Abstract
Humans with salt-sensitive hypertension demonstrate increased morbidity, increased mortality, and renal end-organ damage when compared to normotensive subjects or those with salt-resistant hypertension. Substantial evidence from humans and animals has also demonstrated the role of dietary components other than salt to modulate hypertension. Evidence presented in this review provides support for the view that immunity and inflammation serve to amplify the development of salt-sensitive hypertension and leads to malignant disease accompanied by end-organ damage. Interestingly, salt-sensitive disease is modulated by changes in dietary protein intake, which also influences immune mechanisms. Together, the evidence presented in this review from animal and human studies indicates that changes in dietary protein source have profound effects on the gut microbiota, microbiota-derived metabolites, DNA methylation, gene expression, immune cell activation, the production of cytokines and other factors, and the development of salt-sensitive hypertension and related disease phenotypes.
Keywords: hypertension, kidney, immune cells, microbiota
Introduction
Cardiovascular diseases were listed as the most common cause of mortality in the US in 2019, accounting for over 870,000 deaths1. High blood pressure, or hypertension is a major risk factor for coronary heart disease, heart failure, and stroke; and has been reported to be the largest individual contributing factor to disease and mortality worldwide1. A particularly damaging form of hypertension is salt-sensitive hypertension, in which blood pressure changes in parallel with sodium intake2. Salt-sensitive hypertensives demonstrate increased end-organ damage with particularly high morbidity and mortality3,4. Of interest, salt-sensitive hypertensive subjects demonstrate ventricular hypertrophy, renal damage, and stroke1, but the cause of salt-sensitive hypertension remains unclear. Potential modifiers of salt-sensitive hypertension and associated end-organ damage are mechanisms related to the activation of inflammation and immunity. Multiple reviews have described studies performed over the past five decades that implicate the immune system in hypertension, vascular disease, and renal disease5,6,7,8,9. The present review will focus upon observations in experimental animals and humans demonstrating the role of environmental factors, particularly dietary protein, which modulates inflammation and immunity with resulting alterations in the development of hypertension and related end-organ damage.
Dietary Consumption of Protein and Other Macronutrients in Immunity and Hypertension
Epidemiological studies correlated the consumption of diets with elevated sodium chloride (salt), carbohydrate, saturated fat, cholesterol, and fiber with blood pressure10,11,12,13. The effects of elevated protein diets are somewhat controversial, with evidence supporting protein-induced decreases12,14 and increases15 in blood pressure. In humans with preexisting renal insufficiency, however, it is clear that high protein diets accelerate a decline in renal function16,17, though it is unclear if diets with reduced protein improve outcomes related to chronic kidney disease18. The source of dietary protein is also important: vegetarians have lower blood pressure than omnivores19 while vegetable protein intake inversely correlates with blood pressure20. The Optimal Macronutrient Intake Trial to Prevent Heart Disease (OmniHeart) and the interventional Dietary Approaches to Stop Hypertension (DASH) trials also demonstrated the blood pressure benefit of diets rich in plant protein21,22. Of interest, the DASH-Sodium trial assessed the impact of the reduction of sodium intake on a control diet and demonstrated that hypertensive individuals, African Americans, and those over 45 years of age demonstrated an enhanced blood pressure sensitivity to sodium intake23. Moreover, the generic “animal versus plant” distinction is an oversimplification with the concomitant contribution of other environmental factors including the consumption of nonprotein dietary components24 as well as genetic influences25.
Blood pressure is modulated by the amount of dietary fat26,27, carbohydrate27,28, and protein29 in experimental hypertension. Investigations in our lab indicated that Dahl Salt Sensitive (SS) rats fed a purified, animal-based diet developed a greater degree of salt-sensitive hypertension compared to genetically-identical Dahl SS fed a grain-based diet30,35. Subsequent studies attributed the effect of the animal versus grain diet to the difference in the source of dietary protein (casein versus wheat gluten)31 and revealed the contribution of the immune system32,33,34. Data in humans and animals provide compelling evidence that dietary protein is an important determinant of salt-sensitive hypertension and renal damage.
Dietary Protein and Immune Mechanisms
An approach to modulate inflammatory disease is through dietary modification. A randomized interventional study compared the relationship between dietary protein intake and inflammation. The Metabolic Syndrome Reduction in Navarra (RESMENA) study revealed a direct relationship between dietary protein intake and inflammation. Interestingly, the relationship was specific for increases in animal/meat protein but not for increases in fish or vegetable protein intake36. Additional evidence links increased dietary protein from animal sources to an enhanced incidence of inflammatory bowel disease37, while increased protein in the diet, particularly from red- and processed meats, increases potential relapse of ulcerative colitis38. While increased dietary protein enhances gut inflammation, less is known regarding the potential influence on other organ systems. The link between dietary protein and inflammation holds great interest since immune mechanisms are implicated in the pathology of salt-sensitive hypertension and associated renal end-organ damage.
Immunity in Salt-Sensitive Hypertension
Immune mechanisms have been demonstrated in multiple experimental models of hypertension6,7,8,9, including the Dahl SS rat6,7. Much like salt-sensitive humans, the Dahl SS develops hypertension and end-organ damage when fed high salt6,7. Accompanying the hypertension, Dahl SS also develop renal histological damage and albuminuria6,7. The renal damage parallels the albuminuria observed in salt-sensitive hypertensive humans when compared to salt-resistant subjects6,7.
The damaged kidneys of hypertensive Dahl SS rats after high salt feeding contain increased macrophages, T cells, and B cells when compared to the kidneys of Dahl SS fed low salt6,7. In contrast, infiltration of immune cells is not observed in the kidneys of age-matched, control rats that do not develop hypertension or renal damage when fed high salt. The infiltrating cells in Dahl SS and other rat models of hypertension surround glomeruli, vessels, and tubules in the kidney6,7,39,40, indicating that they may play a role in vascular and tubular changes as well as the development of kidney damage. Supporting these observations, the degree of salt-sensitive hypertension and kidney damage in other rodent models of disease also correlates with immune cell infiltration in the kidney5,6,8,9. Moreover, the localization of immune cells in the renal interstitial spaces adjacent to damaged tubules, vessels, and glomeruli of the Dahl SS is similar to that in hypertensive subjects5. To examine the functional role of immune cells in hypertension, it was shown that treatment with immunosuppressive agents prevented infiltration of T cells in the kidney and attenuated salt-sensitive hypertension and renal damage in Dahl SS rats 6,7,32. Protective effects of immunosuppressive treatment were observed in multiple other models of hypertension in animals5,6,8,9.
Genetic editing strategies in Dahl SS rats targeted individual immune cell types to examine their importance in salt-sensitive hypertension6,7. Null mutation of Rag1 significantly reduced T and B cells in the Dahl SS genetic background (SSRag1−/−) and significantly blunted salt-sensitive hypertension and renal damage compared to Dahl SS controls with intact lymphocytes6,7. Subsequent studies generated Dahl SS with a selective deletion of T cells by inducing a null mutation in CD247 (SSCD247−/−), a gene encoding the CD3 zeta chain6,7. Similar to the SSRag1−/−, the severity of hypertension and renal damage was attenuated in the SSCD247-/−. To confirm the functional role of T cells in salt-sensitive hypertension, reconstitution of T cells by splenocyte transfer to SSCD247−/− restored high salt blood pressure, albuminuria, and renal histological damage to levels similar to that observed in wild type Dahl SS rats fed high salt. These observations are in agreement with reports demonstrating the critical role of T cells for the full development of angiotensin II-mediated hypertension in mice42. Of note, the role of lymphocytes in hypertension has not been universally observed in all laboratories43. Divergent effects of angiotensin II in Rag1−/− mice have been reported from different labs43,44. An explanation for the discrepancy noted in experimental results is unclear but may be due to the influence of environmental effects as described below. With some exceptions, the above-discussed data indicate that adaptive immune mechanisms, mediated by T cells, amplify salt-sensitive hypertension and kidney damage6,7,32.
