Abstract
Purpose
To assess the safety and report the clinical outcomes of synthetic graft augmentation using polypropylene (PP) mesh in the repair of acute Achilles tendon (AT) rupture in patients with preexisting tendinopathy. Methods: Patients who underwent open repair for acute AT rupture at our institution between April 2017 and March 2019 were retrospectively identified. The inclusion criteria were acute AT rupture in patients with preexisting tendinopathy. All patients included in the study underwent acute repair augmented by an inlay PP mesh and had 30 months’ follow-up. Patient characteristics, operative details, and outcomes were analyzed. Continuous data were described by mean, standard deviation, median, and range. The Wilcoxon signed rank test was used to analyze the change in patient-reported outcome measures. The significance level was set at a P-value of .05.
Results
Thirteen patients were included. There were 5 female and 8 male patients, withan average age of 52 years (range 49-56 years). No cases of rerupture or graft-related complications requiring additional treatment occurred during mean follow -up of 38 months. All patients reported good functional outcome, as shown from nonsignificant difference between the preinjury and 38-month postoperative Achilles Tendon Rupture Score (88.5 ± 2.2 vs 89.2 ± 2.2, P = .107) and the excellent postoperative American Orthopedic Foot and Ankle Society Ankle/Hindfoot Scale score (92.22 ± 2.2) at last follow-up. At the end of follow-up, all patients were able to perform single-legged heel rise as the noninvolved side. By average of 16 weeks, all patients returned to their preinjury activity level.
Conclusions
The use of inlay PP mesh to augment the repair of acute AT rupture in patients with preexisting tendinopathy appears to be safe and effective, allowing early return to preinjury activity level with favorable clinical outcomes.
Level of Evidence
Level IV, therapeutic case series.
Ruptures of Achilles tendon (AT) constitute more than 40% of all tendon ruptures that require surgical intervention and often lead to significant morbidity.1 While there is a lot of debate in the management approach for acute AT ruptures, the most reasonable approach is to treat it nonoperatively if tendon apposition could be achieved as detected clinically or by ultrasonography (US).2,3 This is always possible if treatment started early.4,5 However, if a diastasis remains with 20° of plantar flexion, operative treatment is indicated. This operative repair is typically end-to-end tendon repair using one of several different suture materials and configurations, including the Bunnell, Kessler, and Krackow techniques.6
More than two-thirds of AT ruptures are preceded by tendinosis (tendinopathy).7 Achilles tendinopathy is essentially a failed healing response and typically accompanied by histopathologic changes in the tendon structure, including collagen fiber disorganization, increased glycosaminoglycan content, and vascular tissue with coexisting different types of degenerative changes including mucoid, fatty, or hyaline degeneration, and even fibrocartilaginous or bony metaplasia.8 These intratendinous changes could be more profound in ruptured ATs than tendinopathic tendons9 and extend beyond the rupture site to the macroscopically intact AT proximally and distally.10 In this selected group of acute AT ruptures, which have had preexisting tendinopathy, end-to-end tendon repair could be difficult and of weak integrity.11,12 When spontaneous rupture occurs, the degenerated tendon torn ends are frequently ragged (mop ends) and not ideal for a perfect suture.4 Debridement of these ends is usually required, especially in preexisting tendinopathy. This results in patchy tendon ends with an irregular defect even though of negligible length of approximately 1 cm. This leads to a small cross-sectional area of the repair site that is incapable of transmitting larger forces.13 In addition, the unavoidable residual microscopic degeneration in the repaired tendon has been demonstrated to be less stiff and prone to greater strain.14,15
Augmentation of the primary end-to-end reapproximation could facilitate the continuity and strength of the repair construct in selected scenarios of acute AT rupture when repair integrity is questionable.11,12 Moreover, augmentation may be used to improve outcomes.12 Materials used for augmentation could be endogenous or exogenous. Endogenous materials from local tissues such as turned-down gastrocnemius fascial flap may not be an optimal source, especially in cases of preexisting tendinopathy, as the macroscopically intact AT shows pathologic features of a failing healing response as well.10,16 Exogenous materials include allografts and synthetic grafts. However, rapid loss of strength and unavailability in some countries due to increased cost and religious restrictions are the major distinctive handicaps of allografts.12
There are few reports of the use of various synthetic materials to augment the repair of acute AT rupture, such as Dacron vascular graft,17 polypropylene (PP) braid,18 and polyethylene mesh.19 The biomechanical advantages of synthetic graft augmentation have been demonstrated in laboratory studies, including decreasing gapping and increasing repair construct stiffness and ultimate failure load.20,21 However, the theoretic concerns and part of the reason synthetic graft use is limited are the risks of infection and foreign body (FB) reactions.22 The purpose of this preliminary study was to assess the safety and report the clinical outcomes of synthetic graft augmentation using PP mesh in the repair of acute AT rupture in patients with preexisting tendinopathy. We hypothesized that augmented repair using inlay PP mesh would be a safe procedure without major complications and stable enough to allow early return to preinjury activity level.
