Abstract
Population adjustment methods such as matching‐adjusted indirect comparison (MAIC) are increasingly used to compare marginal treatment effects when there are cross‐trial differences in effect modifiers and limited patient‐level data. MAIC is based on propensity score weighting, which is sensitive to poor covariate overlap and cannot extrapolate beyond the observed covariate space. Current outcome regression‐based alternatives can extrapolate but target a conditional treatment effect that is incompatible in the indirect comparison. When adjusting for covariates, one must integrate or average the conditional estimate over the relevant population to recover a compatible marginal treatment effect. We propose a marginalization method based on parametric G‐computation that can be easily applied where the outcome regression is a generalized linear model or a Cox model. The approach views the covariate adjustment regression as a nuisance model and separates its estimation from the evaluation of the marginal treatment effect of interest. The method can accommodate a Bayesian statistical framework, which naturally integrates the analysis into a probabilistic framework. A simulation study provides proof‐of‐principle and benchmarks the method's performance against MAIC and the conventional outcome regression. Parametric G‐computation achieves more precise and more accurate estimates than MAIC, particularly when covariate overlap is poor, and yields unbiased marginal treatment effect estimates under no failures of assumptions. Furthermore, the marginalized regression‐adjusted estimates provide greater precision and accuracy than the conditional estimates produced by the conventional outcome regression, which are systematically biased because the measure of effect is non‐collapsible.
Keywords: causal inference, health technology assessment, indirect treatment comparison, marginal treatment effect, outcome regression, standardization
Highlights.
What is already known
Population adjustment methods such as matching‐adjusted indirect comparison (MAIC) are increasingly used to compare marginal treatment effects where there are cross‐trial differences in effect modifiers and limited patient‐level data.
Current outcome regression‐based alternatives, such as the conventional usage of simulated treatment comparison (STC), target a conditional treatment effect that is incompatible in the indirect comparison. Marginalization methods are required for compatible indirect treatment comparisons.
What is new
We present a marginalization method based on parametric G‐computation that can be easily applied where the outcome regression is a generalized linear model. The method can accommodate a Bayesian statistical framework, which integrates the analysis into a probabilistic framework, typically required for health technology assessment.
We conduct a simulation study that provides proof‐of‐principle and benchmarks the performance of parametric G‐computation against MAIC and the conventional use of STC.
Potential impact for RSM readers outside the authors' field
MAIC is the most widely used method for pairwise population‐adjusted indirect comparisons.
In the scenarios we considered, parametric G‐computation achieves more precise and more accurate estimates than MAIC, particularly when covariate overlap is poor. It yields unbiased treatment effect estimates under correct model specification.
In addition, parametric G‐computation provides lower empirical standard errors and mean square errors than the conventional approach to outcome regression, which, in addition, suffers from non‐collapsibility bias.
G‐computation methods should be considered for population‐adjusted indirect comparisons, particularly where there is limited covariate overlap, and this leads to extreme weights and small effective sample sizes in MAIC.
1. INTRODUCTION
The development of novel pharmaceuticals requires several stages, which include regulatory evaluation and, in several jurisdictions, health technology assessment (HTA). 1 To obtain regulatory approval, a new technology must demonstrate efficacy. Well‐conducted randomized controlled trials (RCTs) are the gold standard design for this purpose due to their internal validity, that is, their potential for limiting bias within the study sample. 2 Evidence supporting regulatory approval is often provided by a two‐arm RCT, typically comparing the new technology to placebo or standard of care. Then, in certain jurisdictions, HTA addresses whether the health care technology should be publicly funded by the health care system. For HTA, manufacturers must convince payers that their product offers the best “value for money” of all available options in the market. This demands more than a demonstration of efficacy 3 and will often require the comparison of treatments that have not been trialed against each other. 4
In the absence of head‐to‐head trials, indirect treatment comparisons (ITCs) are at the top of the hierarchy of evidence to inform treatment and reimbursement decisions, and are very prevalent in HTA. 5 Standard ITCs use indirect evidence obtained from RCTs through a common comparator arm. 5 , 6 These techniques are compatible with both individual patient data (IPD) and aggregate‐level data (ALD). However, they are biased when the distribution of effect measure modifiers differs across trial populations, meaning that relative treatment effects are not constant. 7
Often in HTA, there are: (1) no head‐to‐head trials comparing the interventions of interest; (2) IPD available from the manufacturer's own trial but only published ALD for the comparator(s); and (3) imbalances in effect measure modifiers across studies. Several “pairwise” methods, labeled population‐adjusted indirect comparisons, have been introduced to estimate relative treatment effects in this scenario. These include matching‐adjusted indirect comparison (MAIC), 8 based on inverse propensity score weighting, and simulated treatment comparison (STC), 9 based on outcome modeling/regression. There is a simpler alternative, crude direct post‐stratification, 10 but this fails if any of the covariates are continuous or where there are several covariates for which one must account. 11 Very recently, a relevant outcome modeling‐based approach called multilevel network meta‐regression (ML‐NMR) has been developed. 12 , 13 This incorporates larger networks of treatments and studies. The focus of this article is on the pairwise approaches but ML‐NMR is considered in the discussion.
Recommendations on the use of MAIC and STC in HTA have been provided, defining the relevant terminology and evaluating the theoretical validity of these methods. 7 , 14 However, further research must: (1) examine these methods through comprehensive simulation studies; and (2) develop novel methods for population adjustment. 7 , 14 In addition, recommendations have highlighted the importance of embedding the methods within a Bayesian framework, which allows for the principled propagation of uncertainty to the wider health economic model, 15 and is particularly appealing for “probabilistic sensitivity analysis.” 16 This is a required component in the normative framework of HTA bodies such as NICE, 15 , 16 used to characterize the impact of the uncertainty in the model inputs on decision‐making.
Recently, several simulation studies have been conducted to assess population‐adjusted indirect comparisons. 17 , 18 , 19 , 20 Remiro‐Azócar et al perform a simulation study benchmarking the performance of the typical use of MAIC and STC against the standard ITC for the Cox model and survival outcomes. 17 In this study, MAIC yields unbiased and relatively accurate treatment effect estimates under no failures of assumptions, but the robust sandwich variance may underestimate standard errors where effective sample sizes are small. In the simulation scenarios, there is some degree of overlap between the studies' covariate distributions. Nevertheless, it is well known that weighting methods like MAIC are highly sensitive to poor overlap, are not asymptotically efficient, and incapable of extrapolation. 20 , 21 With poor overlap, extreme weights may produce unstable treatment effect estimates with high variance. A related problem in finite samples is that feasible weighting solutions may not exist 22 due to separation problems where samples sizes are small and the number of covariates is large. 23 , 24
Outcome regression approaches such as STC are appealing as these tend to be more efficient than weighting, providing more stable estimators and allowing for model extrapolation. 25 , 26 We view extrapolation as an advantage because poor overlap, with small effective sample sizes and large percentage reductions in effective sample size, is a pervasive issue in HTA. 27 While extrapolation can also be viewed as a disadvantage if it is not valid, in our case it expands the range of scenarios in which population adjustment can be used.
The aforementioned simulation study 17 demonstrates that the typical usage of STC, as described by HTA guidance and recommendations, 14 produces systematically biased estimates of the marginal treatment effect, with inappropriate coverage rates, because it targets a conditional estimand instead. With the Cox model and survival outcomes, there is bias because the conditional (log) hazard ratio is non‐collapsible. In addition, the conditional estimand cannot be combined in any indirect treatment comparison or compared between studies because non‐collapsible conditional estimands vary across different covariate adjustment sets. This is a recurring problem in meta‐analysis. 28 , 29
The crucial element that has been missing from the typical usage of STC is the marginalization of treatment effect estimates. When adjusting for covariates, one must integrate or average the conditional estimate over the joint covariate distribution to recover a marginal treatment effect that is compatible in the indirect comparison. We propose a simple marginalization method based on parametric G‐computation 30 , 31 or model‐based standardization, 32 , 33 often applied in observational studies in epidemiology and medical research where treatment assignment is non‐random. In meta‐analysis, Vo et al 26 , 34 have used parametric G‐computation to transport RCT results to a specific target population. Our proposal extends these approaches to population‐adjusted indirect comparisons with limited patient‐level data.
Parametric G‐computation can be viewed as an extension to the conventional STC, making use of effectively the same outcome model. It is an outcome regression approach, thereby capable of extrapolation, that targets a marginal treatment effect. It does so by separating the covariate adjustment regression model from the evaluation of the marginal treatment effect of interest. The conditional parameters of the regression are viewed as nuisance parameters, not directly relevant to the research question. The method can be implemented in a Bayesian statistical framework, which explicitly accounts for relevant sources of uncertainty, allows for the incorporation of prior evidence (e.g., expert opinion), and naturally integrates the analysis into a probabilistic framework, typically required for HTA. 15
In this paper, we carry out a simulation study to benchmark the performance of different versions of parametric G‐computation against MAIC and the conventional STC. The simulations provide proof‐of‐principle and are based on scenarios with binary outcomes and continuous covariates, with the log‐odds ratio as the measure of effect. The parametric G‐computation approaches achieve greater precision and accuracy than MAIC and are unbiased under no failures of assumptions. Furthermore, their marginal estimates provide greater precision than the conditional estimates produced by the conventional version of STC. While this precision comparison is irrelevant, because it is made for estimators of different estimands, it supports previous research on non‐collapsible measures of effect. 29 , 32
In section 2, we present the context and data requirements for population‐adjusted indirect comparisons. Section 3 provides a detailed description of the outcome regression methodologies. Section 4 outlines a simulation study, which evaluates the statistical properties of different approaches to outcome regression with respect to MAIC. Section 5 describes the results from the simulation study. An extended discussion of our findings is presented in section 6.
2. CONTEXT
Consider an active treatment A, which needs to be compared to another active treatment B for the purposes of reimbursement. Treatment A is new and being tested for cost‐effectiveness, while treatment B is typically an established intervention, already on the market. Both treatments have been evaluated in a RCT against a common comparator C, for example, standard of care or placebo, but not against each other. Indirect comparisons are performed to estimate the relative treatment effect for a specific outcome. The objective is to perform the analysis that would be conducted in a hypothetical head‐to‐head RCT between A and B, which indirect treatment comparisons seek to emulate.
RCTs have different types of potential target average estimands of interest: marginal or population‐average effects, calibrated at the population level, and conditional effects, calibrated at the individual level. The former are typically, but not necessarily, estimated by an “unadjusted” analysis. This may be a simple comparison of the expected outcomes for each group or a univariable regression including only the main treatment effect. Conditional treatment effects are typically estimated by an “adjusted” analysis (e.g., a multivariable regression of outcome on treatment and covariates), accounting for prognostic variables that are pre‐specified in the protocol or analysis plan, such as prior medical/treatment history, demographics and physiological status. A recurring theme throughout this article is that the terms “conditional and adjusted (likewise marginal and unadjusted) should not be used interchangeably” because marginal need not mean unadjusted and covariate‐adjusted analyses may also target marginal estimands. 29 , 35
The marginal effect would be the average effect, at the population level (conditional on the entire population distribution of covariates), of moving all individuals in the trial population between two hypothetical worlds: from one where everyone receives treatment B to one where everyone receives treatment A. 36 , 37 The conditional effect corresponds to the average treatment effect at the unit level, conditional on the effects of the covariates that have also been included in the model. This would be the average effect of switching the treatment of an individual in the trial population from B to A, fully conditioned on the average combination of subject‐level covariates, or the average effect across sub‐populations of subjects who share the same covariates. Population‐adjusted indirect comparisons are used to inform reimbursement decisions in HTA at the population level. Therefore, marginal treatment effect estimates are required. 35
The indirect comparison between treatments A and B is typically carried out in the “linear predictor” scale; 5 , 6 namely, using additive effects for a given linear predictor, for example, log‐odds ratio for binary outcomes or log hazard ratio for survival outcomes. Indirect treatment comparisons can be “anchored” or “unanchored.” Anchored comparisons make use of a connected treatment network. In this case, this is available through a common comparator C. Unanchored comparisons use disconnected treatment networks or single‐arm trials and require much stronger assumptions than their anchored counterparts. 7 The use of unanchored comparisons where there is connected evidence is discouraged and often labeled as problematic. 7 , 14 This is because it does not respect within‐study randomization and is not protected from imbalances in any covariates that are prognostic of outcome (almost invariably, a larger set of covariates than the set of effect measure modifiers). Hence, our focus is on anchored comparisons.
In the standard anchored scenario, a manufacturer submitting evidence to HTA bodies has access to IPD from its own trial that compares its treatment A against the standard health technology C. The disclosure of proprietary, confidential IPD from industry‐sponsored clinical trials is rare. Hence, individual‐level data on baseline covariates, treatment and outcomes for the competitor's trial, evaluating the relative efficacy or effectiveness of intervention B versus C, are regularly unavailable, for both the submitting company and the national HTA agency assessing the evidence. For this study, only summary outcome measures and marginal moments of the covariates, for example, means with standard deviations for continuous variables or proportions for binary and categorical variables, as found in a table of baseline characteristics in clinical trial publications, are available. We consider, without loss of generality, that IPD are available for a study comparing treatments A and C (denoted AC) and published ALD are available for a study comparing interventions B and C (BC).
Standard ITCs such as the Bucher method 6 assume that there are no differences across trials in effect measure modifiers, effect modifiers for short. The relative effect of a particular intervention, as measured on a specific scale, varies at different levels of the effect modifiers. Within the biostatistics literature, effect modification is usually referred to as heterogeneity or interaction, because effect modifiers are considered to alter the effect of treatment by interacting with it on a specific scale, 38 and are typically detected by examining statistical interactions. 39
Consider that Z denotes a treatment indicator. Active treatment A is denoted Z = 1, active treatment B is denoted Z = 2, and the common comparator C is denoted Z = 0. In addition, S denotes a specific study. The AC study, comparing treatments A and C is denoted S = 1. The BC study is denoted S = 2. The true relative treatment effect between Z and Z ′ in study population S is indicated by and is estimated by .
In standard ITCs, one assumes that the A versus C treatment effect in the AC population is equal to , the effect that would have occurred in the BC population. Note that the Bucher method and most conventional network meta‐analysis methods do not explicitly specify a target population of policy interest (whether this is AC, BC or otherwise). 40 Hence, they cannot account for differences across study populations. The Bucher method is only valid when either: (1) the A versus C treatment effect is homogeneous, such that there is no effect modification; or (2) the distributions of the effect modifiers are the same in both studies.
If the A versus C treatment effect is heterogeneous and the effect modifiers are not equidistributed across trials, relative treatment effects are no longer constant across the trial populations, except in the pathological case where the bias induced by different effect modifiers is in opposite directions and cancels out. Hence, the assumptions of the Bucher method are broken. In this scenario, standard ITC methods are liable to produce biased and overprecise estimates of the treatment effect. 41 These features are undesirable, particularly from the economic modeling point of view, as they impact negatively on the probabilistic sensitivity analysis.
