Abstract
Canine osteoarthritis is a significant cause of pain in many dogs and can therefore compromise animal welfare. As the understanding of the biology and pain mechanisms underpinning osteoarthritis grows, so do the number of treatments available to manage it. Over the last decade, there have been a number of advances in the pharmaceutical treatment options available for dogs with osteoarthritis, as well as an increasing number of clinical trials investigating the efficacy of pre‐existing treatments. This review aims to examine the current evidence behind pharmaceutical treatment options for canine osteoarthritis, including non‐steroidal anti‐inflammatory drugs, piprants, monoclonal antibodies, adjunctive analgesics, structure modifying osteoarthritis drugs and regenerative therapies.
INTRODUCTION
Osteoarthritis (OA) is a significant cause of pain, lameness and morbidity in dogs and many other species, including humans, across the world (Brown et al. 2013b, Knazovicky et al. 2016, Anderson et al. 2018, Cui et al. 2020). It is a multi‐factorial, progressive, degenerative disease of synovial joints, affecting not only the articular cartilage but also other structures within the specific synovial joint (Loeser et al. 2012). Degradation of articular cartilage, subchondral bone sclerosis, osteophytosis, varying degrees of synovitis, meniscal and ligament degeneration are all characteristics of the disease process. However, there is still much to be understood regarding the underlying pathogenesis of OA (Glyn‐Jones et al. 2015).
Canine OA most commonly arises as a result of inciting factors, such as coxofemoral (hip) joint dysplasia, elbow dysplasia, cranial cruciate ligament (CCL) disease, patella luxation, limb malformations and articular fractures (Johnston 1997). A number of risk factors for canine OA have been identified, including genetic predispositions, diet and obesity, all of which can play a role in disease progression (Anderson et al. 2018).
Estimates of the prevalence of dogs presenting with OA to primary care veterinary practices in the UK vary depending on the study, but have recently been reported to be between 2.5% (Anderson et al. 2018) and 6.6% (O'Neill et al. 2014b). A previous study of dogs attending referral hospitals in the USA reported an estimated prevalence of OA of up to 20% in dogs over 1‐year old (Johnston 1997). The true prevalence of the disease, however, is likely to be higher when unreported cases and the discrepancies in recording systems are taken into consideration (O'Neill et al. 2014a). With an estimated UK canine population of around 12.5 million (Pet Food Manufacturers Association 2021) and 77 million in the USA (American Veterinary Medical Association 2017), this represents a significant number of affected dogs, as well as a substantial number of dog owners and caregivers charged with the responsibility (and economic cost) of managing their treatment (Belshaw et al. 2020).
As OA, therefore, presents a welfare problem for many dogs, it is important that information regarding treatment options for these animals is both up to date and evidence based, aiding veterinary practitioners and dog owners in effectively managing these cases. With a plethora of treatment options available, and novel drugs having gained market authorisation for the treatment of canine OA in the last decade, the aim of this review is to examine the recent evidence in the literature underpinning the pharmaceutical treatment options for the management of canine OA. In this review, pharmaceutical treatments of canine OA will be discussed, focussing on novel therapies and updated evidence for existing treatments.
Some of the studies referenced in this review compare pharmaceutical treatments of OA to a placebo. It should be noted that using a placebo as a control in a disease that is known to be painful and for which there are licensed treatments available has ethical implications. In the UK, these would, as a minimum, require an Animal Test Certificate from the Veterinary Medicines Directorate or may require authorisation under the Animal (Scientific Procedures) Act 1986 depending on the nature of the study (Veterinary Medicine Directorate 2018). As an alternative to placebo‐controlled trials, some studies use a medication with known efficacy as a positive control to compare to the treatment group (Reymond et al. 2012). In these cases, the term “non‐inferior” is used if the treatment group is not worse than the positive control (Freise et al. 2013).
Licensing guidelines for the UK market will be used as standard unless stated otherwise. Veterinary practitioners should also be aware of non‐pharmaceutical therapies in the non‐surgical management of canine OA, such as weight management and physiotherapy, enabling a multi‐modal treatment approach to this disease.
PAIN MECHANISMS IN OA
OA is a painful chronic disease. The pain mechanisms involved are complex (Fu et al. 2018). Both peripheral components of pain and central processes are involved, with nociceptive, inflammatory and neuropathic types of pain occurring to varying degrees (White & Hunt 2019). There are several classes of analgesic medications available, with different mechanisms of action, targeting nociception at different steps along the pain pathway (Fig 1). With this in mind, it is important to consider multi‐modal analgesia and management when treating canine OA patients if an insufficient response to one type of medication is shown (Lascelles et al. 2008).
FIG 1.
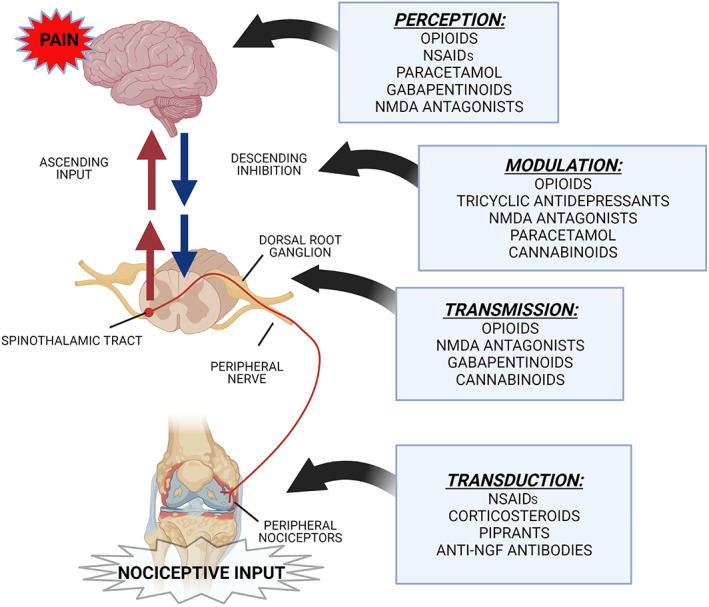
Illustration of an osteoarthritic knee joint showing the four main stages of the pain pathway in osteoarthritis and the targets of certain drugs. Transduction is the conversion of a nociceptive stimulus into electrical impulse. Transmission is the electrical impulse transmitted from peripheral sensory nerves to the central nervous system (CNS). Modulation is how the nociceptive stimulus is processed by the CNS and includes the endogenous opioid system, as well as ascending input and descending inhibitory pathways. Perception is how the brain (particularly the somatosensory cortex) interprets nociceptive inputs resulting in conscious perception of pain. Adapted from Yamaoka & Auckburally (2014)
PHARMACEUTICAL TREATMENTS OF CANINE OA
Non‐steroidal anti‐inflammatory drugs
Non‐steroidal anti‐inflammatory drugs (NSAIDs) have been first‐line analgesics for the management of canine OA pain for many years. Conventional NSAIDs exert their analgesic effects by inhibiting the enzyme cyclooxygenase (COX), which is responsible for the production of prostaglandins from arachidonic acid. There are two peripheral isoforms of COX: COX‐1 and COX‐2. Unwanted side effects caused by the inhibition of COX‐1, such as gastrointestinal and renal effects, have led to the development of preferential and selective COX‐2 inhibitors (Kukanich et al. 2012). The mechanism of action of NSAIDs is outlined in Fig 2.
FIG 2.
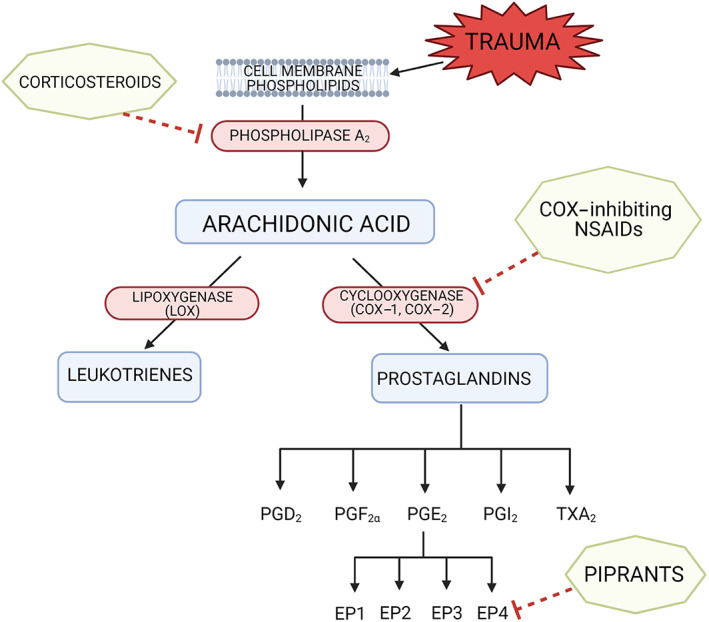
Targets of corticosteroids, COX‐inhibiting NSAIDs and piprants on the pathway of arachidonic acid metabolism. Corticosteroids inhibit phospholipase A2, preventing the conversion of phospholipids to arachidonic acid. NSAIDs inhibit cyclooxygenase, therefore preventing the production of prostaglandins from arachidonic acid. Piprants selectively antagonise the prostaglandin‐E2 EP4 receptor. Adapted from Monteiro & Steagall (2019)
The efficacy of NSAIDs in reducing pain related to OA has been well documented. Of the NSAIDs licensed for veterinary use, previous systematic reviews of treatments for canine OA have shown a high level of evidence in the literature for the efficacy of meloxicam, carprofen and firocoxib (Aragon et al. 2007, Sandersoln et al. 2009). Since the publication of these systematic reviews, four other coxibs have been licensed in the UK for the treatment of OA in dogs, namely robenacoxib (Onsior; Elanco), mavacoxib (Trocoxil; Zoetis), cimicoxib (Cimalgex; Vetoquinol) and enflicoxib (Daxocox; Animalcare Limited). Similar to firocoxib, these NSAIDs are highly selective inhibitors of COX‐2, therefore theoretically reducing the risk of adverse side effects caused by COX‐1 inhibition (Toutain et al. 2018). In humans, coxibs have been shown to increase the risk of myocardial infarction, leading to some products losing their market authorisation (Arora et al. 2020). However, this has not been to date demonstrated in dogs. Due to a lack of sufficiently powered, randomised, blinded, placebo‐controlled studies when comparing the incidence of adverse effects and analgesic efficacy of different NSAIDs, it is not known whether selective COX‐2 inhibitors are superior to preferential COX‐2 inhibitors (Monteiro‐Steagall et al. 2013).
A summary of recent clinical trials involving NSAIDs licensed for use in canine OA is shown in Table 1. One study demonstrated a similar efficacy in reducing pain related to OA with robenacoxib compared to carprofen over a 12‐week treatment period in 188 dogs (Reymond et al. 2012). Clinical trials involving cimicoxib for the treatment of OA in dogs are lacking. However, one study showed cimicoxib to provide analgesia superior to tramadol postoperatively after tibial plateau levelling osteotomy surgery (Piras et al. 2021). Enflicoxib, the most recently licensed of the coxibs, is dosed at weekly intervals, with an initial loading dose of 8 mg/kg, followed by once weekly doses of 4 mg/kg (National Office of Animal Health 2021a). A recent study involving 242 dogs randomised to receive either enflicoxib, mavacoxib or placebo, found an improvement in veterinary and owner assessment of clinical signs related to OA with both NSAIDs compared to placebo over the 6‐week trial period (Salichs et al. 2021). Mavacoxib differs from the other licensed NSAIDs, as it has a much longer half‐life, and the dose regime involves repeating the initial oral dose 14 days after the first treatment, then at monthly intervals (Lees et al. 2015). Two clinical trials examined the clinical efficacy of mavacoxib treatment in 111 and 124 dogs with naturally occurring OA compared to either meloxicam (Walton et al. 2014) or carprofen (Payne‐Johnson et al. 2015), respectively. Both studies found mavacoxib to be non‐inferior to the control NSAID over the study periods of 12 weeks and 134 days with a similar rate of adverse events. Due to its prolonged half‐life, however, there is a potential for increased risk of inadvertent overdose by owners, and an inability to cease daily dosing if unwanted side effects do occur (European Medicines Agency 2008).
Table 1.
