Abstract
Objective:
Gut flora imbalance characterizes patients with chronic kidney disease (CKD). Although biotic supplementation has been proposed to lessen inflammation and oxidative stress and, thus, reduce the risk of progressive kidney damage and cardiovascular disease, the effects remain controversial. We conducted a meta-analysis to assess the therapeutic benefits of biotics in CKD.
Methods:
PubMed, Embase, and Cochrane databases were systematically searched for randomized controlled trials that evaluated any biotic (prebiotic, probiotic, synbiotics) supplements in patients with CKD (CKD, stage 3–4 to end-stage renal disease). Primary endpoints included changes in renal function, markers of inflammation, and oxidative stress. Secondary endpoints included changes in levels of uremic toxins and variations in lipid metabolism.
Results:
Twenty-three eligible studies included 842 participants. In a pooled-analysis, biotics did not change estimated glomerular filtration rate (mean difference [MD] = 0.08, P = .92) or serum albumin (MD = −0.01, P = .86), although prebiotics reduced serum creatinine (standardized mean difference [SMD] = −0.23, P = .009) and blood urea nitrogen (MD = −6.05, P <.00001). Biotics improved total antioxidative capacity (SMD = 0.37, P = .007) and malondialdehyde (SMD = −0.96, P = .006) and reduced the inflammatory marker interleukin-6 (SMD = −0.30, P = .01) although not C-reactive protein (SMD = −0.22, P = .20). Biotic intervention reduced some uremic toxins, including p-cresol sulfate (SMD = −2.18, P <.0001) and indoxyl sulfate (MD = −5.14, P = .0009), which decreased in dialysis-dependent patients. Another toxin, indole-3-acetic acid (MD = −0.22, P = .63), did not change. Lipids were unaffected by biotic intervention (total cholesterol: SMD = −0.01, P = .89; high-density lipoprotein: SMD = −0.08, P = .76; low-density lipoprotein: MD = 3.54, P = .28; triglyceride: MD = −2.26, P = .58).
Conclusion:
The results highlight the favorable influence of biotics on circulating markers of creatinine, oxidant stress (malondialdehyde, total antioxidative capacity), inflammation (interleukin-6), and uremic toxins (p-cresol sulfate) in patients with CKD. Biotics did not affect estimated glomerular filtration rate, albumin, indole-3-acetic acid, or lipids in either predialysis or dialysis patients.
Introduction
CHRONIC KIDNEY DISEASE (CKD) is a global health problem affecting more than 9% of the world population, a percentage that continues to increase each year.1,2 The mortality rates in patients with CKD at every level of renal impairment are much higher than those of the general population (117.9/1000 vs. 47.5/1000).3 The increased risk of death has been linked to a chronic state of inflammation/oxidative stress and malnutrition and a high prevalence of hypertension, diabetes, and cardiovascular disease (CVD).4,5
Reduced renal function causes accumulation of urea that is hydrolyzed by intestinal microenzymes producing ammonia.6 This process results in uremic enterocolitis and leads to an abnormal gut flora composition.7 Increase in uremic toxins caused by CKD-associated intestinal microbiome dysbiosis, in turn, contributes to CKD progression and other untoward consequences of CKD, most notably, CVD.8 As CKD is well established as a strong modifier of the composition and metabolism of intestinal microbiome, there has been considerable interest in interventions to restore normal intestinal biotics, to lessen uremic toxins and inflammatory/oxidative stress factors, and to slow CKD progression and CVD risk. Interventions have included the use of biotics,9 including probiotics, defined as living microorganisms that add to the population of good bacteria10; prebiotics, which are a nondigestive food ingredient that induces the growth and activity of intestinal beneficial bacteria; and synbiotics, which include both. These interventions are thought to act by competing with harmful flora for nutrients, inhibiting their adhesion, and protecting the integrity of the intestinal barrier; they may also limit immune activation and proinflammatory signaling.11 Support for these mechanisms comes from animal studies showing a positive effect of biotics in animal models of CKD.12,13 However, results from human studies have been controversial, and randomized clinical trials (RCTs) have studied only limited populations and reached different conclusions.
Previous meta-analyses have been limited by including only one stage of CKD and/or one type of biotic intervention. For example, Tao et al.,14 Thongprayoon et al.,15 and Liu Tet al.16 explored only the effects of probiotics on patients with CKD stage 3 to end-stage renal disease (ESRD). Rossi et al.17 and March DS et al.18 found biotic supplementation reduced serum indoxyl sulfate (IS) and p-cresol sulfate (p-CS) of dialysis-dependent patients. The effectiveness of biotic intervention on a larger pre-ESRD and dialysis-dependent population is unclear. In view of the limited therapies to slow progressive kidney damage and associated adverse consequences, we conducted a meta-analysis to determine the possible beneficial utility of biotics in patients with CKD across a range of kidney dysfunction.
Materials and Methods
This meta-analysis was performed according to the preferred reporting items for systematic reviews and meta-analyses (PRISMA) guideline.
Data Source and Search Strategy
Two authors independently identified relevant articles by doing a systematic search in PubMed, Embase, and Cochrane databases without time or language restriction up to 31 September 2020. The search strategies are provided in Appendix A. In addition, the authors manually screened the reference lists of relevant articles for additional eligible articles.
Selection Criteria
All eligible articles met the following criteria: (1) randomized-control study on humans; (2) patients diagnosed with at least stage-3 CKD (estimated glomerular filtration rate (eGFR) < 60 mL/min/1.73 m2) through ESRD requiring renal replacement therapy by hemodialysis (HD) or peritoneal dialysis (PD); and (3) in the intervention group, patients were treated with biotic supplements (prebiotics, probiotics, or synbiotics) for at least 4 weeks. The control subjects received placebo or standard treatment. Studies with the following characteristics were excluded: (1) compared different dosages of the same intervention; (2) used the same population data in multiple studies or were repetitive publications; (3) crossover RCTs that failed to provide baseline data or to provide data for each study period; (4) failed to provide data on outcomes of interest (renal function: eGFR, serum creatinine [SCr], blood urea nitrogen [BUN], and albumin; uremic toxin: p-CS, indole-3-acetic acid [IAA], and IS; inflammation markers: interleukin-6 [IL-6] and C-reactive protein [CRP]; antioxidative markers: total antioxidative capacity [TAC] and malondialdehyde [MDA]; lipid metabolism index: total cholesterol [TC], triglyceride [TG], high-density lipoprotein [HDL], and low-density lipoprotein [LDL]).
Data Extraction
Two authors extracted data independently using a data-collecting form. Study characteristics, including title, name of first author, year of publication, country, type of study, sample size, type of intervention, duration, and follow-up period were recorded. Patient characteristics including age, sex, CKD stage, and dialysis type were also recorded.
Data Analysis
Two authors analyzed the data using Review Manager (RevMan, version 5.4; the Nordic Cochrane Center, the Cochrane Collaboration, Copenhagen, Denmark) and Stata/SE (version 15.1; StataCorp LP, College Station, TX). Meta-analyses were conducted for outcomes reported by more than 2 studies. Fixed-effects models were initially applied to combine pooled data, while random-effects models were used when heterogeneity was present. Outcomes were assessed as mean difference (MD) or MD of preintervention value minus postintervention value with 95% confidence interval (CIs) (standardized mean differences [SMDs]). The outcomes are presented as SMD if they were measured in a variety of ways.
After assessment by Cochran’s test I2 statistic, heterogeneity across the studies were defined as insignificant (I2 ≤ 25%), low (25% < I2 ≤ 50%), moderate (50% < I2 ≤ 75%), and high (I2 >75%) heterogeneity. To identify the source of heterogeneity, sensitivity analyses were performed according to the size of the population, dosage, type of biotics, intervention duration, and follow-up period. Subgroup analyses were performed to assess the possibility that heterogeneity stems from ESRD patients undergoing dialysis versus predialysis patients with CKD. Additional sensitivity analyses were conducted to explore the impact of a single article on the results. In addition, 2 reviewers (J.L. and H.Y.) performed quality assessment by using Cochrane Collaboration methodology19 to investigate possible bias of single RCTs, including potential selection bias, performance bias, detection bias, attrition bias, and other sources of bias.
