Summary
Background
The SAVE-MORE trial demonstrated that anakinra treatment in COVID-19 pneumonia with plasma soluble urokinase plasminogen activator (suPAR) levels of 6 ng/mL or more was associated with 0.36 odds for a worse outcome compared to placebo when expressed by the WHO-Clinical Progression Scale (CPS) at day 28. Herein, we report the results of subgroup analyses and long-term outcomes.
Methods
This prospective, double-blind, randomised clinical trial, recruited patients with a confirmed SARS-CoV-2 infection, in need of hospitalisation, lower respiratory tract infection and plasma suPAR ≥6 ng/mL from 37 academic and community hospitals in Greece and Italy. Patients were 1:2 randomised to subcutaneous treatment with placebo or anakinra (100 mg) once daily for 10 days. Pre-defined subgroups of Charlson's comorbidity index (CCI), sex, age, level of suPAR, and time from symptom onset were analysed for the primary endpoint (overall comparison of distribution of frequencies of the scores from the WHO-CPS between treatments on day 28), by multivariable ordinal regression analysis in the intention to treat (ITT) population. This trial is registered with the EU Clinical Trials Register (2020-005828-11) and ClinicalTrials.gov (NCT04680949).
Findings
Patients were enrolled between 23 December 2020 and 31 March 2021; 189 patients in the placebo arm and 405 patients in the anakinra arm were the ITT population. Multivariable analysis showed that anakinra treatment was accompanied by significantly lower odds for worse outcome compared to placebo at day 28 for all studied subgroups (CCI ≥ 2, OR: 0.34, 95% confidence intervals [CI] 0.22–0.50; CCI < 2, OR: 0.38, 95% CI 0.21–0.68; suPAR > 9 ng/mL, OR: 0.35, 95% CI 0.19–0.66; suPAR 6–9 ng/mL, OR: 0.35, 95% CI 0.24–0.52; patients ≥65 years, OR: 0.41, 95% CI 0.25–0.66; and patients <65 years, OR: 0.29, 95% CI 0.19–0.45). The benefit was uniform, irrespective of the time from start of symptoms until the start of the study drug. At days 60 and 90, anakinra treatment had odds of 0.40 (95% CI 0.28–0.57) and 0.46 (95% CI 0.32–0.67) respectively, for a worse outcome compared to placebo. The costs of general ward stay, ICU stay, and drugs were lower with anakinra treatment.
Interpretation
Anakinra represents an important therapeutic tool in the management of COVID-19 that may be administered in all subgroups of patients; benefits are maintained until day 90.
Funding
Hellenic Institute for the Study of Sepsis; Swedish Orphan Biovitrum AB.
Keywords: suPAR, Anakinra, COVID-19, Co-morbidities, Cost, Clinical efficacy, Survival
Research in context.
Evidence before this study
Anakinra has been registered by the European Medicines Agency for the treatment of adults with COVID-19 pneumonia in need of oxygen at risk for progression into severe respiratory failure as this is defined by circulating levels of the biomarker suPAR (soluble urokinase plasminogen activator receptor) 6 ng/mL or more. The registration is based on the results of the double-blind randomized placebo-controlled SAVE-MORE trial. The trial showed 0.36 odds for worse outcome with anakinra treatment compared to placebo as outcome was assessed by the WHO-CPS at day 28. The impact of anakinra treatment on subgroups of patients and long-term outcomes by days 60 and 90 is assessed.
Added value of this study
Anakinra treatment provided similar significant benefit in all subgroups of patients defined by the Charlson's comorbidity index (≥2 or <2), level of suPAR (>9 ng/mL or ≤9 ng/mL), age (≥65 years or <65 years), sex and quartiles time from symptoms onset until start of the study drug (0–7 days; 8–9 days; 10–11 days; and >11 days). Favorable anakinra responses were maintained at days 60 and 90; 90-day survival was prolonged with anakinra treatment.
Implications of all the available evidence
Anakinra represents an important therapeutic tool in the management of COVID-19 being equally effective in all subgroups of patients.
Introduction
The infection by the SARS-CoV-2 coronavirus (COVID-19) has an unpredictable clinical course; patients might abruptly progress into severe respiratory failure (SRF), defined as a respiratory ratio (partial oxygen pressure (PaO2)/fraction of inspired oxygen (FiO2)) below 150 mmHg, requiring non-invasive ventilation (NIV) or mechanical ventilation (MV). Timely identification of patients at risk of progressing to severe disease and early initiation of targeted treatment are of utmost importance.1
We have previously shown that soluble urokinase plasminogen activator receptor (suPAR) serum concentrations can serve as an early predictor of SRF.1 In the case of COVID-19, patient stratification based on circulating suPAR concentrations can be a change in the paradigm of precision medicine for severe infections. This is because suPAR is not just a risk classifier but also a biomarker of the early activation of the interleukin (IL)-1 cascade.2 The SAVE-MORE trial evaluated a novel approach for the management of COVID-19, which depends on timely recognition of patients at risk for unfavorable outcome using suPAR circulating concentrations and subsequent delivery of targeted treatment with the recombinant IL-1 receptor antagonist anakinra for 10 days.3 At day 28, the adjusted proportional odds of having a worse clinical status (assessed by the 11-point World Health Organization Clinical Progression Scale (WHO-CPS)) with anakinra, as compared to placebo, was 0.36. These results prompted the European Medicines Agency (EMA) to approve in December 2021 the use of anakinra guided by suPAR for patients in need of either low-flow or high-flow oxygen.4 One emergency use authorization for anakinra was also recently provided by the Food and Drug Administration of the United States.5 Since suPAR is not commercially available in the United States, an alternative patient identification method was developed to select patients most likely to have suPAR ≥6 ng/mL based on eight commonly measured patient characteristics (Supplementary Table S1).5
The published primary analysis of the SAVE-MORE trial3 reported the efficacy and safety of anakinra treatment up to 28 days. As the scientific interest still needs to understand the disease and if clinical parameters may influence outcomes and treatment effect, we are reporting here subgroups analyses on sex, co-morbidities, levels of suPAR, treatment initiation time since onset of symptoms, as well as long-term efficacy at 60 and 90 days, and the impact of anakinra treatment on cost of hospitalisation.
Methods
Study design and participants
Report of the results of subgroup analyses of this trial is done in accordance to the CONSORT guidelines. SAVE-MORE was a prospective, double-blind randomised clinical trial conducted in 37 academic and community hospitals (29 in Greece and eight in Italy). The protocol and the analysis of the outcome of the patients until day 28 has been recently published.3 The protocol, stratification variables, and the statistical analysis plan (SAP) were discussed, reviewed, and agreed by the Emergency Task Force for COVID-19 (COVID-ETF) of the EMA. Study endpoints were set according to the advice received by COVID-ETF of the EMA. The protocol was approved by the National Ethics Committee of Greece (approval 161/20) and by the Ethics Committee of the National Institute for Infectious Diseases Lazzaro Spallanzani, IRCCS in Rome (01.02.2021).
