Abstract
DNA vaccines whose DNA encodes a variety of antigens from Mycobacterium tuberculosis have been evaluated for immunogenicity and protective efficacy. CD8+ T-cell responses and protection achieved in other infectious disease models have been optimized by using a DNA immunization to prime the immune system and a recombinant virus encoding the same antigen(s) to boost the response. A DNA vaccine (D) and recombinant modified vaccinia virus Ankara (M) in which the DNA encodes early secreted antigenic target 6 and mycobacterial protein tuberculosis 63 synthesized, and each was found to generate specific gamma interferon (IFN-γ)-secreting CD4+ T cells. Enhanced CD4+ IFN-γ T-cell responses were produced by both D-M and M-D immunization regimens. Significantly higher levels of IFN-γ were seen with a D-D-D-M immunization regimen. The most immunogenic regimens were assessed in a challenge study and found to produce protection equivalent to that produced by Mycobacterium bovis BCG. Thus, heterologous prime-boost regimens boost CD4+ as well as CD8+ T-cell responses, and the use of heterologous constructs encoding the same antigen(s) may improve the immunogenicity and protective efficacy of DNA vaccines against tuberculosis and other diseases.
More than 100 years after Koch's discovery of the causative organism, tuberculosis remains a major global public health problem. There are estimated to be 8 to 10 million new cases per annum, and the annual mortality is approximately 3 million. The variability in protective efficacy of the currently available vaccine, Mycobacterium bovis bacillus Calmette-Guérin (BCG) (5), and the advent of multidrug-resistant strains of tuberculosis, mean that there is an urgent need for a better vaccine.
A greater understanding of the nature of protective immunity to tuberculosis would facilitate the development of a vaccine which would mimic this naturally occurring protection. Mycobacterium tuberculosis is an intracellular pathogen, and the predominant immune response involves the cellular arm of the immune system. There is strong evidence from animal and human studies that CD4+ T cells are necessary for the development of protective immunity (1, 15). There is also increasing evidence that CD8+ T cells may play a role (6, 11).
DNA vaccines are potent inducers of cellular immune responses, inducing both CD4+ and CD8+ T cells, and therefore represent a promising delivery system for a tuberculosis vaccine. A number of studies assessing the protective efficacy of DNA vaccines in which the DNA encodes a variety of antigens from M. tuberculosis have shown partial protection against challenge that is equivalent to the protection conferred by BCG (9, 21). However, none of the vaccine candidates tested so far has been found to be consistently superior to BCG.
Although DNA vaccines are good at eliciting both CD4+ and CD8+ T cells, the frequency of response cells they produce may need to be significantly increased in order to confer protection against challenge. Priming an immune response with a DNA vaccine and heterologous boosting of the response with a recombinant modified vaccinia virus Ankara (MVA) expressing the same antigen(s) as the DNA produced high levels of both specific CD8+ cytotoxic T lymphocytes and unprecedented protection against challenge in a rodent model of malaria (17, 19).
The effect of this heterologous prime-boost regimen on CD4+ T cells is unknown. We set out to evaluate a prime-boost regimen in the generation of CD4+ T-cell responses and protection against challenge in a murine model of tuberculosis.
Since secreted antigens that are released from live mycobacteria are thought to be important in the generation of protective immunity, we selected two secreted antigens from M. tuberculosis for inclusion in the vaccines. The first, early secreted antigenic target 6 (ESAT6), is relatively specific for M. tuberculosis and, importantly, is not present in M. bovis BCG (7). ESAT6 is a key antigenic target early in murine infection (3) and is a human cytotoxic T lymphocyte target (11). The second antigen, mycobacterial protein tuberculosis 63 (MPT63), is present in some strains of M. bovis BCG (13). MPT63 induces delayed-type hypersensitivity responses in guinea pigs immunized with it, and guinea pigs infected with M. tuberculosis have antibody responses to MPT63 (8). We made a polyprotein DNA construct and recombinant MVA containing both antigens and assessed the immunogenicity of these constructs, individually and in combination. The most immunogenic vaccine combinations were then assessed in murine challenge experiments with M. tuberculosis.
MATERIALS AND METHODS
M. tuberculosis stocks.
M. tuberculosis (H37Rv) was grown in Dubos medium and incubated at 37°C for 21 to 28 days. The solution was centrifuged, resuspended in tryptic soy broth-glycerol and stored at −70°C after titration. Stock solutions were sonicated before use.
Plasmid DNA constructs.
