Abstract
Given the widespread significance of vicinal diamine units in organic synthesis, pharmaceuticals and functional materials, as well as in privileged molecular catalysts, an efficient and practical strategy that avoids the use of stoichiometric strong oxidants is highly desirable. We herein report the application of ligand-to-metal charge transfer (LMCT) excitation to 1,2-diazidation reactions from alkenes and TMSN3 via a coordination-LMCT-homolysis process with more abundant and greener iron salt as the catalyst. Such a LMCT-homolysis mode allows the generation of electrophilic azidyl radical intermediate from Fe–N3 complexes poised for subsequent radical addition into carbon–carbon double bond. The generated carbon radical intermediate is further captured by iron-mediated azidyl radical transfer, enabling dual carbon–nitrogen bond formation. This protocol provides a versatile platform to access structurally diverse diazides with high functional group compatibility from readily available alkenes without the need of chemical oxidants.
Subject terms: Photocatalysis, Synthetic chemistry methodology
Alkene diazidations represent a promising strategy for the synthesis of 1,2-diamines. Here, the authors report a protocol that enables alkene diazidation via iron-mediated ligand-to-metal charge transfer, providing a versatile platform to access structurally diverse diazides without using external oxidants.
Introduction
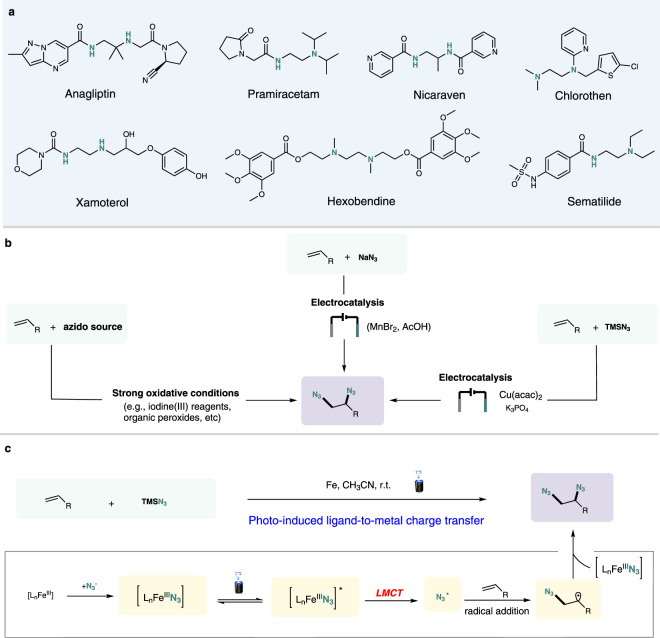
Vicinal diamine units are ubiquitous in nature, and a variety of prevalent natural and synthetic medicines contain diamine functional group structures, which can be easily found in top-selling drugs such as Anagliptin, Pramiracetam, Nicaraven, Hexobendine and so on (Fig. 1a)1,2. The vital importance of diamine structures in chemistry, pharmaceuticals and biology has driven the development of different synthetic pathways to access them from readily available starting materials3–5. Although huge efforts have been made to develop straightforward and efficient alkene diamination strategies, it remains a big challenge to directly incorporate two amino groups into carbon–carbon double bonds, giving rise to 1,2-diamines, particularly free primary 1,2-diamines.
Fig. 1. Representative pharmaceutical compounds and common strategies.
a Representative pharmaceutical compounds containing vicinal diamine moieties. b Prior state of the art of alkenes diazidation reactions. c Photo-induced ligand-to-metal charge transfer enables alkene diazidation. ligand-to-metal charge transfer (LMCT).