Conversely, MAIC and STC target the A versus C treatment effect that would be observed in the BC population, thereby performing an adjusted indirect comparison in such population. MAIC and STC implicitly assume that the target population is the BC population. The estimate of the adjusted A versus B treatment effect is:
| (1) |
where is the estimated relative treatment effect of A versus C (mapped to the BC population), and is the estimated marginal treatment effect of B versus C (in the BC population). The estimate and an estimate of its variance may be directly published or derived non‐parametrically from crude aggregate outcomes made available in the literature. The majority of RCT publications will report an estimate targeting a marginal treatment effect, derived from a simple regression of outcome on a single independent variable, treatment assignment. In addition, the estimate should target a marginal treatment effect for reimbursement decisions at the population level. Therefore, should target a marginal treatment effect that is compatible with . 42
As the relative effects, and , are specific to separate studies, the within‐trial randomization of the originally assigned patient groups is preserved. Because the estimates are based on different study samples (IPD are unavailable for BC), the within‐trial relative effects are assumed statistically independent of each other. Hence, their variances are simply summed to estimate the variance of the A versus B treatment effect. One can also take a Bayesian approach to estimating the indirect treatment comparison, in which case variances would be derived empirically from draws of the posterior density. In our opinion, a Bayesian analysis is helpful because simulation from the posterior distribution provides a framework for probabilistic decision‐making, directly allowing for both statistical estimation and inference, and for principled uncertainty propagation. 5
A reference intervention is required to define the effect modifiers. In the methods considered in this article, we are selecting the effect modifiers of treatment A with respect to C (as opposed to the treatment effect modifiers of B vs. C). This is because we have to adjust for these in order to perform the indirect comparison in the BC population, implicitly assumed to be the target population. If one had access to IPD for the BC study and only published ALD for the AC study, one would have to adjust for the factors modifying the effect of treatment B with respect to C, in order to perform the comparison in the AC population.
In some contexts, a distinction is made between sample‐average and population‐average marginal effects. 11 , 43 In this article, “population‐adjusted” indirect comparisons refer to “sample‐adjusted” indirect comparisons because, due to patient‐level data limitations, the methods contrast treatments in the BC trial sample. Typically, an implicit assumption is that the sample on which inferences are made (as described by its published covariate summaries for ) is exactly the trial's target population. Alternatively, the assumption is that the study sample is a random sample, that is, representative, of such population, ignoring sampling variability in the patients' baseline characteristics and assuming that no random error attributable to such exists. In reality, the subjects of the BC study have been sampled from a, typically more diverse, target population of eligible patients, defined by the trial's inclusion and exclusion criteria.
2.1. Some assumptions
MAIC and the outcome regression methods discussed in this article mostly require the same set of assumptions. A non‐technical description of these is detailed in Appendix S1B, along with some discussion about potential failures of assumptions and their consequences in the context of the simulation study. The assumptions are:
Internal validity of the AC and BC trials, for example appropriate randomization and sufficient sample sizes so that the treatment groups are comparable, no interference, negligible measurement error or missing data, and the absence of non‐compliance.
Consistency under parallel studies such that both trials have identical control treatments, sufficiently similar study designs and outcome measure definitions, and have been conducted in care settings with a high degree of similarity.
Accounting for all effect modifiers of treatment A versus C in the adjustment. This assumption is called the conditional constancy of the A versus C marginal treatment effect, 14 and requires that a sufficiently rich set of baseline covariates has been measured for the AC study and is available in the BC study publication.*
Overlap between the covariate distributions in AC and BC. More specifically, that the ranges of the selected covariates in the AC trial cover their respective moments in the BC population. The overlap assumption (often referred to as “positivity”) can be overcome in outcome regression if one is willing to rely on model extrapolation, assuming correct model specification. 37
Correct specification of the BC population. Namely, that it is appropriately represented by the information available to the analyst, that does not have access to patient‐level data from the BC study. As the full joint distribution of covariates is unavailable for BC, this population is characterized by a combination of the covariate moments published for the BC study, and some assumptions about the covariates' correlation structure and marginal distribution forms.
Correct (typically parametric) model specification. This assumption is different for MAIC and outcome regression. In MAIC, a logistic regression is used to model the trial assignment odds conditional on a selected set of baseline covariates. The weights estimated by the model represent the “trial selection” odds, namely, the odds of being enrolled in the BC trial.† MAIC does not explicitly require an outcome model. On the other hand, outcome regression methods estimate an outcome‐generating mechanism given treatment and the baseline covariates. While MAIC relies on a correctly specified model for the weights given the covariates, outcome regression methods rely on a correctly specified model for the conditional expectation of the outcome given treatment and the covariates.
Most assumptions are causal and untestable, with their justification typically requiring prior substantive knowledge. 44 Nevertheless, we shall assume that they hold throughout the article.
3. METHODOLOGY
3.1. Data structure
For the AC trial IPD, let . Here, x is a matrix of baseline characteristics (covariates), for example, age, gender, comorbidities, baseline severity, of size N × K, where N is the number of subjects in the trial and K is the number of available covariates. For each subject , a row vector x n of K covariates is recorded. Each baseline characteristic can be classed as a prognostic variable (a covariate that affects outcome), an effect modifier, both or none. For simplicity in the notation, it is assumed that all available baseline characteristics are prognostic of the outcome and that a subset of these, x (EM) ⊆ x , is selected as effect modifiers on the linear predictor scale, with a row vector recorded for each subject. We let represent a vector of outcomes, for example, a time‐to‐event or binary indicator for some clinical measurement; and is a treatment indicator (z n = 1 if subject n is under treatment A and z n = 0 if under C). For simplicity, we shall assume that there are no missing values in . The outcome regression methodologies can be readily adapted to address this issue, particularly under a Bayesian implementation, but this is an area for future research.
We let denote the information available for the BC study. No individual‐level information on covariates, treatment or outcomes is available. Here, θ represents a vector of published covariate summaries, for example, proportions or means. For ease of exposition, we shall assume that these are available for all K covariates (otherwise, one would take the intersection of the available covariates), and that the selected effect modifiers are also available such that θ (EM) ⊆ θ . An estimate of the B versus C treatment effect in the BC population, and an estimate of its variance , either published directly or derived from crude aggregate outcomes in the literature, are also available. Note that these are not used in the adjustment mechanism but are ultimately required to perform inference for the indirect comparison in the BC population.
Finally, we let the symbol ρ stand for the dependence structure of the BC covariates. Under certain assumptions about representativeness, this can be retrieved from the AC trial, for example through the observed pairwise correlations, or from external data sources such as registries. This information, together with the published covariate summary statistics, is required to characterize the joint covariate distribution of the BC population. A pseudo‐population of N* subjects is simulated from this joint distribution, such that x * denotes a matrix of baseline covariates of dimensions N* × K, with a row vector of K covariates simulated for each subject . Notice that the value of N* does not necessarily have to correspond to the actual sample size of the BC study; however, the simulated cohort must be sufficiently large so that the sampling distribution is stabilized, minimizing sampling variability. Again, a subset of the simulated covariates, x *(EM) ⊆ x * , makes up the treatment effect modifiers on the linear predictor scale, with a row vector for each subject . In this article, the asterisk superscript represents unobserved quantities that have been constructed in the BC population.
The outcome regression approaches discussed in this article estimate treatment effects with respect to a hypothetical pseudo‐population for the BC study. Before outlining the specific outcome regression methods, we explain how to generate values for the individual‐level covariates x * for the BC population using Monte Carlo simulation.
3.2. Individual‐level covariate simulation
First, the marginal distributions for each covariate are specified. The mean and, if applicable, the standard deviation of the marginals are sourced from the BC report to match the published summary statistics. As the true marginal distributional forms are not known, some parametric assumptions are required. For instance, if it is reasonable to assume that sampling variability for a continuous covariate can be described using a normal distribution, and the covariate's mean and standard deviation are published in the BC report, we can assume that it is marginally normally distributed. Hence, we can also select the family for the marginal distribution using the theoretical validity of the candidate distributions alongside the IPD. For example, the marginal distribution of duration of prior treatment at baseline could be modeled as a log‐normal or Gamma distribution as these distributions are right‐skewed and bounded to the left by zero. Truncated distributions can be used to resemble the inclusion/exclusion criteria for continuous covariates in the BC trial, for example age limits, and avoid deterministic overlap violations.
Second, the correlations between covariates are specified. We suggest two possible data‐generating model structures for this purpose: (1) simulating the covariates from a multivariate Gaussian copula; 12 , 45 or (2) factorizing the joint distribution of the covariates into the product of marginal and conditional distributions. The former approach is perhaps more general‐purpose. The latter is more flexible, defining separate models for each variable, but its specification can be daunting where there are many covariates and interdependencies are complex.
Any multivariate joint distribution can be decomposed in terms of univariate marginal distribution functions and a dependence structure. 46 A Gaussian copula “couples” the marginal distribution functions for each covariate to a multivariate Gaussian distribution function. The main appeal of a copula is that the correlation structure of the covariates and the marginal distribution for each covariate can be modeled separately. We may use the pairwise correlation structure observed in the AC patient‐level data as the dependence structure, while keeping the marginal distributions inferred from the BC summary values and the IPD. Note that the term “Gaussian” does not refer to the marginal distributions of the covariates but to the correlation structure. While the Gaussian copula is sufficiently flexible for most modeling purposes, more complex copula types (e.g., Clayton, Gumbel, Frank) may provide different and more customizable correlation structures. 45
Alternatively, we can account for the correlations by factorizing the joint distribution of covariates in terms of marginal and conditional densities. This strategy is common in implementations of sequential conditional algorithms for parametric multiple imputation. 48 , 49 For instance, consider two baseline characteristics: age, which is a continuous variable, and the presence of a comorbidity c, which is dichotomous. We can factorize the joint distribution of the covariates such that p(age, c) = p(c∣age)p(age).
In this scenario, we draw age i for subject i from a suitable marginal distribution, for example a normal, with the mean and standard deviation sourced from the published BC summaries or official life tables. The mean of c (the conditional proportion of the comorbidity) given the age, can be modeled through a regression: , with where g(·) is an appropriate link function. Here, the coefficients and represent respectively the overall proportion of comorbidity c in the BC population (marginalizing out the age), and the correlation level between comorbidity c and (the centered version of) age. The former coefficient can be directly sourced from the published BC summaries, whereas the latter could be derived from pairwise correlations observed in the AC IPD or from external sources, for example clinical expert opinion, registries or administrative data, applying the selection criteria of the BC trial to subset the data. Figure 1 provides an example of a similar probabilistic structure with three covariates: age and the presence of two comorbidities, c and d. In this example, the distribution of the covariates is factorized such that p(age, c, d) = p(d∣c, age)p(c∣age)p(age).
FIGURE 1.
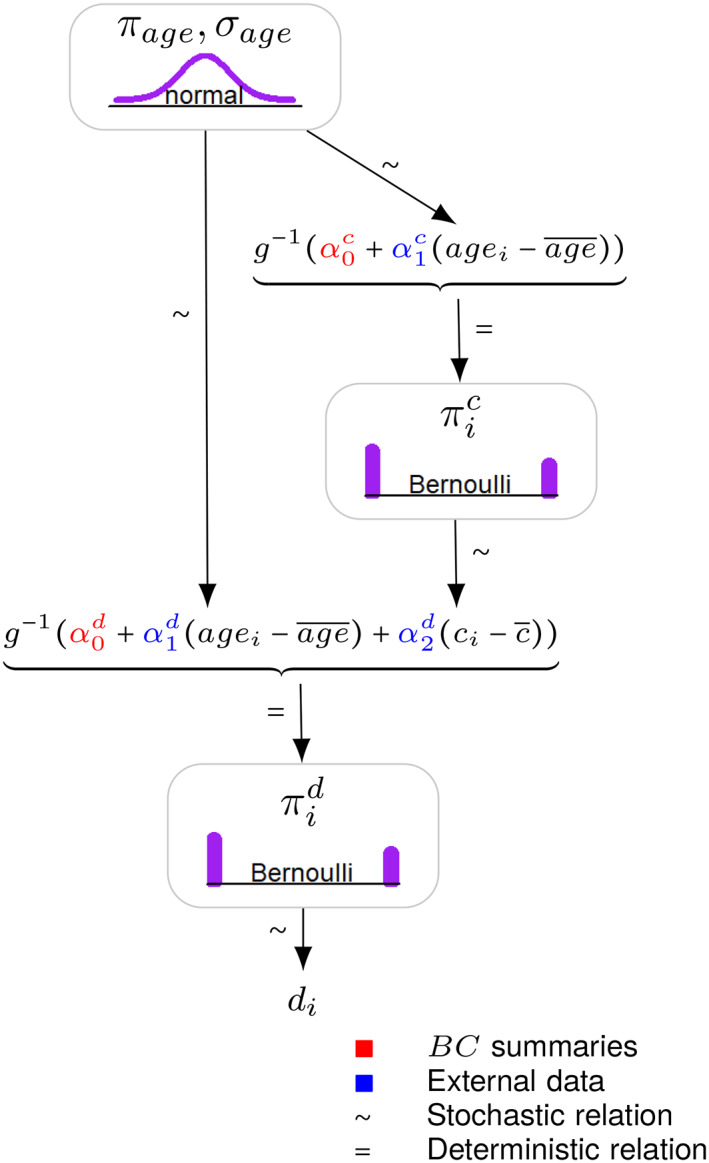
An example of individual‐level Monte Carlo covariate simulation where the joint distribution of three baseline characteristics, age, comorbidity c and comorbidity d, is factorized into the product of marginal and conditional distributions, such that p(age, c, d) = p(d∣c, age)p(c∣age)p(age). The joint distribution is valid because the conditional distributions defining the covariates are compatible: we start with a marginal distribution for age and construct the joint distribution by modeling each additional covariate, one‐by‐one, conditionally on the covariates that have already been simulated. This diagram adopts the convention of Kruschke. 47 [Colour figure can be viewed at wileyonlinelibrary.com]
3.3. Conventional outcome regression
In simulated treatment comparison (STC), 9 IPD from the AC trial are used to fit a regression model describing the observed outcomes y in terms of the relevant baseline characteristics x and the treatment variable z .
STC has different formulations. 7 , 9 , 14 , 50 In the conventional version described by the NICE Decision Support Unit Technical Support Document 18, 7 , 14 the individual‐level covariate simulation step described in section 3.2 is not performed. The covariates are centered at the published mean values θ from the BC population. Under a generalized linear modeling framework, the following regression model is fitted to the observed AC IPD:
| (2) |
where, for a generic subject n, μ n is the expected outcome on the natural scale, for example, the probability scale for binary outcomes, g(·) is an invertible canonical link function, β 0 is the intercept, β 1 is a vector of K regression coefficients for the prognostic variables, β 2 is a vector of interaction coefficients for the effect modifiers (modifying the A vs. C treatment effect) and β z is the A versus C treatment coefficient. In the population adjustment literature, covariates are sometimes centered separately for active treatment and common comparator arms. 51 , 52 We do not recommend this approach because it is likely to break randomization, distorting the balance between treatment arms A and C on covariates that are not accounted for. If these covariates are prognostic of outcome, this would compromise the internal validity of the within‐study treatment effect estimate for A versus C.