Methods
Selection of Patients and Preoperative Evaluation
During the period from April 2017 to March 2019, patients who had an open repair for acute AT rupture at our orthopaedic department were identified retrospectively. This study was approved by our institution’s review board (Faculty of Medicine Ain Shams University Research Ethics Committee [FMASU REC] with reference number FMASU REC 202/2021; FMASU REC operates under Federal Wide Assurance No. FWA 000017585), and all patients provided their written, informed consent. Inclusion criteria were patients with preexisting tendinopathy who presented after 1 week with an acute AT rupture with nonopposing tendon ends on plantarflexion (PF) detected clinically or by US6 and treated with an acute repair augmented by inlay PP mesh. Patients also must have had a minimum of 30 months’ follow-up data available. Patientswith insertional ruptures or an acute AT rupture without a history of prerupture AT pain and with intraoperative appearance of healthy proximal and distal tendon stumps were excluded. The diagnosis of AT was based on history collected at our institution of prerupture AT pain for more than 3 months and/or intraoperative finding by 1 of the authors (H.K.H., A.A.A., and A.M.) of a degenerated tendon at the proximal and/or distal stump. Functional and clinical assessment at baseline presented by preinjury Achilles Tendon Rupture Score (ATRS)23 and then postoperatively until the end of follow-up presented by postoperative ATRS and American Orthopedic Foot and Ankle Society Ankle/Hindfoot Scale (AOFAS).24
A standard preoperative physical examination was performed. Tendon rupture was diagnosed by loss of PF strength with inability to perform single stance heel rise,25 the presence of a palpable gap, and a positive Thompson squeeze test. Uncontrolled diabetes was defined as having a glycosylated hemoglobin (Hb A1c) >6.5%.26
Baseline symptoms and physical activity were evaluated by preoperative collection of preinjury ATRS. The ATRS is an injury-specific and validated patient-reported outcome measure in patients with AT rupture.23
At the beginning of the inclusion period, diagnosis was confirmed by magnetic resonance imaging and the decision on operative treatment was based on whether the tendon ends were found not to approximate well on palpation with the foot in PF.2 Later, US was used to both confirm the diagnosis and assess tendon ends approximation. US was performed by an experienced radiologist.
Surgical Technique
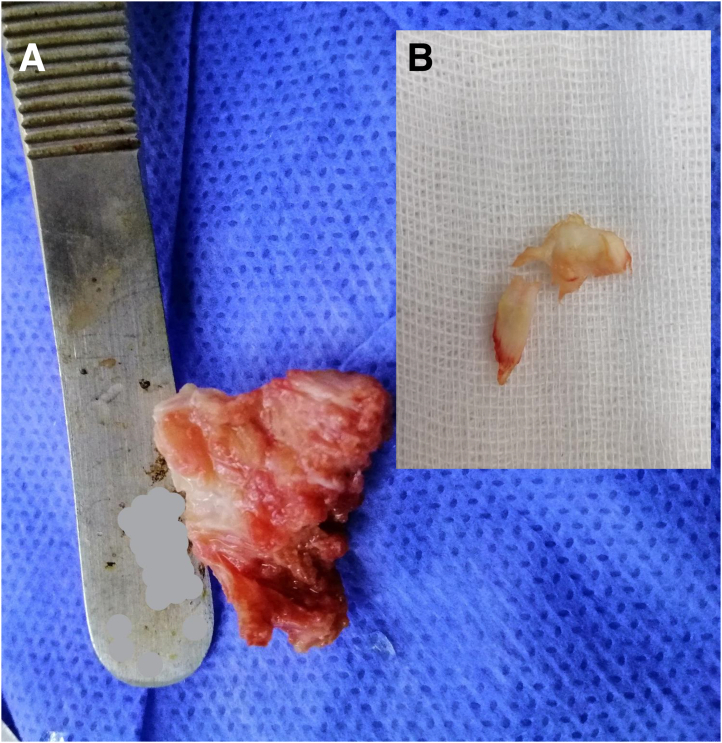
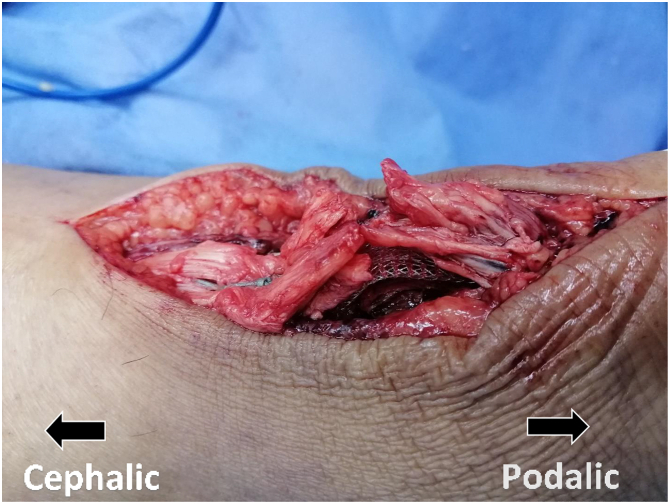
All procedures were performed by one of the authors (H.K.H., A.A.A.E., and A.M.) and with the patient under spinal or general anesthesia. After the prophylactic antibiotic was given, a pneumatic tourniquet was applied. The patients were positioned prone. Both lower extremities were prepped and draped with the ankle hung over edges of the table. An 8-cm posteromedial longitudinal incision was made along the AT. The paratenon was incised, the tendon rupture was exposed, and the friable tendon edges were trimmed. In addition, any devitalized or grossly degenerated tendon tissue at the rupture site was debrided (Fig 1). However, we preserve any healthy, albeit irregular, edges that could prevent gapping and facilitate coverage of the augmenting synthetic graft. The excised tendon tissue was mainly from the central area of the ruptured ends, with an average size of 1 × 1 cm. The quality of this excised tissue was poor, either frayed rupture ends or firm degenerated tissue. It was in the form of calcifications or metaplastic bone in one half of cases. The mean preapproximation tendon defect after debridement measured 10 mm in length (range 5-16 mm).
Fig 1.
Examples of debrided tissue. (A) Metaplastic bone fragment. (B) Mucoid degenerated tendon tissue.
