Abstract
Objective
In 2021 the U.S. FDA issued a Class 1 safety recall notice for specific devices due to a risk of carcinogen exposure. The objective of this study was to evaluate reports of cancer linked to CPAP devices to understand implications for the field of sleep medicine.
Methods
Cases of cancer involving CPAP devices were retrieved from the MAUDE database from 2014 to 2021 and analyzed with descriptive statistics.
Results
A total of 2571 patient injuries were associated with CPAP. Reports of cancer (n = 209; 4.62%) were the second most commonly documented patient problem associated with CPAP, although 1950 (43.13%) patients had a device problem without an associated injury. Of the 209 cancer cases associated with CPAP, 200 (95.7%) of the adverse event reports were received by the FDA in 2021. There were 174 (9.15%) descriptions of the CPAP polyurethane sound abatement foam degrading in association with a cancer diagnosis, but degradation was more commonly not associated with malignancy (n = 1728; 90.85%). Other frequently documented CPAP device problems included broken devices (n = 279; 6.92%), fire (n = 182; 4.51%), and patient–device incompatibility (n = 144; 3.57%).
Conclusion
Malignancy associated with CPAP devices has been reported; however, future studies are required to establish causation. Given 95.7% of those documented cases were reported in 2021, otolaryngologists should be prepared to discuss the risks of carcinogenesis associated with CPAP. The otolaryngology community should also be aware of the potential bandwagon effect and the implications for CPAP compliance.
Level of Evidence
4 Laryngoscope, 132:2270–2274, 2022
Keywords: CPAP, malignancy, sleep medicine, quality improvement, otolaryngology
INTRODUCTION
Obstructive sleep apnea (OSA) affects a large proportion of the general population, with an approximate prevalence of 15%–30%. 1 A consequence of untreated sleep apnea is excessive fatigue during the day, which is known to negatively impact daily function, including decreasing workplace productivity and increasing risk of motor vehicle accidents. 2 , 3 Long‐term consequences of sleep apnea include resistant hypertension, increased stroke risk, and major morbidity, which have been shown to contribute to early mortality. 4 , 5 Studies have consistently described the adverse effects and decreased quality of life associated with untreated OSA. Guidelines from the American Academy of Sleep Medicine recommend continuous positive airway pressure (CPAP) as the gold standard for OSA management. 6 Given the poor outcomes associated with untreated OSA, understanding factors that affect compliance with CPAP is an important clinical question that has health implications for a large number of patients.
The U.S. FDA issued a Class 1 safety recall notice in July of 2021 for CPAP devices due to risk of carcinogen exposure from degradation of the polyurethane sound abatement foam. 7 Class 1 safety recall notices are the most serious type of recall only used in cases of severe injury or death. CPAP devices function by producing airflow through a well‐fitted mask that creates enough pressure to force the upper airway open, thereby reducing hypoxemic events associated with respiration during sleep. The efficacy of CPAP machines is well established in the literature, with numerous studies demonstrating the health benefits of the devices. 8 , 9 These devices are ubiquitous as reflected in the over 15 million devices that were affected by the 2021 Class 1 recall. 7 Additionally, CPAP devices are expected to become more common given that the prevalence of OSA is projected to increase in part due to the rising rates of obesity. 2 However, the association between CPAP devices and carcinogenesis coupled with the Class 1 recall notice has the potential to produce a deleterious effect on CPAP compliance through influencing public perception, similar to publication of the literature, now retracted, linking autism to vaccination. These events underscore the critical nature of understanding the association between malignancy and CPAP to provide proper patient education.
There have been no previous studies, to the best of our knowledge, that have examined the implications of the U.S. FDA Class 1 recall notice on reports of malignancy associated with CPAP. Previous studies have examined adherence to CPAP therapy and all‐cancer incidence, but the literature does not currently include any reports of cancer or deaths related to the degradation of the polyurethane sound abatement foam. 10 This makes sense given that the recall notice was issued on July 22, 2021. The recall notice was based on 1200 adverse events and 100 patient injuries documented in the Manufacturer and User Facility Device Experience (MAUDE) database. We sought to utilize the same MAUDE database to understand the association between CPAP and carcinogenesis as well as to discern previously unidentified national trends. Our hypothesis was that reports of malignancy associated with CPAP increased in 2021 following the U.S. FDA Class 1 recall notice. We hope that the findings presented in this study will help better inform otolaryngologists and sleep specialists to provide proactive recommendations.
