Abstract
Malaria remains a life-threatening health problem and encounters with the increasing of antimalarial drug resistance. Medicinal plants play a critical role in synthesizing novel and potent antimalarial agents. This study aimed to investigate the phytochemical constituents, antiplasmodial activity, and evaluate the toxicity of crude ethanolic extracts of Myristica fragrans, Atractylodes lancea, and Prabchompoothaweep remedy in a mouse model. The phytochemical constituents were characterized by liquid chromatography-mass spectrometry (LC-MS). Antimalarial efficacy against Plasmodium berghei was assessed using 4-day suppressive tests at doses of 200, 400, and 600 mg/kg body weight. Acute toxicity was assessed at a dose of 2000 mg/kg body weight of crude extracts. The 4-day suppression test showed that all crude extracts significantly suppressed parasitemia (p < 0.05) compared to the control group. Higher parasitemia suppression was observed both in Prabchompoothaweep remedy at a dose of 600 mg/kg (60.1%), and A. lancea at a dose of 400 mg/kg (60.1%). The acute oral toxicity test indicated that the LD50 values of all extracts were greater than 2000 mg/kg and that these extracts were not toxic in the mouse model. LC-MS analysis revealed several compounds in M. fragrans, A. lancea, and Prabchompoothaweep remedy. For quantitative analysis, 1,2,6,8-tetrahydroxy-3-methylanthraquinone 2-O-b-D-glucoside, chlorogenic acid, and 3-O-(beta-D-glucopyranosyl-(1->6)-beta-D-glucopyranosyl) ethyl 3-hydroxyoctanoate were found in A. lancea, while (7′x,8′x)-4,7′-epoxy-3,8′-bilign-7-ene-3,5′-dimethoxy-4′,9,9′-triol, edulisin III, and tetra-hydrosappanone A trimethyl ether are found in M. fragrans. 6′-O-Formylmarmin was present in the Prabchompoothaweep remedy, followed by pterostilbene glycinate and amlaic acid. This study showed that the ethanolic extracts of A. lancea and Prabchompoothaweep remedy possess antimalarial activity against Plasmodium berghei. None of the extracts had toxic effects on liver and kidney function. Therefore, the ethanolic extract of A. lancea rhizome and Prabchompoothaweep remedy could be used as an alternative source of new antimalarial agents. Further studies are needed to determine the active compounds in both extracts.
Keywords: antimalarial activity, toxicity, Prabchompoothaweep remedy, Myritica fragrans, Atractylodes lancea, malaria
1. Introduction
Malaria is one of the most serious and life-threatening infectious diseases caused by protozoan parasites of the Plasmodium genus. It is responsible for the high rates of mortality and morbidity in the tropical and subtropical regions of the world, where the climate is suitable for parasite development [1]. According to the World Health Organization report in 2021, there were approximately 241 million cases of malaria which caused 0.6 million deaths worldwide [2]. The mortality rate from malaria has been reduced in recent years due to extensive malaria control through the use of insecticide-impregnated bed nets and treatment with artemisinin derivatives; however, the state of artemisinin resistance, the standard drug for treating malaria, is of great concern [3]. Artemisinin-based combination therapies (ACTs) are the first-line drugs for malaria in a large majority of endemic countries, and intravenous artesunate is usually used for the treatment of severe malaria [4]. Although ACTs act as a fast-acting artemisinin derivative and a slow-acting combined drug, the efficacy of ACTs is limited by long-lasting parasite clearance, contributing to ACT failure [5,6]. Furthermore, Plasmodium falciparum infection has become resistant to almost all available antimalarial drugs, which is estimated to be 10% in Southeast Asia and 93% in Thailand [7]. To manage this pathology, a new antimalarial compound that is safer, more effective than older drugs, and has a novel mode of action is urgently required.
For centuries, plants and herbs have been an important source of drugs being developed to provide a potential treatment for many diseases. Plants contain a large number of bioactive molecules and are a valuable source of pharmacotherapeutics [8,9]. Furthermore, antimalarial drugs, especially quinine and artemisinin, are derived from traditional medicines and plant extracts [10]. Therefore, natural plants are a good source of inspiration in searching for a new antimalarial agent.
Prabchompoothaweep is a traditional Thai medicine that is part of the National List of Essential Medicines (NLEM), which includes 23 herbs [11]. The bioactivity of the ethanolic extract of Prabchompoothaweep remedy, including antiallergic activity, anti-inflammation, and antioxidant activities, has been reported. According to the NLEM, the Prabchompoothaweep remedy is usually suggested to be useful for the treatment of many types of fever, including malaria-like symptoms such as intermittent fever and common cold [12]. In addition, Prabchompoothaweep remedy and two-component plants of Prabchompoothaweep remedy, Myristica fragrans, and Atractylodes lancea, have been reported to show in vitro antimalarial activity. From our previous studies, the in vitro antimalarial activity of the ethanolic extracts of the Prabchompoothaweep remedy, M. fragrans, and A. lancea, displayed antimalarial activity (IC50 = 14.13 µg/mL, 5.96 µg/mL, and 7.73 µg/mL, respectively) (unpublished data). M. fragrans is an aromatic evergreen tropical tree belonging to the Myristicaceae family [13]. M. fragrans has been used to treat several diseases. In particular, the mace part, which is an aril of M. fragrans, has been used for asthma, fever, and gastrointestinal treatment in Ayurvedic medicine [14]. Furthermore, M. fragrans has been suggested to have various medicinal properties, such as antimicrobial, chemoprotective, antioxidant, anti-inflammatory effects, anti-atherosclerosis, and behavioral effects [15]. A. lancea belongs to the Asteraceae (Compositae) family [16]. A. lancea has been used to treat rheumatic diseases, digestive disorders, night blindness, and influenza [17]. The pharmacological properties of rhizomes, including anti-cancer, anti-inflammatory, and antimicrobial activities and activities on the central nervous, cardiovascular, and gastrointestinal systems, have been investigated [18]. Prabchompoothaweep remedy and two-component plants have shown good in vitro antimalarial activity. Therefore, the Prabchompoothweep remedy and its two components are good candidates for further investigation of the in vivo antimalarial activity.
Based on ethnobotanical evidence and our in vitro study of antimalarial activity and toxicity, ethanolic mace extracts of M. fragrans, ethanolic rhizome extract of A. lancea, and ethanolic crude extract of Prabchompoothaweep remedy were found to have good activity against parasite infection without cytotoxicity to Vero cells. Therefore, this study aimed to investigate the potential antimalarial activity and toxicological assessment of two crude extracts from the Prabchompoothaweep remedy in a mouse model. Furthermore, the phytochemical content of selected crude extracts of the Prabchompoothaweep remedy was explored to understand the origin of the bioactivity.
2. Materials and Methods
2.1. Plant Collection
The dried arils (mace) of M. fragrans, dried rhizome of A. lancea, and Prabchompoothaweep remedy were purchased from a traditional Thai drug store in the Nakhon Si Thammarat region of Thailand. The authorization for plant materials complied with the relevant guidelines and regulations of the Plant Varieties Protection, Department of Agriculture, Ministry of Agriculture and Cooperatives, Thailand. The botanical identification of the plant samples was confirmed by a botanist at the School of Pharmacy, Walailak University. Specimens with voucher numbers for M. fragrans (SMD177004003-2) and A. lancea (SMD072010001) were deposited in the School of Medicine, Walailak University.
2.2. Preparation of Plant Extracts
First, the plant samples were powdered using a herb grinder (Jincheng, Model; SF, China). M. fragrans aril powder (60 g), A. lancea rhizome powder (60 g), and Prabchompoothaweep remedy powder (60 g) were soaked in 600 mL of 95% ethanol for 72 h at room temperature (1:10 (w/v) ratio). The mixed solutions were filtered using gauze and Whatman filter No. 1. The unfiltered residues were remacerated in 95% ethanol for 72 h. This procedure was repeated two times. The filtered solutions were combined and concentrated using a rotary evaporator (Buchi® rotary evaporator, Model R-210, Shanghai, China). The residues were then dried in a water bath at 60 °C. Finally, the dried crude extracts of M. fragrans aril, A. lancea rhizome, and Prabchompoothaweep remedy were stored in a refrigerator at 4 °C until use. For animal experiments, each crude extract was dissolved in 7% Tween 80 and 3% ethanol in distilled water to obtain the working concentration.
2.3. Phytochemical Screening
The ethanolic extract was qualitatively investigated to reveal the presence of phytochemical constituents, including flavonoids, terpenoids, alkaloids, tannins, anthraquinones, cardiac glycosides, saponins, and coumarins. These were identified by characteristic color changes using standard procedures [19,20,21].
2.4. Liquid Chromatography-Quadrupole Time-of-Flight Mass Spectrometry (LC-QTOF MS) Analysis
The metabolite profiles of M. fragrans extract, A. lancea extract, and Prabchompoothaweep remedy extract were determined using by an ultra-high performance liquid chromatography (UHPLC) instrument equipped with an electrospray ionization source (ESI). The UHPLC system consisted of a Zorbax Eclipse Plus C18 Rapid Resolution HD column (150 mm length × 2.1 mm inner-diameter, particle size 1.8 µm) with an LC-QTOF MS instrument (1290 Infinity II LC-6545 Quadrupole-TOF, Agilent Technologies, Santa Clara, CA, USA). The mobile phase comprised solvent A (0.1% formic acid in water) and solvent B (acetonitrile). The volume of injection was 2.0 µL, and the column temperature was set at 25 °C. Qualitative analysis of LC-MS/MS was performed in negative ion mode with a scanning range from m/z 100 to 1200 using a Dual AJS ESI ion source. The phytochemical compounds in the extract samples were identified by comparing the retention time, mass data, and fragmentation patterns with known compounds in the library search of the Mass Hunter METLIN database (Agilent Technologies). The compound selection was selected and identified from the peak with 90% similarity in the database.
2.5. Animals and Rodent Parasites
Healthy male Institute of Cancer Research (ICR) mice aged 6–8 weeks, weighing 20–30 g, were purchased from Nomura Siam International Co., Ltd., Bangkok, Thailand. The animals were housed and acclimatized for 7 days under standard and constant laboratory conditions (22 ± 3 °C, 50–60% humidity and 12 h light/dark cycles) with free access to food and clean water. The animal care staff controlled the hygiene by cleaning and removing waste from the cages daily. The mice were handled according to the international guidelines for the animals used in the experiments. The wild-type rodent Plasmodium berghei ANKA strain was obtained from Biodefense and Emerging Infections Research Resources Repository (BEI Resources), National Institute of Allergy and Infectious Diseases (NIAID), and National Institute of Health (NIH), which was received from Thomas F. McCutchan. Mouse donors were injected with P. berghei-infected red blood cells via an intraperitoneal route. When the mouse donors had parasitemia levels of 20–30%, blood was drawn from the heart by cardiac puncture and kept in a heparinized tube for injection into experimental mice.
2.6. Animal Grouping and Dosing
For Peter’s 4-day suppressive test, infected male ICR mice were randomly divided into 12 groups of five mice per group. Group 1 (infected control mice) was administered a mixture of 7% Tween 80 and 3% ethanol in distilled water. Groups 2 and 3 (positive control) received 6 mg/kg body weight of artesunate (Art) and 25 mg/kg body weight of chloroquine (CQ), respectively. Groups 4, 5, and 6 were administered 200, 400, and 600 mg/kg body weight of M. fragrans crude extract, respectively. Groups 7, 8, and 9 were administered 200, 400, and 600 mg/kg body weight A. lancea crude extract, respectively. Groups 10, 11, and 12 were administered 200, 400, and 600 mg/kg body weight of Prabchompoothaweep remedy crude extract, respectively. Dosage selection was chosen based on the results of oral acute toxicity and preliminary results were obtained for the extracts. For oral acute toxicity testing, mice were randomly assigned to five groups of five mice each. Group 1 (untreated control group) received no treatment; Group 2 (negative control group) was treated with a mixture of 7% Tween 80 and 3% ethanol in distilled water; Group 3 was treated with a dose of 2000 mg/kg body weight of M. fragrans crude extract; Group 4 was treated with a dose of 2000 mg/kg body weight of A. lancea crude extract; and Group 5 was treated with a dose of 2000 mg/kg body weight of Prabchompoothaweep remedy crude extract. Acute toxicity in mice was induced by oral administration.
