Abstract
Borrelia burgdorferi, the spirochetal bacterium that causes human Lyme disease, encodes numerous lipoproteins which have the capacity to trigger the release of proinflammatory cytokines from a variety of host cell types, and it is generally believed that these cytokines contribute to the disease process in vivo. We previously reported that low-passage-number infectious B. burgdorferi spirochetes express a novel lipidation-independent activity which induces secretion of the proinflammatory cytokine tumor necrosis factor alpha (TNF-α) by the mouse MC/9 mast cell line. Using RNase protection assays, we determined that mast cells exposed in vitro to low-passage-number, but not high-passage-number, B. burgdorferi spirochetes show increased expression of additional mRNAs representing several chemokines, including macrophage-inflammatory protein 1α (MIP-1α), MIP-1β, and TCA3, as well as the proinflammatory cytokine interleukin-6. Furthermore, mast cell TNF-α secretion can be inhibited by the phosphatidylinositol 3-kinase inhibitor wortmannin and also by preincubation with purified mouse immunoglobulin G1 (IgG1) and IgG2a, but not mouse IgG3, and by a mouse Fc gamma receptor II and III (FcγRII/III)-specific rat monoclonal antibody, suggesting the likely involvement of host FcγRIII in B. burgdorferi-mediated signaling. A role for passively adsorbed rabbit or bovine IgG or serum components in B. burgdorferi-mediated FcγR signaling was excluded in control experiments. These studies confirm that low-passage-number B. burgdorferi spirochetes express a novel activity which upregulates the expression of a variety of host cell chemokine and cytokine genes, and they also establish a novel antibody-independent role for FcγRs in transduction of activation signals by bacterial products.
Lyme disease, the most prevalent arthropod-borne disease in the United States, is a chronic inflammatory disorder caused by Borrelia burgdorferi sensu lato spirochetes (9). Early symptoms of infection include fatigue, joint and muscle pain, and, in approximately 60% of cases, characteristic erythema migrans lesions. If the patient is not treated, secondary pathological symptoms may manifest as arthritis, carditis, and neurologic disorders (48).
Numerous in vitro studies have confirmed that B. burgdorferi spirochetes can directly activate a variety of host cell types, eliciting effects which include proliferation, cytokine or chemokine secretion, and adhesion molecule upregulation (14, 15, 29, 30, 32, 34, 43, 44, 61). It is generally believed that these events provoke heightened inflammatory responses and may contribute to the pathological manifestations seen in Lyme disease. Since activity is enriched in lipoprotein-containing subfractions (44) and studies with recombinant B. burgdorferi outer surface lipoproteins (Osps) indicate that lipidation is required (34, 60, 61), this activity appears to be mediated mainly by bacterial lipoproteins, although some investigators have detected activity in nonlipidated recombinant Osps (17). In a previous report (53), we described a novel lipidation-independent activity (LIA), expressed by low-passage-number infectious B. burgdorferi spirochetes, that induces the synthesis and release of the proinflammatory cytokine tumor necrosis factor alpha (TNF-α) from mast cells. This activity can be destroyed by protease treatment and is expressed on the spirochete's surface (53). In addition, the finding that expression of this activity is lost during in vitro passaging suggests that it is probably encoded on a plasmid.
We now demonstrate that mRNAs for additional mediators, including the chemokines macrophage-inflammatory protein 1α (MIP-1α), MIP-1β, and TCA3 and the cytokine interleukin-6 (IL-6), are upregulated in MC/9 mast cells following in vitro exposure to low-passage-number, but not high-passage-number, B. burgdorferi spirochetes. In addition, we show that B. burgdorferi-mediated mast cell TNF-α secretion is sensitive to inhibition by wortmannin, an irreversible phosphatidylinositol (PI) 3-kinase inhibitor, and can be blocked by mouse immunoglobulin G1 (IgG1) and IgG2a, but not IgG3, antibodies and by the mouse Fc gamma receptor II and III (FcγRII/III)-specific rat monoclonal antibody MAb) 2.4G2, indicating the likely involvement of FcγRIII in B. burgdorferi-mediated cytokine production by B. burgdorferi LIA.
MATERIALS AND METHODS
Borrelia strains.
Low-passage-number (B31-LO) and high-passage-number (B31-HI) strains of B. burgdorferi B31 (5) were obtained from E. Hofmeister (Mayo Clinic, Rochester, Minn.). Spirochetes were grown in 6% rabbit serum-supplemented BSK-II medium and prepared as previously described (53). Clones of B31-LO were derived at in vitro passage +5 by outgrowth at 34°C in BSK-II at a limiting dilution in plastic-sealed, 96-well, round-bottomed plates, using an 80% probability-of-clonality Poisson cutoff. B. burgdorferi B31 clone 5.1 was used in many of the experiments because it consistently expresses high levels of B. burgdorferi LIA. Aliquots of B31-LO, B31-HI, and B31 clone 5.1 spirochetes were frozen at −80°C in BSK-II supplemented with 15% glycerol. To obtain spirochetes for experimentation, scrapings from frozen aliquots were inoculated into 4-, 15-, or 50-ml tubes containing complete BSK-II medium and grown at 34°C for 4 to 7 days.
Reagents.
Lipopolysaccharide (LPS) from Escherichia coli and lipoteichoic acids (LTAs) from Bacillus subtilis, Streptococcus mutans, and Streptococcus sanguis were obtained from Sigma (St. Louis, Mo.). Wortmannin was kindly provided by Hattie Gresham (University of New Mexico). (22). Purified mouse IgG1 (KLH/G1-2-2) and IgG2a (KLH/G2a-1-1) MAbs were obtained from Southern Biotechnology (Birmingham, Ala.). Purified IgG3 MAb (Fructosan/J606) and anti-FcγRII/III (CD32/16) MAb 2.4G2 were obtained from PharMingen (La Jolla, Calif.).
TNF induction and bioassay.