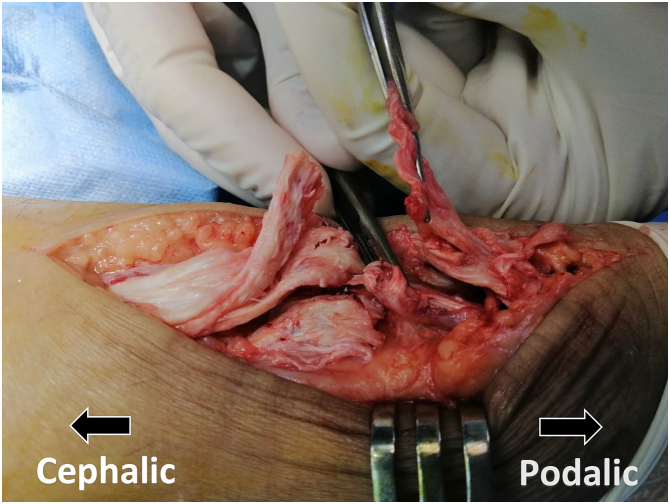
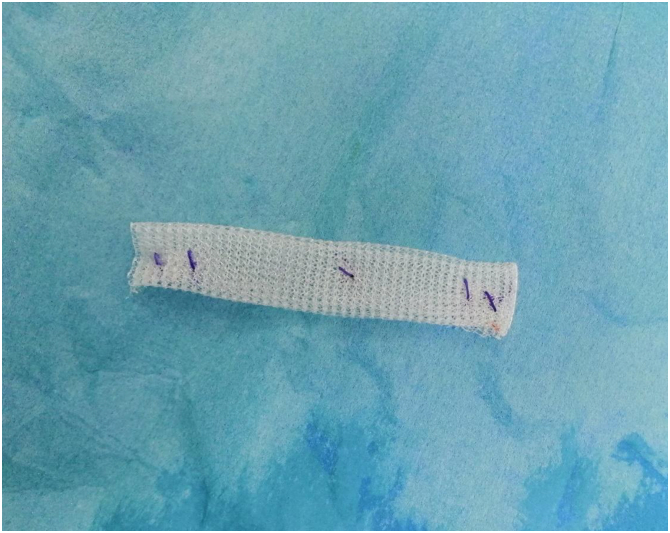
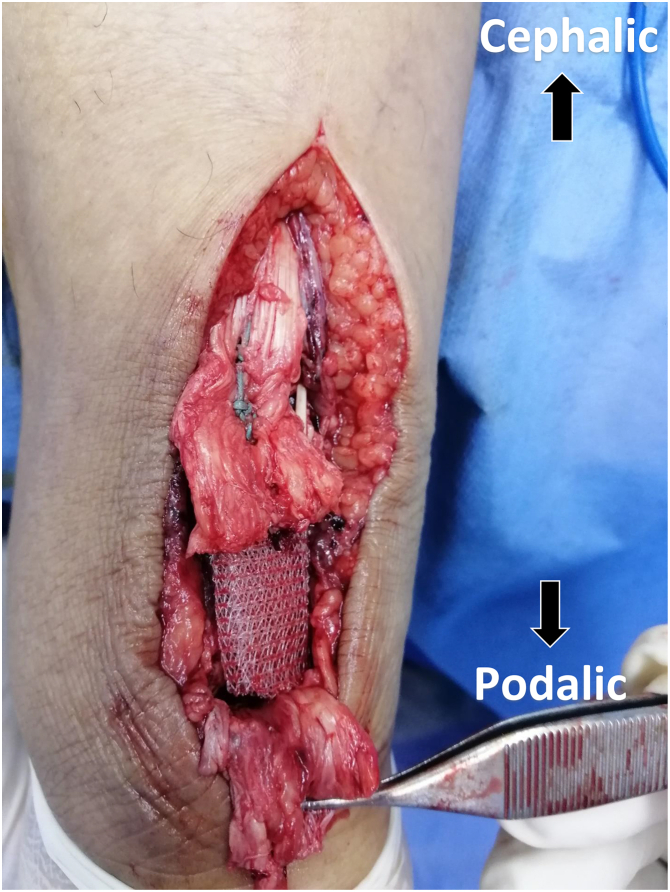
The 2 tendon stumps were split longitudinally in the coronal plane (Fig 2). A 10-cm PP mesh (GTA Mesh PT; GTA Medical, Quistello [Mantua], Italy) was folded 3 times over itself (Fig 3). The mesh was applied inaly inside the split proximal and distal stumps bridging the repair site. The mesh was held in place with a no. 2 nonabsorbable suture (ETHIBOND; Ethicon, Somerville, NJ). The proximal portion was secured first facilitating tensioning adjustments before securing the distal end (Figs 4 and 5).
Fig 2.
Tangential view of patient’s left heel cord showing longitudinal splitting of 2 tendon stumps in the coronal plane.
Fig 3.
PP mesh folded 3 times over itself. (PP, polypropylene.)
Fig 4.
Posterior aspect of patient’s left heel cord showing PP mesh applied inlay inside the split proximal stump and held in place with a no. 2 nonabsorbable suture. (PP, polypropylene.)
Fig 5.
Tangential view of patient’s left heel cord showing PP mesh applied inlay inside the split proximal and distal stumps bridging the repair site. (PP, polypropylene.)
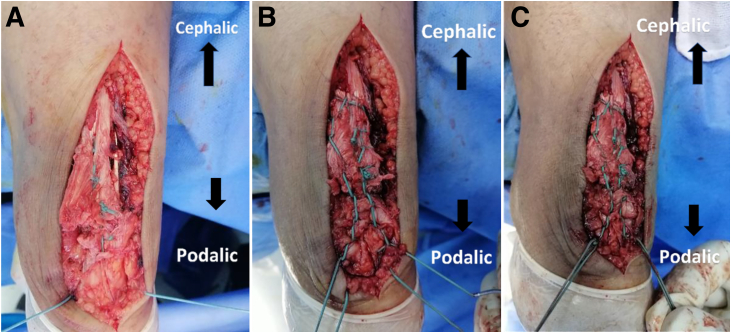
The tendon–graft construct was reinforced with tension suture in a circumferential Krakow stitch configuration, which was distally fixed to the calcaneus through a 2.5-mm drill hole (Fig 6 A-C and 7). The paratenon was closed carefully, as much as we could, using absorbable sutures (Fig 8) followed by skin closure.
Fig 6.