Results
Search Results
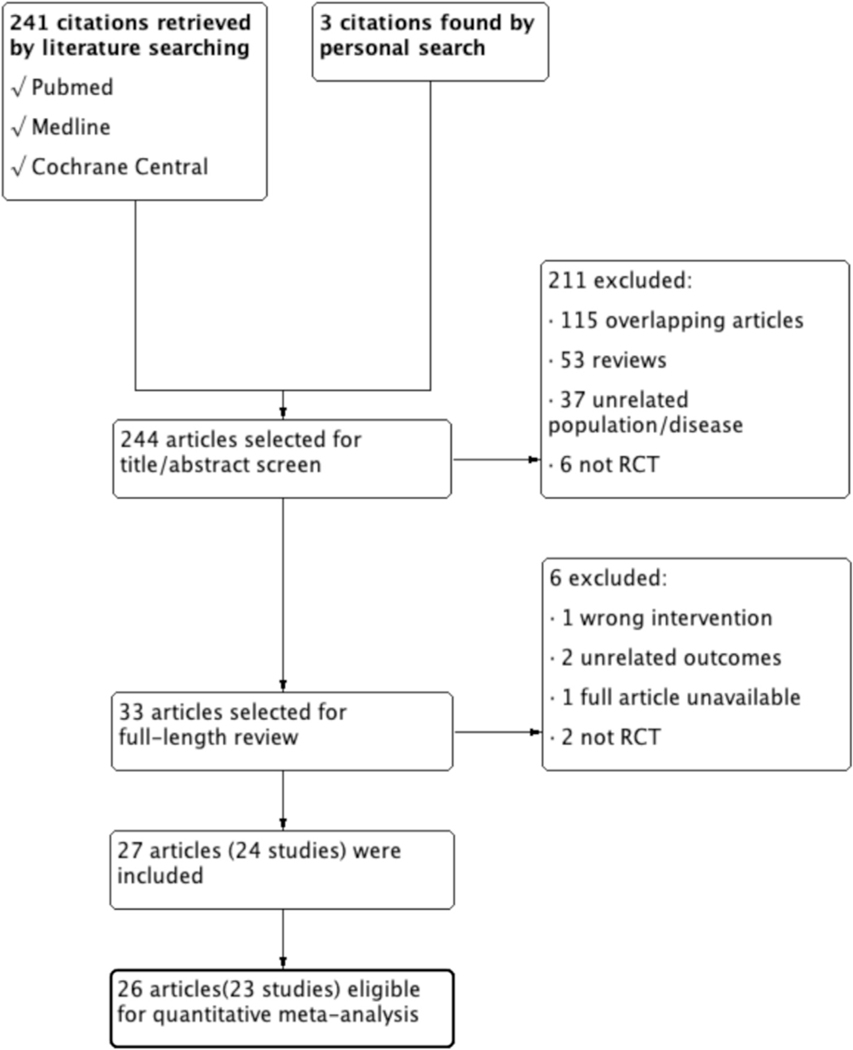
We found 244 articles (241 by electronic search and 3 by manual search). Of these, the review team excluded 211 articles from the initial screening. After assessing the remaining 33 full text articles for eligibility, we eliminated 6 additional articles that failed to meet preset criteria. Reasons for exclusion included the use of various interventions (n = 1); readouts that differed from preset outcomes (n = 2); inability to access the full text (n = 1); and studies that were not RCTs (n = 2). Twenty-four studies were reported in 27 articles, and a subsequent exclusion process yielded 23 randomized controlled trials (in 26 articles). These were then subjected to qualitative and quantitative analyses. A diagram of the selection and exclusion process is illustrated in Figure 1.
Figure 1.
Flow diagram of the literature search process.
Search Characteristics
Of the 23 studies, four were conducted in the United States,20–23 four in Brazil,24–27 three in China,28–30 six in Iran,31–36 two in Italy,37,38 one in Japan,39 one in Australia,40 one in France,41 and one in Mexico.42 In total, the studies enrolled 842 patients with CKD. The mean age spanned 30.6 ± 9.5 to 69.0 ± 10.0 years, and the percentage of males ranged from 27.27% to 86.67%. Study patients were diagnosed with CKD stage 3–4 in 7 RCTs.22,27,36–38,40,41 Fourteen RCTs20,21,23–26,29,31–35,39,42 were performed in patients on maintenance HD for at least 3 months, and 2 articles28,30 reported data in a PD population maintained for at least 1 month. Follow-up duration ranged from 1 to 6 months with a mean of 2.38 months (Supplemental Table 1).
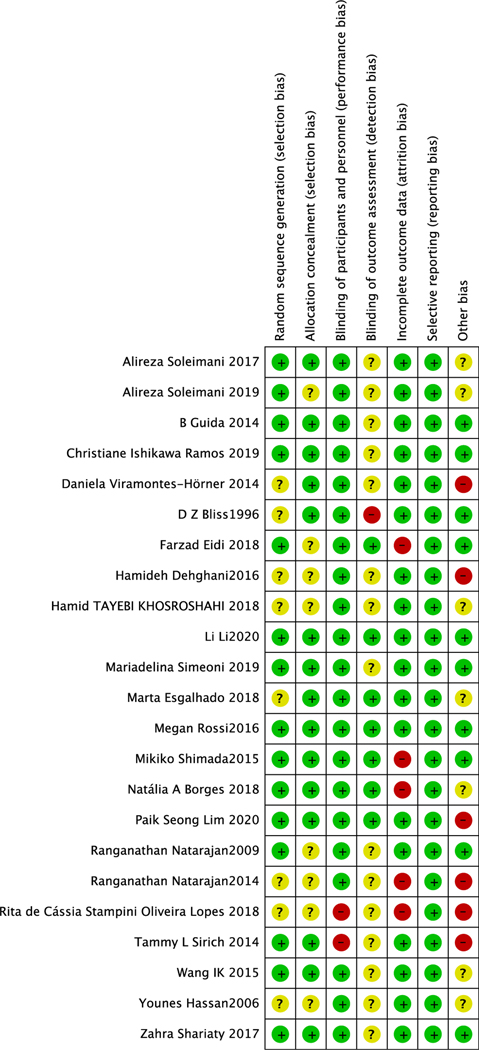
Risk of Bias Among Studies
Fifteen studies20,22,24,27–31,33–35,37,39,40 provided information of random sequence, and 15 studies20,21,24,25,27–30,33,35,37,39,40,42 provided information on allocation concealment. Seven studies were triple-blinded,24,29,30,39,40,43,44 3 studies were single-blinded,21,26,45 and 13 had a double-blind design.22,23,27,28,32–37,41,42 Five articles23,24,26,39,43 reported a high drop-out rate (>21%). Reporting bias was low in all studies. Five studies20,26,29,39,42 had a high bias because of funding source while another 10 studies21,22,27,30,31,33,37,39,40 reported no source of funding. Six of the 15 studies were at low risk of bias,27,30,33,37,40 10 studies had high risk of bias,20,21,23,24,26,29,31,36,39,42 and in the remaining 7 studies, the bias risk was unclear22,25,28,32,34,35,41 (Figure 2).
Figure 2.
Risk of bias in analyzed studies. Unclear risk of bias: “?”, low risk of bias: “−”, and high risk of bias: “+”.