A summary of studies involving pharmaceutical treatments of canine osteoarthritis published from 2008 to 2021
| Class of drug | Drug | Clinical trials | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Author | Type of study | Comparison/control group | Study size (number of dogs) | Study period | Outcomes measured | Findings | Level of evidence (Aragon & Budsberg 2005) | ||
| NSAIDs | Robenacoxib | Reymond et al. (2012) |
Multi‐centre, prospective, randomised, blinded, positive‐controlled non‐inferiority clinical trial Clinical cases of dogs with OA |
Carprofen | 188 | 12 weeks |
Subjective (veterinary and owner assessments) Clinical pathology |
Non‐inferiority in efficacy and tolerability of robenacoxib compared to carprofen | II |
| Mavacoxib | Walton et al. (2014) |
Randomised, positive‐controlled non‐inferiority clinical trial Clinical cases of dogs with OA |
Meloxicam | 111 | 12 weeks |
CMIs: LOAD, HCPI, CBPI Objective (force plate analysis) |
Similar clinical efficacy and adverse event rates | II | |
| Payne‐Johnson et al. (2015) |
Multi‐centre, randomised, blinded, positive‐controlled non‐inferiority clinical trial Clinical cases of dogs with OA |
Carprofen | 124 | 134 days |
Subjective owner and veterinary assessment Clinical pathology |
Mavacoxib non‐inferior to carprofen, similar safety profile | II | ||
| Enflicoxib | Salichs et al. (2021) |
Multi‐centre, randomised, blinded, placebo‐controlled clinical trial Clinical cases of dogs with OA |
Mavacoxib Placebo |
242 | 6 weeks |
Subjective veterinary assessments CMI: CBPI |
Both enflicoxib and mavacoxib groups showed significant improvement compared to placebo. Enflicoxib group had higher success rates | II | |
| Piprants | Grapiprant | Rausch‐Derra et al. (2016) |
Multi‐centre, prospective, randomised, blinded, placebo‐controlled clinical trial Clinical cases of dogs with OA |
Placebo | 265 | 28 days |
Subjective veterinary and owner assessments CMI: CBPI Clinical pathology |
Grapiprant significantly more effective than placebo | II |
| de Salazar Alcalá et al. (2019) |
Randomised, blinded, two‐way crossover study Experimentally induced OA |
Firocoxib Untreated control group |
18 | 24 hours | Objective (force plate analysis) | Firocoxib superior to grapiprant. Grapiprant not statistically different from untreated control | IV | ||
| Budsberg et al. (2019) |
Blinded, three‐way crossover study Experimentally induced OA |
Carprofen L‐766 |
5 | 48 hours |
Subjective (clinical assessment) Objective (force plate analysis) |
Carprofen superior to both grapiprant and L‐766 | IV | ||
| Anti‐NGF mAbs | Ranevetmab | Lascelles et al. (2015) |
Randomised, double‐blinded, placebo controlled, proof of principle clinical pilot study Clinical cases of dogs with OA |
Placebo | 26 | 28 days |
CMI: CSOM, CBPI, LOAD Objective (force plate analysis) |
Significant improvement with anti‐NGF mAb treatment compared to placebo | II |
| Webster et al. (2014) |
Blinded, placebo‐controlled clinical trial Clinical cases of dogs with OA |
Placebo | 11 | 6 weeks | CMI: CBPI | Anti‐NGF mAbs reduced CBPI scores for 4 weeks after treatment | II | ||
| Bedinvetmab | Corral et al. (2021) |
Double‐blinded, randomised, placebo controlled, multi‐centre clinical trial Clinical cases of dogs with OA |
Placebo |
287 (comparative phase) 89 (continuation phase) |
3‐month comparative phase followed by 6 months of continuation phase | CMI: CBPI |
Improvement with bedinvetmab compared to placebo in all assessed time points throughout 3‐month comparative phase Sustained efficacy demonstrated throughout 6‐month continuation phase |
II | |
| Opioid | Tramadol | Budsberg et al. (2018) |
Randomised, blinded, placebo‐controlled crossover study Clinical cases of dogs with OA |
Carprofen Placebo |
40 | 10 days |
CMI: CBPI Objective (force plate analysis) |
Significant improvement with carprofen. No significant difference between tramadol and placebo | II |
| Malek et al. (2012) |
Randomised, prospective, double‐blinded, placebo‐controlled clinical trial Clinical cases of dogs OA |
Carprofen ABT‐116 Placebo |
49 | 2 weeks |
Subjective veterinary assessment CMI: CBPI Objective (force‐plate analysis, accelerometer activity monitor) |
Carprofen or tramadol more effective than ABT‐116 or placebo | II | ||
| Monteiro et al. (2019) |
Randomised, double‐blinded clinical trial Clinical cases of dogs with OA, and 5 healthy dogs as control group |
Reduced dose ketoprofen alone or with tramadol Full dose ketoprofen |
25 | 28 days |
CMI: SVAPS Clinical pathology Gastrointestinal endoscopy |
Reduced dose ketoprofen and tramadol given in conjunction superior to reduced dose ketoprofen alone | II | ||
| Gabapentinoids | Gabapentin | Miles et al. (2020) |
Randomised, observer‐blinded, crossover study Clinical cases of dogs with OA |
Unspecified NSAID + Gabapentin versus Unspecified NSAID + tramadol | 24 | 28 days |
Objective (force plate analysis) Clinical pathology |
Improvement with both tramadol and gabapentin used concurrently with NSAID compared to baseline levels of NSAID alone. Gabapentin plus NSAID more effective than tramadol plus NSAID | II |
| NMDA receptor antagonists | Amantadine | Lascelles et al. (2008) |
Randomised, blinded, placebo‐controlled study Clinical cases of dogs with OA |
Meloxicam plus placebo Meloxicam plus amantadine |
31 | 6 weeks | CMI: CSOM | Meloxicam plus amantadine more effective than meloxicam plus placebo at reducing clinical signs of OA | II |
| Cannabinoid | Cannabidiol (CBD oil) | Gamble et al. (2018) |
Randomised, placebo‐controlled, blinded, crossover study Clinical cases of dogs with OA |
Placebo | 22 | 4 weeks |
Subjective (veterinary assessment, activity scores) CMI: CBPI Clinical pathology |
Decrease pain, increased activity with CBD oil | II |
| Verrico et al. (2020) |
Randomised, double‐blind, placebo‐controlled study Clinical cases of dogs with OA |
Placebo High‐dose CBD Low‐dose CBD Liposomal CBD |
20 | 4 weeks |
Subjective (veterinary and owner assessments) CMI: HCPI Clinical pathology |
Decreased pain with CBD oil treatment | II | ||
| Kogan et al. (2020) |
Pilot open clinical study Clinical cases of dogs with OA |
None | 32 | 90 days |
Subjective (veterinary and owner assessments) CMI: CODI Clinical pathology |
Improvement in clinical signs of OA with CBD treatment. Of 23 dogs, 21 receiving concurrent gabapentin were comfortable reducing or stopping gabapentin | III | ||
| Brioschi et al. (2020) |
Randomised clinical study Clinical cases of dogs with OA |
NSAID plus gabapentin plus amitriptyline with or without CBD oil | 21 | 12 weeks | CMI: CBPI | Improved CBPI scores at certain time points in CBD‐treated group | II | ||
| Mejia et al. (2021) |
Randomised placebo‐controlled, double‐blinded, crossover study Clinical cases of canine OA |
Placebo | 23 | 6 weeks |
CMI: LOAD, CBPI Objective: pressure gait analysis, accelerometery |
No significant difference between CBD group and placebo group | II | ||
| Corticosteroids | Triamcinolone hexacetonide | Alves et al. (2021) |
Randomised, double‐blinded, placebo‐controlled clinical trial Clinical cases of canine OA |
Placebo (IA saline) | 40 (20 per group) | 180 days |
CMI: LOAD, CBPI, HVAS, COI Objective: force plate analysis, pedometer, radiography, digital thermography |
Significant improvement in treated hips up to 90 days follow up after one IA injection compared to placebo | II |
| Candidate Structure Modifying OA Drugs | Intra‐articular hyaluronic acid | Carapeba et al. (2016) |
Randomised, double‐blinded, placebo‐controlled clinical trial Clinical cases of canine OA |
IA saline and carprofen and nutraceutical | 16 (8 per group) | 90 days |
Subjective: veterinary assessment CMI: HCPS, CBPI |
Improvement in outcomes in both groups over 90 days. IA HA group lower scores in some time points compared to control | II |
| Regenerative therapies | Autologous MSCs | Cuervo et al. (2014) |
Multi‐centre, randomised, blinded, parallel group trial Clinical cases of canine hip OA |
PRGF | 39 (19 ADSC, 20 PRGF) | 6 months |
Subjective: VAS, joint mobility Objective: radiography |
Both ADSCs and PRGF reduced pain and improved function | II |
| Vilar et al. (2014) |
Before and after double‐blinded clinical study Clinical cases of dogs with hip OA |
ADSCs alone or with PRGF Non‐treated control |
14 (9 treated, 5 healthy control) | 6 months | Objective: force plate analysis | ADSC therapy led to significant improvement of lameness for 30 days | III | ||
| Mohoric et al. (2016) |
Blinded, placebo controlled, clinical trial Clinical cases of bilateral canine stifle OA |
IA saline | 10 dogs (20 joints) | 1 year |
Subjective owner assessments Radiographs Synovial fluid assessment |
Nine of 10 dogs showed significant improvement up to 1 year after treatment No change in radiographic joint appearance |
II | ||
| Vilar et al. (2016) |
Before and after double‐blinded clinical study Clinical cases of dogs with hip OA |
ADSCs alone or with PRGF Non‐treated control |
14 (9 treated, 5 healthy control) | 6 months |
Subjective: VAS, Bioarth assessment scale Objective: force plate analysis |
Improvement in force plate analysis in ADSC group with or without PRGF at 30 days after treatment, but not at 90 days. Improved subjective measurements for up to 6 months | III | ||
| Srzentić Dražilov et al. (2018) |
Before and after clinical study Clinical cases of dogs with hip OA |
None | 10 | 90 days (5 followed for 1 to 4 years) | Subjective: veterinary assessment | Improvement with treatment compared to baseline over 90‐day period | III | ||
| Allogenic MSCs | Marx et al. (2014) |
Before and after pilot clinical study Clinical cases of canine hip OA |
SVF | 9 (5 ADSC, 4 SVF) | 30 days | Subjective: veterinary assessment | All SVF dogs and four of five ADSC dogs showed improvement | III | |
| Harman et al. (2016) |
Prospective randomised, blinded, placebo‐controlled efficacy study Clinical cases of dogs with hip OA |
Saline placebo | 74 (38 treated, 36 control) | 60 days |
Subjective: veterinary assessments CMI: CSOM |
Improved outcomes with ADSC treatment compared to placebo | II | ||
| Kriston‐Pál et al. (2017) |
Before and after uncontrolled clinical study Clinical cases of elbow OA |
None | 30 (39 joints) | 1 year |
Subjective owner and veterinary assessments Arthroscopic and histology exams |
Thirty of 39 joints had reported clinical improvements for up to 1 year Regenerated cartilage on arthroscopic and histology exam 1 year after treatment |
III | ||
| Shah et al. (2018) |
Before and after uncontrolled clinical study Clinical cases of dogs with OA in various joints |
None | 203 | 10 weeks | Subjective: veterinary assessment | Significant improvement with MSC treatment. IA treatment better outcome than iv treatment. Better outcomes in dogs less than 9 years old | III | ||
| Cabon et al. (2019) |
Before and after, uncontrolled, open‐labelled clinical study Clinical cases of dogs with OA in various joints |
None | 22 | 2 years | Subjective: veterinary and owner assessments | Significant improvement up to 6 months. Eight dogs received second IA injection and showed clinical improvements up to 1 year | III | ||
| PRP | Upchurch et al. (2016) |
Randomised, prospective, double‐blind, placebo‐controlled trial Clinical cases of dogs with OA |
SVF and PRP Saline placebo |
22 (12 control, 10 treated) | 24 weeks |
Subjective: veterinary assessment, VAS CMI: CBPI Objective: force plate analysis |
Improvement in treated groups at some time points compared to placebo | II | |
| Cuervo et al. (2020) |
Before and after study Clinical cases of dogs with OA |
PRP with or without physical therapy | 24 (12 PRP alone, 12 PRP + physical therapy) | 180 days | Objective: force plate analysis | Improvement with PRP, with or without physical therapy but improvement sustained for longer with physical therapy | III | ||
| Venator et al. (2020) | Before and after study. Clinical cases of dogs with OA | None | 12 | 3 months | Objective: force plate analysis | PRP injection improved kinetics for at least 4 weeks, and up to 12 weeks | III | ||
| Alves et al. (2021) |
Double blinded, negatively controlled, randomised clinical trial Clinical cases of dogs with OA |
Saline IA injection | 20 (10 per group) | 180 days | CMI: CBPI, LOAD, COI, HVAS | PRP IA injection lead to improvement in outcomes compared to control | II | ||
| Gene therapy | IL‐10 plasmid DNA therapy | Watkins et al. (2020) |
Prospective, randomised, double‐blind, placebo‐controlled study Clinical cases of dogs with OA |
Placebo (vehicle) | 14 (10 treated, 4 placebo) | 8 weeks | Subjective: VAS, veterinary and owner assessments | Treatment well tolerated, trends towards significant decreases in pain | II |
NSAID Non‐steroidal anti‐inflammatory drugs, OA Osteoarthritis, CMI Clinical metrology instruments, LOAD Liverpool osteoarthritis in dogs scale, HCPI Helsinki chronic pain index, CBPI Canine brief pain inventory, NGF Nerve growth factor, CSOM Client‐specific outcome measures, SVAPS Standardised veterinarian arthritis pain scale, NMDA N‐methyl d‐aspartate, CBD Cannabidiol, CODI Cincinnati Orthopaedic Disability Index, IA Intra‐articular, HVAS Hudson Visual Analogue Scale, COI Canine Orthopaedic Index, HA Hyaluronic acid, MSC Mesenchymal stem cell, PRGF Plasma rich in growth factors, ADSC Adipose‐derived stem cells, VAS Visual analogue score, SVF Stromal vascular fraction, PRP Platelet‐rich plasma, IL‐10 Interleukin 10
All NSAIDs have potential side effects, the most common of which include gastrointestinal signs such as vomiting, diarrhoea and inappetence, with severe side effects, including gastrointestinal ulceration and renal toxicity, being noted on datasheets of medications to happen rarely to very rarely. The true overall incidence of adverse effects, and whether there is a significant difference between licensed NSAIDs in terms of safety, is unknown (Monteiro‐Steagall et al. 2013, Hunt et al. 2015).