Posterior aspect of patient’s left heel cord showing repair reinforced with tension suture. (A) No. 5 nonabsorbable suture was passed through a 2.5-mm transverse calcaneal drill tunnel. (B) Another no. 5 nonabsorbable suture was braided peripherally around the tendon in a Krakow stitch configuration starting from near the tendon insertion and extending 3 cm proximal to the repair site. (C) Each of the calcaneal suture free ends was securely tied to 1 of the 2 suture ends of the tendon Krakow stitch placing knots on the sides of the calcaneal drill hone.
Fig 7.
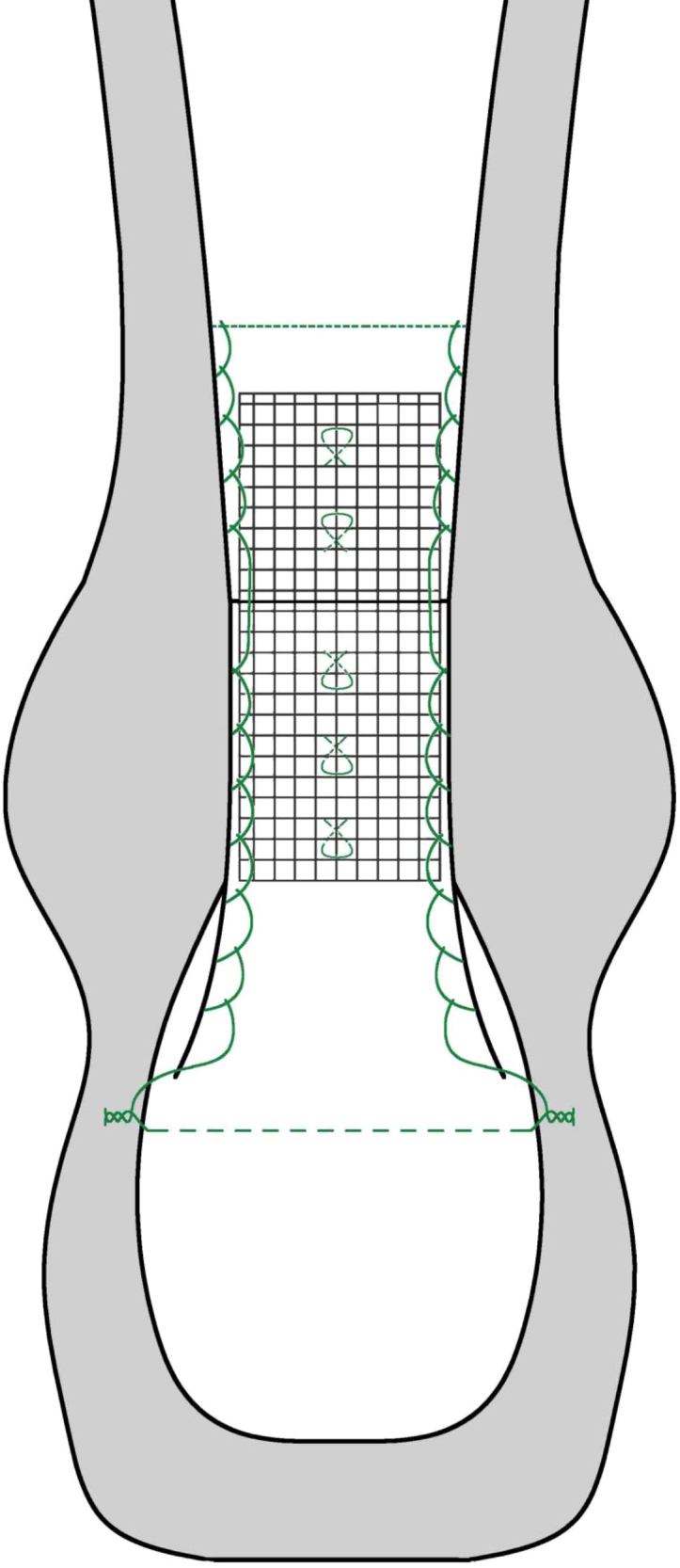
Schematic diagram of our final PP mesh augmented repair. (PP, polypropylene.)
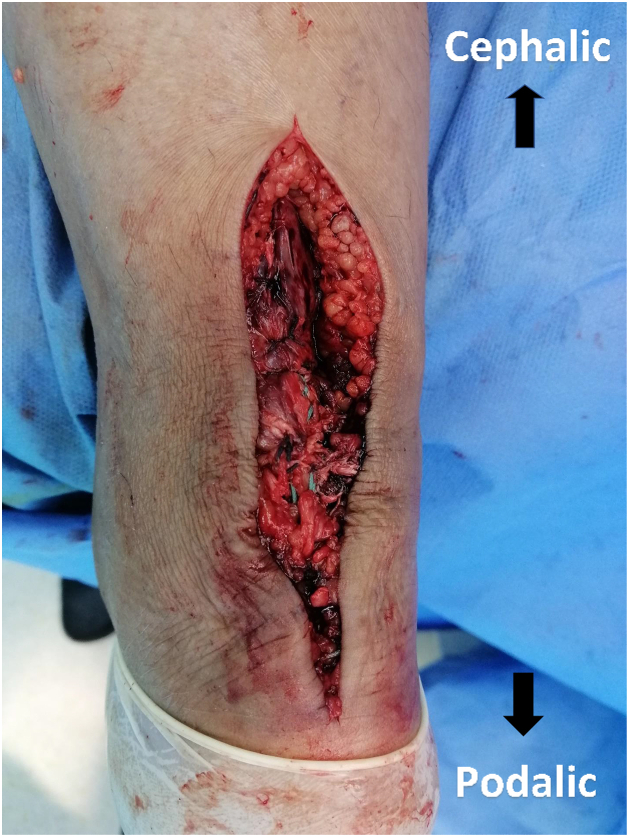
Fig 8.
Posterior aspect of patient’s left heel cord showing repair after paratenon closure.
All patients were placed in a non–weight-bearing, short leg cast in gravity equines for 2 weeks. After 2 weeks, the cast was exchanged for a walking boot with 2-cm heel lift with protected weight-bearing and permitted active PF and dorsiflexion to neutral. After 4 weeks, weight-bearing as tolerated was permitted. After 6 weeks, the heel lift was removed and dorsiflexion stretching started. At 8 weeks, weaning off boots started. According to our hospital protocol, low molecular weight heparin was given to all patients postoperatively for 2 weeks until cast removal.
