Abstract
Background
Glucagon-like peptide-1 receptor agonists (GLP-1 RAs) have been highly recommended for glycemic control and weight reduction. However, evidence has accumulated that GLP-1 RAs treatment is related to an increase in heart rate, which could potentially induce cardiac arrhythmias. This study aims to investigate the association of GLP-1 RAs therapy with incident arrhythmias in diabetic and obese patients.
Methods
MEDLINE, EMBASE, Cochrane Library, and ClinicalTrials.gov were systematically searched from inception up to May 25, 2022. Randomized controlled trials (RCTs) comparing GLP-1 RAs with placebo or active control for adults with type 2 diabetes or obesity were included. The outcomes of interest were prespecified as incident atrial fibrillation (AF), atrial flutter (AFL), ventricular arrhythmias (VAs), and sudden cardiac death (SCD). Mantel-Haenszel relative risk (MH-RR) with a corresponding 95% confidence interval (95% CI) was estimated using a fixed-effects model.
Results
A total of 56 RCTs involving 79,720 participants (44,028 GLP-1 RAs vs 35,692 control: mean age 57.3 years) were included from 7692 citations. GLP-1 RAs use overall did not significantly increase the risk of AF (RR 0.97, 95% CI 0.83–1.12), AFL (RR 0.83, 95% CI 0.59–1.17), VAs (RR 1.24, 95% CI 0.92–1.67), and SCD (RR 0.89, 95% CI 0.67–1.19), compared with controls. In further subgroup analyses, we observed an increasing trend toward incident AF with dulaglutide (RR 1.40, 95% CI 1.03–1.90) while an inverse trend with oral semaglutide (RR 0.43, 95% CI 0.21–0.87). Additionally, higher doses of GLP-1 RAs (RR 1.63, 95% CI 1.11–2.40) and higher baseline BMI (RR 1.60, 95% CI 1.04–2.48) might significantly increase the risk of VAs. No significant differences were identified in other subgroup analyses.
Conclusions
GLP-1 RAs therapy was not associated with an overall higher risk of arrhythmias, demonstrating an assuring cardiovascular safety profile. Further studies are required to determine whether the potential antiarrhythmic or arrhythmogenic effect of GLP-1 RAs is drug-specific and varies from doses or baseline BMI.
Trial registration: PROSPERO Identifier: CRD42022339389.
Supplementary Information
The online version contains supplementary material available at 10.1186/s13098-022-00970-2.
Keywords: GLP-1 RAs, Arrhythmias, Atrial fibrillation, Diabetes mellitus, Obesity
Background
Glucagon-like peptide-1 receptor agonists (GLP-1 RAs) are relatively novel anti-hyperglycemic drugs that mimic the effects of endogenous GLP-1 and contribute to favorable glycemic control and weight loss. Evidence has accumulated that GLP-1 RAs could be also associated with improved cardiovascular outcomes and a reduction in major adverse cardiovascular events (MACEs) [1–3]. They are now highly recommended for patients with type 2 diabetes mellitus (T2DM) and obesity, particularly for those at high risk for cardiovascular diseases. Considering the widespread use of these drugs, their cardiovascular safety and potential adverse effects warrant attention.
Multiple studies have indicated that treatment with GLP-1 RAs could slightly increase heart rate (HR) [4–6], which has been observed across different populations in both short-acting and long-acting agents, implying a potential class effect of these emerging drugs [4]. This observation also raised some concerns about the potential adverse effect of GLP-1 RAs on the risk of incident cardiac arrhythmias.
Cardiac arrhythmia, commonly known as atrial fibrillation (AF), atrial flutter (AFL), and ventricular arrhythmias (VAs), is a severe and prevalent condition, representing a major health burden worldwide. Arrhythmia events had been reported in numerous randomized controlled trials (RCTs) involving GLP-1 RAs, especially in several large-scale cardiovascular outcome trials (CVOTs) [7–12]. REWIND trial revealed that patients with dulaglutide had a significantly higher incidence of AF than patients with placebo (nearly 1.8% vs 1.2%) [8]. Consistently, an aggregate analysis of patient-level data from Harmony registration studies with albiglutide treatment demonstrated a significant increase in the risk of AF and AFL [13]. In other CVOTs (Harmony Outcomes trial [7], LEADER trial [9], ELIXA trial [10], PIONEER-6 trial [11], and SUSTAIN-6 trial [12]), the incidence of AF between GLP-1 RAs and control was not significantly different. LEADER trial showed that liraglutide had a tendency to increase VAs, but the result was not statistically significant [9]. To date, the effects of GLP-1 RAs treatment on arrhythmia events remain uncertain. Given the serious hazard of arrhythmias, it is important to figure out the association between GLP-1 RAs and arrhythmias.
Therefore, we conducted a systematic review and meta-analysis of RCTs to investigate the association between GLP-1 RAs therapy and the risk of arrhythmias. Furthermore, we also examined whether the risks differed across different drug types, treatment dosage, follow-up duration, study designs, and baseline body mass index [BMI].
Methods
This systematic review was conducted according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines [14]. The protocol of this study had been prospectively registered on the PROSPERO website (Number CRD42022339389).
Data sources and searches
A literature search was systematically performed through PubMed, Embase, Cochrane library, and ClinicalTrials.gov. from their inception up to May 25, 2022, without language restrictions. We further extended the search to reference lists of published trials and reviews to maximize the identification of all relevant trials about GLP-1 RAs use. A detailed search strategy is available in Additional file 1: Method S1. The database search was performed on May 26, 2022.
Study selection and main outcomes
According to the predefined inclusion and exclusion criteria (available in Additional file 1: Table S1), two independent reviewers (SW and WL) screened the titles and abstracts of all records, and then evaluated the full texts of eligible studies. Conflicts over study inclusion were resolved through consensus or discussion with other team members. Only RCTs comparing GLP-1 RAs with placebo or active control in adults with diagnosed T2DM or obesity were included. We excluded trials if they: compared different GLP-1 RAs individuals, compounded preparations of GLP-1 RAs with other drugs(e.g., IDegLira), had a short follow-up duration (< 24 weeks), failed to report arrhythmia endpoints, or reported zero events in both experimental and controlled arms. Exploratory or post-hoc analyses of existing trials were also excluded.
The main outcomes of interest were incident cardiac arrhythmias. We selected several frequent types of arrhythmias, including AF, AFL, and VAs (e.g., ventricular extrasystoles, ventricular tachycardia, ventricular fibrillation, ventricular flutter, and torsades de pointes). Since most SCD cases are closely linked to the aforementioned severe VAs, we also included incident SCD as part of the outcome. The SCD variable was predefined as cardiac arrest, sudden death, and sudden cardiac death.
Data extraction and quality assessments
Two investigators (SW and WL) separately extracted relevant data that were confirmed by the senior author (KC). Retrieved data included study characteristics (study names, registration number, year of publication, population size), characteristics of participants (mean age, male proportion, baseline BMI, and glycated hemoglobin [HbA1c]), interventions and comparations (type and dosage of GLP-1 RAs, comparator drugs), and outcome data (incidence of arrhythmias or SCD events). Almost all included studies had reported arrhythmia events as severe adverse events (SAEs) and did not report relevant data in the trial publication, therefore, we searched the results section (adverse event) of the study page on ClinicalTrials.gov or EudraCT.
Two investigators (WS and WL) independently assessed the quality of these eligible RCTs using the revised Cochrane risk-of-bias tool (RoB2) for RCTs [15]. Disagreements were resolved by consensus.
