Graphical Abstract
Graphical Abstract.
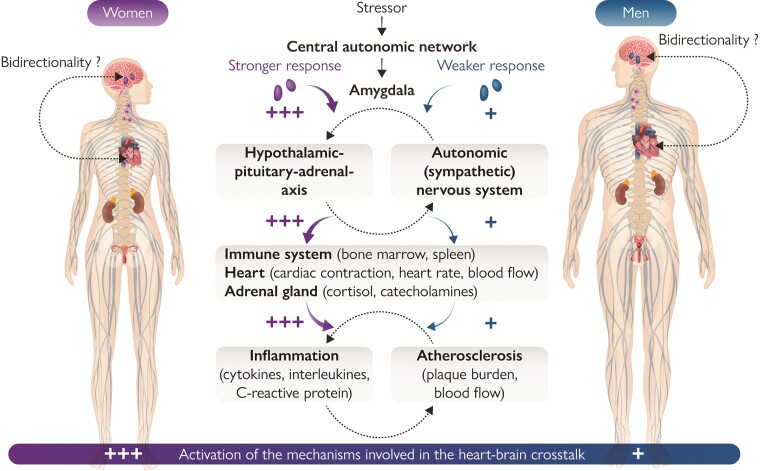
Mechanisms involved in the heart–brain crosstalk. Simplified representation of sex differences seen in the main mechanisms and neurohumoral circuits involved in heart–brain interactions. The intensity of activation is represented by a colour code scale, with red indicating the maximal activation. In brief, specific triggers (e.g. stress, acute myocardial infarction) induce the activation of the amygdala via the central autonomic system. Efferent projections increase the activation of the sympathetic nervous system and initiate neurohormonal output through the hypothalamic–pituitary–adrenal axis leading to catecholamine release, myelopoiesis activation, and release of pro-inflammatory cytokines with deleterious effect on the heart. This pro-inflammatory state initiates and promotes atherosclerosis. Current evidence on the pathophysiology of the specific heart and brain disease discussed in this review has shown that the activation of all these mechanisms is more pronounced in women as compared with men. The bidirectionality of heart–brain interactions is still under investigation.
Keywords: Heart, Brain, Sex, Gender, Ischaemic heart disease, Heart failure, Takotsubo syndrome, Stroke, Depression, Dementia
Abstract
Cardiovascular disease and brain disorders, such as depression and cognitive dysfunction, are highly prevalent conditions and are among the leading causes limiting patient’s quality of life. A growing body of evidence has shown an intimate crosstalk between the heart and the brain, resulting from a complex network of several physiological and neurohumoral circuits. From a pathophysiological perspective, both organs share common risk factors, such as hypertension, diabetes, smoking or dyslipidaemia, and are similarly affected by systemic inflammation, atherosclerosis, and dysfunction of the neuroendocrine system. In addition, there is an increasing awareness that physiological interactions between the two organs play important roles in potentiating disease and that sex- and gender-related differences modify those interactions between the heart and the brain over the entire lifespan. The present review summarizes contemporary evidence of the effect of sex on heart–brain interactions and how these influence pathogenesis, clinical manifestation, and treatment responses of specific heart and brain diseases.
Every affection of the mind that is attended with either pain or pleasure, hope or fear, is the cause of an agitation whose influence extends to the heart.
William Harvey
Introduction
A growing body of evidence demonstrates an intimate and bidirectional crosstalk between heart and brain, resulting from a complex network of several physiological and neurohumoral circuits.1 From a pathophysiological perspective, both organs share common risk factors, such as hypertension, diabetes, smoking, and dyslipidaemia, and are similarly affected by systemic inflammation, ischaemia due to atherosclerosis, and dysfunction of the neuroendocrine system. Moreover, an increasing number of reports shows that physiologic interactions between the two organs can drive the development of cardiovascular as well as cardiometabolic conditions.2–6
Sex-related differences develop and modify the heart–brain axis during the entire lifespan.7,8 Therefore, a deeper understanding of how sex affects heart–brain crosstalk is of paramount importance for patient-tailored prevention and treatment of multiorgan dysfunctions resulting from either cardiac or brain damage. In this context, our review article summarizes the state-of-the-art knowledge of the effect of sex on the heart–brain interactions involved in the development and co-occurrence of specific cardiac and brain conditions with a particular focus on ischaemic heart disease (IHD), heart failure (HF), Takotsubo syndrome (TTS), stroke, depression, and dementia (Table 1).
Table 1.
Sex-specific differences identified in the main pathways transmitting along the heart–brain axis for specific cardiac and brain disorders
| IHD | HF | TTS | Stroke | Depression | Dementia | |
|---|---|---|---|---|---|---|
| Atherosclerosis | + | + | + | |||
| SNS | + | + | + | + | + | |
| Hyperactivation of amygdala and limbic system | + | + | ||||
| HPA axis | + | + | ||||
| Inflammation | + | + | + | |||
| RAAS | + | |||||
| Impaired cerebral blood flow | + | + |
HF: heart failure; HPA: hypothalamic–pituitary–adrenal; IHD: ischaemic heart disease; RAAS: renin–angiotensin–aldosterone system; SNS: sympathetic nervous system; TTS: Takotsubo syndrome.
(Patho)physiological systems regulating heart–brain interactions
In this section, the (patho)physiological systems and pathways involved in heart–brain crosstalk and the related sex differences are described. In addition, a general overview of the main imaging modalities currently available for the evaluation of the heart–brain axis is provided (Figure 1). Indeed, new generation multisystem scanners, such as whole-body positron emission tomography (PET)/computed tomography (CT) and PET/magnetic resonance (MR), offer the unique opportunity to combine molecular with functional and anatomical imaging information, thus allowing a better understanding of the interlinked pathways involved in these complex multisystem interactions.
Figure 1.
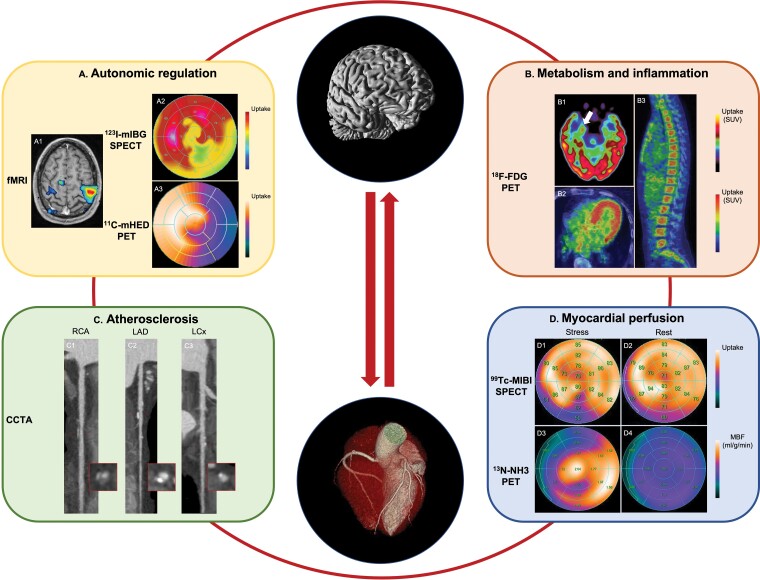
Imaging modalities used to investigate the mechanisms involved in the heart–brain crosstalk. (A) Functional MR illustrates activated regions of the brain (A1). 123I-mIBG-SPECT shows a perfusion defect involving the infero-lateral wall of the left ventricle (A2). 11C-mHED-PET demonstrates reduced tracer uptake in the lateral wall of the left ventricle (A3). The findings shown in A2 and A3 are indicative of cardiac sympathetic denervation and, indirectly, of increased sympathetic tone. The uptake scales used for image visualization are reported on the right. (B) 18F-FDG-PET images show an increased 18F-FDG uptake at the level of the right amygdala (B1 – white arrow), myocardium (B2), and bone marrow of the spine (B3). The SUV scale used for image visualization is reported on the right. (C) Straight multiple curve reconstructions from CCTA show a mixed plaque with positive remodelling of mid RCA (C1), calcified plaque of mid LAD (C2), and spotty calcification of the mid LCx (C3). A cross-section at the level of the corresponding plaque is also shown for each vessel (red box). (D) SPECT images acquired during stress (D1) show a reversible myocardial perfusion defect of the left ventricular inferior wall which is not present at rest (D2). Hypoperfusion is detected as a relative decrease of the uptake of the inferior wall (50–62%) as compared to the myocardial territory with the highest tracer uptake. PET images acquired during stress indicate a low MBF (mL/g/min) in the myocardial territory supplied by the LAD (D3). In the LAD territory MBF did not increase during stress (D3) as compared to rest (D4). The uptake and MBF scales used for image visualization and MBF quantification are reported on the right. CCTA: coronary computed tomography angiography; 11C-mHED: 11C-meta-hydroxyephedrine; 18F-FDG: 18F-fluorodeoxyglucose; fMRI: functional magnetic resonance imaging; 123I-mIBG: 123I-metaiodobenzylguanidine; LAD: left anterior descending coronary artery; LCx: left circumflex coronary artery; MBF: myocardial blood flow; MR: magnetic resonance; 13N-NH3: 13N-ammonia; PET: positron emission tomography; RCA: right coronary artery; SPECT: single-positron emission computed tomography; SUV: standard uptake value; 99Tc-MIBI: 99Technetium-methoxyisobutyl isonitrile.123I-mIBG-SPECT image was provided through the courtesy of Dr Renata Chequer and Prof. François Rouzet from the Nuclear Medicine department of the Bichat Hospital—Assistance Publique Hôpitaux de Paris.