Postoperative Evaluation
As per our clinical practice, the patients were evaluated at 3, 6, 12 months, and every year thereafter. The primary outcome was the assessment of the incidence of perioperative complications and treatment failures with rerupture.
Perioperative complications could be major, including deep infection and venous thromboembolism, or minor, including skin-related complications (wound-healing problems including dehiscence, superficial infection, adhesion, and hypertrophic scar), tendon-related complications (elongation and contracture), retrocalcaneal pain, and sural nerve disturbance. Diagnosis of rerupture, if present, was based on the 3 preoperative physical examination signs of rupture.
Clinical and functional outcomes were evaluated with postoperative AOFAS Ankle/Hindfoot Scale, ATRS, and standing single stance heel rise. For the neutral single stance heel rise test, the patient was instructed to perform a heel rise while standing on the floor without using any manual support. If the patient was unable to elevate the involved heel from the ground to a similar height as the noninvolved side, then the test was graded as having a positive result.25 These were carried out by an independent surgeon. In addition, the time point was recorded when each patient returned to work or to their activity level before injury.
Statistical Analysis
Because ours was a pilot preliminary study, no power analysis was performed. Continuous data were described by mean, standard deviation, median, and range. The Wilcoxon signed rank test was used to analyze the change in patient-reported outcome measures. The significance level was set at a P-value of .05. The analysis was performed with use of IBM SPSS Statistics software, version 21 (IBM Corp., Armonk, NY).
Results
Seventeen patients who underwent acute repair augmented by inlay PP mesh were identified. Two patients were lost to follow-up, and 2 others were missing the preinjury functional score (ATRS). A total of 13 patients were ultimately included. There were 8 male and 5 female patients with an average age of 52 years (range 49-56 years). All female patients were housewives, whereas 5 male patients reported participating in heavy manual labor such as heavy lifting or climbing, and 3 reported participating in light manual labor, such as computer or office work. Eleven patients were classified as having obesity and 2 were classified as having overweight. Nine patients had diabetes mellitus (DM). DM in 6 patients was not controlled. In 11 patients, the injury occurred during activities of daily living, and in 2 others the rupture had resulted from a fall from a height. A history of prerupture AT pain was identified in 8 patients. The average time between injury and surgical treatment was 18 days (range 8-35 days). The right side was affected in 5 patients and the left side in 8 patients (Table 1). The mean follow-up was 38 months (range 32-56 months). At final follow-up, there were no reported reruptures. Regarding perioperative skin complications, wound healing was satisfactory in all cases at 4 weeks, and 2 patients had persistent local discomfort around the hypertrophic scar. There were no soft-tissue infections, scar adherence, or wound dehiscence. Regarding tendon complications, 2 patients suffered from mild ankle joint stiffness upon waking in the morning. There were no clinically notable tendon elongations with no increase in ankle dorsiflexion range in any case.
Table 1.
Baseline Characteristics of the 13 Patients
| Patient | Age, y | Sex | Side | BMI | Other Tendinopathy Risk Factors | Injury to Surgery, d | Preapproximation Tendon Defect After Debridement, mm | Follow Up, mo |
|---|---|---|---|---|---|---|---|---|
| 1 | 50 | F | Left | 33 | Local steroid DM (UC) |
21 | 6 | 32 |
| 2 | 54 | M | Left | 35 | DM (C) | 10 | 8 | 56 |
| 3 | 55 | M | Left | 26 | None | 28 | 15 | 38 |
| 4 | 49 | M | Right | 32 | Local steroid DM (UC) |
14 | 13 | 34 |
| 5 | 51 | F | Right | 31.5 | DM (C) | 24 | 11 | 32 |
| 6 | 54 | M | Right | 28 | None | 21 | 5 | 32 |
| 7 | 50 | M | Left | 33 | DM (C) | 12 | 10 | 44 |
| 8 | 56 | F | Right | 34 | DM (UC) | 18 | 16 | 33 |
| 9 | 53 | M | Left | 34.5 | None | 25 | 8 | 38 |
| 10 | 49 | M | Left | 33.5 | DM (UC) | 35 | 16 | 41 |
| 11 | 53 | F | Left | 32 | DM (UC) | 9 | 10 | 42 |
| 12 | 51 | F | Left | 30 | DM (UC) Local steroid |
8 | 10 | 34 |
| 13 | 49 | M | Right | 31 | None | 10 | 7 | 34 |
BMI, body mass index; C, controlled; DM, diabetes mellitus; F, female; M, male; UC, uncontrolled.
The mean preinjury and postoperative ATRS scores at last follow-up were 88.5 ± 2.2 (median 89; range 85-92) and 89.2 ± 2.2 (median 90; range 86-92), respectively (P = .107). The mean postoperative AOFAS score at last follow-up was 92.2 ± 2.2 (median 92; range 87-97) (Table 2). At the end of follow-up, all patients were able to perform single stance heel rise to a similar height as the noninvolved side. All patients returned to their preinjury activity level and none of them had to change or modify their job. The average time to recovery was 16 weeks (range 14-18).
Table 2.
Final Follow-up AOFAS Score and ATRS Scores Preinjury and at Final Follow-Up of the 13 Patients
| Patient | Preoperative AOFAS Score | Preinjury ATRS Score | Postoperative ATRS Score |
|---|---|---|---|
| 1 | 92 | 86 | 86 |
| 2 | 92 | 90 | 90 |
| 3 | 94 | 92 | 92 |
| 4 | 87 | 86 | 86 |
| 5 | 92 | 89 | 90 |
| 6 | 93 | 90 | 92 |
| 7 | 92 | 89 | 90 |
| 8 | 92 | 85 | 90 |
| 9 | 94 | 91 | 91 |
| 10 | 92 | 88 | 87 |
| 11 | 92 | 88 | 87 |
| 12 | 90 | 86 | 87 |
| 13 | 97 | 90 | 91 |
AOFAS, American Orthopedic Foot and Ankle Society–Ankle/Hindfoot Scale; ATRS, Achilles Tendon Rupture Score.