1,2-Diazidation reaction of alkenes represents a very promising alternative strategy for synthesis of 1,2-diamine compounds for the reason that the resulting vicinal diazide can be readily reduced to free primary 1,2-diamines6,7. Moreover, organic azides have found notable applications in 1,3-dipolar cycloaddition8, inert C–H bond amination9,10, the aza-Wittig reaction11 and Staudinger ligation12, owing to the unique reactivity. The conventional methods of alkene diazidation require stoichiometric quantities of strong oxidizing reagents13–21 such as hypervalent iodines and organic peroxides (Fig. 1b, left). The use of strong and indiscriminate oxidizing agents is incompatible with many sensitive functional groups, limiting their further application in the modern organic synthesis and pharmaceutically relevant studies. As a mild alternative, electrochemistry has provided an attractive strategy for chemical transformations in recent years owing to the avoidance of stoichiometric oxidant or reductant22–24. In this context, Lin and co-workers reported an electrocatalytic 1,2-diazidiation of alkenes, using MnBr2 as the catalyst under acidic conditions in 2017 (Fig. 1b, middle)25. Later, Lin and colleagues discovered that diazidiation of alkenes can be promoted under metal-free conditions by using an aminoxyl catalyst instead of metal catalyst and acidic conditions26. Very recently, Cu-electrocatalytic alkene diazidiation has been developed by Xu group, and the copper catalyst loading could be reduced to the ppm level in this system (Fig. 1b, right)27.
Developing operationally simple and mechanistically distinct catalytic reactivity modes for alkene diazidation remain highly desirable, which would offer more efficient and environmentally sustainable alternatives to established strategies. From the green chemistry points of perspective28–30, there is a longstanding interest in replacing those harmful metals by more and greener earth-abundant elements. Iron is one of the most abundant and safest metal on Earth, and application of Fe complexes in organic synthesis has attracted considerable attention from chemists31–35. Recent research has unlocked ligand-to-metal charge transfer (LMCT) process of Fe complexes through visible-light irradiation36–38. However, this LMCT excitation mode39 remains underexplored in the field of synthetic organic chemistry, in spite of holding great promise for the development of novel and valuable photo-induced transformations. Inspiration for the design of new catalytic modes originates from the throughout understanding of the fundamental reactivity principles of reactive intermediates. Recent work has demonstrated that the use of radicals generated from redox active precursors offers a convenient pathway to alkyl trifluoromethylation by interception of alkyl radical to CuCF3 complexes40–42. we wondered whether the Fe–N3 complexes were capable of a similar reactivity of trapping a radical intermediate. Based on iron catalysis and basic theories of photocatalysis43, we further envisioned that Fe–N3-based complex generated from readily available Fe salts and azido sources could be easily photoexcited by visible-light irradiation and subsequently could undergo Fe(III)−N3 homolysis to release an azide radical through LMCT process. The generated azide radical will readily add to carbon–carbon bond to provide a carbon radical intermediate, followed by interception of Fe(III)−N3 complex in analogy to recent reports in copper catalysis40–42,44–46(Fig. 1c).
Herein, we develop an effective strategy for alkene diazidation via iron-mediated LMCT mode, which provides a versatile platform to access structurally diverse diazides without external oxidants. This diazidation transformation proceeds under mild conditions and the reaction is characterized by its broad substrate scope, good functional group compatibility and operational simplicity.
Results and discussion
Drawing inspiration from photo-induced vicinal dichlorination of alkenes through LMCT excitation of CuCl247, where homolysis of an excited state CuCl2 could generate chlorine atom radicals, we first established the optimum reaction conditions, starting from the identification of the appropriate metal catalysts as shown in Table 1. We initially examined the application of CuCl2 to diazidation reaction of aliphatic alkene 1a with TMSN3. However, none of the desired diazide product was obtained upon visible-light irradiation from blue LEDs (λmax = 440 nm). Only vicinal dichlorination product was generated. Other copper salts such as CuBr, Cu(OAc)2 and Cu(acac)2 further were investigated, and couldn’t catalyze alkene diazidation reaction at all. We next screened a series of cobalt salts (e.g., CoBr2, Co(acac)2, CoCl2-dppe, Co(salen)Cl) or manganese salts (e.g., Mn(OAc)2, Mn(OTf)2, MnBr2, Mn(CO)5Br), respectively. However, no reaction occurred with these metal salts as the photocatalyst. We further studied iron salts and Fe(NO3)3·9H2O proved to be the ideal catalyst, delivering the desired diazide product 2a in 80% isolated yield using CH3CN as solvent at room temperature under irradiation of a 40 W blue kessil light after 24 h (Table 1, entry 1). The use of other solvents such as CH2Cl2, dioxane completely inhibited this reaction (Table 1, entries 2 and 3) and EtOAc led to inferior reaction yield (Table 1, entry 4). The use of other azido sources such as TsN3 and CF3SO2N3 was useless (Table 1, entries 5 and 6). When 38 W White LEDs or 5 W blue LEDs replaced 40 W Blue kessil lamp as light source, this led to decreased reaction yield (Table 1, entries 7 and 8). Control experiments demonstrated that either Fe catalyst or light was essential for this olefin diazidation reaction (entries 9 and 10).