M. tuberculosis (H37Rv) was heat inactivated and DNA extracted (QIAamp; Qiagen, Hilden, Germany). Oligonucleotide primers (Genosys Biotechnologies Ltd., Pampisford, Cambs, United Kingdom) were used to amplify the ESAT6 and MPT63 gene. The PCR products were extracted from agarose gel and purified (QIAquick kit; Qiagen). The tissue plasminogen activator leader sequence was also amplified. The three PCR products were sequenced and then ligated together to form a single coding sequence with the Pk antibody epitope at the 3′ end (TEMPk). The TEMPk fragment was ligated into the plasmid vector pSG2, creating pSG2.TEMPk. This plasmid has the cytomegolovirus promoter with intron A, the bovine growth hormone poly(A) sequence, and the kanamycin resistance gene as a selectable marker. Expression of the TEMPk fusion protein in COS-1 cells was detected by immunofluorescence using antibodies to the Pk tag (Serotech, Oxford, United Kingdom) followed by fluorescein isothiocyanate isomer-labeled secondary antibodies (Sigma). Nuclear staining showed the protein to be in the cytoplasm. Plasmid DNA for injections was purified by anion-exchange chromatography (Qiagen) and diluted in endotoxin-free phosphate-buffered saline (PBS) (Sigma).
Construction of recombinant MVA.
The DNA sequence TEMPk was cloned into the vaccinia virus shuttle vector pSC11. BHK cells were infected with wild-type MVA (A. Mayr, Veterinary Faculty, University of Munich, Munich, Germany) at a multiplicity of infection of 0.05 and then transfected with the recombinant shuttle vector. Recombinant virus was selected for with bromodeoxyuridine and then plaque purified on chicken embryo fibroblasts.
Animals and immunizations.
Female C57BL/6 mice aged 4 to 6 weeks (Harlan Orlac, Shaws Farm, Blackthorn, United Kingdom) were injected with plasmid DNA (25 μg/muscle) into both tibialis muscles, while under anesthesia. Recombinant MVA (106 PFU) was injected intradermally (i.d.). Mice were immunized at 2-week intervals and harvested for immunogenicity assay 2 weeks after the last immunization. For the challenge experiments mice were infected 2 weeks after the last immunization. A BCG control group was immunized with 4 × 105 CFU of M. bovis BCG (Glaxo) i.d. at the time of the first DNA or MVA immunization.
Preparation of splenocytes.
Mice were sacrificed and spleens removed using aseptic technique. Spleens were crushed, and the resulting single-cell suspension was filtered through a strainer (Falcon [70-μm pore size]; Becton Dickson, Paramus, N.J.). Cells were pelleted, and the red blood cells were lysed by using a hypotonic lysis buffer. Cells were then washed and counted. Splenocytes were resuspended in alpha-modified Eagle medium with 10% fetal calf serum, 2 mM glutamine, penicillin (50 U/ml), streptomycin (50 mg/ml), 50 μM 2-mercaptoethanol, and 10 mM HEPES (pH 7.2) (all from Gibco).
Peptides.
Overlapping peptides spanning the length of both antigens were purchased from Research Genetics (Huntsville, Ala.). The peptides were 15 amino acids long and overlapped by 10 amino acids (Table 1).
TABLE 1.
Sequences of ESAT6 and MPT63 peptides
| Peptide | Amino acid no.a | Sequence |
|---|---|---|
| ESAT6 | ||
| 1 | 1 | MTEQQ WNFAG IEAAA |
| 2 | 6 | WNFAG IEAAA SAIQG |
| 3 | 11 | IEAAA SAIQG NVTSI |
| 4 | 16 | SAIQG NVTSI HSLLD |
| 5 | 21 | NVTSI HSLLD EGKQS |
| 6 | 26 | HSLLD EGKQS LTKLA |
| 7 | 31 | EGKQS LTKLA AAWGG |
| 8 | 36 | LTKLA AAWGG SGSEA |
| 9 | 41 | AAWGG SGSEA YQGVQ |
| 10 | 46 | SGSEA YQGVQ QKWDA |
| 11 | 51 | YQGVQ QKWDA TATEL |
| 12 | 56 | QKWDA TATEL NNALQ |
| 13 | 61 | TATEL NNALQ NLART |
| 14 | 66 | NNALQ NLART ISEAG |
| 15 | 71 | NLART ISEAG QAMAS |
| 16 | 76 | ISEAG QAMAS TEGNV |
| 17 | 81 | QAMAS TEGNV TGMFA |
| MPT63 | ||
| 1 | 1 | MKLTT MIKTA VAVVA |
| 2 | 6 | MIKTA VAVVA MAAIA |
| 3 | 11 | VAVVA MAAIA TFAAP |
| 4 | 16 | MAAIA TFAAP VALAA |
| 5 | 21 | TFAAP VALAA YPITG |
| 6 | 26 | VALAA YPITG KLGSE |
| 7 | 31 | YPITG KLGSE LTMTD |
| 8 | 36 | KLGSE LTMTD TVGQV |
| 9 | 41 | LTMTD TVGQV VLGWK |
| 10 | 46 | TVGQV VLGWK VSDLK |
| 11 | 51 | VLGWK VSDLK SSTAV |
| 12 | 56 | VSDLK SSTAV IPGYP |
| 13 | 61 | SSTAV IPGYP VAGQV |
| 14 | 66 | IPGYP VAGQV WEATA |
| 15 | 71 | VAGQV WEATA TVNAI |
| 16 | 76 | WEATA TVNAI RGSVT |
| 17 | 81 | TVNAI RGSVT PAVSQ |
| 18 | 86 | RGSVT PAVSQ FNART |
| 19 | 91 | PAVSQ FNART ADGIN |
| 20 | 96 | FNART ADGIN YRVLW |
| 21 | 101 | ADGIN YRVLW QAAGP |
| 22 | 106 | YRVLW QAAGP DTISG |
| 23 | 111 | QAAGP DTISG ATIPQ |
| 24 | 116 | DTISG ATIPQ GEQST |
| 25 | 121 | ATIPQ GEQST GKIYF |
| 26 | 126 | GEQST GKIYF DVTGP |
| 27 | 131 | GKIYF DVTGP SPTIV |
| 28 | 136 | DVTGP SPTIV AMNNG |
| 29 | 141 | SPTIV AMNNG EDLLI |
| 30 | 146 | AMNNG EDLLI WEP |
Refers to initial amino acid number in sequence of peptide.
