Abstract
In mammals, trait variation is often reported to be greater among males than females. However, to date, mainly only morphological traits have been studied. Energy expenditure represents the metabolic costs of multiple physical, physiological, and behavioral traits. Energy expenditure could exhibit particularly high greater male variation through a cumulative effect if those traits mostly exhibit greater male variation, or a lack of greater male variation if many of them do not. Sex differences in energy expen- diture variation have been little explored. We analyzed a large database on energy expenditure in adult humans (1494 males and 3108 females) to investigate whether humans have evolved sex differences in the degree of interindividual variation in energy expenditure. We found that, even when statistically comparing males and females of the same age, height, and body composition, there is much more variation in total, activity, and basal energy expenditure among males. However, with aging, variation in total energy expenditure decreases, and because this happens more rapidly in males, the magnitude of greater male variation, though still large, is attenuated in older age groups. Considerably greater male variation in both total and activity energy expenditure could be explained by greater male variation in levels of daily activity. The considerably greater male variation in basal energy expenditure is remarkable and may be explained, at least in part, by greater male variation in the size of energy-demanding organs. If energy expenditure is a trait that is of indirect interest to females when choosing a sexual partner, this would suggest that energy expenditure is under sexual selection. However, we present a novel energetics model demonstrating that it is also possible that females have been under stabilizing selection pressure for an intermediate basal energy expenditure to maximize energy available for reproduction.
1. Introduction
Greater variability in males over females has been a topic of interest for at least a century and a half (Darwin, 1871). Empirical studies on intersex differences in trait variability have typically shown greater male variability (GMV), especially for morphological and cognitive traits in mammals. For example, male red deer are more variable in size (Clutton-Brock et al., 1982), male guenons have more variable skull sizes (Cardini and Elton, 2017), and male chimpanzees have greater variation in brain structure (DeCasien et al., 2020). Humans show clear GMV in a range of morphological, physical, and cognitive traits. Morphological traits include birth weight, adult weight and height, body mass index, and brain structure (Lehre et al., 2009; Ritchie et al., 2017; Wierenga et al., 2017, 2020; Forde et al., 2020). Physical performance traits include such as sprinting speed (Lehre et al., 2009) and various measures of muscle strength (Wilmore, 1974; cf. no difference in variability in grip strength; Mathiowetz et al., 1985). Cognitive traits include creativity (Karwowski et al., 2016; He and Wong, 2021), general knowledge (Feingold, 1992), and a gamut of intellectual faculties (Hedges and Nowell, 1995; Strand et al., 2006; Machin and Pekkarinen, 2008; Lohman and Lakin, 2009; Baye and Monseur, 2016). Greater male variability even exists in economically rele- vant decision making such as trading off costs and benefits, and actions in the face of risk ((Thoni and Volk, 2021).
One hypothesis for GMV is heterogamy - the occurrence of homogametic sex chromosomes in one sex and heterogametic sex chromosomes in the other. In the homogametic sex of such species, the traits affected by the sex chromosomes are influenced by the averaging of two parental copies, whereas in the heterogametic sex the effects are not averaged. Consequently, the heterogametic sex is expected to exhibit greater variability in those traits than is the homogametic sex. This explanation has been well evidenced by a meta-analysis of body size measurements for a diverse range of species by Reinhold and Engqvist (2013), who found that in the two taxa represented in the data set where males are heterogametic (mammals and some insects), males are typically more variable in body size, whereas in the two taxa with heterogametic females (birds and butterflies), the females typically exhibit greater variance (but see Wyman and Rowe, 2014; Branch et al., 2020).
However, new work by Zajitschek et al. (2020) on lab-raised wild mice casts doubt on the ubiquity of GMV in mammals. To date, by far the most common type of trait investigated has been morphological, in particular body size (Reinhold and Engqvist, 2013), but of course morphology represents only one facet of an organism. While Zajitschek et al. (2020) found more variance in males for morpho- logical traits, they observed more variance in females than males for eye traits and immunological traits. Moreover, no systematic sex differences in trait variance were found for physiological, behavioral, and hematological traits. The effects of the laboratory environment may influence trait variability - perhaps a more homogenous environment for the study population attenuated sex difference in variance for many traits. Nonetheless, the findings of Zajitschek et al. (2020) suggest that whether differences in trait variability between the sexes represent GMV can depend on the trait type in question.
Why might morphological traits exhibit GMV while many other traits do not? One alternate hypothesis for GMV, though not mutually exclusive to heterogamy, which may answer this question, centers on sexual selection. Across the animal kingdom, the rate at which fertility increases with the number of matings secured is greater for males than females (Janicke et al., 2016), as a result of anisogamy (Bateman, 1948). This underpins the fact that both reproductive fitness and mating success are more variable in males than females (Rowe and Houle, 1996), with some males having a much higher number of offspring than others and many having none (reproductive skew; Trivers, 1972; Clutton-Brock and Vincent, 1991). This includes humans - men have experienced greater reproductive variability than women throughout most of our species’ evolutionary history including the present (Labuda et al., 2010; Betzig, 2012; Wilson et al., 2017). Thus, traits that increase the male’s chances of obtaining successful matings will be particularly strongly selected for, that is, males are under stronger sexual selection than females (Pomiankowski and Møller, 1995; Cuervo and Møller, 2001), perhaps an exception being species that are fully monogamous (Branch et al., 2020). Sexually selected traits are costly to produce, thus they depend on the overall ‘condition’ of the bearer (Cuervo and Møller, 2001; Bonduriansky, 2007). In turn, they will tend to be variable because overall ‘condition’ is likely to be based on numerous loci and thus harbor high genetic variance (Rowe and Houle, 1996; Tomkins et al., 2004). In cases where males are under stronger sexual selection than females, given that highly sexually selected traits tend to be larger in males than females - e.g., body size in mammals (Møller and Pomiankowski, 1993) - and thus more contingent on ‘condition,’ sexual traits are expected to vary more in males than females.
Animals are limited in the energy they can expend and yet doing so is necessary for every process of their bodies from tissue syn- thesis/maintenance and immune function to the beating of the heart and limb movement. Given, therefore, that an animal’s energy expenditure results from the combination of many physiological, morphological, and behavioral adaptations, if enough of those adaptations exhibit GMV (at least some may be sexually selected for, increasing the chance of GMV particularly in mam- mals; Rowe and Houle, 1996; Tomkins et al. 2004; Reinhold and Engqvist, 2013), then in turn we would expect to see GMV in energy expenditure. Indeed, the GMV could be sizeable due to a cumulative effect. Alternatively, energy expenditure may lack GMV because enough of the traits underpinning it do not themselves exhibit GMV.
Testing for differences in trait variability between the sexes is often logistically challenging because sample size must be large, and this may explain why most studies to date have assessed morpho- metric variation, which is relatively easy to quantify. Commonly, GMV is about 10% (i.e., males typically exhibit about 10% more variance than females; Reinhold and Engqvist, 2013), and modeling demonstrates that the total number of animals sampled needs to breach 1000 for that magnitude of GMV in the population to be reliably observed; below this threshold, the chances of detecting GMV can be low (Cardini and Elton, 2017; Branch et al., 2020). Data sets of such size are rare, particularly for measurements of meta- bolism in mammals (Sadowska et al., 2005; Wone et al., 2009). We are aware of just one publicly available data set of energy expendi- ture with a sample size of suitable magnitude for investigating GMV. The International Atomic Energy Agency (IAEA) doubly-labeled water (DKW) database is a worldwide collection of total energy expenditure (TEE) measurements for humans (Speakman et al., 2019) accompanied by measurements of basal energy expenditure (BEE; the minimum amount of energy required for the functioning e.g., breathing, and the maintenance e.g., tissue turnover, of vital systems) and body morphometric and composition variables. We take advantage of this data to investigate whether humans have evolved sex differences in the degree of interindividual variation in energy expenditure. We compared the variability in several aspects of energy expenditure between the sexes, with and without statistically accounting for morphological traits that correlate with energy expenditure and thus might potentially drive the variability in energy expenditure observed. Thus, we were able both to 1) test for GMV in several absolute measures of energy expenditure, and 2) explore whether a putative presence of GMV is explained because of variation in morphological traits that correlate with energy expenditure or is present despite accounting for morphological correlates.