Effects of Biotics on the Primary Outcomes
Renal Function
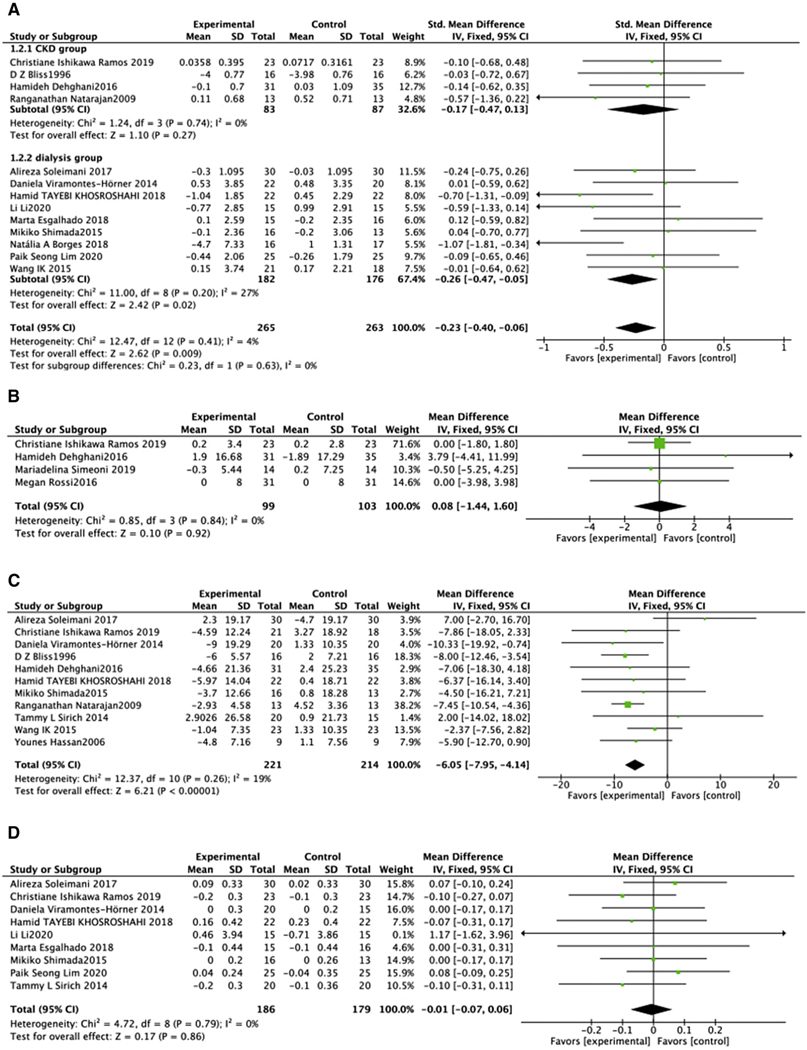
Four parameters of renal function were assessed: SCr, eGFR, serum albumin, and BUN. Biotic intervention did not affect SCr in predialysis cohorts (SMD = −0.17, 95% CI: −0.47, 0.13, P =.27), while Scr decreased in dialysis subgroup (SMD = −0.26, 95% CI: −0.47, −0.05, P 5 .02) (Figure. 3A).21,22,24,25,27–30,32,35,36,39,42 Dialysis population showed moderate heterogeneity which could not be adjusted by sensitivity analysis. Similarly, biotic intervention did not affect eGFR (MD = 0.08, 95% CI: −1.44, 1.60, P = .92). This analysis had no heterogeneity in the predialysis population (Chi2 = 0.85, P = .84, I2 = 0%) (Figure. 3B).27,36,40 Analysis of the effects of biotic intervention on BUN in all subjects revealed a decrease in treated versus untreated groups (MD = −6.05, 95% CI: −7.95, −4.14, P <.00001), with a relative insignificant heterogeneity (I2 = 19%)20–22,27,28,32,34,36,39,41,42 (Figure 3C).
Figure 3.
Effect of biotic intervention on renal function. (A) Effect of biotics on eGFR; (B) effect of biotics on SCr; (C) effect of biotics on BUN; (D) effect of biotics on albuminuria. BUN, blood urine nitrogen; eGFR, estimated glomerular filtration rate; SCr, serum creatinine.
The effect of biotic intervention on serum albumin was assessed in the 9 trials.20,25,27,29,30,32,35,39,42 No significant change in serum albumin level was found (MD = −0.01, 95% CI: −0.07, 0.06, P =.86, I2 = 0%) (Figure. 3D).
Inflammatory Index
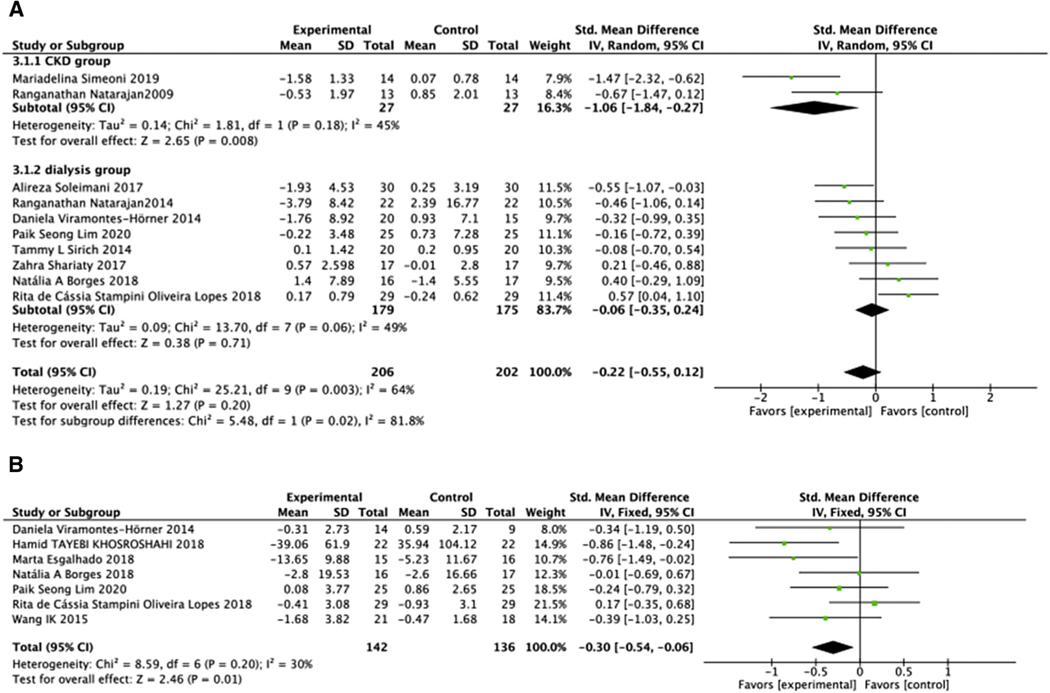
CRP level in 408 patients before dialysis and with ESRD-D in 10 studies20,22–24,26,29,33,35,38,42 showed no significant difference between treated and untreated groups (SMD = −0.22, 95% CI: −0.55, 0.12, P = .20) (Figure 4A). This comparison had moderate intergroup heterogeneity (Chi2 = 25.21, P = .003, I2 = 64%) that was not linked to the type of treatment, duration of treatment, degree of renal impairment, or type of renal replacement. IL-6 was reported only in ESRD-HD. While meta-analysis showed that biotics decreased the IL-6 level (SMD = −0.30, 95% CI: −0.54, −0.06, P = .01), with a low heterogeneity (Chi2 = 8.59, P = .20, I2 = 30%) (Figure 4B).
Figure 4.
Effect of biotic intervention on the inflammatory index. (A) Effect of biotics on CRP; (B) effect of biotics on IL-6. CRP, C-reactive protein; IL-6, interleukin-6.