MATERIALS AND METHODS
This study was deemed exempt from review by the Cleveland Clinic Institutional Review Board due to the public nature of the database. The MAUDE database is maintained by the U.S. FDA. The MAUDE database is derived from adverse event reports collected from both mandatory reporters (e.g., manufacturers) and voluntary reporters (e.g., physicians). 11 The database was queried from January 1, 2014, to October 17, 2021 for all documented adverse events related to the “BZD” product code, which corresponds to Ventilator, Non‐Continuous (Respirator). Cancer was defined by the “Patient Problem” variable, and degradation of the sound abatement foam was defined by the “Device Problem” variable. The “Date Received” variable was used to determine the year in which the adverse event was reported. A single adverse event report could contribute to multiple device problems and patient problems. Descriptive statistics and trend modeling were performed in Tableau Desktop (version 2021.1) and Microsoft Excel (version 16.16).
RESULTS
Patient‐specific adverse events associated with CPAP
A total of 2571 patient problems with 96 distinct categories were associated with CPAP therapy. Table I describes the 10 most common patient problems. The median number of adverse patient events per category was 13 documented reports. Cancer was reported in 209 cases (4.62%) of patients treated with CPAP and was the second most common patient problem after headache, which had 212 (4.69%) adverse event reports. Most frequently, patients had a device problem without an associated injury (n = 1950; 43.13%). Other common injuries included dyspnea with 191 (4.22%) reports, cough with 180 (3.98%) reports, and respiratory problems with 119 (2.63%) reports.
TABLE I.
The Most Common Patient Events Associated with CPAP.
| Category | n = 4521 |
|---|---|
| No consequences or impact to patient | 1950 (43.13%) |
| Headache | 212 (4.69%) |
| Cancer | 209 (4.62%) |
| Dyspnea | 191 (4.22%) |
| Cough | 180 (3.98%) |
| Respiratory problem | 119 (2.63%) |
| Asthma | 88 (1.95%) |
| Respiratory tract infection | 73 (1.61%) |
| Sore throat | 72 (1.59%) |
| Death | 69 (1.53%) |
Temporal trends in malignancy reports
We found that 200 (95.7%) of the 209 cancer cases associated with CPAP were received by the U.S. FDA in 2021, which is shown in Figure 1. For 2021, the date of the report was the same as the year the report was received in 167 cases. The range of reported cancer cases was 200–1 with a median of two cases reported per year. The lowest number of malignancy events was reported in 2015. In 2021, 100% of the adverse event reports for cancer cases related to CPAP therapy were associated with the manufacturer specified in the Class 1 recall notice. Prior to 2021, there were three CPAP manufacturers linked to the nine CPAP‐associated malignancy reports.
Fig. 1.
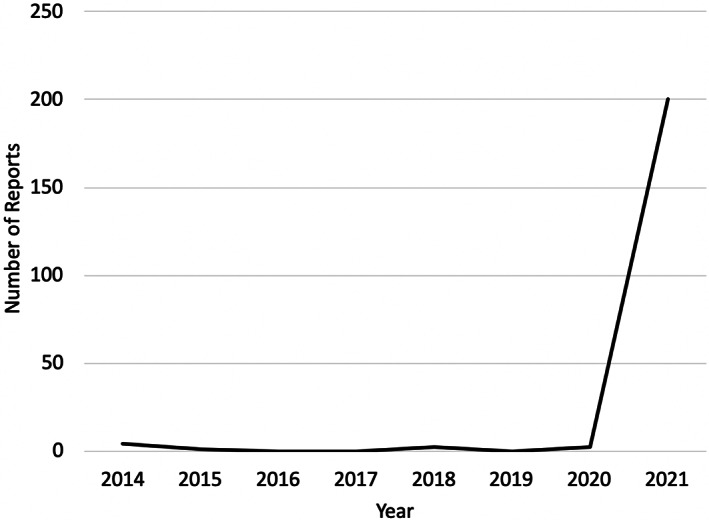
Temporal Trends in Malignancy Reports Associated with CPAP.