Vascular system
The vascular system is an obvious connector between heart and brain since atherosclerosis is the systemic process identified as the culprit for causing both acute myocardial infarction (AMI) and stroke. Atherosclerosis is an inflammatory disease initiated and promoted by endothelial activation and dysfunction, leading to an increased vascular permeability for plasma proteins, upregulation of adhesion molecules, and release of pro-inflammatory cytokines and chemokines.9,10 The involvement of these local and systemic cascades triggers innate and adaptive immunity10,11 and induces a state of hypercoagulability,12 thus increasing the risk of cardiovascular events13,14 and long-term cognitive impairment.15
Thanks to recent technological advances in cardiovascular imaging, it is possible to non-invasively assess atherosclerotic plaques in the coronary arteries, carotids, and aorta16 as well as their effect on myocardial and brain perfusion. While single and dual-energy coronary CT angiography and MR imaging provide mainly anatomical information on plaque morphology and composition,17–19 PET radiotracers such as 18F-sodium fluoride, 68Ga-DOTATATE, 18F-fluorodeoxyglucose (18F-FDG) offer additional details on plaque biology and activity,20,21 thereby allowing a better discrimination between stable and unstable plaques. The current evidence supports a female-specific profile of less obstructive coronary artery disease (CAD) and lower plaque burden, yet with worse clinical outcome.22
Regarding the haemodynamic impact of atherosclerosis, single-photon emission computed tomography (SPECT) imaging is the most commonly used non-invasive modality for the evaluation of myocardial perfusion,23 with a large body of evidence supporting its prognostic role in patients with IHD.24 Nevertheless, radiation dose associated with SPECT remains an issue,25 and its performance in perfusion quantification is limited and not standardized.26 Therefore, PET is considered the reference standard for quantitative measurement of myocardial blood flow by using different radiotracers such as 82Rubidium, 13N-ammonia, 18F-flurpiridaz, or 15O-water.26 Alternative modalities such as stress echocardiography, MR imaging, and CT are currently available for the evaluation of myocardial perfusion.27 In the brain, the main techniques currently dedicated to the evaluation of brain haemodynamics are dynamic CT, PET, SPECT, as well as diffusion and perfusion MR.28,29 Healthy women have been reported to have significantly higher global and regional blood flow than men in both heart and brain.30–34
Neurohumoral system
The autonomic nervous system, the limbic network, and the renin–angiotensin–aldosterone system (RAAS) are all important variables affecting the heart–brain axis, hence representing new important therapeutic targets in cardiovascular and neurological diseases.
Through the sympathetic nervous system (SNS) and parasympathetic nervous system (PNS), the central autonomic network regulates cardiac contraction, heart rate, and blood flow during basal conditions, as well as in response to different triggers, such as acute and chronic stress.35 In particular, sympathetic activation has been detected within the prefrontal cortex, anterior cingulate, left amygdala, as well as the right anterior and left posterior insular cortices.36,37 As such, studies using functional MR imaging (fMRI) techniques to assess connectivity in the brain have demonstrated pathways of heart–brain interactions by identifying the cerebral areas which modulate sympathetic and parasympathetic activity in several conditions, including TSS and hypertension.38–42 While imaging of adrenergic and cholinergic neurotransmission in the brain is not usually performed, the peripheral autonomic system of the human heart can be interrogated by different approaches.43 First, heart rate variability (HRV) and heart rate responses to exercise or pharmacological stress are widely used surrogate parameters of the autonomic activity of the heart (Table 2). In addition, radiolabelled catecholamine analog-based myocardial imaging by SPECT and PET can provide information regarding the integrity of the cardiac SNS by informing on the status of the pre- and post-synaptic nerve function.44 At present, 123I-metaiodobenzylguanidine-(123I-mIBG)-SPECT with planar acquisition is considered the reference standard for the evaluation of cardiac sympathetic dysfunction in several cardiac diseases, such as cardiac arrhythmia, IHD, and HF.45,46 A more sophisticated PET radiotracer is 11C-meta-hydroxyephedrine (11C-mHED), which is characterized by a higher sensitivity and spatial resolution than 123I-mIBG, allowing for the absolute quantification of the regional distribution of cardiac sympathetic neurons.45,47 Besides 123I-mIBG-SPECT and 11C-mHED-PET, 18F-dihydroxyphenylalanine-(18F-DOPA)-PET, originally used to evaluate the striatal dopaminergic dysfunction in degenerative diseases, has been associated with increased sympathetic activity at cardiac level.48,49 Sex differences have been detected at several levels of the autonomic nervous system. In fact, animal and human studies have consistently highlighted that, under physiological conditions, men have a higher baseline sympathetic activity, whereas women display a more pronounced parasympathetic tone while maintaining sympatho-vagal balance.35 Interestingly, this difference attenuates with increasing age,48,50 possibly resulting from changes in sex hormone concentrations which affect the autonomic system at central and peripheral levels.51 These findings have been corroborated by using resting-state fMRI which showed that premenopausal women have a stronger negative resting-state functional connectivity with the default mode network (i.e. area responsible for the suppression of the sympathetic outflow in the central autonomic network) in comparison to age-matched men.52 The sex-dimorphism related to sympatho-vagal balance disappears after menopause confirming that postmenopausal women have weaker parasympathetic activity and increased sympathetic outflow as compared to younger age.52 Of note, the distinct balance of autonomic function between women and men translates clinically into sex-divergent effects of beta-blockers.53,54 Indeed, recent data suggest that women need lower dose of beta-blockers compared to men to reach maximal therapeutic efficacy in HF with reduced ejection fraction (HFrEF).55 Sex hormones also play an important role in modulating sex differences of the sympatho-vagal balance.35 Experimental data have shown that receptors for gonadal hormones are present in areas of the central nervous system involved in the regulation of the autonomic nervous system.35 Accordingly, intravenous or central administration of oestrogens resulted in an enhanced parasympathetic response35,51,56 whereas testosterone triggered the production and reduced the clearance of noradrenaline.35 Therefore, menopause represents an important milestone in female health since it indicates a change in cardiac physiology, as well as an increased risk for cardiovascular diseases.57 Although a disproportionally high sympathetic activity has been associated with unfavourable outcomes in both male and female cardiovascular patients,58 women seem to be more vulnerable to the detrimental effects of sympathetic hyperactivity.59 As such, myocardial 18F-DOPA uptake was shown to be higher in elderly women as compared to men, especially at the level of the left ventricular (LV) apex. This distribution pattern of myocardial 18F-DOPA aligns with the area of LV dysfunction involving the cardiac apex in TTS.48
Table 2.
Heart rate variability and heart rate response: definitions, physiological interpretation, and clinical value
| Definition | Physiological interpretation | Clinical value | |
|---|---|---|---|
| HRV | Variations in the beat-to-beat heart intervals evaluated on electrocardiogram198 |
|
|
| HRR to exercise or pharmacological stress | Maximum percentage change after exercise or pharmacological stress from baseline HR59: [(HRmaximum – HRbaseline)]/HRbaseline * 100 |
AMI: acute myocardial infarction; HF: heart failure; HR: heart rate; HRR: heart rate response; HRV: heart rate variability.
The limbic system comprises different cortical areas and subcortical nuclei of the brain, including the amygdala,60 and mediates most of the vegetative and endocrine functions of the body such as emotions, behaviours, and memory.60 During stress conditions, the amygdala stimulates the hypothalamus via efferent neurons to increase SNS activity and initiate neurohormonal output through release of adrenocorticotropic hormone by the hypothalamic–pituitary–adrenal (HPA) axis.61 In this context, the SNS plays a key role in driving systemic inflammation62 and immune modulation63 through sympathetic nerve fibres terminating in the bone marrow and stimulating turnover and release of myeloid cells.6,64 This effect is further mediated by the HPA axis through catecholamine release, myelopoiesis activation, and a further increase in interleukin (IL)-6 and C-reactive protein (CRP) levels.65,66 The established pro-inflammatory state favours the development of atherosclerosis, thus highlighting the close interdependence between neuroinflammatory circuits and the vascular system.67–69 By administering the glucose analog 18F-FDG, PET imaging enables the evaluation of regional metabolism of heart and brain.6 Notably, 18F-FDG-PET demonstrated higher stress-associated neural activity (SNA) in women during physiological aging, which manifests as an increased resting 18F-FDG uptake at the level of the amygdala.70 This finding may be explained by the greater and prolonged mental stress perceived by women during lifespan compared with men as a reaction to negative emotional episodes.71 Indeed, while both women and men demonstrated resting-state functional connectivity to sensory and emotion-related regions of the brain on resting-state fMRI, men showed higher connectivity to areas involved in the control of emotions.72
Finally, the RAAS is well-represented in both heart and brain, where it regulates blood pressure (BP) and tissue blood flow, as well as immune responses and tissue homeostasis in response to ischaemic injury and SNS activation.73
Immune system and inflammation
Owing to its ability to alter tissue perfusion and neurohumoral activation, inflammation represents the link between heart and brain in different pathological conditions such as stroke and myocardial infarction.74 As inflammatory cells are characterized by elevated glucose metabolism, 18F-FDG-PET can be used to quantify spleen75 and bone marrow activity6 (i.e. indicative of activation of the haematopoietic system), as well as inflammatory responses within the arterial wall.6,43 Nevertheless, due to the low specificity of 18F-FDG in inflammation detection, new targets involved in the regulation of the immune system are currently being investigated.43 Among these, the 18 kD translocator protein (TSPO), expressed on the outer mitochondrial membrane, has shown promising results given that TSPO expression increases in response to immune activation in both microglia and systemic immune system.76 Preliminary data indicate that TSPO-target imaging in patients with myocardial infarction identifies early post-infarct myocardial inflammation as well as the presence of neuroinflammation.77 Clinical data point to significant sex differences in inflammatory and innate immune responses, with women showing higher baseline levels of circulating inflammatory markers78 and more pronounced production of pro-inflammatory cytokines in response to different injuries.79–82 As such, a significant increase in 18F-FDG bone marrow uptake has been reported in women with impaired myocardial perfusion, but not in men.80
The scheme of the Graphical abstract integrates the main systems involved in the heart–brain crosstalk, highlighting the sex differences which affect them.