Biomarkers of Oxidative Stress
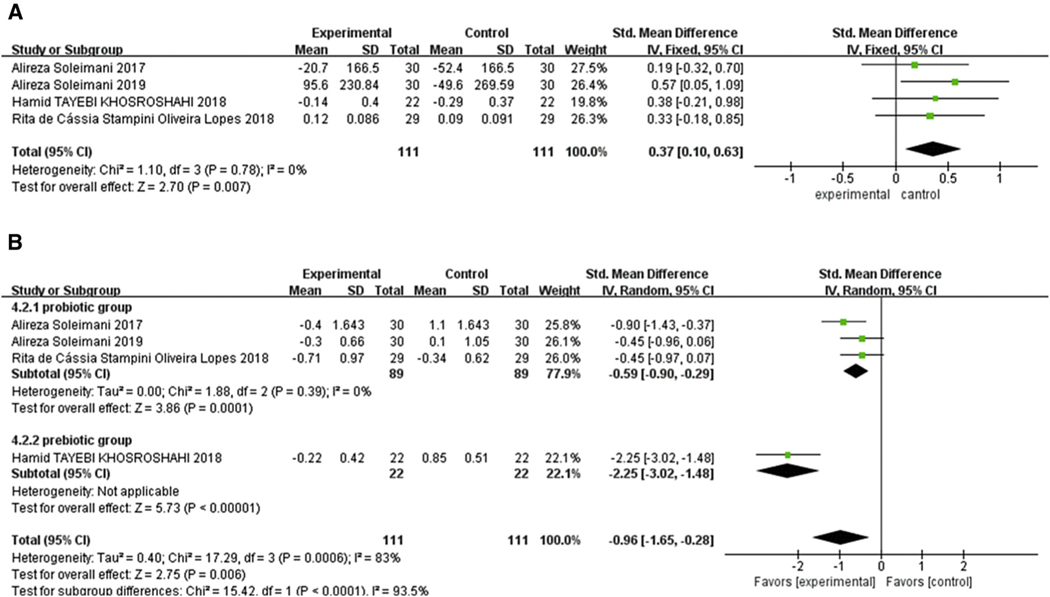
Analysis of oxidative stress markers in 222 patients in 4 studies found that biotic treatment significantly improved TAC (SMD = 0.37, 95% CI: 0.10, 0.63, P =.007). There was no heterogeneity (Chi2 = 1.1, P = .78, I2 = 0%) (Figure 5A).26,32,34,35 Analysis of MDA was also significantly reduced by biotic intervention (SMD = −0.96, 95% CI: −1.65, −0.28, P =.006). Subgroup analysis identified the biotic type as the source of high intergroup heterogeneity (Chi2 = 17.29, P = .00006, I2 = 83%). Both prebiotic subgroup (SMD = −2.25, 95% CI: −3.02 to −1.48, P <.00001) and probiotic and synbiotic subgroup (SMD = −0.59, 95% CI: −0.90 to −0.29, P = .0001, I2 = 0%) showed a significant decrease in MDA level (Figure 5B).
Figure 5.
Effect of biotic intervention on antioxidative capacity. (A) Effect of biotics on TAC; (B) effect of biotics on MDA. MDA, malondialdehyde; TAC, total antioxidative capacity.
Effects of Biotics on the Secondary Outcomes
Uremic Toxins
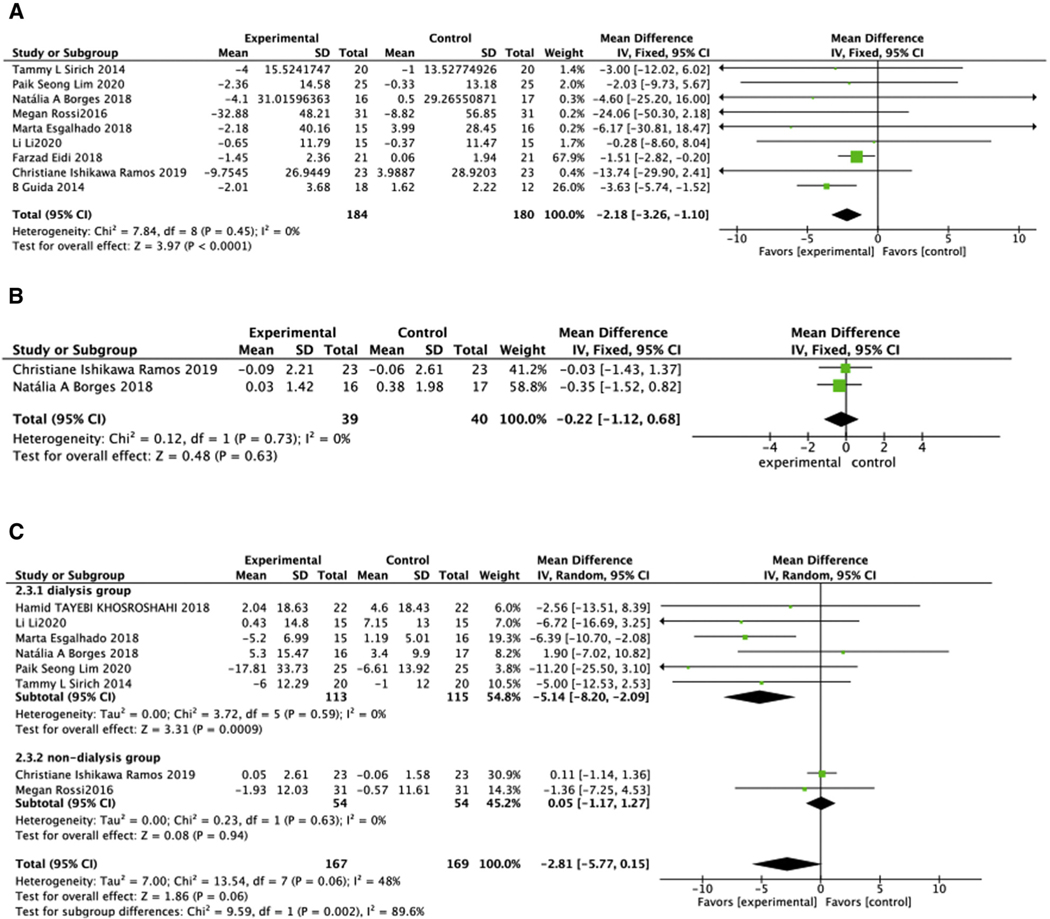
Effects of biotic intervention were analyzed across the entire CKD population (predialysis and ESRD). Biotics significantly reduced serum p-CS concentration (MD = −2.18, 95% CI: −3.26, −1.10, P <.0001) (Figure 6A) across the CKD population20,24,25,27,29–31,37,40 (Figure 6A). IAA level was not affected by biotic intervention (MD = −0.22, 95% CI: −1.12, 0.68, P = .63) (Figure 6B).24,27 Neither p-CS nor IAA analysis showed heterogeneity. In contrast, biotic treatment significantly reduced IS (MD = −2.81, 95% CI: −5.77, 0.15, P =.06, I2 = 48%). This benefit was observed in the dialysis group20,24,25,29,30,32 (MD = −5.14, 95% CI: −8.20, −2.09, P =.0009, I2 = 0%) although not in predialysis-CKD population27,40 (MD = 0.05, 95% CI: −1.17, 1.27, P =.94) (Figure 6C).
Figure 6.
Effect of biotic intervention on uremic toxins. (A) Effect of biotics on p-CS; (B) effect of biotics on IAA; (C) effect of biotics on IS. IAA, indole-3-acetic acid; IS, indoxyl sulfate; p-CS, p-cresol sulfate.
Lipid Metabolism
Available lipid data included TC, TG, LDL, and HDL. Data on TC change were reported for 359 participants in 8 trials.27,29,32,34,35,38,39,42 The pooled analysis found no significance in total cholesterol difference between treated and untreated groups (SMD = −0.01, 95% CI: −0.22, 0.19, P = .89, with no heterogeneity I2 = 0%) (Supplemental Figure 1A). Biotic intervention also did not cause a consistent change in HDL levels (SMD = −0.08, 95% CI: −0.59, 0.43, P = .76). The considerable heterogeneity was not linked to the type of treatment, duration of treatment, degree of renal impairment, or type of renal replacement (Chi2 = 28.02, P <.0001, I2 = 79%)27,32,34,35,42,46 (Supplemental Figure 1B). Assessment of LDL suggested no significant difference between the treated and untreated groups (LDL: MD = 3.54, 95% CI: −2.88, 9.96, P = .28; Supplemental Figure 1C).27,35,42,47 Biotics also did not affect TG levels (Supplemental Figure 1D); MD = −2.26, 95% CI: −10.20, 5.68, P = .58).27,29,32,35,38,42,47 Both analysis of LDL and TG showed no heterogeneity (LDL: P = .74, I2 = 0%; TG: P =.51, I2 = 0%).