Table 1.
Optimization of reaction conditionsa
| Entry | Variation from standard conditions | Yieldb |
|---|---|---|
| 1 | None | 84% (80%) |
| 2 | CH2Cl2 instead of MeCN | 0% |
| 3 | dioxane instead of MeCN | 0% |
| 4 | EtOAc instead of MeCN | 53% |
| 5 | TsN3 instead of TMSN3 | 0% |
| 6 | CF3SO2N3 instead of TMSN3 | 0% |
| 7 | 38 W White LEDs instead of 40 W Blue kessil lamp | 44% |
| 8 | 5 W blue LEDs instead of 40 W Blue kessil lamp | 56% |
| 9 | Without Fe catalyst | 0% |
| 10 | Without light | 0% |
LEDs light-emitting diodes.
aStandard reaction conditions: 1a (0.2 mmol, 1.0 equiv.), TMSN3 (1.0 mmol, 5.0 equiv.), Fe(NO3)3·9H2O (0.24 mmol), MeCN (2 mL), Ar atmosphere, r.t., 24 h.
bIsolated yield.
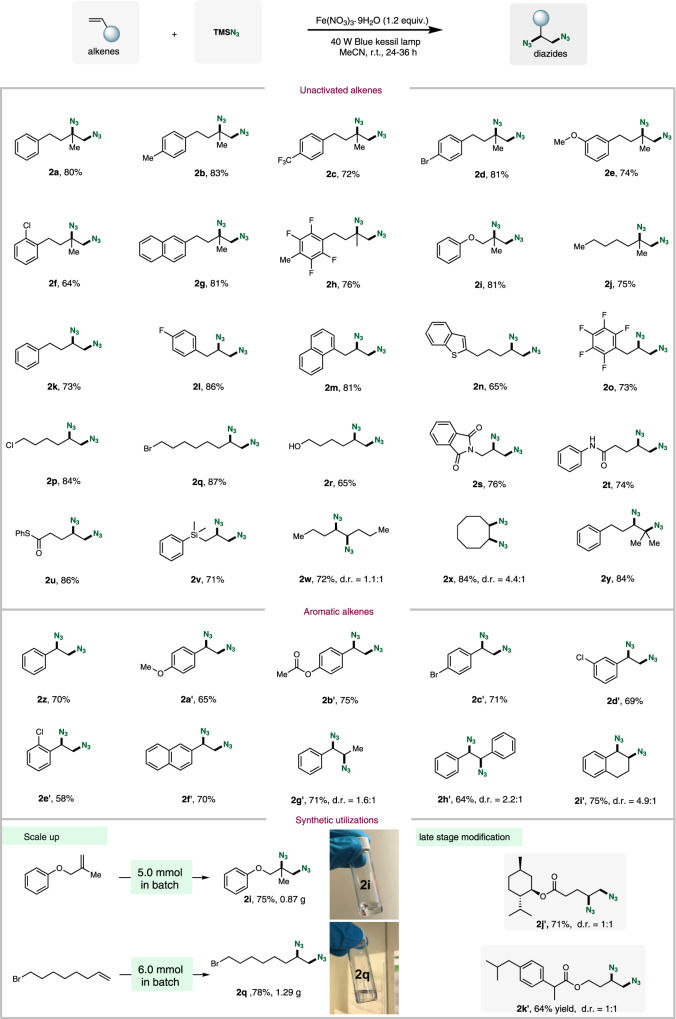
Having established optimized reaction conditions, we set out to explore the scope of alkenes for iron-mediated 1,2-diazidation reaction. A variety of diazide products were effectively synthesized in moderate to high yields from the corresponding alkenes with various functional groups compatible as shown in Fig. 2. First, 1,1-disubstituted type of alkenes were investigated under the optimized reaction conditions. For example, both electron-rich (-CH3, -MeO) and -electron-poor (-CF3) as well as halogen atoms (-Br, -Cl) substituents on the aromatic ring were well tolerated, and the corresponding diazides 2a–f were obtained in good yields. It was found that the substituents of aryl ring on ortho-, meta-, and para-position on the phenyl rings can all work in this transformation. When the aryl ring in 1,1-disubstituted alkenes was replaced by other functional groups (e.g., naphthalene, 2,3,5,6-tetrafluoro-4-methylbenzene, phenol, alkyl group), the diazidation reaction could smoothly occur to provide the desired diazide products 2g–j. Different substitutions (e.g., aromatics, benzothiophene, perfluoroaryl) in α-olefins were well tolerated to give the corresponding diazide products 2k–o in good yields. The diazidation reaction can also tolerate various C(sp3)-bound functional groups, including halogen substituents (2p, 2q), free alcohol (2r), phthalimide (2s), amide (2t), thioester (2u) and silyl group (2v). Internal as well as cyclic alkenes were performed, furnishing the corresponding diazides 2w and 2x with poor diastereomeric ratio. Trisubstituted alkene underwent diazidation reaction to generate the diazide 2y in 84% yield. Various substituted styrenes were also assessed with regarding to this diazidation reaction, smoothly giving rise to the desired products 2z–i’ in good yield. Internal as well as cyclic styrenes smoothly delivered the vicinal diazide products 2g–i’ with low diastereoselectivity.
