitric oxide synthase induction and cellular stress responses after brain injury (Calabrese et al., 2010; Calabrese et al., 2000; Dattilo et al, 2015). Lastly, we used CRISPR to edit the in vivo genome.
Although control CRISPR was also used to exclude potential off-target effects, there may still be some unpredictable off-target effects on neurobehavioral function and protein expression. For further validation of the AHR function, transgenic animals should be considered.
Conclusion
The present study showed kynurenine/AHR signaling routes in ICH. Our results paved new lines of investigation into the effects of the interference of this pathway at an early timepoint after ICH. Kynurenine/AHR-mediated oxidative stress and neuronal impairment could contribute to deleterious outcomes. Overall, this study linked kynurenine with AHR signaling in ICH and identified kynurenine/AHR as a potential therapeutic target in this setting.
Materials and Methods
Data availability
All data are available within the article, and additional data can be acquired from the corresponding author.
Reagents
The AHR antagonist, 6,2,4-trimethoxyflavone (TMF, T4080), AHR agonist, 3-Methylcholanthrene (3-MC, 213942), Dimethyl Sulfoxide (DMSO), Bax-inhibiting Peptide V5 (B1436), Kynurenine (K8625), N-2-hydroxyethylpiperazine-N-2-ethanesulfonic (HEPES), Sucrose, Dithiothreitol (DTT, 43816), EDTA, trypsin inhibitor (10109886001), Leupeptin, Glucose 6-phosphate, Glucose 6-phosphate dehydrogenase, Heme, β-nicotinamide adenine dinucleotide phosphate (NADPH), MgCl2, and Potassium phosphate buffer were from Sigma-Aldrich. Aprotinin was from Roche.
Experimental design and animals
A total of 377 adult male CD-1 mice (weight 30–35 g; Charles River, Wilmington, MA) aged 8–10 weeks were used for the study. All mice were housed under a 12 h light/dark cycle with ad libitum access to food and standard water. All animal experimental procedures were approved by the Institutional Animal Care and Use Committee at Loma Linda University (#8190039) and were compliant with the ARRIVE guidelines.
Mice were randomly assigned to different experimental groups. The information of experimental groups was blinded to researchers who performed surgeries, neurobehavioral assessments, Western blot, immunofluorescence staining, and data analysis. Six separate experiments were conducted (Supplementary Fig. S17). Animal numbers per group are listed in Supplementary Table S1.
Experiment 1. Role of endogenous AHR in oxidative stress and neuronal apoptosis after ICH in mice
In vivo AHR CRISPR Knock out (KO.) was used to explore the role of endogenous AHR in oxidative stress and neuronal apoptosis 48 h after ICH, including neurobehavior, pathological histology changes, and protein biomarkers of oxidative stress and neuronal apoptosis. Mice were randomly divided into four groups (n = 6–10/group): Sham, ICH, ICH+AHR CRISPR (KO.), and ICH+Control CRISPR. Neurobehavior tests (Modified Garcia test and Forelimb Placement test) were performed in each mouse (n = 10/group).
Immunoblots were performed in the ipsilateral hemisphere to assess the protein levels of AHR, oxidative stress markers (4-HNE and HO-1), and intrinsic apoptosis markers (ratio of Bcl-2/Bax). Immunohistochemistry staining of Cleaved Caspase-3 and Terminal deoxynucleotidyl transferase dUTP (TUNEL POD) was performed to evaluate neuronal viability and proapoptotic activity (n = 4/group). In addition, to confirm the knockout efficacy of in vivo AHR CRISPR KO., two more groups (n = 6/group) were added for Western blot: Naive (Control CRISPR) and Naive (AHR CRISPR KO.).
Experiment 2. To evaluate the time course of KYN, AHR, AHR nuclear/cytoplasmic ratio and cellular localization of AHR in the ipsilateral hemisphere after ICH
Mice were randomly assigned to the following six groups (n = 6–12/group): Sham, ICH (6, 24, 48, 72 h and 7 days) after ICH. Western blot was performed to evaluate the temporal changes in the protein expression of total AHR, AHR nuclear/cytoplasmic ratio, and downstream Cytochromes P450 (CYP) 1A1 in each group (n = 4–6/group). Double immunofluorescence staining (n = 4/group) was used to explore the cellular localization of AHR after ICH. Serum and brain endogenous kynurenine, serum tryptophan concentration were measured by ELISA accordingly (n = 4–6/group).
Experiment 3. To assess the short-term treatment value of AHR inhibition after ICH in mice
To evaluate the treatment value of the AHR antagonist TMF that was AHR-dependent, mice were first randomly assigned to six groups (n = 6/group): Vehicle (ICH+DMSO), ICH+TMF, ICH +3-MC, AHR CRISPR (KO.) +Vehicle, AHR CRISPR (KO.) +ICH+TMF, and AHR CRISPR (KO.) +ICH +3-MC. Neurobehavior test, hematoma volume, and hemoglobin assays were performed at 48 h after ICH. Based on our previous study and literature search, TMF (5 mg/kg) and 3-MC (10 mg/kg) were intraperitoneally used for the following experiments (Cuartero et al, 2014).
Next, the mice were randomly divided into three groups (n = 18/group): Sham, Vehicle, and ICH+TMF. Brain water content was measured at 48 h after ICH (n = 6/group). Histological quantification for brain edema using LFB/Cresyl Violet staining and HO-1 activity was also measured (n = 4/group). Eight-OHdG, mitochondrial superoxide (MitoSox), Fluoro-Jade C (F-JC) staining and TUNEL immunofluorescence staining (n = 6/group), and Western blot analysis (n = 6/group) were used at 48 h after ICH.
Experiment 4. Effect of pharmacological AHR inhibition on long-term neuronal degeneration after ICH in mice
The TMF was administrated to evaluate the value of AHR inhibition on neurobehavior and histological morphology changes in ipsilateral hemisphere at day 28 after ICH. Mice were randomly assigned to three groups (n = 15/group): Sham, Vehicle, and ICH+TMF. DMSO or TMF was administered at 2, 24, and 48 h after ICH modeling. Rotarod tests (5RPM and 10RPM) were carried on the pre-modeling day, on the 7th, 14th, and 21st day after ICH. Morris water maze tests were performed between the 23rd and 28th day after ICH, after which all the mice were sacrificed for Nissl staining to determine the neuronal degeneration.