In summary, NSAIDs provide analgesia for dogs with OA pain, and although there are many licensed for use in dogs with OA, no one licensed NSAID has been shown to be consistently superior to another in terms of efficacy or safety.
Piprants
Grapiprant
Recently, a novel class of non‐steroidal, non‐COX inhibiting drugs, the piprants, have been pursued as treatment options for the management of canine OA. This has led to the development of a prostaglandin E2 (PGE2) EP4 receptor antagonist (PRA), grapiprant, as a licensed treatment for dogs with mild to moderate OA pain (Galliprant; Elanco).
EP4 is a receptor through which PGE2, a key mediator of inflammation and pain, exerts its effects (Nakao et al. 2007). By antagonising EP4, grapiprant blocks PGE2 mediated sensitisation of sensory neurons and PGE2 mediated inflammation, thus producing anti‐inflammatory and analgesic effects without inhibiting the production of prostaglandins as a whole (Lin et al. 2006) (Fig 2). Therefore, grapiprant could theoretically reduce the risk of side effects caused by the inhibition of COX enzymes (Kirkby Shaw et al. 2016).
Only one peer‐reviewed clinical trial involving grapiprant use in dogs for the treatment of naturally occurring OA pain has been published to date. This prospective, randomised, blinded, placebo‐controlled study involving 265 client‐owned dogs with OA confirmed by radiography in at least one appendicular joint examined the clinical effects of grapiprant (2 mg/kg once a day orally) compared to a placebo over the course of 28 days treatment (Rausch‐Derra et al. 2016). Outcomes were measured using a clinical metrology instrument (CMI), the canine brief pain inventory (CBPI) (Brown et al. 2008) and veterinary assessments, with safety measured by physical examination, clinical pathology results and owner observations. Grapiprant was generally well tolerated, and it was found to have improved pain scores compared to placebo, with a treatment success rate of 48.1% for grapiprant treated dogs compared to 31.3% for dogs receiving a placebo (P=0.0315). These measurements of treatment success were based on a previous definition by Brown et al. (2013a), using the CBPI and an overall impression score of the same or better on day 28 compared to day 0. Studies comparing the analgesic efficacy of grapiprant to NSAIDs have so far only examined acute pain control for the 24 to 48‐hour period following experimental induction of arthritis or synovitis. Both studies found the NSAID (firocoxib or carprofen) to have superior analgesic properties in that timeframe (Budsberg et al. 2019, De Salazar Alcalá et al. 2019) (Table 1). However, it should be noted that this is a very short timeframe for treatment and is in an acute pain experimental model of induced OA rather that a clinical setting. Further studies comparing the efficacy of grapiprant to that of NSAIDs would be of use in improving the evidence behind its use and as a first‐line treatment for OA pain in dogs.
Potential side effects of grapiprant include vomiting [very common (more than one in 10 animals treated)], diarrhoea and inappetence [common (between one and 10 animals in 100 animals treated)], with these side effects being generally mild and transient. In very rare cases (less than one in 10,000 animals treated), haemorrhagic diarrhoea or haematemesis has been reported (National Office of Animal Health 2021b). The recommended daily dose of grapiprant is 2 mg/kg once a day. In a study examining the safety of daily grapiprant administration at doses up to 50 mg/kg orally once a day for 9 months in healthy Beagles in an experimental setting, it was well tolerated with no renal or hepatic toxicity noted suggesting the safety of long‐term oral administration of grapiprant to dogs (Rausch‐Derra et al. 2015).
Piprants are a new class of drugs that offer a more targeted mechanism of action than COX‐inhibiting NSAIDs (Kirkby Shaw et al. 2016). However, their clinical efficacy in the long‐term treatment of canine OA compared to NSAIDs is, as yet, unknown, and larger clinical trials comparing these drugs would improve the evidence.
Paracetamol and paracetamol/codeine
Paracetamol (acetaminophen) is an analgesic and antipyretic, and is a common first‐line treatment for OA pain in humans (Onakpoya 2020). It has a complex mechanism of action, which is still not completely understood, functioning as both an inhibitor of COX peripherally and centrally, as well as acting on other central antinociception pathways such as serotonergic pathways, the endocannabinoid system and the l‐arginine/NO pathway (Przybyła et al. 2021). Paracetamol on its own is not licensed for use in dogs in the UK, but preparation of paracetamol and codeine phosphate (400 mg paracetamol/9 mg codeine phosphate) is licensed in the UK for the treatment of acute pain of traumatic origin, as a complementary treatment in pain associated with other conditions, and for postoperative analgesia (Pardale‐V, Dechra Veterinary Products). This is licensed for a treatment duration of up to 5 days. Codeine, an opioid, has a low oral bioavailability in dogs and it is unknown whether it effectively contributes to the analgesic effects of this product (Kukanich 2010).
In humans, recent Osteoarthritis Research Society International treatment guidelines no longer recommend the use of paracetamol as a single‐agent in the treatment of knee, hip and polyarticular OA (Bannuru et al. 2019). Previously, the use of paracetamol had been advised as a first‐line treatment, but evidence in recent meta‐analyses of human trials show it to have little to no efficacy in the treatment of OA in humans (Machado et al. 2015, Da Costa et al. 2017, Bannuru et al. 2019). In canines, there are no published studies examining the analgesic efficacy of paracetamol alone, in combination with codeine, or as an adjunctive analgesic with other medications such as NSAIDs in dogs with chronic OA pain. This is a gap in the current evidence base. In practice, paracetamol can be used (off licence) concurrently with an NSAID as an adjunctive analgesia, or as an alternative to an NSAID in those dogs where NSAID use is not tolerated (Pettitt & German 2015).
There is no published data on the analgesic efficacy of paracetamol in canine OA specifically, and use of Pardale‐V (Dechra) of more than 5 days duration, or concurrently with an NSAID, is off licence. This highlights a gap in the current evidence base of pharmaceutical treatments for canine OA.
Antinerve growth factor monoclonal antibodies
Nerve growth factor (NGF) is a soluble signalling protein, released from peripheral tissues in response to noxious stimuli. It has an important role in nociceptor sensitisation in both acute and chronic pain states, including OA, by increasing peripheral sensitisation through phenotypic alterations, increasing the expression of pro‐nociceptive neurotransmitters and inducing inflammatory mediator release in the periphery (Enomoto et al. 2019).
Bedinvetmab
In 2020, a canine‐specific anti‐NGF mAb product, bedinvetmab (Librela; Zoetis), received approval from the European Medicines Agency. This product is licensed in the UK as a once monthly subcutaneous injection for dogs over 12 months of age, at a dose of 0.5 to 1.0 mg/kg.
In a double‐blinded, placebo‐controlled, multi‐centre, randomised controlled trial, Corral et al. (2021) investigated the efficacy of monthly bedinvetmab injections in 287 dogs with OA. The study had a 3‐month comparative phase, where CBPI scores were used as primary outcomes in one group treated with bedinvetmab, compared to another group given a placebo. Improved CBPI scores where observed in all assessed time points in this trial in the bedinvetmab‐treated groups compared to placebo. The study then had a continuation phase, involving 89 dogs that showed clinical improvement with bedinvetmab treatment continuing with monthly injections of bedinvetmab for a further 6 months. Sustained efficacy was shown over this timeframe in terms of CBPI scores, although the study lacked objective outcome measurements such as force plate analysis.
Concurrent use of anti‐NGF mAbs with NSAIDs in humans in clinical trials has shown a rapid progression of OA (Hefti 2020). The concurrent use of bedinvetmab with an NSAID (carprofen) has only been investigated in young, healthy laboratory dogs without OA for a period of 2 weeks (Krautmann et al. 2021), and the long‐term concurrent use of these medications in dogs with OA has not been investigated. Therefore, it cannot be currently recommended to administer bedinvetmab and an NSAID concurrently. The only noted adverse reaction on the datasheet for Librela (Zoetis) is an uncommon mild reaction at the injection site (National Office of Animal Health 2021c).
Initial evidence for the effectiveness of anti‐NGF mAbs shows promise in providing an alternative treatment option for canine OA (Webster et al. 2014, Lascelles et al. 2015, Corral et al. 2021), with bedinvetmab licensed for the alleviation of pain associated with OA in dogs.
Opioids
Tramadol
Tramadol has been used as an analgesic in humans since the 1970s (Schenck & Arend 1978) and exerts its analgesic effects via several mechanisms. It is a mu‐opioid agonist and a serotonin and noradrenaline reuptake inhibitor (Grond & Sablotzki 2004). The effects on the mu‐opioid receptor are predominantly due to the tramadol metabolites, especially the O‐desmethyltramadol (M1) metabolite. Some dogs (and it is unknown what proportion) are unable to produce this metabolite, hence reducing the mu‐opioid analgesic effects in some canines (Kukanich & Papich 2004, Perez Jimenez et al. 2016).
Since 2018, there are now two licensed tramadol hydrochloride products available for use in the reduction of acute and chronic mild soft tissue and musculoskeletal pain in dogs in the UK (Tralieve; Dechra and Tramvetol; Virbac).
The evidence in the literature supporting the use of tramadol as an analgesic in the treatment of canine OA describes mixed results. Budsberg et al. (2018) compared subjective and objective outcome measurements in 40 dogs with OA when treated with either carprofen, tramadol or a placebo for 10 days. A significant improvement in all outcome measures was found with carprofen treatment, but not with placebo or tramadol. It was concluded in this study that tramadol provided no clinical benefit in the treatment of canine OA; however, the time period of the study was short, and the number of dogs in each treatment group was small. Malek et al. (2012), however, found an improvement in owner assessed mobility scores in dogs treated with either tramadol or carprofen compared to ABT‐116 [a transient receptor potential vanilloid 1 (TRPV1) antagonist] or placebo. However, no difference was found between groups on objective kinetic gait analysis. This study involved 49 dogs with OA treated for 2 weeks.
