Abstract
Pancreatic cancer (PC) is exemplified by a complex immune-suppressive, fibrotic tumor microenvironment (TME), oncogenic molecular alterations, and aberrant expression of mucins. The constant crosstalk between cancer cells, cancer-associated fibroblasts (CAFs), and the immune cells mediated by the soluble factors and inflammatory mediators including cytokines, chemokines, reactive oxygen species (ROS) promote the dynamic temporal switch towards an immune-escape phenotype in the neoplastic cells and its microenvironment that bolsters disease progression. Chemokines have been studied in PC pathogenesis, albeit poorly in the context of mucins, tumor glycocalyx, and TME heterogeneity (CAFs and immune cells). With correlative analysis from PC patients’ transcriptome data, support from available literature, and scientific arguments-based speculative extrapolations in terms of disease pathogenesis, we have summarized in this review a comprehensive understanding of chemokine-mucinome interplay during stromal modulation and immune-suppression in PC. Future studies should focus on deciphering the complexities of chemokine-mediated control of glycocalyx maturation, immune infiltration, and CAF-associated immune suppression. Knowledge extracted from such studies will be beneficial to mechanistically correlate the mucin-chemokine abundance in serum versus pancreatic tumors of patients, which may aid in prognostication and stratification of PC patients for immunotherapy.
Keywords: Pancreatic cancer, stroma, mucins, chemokines, tumor-microenvironment
1. Introduction
Inflammation has been indicated as a potential additive stimulus to the neoplastic epithelial tissue in its route towards malignant development [1]. With the recent advancements in pancreatic cancer (PC) pathobiology, the specific contribution of inflammation in concert with oncogenic Kras mutation during PC progression has been widely recognized [2]. The intricate mechanisms that dynamically tune the ability of the immune system to prevent the tumor establishment initially and support tumor progression later are cumulatively known as immunoediting [3], which not only involves the malignant cells but requires biochemical and molecular mediators derived from the complex tumor microenvironment (TME) [4]. Pancreatic tumors manifest an enigmatic stromal reaction that involves cellular interplay between the epithelial, mesenchyme, and immune compartments embedded in a matrix of extracellular proteins like collagens, fibronectin, etc. Emerging studies in cancer immunoediting have identified several mechanisms and interactions originating in the tumor stroma that drive the balance between pro-and anti-tumorigenic immune milieu [4]. The crosstalk between cancer cells, cancer-associated fibroblasts (CAFs), and the immune cells mediated by factors including cytokines, reactive oxygen species (ROS), and small proteins like galectins promote the ability of neoplastic cells to hijack the host inflammatory response, thereby creating an environment that fosters tumor growth and progression [2]. Chemokines, a subfamily of soluble cytokines, can be divided into four major subclasses: CC, CXC, C, CX3C chemokines, of which the CXC chemokines are abundantly expressed in PC. Being secreted by various cell types of the pancreatic TME, the chemokines remain concentrated in the stroma and play pivotal roles in the oncogenic initiation and progression by binding to their cognate G-protein-coupled receptors (GPCRs) in cell-autonomous and non-autonomous manners [5].
Another hallmark of PC is the abundant expression and accumulation of sugar-rich high molecular weight proteins called mucins that mark the onset of epithelial metaplasia [6]. Mucins are multi-domain molecules that are either tethered to the cell membrane (transmembrane mucins like MUC1, MUC4, MUC16) or remain loosely attached to the glycocalyx as a polymeric and multimeric gel (secreted mucins like MUC5AC, MUC6). Other than MUC1, most of these mucins are de novo expressed by the malignant epithelium while remaining absent from the normal pancreatic ductal cells. The mucin family members have been long studied in the context of PC initiation, progression, and metastasis [6]. However, their contributions towards shaping the tumor microenvironment have recently started to be acknowledged in the field [7]. Mucins are studded with sugar-moieties or glycans like sialic acid residues, by virtue of which the cancer cells can directly interact with the oligosaccharides or protein structures (selectins and siglecs) on immune cells, thereby influencing their recruitment, differentiation, functional manifestations in terms of chemokine synthesis in the tumor, establishing a pro-tumorigenic immune milieu [8]. Mucins have also been speculated to scaffold various small molecular-weight proteins like Trefoil factors (TFFs) and cytokines, which not only promote wound healing but can be beneficial for the cancer cells for immune evasion and immune suppression [8].
The epithelial mucins, inflammatory mediators like cytokines/chemokines, and the TME, including the CAFs, immune cells, tumor-associated endothelial cells, extracellular matrix (ECM) proteins, have been shown to mediate profound effects on tumor development, therapeutic response, and clinical outcome. Not many studies have investigated the mutual co-dependencies of these arms in PC pathogenesis. Despite the presence of cancer-associated antigens (or neo-antigens) and several inflammatory immune cell types in the tumor epithelia, the pancreatic tumor microenvironment remains immunosuppressive, which may be due to restricted infiltration of anti-tumor T-cells (CD8+ T cells) in conjunction with the unrestrained infiltration and recruitment of suppressive immune cells like regulatory T-lymphocytes (Tregs), myeloid-derived suppressor cells (MDSCs), and M2 macrophages [2]. Understanding the soluble molecules that drive cellular interaction in the TME, resulting in creating a well-known immunosuppressive microenvironment, will be critical for improving treatment responsiveness and overall outcome in PC patients.
This review summarizes the phenotypic and functional correlation of mucins and mucin-associated proteins with chemokines expression in the pancreatic tumors, elaborates the contribution of mucinome in driving chemokines-associated immune suppression in the PC-TME, and emphasizes the need to comprehensively understand their biological significance in the context of PC pathogenesis.
2. Mucosal chemokines in PC immune modulation
The multifaceted interactions of tumor cells with TME through CXC chemokines are key to tumor growth, immune evasion, and therapeutic resistance. The continuous accumulation of differential chemokines secreted in the TME result in the temporal switch from the proinflammatory, anti-tumorigenic microenvironment to pro-tumorigenic immune escape in PC. Out of all these chemokines, four chemokines, including CXCL14, CXCL17, CCL25, and CCL28, play a vital role in mucosal immunity because of their homeostatic expression patterns in the mucosal tissues and therefore are referred to as mucosal chemokines [9]. In this section, we focus mainly on CXCL17, CCL25, and CCL28 because they have been studied in the context of the inflammatory microenvironment in pancreatic cancer. We have discussed the contribution of CXCL14 in relation to CAFs-mediated immune modulation in Section 3.
CXCL17:
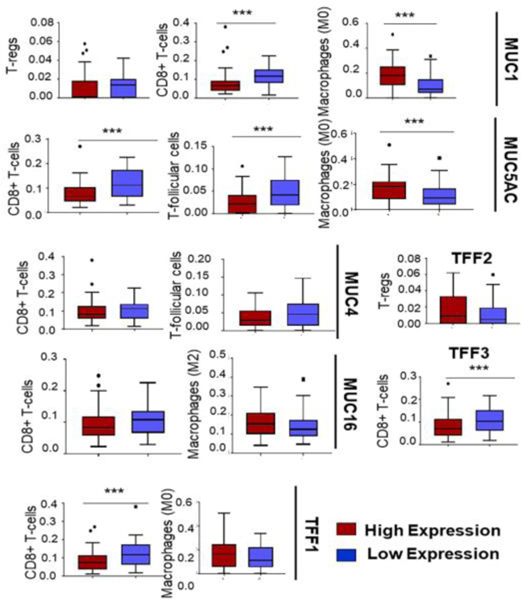
CXCL17 is a mucosal chemokine restricted to epithelium of different organs, macrophages, plasma cells, and endothelial cells of blood vessels and plays an important role in immune homeostasis and anti-tumor immune response. This chemokine is also known as DMC because of its chemoattractant property for immature DCs and monocytes. In the context of cancer progression, this chemokine has been shown to be upregulated in several cancer cell lines, including breast, colorectal, HCC, endometrium, and PC [10]. The downregulation of CXCL17 and ICAM-2 has been shown to induce immune surveillance during the transition from Intraductal Papillary Mucinous Adenoma (IPMA) to Intraductal Papillary Mucinous Adenocarcinoma (IPMC) stages of PC neoplasia. Conversely, they were involved in eliciting anti-tumor immune response during the early stage of IPMNs by expediating the infiltration and accumulation of CD8+ T cells and recruitment of immature myeloid DCs in the tumor [11]. Chemokine-induced downregulation of DC infiltration is one of the immune evasion strategies in different tumors [12, 13]. Since CXCL17 is a homeostatic chemokine restricted to mucosal tissues, and mucin expression progressively increases with disease progression, the sequestration of CXCL17 in the accumulated mucins might be responsible for decreased DC infiltration and immune tolerance. Interestingly, our CIBERSORT analysis from TCGA data of PC patients clearly demonstrates a significant decrease in CD8+ T cells in patients with high expression of MUC1, MUC4, MUC5AC, and MUC16 (Figure 1). In fact, increased expressions of airway mucin MUC5B and concordant levels of CXCL17 chemokine have been reported in patients with idiopathic pulmonary fibrosis (IPF), a lethal lung disease [14]. A recent study showed the overexpression of CXCL17 in different human cancer cell lines, including pancreatic, colon, and lung cancer. Also, the authors demonstrated the recruitment and accumulation of CD11b+Gr-1+F4/80-immature myeloid cells in the subcutaneous tumors of CXCL17 positive cell lines in immunodeficient mice, which in turn increased tumorigenesis and vasculature [15]. Likewise, in another investigation, myeloid-derived cells were found to accumulate in human PC. The authors implied its association with CXCL17 expression and pro-tumorigenic immune environment, thereby suggesting the crucial role of CXCL17 in pancreatic cancer. The Gpr35/CXCR8 has been recently identified as a receptor of CXCL17 [16] and is present on neutrophils, macrophages, and dendritic cells [17]. Therefore, it is quite possible that CXCL17 secretion from a reporter (tdTomato) gene-positive acinar metaplastic cells of the mouse pancreas recruit these immune cells and reshape the immune milieu of TME from an early neoplastic stage. Besides, authors in this study also demonstrated the role of other chemokines, including csf3-csfr3, cxcr2-cxcl1/2/5, ccl8, in mediating immune cells recruitment and activation of immune-suppressive signaling in the early stage of cancer development. An increased tumor-infiltrating Siglec F-positive neutrophil population was found in the RNAseq cluster of the tumors, which may contribute to the immunosuppressive environment. Though the mechanistic rationale remains unclear, further studies will be required to assess the contribution of mucin-chemokine axis mediated mechanism of immune evasion in PC.