Discussion
This meta-analysis of 15 RCTs of 605 subjects with various degrees of kidney dysfunction revealed biotics improve several parameters associated with CKD. Biotics reduced the uremic toxin, P-cresol, and, in the dialysis cohort, IS. Biotic intervention lessened markers of oxidative stress as well as the inflammatory marker IL-6. Biotic intervention also reduced CRP in the predialysis but not dialysis patients. Biotics did not affect markers of renal function in these population. Most of the RCTs used prebiotics, which appeared more effective in reducing the adverse markers than probiotics. Indeed, treatment with prebiotics, but not probiotics, reduced levels of BUN and IL-6 in both predialysis and dialysis populations.
Renal disease, even a mild reduction in kidney function, leads to profound and functional changes in the gastrointestinal structure flora.38 The contributing mechanisms include restricted diet,48 slowed intestinal transit,49 contraction in the total richness of the microbial community,50 and antibiotic exposure.48 The consequences of these modifications are complicated by CKD-induced disruption in the intestinal barrier functions that can lead to bacterial translocation and endotoxemia, which in turn initiate an innate immune response and proinflammatory signaling characterizing the microinflammatory state prevailing in CKD.8 In the last 10 years, the gut microbiota and its bioactive metabolites have been recognized as having a key role in progressive kidney damage and adverse extrarenal consequences, most notably, CVD.51–53 It has been shown that targeting the intestinal microbiome with oral biotics can lessen inflammation and oxidant stress and reduce progressive CKD and CVD. Recently, Sueyoshi et al. showed oral administration of prebiotics for 1 month in CKD rats reduced the uremic toxin IS.54 Biotic treatment also has benefits on CVD through improving colonic epithelial dysfunction, which has been shown to predict incidence of CVD.55 However, other studies have not supported these observations.20,24,27 Thus, the effectiveness of biotic intervention on progressive deterioration of renal function, inflammation, oxidant stress, and their consequences is unsettled.
Biotics intervention attenuated systemic markers of oxidant stress and inflammation, which characterize kidney disease and prevail at all levels of renal dysfunction. Endogenous and exogenous uremic toxins contribute to increasing reactive oxygen species expression in patients with CKD.56 Oxidative stress is a potential trigger for many complications, including CVD,57 endothelial dysfunction,58 anemia,59 and encephalopathy.60 TAC was increased across the entire CKD and ESRD population. Similarly, biotics reduced circulating levels of MDA across the entire CKD population, and all RCTs involving patients with ESRD reported this reduction. Consistent with our finding, Karimi et al.61 and Kwak et al.62 observed that prebiotics improved MDA concentration and augmented TAC in patients with prediabetes and diabetes, respectively. Vaziri et al. were the first to use prebiotics to ameliorate oxidative stress in chronic nephropathy rats.63 Increasing beneficial flora, such as Bifidobacteriaceae and Lactobacilli, after using biotics reduces free radicals generation, which can partly explain this advantageous effect.64,65 Another proposed mechanism to explain the action of biotics on patients with CKD was through hindering lipid peroxidation and improving antioxidative enzymes activity.66
CKD-related oxidant stress is usually accompanied by increased inflammatory markers thought to amplify the common adverse consequences of CKD. The underlying mechanisms involve a biotic imbalance in the gut flora, which disrupts the colonic epithelial barrier, permitting entry of toxic luminal contents into circulation and leading to inflammation.63 Kieffer et al.67 reported prebiotic treatment of rats with CKD reduced blood levels of gut microbe–derived metabolites. Prebiotics and probiotics also serve as substrates for fermentation that produces short-chain fatty acids (SCFAs), including butyrate, which preserve colonic mucosa and support beneficial regulatory T cells.68–70 By inhibiting production of tumor necrosis factor and proinflammatory cytokine, butyrate has been used for treating colonic inflammation in patients with Crohn’s disease.71 Our analysis showed prebiotic supplement significantly reduced IL-6 compared with the control group. CRP was also reduced in the predialysis cohort, although not in the ESRD cohort. Ma et al.72 found that in postmenopausal women, prebiotic intervention reduced IL-6 expression but not levels of CRP. As CRP generation is downstream from IL-6, it is possible that IL-6 may be more sensitive to prebiotics treatment than CRP. Indeed, Cesari M et al. proposed that IL-6 level is a stronger predictor of cardiovascular events than CRP.73 Another reason may relate to high doses (>1 × 109 CFU) of probiotics used in the included studies. Previous reports showed low-dose probiotics have better curative effect on CRP, while high-dose prebiotics are not as effective.74,75 Indeed, another meta-analysis not focusing on CKD showed the CRP decreased with low doses (108 ~109 CFU) of probiotics.76
Meta-analysis reported by Yang HL et al. found decreased IS, p-CS, and BUN after fiber supplementation in patients with CKD, while creatinine unchanged.77 Similarly, our meta-analysis found that biotics do not improve the classical markers of renal function including eGFR or albumin in predialysis patients, reflecting the complexity of factors underlining progressive kidney disease and the short duration of treatment undertaken in these studies. Although biotics would not be expected to affect renal function in ESRD, prebiotic decreased BUN within the ESRD cohort. This reduction may reflect extra-renal influences (e.g., hepatic production of urea). Indeed, biotics reduced the levels of several uremic toxins that reflect both renal elimination and generation and metabolism of molecules. Thus, our analysis showed a beneficial effect of biotics in reducing p-CS in both predialysis and dialysis-requiring patients, which may reflect decreasing p-CS-producing bacteria in the gut of biotic-treated patients, as eGFR remained unchanged. Probiotics contain normal microbiome, at same time, prebiotics promote these non–p-CS-producing flora that may ultimately compete with and replace p-CS-producing bacteria. Another toxin, IS, was also reduced by biotic intervention in dialysis but not predialysis subjects. This distinct effect may relate to lower levels of IS prevailing in predialysis versus dialysis patients and, thus, more easily modulated by therapeutic interventions.20,24,25 Also, while >90% of IS is protein-bound and, therefore, not dialyzable, the small non-protein-bound fraction may be dialyzed and contribute to salutary effects of biotic treatment.78,79 Food intake, especially the ratio of protein and fiber, is an important potential effect on uremic toxin. IS and p-CS were derived from protein fermentation in colon, while fiber limits this process.80 However, this important factor is seldom reported, and our analysis does not include this parameter. Future RCTs should provide the type and amount of nutrition during the course of the studies.
Our analysis showed that type of biotics is a consistent source of between-groups heterogeneity. Furthermore, we showed that prebiotics have greater beneficial effects on BUN, IL-6, and MDA concentrations than probiotics. One reason may be prebiotics not only selectively stimulate the quantity and quality of Bifidobacteria and Lactobacilli species but also inhibit the increase of harmful flora.81 Colon microbes can break fiber into SCFAs, which nourish the intestinal flora growth.82 Thus, under the stimulation of fiber, microbes prefer to use amino acids for growth rather than transforming them into toxic materials.81
This study has some limitations. These include the relatively small sample size and short duration of intervention and follow-up periods. Three to 4-week intervention may be too short to achieve significant changes in humans. Future studies should consider free concentration of uremic toxins, as these are more likely to interact with body tissues than protein-bound toxins.46,83 As SCFA is a vital intermediary in the reduction of urea as well as the improvement of systemic inflammation and oxidative stress,84,85 future studies should report SCFA levels. Another limitation relates to the low-to-moderate heterogeneity among the studies, although the subgroup and sensitivity analysis was conducted to reduce the effect of this limitation. The strength of this meta-analysis is a narrow selection range that includes only RCTs, excluding cross-over RCTs that failed to provide data of baseline and each period. We also assessed a large number of outcomes without restrictions of follow-up period, intervention type, and CKD stage. Through these criteria, we maximized the collected information while minimizing selection bias and other potential bias.