Cloned murine MC/9 mast cells (American Type Culture Collection, Manassas, Va.) (49, 50) were grown in complete Dulbecco's modified Eagle medium containing 50% IL-3-containing WEHI-3 supernatant as previously described (53). To test B. burgdorferi populations for induction of TNF-α release, MC/9 mast cells (105/well) were incubated with washed spirochetes at a spirochete:cell ratio of 100:1 in a total volume of 200 μl at 37°C. After 8 h, 100 μl of supernatant was removed and tested for TNF-α activity as previously described (1). Prior to use in assays, spirochetes were washed several times in Hanks' balanced salt solution (Sigma) by centrifugation (10,000 × g, 5 min), resuspended in mast cell medium, vortexed vigorously to reduce clumping, and counted by dark-field microscopy. Mast cells were also incubated with the calcium ionophore ionomycin (1 μg/ml; Sigma) as a positive control. In several repeated control experiments, antibodies bound to spirochetes were removed by incubation overnight at 4°C in pH 5 isotonic buffer (25 mM Tris, 150 mM NaCl) (12). Following neutralization by washing in pH 7.5 buffer, any additional serum proteins, such as C-reactive protein or serum-associated protein, bound to spirochetes were removed by 15 min of incubation in phosphate-buffered saline containing 1 mM EDTA (49). Following several washes in serum-free HL-1 medium (Bio-Whittaker, Walkersville, Md.), spirochetes were incubated with MC/9 cells in HL-1 medium.
RNase protection assay.
Cytokine mRNA expression was detected by using the Riboquant RNase protection assay (BD Pharmingen, San Diego, Calif.). MC/9 cells (3 × 106/well; 3 wells) coincubated with spirochetes (50:1 ratio) for various time periods at 37°C in 24-well plates were harvested, and total RNA was isolated by using a Qiagen RNeasy kit. Sample RNA (7 to 15 μg) was then hybridized to [α-32P]UTP-labeled murine multicytokine or multichemokine RNA probes sets (mCK-3b [TNF-β, leukotriene β {LTβ}, TNF-α, IL-6, gamma interferon {IFN-γ}, IFN-β, transforming growth factor β1 {TGF-β1}, TGF-β2, TGF-β3, migration-inhibitory factor, ribosomal structural protein L32, and glyceraldehyde-3-phosphate dehydrogenase {GAPDH}] and mCK-5 [lymphotactin {Ltn} RANTES, eotaxin, MIP-1β, MIP-1α, MIP-2, IP-10, macrophage chemoattractant protein 1 {mcp-1}, TCA3, L32, and GAPDH]) according to the manufacturer's instructions. Following RNase treatment to destroy single-stranded RNA species, “protected” cytokine RNA probes were separated on 6% denaturing acrylamide gels and visualized by autoradiography. Bands were quantitated by using ImageQuant software, and levels of cytokine and chemokine mRNAs were expressed relative to levels of constitutively expressed L32 mRNA.
Statistical analysis.
All experimental groups were analyzed in triplicate or quadruplicate, and values presented are means and standard errors of the means. Significant differences between groups were determined by using Student's t test, with P values <0.05 being accepted as significant.
RESULTS
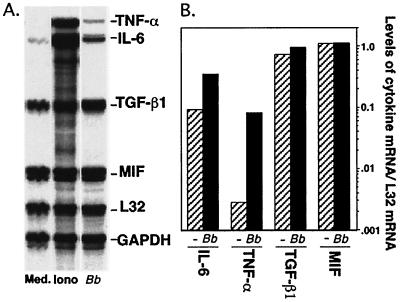
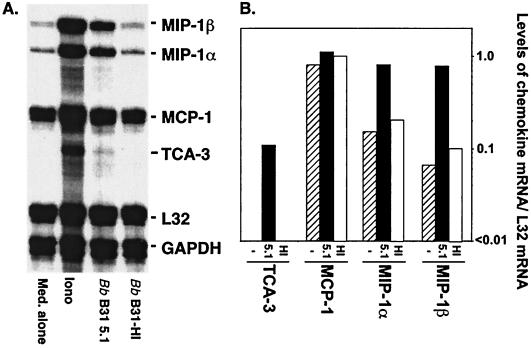
Previous studies demonstrated that low-passage-number B. burgdorferi spirochetes are able to activate MC/9 mast cells to upregulate and/or stabilize message for the proinflammatory cytokine TNF-α at 4 h postchallenge and to secrete bioactive TNF-α at 8 h postchallenge (53). To determine whether B. burgdorferi spirochetes were inducing other genes in MC/9 cells, we employed RNase protection assays to look for upregulation of additional cytokine and chemokine mRNAs. As shown in Fig. 1 and 2, we observed a 10-fold increase in IL-6 mRNA at 4 h and a 10- to 20-fold increase in mRNAs for the chemokines MIP-1α, MIP-1β, and TCA-3 at 1 h following in vitro stimulation with low-passage-number B. burgdorferi spirochetes (B31-LO). In contrast, no increases in chemokine mRNA levels were observed when MC/9 mast cells were stimulated with high-passage-number B. burgdorferi spirochetes (B31-HI), despite the fact that these spirochetes retained the ability to induce comparable levels of spleen cell proliferation to low passage numbers (data not shown), thereby confirming their expression of bioactive lipoproteins. The ability of low-passage- but not high-passage-number B. burgdorferi spirochetes to induce and/or stabilize chemokine MIP-1α, MIP-1β, and TCA-3 mRNAs suggests that induction of these genes is mediated by the same novel LIA previously shown to induce TNF-α (53).
FIG. 1.
Induction of increased IL-6 mRNA expression in MC/9 mast cells exposed in vitro to low-passage-number B. burgdorferi spirochetes. MC/9 cells (3 × 106/24-well plate) were treated with medium alone (Med.), B. burgdorferi B31 clone 5.1 spirochetes (Bb) (100:1 multiplicity), or 1 μM ionomycin (Iono) for 4 h at 37°C. Total RNA was isolated from MC/9 cells by using a Qiagen RNeasy kit, and the presence of cytokine mRNA was determined by using the Riboquant RPA system. Protected cytokine mRNA bands were visualized by autoradiography (A), and bands were quantitated by using ImageQuant software (B). mRNA data presented are relative to mRNA levels for the internal-control L32 housekeeping gene.