Two studies have examined concurrent use of tramadol with an NSAID compared to treatment with an NSAID alone (Monteiro et al. 2019, Miles et al. 2020). Monteiro et al. (2019) found an increased analgesic efficacy in 20 dogs when tramadol was used in conjunction with reduced dose ketoprofen over 28 days. Miles et al. (2020) also found an improvement in objective measurements of lameness such as force plate gait analysis in a group of 18 dogs receiving both tramadol and an NSAID for 28 days compared with NSAID as a sole agent.
With a limited number of clinical studies investigating the efficacy of tramadol for the treatment of canine OA pain, and the known individual variability of the production of the M1 metabolite in dogs (Kukanich & Papich 2004), it is difficult to draw firm conclusions over its clinical effectiveness. Budsberg et al. (2018) suggested that when tramadol was used as a sole analgesic for periods of less than 2 weeks, it provided insufficient analgesia for OA pain. However, there may be a beneficial role when used in conjunction with other analgesics as part of a multi‐modal approach to pain management in some dogs (Monteiro et al. 2019).
Gabapentinoids
Gabapentin and pregabalin
Gabapentin is a synthetic analogue of γ‐aminobutyric acid (GABA) and is used in human medicine as an anti‐epileptic drug, and in the treatment of chronic neuropathic pain and fibromyalgia (Calandre et al. 2016). Its mechanism of action is not fully understood, but it is believed to exert its main effects by selectively inhibiting voltage‐gated calcium channels containing the alpha2delta‐1 subunit, leading to reduced neurotransmitter release and lessening of postsynaptic excitability (Sills 2006). Pregabalin has a similar mechanism of action to gabapentin, but it has a longer half‐life and higher oral bioavailability (Salazar et al. 2009).
Previous pharmacokinetic studies have suggested a dosage of gabapentin in dogs of 10 to 20 mg/kg orally every 8 hours (Kukanich & Cohen 2011) compared to a dosage of pregabalin of 4 mg/kg every 12 hours to achieve the therapeutic plasma concentration seen in humans (Salazar et al. 2009). The therapeutic plasma concentrations in dogs are unknown and there are currently no licensed products of either gabapentin or pregabalin available for veterinary use in the UK.
There are a limited number of published studies involving the use of gabapentin and pregabalin in dogs, and only one published abstract describing a study examining the use of gabapentin as an adjunctive treatment with NSAIDs in canine OA (Miles et al. 2020). This study involved objective measurements of gait analysis of a small cohort of 24 dogs with OA, receiving either tramadol or gabapentin for 4 weeks in addition to an NSAID. Both tramadol and gabapentin led to an improvement in weight bearing (Miles et al. 2020).
The evidence for the use of gabapentinoids in canine OA is currently lacking. More published high‐quality clinical trials are needed to examine the efficacy of gabapentin and pregabalin in the dog, in order to give a greater evidence‐base behind their usage.
N‐methyl d‐aspartate receptor antagonists
Amantadine and memantine
Amantadine, first developed as an antiviral medication and also used to treat Parkinson's disease in humans, exerts its analgesic effects by antagonising N‐methyl d‐aspartate (NMDA) receptors (Fisher et al. 2000). Memantine is also an NMDA receptor antagonist and is a more potent congener of amantadine (Johnson & Kotermanski 2006). Although neither drug is licensed in dogs, suggested doses of amantadine are 3.0 to 5.0 mg/kg orally once a day. A study examining the use of memantine in the treatment of compulsive disorders in dogs suggested doses of memantine of 0.3 to 0.5 mg/kg orally twice a day initially, increasing to 1.0 mg/kg twice a day if necessary (Schneider et al. 2009).
There is only one published study examining the use of amantadine in dogs with OA (Lascelles et al. 2008). This study showed an improvement in subjective veterinary assessment of OA pain, and in the client‐specific outcome measure (CSOM) CMI in 31 dogs when amantadine was given in conjunction with meloxicam, compared to meloxicam with placebo.
NMDA antagonists could be beneficial as adjunctive analgesics in managing chronic OA pain (Lascelles et al. 2008); however, this was found in one small study, and larger clinical trials are required to improve the evidence behind their use. There are no published clinical trials involving the use of memantine in dogs with OA and both the use of amantadine and memantine are off licence in the UK.
Cannabinoids
Cannabidiol (CBD oil)
Cannabinoids have gained attention in recent years for their potential efficacy as analgesics in patients with chronic pain. They have a complex mechanism of action, acting on peripheral, spinal and supra‐spinal sites to exert antinociceptive and antihyperalgesic effects (Richardson 2000, Landa et al. 2016).
Clinical trials investigating the use of CBD oil in dogs with OA have had mixed results, and are all of low sample size, involving up to a total of 32 dogs (Table 1). Two randomised crossover studies comparing CBD oil treatment to placebo found a beneficial effect in reduction of clinical signs by subjective measurements and validated CMIs over study periods of 4 weeks in 20 and 22 dogs, respectively (Gamble et al. 2018, Verrico et al. 2020). However, in another randomised, double‐blinded, crossover study involving 23 dogs, Mejia et al. (2021) found no significant difference between CBD oil treatment and placebo over the course of 6 weeks, based on CMI outcomes and objective pressure gait analysis. Two other published studies have suggested an improvement in clinical signs of OA in dogs treated with CBD oil as part of a multi‐modal analgesic plan (Brioschi et al. 2020), and with gabapentin (Kogan et al. 2020). However, both studies have limitations. The latter study had no control group, and did not use validated pain scoring systems to subjectively measure outcomes (Kogan et al. 2020), and the former involved dogs already receiving an NSAID, gabapentin and amitriptyline (Brioschi et al. 2020). Both also involved small numbers of dogs, with sample sizes of 32 and 23, respectively.
Currently, there is no licence for the use of CBD oil in dogs in the UK, and veterinary surgeons should be aware of the legal position regarding prescribing these medications. A recently published information sheet from the British Small Animal Veterinary Association (BSAVA) outlines the current position in the UK (Wessmann et al. 2016). There is currently very limited available evidence for the efficacy of CBD oil for the treatment of canine OA pain.
Tricyclic antidepressants
Amitriptyline
Amitriptyline is a tricyclic antidepressant (TCA) and commonly used as a treatment for neuropathic pain in humans. TCAs exert their analgesic effects by several mechanisms of action, including NMDA and adrenergic receptor antagonism, serotonin and noradrenaline reuptake inhibition, voltage‐gated sodium channel blockade, and enhancement of adenosine and GABAB receptor activity (Dharmshaktu et al. 2012).
There are currently no published clinical trials investigating the analgesic efficacy of TCAs in dogs with OA pain, and there is no licensed preparation of amitriptyline for dogs.
Corticosteroids
Corticosteroids are thought to have an analgesic effect in OA due to their anti‐inflammatory action (Johnston & Budsberg 1997). Their use in OA management is controversial (Behrens et al. 1975, Murphy et al. 2000), and due to the well‐documented risks of long‐term corticosteroid use they should be used with caution if given long term. Prednoleucotropin, an oral prednisolone and cinchophen combination product has been used for the treatment of OA in dogs (McKellar et al. 1991) but is no longer licensed in the UK.
Intra‐articular (IA) injections of long‐acting preparations of corticosteroids (methylprednisolone acetate, triamcinolone acetonide and triamcinolone hexacetonide) in humans with OA have been shown to have a short‐term benefit in alleviation of pain compared to placebo (Najm et al. 2021). Methylprednisolone acetate is licensed for IA use in the dog for inflammatory conditions (Depo‐medrone V; Zoetis), therefore its use in OA (typically considered a non‐inflammatory condition, although now understood to have an inflammatory component) under the licence is debatable (Sokolove & Lepus 2013). Clinical trials into its effectiveness and safety in naturally occurring OA are lacking. A recent clinical trial found improvements in weight bearing for up to 90 days in a group of 20 dogs following a single IA injection of an unlicensed preparation of the corticosteroid triamcinolone hexacetonide in OA hip joints compared to a control group receiving a placebo (Alves et al. 2021b).
Veterinary practitioners should consider the contraindications for corticosteroids, such a septic arthritis, and safety concerns over potential cartilage damage with long term IA corticosteroids before use (Chunekamrai et al. 1989, Farquhar et al. 1996, Murphy et al. 2000). Long‐term efficacy and safety studies of IA corticosteroid use in clinical trials in dogs are lacking.
Candidate structure modifying OA drugs
Several drugs have been investigated as potential structure modifying OA drugs (SMOADs). SMOADs are defined as drugs that can delay, stabilise or repair OA lesions in affected joints rather than just alleviating the symptoms of OA (Sevalla et al. 2000, Sunaga et al. 2012). However, no evidence currently exists that these drugs are able to stabilise or repair OA lesions in vivo. Examples of these medications include pentosan polysulphate (PPS), polysulphated glycosaminoglycans (PSGAGs), hyaluronic acid (HA) and doxycycline. Little new evidence has emerged regarding the use of these treatments in canine OA over the past decade.
Sodium PPS is licensed for treatment for OA in dogs in the UK [Osteopen; Chanelle Pharma, Cartrophen Vet; Arthropharm (Europe) Ltd]. It has a wide range of pharmacological activities, including anticatabolic activities in articular cartilage, anti‐inflammatory actions, increasing hyaluronan production from synoviocytes and thrombolytic activity that could enhance blood supply to affected joints (Ghosh 1999). Studies investigating the efficacy of PPS as a treatment for OA in dogs have shown improvements in outcomes compared to placebo; however, they are limited and not all based upon objective outcomes (Bouck et al. 1995, Read et al. 1996, Innes et al. 2000, Smith et al. 2001, Budsberg et al. 2007).
The mechanisms by which PSGAGs are believed to be through the inhibition of matrix metalloprotease (MMP) enzymes, therefore having a preventive effect on matrix molecule degradation in articular cartilage (Sevalla et al. 2000). PSGAGs were found to have a moderate level of evidence in a previous systematic review based on two included randomised controlled trials, although it was concluded that further studies were needed (Sandersoln et al. 2009).
Doxycycline has been investigated as a SMOAD due to in vitro studies suggesting a potential to slow cartilage degeneration (Shlopov et al. 1999). However, evidence behind its use as a treatment for canine OA in a clinical setting is limited, and previous systematic reviews have found no evidence for its use in the treatment of canine OA (Aragon et al. 2007, Sandersoln et al. 2009).
A recent small cohort clinical trial in dogs with naturally occurring OA secondary to hip dysplasia, compared eight dogs treated with IA injections of a low molecular weight HA with a control group receiving saline IA injections plus oral carprofen and a nutraceutical containing glucosamine, chondroitin sulphate and collagen (Carapeba et al. 2016) (Table 1). Dogs in both groups showed an improvement from baseline scores when assessed by subjective measurements [CPBI, Helsinki Chronic Pain Scale (HCPS) and veterinary assessments] up to 90 days after treatment. However, a greater improvement was shown in the dogs treated with IA HA. There is currently no licensed preparation of IA HA for use in dogs in the UK, and further evidence behind its use in canine OA is required before conclusions can be drawn as to its effectiveness in clinical cases.
As this review is an update focusing primarily on recent treatments, or updated clinical evidence behind existing treatments, we refer the reader to previous systematic reviews outlining the evidence behind candidate SMOADs (Aragon et al. 2007, Sandersoln et al. 2009).
Mesenchymal stem cells
Mesenchymal stem cells (MSCs) are progenitor cells with the ability to differentiate into numerous cell types, such as cells of connective tissue, bone and cartilage (Caplan 1991). They can be derived from a variety of tissue including adipose tissue and bone marrow, which have both been shown to be a source of MSCs in dogs (Screven et al. 2014).
Although MSC treatment is often termed a regenerative therapy, the exact mechanisms by which MSCs exert their effect are still under investigation. It was previously thought that the main mechanism of action of the stem cells was that, once injected into the site of an OA lesion, they undergo differentiation into chondrocytes, therefore repairing the OA lesion (Scharstuhl et al. 2007). However, it is now thought that the effects of MSCs are exerted primarily through their secreted factors, including extracellular vesicles (EVs) and bioactive molecules such as chemokines, cytokines and growth factors, termed the secretome (Tofiño‐Vian et al. 2018, Villatoro et al. 2019). These paracrine factors have a range of immunomodulatory, anti‐inflammatory, angiogenic and anti‐apoptotic properties (Phinney & Pittenger 2017, Mocchi et al. 2020). Because of this growing knowledge of the importance of these secreted factors in the mechanism of action of MSCs, it has recently been proposed that they should now be known as “Medicinal Signalling Cells” rather than MSCs (Caplan 2017).