2. Materials and methods
2.1. Study subjects
The IAEA DLW database v. 3.1.2 (Speakman et al., 2019) is the largest collection of TEE measurements ever made on humans using the gold-standard stable isotope method of DLW analysis (Lifson et al., 1955; Speakman, 1997). For the present study, the database was restricted to adult subjects >18 years of age. We excluded subjects undergoing interventions involving energy intake, frequent intense physical activity including professional sports training, and those who were pregnant, lactating, or with a diagnosis for disease. Each person was present only once; there were no repeated measures. The data set for analysis comprised TEE measurements for 3108 females and 1494 males. A full set of unique participant identifiers are provided in Supplementary Online Material (SOM) Table S1. Mean age was 52.6 years for females and 47.5 years for males. The percentage of females aged <30 years was 19.7%, >30 and <60 years was 33.7%, and >60 years was 46.6%. The equivalents for males were 22.1, 48.5, and 29.3%, respectively. Total energy expenditure is known to be fairly constant with age as an adult until about 60 years of age and then declines (Pontzer et al., 2021, their Fig. 2A). Given that the ages of the females in the data set are spread across a wider range than are the males, we would expect a measure of GMV to be attenuated. How- ever, we seek to control for age effects on mean TEE within the analyses. Although the data represent 30 countries, 65% of the data are for people in the United States, and the great majority are for people in Western countries (defined as countries in Europe and the ‘Anglosphere’; see SOM Table S2 for the lists of countries designated as Western and non-Western). The participants represented in the database were recruited as volunteers into intervention studies. The data pertain only to control groups or pre-interventions. Not all TEE observations have a paired BEE measure, and therefore the sample size for the BEE and activity energy expenditure (AEE; calculated as 0.9*TEE - BEE) analyses was lower (n ¼ 1063 females and 693 males).
Figure 2.
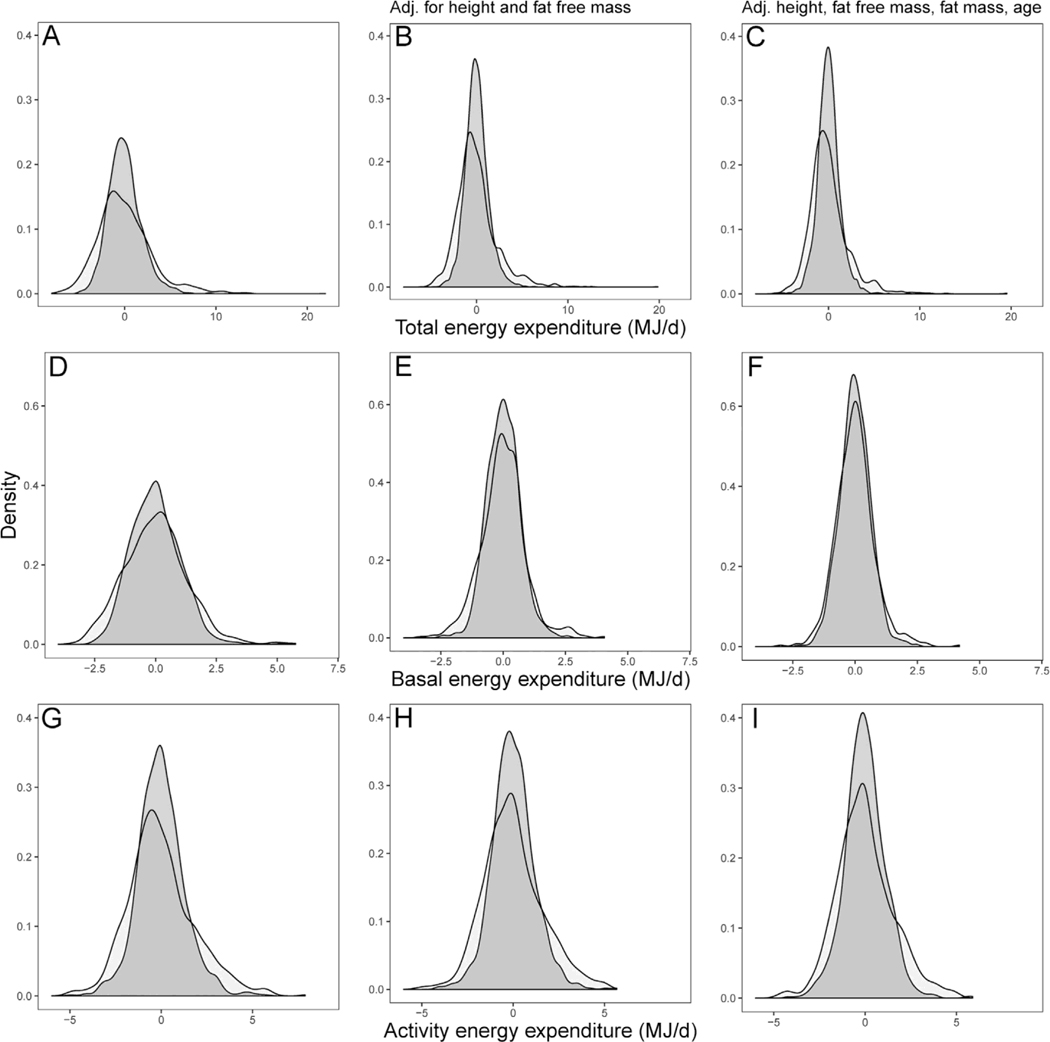
Histograms of total energy expenditure (TEE; AeC), basal energy expenditure (BEE; DeF), and activity energy expenditure (AEE; GeI) for adult males (dark grey) and females (light grey). A) Absolute TEE; B) TEE adjusted for height and fat-free mass (set 1); C) TEE adjusted for height, fat-free mass, fat mass, and age (set 2). D) Absolute BEE; E) BEE adjusted for height and fat-free mass (set 1); F) BEE adjusted for height, fat-free mass, fat mass, and age (set 2). G) Absolute AEE; H) AEE adjusted for height and fat-free mass (set 1); I) AEE adjusted for height, fat-free mass, fat mass, and age (set 2). In each panel, male variance is greater than female variance.
The data reported in this study cannot be deposited in a public repository because they are held by the management group of the IAEA DLW database. The instructions to request access are available at https://doubly-labelled-water-database.iaea.org/dataAnalysisInstructions. Any additional information required to reanalyze the data reported in this article can be requested from the corresponding author upon request. For the syntax needed to reproduce results and figures presented in this article, see SOM S1 for the R script.
2.2. Data
For all study participants, fat-free mass was derived from isotope dilution and fat mass was calculated as the difference between body mass and fat-free mass. For a subset of the TEE measurements (1063 females and 693 males), a paired measure of BEE (i.e., the minimum energy required to keep the body functioning; McNab, 1997; Frappell and Butler, 2004) was taken using indirect calorimetry. To qualify as ‘basal,’ BEE measurements must be performed on resting, postabsorptive subjects within their thermoneutral zone, such that BEE eliminates any source of variation related to behavior, digestion, and thermoregulation. We therefore used this subset of the data to test for GMV not only in BEE but also in AEE. Activity energy expenditure was calculated as 0.9*TEE e BEE, assuming that diet- induced energy expenditure accounts for 10% of the total energy budget (Westerterp, 2004). Total energy expenditure, BEE, and AEE are all reported as MJ•d-1. We also included in our analyses the following traits as variables that differ both on average and in magnitude of variance between the sexes, and can correlate with energy expenditure: height (cm), fat-free mass (kg), fat mass (kg), and age (y) (Heymsfield et al., 2007; Pontzer et al., 2021).
2.3. Statistical analyses
All analyses were conducted in R v. 3.5.3 (R Core Team, 2021). We quantified variance in male and female energy expenditure using Bayesian general linear models based on Monte-Carlo Markov chain (MCMC) models using the ‘MCMCglmm’ package (Hadfield, 2010). Each model included one trait as the response variable (TEE, BEE, or AEE) and sex as an independent variable, with no intercept fitted so that the model returned separate mean estimates for males and females (Schielzeth, 2010). Using the ‘idh’ function, we also allowed the residual variance to be different in males and females. We also ran models with height, fat-free mass, fat mass, or age as the dependent variable (with only sex as the independent variable) to estimate mean and variance differences in males and females for these variables. All models included country as a random effect to account for the unequal sampling distribution across countries.
The first models were run without covariates. We then further investigated the source of variation in TEE, BEE, and AEE by rerun- ning the models and extracting sex-specific means and variances after including three different sets of morphometric covariates. The first set of covariates (‘set 1’) included height and fat-free mass as morphometric covariates, whereas the second set of covariates (‘set 2’) also included fat mass and age; the number of covariates present did not affect sample size. When included as covariates, the three morphometric variables and age were centered so that model estimates were estimated at the center of the distribution of the covariates (Schielzeth, 2010). To make sure that the sex differences in variance were not due to confounding nonlinear age and body composition effects, for example, energy expenditure decreasing with age but only beyond 60 years (Pontzer et al., 2021), the third set of covariates (‘set 3’) included quadratic effects for age, fat mass, and fat-free mass. We also included sex as an interaction term with the linear and quadratic terms for age, fat mass, and fat-free mass. Given that the effect of body composition on TEE, BEE, and AEE might be nonlinear with respect to age, we also included interactions be- tween the two body composition variables (fat mass and fat-free mass) and the linear and quadratic terms for age.