Exploratory concepts for the assessment of heart–brain interaction
Beyond metabolic and perfusion imaging, several brain receptor systems are promising imaging targets to elucidate mechanisms driving heart–brain interactions. Notably, while an enhanced amygdalar metabolic activity was associated with emotional processing, anxiety, and fear, these processes can be attenuated by targeted interventions at the neurotransmitter level, thus suggesting that neuroreceptors are crucial components of the anxiety circuitry.83,84 Among these neuroreceptors, there is a solid body of evidence implicating fast inhibitory ionotropic gamma-aminobutyric acid (GABAA) receptors in fear and mental stress development.85–89 As such, the availability of clinically validated GABAA receptor probes, such as 18F-flumazenil, harbours potential to facilitate heart–brain research and shed light on sex differences in emotional stress processing.90,91 In addition to GABAA receptors, serotonergic, adrenergic, and glutamatergic signalling have been linked to critical neurotransmission in anxiety, mental stress, and stress-induced cardiomyopathy.84,92–94 Notably, advances in translational molecular imaging have channelled the development of suitable radiotracers for the non-invasive assessment of these receptors.95–97
Heart diseases
Ischaemic heart disease
Although tremendous improvements in therapeutic strategies have led to a decline in the overall mortality rate for IHD by ∼30% during the past decade, this occurred far less in women as compared to men.98 Furthermore, mortality rates in women presenting with ST-elevation myocardial infarction (STEMI) are higher than in age-matched men99,100 despite women having less plaque burden and a lower rate of obstructive CAD.22,101 Therefore, the previous assumption that the pathophysiology of IHD is the same for women and men, but with a later onset in females, is an erroneous and over-simplified concept. Since differences in traditional cardiovascular risk factors cannot totally explain the observed sex disparities, sex-specific genetic risk profiles and non-traditional risk conditions have been proposed as complementary mechanisms.
To begin with, genome-wide association studies have recently identified more than 100 genetic loci across the genome correlated with the development of IHD.102,103 In this context, sex-specific variants in several genes have been detected by using polygenic risk scores, demonstrating that the genetic effect of IHD is modified by sex.104 The involvement of the SNS and HPA axis in triggering sex differences in clinical outcomes has also been considered. Clinical results from the large CONFIRM (Coronary CT Angiography Evaluation for Clinical Outcomes: An International Multicenter Registry) registry showed that LV ejection fraction (LVEF) is significantly higher in women as compared with men.105 A strong association between LVEF >65% and increased 6-year mortality risk has been documented in women but not in men after adjusting for age, cardiovascular risk factors, and severity of CAD.106 Interestingly, the prevalence of abnormally low end-systolic volumes was twice as high in older women as compared with younger women or men.106 These findings support the hypothesis that postmenopausal women live under constant sympathetic hyperactivity to compensate for the disadvantage of small left ventricles, hence predisposing them to cardiac vulnerability in high-stress situations. As such, in women presenting with an acute coronary syndrome, sympathetic activity persisted for approximately nine months after the index event and was associated with an unfavourable prognosis.107 Accordingly, chronic psychological stress has recently been listed as a risk factor for incident IHD.108 The Perceived Stress Scale is a validated clinical questionnaire currently used to evaluate individual stress perception.109 Nevertheless, a more reliable and reproducible stress level assessment can be obtained by measuring SNA by 18F-FDG-PET.110 Increased metabolic activity of the amygdala (when referenced to counter-regulatory activity from the medial prefrontal cortex or temporal lobe) has been reported as an independent predictor of future major adverse cardiovascular events (MACE) in a population of patients without known IHD or active cancer.6 Notably, several studies suggested that SNA may represent a key element driving sex differences in IHD pathophysiology through downstream effects on autonomic, immune, and vascular physiology.111 In fact, endothelial dysfunction in response to cumulative mental stress has previously been described in women but less so in men.112 In addition, Vaccarino et al. documented that perceived stress after AMI differs between women and men. In their study, while mental stress-induced myocardial ischaemia was more common in young women with previous myocardial infarction as compared with men, no sex differences were observed after exercise stress testing.113 Second, our group reported a strong association between increased SNA, myocardial dysfunction, and subclinical inflammation in women, but not in men.2,81 Similarly, stress-induced IL-6 and monocyte chemoattractant protein-1 have been identified as predictors of future cardiovascular events in women with existing cardiovascular disease, while this association was not observed in men.114
Heart failure
Women with HF are usually older than men115 and have a better prognosis and reduced mortality after treatment.116 In addition, women are more commonly affected by HF with preserved ejection fraction (HFpEF), which is often associated with diabetes and hypertension. In contrast, HFrEF is more prevalent in men and frequently has an ischaemic aetiology. Overall, the predominance of female sex in HFpEF can be explained by the difference in adaptive ventricular remodelling between women and men in response to increased afterload and aging. As such, postmenopausal women with HFpEF develop more frequently a hypertrophied, stiff, and non-dilated left ventricle as compared to men117 accounting for the higher prevalence of diastolic dysfunction in this demographic group.118
Sex hormones and neurohumoral systems drive the development of HF and related sex differences. Testosterone stimulates RAAS activity and triggers vasoconstriction and cardiac hypertrophy whereas oestrogens attenuate RAAS activity, stimulate vasodilatation, and are associated with a more benign phenotype of cardiac remodelling.119 The hyperactivation of the SNS has also been recognized as a critical mechanism in the development of HF as it correlates with disease progression and poor prognosis.120 Indeed, in the acute phase of HF, the activity of the SNS is enhanced to compensate for the reduced myocardial contractility. However, in the long-term, the persistent and excessive stimulation of the SNS promotes maladaptive cardiac hypertrophy and cell death. Of note, SNS hyperactivation is strongly associated with arterial hypertension, obesity, and diabetes, which are the main determinants of HFpEF in women.121 Enhanced sympathetic outflow as reflected by increased cardiac norepinephrine turnover and pre-synaptic norepinephrine deficits44 can be detected by 123I-mIBG scintigraphy. A pooled analysis of several multicentre cohort studies demonstrated the independent, long-term prognostic value of 123I-mIBG uptake in patients with HF after adjusting for New York Heart Association functional class, LVEF, and natriuretic peptide values.122,123 Similarly, cardiac sympathetic denervation evaluated by 11C-mHED-PET imaging has been associated with the severity of diastolic abnormality, contractile dysfunction, and fibrotic burden in patients with HFpEF.124,125
Finally, both the heart and the brain have an intrinsic RAAS that is activated in HF, also contributing to sympathetic hyperactivity.126 In this context, preliminary data from a rat model of ischaemic cardiomyopathy revealed significant sex differences in the central and peripheral manifestations of ischaemia-induced HF, thereby providing a potential explanation for better outcomes seen in women with HF as compared with men.119 In particular, in the hypothalamic paraventricular nucleus, a key area contributing to neurohumoral excitation in HF, mRNA levels for pro-inflammatory markers, such as tumor necrosis factor-α and IL-1β, increased less in female as compared with male rats. Conversely, plasma norepinephrine levels were lower for female rats suggesting a weaker activation of the SNS.119 Sex specificity in the involvement of the RAAS and sympathetic hyperactivity has also been observed in patients with both HFpEF127,128 and HFrEF.55 In fact, in the PARAGON-HF (Prospective Comparison of Angiotensin Receptor–Neprilysin Inhibitor with Angiotensin Receptor Blockers Global Outcomes in HF with Preserved Ejection Fraction) trial,128,129 among 4796 patients with HFpEF, sex appeared to modify the effect of sacubitril-valsartan versus valsartan, reducing the number of HF hospitalization in women only.128
Takotsubo syndrome
Although contemporary evidence strongly suggests an involvement of the limbic system in the pathophysiology of TTS,40 the exact mechanisms by which a stressful life event translates into the onset of TTS are not fully understood. Notably, recent milestone discoveries have directly linked the amygdala to TSS pathophysiology. As such, enhanced SNA was associated with an increased risk for TTS in a recent study by Radfar and co-workers.5 Further, among individuals who developed TTS, a heightened SNA was present years before disease onset, indicating that SNA precedes the cardiac manifestation of TTS and may represent a promising prevention target.5,130 In a cross-sectional study encompassing 20 female TTS patients and 39 age- and sex-matched healthy controls, a reduced thickness of the insular cortex, as well as a reduced amygdalar gray matter volume were observed in TTS patients but not in controls.38 These anatomical differences were further substantiated by a follow-up study, in which reduced functional connectivity of central brain regions associated with regulation of the limbic system were detected in patients with TTS.40,131–133 Given these findings, it is tempting to hypothesize that sex differences in emotional stress perception and processing via heart–brain interactions may contribute to the higher prevalence of TTS in women.48,107,134,135 Although it should be noted that the relatively low prevalence of male TTS patients constitutes a major challenge for the design of appropriate prospective and sex-specific TTS studies, several recent reports concluded that the link between amygdalar metabolic activity and abnormal cardiac function was particularly accentuated in women.2,81,136,137 Similarly, sympathetic hyperactivity and microvascular dysfunction, both implicated in TSS pathophysiology,138,139 were found to predict MACE in women but not in men.59,140 In concert with the high prevalence of TTS in postmenopausal women, oestrogens were found to attenuate sympathetic responses to mental stress in perimenopausal women.141 Furthermore, through a variety of mechanisms, oestrogens represent a key regulator of endothelial function and vasomotor tone. These mechanisms include, but are not limited to, the attenuation of catecholamine-mediated vasoconstriction142 and the upregulation of endothelial nitric oxide synthase activity143. Consequently, the combination of enhanced baseline sympathetic tone and impaired vasomotor function may render postmenopausal women susceptible to TTS during periods of acute mental or physical stress.135
Brain diseases
Stroke
