Abstract
High mortality rates due to cardiovascular diseases (CVDs) have attracted worldwide attention. It has been reported that mitochondrial dysfunction is one of the most important mechanisms affecting the pathogenesis of CVDs. Mitochondrial DNA (mtDNA) mutations may result in impaired oxidative phosphorylation (OXPHOS), abnormal respiratory chains, and ATP production. In dysfunctional mitochondria, the electron transport chain (ETC) is uncoupled and the energy supply is reduced, while reactive oxygen species (ROS) production is increased. Here, we discussed and analyzed the relationship between mtDNA mutations, impaired mitophagy, decreased OXPHOS, elevated ROS, and CVDs from the perspective of mitochondrial dysfunction. Furthermore, we explored current potential therapeutic strategies for CVDs by eliminating mtDNA mutations (e.g., mtDNA editing and mitochondrial replacement), enhancing mitophagy, improving OXPHOS capacity (e.g., supplement with NAD+, nicotinamide riboside (NR), nicotinamide mononucleotide (NMN), and nano-drug delivery), and reducing ROS (e.g., supplement with Coenzyme Q10 and other antioxidants), and dissected their respective advantages and limitations. In fact, some therapeutic strategies are still a long way from achieving safe and effective clinical treatment. Although establishing effective and safe therapeutic strategies for CVDs remains challenging, starting from a mitochondrial perspective holds bright prospects.
Keywords: cardiovascular disease, mitochondrial dysfunction, mitochondrial DNA mutation, mitophagy, oxidative phosphorylation, reactive oxygen species, therapeutic strategy
1. Introduction
The cardiovascular system plays a crucial role in the normal metabolism of the human body, also known as the circulatory system, which consists of arteries, veins, the heart, and capillaries. Common cardiovascular diseases (CVDs) include heart related heart failure, myocardial hypertrophy, arterial related atherosclerosis, aortic dissection, abdominal aortic aneurysm and other diseases [1]. CVDs constitute a leading worldwide health problem and account for a high proportion of global deaths, with a mortality rate of up to 20% [2]. Thus, it is imperative to explore the pathogenesis of CVDs and develop effective therapeutic strategies.
In fact, mitochondrial dysfunction is considered to be one of the important mechanisms affecting the pathogenesis of CVDs [3,4,5]. Mitochondria are key double-membrane organelles for aerobic respiration in biological cell, and generate energy required by cells through OXPHOS and regulate cell metabolism. The mitochondrial genome (mtDNA) and nuclear genome (nDNA) together control mitochondrial function, and when they are mutated, it may lead to mitochondrial dysfunction, such as excessive production of ROS and reduced OXPHOS capacity. Mitochondria, as a key place for cell metabolism to generate ATP, provide huge energy for the contraction and relaxation of human cardiac myocytes (HCM), and the accumulation of dysfunctional mitochondria will induce CVDs [6]. For example, in heart failure, the heart has a high demand for energy particularly and requires mitochondrial OXPHOS to support continuous ATP in cardiomyocytes [7].
In recent years, a growing number of studies have confirmed that mitochondrial dysfunction is a non negligible cause of CVDs. For instance, mtDNA mutation could disrupt mitochondrial homeostasis, produce oxidative stress, cause a rise in ROS levels, impair OXPHOS and damage energy metabolism, all of which are risk factors for CVDs. Thus, exploration of mtDNA mutation at the genetic level may be a highly advantageous therapeutic approach to identify and predict CVDs [8]. Dysfunctional mitochondria are removed by the autophagy-lysosomal system, however, hyper activation of mitophagy also leads to pathological conditions [9]. Mitochondrial oxidative capacity has been evaluated in relation to CVDs, and specific targeted antioxidant treatments that reduce ROS production and enhance ROS detoxification alleviate oxidative stress caused by mitochondria [10]. In brief, mitochondria can potentially be used as therapeutic targets for cardiovascular health interventions [11].
In this review, we mainly discuss and analyze the relationship between mitochondrial dysfunction (e.g., mtDNA mutations, impaired mitophagy, decreased OXPHOS and elevated ROS) and CVDs, and explore potential therapeutic strategies for CVDs by eliminating mtDNA mutations, enhancing mitophagy, improving OXPHOS capacity and reducing ROS. From the perspective of mitochondrial dysfunction, we aim to provide references for optimal treatment of CVDs.
2. Mitochondrial Dysfunctions and CVDs
The pathogenesis of CVDs is constantly being explored. Although the pathogenesis of CVDs is not fully understood; a study from the perspective of mitochondrial dysfunction and the analysis of the relationship between mitochondrial dysfunction and CVDs will help to better understand and solve the problems related to CVDs. Here, we summarize the relationships between primary CVDs and mitochondrial dysfunction (Table 1 and Figure 1), and further analyzed the four important features of mitochondria in CVDs in detail.
Table 1.
CVDs and corresponding mitochondrial dysfunction.
| CVDs | Mitochondrial Dysfunction | References |
|---|---|---|
| Heart failure | Impaired mitophagy ↓OXPHOS ↑ROS |
[10] review, 2019 [12] experimental, 2010 [13] experimental, 2011 [14] review, 2022 [15] experimental, 2018 [16] experimental, 2020 [17] experimental, 2011 [18] experimental, 2015 |
| Myocardial hypertrophy | mtDNA mutation Impaired mitophagy ↑ROS |
[10] review, 2019 [17] experimental, 2011 [19] experimental, 2022 [20] experimental, 2019 [21] experimental, 2021 [22] experimental, 2021 [23] experimental, 2015 |
| Atherosclerosis | mtDNA mutation Impaired mitophagy ↓OXPHOS ↑ROS |
[24] clinical, 2019 [25] experimental, 2017 [26] experimental, 2018 [27] experimental, 2017 [28] experimental, 2019 [29] experimental, 2021 [30] experimental, 2014 |
| Aortic dissection | ↑ROS | [31] experimental, 2016 [32] experimental, 2020 [33] experimental, 2022 [34] experimental, 2021 |
| Aortic aneurysm | ↑ROS ↓OXPHOS |
[32] experimental, 2020 [35] experimental, 2020 [36] experimental, 2018 [37] experimental, 2022 [38] experimental, 2006 [39] experimental, 2015 [40] experimental, 2013 [41] clinical, 2020 [42] clinical, 2021 |
“↑” indicate a rise and “↓” indicate a decrease.
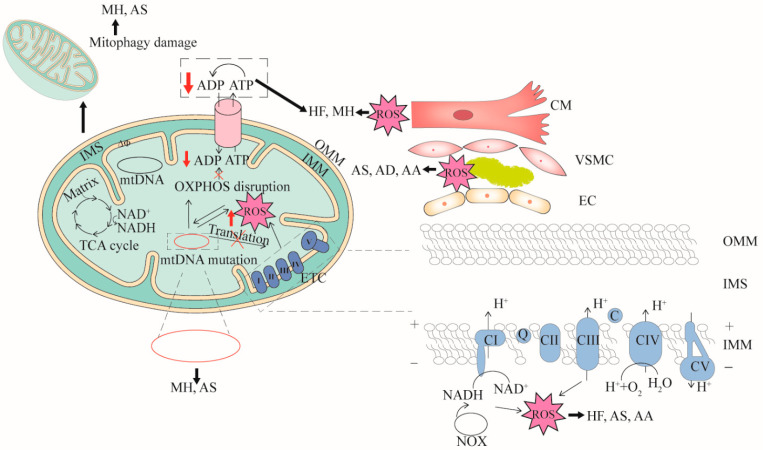
Figure 1.
The relationships between mitochondrial dysfunction and CVDs. Four features (mtDNA mutation, mitophagy damage, decreased OXPHOS and increased ROS) associated with mitochondrial dysfunction are demonstrated. mtDNA mutation can cause dysfunction of mitochondrial respiratory chain complex or cytochrome transcription related to OXPHOS. When OXPHOS is impaired, ATP synthesis is reduced and excess ROS is generated. mtDNA mutation directly affects mitochondrial function or ROS production. In turn, high levels of ROS damage mitochondria. Cardiomyocyte cells (CM), vascular smooth muscle cells (VSMC), and endothelial cells (EC) that are impaired by mitochondrial dysfunction can cause CVDs. mtDNA mutation can cause myocardial hypertrophy (MH) and atherosclerosis (AS), mitophagy damage can cause MH and AS, decreased OXPHOS can cause heart failure (HF), aortic aneurysm (AA) and AS, and increased ROS can cause AS, aortic dissection (AD) and AA. OMM, outer mitochondrial membrane; IMS, inter membrane space; IMM, inner mitochondrial membrane.
2.1. mtDNA Mutation in CVDs
Human mtDNA is a circular double stranded genome with a length of 16,569 bp and consists of 37 genes, which support aerobic respiration and the production of cellular energy through OXPHOS. Unlike nuclear DNA, mtDNA is not protected by histones and does not recombine, resulting in approximately 10–100-fold higher mutation rates [43]. mtDNA mutations include point mutations deletions, fragment deletion and large scale mtDNA rearrangements, which can directly impair OXPHOS [44,45]. A large number of mitochondrial diseases are rooted in mtDNA mutations [46]. Notably, many specific disease mutations in mtDNA have been observed to cause cardiomyopathy, suggesting that mtDNA encoded proteins play a vital role in mitochondrial function of the heart [47]. Heart failure, a complex clinical syndrome that represents the end result of CVDs with multiple etiologies, is a bioenergetic disease with severe mtDNA mutations and mitochondrial dysfunction [14,48]. The MRPL44-disorder causes problems with the translation of a partial protein participating in OXPHOS, and it is associated with the clinical manifestation of cardiomyopathy in infancy [49]. In a mouse model of myocardial infarction (MI), the knockdown of the mouse lncRNA-SNHG8 gene significantly suppressed cardiac tissue injury [50]. Myocardial hypertrophy is a common inherited CVDs, and cardiomyocytes are associated with abnormal mitochondrial structure and dysfunction of mitophagy clearance, which makes it impossible to maintain mtDNA and functional integrity [4]. Hypertrophic cardiomyopathy is the predominant pattern of cardiomyopathy in mtDNA diseases, observed in nearly 40% of patients [51]. The studies have evaluated an association between mtDNA mutations and maternally inherited essential hypertension (MIEH), and these mutations may be one of the pathological mechanisms causing MIEH [45,52]. In addition, mtDNA mutations are also associated with atherosclerosis [53]. For example, four mutation genes including m.A1555 G in the MT-RNR1 gene, m.C3256 T in the MT-TL1 gene, m.G12315A in the MT-TL2 gene and m.G15059A in the MT-CYB gene are associated with atherosclerosis [54].