Patients were recruited among those hospitalized for COVID-19 pneumonia. All patients or their legal representatives provided written informed consent before screening. In summary, patients were eligible if presented with confirmed infection by SARS-CoV-2 by molecular tests, in need for hospitalisation, radiological findings compatible with lower respiratory tract infection and plasma suPAR ≥6 ng/mL.
Randomisation and masking
Patients were randomised in a 1:2 ratio to receive treatment with placebo or anakinra. Stratified randomisation was followed using a computer-generated sequence utilizing as co-variables, severity of pneumonia by WHO, treatment with dexamethasone, body mass index (more than 30 kg/m2 or less than 30 kg/m2) and the country of enrolment (Greece or Italy).
Procedures
Details on interventions have been published.3 Briefly, treatment was either with placebo (0.9% sodium chloride) or 100 mg anakinra by subcutaneous injection once daily for 10 days. Study drug was prepared by an unblinded pharmacist with access to the electronic study system using a separate username and a password. Administration was done by a blind study nurse.
Outcomes
The primary outcome was the overall comparison of the distribution of frequencies of the scores from the 11-point WHO-CPS between the two arms of treatment on day 28. Main secondary endpoints were the changes of WHO-CPS scores at days 14 and 28 from the baseline (before start of the study drug); and the change of sequential organ failure assessment (SOFA) score at day 7 from baseline.
Analysed subgroups were: patients with Charlson's comorbidity index (CCI) ≥2 and patients with CCI <2; patients with suPAR >9 ng/mL and patients with suPAR 6–9 ng/mL; male and female patients; and patients aged ≥65 years and patients aged <65 years. For suPAR, the cut-off of 9 ng/mL was chosen for subgroup analysis in the original study design because unpublished data from the phase II SAVE trial which preceded the SAVE-MORE phase III trial6 showed worse outcomes for patients with suPAR >9 ng/mL. Another subgroup analysis, which was also a study secondary endpoint, was the effect of time from disease onset to the start of the study drug. This was defined as the time from onset of the first COVID-19-associated symptoms to the start of the study drug in days.
The study had three exploratory endpoints: distribution of frequencies of the 11-point WHO-CPS between the two arms of treatment at days 60 and 90 and the comparison of the cost of hospitalisation. Hospitalisation cost was calculated per patient in Euros as the sum of the cost of all administered drugs and the addition of the nominal cost of daily stay in the intensive care unit or in the general ward. The unit price for counted items derived from the official price list of the Greek government for patients participating in Greece and of the Italian government for patients participating in Italy (Supplementary Tables S2 and S3).
Statistical analysis
Target sample sizes were 200 and 400 for treatment with placebo and anakinra, respectively, for 90% power at the 5% significance level; this was calculated based on the findings from the phase 2 SAVE trial.3,6 Analyses of the primary endpoint and of the three secondary endpoints at day 28 within each subgroup were performed for the intent-to-treat population (ITT). Missing data were imputed by last observation carried forward (LOCF). According to the SAP, which was developed with the COVID-ETF of the EMA, the time interval between onset of symptoms and start of the study drug and its impact on the primary endpoint was analysed. The time interval was divided into quartiles and calculated for the entire study population. Separate comparisons were performed for each quartile between patients given placebo and those given anakinra. WHO-CPS is an ordinal 11-point variable ranging from 0 to 10, and comparisons were made by univariable and multivariable ordinal regression analyses using the logit function. Results were expressed as odds ratio (OR) and 95% confidence intervals (CI). As well as analysis within subgroup categories, the categories were tested for the homogeneity of their effect by adding interactions between the treatment group and the subgroup categories to the covariates of the ordinal regression analyses. According to advice from the EMA COVID-ETF, the variables used for stratified randomisation i.e. disease severity, intake of dexamethasone, BMI higher than 30 kg m2 and country were entered as co-variates in the multivariable model. The comparisons of the WHO-CPS at days 60 and 90 were made for the ITT population with imputation of missing values by LOCF. Survival was compared using Cox regression analysis for the ITT population. The proportionality of the hazards was checked using Schoenfeld's partial residuals analysis. The cost of hospitalisation was expressed as means and standard deviation and compared by the Student's t-test. The impact of the allocated intervention on the cost was confirmed by linear regression analysis including the above four co-variates in the equation. Analysis was conducted using IBM SPSS Statistics v28.0. All p values were two sided, and any p value less than 0.05 was considered statistically significant.
This trial is registered with the EU Clinical Trials Register (EudraCT, 2020-005828-11) and ClinicalTrials.gov (NCT04680949).
Role of the funding source
The trial was sponsored by the Hellenic Institute for the Study of Sepsis (HISS) and funded in part by HISS and in part by Swedish Orphan Biovitrum AB (Sobi). HISS was responsible for the design of the study, study conduct, analysis and interpretation of data, and decision to publish. Sobi had no role in study design, data collection, data analysis, data interpretation, or writing of the report.
A.K. and E.J.G.-B. have access to the dataset and have final responsibility for the decision to submit for publication.
Results
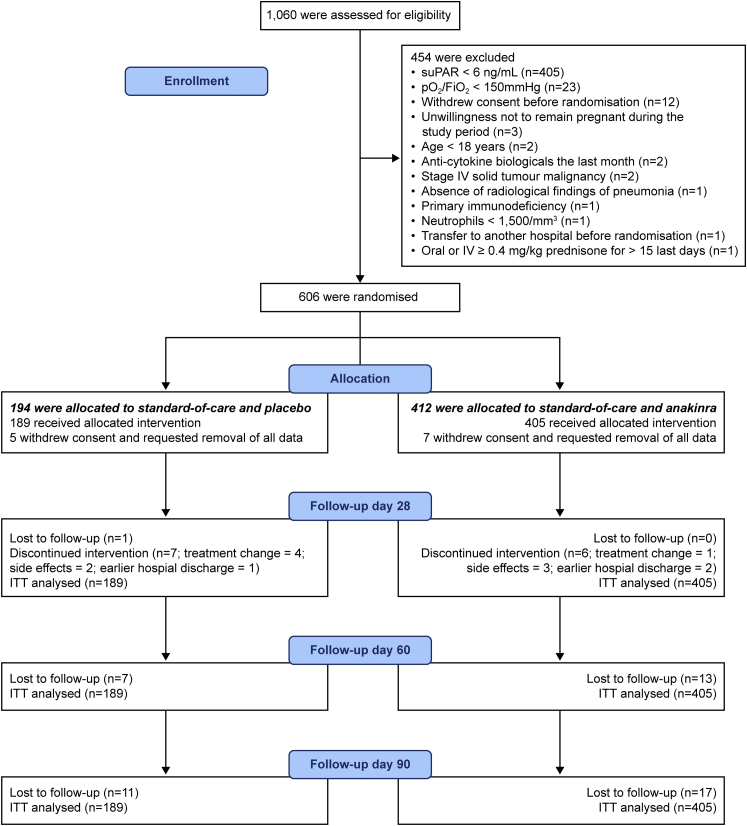
The flow-chart of the SAVE-MORE trial until day 90 of follow-up is provided in Fig. 1. The first patient was enrolled on 23 December 2020; the last patient was enrolled on 31 March 2021 and the last visit of the last patient was on 28 June 2021 when 90-day follow-up was completed. Of the 606 patients randomised, 194 were allocated to standard-of-care (SoC) and placebo, with 412 allocated to SoC and anakinra; five and seven patients withdrew consent and requested removal of all data, so that 189 and 405 patients were included in the ITT population, respectively. Twenty patients were lost to follow-up by day 60 and a further 8 by day 90. Baseline characteristics and co-administered treatments were similar between the two treatment arms, as previously shown.3
Fig. 1.