Data synthesis and analysis
Mantel-Haenszel relative risk (MH-RR) with a corresponding 95% confidence interval (95% CI) was estimated to present the incidence of arrhythmias. RR > 1 would favor that GLP-1 RAs increase the risk of the events compared with controls. For studies with a zero count, 0.5 was added to all cell frequencies of these studies. The cumulative incidence of each endpoint was expressed as events per 10,000 patient-years. Heterogeneity was evaluated by using Cochrane’s Q tests and I2 statistics. It was considered statistically significant that P value < 0.1 for the Q test. For I2 statistics, heterogeneity was regarded as high if I2 value was > 50% [16]. We used a random-effects model when there was high heterogeneity between studies. Otherwise, a fixed-effects model was used. We performed sensitivity analyses with a fixed-effects model by omitting each eligible trial iteratively. Funnel plots and egger’s test for each outcome were examined to assess possible publication bias.
Subgroup analyses were conducted to further investigate the potential risk factors of GLP-1 RAs use on arrhythmias. Subgroup categories were specified as follows: (1) different GLP-1 RAs drugs, including albiglutide, dulaglutide, exenatide, liraglutide, lixisenatide, or semaglutide (since semaglutide is administered subcutaneously or orally, we divided it into two groups); (2) study designs, including CVOT or non-CVOT; (3) follow-up duration (≤ 52 weeks or > 52 weeks); (4) baseline BMI, including higher than the average weight of all included trials, or lower; (5) different dosages (higher or lower). Based on current clinical practice, we considered high dosages of GLP-1 RAs as greater than or equal to: liraglutide, 1.8 mg daily; albiglutide, 50 mg weekly; dulaglutide, 1.5 mg weekly; oral semaglutide, 7 mg daily; subcutaneous semaglutide, 1.0 mg weekly. Other dosages of these drugs were considered low. Exenatide and lixisenatide were excluded from the dosage-effect assessment since they were single dosage. We performed subgroup analyses for each outcome mentioned above in turn.
Data analyses were performed using R (version 4.1.2, 2021-11-01) with the “meta” package (version 5.2-0). All statistical tests were two-sided, and P values < 0.05 were considered statistically significant.
Results
Characteristics of included studies
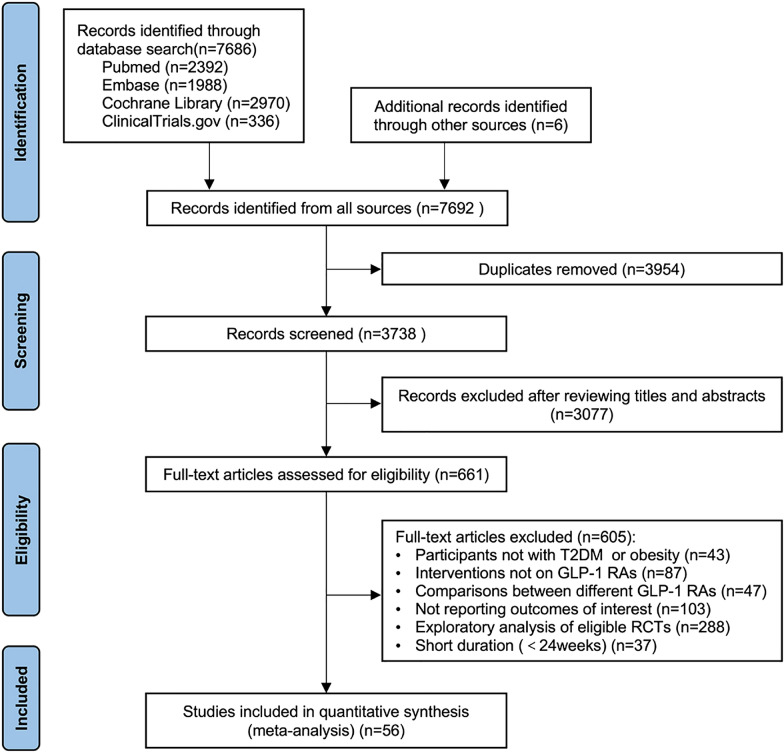
After in-depth literature selection, we included 56 RCTs (including 1 unpublished trial) in quantitative synthesis from a total of 7,692 citations (Fig. 1). GLP-1 RAs used were albiglutide (9 studies), dulaglutide (10 studies), exenatide (7 studies), liraglutide (10 studies), lixisenatide (5 studies), oral semaglutide (5 studies), and sc semaglutide (10 studies). These studies comprised a total population size of 79,720: 44,028 (55.2%) patients treated with GLP-1 RAs and 35,692 (44.8%) controls. The participants were all adult patients with T2DM or obesity. The mean age, baseline BMI, and HbA1c were 57.3 ± 4.8 years, 32.4 kg/m2, and 8.0%, respectively. Across all included studies, the median follow-up duration was 52 weeks (ranging from 24 weeks to 5.4 years), providing 166,579 patient-years. The characteristics of included studies and participants are listed in Table 1 and Additional file 1: Table S2.
Fig. 1.
PRISMA flow diagram. Abbreviations: GLP-1 RAs glucagon-like peptide 1 receptor agonists, RCTs randomized controlled trials, T2DM type 2 diabetes mellitus
Table 1.
Baseline characteristics of studies and participants included
| Trials, year | Drug | Size | Age (years) | BMI (kg/m2) | HbA1c,% | Follow-up duration | Outcomes of interest |
|---|---|---|---|---|---|---|---|
| HARMONY 1, 2014 | Albiglutide | 301 | 55.0 | 34.1 | 8.1 | 52 weeks | AF |
| HARMONY 3, 2014 | Albiglutide | 1012 | 54.5 | 32.7 | 8.1 | 104 weeks | SCD |
| HARMONY 4, 2014 | Albiglutide | 745 | 55.5 | 33.1 | 8.3 | 52 weeks | AF/AFL/VAs |
| HARMONY 8, 2014 | Albiglutide | 495 | 63.3 | 30.4 | 8.2 | 52 weeks | AF/AFL/VAs |
| Rosenstock et al., 2014 | Albiglutide | 566 | 55.6 | NA | 8.5 | 26 weeks | AF/VAs |
| HARMONY 5, 2015 | Albiglutide | 663 | 55.2 | 32.2 | 8.2 | 52 weeks | AF |
| HARMONY 2, 2016 | Albiglutide | 301 | 52.9 | 33.9 | 8.2 | 52 weeks | AF |
| Harmony Outcomes, 2018 | Albiglutide | 9432 | 64.1 | 32.3 | 8.7 | 78 weeks | AF/AFL/VAs/SCD |