2.2. Mitophagy Damage in CVDs
Autophagy plays a positive role in maintaining cellular homeostasis in most cardiovascular-derived cells (e.g., cardiomyocytes, VSMCs) [55], and mitophagy is a kind of selective autophagy [56]. Mitophagy is one of the mitochondrial quality control pathways, and it can control and remove damaged mitochondria in cells [57]. During mitophagy, the damaged mitochondria are sequestered by double membrane vesicles and eventually become hydrolyzed by lysosomes [58]. Therefore, if mitophagy is impaired, the accumulation of dysfunctional mitochondria increases, which may lead to abnormal cell function and CVDs. Reducing mitochondrial dysfunction and lipid accumulation by activating mitophagy can help prevent diabetic cardiomyopathy caused by high fat diet [59]. In contrast, in BMAL1 deficient hESC-derived cardiomyocytes, impaired mitophagy is a key cause for the development of dilated cardiomyopathy [60]. In Hu’s study, constructing mice with overexpression of omentin1 demonstrated that omentin1 activated mitophagy to improve HF [61]. In cardiac ischemia-reperfusion injury associated with disturbed mitochondrial homeostasis, the casein kinase 2α amplifies cardiomyocyte death signals by inhibiting mitophagy [62]. Defective mitophagy in VSMCs affects the progression of atherosclerotic lesions and promotes an unstable phenotype [28]. In addition, mitophagy damage in endothelial cells leads to senescence and apoptosis during atherosclerotic thrombosis [57].
2.3. Mitochondrial OXPHOS Reduction in CVDs
Defects in the genes encoding the OXPHOS complex are responsible for triggering various diseases, especially those with high energy requirements [63]. Dysfunction of OXPHOS is considered as one of the main causes of CVDs [64]. In chronic HF patients, reduced succinyl-CoA levels in myocardial mitochondria cause decreased OXPHOS [65]. In a study of human thoracic aortic aneurysm tissue, when mitochondrial OXPHOS related gene expression is inhibited, although chromatin OXPHOS related genes are increased, the ATP production is still insufficient to maintain contractile activity in human aortic smooth muscle cells (HAoSMCs) [41]. In an another study evaluating the effect of NOTCH1 deletion on the contractile phenotype and mitochondrial dynamics of human HAoSMCs, NOTCH1 deficiency can cause mitochondrial dysfunction in HAoSMCs by reducing mitochondrial fusion, inducing loss of mitochondrial membrane potential, increasing ROS generation, insufficient ATP production, and accompany with an impaired contractile phenotype [42]. PGC-1β deficiency in heart suppresses OXPHOS gene expression, and it can inhibit the transition from pressure overload myocardial hypertrophy to heart failure by modulating PGC-1β activity [13]. miR-27b-3p is thought to be related to OXPHOS. When it is inhibited, OXPHOS is enhanced and inhibits cardiomyocyte hypertrophy [66]. It has been also reported that decreased mitochondrial respiration and OXPHOS damage in epicardial adipose tissue were associated with coronary atherosclerosis severity [24].
2.4. Mitochondrial-Derived ROS Increase in CVDs
Mitochondrial ROS production is closely related to the mitochondrial ETC and NADPH oxidase (NOX). In the process of mitochondrial electron transfer, complex I and complex III are the main sites of ROS generation [67,68]. NADPH acts as a substrate to generate ROS under the action of NOX. The NOX is rich in mitochondria, and under the combined action of ETC and NOX, ROS continuously accumulates [69]. As a toxic by-product, ROS can damage mitochondria and are involved in the pathomechanism of CVDs. In turn, damaged mitochondria induce a large amount of ROS to be released from adjacent mitochondria, which is known as ROS-induced ROS [70]. The increased mitochondrial ROS represents one of the pathogenic mechanisms for vascular diseases [71]. For instance, both Nox2 and Nox4 induce oxidative stress, and the resulting ROS is closely related to ischemia-reperfusion [72]. The degree of atherosclerosis is associated with mitochondrial DNA damage, which associated with increased mitochondrial ROS [73]. It is not difficult to accept that the high reactivity of ROS will break the antioxidant balance that results in increased oxidative modification of the arterial wall. Studies have also shown that increased mitochondrial ROS leads to an increase in apoptotic cells and promotes age-related atherosclerosis [26]. Increased ROS predisposes endothelial cells to mitochondrial dysfunction, vascular inflammation, and accumulation of oxidized low-density lipoprotein, contributing to atherosclerosis and possibly plaque rupture [74]. VSMCs, as the predominant medial effector cells in aortic dissections and aneurysms, are a key factor in AD development. Increased ROS activates multiple hypertrophic signaling kinases and transcription factors, leading to dissection by inducing VSMCs apoptosis through the release of matrix metalloproteinases [31,75].
3. Strategies for Targeting Mitochondria to Treat CVDs
Exploring the mechanism of mitochondrial dysfunction in vascular diseases is a challenge for developing strategies to target mitochondria in CVDs. Here, we have summarized the relationship between four important features of mitochondria (mtDNA mutations, impaired mitophagy, decreased OXPHOS, elevated ROS) and CVDs. In view of these four features, we further sorted out the development and hotspot treatment strategies in recent years, and analyze the advantages and limitations of different treatment strategies (Table 2), hoping to find effective and operable solutions for all kinds of CVDs.
Table 2.
Advantages and limitations of treatment strategies for targeting mitochondria to treat CVDs.
| Features | Strategies | Advantages | Limitations | Examples as Applied | References |
|---|---|---|---|---|---|
| Mutant mtDNA |
mtDNA editing | High specificity; Easy operation |
High cost; Limited availability |
mito-RE, heart related CVDs | [46,76,77,78] |
| Mitochondrial replacement therapy | Reduce the risk of vertical transmission | Ethical and legal issues | [79] | ||
| Impaired mitophagy |
Enhanced mitophagy | Play protective roles | Unclear conditions for mitophagy; A potential cytotoxic |
Berberine and the Pink1/Parkin pathway, HF; Active Ulk1/Rab9-dependent, Cardiomyopathy |
[16,55,80,81] |
| Decreased OXPHOS | Small molecule compounds to improve OXPHOS | Dietary supplements; Wide range of sources |
Lack of clinical trials; Being degraded in advance |
Control of the SIRT3 activity, MI | [82,83,84] |
| Nanomaterials to enhance mitochondrial function | Precise targeting; Noninvasive; High load drug |
Lack of clinical trials; Collaborative targeting against multiple subcellular organelles is limited |
NAD+, HF | [85,86,87] | |
| Increased ROS | Antioxidant | Amounts of clinical trials (CoQ10) | Interaction with statin (CoQ10); Long-term exposure maybe harmful |
CoQ10, HF; Melatonin, AA/AD |
[32,88,89,90] |
HF, heart failure; MI, myocardial infarction; AA, aortic aneurysm; AD, aortic dissection; SIRT3, Sirtuin-3.
3.1. mtDNA Mutation and Treatment in CVDs
3.1.1. mtDNA Editing Therapy
Mitochondrial heterogeneity affects mtDNA stability through copy number alterations and point deletions [91]. Once mtDNA is cleaved and linearized, it is rapidly degraded [92]. By duplicating residual mtDNA, mtDNA can be repopulated to the original level. In general, mitochondrial gene editing may include four potential approaches: mitochondria targeted restriction endonuclease (RE) technology, zinc finger nuclease (ZFN) technology, transcription activator-like effector nuclease (TALEN) technology and CRISPR/Cas9 system. In fact, mtDNA editing is a promising therapeutic modality to treat heteroplasmic or mutant mtDNA diseases. Specific mtDNA was effectively eliminated in heart of mice by using a mitochondria targeted RE [78,93]. Mitochondrial-targeted ZFNs can selectively cleave and degrade pathogenic mtDNA bearing large scale deletions or point mutations [94]. An alternative TALEN has been developed to effectively reduce mutant mtDNA and elevate OXPHOS in cells [95]. TALEN was used to provide a cure for some mitochondrial diseases caused by mtDNA mutations by specifically cleaving and eliminating pathogenic mtDNA mutations [96]. Several studies have reported that CRISPR/Cas9 system mediated mtDNA editing [97,98], and the use of PNPase to target mitochondria and eliminate mtDNA pathogenic mutations is quite promising [99]. Notably, recent studies have found that Ddda-derived cytosine base editor (DdCBE) exhibits higher fidelity and can improve the accuracy of mtDNA [100,101,102]. Efficient and heritable modification of the mouse mitochondrial genome has been shown to be mediated by DdCBE, which is used to potentially generate mtDNA mutation models in humans. This approach could theoretically reduce disease-causing mutational burdens below a threshold, and is a potential strategy to target mtDNA for the treatment of CVDs due to mitochondrial dysfunction and mtDNA mutations.
3.1.2. Mitochondrial Replacement Therapy
mtDNA replacement therapy (MRT) is to use enucleated donor embryos as healthy mtDNA to replace undesired defective/mutated mtDNA to prevent mitochondria from being maternally inherited. MRT is a form of in vitro fertilization (IVF) that includes spindle transfer (ST), prokaryotic transfer (PNT) and polar body transfer (PBT) [103]. In fact, embryos from human nuclear transfer can contain low levels of mutated mtDNA, which may be suitable for treating degenerative diseases caused by mtDNA mutations [104]. This opens up the possibility of MRT for CVDs, a chronic noncommunicable degenerative disease. Hyslop et al. developed a PNT protocol that promotes efficient development at the blastocyst stage, keeping mtDNA residues as low as possible [105]. At present, there have been successful cases of applying MRT strategies [106,107], and offspring will not suffer from mtDNA mutation-related diseases. Therefore, hypertrophic cardiomyopathy, dilated cardiomyopathy, genetically related coronary heart disease and other CVDs can be considered using MRT, as an auxiliary means of human reproduction, to solve the problem from the embryo. However, the scientific knowledge related to MRT is still being explored, and the risks and ethical issues of this technology remain to be resolved [108].