Flow chart of the SAVE-MORE trial until day 90.Abbreviations: FiO2, fraction of inspired oxygen; ITT, intent-to-treat; IV, intravenous; pO2, partial oxygen pressure; suPAR, soluble urokinase plasminogen activator receptor.
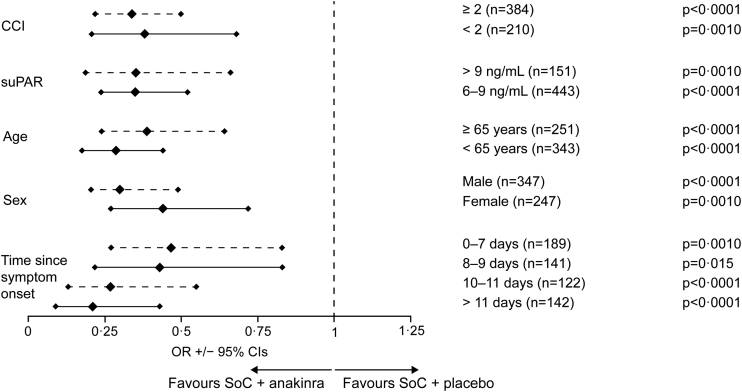
The analysis of the primary endpoint WHO-CPS at day 28 for all subgroups is provided in Fig. 2. Anakinra treatment was associated with decreased risk of having worse WHO-CPS score at day 28 for all studied subgroups. The benefit of anakinra treatment was apparent after multivariable ordinal regression analyses using the stratification factors for randomisation as independent variables i.e., WHO COVID-19 severity, intake of dexamethasone, body mass index (BMI) >30 kg/m2 and country. As shown in Fig. 2 and Supplementary Figs. S1–S12, after adjustment from multivariable analysis anakinra treatment was accompanied by 0.34 odds for worse outcome compared to placebo for patients with CCI ≥2 (95% CI 0.22–0.50); and 0.38 odds for worse outcome compared to placebo for patients with CCI <2 (95% CI 0.21–0.68). The respective odds were 0.35 (95% CI 0.19–0.66) for patients with suPAR >9 ng/mL and 0.35 for patients with suPAR 6–9 ng/mL (95% CI 0.24–0.52); 0.41 (95% CI 0.25–0.66) and 0.29 (95% CI 0.19–0.45) for patients aged ≥65 years and for patients <65 years; and 0.32 (95% CI 0.21–0.49) and 0.44 (95% CI 0.27–0.72) for male and female patients. The benefit of anakinra was consistent irrespective of the time from start of symptoms until the start of the study drug. The calculated quartiles of time were 0–7 days, 8–9 days, 10–11 days and >11 days, respectively. The odds of worse outcome from anakinra treatment was 0.47 (95% CI 0.27–0.83) compared to placebo for patients starting drug between 0 and 7 days from start of symptoms; 0.43 (95% CI 0.22–0.84) for patients starting drug between 8 and 9 days from start of symptoms; 0.27 (95% CI 0.13–0.55) for patients starting drug between 10 and 11 days from start of symptoms; and 0.21 (95% CI 0.09–0.43) for patients starting drug more than 11 days from start of symptoms. In parallel to this analysis within subgroup categories, the categories were tested for the homogeneity of their effect by adding interactions between the treatment group and the subgroup categories to the covariates of the ordinal regression analyses. There was no effect of subgroups on the primary endpoint (Supplementary Fig. S13).
Fig. 2.
Subgroup analysis of the primary endpoint. The primary endpoint of the SAVE-MORE trial is the distribution of the frequencies of the 11-point of the WHO-CPS at day 28. The figure shows the ORs for worse outcome among subgroups treated with SoC and anakinra compared to patients treated with SoC and placebo. Subgroups are defined according to the value of CCI, the levels of suPAR, age, sex and time since onset of symptoms and start of the study drug. The provided ORs are adjusted after multivariable ordinal regression analysis which includes dexamethasone treatment, WHO COVID-19 severity, body mass index and country as co-variates. Only one patient was lost to follow-up by day 28 and this missing value was imputed for analysis by LOCF. Abbreviations: CCI, Charlson's comorbidity index; CI, confidence interval; LOCF, last observation carried forward; OR, odds ratio; SoC, standard-of-care; suPAR, soluble urokinase plasminogen activator receptor; WHO-CPS, World Health Organization Clinical Progression Scale.
The time since hospital admission was analysed post-hoc as a variable which may impact the primary endpoint. Patients were divided into those who started the study drug on the first day of hospital admission (n = 164 patients); on the second day after hospital admission (n = 228 patients); and after the second day from hospital admission (n = 202 patients). Ordinal regression analysis was repeated entering the interaction between group of treatment and subgroup of time since hospital admission as co-variate; there was no subgroup effect on the primary endpoint (Supplementary Table S4).