2.7. Four-Day Suppressive Test (Peter’s Test)
The protocol for a 4-day suppressive test was evaluated according to previous studies [22]. First, all mice were injected with 0.2 mL of 1 × 107 infected blood cells (intraperitoneally); 3 h after infection, the mice in each group were treated with the crude extract as described above and continued to be treated for 3 consecutive days (24, 48, and 72 h after infection). Treatment was administered via oral gavage to mimic the traditional route of administration. On day 5 post-infection, blood was collected from the vascular tail vein to prepare a thin blood smear film. Thin blood smears were stained with 10% Giemsa solution (Biotech Reagent Company Limited, Bangkok, Thailand) to evaluate parasitemia. Parasitemia was observed under a light microscope (Olympus, model: CX-31, Tokyo, Japan) with a 100X objective lens. The percentage of parasitemia was determined from five different fields with an estimated 300 red blood cells per field, and the percentage of parasitemia was calculated using the following formula:
The percentage of parasitemia suppression was calculated using the following formula:
where A is the mean percentage of parasitemia in the infected control group and B is the mean percentage of parasitemia in each treatment group.
2.8. Pack Cell Volume (PCV)
The effectiveness of the crude extracts in preventing hemolysis due to increasing parasite levels was measured using PCV. The tail vein of mice was cut to collect the blood, and the blood was kept in heparinized micro-hematocrit capillary tubes by filling them up to 3/4. One side of the capillary was plugged with clay. The capillary tubes were then centrifuged at 9520× g for 5 min with the sealed ends outwards. The PCV of each mouse was determined on day 0 before infection with P. berghei and day 4 after treatment.
2.9. Acute Toxicity Measurement
The oral acute toxicity of ethanolic crude extracts of M. fragrans, A. lancea, and Prabchompoothaweep remedy was investigated in male ICR mice according to the standard guidelines of the Organization for Economic Co-operation and Development (OECD) [23]. Twenty-five mice were randomly separated into five groups of five, which were explained in the animal grouping and dosing section. On day 1, the mice were not allowed to obtain food and water for 3 h before treatment. Subsequently, the mice in the treatment group were orally administered a single dose of 2000 mg/kg body weight of M. fragrans, A. lancea, or Prabchompoothaweep remedy extract. A mixture of 7% Tween 80 and 3% ethanol in distilled water served as the negative control, and untreated mice served as the control. Three hours after treatment, the mice were noted to have physical and behavioral changes such as muscle tone, mood, sleep, excretion, appetite, and hair erection. The animals were observed daily for 14 days. Food and water intake were recorded daily. The body weight of the mice was measured on days 0 and 14 using a sensitive digital weighing balance (Mettler Toledo, model: ML3002E, Bekasi City, Indonesia). On day 14, the mice were anesthetized with 50 mg/kg body weight sodium pentobarbital (Ceva Sante Animale, Maassluis, The Netherlands) by intraperitoneal injection. After anesthetization, mouse blood was collected for biochemical analysis. Liver and kidney tissues were harvested for histopathological examination using hematoxylin and eosin (H&E) staining.
2.10. Biochemical Analysis
Blood samples from the acute toxicity test group were collected from the heart using a cardiac puncture technique. Blood was centrifuged at 3000× g for 5 min to separate the plasma, which was collected to evaluate liver and kidney function. Liver and kidney functions were tested for biochemical parameters, including aspartate aminotransferase (AST), alanine aminotransferase (ALT), alkaline phosphate (ALP), blood urea nitrogen (BUN), and creatinine levels, using an AU 480 chemistry analyzer (Beckman Coulter, Brea, CA, USA).
2.11. Histopathological Examination
Histopathological investigation was performed using a standard laboratory procedure, as previously reported [24,25,26]. The tissues were fixed in 10% (v/v) formalin at room temperature, dehydrated with a series of alcohol concentrations, cleared with xylene, and embedded in paraffin. After tissue processing, the liver and kidney tissues were cut to 5 µm thickness using a microtome, stained with hematoxylin and eosin solution, and evaluated under a light microscope by two independent observers blinded to the condition groups.
2.12. Statistical Analysis
Statistical analysis was performed with SPSS statistical software version 23 (IBM, Armonk, NY, USA). Quantitative data were presented as means ± standard errors of the means (means ± SEMs). The Kolmogorov–Smirnov test was used to assess the normal distribution of each parameter. Differences in the mean parameters between the groups, such as the percentage of parasitemia, percentage of suppression, food and water consumption, body weight, and liver and lung biochemical parameters, were analyzed with a one-way analysis of variance followed by a post-hoc Tukey’s multiple comparison test. A p-value of less than 0.05 was considered statistically significant for all tests.
3. Results
3.1. Percentage Yield and Phytochemical Screening of Ethanolic Crude Extracts
The percentage yield of the ethanolic mace extract of M. fragrans, rhizome extract of A. lancea, and Prabchompoothaweep remedy was 20.71%, 22.11%, and 5.43%, respectively. The phytochemical constituents included flavonoids, terpenoids, alkaloids, tannins, and coumarins (Table 1).
Table 1.
Phytochemical screening of ethanolic extract of M. fragrans mace, A. lancea rhizome, and Prabchompoothaweep remedy.
| Phytochemical Constituents | M. fragrans | A. lancea | Prabchompoothaweep Remedy |
|---|---|---|---|
| Flavonoid | + | - | - |
| Terpenoids | + | + | + |
| Alkaloids | + | + | + |
| Tannins | - | - | + |
| Anthraquinones | - | - | - |
| Cardiac glycosides | - | - | - |
| Saponins | - | - | - |
| Coumarins | + | - | + |
(+), detected; (-), not detected phytochemical constituents.
3.2. LC-QTOF-MS Analysis
Qualitative analysis of the compounds in extracts of M. fragrans, A. lancea, and Prabchompoothaweep remedy was performed using LC-QTOF-MS in negative mode. Metabolite profiling of the crude extract compounds was performed using a database of well-known compounds in the Library METLIN database. The complete list of compounds detected using LC-QTOF-MS is given in Table 2, Table 3 and Table 4 and supported by Figure 1, Figure 2 and Figure 3.
Table 2.
Compounds identified in the ethanolic M. fragrans extract by LC-QTOF-MS.
| No. | M/Z | RT (min) |
Compounds | Molecular Formula |
Molecular Weight |
|---|---|---|---|---|---|
| 1 | 133.014 | 2.087 | Malic acid | C4H6O5 | 134.021 |
| 2 | 149.009 | 1.886 | Tartaric acid | C4H6O6 | 150.016 |
| 3 | 285.040 | 27.493 | Luteolin | C15H10O6 | 286.047 |
| 4 | 357.134 | 27.969 | (7′x,8′x)-4,7′-Epoxy-3,8′-bilign-7-ene-3,5′-dimethoxy-4′,9,9′-triol | C20H22O6 | 358.141 |
| 5 | 201.149 | 31.852 | 3-Hydroxynonyl acetate | C11H22O3 | 202.156 |
| 6 | 265.056 | 2.1360 | Monoglyceride citrate | C9H14O9 | 266.063 |
| 7 | 373.165 | 38.781 | Sonchifolin | C21H26O6 | 374.172 |
| 8 | 345.134 | 31.690 | Gibberellin A92 | C19H22O6 | 346.141 |
| 9 | 161.045 | 4.429 | 3-Hydroxy-3-methyl-glutaric acid | C6H10O5 | 162.052 |
| 10 | 179.071 | 15.867 | Propyl 2-furanacrylate | C10H12O3 | 180.078 |
| 11 | 207.066 | 18.673 | Sinapyl aldehyde | C11H12O4 | 208.073 |
| 12 | 219.050 | 5.294 | 1-Hydroxypentane-1,2,5-tricarboxylate | C8H12O7 | 220.058 |
| 13 | 329.103 | 35.937 | Isoamericanol A | C18H18O6 | 330.110 |
| 14 | 299.092 | 33.581 | 2,4-Dihydroxy-6,4′-dimethoxychalcone | C17H16O5 | 300.099 |
| 15 | 167.034 | 9.353 | Dihydroxyphenylacetic acid | C8H8O4 | 168.042 |
| 16 | 183.102 | 34.258 | Ascariadole epoxide | C10H16O3 | 184.109 |
| 17 | 375.144 | 31.978 | alpha-Peroxyachifolide | C20H24O7 | 376.152 |
| 18 | 191.019 | 2.124 | Citric acid | C6H8O7 | 192.026 |
| 19 | 287.055 | 21.191 | 3′,4′,5,7-Tetrahydroxyisoflavanone | C15H12O6 | 288.063 |
| 20 | 149.060 | 26.290 | 2-(2-Furanyl)-3-methyl-2-butenal | C9H10O2 | 150.067 |
| 21 | 329.139 | 36.576 | Tetrahydrosappanone A trimethyl ether | C19H22O5 | 330.146 |
| 22 | 371.186 | 34.358 | Tanabalin | C22H28O5 | 372.193 |
| 23 | 315.123 | 39.745 | 5′-Hydroxy-3′,4′,7-trimethoxyflavan | C18H20O5 | 316.130 |
| 24 | 265.144 | 38.918 | Isoleptospermone | C15H22O4 | 266.151 |
| 25 | 237.113 | 20.077 | Benzyl b-L-arabinopyranoside | C13H18O4 | 238.120 |
| 26 | 301.035 | 27.794 | Hieracin | C15H10O7 | 302.042 |
| 27 | 285.040 | 32.491 | Kaempferol | C15H10O6 | 286.047 |
| 28 | 389.160 | 39.444 | Rosmic acid | C21H26O7 | 390.167 |
| 29 | 271.060 | 29.798 | Methylnorlichexanthone | C15H12O5 | 272.068 |
| 30 | 267.071 | 1.936 | 2(α-D-Mannosyl)-D-glycerate | C9H16O9 | 268.078 |
| 31 | 177.040 | 3.139 | L-Sorbosone | C6H10O6 | 178.047 |
| 32 | 303.050 | 16.481 | (±)-Taxifolin | C15H12O7 | 304.058 |
| 33 | 177.019 | 9.265 | Esculetin | C9H6O4 | 178.026 |
| 34 | 359.149 | 35.899 | 6′-O-Formylmarmin | C20H24O6 | 360.156 |
| 35 | 387.144 | 31.364 | Edulisin III | C21H24O7 | 388.151 |
| 36 | 331.118 | 23.709 | 5′,8-Dihydroxy-3′,4′,7-trimethoxyflavan | C18H20O6 | 332.125 |
| 37 | 359.076 | 34.408 | Jaceidin | C18H16O8 | 360.083 |
| 38 | 271.060 | 31.401 | (±)-Naringenin | C15H12O5 | 272.067 |
| 39 | 201.112 | 6.847 | 2,6-Dimethyl-1,8-octanedioic acid | C10H18O4 | 202.120 |
| 40 | 329.232 | 33.243 | 9S,10S,11R-trihydroxy-12Z-octadecenoic acid | C18H34O5 | 330.240 |
| 41 | 311.128 | 39.657 | Gancaonin V | C19H20O4 | 312.135 |
| 42 | 117.018 | 2.688 | Succinic acid | C4H6O4 | 118.026 |
| 43 | 163.039 | 16.068 | m-Coumaric acid | C9H8O3 | 164.047 |
| 44 | 197.045 | 9.904 | 2-Hydroxy-3,4-dimethoxybenzoic acid | C9H10O5 | 198.052 |
| 45 | 133.050 | 2.713 | 2,3-Dihydroxy-2-methylbutanoic acid | C5H10O4 | 134.057 |
| 46 | 281.138 | 10.129 | Bisbynin | C15H22O5 | 282.146 |
| 47 | 221.081 | 13.762 | 2,3-Dihydro-3-hydroxy-6-methoxy-2,2-dimethyl-4H-1-benzopyran-4-one | C12H14O4 | 222.088 |
| 48 | 239.070 | 37.691 | 2,4-Dihydroxychalcone | C15H12O3 | 240.078 |
| 49 | 317.066 | 21.617 | Dihydroisorhamnetin | C16H14O7 | 318.073 |
| 50 | 443.191 | 5.794 | Cynaroside A | C21H32O10 | 444.198 |
| 51 | 371.134 | 20.577 | Citrusin E | C17H24O9 | 372.141 |
| 52 | 447.092 | 18.473 | Kaempferol-7-O-glucoside | C21H20O11 | 448.099 |
| 53 | 205.086 | 32.679 | 2,3-Dihydro-6-methoxy-2,2-dimethyl-4H-1-benzopyran-4-one | C12H14O3 | 206.094 |
| 54 | 343.154 | 31.101 | Safficinolide | C20H24O5 | 344.161 |
| 55 | 353.102 | 32.303 | 1-(3,4-Dihydroxyphenyl)-7-(4-hydroxy-3-methoxyphenyl)-1,6-heptadiene-3,5-dione | C20H18O6 | 354.109 |
| 56 | 313.107 | 33.706 | 7-Hydroxyenterolactone | C18H18O5 | 314.114 |
| 57 | 445.170 | 11.357 | Crosatoside B | C20H30O11 | 446.177 |
| 58 | 331.115 | 24.787 | Mytilin A | C13H20N2O8 | 332.122 |
| 59 | 263.128 | 24.737 | (+)-Abscisic acid | C15H20O4 | 264.135 |
| 60 | 426.227 | 35.285 | Dihydroxyacidissiminol | C25H33NO5 | 427.234 |
| 61 | 343.118 | 22.682 | Diosbulbin B | C19H20O6 | 344.125 |
| 62 | 187.096 | 19.475 | Methyl N-(a-methylbutyryl) glycine | C9H16O4 | 188.104 |
Table 3.