Fig. 2. Scope of alkenes and synthetic utilization.
Standard conditions: alkenes (0.20 mmol, 1.0 equiv), TMSN3 (1.0 mmol, 5.0 equiv), Fe(NO3)3·9H2O (0.24 mmol, 1.2 equiv) and MeCN (2 mL), blue LEDs, 24–36 h, isolated yield. d.r. diastereomeric ratios.
With a widespread exploration of the scope of alkenes in hand, we shifted our attention to the synthetic potential of this diazidation reaction. A gram-scale reaction was performed, and 2i (5.0 mmol, 0.87 g, 75% yield) and 2q (6.0 mmol, 1.29 g 78% yield) were obtained under the respective standard conditions as shown in Fig. 2. It is worth noting that this reaction was found to be broadly applicable to complex alkene substrates such as L-menthol and ibuprofen to deliver vicinal diazide products 2j’ and 2k’, indicating the suitability of this strategy for late-stage modification.
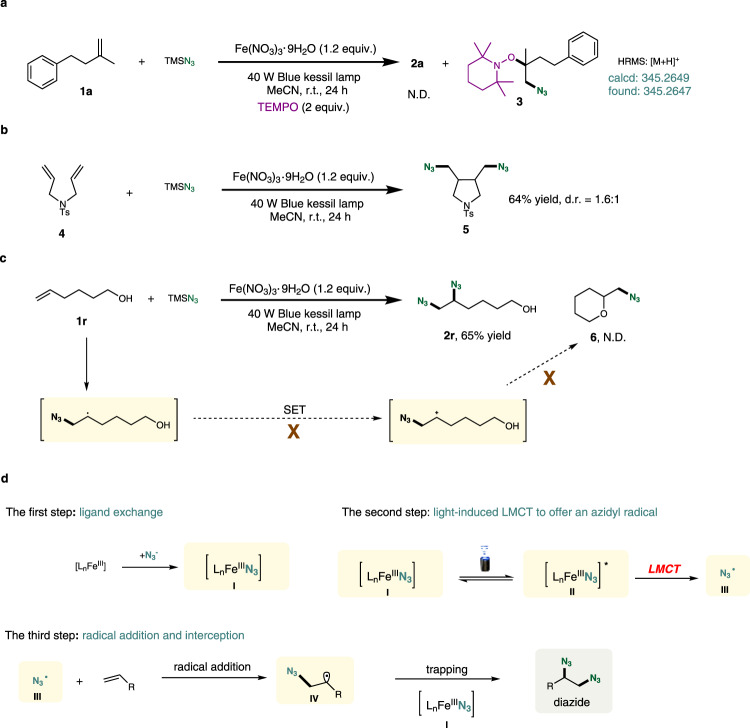
A series of experimental studies were conducted to explore the mechanism of this olefin diazidation reaction (Fig. 3). First, the reaction of 1a with TMSN3 was performed in the presence of a radical scavenger TEMPO (2,2,6,6-tetramethylpiperidine-1-oxyl), under otherwise identical to standard conditions (Fig. 3a). The reaction was completely inhibited without generation of the desired product 2a. Interestingly, the compound 3 was successfully trapped by TEMPO with HRMS analysis, indicating the involvement of radical nature in the reaction process. In a radical clock experiment, N-tosyl diallylamine 4 underwent cyclization upon subjection to the standard condition, delivering radical addition/cyclization cascade product 5 in 64% yield with diastereomeric ratio of 1.6:1 (Fig. 3b). We subsequently conducted diazidation reaction using alkene 1r decorated with free alcohol as the substrate for many times and no product 6 by trapping of a carbocation intermediate was observed by MS analysis, which suggested a radical-polar pathway was impossible. These experimental results further proved the reaction proceeded in a radical pathway.
Fig. 3. Mechanistic investigations and proposed reaction mechanism.
a Radical trapping experiments. b Radical clock experiments. c Investigation of possible intermediates. d Plausible reaction mechanism. N.D. not detected.