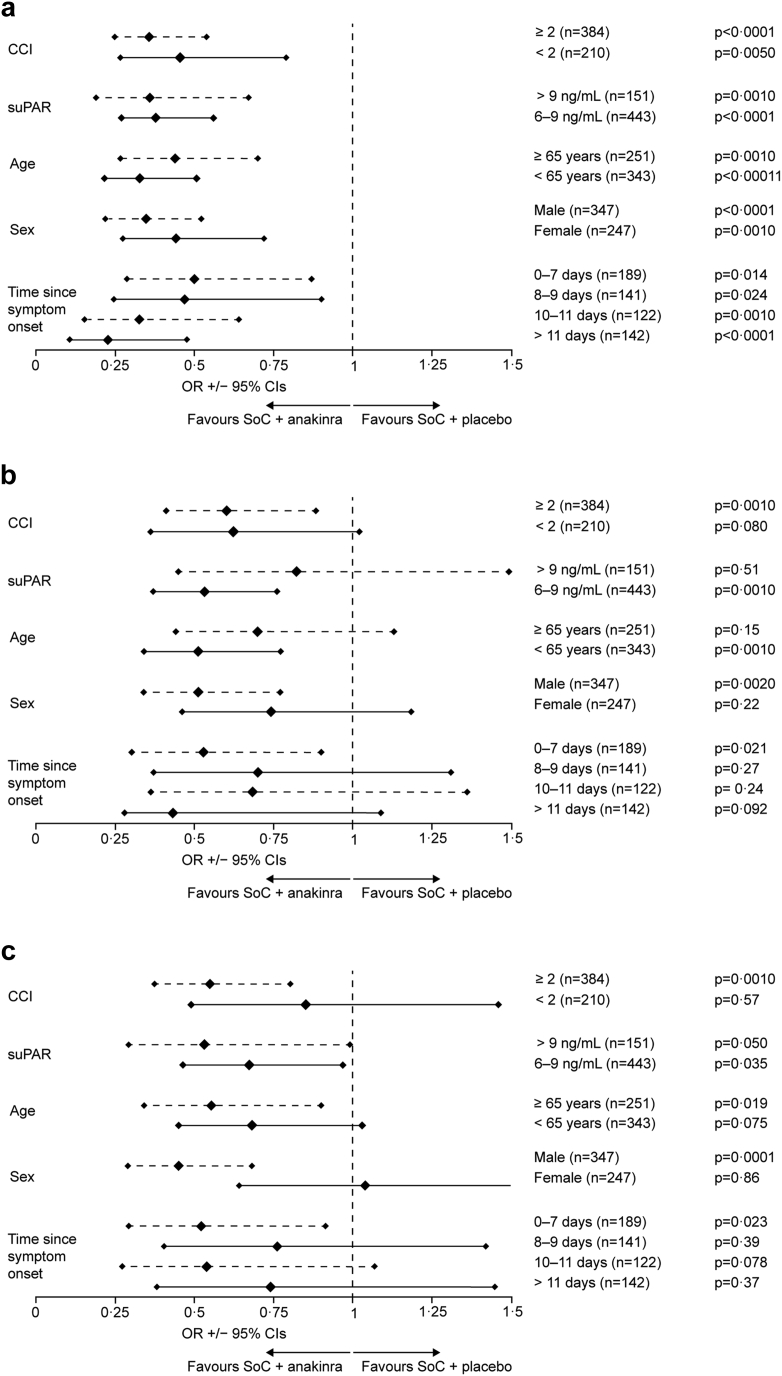
The secondary endpoints analysed for subgroups were the absolute change of WHO-CPS at day 28 from baseline, the absolute change of WHO-CPS at day 14 from baseline and the absolute change of SOFA score at day 7 from baseline. Comparisons were assessed using univariable ordinal regression analysis. Results, which are shown in Fig. 3, are expressed as the odds for worse change of each of the scores with anakinra treatment compared to placebo. The analyses showed statistically significant superiority of anakinra for the absolute change of WHO-CPS at day 28 from baseline for all subgroups. This is expressed as lower odds signified by greater decreases of WHO-CPS among anakinra-treated patients (Fig. 3a). A significant impact of anakinra treatment towards the absolute decrease of WHO-CPS at day 14 from baseline was observed for patients with CCI ≥2, for those with suPAR 6–9 ng/mL, for those aged <65 years, for males, and for those starting the study drug between 0 and 7 days from start of symptoms (Fig. 3b). A significant impact of anakinra treatment towards the absolute decrease of SOFA score at day 7 from the baseline was observed for patients with CCI ≥2, for those with suPAR >9 ng/mL, for those with suPAR 6–9 ng/mL, for those aged ≥65 years, for males, and for those starting the study drug between 0 and 7 days from start of symptoms (Fig. 3c).
Fig. 3.
Subgroup analyses of the secondary endpoints. The main secondary endpoints were: a) the absolute change of the 11-point of the WHO-CPS at day 28 from baseline; b) the absolute change of the 11-point of the WHO-CPS at day 14 from baseline; and c) the absolute change of the SOFA score at day 7. The figure shows the ORs for lower change among subgroups treated with SoC and anakinra compared to patients treated with SoC and placebo. Subgroups are defined according to the value of CCI, the levels of suPAR, age, sex and time since onset of symptoms and start of the study drug. No patient was lost to follow-up by days 7 and 14 and there were no missing values of WHO-CPS at day 14 and of SOFA score at day 7. All patients had valid assessments of the WHO-CPS at day 14 from baseline. One patient was lost to follow-up by day 28 and this missing value was imputed for analysis by LOCF. Abbreviations: CCI, Charlson's comorbidity index; CI, confidence interval; LOCF, last observation carried forward; OR, odds ratio; SoC, standard-of-care; SOFA, sequential organ failure assessment; suPAR, soluble urokinase plasminogen activator receptor; WHO-CPS, World Health Organization Clinical Progression Scale.
The categories were tested for the homogeneity of their effect by adding interactions between the treatment group and the subgroup categories to the covariates of the ordinal regression analyses. There was no effect of subgroups on the secondary endpoints with the exception of the interaction with female sex for the absolute change of SOFA score at day 7 (Supplementary Fig. S14).