Compounds identified in the ethanolic A. lancea extract by LC-QTOF-MS.
| No. | M/Z | RT (min) |
Compounds | Molecular Formula |
Molecular Weight |
|---|---|---|---|---|---|
| 1 | 191.056 | 1.970 | Quinic acid | C7H12O6 | 192.063 |
| 2 | 179.035 | 9.637 | Caffeic acid | C9H8O4 | 180.042 |
| 3 | 177.019 | 9.274 | Esculetin | C9H6O4 | 178.026 |
| 4 | 243.062 | 1.995 | Pseudouridine | C9H12N2O6 | 244.069 |
| 5 | 191.034 | 14.798 | Scopoletin | C10H8O4 | 192.042 |
| 6 | 209.118 | 33.628 | 3-Ethenyl-2,5-dimethyl-4-oxohex-5-en-2-yl acetate | C12H18O3 | 210.125 |
| 7 | 161.024 | 14.673 | 3-Hydroxycoumarin | C9H6O3 | 162.031 |
| 8 | 353.087 | 7.419 | Chlorogenic acid | C16H18O9 | 354.094 |
| 9 | 281.139 | 32.475 | Bisbynin | C15H22O5 | 282.146 |
| 10 | 207.029 | 11.341 | Fraxetin | C10H8O5 | 208.036 |
| 11 | 207.066 | 28.153 | 5-(3′,5′-Dihydroxyphenyl)-gamma-valerolactone | C11H12O4 | 208.073 |
| 12 | 265.144 | 33.653 | Isoleptospermone | C15H22O4 | 266.151 |
| 13 | 193.050 | 15.124 | Scytalone | C10H10O4 | 194.057 |
| 14 | 311.128 | 28.454 | Gancaonin V | C19H20O4 | 312.135 |
| 15 | 153.019 | 8.372 | Gentisic acid | C7H6O4 | 154.026 |
| 16 | 147.029 | 2.721 | D-threo-3-methylmalate | C5H8O5 | 148.036 |
| 17 | 341.108 | 1.907 | Sucrose | C12H22O11 | 342.115 |
| 18 | 353.087 | 8.008 | 5Z-Caffeoylquinic acid | C16H18O9 | 354.094 |
| 19 | 341.087 | 7.156 | Glucocaffeic acid | C15H18O9 | 342.094 |
| 20 | 447.092 | 12.368 | 1,2,6,8-Tetrahydroxy-3-methylanthraquinone 2-O-b-D-glucoside | C21H20O11 | 448.100 |
| 21 | 427.196 | 16.703 | Taraxacolide 1-O-b-D-glucopyranoside | C21H32O9 | 428.204 |
| 22 | 225.112 | 25.472 | 3,7-Dimethyl-2E,6E-decadien-1,10-dioic acid | C12H18O4 | 226.120 |
| 23 | 221.045 | 15.487 | Isofraxidin | C11H10O5 | 222.052 |
| 24 | 128.035 | 2.208 | Pyroglutamic acid | C5H7NO3 | 129.042 |
| 25 | 381.175 | 10.163 | 1,2,10-Trihydroxydihydro-trans-linalyl oxide 7-O-beta-D-glucopyranoside | C16H30O10 | 382.183 |
| 26 | 337.092 | 10.338 | Hydrojuglone glucoside | C16H18O8 | 338.099 |
| 27 | 441.175 | 15.600 | Lusitanicoside | C21H30O10 | 442.183 |
| 28 | 485.199 | 14.673 | Glucosylgalactosyl hydroxylysine | C18H34N2O13 | 486.207 |
| 29 | 401.144 | 8.847 | Benzyl O-(arabinofuranosyl-(1->6)-glucoside) | C18H26O10 | 402.151 |
| 30 | 385.164 | 26.675 | Gingerenone B | C22H26O6 | 386.172 |
| 31 | 335.076 | 13.169 | 4-O-Caffeoylshikimic acid | C16H16O8 | 336.083 |
| 32 | 305.138 | 32.788 | Achillicin | C17H22O5 | 306.146 |
| 33 | 425.144 | 16.414 | 6-(2-Carboxyethyl)-7-hydroxy-2,2-dimethyl-4-chromanone glucoside | C20H26O10 | 426.151 |
| 34 | 353.144 | 26.675 | Isopropyl apiosylglucoside | C14H26O10 | 354.151 |
| 35 | 503.175 | 13.169 | (S)-Multifidol 2-(apiosyl-(1->6)-glucoside) | C22H32O13 | 504.183 |
| 36 | 329.232 | 33.164 | 9S,10S,11R-trihydroxy-12Z-octadecenoic acid | C18H34O5 | 330.239 |
| 37 | 461.238 | 13.270 | xi-Linalool 3-(rhamnosyl-(1->6)-glucoside) | C22H38O10 | 462.245 |
| 38 | 511.238 | 10.789 | 3-O-(beta-D-glucopyranosyl-(1->6)-beta-D-glucopyranosyl) ethyl 3-hydroxyoctanoate | C22H40O13 | 512.245 |
| 39 | 393.133 | 39.996 | Rotenone | C23H22O6 | 394.141 |
| 40 | 447.092 | 15.976 | Kaempferol-7-O-glucoside | C21H20O11 | 448.099 |
| 41 | 461.165 | 9.837 | Verbasoside | C20H30O12 | 462.172 |
| 42 | 479.248 | 10.263 | 3-O-(alpha-L-rhamnopyranosyl-(1-2)-alpha-L-rhamnopyranosyl)-3-hydroxydecanoic acid | C22H40O11 | 480.255 |
| 43 | 529.264 | 18.356 | Cinncassiol D2 glucoside | C26H42O11 | 530.271 |
| 44 | 515.118 | 20.235 | 3″,4″-Diacetylafzelin | C25H24O12 | 516.125 |
| 45 | 128.035 | 2.496 | (r)-(+)-2-Pyrrolidone-5-carboxylic acid | C5H7NO3 | 129.042 |
| 46 | 441.212 | 16.828 | CAY10509 | C23H35FO5S | 442.219 |
| 47 | 299.141 | 32.688 | Bifenazate | C17H20N2O3 | 300.148 |
Table 4.
Compounds identified in the ethanolic extract of Prabchompoothaweep remedy by LC-QTOF-MS.
| No. | M/Z | RT (min) |
Compounds | Molecular Formula |
Molecular Weight |
|---|---|---|---|---|---|
| 1 | 173.045 | 1.988 | Shikimic acid | C7H10O5 | 174.052 |
| 2 | 197.045 | 9.842 | 2-Hydroxy-3,4-dimethoxybenzoic acid | C9H10O5 | 198.052 |
| 3 | 169.014 | 3.816 | Gallic acid | C7H6O5 | 170.021 |
| 4 | 177.019 | 9.216 | Esculetin | C9H6O4 | 178.026 |
| 5 | 137.024 | 7.725 | 3,4-Dihydroxybenzaldehyde | C7H6O3 | 138.031 |
| 6 | 169.014 | 6.497 | 2,4,6-Trihydroxybenzoic acid | C7H6O5 | 170.021 |
| 7 | 243.051 | 3.491 | 1-O-Galloylglycerol | C10H12O7 | 244.058 |
| 8 | 166.050 | 7.976 | 2-Amino-3-methoxy-benzoic acid | C8H9NO3 | 167.058 |
| 9 | 447.129 | 24.625 | Piperenol C | C22H24O10 | 448.136 |
| 10 | 153.019 | 5.320 | 3,4-Dihydroxybenzoic acid | C7H6O4 | 154.026 |
| 11 | 211.061 | 19.401 | Eudesmic acid | C10H12O5 | 212.068 |
| 12 | 187.097 | 19.489 | Methyl N-(a-methylbutyryl) glycine | C9H16O4 | 188.104 |
| 13 | 197.045 | 13.212 | 3,4-O-Dimethylgallic acid | C9H10O5 | 198.052 |
| 14 | 313.056 | 3.290 | Salicyl phenolic glucuronide | C13H14O9 | 314.063 |
| 15 | 161.081 | 7.249 | Potassium 2-(1’-ethoxy) ethoxypropanoate | C7H14O4 | 162.089 |
| 16 | 179.035 | 9.629 | Caffeic acid | C9H8O4 | 180.042 |
| 17 | 151.040 | 9.078 | 4-Acetoxyphenol | C8H8O3 | 152.047 |
| 18 | 290.088 | 2.012 | Sarmentosin epoxide | C11H17NO8 | 291.095 |
| 19 | 191.034 | 22.608 | 5,7-Dihydroxy-4-methylcoumarin | C10H8O4 | 192.042 |
| 20 | 353.087 | 7.713 | 5Z-Caffeoylquinic acid | C16H18O9 | 354.094 |
| 21 | 237.113 | 20.065 | Benzyl b-L-arabinopyranoside | C13H18O4 | 238.120 |
| 22 | 222.040 | 14.340 | (R)-2,3-Dihydro-3,5-dihydroxy-2-oxo-3-indoleacetic acid | C10H9NO5 | 223.047 |
| 23 | 218.103 | 3.516 | Pantothenic acid | C9H17NO5 | 219.110 |
| 24 | 195.102 | 16.169 | Isobutyl 2-furanpropionate | C11H16O3 | 196.109 |
| 25 | 421.186 | 32.869 | Picrasin F | C22H30O8 | 422.193 |
| 26 | 355.030 | 2.137 | (+)-Chebulic acid | C14H12O11 | 356.037 |
| 27 | 153.019 | 8.352 | Gentisic acid | C7H6O4 | 154.026 |
| 28 | 325.056 | 3.992 | Fertaric acid | C14H14O9 | 326.063 |
| 29 | 243.123 | 23.360 | Polyethylene, oxidized | C12H20O5 | 244.130 |
| 30 | 233.045 | 6.735 | 7-Hydroxy-2-methyl-4-oxo-4H-1-benzopyran-5-acetic acid | C12H10O5 | 234.052 |
| 31 | 299.055 | 32.242 | Diosmetin | C16H12O6 | 300.063 |
| 32 | 310.140 | 11.734 | Leonurine | C14H21N3O5 | 311.147 |
| 33 | 300.998 | 15.430 | Ellagic acid | C14H6O8 | 302.005 |
| 34 | 328.118 | 20.604 | N-trans-Feruloyloctopamine | C18H19NO5 | 329.125 |
| 35 | 225.112 | 11.947 | 3,7-Dimethyl-2E,6E-decadien-1,10-dioic acid | C12H18O4 | 226.120 |
| 36 | 163.039 | 13.538 | m-Coumaric acid | C9H8O3 | 164.047 |
| 37 | 321.024 | 7.900 | Digallate | C14H10O9 | 322.032 |
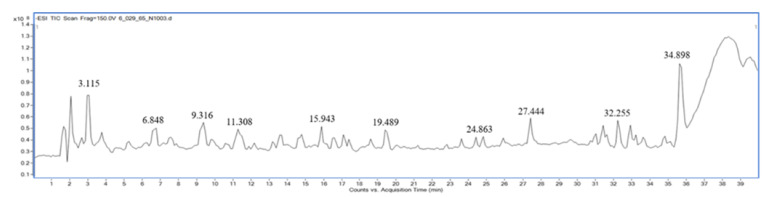