Mechanisms of Immune Activation in Salt-Sensitive Hypertension
The mechanisms activating immunity and inflammation in the kidney in salt-sensitive hypertension and the processes whereby immune cells mediate changes in function are the subject of active investigation. Observations in Dahl SS rats deficient in T cells6,7 demonstrate an initial, immune-independent phase of salt-sensitive hypertension and kidney damage. We speculate that this initial phase, occurring in response to elevated salt intake, is mediated by numerous mechanisms including aberrant regulation of hormonal, neural, paracrine, or autocrine mechanisms which increase renal vascular resistance or enhance renal tubular sodium reabsorption6. The increase in blood pressure leads to elevated renal perfusion pressure that is transmitted to the kidney, an effect that is pronounced when renal vascular autoregulatory mechanisms are impaired as observed in salt-sensitive hypertension45.
We hypothesize (Figure 1)6,7, based upon data from Dahl SS and angiotensin II-hypertensive rats51,52,53, that an initial increase in blood pressure is transmitted to the renal vasculature and mediates the infiltration of immune cells into the kidney. The mechanism is unclear, but it is possible that the elevated perfusion pressure results in tissue damage and triggers the migration of innate and adaptive immune cells into the kidney. The infiltrating immune cells release cytokines, free radicals and/or other molecules that amplify the development of hypertension by increasing epithelial sodium reabsorption, constricting the vasculature, and directly mediating further tissue damage6,7. As previously described, other initiating mechanisms may include increased sympathetic nerve activity, antigens and neoantigens and environmental modifiers of immune cell activation6,7,33,46,47,48,49,50.
FIGURE 1:
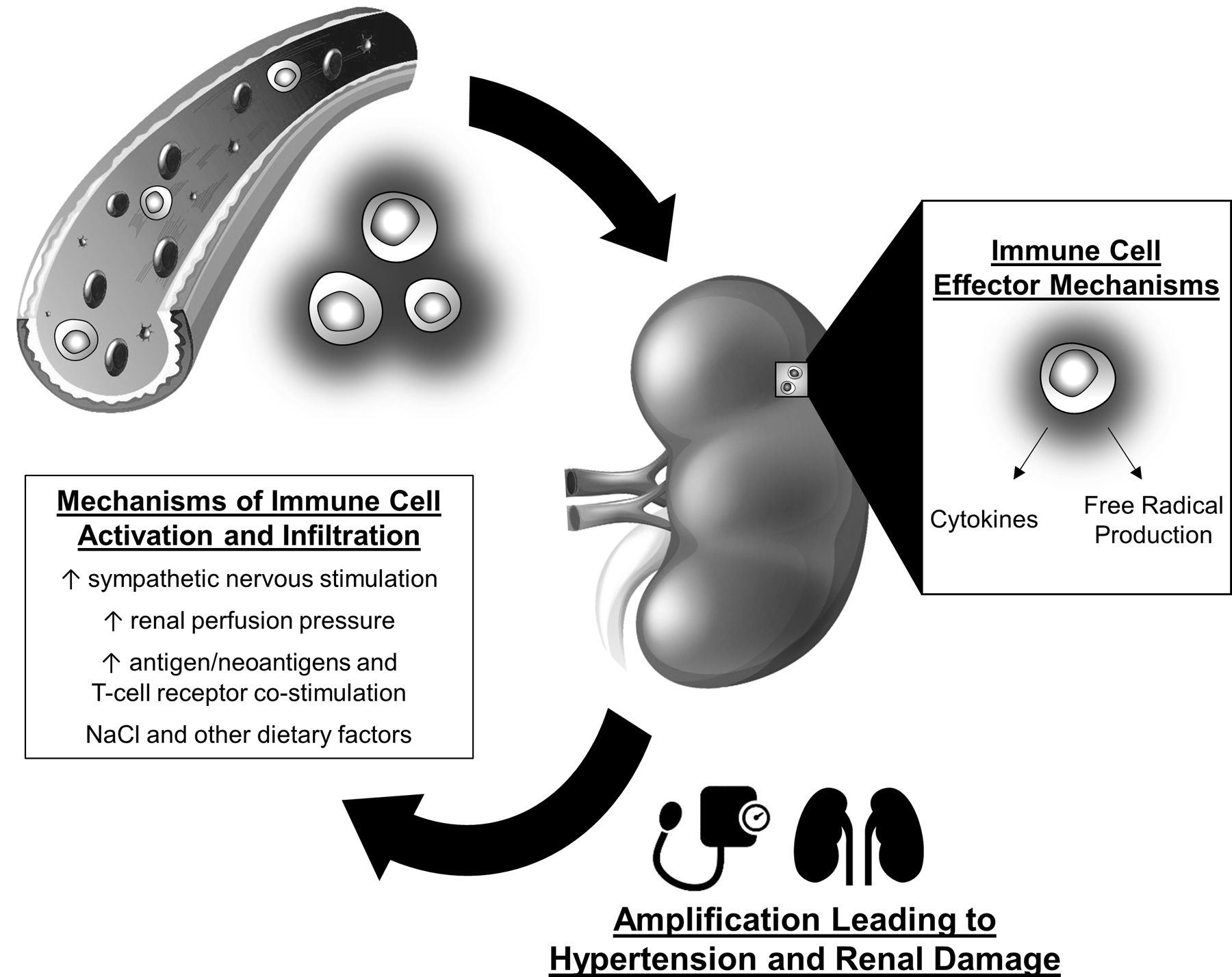
Hypothesized role of immune mechanisms in the amplification of salt-sensitive hypertension. Elevations in sympathetic nerve stimulation, elevated renal perfusion pressure, increased antigens or neoantigens, and environmental factors activate immune cells in hypertension. The immune cells infiltrate target organs, including the kidney, and release free radicals, cytokines, and other molecules that mediate tissue damage and alter physiological function. As a result, the effector actions lead to the amplification of hypertension and tissue damage.
Modulation of Immune Cell Infiltration and Activation Alters Salt-Sensitive Hypertension
The profound influence of T cells and other immune cells to amplify salt-sensitive hypertension and renal damage indicates that mechanisms that modulate the activation or infiltration of these cells into the kidney could alter the progression of disease. Recent data indicate that the source of dietary protein may alter immune activation and salt-sensitive blood pressure. Dahl SS fed diets identical in NaCl but with distinct sources of protein develop dramatic differences in salt-sensitive hypertension and renal damage30,31,34,50,54. Figure 2 summarizes experimental results demonstrating that Dahl SS fed an animal-based diet (SS/Animal) develop more severe salt-sensitive hypertension and renal damage than Dahl SS fed a grain-based diet (SS/Grain). Of note, the altered severity of salt-sensitive disease is associated with differential infiltration of immune cells, (particularly T cells) into the kidney. Moreover, in separate studies, pharmacologic or genetic approaches to suppress the immune system led to a blunting of the effects of an animal-based diet with elevated protein content to amplify salt-sensitive hypertension and renal damage32,34. The remarkable effects of dietary protein consumption on blood pressure led us to hypothesize that changes in the gut microbiota, potentially linked by altered immune activation, can enhance salt-sensitive hypertension.