| Rosenstock et al., 2020 | Albiglutide | 813 | 58.1 | 32.1 | 7.7 | 26 weeks | AF/AFL |
| Ferdinand et al., 2014 | Dulaglutide | 755 | 56.5 | 33.0 | 7.9 | 26 weeks | VAs |
| AWARD-3, 2014 | Dulaglutide | 807 | 55.6 | 33.3 | 7.6 | 52 weeks | AF |
| AWARD-5, 2014 | Dulaglutide | 1098 | 54.0 | 31.3 | 8.1 | 52 weeks | AF/SCD |
| AWARD-2, 2015 | Dulaglutide | 807 | 56.6 | 31.6 | 8.1 | 78 weeks | VAs |
| AWARD-4, 2015 | Dulaglutide | 884 | 59.4 | 32.5 | 8.5 | 52 weeks | AF/AFL/VAs |
| AWARD-9, 2017 | Dulaglutide | 300 | 60.4 | 32.8 | 8.4 | 28 weeks | AF |
| AWARD-7, 2018 | Dulaglutide | 576 | 64.6 | 32.5 | 8.6 | 52 weeks | AF/SCD |
| AWARD-10, 2018 | Dulaglutide | 423 | 57.3 | 32.9 | 8.0 | 24 weeks | AF |
| Chen et al., 2018 | Dulaglutide | 735 | 52.8 | 25.9 | 8.0 | 26 weeks | VAs |
| REWIND, 2019 | Dulaglutide | 9892 | 66.2 | 32.3 | 7.3 | 282 weeks | AF/AFL/VAs/SCD |
| Heine et al., 2005 | Exenatide | 549 | 58.9 | 31.4 | 8.2 | 26 weeks | AF |
| Nauck et al., 2007 | Exenatide | 501 | 58.7 | 30.6 | 8.6 | 52 weeks | AF/AFL |
| NCT00701935, 2008 | Exenatide | 80 | 58.1 | NA | NA | 26 weeks | AF |
| EUREXA, 2012 | Exenatide | 1019 | 56.4 | 32.6 | 7.5 | 208 weeks | AF/AFL |
| DURATION-3, 2010 | Exenatide | 456 | 57.9 | 32.0 | 8.3 | 26 weeks | SCD |
| Inagaki et al., 2012 | Exenatide | 427 | 56.8 | 26.2 | 8.5 | 26 weeks | AF |
| Davies et al., 2013 | Exenatide | 216 | 58.5 | 33.7 | 8.4 | 26 weeks | VAs |
| LEAD-2, 2009 | Liraglutide | 1087 | 56.7 | 31.0 | 8.4 | 26 weeks | AF/AFL/VAs/SCD |
| LEAD-3 Mono, 2009 | Liraglutide | 746 | 53.0 | 33.1 | 8.3 | 104 weeks | AF |
| Pratley et al., 2010 | Liraglutide | 658 | 55.3 | 32.8 | 8.4 | 26 weeks | SCD |
| Charbonnel et al., 2012 | Liraglutide | 650 | 57.3 | 32.7 | 8.2 | 26 weeks | AF |
| MDI-Liraglutide, 2015 | Liraglutide | 124 | 63.7 | 33.7 | 9.0 | 24 weeks | AF |
| SCALE Obesity and Prediabetes, 2015 | Liraglutide | 3723 | 45.1 | 38.3 | 5.6 | 56 weeks | AF/AFL/VAs/SCD |
| Vanderheiden et al., 2016 | Liraglutide | 71 | 54.2 | 41.2 | NA | 26 weeks | AF |
| LEADER, 2016 | Liraglutide | 9340 | 64.3 | 32.5 | 8.7 | 198 weeks | AF/AFL/VAs/SCD |
| Zang et al., 2016 | Liraglutide | 367 | 51.5 | 27.2 | 8.1 | 26 weeks | AF |
| SCALE Insulin, 2020 | Liraglutide | 392 | 56.8 | 35.9 | 8.0 | 56 weeks | AF/AFL |
| GETGOAL-M, 2013 | Lixisenatide | 680 | 54.7 | 33.0 | 8.1 | 24 weeks | AF/VAs |
| GETGOAL-L, 2013 | Lixisenatide | 495 | 57.2 | 32.1 | 8.4 | 24 weeks | AF/AFL |
| GETGOAL-F1, 2014 | Lixisenatide | 482 | 56.1 | 32.5 | 8.0 | 24 weeks | AF |
| ELIXA, 2015 | Lixisenatide | 6063 | 60.3 | 30.2 | 7.7 | 107 weeks | AF/AFL/VAs/SCD |
| GetGoal-Duo-2, 2016 | Lixisenatide | 893 | 59.8 | 32.2 | 7.8 | 26 weeks | AF |
| PIONEER 2, 2019 | Oral semaglutide | 819 | 58.0 | 32.8 | 8.1 | 52 weeks | AF/VAs |
| PIONEER 3, 2019 | Oral semaglutide | 1861 | 58.0 | 32.5 | 8.3 | 78 weeks | AF/AFL/SCD |
| PIONEER 5, 2019 | Oral semaglutide | 324 | 70.0 | 32.4 | 8.0 | 26 weeks | AF |
| PIONEER 6, 2019 | Oral semaglutide | 3182 | 66.0 | 32.3 | 8.2 | 68 weeks | AF/AFL/VAs/SCD |
| PIONEER 8, 2019 | Oral semaglutide | 730 | 61.0 | 31.0 | 8.2 | 52 weeks | AFL/VAs |
| SUSTAIN 6, 2016 | Sc semaglutide | 3297 | 64.6 | 32.8 | 8.7 | 104 weeks | AF/AFL/VAs/SCD |
| SUSTAIN 2, 2017 | Sc semaglutide | 1225 | 55.1 | 32.5 | 8.1 | 56 weeks | AF |
| SUSTAIN 4, 2017 | Sc semaglutide | 1082 | 56.5 | 33.0 | 8.2 | 30 weeks | AF |
| Kaku et al., 2018 | Sc semaglutide | 600 | 58.5 | 26.4 | 8.1 | 56 weeks | AF |
| SUSTAIN China, 2021 | Sc semaglutide | 867 | 53.1 | 28.0 | 8.1 | 30 weeks | AF/VAs |
| STEP 1, 2021 | Sc semaglutide | 1961 | 46.0 | 37.8 | 5.7 | 68 weeks | AF |
| STEP 2, 2021 | Sc semaglutide | 1207 | 55.0 | 35.7 | 8.1 | 68 weeks | AF |
| STEP 4, 2021 | Sc semaglutide | 803 | 46.0 | 34.1 | 5.4 | 68 weeks | AF |
| STEP 6, 2022 | Sc semaglutide | 400 | 51.0 | 31.9 | 6. | 68 weeks | AF |
| SUSTAIN 11, 2022 | Sc semaglutide | 1738 | 61.2 | 31.5 | 8.6 | 52 weeks | AF/AFL/SCD |
AF atrial fibrillation, AFL atrial flutter, BMI body mass index, GLP-1 RAs glucagon-like peptide 1 receptor agonists, NA not available, Sc subcutaneous injection, SCD sudden cardiac death, VAs ventricular arrhythmias
According to the revised RoB2 tool for assessing the quality of RCTs, there was no study with a high risk of bias. Most studies had a low risk or some bias issues in the five domains assessed (Additional file 1: Fig. S1).