The regression in Equation 2 models the conditional outcome mean given treatment and the centered baseline covariates. Because the IPD prognostic variables and the effect modifiers are centered at the published mean values from the BC population, the estimated is directly interpreted as the A versus C treatment effect in the BC population or, more specifically, in a pseudopopulation with the BC covariate means and the AC correlation structure. Typically, analysts set in Equation 1, inputting this coefficient into the health economic decision model. 53 , 54 For uncertainty quantification purposes, the variance of said treatment effect is obtained from the standard error estimate of the treatment coefficient in the fitted model. 7 , 14
An important issue with this approach is that the treatment parameter , extracted from the fitted model, has a conditional interpretation at the individual level, because it is conditional on the baseline covariates included as predictors in the multivariable regression. 17 , 36 However, we require that (and, subsequently, ) estimate a marginal treatment effect, for reimbursement decisions at the population level. In addition, we require that is compatible with the published marginal effect for B versus C, , for comparability in the indirect treatment comparison in Equation 1. Even if the published estimate targets a conditional estimand, this cannot be combined in the indirect treatment comparison because it likely has been adjusted using a different covariate set and model specification than . 29 Therefore, the treatment coefficient does not target the estimand of interest.
With non‐collapsible effect measures such as the odds ratio in logistic regression, marginal and conditional estimands for non‐null effects do not coincide, 54 even with covariate balance and in the absence of confounding. 55 , 56 Targeting the wrong estimand may induce systematic bias, as observed in a recent simulation study. 17 Most applications of population‐adjusted indirect comparisons are in oncology 17 , 27 and are concerned with non‐collapsible measures of treatment effect such as (log) hazard ratios 36 , 55 , 57 or (log) odds ratios. 36 , 55 , 56 , 57 With both collapsible and non‐collapsible measures of effect, maximum‐likelihood estimators targeting distinct estimands will have different standard errors. Therefore, marginal and conditional estimates quantify parametric uncertainty differently, and conflating these will lead to the incorrect propagation of uncertainty to the wider health economic decision model, which will be problematic for probabilistic sensitivity analyses.
3.4. Marginalization via parametric G‐computation
The crucial element that has been missing from the typical application of outcome regression is the marginalization of the A versus C treatment effect estimate. When adjusting for covariates, one must integrate or average the conditional estimate over the joint BC covariate distribution to recover a marginal treatment effect that is compatible in the indirect comparison. Parametric G‐computation 30 , 31 , 59 is an established method for marginalizing regression‐adjusted conditional estimates. We discuss how this methodology can be used in population‐adjusted indirect comparisons.
G‐computation in this context consists of: (1) predicting the conditional outcome expectations under treatments A and C for each subject in the BC population; (2) averaging the predictions to produce marginal outcome means on the natural scale; and (3) back‐transforming the averages to the linear predictor scale, contrasting the linear predictions to estimate the marginal A versus C treatment effect in the BC population. This marginal effect is compatible in the indirect treatment comparison. This procedure is a form of standardization, a technique which has been performed for decades in epidemiology, for example when computing standardized mortality ratios. 60 Parametric G‐computation is often called model‐based standardization 32 , 33 because a parametric model is used to predict the conditional outcome expectations under each treatment. When the covariates and outcome are discrete, the estimation of the conditional expectations could be non‐parametric, in which case G‐computation is numerically identical to crude direct post‐stratification. 10
G‐computation marginalizes the conditional estimates by separating the regression modeling outlined in section 3.3 from the estimation of the marginal treatment effect for A versus C. First, a regression model of the observed outcome y on the covariates x and treatment z is fitted to the AC IPD:
| (3) |
using the notation of the AC trial data. In the context of G‐computation, this regression model is often called the “Q‐model.” Contrary to Equation 2, it is not centered on the mean BC covariates.
Having fitted the Q‐model, the regression coefficients are treated as nuisance parameters. The parameters are applied to the simulated covariates x * to predict hypothetical outcomes for each subject under both possible treatments. Namely, a pair of predicted outcomes, also called potential outcomes, 61 under A and under C, is generated for each subject.
Parametric G‐computation typically relies on maximum‐likelihood estimation to fit the regression model in Equation 3. In this case, the methodology proceeds as follows. We denote the maximum‐likelihood estimate of the regression parameters as . Leaving the simulated covariates x * at their set values, we fix the treatment values, indicated by a vector , for all N*. By plugging treatment A into the maximum‐likelihood regression fit for each simulated individual, we predict the marginal outcome mean, on the natural scale, when all subjects are under treatment A:
| (4) |
| (5) |
Equation (4) follows directly from the law of total expectation. The joint probability density function for the BC covariates is denoted p( x *), and could be replaced by a probability mass function if the covariates are discrete, or by a mixture density if there is a combination of discrete and continuous covariates. Replacing the integral by the summation in Equation (5) follows from using the empirical joint distribution of the simulated covariates as a non‐parametric estimator of the density p( x *). 29
Similar to above, by plugging treatment C into the regression fit for every simulated observation, we predict the marginal outcome mean in the hypothetical scenario in which all units are under treatment C:
| (6) |
| (7) |
To estimate the marginal or population‐average treatment effect for A versus C in the linear predictor scale, one back‐transforms to this scale the average predictions, taken over all subjects on the natural outcome scale, and calculates the difference between the average linear predictions:
| (8) |
If the outcome model in Equation (3) is correctly specified, the estimators of the marginal outcome means under each treatment should be consistent with respect to convergence to their true value, and so should the marginal treatment effect estimate.
For illustrative purposes, consider a logistic regression for binary outcomes. In this case, is the average of the individual probabilities predicted by the regression when all participants are assigned to treatment A. Similarly, is the average probability when everyone is assigned to treatment C. The inverse link function g −1(·) would be the inverse logit function , and the average predictions in the probability scale could be substituted into Equation (8) and transformed to the log‐odds ratio scale, using the logit link function. More interpretable summary measures of the marginal contrast, for example odds ratios, relative risks or risk differences, can also be produced by manipulating the average natural outcome means differently than in Equation (8), mapping these to other scales. For instance, a marginal odds ratio can be estimated as , where g(·) denotes the logit link function. The standard scale commonly used for performing indirect treatment comparisons is the log‐odds ratio scale 5 , 6 , 7 and this linear predictor scale is used to define effect modification, which is scale‐specific. 14 Hence, we assume that the marginal log‐odds ratio is the relative effect measure of interest.
Note that the estimated absolute outcomes and , for example, the average outcome probabilities under each treatment in the case of logistic regression, are sometimes desirable in health economic models without any further processing. 62 In addition, these could be useful in unanchored comparisons, where there is no common comparator group included in the analysis, for example if the competitor trial is an RCT without a common control or a single‐arm trial evaluating the effect of treatment B alone. In the unanchored case, absolute outcome means are compared across studies as opposed to relative effects. However, unanchored comparisons make very strong assumptions which are largely considered impossible to meet (absolute effects are conditionally constant as opposed to relative effects being conditionally constant). 7 , 14
The inclusion of all imbalanced effect modifiers in Equation (3) is required for unbiased estimation of both the marginal and conditional A versus C treatment effects in the BC population. 63 A strong fit of the regression model, evaluated by model checking criteria such as the residual deviance and information criteria, may increase precision. Hence, we could select the model with the lowest information criterion conditional on including all effect modifiers. 63 Model checking criteria should not guide causal decisions on effect modifier status, which should be defined prior to fitting the outcome model. As effect‐modifying covariates are likely to be good predictors of outcome, the inclusion of appropriate effect modifiers should provide an acceptable fit. In addition, note that any model comparison criteria will only provide information about the observed AC data and therefore tell just part of the story. We have no information on the fit of the selected model to the BC patient‐level data.
In Appendix S1C, we develop a parametric G‐computation approach where the nuisance model is a Cox proportional hazards regression. In this setting, and should target marginal log hazard ratios for indirect treatment comparisons in the linear predictor scale. This development is important and useful to practitioners because the most popular outcome types in applications of population‐adjusted indirect comparisons are survival or time‐to‐event outcomes (e.g., overall or progression‐free survival), and the most prevalent measure of effect is the (log) hazard ratio. 27
From a frequentist perspective, it is not easy to derive analytically a closed‐form expression for the standard error of the marginal A versus C treatment effect with non‐linear outcome models. Deriving the asymptotic distribution is not straightforward as the estimate is a non‐linear function of each of the components of . When using maximum‐likelihood estimation to fit the outcome model, standard errors and interval estimates can be obtained using resampling‐based methods such as the traditional non‐parametric bootstrap 64 or the m‐out‐of‐n bootstrap. 65 In our bootstrap implementation, we only resample the IPD of the AC trial due to patient‐level data limitations for the BC study. The standard error would be estimated as the sample standard deviation of the resampled marginal treatment effect estimates. Assuming that the sample size N is reasonably large, we can appeal to the asymptotic normality of the marginal treatment effect and construct Wald‐type normal distribution‐based confidence intervals. Alternatively, one can construct interval estimates using the relevant quantiles of the bootstrapped treatment effect estimates, without necessarily assuming normality. This avoids relying on the adequacy of the asymptotic normal approximation, an approximation which will be inappropriate where the true model likelihood is distinctly non‐normal, 66 and may allow for the more principled propagation of uncertainty.
An alternative to bootstrapping for statistical inference is to simulate the parameters of the multivariable regression in Equation (3) from the asymptotic multivariate normal distribution with means set to the maximum‐likelihood estimator and with the corresponding variance–covariance matrix, iterate over Equations ((4), (5), (6), (7), (8)) and compute the sample variance. This parametric simulation approach is less computationally intensive than bootstrap resampling. It has the same reliance on random numbers and may offer similar performance. 67 It is equivalent to approximating the posterior distribution of the regression parameters, assuming constant non‐informative priors and a large enough sample size. Again, this large‐sample formulation relies on the adequacy of the asymptotic normal approximation.
The choice of the number of bootstrap resamples is important. Given recent advances in computing power, we encourage setting this value as large as possible, in order to maximize the precision and accuracy of the treatment effect estimator, and to minimize the Monte Carlo error in the estimate. A sensible strategy is to increase the number of bootstrap resamples until repeated analyses across different random seeds give similar results, within a specified degree of accuracy.
3.5. Bayesian parametric G‐computation
A Bayesian approach to parametric G‐computation may be beneficial for several reasons. First, the maximum‐likelihood estimates of the outcome regression coefficients may be unstable where the sample size N of the AC IPD is small, the data are sparse or the covariates are highly correlated, for example due to finite‐sample bias or variance inflation. This leads to poor frequentist properties in terms of precision. A Bayesian approach with default shrinkage priors, that is, priors specifying a low likelihood of a very large effect, can reduce variance, stabilize the estimates and improve their accuracy in these cases. 59
Second, we can use external data and/or contextual information on the prognostic effect and effect‐modifying strength of covariates, for example from covariate model parameters reported in the literature, to construct informative prior distributions for β 1 and β 2 , respectively, and skeptical priors (i.e., priors with mean zero, where the variance is chosen so that the probability of a large effect is relatively low) for the conditional treatment effect β z , if necessary. Where meaningful prior knowledge cannot be leveraged, one can specify generic default priors instead. For instance, it is unlikely in practice that conditional odds ratios are outside the range 0.1–10. Therefore, we could use a null‐centered normal prior with standard deviation 1.15, which is equivalent to just over 95% of the prior mass being between 0.1 and 10. As mentioned earlier, this “weakly informative” contextual knowledge may result in shrinkage that improves accuracy with respect to maximum‐likelihood estimators. 59 Finally, it is simpler to account naturally for issues in the AC IPD such as missing data and measurement error within a Bayesian formulation. 68 , 69
In the generalized linear modeling context, consider that we use Bayesian methods to fit the outcome regression model in Equation (3). The difference between Bayesian G‐computation and its maximum‐likelihood counterpart is in the estimated distribution of the predicted outcomes. The Bayesian approach also marginalizes, integrates or standardizes over the joint posterior distribution of the conditional nuisance parameters of the outcome regression, as well as the joint covariate distribution p( x * ). Following Keil et al, 59 Rubin 70 and Saarela et al, 71 we draw a vector of size N* of predicted outcomes under each set intervention z* ∈{0, 1} from its posterior predictive distribution under the specific treatment. This is defined as , where is the posterior distribution of the outcome regression coefficients β , which encode the predictor‐outcome relationships observed in the AC trial IPD. This 59 is given by:
| (9) |
| (10) |
As noted by Keil et al, 59 the posterior predictive distribution is a function only of the observed data , the joint probability density function p( x * ) of the simulated BC pseudo‐population, which is independent of β , the set treatment values z * , and the prior distribution p( β ) of the regression coefficients.
In practice, the integrals in Equations 9 and 10 can be approximated numerically, using full Bayesian estimation via Markov chain Monte Carlo (MCMC) sampling. This is carried out as follows. As per the maximum‐likelihood procedure, we leave the simulated covariates at their set values and fix the value of treatment to create two datasets: one where all simulated subjects are under treatment A and another where all simulated subjects are under treatment C. The outcome regression model in Equation 3 is fitted to the original AC IPD with the treatment actually received. From this model, conditional parameter estimates are drawn from their posterior distribution , given the observed patient‐level data and some suitably defined prior p( β ).
It is relatively straightforward to integrate the model‐fitting and outcome prediction within a single Bayesian computation module using efficient simulation‐based sampling methods such as MCMC. Assuming convergence of the MCMC algorithm, we form realizations of the parameters , where L is the number of MCMC draws after convergence and l indexes each specific draw. Again, these conditional coefficients are nuisance parameters, not of direct interest in our scenario. Nevertheless, the samples are used to extract draws of the conditional expectations for each simulated subject i (the posterior draws of the linear predictor transformed by the inverse link function) from their posterior distribution. The l‐th draw of the conditional expectation for simulated subject i set to treatment A is:
| (11) |
Similarly, the l‐th draw of the conditional expectation for simulated subject i under treatment C is:
| (12) |
The conditional expectations drawn from Equations 11 and 12 are used to impute the individual‐level outcomes under treatment A and under treatment C, as independent draws from their posterior predictive distribution at each iteration of the MCMC chain. For instance, if the outcome model is a normal linear regression with a Gaussian likelihood, one multiplies the simulated covariates and the set treatment for each subject i by the lth random draw of the posterior distribution of the regression coefficients, given the observed IPD and some suitably defined prior, to form draws of the conditional expectation (which is equivalent to the linear predictor because the link function is the identity link in linear regression). Then each predicted outcome would be drawn from a normal distribution with mean equal to and standard deviation equal to the corresponding posterior draw of the error standard deviation. With a logistic regression as the outcome model, one would impute values of a binary response by random sampling from a Bernoulli distribution with mean equal to the expected conditional probability .