FIG. 2.
Low-passage-number but not high-passage-number B. burgdorferi spirochetes induce chemokine MIP-1α, MIP-1β, and TCA-3 mRNAs in MC/9 mast cells MC/9 cells (3 × 106/24-well plate) were treated with medium alone (Med.), 1 μM ionomycin (Iono), low-passage-number B. burgdorferi B31 clone 5.1 spirochetes (Bb B31 5.1), or high-passage-number B31 spirochetes (B31-HI) (both at a multiplicity of 100:1) for 1 h at 37°C. RNA was isolated by using a Qiagen RNeasy kit, and the presence of message was determined by using the Riboquant RPA system. Protected mRNA bands were visualized by autoradiography (A), and chemokine mRNA was quantitated by using ImageQuant software (B). mRNA data presented are relative to mRNA levels for the internal-control L32 housekeeping gene.
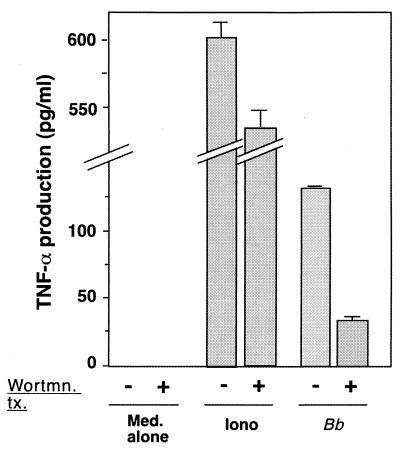
Recent data indicate that B. burgdorferi lipoproteins activate host cell cytokine secretion through interaction with the CD14–Toll-like receptor 2 (TLR2) complex (19, 26). Our failure in the previous study to detect mast TNF-α secretion when cells were incubated with either purified recombinant B. burgdorferi Osp lipoproteins or lipoprotein-expressing high-passage-number B. burgdorferi spirochetes suggests that this CD14- and TLR2-dependent pathway may be missing in MC/9 mast cells, allowing for the detection of the novel activity. Thus far, only a handful of mast cell receptors have been linked to TNF-α secretion. These include the high-affinity Fc epsilon receptor for IgE (FcɛRI) (37, 46), the low-affinity Fc gamma III receptor for IgG (FcγRIII) (25, 59), CD8 (18), CD43 (4), CD48 (31), and the substance P receptor (2). To explore the possible roles of these receptors in TNF-α induction by B. burgdorferi spirochetes, we examined the ability of wortmannin, a specific irreversible inhibitor of PI 3-kinase (38), to affect TNF-α secretion. PI 3-kinase is known to be involved in both FcɛR and FcγR signaling (36, 41). As shown in Fig. 3, wortmannin inhibited B. burgdorferi-mediated TNF-α secretion by MC/9 mast cells. These results indicate that PI 3-kinase is a necessary component in the B. burgdorferi-mediated signaling cascade leading to mast cell TNF-α secretion and suggest the possibility that signaling occurs through either FcɛR or one of the FcγRs.
FIG. 3.
Specific PI 3-kinase inhibitor wortmannin inhibits B. burgdorferi-mediated TNF-α secretion from MC/9 mast cells. Untreated MC/9 cells (−) or MC/9 cells (105/well) pretreated for 15 min with 10 nM wortmannin (Wortmn. tx.) (+) were incubated with either medium alone (Med.), 1 μM ionomycin (Iono), or B. burgdorferi B31 clone 5.1 spirochetes (100:1 multiplicity) (Bb) for 8 h. Supernatants were removed, and TNF-α was measured as described previously (53). The data are the means and standard errors of the means of triplicate determinations. The data presented are from a single experiment representative of three experiments with similar results.
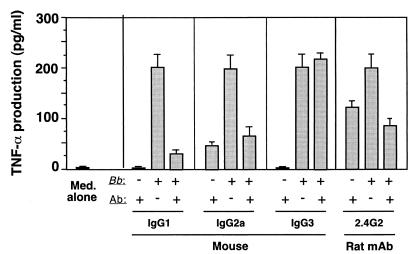
We next examined the possibility that B. burgdorferi was triggering mediator release by interacting with one of the FcγRs. In addition to the high-affinity IgE receptor FcɛRI, murine mast cells also express two low-affinity IgG receptors, FcγRIIb and FcγRIII (6). These two receptors, which have nearly identical binding properties due to a 95% sequence homology in their extracellular domains, are incapable of binding monomeric IgGs but can bind aggregated or antigen-bound mouse IgG1, IgG2a, and IgG2b, but not IgG3 (27). As shown in Fig. 4, prior incubation of MC/9 mast cells with preparations of mouse IgG1 and IgG2a, but not mouse IgG3, antibodies blocked the ability of B. burgdorferi spirochetes to induce TNF-α secretion, presumably by blocking interactions between B. burgdorferi LIA and a low-affinity FcγR. Furthermore, the FcγRII/III-specific MAb 2.4G2 (55) also blocked B. burgdorferi-mediated TNF-α secretion. While these antibody blocking data do not indicate whether FcγRIIb or FcγRIII is the receptor, previous studies have indicated that cross-linking of FcγRIII, but not FcγRIIb, triggers TNF-α production in transfected rat basophilic leukemia (RBL) cells (25), making it more likely that B. burgdorferi spirochetes signal cytokine production by interacting with FcγRIII.
FIG. 4.
Mouse IgG1 and IgG2a antibodies and rat FcγRII/III-specific MAb 2.4G2 block the induction by low-passage-number B. burgdorferi spirochetes of TNF-α secretion in MC/9 mast cells. MC/9 cells (105/well) were (+) or were not (−) treated with 25 μg of murine IgG1, IgG2, or IgG3 antibodies (Ab) or rat MAb 2.4G2/ml for 15 min and then were incubated for 8 h with B. burgdorferi B31 clone 5.1 spirochetes (100:1 multiplicity) or medium (Med.) alone. Supernatants were removed and tested for TNF-α as described previously (53). The data presented are from a single experiment representative of three experiments with similar results. Error bars indicate standard errors of the means.