Atrial fibrillation
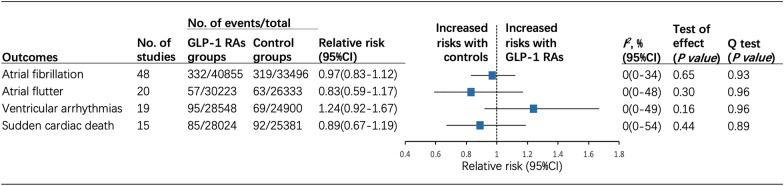
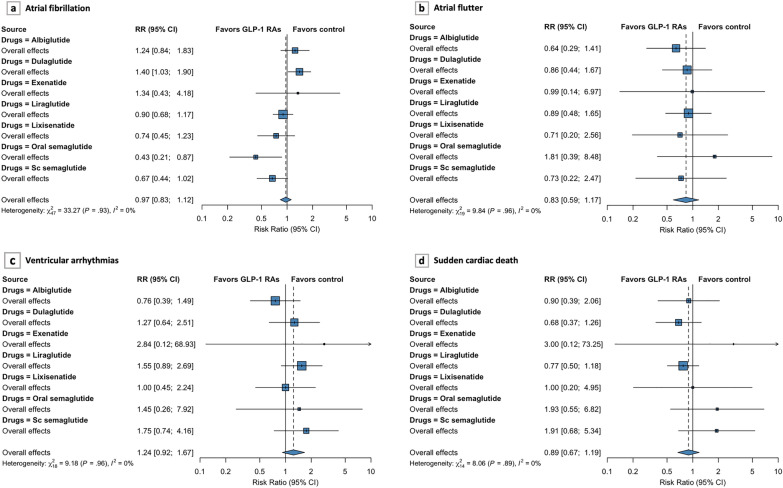
48 trials had reported a total of 651 events of AF as SAEs in 74,351 participants (332 events in GLP-1 RAs group and 319 events in control group) (Fig. 2). Pooled data from all these trials indicated that GLP-1 RAs therapy had no significant association with the risk of incident AF (RR 0.97, 95% CI 0.83–1.12; P = 0.65) (Additional file 1: Fig. S2.1). Regarding the association of AF risk and different GLP-1 RAs agents, dulaglutide displayed an increasing trend toward incident AF (RR 1.40, 95% CI 1.03–1.90; P = 0.03), while oral semaglutide displayed an inverse trend (RR 0.43, 95% CI 0.21–0.87; P = 0.02) (Fig. 3a). Other GLP-1 RAs agents including albiglutide, exenatide, liraglutide, lixisenatide, and sc semaglutide had no significant effect on the risk of AF. Overall, the cumulative incidence of AF was low, averaged at 39 per 10,000 patient-years in GLP-1 RAs treatment. In the subgroup analyses, the incidence of AF did not differ between the GLP-1 RAs and control groups according to different treatment dosage, follow-up duration, baseline BMI, and study designs, with no intergroup heterogeneity (P = 0.72, P = 0.59, P = 0.08, and P = 0.70, respectively) (Table 2 and Additional file 1: Fig. S3).
Fig. 2.
Risk of cardiac arrhythmias in patients randomized to GLP-1 RAs treatment compared with controls across all trials. GLP-1 RAs glucagon-like peptide 1 receptor agonists, CI confidence interval
Fig. 3.
Forest plots of primary analysis for each outcome. a atrial fibrillation, b atrial flutter, c ventricular arrhythmias, d sudden cardiac death. Overall effects are calculated using the fixed‑effect model
Table 2.
Results of subgroup analyses
| Subgroups | No. of patients | No. of studies | RR (95% CI) | aPeffect | Heterogeneity | bPhetero | |
|---|---|---|---|---|---|---|---|
| I2, % | P value | ||||||
| Atrial fibrillation | |||||||
| Dosec | 0.72 | ||||||
| High | 42,809 | 26 | 0.97 (0.81–1.17) | 0.77 | 0 | 0.55 | |
| Low | 22,224 | 19 | 1.04 (0.77–1.40) | 0.81 | 0 | 0.94 | |
| Follow-up duration | 0.59 | ||||||
| ≤ 52 weeks | 25,271 | 32 | 0.89 (0.62–1.26) | 0.50 | 0 | 0.99 | |
| > 52 weeks | 49,080 | 16 | 0.99 (0.84–1.16) | 0.86 | 13 | 0.30 | |
| Baseline BMId | 0.08 | ||||||
| High | 34,143 | 27 | 0.84 (0.67–1.04) | 0.11 | 0 | 0.99 | |
| Low | 39,562 | 19 | 1.10 (0.89–1.35) | 0.38 | 0 | 0.55 | |
| Study designs | 0.70 | ||||||
| CVOTe | 41,206 | 6 | 0.93 (0.84–1.17) | 0.60 | 58 | 0.04 | |
| Non-CVOT | 33,145 | 42 | 0.84 (0.56–1.27) | 0.41 | 0 | 1.00 | |
| Atrial flutter | |||||||
| Dose | 0.34 | ||||||
| High | 33,626 | 10 | 1.04 (0.68–1.58) | 0.86 | 0 | 0.79 | |
| Low | 14,949 | 7 | 0.70 (0.35–1.40) | 0.31 | 0 | 0.63 | |
| Follow-up duration | 0.19 | ||||||
| ≤ 52 weeks | 8355 | 10 | 1.52 (0.58–3.97) | 0.40 | 0 | 1.00 | |
| > 52 weeks | 48,201 | 10 | 0.76 (0.52–1.10) | 0.14 | 0 | 0.66 | |
| Baseline BMI | 0.90 | ||||||
| High | 21,261 | 8 | 0.86 (0.50–1.47) | 0.58 | 0 | 0.79 | |
| Low | 35,295 | 12 | 0.82 (0.53–1.27) | 0.37 | 0 | 0.88 | |
| Study designs | 0.46 | ||||||
| CVOT | 41,206 | 6 | 0.78 (0.53–1.15) | 0.21 | 0 | 0.48 | |
| Non-CVOT | 15,350 | 14 | 1.08 (0.51–2.30) | 0.84 | 0 | 0.98 | |
| Ventricular arrhythmias | |||||||
| Dose | 0.04 | ||||||
| High | 32,205 | 11 | 1.63 (1.11–2.40) | 0.01 | 0 | 0.96 | |
| Low | 16,142 | 9 | 0.83 (0.49–1.41) | 0.49 | 0 | 0.91 | |
| Follow-up duration | 0.47 | ||||||
| ≤ 52 weeks | 7712 | 11 | 0.93 (0.41–2.12) | 0.86 | 0 | 0.93 | |
| > 52 weeks | 45,736 | 8 | 1.29 (0.94–1.78) | 0.12 | 0 | 0.72 | |
| Baseline BMI | 0.13 | ||||||
| High | 20,459 | 9 | 1.60 (1.04–2.48) | 0.03 | 0 | 0.96 | |
| Low | 32,423 | 9 | 1.00 (0.66–1.52) | 1.00 | 0 | 0.89 | |
| Study designs | 0.63 | ||||||
| CVOT | 41,206 | 6 | 1.28 (0.92–1.77) | 0.14 | 0 | 0.50 | |
| Non-CVOT | 12,242 | 13 | 1.04 (0.48–2.24) | 0.92 | 0 | 0.97 | |
| Sudden cardiac death | |||||||
| Dose | 0.39 | ||||||
| High | 33,360 | 10 | 0.84 (0.61–1.17) | 0.31 | 0 | 0.94 | |
| Low | 16,314 | 8 | 1.13 (0.63–2.04) | 0.68 | 0 | 0.76 | |
| Follow-up duration | 0.65 | ||||||
| ≤ 52 weeks | 5603 | 6 | 0.70 (0.24–2.05) | 0.52 | 0 | 0.60 | |
| > 52 weeks | 47,802 | 9 | 0.91 (0.67–1.22) | 0.52 | 0 | 0.84 | |
| Baseline BMI | 0.80 | ||||||
| High | 20,467 | 7 | 0.86 (0.59–1.26) | 0.45 | 0 | 0.58 | |
| Low | 32,938 | 8 | 0.93 (0.60–1.44) | 0.75 | 0 | 0.86 | |
| Study designs | 0.90 | ||||||
| CVOT | 41,206 | 6 | 0.90 (0.66–1.22) | 0.49 | 0 | 0.63 | |
| Non-CVOT | 12,199 | 9 | 0.84 (0.35–2.04) | 0.71 | 0 | 0.80 | |
BMI body mass index, CVOT cardiovascular outcome trial
aPeffect, P-value for test effect
bPhetero, P-value for between-subgroup heterogeneity
COnly studies of albiglutide, dulaglutide, liraglutide, and semaglutide (oral and subcutaneous) had different treatment doses (a single trial may have one or more dose sizes)
dThe cut-off point was 32.38 kg/m2 (mean baseline BMI in all included trials except 2 trials without reporting data on baseline BMI)
eRandom effects model were used to calculate the estimates because of the high heterogeneity
Atrial flutter
A total of 57 patients treated with GLP-1 RAs experienced AFL (n = 30,223), whereas 63 individuals in the control group (n = 26,333). The cumulative incidence of AFL with GLP-1 RAs was 8 per 10,000 patient-years. GLP-1 RAs use did not significantly increase the incidence of AFL (RR 0.83, 95% CI 0.59–1.17; P = 0.96) compared to controls (Additional file 1: Fig. S2.2). The same trend was obtained in the analysis of different groups of GLP-1 RAs receivers (Fig. 3b). There was no significant heterogeneity among all included trials on AFL (I2 = 0%, P = 0.96). Similar nonsignificant association and absence of intergroup heterogeneity were also found in the subgroup analyses (Table 2 and Additional file 1: Fig. S4).