Conclusions
Biotics supplementation reduces circulating several markers of oxidant stress (MDA, TAC) and inflammation (IL-6) in predialysis CKD as well as in ESRD patients requiring dialysis. However, biotics do not affect eGFR, creatinine, albumin, lipids, and other uremic toxins (p-CS, IAA). Studies of longer period and with larger scales are advocated to further understand the relationship between biotics and renal disease.
Supplementary Material
Acknowledgments
The authors thank Yvonne Poindexter, MA, for editorial contributions to the article.
Funding:
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Footnotes
Practical Applications
The effects of biotics, including probiotics, prebiotics, and synbiotics, on patients with CKD are still controversial. Our study indicates that administration of biotics can benefit patients with CKD in terms of oxidative stress, inflammation, and uremic toxins. More evidence is still needed to confirm the effect of biotics.
Credit Authorship Contribution Statement
Jing Liu: Conceptualization, Data curation, Formal analysis, Investigation, Writing - original draft. JianYong Zhong: Conceptualization, Formal analysis, Writing - original draft. HaiChun Yang: Conceptualization, Investigation, Writing - reviewing, Editing - original draft. DongQin Wang: Data curation, Investigation. Ying Zhang: Resources. YuMeng Yang: Resources. GuoLan Xing: Resources, Supervision. Valentina Kon: Conceptualization, Writing, Reviewing, Editing - original draft, Project administration.
Supplementary Data
Supplementary data related to this article can be found at https://doi.org/10.1053/j.jrn.2021.08.005.
Conflicts of Interest: The authors declare no conflict of interest.
References
- 1.Bikbov B, Purcell CA, Levey AS, et al. Global, regional, and national burden of chronic kidney disease, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2020;395:709–733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Saran R, Robinson B, Abbott KC, et al. US renal data System 2019 Annual data report: Epidemiology of kidney disease in the United States. Am J Kidney Dis. 2020;75(1 Suppl 1):A6–A7. [DOI] [PubMed] [Google Scholar]
- 3.Kidney disease statistics for the United States. Available from: https://www.niddk.nih.gov/health-information/health-statistics/kidney-disease. Accessed January 12, 2020.
- 4.Anderson S, Halter JB, Hazzard WR, et al. Prediction, progression, and outcomes of chronic kidney disease in older adults. J Am Soc Nephrol.2009;20:1199–1209. [DOI] [PubMed] [Google Scholar]
- 5.Jha V, Garcia-Garcia G, Iseki K, et al. Chronic kidney disease: global dimension and perspectives. Lancet. 2013;382:260–272. [DOI] [PubMed] [Google Scholar]
- 6.Sud K, Sakhuja V. The gastrointestinal tract in uremia. J Assoc Phys India.1997;45:833–834. [PubMed] [Google Scholar]
- 7.Mills KT, Xu Y, Zhang W, et al. A systematic analysis of worldwide population-based data on the global burden of chronic kidney disease in 2010. Kidney Int. 2015;88:950–957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wu IW, Hsu KH, Lee CC, et al. p-Cresyl sulphate and indoxyl sulphate predict progression of chronic kidney disease. Nephrol Dial Transpl.2011;26:938–947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schrezenmeir J, de Vrese M. Probiotics, prebiotics, and synbiotics–approaching a definition. Am J Clin Nutr. 2001;73(2 Suppl):361s–364s. [DOI] [PubMed] [Google Scholar]
- 10.Gibson GR, Probert HM, Loo JV, Rastall RA, Roberfroid MB. Dietary modulation of the human colonic microbiota: updating the concept of prebiotics. Nutr Res Rev. 2004;17:259–275. [DOI] [PubMed] [Google Scholar]
- 11.van Baarlen P, Troost F, van der Meer C, et al. Human mucosal in vivo transcriptome responses to three lactobacilli indicate how probiotics may modulate human cellular pathways. Proc Natl Acad Sci U S A. 2011;108(Suppl 1):4562–4569. Suppl 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ranganathan N, Patel BG, Ranganathan P, et al. In vitro and in vivo assessment of intraintestinal bacteriotherapy in chronic kidney disease. ASAIO J. 2006;52:70–79. [DOI] [PubMed] [Google Scholar]
- 13.Bowman LM, Holt PG. Selective enhancement of systemic Th1 immunity in immunologically immature rats with an orally administered bacterial extract. Infect Immun. 2001;69:3719–3727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tao S, Tao S, Cheng Y, Liu J, Ma L, Fu P. Effects of probiotic supplements on the progression of chronic kidney disease: a meta-analysis. Nephrology (Carlton). 2019;24:1122–1130. [DOI] [PubMed] [Google Scholar]
- 15.Thongprayoon C, Kaewput W, Hatch ST, et al. Effects of probiotics on inflammation and uremic toxins among patients on dialysis: a systematic review and meta-analysis. Dig Dis Sci. 2019;64:469–479. [DOI] [PubMed] [Google Scholar]
- 16.Liu T, Wang X, Li R, Zhang ZY, Fang J, Zhang X. Effects of probiotic preparations on inflammatory cytokines in chronic kidney disease patients: a systematic review and meta-analysis. Curr Pharm Biotechnol. 2021;22:1338–1349. [DOI] [PubMed] [Google Scholar]
- 17.Rossi M, Klein K, Johnson DW, Campbell KL. Pre-, pro-, and synbiotics: do they have a role in reducing uremic toxins? A systematic review and meta-analysis. Int J Nephrol. 2012;2012:673631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.March DS, Jones AW, Bishop NC, Burton JO. The efficacy of prebiotic, probiotic, and synbiotic supplementation in modulating gut-derived circulatory particles associated with cardiovascular disease in individuals receiving dialysis: a systematic review and meta-analysis of randomized controlled trials. J Ren Nutr. 2020;30:347–359. [DOI] [PubMed] [Google Scholar]
- 19.R M. Cochrane systematic review and meta-analysis of the impact of psychological treatments for people with epilepsy on health-related quality of life. Epilepsia. 2018;59(2):315–332. 2018. [DOI] [PubMed] [Google Scholar]
- 20.Sirich TL, P N, Gardner CD, Hostetter TH, Meyer TW. Effect of increasing dietary fiber on plasma levels of colon-derived solutes in hemodialysis patients 2014;2014:1603–1610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bliss DZ, Stein TP, Schleifer CR, Settle RG. Supplementation with gum Arabic fiber increases fecal nitrogen excretion and lowers serum urea nitrogen concentration in chronic renal failure patients consuming a low-protein diet. Am J Clin Nutr. 1996;63:392–398. [DOI] [PubMed] [Google Scholar]
- 22.Ranganathan N, Friedman EA, Tam P, Rao V, Ranganathan P, Dheer R. Probiotic dietary supplementation in patients with stage 3 and 4 chronic kidney disease: a 6-month pilot scale trial in Canada. Curr Med Res Opin. 2009;25:1919–1930. [DOI] [PubMed] [Google Scholar]
- 23.Natarajan R, Pechenyak B, Vyas U, et al. Randomized controlled trial of strain-specific probiotic formulation (Renadyl) in dialysis patients. Biomed Res Int. 2014;2014:568571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Borges NA, Carmo FL, Stockler-Pinto MB, et al. Probiotic supplementation in chronic kidney disease: a double-blind, randomized, placebo-controlled trial. J Ren Nutr. 2018;28:28–36. [DOI] [PubMed] [Google Scholar]