FIGURE 2:
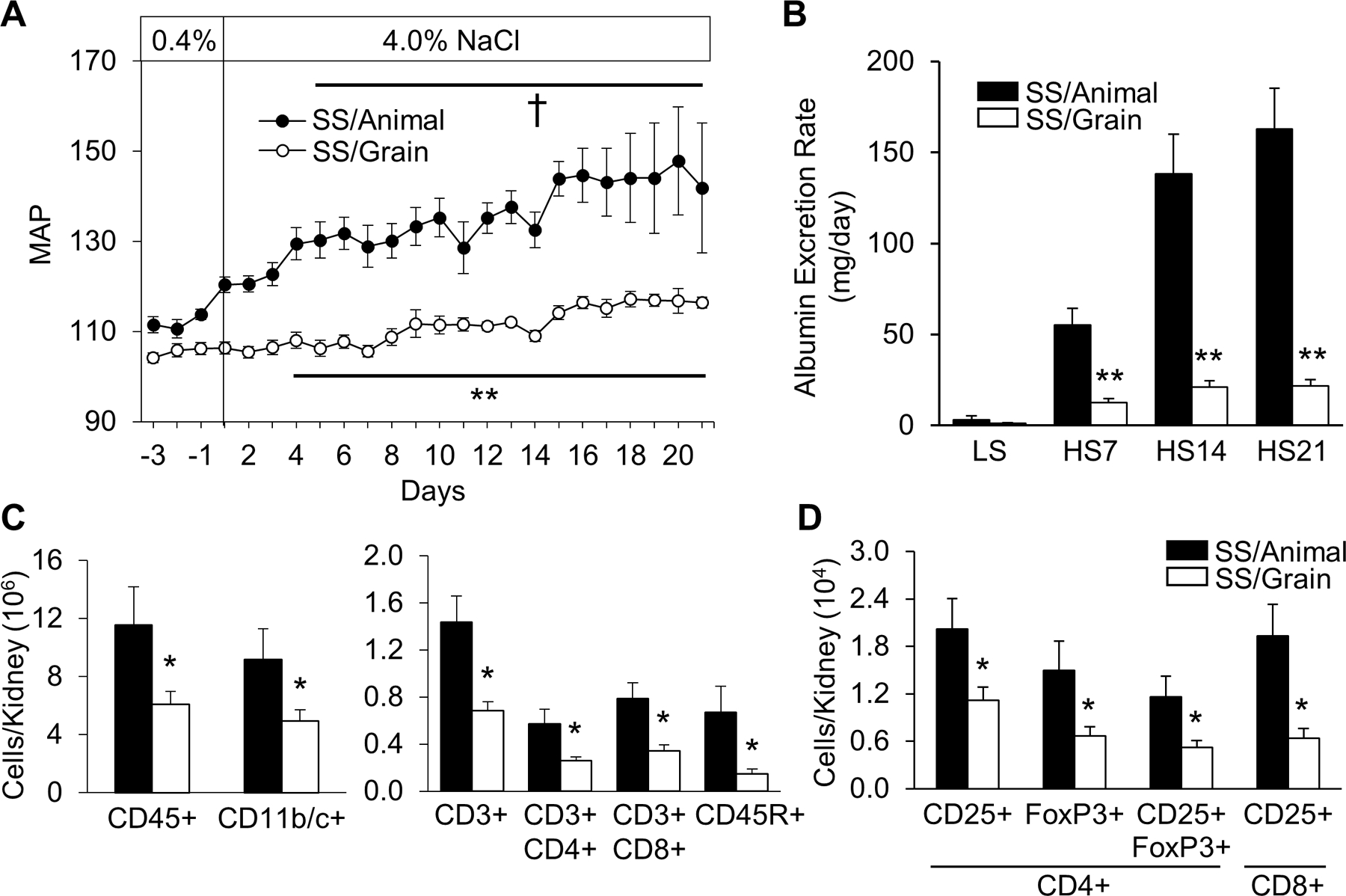
Grain-fed Dahl SS (SS/Grain) demonstrate attenuated salt-induced hypertension (A), renal damage/albuminuria (B), and renal immune cell infiltration (C and D) compared to casein-fed Dahl SS (SS/Animal). Mean arterial pressure: MAP (mmHg). Low salt: LS, 0.4% NaCl; High salt: HS, 4.0% NaCl. CD45: leukocytes, CD11b/c+: monocyte/macrophages, CD3+: T-cells, CD4+: helper T-cells, CD8+: cytotoxic T-cells, CD45R+: B-cells, CD3+CD4+CD25+FoxP3+: T-regulatory cells, CD3+CD8+CD25+: activated cytotoxic T-cells (n=5–7/group, †P<0.05 vs LS Day 0, *P<0.05 and **P<0.001 vs SS/Animal). Reproduced from Abais-Battad et al 33.
Role of Diet and the Gut Microbiota in Hypertension
Human and animal data indicate that the gut microbiota can link diet with hypertension. Both hypertensive humans and animals exhibit gut dysbiosis and decreases in microbial diversity compared to normotensive controls55,56. Studies in animal models of hypertension indicate that pathogenic factors from the gut microbiota participate in disease development. Normotensive Wistar Kyoto rats developed hypertension after transfer of cecal contents from spontaneously hypertensive stroke-prone rats57. Similarly, transplantation of feces from hypertensive humans results in elevated blood pressure in germ-free mice58. Interestingly, the transfer of cecal contents from Dahl SS to Dahl Salt-Resistant (SR) rats did not affect blood pressure, but transfer from Dahl SR to Dahl SS led to an elevation of blood pressure56. Related to end-organ damage in hypertension, the microbiome is also implicated in chronic kidney disease59. Collectively, these studies support the role of the gut microbiota as a determinant of hypertension and end-organ damage.
The gut microbiota and diet are closely linked; diet shapes the gut microbiota and the bacterial metabolites which drive the pathophysiological effects of the diet. Observational evidence illustrated the benefit of plant protein consumption on cardiovascular disease19,24. An inverse relationship between plant protein intake and blood pressure has been observed in humans20, and dietary interventional trials have illustrated the beneficial effects associated with greater plant protein consumption for blood pressure control21,22. Plant-based diets are associated with a distinct microbiota60 and are linked to an improved Firmicutes-to-Bacteroidetes ratio, unique bacterial speciation, and greater fecal short-chain fatty acids61. While diet drives microbial composition, gut bacteria play critical roles in digestion, proteolysis and the fermentation of dietary proteins essential for amino acid balance, utilization, and bioavailability62,63,64. The gut microbiota and diet thus play important roles in host health.
Alterations in the Microbiome Mediate Enhanced Salt-Sensitive Hypertension and Renal Damage in Dahl SS Fed Animal-Based Diets
Significant differences were observed in salt-sensitive hypertension between Dahl SS rats fed an animal- compared to a grain-based diet30,31,33,35. A comparison of the fecal microbiota demonstrated remarkable differences between the two Dahl SS colonies (Figure 3A)54. Significant differences were also noted in the microbiota between the colonies of rats while maintained on a low or high salt diet, consistent with the concept that the microbiota participates in hypertensive disease. To explore the role of changes in the microbiota in disease development, a fecal material transplant (FMT) from the Dahl SS/animal to the Dahl SS/grain. The FMT from rats fed the pro-hypertensive diet amplified the elevation in systolic arterial blood pressure (Figure 3B) and albuminuria (Figure 3C) in the recipient rats. Of great interest, the rats receiving the FMT also demonstrated a specific increase in T cells infiltrating the kidney (Figure 3D). Since T cells amplify salt-sensitive hypertension and renal damage in the Dahl SS6,7,41, altered immune mechanisms may link dietary-induced alterations in the microbiota with salt-sensitive disease. These compelling experiments demonstrate that the microbiota is important in the amplification of salt-sensitive hypertension and related end-organ inflammation and damage in salt-sensitive hypertension, but the mechanisms are unclear.