Device‐specific adverse events associated with CPAP
There were 4,034 reports of CPAP device malfunctions sent to the FDA between 2014 and 2021. In 1902 (47.15%) cases, there were descriptions of the CPAP polyurethane sound abatement foam degrading, which was the most commonly reported CPAP device problem (Table II). The 1902 reports of degradation included 174 (9.15%) cases associated with a cancer diagnosis and 1728 (90.85%) cases not associated with a cancer diagnosis. A total of 98 distinct categories of CPAP device problems were documented in the MAUDE database. Frequently documented CPAP device problems included broken devices (n = 279; 6.92%), fire (n = 182; 4.51%), and patient–device incompatibility (n = 144; 3.57%). Less frequently documented CPAP device problems were self‐activation or keying (n = 3; 0.07%), inadequate instruction for health care professionals (n = 3; 0.07%), and frayed material (n = 3; 0.07%).
TABLE II.
The Most Common Device Problems Associated with CPAP.
| Category | n = 4521 |
|---|---|
| Degraded | 1902 (42.07%) |
| Broken device | 279 (6.17%) |
| Fire | 182 (4.03%) |
| Device operates differently than expected | 151 (3.34%) |
| Patient–device incompatibility | 144 (3.19%) |
| Device emits odor | 80 (1.77%) |
| Mechanical problem | 65 (1.44%) |
| Break | 58 (1.28%) |
| Disconnection | 54 (1.19%) |
| Patient–device interaction problem | 49 (1.08%) |
DISCUSSION
CPAP is considered the gold standard for treatment of OSA, which has an estimated prevalence of 15%–30% in the general population. 1 , 6 The literature on OSA has consistently demonstrated the positive health effects of CPAP therapy, including reducing risks of cognitive impairment, stroke, and metabolic syndrome. 4 However, in July 2021, the U.S. FDA issued a Class 1 recall notice for exposure to toxic material from the degradation of polyurethane sound abatement foam in CPAP devices, which affected more than 15 million devices. 7 No previous study has sought to understand the risk of cancer or death associated with exposure to carcinogenic material from the sound abatement foam in CPAP devices. The objective of our study was to analyze national trends in reporting CPAP‐associated cancer cases to understand the implications of the FDA recall for the field of sleep medicine.
National trends in cases of CPAP‐associated malignancy
To analyze trends in the association of CPAP and tumors, we used the same database from which the Class 1 recall notice was derived. We found that 200 of the 209 reported cancer cases associated with CPAP devices were received by the FDA in the first 9 months of 2021, which means that more than 95% of all documented events were received in 2021. The 200‐fold increase in the number of cases reported emphasizes the impact of the Class 1 recall on the public perception of CPAP therapy. Future implications have yet to be determined; however, it is well established that negative publicity can set back a scientific field many years. This may be best exemplified by decreasing rates of childhood vaccination following the publication of literature associating vaccination with increased risk of developing autism. 12 , 13 Even though the original paper has now been retracted, the anti‐vaccination movement continues to maintain strength and to have significant repercussions for public health efforts. Similarly, in 1999 Jesse Gelsinger died after participating in a gene therapy clinical trial, which had widespread and long‐term negative implications for the entire field. 14 A lot has been learned from these events, in particular that public perception often engenders reality. Given the dramatic increase in reports of CPAP‐associated malignancy cases, our study strongly indicates that health care professionals should take a proactive approach to educating patients about the Class 1 recall notice.