- 25.Esgalhado M, K J, Azevedo R, et al. Could resistant starch supplementation improve inflammatory and oxidative stress biomarkers and uremic toxins levels in hemodialysis patients? A pilot randomized controlled trial. Food Funct. 2018;9(12):6508–6516. [DOI] [PubMed] [Google Scholar]
- 26.Lopes R, de Lima S, da Silva BP, et al. Evaluation of the health benefits of consumption of extruded tannin sorghum with unfermented probiotic milk in individuals with chronic kidney disease. Food Res Int. 2018;107:629–638. [DOI] [PubMed] [Google Scholar]
- 27.Ramos CI, Armani RG, Canziani MEF, et al. Effect of prebiotic (fructooligosaccharide) on uremic toxins of chronic kidney disease patients: a randomized controlled trial. Nephrol Dial Transpl. 2019;34:1876–1884. [DOI] [PubMed] [Google Scholar]
- 28.Wang IK, Wu YY, Yang YF, et al. The effect of probiotics on serum levels of cytokine and endotoxin in peritoneal dialysis patients: a randomised, double-blind, placebo-controlled trial. Benef Microbes. 2015;6:423–430. [DOI] [PubMed] [Google Scholar]
- 29.Lim PS, Wang HF, Lee MC, et al. The efficacy of lactobacillus-containing probiotic supplementation in hemodialysis patients: a randomized, double-blind, placebo-controlled trial. J Ren Nutr. 2020. [DOI] [PubMed] [Google Scholar]
- 30.Li L, Xiong Q, Zhao J, et al. Inulin-type fructan intervention restricts the increase in gut microbiome-generated indole in patients with peritoneal dialysis: a randomized crossover study. Am J Clin Nutr. 2020;111:1087–1099. [DOI] [PubMed] [Google Scholar]
- 31.Eidi F, Gholi FP-R, Ostadrahimi A, Dalili N, Samadian F, Barzegari A. Effect of Lactobacillus Rhamnosus on serum uremic toxins (phenol and P-Cresol) in hemodialysis patients: a double blind randomized clinical trial. Clin Nutr ESPEN. 2018;28:158–164. [DOI] [PubMed] [Google Scholar]
- 32.Khosroshahi HT, Vaziri ND, Abedi B, et al. Effect of high amylose resistant starch (HAM-RS2) supplementation on biomarkers of inflammation and oxidative stress in hemodialysis patients. Hemodial Int. 2018;22:492–500. [DOI] [PubMed] [Google Scholar]
- 33.Shariaty Z, Mahmoodi Shan GR, Farajollahi M, Amerian M, Behnam Pour N. The effects of probiotic supplement on hemoglobin in chronic renal failure patients under hemodialysis: a randomized clinical trial. J Res Med Sci. 2017;22:74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Soleimani A, Motamedzadeh A, Zarrati Mojarrad M, et al. The effects of synbiotic supplementation on metabolic status in diabetic patients undergoing hemodialysis: a randomized, double-blinded, placebo-controlled trial. Probiotics Antimicrob Proteins. 2019;11:1248–1256. [DOI] [PubMed] [Google Scholar]
- 35.Soleimani A, Zarrati Mojarrad M, Bahmani F, et al. Probiotic supplementation in diabetic hemodialysis patients has beneficial metabolic effects. Kidney Int. 2017;91:435–442. [DOI] [PubMed] [Google Scholar]
- 36.Dehghani H, Heidari F, Mozaffari-Khosravi H, Nouri-Majelan N, Dehghani A. Synbiotic supplementations for Azotemia in patients with chronic kidney disease: a randomized controlled trial. Iran J Kidney Dis. 2016;10:351–357. [PubMed] [Google Scholar]
- 37.Guida B, Germanò R, Trio R, et al. Effect of short-term synbiotic treatment on plasma p-cresol levels in patients with chronic renal failure: a randomized clinical trial. Nutr Metab Cardiovasc Dis. 2014;24:1043–1049. [DOI] [PubMed] [Google Scholar]
- 38.Simeoni M, Citraro ML, Cerantonio A, et al. An open-label, randomized, placebo-controlled study on the effectiveness of a novel probiotics administration protocol (ProbiotiCKD) in patients with mild renal insufficiency (stage 3a of CKD). Eur J Nutr 2018. 2019;58:2145–2156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shimada M, Nagano N, Goto S, et al. Effect of polydextrose intake on constipation in Japanese dialysis patients: a triple-blind, randomized, controlled trial. J Nutr Sci Vitaminol (Tokyo). 2015;61:345–353. [DOI] [PubMed] [Google Scholar]
- 40.Rossi M, Johnson DW, Morrison M, et al. Synbiotics easing renal failure by improving gut Microbiology (SYNERGY): a randomized trial. Clin J Am Soc Nephrol. 2016;11:223–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Younes H, Egret N, Hadj-Abdelkader M, et al. Fermentable carbohydrate supplementation alters nitrogen excretion in chronic renal failure. J Ren Nutr. 2006;16:67–74. [DOI] [PubMed] [Google Scholar]
- 42.Viramontes-Horner D, Márquez-Sandoval F, Martín-del-Campo F, et al. Effect of a symbiotic gel (Lactobacillus acidophilus + Bifidobacterium lactis + inulin) on presence and severity of gastrointestinal symptoms in hemodialysis patients. J Ren Nutr. 2015;25:284–291. [DOI] [PubMed] [Google Scholar]
- 43.Eidi F, Poor-Reza Gholi F, Ostadrahimi A, Dalili N, Samadian F, Barzegari A. Effect of Lactobacillus Rhamnosus on serum uremic toxins (phenol and P-Cresol) in hemodialysis patients: a double blind randomized clinical trial. Clin Nutr ESPEN. 2018;28:158–164. [DOI] [PubMed] [Google Scholar]
- 44.Esgalhado M, Kemp JA, Azevedo R, et al. Could resistant starch supplementation improve inflammatory and oxidative stress biomarkers and uremic toxins levels in hemodialysis patients? A pilot randomized controlled trial. Food Funct. 2018;9:6508–6516. [DOI] [PubMed] [Google Scholar]
- 45.Sirich TL, Plummer NS, Gardner CD, Hostetter TH, Meyer TW. Effect of increasing dietary fiber on plasma levels of colon-derived solutes in hemodialysis patients. Clin J Am Soc Nephrol. 2014;9:1603–1610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.von Winckelmann SL, Spriet I, Willems L. Therapeutic drug monitoring of phenytoin in critically ill patients. Pharmacotherapy. 2008;28:1391–1400. [DOI] [PubMed] [Google Scholar]
- 47.Bliss DZ. Supplementation with gum Arabic fiber increases fecal nitrogen excretion and lowers serum urea nitrogen concentration in chronic renal failure patients consuming a low-protein diet. Am J Clin Nutr. 1996;63:392–398. [DOI] [PubMed] [Google Scholar]
- 48.Vaziri ND, Wong J, Pahl M, et al. Chronic kidney disease alters intestinal microbial flora. Kidney Int. 2013;83:308–315. [DOI] [PubMed] [Google Scholar]
- 49.Yang T, Richards EM, Pepine CJ, Raizada MK. The gut microbiota and the brain–gut–kidney axis in hypertension and chronic kidney disease. Nat Rev Nephrol. 2018;14:442–456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jazani NH, Savoj J, Lustgarten M, Lau WL, Vaziri ND. Impact of gut dysbiosis on neurohormonal pathways in chronic kidney disease. Diseases.2019;7:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Meyer TW, Hostetter TH. Uremia. N Engl J Med. 2007;357:1316–1325. [DOI] [PubMed] [Google Scholar]
- 52.Meijers BK, Claes K, Bammens B, et al. p-Cresol and cardiovascular risk in mild-to-moderate kidney disease. Clin J Am Soc Nephrol. 2010;5:1182–1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Meijers B, Bammens B, De Moor B, Verbeke K, Vanrenterghem Y, Evenepoel P. Free p-cresol is associated with cardiovascular disease in hemodialysis patients. Kidney Int. 2008;73:1174–1180. [DOI] [PubMed] [Google Scholar]
- 54.Sueyoshi M, Fukunaga M, Mei M, et al. Effects of lactulose on renal function and gut microbiota in adenine-induced chronic kidney disease rats. Clin Exp Nephrol. 2019;23:908–919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Halcox JP, Donald AE, Ellins E, et al. Endothelial function predicts progression of carotid intima-media thickness. Circulation. 2009;119:1005–1012. [DOI] [PubMed] [Google Scholar]