Figure 1:
Pancreatic cancer (PC) TCGA dataset was subjected to a CIBERSORTx deconvolution to assess the relative abundance of various immune cell types. CIBERSORT or “Cell-type Identification by Estimating Relative Subsets of RNA transcripts”, is a computational method to quantify the relative fractions of 22 immune-related cell-types based on a 547 genesignature containing specific gene expression profile for the various immune cell types. Boxplots represent the relative abundance of various immune cell types, including Tregs, CD8+ T cells, macrophages (M0), and T-follicular cells as a function of expression (high or low) of different mucins and mucin associated trefoil factors (TFFs) within the dataset. The high expression represents the top 25th percentile of expression of a particular mucin or trefoil factor and is shown by the red color box, whereas the low expression represents the bottom 25th percentile as shown in blue color. To keep this article focused, we have illustrated only the cell types with a significant expressional difference among the high and low groups; and elaborated their significance in the text.. Higher expression of the mucins and TFFs mostly correlated with lower infiltration of cytotoxic CD8+T cells and higher infiltration of suppressive Tregs and macrophages, suggesting an association of immunosuppression with mucin expression in human PC. Statistical comparisons were carried out using the non-parametric Mann-Whitney-U test. *** p-value<0.05
CCL25:
CCL25 is the second mucosal chemokine identified as thymus-expressed chemokine (TECK) [18] and is expressed in the intestine in adults [19]. The receptor for this chemokine is CCR9, which is expressed on immature thymocytes, T cells trafficked to the intestine, and IgA-producing plasma cells. The CCL25/CCR9 mediated signaling has been associated with gemcitabine resistance and enhanced proliferation and invasion in PC lines via PI3K-dependent activation of β-catenin [20]. Although CCR9/CCL25 is associated with immune surveillance and mucosal immunity under normal physiological conditions, it has not been explored yet in the immune milieu of pancreatic tumors [21, 22]. CCL25/CCR9 axis has been implicated in the metastasis of different cancers, including melanoma, NSCLC, breast, colon, and hepatocellular carcinoma [23–25]. Pancreatic cancer cells and PanIN lesions demonstrated overexpression of chemokine receptor CCR9, and PC cell proliferation was enhanced upon activation of the CCR9/CCL25 axis [26]. Importantly, CCR9 has recently been identified as an immunosuppressive molecule from the RNAi screens [27]. The authors elaborated the CCR9 relevance in immune suppression using different tumor models. Of note, HLA-matched-tumor infiltrating lymphocytes (TILs) from PC patients displayed augmented killing of CCR9 knockdown PANC-1 cells compared to control cells.
Further investigation into the mechanism of tumor-associated CCR9-specific T cell response revealed that enhanced STAT signaling in T cells by CCR9 knockdown relates with improved effector T cell function and increased T-helper-1 (TH-1) mediated cytokine secretion. However, authors cued the existence of an unknown independent ligand of CCR9 on these T cells as inhibition of its cognate ligand CCL25 using siRNA, or monoclonal antibody did not alter the CCR9 mediated T cell functions in MCF7 breast cancer cells. These findings can be easily extrapolated to comprehend the significance of this axis in PC tumor immune evasion. In fact, the secretion of CCL25 from the pancreatic stellate cells (PSCs) suggests both autocrine and paracrine activation of CCR9 [28]. Hence, the recruitment of immunoregulatory T cells via the CCR9-CCL25 axis in paracrine fashion in the TME may promote immune suppression resulting in PC progression.
CCL28:
CCL28 was the first identified mucosa-associated epithelial chemokine (MEC) and recognized as a ligand for epithelial chemokine receptors CCR10 and CCR3 in the mucosal tissues as exocrine glands, colon, and trachea and also found in acinar epithelial cells and human saliva [29]. The involvement of CCL28 in mucosal immunity and liver inflammation is associated with the recruitment of CCR10 expressing Treg cells. As shown in a recent study, the high levels of CCL28 in the hypoxic TME stimulate the recruitment of FOXP3+ Treg cells through the CCL28-CCR210 axis, which in turn induces immune tolerance and promotes tumorigenesis [30]. Interestingly, MUC1 overexpression has been shown to promote and sustain a hypoxic PC-TME [31], and our CIBERSORT [32] analysis (Figure 1) suggests a significant increase in Tregs in MUC1-high tumors, further supporting the speculation that upregulation of mucins in conjunction with mucosal chemokines lead to higher infiltration of immunosuppressive T cells. Together, these studies emphasize the need for investigating the in-depth mechanisms of mucosal chemokines-mediated immune evasion associated with PC progression.
3. Interplay of mucins and chemokines in driving CAF-mediated immune heterogeneity
PC cells are embedded in an ocean of scar tissue that mirrors “a wound that never heals” and is termed as desmoplasia or fibrotic stroma. The non-malignant cellular components, namely CAFs, immune cells, endothelial cells, and fibrotic tissue, constitute up to 90% of the bulk tumor volume. The tissue-resident pancreatic stellate cells were considered as the main cellular origin of CAFs, until recently, several groups, including ours, have observed that the CAFs originate mostly from the mesenchymal stromal cells recruited to the tumor bed from the adipose tissue and bone marrow. Unlike cutaneous wound healing, fibroblasts in tumors remain constitutively activated, releasing growth factors, chemokines, and ECM components, which eventually results in the vicious cycle of inflamed fibrotic parenchyma development. Apart from cancer cell-directed pro-tumorigenic functions, CAFs contribute towards the cancer-immune escape by promoting the development of a hypoxic immunosuppressive microenvironment. Over the last decade, several groups have tried to target the stroma or CAFs per se, leading to unfavorable outcomes with enhanced tumor aggressiveness and decreased survival. Hence, extensive efforts are underway in delineating the heterogeneity in CAFs and their differential pathological interplay with the immune cells and cancer cells in the malignant milieu. As explained before, mucins have been extensively studied in terms of cancer cell-autonomous functions; however, their paracrine and juxtacrine implications on the other cell types in the TME are unexplored. In this section, we have compiled the literature on the mutual dependence of CAF-heterogeneity and immune-modulation in the TME. Alongside, we have utilized PC patient data from TCGA to derive correlative associations between different mucins, chemokines, and the nature of the immune milieu in the pancreatic tumors.
3.1. CAFs-mediated chemokine regulation and immune modulation in PC:
There are different subpopulations of CAFs with diverse spatial distribution in the tumor stroma and distinct functions. Myofibroblastic CAFs (myCAFs) express high levels of α-SMA, responsible for TGF-β- associated matrix stiffness and are generally localized in juxtracrine position to the tumor cells, whereas inflammatory CAFs (iCAFs) is positioned more distantly from the malignant cells and represent the major source of CAF secretome [33]. In the context of immune-modulation, effector T cells infiltrating the tumor not only need to tease through the dense fibrotic barrier but also overcome the suppressive CAF secretome. Activated CAFs release suppressive chemokines like CCL2, CCL25, CXCL1, CXCL12, IL-6 that can directly or indirectly hamper immune surveillance through the modulation of antigen-presenting cells and impede T-cell effector functions [34]. A recent study showed that CAFs release IL-6 that induced the maturation of MDSCs and M2 macrophages via STAT3 activation, which in turn suppressed T-cell proliferation. Further, iCAF-derived leukemia inhibitory factor (LIF) has also been identified as a major promoter of immune suppression in the PC TME that correlated with tumor progression [35]. Together, these studies suggest that targeting the CAF heterogeneity may reverse the suppression of anti-tumor T-cell activity and improve the efficacy of immunotherapy.