Adipose tissue‐derived MSCs (ADSCs) are the favoured source of MSCs for clinical use in the dog due to the relative ease of accessibility and rapid rate of proliferation in culture (Zhu et al. 2008). MSCs can be autologous, allogenic or xenogenic depending on whether the cells are derived from the same dog that will be the recipient of the cells, from a different donor, or from a different species, respectively (Cuervo et al. 2014, Cabon et al. 2019, Daems et al. 2019). In autologous canine ADSC treatment, subcutaneous adipose tissue is collected from the dog that will be the recipient of the ADSCs, and the cells are cultured and expanded in vitro, followed by IA injection into the affected joint (Cuervo et al. 2014). The process of culturing the cells in vitro ensures a consistent and enriched MSC population, but can take 7 to 10 days, therefore increasing the cost of treatment (Voga et al. 2020). Alternatively, autologous MSC preparations can be performed by minimal manipulation, allowing for a faster preparation of an MSC product patient‐side. These products, such as stromal vascular fraction contain less MSCs per millilitre, and also contain other cell types (Franklin et al. 2018).
Clinical trials involving the use of MSCs in clinical cases of dogs with OA are generally of low sample size and involve different methodologies, but have shown some beneficial outcomes. A systematic review of the treatment of naturally occurring hip OA in dogs with ADSCs has been recently published (Olsson et al. 2021). This systematic review included six clinical trials, and concluded that there was evidence that the use of ADSCs by IA injection led to an improvement in clinical signs associated with OA in dogs and that both autologous and allogenic ADSCs were well tolerated with no adverse events noted. One of these studies involved comparing 38 dogs with OA treated with IA ADSCs compared to 36 given a saline placebo, and found significant improvement in subjective CSOM in the treated group (Harman et al. 2016). Other studies included smaller numbers of dogs with 18 dogs or less. Only two studies utilised objective outcome measurements of lameness with force plate gait analysis (Vilar et al. 2014, Vilar et al. 2016), with the other four studies using subjective outcome measurements, including owner visual analogue scales (VAS) (Cuervo et al. 2014), CSOM (Harman et al. 2016, Srzentić Dražilov et al. 2018) and veterinary assessments (Cuervo et al. 2014, Marx et al. 2014, Harman et al. 2016, Vilar et al. 2016). Clinical trials have also examined the effect of MSC treatment on OA in the stifle joint and elbow joint in the dog (Mohoric et al. 2016, Kriston‐Pál et al. 2017). A larger study involving cases of OA in various joints in 203 dogs found significant improvements on subjective veterinary assessments of clinical signs of OA 10 weeks after either IA (n=128) or intravenous (iv) (n=65) treatment with an allogenic MSC preparation, although these improvements were more significant after IA than iv treatment (Shah et al. 2018). However, there was no control group in this study, and the outcomes were not assessed by a validated CMI.
The length of treatment efficacy after a single IA injection of MSCs differed between studies, and is limited by the length of some studies but has been reported to be from 1 month (Vilar et al. 2014) up to 6 months following a single IA injection (Cuervo et al. 2014, Cabon et al. 2019).
The collection of adipose tissue or bone marrow for autologous MSC preparation involves some risk, with the potential for donor site morbidity to occur, and the need for a general anaesthetic (Redondo et al. 2007, Espinel‐Rupérez et al. 2019). Allogenic MSC preparations can reduce this risk, but potentially hold a greater risk of an adverse immune response to treatment (Oliveira et al. 2017, Cabon et al. 2019). The administration of the product, like other IA products, also requires sedation or general anaesthesia (Redondo et al. 2007). However, minimal adverse effects to MSC treatment in dogs with OA have been reported in studies to date. These include local injection site reactions (Cabon et al. 2019), and a mild skin allergy in one dog (Shah et al. 2018).
A future area for development in MSC treatment research lies in examining EVs released from MSCs, as a potential cell‐free version of treatment. EVs contain a number of biologically active signalling molecules that lead to the beneficial effects of MSCs in OA treatment, and by isolating EVs for use in therapies towards OA there may be less of an immune response towards treatment (Bari et al. 2019, Li et al. 2019). This area of research, however, is still in its infancy.
In veterinary practice, there are currently no defined guidelines for the dose or frequency of MSC treatment in clinical scenarios. Several companies offer assistance in the culture and preparation of MSCs, but there is currently a lack of standardisation of protocols between companies in terms of methods and clinical approach.
Overall, MSCs show potential as an alternative, or adjunctive, treatment option in cases where conventional treatment is not providing adequate outcomes. Commercial systems are available, but there is a need for greater regulation and standardisation of methods. There is also a need for larger, multi‐centre, randomised controlled clinical trials using a standardised clinical approach and method to better evaluate outcomes in clinical cases of canine OA (Sasaki et al. 2019, Olsson et al. 2021).
Platelet‐rich plasma
Platelet‐rich plasma (PRP) is a blood‐derived product, and consists of plasma with a higher concentration of platelets than is present in peripheral blood (Rossi et al. 2019). It can also contain varying amounts of leukocytes (Carr et al. 2015, Alves et al. 2021a). Platelets are integral to blood clotting, and release growth factors such as platelet‐derived growth factor, transforming growth factor beta, epidermal growth factor and vascular endothelial growth factors (Verheul et al. 1997, Coppinger et al. 2004). These growth factors stimulate processes such as angiogenesis, and chondrocyte proliferation, and reduce processes such as chondrocyte apoptosis (Collins et al. 2021).
The clinical use of PRP therapy for canine OA involves drawing a blood sample from the affected dog, which is then processed to produce plasma highly concentrated in platelets (Perego et al. 2020). This is then injected into the IA space of the OA affected joint. Different commercial systems, both external and in‐house, for the processing of canine PRP exists, and, like MSCs, there is currently no standardised method of preparation (Carr et al. 2015, Franklin et al. 2015).
The efficacy of PRP in OA treatment in humans is still under debate, with some systematic reviews on their use in human medicine showing an overall low level of evidence (Gato‐Calvo et al. 2019), and others concluding that PRP was more efficacious than other conservative methods of OA treatment (Hong et al. 2021). In dogs, there are a limited number of clinical trials published, all involving small numbers of dogs (up to 24 dogs), and different methodologies (Cuervo et al. 2020).
Venator et al. (2020) investigated objective force plate analysis in dogs with non‐stabilised CCL rupture treated with a single IA PRP injection. The study found an improvement in kinetics for a minimum of 4 weeks after treatment, but there was no control group to act as a comparison. Upchurch et al. (2016) also found improvements in objective and subjective outcome measures in dogs with hip OA after IA and iv PRP and MSC treatment compared to placebo in 12 dogs. Another study examined objective lameness outcomes in dogs receiving IA PRP compared to another group receiving IA PRP as well as physiotherapy (Cuervo et al. 2020). Both groups improved compared to baseline levels. Five case reports of PRP treatment for refractory OA describe beneficial outcomes, but it is difficult to extrapolate these outcomes to the wider dog population due to the low number of case studies and lack of a control group (Catarino et al. 2020). A recent study by Alves et al. (2021a) compared outcomes of groups of 20 dogs with naturally occurring hip OA receiving either two injections of PRP given 14 days apart, or two IA saline injections as a negative control. Outcomes were measured by four different validated CMIs [LOAD, CBPI, Canine Orthopaedic Index (COI) and Hudson Visual Analogue Scale (HVAS)], and dogs were assessed at intervals up to 180 days after treatment. A significant improvement was found in the PRP group compared to control, lasting for approximately 130 days.
No severe adverse reactions have been reported in the literature to date, although studies have described adverse reactions related to transient pain at the injection site which usually improves within 48 to 72 hours (Alves et al. 2021a). The need for sedation or general anaesthetic to administer the IA treatment also holds some level of risk (Redondo et al. 2007).
Overall, PRP treatment requires further standardisation and regulation of different methods (Carr et al. 2015). Larger scale, multi‐centre, randomised controlled clinical trials are required before firm conclusions over its effectiveness as a treatment for canine OA in practice can be drawn.
Gene therapy
Anti‐inflammatory cytokine plasmid DNA therapy
Anti‐inflammatory cytokines, such as interleukin (IL)‐10 can inhibit the production of pro‐inflammatory cytokines, such as IL‐6, IL‐1β and tumour necrosis factor α, as well as downregulating MMP production and preventing chondrocyte apoptosis (John et al. 2007, Kapoor et al. 2011). Previous studies investigating the use of IL‐10 as an OA therapy have found limited benefits due to the short half‐life in vivo (Chernoff et al. 1995). Recent investigations into novel therapies for OA have included research into the use of gene therapies that encode for anti‐inflammatory cytokines, such as IL‐10. In a recent randomised double‐blinded placebo‐controlled clinical trial in 14 dogs with naturally occurring OA, IA targeted IL‐10 plasmid DNA therapy demonstrated some beneficial effects on reducing clinical signs of OA based on owner and veterinary subjective VAS. The same paper described safety studies, and concluded that the treatment was well tolerated (Watkins et al. 2020).
More work is required before IL‐10 plasmid DNA therapies are available commercially, but this is an area for future development in OA treatment (Schulze‐Tanzil 2021).
Conclusion
A greater understanding of the biology and pain mechanisms of OA has led to a growing number of pharmaceutical treatment options for canine OA in the past decade. With the advent of novel medications such as anti‐NGF mAbs and piprants, as well as a growing number of adjunctive analgesics, and greater availability of regenerative therapies in veterinary medicine, veterinary practitioners now have more therapeutics to offer, hopefully improving the welfare of dogs with this condition. Although a search for a curative treatment of OA is ongoing and, as yet, elusive, there are still exciting areas for future development, such as gene and mRNA therapy (Schulze‐Tanzil 2021).
This review highlights that there is a need for larger scale, randomised controlled clinical trials to improve the evidence underpinning treatments to ensure that both veterinary professionals and animal caregivers can have more confidence in the effectiveness of treatments in clinical cases. It should also be noted that a multi‐modal approach to treatment, incorporating not only different types of pharmaceuticals but also weight management, nutraceuticals, physiotherapy and other complementary therapies is important in the non‐surgical treatment of this whole joint disease (Mlacnik et al. 2006).
Conflict of interest
None of the authors of this article has a financial or personal relationship with other people or organisations that could inappropriately influence or bias the content of the paper.
Acknowledgements
Christine Pye is currently undertaking an MPhil funded by BSAVA Petsavers. Figures in this review were created using Biorender.com.