FIGURE 3:
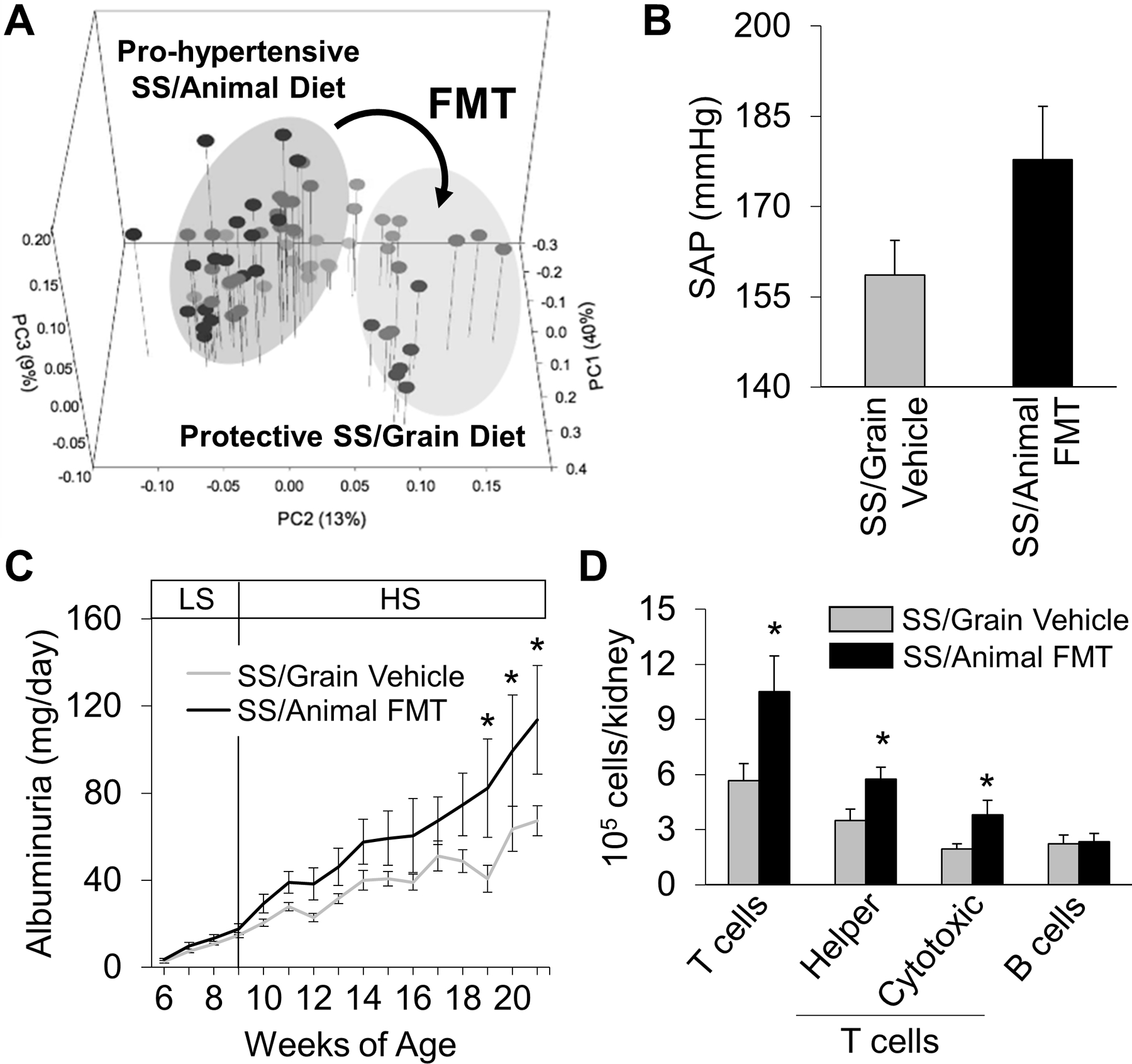
Fecal microbiota transfer (FMT) from SS/Animal into SS/Grain (A) led to a trending increase in systolic arterial pressure in SS/Grain rats receiving FMT after HS (4.0% NaCl) (B), exacerbation of salt-induced albuminuria (C), and increased immune cell infiltration, specifically T cells, into the kidneys (n=5–6/group, *P<.05 vs Vehicle; PC-Principal Coordinate analysis of the weighted Unifrac distance matrix). Reproduced from Abais-Battad and Saravia et al 54.
Though gut dysbiosis in hypertensive humans and experimental animals is associated with increased intestinal inflammation and activation of antigen presenting cells65, the mechanisms of microbiota-mediated inflammatory effects are undefined. Possible mediators include alterations in short-chain fatty acid (SCFA) production67, altered levels of beta-hydroxybuyrate66, trimethylamine N-oxide (TMAO)68,69, changes in LPS58, or other gut-derived metabolites These and other mechanisms remain to be explored, but evidence indicates that SCFA can modulate blood pressure67 while plasma L-carnitine and TMAO levels predict an increased risk for cardiovascular disease and adverse cardiac events68,70,71.
Epigenetic Effects
The mechanisms transducing changes in diet to alter function are unclear, but the microbiota and microbial metabolites may directly or indirectly affect physiological processes. Evidence indicates that epigenetics play a critical role in the development of hypertension and renal disease in humans and animal models of disease73,74. In particular, changes in gut microbial composition have been linked to changes in DNA methylation in metabolic syndrome75, inflammatory bowel disease76, and colorectal cancer77. Since immune cells, T cells in particular, infiltrate the kidney in salt-sensitive hypertension and amplify the development of renal damage and elevation in arterial blood pressure6,7,41, the T cell is a potential target of environmental factors to modulate function by epigenetic mechanism in hypertension. Furthermore, epigenetic modifications have been shown to play a principal role in the regulation of the immune system through the development, activation and function of different individual immune cell types78,79. DNA methylation in the T cell may therefore affect gene expression and modify the cell’s capacity to differentiate and function80.
To examine the mechanisms whereby changes in the diet and microbiota alter T cell activation, a DNA methylation and gene expression analysis was performed on T cells of Dahl SS fed an animal- or plant-based diet33,50. In response to high salt, the methylome of T cells isolated from the kidney of Dahl SS/animal exhibited a significant increase in hypermethylated regions compared to T cells isolated from the kidneys of SS/grain (Figure 4A-B)50. Moreover, a predominant negative correlation was observed between gene expression and DNA methylation, indicating that DNA methylation modulates gene expression in salt-sensitive hypertension50. Finally, the inhibition of DNA methyltransferase blunted salt-induced hypertension and renal damage in the SS rats fed the pro-hypertensive diet, providing a functional role for DNA methylation. These studies demonstrated the potential of diet-induced changes in the microbiota and DNA methylation to modulate the severity of salt-sensitive hypertension.
FIGURE 4:
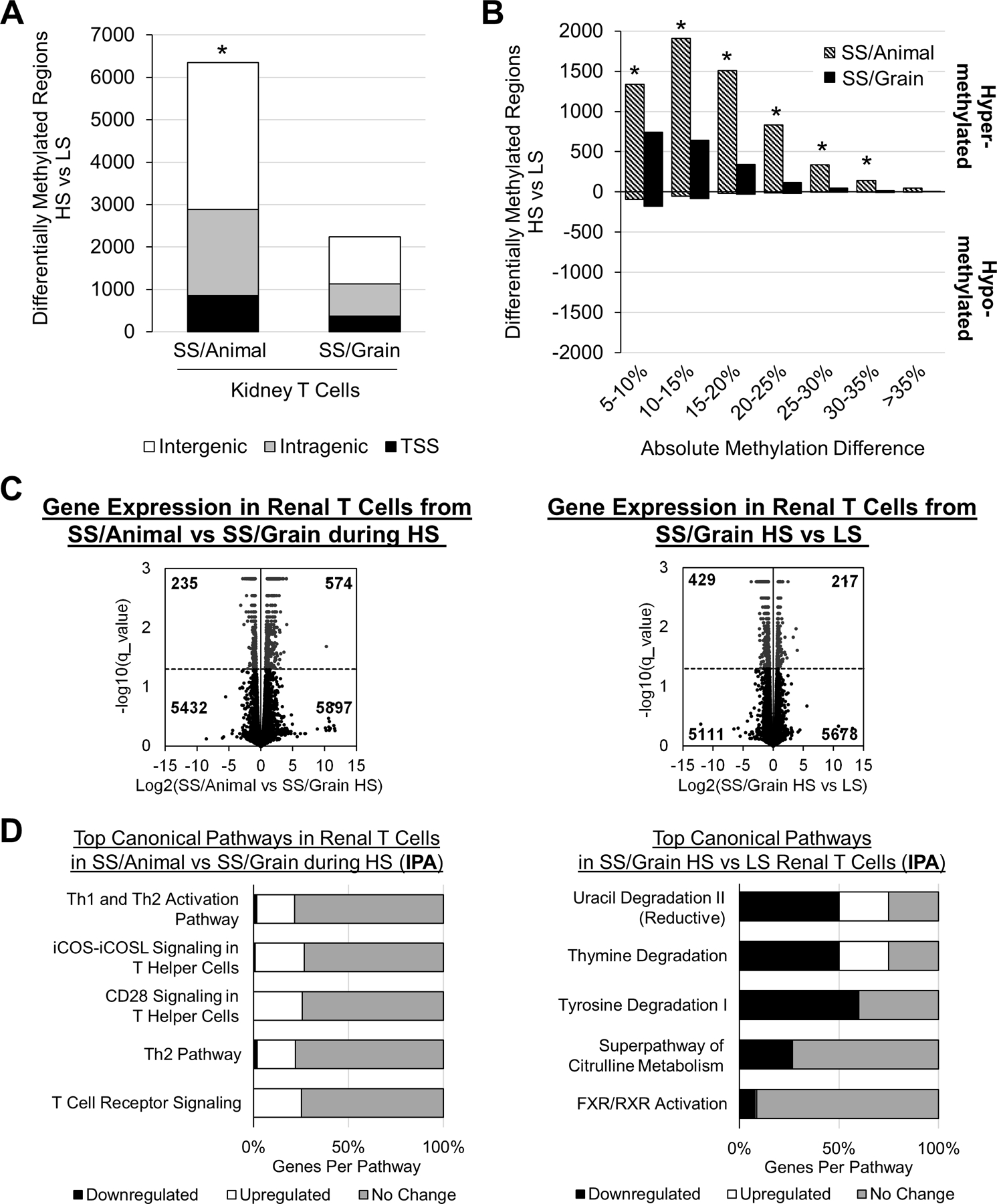
T cells from the kidneys of Dahl SS/Animal exhibit significantly more differentially methylated regions (DMRs) in T cells isolated from the SS/Animal compared to SS/Grain (A). The DMRs in the T cells isolated from the kidney were primarily hypermethylated (B). Comparison of genes up- and downregulated in T cells isolated from the kidney of SS/Animal and SS/Grain rats fed the high salt (4.0% NaCl) diet (C-F) showed upregulation of inflammatory, T-cell signaling pathways in SS/Animal (C-D). Comparison of genes up- and down-regulated in T cells isolated from the kidney of SS/Grain fed the low salt (LS, 0.4% NaCl) or the HS diet demonstrated downregulation of pathways related primarily to metabolic processes during HS (E-F). TSS=transcription start site, n=4 pools/group, 3 rats/pool, *P<.05 vs SS/Grain. Reproduced from Abais-Battad et al 33 and Dasinger et al 50.