3.2. Mitophagy Therapy in CVDs
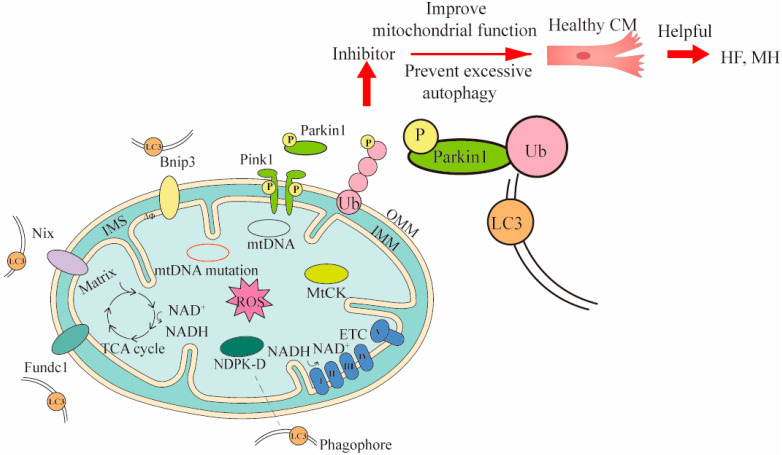
Mitophagy clears dysfunctional mitochondria under normal physiological conditions, and in response to pathological stress [15]. Currently, there are three mechanisms of mitophagy, including mitochondrial outer membrane receptor-mediated, Pink1/Parkin pathway, and lipid receptor-mediated mechanisms (Figure 2) [109]. Notably, the mechanism mediated by the Pink1/Parkin pathway is the most extensively studied. Under normal circumstances, the content of Pink1 is extremely low. When oxidative stress occurs and mitochondria damage is induced, Pink1 is activated and recruits Parkin to the mitochondrial outer membrane for phosphorylation. The phosphorylated Parkin ubiquitinates the substrate protein on the mitochondrial membrane [110]. These ubiquitinated proteins subsequently recruit specific autophagy-related receptors to interact with LC3-II to form autophagosomes [111].
Figure 2.
Three mechanisms of mitophagy and the ways they intervene in treating CVDs. Three mechanisms of mitophagy include mitochondrial outer membrane receptor-mediated (such as Bnip3, Nix and Fundc1), Pink1/Parkin pathway, and lipid receptor-mediated mechanisms (such as MtCK, NDPK-D). The LC3 is located in the phagophore and binds to the corresponding receptor. The LC3 can bind to substances of different mitophagy mechanisms. To demonstrate the mitophagy occurring in CVDs, cardiomyocytes (CM) and Pink1/Parkin pathway were used to intervene in heart failure (HF) and myocardial hypertrophy (MH) by inhibitors. In Pink1/Parkin-dependent mitophagy, the Pink 1 accumulated on the damaged mitochondria is activated and recruits Parkin for phosphorylation. The phosphorylated Parkin binds to the ubiquitin attached to outer OMM, and finally binds to LC3 for mitophagy. The Bnip3 and Nix can directly bind LC3 and promote mitophagy. MtCK and NDPK-D as specific transporters can also directly bind LC3 for mitophagy to eliminate damaged mitochondria. The inhibitor acts on Pink1/Parkin pathway, and then prevents excessive mitophagy and improves mitochondrial function, thereby maintaining the healthy levels of cells associated with CVDs. OMM, outer mitochondrial membrane; IMS, inter membrane space; IMM, inner mitochondrial membrane.
Mitophagy maintains cardiovascular homeostasis and performs significant functions in mitochondrial quality control. It has been shown that phosphorylation of Ser495 in Pink1 by AMPKα2 is necessary for effective mitotic inhibition of the progression of heart failure [15]. Ophiopogonin D’ (OPD’) is toxic to mitochondria, and OPD’-induced mitosis and mitochondrial damage in cardiomyocytes are partly mediated by the dysregulation of the Pink1/Parkin pathway, preventing excessive mitochondrial autophagy [112]. In a study on the improvement of cardiac function by berberine, it was found that the coordinated action of berberine and the Pink1/Parkin pathway enhances mitochondrial phagocytosis and protects patients with heart failure [16]. Ulk1/Rab9-dependent alternative mitophagy is activated during chronic high-fat diet depletion as an important mitochondrial quality control mechanism to protect the heart from the obesity effects of cardiomyopathy [81]. In conclusion, the control of mitophagy has an important role in the clearance of abnormal mitochondria and the protection of cardiomyocytes (Figure 2).
In addition, when abnormal mitochondria undergo fission, they can trigger cardiovascular dysfunction [113,114]. Cytoplasmic GTPase dynamics related protein 1 (Drp1) regulates mitochondrial fission by interacting with proteins located at fission sites such as mitochondrial fission 1 (Fis1), mitochondrial fission factor (Mff), and mitochondrial dynamics proteins of 49 and 51 kDa (MiD49 and MiD51) [115]. A study identified mitochondrial fission inhibitor (mdivi-1) as a cell-permeable quinazolinone derivative inhibitor of Drp1 [116]. In cardiomyocytes treated with mdivi-1, proteolytic cleavage of the OPA1 isoform and decreased expression of Mfn2, altered complex I and complex II protein expression of OXPHOS, and increased superoxide production were observed, which resulted in mitochondrial respiration defects and macro-autophagy inhibition [117]. Taken together, it is speculated that targeting mitochondrial fission or Drp1 may be useful for CVDs therapy.
3.3. Mitochondrial OXPHOS Reduction and Treatment in CVDs
3.3.1. Small Molecule Compounds Enhance Mitochondrial Function
SIRT3 is a mitochondrial protein deacetylase that regulates mitochondrial function and is considered as an emerging drug target for CVDs [118]. SIRT3 can make mitochondrial metabolic pathways and ROS detoxification activate, and increase ATP production [119]. Resveratrol improves mitochondrial OXPHOS in diabetic hearts and prevents the decline of SIRT3 activity in the heart by increasing ETC activity and mitochondrial function [120]. The polyphenolic compound polydatin can initiate SIRT3-regulated mitophagy to prevent MI [84]. Notably, proteolytic targeting chimera technology, as a new strategy of targeted inhibitors, makes it possible to potently target small molecule compounds to enhance mitochondrial function, which may be more beneficial to the treatment of CVDs caused by mitochondrial dysfunction.
When mtDNA is damaged at high levels, increased Poly(ADP-ribose) polymerase (PARP) activity leads to a decrease in NAD+ levels, resulting in impaired NAD+-dependent SIRT3 activation and ultimately cardiac mitochondrial dysfunction [121]. Therefore, targeted improvement of mitochondrial function through nutritional supplementation NAD+ or ketoesters may be useful in patients with heart failure [87]. NAD+ supplementation with nicotinamide riboside (NR) promotes mitophagy in a Pink1-dependent manner [122]. NR can reduce ROS production and maintain normal mitochondrial function in the presence of inflammatory triggers [123]. The effect of nicotinamide mononucleotide (NMN) on the generation of ROS was investigated and it was finally found that NMN can reduce mitochondrial oxidative stress in brain microvascular endothelial cells and improve primary cerebro-microvascular endothelial cell membrane potential and mitochondrial respiration in a sirtuin-dependent manner [124]. Similarly, NMN improves the aorta by reducing oxidative stress [125]. Taken together, it can be seen that NR, NAD+, and NMN have certain therapeutic potential in the treatment of CVDs caused by mitochondrial dysfunction. Among them, NR and NMN still require further preclinical and clinical studies to ensure the safety of the drug [83].
3.3.2. Nanomaterials Targeted Mitochondria to Improve Mitochondrial Function
Many drugs cannot precisely bind to damaged mitochondria, and they are even toxic to other tissues in the body. To solve these problems, precise targeted therapy has attracted much attention. Modification of nanoparticles with different components facilitates mitochondrial directed drug penetration [126]. A team has constructed a non invasive aerosol inhalation delivery system based on antioxidant nano drugs, which can target damaged mitochondria, clear ROS, and improve the targeting ability of nano-drugs to myocardium [85]. Artificial hybrid nanozymes created by protein reconstruction technology and nanotechnology can target mitochondria and scavenge ROS, thereby reducing mitochondrial oxidative damage [127]. Improved formulation of negatively charged peptide nanoparticles enables efficient localization of the drug to mitochondria [128]. Therefore, the novel nano drug delivery system in the human body to effectively treat human CVDs by targeting mitochondria will bring another bright future.
3.4. Reduction or Elimination of Mitochondrial-Derived ROS in CVDs
ROS acts as highly active molecules in vivo, and antioxidants can effectively reduce or eliminate ROS. The in vitro hypoxia/reoxygenation model of H9c2 cells could simulate myocardial ischemia-reperfusion injury, and it found that the experimental group supplemented with vitamin D could inhibit the production of ROS in cardiomyocytes [129]. Melatonin is an indole heterocyclic compound produced by pineal cells in the pineal gland. It can effectively lower ROS production, thereby reducing oxidative stress and VSMC loss, preventing the deterioration of thoracic aortic aneurysm and dissection [32]. Fullerenol nanoparticles are introduced into an alginate hydrogel to form a fullerenol/alginate hydrogel with antioxidant activity. This injectable cell delivery vector can treat myocardial infarction by effectively reducing ROS levels [130]. Cardioprotection of tetrahedral DNA nanostructures can significantly decrease oxidative stress and play a positive role in protecting against myocardial ischemia-reperfusion injury [131]. However, the clinical effects of ROS scavengers in CVDs are not always significant, probably because antioxidants can indiscriminately remove some physiological ROS. Therefore, finding drugs to target damaged mitochondria will improve the clearance of pathological ROS.