| 38 | 359.149 | 34.898 | 6′-O-Formylmarmin | C20H24O6 | 360.156 |
| 39 | 651.083 | 9.316 | Amlaic acid | C27H24O19 | 652.090 |
| 40 | 285.040 | 32.518 | Kaempferol | C15H10O6 | 286.047 |
| 41 | 463.087 | 15.881 | Quercetin 3-galactoside | C21H20O12 | 464.094 |
| 42 | 347.076 | 7.024 | alpha-(1,2-Dihydroxyethyl)-1,2,3,4-tetrahydro-7-hydroxy-9-methoxy-3,4-dioxocyclopenta(c) benzopyran-6-acetaldehyde | C17H16O8 | 348.083 |
| 43 | 431.170 | 33.708 | Melledonal A | C23H28O8 | 432.177 |
| 44 | 261.040 | 17.396 | 2-Acetyl-5,8-dihydroxy-3-methoxy-1,4-naphthoquinone | C13H10O6 | 262.047 |
| 45 | 315.050 | 33.082 | 1,3,5,8-Tetrahydroxy-6-methoxy-2-methylanthraquinone | C16H12O7 | 316.057 |
| 46 | 326.087 | 7.449 | Blepharin | C14H17NO8 | 327.094 |
| 47 | 461.108 | 25.966 | Rhamnetin 3-rhamnoside | C22H22O11 | 462.115 |
| 48 | 161.060 | 18.223 | Allyl benzoate | C10H10O2 | 162.067 |
| 49 | 128.035 | 2.426 | Pyroglutamic acid | C5H7NO3 | 129.042 |
| 50 | 271.060 | 31.302 | (±)-Naringenin | C15H12O5 | 272.067 |
| 51 | 264.066 | 34.710 | Piperolactam A | C16H11NO3 | 265.073 |
| 52 | 272.129 | 29.724 | (2E)-Piperamide-C5:1 | C16H19NO3 | 273.136 |
| 53 | 191.055 | 1.887 | Quinic acid | C7H12O6 | 192.063 |
| 54 | 134.024 | 10.356 | 2-Benzoxazolol | C7H5NO2 | 135.032 |
| 55 | 361.165 | 22.821 | Gibberellin A98 | C20H26O6 | 362.172 |
| 56 | 201.112 | 26.041 | 2,6-Dimethyl-1,8-octanedioic acid | C10H18O4 | 202.120 |
| 57 | 476.040 | 13.688 | Isoterchebin | C41H30O27 | 954.096 |
| 58 | 312.123 | 24.863 | Pterostilbene glycinate | C18H19NO4 | 313.131 |
| 59 | 285.040 | 27.444 | Luteolin | C15H10O6 | 286.047 |
| 60 | 635.088 | 11.308 | 3-O-Galloylhamamelitannin | C27H24O18 | 636.095 |
| 61 | 351.053 | 26.191 | 4′-O-Methyl-(-)-epicatechin-7-O-sulfate | C16H16O7S | 352.061 |
| 62 | 269.045 | 31.453 | Apigenin | C15H10O5 | 270.052 |
| 63 | 343.045 | 36.564 | Aflatoxin GM1 | C17H12O8 | 344.052 |
| 64 | 307.081 | 18.474 | 4R,5R,6S-Trihydroxy-2-hydroxymethyl-2-cyclohexen-1-one 6-(2-hydroxy-6-methylbenzoate) | C15H16O7 | 308.089 |
| 65 | 447.092 | 18.487 | Kaempferol-7-O-glucoside | C21H20O11 | 448.099 |
| 66 | 461.072 | 19.726 | 3-Methylellagic acid 8-rhamnoside | C21H18O12 | 462.079 |
| 67 | 201.018 | 26.341 | 6-Hydroxyangelicin | C11H6O4 | 202.026 |
| 68 | 623.197 | 15.943 | Isoacteoside | C29H36O15 | 624.204 |
| 69 | 211.060 | 5.119 | 3-Hydroxy-4-methoxyphenyllactic acid | C10H12O5 | 212.067 |
| 70 | 301.034 | 27.820 | Hieracin | C15H10O7 | 302.042 |
| 71 | 477.139 | 24.462 | Eugenol O-[3,4,5-Trihydroxybenzoyl-(->6)-b-D-glucopyranoside] | C23H26O11 | 478.146 |
| 72 | 342.134 | 25.665 | N-trans-Feruloyl-4-O-methyldopamine | C19H21NO5 | 343.141 |
| 73 | 547.144 | 21.255 | Puerarin xyloside | C26H28O13 | 548.152 |
| 74 | 251.128 | 15.667 | QH (2) | C14H20O4 | 252.135 |
| 75 | 329.029 | 28.897 | 2,8-Di-O-methylellagic acid | C16H10O8 | 330.036 |
| 76 | 256.133 | 34.309 | Coumaperine | C16H19NO2 | 257.140 |
| 77 | 491.118 | 28.772 | 3′,7-Dimethoxy-4′,5,8-trihydroxyflavone 8-glucoside | C23H24O12 | 492.125 |
| 78 | 281.138 | 10.080 | Bisbynin | C15H22O5 | 282.146 |
| 79 | 403.175 | 32.255 | Myristicanol B | C22H28O7 | 404.183 |
| 80 | 403.123 | 5.921 | Oleoside 11-methyl ester | C17H24O11 | 404.131 |
| 81 | 465.102 | 17.998 | (-)-Epicatechin 7-O-glucuronide | C21H22O12 | 466.110 |
| 82 | 379.175 | 20.403 | 6b-Angeloyl-3b,8b,9b-trihydroxy-7(11)-eremophilen-12,8-olide | C20H28O7 | 380.182 |
| 83 | 593.150 | 17.221 | Saponarin | C27H30O15 | 594.157 |
| 84 | 379.012 | 28.672 | Tectorigenin 7-sulfate | C16H12O9S | 380.019 |
| 85 | 241.071 | 6.046 | Elenaic acid | C11H14O6 | 242.078 |
| 86 | 955.104 | 16.570 | Chebulinic acid | C41H32O27 | 956.111 |
| 87 | 477.102 | 19.000 | Myricetin 3,4′-dimethyl ether 3′-xyloside | C22H22O12 | 478.110 |
| 88 | 497.223 | 19.614 | 2-O-(beta-D-galactopyranosyl-(1->6)-beta-D-galactopyranosyl) 2S-hydroxynonanoic acid | C21H38O13 | 498.230 |
| 89 | 593.129 | 27.795 | 6″-O-p-Coumaroyltrifolin | C30H26O13 | 594.136 |
| 90 | 515.118 | 20.177 | 3″,4″-Diacetylafzelin | C25H24O12 | 516.125 |
| 91 | 161.045 | 4.443 | 3-Hydroxy-3-methyl-glutaric acid | C6H10O5 | 162.052 |
| 92 | 447.092 | 12.385 | 1,2,6,8-Tetrahydroxy-3-methylanthraquinone 2-O-b-D-glucoside | C21H20O11 | 448.099 |
| 93 | 387.107 | 16.945 | 7-Hydroxy-3′,4′,5,6,8-pentamethoxyflavone | C20H20O8 |
388.115 |
| 94 | 769.254 | 18.349 | Leonoside A | C35H46O19 | 770.261 |
| 95 | 637.176 | 13.876 | Quercetin 3,3′-dimethyl ether 7-rutinoside | C29H34O16 | 638.183 |
| 96 | 431.097 | 19.150 | Apigenin 7-O-glucoside | C21H20O10 | 432.104 |
| 97 | 581.222 | 12.498 | (+)-Lyoniresinol 9-glucoside | C28H38O13 | 582.230 |
| 98 | 461.238 | 13.976 | xi-Linalool 3-(rhamnosyl-(1->6)-glucoside) | C22H38O10 | 462.245 |
| 99 | 695.399 | 31.177 | Glucosyl passiflorate | C37H60O12 | 696.407 |
| 100 | 461.165 | 4.543 | Verbasoside | C20H30O12 | 462.172 |
| 101 | 755.238 | 15.292 | Hesperetin 7-(2,6-dirhamnosylglucoside) | C34H44O19 | 756.246 |
| 102 | 435.128 | 19.075 | Phenethyl 6-galloylglucoside | C21H24O10 | 436.135 |
| 103 | 429.152 | 23.685 | 2,3-dinor Fluprostenol | C21H25F3O6 | 430.159 |
| 104 | 651.228 | 24.262 | (-)-Matairesinol 4′-(apiosyl-(1->2)-glucoside) | C31H40O15 | 652.235 |
| 105 | 153.055 | 4.944 | 2-Furanylmethyl propanoate | C8H10O3 | 154.062 |
| 106 | 665.207 | 23.460 | Tetramethylquercetin 3-rutinoside | C31H38O16 | 666.214 |
| 107 | 637.212 | 19.376 | 4′-Hydroxy-5,7,2′-trimethoxyflavanone 4′-rhamnosyl-(1->6)-glucoside | C30H38O15 | 638.219 |
| 108 | 582.259 | 30.238 | N1, N5, N10-Tricoumaroyl spermidine | C34H37N3O6 |
583.266 |
| 109 | 433.149 | 20.854 | Vestitone 7-glucoside | C22H26O9 | 434.156 |
| 110 | 453.248 | 33.457 | Rhodojaponin IV | C24H38O8 | 454.255 |
| 111 | 137.024 | 8.088 | m-Salicylic acid | C7H6O3 | 138.031 |
| 112 | 477.066 | 10.080 | Quercetin 3′-O-glucuronide | C21H18O13 | 478.073 |
| 113 | 787.098 | 14.741 | 1,2’,3,5-Tetra-O-galloylhamamelofuranose | C34H28O22 | 788.105 |
| 114 | 577.154 | 17.647 | Scutellarein 7,4′-dirhamnoside | C27H30O14 | 578.162 |
| 115 | 939.108 | 17.171 | 1,2,3,4,6-Pentakis-O-galloyl-beta-D-glucose | C41H32O26 | 940.115 |
| 116 | 331.081 | 16.795 | 2′,3,5-Trihydroxy-5′,7-dimethoxyflavanone | C17H16O7 | 332.088 |
| 117 | 347.037 | 3.941 | 2-(α-D-Mannosyl)-3-phosphoglycerate | C9H17O12P | 348.045 |
| 118 | 315.159 | 34.910 | Isopulegone caffeate | C19H24O4 | 316.166 |
Figure 1.
LC-MS chromatogram of ethanolic M. fragrans mace extract.
Figure 2.
LC-MS chromatogram of ethanolic A. lancea rhizome extract.
Figure 3.
LC-MS chromatogram of ethanolic extract of Prabchompoothaweep remedy.