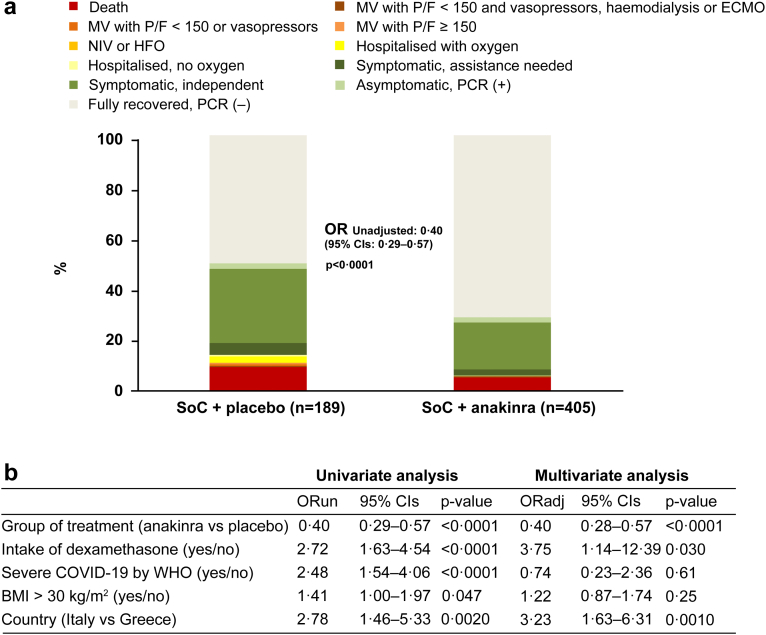
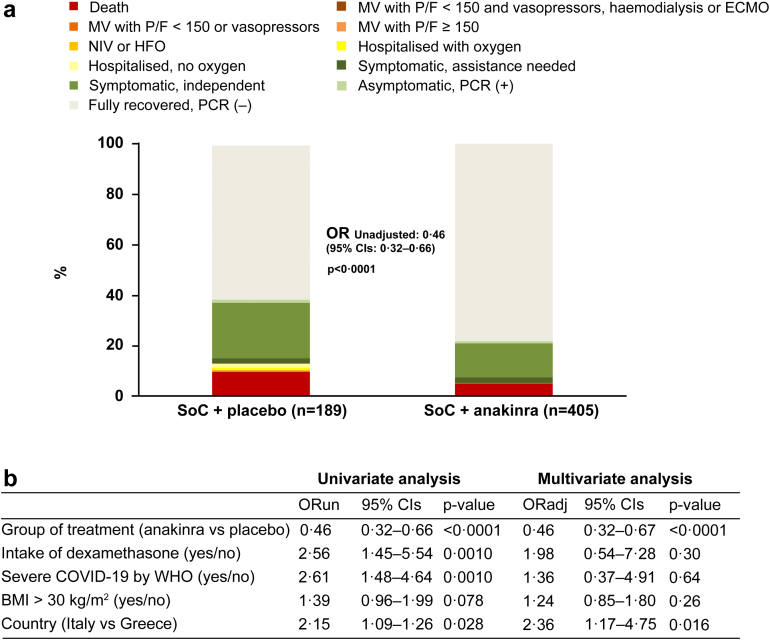
The distribution of the 11-point WHO-CPS in the two treatment arms at day 60 is shown in Fig. 4a. In brief, 71.1% of the anakinra group (288 of 405 patients) fully recovered with no viral RNA detected at day 60 compared to 49.7% of the placebo group (94 of 189 patients); 5.2% (21 of 405 patients) and 9.5% (18 of 189 patients) respectively died. After multivariable ordinal regression analysis, anakinra treatment provided 0.40 odds for worse outcome compared to placebo (Fig. 3b). The benefit of anakinra was maintained until day 90 when 77.8% of the anakinra group (315 of 405 patients) fully recovered with no viral RNA detected compared to 61.4% of the placebo group (116 of 189 patients); 5.4% (22 of 405 patients) and 10.1% (19 of 189 patients) respectively died (Fig. 5).
Fig. 4.
Comparative distribution of WHO-CPS by day 60. a) Comparative allocation of patients treated with SoC and placebo and treated with SoC and anakinra into the 11-point WHO-CPS. The OR for allocation into worse outcomes by anakinra compared to placebo and the respective p-value of comparison is provided. b) Ordinal regression analysis of variables associated with 60-day outcome. Variables entered in the equation are pre-defined by the approved SAP by the COVID-ETF of the EMA. Twenty patients were lost to follow-up by day 60 (7 patients in the placebo group and 13 patients in the anakinra group). The missing values of these patients were imputed for analysis by LOCF. Abbreviations: BMI, body mass index; CI, confidence interval; COVID, Coronavirus disease; CPS, clinical progression scale; ECMO, extracorporeal membrane oxygenation; ETF, Emergency Task Force, HFO, high-flow oxygen; LOCF, last observation carried forward; MV, mechanical ventilation; NIV, non-invasive ventilation; OR, odds ratio; ORun, unadjusted odds ratio; PCR, polymerase chain reaction for SARS-CoV-2; P/F, ratio of partial oxygen pressure to the fraction of inspired oxygen; SoC, standard-of-care; WHO, World Health Organization.
Fig. 5.
Comparative distribution of WHO-CPS by day 90. a) Comparative allocation of patients treated with SoC and placebo and treated with SoC and anakinra into the 11-point WHO-CPS. The OR for allocation into worse outcomes by anakinra compared to placebo and the respective p-value of comparison is provided. b) Ordinal regression analysis of variables associated with 60-day outcome. Variables entered in the equation are pre-defined by the approved SAP by the COVID-ETF of the EMA. Twenty-eight patients were lost to follow-up by day 90 (11 patients in the placebo group and 17 patients in the anakinra group). The missing values of these patients were imputed for analysis by LOCF. Abbreviations: BMI, body mass index; CI, confidence interval; COVID, Coronavirus disease; CPS, clinical progression scale; ECMO, extracorporeal membrane oxygenation; ETF, Emergency Task Force; HFO, high-flow oxygen; LOCF, last observation carried forward; MV, mechanical ventilation; NIV, non-invasive ventilation; OR, odds ratio; ORun, unadjusted odds ratio; PCR, polymerase chain reaction for SARS-CoV-2; P/F, ratio of partial oxygen pressure to the fraction of inspired oxygen; SoC, standard-of-care; WHO, World Health Organization.
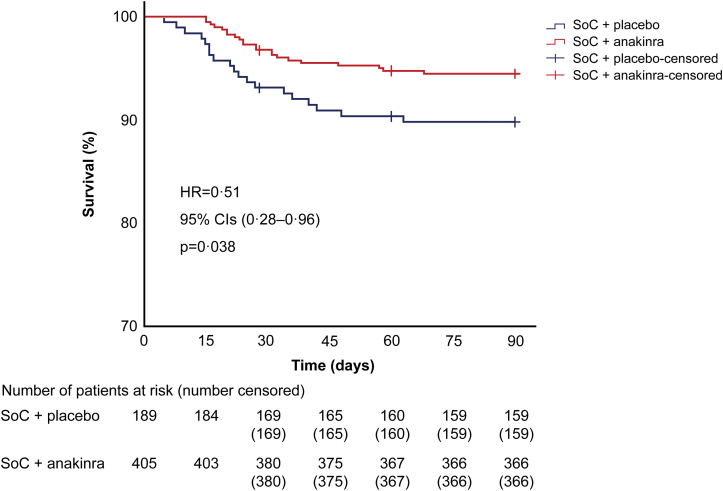
Survival until day 90 was not a pre-specified endpoint. Anakinra treatment significantly reduced the risk of death by day 90 compared to placebo (unadjusted hazard ratio 0.51; 95% CI 0.28–0.96; p = 0.038) (Fig. 6). All patient characteristics were compared between 90-day survivors and non-survivors (Table 1). Age, WHO severity, SOFA score, Charlson's comorbidity index and levels of ferritin and asparagine aminotransferase were significantly different between survivors and non-survivors.
Fig. 6.
Survival analysis by day 90. Kaplan–Meier curves of time to death until day 90 between the two groups of treatment. The crosses indicate the time of censoring for patients who were lost to follow-up. Twenty-eight patients were lost to follow-up by day 90 (11 patients in the placebo group and 17 patients in the anakinra group). The missing values of these patients were censored for analysis at the time to loss of follow-up. Abbreviations: CI, confidence interval; HR, hazard ratio; n, number of patients.