Experiment 5. To determine the role of exogenous kynurenine on AHR expression, mitochondria-mediated oxidative stress, and neuronal apoptosis after ICH
Intraperitoneally administered exogenous kynurenine (10mg/kg) in mice was used to explore the role of kynurenine after ICH. The mice were randomly assigned to the following three groups (n = 16/group): ICH+DMSO, ICH+kynurenine, and ICH+kynurenine+TMF. Intracranial pressure was measured 6 h after sham+Kyn group (n = 4/group). Western blots, 8-OHdG, FJC staining, TUNEL staining, and neurobehavioral tests were performed at 48 h after ICH.
Experiment 6. To determine the potential deleterious molecular mechanism of AHR-mediated oxidative stress and neuronal apoptosis after ICH
RhoA co-immunoprecipitation with AHR was performed to reveal the AHR inhibitory effect on Bax signaling, partly due to RhoA-AHR interaction. Mice were randomly assigned to three groups (n = 4/group): Sham, Vehicle, and ICH+TMF for Western blot of co-immunoprecipitation complex. Besides, Bax-inhibiting Peptide V5 (200 μM intraperitoneally injection) and ARNT CRISPR Activation (ACT) were used to determine the potential molecular signaling of AHR-RhoA-Bax.
Mice were randomly assigned to the following nine groups: Sham, ICH+DMSO, ICH+TMF, ICH+TMF+ARNT CRISPR Activation, ICH+TMF+CRISPR Control, ICH +3-MC, ICH +3-MC+Bax-inhibiting Peptide V5, ICH +3-MC+DMSO, and ICH+Bax-inhibiting Peptide V5 (n = 4–6/group). Western blots were used to evaluate the protein expression changes of AHR, Bax, HO-1, and Bcl-2.
Collagenase-induced ICH model
The collagenase injection ICH model was performed as previously described (Barus et al, 2021; Gautam et al, 2019). Briefly, the mice were weighed and anesthetized with a ketamine-xylazine cocktail (Ketamine 100 mg/kg with Xylazine 10 mg/kg). The mice were placed prone onto the heating pad, which was programmed to maintain a core body temperature around 37°C.
Next, the animals were fixed to a stereotactic frame in a prone position and the position of the Hamilton syringe was adjusted to ensure a proper injection site relative to bregma (right lateral 2.2 mm, rostral 0.2 mm). A burr hole was drilled at the position. Next, the infusion pump was set at a rate of 0.2 μL/min and the needle of the Hamilton syringe was advanced ventrally to a depth of 3.5 mm to infuse 1 μL collagenase (VII collagenase from Clostridium histolyticum, 0.075 U).
The needle was left in situ for 5 min after the end of infusion before retracting at a rate of 1 mm/min. Sham group mice were subjected to a similar procedure but received 0.9% sterile saline injection. We chose buprenorphine (0.03 mg/kg) subcutaneously for postoperative analgesia treatment.
Intracerebroventricular drug delivery route
As previously described (Zhang et al, 2020a), an intracerebroventricular (i.c.v.) injection in the left ventricle was administered by using the coordinates left lateral of bregma = 1.0 mm and ventral depth = 3.2 mm. Using clustered regularly interspaced short palindromic repeat/Cas9 (CRISPR/Cas9) technology, we edited the in vivo genome mediated by homology-independent targeted integration. We used AHR CRISPR/Cas9 KO Plasmid to inhibit AHR expression in the mouse brain.
The AHR Knockout CRISPR (sc-419054; Santa Cruz Biotechnology, Dallas, TX. gRNA sequences: TGAGCTCATATACGCTCTGA; CGGTCTCTGTGTCGCTTAGA; CTCCACTATCCAAGATTACC) was suspended in 20 μL of transfection medium (sc-108062; Santa Cruz Biotechnology) and then activated by using 20 μL transfection reagent (sc-395739; Santa Cruz Biotechnology) to get a final concentration 0.5 μg/μL of CRISPR.
For each of the CRISPRs, a total of 2 μL CRISPR was injected into the left lateral ventricle 48 h before ICH. The efficacy of knockout was validated by testing the protein level of AHR using immunoblotting. Control CRISPR (sc-418922; Santa Cruz Biotechnology) was used to control the off-target effect. In addition, we used ARNT CRISPR Activation Plasmid (m, sc-419204-ACT; Santa Cruz Biotechnology) to activate ARNT expression in the mouse brain and delivered it the same way as mentioned earlier.
Evaluation of kynurenine, tryptophan levels, and k:t ratio
The levels of kynurenine were measured in brain tissue homogenates and serum samples by using mouse kynurenine ELISA kits (LS-F56523; Biosensis) according to the manufacturer's instructions. The levels of tryptophan were measured in serum samples by using Tryptophan ELISA kits (LS-F55484; Biosensis) according to the manufacturer's instructions. The results were quantified by using a microplate reader at 450 nm.
HO-1 activity assay
HO-1 activity, based on the reduction of biliverdin into bilirubin, was used for the present study according to the methods adapted from Kurucz et al (2018), Nath et al (1992), and Datla et al (2007). One hundred twenty μg/mL of mice liver cytosol was used as a source of biliverdin reductase. The bilirubin formed was calculated from the difference between optical densities obtained at 470 and 530 nm. One unit of HO-1activity was defined as the amount of bilirubin (nmol) produced per hour per mg (h/mg) of protein.
Neurobehavioral tests
The modified Garcia neurological score (Garcia et al, 1995) and forelimb placement (Altamentova et al, 2020) test were used to assess short-term neurobehavioral function. The modified Garcia test comprised the 21-point scoring scale adapted from the one developed by Garcia et al and it consisted of seven tests, each of which was assigned a score from 0 to 3 (spontaneous activity, body proprioception, sense of vibrissae touch, limb symmetry, forelimb outstretching, response to whisker stimulus, and climbing).