ELISPOT assays.
The number of gamma interferon (IFN-γ)-secreting peptide-specific T cells was determined using the overlapping peptides in an ELISPOT assay (17). Briefly, 96-well nitrocellulose plates (Milliscreen MAHA; Millipore, Bedford, Mass.) were coated with 15 μg of the anti-mouse IFN-γ monoclonal antibody R4-6A2 (hybridoma purchased from the European Collection of Animal Cell Cultures) per ml. After incubating at 4°C overnight, the wells were washed with PBS and blocked with 100 μl RPMI–10% fetal calf serum for 1 h at room temperature. Splenocytes were added to the wells (106 cells/well) with peptide (final concentration, 2 μg/ml). Concanavalin A (Sigma-Aldrich Co. Ltd., Poole, United Kingdom) was used as a positive control for the assay. Control wells had no peptide. After incubating the plate overnight at 37°C with 5% CO2 in air, it was developed as previously described (17). The spots were counted using a dissecting microscope. Numbers refer to spot-forming cells (SFC) per 106 splenocytes.
Cell depletions.
CD4+ and CD8+ T-cell depletions were performed using anti-CD4 or anti-CD8 monoclonal antibodies conjugated to ferrous beads (Dynal, Oslo, Norway). Splenocytes from immunized mice were restimulated in six-well tissue culture plates with 1 μg of the relevant peptide per ml, and on day 3 of culture 10 U of human interleukin 2 (Lymphocult-T; Biotest, Dreieich, Germany) per ml was added. At days 5 to 7 the restimulated splenocytes were washed twice and incubated on ice for 30 min with one of the two antibodies (bead/cell ratio, 5:1). An ELISPOT assay was then performed as before, using the depleted cell populations. Assays for peptides E1 and E2 were also performed ex vivo. Depletion studies for each peptide response were performed twice.
Challenge experiments.
Mice were infected with 5 × 106 CFU of M. tuberculosis (H37Rv) by intraperitoneal injection, in a category III isolator unit. To assess the baseline level of infection, the liver, lungs, and spleen from two to five mice from each group were harvested and weighed 24 h after infection. The organs from the remaining 7 to 10 mice in each group were harvested 8 weeks after challenge. Organs were homogenized by vortexing with 5-mm-diameter glass beads in 1 ml of sterile PBS, and serial dilutions were plated onto Middlebrook plates (agar 7H10; Difco). Plates were incubated for 21 days at 37°C and colony counts per gram of tissue were then calculated. Data are expressed as mean log10 values per experimental group. Student's t test was used to determine the statistical significance of differences between groups.
RESULTS
Construction of plasmid DNA and recombinant MVA vaccines.
A single coding sequence containing the tissue plasminogen activator leader sequence, ESAT6 and MPT63 genes, and the Pk antibody epitope (TEMPk) was constructed and ligated into the plasmid vector pSG2, creating the DNA vaccine pSG2.TEMPk. Expression of the fusion protein was shown to be in the cytoplasm. The recombinant MVA was purified from a transfection of wild-type MVA and a vaccinia virus shuttle vector containing the sequence TEMPk.
DNA and MVA vaccines both induce peptide-specific IFN-γ-producing CD4+ T cells.