Few published reports have revealed antibody-independent cellular activation via Fc receptors (49, 50). Thus, it was possible that the mast cell cytokine-inducing activity expressed by B. burgdorferi spirochetes grown in medium supplemented with rabbit serum and assayed in medium containing bovine serum was due to binding of bovine or rabbit IgGs or other serum components. To exclude this possibility, TNF-α induction was assayed under serum-free conditions, using spirochetes that had been treated to remove any contaminating serum components. To remove antibodies, B. burgdorferi spirochetes were incubated overnight at 4°C in Tris-buffered isotonic saline, pH 5 (12). Untreated controls were incubated at pH 7.5. Following washing and neutralization with pH 7.5 isotonic saline, pH 5-treated spirochetes were incubated for 15 min in phosphate-buffered saline containing 1 mM EDTA (49) to remove any bound serum-associated protein or C-reactive protein, which also bind FcγRs (49, 50). However, the ability of spirochetes treated in this way to elicit TNF-α release from MC/9 cells under serum-free conditions was comparable to that of untreated spirochetes (pH 5 treated, 262 ± 35 pg/ml; pH 7.5 treated, 256 ± 22 pg/ml [data not shown]), indicating that a bacterial protein, and not a serum contaminant, is responsible for FcγR-mediated signaling of TNF-α release from mast cells.
DISCUSSION
B. burgdorferi lipoproteins have the ability to activate a variety of host cell types. These activation events have been shown to occur through TLR2 (26) in conjunction with CD14 (19). Prior studies in our lab have shown that B. burgdorferi spirochetes can activate mast cells to secrete TNF-α via a lipidation-independent, protease-sensitive moiety that is expressed on the cell surface (53). We considered it likely that this moiety had other mast cell-activating properties. Furthermore, due to the lipidation-independent character of this B. burgdorferi-associated activity, it was likely that B. burgdorferi LIA acted through a receptor distinct from CD14-TLR2.
In this study, using RNase protection assays, we showed that low-passage-number B. burgdorferi spirochetes have the ability to induce mast cells to upregulate and/or stabilize message for the proinflammatory cytokines TNF-α and IL-6 and the chemokines MIP-1α, MIP-1β, and TCA-3 (Fig. 1 and 2). In contrast, high-passage-number spirochetes (≫50 passages in vitro) did not show increased chemokine or cytokine mRNA expression (data not shown) or induce TNF-α secretion (53). Because high-passage-number spirochetes still possess comparable levels of bioactive outer surface lipoproteins, as evidenced by their ability to induce levels of spleen cell proliferation comparable to those induced by low-passage-number spirochetes (data not shown), these findings strengthen prior assertions that this lipidation-independent activity is biochemically distinct.
Because the probe sets we used detected mRNAs for only nine proinflammatory cytokines and nine chemokines, it is possible that additional cytokine and/or chemokine mRNAs are also upregulated by low-passage-number B. burgdorferi. Nevertheless, these findings establish that exposure to B. burgdorferi LIA results in the activation of multiple proinflammatory cytokine and chemokine genes in MC/9 mast cells. TNF-α has a multitude of effects, ranging from neutrophil chemotaxis to macrophage activation to adhesion molecule upregulation (1). IL-6 has many of the same effects and is also a potent inducer of acute-phase proteins (23). The chemokines MIP-1α and MIP-1β are chemotactic for mononuclear cells and T cells (54). MIP-1α is also chemotactic for mast cells and appears to play a role in differentiation of type 1 T cells (28). TCA3 is chemotactic for neutrophils and macrophages (24).
Since prior studies had indicated that B. burgdorferi spirochetes can bind to host cell integrins, including α5β1 and αvβ3 (11), we initially examined the ability of RGD-containing peptides and anti-β1 and -β3 MAbs to block B. burgdorferi-induced TNF-α production by MC/9 mast cells. However, we observed no inhibition of TNF-α production by any of these reagents (data not shown).
A number of different surface molecules have been implicated in the triggering of TNF-α production by mast cells. These include the high-affinity receptor FcɛRI (37, 46), the low-affinity FcγRIII (25, 59), CD48 (31), CD43 (4), and the substance P receptor (2). The ability of the PI 3-kinase inhibitor wortmannin to block B. burgdorferi-mediated TNF-α secretion (Fig. 3) suggested the possible involvement of Fc receptors since signaling through both FcɛRI (41) and FcγRs (36) is wortmannin sensitive.
Mouse cells express three distinct FcγRs, FcγRI, FcγRII, and FcγRIII, each having different binding affinities for the different mouse IgG subclasses (20). FcγRI is the high-affinity IgG receptor, and it binds monomeric IgG2a with high affinity and IgG1, IgG2b, and IgG3 with low affinity (21). FcγRII and FcγRIII are low-affinity receptors with similar IgG binding characteristics because of the 95% amino acid homology in their extracellular domains. They bind aggregated mouse IgG1, IgG2a, and IgG2b, but not IgG3 (27). Murine mast cells do not express FcγRI, but they do express both FcγRIIb and FcγRIII (6). Our finding that mouse IgG1, mouse IgG2a, and the FcγRII/III-specific rat MAb 2.4G2, but not mouse IgG3, are able to block B. burgdorferi-mediated TNF-α production by MC/9 mast cells (Fig. 4) implicated one of the low-affinity FcγRs in this effect. Since these receptors are believed not to bind monomeric mouse IgG (27), blocking by mouse IgG1 and IgG2a preparations may have been mediated by aggregates.