The forelimb placement test examined compound whisker somato sensation and forepaw motor function. The mouse was held without impeding forepaw movement, and then it was approached to the countertop edge while brushing the left whiskers against the table edge. This test was used to determine whether the mice could stereotypically place their forelimb on the table, and the successful reaches of the left-side forelimb was calculated.
The rotarod test was conducted on days 0, 7, 14, and 21 after ICH, aiming at evaluating sensorimotor coordination and balance ability adapted from Harrison et al The rotating speed started with 5 revolutions per minute (rpm) or 10 rpm and accelerated by 2 rpm every 5 s, and the final duration on the rotarod was recorded. Two examiners blinded to the animal groups performed the aforementioned neurobehavioral tests.
To evaluate the spatial learning memory and cognitive function, Morris water maze test (Zhang et al, 2019) was performed from the 23rd to the 28th day after ICH. The time that mice reached the submerged platform was recorded in every 1-min test for 5 consecutive days. On the last day of the 60-s probe trial without platform, the time that mice spent in the southwest quadrant (where the platform was placed earlier) was recorded. Swimming speed and distance, escape latency were recorded by the EthoVision video tracking system (Noldus).
Brain water content measurement
Wet–dry weight measurement for brain edema was performed, as previously reported (Xu et al, 2021). Under deep anesthesia, quick removal of brain tissue was performed. The brain tissue divided into five regions (ipsilateral and contralateral cortex, ipsilateral and contralateral basal ganglion, and cerebellum) was weighed on an electronic analytic balance, and the wet weights for each respective brain section were obtained. Brain specimens were then dried in a 100°C oven for 48 h, and the dry weights were obtained. Brain water content (%) was calculated as (wet weight-dry weight)/wet weight*100%.
Western blots
Western blots were performed as previously reported (Matei et al, 2018). Briefly, the ipsilateral (left) cerebral hemisphere was homogenized in RIPA lysis buffer (Santa Cruz Biotechnology), and it was centrifuged at 15,000 g at 4°C for 20 min. The supernatant was mixed with loading buffer to reach 5 μg/mL protein concentration. The 20 μg protein of each sample was loaded for electrophoresis and was further transferred onto nitrocellulose membranes.
Afterward, the membranes were incubated with the following primary antibodies overnight at 4°C: anti-AHR antibody (1:1000, MA1–514; Thermofisher, Waltham, MA), anti-4-HNE antibody (1:1000, MA5–27570; Thermofisher), anti-iNOS polyclonal antibody (1:1000, PA1–036), anti-HO-1 antibody (1:1000, MA1–112; Thermofisher), anti-Bcl-2 antibody (1:1000, ab59348; Abcam), anti-Bax antibody (1:1000, ab182734; Abcam), anti-CYP1A1 antibody (1:1000, sc-393979; Santa Cruz Biotechnology), anti-ARNT antibody (1:1000, MA1–515; Thermofisher), anti-Cleaved Caspase 3 antibody (1:400, 9661s; Cell Signaling Technology), anti-RhoA antibody (1:500, sc-418; Santa Cruz Biotechnology), anti-β-Actin antibody (1:3000, sc-47778; Santa Cruz Biotechnology), and anti-H3 antibody (1:3000, 17168-1-AP; Proteintech, Rosemont, IL). Western blot bands were quantified as the relative density of bands by using Image J software (NIH, Bethesda, MD).
Nucleus and cytoplasm extracts
The ipsilateral hemisphere of the mouse brain collected in Sham, ICH-24 h, ICH-48 h, ICH-72 h, Vehicle (48 h), ICH+KYN (48 h), and ICH+KYN+TMF (48 h) groups was used for nucleus and cytoplasm isolation by using NE-PER Nuclear and Cytoplasmic Extraction Reagents (78835; Thermofisher) according to the manufacturer's instructions.
Immunoprecipitation assay
Immunoprecipitation was performed by following the manufacturer's guidelines as previously reported (Zhang et al, 2020b). Protein extracts were precipitated by mouse monoclonal anti-RhoA antibody (sc-418; Santa Cruz Biotechnology) and protein A/G PLUS- Agarose (sc-2003; Santa Cruz Biotechnology). Mouse IgG was used as a negative control for immunoprecipitation. After washing and centrifuging, the pellet was collected, re-suspended, and boiled with loading buffer.
Mouse monoclonal anti-AHR antibody (MA1–514; Thermofisher) and anti-RhoA antibody were applied to probe AHR and RhoA by Western blots. IgG (sc-2025; Santa Cruz Biotechnology) was detected as an internal loading control.
Hemoglobin assay
A modified spectrophotometric assay was used to measure hemoglobin content (Choudhri et al, 1997). Mice were euthanized by using deep isoflurane, and right hemispheres were homogenized in 1 mL phosphate-buffered saline (PBS) followed by sonication on ice for 30 s and centrifugation at 14,000 rpm at 4°C for 30 min. Next, we mixed 0.2 mL supernatant aliquots with 0.8 mL Drabkin's reagent (Sigma), allowing them to react for 15 min under room temperature. Lastly, optical density was obtained by using a spectrophotometer at 540 nm and compared with a preset standard curve of known blood volume.
Image-based measurement of hematoma volume
On euthanasia, the mouse was transcardially perfused with ice-cold PBS. The brain tissue was harvested and cut into 1 mm thick slices (−2 mm to +4 mm from Bregma). Consecutive coronal sections were used to digitally quantify the hemorrhage volume by using “Integrated Density” in Image J (v. 1.31 National Institutes of Health). A step-by-step procedure was followed as previously published protocol (Tang et al, 2010).
