Abstract
Coenzyme Q10 (CoQ10) is a natural antioxidant compound that prevents the vascular damage induced by free radicals and the activation of inflammatory signaling pathways. Supplementation with CoQ10 is safe though its bioavailability is generally low, as far as variable depending on the pharmaceutical form of preparation. Recently, the development of phytosome technology has improved the bioavailability of CoQ10 and definitely facilitated its effective use in clinical practice. The present double‐blind, randomized, placebo‐controlled, crossover clinical study aimed to investigate the effect on endothelial reactivity and total antioxidant capacity (TAC) of either acute and chronic supplementation with CoQ10 phytosome in a sample of 20 healthy young nonsmoking subjects. CoQ10 phytosome supplementation acutely improved endothelial reactivity in comparison with baseline and placebo (+4.7% ± 0.9% vs. −0.1 %± 0.3% p < 0.05). Middle‐term supplementation of the tested pharmaceutical formulation of CoQ10 significantly improved mean arterial pressure (−2.2 ± 1.1 mmHg vs. 0.2 ± 0.7 mmHg, p < 0.05 vs. placebo) and TAC (+29.6% ± 3.2% vs. +1.9% ± 0.8%, p < 0.05 vs. placebo). Endothelial reactivity improved compared with baseline following middle‐term dietary supplementation with CoQ10 phytosome (+5.7% ± 1.1%, p < 0.05).
Keywords: coenzyme Q10 , dietary supplementation, endothelial reactivity, phytosome technology, total antioxidant capacity
Abbreviations
- BP
blood pressure
- CoQ10
coenzyme Q10
- CV
cardiovascular
- DBP
diastolic blood pressure
- LDL
low‐density lipoprotein
- MAP
mean arterial pressure
- NO
nitric oxide
- PP
pulse pressure
- PV
pulse volume
- SBP
systolic blood pressure
- TAC
total antioxidant capacity
1. INTRODUCTION
Coenzyme Q10 (CoQ10) is a natural antioxidant compound that offer potential benefit in the management of patients affected by cardiovascular (CV) disease, preventing the damage induced by free radicals and the activation of inflammatory signaling pathways. 1 , 2
Supplementation with CoQ10 is safe, without any known pharmacological interactions. 3 However, its bioavailability is generally low, as far as variable depending on the form of preparation (i.e., tables, powder‐filled capsules, or oil suspensions in soft gel capsules). 1 The development of phytosome technology has recently improved the bioavailability of CoQ10, increasing it by three times compared to standard pharmaceutical formulations and definitely facilitating its effective use in clinical practice. 2
CoQ10 is absorbed slowly (and unpredictably) from the small intestine, because it has a high molecular weight and is not water soluble, passes into the lymphatics, and finally to the blood and tissues. 4 For this reason, CoQ10 plasma level is not clearly related with clinical outcomes, while its dosage is complex and expensive 5 ; thus, the evaluation of the clinical effect of CoQ10 supplementation is preferably to indirectly estimate. A method to estimate the bioavailability and efficacy of CoQ10 is the evaluation of treatment‐related variation in endothelial reactivity, 6 since ubiquinol supplementation is associated with increased nitric oxide (NO) bioavailability and enhances low‐density lipoprotein (LDL) antioxidant protection, both related to endothelial function. 7 , 8
This study aimed to investigate the effect on endothelial reactivity of either acute and chronic supplementation with CoQ10 phytosome in a sample of healthy young nonsmoking subjects. Moreover, we assessed the impact of chronic supplementation with CoQ10 phytosome on the plasma total antioxidant capacity (TAC) that measures the amount of total antioxidants in plasma.
2. MATERIALS AND METHODS
2.1. Study design and participants
This was a double‐blind, randomized, placebo‐controlled, crossover clinical trial aiming to assess the acute and chronic effects of supplementation with CoQ10 phytosome in a group of 20 healthy nonsmoking young volunteers aged 18–40 years and consecutively enrolled in the ambulatory service of CV disease prevention of the Medical and Surgical Sciences Department of the University of Bologna, Bologna, Italy.