Ventricular arrhythmias
164 events of VAs were reported in 19 trials, 95 of which occurred in the GLP-1 RAs group (n = 28,548). The cumulative incidence of VAs with GLP-1 RAs was nearly 14 per 10,000 patient-years. The aggregated data showed that GLP-1 RAs therapy did not increase the risk of VAs compared to the control (RR 1.24, 95% CI 0.92–1.67; P = 0.16) (Additional file 1: Fig. S2.3). When focusing on the treatment dose, an increased incidence of VAs was identified in the high-dose GLP-1 RAs group (RR 1.63, 95% CI 1.11–2.40; P = 0.01) but not in the low-dose group (RR 0.83, 95% CI 0.49–1.41; P = 0.49) with some intergroup heterogeneity (P = 0.04) (Additional file 1: Fig. S5.2). Subgroup analysis by baseline BMI indicated that an increase in VAs incidence was pronounced in those with higher baseline BMI (RR 1.60, 95% CI 1.04–2.48; P = 0.03) but not in those with low BMI (RR 1.00, 95% CI 0.66–1.52; P = 1.00), with no intergroup heterogeneity (P = 0.13) (Additional file 1: Fig. S5.4). Both follow-up duration and study designs were not significantly linked with the incidence of VAs, and had no between-subgroup heterogeneity (P = 0.47, and P = 0.63, respectively) (Table 2). No significant heterogeneity was found across trials on VAs (I2 = 0%, P = 0.96).
Sudden cardiac death
A total of 85 SCD events and 8 per 10,000 patient-years SCD incidence in GLP-1 RAs group were reported. The RRs for SCD ranged from 0.17 to 3.00. The risk of incident SCD was not significantly different between GLP-1 RAs and control (RR 0.89; 95% CI 0.67–1.19; P = 0.51) (Additional file 1: Fig. S2.4). No significant heterogeneity was identified across trials on SCD (I2 = 0%, P = 0.89). In subgroup analyses, GLP-1 RAs were not significantly associated with the risk of SCD when grouped according to different treatment dosage, follow-up duration, baseline BMI, and study designs. Likewise, there is no intergroup heterogeneity (P = 0.39, P = 0.65, P = 0.80, and P = 0.90, respectively) (Table 2 and Additional file 1: Fig. S6).
Sensitivity analyses and publication bias
Sensitivity analyses for each endpoint were conducted in turn. After the iterative omission of each trial, the pooled results for each outcome would not change (Additional file 1: Fig. S7). Considering the variation in the sample size across studies, we performed further sensitivity analyses in the aforementioned subgroups with significant results. After removing the REWIND trial and the POINEER-6 trial respectively, the pooling effect of dulaglutide and oral semaglutide on the risk of AF would lose statistical significance (Additional file 1: Fig. S7.5–6). When we removed the SCALE trial and the SUSTAIIN-6 trial, the previously observed statistically significant association between GLP-1 RAs and VAs in the higher-BMI and higher-doses subgroup would diminish. (Additional file 1: Fig. S7.7–8). Funnel plots for AF, AFL, VAs, and SCD displayed symmetry (Additional file 1: Fig. S8). Egger’s test for each outcome did not reveal significant asymmetry (P = 0.23, P = 0.38, P = 0.49, P = 0.55, respectively).
Discussion
In this systematic review and meta-analysis of 56 RCTs and 79,720 patients with T2DM or obesity, GLP-1 RAs therapy was not associated with an overall higher risk of incident AF, AFL, VAs, and SCD. Apart from VAs, the point estimates in relation to other conditions, all suggested potential benefits of GLP-1 RAs compared to the placebo or active group. Additionally, we observed a potentially protective effect of oral semaglutide on AF but an inverse trend of dulaglutide. When considering VAs, GLP-1 RAs might be associated with an increased risk of VAs in higher dosage and in those with higher baseline BMI.
To our knowledge, this is the largest systematic review and meta-analysis that comprehensively evaluated the relationship between GLP-1 RAs use and arrhythmia outcomes. In accordance with several previous systematic reviews [17–19], our study found that GLP-1 RAs did not increase the risk of cardiac arrhythmias and cemented its cardiovascular safety. A recent network meta-analysis demonstrated differently that GLP-1 RAs might significantly reduce the risk of AF/AFL in diabetic patients compared with other hypoglycemic drugs [20]. Nevertheless, these existing reviews were limited to a few included studies with restrictions on populations and did not include several important studies that have been published recently [21–24].
AF was the most reported arrhythmia event in the present study, consistent with its high prevalence [25]. The incidence of AF in our study was close to a previous cohort study (39 per 10,000 patient-years vs 32 per 10,000 patient-years) [26]. Although our meta-analysis failed to identify a significant association between GLP-1 RAs and AF, it is noted that oral semaglutide had a protective effect on incident AF. Interestingly, subcutaneous semaglutide also seemed to be associated with a small, albeit marginally significant reduction in AF events. These results support the findings of the prior meta-analysis [20], also demonstrating similar efficacy of oral and subcutaneous administration. A slight trend toward an increased risk of AF was observed with dulaglutide, whereas the trend was not robust owing to the overweighting of the REWIND trial. We did not observe a significant association with AF in other GLP-1 RAs agents, suggesting a possible drug-specific effect. Larger studies evaluating the effects of GLP-1 RAs on AF are required to confirm the association.
According to our findings, the incidence of AFL appeared to be lower in the GLP-1RAs group than in the control group, but the difference did not reach statistical significance. In fact, AFL and AF share similar clinical presentations and consequences. AF/AFL has been firmly established to increase the risk of stroke, approximately 13-26% of ischemic strokes are attributed to AF/AFL [27]. A recent cohort study of patients with T2DM observed that GLP-1 RAs treatment could exert a protective effect against ischemic stroke [28]. A speculation was proposed that a reduction in AF/AFL might play an important role in this observation. Owing to the relatively low incidence of AFL, future trials could specify AF and AFL as a composite outcome. And the conjoint analysis of AF/AFL would provide more robust results while obviating possible publication bias for AFL.
The current study found that both higher dose of GLP-1RAs and higher baseline BMI were linked with a nearly 60% higher risk of incident VAs. In practice, GLP-1 RAs are often given in higher doses for weight loss than for glycaemic control. The US Food and Drug Administration (FDA) has approved liraglutide (3.0 mg, daily) and subcutaneous semaglutide (2.4 mg, weekly) for weight management, which are irregular doses for diabetic control. Up-titration of GLP-1 RAs, on the other hand, may be recommended for T2DM patients who do not reach glycemic control targets with lower dosages. Considering the increasing risk of VAs, we should be more careful when treating obese subjects or giving higher dosage to T2DM patients.