References
- Alves, J. C. , Santos, A. & Jorge, P. (2021a) Platelet‐rich plasma therapy in dogs with bilateral hip osteoarthritis. BMC Veterinary Research 17, 207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alves, J. C. , Santos, A. , Jorge, P. , et al. (2021b) The intra‐articular administration of triamcinolone hexacetonide in the treatment of osteoarthritis. Its effects in a naturally occurring canine osteoarthritis model. PLoS One 16, e0245553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Veterinary Medical Association (2017) AVMA Pet Ownership and Demographics Sourcebook. Schaumburg, IL: American Veterinary Medical Association; [Google Scholar]
- Anderson, K. L. , O'Neill, D. G. , Brodbelt, D. C. , et al. (2018) Prevalence, duration and risk factors for appendicular osteoarthritis in a UK dog population under primary veterinary care. Scientific Reports 8, 5641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aragon, C. L. & Budsberg, S. C. (2005) Applications of evidence‐based medicine: cranial cruciate ligament injury repair in the dog. Veterinary Surgery 34, 93‐98 [DOI] [PubMed] [Google Scholar]
- Aragon, C. L. , Hofmeister, E. H. & Budsberg, S. C. (2007) Systematic review of clinical trials of treatments for osteoarthritis in dogs. Journal of the American Veterinary Medical Association 230, 514‐521 [DOI] [PubMed] [Google Scholar]
- Arora, M. , Choudhary, S. , Singh, P. K. , et al. (2020) Structural investigation on the selective COX‐2 inhibitors mediated cardiotoxicity: a review. Life Sciences 251, 117631 [DOI] [PubMed] [Google Scholar]
- Bannuru, R. R. , Osani, M. C. , Vaysbrot, E. E. , et al. (2019) OARSI guidelines for the non‐surgical management of knee, hip, and polyarticular osteoarthritis. Osteoarthritis and Cartilage 27, 1578‐1589 [DOI] [PubMed] [Google Scholar]
- Bari, E. , Perteghella, S. , Catenacci, L. , et al. (2019) Freeze‐dried and GMP‐compliant pharmaceuticals containing exosomes for acellular mesenchymal stromal cell immunomodulant therapy. Nanomedicine (London, England) 14, 753‐765 [DOI] [PubMed] [Google Scholar]
- Behrens, F. , Shepard, N. & Mitchell, N. (2008) Alterations of rabbit articular cartilage by intra‐articular injections of glucocorticoids. The Journal of Bone and Joint Surgery 57, 70‐76 [PubMed] [Google Scholar]
- Belshaw, Z. , Dean, R. & Asher, L. (2020) “You can be blind because of loving them so much”: the impact on owners in the United Kingdom of living with a dog with osteoarthritis. BMC Veterinary Research 16, 1‐10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouck, G. R. , Miller, C. W. & Taves, C. L. (1995) A comparison of surgical and medical treatment of fragmented coronoid process and osteochondritis dissecans of the canine elbow. Veterinary and Comparative Orthopaedics and Traumatology 08, 177‐183 [Google Scholar]
- Brioschi, F. A. , DI Cesare, F. , Gioeni, D. , et al. (2020) Oral transmucosal cannabidiol oil formulation as part of a multimodal analgesic regimen: effects on pain relief and quality of life improvement in dogs affected by spontaneous osteoarthritis. Animals 10, 1505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown, D. C. , Boston, R. C. , Coyne, J. C. , et al. (2008) Ability of the canine brief pain inventory to detect response to treatment in dogs with osteoarthritis. Journal of the American Veterinary Medical Association 233, 1278‐1283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown, D. C. , Bell, M. & Rhodes, L. (2013a) Power of treatment success definitions when the canine brief pain inventory is used to evaluate carprofen treatment for the control of pain and inflammation in dogs with osteoarthritis. American Journal of Veterinary Research 74, 1467‐1473 [DOI] [PubMed] [Google Scholar]
- Brown, D. C. , Boston, R. C. & Farrar, J. T. (2013b) Comparison of force plate gait analysis and owner assessment of pain using the canine brief pain inventory in dogs with osteoarthritis. Journal of Veterinary Internal Medicine 27, 22‐30 [DOI] [PubMed] [Google Scholar]
- Budsberg, S. C. , Bergh, M. S. , Reynolds, L. R. , et al. (2007) Evaluation of pentosan polysulfate sodium in the postoperative recovery from cranial cruciate injury in dogs: a randomized, placebo‐controlled clinical trial. Veterinary Surgery 36, 234‐244 [DOI] [PubMed] [Google Scholar]
- Budsberg, S. C. , Torres, B. T. , Kleine, S. A. , et al. (2018) Lack of effectiveness of tramadol hydrochloride for the treatment of pain and joint dysfunction in dogs with chronic osteoarthritis. Journal of the American Veterinary Medical Association 252, 427‐432 [DOI] [PubMed] [Google Scholar]
- Budsberg, S. C. , Kleine, S. A. , Norton, M. M. , et al. (2019) Comparison of two inhibitors of E‐type prostanoid receptor four and carprofen in dogs with experimentally induced acute synovitis. American Journal of Veterinary Research 80, 1001‐1006 [DOI] [PubMed] [Google Scholar]
- Cabon, Q. , Febre, M. , Gomez, N. , et al. (2019) Long‐term safety and efficacy of single or repeated intra‐articular injection of allogeneic neonatal mesenchymal stromal cells for managing pain and lameness in moderate to severe canine osteoarthritis without anti‐inflammatory pharmacological support: pilot clinical study. Frontiers in Veterinary Science 6, 10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calandre, E. P. , Rico‐Villademoros, F. & Slim, M. (2016) Alpha(2)delta ligands, gabapentin, pregabalin and mirogabalin: a review of their clinical pharmacology and therapeutic use. Expert Review of Neurotherapeutics 16, 1263‐1277 [DOI] [PubMed] [Google Scholar]
- Caplan, A. I. (1991) Mesenchymal stem cells. Journal of Orthopaedic Research 9, 641‐650 [DOI] [PubMed] [Google Scholar]
- Caplan, A. I. (2017) Mesenchymal stem cells: time to change the name! Stem Cells Translational Medicine 6, 1445‐1451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carapeba, G. O. , Cavaleti, P. , Nicácio, G. M. , et al. (2016) Intra‐articular hyaluronic acid compared to traditional conservative treatment in dogs with osteoarthritis associated with hip dysplasia. Evidence‐based Complementary and Alternative Medicine 2016, 2076921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carr, B. J. , Canapp, S. O. Jr. , Mason, D. R. , et al. (2015) Canine platelet‐rich plasma systems: a prospective analysis. Frontiers in Veterinary Science 2, 73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catarino, J. , Carvalho, P. , Santos, S. , et al. (2020) Treatment of canine osteoarthritis with allogeneic platelet‐rich plasma: review of five cases. Open Veterinary Journal 10, 226‐231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chernoff, A. E. , Granowitz, E. V. , Shapiro, L. , et al. (1995) A randomized, controlled trial of IL‐10 in humans. Inhibition of inflammatory cytokine production and immune responses. The Journal of Immunology 154, 5492‐5499 [PubMed] [Google Scholar]
- Chunekamrai, S. , Krook, L. P. , Lust, G. , et al. (1989) Changes in articular cartilage after intra‐articular injections of methylprednisolone acetate in horses. American Journal of Veterinary Research 50, 1733‐1741 [PubMed] [Google Scholar]
- Collins, T. , Alexander, D. & Barkatali, B. (2021) Platelet‐rich plasma: a narrative review. EFORT Open Reviews 6, 225‐235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coppinger, J. A. , Cagney, G. , Toomey, S. , et al. (2004) Characterization of the proteins released from activated platelets leads to localization of novel platelet proteins in human atherosclerotic lesions. Blood 103, 2096‐2104 [DOI] [PubMed] [Google Scholar]
- Corral, M. J. , Moyaert, H. , Fernandes, T. , et al. (2021) A prospective, randomized, blinded, placebo‐controlled multisite clinical study of bedinvetmab, a canine monoclonal antibody targeting nerve growth factor, in dogs with osteoarthritis. Veterinary Anaesthesia and Analgesia 48, 943‐955 [DOI] [PubMed] [Google Scholar]
- Cuervo, B. , Rubio, M. , Sopena, J. , et al. (2014) Hip osteoarthritis in dogs: a randomized study using mesenchymal stem cells from adipose tissue and plasma rich in growth factors. International Journal of Molecular Sciences 15, 13437‐13460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuervo, B. , Rubio, M. , Chicharro, D. , et al. (2020) Objective comparison between platelet rich plasma alone and in combination with physical therapy in dogs with osteoarthritis caused by hip dysplasia. Animals (Basel) 10, 175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui, A. , Li, H. , Wang, D. , et al. (2020) Global, regional prevalence, incidence and risk factors of knee osteoarthritis in population‐based studies. EClinicalMedicine 29‐30, 100587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Da Costa, B. R. , Reichenbach, S. , Keller, N. , et al. (2017) Effectiveness of non‐steroidal anti‐inflammatory drugs for the treatment of pain in knee and hip osteoarthritis: a network meta‐analysis. Lancet 390, e21‐e33 [DOI] [PubMed] [Google Scholar]
- Daems, R. , Van Hecke, L. , Schwarzkopf, I. , et al. (2019) A feasibility study on the use of equine chondrogenic induced mesenchymal stem cells as a treatment for natural occurring osteoarthritis in dogs. Stem Cells International 2019, 4587594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Salazar Alcalá, A. G. , Gioda, L. , Dehman, A. , et al. (2019) Assessment of the efficacy of firocoxib (Previcox®) and grapiprant (Galliprant®) in an induced model of acute arthritis in dogs. BMC Veterinary Research 15, 309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dharmshaktu, P. , Tayal, V. & Kalra, B. S. (2012) Efficacy of antidepressants as analgesics: a review. Journal of Clinical Pharmacology 52, 6‐17 [DOI] [PubMed] [Google Scholar]
- Enomoto, M. , Mantyh, P. W. , Murrell, J. , et al. (2019) Anti‐nerve growth factor monoclonal antibodies for the control of pain in dogs and cats. The Veterinary Record 184, 23‐23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espinel‐Rupérez, J. , Martín‐Ríos, M. D. , Salazar, V. , et al. (2019) Incidence of surgical site infection in dogs undergoing soft tissue surgery: risk factors and economic impact. Veterinary Record Open 6, e000233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- European Medicines Agency . (2008). European Medicines Agency 2008. Trocoxil: EPAR – Scientific Discussion. https://www.ema.europa.eu/en/documents/scientific‐discussion/trocoxil‐epar‐scientific‐discussion_en.pdf. Accessed October 11, 2021
- Farquhar, T. , Todhunter, R. J. , Fubini, S. L. , et al. (1996) Effect of methylprednisolone and mechanical loading on canine articular cartilage in explant culture. Osteoarthritis and Cartilage 4, 55‐62 [DOI] [PubMed] [Google Scholar]
- Fisher, K. , Coderre, T. J. & Hagen, N. A. (2000) Targeting the N‐methyl‐d‐aspartate receptor for chronic pain management. Preclinical animal studies, recent clinical experience and future research directions. Journal of Pain and Symptom Management 20, 358‐373 [DOI] [PubMed] [Google Scholar]
- Franklin, S. P. , Garner, B. C. & Cook, J. L. (2015) Characteristics of canine platelet‐rich plasma prepared with five commercially available systems. American Journal of Veterinary Research 76, 822‐827 [DOI] [PubMed] [Google Scholar]
- Franklin, S. P. , Stoker, A. M. , Bozynski, C. C. , et al. (2018) Comparison of platelet‐rich plasma, stromal vascular fraction (SVF), or SVF with an injectable PLGA nanofiber scaffold for the treatment of osteochondral injury in dogs. The Journal of Knee Surgery 31, 686‐697 [DOI] [PubMed] [Google Scholar]
- Freise, K. J. , Lin, T. L. , Fan, T. M. , et al. (2013) Evidence‐based medicine: the design and interpretation of noninferiority clinical trials in veterinary medicine. Journal of Veterinary Internal Medicine 27, 1305‐1317 [DOI] [PubMed] [Google Scholar]
- Fu, K. , Robbins, S. R. & Mcdougall, J. J. (2018) Osteoarthritis: the genesis of pain. Rheumatology 57, iv43‐iv50 [DOI] [PubMed] [Google Scholar]