Table 1.
Baseline differences between 90-day survivors and non-survivors enrolled in the SAVE-MORE trial.
| 90-day survivors (n = 553) | 90-day non-survivors (n = 41) | p-value | |
|---|---|---|---|
| Male sex, n (%) | 315 (57.0) | 29 (70.7) | 0.10 |
| Female sex, n (%) | 238 (43.0) | 12 (29.3) | 0.10 |
| Age, years, mean (SD) | 61.3 (12.1) | 69.7 (9.2) | <0.0001 |
| Patients from Italy, n (%) | 62 (11.2) | 4 (9.8) | 1.00 |
| Severe COVID-19 at screening, n (%) | 445 (80.2) | 41 (100) | <0.0001 |
| SOFA score, mean (SD) | 2.35 (1.09) | 3.15 (1.15) | <0.0001 |
| BMI >30 kg/m2, n (%) | 203 (36.7) | 13 (31.7) | 0.62 |
| Dexamethasone treatment, n (%) | 472 (85.4) | 38 (92.7) | 0.25 |
| Remdesivir treatment, n (%) | 407 (73.7) | 32 (78.0) | 0.58 |
| suPAR, ng/ml, mean (SD) | 8.20 (1.90) | 8.79 (2.35) | 0.057 |
| White blood cell count (/mm3), mean (SD) | 6546.2 (3616.3) | 6895.6 (3467.9) | 0.28 |
| Creatinine (mg/dl), mean (SD) | 0.81 (0.26) | 0.82 (0.24) | 0.41 |
| AST (U/l), mean (SD) | 47.9 (30.9) | 67.9 (66.1) | <0.0001 |
| ALT (U/l), mean (SD) | 53.3 (46.0) | 55.7 (67.1) | 0.76 |
| D-dimers (μg/l), median (IQR) | 0.52 (0.68) | 0.49 (0.56) | 0.46 |
| Ferritin (ng/ml), median (IQR) | 566.8 (745.5) | 907.8 (1168.2) | 0.0036 |
| CRP (mg/l), median (IQR) | 49.5 (75.5) | 72.8 (59.5) | 0.20 |
| Charlson's comorbidity index, mean (SD) | 2.17 (1.57) | 3.02 (1.21) | <0.0001 |
| Medical history, n (%) | |||
| Arterial hypertension | 190 (34.4) | 24 (58.5) | 0.0036 |
| Coronary heart disease | 35 (6.7) | 5 (12.2) | 0.20 |
| Type 2 diabetes mellitus | 90 (16.3) | 4 (9.8) | 0.38 |
| Atrial fibrillation | 25 (4.5) | 4 (9.8) | 0.13 |
| Chronic obstructive pulmonary disease | 22 (4.0) | 2 (4.9) | 0.68 |
| Chronic heart failure | 16 (2.9) | 2 (4.9) | 0.36 |
| Chronic renal disease | 10 (1.8) | 0 (0) | 1.0 |
| Stroke | 9 (1.6) | 3 (7.3) | 0.043 |
There are no missing values.
Abbreviations: ALT, alanine aminotransferase; AST, aspartate aminotransferase; BMI, body mass index; CRP, C-reactive protein; IQR, interquartile range; n, number of patients; SD, standard deviation; SOFA, sequential organ failure assessment; suPAR, soluble urokinase plasminogen activator receptor.
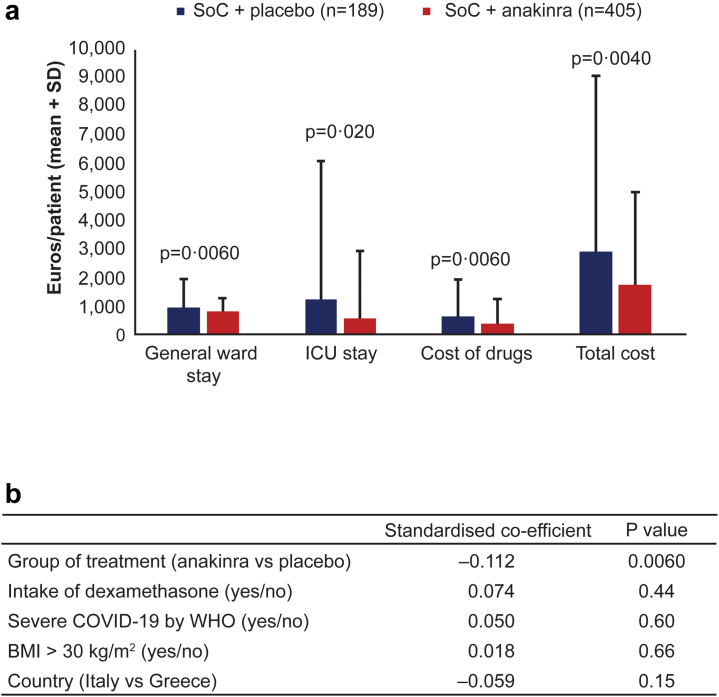
The costs of general ward stay, ICU stay, cost of drugs, and the total cost of hospitalisation until day 90 was significantly lower when patients were treated with anakinra than with placebo (Fig. 7a). This was confirmed by multivariable linear regression using the stratification factors for randomisation as co-variates i.e., disease severity by WHO, intake of dexamethasone, body mass index (BMI) >30 kg/m2 and country. Treatment with anakinra was the only independent variable associated with lower cost (Fig. 7b).
Fig. 7.
Cost of hospitalization until day 90. a) Costs of general ward stay, ICU stay, cost of drugs, and total cost of hospitalisation until day 90 (in Euros) for patients treated with SoC and placebo and for patients treated with SoC and anakinra. The p-values of comparison between the two groups are provided. b) Linear regression analysis of the impact of the group of treatment allocation and each of the four pre-defined variables of stratification i.e. WHO COVID-19 severity, intake of dexamethasone, BMI and country. Abbreviations: BMI, body mass index; ICU, intensive care unit; SD, standard deviation; SoC, standard-of-care.
Discussion
In this study, we performed subgroup analyses on the SAVE-MORE trial primary endpoint at day 283 and evaluated long-term follow-up outcomes at days 60 and 90. At day 28, treatment with anakinra was an independent variable associated with clinical improvement of the 11-point WHO-CPS patient score, in patients with both high and low CCI and in both male and female patients. Similar benefit was shown in patients initiating treatment with anakinra irrespective of the suPAR levels while favourable outcomes were recorded irrespective of duration of disease and timing of administration. We have also shown that anakinra treatment is associated with significantly better outcomes at days 60 and 90, as this is reflected in the WHO-CPS score and mortality. Finally, healthcare associated cost was significantly decreased in patients under anakinra treatment.