The relationship between GLP-1 RAs and SCD has been less well reported, and the current meta-analysis is the first to summarize the SCD events in GLP-1 RAs trials. In our study, GLP-1 RAs treatment appeared to lack of significant effect on SCD events. Further subgroup analyses also supported this finding. In T2DM patients, SCD is partly mediated by the increased presence of coronary heart disease, which facilitates the occurrence of malignant VAs [29], such as ventricular fibrillation and ventricular flutter. However, both VAs and SCD events are difficult to detect since they tend to have immediate onsets and short duration, which may lead to under-reporting. Considering the serious hazard, future trials of GLP-1 RAs should fully detect and report VAs and SCD events.
The pharmacological and physiological mechanisms linking GLP-1RAs and arrhythmias remain unknown. GLP-1 RAs act by activating GLP-1 receptors (GLP-1R) widely distributed outside the pancreas [30]. GLP-1R has also been expressed in the cardiovascular system, within the heart and blood vessels [31, 32]. Some clinical and preclinical studies have demonstrated that the contribution to cardiovascular protection of GLP-1 RAs is mainly ascribed to its modest reduction of blood pressure [33], correction of dyslipidemia [34], improvement of microvascular function [35, 36], and anti-oxidization or anti-inflammation [1, 37]. Since the distributions of GLP-1R in sino-atrial node and ventricular cardiomyocytes [31, 38], a previous study indicated that the direct activations of GLP-1R effectively opposed the effects of β-adrenoceptor stimulation on cardiac ventricular excitability and might be responsible for HR augmentation or arrhythmias [39]. An alternative explanation is that GLP-1RAs may act on the autonomic nervous system. An observational study with 60 T2DM patients showed that liraglutide increased cardiac sympathetic nervous system activity compared to baseline measurements [40]. However, in a study that enrolled 28 T2DM patients, the cardiac sympatho-vagal balance (assessed by HR variability) was not affected after 3 or 6 months of exenatide treatment, despite an increase in HR [41]. Thus, the underlying mechanism by which GLP-1 RAs influence the incidence and development of arrhythmias requires further exploration.
It is well-recognized that both diabetes and obesity are associated with AF and other cardiac arrhythmias, which may in turn increase adverse cardiovascular events and mortality in diabetic and obese individuals [42–44]. Therefore, it is critically important to reduce the risk of cardiac arrhythmias in T2DM and obese patients. Our study showed that GLP-1 RAs might produce antiarrhythmic or arrhythmogenic effects under certain circumstances. However, previous trials mainly reported arrhythmic events as SAEs, further large-scale controlled studies should pre-specify arrhythmias as endpoints to confirm our findings. Additionally, it is possible that GLP-1 RAs are drug-specific and have individual variability in different populations. More evidence is required to assist clinicians in the selection of suitable GLP-1 RAs agents and optimal populations based on the risk classification of arrhythmias. Studies are also needed to help determine the pathophysiological mechanisms by which GLP-1 RAs increase heart rate and generate potential antiarrhythmic or arrhythmogenic effects.
The present systematic review and meta-analysis was strengthened by incorporating recently published trials and larger populations, and by reporting more arrhythmia outcomes. Moreover, we conducted extended and detailed subgroup analyses to investigate the potential risk factors affecting the development of arrhythmias. These made our results more convincing and robust. However, there are several limitations in our study. Firstly, the incidence of arrhythmias was relatively low and even absent from some included studies, leading to wide confidence intervals. Hence, these results might be influenced by low power. Secondly, we included trials involving both diabetic and obese patients, and this population heterogeneity might influence the pooled results. However, diabetes and obesity are both metabolic disorders, and there is a growing recognition that obesity, diabetes, and arrhythmias (especially AF) are closely intertwined epidemics. In addition to T2DM, another key indication for GLP-1 RAs is obesity. Obese patients tend to receive higher-dosage GLP-1 RAs and prolonged treatment duration. To evaluate the cardiovascular safety of GLP-1 RAs and fully understand the utilization of this drug, it is necessary to include these populations. Thirdly, there were some changes mainly related to outcomes in the research protocol registered on the PROSPERO website. To achieve a more comprehensive assessment of arrhythmia outcomes, we added incident AFL as an outcome. Additionally, considering the complexity of the etiologic composition of SCD, we specified VAs and SCD as independent outcomes. We made these alterations before we conducted data extraction and analysis. We do not think that these alterations would change our conclusions; instead, we believe that these alterations would enhance the rigor of our study. Finally, although this was a meta-analysis of numerous RCTs, the results of which were less sensitive and convincing than the analysis of individual-level data.
Conclusions
GLP-1 RAs therapy was overall not significantly associated with incident arrhythmias in diabetic and obese patients, suggesting an assuring cardiovascular safety profile. Oral semaglutide might be associated with a lower risk of incident AF while dulaglutide demonstrated opposite effect. Of note, given the increased risk of VAs, physicians should be more careful when treating obese subjects or prescribing high dosage. Furthermore, the potential antiarrhythmic or arrhythmogenic effect of GLP-1 RAs may be drug-specific and require further investigation.
Supplementary Information
Additional file 1: Method S1. Data sources and search strategies. Table S1. Eligible criteria for included studies. Table S2. Baseline characteristics of included studies and participants. Figure S1. Methodological quality assessment of included studies. Figure S2. Risks of cardiac arrhythmias in patients with GLP-1 RAs treatments compared with control groups. Figure S3. Subgroup analyses on the association of GLP-1 RAs use with incident atrial fibrillation. Figure S4. Subgroup analyses on the association of GLP-1 RAs use with the incidence of atrial flutter. Figure S5. Subgroup analyses on the association of GLP-1 RAs use with the incidence of ventricular arrhythmias. Figure S6. Subgroup analyses on the association of GLP-1 RAs use with the incidence of sudden cardiac death. Figure S7. Sensitivity analyses by omitting each trial one by one of all included studies. Figure S8. Funnel plots for each outcome. References S1.
Acknowledgements
Not applicable.
Abbreviations
- GLP-1 RAs
Glucagon-like peptide-1 receptor agonists
- GLP-1
Glucagon-like peptide-1
- HR
Heart rate
- AF
Atrial fibrillation
- AFL
Atrial flutter
- VAs
Ventricular arrhythmias
- SCD
Sudden cardiac death
- T2DM
Type 2 diabetes mellitus
- BMI
Body mass index
- HbA1c
Glycated hemoglobin
- MACE
Major adverse cardiovascular event
- RR
Relative risk
- CI
Confidence interval
- RCT
Randomized controlled trial
- CVOT
Cardiovascular outcome trial
- SAE
Severe adverse effect
- Sc
Subcutaneous injection
- PRISMA
Preferred Reporting Items for Systematic Reviews and Meta-Analyses
- FDA
Food and Drug Administration
Author contributions
Study concept and design: SW and KC. Literature search: SW and WL. Collection, analysis, or interpretation of data: SW, WL, and ZC. Statistical analysis: SW. Writing of the manuscript: SW. Critical revision of the manuscript for important intellectual content: YD, KC, and SZ. All authors gave final approval and agree to be accountable for all aspects of the work ensuring integrity and accuracy. All authors read and approved the final manuscript.
Funding
None.
Availability of data and materials
All relevant data generated or analyzed in our study are available in the main text and the additional file 1.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Sijin Wu, Email: wusijinpumc@163.com.
Wenzhao Lu, Email: luwzh_yhml@outlook.com.
Zhongli Chen, Email: zhonglichen555@126.com.
Yan Dai, Email: daiy7516@sina.com.
Keping Chen, Email: chenkeping@263.net.
Shu Zhang, Email: zsfuwai@vip.163.com.