Based on the above-mentioned control experiments, a plausible mechanism was presented in Fig. 3d. First, the key group transfer complex I, FeIII–N3, was formed through ligand exchange from Fe salt and TMSN3. The generated FeIII–N3 will become the excited state II by visible-light irradiation, providing an azidyl radical III through LMCT process. The azidyl radical III could undergo radical addition into alkene to furnish carbon radical intermediate IV, followed by interception of I. The iron-mediated azidyl radical transfer finally offered the desired diazides.
In summary, we have developed a mild and practical protocol for alkene diazidation via iron-mediated LMCT mode. The key group transfer agent, FeIII–N3, provides a novel pathway to generate an azidyl radical intermediate through LMCT process without the oxidation conditions. This protocol shows broad alkene scope with high functional group tolerance. Notably, the diazidation reaction represents a nice extension of iron photochemistry into synthetic organic chemistry.
Methods
General procedure for alkene diazidation reaction
A 10 mL Schlenk tube equipped with a magnetic stir bar was charged with Fe(NO3)3·9H2O (0.24 mmol, 100 mg). Then, the tube was evacuated and backfilled with Ar (three times). Alkenes (0.20 mmol, 1.0 equiv.) and TMSN3 (1.0 mmol) in CH3CN (2 mL) were added by syringe under Ar atmosphere. The reaction tube was then sealed and was placed at a distance (app. 5 cm) from a 40 W blue kessil lamp (Fig. S1). The reaction mixture was stirred for 24–36 h at room temperature. After the reaction, the resulting solution was filtered through a cotton plug and washed with EtOAc. The filtrate was removed under reduced pressure and the residue was purified by silica gel column chromatography (ethyl acetate/n-pentane) to afford desired diazides.
Supplementary information
Acknowledgements
Y.S. acknowledges the support from the National Natural Science Foundation of China (Grant No. 61874074) and Science and Technology Project of Shenzhen JCYJ20220531100815034.
Author contributions
M.Z. and J.Z. conceived and designed the project. M.Z. and J.Z. performed and analyzed the experimental data. M.Z., J.Z., Q. L., and Y.S. co-wrote the manuscript.
Peer review
Peer review information
Nature Communications thanks the anonymous reviewers for their contribution to the peer review of this work. Peer reviewer reports are available.
Data availability
The authors declare that all other data supporting the findings of this study are available within the article and Supplementary Information files, and also are available from the corresponding author upon request.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
The online version contains supplementary material available at 10.1038/s41467-022-35344-9.