Producing draws from the posterior predictive distribution of outcomes is fairly simple using dedicated Bayesian software such as BUGS, 72 JAGS 73 or Stan, 74 where the outcome regression and prediction can be implemented simultaneously in the same module. Over the L MCMC draws, these programs typically return a L × N* matrix of simulations from the posterior predictive distribution of outcomes. The lth row of this matrix is a vector of outcome predictions of size N* using the corresponding draw of the regression coefficients from their posterior distribution. We can estimate the marginal treatment effect for A versus C in the BC population by: (1) averaging out the imputed outcome predictions in each draw over the simulated subjects, that is, over the columns, to produce the marginal outcome means on the natural scale; and (2) taking the difference in the sample means under each treatment in a suitably transformed scale. Namely, for the lth draw, the A versus C marginal treatment effect is:
| (13) |
The average, variance and interval estimates of the marginal treatment effect can be derived empirically from draws of the posterior density, that is, by taking the sample mean, variance and the relevant percentiles over the L draws, which approximate the posterior distribution of the marginal treatment effect. The computational expense of the Bayesian approach to G‐computation is expected to be similar to that of the maximum‐likelihood version, given that the latter typically requires bootstrapping for uncertainty quantification. Computational cost can be reduced by adopting approximate Bayesian inference methods such as integrated nested Laplace approximation (INLA) 75 instead of MCMC sampling to draw from the posterior predictive distribution of outcomes.
Note that Equation (13) is the Bayesian version of Equation (8). Other parameters of interest can be obtained, for example, the risk difference by using the identity link function in this equation, but these are typically not of direct relevance in our scenario. Again, where the contrast between two different interventions is not of primary interest, the absolute outcome draws from their posterior predictive distribution under each treatment may be relevant. The average, variance and interval estimates of the absolute outcomes can be derived empirically over the L draws. An argument in favor of a Bayesian approach is that, once the simulations have been conducted, one can obtain a full characterization of uncertainty on any scale of interest.
3.6. Indirect treatment comparison
The estimated marginal treatment effect for A versus C is typically compared with that for B versus C to estimate the marginal treatment effect for A versus B in the BC population. This is the indirect treatment comparison in the BC population performed in Equation (1).
There is some flexibility in this step. Bayesian G‐computation and the indirect comparison can be performed in one step under an MCMC approach. In this case, the estimation of would be integrated within the estimation or simulation of the posterior of , under suitable priors, and a posterior distribution for would be generated. This would require inputting as data the available aggregate outcomes for each treatment group in the published BC study, or reconstructing subject‐level data from these outcomes. For binary outcomes, event counts from the cells of a 2 × 2 contingency table would be required to estimate probabilities of the binary outcome as the incidence proportion for each treatment (dividing the number of subjects with the binary outcome in a treatment group by the total number of subjects in the group), to then estimate a marginal log‐odds ratio for B versus C. For survival outcomes, one can input patient‐level data (with outcome times and event indicators for each subject) reconstructed from digitized Kaplan–Meier curves, for example using the algorithm by Guyot et al. 75
The advantage of this approach is that it directly generates a full posterior distribution for . Hence, its output is perfectly compatible with a probabilistic cost‐effectiveness model. Samples of the posterior are directly incorporated into the decision analysis, so that the relevant economic measures can be evaluated for each sample without further distributional assumptions. 5 If necessary, we can take the expectation over the draws of the posterior density to produce a point estimate of the marginal A versus B treatment effect, in the BC population. Variance and interval estimates are derived empirically from the draws.
Alternatively, we can perform the G‐computation and indirect comparison in two steps. Irrespective of the selected inferential framework, point estimates and can be directly substituted in Equation (1). As the associated variance estimates and are statistically independent, these are summed to estimate the variance of the A versus B treatment effect:
| (14) |
With relatively large sample sizes, interval estimates can be constructed using normal distributions, . This two‐step strategy is simpler and easier to apply but sub‐optimal in terms of integration with probabilistic sensitivity analysis, although one could perform forward Monte Carlo simulation from a normal distribution with mean and variance . Ultimately, it is the distribution of that is relevant for HTA purposes.
4. SIMULATION STUDY
4.1. Aims
The objectives of the simulation study are to benchmark the performance of the different versions of parametric G‐computation, and compare it with that of MAIC and the conventional version of STC across a range of scenarios that may be encountered in practice. We evaluate each estimator on the basis of the following finite‐sample frequentist characteristics: 76 (1) unbiasedness; (2) variance unbiasedness; (3) randomization validity;‡ and (4) precision. The selected performance measures assess these criteria specifically (see section 4.4). The simulation study is reported following the ADEMP (Aims, Data‐generating mechanisms, Estimands, Methods, Performance measures) structure. 76 All simulations and analyses were performed using R software version 3.6.3. 77 The implementation of the methodologies compared in the simulation study, that is, the “M” in ADEMP, is summarized in Table 1. See Appendix S1A for a more a detailed description of the methods and their specific settings. Example R code applying the methods to a simulated example is provided in Appendix S1D.§
TABLE 1.
The implementation of the methodologies compared in the simulation study
| Method | Details |
|---|---|
| Matching‐adjusted indirect comparison (MAIC) |
|
| Simulated treatment comparison (STC) |
|
| Maximum‐likelihood parametric G‐computation |
|
| Bayesian parametric G‐computation |
|
Note: See Appendix S1A for a more a detailed description of the methods and their specific settings. For all methods, the marginal log‐odds ratio for B versus C is estimated directly from the event counts, and its standard error is computed using the delta method. The marginal log‐odds ratio estimate for A versus B and its standard error are obtained by combining the within‐study point estimates. Wald‐type 95% interval estimates are constructed for the marginal A versus B treatment effect using normal distributions.
Abbreviations: IPD, individual patient data; HTA, health technology assessment.
4.2. Data‐generating mechanisms
We consider binary outcomes using the log‐odds ratio as the measure of effect. The binary outcome may be response to treatment or the occurrence of an adverse event. For trials AC and BC, outcome y n for subject n is simulated from a Bernoulli distribution with probabilities of success generated from logistic regression, such that:
Four correlated continuous covariates x n are generated per subject by simulating from a multivariate normal distribution with pre‐specified variable means and covariance matrix. 79 Two of the covariates are purely prognostic variables; the other two () are effect modifiers, modifying the effect of both treatments A and B versus C on the log‐odds ratio scale, and prognostic variables.
The strength of the association between the prognostic variables and the outcome is set to , where k indexes a given covariate. This regression coefficient fixes the conditional odds ratio for the effect of each prognostic variable on the odds of outcome at 2, indicating a strong prognostic effect. The strength of interaction of the effect modifiers is set to , where k indexes a given effect modifier. This fixes the conditional odds ratio for the interaction effect on the odds of the outcome at approximately 1.5. Both active treatments have the same effect modifiers with respect to the common comparator and identical interaction coefficients for each. Therefore, the shared effect modifier assumption 14 holds in the simulation study by design. Pairwise Pearson correlation coefficients between the covariates are set to 0.2, indicating a moderate level of positive correlation.
The binary outcome represents the occurrence of an adverse event. Each active intervention has a very strong conditional treatment effect at baseline (when the effect modifiers are zero) versus the common comparator. Such relative effect is associated with a “major” reduction of serious adverse events in a classification of extent categories by the German national HTA agency. 80 The covariates may represent comorbidities, which are associated with greater rates of the adverse event and, in the case of the effect modifiers, which interact with treatment to render it less effective. The intercept β 0 = −0.62 is set to fix the baseline event percentage at 35% (under treatment C, when the values of the covariates are zero).
The number of subjects in the BC trial is 600, under a 2:1 active treatment versus control allocation ratio. For the BC trial, the individual‐level covariates and outcomes are aggregated to obtain summaries. The continuous covariates are summarized as means and standard deviations, which would be available to the analyst in the published study in a table of baseline characteristics in the RCT publication. The binary outcomes are summarized as overall event counts, for example from the cells of a 2 × 2 contingency table. Typically, the published study only provides this aggregate information to the analyst.
The simulation study investigates two factors in an arrangement with nine scenarios, thus exploring the interaction between these factors. The simulation scenarios are defined by the values of the following parameters:
The number of subjects in the AC trial, N ∈{200, 400, 600} under a 2:1 active intervention versus control allocation ratio. The sample sizes correspond to typical values for a Phase III RCT 81 and for trials included in applications of MAIC submitted to HTA authorities. 27
The degree of covariate imbalance.¶ For both trials, each covariate k follows a normal marginal distribution with mean μ k and standard deviation σ k , such that for subject i. For the BC trial, we fix μ k = 0.6. For the AC trial, we vary the means of the marginal normal distributions such that μ k ∈{0.45, 0.3, 0.15}. The standard deviation of each marginal distribution is fixed at σ k = 0.4 for both trials. This setup corresponds to standardized differences or Cohen effect size indices 82 (the difference in means in units of the pooled standard deviation) of 0.375, 0.75 and 1.125, respectively. This yields strong, moderate and poor covariate overlap; with overlap between the univariate marginal distributions of 85%, 71% and 57%, respectively, when N = 600. To compute the overlap percentages, we have followed a derivation by Cohen 82 for normally‐distributed populations with equal size and equal variance. Note that the percentage overlap between the joint covariate distributions of each study is substantially lower. The strong, moderate and poor covariate overlap scenarios correspond to average percentage reductions in effective sample size of 22%, 60% and 85%, respectively. These percentage reductions are representative of the range encountered in NICE technology appraisals. 17 , 27
4.3. Estimands
The estimand of interest is the marginal log‐odds ratio for A versus B in the BC population. The treatment coefficient is the same for both A versus C and B versus C, and the shared effect modifier assumption holds in the simulation study. Therefore, the true conditional treatment effect for A versus B in the BC population is zero. As the true subject‐level conditional effects are zero for all units, the true marginal log‐odds ratio in the BC population is zero (). This implies a null hypothesis‐like simulation setup of no treatment effect for A versus B, and marginal and conditional estimands in the BC population coincide by design.
Note that the true marginal effect for A versus B in the BC population is a composite of that for A versus C and that for B versus C, both of which are non‐null. These are the same and cancel out. For reference, the true marginal log‐odds ratio in the BC population for the active treatments versus the common comparator ( and ) is computed as −1.25.** All methods perform the same unadjusted analysis (i.e., a simple regression of outcome on treatment) to estimate the marginal treatment effect of B versus C. Because the BC study is a relatively large RCT, this comparison should be unbiased with respect to the true marginal log‐odds ratio in BC. Therefore, any bias in the A versus B comparison should arise from bias in the A versus C comparison, for which marginal and conditional relative treatment effects are non‐null.
4.4. Performance measures
We generate and analyze 2000 Monte Carlo replicates of trial data per simulation scenario. In our implementations of MAIC and G‐computation, a large number of bootstrap resamples or MCMC draws are performed for each of the 2000 replicates (see Appendix S1A). For instance, the analysis for one simulation scenario using Bayesian G‐computation contains 4000 MCMC draws (after burn‐in) times 2000 simulation replicates, which equals a total of 8 million posterior draws. Based on the method and simulation scenario with the highest long‐run variability (MAIC with N = 200 and poor covariate overlap), we consider the degree of precision provided by the Monte Carlo standard errors under 2000 replicates to be acceptable in relation to the size of the effects.††
We evaluate the performance of the outcome regression methods and MAIC on the basis of the following criteria: (1) bias; (2) variability ratio; (3) empirical coverage rate of the interval estimates; (4) empirical standard error (ESE); and (5) mean square error (MSE). These criteria are explicitly defined in a previous simulation study by the authors. 17
With respect to the simulation study aims in section 4.1, the bias in the estimated treatment effect assesses aim 1. This is equivalent to the average estimated treatment effect across simulations because the true treatment effect . The variability ratio evaluates aim 2. This represents the ratio of the average model standard error and the sample standard deviation of the treatment effect estimates (the empirical standard error). Variability ratios greater than (or lesser than) one indicate that model standard errors overestimate (or underestimate) the variability of the treatment effect estimate. It is worth noting that this metric assumes that the correct estimand and corresponding variance are being targeted. A variability ratio of one is of little use if this is not the case, for example if both the model standard errors and the empirical standard errors are taken over estimates targeting the wrong estimand. Coverage targets aim 3, and is estimated as the proportion of simulated datasets for which the true treatment effect is contained within the nominal (100 × [1 − α])% interval estimate of the estimated treatment effect. In this article, α = 0.05 is the nominal significance level. The empirical standard error is the standard deviation of the treatment effect estimates across the 2000 simulated datasets. Therefore, it measures precision or long‐run variability, and evaluates aim 4. The mean square error is equivalent to the average of the squared bias plus the variance across the 2000 simulated datasets. Therefore, it is a summary value of overall accuracy (efficiency), that accounts for both bias (aim 1) and variability (aim 4).
5. RESULTS
Performance metrics for all simulation scenarios are displayed in Figure 2, Figure 3 and Figure 4. Figure 2 displays the results for the three data‐generating mechanisms under N = 200. Figure 3 presents the results for the three scenarios with N = 400. Figure 4 depicts the results for the three scenarios with N = 600. From top to bottom, each figure considers the scenario with strong overlap first, followed by the moderate and poor overlap scenarios. For each scenario, there is a box plot of the point estimates of the A versus B marginal treatment effect across the 2000 simulated datasets. Below, is a summary tabulation of the performance measures for each method. Each performance measure is followed by its Monte Carlo standard error, presented in parentheses, which quantifies the simulation uncertainty.
FIGURE 2.
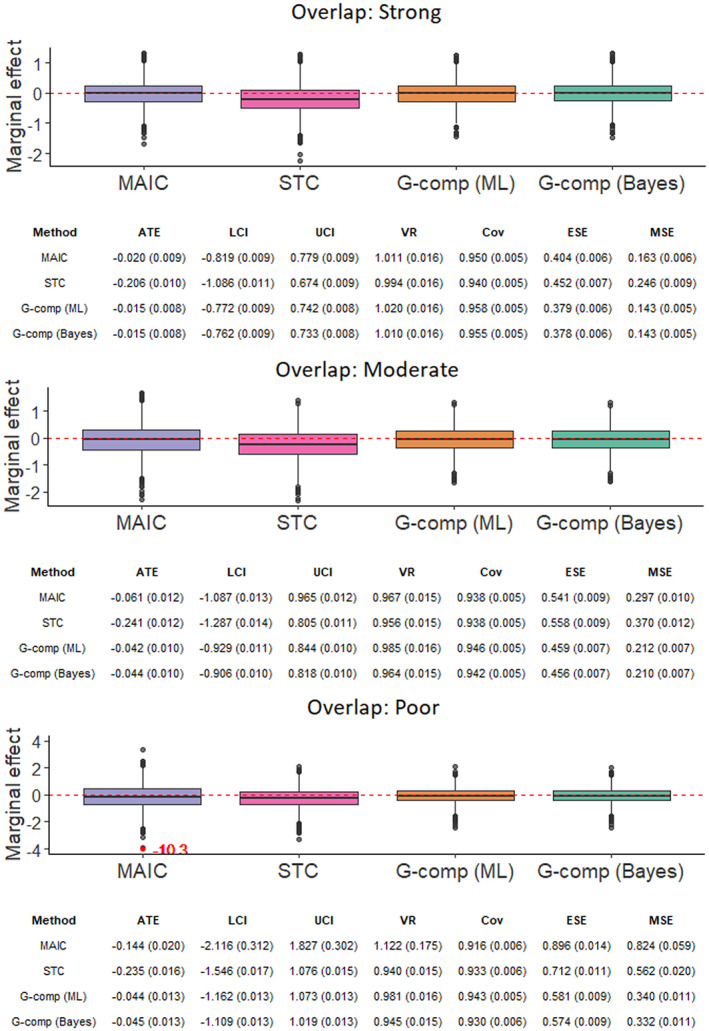
Point estimates and performance metrics across all methods for each simulation scenario with N = 200. The model standard error for the matching‐adjusted indirect comparison outlier in the poor overlap scenario has an inordinate influence on the variability ratio; removing it reduces the variability ratio to 0.980 (0.019). Note that the version of STC evaluated does not actually target a marginal effect. [Colour figure can be viewed at wileyonlinelibrary.com]
FIGURE 3.