References
- 1.Nauck MA, Meier JJ, Cavender MA, Abd El Aziz M, Drucker DJ. Cardiovascular actions and clinical outcomes with glucagon-like peptide-1 receptor agonists and dipeptidyl peptidase-4 inhibitors. Circulation. 2017;136:849–70. doi: 10.1161/CIRCULATIONAHA.117.028136. [DOI] [PubMed] [Google Scholar]
- 2.Bethel MA, Patel RA, Merrill P, Lokhnygina Y, Buse JB, et al. Cardiovascular outcomes with glucagon-like peptide-1 receptor agonists in patients with type 2 diabetes: a meta-analysis. Lancet Diabetes Endocrinol. 2018;6:105–113. doi: 10.1016/S2213-8587(17)30412-6. [DOI] [PubMed] [Google Scholar]
- 3.Giugliano D, Scappaticcio L, Longo M, Caruso P, Maiorino MI, et al. GLP-1 receptor agonists and cardiorenal outcomes in type 2 diabetes: an updated meta-analysis of eight CVOTs. Cardiovasc Diabetol. 2021;20:189. doi: 10.1186/s12933-021-01366-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lorenz M, Lawson F, Owens D, Raccah D, Roy-Duval C, et al. Differential effects of glucagon-like peptide-1 receptor agonists on heart rate. Cardiovasc Diabetol. 2017;16:6. doi: 10.1186/s12933-016-0490-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ferdinand KC, White WB, Calhoun DA, Lonn EM, Sager PT, et al. Effects of the once-weekly glucagon-like peptide-1 receptor agonist dulaglutide on ambulatory blood pressure and heart rate in patients with type 2 diabetes mellitus. Hypertension. 2014;64:731–737. doi: 10.1161/HYPERTENSIONAHA.114.03062. [DOI] [PubMed] [Google Scholar]
- 6.Sun F, Wu S, Guo S, Yu K, Yang Z, et al. Impact of GLP-1 receptor agonists on blood pressure, heart rate and hypertension among patients with type 2 diabetes: a systematic review and network meta-analysis. Diabetes Res Clin Pract. 2015;110:26–37. doi: 10.1016/j.diabres.2015.07.015. [DOI] [PubMed] [Google Scholar]
- 7.Hernandez AF, Green JB, Janmohamed S, D'Agostino RB, Sr, Granger CB, et al. Albiglutide and cardiovascular outcomes in patients with type 2 diabetes and cardiovascular disease (Harmony Outcomes): a double-blind, randomised placebo-controlled trial. Lancet. 2018;392:1519–1529. doi: 10.1016/S0140-6736(18)32261-X. [DOI] [PubMed] [Google Scholar]
- 8.Gerstein HC, Colhoun HM, Dagenais GR, Diaz R, Lakshmanan M, et al. Dulaglutide and cardiovascular outcomes in type 2 diabetes (REWIND): a double-blind, randomised placebo-controlled trial. Lancet. 2019;394:121–130. doi: 10.1016/S0140-6736(19)31149-3. [DOI] [PubMed] [Google Scholar]
- 9.Marso SP, Daniels GH, Brown-Frandsen K, Kristensen P, Mann JF, et al. Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2016;375:311–322. doi: 10.1056/NEJMoa1603827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pfeffer MA, Claggett B, Diaz R, Dickstein K, Gerstein HC, et al. Lixisenatide in patients with type 2 diabetes and acute coronary syndrome. N Engl J Med. 2015;373:2247–2257. doi: 10.1056/NEJMoa1509225. [DOI] [PubMed] [Google Scholar]
- 11.Husain M, Birkenfeld AL, Donsmark M, Dungan K, Eliaschewitz FG, et al. Oral Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med. 2019;381:841–851. doi: 10.1056/NEJMoa1901118. [DOI] [PubMed] [Google Scholar]
- 12.Marso SP, Bain SC, Consoli A, Eliaschewitz FG, Jodar E, et al. Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med. 2016;375:1834–1844. doi: 10.1056/NEJMoa1607141. [DOI] [PubMed] [Google Scholar]
- 13.Fisher M, Petrie MC, Ambery PD, Donaldson J, Ye J, et al. Cardiovascular safety of albiglutide in the Harmony programme: a meta-analysis. Lancet Diabetes Endocrinol. 2015;3:697–703. doi: 10.1016/S2213-8587(15)00233-8. [DOI] [PubMed] [Google Scholar]
- 14.Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ. 2009;339:b2700. doi: 10.1136/bmj.b2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Higgins JPT, Savović J, Page MJ, Elbers RG, Sterne JAC. Chapter 8: assessing risk of bias in a randomized trial. In: Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (eds) Cochrane handbook for systematic reviews of interventions version 6.0 (updated July 2019). Cochrane. 2019. Available from www.training.cochrane.org/handbook.
- 16.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Boulmpou A, Patoulias D, Papadopoulos CE, Teperikidis E, Doumas M, et al. Meta-analysis of cardiovascular outcome trials assessing the impact of glucagon-like peptide-1 receptor agonists on major cardiac arrhythmias. Acta cardiologica. 2022 doi: 10.1080/00015385.2022.2087839. [DOI] [PubMed] [Google Scholar]
- 18.Monami M, Nreu B, Scatena A, Giannini S, Andreozzi F, et al. Glucagon-like peptide-1 receptor agonists and atrial fibrillation: a systematic review and meta-analysis of randomised controlled trials. J Endocrinol Invest. 2017;40:1251–1258. doi: 10.1007/s40618-017-0698-7. [DOI] [PubMed] [Google Scholar]
- 19.Nreu B, Dicembrini I, Tinti F, Sesti G, Mannucci E, et al. Major cardiovascular events, heart failure, and atrial fibrillation in patients treated with glucagon-like peptide-1 receptor agonists: an updated meta-analysis of randomized controlled trials. Nutr Metab Cardiovasc Dis. 2020;30:1106–1114. doi: 10.1016/j.numecd.2020.03.013. [DOI] [PubMed] [Google Scholar]
- 20.Shi W, Zhang W, Zhang D, Ren G, Wang P, et al. Comparison of the effect of glucose-lowering agents on the risk of atrial fibrillation: a network meta-analysis. Heart Rhythm. 2021;18:1090–1096. doi: 10.1016/j.hrthm.2021.03.007. [DOI] [PubMed] [Google Scholar]
- 21.Wilding JPH, Batterham RL, Calanna S, Davies M, Van Gaal LF, et al. Once-weekly semaglutide in adults with overweight or obesity. N Engl J Med. 2021;384:989–1002. doi: 10.1056/NEJMoa2032183. [DOI] [PubMed] [Google Scholar]
- 22.Rubino D, Abrahamsson N, Davies M, Hesse D, Greenway FL, et al. Effect of continued weekly subcutaneous Semaglutide vs placebo on weight loss maintenance in adults with overweight or obesity: the step 4 randomized CLINICAL trial. JAMA. 2021;325:1414–1425. doi: 10.1001/jama.2021.3224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kadowaki T, Isendahl J, Khalid U, Lee SY, Nishida T, et al. Semaglutide once a week in adults with overweight or obesity, with or without type 2 diabetes in an east Asian population (STEP 6): a randomised, double-blind, double-dummy, placebo-controlled, phase 3a trial. Lancet Diabetes Endocrinol. 2022;10:193–206. doi: 10.1016/S2213-8587(22)00008-0. [DOI] [PubMed] [Google Scholar]
- 24.Kellerer M, Kaltoft MS, Lawson J, Nielsen LL, Strojek K, et al. Effect of once-weekly semaglutide versus thrice-daily insulin aspart, both as add-on to metformin and optimized insulin glargine treatment in participants with type 2 diabetes (SUSTAIN 11): a randomized, open-label, multinational, phase 3b trial. Diabetes Obes Metab. 2022;24:1788–1799. doi: 10.1111/dom.14765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rahman F, Kwan GF, Benjamin EJ. Global epidemiology of atrial fibrillation. Nat Rev Cardiol. 2014;11:639–654. doi: 10.1038/nrcardio.2014.118. [DOI] [PubMed] [Google Scholar]