References
- 1.Lucet D, Le Gall T, Mioskowski C. The chemistry of vicinal diamines. Angew. Chem. Int. Ed. 1998;37:2580–2627. doi: 10.1002/(SICI)1521-3773(19981016)37:19<2580::AID-ANIE2580>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 2.Cardona F, Goti A. Metal-catalysed 1, 2-diamination reactions. Nat. Chem. 2009;1:269–275. doi: 10.1038/nchem.256. [DOI] [PubMed] [Google Scholar]
- 3.Makai S, Falk E, Morandi B. Direct synthesis of unprotected 2-azidoamines from alkenes via an iron-catalyzed difunctionalization reaction. J. Am. Chem. Soc. 2020;142:21548–21555. doi: 10.1021/jacs.0c11025. [DOI] [PubMed] [Google Scholar]
- 4.Muniz K, Barreiro L, Romero RM, Martinez C. Catalytic asymmetric diamination of styrenes. J. Am. Chem. Soc. 2017;139:4354–4357. doi: 10.1021/jacs.7b01443. [DOI] [PubMed] [Google Scholar]
- 5.Zhu Y, Cornwall RG, Du H, Zhao B, Shi Y. Catalytic diamination of olefins via N–N bond activation. Acc. Chem. Res. 2014;47:3665–3678. doi: 10.1021/ar500344t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Minisci F. Free-radical additions to olefins in the presence of redox systems. Acc. Chem. Res. 1975;8:165–171. doi: 10.1021/ar50089a004. [DOI] [Google Scholar]
- 7.Yuan YA, Lu DF, Chen YR, Xu H. Iron‐catalyzed direct diazidation for a broad range of olefins. Angew. Chem. Int. Ed. 2016;55:534–538. doi: 10.1002/anie.201507550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kolb HC, Finn M, Sharpless KB. Click chemistry: diverse chemical function from a few good reactions. Angew. Chem. Int. Ed. 2001;40:2004–2021. doi: 10.1002/1521-3773(20010601)40:11<2004::AID-ANIE2004>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 9.Hennessy ET, Betley TA. Complex N-heterocycle synthesis via iron-catalyzed, direct C–H bond amination. Science. 2013;340:591–595. doi: 10.1126/science.1233701. [DOI] [PubMed] [Google Scholar]
- 10.Jin L-M, Xu P, Xie J, Zhang XP. Enantioselective intermolecular radical C–H amination. J. Am. Chem. Soc. 2020;142:20828–20836. doi: 10.1021/jacs.0c10415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Palacios F, Alonso C, Aparicio D, Rubiales G, Jesús M. The aza-Wittig reaction: an efficient tool for the construction of carbon–nitrogen double bonds. Tetrahedron. 2007;63:523–575. doi: 10.1016/j.tet.2006.09.048. [DOI] [Google Scholar]
- 12.Schilling CI, Jung N, Biskup M, Schepers U, Bräse S. Bioconjugation via azide–Staudinger ligation: an overview. Chem. Soc. Rev. 2011;40:4840–4871. doi: 10.1039/c0cs00123f. [DOI] [PubMed] [Google Scholar]
- 13.Arimoto M, Yamaguchi H, Fujita E, Nagao Y, Ochiai M. Diazidation of allylsilanes with a combination of iodosylbenzene and trimethylsilyl azide, and synthesis of allyl azides. Chem. Pharm. Bull. 1989;37:3221–3224. doi: 10.1248/cpb.37.3221. [DOI] [Google Scholar]
- 14.Fristad WE, Brandvold TA, Peterson JR, Thompson SR. Conversion of alkenes to 1, 2-diazides and 1, 2-diamines. J. Org. Chem. 1985;50:3647–3649. doi: 10.1021/jo00219a049. [DOI] [Google Scholar]
- 15.Fumagalli G, Rabet PT, Boyd S, Greaney MF. Three‐component azidation of styrene‐type double bonds: light‐switchable behavior of a copper photoredox catalyst. Angew. Chem. Int. Ed. 2015;54:11481–11484. doi: 10.1002/anie.201502980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu W, et al. Iron-catalyzed enantioselective radical carboazidation and diazidation of α, β-unsaturated carbonyl compounds. J. Am. Chem. Soc. 2021;143:11856–11863. doi: 10.1021/jacs.1c05881. [DOI] [PubMed] [Google Scholar]