Our analysis demonstrated that regardless the timing of anakinra administration, intervention significantly improved 28-day clinical outcomes. Ordinal regression analyses including the interaction between subgroups and allocated treatment as co-variate, did not show any effect of time since onset of symptoms on 28-day and 14-day outcome. This is of particular importance, since disease progression and timing of acute deterioration seem to be characterised by diverse immune phenotypes that exhibit totally different clinical trajectories, despite initial similarities upon presentation.7,8 Anakinra treatment has been evaluated in 44 paediatric patients with secondary hemophagocytic lymphohistiocytosis (HLH), an analogue of cytokine storm syndrome. Ten patients did not have any known underlying disease whereas 34 patients had underlying disorders of rheumatic or malignant origin. Six (60%) and 13 (38%) patients respectively had HLH associated with acute infection. Earlier initiation of anakinra (within 5 days of hospitalization) was associated with reduced mortality (p = 0.046)9 especially in patients with underlying rheumatologic disease. However, none of these infections were caused by SARS-CoV-2.
Early identification of temporal activation of underlying pathophysiological pathways that drive systemic inflammation, using a molecular biomarker like suPAR, is therefore pivotal to ensure optimal outcomes.1 Of note, it seems that early improvement is profound in patients with CCI 2 or more, suggesting that, despite the current general notion that co-morbidities could drive outcomes in these patients due to secondary complications, it is COVID-19 itself and the consequent hyper-inflammatory response that are responsible for poor outcomes and are successfully targeted by anakinra. A recent publication from the International Study of Inflammation in COVID-19 included 2044 patients from both the United States and Europe. The primary outcome was to identify variables associated with the composite of in-hospital death, need for mechanical ventilation, or need for renal replacement therapy. Analysis showed that the risk for the composite outcome among patients with a medical history of type 2 diabetes mellitus increased dramatically with increasing levels of suPAR, reaching 53.8% among individuals with admission suPAR 14.8 ng/mL or more.10
To the best of our knowledge, this report is the first systematic approach to assess long term immunomodulatory intervention with anakinra in patients with COVID-19. Other immunomodulatory treatment strategies for COVID-19 include the use of tocilizumab or baricitinib according to current international recommendations.11,12 A longer follow-up of the CORIMUNO-TOCI-1 trial,13 which examined patients receiving tocilizumab and supplemental oxygen (rate, ≥3 L/min), but did not require high-flow or mechanical ventilation,14 showed a survival benefit at day 90 only in patients admitted with levels of C-reactive protein (CRP) higher than 150 mg/L. Similarly, a retrospective study of hospitalised adult patients with COVID-19 using a large US-based multicentre COVID-19 database including 1510 patients receiving at least one dose of tocilizumab, only showed temporal benefit, especially in patients on non-invasive high-flow supplemental oxygen. However, the benefit of treatment faded over time, while long-term adverse events, especially superinfections, were worrisome.15,16 Baseline levels of circulating ferritin and aspartate aminotransferase (AST) were higher among 90-day non-survivors. Ferritin and AST are among the traits of cytokine storm of COVID-19, which is a well-recognised driver of death in COVID-19. Survival benefit coming from anakinra treatment in these patients may come from attenuation of the cytokine storm.17
The COVID-19 pandemic increased healthcare expenditure worldwide. A recent study from the United States in patients hospitalised with COVID-19 reported an overall median cost and cost/day of $11,267, and $1772, respectively. Overall median ICU cost, and cost/day were $13,443, and $2902, respectively. Patients requiring mechanical ventilation had the highest hospital and ICU median costs ($47,454 and $41,510), respectively.18 In Spain, the impact of COVID-19 on the hospital's budget for the 3 months was calculated at €15,633,180, 97.4% of which was related to health care and hospitalisation. The mean cost per patient was €10,744.19 In our study, use of anakinra reduced the total cost of hospitalisation by almost 40%. This is of particular importance in a current era of stringent budgetary pressure, with expenditures needing to be re-allocated to ensure best cost-effective quality of care for all.
The main limitation of the SAVE-MORE trial is the selection of the best treatment candidate using the biomarker suPAR which may not be available in some countries. Two different alternatives to suPAR have been suggested to early recognize the patient who will get most of anakinra treatment benefit. The first alternative is a score which integrates AST, neutrophil to lymphocyte ratio, CRP and ferritin3; and the second alternative is the SCOPE score which integrates D-dimers, CRP, ferritin and IL-6.20 Recently, the Food and Drug Administration of the United States has suggested a score composed by eight commonly measured variables in everyday routine practice; patients meeting at least three of these variables are at high likelihood to have suPAR ≥6 ng/mL.5
In summary, anakinra use administered early in patients at risk for SRF as defined by suPAR 6 ng/mL or more, improved outcomes at day 60 and 90 post-COVID, irrespective of timing of administration, baseline co-morbidities and disease severity. Anakinra represents an important therapeutic tool in the management of COVID-19.
Contributors
K.A. contributed to data acquisition and drafting the manuscript, revised the manuscript for intellectual content and approve the final version for submission. A.K. supervised the study, revised the manuscript for intellectual content and approved the final version for submission. K.A. has accessed and verified the data, and she is responsible for the decision to submit the manuscript. I.-M.G., E.C., S.M., G.A., A.F., M.F., A.R., Io.K., G.C., G.B., Il.K., Z.A., F.C., F.S.S., P.B., E.N., V.T., A.S., S.I., L.D., K.D., Gl.T., M.C., M.B., V.K., An.A., Ge.T., C.S., O.-M.S., M.S., M.D., G.D., A.M., I.P., Ai.A., M.N., K.L., S.S., N.K.G., V.P., M.G.N., P.P., V.S., H.M. and G.N.D. contributed to data acquisition, revised the manuscript for intellectual content and approved the final version for submission. E.J.G.-B. conceptualised the study, analysed the data, drafted the manuscript, revised the manuscript for intellectual content and approved the final version for submission. E.J.G.-B. has accessed and verified the data, and he is responsible for the decision to submit the manuscript.
Data sharing statement
Data are available with publication by the corresponding author by a signed data access agreement.