The known properties of these two FcγRs, however, suggest that it is more likely that FcγRIII has a role in B. burgdorferi-mediated signaling. In contrast to FcγRIIb, FcγRIII acts mainly as a cell-activating receptor (20). Like FcɛRI, FcγRIII is a multisubunit receptor and it shares a common γ chain with FcɛRI (40). The γ chain is thought to be the major signaling molecule since it bears an immunoreceptor tyrosine-based activation motif in its cytoplasmic tail (7, 8). Cross-linking of FcγRIII (or FcɛRI) leads to cell activation which is accompanied by tyrosine phosphorylation (8) and Ca2+ mobilization (10). FcγRIIb, in contrast, appears to function as an inhibitory or regulatory receptor (39). Its single α chain bears an immunoreceptor tyrosine-based inhibitory motif (13). Cross-linking of FcγRIIb does not induce tyrosine phosphorylation or Ca2+ mobilization (56) and is known to downregulate B-cell activation (3). Perhaps most relevant to this study, transfection of FcγRIII, but not FcγRIIb, into nonexpressing rat RBL mast cells rendered these cells capable of TNF-α secretion when cross-linked with the FcγRII/III-specific MAb 2.4G2 (25). Furthermore, the finding that cross-linking of FcγRIIb downregulates PI 3-kinase activity (41) also argues against a role for FcγRIIb in signaling since PI 3-kinase is necessary for B. burgdorferi-induced mast cell TNF-α production by mast cells (Fig. 3).
The nature of the physical association between B. burgdorferi LIA and FcγR resulting in host cell signaling and TNF-α production is unknown. By analogy with antibody-mediated activation (20), it probably requires receptor cross-linking. The fact that activity is destroyed by limited proteolysis of live organisms and remains associated with pelleted bacteria despite intense sonication (53) is consistent with a requirement for arrayed, surface-expressed B. burgdorferi LIA for efficient FcγR cross-linking.
Recent studies have demonstrated that B. burgdorferi lipoproteins activate cells by signaling through CD14-TLR2 receptors (19, 26, 61). It is likely that our ability to efficiently detect B. burgdorferi LIA in MC/9 mast cells is a consequence of the absence of this activation pathway in this cell line. In support of this hypothesis, MC/9 cells fail to make TNF-α when stimulated with several different forms of LTA, which is known to activate cells via the TLR2 pathway (47; J. Talkington and S. P. Nickell, unpublished observations). LPS, which appears to signal through TLR4 (42), also did not induce TNF-α production in MC/9 cells (53), suggesting that the TLR4-dependent activation pathway is also absent in these cells. In vivo studies suggest that LPS may not be a significant inducer of mast cell TNF-α (16). Interestingly, despite the insensitivity of MC/9 mast cells to direct LPS stimulation, such costimulation significantly augmented B. burgdorferi-induced TNF-α production by these cells (212 ± 28 versus 118 ± 16 pg/ml [pooled data from four experiments]). While the mechanism responsible for such LPS augmentation of cytokine production is not known, it has also been observed in bone marrow-derived mast cells activated by FcɛRI cross-linking or c-kit stimulation (33). In contrast, B. burgdorferi-mediated TNF-α production in MC/9 cells was not augmented by LTA.
While this is not the first report of host-pathogen signaling through FcRs (45, 62), to our knowledge it is the first report of direct FcR signaling by a bacterial pathogen. Current views of Lyme disease pathology hypothesize that bioactive bacterial products contribute to tissue damage by provoking the release of proinflammatory cytokines or chemokines and other inflammatory mediators from host cells. Our finding that interaction between FcγRs and B. burgdorferi-associated proteins leads to host cell cytokine production raises the possibility that such signaling contributes to pathological events in vivo. However, considering the very high biological potency of lipoproteins (60), it is likely that lipoprotein-mediated effects significantly outweigh lipidation-independent effects in vivo. This is supported by preliminary studies which found that levels of B. burgdorferi spirochete-induced TNF-α produced in vitro by bone marrow-derived macrophages from either common Fc γ-chain-deficient mice [C57BL/6 (B6)-Fcer1g], which lack expression of FcɛRI, FcγRI, and FcγRIII (51, 58), or FcγRIIb-deficient mice (B6-Fcgr2) (52) were not significantly reduced compared to wild-type FcγR-expressing B6 mice (Talkington and Nickell, unpublished observations), suggesting that lipoprotein-mediated signaling via TLR2 is dominant over lipidation-independent signaling via FcγRs. Additionally, while our data suggest that positive signaling for TNF-α production by B. burgdorferi LIA occurs through FcγRIII, similar interactions between B. burgdorferi LIA and FcγRIIb may also occur, with possible regulatory consequences. It is perhaps of some relevance to the present work that recent studies have demonstrated a role for the Fc receptor common γ chain in the development of inflammation and cartilage damage in a mouse model of experimental antigen-induced arthritis (57). Also, a genetic polymorphism in human FcγRIII has recently been linked to arthritis susceptibility (35). Ultimately, the relevance of B. burgdorferi-FcγR signaling events to outcome will have to be determined empirically. In studies under way, we are addressing whether FcγR signaling can modify B. burgdorferi lipoprotein-mediated cytokine production in vitro and whether the course of B. burgdorferi infection differs in FcγR-deficient and wild-type mice.
ACKNOWLEDGMENTS
This work was supported by dedicated health research funds of The University of New Mexico School of Medicine.
We thank Carolyn Mold and Hattie Gresham for helpful discussions, for comments on the manuscript, and for providing reagents.