- Gamble, L. J. , Boesch, J. M. , Frye, C. W. , et al. (2018) Pharmacokinetics, safety, and clinical efficacy of cannabidiol treatment in osteoarthritic dogs. Frontiers in Veterinary Science 5, 165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gato‐Calvo, L. , Magalhaes, J. , Ruiz‐Romero, C. , et al. (2019) Platelet‐rich plasma in osteoarthritis treatment: review of current evidence. Therapeutic Advances in Chronic Disease 10, 2040622319825567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh, P. (1999) The pathobiology of osteoarthritis and the rationale for the use of pentosan polysulfate for its treatment. Seminars in Arthritis and Rheumatism 28, 211‐267 [DOI] [PubMed] [Google Scholar]
- Glyn‐Jones, S. , Palmer, A. , Agricola, R. , et al. (2015) Osteoarthritis. The Lancet 386, 376‐387 [DOI] [PubMed] [Google Scholar]
- Grond, S. & Sablotzki, A. (2004) Clinical pharmacology of tramadol. Clinical Pharmacokinetics 43, 879‐923 [DOI] [PubMed] [Google Scholar]
- Harman, R. , Carlson, K. , Gaynor, J. , et al. (2016) A prospective, randomized, masked, and placebo‐controlled efficacy study of intraarticular allogeneic adipose stem cells for the treatment of osteoarthritis in dogs. Frontiers in Veterinary Science 3, 81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hefti, F. (2020) Pharmacology of nerve growth factor and discovery of tanezumab, an anti‐nerve growth factor antibody and pain therapeutic. Pharmacological Research 154, 104240 [DOI] [PubMed] [Google Scholar]
- Hong, M. , Cheng, C. , Sun, X. , et al. (2021) Efficacy and safety of intra‐articular platelet‐rich plasma in osteoarthritis knee: a systematic review and meta‐analysis. BioMed Research International 2021, 2191926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunt, J. R. , Dean, R. S. , Davis, G. N. , et al. (2015) An analysis of the relative frequencies of reported adverse events associated with NSAID administration in dogs and cats in the United Kingdom. Veterinary Journal 206, 183‐190 [DOI] [PubMed] [Google Scholar]
- Innes, J. F. , Barr, A. R. & Sharif, M. (2000) Efficacy of oral calcium pentosan polysulphate for the treatment of osteoarthritis of the canine stifle joint secondary to cranial cruciate ligament deficiency. The Veterinary Record 146, 433‐437 [DOI] [PubMed] [Google Scholar]
- John, T. , Müller, R. , Oberholzer, A. , et al. (2007) Interleukin‐10 modulates pro‐apoptotic effects of TNF‐α in human articular chondrocytes in vitro. Cytokine 40, 226‐234 [DOI] [PubMed] [Google Scholar]
- Johnson, J. W. & Kotermanski, S. E. (2006) Mechanism of action of memantine. Current Opinion in Pharmacology 6, 61‐67 [DOI] [PubMed] [Google Scholar]
- Johnston, S. A. (1997) Osteoarthritis. Joint anatomy, physiology, and pathobiology. The Veterinary Clinics of North America. Small Animal Practice 27, 699‐723 [DOI] [PubMed] [Google Scholar]
- Johnston, S. A. & Budsberg, S. C. (1997) Nonsteroidal anti‐inflammatory drugs and corticosteroids for the management of canine osteoarthritis. Veterinary Clinics of North America: Small Animal Practice 27, 841‐862 [DOI] [PubMed] [Google Scholar]
- Kapoor, M. , Martel‐Pelletier, J. , Lajeunesse, D. , et al. (2011) Role of proinflammatory cytokines in the pathophysiology of osteoarthritis. Nature Reviews Rheumatology 7, 33 [DOI] [PubMed] [Google Scholar]
- Kirkby Shaw, K. , Rausch‐Derra, L. C. & Rhodes, L. (2016) Grapiprant: an EP 4 prostaglandin receptor antagonist and novel therapy for pain and inflammation. Veterinary Medicine and Science 2, 3‐9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knazovicky, D. , Helgeson, E. S. , Case, B. , et al. (2016) Widespread somatosensory sensitivity in naturally occurring canine model of osteoarthritis. Pain 157, 1325‐1332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kogan, L. , Hellyer, P. & Downing, R. (2020) The use of cannabidiol‐rich hemp oil extract to treat canine osteoarthritis‐related pain: a pilot study. AHVMA Journal 58, 1‐10 [Google Scholar]
- Krautmann, M. , Walters, R. , Cole, P. , et al. (2021) Laboratory safety evaluation of bedinvetmab, a canine anti‐nerve growth factor monoclonal antibody, in dogs. The Veterinary Journal 276, 105733 [DOI] [PubMed] [Google Scholar]
- Kriston‐Pál, É. , Czibula, Á. , Gyuris, Z. , et al. (2017) Characterization and therapeutic application of canine adipose mesenchymal stem cells to treat elbow osteoarthritis. Canadian Journal of Veterinary Research 81, 73‐78 [PMC free article] [PubMed] [Google Scholar]
- Kukanich, B. (2010) Pharmacokinetics of acetaminophen, codeine, and the codeine metabolites morphine and codeine‐6‐glucuronide in healthy greyhound dogs. Journal of Veterinary Pharmacology and Therapeutics 33, 15‐21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kukanich, B. & Cohen, R. L. (2011) Pharmacokinetics of oral gabapentin in greyhound dogs. Veterinary Journal 187, 133‐135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kukanich, B. & Papich, M. G. (2004) Pharmacokinetics of tramadol and the metabolite O‐desmethyltramadol in dogs. Journal of Veterinary Pharmacology and Therapeutics 27, 239‐246 [DOI] [PubMed] [Google Scholar]
- Kukanich, B. , Bidgood, T. & Knesl, O. (2012) Clinical pharmacology of nonsteroidal anti‐inflammatory drugs in dogs. Veterinary Anaesthesia and Analgesia 39, 69‐90 [DOI] [PubMed] [Google Scholar]
- Landa, L. , Sulcova, A. & Gbelec, P. (2016) The use of cannabinoids in animals and therapeutic implications for veterinary medicine: a review. Veterinární Medicína 61, 111‐122 [Google Scholar]
- Lascelles, B. D. X. , Gaynor, J. S. , Smith, E. S. , et al. (2008) Amantadine in a multimodal analgesic regimen for alleviation of refractory osteoarthritis pain in dogs. Journal of Veterinary Internal Medicine 22, 53‐59 [DOI] [PubMed] [Google Scholar]
- Lascelles, B. D. X. , Knazovicky, D. , Case, B. , et al. (2015) A canine‐specific anti‐nerve growth factor antibody alleviates pain and improves mobility and function in dogs with degenerative joint disease‐associated pain. BMC Veterinary Research 11, 101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lees, P. , Pelligand, L. , Elliott, J. , et al. (2015) Pharmacokinetics, pharmacodynamics, toxicology and therapeutics of mavacoxib in the dog: a review. Journal of Veterinary Pharmacology and Therapeutics 38, 1‐14 [DOI] [PubMed] [Google Scholar]
- Li, J. J. , Hosseini‐Beheshti, E. , Grau, G. E. , et al. (2019) Stem cell‐derived extracellular vesicles for treating joint injury and osteoarthritis. Nanomaterials (Basel, Switzerland) 9, 261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin, C.‐R. , Amaya, F. , Barrett, L. , et al. (2006) Prostaglandin E2 receptor EP4 contributes to inflammatory pain hypersensitivity. Journal of Pharmacology and Experimental Therapeutics 319, 1096‐1103 [DOI] [PubMed] [Google Scholar]
- Loeser, R. F. , Goldring, S. R. , Scanzello, C. R. , et al. (2012) Osteoarthritis: a disease of the joint as an organ. Arthritis and Rheumatism 64, 1697‐1707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machado, G. C. , Maher, C. G. , Ferreira, P. H. , et al. (2015) Efficacy and safety of paracetamol for spinal pain and osteoarthritis: systematic review and meta‐analysis of randomised placebo controlled trials. BMJ 350, h1225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malek, S. , Sample, S. J. , Schwartz, Z. , et al. (2012) Effect of analgesic therapy on clinical outcome measures in a randomized controlled trial using client‐owned dogs with hip osteoarthritis. BMC Veterinary Research 8, 185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marx, C. , Silveira, M. D. , Selbach, I. , et al. (2014) Acupoint injection of autologous stromal vascular fraction and allogeneic adipose‐derived stem cells to treat hip dysplasia in dogs. Stem Cells International 2014, 391274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKellar, Q. A. , Pearson, T. , Galbraith, E. A. , et al. (1991) Pharmacokinetics and clinical efficacy of a cinchophen and prednisolone combination in the dog. Journal of Small Animal Practice 32, 53‐58 [Google Scholar]
- Mejia, S. , Duerr, F. M. , Griffenhagen, G. , et al. (2021) Evaluation of the effect of cannabidiol on naturally occurring osteoarthritis‐associated pain: a pilot study in dogs. Journal of the American Animal Hospital Association 57, 81‐90 [DOI] [PubMed] [Google Scholar]
- Miles, J. , Bøjesen, J. , Christensen, P. , et al. (2020) Tramadol and gabapentin improve peak vertical force in osteoarthritic dogs already receiving non‐steroidal anti‐inflammatory drugs. BSAVA Congress Proceedings 2020. British Small Animal Veterinary Association
- Mlacnik, E. , Bockstahler, B. A. , Müller, M. , et al. (2006) Effects of caloric restriction and a moderate or intense physiotherapy program for treatment of lameness in overweight dogs with osteoarthritis. Journal of the American Veterinary Medical Association 229, 1756‐1760 [DOI] [PubMed] [Google Scholar]
- Mocchi, M. , Dotti, S. , Bue, M. D. , et al. (2020) Veterinary regenerative medicine for musculoskeletal disorders: can mesenchymal stem/stromal cells and their secretome be the new frontier? Cell 9(6), 1453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohoric, L. , Zorko, B. , Ceh, K. , et al. (2016) Blinded placebo study of bilateral osteoarthritis treatment using adipose derived mesenchymal stem cells. Slovenian Veterinary Research 53(3), 167‐174 [Google Scholar]
- Monteiro, B. & Steagall, P. V. (2019) Antiinflammatory drugs. Veterinary Clinics of North America: Small Animal Practice 49, 993‐1011 [DOI] [PubMed] [Google Scholar]
- Monteiro, B. P. , Lambert, C. , Bianchi, E. , et al. (2019) Safety and efficacy of reduced dosage ketoprofen with or without tramadol for long‐term treatment of osteoarthritis in dogs: a randomized clinical trial. BMC Veterinary Research 15, 213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monteiro‐Steagall, B. P. , Steagall, P. V. M. & Lascelles, B. D. X. (2013) Systematic review of nonsteroidal anti‐inflammatory drug‐induced adverse effects in dogs. Journal of Veterinary Internal Medicine 27, 1011‐1019 [DOI] [PubMed] [Google Scholar]
- Murphy, D. J. , Todhunter, R. J. , Fubini, S. L. , et al. (2000) The effects of methylprednisolone on normal and monocyte‐conditioned medium‐treated articular cartilage from dogs and horses. Veterinary Surgery 29, 546‐557 [DOI] [PubMed] [Google Scholar]
- Najm, A. , Alunno, A. , Gwinnutt, J. M. , et al. (2021) Efficacy of intra‐articular corticosteroid injections in knee osteoarthritis: a systematic review and meta‐analysis of randomized controlled trials. Joint, Bone, Spine 88, 105198 [DOI] [PubMed] [Google Scholar]
- Nakao, K. , Murase, A. , Ohshiro, H. , et al. (2007) CJ‐023,423, a novel, potent and selective prostaglandin EP4 receptor antagonist with antihyperalgesic properties. Journal of Pharmacology and Experimental Therapeutics 322, 686‐694 [DOI] [PubMed] [Google Scholar]
- National Office of Animal Health . (2021a). NOAH compendium, Datasheet for Daxocox tablets for dogs (Animalcare Limited). https://www.noahcompendium.co.uk/?id=‐479652. Accessed October 11, 2021
- National Office of Animal Health . (2021b). NOAH compendium, Datasheet for Galliprant (Elanco) tablets for dogs. https://www.noahcompendium.co.uk/?id=‐472523. Accessed October 8, 2021
- National Office of Animal Health . (2021c). NOAH compendium, Datasheet for Librela® solution for injection for dogs by Zoetis UK Ltd. https://www.noahcompendium.co.uk/?id=‐478735. Accessed May 25, 2021
- Oliveira, R. L. , Chagastelles, P. C. , Sesterheim, P. , et al. (2017) In vivo immunogenic response to allogeneic mesenchymal stem cells and the role of preactivated mesenchymal stem cells cotransplanted with allogeneic islets. Stem Cells International 2017, 9824698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsson, D. C. , Teixeira, B. L. , Jeremias, T. D. S. , et al. (2021) Administration of mesenchymal stem cells from adipose tissue at the hip joint of dogs with osteoarthritis: a systematic review. Research in Veterinary Science 135, 495‐503 [DOI] [PubMed] [Google Scholar]
- Onakpoya, I. J. (2020) Paracetamol as first line for treatment of knee and hip osteoarthritis. BMJ Evidence‐Based Medicine 25, 40 [DOI] [PubMed] [Google Scholar]