3.3. Four-Day Suppressive Test
The antimalarial activity of M. fragrans extract, A. lancea extract, and Prabchompoothaweep remedy extract against P. berghei ANKA was measured using a 4-day suppressive test. Animals in each condition were treated with daily doses of crude extracts at 200, 400, and 600 mg/kg body weight by an oral route. The results showed that mice treated with extracts of M. fragrans and Prabchompoothaweep remedy showed significant suppression of parasitemia in a dose-dependent response (M. fragrans: 38.32, 44.17, and 46.86, respectively; and Prabchompoothaweep remedy: 39.18, 48.35, and 60.11, respectively) compared to the negative control group (p < 0.05). The A. lancea group also showed suppressed parasites compared to the negative control group (p < 0.05), especially at a dose of 400 mg/kg body weight. Parasite levels decreased after treatment with the ethanolic extract of M. fragrans, A. lancea, and Prabchompoothaweep remedy. However, all treatment groups in the crude extract did not completely suppress parasitemia, whereas the parasites were suppressed by more than 95% in the positive control groups (6 mg/kg body weight artesunate and 25 mg/kg body weight chloroquine). Parasite levels and parasite suppression are shown in Table 5.
Table 5.
Effect of ethanolic extracts of M. fragrans, A. lancea, and Prabchompoothaweep remedy on parasite level and parasite suppression in the 4-day suppressive test.
| Group | Dose (mg/kg) | % Parasitemia | % Suppression |
|---|---|---|---|
| 7% Tween 80 | - | 40.45 ± 2.15 b,c,d,e,f,g,h,i,j,k,l | - |
| Artesunate | 6 | 2.18 ± 0.50 a,d,e,f,g,h,i,j,k,l | 95.32 ± 0.57 d,e,f,g,h,i,j,k,l |
| Chloroquine | 25 | 0.27 ± 0.15 a,d,e,f,g,h,i,j,k,l | 99.34 ± 0.37 d,e,f,g,h,i,j,k,l |
| M. fragrans | 200 | 24.94 ± 2.50 a,b,c,h,l | 38.32 ± 6.18 b,c,h,i,k,l |
| 400 | 22.36 ± 1.26 a,b,c,h,l | 44.17 ± 3.12 b,c,h,l | |
| 600 | 21.48 ± 0.73 a,b,c,h,l | 46.86 ± 1.80 b,c,h,l | |
| A. lancea | 200 | 21.53 ± 2.47 a,b,c,h,l | 46.75 ± 6.11 b,c,h,j,k,l |
| 400 | 16.13 ± 0.41 a,b,c,d,e,f,g,i,j,k | 60.09 ± 1.03 b,c,d,e,f,g,i,j,k | |
| 600 | 20.91 ± 1.15 a,b,c,h,l | 48.29 ± 2.86 b,c,d,h,l | |
| Prabchompoothaweep remedy | 200 | 24.60 ± 1.03 a,b,c,h,l | 39.18 ± 2.56 b,c,h,l |
| 400 | 20.88 ± 3.08 a,b,c,h,l | 48.35 ± 7.62 b,c,d,h,l | |
| 600 | 16.13 ± 0.58 a,b,c,d,e,f,g,i,j,k | 60.11 ± 1.44 b,c,d,e,f,g,i,j,k |
Data are presented as mean ± SEM (n = 5 per group), p < 0.05. a Compared to the negative control, b compared to artesunate, c compared to chloroquine, d compared to 200 mg/kg of M. fragrans, e compared to 400 mg/kg of M. fragrans, f compared to 600 mg/kg of M. fragrans, g compared to 200 mg/kg of A. lancea, h compared to 400 mg/kg of A. lancea, i compared to 600 mg/kg of A. lancea, j compared to 200 mg/kg of Prabchompoothaweep remedy, k compared to 400 mg/kg of Prabchompoothaweep remedy, and l compared to 600 mg/kg of Prabchompoothaweep remedy.
3.4. PCV
The effects of ethanolic extracts of M. fragrans, A. lancea, and Prabchompoothaweep remedy on PCVs are presented in Table 6. In the positive treatment control group (artesunate and chloroquine), there was a significant decrease in PCV compared with the negative control group (p > 0.05). The PCV loss was protected by 200, 400, and 600 mg/kg doses of crude extracts compared to the negative control group. However, the protection of crude extracts did not significantly reduce PCV loss at any dose of crude extract compared to the negative control group (p < 0.05).
Table 6.
Effect of ethanolic extracts of M. fragrans, A. lancea, and Prabchompoothaweep remedy on pack cell volume in the 4-day suppressive test.
| Group | Dose (mg/kg) |
Day 0 | Day 4 | % Change |
|---|---|---|---|---|
| 7% Tween 80 | - | 49.60 ± 1.01 | 45.00 ± 1.89 | −10.35 ± 3.67% b,c |
| Artesunate | 6 | 52.20 ± 1.32 | 54.80 ± 1.16 | 4.74 ± 1.43% a,d,e,f,g,h,i,j,k,l |
| Chloroquine | 25 | 51.80 ± 0.43 | 53.40 ± 1.47 | 2.87 ± 2.36% a,d,j |
| M. fragrans | 200 | 54.00 ± 0.89 | 49.80 ± 2.03 | −8.58 ± 3.89% b,c |
| 400 | 51.20 ± 1.16 | 48.40 ± 0.80 | −5.78 ± 1.52% b | |
| 600 | 51.00 ± 1.09 | 48.80 ± 1.93 | −4.64 ± 4.05% b | |
| A. lancea | 200 | 52.00 ± 2.73 | 49.40 ± 2.17 | −5.58 ± 8.67% b |
| 400 | 52.40 ± 1.01 | 50.00 ± 1.67 | −4.86 ± 2.15% b | |
| 600 | 51.20 ± 1.16 | 48.80 ± 1.46 | −5.01 ± 3.73% b | |
| Prabchompoothaweep remedy | 200 | 52.60 ± 1.35 | 48.20 ± 2.13 | −9.25 ± 3.46% b,c |
| 400 | 51.80 ± 1.83 | 49.40 ± 1.35 | −4.87 ± 3.02% b | |
| 600 | 50.80 ± 2.63 | 48.60 ± 1.62 | −4.47 ± 2.65% b |
Data are presented as mean ± SEM (n = 5 per group), p < 0.05. a Compared to the negative control, b compared to artesunate, c compared to chloroquine, d compared to 200 mg/kg of M. fragrans, e compared to 400 mg/kg of M. fragrans, f compared to 600 mg/kg of M. fragrans, g compared to 200 mg/kg of A. lancea, h compared to 400 mg/kg of A. lancea, i compared to 600 mg/kg of A. lancea, j compared to 200 mg/kg of Prabchompoothaweep remedy, k compared to 400 mg/kg Prabchompoothaweep remedy, and l compared to 600 mg/kg of Prabchompoothaweep remedy.
3.5. Acute Oral Toxicity Test
3.5.1. Physical Activity and Behavior, Food and Water Uptake, and Body Weight
On the first day of the experiment, mice were administered a single dose of 2000 mg/kg M. fragrans ethanolic extract, A. lancea ethanolic extract, or Prabchompoothaweep remedy ethanolic extract. Physical activity and behavioral changes were observed for 14 consecutive days after treatment. The results showed no signs or symptoms of toxicity, such as rigidity, mood changes, ataxia, abnormal sleep, diarrhea, vomiting, consumption changes, and hair erection, during the experiment period. The mice in the acute toxicity test did not show mortality within the first 24 h or 14 days of treatment. Therefore, lethal doses of M. fragrans extracts, A. lancea extracts, or Prabchompoothaweep remedy extracts are greater than 2000 mg/kg body weight. According to water and food consumption in acute toxicity tests after treatment with ethanolic extracts, the mean water and food consumption of mice in the treatment groups treated with a single dose of 2000 mg/kg body weight of M. fragrans extract, A. lancea extract, Prabchompoothaweep remedy extract, and those in the 7% Tween 80 group (negative control group) did not show significant differences compared to those of mice in the control group (untreated group) (p > 0.05) (Table 7). Furthermore, the body weight changes in mice treated with 2000 mg/kg crude extracts and 7% Tween 80 were not significantly different from those in the control group (p > 0.05) (Table 8) at week 2 after receiving crude extracts.
Table 7.
Effect of ethanolic extracts of M. fragrans, A. lancea, and Prabchompoothaweep remedy on food and water uptake in the acute toxicity test at week 1 and week 2 after treatment.
| Food Consumption (g) | Week 1 | Week 2 |
| Normal mice | 25.0 ± 3.5 | 21.6 ± 2.7 |
| 7% Tween 80 | 22.1 ± 1.7 | 20.8 ± 2.5 |
| M. fragrans 2000 mg/kg | 20.8 ± 2.0 | 20.7 ± 0.8 |
| A. lancea 2000 mg/kg | 22.8 ± 2.5 | 22.4 ± 2.3 |
| Prabchompoothaweep remedy 2000 mg/kg |
23.6 ± 3.9 | 22.0 ± 1.9 |
| Water Consumption (mL) | Week 1 | Week 2 |
| Normal mice | 122.2 ± 4.7 | 125.8 ± 7.9 |
| 7% Tween 80 | 122.4 ± 8.3 | 126.7 ± 8.3 |
| M. fragrans 2000 mg/kg | 122.4 ± 3.5 | 127.7 ± 3.5 |
| A. lancea 2000 mg/kg | 126.0 ± 4.8 | 130.4 ± 5.0 |
| Prabchompoothaweep remedy 2000 mg/kg |
125.0 ± 2.6 | 130.5 ± 4.8 |
Data are presented as mean ± SEM (n = 5 per group).
Table 8.
Effect of ethanolic extracts of M. fragrans, A. lancea, and Prabchompoothaweep remedy on body weight changes in the acute toxicity test on day 0 and day 14 after treatment.
| Group | Mean Body Weight | ||
|---|---|---|---|
| Day 0 | Day 14 | % Change | |
| Normal mice | 33.4 ± 1.5 | 39.2 ± 2.2 | 14.6 ± 1.7% |
| 7% Tween 80 | 32.5 ± 1.4 | 36.5 ± 1.5 | 11.1 ± 1.3% |
| M. fragrans 2000 mg/kg | 32.8 ± 1.2 | 38.0 ± 2.4 | 13.4 ± 3.3% |
| A. lancea 2000 mg/kg | 33.0 ± 1.6 | 38.0 ± 2.7 | 13.0 ± 2.6% |
| Prabchompoothaweep remedy 2000 mg/kg |
32.1 ± 1.1 | 36.8 ± 1.4 | 12.6 ± 1.5% |
Data are presented as mean ± SEM (n = 5 per group).
3.5.2. Biochemical Assessment of Liver and Kidney Functions
The levels of liver function, such as AST, ALT, and ALP, in mice that received a single 2000 mg/kg dose of M. fragrans extract, A. lancea extract, Prabchompoothaweep remedy extract, and those of the 7% Tween 80 group (negative control group) did not show statistically significant differences compared to the control group (untreated group) (p > 0.05) at the end of this study. Furthermore, the level of biochemical parameters of kidney functions, such as creatinine and BUN, in mice treated with 2000 mg/kg body weight of crude extracts and 7% Tween 80 showed no significant difference from those in mice in the control group (p > 0.05) (Table 9).
Table 9.
Effect of ethanolic extracts of M. fragrans, A. lancea, and Prabchompoothaweep remedy on kidney and liver functions in the acute toxicity test.
| Parameters | Normal Mice | 7% Tween 80 | M. fragrans | A. lancea | Prabchompoothaweep Remedy |
|---|---|---|---|---|---|
| Liver Function Test | |||||
| AST (U/L) | 83.80 ± 7.13 | 83.00 ± 9.18 | 87.75 ± 12.57 | 94.60 ± 8.77 | 92.00 ± 5.17 |
| ALT (U/L) | 36.80 ± 8.08 | 38.80 ± 3.70 | 31.75 ± 6.96 | 34.60 ± 5.57 | 34.60 ± 7.05 |
| ALP (U/L) | 92.10 ± 11.35 | 91.04 ± 7.86 | 90.50 ± 8.96 | 88.40 ± 7.03 | 89.20 ± 12.79 |
| Kidney Function Test | |||||
| BUN (mg/dL) | 26.42 ± 3.86 | 31.04 ± 3.96 | 25.45 ± 2.30 | 25.56 ± 3.89 | 25.26 ± 2.12 |
| Creatinine (mg/dL) | 0.66 ± 0.04 | 0.69 ± 0.07 | 0.66 ± 0.03 | 0.65 ± 0.04 | 0.61 ± 0.06 |
Data are presented as mean ± SEM (n = 5 per group).