C57B1/6 mice were immunized with DNA (i.m.), MVA (i.d.), or a combination of the two. Using overlapping peptides which span the length of both antigens (Table 1), and an IFN-γ ELISPOT assay, we identified responses to several peptides in the splenocytes of immunized mice. Responses to two peptides within ESAT6 (E1 and E2) and four peptides within MPT63 (M3, -15, -27, and -28) were seen (Table 2). To assess the phenotype of the cells responding to these peptides, CD4+ and CD8+ cells were depleted by using magnetic beads. The responses to all six peptides were fully abrogated when CD4+ T cells were depleted and were unaffected when CD8+ T cells were depleted (Table 2). Assays were performed both ex vivo (peptides E1 and E2) and after culturing the cells with peptide for 5 to 7 days (all peptides [see Materials and Methods]).
TABLE 2.
Peptides showing T-cell responses identified in ESAT6 and MPT63
| Antigen | Peptide | Sequence | Undepleted responsea | CD8 Depletiona | CD4 Depletiona |
|---|---|---|---|---|---|
| ESAT 6 | 1 | MTEQQ WNFAG IEAAA | 150 | 150 | 0 |
| 2 | WNFAG IEAAA SAIQG | 100 | 100 | 0 | |
| MPT63 | 3 | VAVVA MAAIA TFAAP | 45 | 40 | 0 |
| 15 | VAGQV WEATA TVNAI | 100 | 100 | 0 | |
| 27 | GKIYF DVTGP SPTIV | 50 | 50 | 0 | |
| 28 | DVTGP SPTIV AMNGM | 50 | 45 | 0 |
Results reported as SFC per 106 splenocytes.
Homologous boosting of DNA- and MVA-induced responses.
The highest frequency of IFN-γ secreting T cells (SFC) was to the first ESAT6 peptide, E1 (Table 3). A single dose of DNA failed to generate any detectable responses, but when repeated twice or three times at two weekly intervals, consistent responses were seen. After three immunizations, the number of SFC was more than double that seen after two immunizations; the mean response to E1 after two doses was 30 SFC per 106 splenocytes, and after three doses it was 75 SFC per 106 splenocytes. In contrast, a single immunization with MVA generated weak responses (mean ratio of SFC per 106 splenocytes to E1, 20), which were modestly improved following a second dose of MVA virus (mean ratio of SFC per 106 splenocytes to E1, 30).
TABLE 3.
Summary of peptide-specific T-cell responses to the two constructs
| Vaccination regimen | No. of peptide-specific SFCa
|
|||||
|---|---|---|---|---|---|---|
| E1 | E2 | M3 | M15 | M27 | M28 | |
| D | ||||||
| DD | 30 | 5 | 5 | 5 | 5 | |
| DDD | 75 | 50 | 15 | 40 | 45 | |
| M | 20 | 10 | 5 | 5 | 5 | |
| MM | 30 | 10 | 5 | 10 | 10 | |
| DM | 130 | 26 | 12 | 17 | 5 | 10 |
| MD | 130 | 70 | 10 | 10 | 10 | 5 |
| DDDM | 360 | 250 | 25 | 100 | 30 | 50 |
Numbers represent means of SFC per 106 splenocytes for 3 to 10 mice per group. Standard error is < 20%.
Heterologous prime-boost regimens increased magnitude of response seen.
Having detected weak responses using the vaccines individually, the role of heterologous prime-boost regimes, i.e., using either the DNA or MVA construct to prime the response and the second construct to boost 2 weeks later, was assessed. Heterologous boosting, either with DNA followed by MVA (DM) or MVA followed by DNA (MD), produced stronger responses than homologous boosting of either DD or MM (Table 3). The mean response to peptide E1 was increased by more than fourfold, to 130 SFC per 106 splenocytes, and this occurred regardless of the order in which the two vaccines were given. The response to peptide E2 was slightly stronger when MVA was followed by DNA rather than the reverse order. The responses to peptides M3 and M15 were stronger with a heterologous boost, regardless of the order in which the vaccines were given, while the responses to peptides M27 and M28 were weak and not boosted.
The strongest response was seen when DNA was given three times and then boosted with MVA once (DDDM). In this case the mean response to E1 was increased almost fivefold, from 75 to 360 SFC per 106 splenocytes. The responses to the other peptides were also higher after DDDM compared with DM.
Challenge experiments.
Heterologous prime-boost regimens generated the highest levels of IFN-γ-secreting CD4+ T cells, and therefore the protective efficacy of these regimens was assessed in challenge experiments. The first challenge experiment compared the protective efficacy of DNA prime-MVA boost (DM) with MVA prime-DNA boost (MD), supplemented in each case with a second MVA boost. The second challenge experiment assessed the protection conferred by three sequential immunizations with DNA followed by a single MVA immunization (DDDM). In both experiments, BCG was used as a positive control. The immunogenicity of each vaccine regimen was assessed in two to three mice before the remainder of the group was challenged. In the first experiment, the immune responses were not as strong as previously seen (average response to E1, 25 SFC per 106 splenocytes). Therefore, in this case a second dose of MVA was administered to both groups prior to challenge. In the second challenge experiment, the average response to the dominant peptide, E1, was 225 SFC per 106 splenocytes.