Although the association between genetic risk score and incident stroke has been demonstrated in both women and men, the absolute risk of incident stroke is lower in women.144 Sex differences in stroke epidemiology have been reported with a specific trend over the lifespan. During youth and early adulthood, stroke incidence is lower in women than men. In the middle-aged, stroke rates start to increase in women145,146 and progressively grow with aging. The increasing risk of stroke in women above 65 years compared with younger women is partially explained by the loss of neuroprotective effect of sex hormones in the postmenopause period, owing to their ability to maintain vascular endothelial function and attenuate inflammatory responses.147,148 Similarly, low levels of testosterone in men have been associated with increased systemic inflammation and endothelial dysfunction, thus promoting the development of atherosclerosis as well as increasing the risk for stroke.149
Current evidence supports a deep connection between the heart and brain in patients affected by ischaemic stroke. First, stroke and IHD share the same risk factors.150 Moreover, ischaemic stroke is caused by IHD in about 20% of cases. In this context, stroke due to atrial fibrillation is more common in women as compared with men, particularly at an older age. Stroke is also associated with worse outcomes in women, as shown by the higher all-cause mortality rate in this population.151 Furthermore, a strong interaction between stroke and HF is well established given that HF induces a state of hypercoagulability thereby leading to decreased blood flow velocity, endothelial dysfunction, enhanced platelet aggregation, as well as reduced fibrinolysis152 all of which increase stroke risk and, consequently, morbidity and mortality of HF patients.152 Second, cardiac complications represent the second leading cause of mortality after stroke.153 Indeed, after the index event, patients may present with a broad range of cardiovascular signs and symptoms (stroke–heart syndrome), including electrocardiogram alterations, elevation of cardiac biomarkers, cardiac dysfunction, arrhythmia, and myocardial infarction.36 The extent and burden of cardiac complications after stroke correlates with the site of the brain injury and the severity of the index event.154,155 Notably, several large population-based studies showed a sex-specific risk of MACE after stroke156,157 with a higher incidence of MACE, cardiovascular mortality, and HF in women as compared to men.157
In addition to neurological dysfunction, ischaemic stroke is also associated with an increased risk of acute cardiac events and chronic HF. The hypothesis supporting the pathophysiology of the stroke–heart syndrome is based on the concept that stroke damages specific brain areas of the central autonomic network. As with strong emotions, such as fear, this may lead to an overactivated stress response that triggers the autonomic nervous system and the HPA axis.36,150,153 As such, the excessive release of cortisol and catecholamines has a detrimental effect on the heart, causing cardiomyocyte necrosis, hypertrophy, and myocardial fibrosis.150 In a population of 222 consecutive patients admitted due to ischaemic stroke, high troponin I levels were significantly associated with elevation of circulating catecholamines, supporting the concept of an hyperactivation of the sympathoadrenal system.158 Inflammation represents an additional linking factor since the local inflammatory response induced by stroke extends into the systemic circulation, hence yielding secondary cardiac damage.150 In an animal model of stroke induced by transient ligation of the middle cerebral artery, PET imaging showed an association between neuroinflammation and cardiac inflammation, as detected by an increased TSPO uptake as well as by a persisting decline in cardiac contractility.159 At a molecular level, the activation of SNS and HPA axis translates into the activation of forkhead box O (FOXO) genes. FOXO genes have recently been identified as potential molecular target for cardiac dysfunction since they are associated with an increased risk of myocardial infarction.150 It is likely that the known sex differences in stress response, autonomic function, and the related inflammatory response may disproportionally affect women after stroke.160,161
Depression
While depression is evenly distributed between both sexes during childhood, sex and gender imbalance starts at the age of twelve and peaks during puberty, with young women being up to three-fold more often affected than young men.162,163 Thereafter, the well-known female-male ratio of 2:1 remains stable over the entire adult lifespan. Current evidence supports an association between depression and cardiovascular disease. The prevalence of cardiac comorbidities among adult patients with depression is approximately three-fold greater than in the general population without mood disorders.164,165 On the other hand, the presence of depressive symptoms is independently associated with higher cardiac and all-cause mortality, re-hospitalization, and quality of life after AMI.166 Therefore, the European Society of Cardiology recently listed depression as a modifiable cardiovascular risk factor in patients with CAD.108 Of note, anti-depressant medication, such as escitalopram and sertraline, has been shown to be an effective therapeutic strategy to improve long-term cardiovascular outcomes in both sexes.167
The prevalence of depression after AMI is higher in women than men.166,168 This likely occurs as the result of the combined effect of sex-related differences in several mechanisms involved in heart–brain crosstalk. To begin with, a number of fMRI studies have linked anxiety and depressive disorders to the hyperactivation of the amygdala, insula, and anterior cingulate cortex.169 In particular, trait anxiety in post-puberal females has been shown to be mediated by elevated perfusion in the left amygdala.170 Second, depression has been associated with a greater activation of the SNS, as demonstrated by a reduced HRV in patients with depressive disorders.171,172 Again, this phenomenon is more pronounced in women with depression as compared with men173 and appears to mediate the detrimental interaction between depression and cardiovascular health.174 Next, reduced HRV has been linked to peripheral inflammation, another potential mechanism connecting depression and cardiovascular disease.175 A Mendelian randomization analysis reported that triglycerides, IL-6, and CRP are likely to be causally linked to depression, thus representing promising future targets for pharmacological therapies.176 Accordingly, incremented levels of inflammatory markers, such as CRP and IL-6, are commonly being measured in patients with depression, and this finding is more pronounced in women.177,178 Finally, sex hormones, such as progesterone, testosterone, and high oestrogen levels, are known to be anti-inflammatory and protective in terms of depression.179 Conversely, lower oestrogen levels have been shown to exert detrimental pro-inflammatory effects during hormonal transition periods such as post-partum and peri-menopause,180–182 explaining the higher vulnerability of women to the coexistence of depression and cardiovascular disease. In men with depressive disorders, testosterone treatment has been associated with a moderate anti-depressant effect as compared with placebo.183
Dementia
Dementia overall affects women twice as often as men.184 Apolipoprotein E epsilon 4 (ApoE4) is the strongest genetic risk factor for Alzheimer’s disease. Although the frequency of ApoE4 genotype is similar between women and men, the risk of Alzheimer’s disease in ApoE4 carriers is higher in women than men between 65 and 75 years.185
The heart–brain axis has been identified as a potential contributor to the pathogenesis and progression of degenerative diseases. Indeed, cognitive impairment is more common in patients with previous myocardial infarction and chronic HF.186,187 Similarly, atherosclerosis has been identified as a risk factor for Alzheimer’s disease, with endothelial dysfunction and impaired microcirculation being strictly connected to the functional decline.188,189 In this context, ApoE has been reported to be the link between neuroinflammation and atherosclerosis in Alzheimer patients.190 In addition, TSPO-target whole-body molecular imaging confirmed the role of inflammation as the critical connector between the heart and the brain after cardiac injury by detecting microglia activation in the early phase of post-myocardial infarction and in the late phase of chronic HF.77 This is of paramount importance since inflammation represents a potential therapeutic target for decelerating cognitive decline.77
Current evidence suggests also that several heart conditions are linked to dementia in a sex-specific manner.191 First, hypertension has been associated with vascular dementia and Alzheimer’s disease.192 Indeed, both elevated BP and high variability in BP compromise the structural integrity of the cerebral microvasculature by impairing cerebral blood supply and promoting neuroinflammation through disruption of the blood–brain barrier.193,194 The LIFE-Adult study demonstrated independent and significant associations between white matter lesions and age as well as high BP, stroke, and HF. HF patients had a 2.5-fold increased likelihood of white matter lesions than those without HF. In addition, white matter lesions increased with the duration of HF.195 Regional cerebral hypoperfusion has been identified in several brain areas of patients with HF, affecting autonomic, mood, and cognitive regulatory cerebral sites.152,196 Of note, women suffering from hypertension have shown worse cognitive performance than normotensive women in the postmenopausal period.197
Conclusion and outlook
The crosstalk between heart and brain is complex, multifactorial, and still insufficiently defined. An additional layer of complexity is added by sex and gender differences that characterize the heart–brain axis, partially explaining sexual dimorphism in epidemiology, pathogenesis, clinical manifestation, and treatment responses of specific heart and brain diseases. Sex hormones, neurohumoral activity, and systemic inflammation are potential pathways mediating sex differences in heart–brain interactions. As many of these pathways represent pharmacological targets, further investigation of molecular mechanisms that regulate the heart–brain axis offers the possibility to interrupt pathogenetic transmission, thereby leading to novel individualized treatment approaches. However, many knowledge gaps remain (Table 3). As such, the effect of socio-cultural gender on heart–brain interactions is largely unknown, although increasing evidence emphasizes the importance of both, biological attributes and gender as major modifiers of health and disease.8 As the mechanisms contributing to the excess risk in women with myocardial infarction remain largely unclear, gender-specific research identifying novel targets reflecting women’s biological systems and behaviour is also urgently needed. As such, the brain’s stress network and its downstream consequences is a promising signalling pathway given the predisposition of women to mental stress-induced ischaemia and sympathetic over-activity. Indeed, designing therapies and interventions that can interrupt a vicious cycle between stress and cardiovascular events is an attractive strategy to target cardiovascular health inequalities between women and men linked to modifiable risk factors. Future research will also have to investigate the directionality and causality of heart–brain interactions, thus, the currently evolving field of neurocardiology promises to be very active in the upcoming years.