- 56.Vaziri ND. Oxidative stress in uremia: nature, mechanisms, and potential consequences. Semin Nephrol. 2004;24:469–473. [DOI] [PubMed] [Google Scholar]
- 57.Vaziri ND, Ni Z, Oveisi F, Liang K, Pandian R. Enhanced nitric oxide inactivation and protein nitration by reactive oxygen species in renal insufficiency. Hypertension. 2002;39:135–141. [DOI] [PubMed] [Google Scholar]
- 58.Hasdan G, Benchetrit S, Rashid G, Green J, Bernheim J, Rathaus M. Endothelial dysfunction and hypertension in =/6 nephrectomized rats are mediated by vascular superoxide. Kidney Int. 2002;61:586–590. [DOI] [PubMed] [Google Scholar]
- 59.Giardini O, Taccone-Gallucci M, Lubrano R, et al. Effects of alpha-tocopherol administration on red blood cell membrane lipid peroxidation in hemodialysis patients. Clin Nephrol. 1984;21:174. [PubMed] [Google Scholar]
- 60.Deng G, Vaziri ND, Jabbari B, Ni Z, Yan XX. Increased tyrosine nitration of the brain in chronic renal insufficiency: reversal by antioxidant therapy and angiotensin-converting enzyme inhibition. J Am Soc Nephrol. 2001;12:1892–1899. [DOI] [PubMed] [Google Scholar]
- 61.Karimi P, Farhangi MA, Sarmadi B, et al. The therapeutic potential of resistant starch in modulation of insulin resistance, endotoxemia, oxidative stress and antioxidant biomarkers in women with type 2 diabetes: a randomized controlled clinical trial. Ann Nutr Metab. 2016;68:85–93. [DOI] [PubMed] [Google Scholar]
- 62.Kwak JH, Paik JK, Kim HI, et al. Dietary treatment with rice containing resistant starch improves markers of endothelial function with reduction of postprandial blood glucose and oxidative stress in patients with prediabetes or newly diagnosed type 2 diabetes. Atherosclerosis. 2012;224: 457–464. [DOI] [PubMed] [Google Scholar]
- 63.Vaziri ND, Liu SM, Lau WL, et al. High amylose resistant starch diet ameliorates oxidative stress, inflammation, and progression of chronic kidney disease. PLoS One. 2014;9:e114881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Salehi-Abargouei A, Ghiasvand R, Hariri M. Prebiotics, Prosynbiotics and synbiotics: can they reduce plasma oxidative stress parameters? A systematic review. Probiotics Antimicrob Proteins. 2017;9:1–11. [DOI] [PubMed] [Google Scholar]
- 65.Si X, Zhou Z, Strappe P, Blanchard C. A comparison of RS4-type resistant starch to RS2-type resistant starch in suppressing oxidative stress in high-fat-diet-induced obese rats. Food Funct. 2017;8:232–240. [DOI] [PubMed] [Google Scholar]
- 66.Yadav H, Jain S, Sinha PR. Oral administration of dahi containing probiotic Lactobacillus acidophilus and Lactobacillus casei delayed the progression of streptozotocin-induced diabetes in rats. J Dairy Res. 2008;75:189–195. [DOI] [PubMed] [Google Scholar]
- 67.Kieffer DA, Piccolo BD, Vaziri ND, et al. Resistant starch alters gut microbiome and metabolomic profiles concurrent with amelioration of chronic kidney disease in rats. Am J Physiol Ren Physiol. 2016;310:F857–F871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wong JM, de Souza R, Kendall CW, Emam A, Jenkins DJ. Colonic health: fermentation and short chain fatty acids. J Clin Gastroenterol. 2006;40:235–243. [DOI] [PubMed] [Google Scholar]
- 69.Smith PM, Howitt MR, Panikov N, et al. The microbial metabolites, short-chain fatty acids, regulate colonic Treg cell homeostasis. Science. 2013;341:569–573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Roediger W. Utilization of nutrients by isolated epithelial cells of the rat colon. Gastroenterology. 1982;83:424–429. [PubMed] [Google Scholar]
- 71.Segain JP, Raingeard de la Blétière D, Bourreille A, et al. Butyrate inhibits inflammatory responses through NFkappaB inhibition: implications for Crohn’s disease. Gut. 2000;47:397–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ma Y, Hébert JR, Li W, et al. Association between dietary fiber and markers of systemic inflammation in the Women’s Health Initiative Observational Study. Nutrition. 2008;24:941–949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Cesari M, Penninx BW, Newman AB, et al. Inflammatory markers and onset of cardiovascular events: results from the Health ABC study. Circulation. 2003;108:2317–2322. [DOI] [PubMed] [Google Scholar]
- 74.Alipour B, Homayouni-Rad A, Vaghef-Mehrabany E, et al. Effects of Lactobacillus casei supplementation on disease activity and inflammatory cytokines in rheumatoid arthritis patients: a randomized double-blind clinical trial. Int J Rheum Dis. 2014;17:519–527. [DOI] [PubMed] [Google Scholar]
- 75.Gill HS, Rutherfurd KJ, Cross ML, Gopal PK. Enhancement of immunity in the elderly by dietary supplementation with the probiotic Bifidobacterium lactis HN019. Am J Clin Nutr. 2001;74:833–839. [DOI] [PubMed] [Google Scholar]
- 76.Mazidi M, Rezaie P, Ferns GA, Vatanparast H. Impact of probiotic administration on serum C-reactive protein concentrations: systematic review and meta-analysis of randomized control trials. Nutrients. 2017;9:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Yang HL, Feng P, Xu Y, Hou YY, Ojo O, Wang XH. The role of dietary fiber supplementation in regulating uremic toxins in patients with chronic kidney disease: a meta-analysis of randomized controlled trials. J Ren Nutr. 2021;31:438–447. [DOI] [PubMed] [Google Scholar]
- 78.Jourde Chiche N, Dou L, Cerini C, Dignat-George F, Brunet P. Vascular incompetence in dialysis patients—protein bound uremic toxins and endothelial dysfunction. Semin Dial. 2011;24:327–337. [DOI] [PubMed] [Google Scholar]
- 79.Deltombe O, Van Biesen W, Glorieux G, Massy Z, Dhondt A, Eloot S. Exploring protein binding of uremic toxins in patients with different stages of chronic kidney disease and during hemodialysis. Toxins.2015;7:3933–3946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Rossi M, Johnson DW, Xu H, et al. Dietary protein-fiber ratio associates with circulating levels of indoxyl sulfate and p-cresyl sulfate in chronic kidney disease patients. Nutr Metab Cardiovasc Dis. 2015;25:860–865. [DOI] [PubMed] [Google Scholar]
- 81.Silk DB, Davis A, Vulevic J, Tzortzis G, Gibson GR. Clinical trial: the effects of a trans-galactooligosaccharide prebiotic on faecal microbiota and symptoms in irritable bowel syndrome. Aliment Pharmacol Ther.2009;29:508–518. [DOI] [PubMed] [Google Scholar]
- 82.Evenepoel P, Meijers BK, Bammens BR, Verbeke K. Uremic toxins originating from colonic microbial metabolism. Kidney Int Suppl.2009;(114):S12–S19. [DOI] [PubMed] [Google Scholar]
- 83.Schmidt S, Gonzalez D, Derendorf H. Significance of protein binding in pharmacokinetics and pharmacodynamics. J Pharm Sci. 2010;99:1107–1122. [DOI] [PubMed] [Google Scholar]
- 84.Kalina U, Koyama N, Hosoda T. Enhanced production of IL 18 in butyrate treated intestinal epithelium by stimulation of the proximal promoter region. Eur J Immunol. 2002;32:2635–2643. [DOI] [PubMed] [Google Scholar]
- 85.Vitali B, Ndagijimana M, Cruciani F, et al. Impact of a synbiotic food on the gut microbial ecology and metabolic profiles. Bmc Microbiol. 2010;10:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.