- O'Neill, D. G. , Church, D. B. , McGreevy, P. D. , et al. (2014a) Approaches to canine health surveillance. Canine Genetics and Epidemiology 1, 2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Neill, D. G. , Church, D. B. , McGreevy, P. D. , et al. (2014b) Prevalence of disorders recorded in dogs attending primary‐care veterinary practices in England. PLoS One 9, e90501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payne‐Johnson, M. , Becskei, C. , Chaudhry, Y. , et al. (2015) Comparative efficacy and safety of mavacoxib and carprofen in the treatment of canine osteoarthritis. Veterinary Record 176, 284‐284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perego, R. , Spada, E. , Baggiani, L. , et al. (2020) Efficacy of a semi automated commercial closed system for autologous leukocyte‐ and platelet‐rich plasma (l‐prp) production in dogs: a preliminary study. Animals (Basel) 10, 1342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez Jimenez, T. E. , Mealey, K. L. , Grubb, T. L. , et al. (2016) Tramadol metabolism to O‐desmethyl tramadol (M1) and N‐desmethyl tramadol (M2) by dog liver microsomes: species comparison and identification of responsible canine cytochrome P‐450s (CYPs). Drug Metabolism and Disposition 44, 1963‐1972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pet Food Manufacturers Association . (2021) Pet population 2021. https://www.pfma.org.uk/pet‐population‐2021. Accessed October 8, 2021
- Pettitt, R. A. & German, A. J. (2015) Investigation and management of canine osteoarthritis. In Practice 37, 1‐8 [Google Scholar]
- Phinney, D. G. & Pittenger, M. F. (2017) Concise review: MSC‐derived exosomes for cell‐free therapy. Stem Cells 35, 851‐858 [DOI] [PubMed] [Google Scholar]
- Piras, L. A. , Mancusi, D. , Olimpo, M. , et al. (2021) Post‐operative analgesia following TPLO surgery: a comparison between cimicoxib and tramadol. Research in Veterinary Science 136, 351‐359 [DOI] [PubMed] [Google Scholar]
- Przybyła, G. W. , Szychowski, K. A. & Gmiński, J. (2021) Paracetamol – an old drug with new mechanisms of action. Clinical and Experimental Pharmacology and Physiology 48, 3‐19 [DOI] [PubMed] [Google Scholar]
- Rausch‐Derra, L. C. , Huebner, M. & Rhodes, L. (2015) Evaluation of the safety of long‐term, daily oral administration of grapiprant, a novel drug for treatment of osteoarthritic pain and inflammation, in healthy dogs. American Journal of Veterinary Research 76, 853‐859 [DOI] [PubMed] [Google Scholar]
- Rausch‐Derra, L. , Huebner, M. , Wofford, J. , et al. (2016) A prospective, randomized, masked, placebo‐controlled multisite clinical study of grapiprant, an EP4 prostaglandin receptor antagonist (PRA), in dogs with osteoarthritis. Journal of Veterinary Internal Medicine 30, 756‐763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Read, R. A. , Cullis‐Hill, D. & Jones, M. P. (1996) Systemic use of pentosan polysulphate in the treatment of osteoarthritis. The Journal of Small Animal Practice 37, 108‐114 [DOI] [PubMed] [Google Scholar]
- Redondo, J. I. , Rubio, M. , Soler, G. , et al. (2007) Normal values and incidence of cardiorespiratory complications in dogs during general anaesthesia. A review of 1281 cases. Journal of Veterinary Medicine Series A 54, 470‐477 [DOI] [PubMed] [Google Scholar]
- Reymond, N. , Speranza, C. , Gruet, P. , et al. (2012) Robenacoxib vs. carprofen for the treatment of canine osteoarthritis; a randomized, noninferiority clinical trial. Journal of Veterinary Pharmacology and Therapeutics 35, 175‐183 [DOI] [PubMed] [Google Scholar]
- Richardson, J. D. (2000) Cannabinoids modulate pain by multiple mechanisms of action. The Journal of Pain 1, 2‐14 14622836 [Google Scholar]
- Rossi, L. A. , Murray, I. R. , Chu, C. R. , et al. (2019) Classification systems for platelet‐rich plasma. The Bone & Joint Journal 101‐B, 891‐896 [DOI] [PubMed] [Google Scholar]
- Salazar, V. , Dewey, C. W. , Schwark, W. , et al. (2009) Pharmacokinetics of single‐dose oral pregabalin administration in normal dogs. Veterinary Anaesthesia and Analgesia 36, 574‐580 [DOI] [PubMed] [Google Scholar]
- Salichs, M. , Badiella, L. , Sarasola, P. , et al. (2021) Efficacy and safety of enflicoxib for treatment of canine osteoarthritis: a 6‐week randomised, controlled, blind, multicentre clinical trial. Veterinary Record n/a, e949 [DOI] [PubMed] [Google Scholar]
- Sandersoln, R. O. , Beata, C. , Flipo, R. M. , et al. (2009) Systematic review of the management of canine osteoarthritis. Veterinary Record 164, 418‐424 [DOI] [PubMed] [Google Scholar]
- Sasaki, A. , Mizuno, M. , Mochizuki, M. , et al. (2019) Mesenchymal stem cells for cartilage regeneration in dogs. World Journal of Stem Cells 11, 254‐269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scharstuhl, A. , Schewe, B. , Benz, K. , et al. (2007) Chondrogenic potential of human adult mesenchymal stem cells is independent of age or osteoarthritis etiology. Stem Cells 25, 3244‐3251 [DOI] [PubMed] [Google Scholar]
- Schenck, E. G. & Arend, I. (1978) The effect of tramadol in an open clinical trial (author's transl). Arzneimittel‐Forschung 28, 209‐212 [PubMed] [Google Scholar]
- Schneider, B. M. , Dodman, N. H. & Maranda, L. (2009) Use of memantine in treatment of canine compulsive disorders. Journal of Veterinary Behavior 4, 118‐126 [Google Scholar]
- Schulze‐Tanzil, G. (2021) Experimental therapeutics for the treatment of osteoarthritis. Journal of Experimental Pharmacology 13, 101‐125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Screven, R. , Kenyon, E. , Myers, M. J. , et al. (2014) Immunophenotype and gene expression profile of mesenchymal stem cells derived from canine adipose tissue and bone marrow. Veterinary Immunology and Immunopathology 161, 21‐31 [DOI] [PubMed] [Google Scholar]
- Sevalla, K. , Todhunter, R. J. , Vernier‐Singer, M. , et al. (2000) Effect of polysulfated glycosaminoglycan on DNA content and proteoglycan metabolism in normal and osteoarthritic canine articular cartilage explants. Veterinary Surgery 29, 407‐414 [DOI] [PubMed] [Google Scholar]
- Shah, K. , Drury, T. , Roic, I. , et al. (2018) Outcome of allogeneic adult stem cell therapy in dogs suffering from osteoarthritis and other joint defects. Stem Cells International 2018, 7309201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shlopov, B. V. , Smith, G. N. Jr. , Cole, A. A. , et al. (1999) Differential patterns of response to doxycycline and transforming growth factor β1 in the down‐regulation of collagenases in osteoarthritic and normal human chondrocytes. Arthritis and Rheumatism 42, 719‐727 [DOI] [PubMed] [Google Scholar]
- Sills, G. J. (2006) The mechanisms of action of gabapentin and pregabalin. Current Opinion in Pharmacology 6, 108‐113 [DOI] [PubMed] [Google Scholar]
- Smith, J. , Hannon, R. , Brunnberg, L. , et al. (2001) A randomised double blind comparator clinical study of the efficacy of sodium pentosan polysulfate injection and carprofen capsules in arthritic dogs. Journal of the Osteoarthritis Research Society International 9, S21‐S22 [Google Scholar]
- Sokolove, J. & Lepus, C. M. (2013) Role of inflammation in the pathogenesis of osteoarthritis: latest findings and interpretations. Therapeutic Advances in Musculoskeletal Disease 5, 77‐94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srzentić Dražilov, S. , Mrkovački, J. , Spasovski, V. , et al. (2018) The use of canine mesenchymal stem cells for the autologous treatment of osteoarthritis. Acta Veterinaria Hungarica 66, 376‐389 [DOI] [PubMed] [Google Scholar]
- Sunaga, T. , Oh, N. , Hosoya, K. , et al. (2012) Inhibitory effects of pentosan polysulfate sodium on MAP‐kinase pathway and NF‐κB nuclear translocation in canine chondrocytes in vitro. The Journal of Veterinary Medical Science 74, 707‐711 [DOI] [PubMed] [Google Scholar]
- Tofiño‐Vian, M. , Guillén, M. I. & Alcaraz, M. J. (2018) Extracellular vesicles: a new therapeutic strategy for joint conditions. Biochemical Pharmacology 153, 134‐146 [DOI] [PubMed] [Google Scholar]
- Toutain, C. E. , Brossard, P. , King, S. B. , et al. (2018) Six‐month safety evaluation of robenacoxib tablets (Onsior™) in dogs after daily oral administrations. BMC Veterinary Research 14, 242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Upchurch, D. A. , Renberg, W. C. , Roush, J. K. , et al. (2016) Effects of administration of adipose‐derived stromal vascular fraction and platelet‐rich plasma to dogs with osteoarthritis of the hip joints. American Journal of Veterinary Research 77, 940‐951 [DOI] [PubMed] [Google Scholar]
- Venator, K. P. , Frye, C. W. , Gamble, L. J. , et al. (2020) Assessment of a single intra‐articular stifle injection of pure platelet rich plasma on symmetry indices in dogs with unilateral or bilateral stifle osteoarthritis from long‐term medically managed cranial cruciate ligament disease. Veterinary Medicine (Auckland, N.Z.) 11, 31‐38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verheul, H. M. , Hoekman, K. , Luykx‐De Bakker, S. , et al. (1997) Platelet: transporter of vascular endothelial growth factor. Clinical Cancer Research 3, 2187‐2190 [PubMed] [Google Scholar]
- Verrico, C. D. , Wesson, S. , Konduri, V. , et al. (2020) A randomized, double‐blind, placebo‐controlled study of daily cannabidiol for the treatment of canine osteoarthritis pain. Pain 161, 2191‐2202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veterinary Medicine Directorate . (2018). Guidance: animal test certificates. https://www.gov.uk/guidance/animal‐test‐certificates. Accessed October 22, 2021
- Vilar, J. M. , Batista, M. , Morales, M. , et al. (2014) Assessment of the effect of intraarticular injection of autologous adipose‐derived mesenchymal stem cells in osteoarthritic dogs using a double blinded force platform analysis. BMC Veterinary Research 10, 143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vilar, J. M. , Cuervo, B. , Rubio, M. , et al. (2016) Effect of intraarticular inoculation of mesenchymal stem cells in dogs with hip osteoarthritis by means of objective force platform gait analysis: concordance with numeric subjective scoring scales. BMC Veterinary Research 12, 1‐10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villatoro, A. J. , Alcoholado, C. , Martín‐Astorga, M. C. , et al. (2019) Comparative analysis and characterization of soluble factors and exosomes from cultured adipose tissue and bone marrow mesenchymal stem cells in canine species. Veterinary Immunology and Immunopathology 208, 6‐15 [DOI] [PubMed] [Google Scholar]
- Voga, M. , Adamic, N. , Vengust, M. , et al. (2020) Stem cells in veterinary medicine‐current state and treatment options. Frontiers in Veterinary Science 7, 278‐278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walton, M. B. , Cowderoy, E. C. , Wustefeld‐Janssens, B. , et al. (2014) Mavacoxib and meloxicam for canine osteoarthritis: a randomised clinical comparator trial. Veterinary Record 175, 280‐280 [DOI] [PubMed] [Google Scholar]
- Watkins, L. R. , Chavez, R. A. , Landry, R. , et al. (2020) Targeted interleukin‐10 plasmid DNA therapy in the treatment of osteoarthritis: toxicology and pain efficacy assessments. Brain, Behavior, and Immunity 90, 155‐166 [DOI] [PubMed] [Google Scholar]
- Webster, R. P. , Anderson, G. I. & Gearing, D. P. (2014) Canine brief pain inventory scores for dogs with osteoarthritis before and after administration of a monoclonal antibody against nerve growth factor. American Journal of Veterinary Research 75, 532‐535 [DOI] [PubMed] [Google Scholar]
- Wessmann, A. , Stalin, K. & Kisielewicz, C . (2016) Use of cannabidiol (CBD) in dogs and cats. Scientific information sheets. British Small Animal Veterinary Association
- White, K. & Hunt, J. (2019) Chronic and osteoarthritic pain. In: BSAVA Guide to Pain Management in Small Animal Practice. Ed. Self I., Gloucester, UK: British Small Animal Veterinary Association; [Google Scholar]
- Yamaoka, T. T. & Auckburally, A. (2014) Adjunctive analgesics. The Veterinary Nurse 5, 474‐481 [Google Scholar]
- Zhu, Y. , Liu, T. , Song, K. , et al. (2008) Adipose‐derived stem cell: a better stem cell than BMSC. Cell Biochemistry and Function 26, 664‐675 [DOI] [PubMed] [Google Scholar]