Table 3.
Knowledge gaps in heart–brain interactions
|
Abbreviations
- AMI
acute myocardial infarction
- BP
blood pressure
- CAD
coronary artery disease
- CRP
C-reactive protein
- 11C-mHED
11C-meta-hydroxyephedrine
- CONFIRM
Coronary CT Angiography Evaluation for Clinical Outcomes: An International Multicenter Registry
- CT
computed tomography
- 18F-DOPA
18F-dihydroxyphenylalanine
- 18F-FDG
18F-fluorodeoxyglucose
- fMRI
functional magnetic resonance imaging
- FOXO
forkhead box O
- GABAA
gamma-aminobutyric acid
- HF
heart failure
- HFpEF
heart failure with preserved ejection fraction
- HFrEF
heart failure with reduced ejection fraction
- HPA axis
hypothalamic–pituitary–adrenal axis
- HRV
hear rate variability
- 123I-mIBG
123I-metaiodobenzylguanidine
- IHD
ischaemic heart disease
- IL
interleukin
- LV
left ventricular
- LVEF
left ventricular ejection fraction
- MACE
major adverse cardiovascular events
- MR
magnetic resonance
- PET
positron emission tomography
- PNS
parasympathetic nervous system
- RAAS
renin–angiotensin–aldosterone system
- SNA
stress-associated neural activity
- SNS
sympathetic nervous system
- SPECT
single-photon emission computed tomography
- STEMI
ST-elevation myocardial infarction
- 99Tc-MIBI
99Technetium-methoxyisobutyl isonitrile
- TSPO
18kD translocator protein
- TTS
Takotsubo syndrome.
Contributor Information
Alexia Rossi, Department of Nuclear Medicine, University Hospital Zurich, Raemistrasse 100, 8091 Zurich, Switzerland; Center for Molecular Cardiology, University of Zurich, Schlieren, Switzerland.
Nidaa Mikail, Department of Nuclear Medicine, University Hospital Zurich, Raemistrasse 100, 8091 Zurich, Switzerland; Center for Molecular Cardiology, University of Zurich, Schlieren, Switzerland.
Susan Bengs, Department of Nuclear Medicine, University Hospital Zurich, Raemistrasse 100, 8091 Zurich, Switzerland; Center for Molecular Cardiology, University of Zurich, Schlieren, Switzerland.
Ahmed Haider, Department of Nuclear Medicine, University Hospital Zurich, Raemistrasse 100, 8091 Zurich, Switzerland; Center for Molecular Cardiology, University of Zurich, Schlieren, Switzerland; Division of Nuclear Medicine and Molecular Imaging, Department of Radiology, Massachusetts General Hospital, and Harvard Medical School, Boston, MA, USA.
Valerie Treyer, Department of Nuclear Medicine, University Hospital Zurich, Raemistrasse 100, 8091 Zurich, Switzerland.
Ronny Ralf Buechel, Department of Nuclear Medicine, University Hospital Zurich, Raemistrasse 100, 8091 Zurich, Switzerland.
Susanne Wegener, Department of Neurology, University Hospital Zurich and University of Zurich, Zurich, Switzerland.
Katrin Rauen, Department of Geriatric Psychiatry, Psychiatric Hospital, Zurich, Switzerland; Institute for Stroke and Dementia Research, University Hospital, Ludwig Maximilian University of Munich, Munich, Germany.
Ahmed Tawakol, Cardiovascular Imaging Research Center, Cardiology Division, Massachusetts General Hospital and Harvard Medical School, Boston, MA, USA.
C Noel Bairey Merz, Barbra Streisand Women's Heart Center, Smidt Heart Institute, Cedars-Sinai Medical Center, Los Angeles, CA, United States.
Vera Regitz-Zagrosek, Charité, Universitätsmedizin Berlin, Berlin, Germany; University of Zurich, Zurich, Switzerland.
Catherine Gebhard, Department of Nuclear Medicine, University Hospital Zurich, Raemistrasse 100, 8091 Zurich, Switzerland; Center for Molecular Cardiology, University of Zurich, Schlieren, Switzerland.
Funding
Susan Bengs received research funding from the University of Zurich (UZH) Foundation and the Swiss Heart Foundation. Ahmed Haider was supported by the University of Zurich (UZH) Foundation. Susanne Wegener reports research funding from Swiss National Science Foundation PP00P3_170683 and UZH Clinical Research Priority Program (CRPP) Stroke. Noel Bairey Merz reports research funding from the National Institutes of Aging U54AG065141; the Barbra Streisand Women’s Cardiovascular Research and Education Program; the Linda Joy Pollin Women’s Heart Health Program; the Erika Glazer Women’s Heart Health Project; and the Adelson Family Foundation, Cedars-Sinai Medical Center, Los Angeles, California. Vera Regitz-Zagrosek reports research funding from GE Academy H2020-SwafS-2018-2020/EU: 824585; Gendage, BMBF, 01GL1716A; and Gender/Sex bei MS, BMG, ZMV I 1 - 25 20 FSB 431. Ahmed Tawakol reports research funding from the US National Institutes of Health (Grants R01HL152957, R01HL149516, R56 AR077187, R33HL141047, R01HL137913); and the Harvard Osher Center for Integrative Medicine. Catherine Gebhard reports research funding from the Swiss National Science Foundation (SNSF); the Olga Mayenfisch Foundation, Switzerland; the OPO Foundation, Switzerland; the Novartis Foundation, Switzerland; the Swiss Heart Foundation; the Helmut Horten Foundation, Switzerland; the EMDO Foundation, Switzerland; the Iten-Kohaut Foundation, Switzerland, the UZH Foundation, the University Hospital Zurich (USZ) Foundation, and from the LOOP, Zurich.
Conflict of interest
none declared.
References
- 1. Tahsili-Fahadan P, Geocadin RG. Heart-brain axis: effects of neurologic injury on cardiovascular function. Circ Res 2017;120:559–572. [DOI] [PubMed] [Google Scholar]
- 2. Fiechter M, Roggo A, Burger IA, Bengs S, Treyer V, Becker A, et al. Association between resting amygdalar activity and abnormal cardiac function in women and men: a retrospective cohort study. Eur Heart J Cardiovasc Imaging 2019;20:625–632. [DOI] [PubMed] [Google Scholar]
- 3. Ishai A, Osborne MT, Tung B, Wang Y, Hammad B, Patrich T, et al. Amygdalar metabolic activity independently associates with progression of visceral adiposity. J Clin Endocrinol Metab 2019;104:1029–1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Osborne MT, Ishai A, Hammad B, Tung B, Wang Y, Baruch A, et al. Amygdalar activity predicts future incident diabetes independently of adiposity. Psychoneuroendocrinology 2019;100:32–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Radfar A, Abohashem S, Osborne MT, Wang Y, Dar T, Hassan MZO, et al. Stress-associated neurobiological activity associates with the risk for and timing of subsequent Takotsubo syndrome. Eur Heart J 2021;42:1898–1908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Tawakol A, Ishai A, Takx RA, Figueroa AL, Ali A, Kaiser Y, et al. Relation between resting amygdalar activity and cardiovascular events: a longitudinal and cohort study. Lancet 2017;389:834–845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Trevisan C, Sergi G, Maggi S. Gender differences in brain-heart connection. In: Govoni SPoliti P and Vanoli E, editors. Brain and heart dynamics. Cham: Springer International Publihing; 2020. p1–15. [Google Scholar]
- 8. Mauvais-Jarvis F, Bairey Merz N, Barnes PJ, Brinton RD, Carrero JJ, DeMeo DL, et al. Sex and gender: modifiers of health, disease, and medicine. Lancet 2020;396:565–582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Libby P. Inflammation in atherosclerosis. Arterioscler Thromb Vasc Biol 2012;32:2045–2051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Gimbrone MA Jr, Garcia-Cardena G. Endothelial Cell Dysfunction and the Pathobiology of Atherosclerosis. Circ Res 2016;118:620–636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Libby P, Lichtman AH, Hansson GK. Immune effector mechanisms implicated in atherosclerosis: from mice to humans. Immunity 2013;38:1092–1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Bester J, Pretorius E. Effects of IL-1beta, IL-6 and IL-8 on erythrocytes, platelets and clot viscoelasticity. Sci Rep 2016;6:32188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lindsberg PJ, Grau AJ. Inflammation and infections as risk factors for ischemic stroke. Stroke 2003;34:2518–2532. [DOI] [PubMed] [Google Scholar]
- 14. Emerging Risk Factors C, Kaptoge S, Di Angelantonio E, Lowe G, Pepys MB, Thompson SG, et al. C-reactive protein concentration and risk of coronary heart disease, stroke, and mortality: an individual participant meta-analysis. Lancet 2010;375:132–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Noble JM, Manly JJ, Schupf N, Tang MX, Mayeux R, Luchsinger JA. Association of C-reactive protein with cognitive impairment. Arch Neurol 2010;67:87–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Daghem M, Bing R, Fayad ZA, Dweck MR. Noninvasive imaging to assess atherosclerotic plaque composition and disease activity: coronary and carotid applications. JACC Cardiovasc Imaging 2020;13:1055–1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Bos D, Arshi B, van den Bouwhuijsen QJA, Ikram MK, Selwaness M, Vernooij MW, et al. Atherosclerotic carotid plaque composition and incident stroke and coronary events. J Am Coll Cardiol 2021;77:1426–1435. [DOI] [PubMed] [Google Scholar]