- 17.Lu M-Z, Wang C-Q, Loh T-P. Copper-catalyzed vicinal oxyazidation and diazidation of styrenes under mild conditions: access to alkyl azides. Org. Lett. 2015;17:6110–6113. doi: 10.1021/acs.orglett.5b03130. [DOI] [PubMed] [Google Scholar]
- 18.Shee, M. & Singh, N. P. Chemical versatility of azide radical: journey from a transient species to synthetic accessibility in organic transformations. Chem. Soc. Rev. 51, 2255–2312 (2022). [DOI] [PubMed]
- 19.Xu L, Chen J, Chu L. Solvent-tuned chemoselective carboazidation and diazidation of alkenes via iron catalysis. Org. Chem. Front. 2019;6:512–516. doi: 10.1039/C8QO01142G. [DOI] [Google Scholar]
- 20.Zhou H, et al. Copper-catalyzed ligand-free diazidation of olefins with TMSN3 in CH3CN or in H2O. Org. Lett. 2017;19:6120–6123. doi: 10.1021/acs.orglett.7b02982. [DOI] [PubMed] [Google Scholar]
- 21.Lv D, et al. Iron‐catalyzed radical asymmetric aminoazidation and diazidation of styrenes. Angew. Chem. Int. Ed. 2021;60:12455–12460. doi: 10.1002/anie.202017175. [DOI] [PubMed] [Google Scholar]
- 22.Yan M, Kawamata Y, Baran PS. Synthetic organic electrochemical methods since 2000: on the verge of a renaissance. Chem. Rev. 2017;117:13230–13319. doi: 10.1021/acs.chemrev.7b00397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ma C, et al. Recent advances in organic electrosynthesis employing transition metal complexes as electrocatalysts. Sci. Bull. 2021;66:2412–2429. doi: 10.1016/j.scib.2021.07.011. [DOI] [PubMed] [Google Scholar]
- 24.Savéant J-M. Molecular catalysis of electrochemical reactions. Mechanistic aspects. Chem. Rev. 2008;108:2348–2378. doi: 10.1021/cr068079z. [DOI] [PubMed] [Google Scholar]
- 25.Fu N, Sauer GS, Saha A, Loo A, Lin S. Metal-catalyzed electrochemical diazidation of alkenes. Science. 2017;357:575–579. doi: 10.1126/science.aan6206. [DOI] [PubMed] [Google Scholar]
- 26.Siu JC, Parry JB, Lin S. Aminoxyl-catalyzed electrochemical diazidation of alkenes mediated by a metastable charge-transfer complex. J. Am. Chem. Soc. 2019;141:2825–2831. doi: 10.1021/jacs.8b13192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cai C-Y, Zheng Y-T, Li J-F, Xu H-C. Cu-electrocatalytic diazidation of alkenes at ppm catalyst loading. J. Am. Chem. Soc. 2022;144:11980–11985. doi: 10.1021/jacs.2c05126. [DOI] [PubMed] [Google Scholar]
- 28.Li C-J, Anastas PT. Green chemistry: present and future. Chem. Soc. Rev. 2012;41:1413–1414. doi: 10.1039/c1cs90064a. [DOI] [PubMed] [Google Scholar]
- 29.Li C-J, Trost BM. Green chemistry for chemical synthesis. Proc. Natl Acad. Sci. USA. 2008;105:13197–13202. doi: 10.1073/pnas.0804348105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Han J, et al. Photoinduced manganese-catalysed hydrofluorocarbofunctionalization of alkenes. Nat. Synth. 2022;1:475–486. doi: 10.1038/s44160-022-00074-9. [DOI] [Google Scholar]
- 31.Cheng L, et al. Iron-catalyzed arene C-H hydroxylation. Science. 2021;374:77–81. doi: 10.1126/science.abj0731. [DOI] [PubMed] [Google Scholar]
- 32.Liu L, et al. General method for iron-catalyzed multicomponent radical cascades–cross-couplings. Science. 2021;374:432–439. doi: 10.1126/science.abj6005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ma W, et al. Iron-catalyzed anti-Markovnikov hydroamination and hydroamidation of allylic alcohols. J. Am. Chem. Soc. 2019;141:13506–13515. doi: 10.1021/jacs.9b05221. [DOI] [PubMed] [Google Scholar]