Pancreatic CAFs abundantly express TGF-β, a pleiotropic cytokine, and major immunosuppression. It has been extensively associated with tumor cell growth, extracellular matrix deposition, suppression of T cell recruitment, and abrogation of the cytotoxic effector functions of tumor-infiltrating T cells [36]. Several recent studies identified that tumors bearing a high population of TGF-β–expressing CAFs are associated with poor response to immunotherapies, and inhibition of the same leads to increased T cell infiltration. A recent literature review compressively described the heterogeneous CAFs as the crucial drivers of immunosuppression in solid tumors, including PC [37]. Interestingly, TGF-β transcriptionally activates the expression of α-SMA in the CAF-precursor cells and activates pancreatic CAFs [38]. Our recent unpublished work on secreted mucin, MUC5AC, for the first time, indicates the role of mucin in CAF heterogeneity by modulation of inflamed adipose tissue-derived mesenchymal stem cells (manuscript under review). Briefly, we observed that MUC5AC-high patient tumors exhibit a higher population of α-SMA+ CAFs with concurrent upregulation of CXCL5 expression. Furthermore, in our CIBERSORT analysis, high MUC5AC expression was associated with low CD8+ T cells, suggesting a suppressive phenotype. Hence, the mutual codependence of mucin-chemokine interplay on CAF heterogeneity should be extensively studied, leading to the better elucidation of the immune-phenotype in the tumors.
3.2. Mucin and mucin-associated proteins in chemokine regulation and immune modulation in PC:
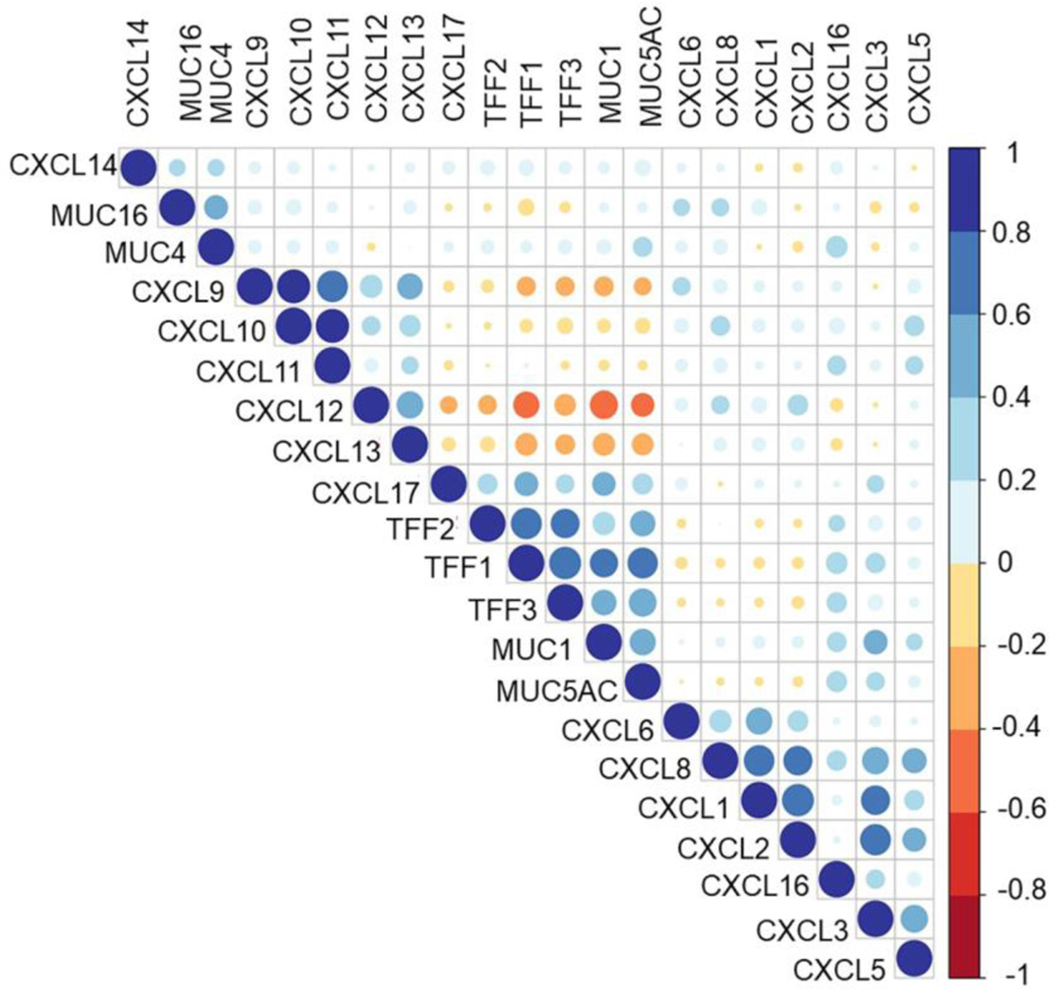
Envisioning the association of mucins with differential chemokines during immune modulation in PC TME and owing to the dearth of literature supporting the same, we investigated the expressional correlation of CXC chemokines and the abundantly expressed mucins and mucin-associated proteins TFFs from the PC patients’ transcriptome available in TCGA. We observed a strong positive correlation of the CXCL1,2,5,8, the cognate ligands for CXCR2, with most transmembrane and secreted mucins and TFFs (Figure 2). The CXCR2 ligands have been extensively studied in PC immune modulation. It was shown that Cxcl5 is highly overexpressed by the PC cells and is regulated by oncogenic Kras and NF-κB activation [39]. Steele et al. showed that the CXCR2 axis is important in immune modulation of pancreatic cancer and that inhibition of CXCR2 reduces metastasis and improves chemotherapy and immunotherapy response [40]. Furthermore, CXCR2 inhibition by genetic ablation prevented neutrophil accumulation in pancreatic tumors and led to a T cell-dependent suppression of tumor growth. In the absence of neutrophils, activated and functional T cells infiltrated pancreatic tumors that are otherwise devoid of effector T cells. Thus, the CXCR2-ligand axis helps establish an immunosuppressive microenvironment in PC, highlighting the potential utility of targeting this axis as a novel therapy for this deadly disease. Interestingly, the CIBERSORT analysis from TCGA data of PC patients’ transcriptome suggested a potential role of mucins and TFFs including MUC1, MUC4, and MUC5AC in suppressing CD8+ T cells and T-follicular helper cells infiltration in the patient tumors (Figure 1), supporting the speculation that mucins may participate in immune suppression via chemokines. Mucins, being highly glycosylated bulky molecules, can participate in immune evasion of tumor cells by occluding the receptors of activated immune cells. Other than the multi-domain protein backbone, mucins are studded with sugar moieties (glycans) and small interacting partners like galectins. Mucins can employ these unique biologically reactive components to participate in immune modulation, and it has been discussed in detail in Section 4. Recent unpublished work from our group has shown that secreted mucins can scaffold and enrich chemokines (especially CXCR2 ligands in PC) by virtue of their sialic acid residues. This phenomenon of micro-enrichment of the chemokines is a non-random and organ-specific process. To our understanding, secreted gel-forming mucins allow the local accumulation of chemokines near the chemokine receptors of cancer cells as well as immune cells, which in turn promote tumor aggressiveness and immune suppression.
Figure 2:
Correlation plot representing spearman correlations between transcriptomic expression of mucins, trefoil factors, and CXC chemokines in pancreatic cancer (PC)- TCGA patient data. Darker and bigger circles represent stronger correlations, with blue color representing positive and red representing negative correlations.
Next, in our correlation analysis, there was a differential pattern of correlation of CXCL9, 10,11,12, with MUC1 and MUC5AC, compared to that with MUC4 and MUC16. While these ligands were negatively correlated with MUC1, MUC5AC, and TFFs, they showed a moderate positive correlation with MUC4 and MUC16. Several chemokines like CXCL9, CXCL10, and CXCL11, CXCL13 have been shown to inhibit angiogenesis and limit tumor progression by recruiting innate and adaptive immune cells like NK cells and CD8 T cells in different malignancies [41]. However, upon priming in the tumor milieu and interaction with mucin-associated glycans, the immune-surveillance phenotype eventually shifts towards immune escape. CXCL12/ CXCR4 axis has also been reported to promote PC invasiveness, stromal modulation, recruitment of endothelial cells, and bone-marrow-derived CAF-precursor cells [42]. A recent study in PC suggested that iCAFs are the primary source of CXCL12 in pancreatic tumors and blocking the CXCL12/CXCR4 axis led to the infiltration of anti-tumor T-cells resulting in retarded tumor growth [43]. Hence, understanding the variety of glycans on different mucins along the course of the disease progression and establishing a comprehensive correlation between the mucins, CAF subtype, and chemokine profile of the tumors may help delineate the immune landscape and determine immunotherapy response in the PC patients.
In addition to mucins, Trefoil factors (TFFs) family peptides (TFF1, TFF2, and TFF3) also play a crucial role in immune modulation and disease progression in the PC-TME [44]. TFF2 secretion from the myeloid and lymphoid cells elicit a range of inflammatory reaction and immune cell recruitment [45]. In terms of their activity by chemokine-mediated signaling pathways, TFF2 has been shown to bind CXCR4 on epithelial cancer cells and lymphocytes and elicit various functions such as cell migration and T-cell survival. However, this engagement has not been validated in immune cell recruitment. Nevertheless, congruent expression profiles of TFF2 and chemokine receptors CXCR4 and CXCR7 in lymphoid tissues, including spleen, bone marrow, lymph node, and thymus, and their involvement in monocyte migration suggest a possible chemotactic interaction. Further evidence comes from the attenuating effect of TFF2 on CXCR4-CXCL12-mediated chemotaxis [46]. In fact, the dysregulated immune response by macrophages and lymphocytes in TFF2 deficient mice might explain the critical role of the TFF-CXCR4 axis. TFFs’ involvement in enhancing TNF-α mediated IL-6, and IL-8 secretion from bronchial epithelial cells has been explained by their binding to TNFα-induced CXCR4 [47]. Furthermore, lower infiltration of CD8 T cells and higher abundance of Tregs in high TFF1,2,3 -expressing tumors (Figure 1) further support the immuno-suppressive function of TFFs in the mucosal neoplasia. However, in-depth studies are warranted to understand the role of TFFs in chemokine-mediated immune cell modulation in the PC-TME.