3.5.3. Histological Examination of Liver and Kidney Tissues
Histopathological examination of the liver and kidney samples is shown in Figure 4. The liver tissue morphology of the mice that received a single 2000 mg/kg dose of M. fragrans extract, A. lancea extract, and Prabchompoothaweep remedy extract manifested normal hepatocytes containing a red–pink cytoplasm and normal structures in the hepatic sinusoids and central vein. The sinusoidal vasodilation or inflammatory infiltration was not observed in the H&E staining of the liver tissue. Furthermore, the kidney morphology of the mice treated with a single dose of the crude extract revealed a normal structure of the glomerulus, Bowman’s capsule, and kidney epithelial cells compared to those of the control group (Figure 4f) and the 7% Tween 80 group (Figure 4g).
Figure 4.
Histopathological examination of liver and kidney tissues from ICR mice that administrated with ethanolic extract from M. fragrans, A. lancea, and Prabchompoothaweep remedy in acute toxicity test: (a) histology of the liver tissue of control group, (b) histology of the liver tissue of 7% Tween 80 group, (c) histology of the liver tissue of M. fragrans treated mice, (d) histology of the liver tissue of A. lancea treated mice, (e) histology of the liver tissue of Prabchompoothaweep remedy treated mice, (f) histology of the kidney tissue of control group, (g) histology of the kidney tissue of 7% Tween 80 group, (h) histology of the kidney tissue of M. fragrans treated mice, (i) histology of the kidney tissue of A. lancea treated mice, (j) histology of the kidney tissue of Prabchompoothaweep remedy treated mice. All images were acquired at 20× magnification. Bar = 200 µm. CV, central vein; H, hepatocyte; T, tubule; G, glomerulus.
4. Discussion
Antimalarial treatment remains a public health concern in several countries. The use of traditional medicine that is safe, effective, and cost-efficient is a way to ensure that all patients have access to treatment [8]. From 2014 to 2023, the World Health Organization’s traditional medicine strategy has become popular worldwide and constantly increased each year [27]. Furthermore, natural plants are important sources of bioactive compounds, and many studies have focused on finding new substances to solve the antimalarial drug problem [24]. Therefore, this study focused on natural plants to stimulate the development of a new, effective antimalarial agent. In our previous report, in vitro studies showed that the ethanolic extracts of the mace of M. fragrans, rhizome of A. lancea, and Prabchompoothaweep remedy had anti-plasmodium activity against the P. falciparum K1 strain, with IC50 values of 5.96, 7.37, and 14.13 µg/mL, respectively (unpublished data). All IC50 values of the crude extracts were categorized as a good or promising activity for antimalarial effects [28]. A selectivity index (SI), which is calculated from the ratio between the toxic concentration to human cells (CC50) and the effective concentration to prevent parasite growth (IC50), which is lower than two, indicates the general toxicity of the compound [29]. These results showed that the ethanolic extract of the mace of M. fragrans, rhizome of A. lancea, and Prabchompoothaweep remedy exhibited SI values higher than two. Because the ethanolic extracts of M. fragrans, A. lancea, and Prabchompoothaweep remedy showed strong in vitro therapeutic effects with promising antimalarial activity and low toxicity to human cells, these two plants and one remedy were considered for in vivo antimalarial evaluation in this study. An in vivo model is commonly used to investigate the effects of a prodrug, the elimination of parasites by the immune system and the safety of the drug before processing into the clinical phase [30]. Mouse models have been used to identify a large number of conventional antimalarial agents, including chloroquine, halofantrine, mefloquine, and artemisinin derivatives [31]. In this study, ICR mice were inoculated with the wild-type P. berghei ANKA strain, a common model for the induction of malaria in mice and evaluation of antimalarial effects. The P. berghei ANKA strain is a suitable parasite that has higher accessibility and can sequester within the blood microcirculation. In this study, we used the 4-day suppressive test because it is a commonly used method for testing the antimalarial effects of candidate compounds in early infection. Moreover, this model shows the most reliable parameters, such as percentage of suppression of blood parasitemia [32].
In the present study, the 4-day suppressive test showed inhibition of parasitemia, which showed a high percentage in mice receiving 600 mg/kg M. fragrans (46.86%), 600 mg/kg Prabchompoothaweep remedy (60.11%), while A. lancea showed a high percentage of suppression in mice receiving 400 mg/kg (60.09%). A. lancea showed a high percentage of suppression in mice at a dose of 400 mg/kg because of its immunomodulatory property. Normally, cytokines play a major role in modulating the symptoms of malaria, parasitemia load, and the severity of malaria disease [33]. Moreover, the pro-inflammatory cytokines such as TNF-α, and IL-6 have been associated with severe malaria and death [34]. A previous study found that the low concentration of atractylodin, which is a bioactive compound of A. Lancea, significantly inhibited the expression of both TNF and IL-6, while the high concentration of atractylodin significantly suppressed only IL-6 expression [35]. Consistent with our results, the crude extract was identified as a considered active when parasitemia suppression was more than 30% [31]. Therefore, it can be implied that these crude extracts are active in schizonticide activity against P. berghei ANKA-infected mice. The antimalarial effects of the crude extract are associated with bioactive compounds such as polyphenols, flavonoids, alkaloids, terpenoids, and saponins [36]. Therefore, the antimalarial effect of ethanolic crude extracts could be due to a single or combined mechanism of action of these active compounds [37].
The results of phytochemical screening revealed that the ethanolic extract of M. fragrans, A. lancea, and Prabchompoothaweep remedy is rich in several plant secondary metabolites. M. fragrans extract contained flavonoids, terpenoids, alkaloids, and coumarins, while the extract of A. lancea contained terpenoids, alkaloids, and coumarins, and Prabchompoothaweep remedy contained terpenoid, alkaloids, tannins, and coumarins, all of which are associated with antimalarial activity. These results were consistent with a previous study on secondary plant metabolites. They have shown antimalarial activities posed by the classes of alkaloids, terpenes, flavonoids, xanthones, anthraquinones, phenolic compounds, sesquiterpenes, and other compounds [38,39]. The phytochemical constituents of the ethanolic extracts of M. fragrans, A. lancea, and Prabchompoothaweep remedy may have a single or synergistic effect to provide antimalarial properties through various mechanisms. In this context, flavonoids have been shown to prevent the transportation of L-glutamine and myoinositol into infected red blood cells, which play a role in parasite growth [40], while terpenoids (e.g., artemisinin) may exert their effect by the endoperoxidation that forms potentially toxic heme-adducts. Alkaloids (e.g., quinine) act as antimalarial agents by inhibiting protein synthesis and preventing heme (toxic) from being converted into hemozoin pigments (non-toxic) in parasite food vacuole [41]. Consequently, tannins also exhibit antimalarial effects by scavenging free radicals. Furthermore, coumarin compounds might contribute to antiplasmodial activity by controlling oxidative enzymes, such as superoxide dismutase, and inhibiting DNA synthesis. The antioxidant effects can disrupt heme polymerization, which oxidizes heme before heme polymerization, and unpolymerized heme is toxic to intraerythrocytic parasites [10]. Furthermore, phytochemical constituents, such as steroids, flavonoids, and other components, might act as antimalarial agents not only by directly attacking parasites but also by indirectly modulating the immune system of the host [42]. Therefore, the antiplasmodial activity observed in plants could have been derived from a single or synergistic effect of these metabolites.
Qualitative analysis of M. fragrans mace extracts presented many compounds. M. fragrans is an important source of secondary compounds consisting of coumarin (edulisin III), and flavonoids (kaempferol, (7′x,8′x)-4,7′-epoxy-3,8′-bilign-7-ene-3,5′-dimethoxy-4′,9,9′-triol), including citric acid and propyl 2-furanacrylate (fatty acid esters). Analysis of A. lancea rhizome extracts revealed the presence of polyphenols (chlorogenic acid), hydroxyanthraquinones (1,2,6,8-Tetrahydroxy-3-methylanthraquinone 2-O-b-D-glucoside), sesquiterpene lactone (taraxacolide 1-O-b-D-glucopyranoside), and salicylic acid. Furthermore, the Prabchompoothaweep remedy extracts allowed us to putatively identify 10 major peaks. The results showed the presence of flavonoid (luteolin), coumarins (6′-O-formylmarmin), and phenolic compounds (caffeic acid, eudesmic acid, gallic acid, and ellagic acid) constituents in the remedy. Some constituents analyzed by LC-MS are biologically active compounds. Luteolin has been shown to possess anti-inflammatory, antiallergy, anticancer, and antioxidant activity [43]. In addition, vanillic acid has been shown to exhibit anti-inflammatory and antioxidant effects both in vitro and in vivo in a carrageenan-induced inflammation model, and it has also shown anticancer, antifungal, antibacterial, and anti-viral effects [44,45]. Kaempferol has several pharmacological effects, including antioxidant and antibacterial activities [46]. Caffeic acid has potential as an antioxidant, anti-inflammatory, and antineoplastic agent [47]. Gallic acid exhibits antibacterial, anticancer, and antiplasmodial activities [48]. Among the identified compounds, ellagic acid has been reported to have anti-plasmodium properties. A previous study by Verotta et al. found that ellagic acid isolated from Tristaniopsis callobuxus (Myrtaceae) showed significant antiplasmodial activity against the resistant strain of Plasmodium, with an IC50 between 0.331 and 0.480 µM [49]. A study by Banzouzi et al. suggested that ellagic acid has also shown anti-plasmodium in mice infected with Plasmodium vinckei pettri using the Peter’s test [50]. Furthermore, Soh et al. found that ellagic acid inhibits parasitemia in a dose-dependent manner, with 50% suppression in mice receiving 1 mg/kg and 100% suppression in mice receiving 50 and 100 mg/kg via the intraperitoneal route [51]. In addition to antimalarial activity, ellagic acid also shows antioxidant properties and anti-inflammation that could prolong the survival rate after the administration of T. albida in experimental cerebral malaria (ECM) [52]. Flavonoid compounds also have antimalarial effects by stimulating the immune system, inhibiting the synthesis of fatty acids in parasites and preventing protein synthesis [53]. Therefore, the selected crude extract of the Prabchompoothaweep remedy might be responsible, at least partially, for antimalarial property, which is produced by a single phytoconstituent or the synergistic effect of these compounds, as mentioned above. However, further studies are needed to isolate, identify, and characterize active compounds, as well as to understand the mechanism of inhibition.
A decrease in PCV is one of the characteristics of malaria infection in mice. PCV was determined to investigate the effectiveness of the ethanolic crude extract in inhibiting erythrocyte damage caused by an increase in parasitemia [54]. To prevent PCV reduction, plants with antimalarial activity are expected to maintain PCV during mouse infection. Surprisingly, in a 4-day suppressive test, the ethanolic extract of M. fragrans, A. lancea, and Prabchompoothaweep remedy at all doses prevented PCV loss compared to the negative control group. It is possible that phenols and other metabolites in plants have antioxidant effects and membrane protection. Phenolic compounds have excellent antioxidant effects due to their hydroxyl groups, which can donate electrons to reactive oxygen species (ROS) [55]. The protective effect of the crude extracts was consistent with the results of studies by Wannang et al. [56], Saba et al. [57], and Misganaw et al. [58]. Moreover, the prevention of PCV reduction may be due to the absence of saponins in this crude extract. Normally, saponins act as phytodetergents, leading to cholesterol release from the cell membrane and promoting the permeability of the red blood cell membrane with strong hemolytic activity [59]. The effect of the plant extract on PCV loss may be due to the elimination of parasites from infected erythrocytes before hemolysis. Furthermore, the activation of the immune system and release of free radicals and ROS caused by malaria infection contribute to the degradation of hemoglobin and development of anemia [60]. In addition, the antioxidant activity of crude extracts, especially polyphenolic compounds, may protect red blood cells (RBCs) from ROS and promote the survival rate of both normal and infected RBCs during malaria infection.