- 18. Danad I, Fayad ZA, Willemink MJ, Min JK. New applications of cardiac computed tomography: dual-energy, spectral, and molecular CT imaging. JACC Cardiovasc Imaging 2015;8:710–723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Williams MC, Moss AJ, Dweck M, Adamson PD, Alam S, Hunter A, et al. Coronary artery plaque characteristics associated with adverse outcomes in the SCOT-HEART study. J Am Coll Cardiol 2019;73:291–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Varasteh Z, De Rose F, Mohanta S, Li Y, Zhang X, Miritsch B, et al. Imaging atherosclerotic plaques by targeting Galectin-3 and activated macrophages using ((89)Zr)-DFO- Galectin3-F(ab’)2 mAb. Theranostics 2021;11:1864–1876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Demirdelen S, Mannes PZ, Aral AM, Haddad J, Leers SA, Gomez D, et al. Divergence of acetate uptake in proinflammatory and inflammation-resolving macrophages: implications for imaging atherosclerosis. J Nucl Cardiol 2021. Published online ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Truong QA, Rinehart S, Abbara S, Achenbach S, Berman DS, Bullock-Palmer R, et al. Coronary computed tomographic imaging in women: an expert consensus statement from the Society of Cardiovascular Computed Tomography. J Cardiovasc Comput Tomogr 2018;12:451–466. [DOI] [PubMed] [Google Scholar]
- 23. Fazel R, Krumholz HM, Wang Y, Ross JS, Chen J, Ting HH, et al. Exposure to low-dose ionizing radiation from medical imaging procedures. N Engl J Med 2009;361:849–857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Shaw LJ, Berman DS, Maron DJ, Mancini GB, Hayes SW, Hartigan PM, et al. Optimal medical therapy with or without percutaneous coronary intervention to reduce ischemic burden: results from the Clinical Outcomes Utilizing Revascularization and Aggressive Drug Evaluation (COURAGE) trial nuclear substudy. Circulation 2008;117:1283–1291. [DOI] [PubMed] [Google Scholar]
- 25. Gimelli A, Achenbach S, Buechel RR, Edvardsen T, Francone M, Gaemperli O, et al. Strategies for radiation dose reduction in nuclear cardiology and cardiac computed tomography imaging: a report from the European Association of Cardiovascular Imaging (EACVI), the Cardiovascular Committee of European Association of Nuclear Medicine (EANM), and the European Society of Cardiovascular Radiology (ESCR). Eur Heart J 2018;39:286–296. [DOI] [PubMed] [Google Scholar]
- 26. Schofield R, Menezes L, Underwood SR. Nuclear cardiology: state of the art. Heart 2021. Published online ahead of print. [DOI] [PubMed] [Google Scholar]
- 27. Dewey M, Siebes M, Kachelriess M, Kofoed KF, Maurovich-Horvat P, Nikolaou K, et al. Clinical quantitative cardiac imaging for the assessment of myocardial ischaemia. Nat Rev Cardiol 2020;17:427–450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Donahue MJ, Achten E, Cogswell PM, De Leeuw FE, Derdeyn CP, Dijkhuizen RM, et al. Consensus statement on current and emerging methods for the diagnosis and evaluation of cerebrovascular disease. J Cereb Blood Flow Metab 2018;38:1391–1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. European Association of Nuclear Medicine . Brain Imaging. A Technologist’s Guide. https://www.eanm.org/content-eanm/uploads/2016/11/EANM_2016_TechGuide-BrainImaging.pdf.
- 30. Parkes LM, Rashid W, Chard DT, Tofts PS. Normal cerebral perfusion measurements using arterial spin labeling: reproducibility, stability, and age and gender effects. Magn Reson Med 2004;51:736–743. [DOI] [PubMed] [Google Scholar]
- 31. Ghisleni C, Bollmann S, Biason-Lauber A, Poil SS, Brandeis D, Martin E, et al. Effects of steroid hormones on sex differences in cerebral perfusion. PLoS One 2015;10:e0135827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Chareonthaitawee P, Kaufmann PA, Rimoldi O, Camici PG. Heterogeneity of resting and hyperemic myocardial blood flow in healthy humans. Cardiovasc Res 2001;50:151–161. [DOI] [PubMed] [Google Scholar]
- 33. Nickander J, Themudo R, Sigfridsson A, Xue H, Kellman P, Ugander M. Females have higher myocardial perfusion, blood volume and extracellular volume compared to males - an adenosine stress cardiovascular magnetic resonance study. Sci Rep 2020;10:10380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Patel MB, Bui LP, Kirkeeide RL, Gould KL. Imaging microvascular dysfunction and mechanisms for female-male differences in CAD. JACC Cardiovasc Imaging 2016;9:465–482. [DOI] [PubMed] [Google Scholar]
- 35. Dart AM, Du XJ, Kingwell BA. Gender, sex hormones and autonomic nervous control of the cardiovascular system. Cardiovasc Res 2002;53:678–687. [DOI] [PubMed] [Google Scholar]
- 36. Scheitz JF, Nolte CH, Doehner W, Hachinski V, Endres M. Stroke-heart syndrome: clinical presentation and underlying mechanisms. Lancet Neurol 2018;17:1109–1120. [DOI] [PubMed] [Google Scholar]
- 37. Beissner F, Meissner K, Bar KJ, Napadow V. The autonomic brain: an activation likelihood estimation meta-analysis for central processing of autonomic function. J Neurosci 2013;33:10503–10511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Hiestand T, Hänggi J, Klein C, Topka MS, Jaguszewski M, Ghadri JR, et al. Takotsubo syndrome associated with structural brain alterations of the limbic system. J Am Coll Cardiol 2018;71:809–811. [DOI] [PubMed] [Google Scholar]
- 39. Macey PM, Ogren JA, Kumar R, Harper RM. Functional imaging of autonomic regulation: methods and key findings. Front Neurosci 2015;9:513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Templin C, Hänggi J, Klein C, Topka MS, Hiestand T, Levinson RA, et al. Altered limbic and autonomic processing supports brain-heart axis in Takotsubo syndrome. Eur Heart J 2019;40:1183–1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Macefield VG, Henderson LA. Identification of the human sympathetic connectome involved in blood pressure regulation. Neuroimage 2019;202:116119. [DOI] [PubMed] [Google Scholar]
- 42. Hiestand T, Hanggi J, Klein C, Topka MS, Jaguszewski M, Ghadri JR, et al. Takotsubo syndrome associated with structural brain alterations of the limbic system. J Am Coll Cardiol 2018;71:809–811. [DOI] [PubMed] [Google Scholar]
- 43. Bengel FM, Hermanns N, Thackeray JT. Radionuclide imaging of the molecular mechanisms linking heart and brain in ischemic syndromes. Circ Cardiovasc Imaging 2020;13:e011303. [DOI] [PubMed] [Google Scholar]
- 44. Gimelli A, Liga R, Agostini D, Bengel FM, Ernst S, Hyafil F, et al. The role of myocardial innervation imaging in different clinical scenarios: an expert document of the European Association of Cardiovascular Imaging and Cardiovascular Committee of the European Association of Nuclear Medicine. Eur Heart J Cardiovasc Imaging 2021;22:480–490. [DOI] [PubMed] [Google Scholar]
- 45. Zelt JGE, deKemp RA, Rotstein BH, Nair GM, Narula J, Ahmadi A, et al. Nuclear imaging of the cardiac sympathetic nervous system: a disease-specific interpretation in heart failure. JACC Cardiovasc Imaging 2020;13:1036–1054. [DOI] [PubMed] [Google Scholar]
- 46. Mehta PK, Thomson LEJ, Slomka PJ, Hayes SW, Friedman JD, Swift A, et al. Cardiac sympathetic activity by 123I-meta-iodobenzylguanidine imaging in women with coronary microvascular dysfunction: a pilot study. JACC Cardiovasc Imaging 2021;14:1873–1875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Matsunari I, Aoki H, Nomura Y, Takeda N, Chen WP, Taki J, et al. Iodine-123 metaiodobenzylguanidine imaging and carbon-11 hydroxyephedrine positron emission tomography compared in patients with left ventricular dysfunction. Circ Cardiovasc Imaging 2010;3:595–603. [DOI] [PubMed] [Google Scholar]
- 48. Burger IA, Lohmann C, Messerli M, Bengs S, Becker A, Maredziak M, et al. Age- and sex-dependent changes in sympathetic activity of the left ventricular apex assessed by 18F-DOPA PET imaging. PLoS One 2018;13:e0202302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Goyal H, Sharma A, Patel C, Deepak KK, Tripathi M, Gupta P, et al. Assessment of myocardial sympathetic innervation with 18F-FDOPA-PET/CT in patients with autonomic dysfunction: feasibility study in IPD patients. J Nucl Cardiol 2021. Published online ahead of print. [DOI] [PubMed] [Google Scholar]
- 50. Umetani K, Singer DH, McCraty R, Atkinson M. Twenty-four hour time domain heart rate variability and heart rate: relations to age and gender over nine decades. J Am Coll Cardiol 1998;31:593–601. [DOI] [PubMed] [Google Scholar]
- 51. Saleh TM, Connell BJ. Centrally mediated effect of 17beta-estradiol on parasympathetic tone in male rats. Am J Physiol 1999;276:R474–R481. [DOI] [PubMed] [Google Scholar]
- 52. Sie JH, Chen YH, Shiau YH, Chu WC. Gender- and age-specific differences in resting-state functional connectivity of the central autonomic network in adulthood. Front Hum Neurosci 2019;13:369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Luzier AB, Killian A, Wilton JH, Wilson MF, Forrest A, Kazierad DJ. Gender-related effects on metoprolol pharmacokinetics and pharmacodynamics in healthy volunteers. Clin Pharmacol Ther 1999;66:594–601. [DOI] [PubMed] [Google Scholar]