Furthermore, we found a strong correlation of mucins and TFFs with mucosal chemokine CXCL14 and CXCL17 (Figure 2), supporting the literature explained in Section 2. Studies on CXCL14 have shown paradoxical effects across multiple malignancies and under in vitro and in vivo contexts. While overexpression of CXCL14 enhanced immune surveillance and decreased tumor growth in squamous carcinoma, CXCL14 synthesized by the CAFs was shown to enhance tumorigenesis, stromal maturation, and neovascularization [48, 49]. A strong positive correlation of CXCL14 with the MUCs and TFFs provides a strong rationale for exploring the role of CXCL14 in mediating CD8 T cell response. Indeed, MUC1 and MUC5AC high pancreatic tumors showed significantly high CD8 T cell infiltration (Figure 1). Discussion on mucosal chemokines and their pivotal roles in TME-modulation have been detailed in Section 2.
4. Mucin-associated glycans in immune modulation in PC TME
The aberrant and deregulated glycosylation is the hallmark of cancer progression, and these overexpressed altered glycans are used by tumor cells for immune evasion through binding to inhibitory receptors on immune cells. A variety of altered glycosylation alterations have been observed in PC patients, which include sialyl LewisA (present in CA19–9, a widely used biomarker in pancreatic cancer), sialyl lewisX, hypersialylation, increased truncated O-glycans (Tn and STn), proteoglycans, galectins, and fucosylated N-glycans [50]. These aberrantly expressed glycans and glycan-binding proteins present in the glycocalyx are the first points of contact between the immune cells and their targets, so it is obvious that they are involved in different biological processes of the cancer cell, including signaling, cell-matrix interactions, metastasis, angiogenesis, and immune modulation [51]. In this section, we have elaborated the role of glycans on mucins and mucin-associated proteins in driving an interplay between cancer cells, chemokines, and TME immune milieu in PC.
4.1. Regulation of mucin glycome by the inflamed tissue parenchyma:
The cytokines and chemokines released from the various cells of the TME are exploited by the tumor cells for evading the anti-tumor immune response. Several studies demonstrate the increased levels of cytokines in pancreatic cancer cell lines [52]. Indeed, enhanced levels of cytokines including IL-8, IL-10, Il-12, IL-18, and TGFβ in the sera of PC patients correlated with their poor prognosis [53]. However, there is not much clarity on the mechanism of chemokine/cytokine-induced alterations in tumor-associated glycans and vice-versa. Numerous studies have demonstrated that cytokines upregulate glycosyltransferases, resulting in altered glycan levels in various malignancies. Sialylated (SA) and lewis-type (SLeX and Sley) glycan levels were increased by IL-1 stimulation in PC cell lines while IL-6 and TNFα treatments resulted in augmented expressions of SLex and Ley [54]. In contrast, another recent study showed that the proinflammatory cytokines IL-6 and CXC family chemokine IL-8 (CXCL8) increased sialic acid (SA) content on RAW7 macrophages while no changes in SA expression was seen on tumor cells [55]. Nonetheless, IL-6 and IL-8 mediated enhancement in the levels of SLex, and sulfated SLex in the mucins, especially MUC4 of human bronchial tissues of cystic fibrosis patients, substantiate the association of mucins with modulated glycosylation patterns in PC [56]). A very important aspect to consider here is that the chemokine/cytokine and their receptor network are quite promiscuous. Therefore, predicting their correlation with differential expression patterns of target antigens and prognosis in patients is quite difficult. A better understanding of the effect of a chemokine on both tumor cells and immune cells is required for extrapolating these findings with the clinical outcome in PC patients. The transcriptional co-regulation of proinflammatory cytokines, as well as mucins like MUC2, MUC4, and MUC5AC by NF-kB in airway pathologies and solid malignancies, hint towards their involvement in inflammation associated with tumor aggressiveness [6, 57]. These inflammatory cytokines, including IL-1β, IL-6, and TNF-α, increase the levels of tumor-associated sialylated glycans SLe(X), Le(y), SLe(a), and α2,6-sialic acid through increased expression of sialyltransferases and fructosyltransferases involved in glycan synthesis [54]. A high level of glycosylation in the tandem repeats in the extracellular domains of transmembrane mucins facilitate their interaction with chemokine receptors present in the glycocalyx. A wide variety of oligosaccharide structures with different types of glycans (composition) are attached to the mucins and provide them the cell-specific function. For instance, there is enormous variability in the glycosylation of MUC1 and MUC16 in the breast and pancreatic tissues [58]. Besides, different types of sugar molecules, such as sialyl-Lewis a, sialyl-Lewis c, sialyl-lewis x, and sialyl-Tn, have been shown to be present on MUC1 of pancreatic and colon cancer cell lines [59]. In fact, carbohydrate structures sLea and sLex on a soluble form of tumor-associated MUC1 may bind to P-selectins of endothelial cells and help leucocyte recruitment. Alternatively, the soluble form of MUC1 with these carbohydrates may mimic selectins and evade leucocyte trafficking, which might explain the immune evasion by mucins in cancer. In a nutshell, these studies highlight the importance of inflammatory tissue parenchyma in modulating the sialylation and O-glycan profile in PC tumors, which in turn are thought to induce immune escape through binding to lectin receptors on various immune cells and induce tolerance in TAMs, tumor-resident DCs, and cytotoxic NK cells [60].
4.2. Role of mucin-associated glycans in immune modulation:
There have been several studies to understand the mechanistic insights on glycan-mediated immune suppression in cancer. The aberrantly expressed sialylated glycans and glycan-binding proteins such as galectins and siglecs present on cancer cells are involved in immune modulation via engaging inhibitory receptors present on different immune cells such as T-cells, B-cells, monocytes, and NK cells [61]. There is a lot of diversity in the expression of these siglec receptors on different subsets of immune cells, as exemplified by the expression of siglec-7 on all human NK cells and restricted expression of siglec-9 on a specific subset of NK cells but the wider expression on myeloid cells [62, 63]. Binding of DCs to sialylated antigens induced immune tolerance by promoting IL-10 secretion and Treg induction [64]. This variation in the binding and expression of siglecs on immune cell subsets may dictate their function. Apart from this, there is a high level of heterogeneity in the degree of sialylation in different cancers and within a specific cancer type, as exemplified by different lung cancer patients displaying variability in hyper sialylation of ligands of siglec-7 and siglec-9 [65]. There is a need for extensive research on how these differences impact cancer progression and enhanced sialylation contributes to immune evasion in PC. A recent study in PC showed increased sialic acid (sialylation) content from PC tumor cells bound to siglec-7 and siglec-9 on myeloid cells [66]. Specifically, in PC, siglec-9 engagement induced switching of myeloid cells towards TAM phenotype and thus resulted in immunosuppression through increased PD-L1 expression and IL-10 production [66]. Indeed, hyper sialyation in the TME is associated with poor clinical outcomes in PC patients. In line with this, previous studies have shown the interaction of a cancer-specific truncated, sialylated Olinked glycan (STn) isoform of MUC1 with siglec-9 present on myeloid cells [67] and siglec-7 [68]. The released chemokines CXCL5, CCl2, CCl3, CXCL1, CXCL8 might facilitate the immune cell recruitment and immune modulation in the TME as seen by the recruitment of TAMs expressing CD206, CD163, IDO, and PD-L1. Similarly, the cleaved soluble form of MUC16 binds to siglec-9 on human NK cells and T-cells, thereby resulting in the suppression of their functional activation [69]. Taken together, the above-mentioned studies establish a direct link between mucin-associated sialylation and immune modulation in PC. Therefore, exploiting the immunomodulatory properties of tumor-associated sialylated moieties of mucins should be considered as an amenable approach for targeting PC.