We also wanted to test whether variance in TEE changed according to age (heterogeneity of variance) in males and females separately; a statistical effect that is not accounted for by including linear or quadratic terms for age, or interactions between age and other terms, in the models. To do this, we recoded age as a four-level categorical variable with roughly equal sample sizes in each age category. We then reran the model for TEE with the third set of covariates while estimating separate residual variances for each of the four age categories in males and females. Finally, we also wanted to test whether variance in TEE was different in Western countries compared to the rest of the countries sampled. To do this, we recoded country as a two-level categorical variable (Western country or non-Western country) and reran the model for TEE with the third set of covariates while estimating separate residual variances in males and females for Western countries and non-Western countries. The ethnic makeup of Western and non-Western countries based on the available data are presented in SOM Table S3. In brief, the majority of participants in the non-Western group are African, whereas a quarter are Asian; in the Western group, the great majority are Caucasian and nearly a fifth are African American.
Models were run using MCMCglmm default priors. Posterior distributions were estimated from 260,000 MCMC iterations sampled at 200 iteration intervals following an initial burn-in period of 60,000 iterations. Autocorrelations were less than 0.1 for all estimates, which yielded effective sample sizes close to 1000 for all estimates (range ¼ 833e1361). We visually inspected plots of the traces and posterior distributions as well as calculated the autocorrelation among samples to make sure that all models had properly converged.
From each model, we calculated the posterior mode and 95% highest posterior density credible intervals (CIs) for the means and variances in males and females. We also wanted to standardize the variance in each sex with respect to the observed mean differences. To do so, we calculated the relative variance by dividing the sex- specific variance by the sex-specific mean estimates in each of the 1000 posterior MCMC samples, and then calculating the posterior mode and 95% CIs. Treating these as 95% confidence intervals, in cases where the CIs for the relative variances of the two sexes do not overlap, the evidence of a difference in variance between the sexes is strong (Belia et al., 2005). We also investigated differences in variation in each trait between the sexes by calculating the ratio of the male:female variance. Note that the sample size is larger in females than males. To assess whether this imbalance affected the results, we reran the third set of models after randomly deleting observations in females so that the sample size is the same as in males.
Finally, to gain a visual representation of the trait variance in males vs. females, we reran each model above using standard multiple linear regression using the lm() function in R, with the caveat that these models did not account for the country of data collection. Residuals generated from each model were then used to graphically represent the effects of accounting for morphometric variables on the variance in energy expenditure for males and females. Thus, for example, in the case of TEE, we plotted a histogram of TEE for each sex separately, then one of TEE accounting for height and fat-free body mass (set 1), and finally one of TEE ac- counting for height, fat-free mass, fat mass, and age (set 2).
3. Results
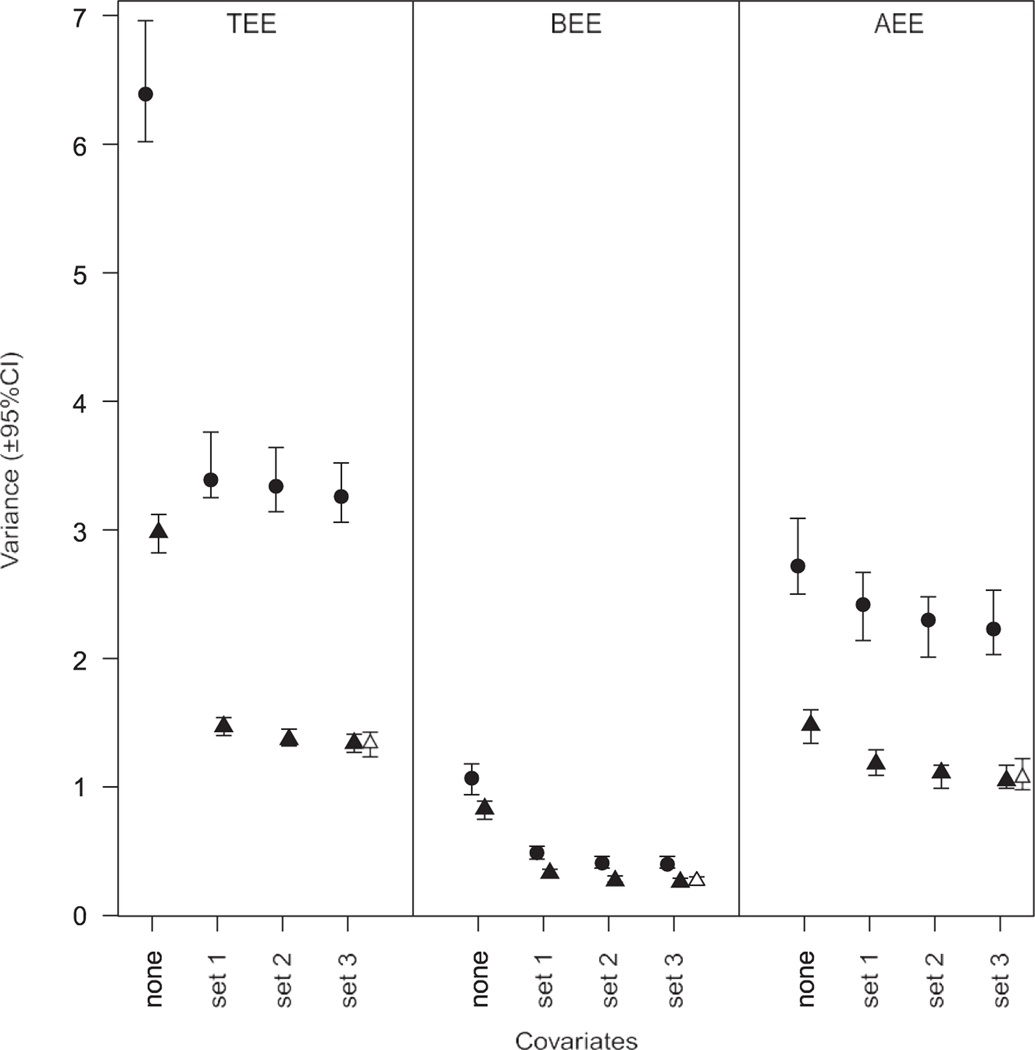
Overall, TEE was 2.20 times more variable in males than females (Table 1; Figs. 1A and 2A). Males also have higher mean TEE than females, yet relative variance (standardizing for mean differences) was still 1.67 times higher in males than females (Table 1). Height and fat-free mass - two strong determinants of TEE - were also more variable in males than females (Table 1). Accordingly, including height and fat-free mass within the model (set 1) decreased the overall residual variance in TEE. Nonetheless, TEE (adjusted for height and fat-free mass) was 2.38 times more variable in males than females (Table 1; Figs. 1A and 2B). By contrast, variance in fat mass and age was lower in males than females in our sample (Table 1). Adding fat mass and age as covariates (set 2) had negligible effect on residual variance in TEE (Table 1; Figs. 1A and 2C). The 95% CIs around the estimates of variance for males and females do not overlap, providing strong support that male variance is greater than female variance in TEE (Fig. 1A).
Table 1.
Variances, relative variance (standardizing for mean differences), and the male:female variance ratio of height, fat-free mass, fat mass, age, total energy expenditure (TEE), basal energy expenditure (BEE), and activity energy expenditure (AEE).