Discussion
This series reported on 13 patients with acute AT rupture on top of tendinopathy who underwent augmented repair by inlay PP mesh. All patients regained functional PF strength with no reported reruptures. Also, there were no soft-tissue infections, and it seems that there was no FB reaction in any of our cases, which could be inferred from the improved postoperative patient-reported outcomes, the satisfactory wound healing, and that none of them developed retrocalcaneal pain or adherent scar.
Augmentation of acute AT rupture repair has been suggested to attain high tensile strength, permitting early functional rehabilitation and a decrease in rerupture rate.16 However, augmentation adds potential morbidity to open surgical repair and should be justified.16 The previously suggested theoretical aim of augmentation per se could not justify its addition to operative repair of any acute AT rupture, especially that accelerated rehabilitation with early recovery and decreased rerupture rate are achievable even by nonoperative treatment.1 Until now, there has been no consensus regarding the surgical indication for augmentation in the treatment of acute AT ruptures, and surgeons are unable to appreciate its clinical relevance.16 Accelerated rehabilitation could threaten the repair of selected cases of acute AT rupture that have had preexisting tendinopathy.11,12 Debridement of degenerated, devitalized, and friable tissues leaves uneven, thin tendon ends with an irregular defect, leading to a small cross-sectional area of the repair site that is incapable of transmitting larger forces.13 In addition, the unavoidable residual microscopic, degenerated tissue proximal and distal to the repair has been demonstrated to be less stiff and prone to greater strain.14,15 These worse mechanical properties could lead to rerupture or tendon elongation with accelerated rehabilitation. However, although rerupture remains uncommon, tendon elongation and possible weakness in end-range PF may be a more likely occurrence.27 In our study, we included a selected group of acute AT ruptures which is on top of tendinopathy. In addition, we did not exclude patients with risk factors with preexisting tendinopathy, like old age, high body mass index, DM, or local steroid injection.28 Debrided tissues in our cases measured 1 × 1 cm on average, removed mainly from the central area of ruptured ends, and were of poor quality in the form of frayed ends and/or firm degenerated tissue.
Most studies on augmented repair of acute AT rupture used endogenous material from local tissues such as gastrocnemius fascial flap or plantaris tendon if available. Controlled studies demonstrated that there was no difference in AT elongation, PF strength, and rerupture rate between these locally augmented repairs and primary end-to-end repair of acute AT rupture.16 The sacrificed local tissue could weaken PF strength. Moreover, this seeming to be macroscopically intact adjacent AT tissue shows tendinopathic changes as well.10 In our study, we used exogenous synthetic graft for augmentation, which was a synthetic graft, especially since allografts are unavailable in our country. In our study, there were no reported reruptures. Also, all patients regained functional PF strength, revealed by adequate heel rise performance compared with the noninvolved side. The regained functional PF strength also could be inferred from the absence of clinically notable tendon elongation in all our cases. However, due to the retrospective nature of our study, we had not measured quantitatively PF strength, as it is not performed during routine care of AT repair.
In acute AT augmented repair, the synthetic graft could be applied by means of onlay,19 inlay,18 or any of a variety of weaving techniques.17 Because of concerns of soft-tissue infection and FB reaction, we chose to apply synthetic graft by inlay technique, where the graft was fully covered by the repaired tendon. In our study, there were no soft-tissue infections, although nearly two and one-thirds of patients had DM and were female, respectively. FB reaction is mainly a histopathologic diagnosis. However, it seems that there was no FB reaction in any of our cases, which could be inferred from the regained physical activity measured by ATRS, the satisfactory wound healing, and that none of our patients developed retrocalcaneal pain or adherent scar. However, we acknowledge that this a midterm 38 months’ follow-up study and that longer follow-up studies are required to assess the risk of this complication. While FB reaction is reported frequently with the use of synthetic grafts in other body regions,29 it is scarcely reported in AT repair. A case with reactive inflammatory reaction 11 years after LIGASTIC synthetic ligament reconstruction of AT rerupture has been reported.30 Another case with 1-year postoperative presentation of pain, difficulty in walking, and adherent scar after onlay Artelon-augmented repair of acute AT rupture has been reported.31 Unlike the fully covered inlay synthetic graft in our technique, the synthetic tendon was bridging the maximally reapproximated tendon gap in the first case and was wrapped around the repair in the second case.
The potential safety of inlay PP mesh–augmented AT repair has also been demonstrated in several reports. Ozaki et al.32 and Choksey et al.33 reported minimal signs of FB reaction and no wound-healing problems nor infections in 6 and 5 chronic ruptures after an average follow-up of 3 and 2 years, respectively. However, it should be noted that in these 2 case series, an inlay PP mesh was applied to bridge a residual maximally reapproximated gap of chronic AT rupture. This might increase the risk of graft-related complications than our series. Trying to avoid this risk in neglected AT rupture reconstruction, Fridman et al.22 wrapped the inlay PP mesh graft with resected plantaris tendon. They reported better findings with no signs of FB reactions. Technically, they reinforced the tendon–graft construct with calcaneus-based woven sutures like our technique.