4.3. Role of glycan-binding proteins in immune modulation in PC TME:
Galectins comprise a versatile family of glycan-binding proteins that play crucial roles in immunoregulation. These proteins modulate the function of various cytokines and chemokines as they are simultaneously upregulated under inflammatory conditions. In cancer, galectins help tumor cells escape from T-cell-mediated immune response. Currently, several studies have highlighted the formation of heterodimers of different chemokine and galectins with functional relevance, as evident from Galectin-3 (Gal-3)-CXCL12 pair in chronic inflammation [70]. Besides, the results also indicate the promiscuity in the binding of various chemokines with different galectins. However, there is limited understanding on whether these chemokines could regulate the galectin functions. The enhanced levels of Gal-3 in early-stage pancreatic tumors, though not associated with advanced disease, and increased expression of Gal-1 in CAFs and ECM components suggest their differential role in cancer onset, metastasis, and desmoplasia, respectively [71]. Since Gal1 is known to induce apoptosis in activated T-cells, it is quite possible that Gal-1 could help PC tumors to escape the immune response. Its expression in the CAFs might help in the remodeling of stroma and thereby modulate the immune microenvironment. Stromal derived Gal-1 induced apoptosis of T-cells in a paracrine manner and favored Th2 cytokine versus Th1 response substantiating the observations that Gal-1 contributes to pancreatic tumor immune escape [72, 73]. Furthermore, chemokine antibody-array analysis of Gal-1 treated PSCs revealed significantly higher secretion of chemokine CXCL12 via NF-kB activation, which was reversed by galectin knockdown [74]. These findings provide novel insight into the mechanism of galectin-mediated modulation of TME and disease progression through chemokines. In contrast to previous studies showing Gal-3’s implication in the early stages of PC, a recent study demonstrated the Gal-3 mediated secretion of chemokines IL-8, CXCL-1, CCl2, and IL-6, GM-CSF from PSCs via integrin signaling [75] and PC progression. In fact, the authors showed the involvement of the NF-kB pathway in Gal-3 mediated CXCL8 production. The above-mentioned studies describe the regulation of immune modulation through galectin-chemokine interactions. However, there are unanswered questions that need to be addressed in the context of galectins-chemokine interactions. For example, how these interactions regulate immune cell differentiation and maturation, and what factors are driving the pairing of these galectins and chemokines in the cancer microenvironment. In addition, the role of these interactions in other cell types like endothelial cells and platelets is yet elusive, which might be crucial in tumor-immune cell crosstalk and immune modulation in the TME.
Mucins, the primary architect of the cancer glycocalyx, have so far been extensively studied in driving cancer cell-autonomous signaling; however, their biophysical and rheological properties in reshaping the composition and functional properties of the glycocalyx remain unexplored in PC. Our group and others have shown the crucial contribution of mucin-galectin interaction is driving PC pathogenesis [76]. It has been shown that transmembrane mucins like MUC1, MUC4, and MUC16 interact with Gal-3 in a glycocalyx-mediated fashion [77, 78]. Such interactions participate in barrier function on the normal epithelium (gastric mucosa) while promoting cell signaling and invasiveness in malignant settings. Furthermore, a recent study has shown that inflammatory cytokine IL-17 leads to MUC5AC and Gal-3 interactions in the airway epithelium during asthma [79]. Overall, from these studies, it is tempting to speculate that the polymeric gel of secreted mucins and extended sugar-rich extracellular domains of membrane-tethered mucins may bring the galectins and chemokines in close biological proximity to facilitate their interactions via a glycocalyx-mediated mechanism which further leads to immune modulation as explained in the previous sections.
Apart from mucins and galectins, proteoglycans also form an important layer of glycocalyx, and participate in many cellular functions such as proliferation, cell adhesion, inflammation, tumorigenesis, and interaction with pathogens. Chemokines interact with glycosaminoglycans (GAGs) of proteoglycans and form a chemokine micro-gradient that directs the cell migration in the context of immune cell trafficking, immune surveillance, and immune cell maturation. Particularly in cancer, the modulation of these gradients due to the reshuffling of glycocalyx has been shown to result in immune cell recruitment. For instance, telomeric protein the telomeric repeat binding factor 2 (TRF2) mediated synthesis of heparin sulfate proteoglycans (HSPG) gene led to reshaping the glycocalyx in cancer conditions, which triggered the recruitment and activation of MDSCs via the TLR2/MyD88/IL-6/JAK1/2 axis as well as NK and T-cell inhibition and thereby induction of immunosuppressive TME [80]. Thus, glycocalyx reshuffling by proteoglycans in the cancer cells directly links to the MDSC recruitment in the TME.
5. Conclusions and Perspectives
The complex desmoplastic stroma is a hallmark of PC that distinguishes it from other solid malignancies. The PC TME is not merely an amalgamation of various cell types surrounded by the stiff matrix; rather, it facilitates the dynamic crosstalk between malignant and non-malignant compartments like CAFs, immune cells, and endothelial cells. Such diverse interactions are mediated by soluble chemotactic molecules like chemokines, and cancer cell-associated glycocalyx or mucinome, which cumulatively led to the disease aggressiveness, immunometabolic landscape of the tumor, and responses to chemotherapy and immunotherapy. The field of co-regulation of mucins and chemokine has not been explored much in the context of heterogeneity in CAFs or diversity of the immune milieu of pancreatic TME. Nevertheless, various studies in other solid malignancies and benign pathologies suggest that mucins and mucin-associated proteins like TFFs, and galectins might modulate the immune landscape in PC via mucin and chemokine crosstalk. The specific expression patterns of mucosal chemokines enable immune homeostasis in the mucosal tissues. However, the intricate mechanisms responsible for switching their role in defense functions to pathogenic ones in the cancer are elusive. Though several studies show their implications in tumor progression and metastasis, extensive efforts are required to characterize their contribution to immune cells recruitment and immune modulation.
The ability of mucins to sequester or scaffold the chemokines may explain an enhanced micro-accumulation of these chemokines near the chemokine receptors to promote immune tolerance or the ability of mucins to occlude the chemokine receptors on cancer cells to facilitate immune escape. In these aspects, the glycan moieties on the mucins play pivotal roles in mediating direct or indirect routes towards immune suppression. The bioinformatics analysis from PC patients’ transcriptome suggested a differential correlation of each mucin with different chemokines. However, overexpression of each of these mucins was associated with an immunosuppressive tumor milieu. An interesting yet elusive aspect that deserves attention is the mutually exclusive and complementary correlative patterns between the mucin family members and chemokines. For example, MUC1 and MUC5AC were strongly correlated with the mucosal chemokine CXCL17, whereas MUC4 showed a weak-positive correlation and MUC16 exhibited a negative correlation. On the other hand, MUC1, TFFs, and MUC5AC exhibited strong negative correlations with CXCL9, 10,11,12, and 13, which were positively correlated with MUC16. From these observations, it is tempting to speculate the few possibilities: 1) mucins may have partially compensatory mechanisms, but they definitely form unique functional clusters with other family members. Hence, studying the mucins as a family and not a single molecule may be crucial in understanding their role in disease pathology; 2) mucins may have a temporal shift in abundance, glycan composition, and hence in the corresponding interactions as the tumor glycocalyx matures along with the disease progression. Hence, studying the stage-specific contribution of mucin-chemokine axis will be beneficial in understanding the immune landscape; and 3) mucins and associated chemokines may have co-regulatory circuits from the advent of metaplasia to oncogenesis and distant dissemination. This may explain why MUC1 and MUC5AC exhibited similar patterns of correlation with chemokines and associations with immune infiltration. Since MUC1 is expressed by the normal ductal epithelial cells and MUC5AC is expressed de novo at early PanIN stages (and cells undergoing acinar-to ductal metaplasia in the murine pancreas), they may be strongly correlated with the mucosal chemokine CXCL17, which is also abundantly expressed in the normal gastric mucosa and CXCR2 ligands like CXCL5, which is also expressed in the malignant pancreas from an early stage. On the other hand, MUC 16 starts expressing later in the oncogenic cascade, and its expression on the malignant cells amplifies as the disease progresses towards a poorly differentiated and invasive phenotype. This may justify its negative association with the early expressed chemokines and strong positive association with the chemokines implicated in cellular chemotaxis and trafficking, like CXCLs 9, 10, 12.
Although, there is better clarity on the contribution of glycans in immune regulation by inducing tolerance associated with cancer progression. However, the TME modulation by crosstalk between mucin-associated glycans and chemokine cascade is a relatively new field and needs an in-depth understanding of alterations observed in mucin glycans in various malignancies, including PC. Mucins possess a vast variety of O-linked glycans, which are aberrantly expressed in PC. The alterations in their composition may have differential potential to engage immune inhibitory receptors such as siglecs and thereby induce immune tolerance. Besides, limited attempts have been made to explore the chemokine-mediated regulation of mucin glycome in inflamed and transformed pancreatic parenchyma. Nevertheless, elucidating the mechanisms involved in mucin glycome-chemokine interplay will help address the pathological challenges in PC, early disease detection by identifying novel biomarkers and amenable targets for developing better immune modulation strategies in PC.
The perpetual abundance of mucins in the pancreatic tumors from an early stage of oncogenesis and their unique physicochemical nature and rheological properties warrant the need for investigating the mucin-chemokine axis during the co-evolving events of oncogenesis, stromagenesis, and immunoediting. With the recent advances in single-cell RNA sequencing, spatial transcriptomics, lineage tracing experiments, TME-directed murine models, the definite source and pathological significance of each of these chemokines should be studied. Further, mucins being abundantly present in the patient sera, serving as potential biomarkers, the critical association of mucin-chemokine interplay can be extrapolated from peripheral circulation to the primary tumor that may lead to a better understanding of intra-tumoral immune milieu and help in patient stratification for immunotherapies.