The challenge was conducted 2 weeks after the last subunit vaccine immunization, shortly after the T-cell response had reached a plateau (unpublished data).
Eight-week challenge results.
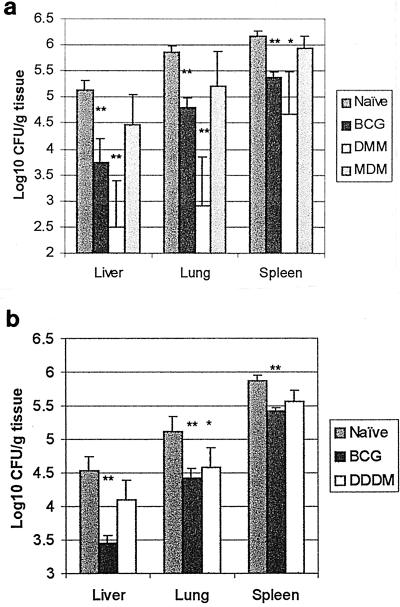
To assess the efficacy of the immunization regimens at 8 weeks, organs from all mice remaining at 8 weeks were harvested, and CFU counts were determined. In both challenge experiments, as expected, the CFU counts in the BCG group were significantly lower than in the naïve group in all three organs (Fig. 1). In the first challenge experiment, the CFU counts in all three organs in the DMM group were significantly lower than the naïve group. The CFU counts in the MDM group in all three organs were not significantly different from the naïve control group. However, in the second challenge experiment, the lung was the only organ in which the CFU counts in the DDDM group were significantly lower than the naïve control group. The CFU counts in liver and spleen were not significantly different between these two groups. The DMM-MDM-DDDM group CFU counts were not significantly different from the BCG group in any organ, in either experiment.
FIG. 1.
Eight week CFU results. Data represent the mean and standard error (error bar) of 7 to 15 mice per group. (a) Protective efficacy of DMM and MDM compared with BCG. (b) Protective efficacy of DDDM compared with BCG. ∗, P < 0.05; ∗∗, P < 0.01. Significance determined by Student's t test for immunized groups compared with naïve control group.
Twenty-four-hour bacterial counts.
To assess the baseline level of infection, organs from two to five mice from each group were harvested 24 h after infection. The CFU counts in liver and spleen in the BCG group were significantly lower than in the naïve control group (Table 4) at this time point in both challenge experiments. The CFU counts in lung were not significantly different between these two groups.
TABLE 4.
Twenty-four-hour bacterial counts
| Challenge no. and regimen | Mean (SE) of log10 CFU counts/g of:
|
||
|---|---|---|---|
| Liver | Lung | Spleen | |
| 1 | |||
| DMM | 5.64 (0.05) | 4.78 (0.08) | 6.12 (0.29) |
| MDM | 5.49 (0.09) | 4.3 (0.09) | 6.41 (0.02) |
| BCG | 4.85 (0.23)a | 4.2 (0.31) | 5.23 (0.40)a |
| Naive | 5.77 (0.16) | 4.5 (0.21) | 6.5 (0.15) |
| 2 | |||
| DDDM | 5.10 (0.20) | 3.91 (0.21)a | 5.65 (0.24)a |
| BCG | 4.90 (0.13)a | 4.43 (0.14) | 5.86 (0.11)a |
| Naive | 5.38 (0.08) | 4.51 (0.04) | 6.25 (0.09) |
P < 0.05 (Student's t test for comparison of immunized groups and naïve control group.
When the DM-MD-DDDM groups were compared with the naïve control group at 24 h, there was no significant difference between the DM or MD group counts compared to the naïve group counts in any organ. However, in the DDDM group in the second experiment, the CFU counts in spleen and lung were significantly lower than in the naïve control group. The CFU counts in liver were not significantly different between these two groups.
There were no significant differences between the DM-MD-DDDM group and the BCG group in any organ in either experiment at this early 24-h time point.
DISCUSSION
We report immunogenicity and challenge studies with M. tuberculosis using a prime-boost immunization regime. DNA vaccination is known to induce a TH1-type immune response, and therefore we chose the quantification of a TH1 cytokine, IFN-γ, in an ELISPOT assay as our functional outcome measure. This assay is a very sensitive method of quantifying T-cell function (10). Proliferation assays are an alternative measure of CD4+ T-cell function but do not always correlate with ELISPOT results (Flanagan et al., submitted for publication). There are two reasons why measuring IFN-γ production is a more relevant outcome measure in an M. tuberculosis challenge model. IFN-γ is an essential component of the protective immune response to tuberculosis, as IFN-γ knockout mice are much more susceptible to challenge with M. tuberculosis than their wild-type counterparts (4). In addition, a human mutation in the IFN-γ receptor gene confers susceptibility to atypical mycobacterial infection (14).