Enrolled volunteers did not have any serious or disabling diseases (e.g., severe organ failure, previous major CV event, active viral hepatitis, inflammatory bowel disease, malignancy, and dementia). Further exclusion criteria were body mass index (BMI) ≥ 30 kg/m2, treatment with drugs, and/or dietary supplements with antioxidant/anti‐inflammatory effect and known gastrointestinal disorders potentially affecting the absorption of CoQ10.
Enrolled subjects were adhering to a low‐fat low‐sodium Mediterranean diet for 4 weeks before being randomized to receive CoQ10 supplement or placebo. At baseline and on the day of crossover, the immediate acute effect of dietary supplementation with CoQ10 phytosome was assessed at baseline and 2 h after receiving a double dose of either active treatment or placebo. Study's participants received adequate doses of either CoQ10 phytosome or placebo to complete the one of two 4‐week treatment sequences. The crossover to the second treatment was preceded by a 2‐week wash‐out period. Study design and timeline is described in detail in Figure 1.
FIGURE 1.
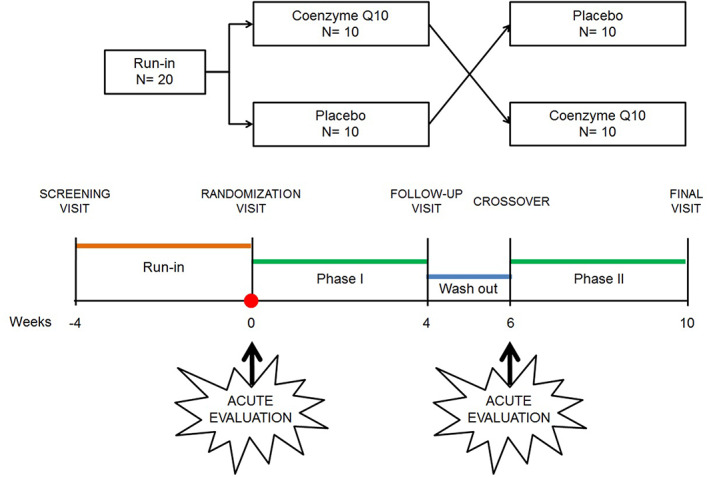
Study design and time‐line
At each follow‐up visit, patients were evaluated for clinical status, TAC and by the execution of a physical examination and hemodynamic analyses.
The study fully complied with the ethical guidelines of the Declaration of Helsinki and with The International Council for Harmonization of Technical Requirements for Registration of Pharmaceuticals for Human Use (ICH) Harmonized Tripartite Guideline for Good Clinical Practice (GCP). The study protocol was approved by the Ethical Committee of the University of Bologna. All patients signed a written informed consent to participate.
2.2. Treatment
After 4‐week period of diet standardization, enrolled subjects were randomized to receive either indistinguishable pills of placebo or 150 mg CoQ10 phytosome (equivalent to 30 mg CoQ10; Ubiqsome®, Indena S.p.A., Milan). For the entire duration of the study, patients were instructed to take a pill of the assigned treatment once daily (except on acute evaluation, when they received a double dose of the treatment).
Randomization was performed centrally, by computer‐generated codes. Participants and investigators were blinded to the group assignment. Randomization codes were kept in a sealed envelope that was opened after study completion and data analysis.
At the end of the clinical trial, all unused pills were retrieved for inventory. Treatment compliance was assessed by counting the number of returned pills.
2.3. Assessments
2.3.1. Clinical data and other measurements
Information gathered in the patients history included presence of CV disease and other systemic diseases, allergies, and medications. Validated semi‐quantitative questionnaires including food frequency questionnaire (FFQ) were used to assess demographic variables, recreational physical activity, and dietary and smoking habits. 9
Plasma TAC was measured using a commercial kit as per the manufacturer's instructions (Biovision Company, Milpitas, CA).
2.3.2. Blood pressure measurements
Blood pressure (BP) was measured in accordance with the recommendations of the International Guidelines for the management of arterial hypertension. 10 Resting systolic (SBP) and diastolic BP (DBP) were measured with a validated oscillometric device and a cuff of the appropriate size applied on the right upper arm. To improve detection accuracy, three BP readings were sequentially obtained at 1‐min intervals. The first reading was discarded, and the average between the second and the third reading was recorded as study variable.