Fortunately, the antioxidant CoQ10 has been used in the clinical treatment of CVDs and has good curative effect. Ubiquinone, the oxidized form of CoQ10, transports electrons in the mitochondrial ETC and plays a crucial role in mitochondrial energy production. CoQ10 can transport H+ to thermally dissipate chemosmotic gradients via uncoupling proteins (UCP-1, 2 and 3). After uncoupling, the reduction level of electron carriers is reduced, thereby reducing the production of ROS [132]. Moreover, the reduced form of CoQ10 is also an active agent involved in antioxidant function, which can scavenge ROS production due to mitochondrial dysfunction [133]. Meanwhile, CoQ10 helps recycle other antioxidants such as radical forms of vitamin C and vitamin E [134]. CoQ10 has been shown to increase ATP production in cardiomyocytes, enhance oxidative effects, and improve endothelial function and lipid profile [135]. Comparing CoQ10 with placebo, the therapeutic effect of CoQ10 was more significant in the long term [136]. In addition, substantial clinical evidence suggests that CoQ10 supplementation (≥200 mg/day) contributes to cardiac health in patients affected by coronary heart disease and heart failure [90]. The safety profile of CoQ10 can be used as adjunctive therapy in congestive heart failure and may be helpful in patients who cannot tolerate mainstream drugs [137]. CoQ10 supplementation is safe and well tolerated with few drug interactions and side effects [138]. Similarly, the MitoQ was clinically demonstrated for its antioxidant effects on mitochondrial-derived ROS. The MitoQ can increase the resistance of aging mice to mitochondrial-derived ROS and protect against the imbalance of mitochondrial homeostasis due to aging. It is a novel strategy to treat and prevent age-related CVDs [139]. Of course, the task of applying more safe and effective new antioxidants to the clinical treatment of CVDs is a long way to go and needs to be continuously explored.
4. Conclusions and Perspective
As an important component of the cell, mitochondria contain genetic material, produce energy, and participate in a wide variety of metabolic activities in the cell. It can be seen that if the mitochondrial dysfunction occurs, the normal replication of mtDNA, energy production, and other functions will be affected, which may cause diseases. Here, we mainly analyzed the relationship between the heart and arterial-related CVDs, and mitochondrial dysfunction. Mitochondrial dysfunction in cardiomyocytes, vascular smooth muscle cells, and endothelial cells causes a wide variety of CVDs; and has attracted more and more scientists. With the deepening of CVDs pathogenesis related studies, we summarized the mitochondrial dysfunction causing CVDs into four important characteristics, including mtDNA mutations, impaired mitophagy, decreased OXPHOS, and mitochondrial-derived ROS increase. In multiple animal and human models, many relevant intervention experiments have been designed according to mitochondrial dysfunction, constantly exploring more effective CVDs related therapeutic strategies. According to the four important characteristics of mitochondrial dysfunction, the related treatment strategies of CVDs were sorted out. Exploring the significance, advantages, and current limitations of different mitochondrial targeted therapy strategies can provide more ideas and options for the treatment of different CVDs. Although each of these strategies for ameliorating mitochondrial dysfunction has its own characteristics, combination therapy may be more effective. It is well known that CVDs are quite complex, and their pathological mechanisms are even more complex and diverse. On the basis of continuously deepening the pathological mechanisms, mitochondrial targets can be found more accurately. In the case of harmless to human body, mitochondrial targeted therapy for CVDs may improve the efficiency and safety of treatment, and contribute to the development of human health.
Abbreviations
CVDs: cardiovascular diseases; mtDNA, mitochondrial DNA; OXPHOS, oxidative phosphorylation; ETC, electron transport chain; ROS, reactive oxygen species; NR, nicotinamide riboside; NMN, nicotinamide mononucleotide; HCM, human cardiac myocytes; CM, cardiomyocyte cells; VSMCs, vascular smooth muscle cells; EC, endothelial cells; HF, heart failure; AS, atherosclerosis; MH, myocardial hypertrophy; AA, aortic aneurysm; AD, aortic dissection; MI, myocardial infarction; OMM, outer mitochondrial membrane; IMM, inner mitochondrial membrane; MIEH, maternally inherited essential hypertension; HAoSMCs, human aortic smooth muscle cells; NOX, NADPH oxidase; ZFN, zinc finger nuclease; TALEN, transcription activator-like effector nuclease; DdCBE, Ddda-derived cytosine base editor; MRT, mtDNA replacement therapy; PNT, prokaryotic transfer; Drp1, dynamics-related protein 1.
Author Contributions
Conceptualization, J.L. and Y.L. (Yongzhi Li); investigation, Y.L. (Yu Liu), Y.H., P.A. and Y.L. (Yongting Luo); writing—original draft preparation, Y.L. (Yu Liu) and Y.H.; writing—review and editing, P.A., Y.L. (Yongting Luo), J.L. and Y.L. (Yongzhi Li); visualization, C.X. and L.J.; supervision, J.L. and Y.L. (Yongzhi Li); project administration, J.L. and Y.L. (Yongzhi Li); funding acquisition, J.L. and Y.L. (Yongzhi Li). All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This work was supported by the Beijing Advanced Innovation Center for Food Nutrition and Human Health, and the space health research foundation of Astronaut Health Center (AHCC2020ZX002, 413FZ2, WSQW2001).
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Arnett D.K., Blumenthal R.S., Albert M.A., Buroker A.B., Goldberger Z.D., Hahn E.J., Himmelfarb C.D., Khera A., Lloyd-Jones D., McEvoy J.W., et al. 2019 ACC/AHA guideline on the primary prevention of cardiovascular disease: Executive summary: A report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J. Am. Coll. Cardiol. 2019;74:1376–1414. doi: 10.1016/j.jacc.2019.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Roth G.A., Abate D., Abate K.H., Abay S.M., Abbafati C., Abbasi N., Abbastabar H., Abd-Allah F., Abdela J., Abdelalim A., et al. Global, regional, and national age-sex-specific mortality for 282 causes of death in 195 countries and territories, 1980–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2018;392:1736–1788. doi: 10.1016/S0140-6736(18)32203-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kumar A.A., Kelly D.P., Chirinos J.A. Mitochondrial Dysfunction in Heart Failure With Preserved Ejection Fraction. Circulation. 2019;139:1435–1450. doi: 10.1161/CIRCULATIONAHA.118.036259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ranjbarvaziri S., Kooiker K.B., Ellenberger M., Fajardo G., Zhao M., Roest A.S.V., Woldeyes R.A., Koyano T.T., Fong R., Ma N., et al. Altered Cardiac Energetics and Mitochondrial Dysfunction in Hypertrophic Cardiomyopathy. Circulation. 2021;144:1714–1731. doi: 10.1161/CIRCULATIONAHA.121.053575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Eirin A., Lerman A., Lerman L.O. Mitochondrial injury and dysfunction in hypertension-induced cardiac damage. Eur. Hear. J. 2014;35:3258–3266. doi: 10.1093/eurheartj/ehu436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Manolis A.S., Manolis T.A., Apostolaki N.E., Apostolopoulos E.J., Melita H., Katsiki N. Mitochondrial dysfunction in cardiovascular disease: Current status of translational research/clinical and therapeutic implications. Med. Res. Rev. 2020;41:275–313. doi: 10.1002/med.21732. [DOI] [PubMed] [Google Scholar]
- 7.Lopaschuk G.D., Karwi Q.G., Tian R., Wende A.R., Abel E.D. Cardiac energy metabolism in heart failure. Circ. Res. 2021;128:1487–1513. doi: 10.1161/CIRCRESAHA.121.318241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dabravolski S.A., Khotina V.A., Sukhorukov V.N., Kalmykov V.A., Mikhaleva L.M., Orekhov A.N. The Role of Mitochondrial DNA Mutations in Cardiovascular Diseases. Int. J. Mol. Sci. 2021;23:952. doi: 10.3390/ijms23020952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Palikaras K., Lionaki E., Tavernarakis N. Mechanisms of mitophagy in cellular homeostasis, physiology and pathology. Nat. Cell Biol. 2018;20:1013–1022. doi: 10.1038/s41556-018-0176-2. [DOI] [PubMed] [Google Scholar]
- 10.Peoples J.N., Saraf A., Ghazal N., Pham T.T., Kwong J.Q. Mitochondrial dysfunction and oxidative stress in heart disease. Exp. Mol. Med. 2019;51:1–13. doi: 10.1038/s12276-019-0355-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zampino M., Spencer R.G., Fishbein K.W., Simonsick E.M., Ferrucci L. Cardiovascular Health and Mitochondrial Function: Testing an Association. J. Gerontol. Ser. A. 2021;76:361–367. doi: 10.1093/gerona/glaa297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kuroda J., Ago T., Matsushima S., Zhai P., Schneider M.D., Sadoshima J. NADPH oxidase 4 (Nox4) is a major source of oxidative stress in the failing heart. Proc. Natl. Acad. Sci. USA. 2010;107:15565–15570. doi: 10.1073/pnas.1002178107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Riehle C., Wende A.R., Zaha V.G., Pires K.M., Wayment B., Olsen C., Bugger H., Buchanan J., Wang X., Moreira A.B., et al. PGC-1β Deficiency Accelerates the Transition to Heart Failure in Pressure Overload Hypertrophy. Circ. Res. 2011;109:783–793. doi: 10.1161/CIRCRESAHA.111.243964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wu C., Zhang Z., Zhang W., Liu X. Mitochondrial dysfunction and mitochondrial therapies in heart failure. Pharmacol. Res. 2021;175:106038. doi: 10.1016/j.phrs.2021.106038. [DOI] [PubMed] [Google Scholar]