Transcriptomic Effects
Recent studies demonstrated that high salt intake or alterations in other dietary factors can alter immune cell activation. In particular, elevated extracellular sodium concentration induced T helper 17 (Th17) cell polarization from naïve T cells of mouse or human origin46,47. High-salt feeding was shown to alter the gut microbiome and modulate the induction of Th17 cells in mice and humans48. Since Il-17 released from Th17 cells is pro-hypertensive, elevated sodium concentrations may have a feed-forward effect on blood pressure. Interestingly, evidence indicates that large amounts of sodium can be stored in the skin81; while elevated skin sodium content has been reported to activate macrophages49.
We examined gene expression in T cells of Dahl SS fed an animal- or plant-based diet33. A transcriptomic analysis of T cells isolated from the kidney demonstrated a shift in expression from genes related to inflammation in the Dahl SS/Animal to genes related to metabolism in SS/Grain (Figure 4C-F)33. An IPA analysis indicated significant upregulation in pro-inflammatory pathways in T cells isolated from the kidneys of Dahl SS/Animal. In contrast, downregulated gene expression in metabolic pathways was observed in the T cells of the Dahl SS/Grain when fed high salt. These studies therefore demonstrate the potential influence of diet-induced changes in the microbiota, DNA methylation, and gene expression to modulate immune cell function and ultimately the severity of salt-sensitive hypertension and associated renal end-organ damage.
To place these animal studies into a translational context, diets rich in vegetable and plant protein are beneficial for blood pressure20,21,22. A potential mechanism is the direct relationship between dietary protein intake and inflammation which is specific for increases in animal/meat protein but not for increases in fish or vegetable protein intake36. Similarly, changes in sodium intake have been associated with changes in inflammation. A sustained reduction in sodium intake in normal individuals associated with decreased total numbers of peripheral monocytes82. The levels of pathogenic IL-6 and IL-23 also decreased with reduced salt intake, whereas protective IL-10 increased82. These results in animals and humans indicate that changes in dietary protein source, as well as alterations in sodium intake, have profound effects on the microbiota, DNA methylation, gene expression, the activation of immune cells, and the development of salt-sensitive hypertension and related disease phenotypes.
Perspectives and Conclusions
Figure 5 illustrates a hypothesis whereby changes in protein source can alter salt-sensitive hypertension. Observations in rats and humans indicate that changes in the dietary protein source can modulate salt-sensitive hypertension. Many mechanisms may mediate these effects, but we demonstrated that ingestion of a pro-hypertensive diet containing animal-based protein shifts the gut microbiota. Further experiments demonstrated that microbiota transfer from animals fed the pro-hypertensive diet can confer an elevation of arterial pressure and increased end-organ damage to animals fed a plant-based diet. We speculate that the altered microbial community leads to the production and release of metabolites that act upon host physiology. The metabolites may directly affect function by specific interactions or indirectly alter function via effects on gene transcription. Specifically, we observed profound changes in DNA methylation and gene expression in T cells. Since T cells amplify salt-sensitive hypertension in the Dahl SS, metabolites which alter T cell function and number could profoundly affect the salt-sensitive disease phenotype. Rats fed the pro-hypertensive, animal-based diet, in comparison to rats fed the grain-based diet, have increased DNA methylation and upregulation of pro-inflammatory genes in the T cells that infiltrate the kidney. By contrast, T cells isolated from the kidneys of rats fed the grain-based diet demonstrate a downregulation of genes in metabolic pathways when comparing the low and high salt conditions. Finally, we observed increased infiltration of T cells and other immune cells into the kidney of Dahl SS fed the animal-based diets that develop the greatest degree of salt-sensitive hypertension and renal end-organ damage. These results provide a potential explanation for the deleterious effects of some diets to amplify salt-sensitive disease.
FIGURE 5:
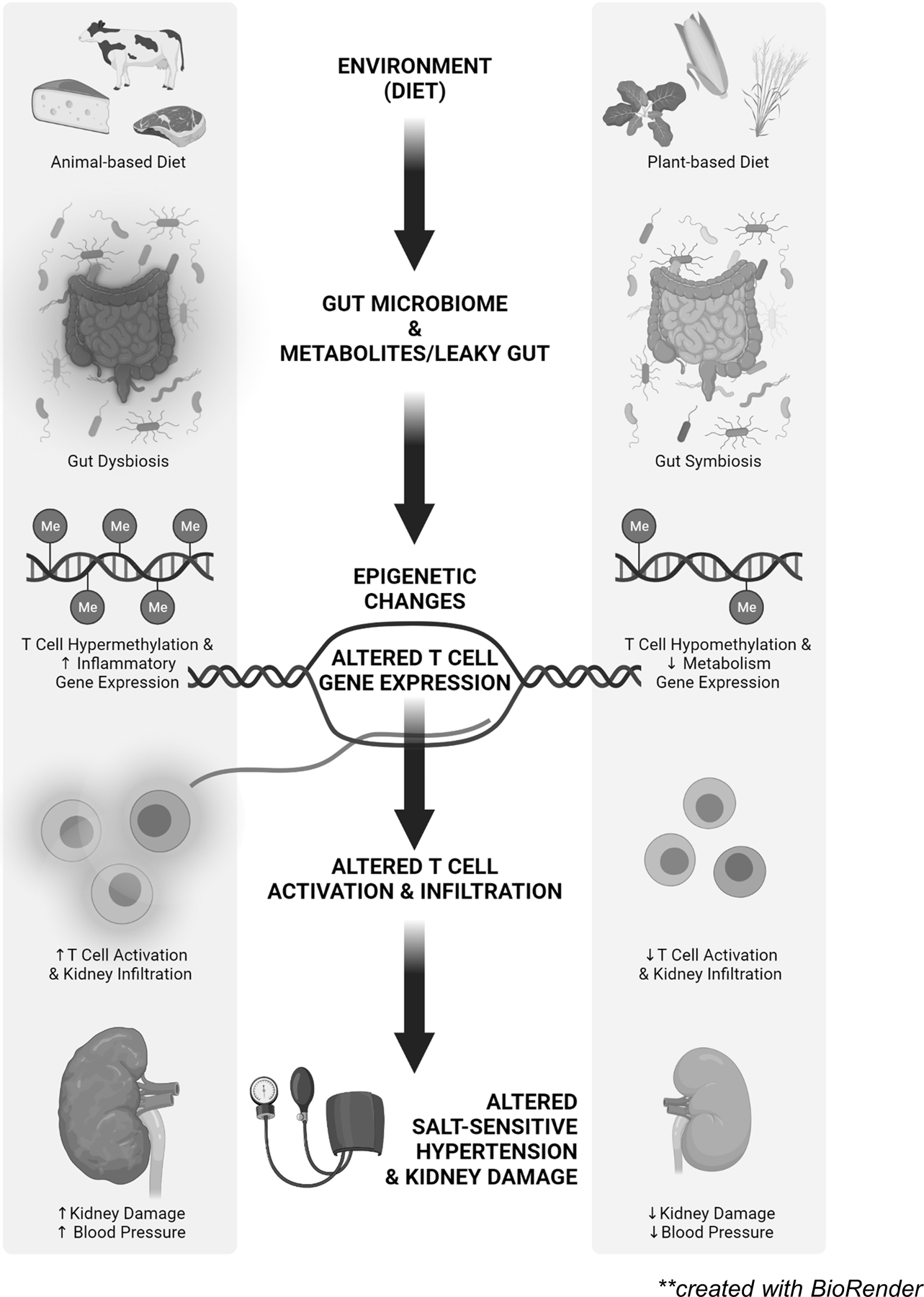
Hypothesized effects of animal- and plant-based diets to alter the gut microbiome and gut metabolites with downstream effects on DNA methylation, gene expression, immune activation, and salt-sensitive hypertension and renal damage. Created with BioRender.com.