Pulse pressure (PP) was calculated as the difference between SBP and DBP (PP = SBP − DBP). Mean arterial pressure (MAP) was obtained by adding one‐third of PP to DBP (MAP = 1/3PP + DBP).
2.3.3. Endothelial reactivity
Following the current guidelines, 11 during the clinical study endothelial reactivity (ER) was evaluated though Endocheck® (BC Biomedical Laboratories Ltd, Vancouver, BC, Canada), a method embedded within the Vicorder® device which guarantees a very good intra‐ and inter‐operator reliability. 12 The measurement was carried out with patients in supine position and in abstinence from cigarette smoking and caffeinated beverages for at least 12 h. After a 10‐min rest, the brachial pulse volume (PV) waveforms were recorded at baseline for 10 s and during reactive hyperemia. 13 The percent PV displacement was calculated as the percentage change from baseline to peak dilatation. 14 All of the hemodynamic measurements were performed by a trained physician who was blinded to the treatment groups.
2.3.4. Assessment of safety and tolerability
Safety and tolerability were evaluated through a continuous monitoring during the study, in order to detect any adverse event, clinical safety, laboratory findings, vital sign measurements, and physical examinations. A blinded, independent expert clinical event committee was appointed by the principal investigator in order to categorize the adverse events that could possibly be experienced during the trial as not related, unlikely related, possibly related, probably related, or definitely related to the tested treatment. 15
2.4. Statistical analysis
Data were analyzed using intention to treat by means of the Statistical Package for Social Sciences (SPSS) version 25.0 (IBM Corporation, Armonk, NY) for Windows.
Efficacy analyses were performed considering the intention‐to‐treat (ITT) population. A sensitivity analysis of the primary variable was also planned in the per‐protocol population (PPP).
A full descriptive analysis of the collected parameters was carried out. Categorical variables were expressed as absolute number and percentage and compared with the Fisher corrected chi‐square test (for nominal variables) and the Wilcoxon‐Rank test (for ordinal variables). The Duncan test was always carried out to exclude extreme values. As they were all normally distributed, continuous variables were expressed as mean ± standard deviation (SD) and compared by two‐way analysis of variance (ANOVA) for crossover design followed by Tukey's post hoc test.
The minimum level of statistical significance was set to p < 0.05 two‐tailed.
3. RESULTS
All enrolled subjects (mean age 26.8 ± 1.3 years old; Male: 12, Women: 8) completed the clinical trial according to the study design (dropout rate = 0%). No protocol violations were reported.
Compliance to treatment was 100% either in the active treated group and in the placebo group.
CoQ10 phytosome supplementation acutely improved endothelial reactivity in comparison with placebo and baseline (Table 1).
TABLE 1.
CoQ10 acute effect (2 h after ingestion) on PV
| Placebo | CoQ10 phytosome | |||
|---|---|---|---|---|
| Baseline | 2‐h follow‐up | Baseline | 2‐h follow‐up | |
| Endothelial reactivity (%) | 64.9 ± 5.4 | 64.8 ± 5.3 | 64.6 ± 5.7 | 69.3 ± 6.1*,** |
Note: *p < 0.05 versus baseline; **p < 0.05 versus placebo.
The tested pharmaceutical formulation of CoQ10 significantly improved MAP and TAC compared to placebo and baseline values. PV improved than baseline following dietary supplementation with CoQ10 phytosome (Table 2).
TABLE 2.