- 15.Wang B., Nie J., Wu L., Hu Y., Wen Z., Dong L., Zou M.-H., Chen C., Wang D.W. AMPKα2 Protects Against the Development of Heart Failure by Enhancing Mitophagy via PINK1 Phosphorylation. Circ. Res. 2018;122:712–729. doi: 10.1161/CIRCRESAHA.117.312317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Abudureyimu M., Yu W., Cao R.Y., Zhang Y., Liu H., Zheng H. Berberine promotes cardiac function by upregulating PINK1/Parkin-mediated mitophagy in heart failure. Front. Physiol. 2020;11:565751. doi: 10.3389/fphys.2020.565751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dai D.-F., Johnson S.C., Villarin J.J., Chin M.T., Nieves-Cintrón M., Chen T., Marcinek D.J., Dorn G.W., Kang Y.J., Prolla T.A. Mitochondrial oxidative stress mediates angiotensin II–induced cardiac hypertrophy and Gαq overexpression–induced heart failure. Circ. Res. 2011;108:837–846. doi: 10.1161/CIRCRESAHA.110.232306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wu S.-P., Kao C.-Y., Wang L., Creighton C.J., Yang J., Donti T.R., Harmancey R., Vasquez H.G., Graham B.H., Bellen H.J., et al. Increased COUP-TFII expression in adult hearts induces mitochondrial dysfunction resulting in heart failure. Nat. Commun. 2015;6:8245. doi: 10.1038/ncomms9245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yan M., Li Y., Luo Q., Zeng W., Shao X., Li L., Wang Q., Wang D., Zhang Y., Diao H., et al. Mitochondrial damage and activation of the cytosolic DNA sensor cGAS–STING pathway lead to cardiac pyroptosis and hypertrophy in diabetic cardiomyopathy mice. Cell Death Discov. 2022;8:1–12. doi: 10.1038/s41420-022-01046-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kumar V., Sanawar R., Jaleel A., Kumar T.R.S., Kartha C.C. Chronic Pressure Overload Results in Deficiency of Mitochondrial Membrane Transporter ABCB7 Which Contributes to Iron Overload, Mitochondrial Dysfunction, Metabolic Shift and Worsens Cardiac Function. Sci. Rep. 2019;9:1–16. doi: 10.1038/s41598-019-49666-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zou R., Tao J., Qiu J., Shi W., Zou M., Chen W., Li W., Zhou N., Wang S., Ma L., et al. Ndufs1 Deficiency Aggravates the Mitochondrial Membrane Potential Dysfunction in Pressure Overload-Induced Myocardial Hypertrophy. Oxidative Med. Cell. Longev. 2021;2021:1–21. doi: 10.1155/2021/5545261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fu Y.-L., Tao L., Peng F.-H., Zheng N.-Z., Lin Q., Cai S.-Y., Wang Q. GJA1-20k attenuates Ang II-induced pathological cardiac hypertrophy by regulating gap junction formation and mitochondrial function. Acta Pharmacol. Sin. 2021;42:536–549. doi: 10.1038/s41401-020-0459-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Matsuda S., Umemoto S., Yoshimura K., Itoh S., Murata T., Fukai T., Matsuzaki M. Angiotensin Ⅱ Activates MCP-1 and Induces Cardiac Hypertrophy and Dysfunction via Toll-like Receptor 4. J. Atheroscler. Thromb. 2015;22:833–844. doi: 10.5551/jat.27292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nakajima T., Yokota T., Shingu Y., Yamada A., Iba Y., Ujihira K., Wakasa S., Ooka T., Takada S., Shirakawa R., et al. Impaired mitochondrial oxidative phosphorylation capacity in epicardial adipose tissue is associated with decreased concentration of adiponectin and severity of coronary atherosclerosis. Sci. Rep. 2019;9:3535. doi: 10.1038/s41598-019-40419-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vilne B., Skogsberg J., Foroughi Asl H., Talukdar H.A., Kessler T., Björkegren J.L., Schunkert H. Network analysis reveals a causal role of mitochondrial gene activity in atherosclerotic lesion formation. Arteriosclerosis. 2017;267:39–48. doi: 10.1016/j.atherosclerosis.2017.10.019. [DOI] [PubMed] [Google Scholar]
- 26.Jacinto T.A., Meireles G.S., Dias A.T., Aires R., Porto M.L., Gava A.L., Vasquez E.C., Pereira T.M.C., Campagnaro B.P., Meyrelles S.S. Increased ROS production and DNA damage in monocytes are biomarkers of aging and atherosclerosis. Biol. Res. 2018;51:1–13. doi: 10.1186/s40659-018-0182-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sergin I., Evans T.D., Zhang X., Bhattacharya S., Stokes C.J., Song E., Ali S., Dehestani B., Holloway K.B., Micevych P.S., et al. Exploiting macrophage autophagy-lysosomal biogenesis as a therapy for atherosclerosis. Nat. Commun. 2017;8:15750. doi: 10.1038/ncomms15750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nahapetyan H., Moulis M., Grousset E., Faccini J., Grazide M.-H., Mucher E., Elbaz M., Martinet W., Vindis C. Altered mitochondrial quality control in Atg7-deficient VSMCs promotes enhanced apoptosis and is linked to unstable atherosclerotic plaque phenotype. Cell Death Dis. 2019;10:1–15. doi: 10.1038/s41419-019-1400-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Qiu J., Fu Y., Chen Z., Zhang L., Li L., Liang D., Wei F., Wen Z., Wang Y., Liang S. BTK Promotes Atherosclerosis by Regulating Oxidative Stress, Mitochondrial Injury, and ER Stress of Macrophages. Oxidative Med. Cell. Longev. 2021;2021:1–15. doi: 10.1155/2021/9972413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang Y., Wang G.Z., Rabinovitch P.S., Tabas I. Macrophage mitochondrial oxidative stress promotes atherosclerosis and nuclear factor-κB–mediated inflammation in macrophages. Circ. Res. 2014;114:421–433. doi: 10.1161/CIRCRESAHA.114.302153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liu W., Wang B., Wang T., Liu X., He X., Liu Y., Li Z., Zeng H. Ursodeoxycholic Acid Attenuates Acute Aortic Dissection Formation in Angiotensin II-Infused Apolipoprotein E-Deficient Mice Associated with Reduced ROS and Increased Nrf2 Levels. Cell. Physiol. Biochem. 2016;38:1391–1405. doi: 10.1159/000443082. [DOI] [PubMed] [Google Scholar]
- 32.Xia L., Sun C., Zhu H., Zhai M., Zhang L., Jiang L., Hou P., Li J., Li K., Liu Z., et al. Melatonin protects against thoracic aortic aneurysm and dissection through SIRT1-dependent regulation of oxidative stress and vascular smooth muscle cell loss. J. Pineal Res. 2020;69:e12661. doi: 10.1111/jpi.12661. [DOI] [PubMed] [Google Scholar]
- 33.Shi F., Wang Z., Wu Q., Zhong X., Zhang M., Li B., Ren W., Yuan S., Chen Y. Iron deficiency promotes aortic media degeneration by activating endoplasmic reticulum stress-mediated IRE1 signaling pathway. Pharmacol. Res. 2022;183:106366. doi: 10.1016/j.phrs.2022.106366. [DOI] [PubMed] [Google Scholar]
- 34.Qiu L., Yi S., Yu T., Hao Y. Sirt3 Protects Against Thoracic Aortic Dissection Formation by Reducing Reactive Oxygen Species, Vascular Inflammation, and Apoptosis of Smooth Muscle Cells. Front. Cardiovasc. Med. 2021;8:380. doi: 10.3389/fcvm.2021.675647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tsai S.-H., Hsu L.-A., Tsai H.-Y., Yeh Y.-H., Lu C.-Y., Chen P.-C., Wang J.-C., Chiu Y.-L., Lin C.-Y., Hsu Y.-J. Aldehyde dehydrogenase 2 protects against abdominal aortic aneurysm formation by reducing reactive oxygen species, vascular inflammation, and apoptosis of vascular smooth muscle cells. FASEB J. 2020;34:9498–9511. doi: 10.1096/fj.201902550RRR. [DOI] [PubMed] [Google Scholar]
- 36.Van Der Pluijm I., Burger J., Van Heijningen P.M., Ijpma A., Van Vliet N., Milanese C., Schoonderwoerd K., Sluiter W., Ringuette L.-J., Dekkers D.H.W., et al. Decreased mitochondrial respiration in aneurysmal aortas of Fibulin-4 mutant mice is linked to PGC1A regulation. Cardiovasc. Res. 2018;114:1776–1793. doi: 10.1093/cvr/cvy150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Landis B.J., Lai D., Guo D.-C., Corvera J.S., Idrees M.T., Stadler H.W., Cuevas C., Needler G.U., Vujakovich C.E., Milewicz D.M., et al. Identification of a common polymorphism in COQ8B acting as a modifier of thoracic aortic aneurysm severity. Hum. Genet. Genom. Adv. 2022;3:100057. doi: 10.1016/j.xhgg.2021.100057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Thomas M., Gavrila D., McCormick M.L., Miller F.J., Jr., Daugherty A., Cassis L.A., Dellsperger K.C., Weintraub N.L. Deletion of p47 phox attenuates angiotensin II–induced abdominal aortic aneurysm formation in apolipoprotein E–deficient mice. Circulation. 2006;114:404–413. doi: 10.1161/CIRCULATIONAHA.105.607168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yuan K., Liang W., Zhang J. A comprehensive analysis of differentially expressed genes and pathways in abdominal aortic aneurysm. Mol. Med. Rep. 2015;12:2707–2714. doi: 10.3892/mmr.2015.3709. [DOI] [PubMed] [Google Scholar]