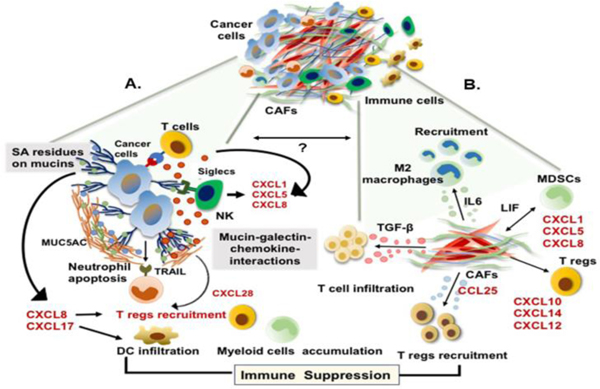
Figure 3:
Schematic representation demonstrating the co-evolving mechanisms of immune suppression in PC-TME. Immunosuppression is mediated by two exclusive yet inter-dependent mechanisms, which includes: (A) Immune evasion by cancer cells; cancer glycocalyx comprising of the mucins and mucin-associated molecules like galectins sequester the chemokines enhancing its local enrichment near the chemokine receptor on immune cells and result in Treg recruitment, mucin-glycans directly interact with the inhibitory siglecs of T cells and NK cells leading to their impaired function, or chemokines released into the TME by sialic acid residues of glycans decrease DC infiltration. Cumulatively, these phenomena result in an immunosuppressive TME. Additionally, certain mucins like MUC5AC can induce TRAILmediated apoptosis of tumor-infiltrating neutrophils, which further contribute to the immune escape, and (B) CAFs-mediated alterations in immune cell phenotype; CAFs secretome is a rich reservoir of immunosuppressive chemokines like IL-6, LIF, TGF-β, CCL25, CXCL12, CXCL8, which promote the maturation of M2 macrophages, recruitment of MDSCs, and infiltration of Tregs in the tumor milieu, leading to the development of tumor-promoting, immune-suppressive TME. Although no study has yet suggested a direct link between aberrant mucin expression/ glycosylation and TME development, to our understanding, these two events co-evolve and rely on soluble factors like chemokines to fine-tune the mutual co-dependence.
Funding:
The authors/work on this manuscript were supported, in parts, by grants from the NIH R01 CA247471, RO1 CA210637, RO1 CA206444, RO1 CA183459, UO1 CA200466, PO1 CA217798, R44 CA235991.
Footnotes
Conflicts of Interest: SKB is one of the founders of Sanguine Diagnostics and Therapeutics, Inc. Other authors have no conflicts of interest to declare.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Del Poggetto E, Ho IL, Balestrieri C, Yen EY, Zhang S, Citron F, Shah R, Corti D, Diaferia GR, Li CY, Loponte S, Carbone F, Hayakawa Y, Valenti G, Jiang S, Sapio L, Jiang H, Dey P, Gao S, Deem AK, Rose-John S, Yao W, Ying H, Rhim AD, Genovese G, Heffernan TP, Maitra A, Wang TC, Wang L, Draetta GF, Carugo A, Natoli G, Viale A, Epithelial memory of inflammation limits tissue damage while promoting pancreatic tumorigenesis, Science 373(6561) (2021) eabj0486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Li KY, Yuan JL, Trafton D, Wang JX, Niu N, Yuan CH, Liu XB, Zheng L, Pancreatic ductal adenocarcinoma immune microenvironment and immunotherapy prospects, Chronic Dis Transl Med 6(1) (2020) 6–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Padoan A, Plebani M, Basso D, Inflammation and Pancreatic Cancer: Focus on Metabolism, Cytokines, and Immunity, Int J Mol Sci 20(3) (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Ho WJ, Jaffee EM, Zheng L, The tumour microenvironment in pancreatic cancer - clinical challenges and opportunities, Nat Rev Clin Oncol 17(9) (2020) 527–540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Hughes CE, Nibbs RJB, A guide to chemokines and their receptors, FEBS J 285(16) (2018) 2944–2971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Ganguly K, Rauth S, Marimuthu S, Kumar S, Batra SK, Unraveling mucin domains in cancer and metastasis: when protectors become predators, Cancer Metastasis Rev 39(3) (2020) 647–659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Bhatia R, Gautam SK, Cannon A, Thompson C, Hall BR, Aithal A, Banerjee K, Jain M, Solheim JC, Kumar S, Batra SK, Cancer-associated mucins: role in immune modulation and metastasis, Cancer Metastasis Rev 38(1–2) (2019) 223–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Hollingsworth MA, Swanson BJ, Mucins in cancer: protection and control of the cell surface, Nat Rev Cancer 4(1) (2004) 45–60. [DOI] [PubMed] [Google Scholar]
- [9].Hernandez-Ruiz M, Zlotnik A, Mucosal Chemokines, J Interferon Cytokine Res 37(2) (2017) 62–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Xiao S, Xie W, Zhou L, Mucosal chemokine CXCL17: What is known and not known, Scand J Immunol 93(2) (2021) e12965. [DOI] [PubMed] [Google Scholar]
- [11].Hiraoka N, Yamazaki-Itoh R, Ino Y, Mizuguchi Y, Yamada T, Hirohashi S, Kanai Y, CXCL17 and ICAM2 are associated with a potential anti-tumor immune response in early intraepithelial stages of human pancreatic carcinogenesis, Gastroenterology 140(1) (2011) 310–21. [DOI] [PubMed] [Google Scholar]
- [12].Shurin GV, Ferris RL, Tourkova IL, Perez L, Lokshin A, Balkir L, Collins B, Chatta GS, Shurin MR, Loss of new chemokine CXCL14 in tumor tissue is associated with low infiltration by dendritic cells (DC), while restoration of human CXCL14 expression in tumor cells causes attraction of DC both in vitro and in vivo, J Immunol 174(9) (2005) 5490–8. [DOI] [PubMed] [Google Scholar]
- [13].Hubert P, Herman L, Maillard C, Caberg JH, Nikkels A, Pierard G, Foidart JM, Noel A, Boniver J, Delvenne P, Defensins induce the recruitment of dendritic cells in cervical human papillomavirus-associated (pre)neoplastic lesions formed in vitro and transplanted in vivo, FASEB J 21(11) (2007) 2765–75. [DOI] [PubMed] [Google Scholar]
- [14].Burkhardt AM, Tai KP, Flores-Guiterrez JP, Vilches-Cisneros N, Kamdar K, Barbosa-Quintana O, Valle-Rios R, Hevezi PA, Zuniga J, Selman M, Ouellette AJ, Zlotnik A, CXCL17 is a mucosal chemokine elevated in idiopathic pulmonary fibrosis that exhibits broad antimicrobial activity, J Immunol 188(12) (2012) 6399–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Matsui A, Yokoo H, Negishi Y, Endo-Takahashi Y, Chun NA, Kadouchi I, Suzuki R, Maruyama K, Aramaki Y, Semba K, Kobayashi E, Takahashi M, Murakami T, CXCL17 expression by tumor cells recruits CD11b+Gr1 high F4/80- cells and promotes tumor progression, PLoS One 7(8) (2012) e44080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Maravillas-Montero JL, Burkhardt AM, Hevezi PA, Carnevale CD, Smit MJ, Zlotnik A, Cutting edge: GPR35/CXCR8 is the receptor of the mucosal chemokine CXCL17, J Immunol 194(1) (2015) 29–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Schlesinger Y, Yosefov-Levi O, Kolodkin-Gal D, Granit RZ, Peters L, Kalifa R, Xia L, Nasereddin A, Shiff I, Amran O, Nevo Y, Elgavish S, Atlan K, Zamir G, Parnas O, Singlecell transcriptomes of pancreatic preinvasive lesions and cancer reveal acinar metaplastic cells’ heterogeneity, Nat Commun 11(1) (2020) 4516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Vicari AP, Figueroa DJ, Hedrick JA, Foster JS, Singh KP, Menon S, Copeland NG, Gilbert DJ, Jenkins NA, Bacon KB, Zlotnik A, TECK: a novel CC chemokine specifically expressed by thymic dendritic cells and potentially involved in T cell development, Immunity 7(2) (1997) 291–301. [DOI] [PubMed] [Google Scholar]
- [19].Wurbel MA, Philippe JM, Nguyen C, Victorero G, Freeman T, Wooding P, Miazek A, Mattei MG, Malissen M, Jordan BR, Malissen B, Carrier A, Naquet P, The chemokine TECK is expressed by thymic and intestinal epithelial cells and attracts double- and singlepositive thymocytes expressing the TECK receptor CCR9, Eur J Immunol 30(1) (2000) 262–71. [DOI] [PubMed] [Google Scholar]
- [20].Lee S, Heinrich EL, Li L, Lu J, Choi AH, Levy RA, Wagner JE, Yip ML, Vaidehi N, Kim J, CCR9-mediated signaling through beta-catenin and identification of a novel CCR9 antagonist, Mol Oncol 9(8) (2015) 1599–611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Svensson M, Agace WW, Role of CCL25/CCR9 in immune homeostasis and disease, Expert Rev Clin Immunol 2(5) (2006) 759–73. [DOI] [PubMed] [Google Scholar]
- [22].Wang C, Liu Z, Xu Z, Wu X, Zhang D, Zhang Z, Wei J, The role of chemokine receptor 9/chemokine ligand 25 signaling: From immune cells to cancer cells, Oncol Lett 16(2) (2018) 2071–2077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Niu Y, Tang D, Fan L, Gao W, Lin H, CCL25 promotes the migration and invasion of non-small cell lung cancer cells by regulating VEGF and MMPs in a CCR9-dependent manner, Exp Ther Med 19(6) (2020) 3571–3580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Zhang Z, Sun T, Chen Y, Gong S, Sun X, Zou F, Peng R, CCL25/CCR9 Signal Promotes Migration and Invasion in Hepatocellular and Breast Cancer Cell Lines, DNA Cell Biol 35(7) (2016) 348–57. [DOI] [PubMed] [Google Scholar]