| Trait | Covariates | Variance (95% CI) | CV (95% CI) | Variance ratio (95% CI) | ||
|---|---|---|---|---|---|---|
|
|
|
|||||
| Males | Females | Males | Females | |||
| Height (cm) | None | 48.92 (45.96e52.96) | 43.34 (41.45e45.65) | 0.28 (0.26e0.31) | 0.30 (0.26e0.28) | 1.12 (1.04e1.24) |
| Fat-free mass (kg) | None | 69.79 (64.42e74.12) | 43.98 (41.31e45.57) | 1.23 (1.15e1.34) | 1.06 (0.97e1.12) | 1.59 (1.48e1.75) |
| Fat mass (kg) | None | 107.29 (100.75e115.45) | 142.59 (133.79e148.52) | 6.54 (5.70e8.41) | 6.78 (5.90e8.07) | 0.75 (0.69e0.83) |
| Age (y) | None | 245.36 (224.87e261.2) | 327.1 (312.34e345.32) | 6.74 (5.44e7.88) | 8.49 (6.99e9.78) | 0.73 (0.68e0.82) |
| TEE (MJ d−1) | None | 6.54 (6.03e7.01) | 2.97 (2.83e3.12) | 0.50 (0.45e0.55) | 0.30 (0.27e0.32) | 2.18 (1.98e2.37) |
| TEE (MJ d−1) | Set 1 | 3.45 (3.22e3.73) | 1.46 (1.40e1.54) | 0.31 (0.28e0.34) | 0.13 (0.12e0.14) | 2.35 (2.16e2.59) |
| TEE (MJ d−1) | Set 2 | 3.37 (3.12e3.61) | 1.38 (1.31e1.45) | 0.31 (0.28e0.33) | 0.12 (0.12e0.14) | 2.43 (2.22e2.67) |
| TEE (MJ d−1) | Set 3 | 3.31 (3.06e3.54) | 1.33 (1.28e1.41) | 0.29 (0.27e0.32) | 0.12 (0.11e0.13) | 2.38 (2.24e2.68) |
| BEE (MJ d−1) | None | 1.07 (0.95e1.19) | 0.82 (0.75e0.89) | 0.15 (0.13e0.17) | 0.14 (0.12e0.15) | 1.26 (1.10e1.50) |
| BEE (MJ d−1) | Set 1 | 0.49 (0.44e0.55) | 0.33 (0.30e0.36) | 0.08 (0.07e0.09) | 0.05 (0.05e0.06) | 1.56 (1.31e1.75) |
| BEE (MJ d−1) | Set 2 | 0.41 (0.37e0.45) | 0.28 (0.26e0.31) | 0.06 (0.06e0.07) | 0.04 (0.04e0.05) | 1.45 (1.28e1.68) |
| BEE (MJ d−1) | Set 3 | 0.39 (0.37e0.45) | 0.26 (0.24e0.29) | 0.06 (0.05e0.07) | 0.04 (0.04e0.05) | 1.50 (1.31e1.72) |
| AEE (MJ d−1) | None | 2.83 (2.44e3.04) | 1.45 (1.35e1.60) | 0.71 (0.57e0.85) | 0.46 (0.37e0.60) | 1.86 (1.64e2.16) |
| AEE (MJ d−1) | Set 1 | 2.38 (2.16e2.66) | 1.16 (1.07e1.27) | 0.71 (0.58e0.85) | 0.33 (0.28e0.40) | 1.98 (1.76e2.31) |
| AEE (MJ d−1) | Set 2 | 2.28 (2.01e2.50) | 1.08 (0.99e1.17) | 0.70 (0.59e0.90) | 0.32 (0.28e0.41) | 2.04 (1.79e2.37) |
| AEE (MJ d−1) | Set 3 | 2.22 (2.03e2.51) | 1.08 (0.98e1.16) | 0.70 (0.55e0.88) | 0.34 (0.27e0.40) | 2.10 (1.83e2.37) |
For all traits, variances were estimated in the raw data (no covariates). For TEE, BEE, and AEE, variances were also estimated in the residuals after accounting for three sets of covariates including height and fat-free mass (set 1); height, fat-free mass, fat mass, and age (set 2); and height, fat-free mass, fat mass, age, and sex-specific nonlinear age and body composition effects (set 3). Variance ratios >1 and <1, respectively, imply greater male and greater female variance. Also shown are the 95% credible intervals (CIs).
Figure 1.
Male (closed circles) and female (closed triangles) variance for A) total energy expenditure (TEE), B) basal energy expenditure (BEE), and C) activity energy expenditure (AEE). While within sex variance differs considerably between TEE, BEE, and AEE, in each case, male variance is greater than female variance. Values shown are the posterior modes with 95% highest posterior density credible intervals (CI) for the sex-specific residual variance estimates extracted from models that did not include covariates (‘none’), and models that included three sets of covariates such as height and fat-free mass (set 1), height, fat-free mass, fat mass, and age (set 2), and height, fat-free mass, fat mass, age, and sex-specific nonlinear age and body composition effects (set 3). The open symbols show the variance estimates in females obtained from an analysis restricted to a data set in which sample size was randomly reduced to equal that of males.
Variance in BEE and AEE within each sex was lower than in TEE, especially for BEE (Table 1; Fig. 1B, C). Nonetheless, GMV in these traits was still sizeable. Overall, BEE and AEE were, respectively, 1.30 and 1.95 times more variable in males than females (Table 1). Moreover, similarly to TEE, the fold-difference in variance slightly increased when including covariates (Table 1; Figs. 1B, C and 2D–I). In each of these cases, the 95% CIs around the variance estimates for males and females do not overlap, clarifying the strong evidence that male variance is greater than female variance in BEE and AEE.
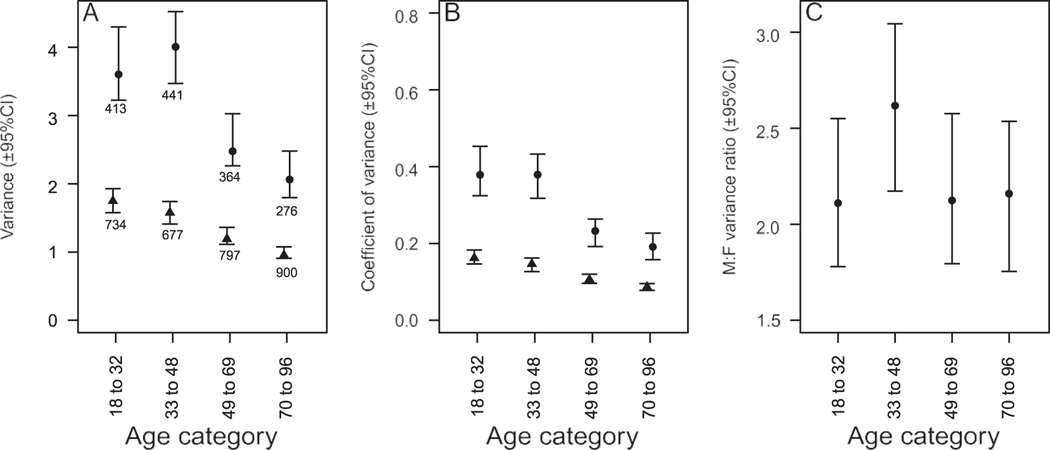
The model for TEE with age-specific residual variances revealed that, although there is a somewhat more pronounced decrease in TEE variance in males than females (Fig. 3A, B), there were no clear age differences in the male:female variance ratios (Fig. 3C).
Figure 3.
Age-specific A) variance and B) coefficient of variance in total energy expenditure in males (circles) and females (triangles) and C) male:female variance ratio. The amount of greater male variability (GMV) remains fairly constant across age categories. Values shown are the posterior modes with 95% highest posterior density credible intervals (95% CIs) extracted from a single model that included multiple covariates and sex-specific nonlinear aging and body condition effects. Numbers shown below the symbols are the sample sizes for each category.
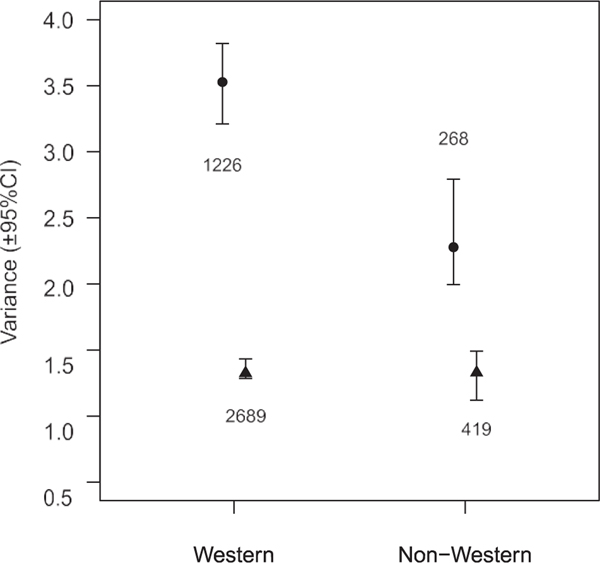
There was a markedly lower TEE variance in males from non- Western countries compared to Western countries but no difference in females, resulting in a reduced but still considerable GMV in non-Western compared to Western countries (Fig. 4).
Figure 4.
Male (circles) and female (triangles) variance for total energy expenditure disaggregated into Western countries and non-Western countries. Values shown are the posterior modes with 95% highest posterior density credible intervals (CIs) for the sex-specific residual variance estimates extracted from models that included height, fat-free mass, fat mass, age, and sex-specific nonlinear age and body composition ef- fects, and country as a random factor. Numbers shown by the symbols are the sample sizes for each category. Variance in total energy expenditure among males is much higher in Western than non-Western countries, whereas the variance among females is similar in Western and non-Western countries; thus, the magnitude of greater male variability is decreased in non-Western countries.