Though much work has advanced this field, many questions remain. Fundamentally, the bacterial species influenced by changes in diet, the metabolites or other cellular products produced by those species, and the rapidity of reversibility of diet-induced effects on the microbiota and microbiota-derived metabolites are unknown. Similarly, the potential approaches to manipulate these parameters to treat salt-sensitive hypertension will require intense effort. This review has focused upon the potential role of T cells in the kidney in salt-sensitive hypertension, but effects could be mediated in multiple cell types in different tissues to mediate the biological effects of altered dietary protein sources on hypertension. Moreover, the mechanisms transducing infiltration, activation, and action of innate and adaptive immune mechanisms in hypertension and end-organ damage are unclear. Despite these challenges, this field provides great potential for the development of improved therapy for this life-threatening disease.
Sources of Funding
The authors’ work is supported by NIH Grants HL137748, HL116264, 19CDA34660184, and the Georgia Research Alliance.
Footnotes
Conflict of Interest
The authors declare that they have no conflict of interest with the work presented in this manuscript.
Disclosures
None
Bibliography
- 1.Tsao CW, Aday AW, Almarzooq ZI, Alonso A, Beaton AZ, Bittencourt MS, Boehme AK, Buxton AE, Carson AP, Commodore Mensah Y, et al. ; on behalf of the American Heart Association Council on Epidemiology and Prevention Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics—2022 update: a report from the American Heart Association. Circulation 2022;145:e153–e639. [DOI] [PubMed] [Google Scholar]
- 2.Elijovich F, Weinberger MH, Anderson CA, Appel LJ, Bursztyn M, Cook NR, Dart RA, Newton-Cheh CH, Sacks FM, Laffer CL. Salt sensitivity of blood pressure: a scientific statement from the American Heart Association. Hypertension 2016; 68:e7–e46. [DOI] [PubMed] [Google Scholar]
- 3.Morimoto A, Uzu T, Fujii T, Nishimura M, Kuroda S, Nakamura S, Inenaga T, Kimura G. Sodium sensitivity and cardiovascular events in patients with essential hypertension. Lancet 1997; 350:1734–1737. [DOI] [PubMed] [Google Scholar]
- 4.Weinberger MH, Fineberg NS, Fineberg SE, Weinberger M. Salt sensitivity, pulse pressure, and death in normal and hypertensive humans. Hypertension 2001; 37:429–432. [DOI] [PubMed] [Google Scholar]
- 5.Hughson MD, Gobe GC, Hoy WE, Manning RD, Douglas-Denton R, Bertram JF. Associations of glomerular number and birth weight with clinicopathological features of African Americans and whites. Am J Kidney Dis 2008; 52:18–28. [DOI] [PubMed] [Google Scholar]
- 6.Mattson DL. Immune Mechanisms of Salt-Sensitive Hypertension and Renal End-Organ Damage. Nature Rev Nephrol 2019; 15:290–300. [DOI] [PubMed] [Google Scholar]
- 7.Mattson DL, Dasinger JH, Abais-Battad JM. Amplification of salt-sensitive hypertension and kidney damage by immune mechanisms. Am J Hypertension 34:3–14, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rodriguez-Iturbe B, Pons H, Johnson RJ. Role of the Immune System in Hypertension. Physiological Reviews 2017; 97:1127–1164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Norlander AE, Madhur MS, Harrison DG. The immunology of hypertension. J Exp Med 2018; 215:21–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hajjar I, Kotchen T. Regional variations of blood pressure in the United States are associated with regional variations in dietary intakes: the NHANES-III data. J Nutr 2003;133:211–214. [DOI] [PubMed] [Google Scholar]
- 11.Kesteloot H, Joossens JV. Relationship of serum sodium, potassium, calcium, and phosphorus with blood pressure. Belgian Interuniversity Research on Nutrition and Health. Hypertension 1988;12:589–593. [DOI] [PubMed] [Google Scholar]
- 12.Stamler J, Caggiula A, Grandits GA, Kjelsberg M, Cutler JA. Relationship to blood pressure of combinations of dietary macronutrients. Findings of the Multiple Risk Factor Intervention Trial (MRFIT). Circulation 1996;94:2417–2423. [DOI] [PubMed] [Google Scholar]
- 13.Aljuraiban GS, Griep LMO, Chan Q, Daviglus ML, Stamler J, Van Horn L, Elliott P, Frost GS. Total, insoluble and soluble dietary fibre intake in relation to blood pressure: the INTERMAP Study. Br J Nutr 2015;114:1480–1486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vasdev S, Stuckless J. Antihypertensive effects of dietary protein and its mechanism. Int J Angiol 2010;19:e7–e20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hajjar IM, Grim CE, George V, Kotchen TA. Impact of diet on blood pressure and age-related changes in blood pressure in the US population: analysis of NHANES III. Arch Intern Med 2001;161:589–593. [DOI] [PubMed] [Google Scholar]
- 16.Malhotra R, Lipworth L, Cavanaugh KL, Young BA, Tucker KL, Carithers TC, Taylor HA, Correa A, Kabagambe EK, Ikizler TA. Protein Intake and Long-term Change in Glomerular Filtration Rate in the Jackson Heart Study. J Ren Nutr 2018;28:245–250. [DOI] [PubMed] [Google Scholar]
- 17.Watanabe D, Machida S, Matsumoto N, Shibagaki Y, Sakurada T. Age Modifies the Association of Dietary Protein Intake with All-Cause Mortality in Patients with Chronic Kidney Disease. Nutrients 2018;10:1744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hahn D, Hodson EM, Fouque D. Low protein diets for non‐diabetic adults with chronic kidney disease. Cochrane Database of Systematic Reviews 2020, Issue 10. Art. No.: CD001892. DOI: 10.1002/14651858.CD001892.pub5. Accessed 01 July 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sacks FM, Rosner B, Kass EH. Blood pressure in vegetarians. Am J Epidemiol 1974;100:390–398. [DOI] [PubMed] [Google Scholar]
- 20.Elliott P, Stamler J, Dyer AR, Appel L, Dennis B, Kesteloot H, Ueshima H, Okayama A, Chan Q, Garside DB, Zhou B. Association between protein intake and blood pressure: the INTERMAP Study. Arch Intern Med 2006;166:79–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Appel LJ, Moore TJ, Obarzanek E, Vollmer WM, Svetkey LP, Sacks FM, Bray GA, Vogt TM, Cutler JA, Windhauser MM, Lin PH, Karanja N. A clinical trial of the effects of dietary patterns on blood pressure. DASH Collaborative Research Group. N Engl J Med 1997;336:1117–1124. [DOI] [PubMed] [Google Scholar]
- 22.Appel LJ, Sacks FM, Carey VJ, Obarzanek E, Swain JF, Miller ER 3rd, Conlin PR, Erlinger TP, Rosner BA, Laranjo NM, et al. ; OmniHeart Collaborative Research Group. Effects of protein, monounsaturated fat, and carbohydrate intake on blood pressure and serum lipids: results of the OmniHeart randomized trial. JAMA 2005;294:2455–2464. [DOI] [PubMed] [Google Scholar]
- 23.Vollmer WM, Sacks FM, Ard J, Appel LJ, Bray GA, Simons-Morton DG, Conlin PR, Svetkey LP, Erlinger TP, Moore TJ, et al. Effects of Diet and Sodium Intake on Blood Pressure: Subgroup Analysis of the DASH-Sodium Trial. Ann Intern Med 2001;135:1019–1028. [DOI] [PubMed] [Google Scholar]