- 40.Guzik B., Sagan A., Ludew D., Mrowiecki W., Chwała M., Bujak-Gizycka B., Filip G., Grudzien G., Kapelak B., Żmudka K., et al. Mechanisms of oxidative stress in human aortic aneurysms—Association with clinical risk factors for atherosclerosis and disease severity. Int. J. Cardiol. 2013;168:2389–2396. doi: 10.1016/j.ijcard.2013.01.278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Li Y., Ren P., Dawson A., Vasquez H.G., Ageedi W., Zhang C., Luo W., Chen R., Li Y., Kim S., et al. Single-Cell Transcriptome Analysis Reveals Dynamic Cell Populations and Differential Gene Expression Patterns in Control and Aneurysmal Human Aortic Tissue. Circulation. 2020;142:1374–1388. doi: 10.1161/CIRCULATIONAHA.120.046528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Abudupataer M., Zhu S., Yan S., Xu K., Zhang J., Luo S., Ma W., Alam F., Tang Y., Huang H., et al. Aorta smooth muscle-on-a-chip reveals impaired mitochondrial dynamics as a therapeutic target for aortic aneurysm in bicuspid aortic valve disease. Elife. 2021;10:69310. doi: 10.7554/eLife.69310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Luo J., Shen S., Xia J., Wang J., Gu Z. Mitochondria as the Essence of Yang Qi in the Human Body. Phenomics. 2022;2:336–348. doi: 10.1007/s43657-022-00060-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhu Z., Wang X. Mitochondrial DNA and Diseases. Volume 1038. Springer; Singapore: 2017. Significance of Mitochondria DNA Mutations in Diseases; pp. 219–230. (Advances in Experimental Medicine and Biology Book Series). [DOI] [PubMed] [Google Scholar]
- 45.Zhu Y., You J., Xu C., Gu X. Associations of mitochondrial DNA 3777–4679 region mutations with maternally inherited essential hypertensive subjects in China. BMC Med. Genet. 2020;21:1–9. doi: 10.1186/s12881-020-01045-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Siva M.A., Mahalakshmi R., Bhakta-Guha D., Guha G. Gene therapy for the mitochondrial genome: Purging mutations, pacifying ailments. Mitochondrion. 2018;46:195–208. doi: 10.1016/j.mito.2018.06.002. [DOI] [PubMed] [Google Scholar]
- 47.DiMauro S., Schon E.A. Mitochondrial Respiratory-Chain Diseases. N. Engl. J. Med. 2003;348:2656–2668. doi: 10.1056/NEJMra022567. [DOI] [PubMed] [Google Scholar]
- 48.Bray A.W., Ballinger S.W. Mitochondrial DNA mutations and cardiovascular disease. Curr. Opin. Cardiol. 2017;32:267–274. doi: 10.1097/HCO.0000000000000383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Friederich M.W., Geddes G.C., Wortmann S.B., Punnoose A., Wartchow E., Knight K.M., Prokisch H., Creadon-Swindell G., Mayr J.A., Van Hove J.L. Pathogenic variants in MRPL44 cause infantile cardiomyopathy due to a mitochondrial translation defect. Mol. Genet. Metab. 2019;133:362–371. doi: 10.1016/j.ymgme.2021.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tang J., Tang Q.-X., Liu S. METTL3-modified lncRNA-SNHG8 binds to PTBP1 to regulate ALAS2 expression to increase oxidative stress and promote myocardial infarction. Mol. Cell. Biochem. 2022 doi: 10.1007/s11010-022-04570-6. [DOI] [PubMed] [Google Scholar]
- 51.Lioncino M., Monda E., Caiazza M., Fusco A., Cirillo A., Dongiglio F., Simonelli V., Sampaolo S., Ruggiero L., Scarano G. Cardiovascular involvement in mtDNA disease: Diagnosis, management, and therapeutic options. Heart Fail. Clin. 2022;18:51–60. doi: 10.1016/j.hfc.2021.07.003. [DOI] [PubMed] [Google Scholar]
- 52.Zhu Y., Gu X., Xu C. Mitochondrial DNA 7908–8816 region mutations in maternally inherited essential hypertensive subjects in China. BMC Med. Genom. 2018;11:1–11. doi: 10.1186/s12920-018-0408-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Markin A., Khotina V., Zabudskaya X., Bogatyreva A., Starodubova A., Ivanova E., Nikiforov N., Orekhov A. Disturbance of Mitochondrial Dynamics and Mitochondrial Therapies in Atherosclerosis. Life. 2021;11:165. doi: 10.3390/life11020165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sobenin I.A., Sazonova M.A., Postnov A.Y., Bobryshev Y.V., Orekhov A.N. Changes of mitochondria in atherosclerosis: Possible determinant in the pathogenesis of the disease. Atherosclerosis. 2013;227:283–288. doi: 10.1016/j.atherosclerosis.2013.01.006. [DOI] [PubMed] [Google Scholar]
- 55.Bravo-San Pedro J.M., Kroemer G., Galluzzi L. Autophagy and Mitophagy in Cardiovascular Disease. Circ. Res. 2017;120:1812–1824. doi: 10.1161/CIRCRESAHA.117.311082. [DOI] [PubMed] [Google Scholar]
- 56.Doblado L., Lueck C., Rey C., Samhan-Arias A.K., Prieto I., Stacchiotti A., Monsalve M. Mitophagy in Human Diseases. Int. J. Mol. Sci. 2021;22:3903. doi: 10.3390/ijms22083903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Poznyak A.V., Nikiforov N.G., Wu W.-K., Kirichenko T.V., Orekhov A.N. Autophagy and Mitophagy as Essential Components of Atherosclerosis. Cells. 2021;10:443. doi: 10.3390/cells10020443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ashrafi G., Schwarz T.L. The pathways of mitophagy for quality control and clearance of mitochondria. Cell Death Differ. 2013;20:31–42. doi: 10.1038/cdd.2012.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Tong M., Saito T., Zhai P., Oka S.-I., Mizushima W., Nakamura M., Ikeda S., Shirakabe A., Sadoshima J. Mitophagy Is Essential for Maintaining Cardiac Function During High Fat Diet-Induced Diabetic Cardiomyopathy. Circ. Res. 2019;124:1360–1371. doi: 10.1161/CIRCRESAHA.118.314607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Li E., Li X., Huang J., Xu C., Liang Q., Ren K., Bai A., Lu C., Qian R., Sun N. BMAL1 regulates mitochondrial fission and mitophagy through mitochondrial protein BNIP3 and is critical in the development of dilated cardiomyopathy. Protein Cell. 2020;11:661–679. doi: 10.1007/s13238-020-00713-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hu J., Liu T., Fu F., Cui Z., Lai Q., Zhang Y., Yu B., Liu F., Kou J., Li F. Omentin1 ameliorates myocardial ischemia-induced heart failure via SIRT3/FOXO3a-dependent mitochondrial dynamical homeostasis and mitophagy. J. Transl. Med. 2022;20:447. doi: 10.1186/s12967-022-03642-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zhou H., Zhu P., Wang J., Zhu H., Ren J., Chen Y. Pathogenesis of cardiac ischemia reperfusion injury is associated with CK2α-disturbed mitochondrial homeostasis via suppression of FUNDC1-related mitophagy. Cell Death Differ. 2018;25:1080–1093. doi: 10.1038/s41418-018-0086-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ghezzi D., Zeviani M. Human diseases associated with defects in assembly of OXPHOS complexes. Essays Biochem. 2018;62:271–286. doi: 10.1042/ebc20170099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Stamerra C.A., Di Giosia P., Giorgini P., Ferri C., Sukhorukov V.N., Sahebkar A. Mitochondrial Dysfunction and Cardiovascular Disease: Pathophysiology and Emerging Therapies. Oxidative Med. Cell. Longev. 2022;2022:1–16. doi: 10.1155/2022/9530007. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 65.Takada S., Maekawa S., Furihata T., Kakutani N., Setoyama D., Ueda K., Nambu H., Hagiwara H., Handa H., Fumoto Y. Succinyl-CoA-based energy metabolism dysfunction in chronic heart failure. Proc. Natl. Acad. Sci. USA. 2022;119:e2203628119. doi: 10.1073/pnas.2203628119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Li G., Shao Y., Guo H.C., Zhi Y., Qiao B., Ma K., Du J., Lai Y.Q., Li Y. MicroRNA-27b-3p down-regulates FGF1 and aggravates pathological cardiac remodelling. Cardiovasc. Res. 2021;118:2139–2151. doi: 10.1093/cvr/cvab248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Chen Q., Vazquez E.J., Moghaddas S., Hoppel C.L., Lesnefsky E.J. Production of reactive oxygen species by mitochondria: Central role of complex III. J. Biol. Chem. 2003;278:36027–36031. doi: 10.1074/jbc.M304854200. [DOI] [PubMed] [Google Scholar]
- 68.Brand M.D. Mitochondrial generation of superoxide and hydrogen peroxide as the source of mitochondrial redox signaling. Free Radic. Biol. Med. 2016;100:14–31. doi: 10.1016/j.freeradbiomed.2016.04.001. [DOI] [PubMed] [Google Scholar]
- 69.Moghadam Z.M., Henneke P., Kolter J. From Flies to Men: ROS and the NADPH Oxidase in Phagocytes. Front. Cell Dev. Biol. 2021;9:628991. doi: 10.3389/fcell.2021.628991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Madamanchi N., Runge M.S. Mitochondrial Dysfunction in Atherosclerosis. Circ. Res. 2007;100:460–473. doi: 10.1161/01.RES.0000258450.44413.96. [DOI] [PubMed] [Google Scholar]
- 71.López-Acosta O., Fortis-Barrera M.D.L.A., Barrios-Maya M.A., Ramírez A.R., Aguilar F.J.A., El-Hafidi M. Reactive Oxygen Species from NADPH Oxidase and Mitochondria Participate in the Proliferation of Aortic Smooth Muscle Cells from a Model of Metabolic Syndrome. Oxidative Med. Cell. Longev. 2018;2018:1–10. doi: 10.1155/2018/5835072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Matsushima S., Kuroda J., Ago T., Zhai P., Park J.Y., Xie L.-H., Tian B., Sadoshima J. Increased Oxidative Stress in the Nucleus Caused by Nox4 Mediates Oxidation of HDAC4 and Cardiac Hypertrophy. Circ. Res. 2013;112:651–663. doi: 10.1161/CIRCRESAHA.112.279760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Bennett M., Yu E., Reinhold Y., Starks L., Uryga A., Foote K., Finigan A., Figg N., Pung Y.-F., Logan A., et al. Mitochondrial respiration is reduced in atherosclerosis, promoting necrotic core formation and reducing relative fibrous cap thickness. Arterioscler. Thromb. Vasc. Biol. 2017;37:2322–2332. doi: 10.17863/cam.22045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Chistiakov D.A., Shkurat T.P., Melnichenko A.A., Grechko A.V., Orekhov A.N. The role of mitochondrial dysfunction in cardiovascular disease: A brief review. Ann. Med. 2018;50:121–127. doi: 10.1080/07853890.2017.1417631. [DOI] [PubMed] [Google Scholar]