The DNA vaccine and recombinant MVA generated specific IFN-γ-secreting CD4+ T cells in response to the same peptides. No IFN-γ-secreting CD8+ T-cell responses to these constructs were observed. As the peptides used to assess the responses spanned the length of both antigens, this effectively excludes the presence of a CD8 epitope for this mouse strain. These constructs therefore allowed us to assess the effect of prime-boost regimens on CD4+ T cells. Heterologous prime-boost regimens with the two vaccines generated stronger CD4+ T-cell responses than homologous boosting. Interestingly, the order in which the two vaccines were given made no clear difference to the strength of the immune responses generated. Priming with DNA and boosting with MVA or priming with MVA and boosting with DNA both produced a three to fourfold increase in the number of IFN-γ-secreting CD4+ T cells specific for the first ESAT6 peptide. This contrasts with published work on CD8+ T-cell responses, in which DNA prime followed by MVA boost is the only order in which high levels of immunogenicity and protection are seen (17).
At 8 weeks, levels of protection with DNA-MVA immunization regimens were equivalent to those obtained with BCG, and the protection in the BCG-immunized group is in the same order as that previously published (21). We are confident that the colonies seen on Middlebrook media were M. tuberculosis colonies and not BCG colonies, as no colonies could be detected in organ homogenates from mice immunized with BCG alone at 4 weeks after immunization.
In the first challenge experiment, the group immunized with DNA-MVA showed levels of protection equivalent to that produced by BCG in all three organs. In the second experiment, protection in the DNA-MVA-immunized group was only seen in the lungs at 8 weeks. Previous authors have observed varying protective effects in different organs depending on the time from challenge to harvesting. Zhu et al. reported protection in the lungs after DNA immunization 4 weeks after challenge but only observed protection in the lungs and spleen 12 weeks after challenge (23). As the primary route of infection in humans is the pulmonary route, the lung is the most relevant organ in which to identify protection. More relevant aerosol models of M. tuberculosis challenge have been developed, and it will be important to see whether vaccines that confer protection against a systemic route of challenge remain protective against an aerosol challenge and whether protection in the lungs is maintained.
DNA priming seemed to be necessary for protection to occur in the challenge experiments, as in the first challenge experiment, protection was seen in the DMM group but not the MDM group. Note that the lack of protection in the MDM group at 24 h and at 8 weeks effectively rules out a nonspecific protective effect of the subunit vaccines administered up to 2 weeks before challenge. It is uncertain why protection was achieved in the DMM but not the MDM groups when the immunization order (Table 3) appeared not to affect immunogenicity. The difference, however, may relate to the timing of the second MVA boost, as the two MVA doses were given a month apart in the MDM group. It may be that within this interval an antibody response to the MVA abrogated the boosting effect.
The protection against challenge seen in the BCG-immunized group at 24 h has not to our knowledge been assessed before. It is not known whether this 24-h effect may be an early specific antituberculous effect of BCG. Although a nonspecific effect is possible, the challenge with M. tuberculosis was given 4 to 6 weeks after BCG immunization, by which time any nonspecific immune activation of NK cells might be expected to have worn off. Alternatively, BCG may induce a TH1 bias, which facilitates the eradication of intracellular organisms, including M. tuberculosis via TH1-type cytokines (IFN-γ, tumor necrosis factor, interleukin 14). The 24-h protective effect was seen in the liver and spleen but not the lungs. This differential organ effect may be a function of the intraperitoneal route of challenge, as the absolute CFU count in the lungs in all groups is lower than those in the liver and spleen at this time point. After an intraperitoneal challenge, a tuberculous peritonitis occurs, which explains why the counts in the liver and spleen are higher than those in the lung at this time point. It would be interesting to determine whether a protective effect at 24 h can be seen in the lungs after an aerosol challenge. In the second challenge experiment, an early protective effect was also observed in the spleen and lungs of DDDM-immunized mice. This may also be a nonspecific effect of the DNA (22). Further work on the mechanism of this early protective effect is needed.
The mechanism by which the response to a DNA priming vaccination can be boosted by a subsequent immunization with a recombinant virus encoding the same antigen has not been fully elucidated. It may relate to the induction by DNA of memory T cells to an immunodominant epitope(s) that expand rapidly on exposure to a recombinant virus carrying the same epitope (18). It is possible that the mechanisms involved in the boosting of CD8+ T cells are different to those involved in the boosting of CD4+ T cells.