- [25].Xu B, Deng C, Wu X, Ji T, Zhao L, Han Y, Yang W, Qi Y, Wang Z, Yang Z, Yang Y, CCR9 and CCL25: A review of their roles in tumor promotion, J Cell Physiol 235(12) (2020) 9121–9132. [DOI] [PubMed] [Google Scholar]
- [26].Shen X, Mailey B, Ellenhorn JD, Chu PG, Lowy AM, Kim J, CC chemokine receptor 9 enhances proliferation in pancreatic intraepithelial neoplasia and pancreatic cancer cells, J Gastrointest Surg 13(11) (2009) 1955–62; discussion 1962. [DOI] [PubMed] [Google Scholar]
- [27].Khandelwal N, Breinig M, Speck T, Michels T, Kreutzer C, Sorrentino A, Sharma AK, Umansky L, Conrad H, Poschke I, Offringa R, Konig R, Bernhard H, Machlenkin A, Boutros M, Beckhove P, A high-throughput RNAi screen for detection of immune-checkpoint molecules that mediate tumor resistance to cytotoxic T lymphocytes, EMBO Mol Med 7(4) (2015) 450–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Heinrich EL, Arrington AK, Ko ME, Luu C, Lee W, Lu J, Kim J, Paracrine Activation of Chemokine Receptor CCR9 Enhances The Invasiveness of Pancreatic Cancer Cells, Cancer Microenviron 6(3) (2013) 241–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Wang W, Soto H, Oldham ER, Buchanan ME, Homey B, Catron D, Jenkins N, Copeland NG, Gilbert DJ, Nguyen N, Abrams J, Kershenovich D, Smith K, McClanahan T, Vicari AP, Zlotnik A, Identification of a novel chemokine (CCL28), which binds CCR10 (GPR2), J Biol Chem 275(29) (2000) 22313–23. [DOI] [PubMed] [Google Scholar]
- [30].Facciabene A, Peng X, Hagemann IS, Balint K, Barchetti A, Wang LP, Gimotty PA, Gilks CB, Lal P, Zhang L, Coukos G, Tumour hypoxia promotes tolerance and angiogenesis via CCL28 and T(reg) cells, Nature 475(7355) (2011) 226–30. [DOI] [PubMed] [Google Scholar]
- [31].Chaika NV, Gebregiworgis T, Lewallen ME, Purohit V, Radhakrishnan P, Liu X, Zhang B, Mehla K, Brown RB, Caffrey T, Yu F, Johnson KR, Powers R, Hollingsworth MA, Singh PK, MUC1 mucin stabilizes and activates hypoxia-inducible factor 1 alpha to regulate metabolism in pancreatic cancer, Proc Natl Acad Sci U S A 109(34) (2012) 13787–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Newman AM, Liu CL, Green MR, Gentles AJ, Feng W, Xu Y, Hoang CD, Diehn M, Alizadeh AA, Robust enumeration of cell subsets from tissue expression profiles, Nat Methods 12(5) (2015) 453–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Ohlund D, Handly-Santana A, Biffi G, Elyada E, Almeida AS, Ponz-Sarvise M, Corbo V, Oni TE, Hearn SA, Chio E.J. Lee II, Hwang CI, Tiriac H, Baker LA, Engle DD, Feig C, Kultti A, Egeblad M, Fearon DT, Crawford JM, Clevers H, Park Y, Tuveson DA, Distinct populations of inflammatory fibroblasts and myofibroblasts in pancreatic cancer, J Exp Med 214(3) (2017) 579–596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Gorchs L, Kaipe H, Interactions between Cancer-Associated Fibroblasts and T Cells in the Pancreatic Tumor Microenvironment and the Role of Chemokines, Cancers (Basel) 13(12) (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Fu XL, Duan W, Su CY, Mao FY, Lv YP, Teng YS, Yu PW, Zhuang Y, Zhao YL, Interleukin 6 induces M2 macrophage differentiation by STAT3 activation that correlates with gastric cancer progression, Cancer Immunol Immunother 66(12) (2017) 1597–1608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Monteran L, Erez N, The Dark Side of Fibroblasts: Cancer-Associated Fibroblasts as Mediators of Immunosuppression in the Tumor Microenvironment, Front Immunol 10 (2019) 1835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Mhaidly R, Mechta-Grigoriou F, Fibroblast heterogeneity in tumor micro-environment: Role in immunosuppression and new therapies, Semin Immunol 48 (2020) 101417. [DOI] [PubMed] [Google Scholar]
- [38].Desmouliere A, Geinoz A, Gabbiani F, Gabbiani G, Transforming growth factor-beta 1 induces alpha-smooth muscle actin expression in granulation tissue myofibroblasts and in quiescent and growing cultured fibroblasts, J Cell Biol 122(1) (1993) 103–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Geismann C, Schafer H, Gundlach JP, Hauser C, Egberts JH, Schneider G, Arlt A, NF-kappaB Dependent Chemokine Signaling in Pancreatic Cancer, Cancers (Basel) 11(10) (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Steele CW, Karim SA, Leach JDG, Bailey P, Upstill-Goddard R, Rishi L, Foth M, Bryson S, McDaid K, Wilson Z, Eberlein C, Candido JB, Clarke M, Nixon C, Connelly J, Jamieson N, Carter CR, Balkwill F, Chang DK, Evans TRJ, Strathdee D, Biankin AV, Nibbs RJB, Barry ST, Sansom OJ, Morton JP, CXCR2 Inhibition Profoundly Suppresses Metastases and Augments Immunotherapy in Pancreatic Ductal Adenocarcinoma, Cancer Cell 29(6) (2016) 832–845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Turner MD, Nedjai B, Hurst T, Pennington DJ, Cytokines and chemokines: At the crossroads of cell signalling and inflammatory disease, Biochim Biophys Acta 1843(11) (2014) 2563–2582. [DOI] [PubMed] [Google Scholar]
- [42].Zhou W, Guo S, Liu M, Burow ME, Wang G, Targeting CXCL12/CXCR4 Axis in Tumor Immunotherapy, Curr Med Chem 26(17) (2019) 3026–3041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Feig C, Jones JO, Kraman M, Wells RJ, Deonarine A, Chan DS, Connell CM, Roberts EW, Zhao Q, Caballero OL, Teichmann SA, Janowitz T, Jodrell DI, Tuveson DA, Fearon DT, Targeting CXCL12 from FAP-expressing carcinoma-associated fibroblasts synergizes with anti-PD-L1 immunotherapy in pancreatic cancer, Proc Natl Acad Sci U S A 110(50) (2013) 20212–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Jahan R, Shah A, Kisling SG, Macha MA, Thayer S, Batra SK, Kaur S, Odyssey of trefoil factors in cancer: Diagnostic and therapeutic implications, Biochim Biophys Acta Rev Cancer 1873(2) (2020) 188362. [DOI] [PubMed] [Google Scholar]
- [45].Hoffmann W, Trefoil Factor Family (TFF) Peptides and Their Links to Inflammation: A Reevaluation and New Medical Perspectives, Int J Mol Sci 22(9) (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Dubeykovskaya Z, Dubeykovskiy A, Solal-Cohen J, Wang TC, Secreted trefoil factor 2 activates the CXCR4 receptor in epithelial and lymphocytic cancer cell lines, J Biol Chem 284(6) (2009) 3650–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Graness A, Chwieralski CE, Reinhold D, Thim L, Hoffmann W, Protein kinase C and ERK activation are required for TFF-peptide-stimulated bronchial epithelial cell migration and tumor necrosis factor-alpha-induced interleukin-6 (IL-6) and IL-8 secretion, J Biol Chem 277(21) (2002) 18440–6. [DOI] [PubMed] [Google Scholar]
- [48].Wente MN, Mayer C, Gaida MM, Michalski CW, Giese T, Bergmann F, Giese NA, Buchler MW, Friess H, CXCL14 expression and potential function in pancreatic cancer, Cancer Lett 259(2) (2008) 209–17. [DOI] [PubMed] [Google Scholar]
- [49].Augsten M, Hagglof C, Olsson E, Stolz C, Tsagozis P, Levchenko T, Frederick MJ, Borg A, Micke P, Egevad L, Ostman A, CXCL14 is an autocrine growth factor for fibroblasts and acts as a multi-modal stimulator of prostate tumor growth, Proc Natl Acad Sci U S A 106(9) (2009) 3414–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Munkley J, The glycosylation landscape of pancreatic cancer, Oncol Lett 17(3) (2019) 2569–2575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Pinho SS, Reis CA, Glycosylation in cancer: mechanisms and clinical implications, Nat Rev Cancer 15(9) (2015) 540–55. [DOI] [PubMed] [Google Scholar]
- [52].Roshani R, McCarthy F, Hagemann T, Inflammatory cytokines in human pancreatic cancer, Cancer Lett 345(2) (2014) 157–63. [DOI] [PubMed] [Google Scholar]
- [53].Bellone G, Smirne C, Mauri FA, Tonel E, Carbone A, Buffolino A, Dughera L, Robecchi A, Pirisi M, Emanuelli G, Cytokine expression profile in human pancreatic carcinoma cells and in surgical specimens: implications for survival, Cancer Immunol Immunother 55(6) (2006) 684–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Bassaganas S, Allende H, Cobler L, Ortiz MR, Llop E, de Bolos C, Peracaula R, Inflammatory cytokines regulate the expression of glycosyltransferases involved in the biosynthesis of tumor-associated sialylated glycans in pancreatic cancer cell lines, Cytokine 75(1) (2015) 197–206. [DOI] [PubMed] [Google Scholar]
- [55].Zhang Y, Llapashtica K, Shinde S, Sellergren B, El-Schich Z, Wingren A, Determination of cytokine regulated glycan expression by using molecularly imprinted polymers targeting sialic acid, Journal of Cancer Metastasis and Treatment 2019 (2019).