The bandwagon effect is defined by a phenomenon where people choose a particular belief or action because other people are doing so (e.g., choosing a diet because it is popular), and this effect in part explains the widespread repercussions of negative media events. The ability of the bandwagon effect to overwhelm even closely held principles has been described. 15 In this way, the dramatic increase in reporting of malignancy associated with CPAP in our study may be indicative of the bandwagon effect giving rise to a perception in the general public that CPAP therapy causes cancer. Per the bandwagon effect, this may occur even in the absence of prospective literature assessing the risk of carcinogenesis related to sound abatement foam in CPAP devices. Furthermore, patients may not discern that the Class 1 recall was limited to certain devices from specific manufacturers. The literature already consistently raises concern regarding poor compliance rates with CPAP therapy. 16 , 17 Further concern over the risk of cancer in patients who are marginally tolerating CPAP could lead to increased abandonment of this therapy, worsened compliance rates, and increased loss to follow‐up. This points to the critical nature of providing education to OSA patients to reduce the risk of patients making an uninformed decision regarding compliance with CPAP therapy. In addition, the American Academy of Sleep Medicine (AASM) recently published guidelines on referral to sleep surgeons. 18 The guidelines make a strong recommendation for referral to a sleep surgeon in any patient with a BMI < 40 who is struggling or not‐accepting CPAP. In the setting of the Class 1 recall notice, the AASM recommendation should be taken to heart by physicians prescribing CPAP and efforts to create an easy workflow for referral should be established.
Degradation of CPAP polyurethane sound abatement foam
Our study determined that, between 2014 and 2021, there were 174 reports of CPAP polyurethane sound abatement foam degradation in association with a cancer diagnosis. However, during the same time period, there were more than 1700 cases of foam degradation that were not associated with malignancy, and there were more than 15,000,000 CPAP devices that were affected by the 2021 recall. Comparatively, the prevalence of all cancer in the U.S. population is 18.2% in women and 22.4% in men. 19 Although this may provide a general starting point for understanding the risk of cancer among CPAP users, it should be noted that the MAUDE database is not comprehensive as it is derived from mandatory (e.g., manufacturers') and voluntary (e.g., physicians') reports. Additionally, other studies have attempted to quantify the malignancy risk in OSA patients treated with CPAP. In particular, one study following CPAP adherent patients over a 5‐year period did not find a positive or negative change in all‐cancer incidence. 10 Although this study did not specifically examine the risk associated with degradation of the sound abatement foam, the study likely included a high proportion of the CPAP devices affected by the recall given that these devices are the most popular brands. Future studies are required to establish if exposure to carcinogenic material in CPAP devices causes a higher rate of malignancy.
Future studies examining CPAP‐associated malignancy will be a valuable addition to the literature but may take years of coordinated efforts to come to fruition. Additionally, carcinogenesis is determined in a laboratory setting and does not always correlate with clinical findings. 20 However, this uncertainty does not mean physicians should disregard the carcinogenic nature of certain materials. Rather, we propose that the goal be proper patient education to make an informed decision. As indicated in the Class 1 recall notice, if alternatives are not available, patients should consult with physicians to “determine if the benefit of continuing therapy with your device outweighs the risks identified.” 7 Therefore, otolaryngologists and sleep specialists should be prepared to facilitate informed decision‐making on an individualized basis. Joint academic recommendations regarding management of patients affected by the CPAP recall have already been published. 21 These recommendations are particularly important given that a timeline for replacement remains indeterminate and may continue to be indeterminate in the context of COVID‐19 and the scope of the overall recall. We hope that the combination of our study and the Class 1 recall notice will help raise awareness regarding the value of taking a proactive approach to educating patients on the association between CPAP therapy and malignancy.