Immunohistochemistry and Immunofluorescence
Mice were sacrificed under deep anesthesia at 48 h (except for Nissl staining) after ICH by transcardial perfusing with 0.01 M PBS and subsequent 10% neutral buffered formalin. The brain was extracted from the skull and was further fixed in 10% neutral buffered formalin for 24 h, followed by 4% paraformaldehyde for 24 h. Lastly, they were dehydrated by being successively immersed in serial 15% and 30% sucrose solutions for 2 days. Coronal brain slices 10–20 μm were cut by using a cryostat (Leica CM3050S-3-1-1, Bannockburn, IL), and standard immunohistochemistry and immunofluorescence protocol was followed. The brain slices were blocked with 5% normal donkey serum in 0.1% Triton X-100 for 1 h at room temperature, followed by overnight incubation at 4°C with the following measurements:
Measurement of oxidative stress
Immunohistochemistry staining was used to identify the brain levels of 8-OHdG (Mouse monoclonal Anti-DNA/RNA Damage antibody, ab2623; Abcam) and MitoSox (MitoSox™ Red Mitochondrial Superoxide Indicator, for live-cell imaging, M36008; Thermofisher). Freshly prepared frozen 10 μm brain slices were placed on the microscope slides (Genesee Scientific). The slides were immersed in the pH 6.0 antigen retrieval solution and heated with a microwave for 15 min to expose the antigen. The sections were then blocked with endogenous peroxidase for 10 min with 3% H2O2 followed by the incubation of the 8-OHdG antibody or MitoSox antibody at room temperature.
The slides were observed under the microscope under a magnification of 400 × . The fluorescence intensity was quantified as the average of four brain slices randomly selected within the ipsilateral hemisphere around the hematoma area by using ImageJ 1.52p (National Institutes of Health).
Measurements of neuronal damage
Neuronal degeneration was evaluated though FJC staining at 48 h after ICH by using a modified FJC Ready-to-Dilute Staining Kit (Biosensis) according to the manufacturer's instructions. Using a fluorescence microscope (DMi8; Leica Microsystems, Wetzlar, Germany), FJC-positive cells were counted in four brain slices under 200 × magnification Data were presented as the average number of FJC-positive cells in the fields as cells/mm2.
Neuronal viability was evaluated through TUNEL staining at 48 h after ICH by using an in situ Apoptosis Detection Kit, POD (11684817910; Roche) according to the manufacturer's instructions. Using a fluorescence microscope (DMi8; Leica Microsystems), TUNEL-positive neurons were counted in four brain slices from randomly selected regions of the ipsilateral hemisphere cortex under 200 × magnification. Data were presented as the average number of TUNEL-positive neurons/field.
We also performed immunohistochemistry staining by using POD kit (11684817910; Roche) according to the manufacturer's instructions. A diaminobenzidine/hydrogen peroxide solution (DAB-buffer tablets, Merck, Germany) was used to elicit a color reaction, and the slices were counter-stained with anti-NeuN antibodies (ImmPRESS®-AP Horse Anti-Mouse IgG Polymer Detection Kit, MP-5402; Vector). In addition, anti-cleaved Caspase 3 and anti-3-nitrotyrosine (anti-3-Nitrotyrosine antibody, 1:1000, ab61392) were used for immunohistochemistry staining detection for apoptosis and oxidative stress before being mounted by using DPX mountant for histology (Sigma-Aldrich) with a Zeiss microscope equipped with a digital color camera under 400 × magnification.
Nissl staining
Hippocampus injury was evaluated by using Nissl staining at the 28th day after ICH. The hippocampal coronal sections were cut into 20 μm by using a Leica CM1860 cryostat microtome (Leica Biosystems, Nussloch, Germany) and mounted on microscope slides (Genesee Scientific). Nissl staining was performed by using 0.5% crystal violet as previously described. The hippocampal regions, including CA1, CA3, and DG, per brain slice were observed under a light microscope at 400 × magnification.
LFB/cresyl violet staining
Brain edema around hematoma was evaluated though LFB/Cresyl violet staining at 48 h after ICH by using an LFB Stain Kit (Myelin Stain, AB150675; Abcam) according to the manufacturer's instructions. All the morphological results were photographed by using a Zeiss microscope equipped with a digital color camera at 10 × magnification.
Immunofluorescence
For the detection of cellular localization of AHR in mice, immunofluorescence staining was performed on frozen brain sections as previously reported (Zhang et al, 2015). The brain sections were incubated at 4°C overnight with primary antibodies listed as follows: rabbit polyclonal Anti-AHR antibody (1:100, ab84833; Abcam), Anti-GFAP (1:100; Santa Cruz Biotechnology), Anti-NeuN (marker of neuron, 1:100, ab104224; Abcam), and goat anti-Iba-1 (marker of microglia, 1:100, ab5076; Abcam).
This was followed by incubation with fluorescence-conjugated secondary antibodies (1:200; Jackson Immunoresearch, West Grove, PA) for 1–2 h at room temperature. The stained slices were visualized by using a fluorescence microscope (Olympus BX51; Olympus Optical Co. Ltd), and images were captured by software MagnaFire SP 2.1B (Olympus, Melville, NY). Colocalization AHR/GFAP images were analyzed by using Image J (v. 1.31 National Institutes of Health).
The original Olympus Image output data for the different fluorescent wavelengths were opened in ImageJ by using Bio-Formats plugins. Each image was inverted to grayscale to calculate the minimum and maximum gray values (i.e., pixel intensity) in each image. These values set a basis for quantifying and comparing the fluorescent intensities. For each image, 10 different locations were selected randomly for ImageJ analysis.
A step-by-step procedure was followed as previously published protocol (Nazem-Bokaee et al, 2019). Colocalization AHR/NeuN and AHR/Iba-1 were analyzed by using Image J Trainable Weka segmentation plugin. The images were corrected for the background noise and were segmented. A few colocalization areas and backgrounds were selected in each image for the plugin to train itself. Then, the probability map of the segmented area was used for highlighting and analysis. A step-by-step procedure was followed as previously published protocol (Arganda-Carreras et al, 2017). All fluorescent images were analyzed ten fields per section, with at least three sections per mouse (with at least 100 μm between sections) using samples obtained from four separate mice for the analysis of two groups.