- 75.Cheng X.-M., Hu Y.-Y., Yang T., Wu N., Wang X.-N. Reactive Oxygen Species and Oxidative Stress in Vascular-Related Diseases. Oxidative Med. Cell. Longev. 2022;2022:1–11. doi: 10.1155/2022/7906091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Yang X., Jiang J., Li Z., Liang J., Xiang Y. Strategies for mitochondrial gene editing. Comput. Struct. Biotechnol. J. 2021;19:3319–3329. doi: 10.1016/j.csbj.2021.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Zekonyte U., Bacman S.R., Moraes C.T. DNA-editing enzymes as potential treatments for heteroplasmic mtDNA diseases. J. Intern. Med. 2020;287:685–697. doi: 10.1111/joim.13055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Bacman S.R., Williams S.L., Garcia S., Moraes C.T. Organ-specific shifts in mtDNA heteroplasmy following systemic delivery of a mitochondria-targeted restriction endonuclease. Gene Ther. 2010;17:713–720. doi: 10.1038/gt.2010.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Sendra L., García-Mares A., Herrero M.J., Aliño S.F. Mitochondrial DNA Replacement Techniques to Prevent Human Mitochondrial Diseases. Int. J. Mol. Sci. 2021;22:551. doi: 10.3390/ijms22020551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Paz M.V., Cotán D., Garrido-Maraver J., Cordero M.D., Oropesa-Ávila M., de La Mata M., Pavón A.D., de Lavera I., Alcocer-Gómez E., Sánchez-Alcázar J.A. Targeting autophagy and mitophagy for mitochondrial diseases treatment. Expert Opin. Ther. Targets. 2016;20:487–500. doi: 10.1517/14728222.2016.1101068. [DOI] [PubMed] [Google Scholar]
- 81.Tong M., Saito T., Zhai P., Oka S.-I., Mizushima W., Nakamura M., Ikeda S., Shirakabe A., Sadoshima J. Alternative Mitophagy Protects the Heart Against Obesity-Associated Cardiomyopathy. Circ. Res. 2021;129:1105–1121. doi: 10.1161/CIRCRESAHA.121.319377. [DOI] [PubMed] [Google Scholar]
- 82.Luna-Castillo K., Lin S., Muñoz-Valle J., Vizmanos B., López-Quintero A., Márquez-Sandoval F. Functional Food and Bioactive Compounds on the Modulation of the Functionality of HDL-C: A Narrative Review. Nutrients. 2021;13:1165. doi: 10.3390/nu13041165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Yoshino J., Baur J.A., Imai S.-I. NAD+ Intermediates: The Biology and Therapeutic Potential of NMN and NR. Cell Metab. 2018;27:513–528. doi: 10.1016/j.cmet.2017.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Zhang M., Zhao Z., Shen M., Zhang Y., Duan J., Guo Y., Zhang D., Hu J., Lin J., Man W., et al. Polydatin protects cardiomyocytes against myocardial infarction injury by activating Sirt3. Biochim. Et Biophys. Acta Mol. Basis Dis. 2017;1863:1962–1972. doi: 10.1016/j.bbadis.2016.09.003. [DOI] [PubMed] [Google Scholar]
- 85.Liu C., Chen L., Ma Y., Hu K., Wu P., Pan L., Chen H., Li L., Hu H., Zhang J. Pulmonary circulation-mediated heart targeting for the prevention of heart failure by inhalation of intrinsically bioactive nanoparticles. Theranostics. 2021;11:8550–8569. doi: 10.7150/thno.61875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Liew S.S., Qin X., Zhou J., Li L., Huang W., Yao S.Q. Smart design of nanomaterials for mitochondria-targeted nanotherapeutics. Angew. Chem. Int. Ed. 2020;60:2232–2256. doi: 10.1002/anie.201915826. [DOI] [PubMed] [Google Scholar]
- 87.O’Brien K.D., Tian R. Boosting mitochondrial metabolism with dietary supplements in heart failure. Nat. Rev. Cardiol. 2021;18:685–686. doi: 10.1038/s41569-021-00610-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Di Lorenzo A., Iannuzzo G., Parlato A., Cuomo G., Testa C., Coppola M., D’Ambrosio G., Oliviero D.A., Sarullo S., Vitale G., et al. Clinical Evidence for Q10 Coenzyme Supplementation in Heart Failure: From Energetics to Functional Improvement. J. Clin. Med. 2020;9:1266. doi: 10.3390/jcm9051266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Pitceathly R.D.S., Keshavan N., Rahman J., Rahman S. Moving Towards Clinical Trials for Mitochondrial Diseases. J. Inherit. Metab. Dis. 2021;44:22–41. doi: 10.1002/jimd.12281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Martelli A., Testai L., Colletti A., Cicero A.F.G. Coenzyme Q10: Clinical Applications in Cardiovascular Diseases. Antioxidants. 2020;9:341. doi: 10.3390/antiox9040341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Wang W., Wang X. New potentials of mitochondrial DNA editing. Cell Biol. Toxicol. 2020;36:391–393. doi: 10.1007/s10565-020-09549-x. [DOI] [PubMed] [Google Scholar]
- 92.Peeva V., Blei D., Trombly G., Corsi S., Szukszto M.J., Rebelo-Guiomar P., Gammage P.A., Kudin A.P., Becker C., Altmüller J., et al. Linear mitochondrial DNA is rapidly degraded by components of the replication machinery. Nat. Commun. 2018;9:1–11. doi: 10.1038/s41467-018-04131-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Bacman S.R., Williams S.L., Duan N., Moraes C.T. Manipulation of mtDNA heteroplasmy in all striated muscles of newborn mice by AAV9-mediated delivery of a mitochondria-targeted restriction endonuclease. Gene Ther. 2012;19:1101–1106. doi: 10.1038/gt.2011.196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Gammage P.A., Rorbach J., Vincent A.I., Rebar E.J., Minczuk M. Mitochondrially targeted ZFN s for selective degradation of pathogenic mitochondrial genomes bearing large-scale deletions or point mutations. EMBO Mol. Med. 2014;6:458–466. doi: 10.1002/emmm.201303672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Pereira C.V., Bacman S.R., Arguello T., Zekonyte U., Williams S.L., Edgell D.R., Moraes C.T. mitoTev-TALE: A monomeric DNA editing enzyme to reduce mutant mitochondrial DNA levels. EMBO Mol. Med. 2018;10:e8084. doi: 10.15252/emmm.201708084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Bacman S.R., Williams S.L., Pinto M., Peralta S., Moraes C.T. Specific elimination of mutant mitochondrial genomes in patient-derived cells by mitoTALENs. Nat. Med. 2013;19:1111–1113. doi: 10.1038/nm.3261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Wang B., Lv X., Wang Y., Wang Z., Liu Q., Lu B., Liu Y., Gu F. CRISPR/Cas9-mediated mutagenesis at microhomologous regions of human mitochondrial genome. Sci. China Life Sci. 2021;64:1463–1472. doi: 10.1007/s11427-020-1819-8. [DOI] [PubMed] [Google Scholar]
- 98.Bian W.-P., Chen Y.-L., Luo J.-J., Wang C., Xie S.-L., Pei D.-S. Knock-In Strategy for Editing Human and Zebrafish Mitochondrial DNA Using Mito-CRISPR/Cas9 System. ACS Synth. Biol. 2019;8:621–632. doi: 10.1021/acssynbio.8b00411. [DOI] [PubMed] [Google Scholar]
- 99.Hussain S.-R.A., Yalvac M.E., Khoo B., Eckardt S., McLaughlin K.J. Adapting CRISPR/Cas9 System for Targeting Mitochondrial Genome. Front. Genet. 2021;12:627050. doi: 10.3389/fgene.2021.627050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Guo J., Chen X., Liu Z., Sun H., Zhou Y., Dai Y., Ma Y., He L., Qian X., Wang J., et al. DdCBE mediates efficient and inheritable modifications in mouse mitochondrial genome. Mol. Ther. Nucleic Acids. 2022;27:73–80. doi: 10.1016/j.omtn.2021.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Silva-Pinheiro P., Nash P.A., Van Haute L., Mutti C.D., Turner K., Minczuk M. In vivo mitochondrial base editing via adeno-associated viral delivery to mouse post-mitotic tissue. Nat. Commun. 2022;13:1–9. doi: 10.1038/s41467-022-28358-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Mok B.Y., De Moraes M.H., Zeng J., Yeh M.M., Kotrys A.V., Raguram A., Hsu F., Radey M.C., Peterson S.B., Mootha V.K., et al. A bacterial cytidine deaminase toxin enables CRISPR-free mitochondrial base editing. Nature. 2020;583:631–637. doi: 10.1038/s41586-020-2477-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Sharma H., Singh D., Mahant A., Sohal S.K., Kesavan A.K. Samiksha Development of mitochondrial replacement therapy: A review. Heliyon. 2020;6:e04643. doi: 10.1016/j.heliyon.2020.e04643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Greggains G.D., Lister L.M., Tuppen H.A.L., Zhang Q., Needham L.H., Prathalingam N., Hyslop L.A., Craven L., Polanski Z., Murdoch A.P., et al. Therapeutic potential of somatic cell nuclear transfer for degenerative disease caused by mitochondrial DNA mutations. Sci. Rep. 2014;4:3844. doi: 10.1038/srep03844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Hyslop L.A., Blakeley P., Craven L., Richardson J., Fogarty N.M., Fragouli E., Lamb M., Wamaitha S.E., Prathalingam N., Zhang Q., et al. Towards clinical application of pronuclear transfer to prevent mitochondrial DNA disease. Nature. 2016;534:383–386. doi: 10.1038/nature18303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Kang E., Wu J., Gutierrez N.M., Koski A., Tippner-Hedges R., Agaronyan K., Platero-Luengo A., Martinez-Redondo P., Ma H., Lee Y., et al. Mitochondrial replacement in human oocytes carrying pathogenic mitochondrial DNA mutations. Nature. 2016;540:270–275. doi: 10.1038/nature20592. [DOI] [PubMed] [Google Scholar]
- 107.Adashi E.Y., Caplan A.L., Capron A., Chapman A.R., Cho M., Clayton E.W., Cohen I.G., Cook-Deegan R., Faden R.R., Friedmann T., et al. In support of mitochondrial replacement therapy. Nat. Med. 2019;25:870–871. doi: 10.1038/s41591-019-0477-4. [DOI] [PubMed] [Google Scholar]