Comparison of our results with other studies in the literature could be difficult, as there is a relative paucity of studies that used inlay synthetic graft augmentation of acute rupture, and we do not know of other studies to include only acute AT rupture on top of tendinopathy. We believe this is an important and underestimated prognostic variable to predict the outcome after acute AT rupture repair. However, Giannini et al.18 conducted a case series on 15 patients with acute AT ruptures who underwent inlay augmented repair with a 8-mm PP braid. The average age of the patients was 37 years, and they had no risk factors of AT tendinopathy. After 18 months’ follow up, patient-reported outcomes improved with 100% return to preinjury activity level. The authors assessed muscle strength in 6 cases and revealed no statistically significant difference with the contralateral normal side. There were no complications, except one case in which the synthetic graft end stuck out from the tendon, requiring implant removal, which was attributed to inadequate surgical technique. Histologic assessment of the retrieved graft showed fibrous tissue rich in growth cells grown onto periphery of the graft. This induced collagenous tissue formation also was demonstrated in a PP mesh–AT complex in rabbits.20 Clinical results presented in our longer follow up case series were favorable and comparable with the study of Giannini et al.18 This was evident in the nonsignificant difference between the preinjury and 38-month postoperative ATRS and the excellent postoperative AOFAS score (mean 92.2; range 87-97). Because our patient series was made up of acute AT rupture on top of tendinopathy, the elevated age and more comorbidities of our patient series are not surprising.
Regarding the timing of recovery, our patients returned to work or their level of activity before injury at an average of 16 weeks. This early recovery of functional capacity could be explained by that the augmented repair was stable enough to allow an accelerated functional rehabilitation protocol with early weight-bearing and range of motion. This looks comparable with the published literature on the timing of functional capability recovery post-AT rupture.34 However, in concept, this study patient population should demonstrate substantially worse outcomes than other series because of older age and more morbidity of this group.
The strengths of this study were that first, eligible cases were defined by strict inclusion criteria to avoid measurement bias and incompleteness of follow-up. Second, it included patients with different characteristics (e.g., diabetes) and cointerventions (e.g., previous steroid injection). Therefore, the results can be applied to common clinical practice.
Limitations
However, there were limitations in this study. First, it included a small number of patients with no comparative or control group. However, this is considered a pilot preliminary study, and its primary aim was to assess the safety of the procedure and the risk of major complications as wound complications and FB reactions. This study should be followed by a full-scale effectiveness study and controlled comparative studies. Second, due to the retrospective design, an objective imaging assessment of the healing tendon was not performed. In addition, selection bias might be present, since only patients with complete medical records and a minimum of 30 months of follow-up were included.
Conclusions
The use of inlay PP mesh to augment the repair of acute AT rupture in patients with preexisting tendinopathy appears to be safe and effective, allowing early return to preinjury activity level with favorable clinical outcomes.
Footnotes
The authors report that they have no conflicts of interest in the authorship and publication of this article. Full ICMJE author disclosure forms are available for this article online, as supplementary material.
Supplementary Data
References
- 1.Willits K., Amendola A., Bryant D., et al. Operative versus nonoperative treatment of acute Achilles tendon ruptures: A multicenter randomized trial using accelerated functional rehabilitation. J Bone Joint Surg Am. 2010;92:2767–2775. doi: 10.2106/JBJS.I.01401. [DOI] [PubMed] [Google Scholar]
- 2.Wallace R.G., Heyes G.J., Michael A.L. The non-operative functional management of patients with a rupture of the tendo Achillis leads to low rates of re-rupture. J Bone Joint Surg Br. 2011;93:1362–1366. doi: 10.1302/0301-620X.93B10.26187. [DOI] [PubMed] [Google Scholar]
- 3.Westin O., Nilsson Helander K., Gravare Silbernagel K., et al. Acute ultrasonography investigation to predict reruptures and outcomes in patients with an Achilles tendon rupture. Orthop J Sports Med. 2016;4 doi: 10.1177/2325967116667920. 2325967116667920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chalmers J. Review article: Treatment of Achilles tendon ruptures. J Orthop Surg (Hong Kong) 2000;8:97–99. doi: 10.1177/230949900000800118. [DOI] [PubMed] [Google Scholar]
- 5.Young S.W., Patel A., Zhu M., et al. Weight-bearing in the nonoperative treatment of acute achilles tendon ruptures: A randomized controlled trial. J Bone Joint Surg Am. 2014;96:1073–1079. doi: 10.2106/JBJS.M.00248. [DOI] [PubMed] [Google Scholar]
- 6.Saltzman C.L., Tearse D.S. Achilles tendon injuries. J Am Acad Orthop Surg. 1998;6:316–325. doi: 10.5435/00124635-199809000-00007. [DOI] [PubMed] [Google Scholar]
- 7.Park Y.H., Kim T.J., Choi G.W., et al. Achilles tendinosis does not always precede Achilles tendon rupture. Knee Surg Sports Traumatol Arthrosc. 2019;27:3297–3303. doi: 10.1007/s00167-018-5172-3. [DOI] [PubMed] [Google Scholar]
- 8.Maffulli N., Longo U.G., Kadakia A., et al. Achilles tendinopathy. Foot Ankle Surg. 2020;26:240–249. doi: 10.1016/j.fas.2019.03.009. [DOI] [PubMed] [Google Scholar]
- 9.Tallon C., Maffulli N., Ewen S.W. Ruptured Achilles tendons are significantly more degenerated than tendinopathic tendons. Med Sci Sports Exerc. 2001;33:1983–1990. doi: 10.1097/00005768-200112000-00002. [DOI] [PubMed] [Google Scholar]