Intracranial pressure measurement
Under anesthesia, mice were placed in the prone position and the atlantooccipital membrane was exposed by a midline incision. A PE-50 catheter (Becton Dickinson, Franklin Lakes, NJ) filled with saline was gently inserted at 2 to 3 mm depth into the cisterna magna and fixed with dental cement and cyanoacrylate glue to prevent the potential CSF leakage. The data were recorded after ICP equilibration by using WINDAQ data acquisition system (DATAQ, Akron, OH).
Statistical analysis
All data analysis was performed by using GraphPad Prism 8.2.1 (GraphPad Software), and data were expressed as mean ± SD. We used D'Agostino-Pearson omnibus normality test and Shapiro-Wilk normality test. Multiple comparisons were statistically analyzed with one-way ANOVA or two-way ANOVA followed by post hoc Tukey method. Correlation analysis was performed by the use of a nonparametric Spearman correlation, and a linear regression of the data was displayed. Wilcoxon signed-rank test, Kruskal–Wallis test, and Mann–Whitney test were used for nonparametric comparison. Values of p < 0.05 were considered statistically significant. In each figure, the mean value of every group labeled with a specific symbol is significantly different (p < 0.05) from the mean value of the reference group, which is indicated in each case.
Supplementary Material
Acknowledgment
The authors acknowledge the animal care facility of Loma Linda University for keeping and raising the mice.
Abbreviations Used
- 3-MC
3-methylcholanthrene
- 4-HNE
4-Hydroxynonena
- AHR
aryl hydrocarbon receptor
- ARNT
AHR nuclear translocator
- BDNF
brain-derived neurotrophic factor
- CA
cornu ammonis
- CRISPR
clustered regularly interspaced short palindromic repeat
- DG
dentate gyrus
- FJC
fluoro-jade c
- GFAP
glial fibrillary acidic protein
- H&E
hematoxylin, and eosin
- HO-1
heme oxygenase 1
- ICH
intracerebral hemorrhage
- IDO
indole 2,3-dioxygenase
- KO
knockout
- LFB
luxol fast blue
- MCAO
middle cerebral artery occlusion
- NMDA
N-methyl-D-aspartate
- ROS
reactive oxygen species
- TDO
tryptophan 2,3-dioxygenase
- TMF
6,2,4-trimethoxyflavone
Authors' Contributions
J.Z. and J.T. developed the concept, supervised the study, and edited the article. R.R. and Y.F. designed and conducted the experiments, analyzed the data, and wrote the article. R.R. and P.S. performed the animal study and collected the data. Q.L. and C.L. conducted neurobehavioral tests. J.H.Z. edited the article. All authors reviewed the article. The electronic laboratory notebook was not used.
Author's Disclosure Statement
The authors have no conflict of interest to declare.
Funding Information
This work was supported by grants from the National Institutes of Health (R01NS091042) of John H. Zhang, and the Program of Science and Technology Development of Zhejiang Province (LQ22H090017) and Health Commission of Zhejiang Province Young Talent supporting program (2022RC160) of Reng Ren.
Supplementary Material
References
- Al-Karagholi MA, Hansen JM, Abou-Kassem D, et al. . Phase 1 study to access safety, tolerability, pharmacokinetics, and pharmacodynamics of kynurenine in healthy volunteers. Pharmacol Res Perspect 2021;9(2):e00741. doi: 10.1002/prp2.741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altamentova S, Rumajogee P, Hong J, et al. Methylprednisolone reduces persistent post-ischemic inflammation in a rat hypoxia-ischemia model of perinatal stroke. Transl Stroke Res 2020;11(5):1117–1136. doi: 10.1007/s12975-020-00792-2. [DOI] [PubMed] [Google Scholar]
- Androutsopoulos VP, Tsatsakis AM, Spandidos DA. Cytochrome p450 cyp1a1: Wider roles in cancer progression and prevention. BMC Cancer 2009;9:187. doi: 10.1186/1471-2407-9-187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arganda-Carreras I, Kaynig V, Rueden C, et al. Trainable weka segmentation: A machine learning tool for microscopy pixel classification. Bioinformatics 2017;33(15):2424–2426. doi: 10.1093/bioinformatics/btx180. [DOI] [PubMed] [Google Scholar]
- Barus R, Bergeron S, Auger F, et al. Sex differences in cognitive impairment induced by cerebral microhemorrhage. Transl Stroke Res 2021;12(2):316–330. doi: 10.1007/s12975-020-00820-1. [DOI] [PubMed] [Google Scholar]
- Brinkmann V, Ale-Agha N, Haendeler J, et al. The aryl hydrocarbon receptor (ahr) in the aging process: Another puzzling role for this highly conserved transcription factor. Front Physiol 2020;10:1561. doi: 10.3389/fphys.2019.01561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calabrese V, Copani A, Testa D, et al. Nitric oxide synthase induction in astroglial cell cultures: Effect on heat shock protein 70 synthesis and oxidant/antioxidant balance. J Neurosci Res 2000;60(5):613–622. doi: 10.1002/(SICI)1097-4547(20000601)60:5 <613::AID-JNR6>3.0.CO;2–8. [DOI] [PubMed] [Google Scholar]
- Calabrese V, Cornelius C, Dinkova-Kostova AT, et al. Cellular stress responses, the hormesis paradigm, and vitagenes: Novel targets for therapeutic intervention in neurodegenerative disorders. Antioxid Redox Signal 2010;13(11):1763–1811. doi: 10.1089/ars.2009.3074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Changsirivathanathamrong D, Wang Y, Rajbhandari D, et al. Tryptophan metabolism to kynurenine is a potential novel contributor to hypotension in human sepsis. Crit Care Med 2011;39(12):2678–2683. doi: 10.1097/CCM.0b013e31822827f2. [DOI] [PubMed] [Google Scholar]