REFERENCES
- 1.Aggarwal B B, Kohr W J, Hass P E, Moffat B, Spencer S A, Henzel W J, Bringman T S, Nedwin G E, Goeddel D V, Harkins R N. Human tumor necrosis factor. Production, purification, and characterization. J Biol Chem. 1985;260:2345–2354. [PubMed] [Google Scholar]
- 2.Ansel J C, Brown J R, Payan D G, Brown M A. Substance P selectively activates TNF-alpha gene expression in murine mast cells. J Immunol. 1993;150:4478–4485. [PubMed] [Google Scholar]
- 3.Ashman R F, Peckham D, Stunz L L. Fc receptor off-signal in the B cell involves apoptosis. J Immunol. 1996;157:5–11. [PubMed] [Google Scholar]
- 4.Babina M, Weber S, Mammeri K, Henz B M. Signal transduction via CD43 (leukosialin, sialophorin) and associated biological effects in human mast cell line (HMC-1) Biochem Biophys Res Commun. 1998;243:163–169. doi: 10.1006/bbrc.1998.8083. [DOI] [PubMed] [Google Scholar]
- 5.Barbour A G. Isolation and cultivation of Lyme disease spirochetes. Yale J Biol Med. 1984;57:521–525. [PMC free article] [PubMed] [Google Scholar]
- 6.Benhamou M, Bonnerot C, Fridman W H, Daeron M. Molecular heterogeneity of murine mast cell Fc gamma receptors. J Immunol. 1990;144:3071–3077. [PubMed] [Google Scholar]
- 7.Blank U, Ra C, Miller L, White K, Metzger H, Kinet J P. Complete structure and expression in transfected cells of high affinity IgE receptor. Nature. 1989;337:187–189. doi: 10.1038/337187a0. [DOI] [PubMed] [Google Scholar]
- 8.Bonnerot C, Amigorena S, Choquet D, Pavlovich R, Choukroun V, Fridman W H. Role of associated gamma-chain in tyrosine kinase activation via murine Fc gamma RIII. EMBO J. 1992;11:2747–2757. doi: 10.1002/j.1460-2075.1992.tb05340.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Burgdorfer W, Barbour A G, Hayes S F, Benach J L, Grunwaldt E, Davis J P. Lyme disease—a tick-borne spirochetosis? Science. 1982;216:1317–1319. doi: 10.1126/science.7043737. [DOI] [PubMed] [Google Scholar]
- 10.Cassatella M A, Anegon I, Cuturi M C, Griskey P, Trinchier G, Perussia B. Fc gamma R(CD16) interaction with ligand induces Ca2+ mobilization and phosphoinositide turnover in human natural killer cells. Role of Ca2+ in Fc gamma R(CD16)-induced transcription and expression of lymphokine genes. J Exp Med. 1989;169:549–567. doi: 10.1084/jem.169.2.549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Coburn J, Magoun L, Bodary S C, Leong J M. Integrins αvβ3 and α5β1 mediate attachment of Lyme disease spirochetes to human cells. Infect Immun. 1998;66:1946–1952. doi: 10.1128/iai.66.5.1946-1952.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Coligan J E, Kruisbeek A M, Margulies D H, Shevach E M, Strober W, editors. Current protocols in immunology. New York, N.Y: Greene Publishing Associates and John Wiley & Sons; 1994. pp. 2.7.1–2.7.12. [Google Scholar]
- 13.Daeron M, Latour S, Malbec O, Espinosa E, Pina P, Pasmans S, Fridman W H. The same tyrosine-based inhibition motif, in the intracytoplasmic domain of Fc gamma RIIB, regulates negatively BCR-, TCR-, and FcR-dependent cell activation. Immunity. 1995;3:635–646. doi: 10.1016/1074-7613(95)90134-5. [DOI] [PubMed] [Google Scholar]
- 14.DeFosse D L, Johnson R C. In vitro and in vivo induction of tumor necrosis factor alpha by Borrelia burgdorferi. Infect Immun. 1992;60:1109–1113. doi: 10.1128/iai.60.3.1109-1113.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ebnet K, Brown K D, Siebenlist U K, Simon M M, Shaw S. Borrelia burgdorferi activates nuclear factor-kappa B and is a potent inducer of chemokine and adhesion molecule gene expression in endothelial cells and fibroblasts. J Immunol. 1997;158:3285–3292. [PubMed] [Google Scholar]
- 16.Gatti S, Faggioni R, Sironi M, Erroi A, Ghezzi P. Mast cells do not contribute to the rapid appearance of TNF in the serum of LPS-treated mice: a study with mast cell-deficient mice. Int J Immunopharmacol. 1993;15:551–555. doi: 10.1016/0192-0561(93)90071-6. [DOI] [PubMed] [Google Scholar]
- 17.Haupl T, Landgraf S, Netusil P, Biller N, Capiau C, Desmons P, Hauser P, Burmester G R. Activation of monocytes by three OspA vaccine candidates: lipoprotein OspA is a potent stimulator of monokines. FEMS Immunol Med Microbiol. 1997;19:15–23. doi: 10.1111/j.1574-695X.1997.tb01068.x. [DOI] [PubMed] [Google Scholar]
- 18.Hirji N S, Lin T J, Gilchrist M, Nault G, Nohara O, Grill B J, Belosevic M, Stenton G R, Schreiber A D, Befus A D. Novel CD8 molecule on macrophages and mast cells: expression, function and signaling. Int Arch Allerg Immunol. 1999;118:180–182. doi: 10.1159/000024060. [DOI] [PubMed] [Google Scholar]
- 19.Hirschfeld M, Kirschning C J, Schwandner R, Wesche H, Weis J H, Wooten R M, Weis J J. Cutting edge: inflammatory signaling by Borrelia burgdorferi lipoproteins is mediated by toll-like receptor 2. J Immunol. 1999;163:2382–2386. [PubMed] [Google Scholar]
- 20.Hulett M D, Hogarth P M. Molecular basis of Fc receptor function. Adv Immunol. 1994;57:1–127. doi: 10.1016/s0065-2776(08)60671-9. [DOI] [PubMed] [Google Scholar]
- 21.Hulett M D, Osman N, McKenzie I F, Hogarth P M. Chimeric Fc receptors identify functional domains of the murine high affinity receptor for IgG. J Immunol. 1991;147:1863–1868. [PubMed] [Google Scholar]