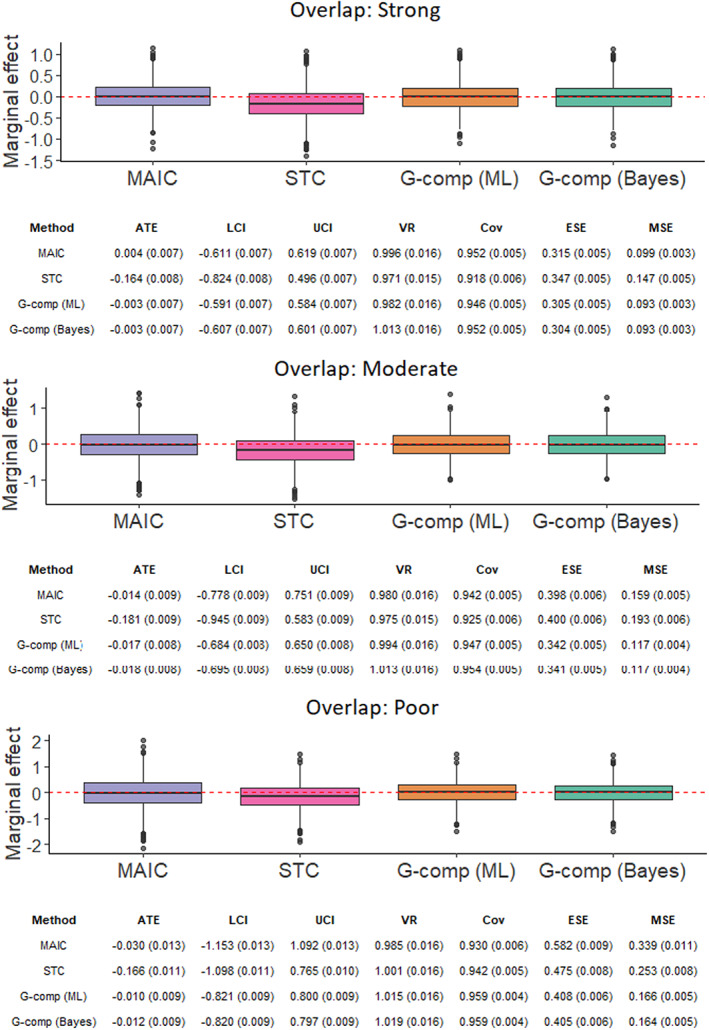
Point estimates and performance metrics across all methods for each simulation scenario with N = 400 [Colour figure can be viewed at wileyonlinelibrary.com]
FIGURE 4.
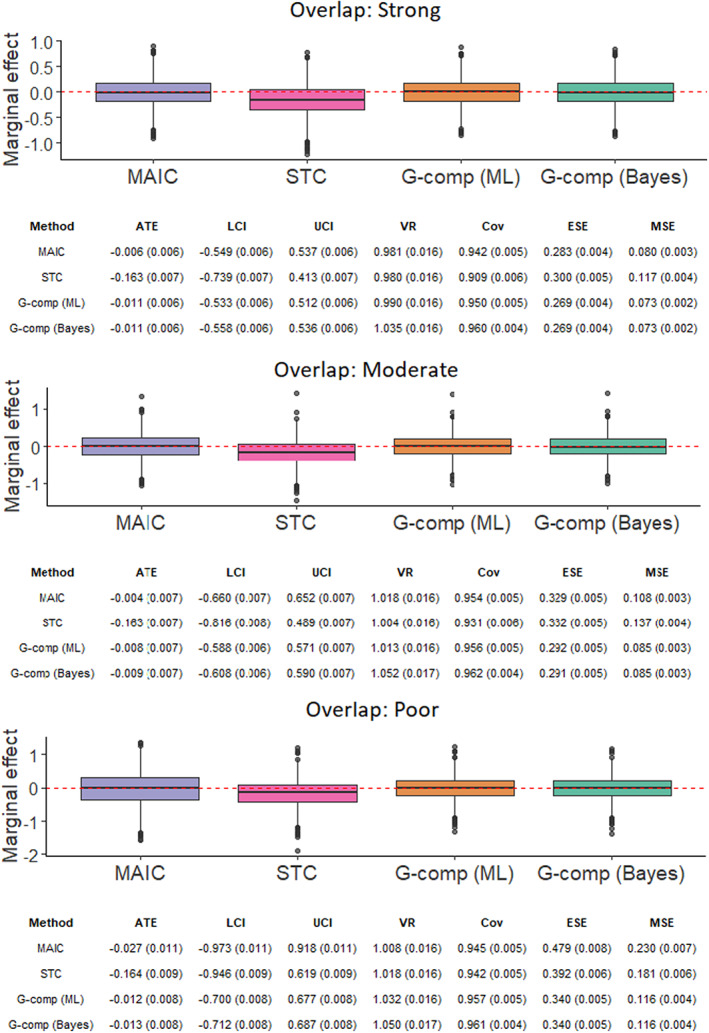
Point estimates and performance metrics across all methods for each simulation scenario with N = 600 [Colour figure can be viewed at wileyonlinelibrary.com]
In the figures, ATE is the average marginal treatment effect estimate for A versus B across the simulated datasets (this is equal to the bias as the true effect is zero). LCI is the average lower bound of the 95% interval estimate. UCI is the average upper bound of the 95% interval estimate. VR, ESE and MSE are the variability ratio, empirical standard error and mean square error, respectively. Cov is the empirical coverage rate of the 95% interval estimates. G‐comp (ML) stands for the maximum‐likelihood version of parametric G‐computation and G‐comp (Bayes) denotes its Bayesian counterpart using MCMC estimation.
Weight estimation cannot be performed for 4 of the 18,000 replicates in MAIC, where there are no feasible weighting solutions. This issue occurs in the most extreme scenario, corresponding to N = 200 and poor covariate overlap. Feasible weighting solutions do not exist due to separation problems, that is, there is a total lack of covariate overlap. Because MAIC is incapable of producing an estimate in these cases, the affected replicates are discarded altogether (the scenario in question analyzes 1996 simulated datasets for MAIC).
5.1. Unbiasedness of treatment effect estimates
The impact of the bias largely depends on the uncertainty in the estimated treatment effect, quantified by the empirical standard error. We compute standardized biases (bias as a percentage of the empirical standard error). With N = 200, MAIC has standardized biases of magnitude 11.3% and 16.1% under moderate and poor covariate overlap, respectively. Otherwise, the magnitude of the standardized bias is below 10%. Similarly, under N = 200, the maximum‐likelihood version of parametric G‐computation has standardized biases of magnitude 13.3% and 24.8% in the scenarios with moderate and poor overlap, respectively. In all other scenarios, the standardized bias has magnitude below 10%. For Bayesian parametric G‐computation, standardized biases never have a magnitude above 10% and troublesome biases are not produced in any of the simulation scenarios. The maximum absolute value of the standardized bias is 9.7% in a scenario with N = 200 and moderate covariate overlap.
To evaluate whether the bias in MAIC and parametric G‐computation has any practical significance, we investigate whether the coverage rates are degraded by it. Coverage is not affected for maximum‐likelihood parametric G‐computation, where empirical coverage rates for all simulation scenarios are very close to the nominal coverage rate, 0.95 for 95% interval estimates. In the case of MAIC, there is discernible undercoverage in the scenario with N = 200 and poor covariate overlap (empirical coverage rate of 0.916). This is the scenario with the lowest effective sample size after weighting. Hence, the results are probably related to small‐sample bias in the weighted logistic regression. 83 This bias for MAIC was not observed in the more extreme scenarios of a recent simulation study, 17 which considered survival outcomes and the Cox proportional hazards regression as the outcome model. In absolute terms, the bias of MAIC is greater than that of both versions of parametric G‐computation where the number of patients in the AC trial is small (N = 200) or covariate overlap is poor. In fact, when both of these apply, the bias of MAIC is important (−0.144). Otherwise, with N = 400 or greater, and moderate or strong overlap, the aforementioned methods produce similarly low levels of bias.
STC generates problematic negative biases in all nine scenarios considered in this simulation study, with a standardized bias of magnitude greater than 30% in all cases. STC consistently produces the highest bias of all methods, and the magnitude of the bias appears to increase under the smallest sample size (N = 200). The systematic bias in STC is due to the divergence of the conditional estimates produced for A versus C from the corresponding marginal estimand that should be targeted. This is a result of the non‐collapsibility of the (log) odds ratio.
5.2. Unbiasedness of variance estimates
In MAIC, the variability ratio of treatment effect estimates is close to one under all simulation scenarios except one. That is the scenario with N = 200 and poor covariate overlap, where the variability ratio is 1.122. This high value is attributed to the undue influence of an outlier (as seen in the box plot of point estimates) on the average model standard error. Once the outlier is removed, the variability ratio decreases to 0.98, just outside from being within Monte Carlo error of one but not statistically significantly different. This suggests very little bias in the standard error estimates in this scenario, that is, that the model standard errors tend to coincide with the empirical standard error. In a previous simulation study, 17 robust sandwich standard errors underestimated the variability of estimates in MAIC under small sample sizes and poor covariate overlap. The non‐parametric bootstrap seems to provide more conservative variance estimation in these extreme settings.
In STC, variability ratios are generally close to one with N = 400 and N = 600. Any bias in the estimated variances appears to be negligible, although there is a slight decrease in the variability ratios when the AC sample size is small (N = 200). Recall that this metric assumes that the correct estimand and corresponding variance are being targeted. However, in our application of STC, both model standard errors and empirical standard errors are taken over an incompatible indirect treatment comparison.
In maximum‐likelihood parametric G‐computation, variability ratios are generally very close to one. In Bayesian parametric G‐computation, variability ratios are generally close to one but are slightly above it in some scenarios with N = 600 (1.05 and 1.052 with moderate and poor covariate overlap, respectively). This suggests some overestimation of the empirical standard error by the model standard errors.
5.3. Randomization validity
From a frequentist viewpoint, 84 95% interval estimates are randomization‐valid if these are guaranteed to include the true treatment effect 95% of the time. Namely, the empirical coverage rate should be approximately equal to the nominal coverage rate, in this case 0.95 for 95% interval estimates, to obtain appropriate type I error rates for testing a “no effect” null hypothesis. Theoretically, the empirical coverage rate is statistically significantly different to 0.95 if, roughly, it is less than 0.94 or more than 0.96, assuming 2000 independent simulations per scenario. These values differ by approximately two standard errors from the nominal coverage rate. Poor coverage rates are a decomposition of both the bias and the standard error used to compute the Wald‐type interval estimates. In the simulation scenarios, none of the methods lead to overly conservative inferences but there are some issues with undercoverage.
Empirical coverage rates for MAIC are significantly different from the advertised nominal coverage rate in three scenarios. In the three, the coverage rate is below 0.94 (empirical coverage rates of 0.938, 0.93 and 0.916). The last two of these occur in scenarios with poor covariate overlap, with the latter corresponding to the smallest effective sample size after weighting (N = 200). This is the scenario which integrates the two most important determinants of small‐sample bias, in which MAIC has exhibited discernible bias. In this case, undercoverage is bias‐induced. On the other hand, in our previous simulation study, 17 undercoverage was induced by the robust sandwich variance underestimating standard errors.
In the conventional version of STC, coverage rates are degraded by the bias induced by the non‐collapsibility of the log‐odds ratio. Almost invariably, there is undercoverage. Interestingly, the empirical coverage does not markedly deteriorate—coverage percentages never fall below 90%, that is, never at least double the nominal rate of error. In general, both versions of parametric G‐computation exhibit appropriate coverage. Only one scenario provides rates below 0.94 (Bayesian G‐computation with N = 200 and poor overlap, with an empirical coverage rate of 0.93). No scenarios have empirical coverage above 0.96. Coverage rates for the maximum‐likelihood implementation are always appropriate, with most empirical coverage percentages within Monte Carlo error of 95%.
5.4. Precision and efficiency
Both versions of parametric G‐computation have reduced empirical standard errors compared to MAIC across all scenarios. Interestingly, conventional STC is even less precise than MAIC in most scenarios (all the scenarios with moderate or strong overlap, where reductions in effective sample size after weighting are tolerable). Several trends are revealed upon comparison of the ESEs, and upon visual inspection of the spread of the point estimates in the box plots. As expected, the ESE increases for all methods (i.e., estimates are less precise) as the number of subjects in the AC trial is lower. The decrease in precision is more substantial for MAIC than for the outcome regression methods.
The degree of covariate overlap has an important influence on the ESE and population adjustment methods incur losses of precision when covariate overlap is poor. Again, this loss of precision is more substantial for MAIC than for the outcome regression approaches. Where overlap is poor, there exists a subpopulation in BC that does not overlap with the AC population. Therefore, inferences in this subpopulation rely largely on extrapolation. Outcome regression approaches require greater extrapolation when the covariate overlap is weaker, thereby incurring a loss of precision.
Where covariate overlap is strong, both versions of parametric G‐computation display very similar ESEs than MAIC. As mentioned earlier, conventional STC offers even lower precision than MAIC in these cases. To illustrate this, consider the scenario with N = 200 and moderate overlap, where MAIC is expected to have a low effective sample size after weighting and perform comparatively worse than outcome regression. Even in this scenario, MAIC appears to be more precise (empirical standard error of 0.541) than conventional STC (empirical standard error of 0.558). As overlap decreases, precision is reduced more markedly for MAIC compared to the outcome regression methods. Under poor overlap, MAIC considerably increases the ESE compared to the conventional STC.
In MAIC, extrapolation is not even possible. Where covariate overlap is poor, the observations in the AC IPD that are not covered by the ranges of the selected covariates in BC are assigned weights that are very close to zero. The relatively small number of individuals in the overlapping region of the covariate space are assigned inflated weights, dominating the reweighted sample. These extreme weights lead to large reductions in effective sample size and affect very negatively the precision of estimates.
Similar to the trends observed for the ESE, the MSE is also very sensitive to the value of N and to the level of covariate overlap. The MSE decreases for all methods as N and the level of overlap increase. The accuracy of MAIC and the G‐computation methods is comparable when the AC sample size is high or covariate overlap is strong. As the AC sample size and overlap decrease, the relative accuracy of MAIC with respect to both approaches to G‐computation is markedly reduced. Accuracy for the conventional version of STC is comparatively poor and this is driven by bias.