- Chen WC, Chang LH, Huang SS, et al. . Aryl hydrocarbon receptor modulates stroke-induced astrogliosis and neurogenesis in the adult mouse brain. J Neuroinflammation 2019;16(1):187. doi: 10.1186/s12974-019-1572-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choudhri TF, Hoh BL, Solomon RA, et al. Use of a spectrophotometric hemoglobin assay to objectively quantify intracerebral hemorrhage in mice. Stroke 1997;28(11):2296–2302. doi: 10.1161/01.str.28.11.2296. [DOI] [PubMed] [Google Scholar]
- Colpo GD, Venna VR, Mccullough LD, et al. Systematic review on the involvement of the kynurenine pathway in stroke: Pre-clinical and clinical evidence. Front Neurol 2019;10:778. doi: 10.3389/fneur.2019.00778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuartero MI, Ballesteros I, De La Parra J, et al. L-kynurenine/aryl hydrocarbon receptor pathway mediates brain damage after experimental stroke. Circulation 2014;130(23):2040–2051. doi: 10.1161/CIRCULATIONAHA.114.011394. [DOI] [PubMed] [Google Scholar]
- Darlington LG, Mackay GM, Forrest CM, et al. Altered kynurenine metabolism correlates with infarct volume in stroke. Eur J Neurosci 2007;26(8):2211–2221. doi: 10.1111/j.1460-9568.2007.05838.x. [DOI] [PubMed] [Google Scholar]
- Datla SR, Dusting GJ, Mori TA, et al. Induction of heme oxygenase-1 in vivo suppresses nadph oxidase derived oxidative stress. Hypertension 2007;50(4):636–642.doi: 10.1161/HYPERTENSIONAHA.107.092296. [DOI] [PubMed] [Google Scholar]
- Dattilo S, Mancuso C, Koverech G, et al. Heat shock proteins and hormesis in the diagnosis and treatment of neurodegenerative diseases. Immun Ageing 2015;12:20. doi: 10.1186/s12979-015-0046-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Re DP, Miyamoto S, Brown JH. Rhoa/rho kinase up-regulate bax to activate a mitochondrial death pathway and induce cardiomyocyte apoptosis. J Biol Chem 2007;282(11):8069–8078. doi: 10.1074/jbc.M604298200. [DOI] [PubMed] [Google Scholar]
- Dietrich C. Antioxidant functions of the aryl hydrocarbon receptor. Stem Cells Int 2016;2016:7943495; doi: 10.1155/2016/7943495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan X, Wen Z, Shen H, et al. Intracerebral hemorrhage, oxidative stress, and antioxidant therapy. Oxid Med Cell Longev 2016;2016:1203285. doi: 10.1155/2016/1203285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez-Salguero PM, Ward JM, Sundberg JP, et al. Lesions of aryl-hydrocarbon receptor-deficient mice. Vet Pathol 1997;34(6):605–614. doi: 10.1177/030098589703400609. [DOI] [PubMed] [Google Scholar]
- Garcia JH, Wagner S, Liu KF, et al. Neurological deficit and extent of neuronal necrosis attributable to middle cerebral artery occlusion in rats. Statistical validation. Stroke 1995;26(4):627–634. discussion 635; doi: 10.1161/01.str.26.4.627. [DOI] [PubMed] [Google Scholar]
- Gautam J, Miner JH, Yao Y. Loss of endothelial laminin alpha5 exacerbates hemorrhagic brain injury. Transl Stroke Res 2019;10(6):705–718. doi: 10.1007/s12975-019-0688-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hale MD, Galligan TM, Rainwater TR, et al. Ahr and cyp1a expression link historical contamination events to modern day developmental effects in the american alligator. Environ Pollut 2017;230:1050–1061. doi: 10.1016/j.envpol.2017.07.065. [DOI] [PubMed] [Google Scholar]
- Hu X, Tao C, Gan Q, et al. Oxidative stress in intracerebral hemorrhage: Sources, mechanisms, and therapeutic targets. Oxid Med Cell Longev 2016;2016:3215391. doi: 10.1155/2016/3215391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y, He J, Liang H, et al. Aryl hydrocarbon receptor regulates apoptosis and inflammation in a murine model of experimental autoimmune uveitis. Front Immunol 2018;9:1713. doi: 10.3389/fimmu.2018.01713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joundi RA, Smith EE, Yu AYX, et al. Temporal trends in case fatality, discharge destination, and admission to long-term care after acute stroke. Neurology 2021;96(16):e2037–e2047. doi: 10.1212/WNL.0000000000011791. [DOI] [PubMed] [Google Scholar]
- Juricek L, Coumoul X. The aryl hydrocarbon receptor and the nervous system. Int J Mol Sci 2018;19(9):2504. doi: 10.3390/ijms19092504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kajta M, Wnuk A, Rzemieniec J, et al. Triclocarban disrupts the epigenetic status of neuronal cells and induces ahr/car-mediated apoptosis. Mol Neurobiol 2019;56:3113–3131. doi: 10.1007/s12035-018-1285-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kajta M, Wojtowicz AK, Mackowiak M, et al. Aryl hydrocarbon receptor-mediated apoptosis of neuronal cells: A possible interaction with estrogen receptor signaling. Neuroscience 2009;158(2):811–822. doi: 10.1016/j.neuroscience.2008.10.045. [DOI] [PubMed] [Google Scholar]
- Kou Z, Dai W. Aryl hydrocarbon receptor: Its roles in physiology. Biochem Pharmacol 2021;185:114428. doi: 10.1016/j.bcp.2021.114428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurucz A, Bombicz M, Kiss R, et al. Heme oxygenase-1 activity as a correlate to exercise-mediated amelioration of cognitive decline and neuropathological alterations in an aging rat model of dementia. Biomed Res Int 2018;2018:7212861. doi: 10.1155/2018/7212861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larkin PB, Sathyasaikumar KV, Notarangelo FM, et al. Tryptophan 2,3-dioxygenase and indoleamine 2,3-dioxygenase 1 make separate, tissue-specific contributions to basal and inflammation-induced kynurenine pathway metabolism in mice. Biochim Biophys Acta 2016;1860(11 Pt A):2345–2354. doi: 10.1016/j.bbagen.2016.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leasure AC, Kuohn LR, Vanent KN, et al. Association of serum il-6 (interleukin 6) with functional outcome after intracerebral hemorrhage. Stroke 2021;52(5):1733–1740. doi: 10.1161/STROKEAHA.120.032888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim-Hing K, Rincon F. Secondary hematoma expansion and perihemorrhagic edema after intracerebral hemorrhage: From bench work to practical aspects. Front Neurol 2017;8:74. doi: 10.3389/fneur.2017.00074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin J, Zhao HS, Qin L, et al. Atrazine triggers mitochondrial dysfunction and oxidative stress in quail (coturnix c. Coturnix) cerebrum via activating xenobiotic-sensing nuclear receptors and modulating cytochrome p450 systems. J Agric Food Chem 2018;66(25):6402–6413. doi: 10.1021/acs.jafc.8b01413. [DOI] [PubMed] [Google Scholar]