- 54. Mauvais-Jarvis F, Berthold HK, Campesi I, Carrero JJ, Dakal S, Franconi F, et al. Sex- and gender-based pharmacological response to drugs. Pharmacol Rev 2021;73:730–762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Santema BT, Ouwerkerk W, Tromp J, Sama IE, Ravera A, Regitz-Zagrosek V, et al. Identifying optimal doses of heart failure medications in men compared with women: a prospective, observational, cohort study. Lancet 2019;394:1254–1263. [DOI] [PubMed] [Google Scholar]
- 56. Saleh MC, Connell BJ, Saleh TM. Medullary and intrathecal injections of 17β-estradiol in male rats. Brain Res 2000;867:200–209. [DOI] [PubMed] [Google Scholar]
- 57. Moodithaya S, Avadhany ST. Gender differences in age-related changes in cardiac autonomic nervous function. J Aging Res 2012;2012:679345 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. La Rovere MT, Bigger JT, Marcus FI, Mortara A, Schwartz PJ. Baroreflex sensitivity and heart-rate variability in prediction of total cardiac mortality after myocardial infarction. ATRAMI (Autonomic Tone and Reflexes After Myocardial Infarction) Investigators. Lancet 1998;351:478–484. [DOI] [PubMed] [Google Scholar]
- 59. Gebhard CE, Maredziak M, Portmann A, Bengs S, Haider A, Fiechter M, et al. Heart rate reserve is a long-term risk predictor in women undergoing myocardial perfusion imaging. Eur J Nucl Med Mol Imaging 2019;46:2032–2041. [DOI] [PubMed] [Google Scholar]
- 60. Catani M, Dell’acqua F, de Schotten MT. A revised limbic system model for memory, emotion and behaviour. Neurosci Biobehav Rev 2013;37:1724–1737. [DOI] [PubMed] [Google Scholar]
- 61. Osborne MT, Shin LM, Mehta NN, Pitman RK, Fayad ZA, Tawakol A. Disentangling the links between psychosocial stress and cardiovascular disease. Circ Cardiovasc Imaging 2020;13:e010931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Karakas M, Haase T, Zeller T. Linking the sympathetic nervous system to the inflammasome: towards new therapeutics for atherosclerotic cardiovascular disease. Eur Heart J 2018;39:70–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Bellinger DL, Millar BA, Perez S, Carter J, Wood C, ThyagaRajan S, et al. Sympathetic modulation of immunity: relevance to disease. Cell Immunol 2008;252:27–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Heidt T, Sager HB, Courties G, Dutta P, Iwamoto Y, Zaltsman A, et al. Chronic variable stress activates hematopoietic stem cells. Nat Med 2014;20:754–758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Cosentino M, Marino F, Maestroni GJ. Sympathoadrenergic modulation of hematopoiesis: a review of available evidence and of therapeutic perspectives. Front Cell Neurosci 2015;9:302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Hinterdobler J, Schott S, Jin H, Meesmann A, Steinsiek AL, Zimmermann AS, et al. Acute mental stress drives vascular inflammation and promotes plaque destabilization in mouse atherosclerosis. Eur Heart J 2021;42:4077–4088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Xiao H, Li H, Wang JJ, Zhang JS, Shen J, An XB, et al. IL-18 cleavage triggers cardiac inflammation and fibrosis upon β-adrenergic insult. Eur Heart J 2018;39:60–69. [DOI] [PubMed] [Google Scholar]
- 68. Kang DO, Eo JS, Park EJ, Nam HS, Song JW, Park YH, et al. Stress-associated neurobiological activity is linked with acute plaque instability via enhanced macrophage activity: a prospective serial 18F-FDG-PET/CT imaging assessment. Eur Heart J 2021;42:1883–1895. [DOI] [PubMed] [Google Scholar]
- 69. Moazzami K, Wittbrodt MT, Lima BB, Nye JA, Mehta PK, Pearce BD, et al. Higher activation of the rostromedial prefrontal cortex during mental stress predicts major cardiovascular disease events in individuals with coronary artery disease. Circulation 2020;142:455–465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Haider A, Bengs S, Diggelmann F, Epprecht G, Etter D, Beeler AL, et al. Age- and sex-dependent changes of resting amygdalar activity in individuals free of clinical cardiovascular disease. J Nucl Cardiol 2021;28:427–432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Mazure CM, Weinberger AH, Pittman B, Sibon I, Swendsen J. Gender and stress in predicting depressive symptoms following stroke. Cerebrovasc Dis 2014;38:240–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Engman J, Linnman C, Van Dijk KR, Milad MR. Amygdala subnuclei resting-state functional connectivity sex and estrogen differences. Psychoneuroendocrinology 2016;63:34–42. [DOI] [PubMed] [Google Scholar]
- 73. Forrester SJ, Booz GW, Sigmund CD, Coffman TM, Kawai T, Rizzo V, et al. Angiotensin II signal transduction: an update on mechanisms of physiology and pathophysiology. Physiol Rev 2018;98:1627–1738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Courties G, Moskowitz MA, Nahrendorf M. The innate immune system after ischemic injury: lessons to be learned from the heart and brain. JAMA Neurol 2014;71:233–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Emami H, Singh P, MacNabb M, Vucic E, Lavender Z, Rudd JH, et al. Splenic metabolic activity predicts risk of future cardiovascular events: demonstration of a cardiosplenic axis in humans. JACC Cardiovasc Imaging 2015;8:121–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Tuisku J, Plaven-Sigray P, Gaiser EC, Airas L, Al-Abdulrasul H, Bruck A, et al. Effects of age, BMI and sex on the glial cell marker TSPO — a multicentre [11C]PBR28 HRRT PET study. Eur J Nucl Med Mol Imaging 2019;46(11):2329–2338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Thackeray JT, Hupe HC, Wang Y, Bankstahl JP, Berding G, Ross TL, et al. Myocardial inflammation predicts remodeling and neuroinflammation after myocardial infarction. J Am Coll Cardiol 2018;71(3):263–275. [DOI] [PubMed] [Google Scholar]
- 78. Khera A, McGuire DK, Murphy SA, Stanek HG, Das SR, Vongpatanasin W, et al. Race and gender differences in C-reactive protein levels. J Am Coll Cardiol 2005;46:464–469. [DOI] [PubMed] [Google Scholar]
- 79. Sullivan S, Hammadah M, Wilmot K, Ramadan R, Pearce BD, Shah A, et al. Young women with coronary artery disease exhibit higher concentrations of interleukin-6 at baseline and in response to mental stress. J Am Heart Assoc 2018;7:e010329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Fiechter M, Haider A, Bengs S, Maredziak M, Burger IA, Roggo A, et al. Sex differences in the association between inflammation and ischemic heart disease. Thromb Haemost 2019;119:1471–1480. [DOI] [PubMed] [Google Scholar]
- 81. Fiechter M, Haider A, Bengs S, Maredziak M, Burger IA, Roggo A, et al. Sex-dependent association between inflammation, neural stress responses, and impaired myocardial function. Eur J Nucl Med Mol Imaging 2020;47:2010–2015. [DOI] [PubMed] [Google Scholar]
- 82. Diggelmann F BS, Haider A, Epprecht G, Beeler AL, Etter D, Wijnen WJ, et al. Potential impact of statins on neuronal stress responses in patients at risk for cardiovascular disease. J Pers Med 2021;11:261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Janak PH, Tye KM. From circuits to behaviour in the amygdala. Nature 2015;517:284–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Craske MG, Stein MB, Eley TC, Milad MR, Holmes A, Rapee RM, et al. Anxiety disorders. Nat Rev Dis Primers 2017;3:17024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Ehrlich I, Humeau Y, Grenier F, Ciocchi S, Herry C, Lüthi A. Amygdala inhibitory circuits and the control of fear memory. Neuron 2009;62:757–771. [DOI] [PubMed] [Google Scholar]
- 86. Babaev O, Piletti Chatain C, Krueger-Burg D. Inhibition in the amygdala anxiety circuitry. Exp Mol Med 2018;50:1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Benham RS, Engin E, Rudolph U. Diversity of neuronal inhibition: a path to novel treatments for neuropsychiatric disorders. JAMA Psychiatry 2014;71:91–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Rudolph U, Knoflach F. Beyond classical benzodiazepines: novel therapeutic potential of GABAA receptor subtypes. Nat Rev Drug Discov 2011;10:685–697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Schoch P, Richards JG, Häring P, Takacs B, Stähli C, Staehelin T, et al. Co-localization of GABA receptors and benzodiazepine receptors in the brain shown by monoclonal antibodies. Nature 1985;314:168–171. [DOI] [PubMed] [Google Scholar]
- 90. Geuze E, van Berckel BNM, Lammertsma AA, Boellaard R, de Kloet CS, Vermetten E, et al. Reduced GABAA benzodiazepine receptor binding in veterans with post-traumatic stress disorder. Mol Psychiatry 2008;13:74–83. [DOI] [PubMed] [Google Scholar]