Effect of CoQ10 on blood pressure, pulse volume, and total antioxidant capacity
| Baseline/end wash‐out | End of treatment | |||
|---|---|---|---|---|
| Placebo ➔ CoQ10 phytosome | CoQ10 phytosome ➔ placebo | Placebo ➔ CoQ10 phytosome | CoQ10 phytosome ➔ placebo | |
| SBP (mmHg) | 122.8 ± 4.1 | 122.0 ± 5.5 | 121.5 ± 3.3 | 123.1 ± 2.4 |
| DBP(mmHg) | 82.8 ± 1.9 | 82.3 ± 1.6 | 83.9 ± 1.7 | 83.2 ± 1.4 |
| PP (mmHg) | 39.5 ± 1.6 | 39.4 ± 1.8 | 37.9 ± 1.1 | 40.2 ± 1.6 |
| MAP (mmHg) | 96.4 ± 3.7 | 96.2 ± 3.6 | 96.5 ± 3.6 | 94.3 ± 3.0*,** |
| Endothelial reactivity (%) | 64.7 ± 5.6 | 64.1 ± 5.4 | 64.8 ± 5.8 | 68.5 ± 6.2* |
| TAC (pg/ml) | 10.1 ± 1.3. | 10.3 ± 1.4 | 10.4 ± 1.5 | 13.2 ± 1.4*,** |
Note: *p < 0.05 versus baseline; **p < 0.05 versus placebo.
Abbreviations: CoQ10, coenzyme Q10; DBP, diastolic blood pressure; MAP, mean arterial pressure; PP, pulse pressure; SBP, systolic blood pressure; TAC, total antioxidant capacity.
4. DISCUSSION
Endothelial function of the arterial vasculature is an important early marker of atherosclerosis, reflecting the ability of the endothelial layer to release NO, modulating smooth muscle tone in the arterial wall of the conduit arteries. 16 The effect of CoQ10 supplementation on the modulation of endothelial function has been previously evaluated in patients with Type 2 diabetes mellitus, coronary artery disease, and in elderly people. 17 These studies had already showed that flow‐mediated dilation, or nitroglycerin‐mediated dilation and the extracellular superoxide dismutase activity increased in most of the subjects treated with CoQ10, and this effect had been attributed to the antioxidant and anti‐inflammatory activity of this compound. 18 , 19 , 20 Indeed, endothelial dysfunction is primarily driven by increased bioavailability of oxidizing reactive oxygen species (ROS) as a consequence of NO deficiency and, then, increased vascular smooth muscle growth within the endothelium. 21 , 22
In our study, either acute and chronic supplementation with CoQ10 phytosome was effective in improving plasma TAC and endothelial reactivity in healthy young not smoking subjects. The observed result was enhanced by the use of a specific phytosome delivery formulation (patented as Ubiqsome®) that has already been previously showed improve the oral absorption of coenzyme CoQ10 and optimize the physiological plasma levels of CoQ10 after just a single dose. 23 , 24
The relevance of these findings cannot be underestimated, since they collectively support the use of Ubiqsome® in clinical practice, even in the short term and in the absence of known vascular damage. In particular, a meta‐analysis of 41 studies involving more than 18000 participants showed that an even small improvement in endothelial function is associated to a significant reduction in CV disease risk. 25
Despite the relevant findings and the practical implications, this study is not without limitations. We acknowledge the small sample size and the relatively short follow‐up that does not clarify on the possible occurrence of adaptation phenomena (which however have never been documented for CoQ10 before). Furthermore, possible changes of signaling molecules such as bradykinin, adenosine, vascular endothelial growth factor, serotonin, or NO synthase—also related to endothelial function—were not investigated, but our observation was limited to the instrumental assessment of ER. Finally, as per study's design, the effect of supplementation with a nonphytosome formulation of CoQ10 was not assessed in the context of the present clinical trial. Further research directions should also explore whether the positive effect of CoQ10 supplementation we observed is demonstrable in individuals with endothelial dysfunction a priori.
In conclusion, CoQ10 phytosome exerts beneficial effects on endothelial reactivity in healthy young subjects. Further studies are needed that confirms our observations in the long term by directly comparing different CoQ10 pharmaceutical formulations.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
ACKNOWLEDGMENTS
This research was funded by institutional funding of the University of Bologna. Indena S.p.A. (Milan, Italy) kindly provided the tested products. Open Access Funding provided by Universita degli Studi di Bologna within the CRUI‐CARE Agreement.