- 34.Wang Q, et al. Iron-catalysed reductive cross-coupling of glycosyl radicals for the stereoselective synthesis of C-glycosides. Nat. Synth. 2022;1:235–244. doi: 10.1038/s44160-022-00024-5. [DOI] [Google Scholar]
- 35.Kang YC, Treacy SM, Rovis T. Iron-catalyzed photoinduced LMCT: A 1 °C–H abstraction enables skeletal rearrangements and C (sp3)–H alkylation. ACS Catal. 2021;11:7442–7449. doi: 10.1021/acscatal.1c02285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kjær KS, et al. Luminescence and reactivity of a charge-transfer excited iron complex with nanosecond lifetime. Science. 2019;363:249–253. doi: 10.1126/science.aau7160. [DOI] [PubMed] [Google Scholar]
- 37.Zhang Q, et al. Iron-catalyzed photoredox functionalization of methane and heavier gaseous alkanes: scope, kinetics, and computational studies. Org. Lett. 2022;24:1901–1906. doi: 10.1021/acs.orglett.2c00224. [DOI] [PubMed] [Google Scholar]
- 38.Wang S, et al. Decarboxylative tandem C-N coupling with nitroarenes via SH2 mechanism. Nat. Commun. 2022;13:2432. doi: 10.1038/s41467-022-30176-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hu A, et al. δ-Selective functionalization of alkanols enabled by visible-light-induced ligand-to-metal charge transfer. J. Am. Chem. Soc. 2018;140:1612–1616. doi: 10.1021/jacs.7b13131. [DOI] [PubMed] [Google Scholar]
- 40.Xiao H, et al. Copper-catalyzed late-stage benzylic C (sp3)–H trifluoromethylation. Chem. 2019;5:940–949. doi: 10.1016/j.chempr.2019.02.006. [DOI] [Google Scholar]
- 41.Guo S, AbuSalim DI, Cook SP. Aqueous benzylic C–H trifluoromethylation for late-stage functionalization. J. Am. Chem. Soc. 2018;140:12378–12382. doi: 10.1021/jacs.8b08547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Xiao H, Shen H, Zhu L, Li C. Copper-catalyzed radical aminotrifluoromethylation of alkenes. J. Am. Chem. Soc. 2019;141:11440–11445. doi: 10.1021/jacs.9b06141. [DOI] [PubMed] [Google Scholar]
- 43.Prier CK, Rankic DA, MacMillan DW. Visible light photoredox catalysis with transition metal complexes: applications in organic synthesis. Chem. Rev. 2013;113:5322–5363. doi: 10.1021/cr300503r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zeng X, et al. Copper-catalyzed decarboxylative difluoromethylation. J. Am. Chem. Soc. 2019;141:11398–11403. doi: 10.1021/jacs.9b05363. [DOI] [PubMed] [Google Scholar]
- 45.Zeng X, et al. Copper-catalyzed deaminative difluoromethylation. Angew. Chem. Int. Ed. 2020;59:16398–16403. doi: 10.1002/anie.202006048. [DOI] [PubMed] [Google Scholar]
- 46.Zeng XJ, et al. Copper-catalyzed, chloroamide-directed benzylic C–H diflfluoromethylation. J. Am. Chem. Soc. 2019;141:19941–19949. doi: 10.1021/jacs.9b11549. [DOI] [PubMed] [Google Scholar]
- 47.Lian P, Long W, Li J, Zheng Y, Wan X. Visible‐light‐induced vicinal dichlorination of alkenes through LMCT excitation of CuCl2. Angew. Chem. Int. Ed. 2020;59:23603–23608. doi: 10.1002/anie.202010801. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The authors declare that all other data supporting the findings of this study are available within the article and Supplementary Information files, and also are available from the corresponding author upon request.