4. Discussion
Our study represents a first exploration of GMV in energy expenditure, a trait that captures the net effect of many morpho- logical, physiological, and behavioral factors. Our results indicate considerable GMV in human energy expenditure in terms of TEE, BEE, and AEE (Table 1; Figs. 1 and 2), although the data cannot distinguish between the two prominent explanations for GMV, heterogamy and sexual selection, since both explanations predict greater trait variance in males than females. We also found GMV in key measures of body condition associated with energy expenditure: height and in particular fat-free mass (Heymsfield et al., 2007; Pontzer et al., 2021; Table 1). Height and fat-free mass correlate strongly with energy expenditure in humans (Cameron et al., 2016; Hopkins et al., 2016; Thomas et al., 2019), raising the possibility that GMV in energy expenditure is simply a result of the GMV in those morphometric traits. However, while statistically accounting for height and fat-free mass considerably reduced the within-sex variance in all three measures of energy expenditure in both males and females (with no clear, systematic, additional reduction in variance when accounting for fat mass and age), the variance ratio between males and females did not systematically decrease. In fact, statistically accounting for these morphometric variables and age resulted in a slight increase in the male:female variance ratio in all three measures of energy expenditure. In other words, remarkably, even when attempting to compare, with statistics, males and females of the same height, fat-free mass, fat mass, and age, males exhibit far more variation in TEE, BEE, and AEE than do females.
The fact that the considerable GMV in energy expenditure is not explained by variation in age, body morphometrics, and condition - key correlates of energy expenditure (Heymsfield et al., 2007; Pontzer et al., 2021) - indicates that GMV in energy expenditure is affected by other factors. It has long been known that behavioral traits are important drivers of energy expenditure. Indeed, when Lavoisier (1743e1794) first started measuring metabolic rate more than 225 years ago, it became immediately clear that organisms spend a lot more energy when active than when resting (Lighton, 2008). Activity levels are more variable in males than females consistently across diverse cultures (Althoff et al., 2017), along with hours slept per night (Ban and Lee, 2001), hours spent sitting (Parsons et al., 2009), and aerobic capacity (Olds et al., 2006), which probably explains why males have more vari- able AEE than do females. In turn, this is probably reflected in TEE given that AEE constitutes 33% of TEE in the current sample of adult humans (Careau et al., 2021).
In contrast to AEE, BEE by definition all but eliminates the direct effect of behavior on metabolic measurements. The fact that we observed considerable GMV in BEE, and even after factoring out body size and composition, is particularly surprising (though a similar finding can be calculated for resting energy expenditure in 104 adult males and 155 adult females having adjusted for fat-free mass; Müller et al., 2011, their Table 1). It suggests that males are more variable than females in the maintenance costs of some of the physiological components that underpin BEE and which are not reflected in measures of fat-free mass. Although hormonal differ- ences could be a factor (Wu and O’Sullivan 2011; Wang and Xu, 2019), the proximate explanation must be the energy expenditures of the various physiological components of the body. More than 80% of the interindividual variance in BEE in humans is explained by the major body systems (Müller et al., 2018), and the remaining factors probably include the immune system (Buttgereit et al., 2000; Wolowczuk et al., 2008) and the digestive systems, including the influence of the gut microbiota on anaerobic resting metabolism (Riedl et al., 2017; Müller et al., 2018). Although there is no evidence that the mass-independent energy expenditures of various individual organs exhibit GMV (Müller et al., 2013), key elements of the cardiorespiratory system such as heart mass and lung vital capacity vary in size more in males than females (Lauer et al., 1992; Müller et al., 2011; Wierenga et al., 2017), as do two other energy- demanding systems (Müller et al., 2013), the brain (Wierenga et al., 2017) and the kidneys (Gong et al., 2012; cf. Müller et al., 2011), though probably not the liver (Chouker et al., 2004; Müller et al., 2011; Patzak et al., 2014). The spleen also exhibits GMV (Spielmann et al., 2005; Hosey et al., 2006; Müller et al., 2011), as perhaps does ‘residual mass’ which includes bone, skin, stomach, intestines, and glands (Müller et al., 2013). There is also evidence that mitochondrial energetics in response to low metabolic de- mands vary more in males, as does the abundance of different mitochondrial proteins in skeletal muscle, although sample sizes are fairly small and such studies are in vitro; thus extrapolation of the findings to resting muscles must be tentative (Miotto et al., 2018; Monaco et al., 2020). Blood parameters more often show GMV than the reverse (Lehre et al., 2009), though two reported measures in that study which one might a priori posit show GMV but exhibit the reverse are thyroid-stimulating hormone and tetraiodothyronine. Core temperature, albeit subtly, also exhibits GMV (Chamberlain et al., 1995).
Even though activity is eliminated from the BEE measurements, GMV in activity might still have an indirect role to play in generating GMV in BEE due to training effects. For example, regular exercise is known to increase heart size, mitochondrial count, and blood volume (McArdle et al., 2015), decrease levels of certain hormones and cytokines (Node et al., 2010; Silverman and Deuster, 2014; Pontzer, 2018), and improve mitochondrial oxidative capacity (Cardinale et al., 2018). One possibility, then, is that males have evolved to exhibit considerably more interindividual variation in AEE than have females, and that this drives greater variability in both BEE and TEE. High energy expenditure is related to various traits that are arguably attractive to females. High BEE for a given size and body condition could positively correlate with aerobic fitness (Poehlman et al., 1989), cognitive capacity (Goncerzewicz et al., 2022), or organ function (Müller et al., 2018). High AEE is associated with high levels of physical activity, and also strength and muscle mass, characteristics known to be attractive to females or at least asso- ciated with gaining access to females (Schulte-Hostedde et al., 2008; Neave et al., 2011; Lidborg et al., 2022), in part because these characteristics signal physical fitness (Sharp et al., 1992), athletic ability, and thus competitiveness (Hugill et al., 2010), and also access to high levels of energy resources (Bonduriansky, 2007). If so, then energy expenditure does have a sexual signal component, which would associate with greater variation in males. In turn, by viewing everyday energy expenditure in adults ultimately as reproductive investment (directly and indirectly; Key and Ross, 1999), some males are investing considerably more energy in (anticipated) reproduction than are others, whereas in contrast, the variation between females in terms of energy investment in their potential reproduction is much smaller.
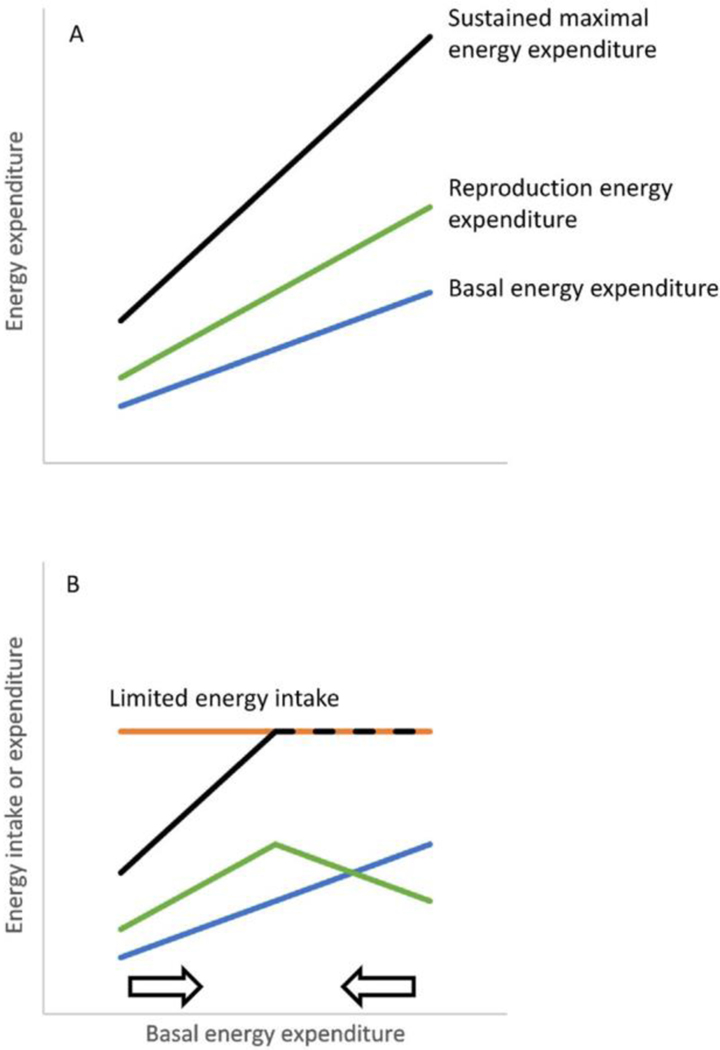
However, an alternative explanation for GMV in energy expenditure arises through consideration not of why male variation is greater but why female variation is lower. That is, why might females have undergone stabilizing selection - both low and high energy expenditures being selected against over time? Maximal sustained energy expenditure is intrinsically constrained at a fixed multiple of BEE, both in animals (Drent and Daan, 1980; Peterson et al., 1990) and humans (Hammond and Diamond, 1997; Thurber et al., 2019). Thus, people with a higher BEE will tend to be those with a higher maximal sustained energy expenditure. For females, this could be advantageous during lactation because it would allow them to expend more energy on reproduction (Fig. 5A). However, if there is an external constraint on sustained energy expenditure due to limited food supply, then the energy available for female reproduction would follow a peaked function with BEE (Fig. 5B). In turn, females with either high or low BEE would be selected against because this would be associated with submaximal energy being expended on reproduction. Because BEE is the dominant component of nonreproductive energy expenditure, reduced variation in BEE results in reduced variation in TEE when females are not reproducing.