Cicero AFG, Fogacci F, Di Micoli A, Veronesi M, Borghi C. Noninvasive instrumental evaluation of coenzyme Q10 phytosome on endothelial reactivity in healthy nonsmoking young volunteers: A double‐blind, randomized, placebo‐controlled crossover clinical trial. BioFactors. 2022;48:1160–1165. 10.1002/biof.1839
Funding information Universita di Bologna, Grant/Award Number: RFO2017
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. Raizner AE, Quiñones MA. Coenzyme Q10 for patients with cardiovascular disease: JACC focus seminar. J Am Coll Cardiol. 2021;77:609–19. 10.1016/j.jacc.2020.12.009 [DOI] [PubMed] [Google Scholar]
- 2. Martelli A, Testai L, Colletti A, Cicero AFG. Coenzyme Q10: clinical applications in cardiovascular diseases. Antioxidants. 2020;9:341. 10.3390/antiox9040341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Saini R. Coenzyme Q10: the essential nutrient. J Pharm Bioallied Sci. 2011;3:466–7. 10.4103/0975-7406.84471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Garrido‐Maraver J, Cordero MD, Oropesa‐Ávila M, Fernández Vega A, de la Mata M, et al. Coenzyme q10 therapy. Mol Syndromol. 2014;5:187–97. 10.1159/000360101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Pastor‐Maldonado CJ, Suárez‐Rivero JM, Povea‐Cabello S, Álvarez‐Córdoba M, Villalón‐García I, et al. Coenzyme Q10: novel formulations and medical trends. Int J Mol Sci. 2020;21:8432. 10.3390/ijms21228432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Park SY, Pekas EJ, Headid RJ 3rd, Son WM, Wooden TK, et al. Acute mitochondrial antioxidant intake improves endothelial function, antioxidant enzyme activity, and exercise tolerance in patients with peripheral artery disease. Am J Physiol Heart Circ Physiol. 2020;319:H456–67. 10.1152/ajpheart.00235.2020 [DOI] [PubMed] [Google Scholar]
- 7. Sabbatinelli J, Orlando P, Galeazzi R, Silvestri S, Cirilli I, et al. Ubiquinol ameliorates endothelial dysfunction in subjects with mild‐to‐moderate dyslipidemia: a randomized clinical trial. Nutrients. 2020;12:1098. 10.3390/nu12041098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Chang X, Zhao Z, Zhang W, Liu D, Ma C, et al. Natural antioxidants improve the vulnerability of Cardiomyocytes and vascular endothelial cells under stress conditions: a focus on mitochondrial quality control. Oxid Med Cell Longev. 2021;2021:6620677. 10.1155/2021/6620677 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 9. Cicero AFG, Fogacci F, Veronesi M, Strocchi E, Grandi E, et al. A randomized placebo‐controlled clinical trial to evaluate the medium‐term effects of oat fibers on human health: the Beta‐Glucan effects on lipid profile, Glycemia and inTestinal health (BELT) study. Nutrients. 2020;12:686. 10.3390/nu12030686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Williams B, Mancia G, Spiering W, Agabiti Rosei E, Azizi M, et al. 2018 ESC/ESH guidelines for the management of arterial hypertension: the task force for the management of arterial hypertension of the European Society of Cardiology and the European Society of Hypertension: the task force for the management of arterial hypertension of the European Society of Cardiology and the European Society of Hypertension. J Hypertens. 2018;36:1953–2041. 10.1097/HJH.0000000000001940 [DOI] [PubMed] [Google Scholar]
- 11. Corretti MC, Anderson TJ, Benjamin EJ, Celermajer D, Charbonneau F, et al. Guidelines for the ultrasound assessment of endothelial‐dependent flow‐mediated vasodilation of the brachial artery: a report of the international brachial artery reactivity task force. J Am Coll Cardiol. 2002;39:257–65. 10.1016/s0735-1097(01)01746-6 [DOI] [PubMed] [Google Scholar]
- 12. McGreevy C, Barry M, Bennett K, Williams D. Repeatability of the measurement of aortic pulse wave velocity (aPWV) in the clinical assessment of arterial stiffness in community‐dwelling older patients using the Vicorder(®) device. Scand J Clin Lab Invest. 2013;73:269–73. 10.3109/00365513.2013.770162 [DOI] [PubMed] [Google Scholar]