- 24.Richter CK, Skulas-Ray AC, Champagne CM, Kris-Etherton PM. Plant protein and animal proteins: do they differentially affect cardiovascular disease risk? Adv Nutr 2015;6:712–728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sun D, Zhou T, Li X, Heianza Y, Liang Z, Bray GA, Sacks FM, Qi L. Genetic Susceptibility, Dietary Protein Intake, and Changes of Blood Pressure: The POUNDS Lost Trial. Hypertension 74:1460–1467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nagae A, Fujita M, Kawarazaki H, Matsui H, Ando K, Fujita T. Effect of high fat loading in Dahl salt-sensitive rats. Clin Exp Hypertens 2009;31:451–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang HY, Reddy S, Kotchen TA. A high sucrose, high linoleic acid diet potentiates hypertension in the Dahl salt sensitive rat. Am J Hypertens 1999; 12(2pt 1):183–187. [DOI] [PubMed] [Google Scholar]
- 28.Preuss HG, Knapka JJ, MacArthy P, Yousufi AK, Sabnis SG, Antonovych TT. High sucrose diets increase blood pressure of both salt-sensitive and salt-resistant rats. Am J Hypertens 1992;5(9):585–591. [DOI] [PubMed] [Google Scholar]
- 29.Nevala R, Vaskonen T, Vehniainen J, Korpela R, Vapaatalo H. Soy based diet attenuates the development of hypertension when compared to casein based diet in spontaneously hypertensive rat. Life Sci 2000;66:115–124. [DOI] [PubMed] [Google Scholar]
- 30.Mattson DL, Kunert MP, Kaldunski ML, Greene AS, Roman RJ, Jacob HJ, Cowley AW Jr. Influence of diet and genetics on hypertension and renal disease in Dahl salt-sensitive rats. Physiological Genomics 2004; 16:194–203. [DOI] [PubMed] [Google Scholar]
- 31.Mattson DL, Meister CJ, Marcelle M. Dietary protein source determines the degree of hypertension in renal disease in the Dahl salt-sensitive rat. Hypertension 2005; 45:736–741. [DOI] [PubMed] [Google Scholar]
- 32.De Miguel C, Lund H, Mattson DL. High dietary protein exacerbates hypertension and renal damage in Dahl salt-sensitive (SS) rats by increasing infiltrating immune cells. Hypertension 2011; 57:269–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Abais-Battad JM, Alsheikh AJ, Pan X, Fehrenbach DJ, Dasinger JH, Lund H, Roberts M, Kriegel AJ, Cowley AW, Kidambi S, et al. Transcriptomic Analysis in Renal T Lymphocytes Exposes Sodium-Independent Dietary Differences in Dahl SS Rats. Hypertension 2019; 44:854–863. [Google Scholar]
- 34.Abais-Battad JM, Lund H, Fehrenbach DJ, Dasinger JH, Mattson DL. Rag1-null Dahl SS rats reveal adaptive immune mechanisms exacerbate high protein-induced hypertension and renal injury. Am J Physiol 2018; 315:R28–R35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Geurts AM, Mattson DL, Liu P, Cabacungan E, Skelton MM, Kurth TM et al. Maternal diet during gestation and lactation modifies the severity of salt-induced hypertension and renal injury in Dahl salt-sensitive rats. Hypertension 2015;65:447–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lopez-Legarrea P, de la Iglesia R, Abete I, Navas-Carretero S, Martinez JA, Zulet MA. The protein type within a hypocaloric diet affects obesity-related inflammation: the RESMENA project. Nutrition 2014;30:424–429. [DOI] [PubMed] [Google Scholar]
- 37.Jantchou P, Morois S, Clavel-Chapelon F, Boutron-Ruault MC, Carbonnel F. Animal protein intake and risk of inflammatory bowel disease: The E3N prospective study. Am J Gastroenterol 2010;105:2195–2201. [DOI] [PubMed] [Google Scholar]
- 38.Jowett SL, Seal CJ, Pearce MS, Phillips E, Gregory W, Barton JR et al. Influence of dietary factors on the clinical course of ulcerative colitis: a prospective cohort study. Gut 2004;53:1479–1484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Guyonnet L, Czopek A, Farrah TE, Baudrie V, Bonnin P, Chipont A, Lenoir O, Sennlaub F, Roubeix C, Webb DJ, et al. Deletion of the myeloid endothelin-B receptor confers long-term protection from angiotensin II-mediated kidney, eye and vessel injury. Kidney International 2020; 98:1193–1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Benson LN, Liu Y, Wang X, Xiong Y, Rhee SW, Guo Y, Deck KS, Mora CJ, Li L-X, Huang L et al. The IFNγ-PDL1 Pathway Enhances CD8T-DCT Interaction to Promote Hypertension. Circulation Research 2022; 130:1550–1564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fehrenbach DJ, Dasinger JH, Lund H, Zemaj J, Mattson DL. Splenocyte transfer exacerbates salt-sensitive hypertension in rats. Exp Physiol 105:864–875, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Guzik TJ, Hoch NE, Brown KA, McCann LA, Rahman A, Dikalov S, Goronzy J, Weyand C, Harrison DG. Role of the T cell in the genesis of angiotensin II induced hypertension and vascular dysfunction. J Exp Med 2007; 204:2449–2460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Seniuk A, Thiele JL, Stubbe A, Oser P, Rosendahl A, Bode M, Meyer-Schwesinger C, Wenzel U, Ehmke H. B6.Rag1 Knockout Mice Generated at the Jackson Laboratory in 2009 Show a Robust Wild-Type Hypertensive Phenotype in Response to Ang II (Angiotensin II). Hypertension 2020; 75:1110–1116. [DOI] [PubMed] [Google Scholar]
- 44.Ji H, Pai AV, West CA, Wu X, Speth RC, Sandberg K. Loss of Resistance to Angiotensin II-Induced Hypertension in the Jackson Laboratory Recombination-Activating Gene Null Mouse on the C57BL/6J Background. Hypertension 2017; 69:1121–1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Burke M, Pabbidi MR, Farley J, Roman RJ. Molecular mechanisms of renal blood flow autoregulation. Curr Vasc Pharmacol 2014; 12:845–858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kleinewietfeld M, Manzel A, Titze J, Kvakan H, Yosef N, Linker RA, Muller DN, Hafler DA. Sodium chloride drives autoimmune disease by the induction of pathogenic TH17 cells. Nature 2013; 496:518–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wu C, Yosef N, Thalhamer T, Zhu C, Xiao S, Kishi Y, Regev A, Kuchroo VK. Induction of pathogenic TH17 cells by inducible salt-sensing kinase SGK1. Nature 2013; 496:513–517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wilck N, Matus MG, Kearney SM, Olesen SW, Forslund K, Bartolomaeus H, Haase S, Mähler A, Balogh A, Markó L, et al. Salt-responsive gut commensal modulates TH17 axis and disease. Nature 2017; 551:585–589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jantsch J, Schatz V, Friedrich D, Schröder A, Kopp C, Siegert I, Maronna A, Wendelborn D, Linz P, Binger KJ, et al. Cutaneous Na+ storage strengthens the antimicrobial barrier function of the skin and boosts macrophage-driven host defense. Cell Metab 2015; 21:493–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Dasinger JH, Alsheikh AJ, Abais-Battad JM, Pan X, Fehrenbach DJ, Lund H, Roberts ML, Cowley AW Jr, Kidambi S, Kotchen TA, et al. Epigenetic Modifications in T Cells: The Role of DNA Methylation in Salt-Sensitive Hypertension. Hypertension 2020; 75:372–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mori T, Polichnowski A, Glocka P, Kaldunski M, Ohsaki Y, Liang M, Cowley AW Jr. High perfusion pressure accelerates renal injury in salt-sensitive hypertension. J Am Soc Nephrol 2008; 19:1472–1482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Evans LC, Petrova G, Kurth T, Yang C, Bukowy JD, Mattson DL, Cowley AW Jr. Increased perfusion pressure drives renal T-cell infiltration in the Dahl salt-sensitive rat. Hypertension 2017; 70:543–551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Shimada S, Abais-Battad JM, Alsheikh AJ, Yang C, Stumpf M, Kurth T, Mattson DL, Cowley AW Jr. Renal perfusion pressure determines infiltration of leukocytes in the kidney of rats with angiotensin II induced hypertension. Hypertension 2020; 76:849–858, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Abais-Battad JM, Saravia FL, Lund H, Dasinger JH, Fehrenbach DJ, Alsheikh AJ, Zemaj J, Kirby JR, Mattson DL. Dietary Influences on the Dahl SS rat gut microbiota and its effects on salt-sensitive hypertension and renal damage. Acta Physiol 2021. 