- Lindsey S, Papoutsakis ET. The evolving role of the aryl hydrocarbon receptor (ahr) in the normophysiology of hematopoiesis. Stem Cell Rev Rep 2012;8(4):1223–1235. doi: 10.1007/s12015-012-9384-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marszalek-Grabska M, Walczak K, Gawel K, et al. Kynurenine emerges from the shadows - current knowledge on its fate and function. Pharmacol Ther 2021;225:107845. doi: 10.1016/j.pharmthera.2021.107845. [DOI] [PubMed] [Google Scholar]
- Matei N, Camara J, Mcbride D, et al. Intranasal wnt3a attenuates neuronal apoptosis through frz1/piwil1a/foxm1 pathway in mcao rats. J Neurosci 2018;38(30):6787–6801. doi: 10.1523/JNEUROSCI.2352-17.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matikainen T, Perez GI, Jurisicova A, et al. Aromatic hydrocarbon receptor-driven bax gene expression is required for premature ovarian failure caused by biohazardous environmental chemicals. Nat Genet 2001;28(4):355–360. doi: 10.1038/ng575. [DOI] [PubMed] [Google Scholar]
- Mekjaruskul C, Jay M, Sripanidkulchai B. Pharmacokinetics, bioavailability, tissue distribution, excretion, and metabolite identification of methoxyflavones in kaempferia parviflora extract in rats. Drug Metab Dispos 2012;40(12):2342–2353. doi: 10.1124/dmd.112.047142. [DOI] [PubMed] [Google Scholar]
- Melhem NJ, Chajadine M, Gomez I, et al. Endothelial cell indoleamine 2, 3-dioxygenase 1 alters cardiac function after myocardial infarction through kynurenine. Circulation 2021;143(6):566–580. doi: 10.1161/CIRCULATIONAHA.120.050301. [DOI] [PubMed] [Google Scholar]
- Metghalchi S, Ponnuswamy P, Simon T, et al. Indoleamine 2,3-dioxygenase fine-tunes immune homeostasis in atherosclerosis and colitis through repression of interleukin-10 production. Cell Metab 2015;22(3):460–471. doi: 10.1016/j.cmet.2015.07.004. [DOI] [PubMed] [Google Scholar]
- Mulero-Navarro S, Fernandez-Salguero PM. New trends in aryl hydrocarbon receptor biology. Front Cell Dev Biol 2016;4:45. doi: 10.3389/fcell.2016.00045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murthy SB, Moradiya Y, Dawson J, et al. Perihematomal edema and functional outcomes in intracerebral hemorrhage: Influence of hematoma volume and location. Stroke 2015;46(11):3088–3092. doi: 10.1161/STROKEAHA.115.010054. [DOI] [PubMed] [Google Scholar]
- Nath KA, Balla G, Vercellotti GM, et al. Induction of heme oxygenase is a rapid, protective response in rhabdomyolysis in the rat. J Clin Invest 1992;90(1):267–270. doi: 10.1172/JCI115847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Natividad JM, Agus A, Planchais J, et al. Impaired aryl hydrocarbon receptor ligand production by the gut microbiota is a key factor in metabolic syndrome. Cell Metab 2018;28(5):737–749.e4. doi: 10.1016/j.cmet.2018.07.001. [DOI] [PubMed] [Google Scholar]
- Nazem-Bokaee H, Chen D, O'donnell SM, et al. New insights into the performance characteristics of the planova-series hollow-fiber parvovirus filters using confocal and electron microscopy. Biotechnol Bioeng 2019;116(8):2010–2017. doi: 10.1002/bit.26991. [DOI] [PubMed] [Google Scholar]
- Niinisalo P, Raitala A, Pertovaara M, et al. Indoleamine 2,3-dioxygenase activity associates with cardiovascular risk factors: The health 2000 study. Scand J Clin Lab Invest 2008;68(8):767–770. doi: 10.1080/00365510802245685. [DOI] [PubMed] [Google Scholar]
- Pedersen ER, Midttun O, Ueland PM, et al. Systemic markers of interferon-gamma-mediated immune activation and long-term prognosis in patients with stable coronary artery disease. Arterioscler Thromb Vasc Biol 2011;31(3):698–704. doi: 10.1161/ATVBAHA.110.219329. [DOI] [PubMed] [Google Scholar]
- Pedersen ER, Tuseth N, Eussen SJ, et al. Associations of plasma kynurenines with risk of acute myocardial infarction in patients with stable angina pectoris. Arterioscler Thromb Vasc Biol 2015;35(2):455–462. doi: 10.1161/ATVBAHA.114.304674. [DOI] [PubMed] [Google Scholar]
- Pertovaara M, Raitala A, Juonala M, et al. Indoleamine 2,3-dioxygenase enzyme activity correlates with risk factors for atherosclerosis: The cardiovascular risk in young finns study. Clin Exp Immunol 2007;148(1):106–111. doi: 10.1111/j.1365-2249.2007.03325.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pulvers JN, Watson JDG. If time is brain where is the improvement in prehospital time after stroke? Front Neurol 2017;8:617. doi: 10.3389/fneur.2017.00617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren R, Lu Q, Sherchan P, et al. . Inhibition of aryl hydrocarbon receptor attenuates hyperglycemia-induced hematoma expansion in an intracerebral hemorrhage mouse model. J Am Heart Assoc 2021;10(20):e022701. doi: 10.1161/JAHA.121.022701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothhammer V, Quintana FJ. The aryl hydrocarbon receptor: An environmental sensor integrating immune responses in health and disease. Nat Rev Immunol 2019;19(3):184–197. doi: 10.1038/s41577-019-0125-8. [DOI] [PubMed] [Google Scholar]