Figure 5.
Conceptual model of energy availability during pregnancy in relation to basal energy expenditure (BEE). A) Sustained maximal energy expenditure is a multiple of BEE. Consequently, the energy potentially available for reproduction (calculated as sustained maximal energy expenditure minus BEE) is higher in females with a higher BEE. B) If food availability is limited, then energy intake can create a limit to sustained maximal energy expenditure (dashed line) and in turn, energy available for reproduction is not only low for females with a low BEE but also for females with a high BEE; it is highest when BEE is an intermediate value. The arrows denote selection against the extremes of low BEE and high BEE.
Although our study indicates that GMV is a robust phenomenon in human energy expenditure, we cannot assume that the magnitude of GMV is consistent across all human populations and cultures. Indeed, there is a tentative indication in the present data that people from non-Western countries exhibit a still substantial but reduced GMV, due to a reduction in male variability (Fig. 4). It could be that there are features of Western cultures/societies that serve to exacerbate or attenuate GMV although what these could be are not immediately obvious (perhaps, for example, more time and money enable the sexes to pursue hobbies and lifestyles, e.g., Stoet and Geary, 2018, that contrast in terms of energy expenditure). Cultural variations in the magnitude of GMV might indicate that heterogamy is at best only part of the underlying mechanism but do not offer evidence for or against sexual selection as a predominant mechanism underlying GMV because sexual selection can have strong cultural components (Nakahashi, 2017).
5. Conclusions
The large data set at our disposal enabled us not only to robustly test for GMV in humans in an as yet untested suite of traits - various aspects of energy expenditure - but also to investigate whether GMV in energy expenditure is explained by GMV in correlated morphological traits. In our sample, they are not, and while GMV in behavior likely directly explains much of the GMV in activity and thus TEE, we are left with the intriguing possibility that males exhibit much more variation than do females in the energy demands of their major body systems, the most likely proximate drivers being greater variation in the size of those organs perhaps coupled with greater variation in mitochondrial efficiency. There are several potential explanations for this GMV in humans. Given that men experience greater reproductive variability than women, and that TEE variance decreases with age more in men than women, one possibility is that these traits are under sexual selection, and indeed energy expenditure is related to other traits known to be assessed by women during mate choice such as physical capacity - characteristics that can be expected to correlate with reproductive success. We also suggest another, novel explanation for GMV in energy expenditure based on putative restricted energy availability experienced by females during the height of reproduction.
Supplementary Material
Acknowledgments
The DLW database, which can be found at https://doubly-labelled-waterdatabase.iaea.org/home, is hosted by the IAEA and generously supported by Taiyo Nippon Sanso and SERCON. We are grateful to the IAEA and these companies for their support and especially to Takashi Oono for his tremendous efforts at fundraising on our behalf. The authors also gratefully acknowledge funding from the Chinese Academy of Sciences (CAS153E11KYSB20190045) to J.R.S. and the US National Science Foundation (BCS-1824466) awarded to H.P. JAY is supported by the Intramural Research Program of the NICHD (ZIAHD00641).
Footnotes
Supplementary Online Material
Supplementary online material to this article can be found on- line at https://doi.org/10.1016/j.jhevol.2022.103229.
Conflicts of interest
The authors have no conflicts of interest to declare.
References
- Althoff T, Sosi c R, Hicks JL, King AC, Delp SL, Leskovec J, 2017. Large-scale physical activity data reveal worldwide activity inequality. Nature 547, 336e339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ban DJ, Lee TJ, 2001. Sleep duration, subjective sleep disturbances and associated factors among university students in Korea. J. Korean Med. Sci. 16, 475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bateman AJ, 1948. Intra-sexual selection in drosophila. Heredity 2, 349e368. [DOI] [PubMed] [Google Scholar]
- Baye A, Monseur C, 2016. Gender differences in variability and extreme scores in an international context. Large-scale Assess. Educ 4, 1. [Google Scholar]
- Belia S, Fidler F, Williams J, Cumming G, 2005. Researchers misunderstand confidence intervals and standard error bars. Psychol. Methods 10, 389. [DOI] [PubMed] [Google Scholar]
- Betzig L, 2012. Means, variances, and ranges in reproductive success: Comparative evidence. Evol. Hum. Behav. 33, 309e317. [Google Scholar]
- Bonduriansky R, 2007. The evolution of condition-dependent sexual dimorphism. Am. Nat. 169, 9e19. [DOI] [PubMed] [Google Scholar]
- Branch CL, Sonnenberg BR, Pitera AM, Benedict LM, Kozlovsky DY, Bridge ES, Pravosudov VV, 2020. Testing the greater male variability phe- nomenon: Male mountain chickadees exhibit larger variation in reversal learning performance compared with females. Proc. R. Soc. B Biol. Sci. 287, 20200895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buttgereit F, Burmester G-R, Brand MD, 2000. Bioenergetics of immune func- tions: Fundamental and therapeutic aspects. Immunol. Today 21, 194e199. [DOI] [PubMed] [Google Scholar]
- Cameron JD, Sigal RJ, Kenny GP, Alberga AS, Prud’homme D, Phillips P, Doucette S, Goldfield G, 2016. Body composition and energy intakedskeletal muscle mass is the strongest predictor of food intake in obese adolescents: The HEARTY trial. Appl. Physiol. Nutr. Metabol. 41, 611e617. [DOI] [PubMed] [Google Scholar]
- Cardinale DA, Larsen FJ, Schiffer TA, Morales-Alamo D, Ekblom B, Calbet JA, Holmberg H-C, Boushel R, 2018. Superior intrinsic mitochondrial respiration in women than in men. Front. Physiol. 9, 1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardini A, Elton S, 2017. Is there a “Wainer’s rule”? Testing which sex varies most as an example analysis using GueSDat, the free Guenon Skull Database. Hystrix 28, 147e156. [Google Scholar]
- Careau V, Halsey LG, Pontzer H, Ainslie PN, Andersen LF, Anderson LJ, Arab L, Baddou I, Bedu-Addo K, Blaak EE, Blanc S, Bonomi AG, Bouten CVC, Buchowski MS, Butte NF, Camps SGJA, Close GL, Cooper JA, Das SK, Cooper R, Dugas LR, Eaton SD, Ekelund U, Entringer S, Forrester T, Fudge BW, Goris AH, Gurven M, Hambly C, El Hamdouchi A, Hoos MB, Hu S, Joonas N, Joosen AM, Katzmarzyk P, Kempen KP, Kimura M, Kraus WE, Kushner RF, Lambert EV, Leonard WR, Lessan N, Martin CK, Medin AC, Meijer EP, Morehen JC, Morton JP, Neuhouser ML, Nicklas TA, Ojiambo RM, Pietila€inen KH, Pitsiladis YP, Plange-Rhule J, Plasqui G, Prentice RL, Rabinovich RA, Racette SB, Raichlen DA, Ravussin E, Reilly JJ, Reynolds RM, Roberts SB, Schuit AJ, Sjo€din AM, Stice E, Urlacher SS, Valenti G, Van Etten LM, Van Mil EA, Wells JCK, Wilson G, Wood BM, Yanovski J, Yoshida T, Zhang X, Murphy-Alford AJ, Loechl CU, Luke AH, Rood J, Sagayama H, Schoeller DA, Wong WW, Yamada Y, Speakman JR, 2021. Energy compensation and adiposity in humans. Curr. Biol. 31, 4659e4666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chamberlain JM, Terndrup TE, Alexander DT, Silverstone FA, Wolf-Klein G, O’Donnell R, Grandner J, 1995. Determination of normal ear temperature with an infrared emission detection thermometer. Ann. Emerg. Med. 25, 15e20. [DOI] [PubMed] [Google Scholar]
- Chouker A, Martignoni A, Dugas M, Eisenmenger W, Schauer R, Kaufmann I, Schelling G, Lo€he F, Jauch KW, Peter K, 2004. Estimation of liver size for liver transplantation: The impact of age and gender. Liver Transpl 10, 678e685. [DOI] [PubMed] [Google Scholar]