- 10.Maffulli N., Longo U.G., Maffulli G.D., et al. Marked pathological changes proximal and distal to the site of rupture in acute Achilles tendon ruptures. Knee Surg Sports Traumatol Arthrosc. 2011;19:680–687. doi: 10.1007/s00167-010-1193-2. [DOI] [PubMed] [Google Scholar]
- 11.Yinger K., Mandelbaum B.R., Almekinders L.C. Achilles rupture in the athlete. Current science and treatment. Clin Podiatr Med Surg. 2002;19:231–250. doi: 10.1016/s0891-8422(02)00002-2. v. [DOI] [PubMed] [Google Scholar]
- 12.Lee D.K. A preliminary study on the effects of acellular tissue graft augmentation in acute Achilles tendon ruptures. J Foot Ankle Surg. 2008;47:8–12. doi: 10.1053/j.jfas.2007.08.015. [DOI] [PubMed] [Google Scholar]
- 13.Zellers J.A., Pohlig R.T., Cortes D.H., et al. Achilles tendon cross-sectional area at 12 weeks post-rupture relates to 1-year heel-rise height. Knee Surg Sports Traumatol Arthrosc. 2020;28:245–252. doi: 10.1007/s00167-019-05608-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Arya S., Kulig K. Tendinopathy alters mechanical and material properties of the Achilles tendon. J Appl Physiol (1985) 2010;108:670–675. doi: 10.1152/japplphysiol.00259.2009. [DOI] [PubMed] [Google Scholar]
- 15.Finnamore E., Waugh C., Solomons L., et al. Transverse tendon stiffness is reduced in people with Achilles tendinopathy: A cross-sectional study. PLoS One. 2019;14 doi: 10.1371/journal.pone.0211863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang Y.J., Zhang C., Wang Q., et al. Augmented versus nonaugmented repair of acute achilles tendon rupture: A systematic review and meta-analysis. Am J Sports Med. 2018;46:1767–1772. doi: 10.1177/0363546517702872. [DOI] [PubMed] [Google Scholar]
- 17.Lieberman J.R., Lozman J., Czajka J., et al. Repair of Achilles tendon ruptures with Dacron vascular graft. Clin Orthop Relat Res. 1988;234:204–208. [PubMed] [Google Scholar]
- 18.Giannini S., Girolami M., Ceccarelli F., et al. Surgical repair of Achilles tendon ruptures using polypropylene braid augmentation. Foot Ankle Int. 1994;15:372–375. doi: 10.1177/107110079401500705. [DOI] [PubMed] [Google Scholar]
- 19.Fernandez-Fairen M., Gimeno C. Augmented repair of Achilles tendon ruptures. Am J Sports Med. 1997;25:177–181. doi: 10.1177/036354659702500206. [DOI] [PubMed] [Google Scholar]
- 20.Hosey G., Kowalchick E., Tesoro D., et al. Comparison of the mechanical and histologic properties of Achilles tendons in New Zealand white rabbits secondarily repaired with Marlex mesh. J Foot Surg. 1991;30:214–233. [PubMed] [Google Scholar]
- 21.Humadi S. A comparative study between kessler suture versus polypropylene mesh implantation to repair tenotomized common calcaneal tendon in rabbits. Iraqi J Veterinary Sci. 2019;33:289–296. [Google Scholar]
- 22.Fridman R., Rahimi F., Lucas P., Daugherty R., Hoffmann H. Repair of neglected Achilles tendon rupture with monofilament polypropylene mesh: A case study of 12 patients. Foot Ankle J. 2008;1:2. [Google Scholar]
- 23.Nilsson-Helander K., Thomee R., Silbernagel K.G., et al. The Achilles tendon Total Rupture Score (ATRS): Development and validation. Am J Sports Med. 2007;35:421–426. doi: 10.1177/0363546506294856. [DOI] [PubMed] [Google Scholar]
- 24.Kitaoka H.B., Alexander I.J., Adelaar R.S., et al. Clinical rating systems for the ankle-hindfoot, midfoot, hallux, and lesser toes. Foot Ankle Int. 1994;15:349–353. doi: 10.1177/107110079401500701. [DOI] [PubMed] [Google Scholar]
- 25.Mullaney M.J., McHugh M.P., Tyler T.F., et al. Weakness in end-range plantar flexion after Achilles tendon repair. Am J Sports Med. 2006;34:1120–1125. doi: 10.1177/0363546505284186. [DOI] [PubMed] [Google Scholar]
- 26.Navarro-Perez J., Orozco-Beltran D., Gil-Guillen V., et al. Mortality and cardiovascular disease burden of uncontrolled diabetes in a registry-based cohort: The ESCARVAL-risk study. BMC Cardiovasc Disord. 2018;18:180. doi: 10.1186/s12872-018-0914-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Orishimo K.F., Schwartz-Balle S., Tyler T.F., et al. Can weakness in end-range plantar flexion after Achilles tendon repair be prevented? Orthop J Sports Med. 2018;6 doi: 10.1177/2325967118774031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.van der Vlist A.C., Breda S.J., Oei E.H.G., et al. Clinical risk factors for Achilles tendinopathy: A systematic review. Br J Sports Med. 2019;53:1352–1361. doi: 10.1136/bjsports-2018-099991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Spaans A.J., van Heeswijk E.J., Arnold D.E., et al. Foreign body reaction associated with polyethylene mesh interposition used for treatment of trapeziometacarpal osteoarthritis: Report of 8 cases. J Hand Surg Am. 2014;39:2016–2019. doi: 10.1016/j.jhsa.2014.07.038. [DOI] [PubMed] [Google Scholar]
- 30.Basiglini L., Iorio R., Vadala A., et al. Achilles tendon surgical revision with synthetic augmentation. Knee Surg Sports Traumatol Arthrosc. 2010;18:644–647. doi: 10.1007/s00167-010-1085-5. [DOI] [PubMed] [Google Scholar]
- 31.Mohamed A., Oliva F., Nardoni S., et al. Failed synthetic graft after acute Achilles tendon repair. Muscles Ligaments Tendons J. 2017;7:396–402. doi: 10.11138/mltj/2017.7.2.396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ozaki J., Fujiki J., Sugimoto K., et al. Reconstruction of neglected Achilles tendon rupture with Marlex mesh. Clin Orthop Relat Res. 1989;238:204–208. [PubMed] [Google Scholar]
- 33.Choksey A., Soonawalla D., Murray J. Repair of neglected Achilles tendon ruptures with Marlex mesh. Injury. 1996;27:215–217. doi: 10.1016/0020-1383(95)00214-6. [DOI] [PubMed] [Google Scholar]
- 34.Zellers J.A., Carmont M.R., Gravare Silbernagel K. Return to play post-Achilles tendon rupture: A systematic review and meta-analysis of rate and measures of return to play. Br J Sports Med. 2016;50:1325–1332. doi: 10.1136/bjsports-2016-096106. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.