Patient safety and device‐specific adverse events
We identified a total of 2,571 patient injuries that were associated with CPAP therapy. This number is relatively small compared with the 15,000,000 devices affected by the Class 1 recall notice. The difference between the number of devices recalled and the number of patient injuries underscores the safety of CPAP devices. This finding is supported by previous literature demonstrating that severe side effects do not occur frequently and that most side effects are mild and can resolve with time. 17 , 22 Many patients in our study had device problems without an associated injury, which makes sense given the noninvasive nature of CPAP therapy. We did determine that the most frequent device problems included broken devices, fires, and patient–device incompatibility. Fire is a documented risk of CPAP but only in high oxygen environments, which does not reflect the standard CPAP patient. 23 Overall, it is well established that the adverse events associated with CPAP do not constitute a disproportionate risk and that the benefits outweigh the risks of no treatment. Patient–device incompatibility is a frequently described issue related to CPAP therapy. Patients often find CPAP devices to be uncomfortable during sleep, and improper fitting can contribute to dry eyes, nasal congestion, and other side effects, all of which can decrease patient compliance. 16 , 17 , 24 Knowing the most common CPAP device problems can provide actionable targets for quality improvement projects focused on optimizing patient outcomes by improving patient compliance.
Limitations
The principle limitation of our study is the inherent retrospective nature of the MAUDE database, which precludes determination of causation. The retrospective design may also contribute to selection bias. There is a risk of reporting bias because the MAUDE database is derived in part from voluntary reporters who may indiscriminately document certain adverse events and not others. In this way, the results of the MAUDE database may represent an under‐reporting of adverse events and may influence the precision of our findings. Given the lack of literature identifying the specific malignancy associated with degradation of the sound abatement foam, it was not possible to limit reports based on the type of cancer. The MAUDE database does not contain demographic variables and does not report the total number of devices on the market. As a result, we cannot comment on the incidence or prevalence of a specific adverse event. Despite these limitations, our study offers novel insight into the implications of the Class 1 recall notice for CPAP devices and can help better inform otolaryngologists managing CPAP patients.
CONCLUSION
In light of the recent Class 1 safety recall notice for CPAP devices, it is important to evaluate reports of cancer linked to CPAP devices to understand the implications of the recall notice. We found a dramatic rise in reports of CPAP‐associated malignancy with 95.7% of the documented cases received by the FDA in 2021. Degradation of the CPAP polyurethane sound abatement foam was frequently reported, but only a minority of cases were associated with a cancer diagnosis. Future studies are required to evaluate causation, but otolaryngologists should be prepared to educate patients on the risks of carcinogenesis associated with CPAP. Additionally, the otolaryngology community should be aware of the potential bandwagon effect and the implications of public perception for CPAP compliance.
Editor's Note: This Manuscript was accepted for publication on March 18, 2022.
This article will be presented at the 2022 COSM Annual Meeting as part of the 102nd ABEA Annual Meeting; April 29–May 1, 2022; Dallas, Texas, USA.
The authors have no funding, financial relationships, or conflicts of interest to disclose.
REFERENCES
- 1. Arnardottir ES, Bjornsdottir E, Olafsdottir KA, Benediktsdottir B, Gislason T. Obstructive sleep apnoea in the general population: highly prevalent but minimal symptoms. Eur Respir J. 2016;47:194‐202. [DOI] [PubMed] [Google Scholar]
- 2. Young T, Peppard PE, Gottlieb DJ. Epidemiology of obstructive sleep apnea: a population health perspective. Am J Respir Crit Care Med. 2002;165:1217‐1239. [DOI] [PubMed] [Google Scholar]
- 3. Mulgrew AT, Ryan CF, Fleetham JA, et al. The impact of obstructive sleep apnea and daytime sleepiness on work limitation. Sleep Med. 2007;9:42‐53. [DOI] [PubMed] [Google Scholar]
- 4. Harding SM. Complications and consequences of obstructive sleep apnea. Curr Opin Pulm Med. 2000;6:485‐489. [DOI] [PubMed] [Google Scholar]
- 5. Tien DA, Kominsky A. Managing snoring: when to consider surgery. Cleve Clin J Med. 2014;81:613‐619. [DOI] [PubMed] [Google Scholar]
- 6. Morgenthaler TI, Aurora RN, Brown T, et al. Practice parameters for the use of autotitrating continuous positive airway pressure devices for titrating pressures and treating adult patients with obstructive sleep apnea syndrome: an update for 2007. An American Academy of sleep medicine report. Sleep. 2008;31:141‐147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Class 1 Device Recall DreamStation, DreamStation Go, Dorma 400, Dorma 500, and REMstar SE Auto. Available at: https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfres/res.cfm?id=187815.