- Clutton-Brock TH, Vincent ACJ, 1991. Sexual selection and the potential reproductive rates of males and females. Nature 351, 58e60. [DOI] [PubMed] [Google Scholar]
- Clutton-Brock TH, Guinness FE, Albon SD, 1982. Red Deer: Behaviour and Ecology of Two Sexes. Cambridge University Press, Cambridge, UK. [Google Scholar]
- Cuervo JJ, Møller AP, 2001. Components of phenotypic variation in avian ornamental and non-ornamental feathers. Evol. Ecol. 15, 53e72. [Google Scholar]
- Darwin C, 1871. The Descent of Man, and Selection in Relation to Sex. John Murray, London. [Google Scholar]
- DeCasien AR, Sherwood CC, Schapiro SJ, Higham JP, 2020. Greater variability in chimpanzee (Pan troglodytes) brain structure among males. Proc. R. Soc. B Biol. Sci. 287, 20192858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drent R, Daan S, 1980. The prudent parent: Energetic adjustments in avian breeding. Ardea 68, 225e252. [Google Scholar]
- Feingold A, 1992. Sex differences in variability in intellectual abilities: A new look at an old controversy. Rev. Educat. Res. 61, 61e84. [Google Scholar]
- Forde NJ, Jeyachandra J, Joseph M, Jacobs GR, Dickie E, Satterthwaite TD, Shinohara RT, Ameis SH, Voineskos AN, 2020. Sex differences in variability of brain structure across the lifespan. Cereb. Cortex 30, 5420e5430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frappell PB, Butler PJ, 2004. Minimal metabolic rate, what it is, its usefulness, and its relationship to the evolution of endothermy: A brief synopsis. Phys. Biochem. Zool. 77, 865e868. [DOI] [PubMed] [Google Scholar]
- Goncerzewicz A, Go’rkiewicz T, Dzik JM, Ję drzejewska-Szmek J, Knapska E, Konarzewski M, 2022. Brain size, gut size and cognitive abilities: The energy trade-offs tested in artificial selection experiment. Proc. R. Soc. B 289, 20212747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong H, Hwang J, Choi DK, Lee SR, Hong YK, Hong JY, Park DS, Jeon HG, 2012. Relationship among total kidney volume, renal function and age. J. Urol. 187, 344e349. [DOI] [PubMed] [Google Scholar]
- Hadfield JD, 2010. MCMC methods for multi-response generalized linear mixed models: The MCMCglmm R package. J. Stat. Softw. 33, 1e22. [Google Scholar]
- Hammond KA, Diamond J, 1997. Maximal sustained energy budgets in humans and animals. Nature 386, 457e462. [DOI] [PubMed] [Google Scholar]
- He W. j., Wong W. c., 2021. Gender differences in the distribution of creativity scores: Domain-specific patterns in divergent thinking and creative problem solving. Front. Psychol. 12, 571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedges L, Nowell A, 1995. Sex differences in mental test scores, variability, and numbers of high-scoring individuals. Science 269, 41e45. [DOI] [PubMed] [Google Scholar]
- Heymsfield S, Childers D, Beetsch J, Allison D, Pietrobelli A, 2007. Body size and human energy requirements: Reduced mass-specific resting energy expenditure in tall adults. J. Appl. Physiol. 103, 1543e1550. [DOI] [PubMed] [Google Scholar]
- Hopkins M, Finlayson G, Duarte C, Whybrow S, Ritz P, Horgan G, Blundell J, Stubbs R, 2016. Modelling the associations between fat-free mass, resting metabolic rate and energy intake in the context of total energy balance. Int. J. Obes. 40, 312. [DOI] [PubMed] [Google Scholar]
- Hosey RG, Mattacola CG, Kriss V, Armsey T, Quarles J, Jagger J, 2006. Ultrasound assessment of spleen size in collegiate athletes. Br. J. Sports Med. 40, 251e254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hugill N, Fink B, Neave N, 2010. The role of human body movements in mate selection. Evol. Psychol. 8, 66e89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janicke T, H€aderer IK, Lajeunesse MJ, Anthes N, 2016. Darwinian sex roles confirmed across the animal kingdom. Sci. Adv. 2, e1500983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karwowski M, Jankowska DM, Gralewski J, Gajda A, Wi’sniewska E, Lebuda I, 2016. Greater male variability in creativity: A latent variables approach. Think. Skills Creat. 22, 159e166. [Google Scholar]
- Key C, Ross C, 1999. Sex differences in energy expenditure in non-human primates. Proc. R. Soc. B Biol. Sci. 266, 2479e2485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labuda D, Lefebvre J-F, Nadeau P, Roy-Gagnon M-H, 2010. Female-to-male breeding ratio in modern humans: An analysis based on historical re- combinations. Am. J. Hum. Genet. 86, 353e363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauer MS, Levy D, Anderson KM, Plehn JF, 1992. Is there a relationship between exercise systolic blood pressure response and left ventricular mass? The Framingham Heart Study. Ann. Intern. Med. 116, 203e210. [DOI] [PubMed] [Google Scholar]
- Lehre AC, Lehre KP, Laake P, Danbolt NC, 2009. Greater intrasex phenotype variability in males than in females is a fundamental aspect of the gender differences in humans. Dev. Psychobiol. 51, 198e206. [DOI] [PubMed] [Google Scholar]
- Lidborg LH, Cross CP, Boothroyd LG, 2022. A meta-analysis of the association between male dimorphism and fitness outcomes in humans. eLife 11, e65031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lifson N, Gordon GB, McClintock R, 1955. Measurement of total carbon dioxide production by means of D18O. J. Appl. Physiol. 7, 704e710. [DOI] [PubMed] [Google Scholar]
- Lighton J, 2008. Measuring Metabolic Rates. Oxford University Press, Oxford. [Google Scholar]
- Lohman DF, Lakin JM, 2009. Consistencies in sex differences on the cognitive abilities test across countries, grades, test forms, and cohorts. Br. J. Educ. Psychol. 79, 389e407. [DOI] [PubMed] [Google Scholar]
- Machin S, Pekkarinen T, 2008. Global sex differences in test score variability. Science 322, 1331e1332. [DOI] [PubMed] [Google Scholar]
- Mathiowetz V, Kashman N, Volland G, Weber K, Dowe M, Rogers S,1985. Grip and pinch strength: Normative data for adults. Arch. Phys. Med. Rehabil. 66, 69e74. [PubMed] [Google Scholar]
- McArdle W, Katch F, Katch V, 2015. Training for anaerobic and aerobic power. In: McArdle W, Katch F, Katch V. (Eds.), Exercise Physiology. Wolters Kluwer, Baltimore and Philadelphia, pp. 461e497. [Google Scholar]
- McNab BK, 1997. On the utility of uniformity in the definition of basal rate of metabolism. Physiol. Zool. 70, 718e720. [DOI] [PubMed] [Google Scholar]
- Miotto PM, McGlory C, Holloway TM, Phillips SM, Holloway GP, 2018. Sex differences in mitochondrial respiratory function in human skeletal muscle. Am. J. Physiol. Regul. Integr. Comp. Physiol. 314, R909eR915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Møller AP, Pomiankowski A, 1993. Fluctuating asymmetry and sexual selection. Genetica 89, 267. [Google Scholar]
- Monaco CM, Bellissimo CA, Hughes MC, Ramos SV, Laham R, Perry CG, Hawke TJ, 2020. Sexual dimorphism in human skeletal muscle mitochondrial bioenergetics in response to type 1 diabetes. Am. J. Physiol. Endocrinol. Metab. 318, E44eE51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller MJ, Langemann D, Gehrke I, Later W, Heller M, Gluer C, Heymsfield S, Bosy-Westphal A, 2011. Effect of constitution on mass of individual organs and their association with metabolic rate in humansda detailed view on allometric scaling. PLoS One 6, e22732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller MJ, Wang Z, Heymsfield SB, Schautz B, Bosy-Westphal A, 2013. Advances in the understanding of specific metabolic rates of major organs and tissues in humans. Curr. Opin. Clin. Nutr. Metab. Care 16, 501e508. [DOI] [PubMed] [Google Scholar]
- Müller MJ, Geisler C, Hübers M, Pourhassan M, Braun W, Bosy-Westphal A, 2018. Normalizing resting energy expenditure across the life course in humans: Challenges and hopes. Eur. J. Clin. Nutr. 72, 628e637. [DOI] [PubMed] [Google Scholar]
- Nakahashi W, 2017. Cultural sexual selection in monogamous human populations. R. Soc. Open Sci. 4, 160946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neave N, McCarty K, Freynik J, Caplan N, Honekopp J, Fink B, 2011. Male dance moves that catch a woman’s eye. Biol. Lett. 7, 221e224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Node K, Michishita R, Tsuruta T, Shono N, Inoue T, 2010. Effect of exercise therapy on monocyte and neutrophil counts in overweight women. Am. J. Med. Sci. 339, 152e156. [DOI] [PubMed] [Google Scholar]