Where covariate overlap is strong or moderate, the parametric G‐computation methods have the highest accuracy, followed by MAIC and STC. Where overlap is poor, both versions of parametric G‐computation are considerably more accurate than MAIC, with much smaller mean square errors. MAIC also provides less accurate estimates than STC in terms of mean square error. The variability of estimates under MAIC increases considerably in these scenarios. The precision is sufficiently poor to offset the loss of bias with respect to STC.
6. DISCUSSION
6.1. Summary of results
The parametric G‐computation methods and MAIC can yield unbiased estimates of the marginal A versus B treatment effect in the BC population. Conventional STC targets a conditional treatment effect for A versus C that is incompatible in the indirect comparison. Bias is produced when the measure of effect is non‐collapsible. Across all scenarios, both versions of G‐computation largely eliminate the bias induced by effect modifier imbalances. There is some negative bias in MAIC and parametric G‐computation where the sample size N is small. In the case of MAIC, this is problematic where covariate overlap is poor. MAIC did not display these biases using Cox proportional hazards regression as the outcome model in the time‐to‐event setting. 17 The difference in results is likely due to logistic regression being more prone to small‐sample bias than Cox regression. 83
As for precision, the G‐computation approaches have reduced variability compared to MAIC. The superior precision is demonstrated by their lower empirical standard errors across all scenarios. Because the methods are generally unbiased, precision is the driver of comparative accuracy. The simulation study confirms that, under correct model specification, parametric G‐computation methods have lower mean square errors than weighting and are therefore more efficient. The differences in performance are exacerbated where covariate overlap is poor and sample sizes are low. In these cases, the effective sample size after weighting is small, and this leads to inflated variances and wider interval estimates for MAIC.
For the conventional STC, outcome regression may have decreased precision relative to MAIC, as dictated by the empirical standard errors. On the other hand, the marginalized outcome regression methods are more precise than both MAIC and conventional STC. From a frequentist perspective, the standard error of the estimator of a conditional log‐odds ratio for A versus C, targeted by conventional STC, is larger than the standard error of a regression‐adjusted estimate of the marginal log‐odds ratio for A versus C, produced by G‐computation. This precision comparison likely lacks relevance, because one is comparing estimators that target different estimands. Nevertheless, it supports previous findings on non‐collapsible measures of effect when adjusting for prognostic covariates. 29 , 32 When we marginalize and compare estimators targeting like‐for‐like marginal estimands, we find that outcome regression is no longer detrimental for precision and efficiency compared to weighting.
The robust sandwich variance estimator for MAIC underestimates variability and produces narrow interval estimates under small effective sample sizes. 17 This simulation study demonstrates that the bootstrap procedure provides more conservative variance estimation in the more extreme settings. This implies that the bootstrap approach should be preferred for statistical inference where there are violations of the overlap assumption and small sample sizes.
6.2. Extrapolation capabilities and precision considerations
Some care is required to translate the results of this simulation study into real applications, where effective sample sizes and percentage reductions in effective sample size may be lower and higher, respectively, than those considered. 27 In these situations, covariate overlap is poor and this leads to a high loss of precision in MAIC. G‐computation methods should be considered because they are substantially more statistically efficient. This is particularly the case where the outcome model is a logistic regression, more prone to small‐sample bias, 20 , 83 imprecision and inefficiency 85 than other models, for example, the Cox regression. In addition, where sample sizes are small and the number of covariates is large, feasible weighting solutions may not exist for MAIC due to separation problems. 22 This is observed in one of the scenarios of this simulation study (N = 200 with poor overlap) and elsewhere. 20 An advantage of outcome regression is that it can be applied in these settings. MAIC cannot extrapolate beyond the covariate space observed in the IPD. Therefore, it cannot overcome the failure of assumptions that is the lack of covariate overlap and is incapable of producing an estimate.
Moreover, we note that MAIC requires accounting for all effect modifiers (balanced and imbalanced), as excluding balanced covariates from the weighting procedure does not ensure balance after the weighting. On the other hand, outcome regression methods do not necessarily require the inclusion of the effect modifiers that are in balance, for instance when the outcome model is a linear regression. This may mitigate losses of precision further, particularly where the number of potential effect modifiers is large.
With limited overlap, outcome regression methods can use the linearity assumption to extrapolate beyond the AC population, provided the true relationship between the covariates and the outcome is adequately captured. We view this as a desirable attribute because poor overlap, with small effective sample sizes and large percentage reductions in effective sample size, is a pervasive issue in health technology appraisals. 27 Nevertheless, where overlap is more considerable, one may wish to restrict inferences to the region of overlap and avoid relying on a model for extrapolation outside this region. 86
It is worth noting that we have used the standard MAIC formulation proposed by Signorovitch et al 8 , 14 , 17 , 87 and that our conclusions are based on this approach. Nevertheless, MAIC is a rapidly developing methodology with novel implementations. An alternative formulation based on entropy balancing has been presented. 51 , 52 , 87 This approach is similar to the original version with a subtle modification to the weight estimation procedure. While it has some interesting computational properties, Phillippo et al 87 have recently shown that the standard method of moments and entropy balancing produce weights that are mathematically equivalent (up to optimization error or a normalizing constant). Jackson et al 22 propose a distinct weight estimation procedure that satisfies the conventional method of moments and maximizes the effective sample size. A larger effective sample size translates into minimizing the variance of the weights, with more stable weights producing a gain in precision at the expense of introducing some bias.
6.3. Model specification assumptions
Note that the model extrapolation uncertainty is not reflected in the interval estimates for the outcome regression approaches and that some consider weighting approaches to give a “more honest reflection of the overall uncertainty.” 60 The gain in efficiency produced by outcome regression must be counterbalanced against the potential for model misspecification bias.
This simulation study only considers a best‐case scenario with correct parametric model specification. To predict the outcomes in G‐computation, we use the logistic regression model implied by the data‐generating mechanism. In real applications, parametric modeling assumptions are difficult to hold because, unlike in simulations, the correct specification is unknown, particularly where there are many covariates and complex relationships exist between them. For instance, assumptions will not hold if only linear relationships are considered and the selected covariates have non‐linear interactions with treatment on the linear predictor scale.
Weighting methods are often perceived to rely on less demanding parametric assumptions, yet model misspecification is also a potential issue. Note that, even though the data‐generating mechanism of the simulation study does not specify a trial assignment model, the logistic regression for weight estimation is the “best‐case” model because the “right” subset of covariates is selected as effect modifiers.‡‡ The balancing property of the weights 88 , 89 holds with respect to the effect modifier means—conditional on the weights, all effect modifier means are balanced between the two studies. More precisely, the estimated weights are scores that, when applied to the AC IPD, form a pseudo‐population that has balanced effect modifier means with respect to the BC population. Therefore, there is conditional exchangeability over trial assignment and one can achieve unbiased estimation of treatment effects in the BC population.
The simulation study presented in this article demonstrates proof‐of‐concept for the outcome regression methods and for MAIC but does not investigate how robust the methods are to failures in assumptions. Future simulation studies should explore performance in scenarios where assumptions are violated, in order to draw more accurate conclusions with respect to practical applications and limitations. Conditional exchangeability (“no omitted effect modifiers”) is a fundamental assumption for all methods. In practice, there may be incomplete information on effect modifiers for one or both trials, and background knowledge or theory about effect modification is often weak.
The general‐purpose nature of the proposed G‐computation approaches may provide some degree of robustness against model misspecification because the covariate‐adjusted outcome model does not necessarily need to be parametric. Non‐ and semi‐parametric regression techniques or other data‐adaptive estimation approaches are viable to detect (higher‐order) interactions, product terms and non‐linear relationships between regressors, and offer more flexible functions to predict the conditional outcome expectations. These make weaker modeling assumptions than parametric regressions but are more prone to overfitting, particularly with small sample sizes.
6.4. Limitations
Care must be taken where sample sizes are small in population‐adjusted indirect comparisons. Low sample sizes cause substantial issues for the accuracy of MAIC due to unstable weights. As the sponsor company is directly responsible for setting the value of N, the AC trial should be as large as possible to maximize precision and accuracy. The sample size requirements for indirect comparisons, and more generally for economic evaluation, are considerably larger than those required to demonstrate an effect for the main clinical outcome in a single RCT. However, trials are usually powered for the main clinical comparison, even if there is a prospective indirect, potentially adjusted, comparison down the line. Ideally, if the manufacturer intends to use standard or population‐adjusted indirect comparisons for reimbursement purposes, its clinical study should be powered for the relevant methods.
Note that sponsors tend to run multiple RCTs instead of one larger RCT for marketing authorization applications. If there are many different IPD RCTs, it is necessary to fit the covariate‐adjusted regression to each patient‐level dataset and marginalize against the BC pseudo‐population in G‐computation. Similarly, one would apply MAIC to each study individually, reweighting each patient‐level dataset against the BC study report. Then, a meta‐analysis of effect measure estimates can be performed in the same population using the marginalized or weighted results from the IPD studies and the original effect estimate published in the ALD study.
The population adjustment methods outlined in this article are only applicable to pairwise indirect comparisons, and not easily generalizable to larger network structures of treatments and studies. This is because the methods have been developed in the two‐study scenario seen in this paper, very common in HTA submissions, where there is one AC study with IPD and another BC study with ALD. In this very sparse network, indirect comparisons are vulnerable to bias induced by effect modifier imbalances. In larger networks, multiple pairwise comparisons do not necessarily generate a consistent set of relative effect estimates for all treatments. This is because the comparisons must be undertaken in the ALD populations.
Another issue is that the ALD population(s) may not correspond precisely to the target population for the decision. Marginal estimands in different populations may not match if there are differences in the distribution of effect modifiers. This is a problem of external validity: if populations are non‐exchangeable, an internally valid estimate of the marginal estimand in one population is not necessarily unbiased for the marginal estimand in the other(s). 89 , 90 To address this, one suggestion would be for the decision‐maker to define a target population for a specific disease into which all manufacturers should conduct their indirect comparisons. The outcome regression approaches discussed in this article could be applied to produce marginal effects in any target population. The target could be represented by the joint covariate distribution of a registry, cohort study or some other observational dataset, and one would marginalize over this distribution. Similarly, MAIC can reweight the IPD with respect to a different population than that of the BC study.
Recently, a novel population adjustment method named multilevel network meta‐regression (ML‐NMR) has been introduced. 12 , 13 ML‐NMR generalizes IPD network meta‐regression to include aggregate‐level data, reducing to this method when IPD are available for all studies. ML‐NMR is a timely addition; it is applicable in treatment networks of any size with the aforementioned two‐study scenario as a special case. This is important because a recent review 27 finds that 56% of NICE technology appraisals include larger networks, where the methods discussed in this article cannot be readily applied.
ML‐NMR is an outcome regression approach, with the outcome model of interest being identical to that of parametric G‐computation. While the methods share the same assumptions in the two‐study scenario, ML‐NMR generalizes the regression to handle larger networks. Like Bayesian G‐computation, ML‐NMR has been developed under a Bayesian framework and estimates the outcome model using MCMC. It also makes parametric assumptions to characterize the marginal covariate distributions in BC and reconstructs the joint covariate distribution using a copula. The methods average over the BC population in different ways; Bayesian G‐computation simulates individual‐level covariates from their approximate joint distribution and ML‐NMR uses numerical integration over the approximate joint distribution (quasi‐Monte Carlo methods).
In its original publication, 12 , 13 ML‐NMR targets a conditional treatment effect (avoiding the compatibility issues of conventional STC), because the effect estimate is derived from the treatment coefficient of a covariate‐adjusted multivariable regression. However, ML‐NMR can directly calculate marginalization integrals akin to those required for Bayesian G‐computation (Equations 9 and 10). Phillippo et al have recently demonstrated that ML‐NMR can be adapted to target marginal treatment effects. 61 We previously mentioned that pairwise population‐adjusted indirect comparisons target marginal estimands that are specific to the BC study. These may not be directly relevant for HTA decision‐making. On the other hand, ML‐NMR can potentially estimate marginal effects in any target population, presenting novel and exciting opportunities for evidence synthesis.
7. CONCLUDING REMARKS
The traditional regression adjustment approach in population‐adjusted indirect comparisons targets a conditional treatment effect for A versus C. We have showed empirically that this effect is incompatible in the indirect treatment comparison, producing biased estimation where the measure of effect is non‐collapsible. In addition, this effect is not of interest in our scenario because we seek marginal effects for policy decisions at the population level. We have proposed approaches for marginalizing the conditional estimates produced by covariate‐adjusted regressions. These are applicable to a wide range of outcome models and target marginal treatment effects for A versus C that have no compatibility issues in the indirect treatment comparison.
At present, MAIC is the most commonly used population‐adjusted indirect comparison method. 27 We have demonstrated that the novel marginalized outcome regression approaches achieve greater statistical precision than MAIC and are unbiased under no failures of assumptions. Hence, the development of these approaches is appealing and impactful. As observed in the simulation study, outcome regression provides more stable estimators and is more efficient than weighting under correct model specification. This is particularly the case where covariate overlap is poor and effective sample sizes are small. These results support previous findings comparing outcome regression and weighting in different contexts. 24 , 26 , 91 , 92
We can now capitalize on the advantages offered by outcome regression with respect to weighting in our scenario, for example extrapolation capabilities and increased precision. Outcome regression methods are also appealing because they make different modeling assumptions than weighting. Sometimes, identifying the variables that affect outcome is more straightforward than identifying the factors that drive trial assignment. This is not typically the case in the standard use of propensity score weighting in observational studies, where one identifies the factors that drive treatment (as opposed to trial) assignment. Nevertheless, in our context, the factors driving selection into different RCTs are often arbitrary. In these cases, the proposed G‐computation approaches may be useful. In addition, researchers could develop augmented or doubly robust methods 37 , 59 , 93 that combine the outcome model with the trial assignment model for the weights. These approaches are attractive due to their increased flexibility and robustness to model misspecification. 93 , 94
Furthermore, we have shown that the marginalized regression‐adjusted estimates provide greater statistical precision than the conditional estimates produced by the conventional version of STC. While this precision comparison is irrelevant, because it is made for estimators of different estimands, it supports previous research on non‐collapsible measures of effect. 29 , 32
Marginal and conditional effects are regularly conflated in the literature on population‐adjusted indirect comparisons, with many simulation studies comparing the bias, precision and efficiency of estimators of different effect measures. The implications of this conflation must be acknowledged in order to provide meaningful comparisons of methods. We have built on previous research conducted by the original authors of STC, who have also suggested the use of a preliminary “covariate simulation” step. 50 , 96 Nevertheless, up until now, there was no consensus on how to marginalize the conditional effect estimates. For instance, in a previous simulation study, 17 we discouraged the “covariate simulation” approach when attempting to average on the linear predictor scale. Averaging on the linear predictor scale, that is, computing the conditional linear prediction under each treatment for every simulated subject and averaging the linear predictions across all subjects, then calculating the difference between the average predictions, reduces to the conventional version of STC (i.e., to “plugging in” the mean BC covariate values). As discussed in the next paragraph, it is equivalent to averaging “predictions at the mean” 97 or estimating the “mean at mean covariates,” 98 hence producing conditional effect estimates for A versus C, as opposed to marginal estimates. We hope to have established some clarity.