232:e13506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yang T, Santisteban MM, Rodriguez V, Li E, Ahmari N, Carvajal JM, Zadeh M, Gong M, Qi Y, Zubcevic J, Sahay B, Pepine CJ, Raizada MK, Mohamadzadeh M. Gut dysbiosis is linked to hypertension. Hypertension 2015;65:1331–1340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mell B, Jala VR, Mathew AV, Byun J, Waghulde H, Zhang Y, Haribabu B, Vijay-Kumar M, Pennathur S, Joe B. Evidence for a link between gut microbiota and hypertension in the Dahl rat. Physiol Genomics 2015;47:187–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Adnan S, Nelson JW, Ajami NJ, Venna VR, Petrosino JF, Bryan RM Jr, Durgan DJ. Alterations in the gut microbiota can elicit hypertension in rats. Physiol Genomics 2017;49:96–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Li J, Zhao F, Wang Y, Chen J, Tao J, Tian G, Wu S, Liu W, Cui Q, Geng B, Zhang W, et al. Gut microbiota dysbiosis contributes to the development of hypertension. Microbiome 2017;5:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hobby GP, Karaduta O, Dusio GF, Singh M, Zybailov BL, Arthur JM. Chronic kidney disease and the gut microbiome. Am J Physiol 2019; 316:F1211–F1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.David LA, Maurice CF, Carmody RN, Gootenberg DB, Button JE, Wolfe BE, Ling AV, Devlin AS, Varma Y, Fischbach MA, et al. Diet rapidly and reproducibly alters the human gut microbiome. Nature 2014;505:559–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.De Filippo C, Cavalieri D, Di Paola M, Ramazzotti M, Poullet JB, Massart S, Collini S, Pieraccini G, Lionetti P. Impact of diet in shaping gut microbiota revealed by a comparative study in children from Europe and rural Africa. Proc Natl Acad Sci U S A 2010;107:14691–14696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Macfarlane GT, Cummings JH, Allison C. Protein degradation by human intestinal bacteria. J Gen Microbiol 1986;132:1647–1656. [DOI] [PubMed] [Google Scholar]
- 63.Dai ZL, Zhang J, Wu G, Zhu WY. Utilization of amino acids by bacteria from the pig small intestine. Amino Acids 2010;39:1201–1215. [DOI] [PubMed] [Google Scholar]
- 64.Davila AM, Blachier F, Gotteland M, Andriamihaja M, Benetti PH, Sanz Y, Tomé D. Intestinal luminal nitrogen metabolism: role of the gut microbiota and consequences for the host. Pharmacol Res 2013;68:95–107. [DOI] [PubMed] [Google Scholar]
- 65.Ferguson JF, Aden LA, Barbaro NR, Van Beusecum JP, Xiao L, Simmons AJ, Warden C, Pasic L, Himmel LE, Washington MK, et al. High dietary salt-induced dendritic cell activation underlies microbial dysbiosis-associated hypertension. JCI Insight 2019;5:e126241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Chakraborty S, Galla S, Cheng X, Yeo JY, Mell B, Singh V, Yeoh B, Saha P, Mathew AV, Vijay-Kumar M, Joe B. Salt-Responsive Metabolite, beta-Hydroxybutyrate, Attenuates Hypertension. Cell Rep 2018;25:677–689.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Pluznick JL. Microbial Short-Chain Fatty Acids and Blood Pressure Regulation. Curr Hypertens Rep 19: 25, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Koeth RA, Wang Z, Levison BS, Buffa JA, Org E, Sheehy BT, Britt EB, Fu X, Wu Y, Li L, et al. Intestinal microbiota metabolism of L-carnitine, a nutrient in red meat, promotes atherosclerosis. Nat Med 2013; 19:576–585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wang Z, Klipfell E, Bennett BJ, Koeth R, Levison BS, Dugar B, Feldstein AE, Britt EB, Fu X, Chung YM, Wu Y, Schauer P, Smith JD, Allayee H, Tang WH, DiDonato JA, Lusis AJ, Hazen SL. Gut flora metabolism of phosphatidylcholine promotes cardiovascular disease. Nature 2011; 472:57–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Heianza Y, Ma W, Manson JE, Rexrode KM, Qi L. Gut Microbiota Metabolites and Risk of Major Adverse Cardiovascular Disease Events and Death: A Systematic Review and Meta-Analysis of Prospective Studies. J Am Heart Assoc 2017; 6:e004947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Li XS, Obeid S, Klingenberg R, Gencer B, Mach F, Raber L, Windecker S, Rodondi N, Nanchen D, Muller O, et al. Gut microbiota-dependent trimethylamine N-oxide in acute coronary syndromes: a prognostic marker for incident cardiovascular events beyond traditional risk factors. Eur Heart J 2017; 38:814–824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ufnal M, Jazwiec R, Dadlez M, Drapala A, Sikora M, Skrzypecki J. Trimethylamine-N-oxide: a carnitine-derived metabolite that prolongs the hypertensive effect of angiotensin II in rats. Can J Cardiol 2014; 30:1700–1705. [DOI] [PubMed] [Google Scholar]
- 73.Kato N, Loh M, Takeuchi F, Verweij N, Wang X, Zhang W, Kelly TN, Saleheen D, Lehne B, Leach IM, et al. Trans-ancestry genome-wide association study identifies 12 genetic loci influencing blood pressure and implicates a role for DNA methylation. Nat Genet 2015; 47:1282–1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Liu P, Liu Y, Liu H, Pan X, Li Y, Usa K, Mishra MK, Nie J, Liang M. Role of DNA De Novo (De)Methylation in the Kidney in Salt-Induced Hypertension. Hypertension, 2018; 72:1160–1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.van der Vossen EWJ, Bastos D, Stols-Gonçalves D, de Goffau MC, Davids M, Pereira JPB, Li Yim AYF, Henneman P, Netea MG, de Vos WM, et al. Effects of fecal microbiota transplant on DNA methylation in subjects with metabolic syndrome. Gut Microbes 2021; 13:e1993513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ryan FJ, Ahern AM, Fitzgerald RS, Laserna-Mendieta EJ, Power EM, Clooney AG, O’Donoghue KW, McMurdie PJ, Iwai S, Crits-Christoph A, et al. Colonic microbiota is associated with inflammation and host epigenomic alterations in inflammatory bowel disease. Nat Commun 2020; 11:1512–1523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Wang Q, Ye J, Fang D, Lv L, Wu W, Shi D, Li Y, Yang L, Bian X, Wu J, et al. Multi-omic profiling reveals associations between the gut mucosal microbiome, the metabolome, and host DNA methylation associated gene expression in patients with colorectal cancer. BMC Microbiol 2020; 20:83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Morales-Nebreda L, McLafferty FS, Singer BD. DNA methylation as a transcriptional regulator of the immune system. Transl Res 2019; 204:1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Schmidl C, Delacher M, Huehn J, Feuerer M. Epigenetic mechanisms regulating T-cell responses. J Allergy Clin Immunol 2018; 142:728–743. [DOI] [PubMed] [Google Scholar]
- 80.Tsagaratou A, Äijö T, Lio CW, Yue X, Huang Y, Jacobsen SE, Lähdesmäki H, Rao A. Dissecting the dynamic changes of 5-hydroxymethylcytosine in T-cell development and differentiation. Proc Natl Acad Sci U S A 2014; 111:E3306–3315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Titze J Sodium balance is not just a renal affair. Curr Opin Nephrol Hypertens 2014; 23:101–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Yi B, Titze J, Rykova M, Feuerecker M, Vassilieva G, Nichiporuk I, Schelling G, Morukov B, Choukèr A. Effects of dietary salt levels on monocytic cells and immune responses in healthy human subjects: a longitudinal study. Transl Res 2015; 166:103–110. [DOI] [PMC free article] [PubMed] [Google Scholar]