- [56].Groux-Degroote S, Krzewinski-Recchi MA, Cazet A, Vincent A, Lehoux S, Lafitte JJ, Van Seuningen I, Delannoy P, IL-6 and IL-8 increase the expression of glycosyltransferases and sulfotransferases involved in the biosynthesis of sialylated and/or sulfated Lewisx epitopes in the human bronchial mucosa, Biochem J 410(1) (2008) 213–23. [DOI] [PubMed] [Google Scholar]
- [57].Mejias-Luque R, Linden SK, Garrido M, Tye H, Najdovska M, Jenkins BJ, Iglesias M, Ernst M, de Bolos C, Inflammation modulates the expression of the intestinal mucins MUC2 and MUC4 in gastric tumors, Oncogene 29(12) (2010) 1753–62. [DOI] [PubMed] [Google Scholar]
- [58].van Putten JPM, Strijbis K, Transmembrane Mucins: Signaling Receptors at the Intersection of Inflammation and Cancer, J Innate Immun 9(3) (2017) 281–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Burdick MD, Harris A, Reid CJ, Iwamura T, Hollingsworth MA, Oligosaccharides expressed on MUC1 produced by pancreatic and colon tumor cell lines, J Biol Chem 272(39) (1997) 24198–202. [DOI] [PubMed] [Google Scholar]
- [60].Cornelissen LA, Van Vliet SJ, A Bitter Sweet Symphony: Immune Responses to Altered O-glycan Epitopes in Cancer, Biomolecules 6(2) (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Lubbers J, Rodriguez E, van Kooyk Y, Modulation of Immune Tolerance via Siglec-Sialic Acid Interactions, Front Immunol 9 (2018) 2807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Daly J, Carlsten M, O’Dwyer M, Sugar Free: Novel Immunotherapeutic Approaches Targeting Siglecs and Sialic Acids to Enhance Natural Killer Cell Cytotoxicity Against Cancer, Front Immunol 10 (2019) 1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Gonzalez-Gil A, Schnaar RL, Siglec Ligands, Cells 10(5) (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Perdicchio M, Ilarregui JM, Verstege MI, Cornelissen LA, Schetters ST, Engels S, Ambrosini M, Kalay H, Veninga H, den Haan JM, van Berkel LA, Samsom JN, Crocker PR, Sparwasser T, Berod L, Garcia-Vallejo JJ, van Kooyk Y, Unger WW, Sialic acid-modified antigens impose tolerance via inhibition of T-cell proliferation and de novo induction of regulatory T cells, Proc Natl Acad Sci U S A 113(12) (2016) 3329–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Fraschilla I, Pillai S, Viewing Siglecs through the lens of tumor immunology, Immunol Rev 276(1) (2017) 178–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Rodriguez E, Boelaars K, Brown K, Eveline Li RJ, Kruijssen L, Bruijns SCM, van Ee T, Schetters STT, Crommentuijn MHW, van der Horst JC, van Grieken NCT, van Vliet SJ, Kazemier G, Giovannetti E, Garcia-Vallejo JJ, van Kooyk Y, Sialic acids in pancreatic cancer cells drive tumour-associated macrophage differentiation via the Siglec receptors Siglec7 and Siglec-9, Nat Commun 12(1) (2021) 1270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Beatson R, Tajadura-Ortega V, Achkova D, Picco G, Tsourouktsoglou TD, Klausing S, Hillier M, Maher J, Noll T, Crocker PR, Taylor-Papadimitriou J, Burchell JM, The mucin MUC1 modulates the tumor immunological microenvironment through engagement of the lectin Siglec-9, Nat Immunol 17(11) (2016) 1273–1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Malaker SA, Pedram K, Ferracane MJ, Bensing BA, Krishnan V, Pett C, Yu J, Woods EC, Kramer JR, Westerlind U, Dorigo O, Bertozzi CR, The mucin-selective protease StcE enables molecular and functional analysis of human cancer-associated mucins, Proc Natl Acad Sci U S A 116(15) (2019) 7278–7287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Belisle JA, Horibata S, Jennifer GA, Petrie S, Kapur A, Andre S, Gabius HJ, Rancourt C, Connor J, Paulson JC, Patankar MS, Identification of Siglec-9 as the receptor for MUC16 on human NK cells, B cells, and monocytes, Mol Cancer 9 (2010) 118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Eckardt V, Miller MC, Blanchet X, Duan R, Leberzammer J, Duchene J, Soehnlein O, Megens RT, Ludwig AK, Dregni A, Faussner A, Wichapong K, Ippel H, Dijkgraaf I, Kaltner H, Doring Y, Bidzhekov K, Hackeng TM, Weber C, Gabius HJ, von Hundelshausen P, Mayo KH, Chemokines and galectins form heterodimers to modulate inflammation, EMBO Rep 21(4) (2020) e47852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Berberat PO, Friess H, Wang L, Zhu Z, Bley T, Frigeri L, Zimmermann A, Buchler MW, Comparative analysis of galectins in primary tumors and tumor metastasis in human pancreatic cancer, J Histochem Cytochem 49(4) (2001) 539–49. [DOI] [PubMed] [Google Scholar]
- [72].Tang D, Gao J, Wang S, Yuan Z, Ye N, Chong Y, Xu C, Jiang X, Li B, Yin W, Miao Y, Wang D, Jiang K, Apoptosis and anergy of T cell induced by pancreatic stellate cells-derived galectin-1 in pancreatic cancer, Tumour Biol 36(7) (2015) 5617–26. [DOI] [PubMed] [Google Scholar]
- [73].Tang D, Yuan Z, Xue X, Lu Z, Zhang Y, Wang H, Chen M, An Y, Wei J, Zhu Y, Miao Y, Jiang K, High expression of Galectin-1 in pancreatic stellate cells plays a role in the development and maintenance of an immunosuppressive microenvironment in pancreatic cancer, Int J Cancer 130(10) (2012) 2337–48. [DOI] [PubMed] [Google Scholar]
- [74].Qian D, Lu Z, Xu Q, Wu P, Tian L, Zhao L, Cai B, Yin J, Wu Y, Staveley-O’Carroll KF, Jiang K, Miao Y, Li G, Galectin-1-driven upregulation of SDF-1 in pancreatic stellate cells promotes pancreatic cancer metastasis, Cancer Lett 397 (2017) 43–51. [DOI] [PubMed] [Google Scholar]
- [75].Zhao W, Ajani JA, Sushovan G, Ochi N, Hwang R, Hafley M, Johnson RL, Bresalier RS, Logsdon CD, Zhang Z, Song S, Galectin-3 Mediates Tumor Cell-Stroma Interactions by Activating Pancreatic Stellate Cells to Produce Cytokines via Integrin Signaling, Gastroenterology 154(5) (2018) 1524–1537 e6. [DOI] [PubMed] [Google Scholar]
- [76].Senapati S, Das S, Batra SK, Mucin-interacting proteins: from function to therapeutics, Trends Biochem Sci 35(4) (2010) 236–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Senapati S, Chaturvedi P, Chaney WG, Chakraborty S, Gnanapragassam VS, Sasson AR, Batra SK, Novel INTeraction of MUC4 and galectin: potential pathobiological implications for metastasis in lethal pancreatic cancer, Clin Cancer Res 17(2) (2011) 267–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Argueso P, Guzman-Aranguez A, Mantelli F, Cao Z, Ricciuto J, Panjwani N, Association of cell surface mucins with galectin-3 contributes to the ocular surface epithelial barrier, J Biol Chem 284(34) (2009) 23037–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Mammen MJ, Ali J, Aurora A, Sharma UC, Aalinkeel R, Mahajan SD, Sands M, Schwartz SA, IL-17 Is a Key Regulator of Mucin-Galectin-3 Interactions in Asthma, Int J Cell Biol 2021 (2021) 9997625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Cherfils-Vicini J, Iltis C, Cervera L, Pisano S, Croce O, Sadouni N, Gyorffy B, Collet R, Renault VM, Rey-Millet M, Leonetti C, Zizza P, Allain F, Ghiringhelli F, Soubeiran N, Shkreli M, Vivier E, Biroccio A, Gilson E, Cancer cells induce immune escape via glycocalyx changes controlled by the telomeric protein TRF2, EMBO J 38(11) (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]