The readers may notice that the outcome regression fitted in the conventional STC (section 3.3) is different to that fitted in outcome regression with “covariate simulation” (e.g., parametric G‐computation in section 3.4). In the conventional outcome regression described in section 3.3 and by the NICE technical support document, 14 the IPD covariates are centered by plugging in the mean BC covariate values. In the Q‐model required for G‐computation, outlined in section 3.4, the covariates are not centered and the regression fit is used to make predictions for the simulated covariates. The underlying reason for this has been described for generalized linear models with non‐linear link functions, such as logistic or Poisson regression. 97 , 98 , 99 On the natural scale, averaging the individual outcome predictions made at the centered covariates of the sample does not consistently estimate the marginal mean response for the centered sample. In the words of Bartlett, 97 “prediction at the mean” value of the baseline covariates for a treatment group does not result in the “marginal mean” under such treatment. Similarly, in the words of Qu and Luo, 98 the “mean at mean covariates” of the study sample is generally not equivalent to the marginal response over the subjects in the sample. The former results in a conditional estimate whereas the latter produces a marginal population‐level estimate, of interest in our scenario.
It is important to acknowledge that the methods discussed in this article are required due to a suboptimal scenario, where patient‐level data on covariates are unavailable for the BC study. Ideally, these should be made freely available or, at least, made available for the purpose of evidence synthesis by the sponsor company. Raw patient‐level data are always the preferred input for statistical inference, allowing for the testing of assumptions. 100 We note that the manufacturer itself could facilitate inference by using the IPD to create fully artificial covariate datasets. 101 The release of such datasets would not involve a violation of privacy or confidentiality, avoiding the need for the “covariate simulation” step and removing the risk of misspecifying the joint covariate distribution of the BC population. Alternatively, Bonofiglio et al 102 have recently proposed a framework where access to covariate correlation summaries is made possible through distributed computing. It is unclear whether access to such framework would be granted to a competitor submitting evidence for reimbursement to HTA bodies, albeit the summaries could be reported in clinical trial publications.
The presented marginalization methods have been developed in a very specific context, common in HTA, where access to patient‐level data is limited and an indirect comparison is required. However, their principles are applicable to estimate marginal treatment effects in any situation. For instance, in scenarios which require marginalizing out regression‐adjusted estimates over the study sample in which they have been computed. Alternatively, the frameworks can be used to transport the results of a randomized experiment to any other external target population; not necessarily that of the BC trial. In both cases, the required assumptions are weaker than those required for population‐adjusted indirect comparisons.
Finally, we have assumed that the AC study is a randomized trial. Our approach to parametric G‐computation could be extended to the situation where the AC trial is an observational study by including all confounders of the treatment‐outcome relationship in the outcome model. In this scenario, one must overcome the limited internal validity of the study design. Because treatment assignment is non‐random, additional assumptions would be required, for example, conditional exchangeability within the study arms (“no unmeasured confounding”) and the associated overlap/positivity condition. 103 , 104 These assumptions are similar to those discussed in section 2.1 but would be expected to hold across treatment arms in the IPD study in addition to across study populations.
AUTHOR CONTRIBUTION
All authors contributed to the conception and design of the article, and to the analysis and interpretation of data. All authors drafted the manuscript, critically revised it for important intellectual content and provided final approval of the version to be published.
FUNDING INFORMATION
Funding agreements ensure the authors' independence in developing the methodology, designing the simulation study, interpreting the results, writing, and publishing the article.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
Supporting information
APPENDIX S1 Supplementary Information
ACKNOWLEDGMENTS
The authors thank the peer reviewers of a previous article of theirs. 17 Their comments were extremely insightful and helped improved the underlying motivation of this article. The authors are hugely thankful to Tim Morris, whose comments on G‐computation and marginalization helped motivate the article. Finally, the authors are grateful to David Phillippo, who has provided very valuable feedback to our work, and helped in substantially improving our research. This article is based on research supported by Antonio Remiro‐Azócar's PhD scholarship from the Engineering and Physical Sciences Research Council of the United Kingdom. Anna Heath is supported by the Canada Research Chair in Statistical Trial Design and funded by the Discovery Grant Program of the Natural Sciences and Engineering Research Council of Canada (RGPIN‐2021‐03366).
Remiro‐Azócar A, Heath A, Baio G. Parametric G‐computation for compatible indirect treatment comparisons with limited individual patient data. Res Syn Meth. 2022;13(6):716‐744. doi: 10.1002/jrsm.1565
Funding information Canada Research Chairs, Grant/Award Number: Anna Heath: Statistical Trial Design; Engineering and Physical Sciences Research Council, Grant/Award Number: Antonio Remiro‐Azocar: PhD scholarship; Natural Sciences and Engineering Research Council of Canada, Grant/Award Number: Anna Heath: Discovery Grant Program RGPIN‐2021‐03
Endnotes
In the anchored scenario, we are interested in a comparison of relative outcomes or effects, not absolute outcomes. Hence, an anchored comparison only requires conditioning on the effect modifiers, the covariates that explain the heterogeneity of the A versus C treatment effect. This assumption is named the conditional constancy of relative effects, 7 , 14 that is, given the selected effect‐modifying covariates, the marginal A versus C treatment effect is constant across the AC and BC populations. There are other formulations of this assumption, 11 , 43 such as trial assignment/selection being conditionally ignorable, unconfounded or exchangeable for such treatment effect, that is, conditionally independent of the treatment effect, given the selected effect modifiers. One can consider that being in population AC or population BC does not carry over any information about the marginal A versus C treatment effect, once we condition on the treatment effect modifiers. This means that after adjusting for these effect modifiers, treatment effect heterogeneity and trial assignment are conditionally independent. Another advantage of outcome regression with respect to weighting is that, by being less sensitive to overlap issues, it allows for the inclusion of larger numbers of effect modifiers. This makes it easier to satisfy the conditional constancy of relative effects.
Note that the “matching‐adjusted” term in MAIC is a misnomer, as the indirect comparison is actually “weighting‐adjusted.”
In a sufficiently large number of repetitions (100 × (1 − α))% interval estimates based on normal distributions should contain the true value (100 × (1 − α))% of the time, for a nominal significance level α.
The files required to run the simulations are available at http://github.com/remiroazocar/Gcomp_indirect_comparisons_simstudy.
In the simulation study, covariate balance is a proxy for covariate overlap. Imbalance refers to the difference in covariate distributions across studies, as measured by the difference in (standardized) average values. Imbalances in effect measure modifiers across studies induce bias in the standard indirect comparison and motivate the use of population adjustment. Overlap describes how similar the covariate ranges are across studies—there is complete overlap if the ranges are identical. Lack of complete overlap hinders the use of population adjustment.
This has been calculated by simulating two potential cohorts of 500,000 subjects, with the BC covariate distribution and the outcome‐generating mechanism in section 4.2. One cohort is under the active treatment and the other is under the common comparator. The number of simulated subjects is sufficiently large to minimize sampling variability. The two cohorts are concatenated and a simple logistic regression is fitted, regressing the simulated binary outcomes on an indicator variable for treatment assignment. The treatment coefficient estimates the average difference in the potential outcomes on the log‐odds ratio scale, and serves as the log of the true marginal odds ratio for the two interventions under consideration. This is because the outcomes have been generated according to the true data‐generating mechanism, where the true conditional effects are explicit, and which uses the correct conditional model by definition. Due to the non‐collapsibility of the odds ratio, this simulation‐based approach is necessary to determine the true marginal effect for A versus C and B versus C.
Conservatively, we assume that and that the variance across simulations of the estimated treatment effect is always less than 2.92. Given that the MCSE of the bias is equal to , where N sim = 2000 is the number of simulations, it is at most 0.038 under 2000 simulations. We consider the degree of precision provided by the MCSE of the bias to be acceptable in relation to the size of the effects. If the empirical coverage rate of the methods is 95%, N sim = 2000 implies that the MCSE of the coverage is , with the worst‐case MCSE being 1.12% under 50% coverage. We also consider this degree of precision to be acceptable. Hence, the simulation study is conducted under N sim = 2000.
The MAIC implementation is optimal in terms of precision and accuracy because the trial assignment model only balances the two covariates that interact with treatment. Nevertheless, these are not the only two covariates that are associated with trial assignment. Consider balancing the full set of covariates that predict trial assignment (a total of four covariates, including the two predictors with only main effects in the data‐generating outcome model). Variance would be increased without improving the potential for bias reduction in the BC population. The behavior of MAIC would be more unstable because of weaker overlap. More extreme weights would be produced, and finite‐sample or “chance” overlap violations would be more likely, particularly with small AC sample sizes.
DATA AVAILABILITY STATEMENT
The files required to generate the data, run the simulations, and reproduce the results are available at http://github.com/remiroazocar/Gcomp_indirect_comparisons_simstudy.
REFERENCES
- 1. Vreman RA, Naci H, Goettsch WG, et al. Decision making under uncertainty: comparing regulatory and health technology assessment reviews of medicines in the United States and Europe. Clin Pharmacol Therap. 2020;108(2):350‐357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Temple R, Ellenberg SS. Placebo‐controlled trials and active‐control trials in the evaluation of new treatments. Part 1: ethical and scientific issues. Ann Intern Med. 2000;133(6):455‐463. [DOI] [PubMed] [Google Scholar]
- 3. Paul JE, Trueman P. ‘Fourth hurdle reviews’, NICE, and database applications. Pharmacoepidemiol Drug Saf. 2001;10(5):429‐438. [DOI] [PubMed] [Google Scholar]
- 4. Sutton A, Ades A, Cooper N, Abrams K. Use of indirect and mixed treatment comparisons for technology assessment. Pharmacoeconomics. 2008;26(9):753‐767. [DOI] [PubMed] [Google Scholar]
- 5. Dias S, Sutton AJ, Ades A, Welton NJ. Evidence synthesis for decision making 2: a generalized linear modeling framework for pairwise and network meta‐analysis of randomized controlled trials. Med Decis Making. 2013;33(5):607‐617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bucher HC, Guyatt GH, Griffith LE, Walter SD. The results of direct and indirect treatment comparisons in meta‐analysis of randomized controlled trials. J Clin Epidemiol. 1997;50(6):683‐691. [DOI] [PubMed] [Google Scholar]
- 7. Phillippo DM, Ades AE, Dias S, Palmer S, Abrams KR, Welton NJ. Methods for population‐adjusted indirect comparisons in health technology appraisal. Med Decis Making. 2018;38(2):200‐211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Signorovitch JE, Wu EQ, Andrew PY, et al. Comparative effectiveness without head‐to‐head trials. Pharmacoeconomics. 2010;28(10):935‐945. [DOI] [PubMed] [Google Scholar]
- 9. Caro JJ, Ishak KJ. No head‐to‐head trial? Simulate the missing arms. Pharmacoeconomics. 2010;28(10):957‐967. [DOI] [PubMed] [Google Scholar]
- 10. Miettinen OS. Standardization of risk ratios. Am J Epidemiol. 1972;96(6):383‐388. [DOI] [PubMed] [Google Scholar]
- 11. Stuart EA, Cole SR, Bradshaw CP, Leaf PJ. The use of propensity scores to assess the generalizability of results from randomized trials. J R Stat Soc A Stat Soc. 2011;174(2):369‐386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Phillippo DM, Dias S, Ades A, et al. Multilevel network meta‐regression for population‐adjusted treatment comparisons. J R Stat Soc A Stat Soc. 2020;183:1189‐1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Phillippo DM. Calibration of Treatment Effects in Network Meta‐Analysis Using Individual Patient Data. PhD thesis. University of Bristol; 2019. [Google Scholar]
- 14. Phillippo D, Ades T, Dias S, Palmer S, Abrams KR, Welton N. NICE DSU technical support document 18: methods for population‐adjusted indirect comparisons in submissions to NICE. 2016.