There has been surprisingly little analysis of the capacity of recombinant viral vectors to induce CD4 T-cells responses despite the established utility of poxvirus recombinants in inducing CD8 T cells. Further work is required to analyze the relevant antigen processing pathways involved in the major histocompatibility complex class II loading of antigens expressed intracellularly by recombinant MVA. This virus permits late-stage viral antigen expression but is effectively replication incompetent in mammalian cells (2). To our knowledge, this is the first time that DNA-poxvirus prime-boost immunization regimens generating enhanced IFN-γ-secreting CD4+ T cells have been reported. The effectiveness of this immunization regimen (18) at inducing high-level CD8+ T-cell responses against infectious organisms and tumors in mice and striking immunogenicity in primates has led to ongoing clinical trials of DNA-MVA immunization approaches. The capacity of this immunization approach, identified here, to generate TH1-type CD4+ T-cell as well as CD8+ T-cell responses should enhance its utility in several diseases. For example, in human immunodeficiency virus infection (16) and malaria (20, 12) as well as in tuberculosis the development and maintenance of TH1-type CD4+ T cells is likely to contribute to protective immunity. Further analysis of the mechanisms of the enhanced immunogenicity of this immunization approach should facilitate the design of effective vaccines for these increasingly important infectious diseases.
ACKNOWLEDGMENTS
We thank Jiang-Ting Hu and Carolyn Hannan for assistance, Pauline Henbest for animal welfare, E. A. Gould for use of facilities, and M. J. Colston for advice.
Helen McShane is supported by an MRC Clinical Training Fellowship. Adrian Hill is a Wellcome Trust Principal Research Fellow.
REFERENCES
- 1.Barnes P F, Bloch A B, Davidson P T, Snider D E., Jr Tuberculosis in patients with human immunodeficiency virus infection. N Engl J Med. 1991;324:1644–1650. doi: 10.1056/NEJM199106063242307. [DOI] [PubMed] [Google Scholar]
- 2.Blanchard T J, Alcami A, Andrea P, Smith G L. Modified vaccinia virus Ankara undergoes limited replication in human cells and lacks several immunomodulatory proteins: implications for use as a human vaccine. J Gen Virol. 1998;79:1159–1167. doi: 10.1099/0022-1317-79-5-1159. [DOI] [PubMed] [Google Scholar]
- 3.Brandt L, Oettinger T, Holm A, Andersen A B, Andersen P. Key epitopes on the ESAT-6 antigen recognized in mice during the recall of protective immunity to Mycobacterium tuberculosis. J Immunol. 1996;157:3527–3533. [PubMed] [Google Scholar]
- 4.Cooper A M, Dalton D K, Stewart T A, Griffin J P, Russell D G, Orme I M. Disseminated tuberculosis in interferon gamma gene-disrupted mice. J Exp Med. 1993;178:2243–2247. doi: 10.1084/jem.178.6.2243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fine P E, Rodrigues L C. Modern vaccines. Mycobacterial diseases. Lancet. 1990;335:1016–1020. doi: 10.1016/0140-6736(90)91074-k. [DOI] [PubMed] [Google Scholar]
- 6.Flynn J L, Goldstein M M, Triebold K J, Koller B, Bloom B R. Major histocompatibility complex class I-restricted T cells are required for resistance to Mycobacterium tuberculosis infection. Proc Natl Acad Sci USA. 1992;89:12013–12017. doi: 10.1073/pnas.89.24.12013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Harboe M, Oettinger T, Wiker H G, Rosenkrands L, Andersen P. Evidence for occurrence of the ESAT-6 protein in Mycobacterium tuberculosis and virulent Mycobacterium bovis and for its absence in Mycobacterium bovis BCG. Infect Immun. 1996;64:16–22. doi: 10.1128/iai.64.1.16-22.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Horwitz M A, Lee B W, Dillon B J, Harth G. Protective immunity against tuberculosis induced by vaccination with major extracellular proteins of Mycobacterium tuberculosis. Proc Natl Acad Sci USA. 1995;92:1530–1534. doi: 10.1073/pnas.92.5.1530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Huygen K, Content J, Denis O, Montgomery D L, Yawman A M, Deck R R, DeWitt C M, Orme L M, Baldwin S, D'Souza C, Drowart A, Lozes E, Vandenbussche P, Van Vooren J P, Liu M A, Ulmer J B. Immunogenicity and protective efficacy of a tuberculosis DNA vaccine. Nat Med. 1996;2:893–898. doi: 10.1038/nm0896-893. [DOI] [PubMed] [Google Scholar]