- Rzemieniec J, Bratek E, Wnuk A, et al. Neuroprotective effect of 3,3'-diindolylmethane against perinatal asphyxia involves inhibition of the ahr and nmda signaling and hypermethylation of specific genes. Apoptosis 2020;25(9–10.: 747–762. doi: 10.1007/s10495-020-01631-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sauzeau V, Carvajal-Gonzalez JM, Riolobos AS, et al. Transcriptional factor aryl hydrocarbon receptor (ahr) controls cardiovascular and respiratory functions by regulating the expression of the vav3 proto-oncogene. J Biol Chem 2011;286(4):2896–2909. doi: 10.1074/jbc.M110.187534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savitz J. The kynurenine pathway: A finger in every pie. Mol Psychiatry 2020;25(1):131–147. doi: 10.1038/s41380-019-0414-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonni S, Lioutas VA, Selim MH. New avenues for treatment of intracranial hemorrhage. Curr Treat Options Cardiovasc Med 2014;16(1):277. doi: 10.1007/s11936-013-0277-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stejskalova L, Vecerova L, Perez LM, et al. Aryl hydrocarbon receptor and aryl hydrocarbon nuclear translocator expression in human and rat placentas and transcription activity in human trophoblast cultures. Toxicol Sci 2011;123(1):26–36. doi: 10.1093/toxsci/kfr150. [DOI] [PubMed] [Google Scholar]
- Stone TW, Darlington LG. The kynurenine pathway as a therapeutic target in cognitive and neurodegenerative disorders. Br J Pharmacol 2013;169(6):1211–1227. doi: 10.1111/bph.12230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szelest M, Walczak K, Plech T. A new insight into the potential role of tryptophan-derived ahr ligands in skin physiological and pathological processes. Int J Mol Sci 2021;22(3):1104. doi: 10.3390/ijms22031104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang XN, Berman AE, Swanson RA, et al. Digitally quantifying cerebral hemorrhage using photoshop and image j. J Neurosci Methods 2010;190(2):240–243. doi: 10.1016/j.jneumeth.2010.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong D, Reeves MJ, Hernandez AF, et al. Times from symptom onset to hospital arrival in the get with the guidelines—stroke program 2002 to 2009: Temporal trends and implications. Stroke 2012;43(7):1912–1917. doi: 10.1161/STROKEAHA.111.644963. [DOI] [PubMed] [Google Scholar]
- Tschoe C, Bushnell CD, Duncan PW, et al. Neuroinflammation after intracerebral hemorrhage and potential therapeutic targets. J Stroke 2020;22(1):29–46. doi: 10.5853/jos.2019.02236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vecsei L, Szalardy L, Fulop F, et al. Kynurenines in the cns: Recent advances and new questions. Nat Rev Drug Discov 2013;12(1):64–82. doi: 10.1038/nrd3793. [DOI] [PubMed] [Google Scholar]
- Wang Y, Liu H, Mckenzie G, et al. Kynurenine is an endothelium-derived relaxing factor produced during inflammation. Nat Med 2010;16(3):279–285. doi: 10.1038/nm.2092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wojtowicz AK, Sitarz-Glownia AM, Szczesna M, et al. The action of di-(2-ethylhexyl) phthalate (dehp) in mouse cerebral cells involves an impairment in aryl hydrocarbon receptor (ahr) signaling. Neurotox Res 2019;35(1):183–195. doi: 10.1007/s12640-018-9946-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie J, Hong E, Ding B, et al. Inhibition of nox4/ros suppresses neuronal and blood-brain barrier injury by attenuating oxidative stress after intracerebral hemorrhage. Front Cell Neurosci 2020;14:578060. doi: 10.3389/fncel.2020.578060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu P, Hong Y, Xie Y, et al. Trem-1 exacerbates neuroinflammatory injury via nlrp3 inflammasome-mediated pyroptosis in experimental subarachnoid hemorrhage. Transl Stroke Res 2021;12(4):643–659. doi: 10.1007/s12975-020-00840-x. [DOI] [PubMed] [Google Scholar]
- Yi T, Wang J, Zhu K, et al. Aryl hydrocarbon receptor: A new player of pathogenesis and therapy in cardiovascular diseases. Biomed Res Int 2018;2018:6058784. doi: 10.1155/2018/6058784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang C, Jiang M, Wang WQ, et al. Selective mglur1 negative allosteric modulator reduces blood-brain barrier permeability and cerebral edema after experimental subarachnoid hemorrhage. Transl Stroke Res 2020;11(4):799–811. doi: 10.1007/s12975-019-00758-z. [DOI] [PubMed] [Google Scholar]
- Zhang Q, Yang H, Gao H, et al. Psd-93 interacts with syngap and promotes syngap ubiquitination and ischemic brain injury in mice. Transl Stroke Res 2020;11(5):1137–1147. doi: 10.1007/s12975-020-00795-z. [DOI] [PubMed] [Google Scholar]
- Zhang T, Wu P, Budbazar E, et al. Mitophagy reduces oxidative stress via keap1 (kelch-like epichlorohydrin-associated protein 1)/nrf2 (nuclear factor-e2-related factor 2)/phb2 (prohibitin 2) pathway after subarachnoid hemorrhage in rats. Stroke 2019;50(4):978–988. doi: 10.1161/STROKEAHA.118.021590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Chen Y, Wu J, et al. Activation of dopamine d2 receptor suppresses neuroinflammation through alphab-crystalline by inhibition of nf-kappab nuclear translocation in experimental ich mice model. Stroke 2015;46(9):2637–2646. doi: 10.1161/STROKEAHA.115.009792. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data are available within the article, and additional data can be acquired from the corresponding author.