- 15. Baio G. Bayesian Methods in Health Economics. CRC Press; 2012. [Google Scholar]
- 16. Claxton K, Sculpher M, McCabe C, et al. Probabilistic sensitivity analysis for NICE technology assessment: not an optional extra. Health Econ. 2005;14(4):339‐347. [DOI] [PubMed] [Google Scholar]
- 17. Remiro‐Azócar A, Heath A, Baio G. Methods for population adjustment with limited access to individual patient data: a review and simulation study. Res Synth Meth. 2021:12(6):750‐775. [DOI] [PubMed] [Google Scholar]
- 18. Cheng D, Ayyagari R, Signorovitch J. The statistical performance of matching‐adjusted indirect comparisons. Ann App Stat. 2019;14(4):1806‐1833. [Google Scholar]
- 19. Hatswell AJ, Freemantle N, Baio G. The effects of model misspecification in unanchored matching‐adjusted indirect comparison (MAIC): results of a simulation study. Value Health. 2020;23:751‐759. [DOI] [PubMed] [Google Scholar]
- 20. Phillippo DM, Dias S, Ades A, Welton NJ. Assessing the performance of population adjustment methods for anchored indirect comparisons: a simulation study. Stat Med. 2020;39(30):4885‐4911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Stuart EA. Matching methods for causal inference: a review and a look forward. Stat Sci. 2010;25(1):1‐21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Jackson D, Rhodes K, Ouwens M. Alternative weighting schemes when performing matching‐adjusted indirect comparisons. Res Synth Meth. 2020;12:333‐346. [DOI] [PubMed] [Google Scholar]
- 23. van der Laan M, Rose S. Targeted Learning: Causal Inference for Observational and Experimental Data. Springer Science & Business Media; 2011. [Google Scholar]
- 24. Neugebauer R, van der Laan M. Why prefer double robust estimators in causal inference? Journal of Statistical Planning and Inference. 2005;129(1–2):405‐426. [Google Scholar]
- 25. Robins JM, Mark SD, Newey WK. Estimating exposure effects by modelling the expectation of exposure conditional on confounders. Biometrics. 1992;48:479‐495. [PubMed] [Google Scholar]
- 26. Vo TT, Porcher R, Vansteelandt S. Assessing the impact of case‐mix heterogeneity in individual participant data meta‐analysis: novel use of I 2 statistic and prediction interval. Res Meth Med Health Sci. 2021;2(1):12‐30. [Google Scholar]
- 27. Phillippo DM, Dias S, Elsada A, Ades A, Welton NJ. Population adjustment methods for indirect comparisons: a review of National Institute for health and care excellence technology appraisals. Int J Technol Assess Health Care. 2019;35:1‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Hauck WW, Anderson S, Marcus SM. Should we adjust for covariates in nonlinear regression analyses of randomized trials? Control Clin Trials. 1998;19(3):249‐256. [DOI] [PubMed] [Google Scholar]
- 29. Daniel R, Zhang J, Farewell D. Making apples from oranges: comparing noncollapsible effect estimators and their standard errors after adjustment for different covariate sets. Biom J. 2020;63:528‐557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Robins J. A new approach to causal inference in mortality studies with a sustained exposure period—application to control of the healthy worker survivor effect. Math Model. 1986;7(9–12):1393‐1512. [Google Scholar]
- 31. Robins J. A graphical approach to the identification and estimation of causal parameters in mortality studies with sustained exposure periods. J Chronic Dis. 1987;40:139S‐161S. [DOI] [PubMed] [Google Scholar]
- 32. Moore KL, Laan v dMJ. Covariate adjustment in randomized trials with binary outcomes: targeted maximum likelihood estimation. Stat Med. 2009;28(1):39‐64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Austin PC. Absolute risk reductions, relative risks, relative risk reductions, and numbers needed to treat can be obtained from a logistic regression model. J Clin Epidemiol. 2010;63(1):2‐6. [DOI] [PubMed] [Google Scholar]
- 34. Vo TT, Porcher R, Chaimani A, Vansteelandt S. A novel approach for identifying and addressing case‐mix heterogeneity in individual participant data meta‐analysis. Res Synth Methods. 2019;10(4):582‐596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Remiro‐Azócar A. Target estimands for population‐adjusted indirect comparisons. In press, Stat Med. 2022. [DOI] [PubMed] [Google Scholar]
- 36. Austin PC. An introduction to propensity score methods for reducing the effects of confounding in observational studies. Multivar Behav Res. 2011;46(3):399‐424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Hernán MA, Robins JM. Causal Inference: What if. Chapman & Hall; 2020. [Google Scholar]
- 38. VanderWeele TJ. Concerning the consistency assumption in causal inference. Epidemiology. 2009;20(6):880‐883. [DOI] [PubMed] [Google Scholar]
- 39. Rothman KJ, Greenland S, Walker AM. Concepts of interaction. Am J Epidemiol. 1980;112(4):467‐470. [DOI] [PubMed] [Google Scholar]
- 40. Manski CF. Meta‐Analysis for Medical Decisions. 2019. National Bureau of Economic Research. [Google Scholar]
- 41. Song F, Altman DG, Glenny AM, Deeks JJ. Validity of indirect comparison for estimating efficacy of competing interventions: empirical evidence from published meta‐analyses. BMJ. 2003;326(7387):472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Remiro‐Azócar A, Heath A, Baio G. Conflating marginal and conditional treatment effects: comments on ‘Assessing the performance of population adjustment methods for anchored indirect comparisons: a simulation study’. Stat Med. 2021;40(11):2753‐2758. [DOI] [PubMed] [Google Scholar]
- 43. Cole SR, Stuart EA. Generalizing evidence from randomized clinical trials to target populations: the ACTG 320 trial. Am J Epidemiol. 2010;172(1):107‐115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Remiro‐Azócar A, Heath A, Baio G. Effect modification in anchored indirect treatment comparisons: ‘Matching‐adjusted indirect comparisons: Application to time‐to‐event data’. Stat Med. 2022;41(8):1541‐1553. [DOI] [PubMed] [Google Scholar]
- 45. Nelsen RB. An Introduction to Copulas. Springer Science & Business Media; 2007. [Google Scholar]
- 46. Sklar M. Fonctions de repartition an dimensions et leurs marges. Publications de l'Institut de statistique de l'Université de Paris. 1959;8:229‐231. [Google Scholar]
- 47. Kruschke J. Doing Bayesian Data Analysis: A Tutorial with R, JAGS, and Stan. Academic Press; 2014. [Google Scholar]
- 48. Royston P. Multiple imputation of missing values. Stata J. 2004;4(3):227‐241. [Google Scholar]
- 49. Buuren Sv, Groothuis‐Oudshoorn K. mice: Multivariate imputation by chained equations in R. J Stat Softw 2010: 1–68, 45. [Google Scholar]
- 50. Ishak KJ, Proskorovsky I, Benedict A. Simulation and matching‐based approaches for indirect comparison of treatments. Pharmacoeconomics. 2015;33(6):537‐549. [DOI] [PubMed] [Google Scholar]
- 51. Petto H, Kadziola Z, Brnabic A, Saure D, Belger M. Alternative weighting approaches for anchored matching‐adjusted indirect comparisons via a common comparator. Value Health. 2019;22(1):85‐91. [DOI] [PubMed] [Google Scholar]
- 52. Belger M, Brnabic A, Kadziola Z, Petto H, Faries D. Inclusion of multiple studies in matching adjusted indirect comparisons (MAIC). Value Health. 2015;18(3):A33. [Google Scholar]
- 53. Grimm SE, Armstrong N, Ramaekers BL, et al. Nivolumab for treating metastatic or unresectable urothelial cancer: an evidence review group perspective of a NICE single technology appraisal. Pharmacoeconomics. 2019;37(5):655‐667. [DOI] [PubMed] [Google Scholar]
- 54. Ren S, Squires H, Hock E, Kaltenthaler E, Rawdin A, Alifrangis C. Pembrolizumab for locally advanced or metastatic urothelial cancer where cisplatin is unsuitable: an evidence review group perspective of a NICE single technology appraisal. Pharmacoeconomics. 2019;37(9):1073‐1080. [DOI] [PubMed] [Google Scholar]
- 55. Janes H, Dominici F, Zeger S. On quantifying the magnitude of confounding. Biostatistics. 2010;11(3):572‐582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Greenland S. Interpretation and choice of effect measures in epidemiologic analyses. Am J Epidemiol. 1987;125(5):761‐768. [DOI] [PubMed] [Google Scholar]
- 57. Greenland S, Robins JM, Pearl J. Confounding and collapsibility in causal inference. Stat Sci. 1999;14:29‐46. [Google Scholar]
- 58. Austin PC. The use of propensity score methods with survival or time‐to‐event outcomes: reporting measures of effect similar to those used in randomized experiments. Stat Med. 2014;33(7):1242‐1258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Keil AP, Daza EJ, Engel SM, Buckley JP, Edwards JK. A Bayesian approach to the g‐formula. Stat Methods Med Res. 2018;27(10):3183‐3204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Vansteelandt S, Keiding N. Invited commentary: G‐computation–lost in translation? Am J Epidemiol. 2011;173(7):739‐742. [DOI] [PubMed] [Google Scholar]
- 61. Imbens GW, Rubin DB. Causal Inference in Statistics, Social, and Biomedical Sciences. Cambridge University Press; 2015. [Google Scholar]
- 62. Phillippo DM, Dias S, Ades AE, Welton NJ. Target estimands for efficient decision making: response to comments on “Assessing the performance of population adjustment methods for anchored indirect comparisons: a simulation study”. Stat Med. 2021;40(11):2759‐2763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Zhang Z, Nie L, Soon G, Hu Z. New methods for treatment effect calibration, with applications to non‐inferiority trials. Biometrics. 2016;72(1):20‐29. [DOI] [PubMed] [Google Scholar]
- 64. Efron B, Tibshirani R. Bootstrap methods for standard errors, confidence intervals, and other measures of statistical accuracy. Stat Sci. 1986;1:54‐75. [Google Scholar]
- 65. Varadhan R, Henderson NC, Weiss CO. Cross‐design synthesis for extending the applicability of trial evidence when treatment effect is heterogeneous: part I. methodology. Commun Stat. 2016;2(3–4):112‐126. [Google Scholar]
- 66. Rubin DB, Schenker N. Logit‐based interval estimation for binomial data using the Jeffreys prior. Sociol Methodol. 1987;17:131‐144. [Google Scholar]
- 67. Aalen OO, Farewell VT, De Angelis D, Day NE, Nöel GO. A Markov model for HIV disease progression including the effect of HIV diagnosis and treatment: application to AIDS prediction in England and Wales. Stat Med. 1997;16(19):2191‐2210. [DOI] [PubMed] [Google Scholar]
- 68. Keil AP, Daniels JL, Hertz‐Picciotto I. Autism spectrum disorder, flea and tick medication, and adjustments for exposure misclassification: the CHARGE (CHildhood Autism Risks from Genetics and Environment) case–control study. Environ Health. 2014;13(1):1‐10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Josefsson M, Daniels MJ. Bayesian semi‐parametric G‐computation for causal inference in a cohort study with MNAR dropout and death. J R Stat Soc Ser C Appl Stat. 2021;70(2):398‐414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Rubin DB. Bayesian inference for causal effects: the role of randomization. Annal Stat. 1978;6:34‐58. [Google Scholar]
- 71. Saarela O, Arjas E, Stephens DA, Moodie EE. Predictive Bayesian inference and dynamic treatment regimes. Biom J. 2015;57(6):941‐958. [DOI] [PubMed] [Google Scholar]
- 72. Lunn D, Jackson C, Best N, Thomas A, Spiegelhalter D. The BUGS Book: A Practical Introduction to Bayesian Analysis. CRC Press; 2012. [Google Scholar]
- 73. Plummer M. JAGS: A Program for Analysis of Bayesian Graphical Models Using Gibbs Sampling. Proceedings of the 3rd International Workshop on Distributed Statistical Computing (DSC 2003) ; 2003: 1‐10.
- 74. Carpenter B, Gelman A, Hoffman MD, Lee D, Goodrich B, Betancourt M, Brubaker M, Guo J, Li P, Riddell A Stan: A probabilistic programming language. J Stat Softw 2017; 76(1):1183‐1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Rue H, Martino S, Chopin N. Approximate Bayesian inference for latent Gaussian models by using integrated nested Laplace approximations. J R Stat Soc Series B Stat Methodology. 2009;71(2):319‐392. [Google Scholar]
- 76. Guyot P, Ades A, Ouwens MJ, Welton NJ. Enhanced secondary analysis of survival data: reconstructing the data from published Kaplan‐Meier survival curves. BMC Med Res Methodol. 2012;12(1):9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Morris TP, White IR, Crowther MJ. Using simulation studies to evaluate statistical methods. Stat Med. 2019;38(11):2074‐2102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Team RC R: A Language and Environment for Statistical Computing. 2013. R Foundation for Statistical Computing. [Google Scholar]
- 79. Ripley BD. Stochastic Simulation. Wiley; 2009. [Google Scholar]
- 80. Skipka G, Wieseler B, Kaiser T, et al. Methodological approach to determine minor, considerable, and major treatment effects in the early benefit assessment of new drugs. Biom J. 2016;58(1):43‐58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Stanley K. Design of randomized controlled trials. Circulation. 2007;115(9):1164‐1169. [DOI] [PubMed] [Google Scholar]
- 82. Cohen J. Statistical Power Analysis for the Behavioral Sciences. Academic Press; 2013. [Google Scholar]
- 83. Vittinghoff E, McCulloch CE. Relaxing the rule of ten events per variable in logistic and cox regression. Am J Epidemiol. 2007;165(6):710‐718. [DOI] [PubMed] [Google Scholar]
- 84. Neyman J. On the two different aspects of the representative method: the method of stratified sampling and the method of purposive selection. J R Stat Soc. 1934;97(4):558‐625. [Google Scholar]
- 85. Annesi I, Moreau T, Lellouch J. Efficiency of the logistic regression and cox proportional hazards models in longitudinal studies. Stat Med. 1989;8(12):1515‐1521. [DOI] [PubMed] [Google Scholar]
- 86. Ho DE, Imai K, King G, Stuart EA. Matching as nonparametric preprocessing for reducing model dependence in parametric causal inference. Political Anal. 2007;15(3):199‐236. [Google Scholar]
- 87. Phillippo DM, Dias S, Ades A, Welton NJ. Equivalence of entropy balancing and the method of moments for matching‐adjusted indirect comparison. Res Synth Methods. 2020;11:568‐572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Dehejia RH, Wahba S. Causal effects in nonexperimental studies: reevaluating the evaluation of training programs. J Am Stat Assoc. 1999;94(448):1053‐1062. [Google Scholar]
- 89. Waernbaum I. Propensity score model specification for estimation of average treatment effects. J Stat Plan Inference. 2010;140(7):1948‐1956. [Google Scholar]
- 90. Westreich D, Edwards JK, Lesko CR, Cole SR, Stuart EA. Target validity and the hierarchy of study designs. Am J Epidemiol. 2019;188(2):438‐443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Imai K, King G, Stuart EA. Misunderstandings between experimentalists and observationalists about causal inference. J R Stat Soc A Stat Soc. 2008;171(2):481‐502. [Google Scholar]
- 92. Lunceford JK, Davidian M. Stratification and weighting via the propensity score in estimation of causal treatment effects: a comparative study. Stat Med. 2004;23(19):2937‐2960. [DOI] [PubMed] [Google Scholar]
- 93. Laan V dMJ, Laan M, Robins JM. Unified Methods for Censored Longitudinal Data and Causality. Springer Science & Business Media; 2003. [Google Scholar]
- 94. Bang H, Robins JM. Doubly robust estimation in missing data and causal inference models. Biometrics. 2005;61(4):962‐973. [DOI] [PubMed] [Google Scholar]
- 95. Kang JD, Schafer JL. Demystifying double robustness: a comparison of alternative strategies for estimating a population mean from incomplete data. Stat Sci 2007; 22(4): 523–539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Ishak K, Rael M, Phatak H, Masseria C, Lanitis T. Simulated treatment comparison of time‐to‐event (and other non‐linear) outcomes. Value Health. 2015;18(7):A719. [Google Scholar]
- 97. Bartlett JW. Covariate adjustment and estimation of mean response in randomised trials. Pharm Stat. 2018;17(5):648‐666. [DOI] [PubMed] [Google Scholar]
- 98. Qu Y, Luo J. Estimation of group means when adjusting for covariates in generalized linear models. Pharm Stat. 2015;14(1):56‐62. [DOI] [PubMed] [Google Scholar]
- 99. Lane PW, Nelder JA. Analysis of covariance and standardization as instances of prediction. Biometrics. 1982;38:613‐621. [PubMed] [Google Scholar]
- 100. Chan AW, Song F, Vickers A, et al. Increasing value and reducing waste: addressing inaccessible research. Lancet. 2014;383(9913):257‐266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Nowok B, Raab GM, Dibben C. synthpop: Bespoke creation of synthetic data in R. J Stat Softw 2016; 74(11): 1–26. [Google Scholar]
- 102. Bonofiglio F, Schumacher M, Binder H. Recovery of original individual person data (IPD) inferences from empirical IPD summaries only: applications to distributed computing under disclosure constraints. Stat Med. 2020;39(8):1183‐1198 [DOI] [PubMed] [Google Scholar]
- 103. Faria R, Hernandez Alava M, Manca A, Wailoo A. NICE DSU Technical Support Document 17: the Use of Observational Data to Inform Estimates of Treatment Effectiveness for Technology Appraisal: Methods for Comparative Individual Patient Data. NICE Decision Support Unit; 2015. [Google Scholar]
- 104. Robins JM, Hernán MA. Estimation of the Causal Effects of Time‐Varying Exposures. Vol 553; 2009:599. Chapman & Hall/CRC. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
APPENDIX S1 Supplementary Information
Data Availability Statement
The files required to generate the data, run the simulations, and reproduce the results are available at http://github.com/remiroazocar/Gcomp_indirect_comparisons_simstudy.