- 26.Chan YH, Chao TF, Chen SW, Lee HF, Li PR, et al. The risk of incident atrial fibrillation in patients with type 2 diabetes treated with sodium glucose cotransporter-2 inhibitors, glucagon-like peptide-1 receptor agonists, and dipeptidyl peptidase-4 inhibitors: a nationwide cohort study. Cardiovasc Diabetol. 2022;21:118. doi: 10.1186/s12933-022-01549-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Seiffge DJ, Werring DJ, Paciaroni M, Dawson J, Warach S, et al. Timing of anticoagulation after recent ischaemic stroke in patients with atrial fibrillation. Lancet Neurol. 2019;18:117–126. doi: 10.1016/S1474-4422(18)30356-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yang YS, Chen HH, Huang CN, Hsu CY, Hu KC, et al. GLP-1RAs for ischemic stroke prevention in patients with type 2 diabetes without established atherosclerotic cardiovascular disease. Diabetes Care. 2022;45:1184–1192. doi: 10.2337/dc21-1993. [DOI] [PubMed] [Google Scholar]
- 29.Siscovick DS, Sotoodehnia N, Rea TD, Raghunathan TE, Jouven X, et al. Type 2 diabetes mellitus and the risk of sudden cardiac arrest in the community. Rev Endocr Metab Disord. 2010;11:53–59. doi: 10.1007/s11154-010-9133-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Campbell JE, Drucker DJ. Pharmacology, physiology, and mechanisms of incretin hormone action. Cell Metab. 2013;17:819–837. doi: 10.1016/j.cmet.2013.04.008. [DOI] [PubMed] [Google Scholar]
- 31.Baggio LL, Yusta B, Mulvihill EE, Cao X, Streutker CJ, et al. GLP-1 Receptor expression within the human heart. Endocrinology. 2018;159:1570–1584. doi: 10.1210/en.2018-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pujadas G, Drucker DJ. Vascular biology of glucagon receptor superfamily peptides: mechanistic and clinical relevance. Endocr Rev. 2016;37:554–583. doi: 10.1210/er.2016-1078. [DOI] [PubMed] [Google Scholar]
- 33.Kim M, Platt MJ, Shibasaki T, Quaggin SE, Backx PH, et al. GLP-1 receptor activation and Epac2 link atrial natriuretic peptide secretion to control of blood pressure. Nat Med. 2013;19:567–575. doi: 10.1038/nm.3128. [DOI] [PubMed] [Google Scholar]
- 34.Sun F, Wu S, Wang J, Guo S, Chai S, et al. Effect of glucagon-like peptide-1 receptor agonists on lipid profiles among type 2 diabetes: a systematic review and network meta-analysis. Clin Ther. 2015;37:225–41.e8. doi: 10.1016/j.clinthera.2014.11.008. [DOI] [PubMed] [Google Scholar]
- 35.Sjoberg KA, Rattigan S, Jeppesen JF, Lundsgaard AM, Holst JJ, et al. Differential effects of glucagon-like peptide-1 on microvascular recruitment and glucose metabolism in short- and long-term insulin resistance. J Physiol. 2015;593:2185–2198. doi: 10.1113/JP270129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.De Ciuceis C, Agabiti-Rosei C, Rossini C, Caletti S, Coschignano MA, et al. Microvascular density and circulating endothelial progenitor cells before and after treatment with incretin mimetics in diabetic patients. High Blood Press Cardiovasc Prev. 2018;25:369–378. doi: 10.1007/s40292-018-0279-7. [DOI] [PubMed] [Google Scholar]
- 37.Smits MM, Muskiet MH, Tonneijck L, Kramer MH, Diamant M, et al. GLP-1 receptor agonist exenatide increases capillary perfusion independent of nitric oxide in healthy overweight men. Arterioscler Thromb Vasc Biol. 2015;35:1538–1543. doi: 10.1161/ATVBAHA.115.305447. [DOI] [PubMed] [Google Scholar]
- 38.Pyke C, Heller RS, Kirk RK, Orskov C, Reedtz-Runge S, et al. GLP-1 receptor localization in monkey and human tissue: novel distribution revealed with extensively validated monoclonal antibody. Endocrinology. 2014;155:1280–1290. doi: 10.1210/en.2013-1934. [DOI] [PubMed] [Google Scholar]
- 39.Ang R, Mastitskaya S, Hosford PS, Basalay M, Specterman M, et al. Modulation of cardiac ventricular excitability by GLP-1 (Glucagon-Like Peptide-1) Circ Arrhythm Electrophysiol. 2018;11:e006740. doi: 10.1161/CIRCEP.118.006740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nakatani Y, Kawabe A, Matsumura M, Aso Y, Yasu T, et al. Effects of GLP-1 receptor agonists on heart rate and the autonomic nervous system using holter electrocardiography and power spectrum analysis of heart rate variability. Diabetes Care. 2016;39:e22–e23. doi: 10.2337/dc15-1437. [DOI] [PubMed] [Google Scholar]
- 41.Cacciatori V, Zoppini G, Bellavere F, Rigolon R, Thomaseth K, et al. Long-acting GLP-1 receptor agonist exenatide influence on the autonomic cardiac sympatho-vagal balance. J Endocr Soc. 2018;2:53–62. doi: 10.1210/js.2017-00300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wang A, Green JB, Halperin JL, Piccini JP., Sr Atrial fibrillation and diabetes mellitus: JACC review topic of the week. J Am Coll Cardiol. 2019;74:1107–1115. doi: 10.1016/j.jacc.2019.07.020. [DOI] [PubMed] [Google Scholar]
- 43.Sabbag A, Sidi Y, Kivity S, Beinart R, Glikson M, et al. Obesity and exercise-induced ectopic ventricular arrhythmias in apparently healthy middle aged adults. Eur J Prev Cardiol. 2016;23:511–517. doi: 10.1177/2047487315591442. [DOI] [PubMed] [Google Scholar]
- 44.Karam BS, Chavez-Moreno A, Koh W, Akar JG, Akar FG. Oxidative stress and inflammation as central mediators of atrial fibrillation in obesity and diabetes. Cardiovasc Diabetol. 2017;16:120. doi: 10.1186/s12933-017-0604-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Method S1. Data sources and search strategies. Table S1. Eligible criteria for included studies. Table S2. Baseline characteristics of included studies and participants. Figure S1. Methodological quality assessment of included studies. Figure S2. Risks of cardiac arrhythmias in patients with GLP-1 RAs treatments compared with control groups. Figure S3. Subgroup analyses on the association of GLP-1 RAs use with incident atrial fibrillation. Figure S4. Subgroup analyses on the association of GLP-1 RAs use with the incidence of atrial flutter. Figure S5. Subgroup analyses on the association of GLP-1 RAs use with the incidence of ventricular arrhythmias. Figure S6. Subgroup analyses on the association of GLP-1 RAs use with the incidence of sudden cardiac death. Figure S7. Sensitivity analyses by omitting each trial one by one of all included studies. Figure S8. Funnel plots for each outcome. References S1.
Data Availability Statement
All relevant data generated or analyzed in our study are available in the main text and the additional file 1.