- 13. Day LM, Maki‐Petaja KM, Wilkinson IB, McEniery CM. Assessment of brachial artery reactivity using the endocheck: repeatability, reproducibility and preliminary comparison with ultrasound. Artery Res. 2013;7:119–20. [Google Scholar]
- 14. Cicero AFG, Caliceti C, Fogacci F, Giovannini M, Calabria D, et al. Effect of apple polyphenols on vascular oxidative stress and endothelium function: a translational study. Mol Nutr Food Res. 2017;61. 10.1002/mnfr.201700373 [DOI] [PubMed] [Google Scholar]
- 15. Cicero AFG, Fogacci F, Bove M, Giovannini M, Borghi C. Impact of a short‐term synbiotic supplementation on metabolic syndrome and systemic inflammation in elderly patients: a randomized placebo‐controlled clinical trial. Eur J Nutr. 2012;60:655–63. 10.1007/s00394-020-02271-8 [DOI] [PubMed] [Google Scholar]
- 16. Thijssen DHJ, Bruno RM, van Mil ACCM, Holder SM, Faita F, et al. Expert consensus and evidence‐based recommendations for the assessment of flow‐mediated dilation in humans. Eur Heart J. 2019;40:2534–47. 10.1093/eurheartj/ehz350 [DOI] [PubMed] [Google Scholar]
- 17. Gutierrez‐Mariscal FM, de la Cruz‐Ares S, Torres‐Peña JD, Alcalá‐Diaz JF, Yubero‐Serrano EM, et al. Coenzyme Q10 and cardiovascular diseases. Antioxidants (Basel). 2021;10:906. 10.3390/antiox10060906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. González‐Guardia L, Yubero‐Serrano EM, Delgado‐Lista J, Perez‐Martinez P, Garcia‐Rios A, et al. Effects of the Mediterranean diet supplemented with coenzyme q10 on metabolomic profiles in elderly men and women. J Gerontol A Biol Sci Med Sci. 2015;70:78–84. 10.1093/gerona/glu098 [DOI] [PubMed] [Google Scholar]
- 19. Tiano L, Belardinelli R, Carnevali P, Principi F, Seddaiu G, et al. Effect of coenzyme Q10 administration on endothelial function and extracellular superoxide dismutase in patients with ischaemic heart disease: a double‐blind, randomized controlled study. Eur Heart J. 2007;28:2249–55. 10.1093/eurheartj/ehm267 [DOI] [PubMed] [Google Scholar]
- 20. Yubero‐Serrano EM, Gonzalez‐Guardia L, Rangel‐Zuñiga O, Delgado‐Lista J, Gutierrez‐Mariscal FM, et al. Mediterranean diet supplemented with coenzyme Q10 modifies the expression of proinflammatory and endoplasmic reticulum stress‐related genes in elderly men and women. J Gerontol A Biol Sci Med Sci. 2012;67:3–10. 10.1093/gerona/glr167 [DOI] [PubMed] [Google Scholar]
- 21. Akhigbe R, Ajayi A. The impact of reactive oxygen species in the development of cardiometabolic disorders: a review. Lipids Health Dis. 2021;20:23. 10.1186/s12944-021-01435-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Rabanal‐Ruiz Y, Llanos‐González E, Alcain FJ. The use of coenzyme Q10 in cardiovascular diseases. Antioxidants (Basel). 2021;10:755. 10.3390/antiox10050755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Petrangolini G, Ronchi M, Frattini E, de Combarieu E, Allegrini P, et al. A new food‐grade coenzyme Q10 formulation improves bioavailability: single and repeated pharmacokinetic studies in healthy volunteers. Curr Drug Deliv. 2019;16:759–67. 10.2174/1567201816666190902123147 [DOI] [PubMed] [Google Scholar]
- 24. Rizzardi N, Liparulo I, Antonelli G, Orsini F, Riva A, et al. Coenzyme Q10 phytosome formulation improves CoQ10 bioavailability and mitochondrial functionality in cultured cells. Antioxidants (Basel). 2021;10:927. 10.3390/antiox10060927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Matsuzawa Y, Kwon TG, Lennon RJ, Lerman LO, Lerman A. Prognostic value of flow‐mediated vasodilation in brachial artery and fingertip artery for cardiovascular events: a systematic review and meta‐analysis. J Am Heart Assoc. 2015;4:e002270. 10.1161/JAHA.115.002270 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