- 108.Tachibana M., Kuno T., Yaegashi N. Mitochondrial replacement therapy and assisted reproductive technology: A paradigm shift toward treatment of genetic diseases in gametes or in early embryos. Reprod. Med. Biol. 2018;17:421–433. doi: 10.1002/rmb2.12230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Qiu Z., Wei Y., Song Q., Du B., Wang H., Chu Y., Hu Y. The Role of Myocardial Mitochondrial Quality Control in Heart Failure. Front. Pharmacol. 2019;10:1404. doi: 10.3389/fphar.2019.01404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.McWilliams T.G., Muqit M.M. PINK1 and Parkin: Emerging themes in mitochondrial homeostasis. Curr. Opin. Cell Biol. 2017;45:83–91. doi: 10.1016/j.ceb.2017.03.013. [DOI] [PubMed] [Google Scholar]
- 111.Bansal M., Moharir S.C., Swarup G. Autophagy receptor optineurin promotes autophagosome formation by potentiating LC3-II production and phagophore maturation. Commun. Integr. Biol. 2018;11:1–4. doi: 10.1080/19420889.2018.1467189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Lei S., Feng Y., Huang P., Chen B., Bao K., Wu Q., Zhang H., Huang X. Ophiopogonin D’-induced mitophagy and mitochondrial damage are associated with dysregulation of the PINK1/Parkin signaling pathway in AC16 cells. Toxicology. 2022;477:153275. doi: 10.1016/j.tox.2022.153275. [DOI] [PubMed] [Google Scholar]
- 113.Quiles J.M., Gustafsson B. The role of mitochondrial fission in cardiovascular health and disease. Nat. Rev. Cardiol. 2022;19:723–736. doi: 10.1038/s41569-022-00703-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Jin J.-Y., Wei X.-X., Zhi X.-L., Wang X.-H., Meng D. Drp1-dependent mitochondrial fission in cardiovascular disease. Acta Pharmacol. Sin. 2021;42:655–664. doi: 10.1038/s41401-020-00518-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Losón O.C., Song Z., Chen H., Chan D.C. Fis1, Mff, MiD49, and MiD51 mediate Drp1 recruitment in mitochondrial fission. Mol. Biol. Cell. 2013;24:659–667. doi: 10.1091/mbc.e12-10-0721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Cassidy-Stone A., Chipuk J.E., Ingerman E., Song C., Yoo C., Kuwana T., Kurth M.J., Shaw J.T., Hinshaw J.E., Green D.R., et al. Chemical Inhibition of the Mitochondrial Division Dynamin Reveals Its Role in Bax/Bak-Dependent Mitochondrial Outer Membrane Permeabilization. Dev. Cell. 2008;14:193–204. doi: 10.1016/j.devcel.2007.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Aishwarya R., Alam S., Abdullah C.S., Morshed M., Nitu S.S., Panchatcharam M., Miriyala S., Kevil C.G., Bhuiyan S. Pleiotropic effects of mdivi-1 in altering mitochondrial dynamics, respiration, and autophagy in cardiomyocytes. Redox Biol. 2020;36:101660. doi: 10.1016/j.redox.2020.101660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Sun W., Liu C., Chen Q., Liu N., Yan Y., Liu B. SIRT3: A New Regulator of Cardiovascular Diseases. Oxidative Med. Cell. Longev. 2018;2018:1–11. doi: 10.1155/2018/7293861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Zhang J., Xiang H., Liu J., Chen Y., He R.-R., Liu B. Mitochondrial Sirtuin 3: New emerging biological function and therapeutic target. Theranostics. 2020;10:8315–8342. doi: 10.7150/thno.45922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Bagul P.K., Katare P.B., Bugga P., Dinda A.K., Banerjee S.K. SIRT-3 Modulation by Resveratrol Improves Mitochondrial Oxidative Phosphorylation in Diabetic Heart through Deacetylation of TFAM. Cells. 2018;7:235. doi: 10.3390/cells7120235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Lauritzen K.H., Olsen M.B., Ahmed M.S., Yang K., Rinholm J.E., Bergersen L.H., Esbensen Q.Y., Sverkeli L.J., Ziegler M., Attramadal H., et al. Instability in NAD+ metabolism leads to impaired cardiac mitochondrial function and communication. eLife. 2021;10:e59828. doi: 10.7554/eLife.59828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Yang B., Dan X., Hou Y., Lee J., Wechter N., Krishnamurthy S., Kimura R., Babbar M., Demarest T., McDevitt R., et al. NAD + supplementation prevents STING-induced senescence in ataxia telangiectasia by improving mitophagy. Aging Cell. 2021;20:e13329. doi: 10.1111/acel.13329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Zhou B., Wang D.D.-H., Qiu Y., Airhart S., Liu Y., Stempien-Otero A., O’Brien K.D., Tian R. Boosting NAD level suppresses inflammatory activation of PBMCs in heart failure. J. Clin. Investig. 2020;130:6054–6063. doi: 10.1172/JCI138538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Tarantini S., Valcarcel-Ares M.N., Toth P., Yabluchanskiy A., Tucsek Z., Kiss T., Hertelendy P., Kinter M., Ballabh P., Süle Z., et al. Nicotinamide mononucleotide (NMN) supplementation rescues cerebromicrovascular endothelial function and neurovascular coupling responses and improves cognitive function in aged mice. Redox Biol. 2019;24:101192. doi: 10.1016/j.redox.2019.101192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.De Picciotto N.E., Gano L.B., Johnson L.C., Martens C.R., Sindler A.L., Mills K.F., Imai S., Seals D.R. Nicotinamide mononucleotide supplementation reverses vascular dysfunction and oxidative stress with aging in mice. Aging Cell. 2016;15:522–530. doi: 10.1111/acel.12461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Charles C., Cohen-Erez I., Kazaoka B., Melnikov O., Stein D.E., Sensenig R., Rapaport H., Orynbayeva Z. Mitochondrial responses to organelle-specific drug delivering nanoparticles composed of polypeptide and peptide complexes. Nanomedicine. 2020;15:2917–2932. doi: 10.2217/nnm-2020-0266. [DOI] [PubMed] [Google Scholar]
- 127.Zhang Y., Khalique A., Du X., Gao Z., Wu J., Zhang X., Zhang R., Sun Z., Liu Q., Xu Z., et al. Biomimetic Design of Mitochondria-Targeted Hybrid Nanozymes as Superoxide Scavengers. Adv. Mater. 2021;33:e2006570. doi: 10.1002/adma.202006570. [DOI] [PubMed] [Google Scholar]
- 128.Cohen-Erez I., Rapaport H. Negatively charged polypeptide-peptide nanoparticles showing efficient drug delivery to the mitochondria. Colloids Surfaces B: Biointerfaces. 2018;162:186–192. doi: 10.1016/j.colsurfb.2017.11.048. [DOI] [PubMed] [Google Scholar]
- 129.Lee T.-L., Lee M.-H., Chen Y.-C., Lee Y.-C., Lai T.-C., Lin H.Y.-H., Hsu L.-F., Sung H.-C., Lee C.-W., Chen Y.-L. Vitamin D attenuates ischemia/reperfusion-induced cardiac injury by reducing mitochondrial fission and mitophagy. Front. Pharmacol. 2020;11:604700. doi: 10.3389/fphar.2020.604700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Hao T., Li J., Yao F., Dong D., Wang Y., Yang B., Wang C. Injectable Fullerenol/Alginate Hydrogel for Suppression of Oxidative Stress Damage in Brown Adipose-Derived Stem Cells and Cardiac Repair. ACS Nano. 2017;11:5474–5488. doi: 10.1021/acsnano.7b00221. [DOI] [PubMed] [Google Scholar]
- 131.Zhang M., Zhu J., Qin X., Zhou M., Zhang X., Gao Y., Zhang T., Xiao D., Cui W., Cai X. Cardioprotection of Tetrahedral DNA Nanostructures in Myocardial Ischemia-Reperfusion Injury. ACS Appl. Mater. Interfaces. 2019;11:30631–30639. doi: 10.1021/acsami.9b10645. [DOI] [PubMed] [Google Scholar]
- 132.Napolitano G., Fasciolo G., Venditti P. Mitochondrial Management of Reactive Oxygen Species. Antioxidants. 2021;10:1824. doi: 10.3390/antiox10111824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Gutierrez-Mariscal F.M., Larriva A.P.A.-D., Limia-Perez L., Romero-Cabrera J.L., Yubero-Serrano E.M., López-Miranda J. Coenzyme Q10 Supplementation for the Reduction of Oxidative Stress: Clinical Implications in the Treatment of Chronic Diseases. Int. J. Mol. Sci. 2020;21:7870. doi: 10.3390/ijms21217870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Dhanasekaran M., Ren J. The Emerging Role of Coenzyme Q-10 in Aging, Neurodegeneration, Cardiovascular Disease, Cancer and Diabetes Mellitus. Curr. Neurovascular Res. 2005;2:447–459. doi: 10.2174/156720205774962656. [DOI] [PubMed] [Google Scholar]
- 135.Szczepańska E., Białek-Dratwa A., Janota B., Kowalski O. Dietary Therapy in Prevention of Cardiovascular Disease (CVD)—Tradition or Modernity? A Review of the Latest Approaches to Nutrition in CVD. Nutrients. 2022;14:2649. doi: 10.3390/nu14132649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Mortensen A.L., Rosenfeldt F., Filipiak K.J. Effect of coenzyme Q10 in Europeans with chronic heart failure: A sub-group analysis of the Q-SYMBIO randomized double-blind trial. Cardiol. J. 2019;26:147–156. doi: 10.5603/CJ.a2019.0022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Jafari M., Mousavi S.M., Asgharzadeh A., Yazdani N. Coenzyme Q10 in the treatment of heart failure: A systematic review of systematic reviews. Indian Hear. J. 2018;70:S111–S117. doi: 10.1016/j.ihj.2018.01.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Renke G., Pereira M.B., Renke A. Coenzyme Q10 for Diabetes and Cardiovascular Disease: Useful or Useless? Curr Diabetes Rev. 2022 doi: 10.2174/1573399818666220429101336. [DOI] [PubMed] [Google Scholar]
- 139.Gioscia-Ryan R.A., LaRocca T.J., Sindler A.L., Zigler M.C., Murphy M.P., Seals D.R. Mitochondria-targeted antioxidant (MitoQ) ameliorates age-related arterial endothelial dysfunction in mice. J. Physiol. 2014;592:2549–2561. doi: 10.1113/jphysiol.2013.268680. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.