- 10.Lalvani A, Brookes R, Hambleton S, Britton W J, Hill A V, McMichael A J. Rapid effector function in CD8+ memory T cells. J Exp Med. 1997;186:859–865. doi: 10.1084/jem.186.6.859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lalvani A, Brookes R, Wilkinson R J, Malin A S, Pathan A A, Andersen P, Dockrell H, Pasvol G, Hill A V. Human cytolytic and interferon gamma-secreting CD8+ T lymphocytes specific for Mycobacterium tuberculosis. Proc Natl Acad Sci USA. 1998;95:270–275. doi: 10.1073/pnas.95.1.270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lalvani A, Moris P, Voss G, Pathan A A, Kester K E, Brookes R, Lee E, Koutsoukos M, Plebanski M, Delchambre M, Flanagan K L, Carton C, Slaoui M, Van H C, Ballou W R, Hill A V, Cohen J. Potent induction of focused Th1-type cellular and humoral immune responses by RTS, S/SBAS2, a recombinant Plasmodium falciparum malaria vaccine. J Infect Dis. 1999;180:1656–1664. doi: 10.1086/315074. [DOI] [PubMed] [Google Scholar]
- 13.Manca C, Lyashchenko K, Wiker H G, Usai D, Colangeli R, Gennaro M L. Molecular cloning, purification, and serological characterization of MPT63, a novel antigen secreted by Mycobacterium tuberculosis. Infect Immun. 1997;65:16–23. doi: 10.1128/iai.65.1.16-23.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Newport M J, Huxley C M, Huston S, Hawrylowicz C M, Oostra B A, Williamson R, Levin M. A mutation in the interferon-gamma-receptor gene and susceptibility to mycobacterial infection. N Engl J Med. 1996;335:1941–1949. doi: 10.1056/NEJM199612263352602. [DOI] [PubMed] [Google Scholar]
- 15.Orme I M. The kinetics of emergence and loss of mediator T lymphocytes acquired in response to infection with Mycobacterium tuberculosis. J Immunol. 1987;138:293–298. [PubMed] [Google Scholar]
- 16.Rosenberg E S, Billingsley J M, Caliendo A M, Boswell S L, Sax P E, Kalams S A, Walker B D. Vigorous HIV-1-specific CD4+ T cell responses associated with control of viremia. Science. 1997;278:1447–1450. doi: 10.1126/science.278.5342.1447. [DOI] [PubMed] [Google Scholar]
- 17.Schneider J, Gilbert S C, Blanchard T J, Hanke T, Robson K J, Hannan C M, Becker M, Sinden R, Smith G L, Hill A V. Enhanced immunogenicity for CD8+ T cell induction and complete protective efficacy of malaria DNA vaccination by boosting with modified vaccinia virus Ankara. Nat Med. 1998;4:397–402. doi: 10.1038/nm0498-397. [DOI] [PubMed] [Google Scholar]
- 18.Schneider J, Gilbert S C, Hannan C M, Degano P, Prieur E, Sheu E G, Plebanski M, Hill A V S. Induction of CD8+ T cells using heterologous prime-boost immunisation strategies. Immunol Rev. 1999;170:29–38. doi: 10.1111/j.1600-065x.1999.tb01326.x. [DOI] [PubMed] [Google Scholar]
- 19.Sedegah M, Jones T R, Kaur M, Hedstrom R, Hobart P, Tine J A, Hoffman S L. Boosting with recombinant vaccinia increases immunogenicity and protective efficacy of malaria DNA vaccine. Proc Natl Acad Sci USA. 1998;95:7648–7653. doi: 10.1073/pnas.95.13.7648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stoute J A, Slaoui M, Heppner D G, Momin P, Kester K E, Desmons P, Wellde B T, Garcon N, Krzych U, Marchand M. A preliminary evaluation of a recombinant circumsporozoite protein vaccine against Plasmodium falciparum malaria. RTS, S Malaria Vaccine Evaluation Group. N Engl J Med. 1997;336:86–91. doi: 10.1056/NEJM199701093360202. [DOI] [PubMed] [Google Scholar]
- 21.Tascon R E, Colston M J, Ragno S, Stavropoulos E, Gregory D, Lowrie D B. Vaccination against tuberculosis by DNA injection. Nat Med. 1996;2:888–892. doi: 10.1038/nm0896-888. [DOI] [PubMed] [Google Scholar]
- 22.Tighe H, Corr M, Roman M, Raz E. Gene vaccination: plasmid DNA is more than just a blueprint. Immunol Today. 1998;19:89–97. doi: 10.1016/s0167-5699(97)01201-2. [DOI] [PubMed] [Google Scholar]
- 23.Zhu X, Venkataprasad N, Thangaraj H S, Hill M, Singh M, Ivanyi J, Vordermeier H M. Functions and specificity of T cells following nucleic acid vaccination of mice against Mycobacterium tuberculosis infection. J Immunol. 1997;158:5921–5926. [PubMed] [Google Scholar]