The toxicity of the plant extracts was assessed using an oral acute toxicity test. Under these conditions, the mice received a single dose of 2000 mg/kg of the ethanolic extract of M. fragrans, A. lancea, and Prabchompoothaweep remedy, where a single high dose is suggested for acute toxicity testing [23]. The results of acute toxicity of all crude extracts revealed that there was no mortality and no signs of toxicity over 14 days. Therefore, the approximate median lethal dose (LD50) of the crude extracts was greater than 2000 mg/kg. According to the OECD’s Globally Harmonized System of Classification, crude extracts presented a low acute toxicity hazard with a category 5 classification. The results observed in the acute toxicity study with M. fragrans and A. lancea remedies are consistent with those of a previous study, indicating the safety profiles of this crude extract in a broad range of dose levels (1000−5000 mg/kg body weight) [17,61]. Food and water uptake were recorded to monitor toxicity because these parameters can be used to identify the harmful effects of crude extracts [62]. These results indicated that food and water consumption were not significantly different between the treatment and control groups. Body weight loss is a sensitive toxicity index after exposure to toxic compounds. In the acute toxicity test study, all treatments with M. fragrans, A. lancea, and Prabchompoothaweep remedy did not show significant differences (p < 0.05) in body weight loss compared with the control group on day 14. This observation suggests that the crude extracts did not disturb metabolism in these animals. In addition, the functions of the liver and kidneys were examined using biochemical analyses. Liver abnormalities were indicated by AST, ALT, and ALP levels. Damage to liver cells depends on the levels of AST, ALT, and ALP [63,64]. In all liver marker enzyme activities assessed, AST, ALT, and ALP levels were not significantly different between the treatment groups and the untreated control group. The levels of BUN and creatinine were analyzed for kidney function [65]. These results show that the levels of BUN and creatinine in mice receiving ethanolic extracts of M. fragrans, A. lancea, and Prabchompoothaweep remedy have normal functions in kidney organs that were not different from those in the untreated control group. Additionally, histopathological evaluation of kidney and liver tissue after treatment with the ethanolic extract of M. fragrans, A. lancea, and Prabchompoothaweep remedy did not show any abnormalities. Therefore, the results suggest that oral administration of crude extracts is neither harmful nor unsafe.
5. Conclusions
This study is the first to report the antimalarial activity of ethanolic extracts of M. fragrans mace and A. lancea rhizomes in a mouse model. The ethanolic crude extracts contained several phytoconstituents with important medicinal properties and antimalarial activity. The extracts significantly suppressed parasitemia. Moreover, the crude extracts also showed no adverse health effects on behavioral changes or liver or kidney function in the acute toxicity test. The overall results of this study illustrated that the use of rhizome extracts of A. lancea at 400 mg/kg body weight and extract of Prabchompoothaweep remedy at 600 mg/kg body weight could be developed as a new antimalarial drug treatment. More studies are required to isolate and identify the active compounds and to understand their mechanism of action.
Acknowledgments
This work was supported by Walailak University Ph.D. Scholarships for High-Potential Candidates to Enroll in Doctoral Programs (Contract No. HP005/2021).
Author Contributions
Conceptualization, W.P., P.C. and C.P.; methodology, W.P., A.P., P.C. and C.P.; formal analysis, W.P., P.C. and C.P.; investigation, W.P., A.P., P.C. and C.P.; resources, P.C. and C.P.; data curation, W.P., P.C., A.W.S. and C.P.; writing—original draft preparation, W.P.; writing—review and editing, P.C., A.W.S. and C.P.; visualization, W.P., P.C., A.P. and C.P.; supervision, P.C. and C.P.; project administration, C.P.; funding acquisition, W.P. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
The study protocol was reviewed and approved by the Human Ethics Committee of Walailak University before recruitment (approval number: WUEC-22152-01) and followed the tenets of the Declaration of Helsinki. Written informed consent was obtained from all participants before data and blood sample collection. Animal care requirements were considered during the experiments as required by the National Guidelines for Handling Laboratory Animals. The 4-day suppressive test and oral acute toxicity tests were approved and authorized with permit numbers (WU-ACUC-64027) from the Walailak University Ethical Review Committee before carrying out the experiments.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data associated with this study have been included in this published article. Additional files are available from the corresponding authors upon request.
Conflicts of Interest
The authors declare no competing interests regarding the publication of this paper.
Funding Statement
This research was financially supported by Walailak University Graduate Research Fund (contract no. 2022/07).
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Moxon C.A., Gibbins M.P., McGuinness D., Milner D.A., Marti M. New Insights into Malaria Pathogenesis. Annu. Rev. Pathol. 2020;15:315–343. doi: 10.1146/annurev-pathmechdis-012419-032640. [DOI] [PubMed] [Google Scholar]
- 2.World Health Organization World Malaria Report. 2021. [(accessed on 20 May 2022)]. Available online: https://www.who.int/teams/global-malaria-programme/reports/world-malaria-report-2021.
- 3.World Health Organization Artemisinin Resistance and Artemisinin-Based Combination Therapy Efficacy: Status Report. 2018. [(accessed on 20 May 2022)]. Available online: https://apps.who.int/iris/handle/10665/274362.
- 4.Ashley E.A., Dhorda M., Fairhurst R.M., Amaratunga C., Lim P., Suon S., Sreng S., Anderson J.M., Mao S., Sam B., et al. Spread of artemisinin resistance in Plasmodium falciparum malaria. N. Engl. J. Med. 2014;371:411–423. doi: 10.1056/NEJMoa1314981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nsanzabana C. Resistance to Artemisinin Combination Therapies (ACTs): Do Not Forget the Partner Drug! Trop. Med. Infect. Dis. 2019;4:26. doi: 10.3390/tropicalmed4010026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fairhurst R.M., Dondorp A.M. Artemisinin-resistant Plasmodium falciparum malaria. Microbiol. Spectr. 2016;4:409–429. doi: 10.1128/microbiolspec.EI10-0013-2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.World Health Oragnization World Malaria Report. 2019. [(accessed on 20 May 2022)]. Available online: https://www.who.int/publications/i/item/9789241565721.
- 8.Haidara M., Haddad M., Denou A., Marti G., Bourgeade-Delmas S., Sanogo R., Bourdy G., Aubouy A. In vivo validation of anti-malarial activity of crude extracts of Terminalia macroptera, a Malian medicinal plant. Malar. J. 2018;17:68. doi: 10.1186/s12936-018-2223-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mojab F. Antimalarial natural products: A review. Avicenna J. Phytomed. 2012;2:52–62. [PMC free article] [PubMed] [Google Scholar]
- 10.Alehegn A.A., Yesuf J.S., Birru E.M. Antimalarial activity of crude extract and solvent fractions of the leaves of Bersama abyssinica fresen. (Melianthaceae) against Plasmodium berghei infection in Swiss albino mice. Evid.-Based Complement. Altern. Med. 2020;2020:9467359. doi: 10.1155/2020/9467359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Leangpanich S., Itharat A., Chanvimalueng W., Mukkasombat N. A preliminary study on efficacy of Prapchompoothaweep remedy for treatment of allergic rhinitis patients and their quality of life after the treatment. TMJ. 2019;19:537–546. [Google Scholar]
- 12.Jai-aue A., Makchuchit S., Juckmeta T., Itharat A. Anti-allergic, anti-inflammatory and antioxidant activities of the different extracts of Thai traditional remedy called prabchompoothaweep for allergic rhinitis treatment. J. Med. Assoc. Thail. 2014;97:140–1488. [PubMed] [Google Scholar]
- 13.Ha M.T., Vu N.K., Tran T.H., Kim J.A., Woo M.H., Min B.S. Phytochemical and pharmacological properties of Myristica fragrans Houtt.: An updated review. Arch. Pharm. Res. 2020;43:1067–1092. doi: 10.1007/s12272-020-01285-4. [DOI] [PubMed] [Google Scholar]
- 14.Arumugam G., Purushotham B., Swamy M.K. Natural Bio-Active Compounds. Springer; New York, NY, USA: 2019. Myristica fragrans Houtt.: Botanical, pharmacological, and toxicological aspects; pp. 81–106. [Google Scholar]
- 15.Latha P., Sindhu P., Suja S., Geetha B., Pushpangadan P., Rajasekharan S. Pharmacology and chemistry of Myristica fragrans Houtt.—A review. JOSAC. 2005;14:94–101. [Google Scholar]
- 16.Zhang W.-J., Zhao Z.-Y., Chang L.-K., Cao Y., Wang S., Kang C.-Z., Wang H.-Y., Zhou L., Huang L.-Q., Guo L.-P. Atractylodis Rhizoma: A review of its traditional uses, phytochemistry, pharmacology, toxicology and quality control. J. Ethnopharmacol. 2021;266:113415. doi: 10.1016/j.jep.2020.113415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Koonrungsesomboon N., Na-Bangchang K., Karbwang J. Therapeutic potential and pharmacological activities of Atractylodes lancea (Thunb.) DC. Asian Pac. J. Trop. Med. 2014;7:421–428. doi: 10.1016/S1995-7645(14)60069-9. [DOI] [PubMed] [Google Scholar]
- 18.Na-Bangchang K., Kulma I., Plengsuriyakarn T., Tharavanij T., Kotawng K., Chemung A., Muhamad N., Karbwang J. Phase I clinical trial to evaluate the safety and pharmacokinetics of capsule formulation of the standardized extract of Atractylodes lancea. J. Tradit. Complement. Med. 2021;11:343–355. doi: 10.1016/j.jtcme.2021.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Malar C.G.R., Chellaram C. Phytochemical Screening, Quantification of Total Phenols, Total Flavonoids and Antimicrobial Activity of Stem Extracts of Salacia Oblonga. Indian J. Sci. Technol. 2018;11:1–8. doi: 10.17485/ijst/2018/v11i23/125632. [DOI] [Google Scholar]
- 20.Shad A.A., Ahmad S., Ullah R., AbdEl-Salam N.M., Fouad H., Rehman N.U., Hussain H., Saeed W. Phytochemical and biological activities of four wild medicinal plants. Sci. World J. 2014;2014:857363. doi: 10.1155/2014/857363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shaikh J.R., Patil M. Qualitative tests for preliminary phytochemical screening: An overview. Int. J. Chem. Stud. 2020;8:603–608. doi: 10.22271/chemi.2020.v8.i2i.8834. [DOI] [Google Scholar]
- 22.Peters W., Portus J., Robinson B. The chemotherapy of rodent malaria, XXII: The value of drug-resistant strains of P. berghei in screening for blood schizontocidal activity. Ann. Trop. Med. Parasitol. 1975;69:155–171. doi: 10.1080/00034983.1975.11686997. [DOI] [PubMed] [Google Scholar]
- 23.Organisation for Economic Co-operation and Development (OECD) Test No. 425: Acute Oral Toxicity: Up-and-Down Procedure. OECD Publishing; Paris, France: 2008. [Google Scholar]
- 24.Phuwajaroanpong A., Chaniad P., Horata N., Muangchanburee S., Kaewdana K., Punsawad C. In vitro and in vivo antimalarial activities and toxicological assessment of Pogostemon cablin (Blanco) Benth. J. Evid.-Based Integr. Med. 2020;25:2515690X20978387. doi: 10.1177/2515690X20978387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Viriyavejakul P., Khachonsaksumet V., Punsawad C. Liver changes in severe Plasmodium falciparum malaria: Histopathology, apoptosis and nuclear factor kappa B expression. Malar. J. 2014;13:106. doi: 10.1186/1475-2875-13-106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wichapoon B., Punsawad C., Viriyavejakul P. Expression of cleaved caspase-3 in renal tubular cells in Plasmodium falciparum malaria patients. Nephrology. 2017;22:79–84. doi: 10.1111/nep.12715. [DOI] [PubMed] [Google Scholar]
- 27.World Health Organization WHO Traditional Medicine Strategy: 2014–2023. 2013. [(accessed on 3 June 2022)]. Available online: https://www.who.int/publications/i/item/9789241506096.