- 8. Jonas DE, Amick HR, Feltner C, et al. Screening for obstructive sleep apnea in adults: evidence report and systematic review for the US preventive services task force. JAMA. 2017;317:415‐433. [DOI] [PubMed] [Google Scholar]
- 9. Sullivan CE, Issa FG, Berthon‐Jones M, Eves L. Reversal of obstructive sleep apnoea by continuous positive airway pressure applied through the nares. Lancet. 1981;1:862‐865. [DOI] [PubMed] [Google Scholar]
- 10. Justeau G, Bailly S, Gervès‐Pinquié C, et al. Cancer risk in patients with sleep apnoea following adherent 5‐year CPAP therapy. Eur Respir J. 2021;2101935. 10.1183/13993003.01935-2021 [DOI] [PubMed] [Google Scholar]
- 11. MAUDE . Manufacturer and User Facility Device Experience. Available at: https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfmaude/search.cfm. Accessed 6/12/2021.
- 12. Hussain A, Ali S, Ahmed M, Hussain S. The anti‐vaccination movement: a regression in modern medicine. Cureus. 2018;10:e2919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Zerbo O, Modaressi S, Goddard K, et al. Vaccination patterns in children after autism Spectrum disorder diagnosis and in their younger siblings. JAMA Pediatr. 2018;172:469‐475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Wilson JM. Lessons learned from the gene therapy trial for ornithine transcarbamylase deficiency. Mol Genet Metab. 2009;96:151‐157. [DOI] [PubMed] [Google Scholar]
- 15. O'Connor N, Clark S. Beware bandwagons! The bandwagon phenomenon in medicine, psychiatry and management. Australas Psychiatry. 2019;27:603‐606. [DOI] [PubMed] [Google Scholar]
- 16. Rotenberg BW, Murariu D, Pang KP. Trends in CPAP adherence over twenty years of data collection: a flattened curve. J Otolaryngol Head Neck Surg. 2016;45:43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ulander M, Johansson MS, Ewaldh AE, Svanborg E, Broström A. Side effects to continuous positive airway pressure treatment for obstructive sleep apnoea: changes over time and association to adherence. Sleep Breath. 2014;18:799‐807. [DOI] [PubMed] [Google Scholar]
- 18. Kent D, Stanley J, Aurora RN, et al. Referral of adults with obstructive sleep apnea for surgical consultation: an American Academy of sleep medicine systematic review, meta‐analysis, and GRADE assessment. J Clin Sleep Med. 2021;17:2507‐2531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Mattiuzzi C, Lippi G. Current cancer epidemiology. J Epidemiol Glob Health. 2019;9:217‐222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lorger M, Lee H, Forsyth JS, Felding‐Habermann B. Comparison of in vitro and in vivo approaches to studying brain colonization by breast cancer cells. J Neuro‐Oncol. 2011;104:689‐696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Owens RL, Wilson KC, Gurubhagavatula I, Mehra R. Philips respironics recall of positive airway pressure and noninvasive ventilation devices: a brief statement to inform response efforts and identify key steps forward. Am J Respir Crit Care Med. 2021;204:887‐890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Venessa LP, Sandeep S. Continuous positive airway pressure StatPearls. 2021.
- 23. Gowardman JR, Moriarty B. Explosion and fire in the expiratory limb of a fisher and Paykel “three in one” respiratory care system. Anaesth Intensive Care. 1998;26:427‐430. [DOI] [PubMed] [Google Scholar]
- 24. Russell JO, Gales J, Bae C, Kominsky A. Referral patterns and positive airway pressure adherence upon diagnosis of obstructive sleep apnea. Otolaryngol Head Neck Surg. 2015;153:881‐887. [DOI] [PubMed] [Google Scholar]