- 22.Ishizuka T, Kawasome H, Terada N, Takeda K, Gerwins P, Keller G M, Johnson G I, Gelfand E W. Stem cell factor augments FcɛRI-mediated TNF-α production and stimulates MAP kinases via a different pathway in MC/9 mast cells. J Immunol. 1998;161:3624–3630. [PubMed] [Google Scholar]
- 23.Kopf M, Gros G L, Coyle A J, Kosko-Vilbois M, Brombacher F. Immune responses of IL-4-, IL-5- and IL-6-deficient mice. Immunol Rev. 1995;148:45–69. doi: 10.1111/j.1600-065x.1995.tb00093.x. [DOI] [PubMed] [Google Scholar]
- 24.Laning J, Kawasaki H, Tanaka E, Luo Y, Dorf M E. Inhibition of in vivo tumor growth by the beta chemokine, TCA-3. J Immunol. 1994;153:4625–4635. [PubMed] [Google Scholar]
- 25.Latour S, Bonnerot C, Fridman W H, Daeron M. Induction of tumor necrosis factor-alpha production by mast cells via Fc gamma R. Role of the Fc gamma RIII gamma subunit. J Immunol. 1992;149:2155–2162. [PubMed] [Google Scholar]
- 26.Lien E, Sellati T J, Yoshimura A, Flo T H, Rawadi G, Finberg R W, Carroll J D, Espevik T, Ingalls R R, Radolf J D, Golenbock D T. Toll-like receptor 2 functions as a pattern recognition receptor for diverse bacterial products. J Biol Chem. 1999;274:33419–33425. doi: 10.1074/jbc.274.47.33419. [DOI] [PubMed] [Google Scholar]
- 27.Lopez A F, Battye F L, Vadas M A. Fc receptors on mouse neutrophils and eosinophils: antigenic characteristics, isotype specificity and relative cell membrane density measured by flow cytometry. Immunology. 1985;55:125–133. [PMC free article] [PubMed] [Google Scholar]
- 28.Lukacs N W, Kunkel S L, Streiter R M, Warmington K, Chensue S W. The role of MIP-1α in Schistosoma mansoni egg induced granulomatous inflammation. J Exp Med. 1993;177:1551–1559. doi: 10.1084/jem.177.6.1551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ma Y, Seiler K P, Tai K-F, Yang L, Woods M, Weis J J. Outer surface lipoproteins of Borrelia burgdorferi stimulate nitric oxide production by the cytokine-inducible pathway. Infect Immun. 1994;62:3663–3671. doi: 10.1128/iai.62.9.3663-3671.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ma Y, Weis J J. Borrelia burgdorferi outer surface lipoproteins OspA and OspB possess B-cell mitogenic and cytokine-stimulatory properties. Infect Immun. 1993;61:3843–3853. doi: 10.1128/iai.61.9.3843-3853.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Malaviya R, Gao Z, Thankavel K, van der Merwe P A, Abraham S N. The mast cell tumor necrosis factor alpha response to FimH-expressing Escherichia coli is mediated by the glycosylphosphatidylinositol-anchored molecule CD48. Proc Natl Acad Sci USA. 1999;96:8110–8115. doi: 10.1073/pnas.96.14.8110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Marietta E V, Weis J J, Weis J H. CD28 expression by mouse mast cells is modulated by lipopolysaccharide and outer surface protein A lipoprotein from Borrelia burgdorferi. J Immunol. 1997;159:2840–2848. [PubMed] [Google Scholar]
- 33.Moon T C, Murakami M, Ashraf M D, Kudo I, Chang H W. Regulation of cyclooxygenase-2 and endogenous cytokine expression by bacterial lipopolysaccharide that acts in synergy with c-kit ligand and Fcɛ receptor I crosslinking in cultured mast cells. Cell Immunol. 1998;185:146–152. doi: 10.1006/cimm.1998.1284. [DOI] [PubMed] [Google Scholar]
- 34.Morrison T B, Weis J H, Weis J J. Borrelia burgdorferi outer surface protein A (OspA) activates and primes human neutrophils. J Immunol. 1997;158:4838–4845. [PubMed] [Google Scholar]
- 35.Nieto A, Caliz R, Pascual M, Matraran L, Garcia S, Martin J. Involvement of Fcγ receptor IIIA genotypes in susceptibility to rheumatoid arthritis. Arthritis Rheum. 2000;43:735–739. doi: 10.1002/1529-0131(200004)43:4<735::AID-ANR3>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 36.Ninomiya N, Hazeki K, Fukui Y, Seya T, Okada T, Hazeki O, Ui M. Involvement of phosphatidylinositol 3-kinase in Fc gamma receptor signaling. J Biol Chem. 1994;269:22732–22737. [PubMed] [Google Scholar]
- 37.Ohno I, Tanno Y, Yamauchi K, Takishima T. Gene expression and production of tumour necrosis factor by a rat basophilic leukaemia cell line (RBL-2H3) with IgE receptor triggering. Immunology. 1990;70:88–93. [PMC free article] [PubMed] [Google Scholar]
- 38.Okada T, Kawano Y, Sakakibara T, Hazeki O, Ui M. Essential role of phosphatidylinositol 3-kinase in insulin-induced glucose transport and antilipolysis in rat adipocytes. Studies with a selective inhibitor wortmannin. J Biol Chem. 1994;269:3568–3573. [PubMed] [Google Scholar]
- 39.Ono M, Bolland S, Tempst P, Ravetch J. Role of the inositol phosphatase SHIP in negative regulation of the immune system by the receptor FcγRIIb. Nature. 1996;383:263–266. doi: 10.1038/383263a0. [DOI] [PubMed] [Google Scholar]
- 40.Orloff D G, Ra C S, Frank S J, Klausner R D, Kinet J P. Family of disulphide-linked dimers containing the zeta and eta chains of the T-cell receptor and the gamma chain of Fc receptors. Nature. 1990;347:189–191. doi: 10.1038/347189a0. [DOI] [PubMed] [Google Scholar]
- 41.Pelletier C, Guerin-Marchand C, Iannascoli B, Marchand F, David B, Weyer A, Blank U. Specific signaling pathways in the regulation of TNF-alpha mRNA synthesis and TNF-alpha secretion in RBL-2H3 mast cells stimulated through the high affinity IgE receptor. Inflamm Res. 1998;47:493–500. doi: 10.1007/s000110050364. [DOI] [PubMed] [Google Scholar]