- 28.Kaharudin F.A., Zohdi R.M., Mukhtar S.M., Sidek H.M., Bihud N.V., Rasol N.E., Ahmad F.B., Ismail N.H. In vitro antiplasmodial and cytotoxicity activities of crude extracts and major compounds from Goniothalamus lanceolatus. J. Ethnopharmacol. 2020;254:112657. doi: 10.1016/j.jep.2020.112657. [DOI] [PubMed] [Google Scholar]
- 29.Koch A., Tamez P., Pezzuto J., Soejarto D. Evaluation of plants used for antimalarial treatment by the Maasai of Kenya. J. Ethnopharmacol. 2005;101:95–99. doi: 10.1016/j.jep.2005.03.011. [DOI] [PubMed] [Google Scholar]
- 30.Haddadi M., Mousavi M.J., Mohseni S., Mardani G. In vitro ADME Screening Instead of in vivo Studies in Preclinical Safety. Biomed. J. Sci. Tech. Res. 2020;24:18371–18376. doi: 10.26717/BJSTR.2020.24.004071. [DOI] [Google Scholar]
- 31.Fidock D.A., Rosenthal P.J., Croft S.L., Brun R., Nwaka S. Antimalarial drug discovery: Efficacy models for compound screening. Nat. Rev. Drug Discov. 2004;3:509–520. doi: 10.1038/nrd1416. [DOI] [PubMed] [Google Scholar]
- 32.Mekonnen L.B. In vivo antimalarial activity of the crude root and fruit extracts of Croton macrostachyus (Euphorbiaceae) against Plasmodium berghei in mice. J. Tradit. Complement. Med. 2015;5:168–173. doi: 10.1016/j.jtcme.2014.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Farrington L., Vance H., Rek J., Prahl M., Jagannathan P., Katureebe A., Arinaitwe E., Kamya M.R., Dorsey G., Feeney M.E. Both inflammatory and regulatory cytokine responses to malaria are blunted with increasing age in highly exposed children. Malar. J. 2017;16:499. doi: 10.1186/s12936-017-2148-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Afolayan F.I.D., Adegbolagun O., Mwikwabe N.N., Orwa J., Anumudu C. Cytokine modulation during malaria infections by some medicinal plants. Sci. Afr. 2020;8:e00428. doi: 10.1016/j.sciaf.2020.e00428. [DOI] [Google Scholar]
- 35.Kulma I., Panrit L., Plengsuriyakarn T., Chaijaroenkul W., Warathumpitak S., Na-Bangchang K. A randomized placebo-controlled phase I clinical trial to evaluate the immunomodulatory activities of Atractylodes lancea (Thunb) DC. in healthy Thai subjects. BMC Complement. Med. Ther. 2021;21:61. doi: 10.1186/s12906-020-03199-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ungogo M.A., Ebiloma G.U., Ichoron N., Igoli J.O., de Koning H.P., Balogun E.O. A review of the antimalarial, antitrypanosomal, and antileishmanial activities of natural compounds Isolated from nigerian flora. Front. Chem. 2020;8:617448. doi: 10.3389/fchem.2020.617448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rasoanaivo P., Wright C.W., Willcox M.L., Gilbert B. Whole plant extracts versus single compounds for the treatment of malaria: Synergy and positive interactions. Malar. J. 2011;10:S4. doi: 10.1186/1475-2875-10-S1-S4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mazid M., Khan T., Mohammad F. Role of secondary metabolites in defense mechanisms of plants. Biol. Med. 2011;3:232–249. [Google Scholar]
- 39.Batista R., De Jesus Silva A., Jr., De Oliveira A.B. Plant-derived antimalarial agents: New leads and efficient phytomedicines. Part II. Non-alkaloidal natural products. Molecules. 2009;14:3037–3072. doi: 10.3390/molecules14083037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Saxena M., Saxena J., Nema R., Singh D., Gupta A. Phytochemistry of medicinal plants. J. Pharmacogn. Phytochem. 2013;1:168–182. [Google Scholar]
- 41.Soares J.B.R.C., Menezes D., Vannier-Santos M.A., Ferreira-Pereira A., Almeida G.T., Venancio T.M., Verjovski-Almeida S., Zishiri V.K., Kuter D., Hunter R., et al. Interference with hemozoin formation represents an important mechanism of schistosomicidal action of antimalarial quinoline methanols. PLoS Negl. Trop. Dis. 2009;3:e477. doi: 10.1371/journal.pntd.0000477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Masihi K.N. Fighting infection using immunomodulatory agents. Expert Opin. Biol. Ther. 2001;1:641–653. doi: 10.1517/14712598.1.4.641. [DOI] [PubMed] [Google Scholar]
- 43.Lin Y., Shi R., Wang X., Shen H.-M. Luteolin, a flavonoid with potential for cancer prevention and therapy. Curr. Cancer Drug Targets. 2008;8:634–646. doi: 10.2174/156800908786241050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Arya S.S., Rookes J.E., Cahill D.M., Lenka S.K. Vanillin: A review on the therapeutic prospects of a popular flavouring molecule. Adv. Trad. Med. 2021;21:1–17. doi: 10.1007/s13596-020-00531-w. [DOI] [Google Scholar]
- 45.Tai A., Sawano T., Yazama F., Ito H. Evaluation of antioxidant activity of vanillin by using multiple antioxidant assays. Biochim. Biophys. Acta Gen. Subj. 2011;1810:170–177. doi: 10.1016/j.bbagen.2010.11.004. [DOI] [PubMed] [Google Scholar]
- 46.Calderón-Montaño J.M., Burgos-Morón E., Pérez-Guerrero C., López-Lázaro M. A review on the dietary flavonoid kaempferol. Mini-Rev. Med. Chem. 2011;11:298–344. doi: 10.2174/138955711795305335. [DOI] [PubMed] [Google Scholar]
- 47.Aijaz M., Keserwani N., Yusuf M., Ansari N.H., Ushal R., Kalia P. Chemical, biological, and pharmacological prospects of caffeic acid. Biointerface Res. Appl. Chem. 2022;13:324. doi: 10.33263/BRIAC134.324. [DOI] [Google Scholar]
- 48.Chaniad P., Phuwajaroanpong A., Plirat W., Techarang T., Chukaew A., Punsawad C. In vivo assessment of the antimalarial activity and acute oral toxicity of an ethanolic seed extract of Spondias pinnata (Lf) Kurz. BMC Complement. Med. Ther. 2022;22:72. doi: 10.1186/s12906-022-03546-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Verotta L., Dell’Agli M., Giolito A., Guerrini M., Cabalion P., Bosisio E. In vitro antiplasmodial activity of extracts of Tristaniopsis species and identification of the active constituents: Ellagic acid and 3, 4, 5-trimethoxyphenyl-(6′-O-galloyl)-O-β-D-glucopyranoside. J. Nat. Prod. 2001;64:603–607. doi: 10.1021/np000306j. [DOI] [PubMed] [Google Scholar]
- 50.Banzouzi J.-T., Prado-Gotor R., Menan H., Valentin A., Roumestan C., Mallie M., Pelissier Y., Blache Y. In vitro antiplasmodial activity of extracts of Alchornea cordifolia and identification of an active constituent: Ellagic acid. J. Ethnopharmacol. 2002;81:399–401. doi: 10.1016/S0378-8741(02)00121-6. [DOI] [PubMed] [Google Scholar]
- 51.Soh P.N., Witkowski B., Olagnier D., Nicolau M.-L., Garcia-Alvarez M.-C., Berry A., Benoit-Vical F. In vitro and in vivo properties of ellagic acid in malaria treatment. Antimicrob. Agents Chemother. 2009;53:1100–1106. doi: 10.1128/AAC.01175-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Abiodun O.O., Rodríguez-Nogales A., Algieri F., Gomez-Caravaca A.M., Segura-Carretero A., Utrilla M.P., Rodriguez-Cabezas M.E., Galvez J. Antiinflammatory and immunomodulatory activity of an ethanolic extract from the stem bark of Terminalia catappa L. (Combretaceae): In vitro and in vivo evidences. J. Ethnopharmacol. 2016;192:309–319. doi: 10.1016/j.jep.2016.07.056. [DOI] [PubMed] [Google Scholar]
- 53.Kırmızıbekmez H., Çalıs I., Perozzo R., Brun R., Dönmez A.A., Linden A., Rüedi P., Tasdemir D. Inhibiting activities of the secondary metabolites of Phlomis brunneogaleata against parasitic protozoa and plasmodial enoyl-ACP reductase, a crucial enzyme in fatty acid biosynthesis. Planta Med. 2004;70:711–717. doi: 10.1055/s-2004-827200. [DOI] [PubMed] [Google Scholar]
- 54.Okokon J., Ita B., Udokpoh A. The in-vivo antimalarial activities of Uvaria chamae and Hippocratea africana. Ann. Trop. Med. Parasitol. 2006;100:585–590. doi: 10.1179/136485906X118512. [DOI] [PubMed] [Google Scholar]
- 55.Nicholas S. Antioxidants and Reactive Oxygen Species in Plants. Wiley Online Library; Hoboken, NJ, USA: 2008. [Google Scholar]
- 56.Wannang N.N., Jimam N.S., Omale S., Dapar M.L., Gyang S.S., Aguiyi J.C. Effects of Cucumis metuliferus (Cucurbitaceae) fruits on enzymes and haematological parameters in albino rats. Afr. J. Biotechnol. 2007;6:2515–2518. doi: 10.5897/AJB2007.000-2400. [DOI] [Google Scholar]
- 57.Saba A.B., Oridupa O.A., Ofuegbe S.O. Evaluation of haematological and serum electrolyte changes in Wistar rats administered with ethanolic extract of whole fruit of Lagenaria breviflora Robert. J. Med. Plants Res. 2009;3:758–762. [Google Scholar]
- 58.Misganaw D., Engidawork E., Nedi T. Evaluation of the anti-malarial activity of crude extract and solvent fractions of the leaves of Olea europaea (Oleaceae) in mice. BMC Complement. Altern. Med. 2019;19:171. doi: 10.1186/s12906-019-2567-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kaur K., Jain M., Kaur T., Jain R. Antimalarials from nature. Bioorg. Med. Chem. 2009;17:3229–3256. doi: 10.1016/j.bmc.2009.02.050. [DOI] [PubMed] [Google Scholar]
- 60.Greve B., Kremsner P., Lell B., Luckner D., Schmid D. Malarial anaemia in African children associated with high oxygen-radical production. Lancet. 2000;355:40–41. doi: 10.1016/s0140-6736(99)04761-3. [DOI] [PubMed] [Google Scholar]
- 61.Anaduaka E.G., Okagu I.U., Uchendu N.O., Ezeanyika L.U.S., Nwanguma B.C. Hepato-renal toxicity of Myristica fragrans Houtt. (Myristicaceae) seed extracts in rats. J. King Saud Univ. Sci. 2022;34:101694. doi: 10.1016/j.jksus.2021.101694. [DOI] [Google Scholar]
- 62.Osagie-Eweka S.E., Orhue N.E.J., Omogbai E.K.I., Amaechina F.C. Oral acute and sub-chronic toxicity assessment of aqueous leaf extract of Simarouba glauca DC (Paradise tree) Toxicol. Rep. 2021;8:239–247. doi: 10.1016/j.toxrep.2021.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Anosike C.A., Ugwu U.B., Nwakanma O. Effect of ethanol extract of Pyrenacantha staudtii leaves on carbontetrachloride induced hepatotoxicity in rats. Biokemistri. 2008;20:17–22. doi: 10.4314/biokem.v20i1.56433. [DOI] [Google Scholar]
- 64.Nigatu T.A., Afework M., Urga K., Ergete W., Makonnen E. Toxicological in vestigation of acute and chronic treatment with Gnidia stenophylla Gilg root extract on some blood parameters and histopathology of spleen, liver and kidney in mice. BMC Res. Notes. 2017;10:625. doi: 10.1186/s13104-017-2964-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Reddy M.K., Gupta S.K., Jacob M.R., Khan S.I., Ferreira D. Antioxidant, antimalarial and antimicrobial activities of tannin-rich fractions, ellagitannins and phenolic acids from Punica granatum L. Planta Med. 2007;53:461–467. doi: 10.1055/s-2007-967167. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data associated with this study have been included in this published article. Additional files are available from the corresponding authors upon request.