- Olds T, Tomkinson G, Le’ger L, Cazorla G, 2006. Worldwide variation in the performance of children and adolescents: An analysis of 109 studies of the 20-m shuttle run test in 37 countries. J. Sports Sci. 24, 1025e1038. [DOI] [PubMed] [Google Scholar]
- Parsons T, Thomas C, Power C, 2009. Estimated activity patterns in British 45 year olds: Cross-sectional findings from the 1958 British birth cohort. Eur. J. Clin. Nutr. 63, 978e985. [DOI] [PubMed] [Google Scholar]
- Patzak M, Porzner M, Oeztuerk S, Mason RA, Wilhelm M, Graeter T, Kratzer W, Haenle MM, Akinli AS, Group ES, 2014. Assessment of liver size by ultrasonography. J. Clin. Ultrasound 42, 399e404. [DOI] [PubMed] [Google Scholar]
- Peterson CC, Nagy KA, Diamond J, 1990. Sustained metabolic scope. Proc. Natl. Acad. Sci. USA 87, 2324e2328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poehlman ET, Melby CL, Badylak SF, Calles J, 1989. Aerobic fitness and resting energy expenditure in young adult males. Metabolism 38, 85e90. [DOI] [PubMed] [Google Scholar]
- Pomiankowski A, Møller AP, 1995. A resolution of the lek paradox. Proc. R. Soc. B 260, 21e29. [Google Scholar]
- Pontzer H, 2018. Energy constraint as a novel mechanism linking exercise and health. Physiology 33, 384e393. [DOI] [PubMed] [Google Scholar]
- Pontzer H, Yamada Y, Sagayama H, Ainslie PN, Andersen LF, Anderson LJ, Arab L, Baddou I, Bedu-Addo K, Blaak EE, et al. 2021. Daily energy expenditure through the human life course. Science 373, 808e812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- R Core Team, 2021. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna. URL. http://www.R-project.org/. [Google Scholar]
- Reinhold K, Engqvist L, 2013. The variability is in the sex chromosomes. Evolution 67, 3662e3668. [DOI] [PubMed] [Google Scholar]
- Riedl RA, Atkinson SN, Burnett CML, Grobe JL, Kirby JR, 2017. The gut microbiome, energy homeostasis, and implications for hypertension. Curr. Hypertens. Rep. 19, 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritchie SJ, Cox SR, Shen X, Lombardo MV, Reus LM, Alloza C, Harris MA, Alderson H, Hunter S, Neilson E, Liewald DC, Auyeung B, Whalley HC, Lawrie SM, Gale CR, Bastin ME, McIntosh AM, Deary IJ, 2017. Sex differences in the adult human brain: Evidence from 5216 UK Biobank participants. Cereb. Cortex 28, 2959e2975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowe L, Houle D, 1996. The lek paradox and the capture of genetic variance by condition dependent traits. Proc. R. Soc. B 263, 1415e1421. [Google Scholar]
- Sadowska ET, Labocha MK, Baliga K, Stanisz A, Wro’blewska AK, Jagusiak W, Koteja P, 2005. Genetic correlations between basal and maximum metabolic rates in a wild rodent: Consequences for evolution of endothermy. Evolution 59, 672e681. [PubMed] [Google Scholar]
- Schielzeth H, 2010. Simple means to improve the interpretability of regression coefficients. Methods Ecol. Evol. 1, 103e113. [Google Scholar]
- Schulte-Hostedde AI, Eys MA, Johnson K, 2008. Female mate choice is influenced by male sport participation. Evol. Psychol. 6, 147470490800 600113. [Google Scholar]
- Sharp TA, Reed GW, Sun M, Abumrad N, Hill J, 1992. Relationship between aerobic fitness level and daily energy expenditure in weight-stable humans. Am. J. Physiol. Endocrinol. Metab. 263, E121eE128. [DOI] [PubMed] [Google Scholar]
- Silverman MN, Deuster PA, 2014. Biological mechanisms underlying the role of physical fitness in health and resilience. Interface Focus 4, 20140040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Speakman JR, 1997. Doubly Labelled Water: Theory and Practice. Chapman & Hall, London. [Google Scholar]
- Speakman JR, Pontzer H, Rood J, Sagayama H, Schoeller DA, Westerterp KR, Wong WW, Yamada Y, Loechl C, Murphy-Alford AJ, 2019. The international atomic energy agency international doubly labelled water database: Aims, scope and procedures. Ann. Nutr. Metab. 75, 114e118. [DOI] [PubMed] [Google Scholar]
- Spielmann AL, DeLong DM, Kliewer MA, 2005. Sonographic evaluation of spleen size in tall healthy athletes. Am. J. Roentgenol. 184, 45e49. [DOI] [PubMed] [Google Scholar]
- Strand S, Deary IJ, Smith P, 2006. Sex differences in cognitive abilities test scores: A UK national picture. Br. J. Educ. Psychol. 76, 463e480. [DOI] [PubMed] [Google Scholar]
- Stoet G, Geary DC, 2018. The gender-equality paradox in science, technology, engineering, and mathematics education. Psychol. Sci. 29, 581e593. [DOI] [PubMed] [Google Scholar]
- Thomas DM, Watts K, Friedman S, Schoeller DA, 2019. Modelling the metabolism: Allometric relationships between total daily energy expenditure, body mass, and height. Eur. J. Clin. Nutr. 73, 763e769. [DOI] [PubMed] [Google Scholar]
- Thurber C, Dugas LR, Ocobock C, Carlson B, Speakman JR, Pontzer H, 2019. Extreme events reveal an alimentary limit on sustained maximal human energy expenditure. Sci. Adv. 5, eaaw0341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thoni C, Volk S, 2021. Converging evidence for greater male variability in time, risk, and social preferences. Proc. Natl. Acad. Sci. USA 118, e2026112118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomkins JL, Radwan J, Kotiaho JS, Tregenza T, 2004. Genic capture and resolving the lek paradox. Trends Ecol. Evol. 19, 323e328. [DOI] [PubMed] [Google Scholar]
- Trivers RL, 1972. Parental investment and sexual selection. In: Campbell B. (Ed.), Sexual Selection and the Descent of Man: 1871–1971. Aldine Press, Chicago, pp. 136e179. [Google Scholar]
- Wang C, Xu Y, 2019. Mechanisms for sex differences in energy homeostasis. J. Mol. Endocrinol. 62, R129eR143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westerterp KR, 2004. Diet induced thermogenesis. Nutr. Metab. 1, 1e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wierenga LM, Doucet GE, Dima D, Agartz I, Aghajani M, Akudjedu TN, Albajes-Eizagirre A, Alnæs D, Alpert KI, Andreassen OA, 2020. Greater male than female variability in regional brain structure across the lifespan. Hum. Brain Mapp. 2020, 1e30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wierenga LM, Sexton JA, Laake P, Giedd JN, Tamnes CK, 2017. A key characteristic of sex differences in the developing brain: Greater variability in brain structure of boys than girls. Cereb. Cortex 28, 2741e2751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilmore JH, 1974. Alterations in strength, body composition and anthropometric measurements consequent to a 10-week weight training program. Med. Sci. Sports Exerc. 6, 133e138. [PubMed] [Google Scholar]
- Wilson ML, Miller CM, Crouse KN, 2017. Humans as a model species for sexual selection research. Proc. R. Soc. B 284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolowczuk I, Verwaerde C, Viltart O, Delanoye A, Delacre M, Pot B, Grangette C, 2008. Feeding our immune system: Impact on metabolism. Clin. Dev. Immunol. 2008, 1e19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wone B, Sears MW, Labocha MK, Donovan ER, Hayes JP, 2009. Genetic variances and covariances of aerobic metabolic rates. Proc. R. Soc. B 276, 3695e3704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu BN, O’Sullivan AJ, 2011. Sex differences in energy metabolism need to be considered with lifestyle modifications in humans. J. Nutr. Metab. 2011, 391809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyman MJ, Rowe L, 2014. Male bias in distributions of additive genetic, residual, and phenotypic variances of shared traits. Am. Nat. 184, 326e337. [DOI] [PubMed] [Google Scholar]
- Zajitschek SRK, Zajitschek F, Bonduriansky R, Brooks RC, Cornwell W, Falster DS, Lagisz M, Mason J, Senior AM, Noble DWA, Nakagawa S, 2020. Sexual dimorphism in trait variability and its eco-evolutionary and statistical implications. eLife 9, e63170. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.