- 42.Poltorak A, He X, Smirnova I, Liu M Y, Huffel C V, Du X, Birdwell D, Alejos E, Silva M, Galanos C, Freudenberg M, Ricciardi-Castagnoli P, Layton B, Beutler B. Defective LPS signaling in C3H/HeJ and C57BL/10ScCr mice: mutations in Tlr4 gene. Science. 1998;282:2085–2088. doi: 10.1126/science.282.5396.2085. [DOI] [PubMed] [Google Scholar]
- 43.Radolf J D, Arndt L L, Atkins D R, Curety L L, Levi M E, Shen Y, Davis L S, Norgard M V. Treponema pallidum and Borrelia burgdorferi lipoproteins and synthetic lipopeptides activate monocytes/macrophages. J Immunol. 1995;154:2866–2877. [PubMed] [Google Scholar]
- 44.Radolf J D, Norgard M V, Brandt M E, Isaacs R D, Thompson P A, Beutler B. Lipoproteins of Borrelia burgdorferi and Treponema pallidum activate cachetin/tumor necrosis factor synthesis. J Immunol. 1991;147:1968–1974. [PubMed] [Google Scholar]
- 45.Ravane K, Castelle C, Defrance T, Wild T F, Charron D, Lotteau V, Rabourdin-Combe C. Measles virus nucleocapsid protein binds to FcγRII and inhibits human B cell antibody production. J Exp Med. 1997;186:269–278. doi: 10.1084/jem.186.2.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Richards A L, Okuno T, Takagaki Y, Djeu J Y. Natural cytotoxic cell-specific cytotoxic factor produced by IL-3-dependent basophilic/mast cells. Relationship to TNF. J Immunol. 1988;141:3061–3066. [PubMed] [Google Scholar]
- 47.Schwandner R, Dzarski R, Wesche H, Rothe M, Kirschning C J. Peptidoglycan- and lipoteichoic acid-induced cell activation is mediated by Toll-like receptor 2. J Biol Chem. 1999;274:17406–17409. doi: 10.1074/jbc.274.25.17406. [DOI] [PubMed] [Google Scholar]
- 48.Steere A C, Schoen R T, Taylor E. The clinical evolution of Lyme arthritis. Ann Intern Med. 1987;107:725–731. doi: 10.7326/0003-4819-107-5-725. [DOI] [PubMed] [Google Scholar]
- 49.Stein M P, Edberg J C, Kimberly R P, Mangan E K, Bharadwaj D, Mold C, Clos T W D. C-reactive protein binding to FcγRIIa on human monocytes and neutrophils is allele-specific. J Clin Investig. 2000;105:369–376. doi: 10.1172/JCI7817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Stein M P, Mold C, Clos T W D. C-reactive protein binding to murine leukocytes requires Fc gamma receptors. J Immunol. 2000;164:1514–1520. doi: 10.4049/jimmunol.164.3.1514. [DOI] [PubMed] [Google Scholar]
- 51.Takai T, Li M, Sylvestre D, Clynes R, Ravetch J. FcR γ chain deletion results in pleiotrophic effector cell defects. Cell. 1994;76:519–529. doi: 10.1016/0092-8674(94)90115-5. [DOI] [PubMed] [Google Scholar]
- 52.Takai T, Ono M, Hikida M, Ohmori H, Ravetch J. Augmented humoral and anaphylactic responses in FcgRII-deficient mice. Nature. 1996;379:346–349. doi: 10.1038/379346a0. [DOI] [PubMed] [Google Scholar]
- 53.Talkington J, Nickell S P. Borrelia burgdorferi spirochetes induce mast cell activation and cytokine release. Infect Immun. 1999;67:1107–1115. doi: 10.1128/iai.67.3.1107-1115.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Taub D D, Conlon K, Lloyd A L, Oppenheim J J, Kelvin D J. Preferential migration of activated CD4+ and CD8+ T cells in response to MIP-1 alpha and MIP-1 beta. Science. 1993;260:355–358. doi: 10.1126/science.7682337. [DOI] [PubMed] [Google Scholar]
- 55.Unkeless J C, Kaplan G, Plutner H, Cohn Z A. Fc receptor variants of a macrophage cell line. Proc Natl Acad Sci USA. 1979;76:1400–1404. doi: 10.1073/pnas.76.3.1400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.van den Herik-Oudijk I E, Westerdaal N A, Henriquez N V, Capel P J, van de Winkel J G. Functional analysis of human Fc gamma RII (CD32) isoforms expressed in B lymphocytes. J Immunol. 1994;152:574–585. [PubMed] [Google Scholar]
- 57.van Lent P L, van Vuuren A J, Blom A B, Holthuysen A E, van de Putte L B, van de Winkel J G, van den Berg W B. Role of Fc receptor γ chain in inflammation and cartilage damage during experimental antigen-induced arthritis. Arthritis Rheum. 2000;43:740–752. doi: 10.1002/1529-0131(200004)43:4<740::AID-ANR4>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 58.van Vugt M J, Heijnen A F, Capel P J, Park S Y, Ra C, Saito T, Verbeek J S, van de Winkel J G. FcR gamma-chain is essential for both surface expression and function of human Fc gamma RI (CD64) in vivo. Blood. 1996;87:3593–3599. [PubMed] [Google Scholar]
- 59.Watanabe N, Akikusa B, Park S Y, Ohno H, Fossati L, Vecchietti G, Gessner J E, Schmidt R E, Verbeek J S, Ryffel B, Iwamoto I, Izui S, Saito T. Mast cells induce autoantibody-mediated vasculitis syndrome through tumor necrosis factor production upon triggering Fcγ receptors. Blood. 1999;94:3855–3863. [PubMed] [Google Scholar]
- 60.Weis J J, Ma Y, Erdile L F. Biological activities of native and recombinant Borrelia burgdorferi outer surface protein A: dependence on lipid modification. Infect Immun. 1994;62:4632–4636. doi: 10.1128/iai.62.10.4632-4636.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wooten R M, Modur V R, McIntyre T M, Weis J J. Borrelia burgdorferi outer membrane protein A induces nuclear translocation of nuclear factor-κB and inflammatory activation in human endothelial cells. J Immunol. 1996;157:4584–4590. [PubMed] [Google Scholar]
- 62.Yang Z, Delgado R, Xu L, Todd R F, Nabel E G, Sanchez A, Nabel G J. Distinct cellular interactions of secreted and transmembrane Ebola virus glycoproteins. Science. 1998;279:1034–1037. doi: 10.1126/science.279.5353.1034. [DOI] [PubMed] [Google Scholar]