Declaration of interests
A.An. is partly funded by the Italian Ministry of Health under “Fondi Ricerca Corrente” – L1P1. L.D. has received consultation honoraria from Sobi. M.B. has received funds for research grants and/or advisor/consultant and/or speaker/chairman from Angelini, Astellas, Bayer, Biomerieux, Cidara, Cipla, Gilead, Menarini, MSD, Pfizer, Roche, Shionogi and Nabriva. M.G.N. was supported by an ERC Advanced Grant (833247) and a Spinoza Grant of the Netherlands Organization for Scientific Research. P.P. has received honoraria from GILEAD Sciences, Janssen, and MSD. H.M. reports receiving honoraria, consulting fees and non-financial support from healthcare companies, including Amgen, Angelini, Bayer, Mylan, MSD, Pfizer, and Servier. G.N.D. is an advisor or lecturer for Ipsen, Pfizer, Genkyotex, Novartis, Sobi, received research grants from Abbvie, Gilead and has served as PI in studies for Abbvie, Novartis, Gilead, Novo Nordisk, Genkyotex, Regulus Therapeutics Inc, Tiziana Life Sciences, Bayer, Astellas, Pfizer, Amyndas Pharmaceuticals, CymaBay Therapeutics Inc., Sobi and Intercept Pharmaceuticals. E.J.G.-B. has received honoraria from Abbott CH, bioMérieux, Brahms GmbH, GSK, InflaRx GmbH, Sobi and Xbiotech Inc; independent educational grants from Abbott CH, bioMérieux Inc, InflaRx GmbH, Johnson & Johnson, MSD, Sobi and Xbiotech Inc. and funding from the Horizon 2020 Marie Skłodowska-Curie International Training Network “the European Sepsis Academy” (granted to the National and Kapodistrian University of AthensNational and Kapodistrian University of Athens), the Horizon 2020 European Grants ImmunoSep and RISCinCOVID and the Horizon Health grant EPIC-CROWN-2 (granted to the Hellenic Institute for the Study of Sepsis). The other authors do not disclose any conflict of interest.
Acknowledgments
This study was funded by the Hellenic Institute for the Study of Sepsis and Swedish Orphan Biovitrum AB.
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.eclinm.2022.101785.
Appendix A. Supplementary data
References
- 1.Rovina N., Akinosoglou K., Eugen-Olsen J., Hayek S., Reiser J., Giamarellos-Bourboulis E.J. Soluble urokinase plasminogen activator receptor (suPAR) as an early predictor of severe respiratory failure in patients with COVID-19 pneumonia. Crit Care. 2020;24(1):187. doi: 10.1186/s13054-020-02897-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Renieris G., Karakike E., Gkavogianni T., et al. IL-1 mediates tissue specific inflammation and severe respiratory failure in COVID-19. J Innate Immun. 2022 doi: 10.1159/000524560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kyriazopoulou E., Poulakou G., Milionis H., et al. Early treatment of COVID-19 with anakinra guided by soluble urokinase plasminogen receptor plasma levels: a double-blind, randomized controlled phase 3 trial. Nat Med. 2021;27(10):1752–1760. doi: 10.1038/s41591-021-01499-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.European Medicines Agency. https://www.ema.europa.eu/en/news/ema-recommends-approval-use-kineret-adults-covid-19
- 5.Fact sheet for healthcare providers: emergency use authorization for kineret. https://www.fda.gov/media/163075/download
- 6.Kyriazopoulou E., Panagopoulos P., Metallidis S., et al. An open label trial of anakinra to prevent respiratory failure in COVID-19. Elife. 2021;10 doi: 10.7554/eLife.66125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lucas C., Wong P., Klein J., et al. Longitudinal analyses reveal immunological misfiring in severe COVID-19. Nature. 2020;584(7821):463–469. doi: 10.1038/s41586-020-2588-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Giamarellos-Bourboulis E.J., Netea M.G., Rovina N., et al. Complex immune dysregulation in COVID-19 patients with severe respiratory failure. Cell Host Microbe. 2020;27(6):992–1000.e3. doi: 10.1016/j.chom.2020.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Eloseily E.M., Weiser P., Crayne C.B., et al. Benefit of anakinra in treating pediatric secondary hemophagocytic lymphohistiocytosis. Arthritis Rheumatol. 2020;72(2):326–334. doi: 10.1002/art.41103. [DOI] [PubMed] [Google Scholar]
- 10.Vasbinder A., Anderson E., Shadid H., et al. Inflammation, hyperglycemia, and adverse outcomes in individuals with diabetes mellitus hospitalized for COVID-19. Diabetes Care. 2022;45(3):692–700. doi: 10.2337/dc21-2102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bhimraj A., Morgan R.L., Shumaker A.H., et al. Infectious diseases society of America guidelines on the treatment and management of patients with COVID-19. 2021. https://www.idsociety.org/practice-guideline/covid-19-guideline-treatment-and-management/#toc-9 [DOI] [PMC free article] [PubMed]
- 12.Bartoletti M., Azap O., Barac A., et al. ESCMID COVID-19 living guidelines: drug treatment and clinical management. Clin Microbiol Infect. 2022;28(2):222–238. doi: 10.1016/j.cmi.2021.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hermine O., Mariette X., Tharaux P.L., et al. Effect of tocilizumab vs usual care in adults hospitalized with COVID-19 and moderate or severe pneumonia: a randomized clinical trial. JAMA Intern Med. 2021;181(1):32–40. doi: 10.1001/jamainternmed.2020.6820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mariette X., Hermine O., Tharaux P.L., et al. Effectiveness of tocilizumab in patients hospitalized with COVID-19: a follow-up of the CORIMUNO-TOCI-1 randomized clinical trial. JAMA Intern Med. 2021;181(9):1241–1243. doi: 10.1001/jamainternmed.2021.2209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nigo M., Rasmy L., May S.B., et al. Real-world long-term assessment of the efficacy of tocilizumab in patients with COVID-19: results from a large de-identified multicenter electronic health record dataset in the United States. Int J Infect Dis. 2021;113:148–154. doi: 10.1016/j.ijid.2021.09.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chamorro-de-Vega E., Rodriguez-Gonzalez C.G., Manrique-Rodriguez S., et al. Clinical course of severe patients with COVID-19 treated with tocilizumab: report from a cohort study in Spain. Expert Rev Clin Pharmacol. 2021;14(2):249–260. doi: 10.1080/17512433.2021.1875819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Canna S.W., Cron R.Q. Highways to hell: mechanism-based management of cytokine storm syndrome. J Allergy Clin Immunol. 2020;146(5):949–959. doi: 10.1016/j.jaci.2020.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ohsfeldt R.L., Choong C.K., Mc Collam P.L., Abedtash H., Kelton K.A., Burge R. Inpatient hospital costs for COVID-19 patients in the United States. Adv Ther. 2021;38(11):5557–5595. doi: 10.1007/s12325-021-01887-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Carrera-Hueso F.J., Alvarez-Arroyo L., Poquet-Jornet J.E., et al. Hospitalization budget impact during the COVID-19 pandemic in Spain. Health Econ Rev. 2021;11(1):43. doi: 10.1186/s13561-021-00340-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Giamarellos-Bourboulis E.J., Poulakou G., de Nooijer A., et al. Development and validation of SCOPE score: a clinical score to predict COVID-19 pneumonia progression to severe respiratory failure. Cell Rep Med. 2022;3(3):100560. doi: 10.1016/j.xcrm.2022.100560. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.