- 91. Malizia AL, Cunningham VJ, Bell CJ, Liddle PF, Jones T, Nutt DJ. Decreased brain GABA(A)-benzodiazepine receptor binding in panic disorder: preliminary results from a quantitative PET study. Arch Gen Psychiatry 1998;55:715–720. [DOI] [PubMed] [Google Scholar]
- 92. Calhoon GG, Tye KM. Resolving the neural circuits of anxiety. Nat Neurosci 2015;18:1394–1404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Maron E, Shlik J. Serotonin function in panic disorder: important, but why? Neuropsychopharmacology 2006;31:1–11. [DOI] [PubMed] [Google Scholar]
- 94. Nesse RM, Cameron OG, Curtis GC, McCann DS, Huber-Smith MJ. Adrenergic function in patients with panic anxiety. Arch Gen Psychiatry 1984;41:771–776. [DOI] [PubMed] [Google Scholar]
- 95. Frick A, Ahs F, Palmquist AM, Pissiota A, Wallenquist U, Fernandez M, et al. Overlapping expression of serotonin transporters and neurokinin-1 receptors in posttraumatic stress disorder: a multi-tracer PET study. Mol Psychiatry 2016;21:1400–1407. [DOI] [PubMed] [Google Scholar]
- 96. Goto T, Kikuchi S, Mori K, Nakayama T, Fukuta H, Seo Y, et al. Cardiac β-adrenergic receptor downregulation, evaluated by cardiac PET, in chronotropic incompetence. J Nucl Med 2021;62:996–998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Miyazaki T, Nakajima W, Hatano M, Shibata Y, Kuroki Y, Arisawa T, et al. Visualization of AMPA receptors in living human brain with positron emission tomography. Nat Med 2020;26:281–288. [DOI] [PubMed] [Google Scholar]
- 98. Wilmot KA, O’Flaherty M, Capewell S, Ford ES, Vaccarino V. Coronary heart disease mortality declines in the United States from 1979 through 2011: evidence for stagnation in young adults, especially women. Circulation 2015;132:997–1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Ya’qoub L, Lemor A, Dabbagh M, O’Neill W, Khandelwal A, Martinez SC, et al. Ethnic, and sex disparities in patients with STEMI and cardiogenic shock. JACC Cardiovasc Interv 2021;14:653–660. [DOI] [PubMed] [Google Scholar]
- 100. Dagan M, Dinh DT, Stehli J, Tan C, Brennan A, Warren J, et al. Sex disparity in secondary prevention pharmacotherapy and clinical outcomes following acute coronary syndrome. Eur Heart J Qual Care Clin Outcomes 2021:qcab007. Published online ahead of print. [DOI] [PubMed] [Google Scholar]
- 101. Williams MC, Kwiecinski J, Doris M, McElhinney P, D’Souza MS, Cadet S, et al. Sex-specific computed tomography coronary plaque characterization and risk of myocardial infarction. JACC Cardiovasc Imaging 2021;14:1804–1814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Nikpay M, Goel A, Won HH, Hall LM, Willenborg C, Kanoni S, et al. A comprehensive 1,000 Genomes-based genome-wide association meta-analysis of coronary artery disease. Nat Genet 2015;47:1121–1130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. van der Harst P, Verweij N. Identification of 64 novel genetic loci provides an expanded view on the genetic architecture of coronary artery disease. Circ Res 2018;122:433–443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Silander K, Alanne M, Kristiansson K, Saarela O, Ripatti S, Auro K, et al. Gender differences in genetic risk profiles for cardiovascular disease. PLoS One 2008;3:e3615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Gebhard C, Buechel RR, Stahli BE, Gransar H, Achenbach S, Berman DS, et al. Impact of age and sex on left ventricular function determined by coronary computed tomographic angiography: results from the prospective multicentre CONFIRM study. Eur Heart J Cardiovasc Imaging 2017;18:990–1000. [DOI] [PubMed] [Google Scholar]
- 106. Gebhard C, Maredziak M, Messerli M, Buechel RR, Lin F, Gransar H, et al. Increased long-term mortality in women with high left ventricular ejection fraction: data from the CONFIRM (COronary CT Angiography EvaluatioN For Clinical Outcomes: an InteRnational Multicenter) long-term registry. Eur Heart J Cardiovasc Imaging 2020;21:363–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Hogarth AJ, Graham LN, Mary DA, Greenwood JP. Gender differences in sympathetic neural activation following uncomplicated acute myocardial infarction. Eur Heart J 2009;30:1764–1770. [DOI] [PubMed] [Google Scholar]
- 108. Piepoli MF, Hoes AW, Agewall S, Albus C, Brotons C, Catapano AL, et al. 2016 European Guidelines on cardiovascular disease prevention in clinical practice: The Sixth Joint Task Force of the European Society of Cardiology and Other Societies on Cardiovascular Disease Prevention in Clinical Practice (constituted by representatives of 10 societies and by invited experts). Developed with the special contribution of the European Association for Cardiovascular Prevention & Rehabilitation (EACPR). Eur Heart J 2016;37:2315–2381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Cohen S, Kamarck T, Mermelstein R. A global measure of perceived stress. J Health Soc Behav 1983;24:385–396. [PubMed] [Google Scholar]
- 110. Schaefer SM, Abercrombie HC, Lindgren KA, Larson CL, Ward RT, Oakes TR, et al. Six-month test-retest reliability of MRI-defined PET measures of regional cerebral glucose metabolic rate in selected subcortical structures. Hum Brain Mapp 2000;10:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Vaccarino V, Shah AJ, Mehta PK, Pearce B, Raggi P, Bremner JD, et al. Brain-heart connections in stress and cardiovascular disease: Implications for the cardiac patient. Atherosclerosis 2021;328:74–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Martin EA, Tan SL, MacBride LR, Lavi S, Lerman LO, Lerman A. Sex differences in vascular and endothelial responses to acute mental stress. Clin Auton Res 2008;18:339–345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Vaccarino V, Shah AJ, Rooks C, Ibeanu I, Nye JA, Pimple P, et al. Sex differences in mental stress-induced myocardial ischemia in young survivors of an acute myocardial infarction. Psychosom Med 2014;76:171–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Sullivan S, Young A, Hammadah M, Lima BB, Levantsevych O, Ko YA, et al. Sex differences in the inflammatory response to stress and risk of adverse cardiovascular outcomes among patients with coronary heart disease. Brain Behav Immun 2020;90:294–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Dewan P, Rorth R, Jhund PS, Shen L, Raparelli V, Petrie MC, et al. Differential impact of heart failure with reduced ejection fraction on men and women. J Am Coll Cardiol 2019;73:29–40. [DOI] [PubMed] [Google Scholar]
- 116. Lam CSP, Arnott C, Beale AL, Chandramouli C, Hilfiker-Kleiner D, Kaye DM, et al. Sex differences in heart failure. Eur Heart J 2019;40:3859–3868c. [DOI] [PubMed] [Google Scholar]
- 117. De Simone G, Devereux RB, Chinali M, Roman MJ, Barac A, Panza JA, et al. Sex differences in obesity-related changes in left ventricular morphology: the Strong Heart Study. J Hypertens 2011;29:1431–1438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Sotomi Y, Hikoso S, Nakatani D, Mizuno H, Okada K, Dohi T, et al. Sex differences in heart failure with preserved ejection fraction. J Am Heart Assoc 2021;10:e018574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Yu Y, Wei SG, Weiss RM, Felder RB. Sex differences in the central and peripheral manifestations of ischemia-induced heart failure in rats. Am J Physiol Heart Circ Physiol 2019;316:H70–H79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Triposkiadis F, Karayannis G, Giamouzis G, Skoularigis J, Louridas G, Butler J. The sympathetic nervous system in heart failure physiology, pathophysiology, and clinical implications. J Am Coll Cardiol 2009;54:1747–1762. [DOI] [PubMed] [Google Scholar]
- 121. Tadic M, Cuspidi C, Plein S, Belyavskiy E, Heinzel F, Galderisi M. Sex and heart failure with preserved ejection fraction: from pathophysiology to clinical studies. J Clin Med 2019;8:792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Nakata T, Nakajima K, Yamashina S, Yamada T, Momose M, Kasama S, et al. A pooled analysis of multicenter cohort studies of 123I-mIBG imaging of sympathetic innervation for assessment of long-term prognosis in heart failure. JACC Cardiovasc Imaging 2013;6:772–784. [DOI] [PubMed] [Google Scholar]
- 123. Jacobson AF, Senior R, Cerqueira MD, Wong ND, Thomas GS, Lopez VA, et al. Myocardial iodine-123 meta-iodobenzylguanidine imaging and cardiac events in heart failure. Results of the prospective ADMIRE-HF (AdreView Myocardial Imaging for Risk Evaluation in Heart Failure) study. J Am Coll Cardiol 2010;55:2212–2221. [DOI] [PubMed] [Google Scholar]
- 124. Aikawa T, Naya M, Obara M, Oyama-Manabe N, Manabe O, Magota K, et al. Regional interaction between myocardial sympathetic denervation, contractile dysfunction, and fibrosis in heart failure with preserved ejection fraction: (11)C-hydroxyephedrine PET study. Eur J Nucl Med Mol Imaging 2017;44:1897–1905. [DOI] [PubMed] [Google Scholar]
- 125. Aikawa T, Naya M, Obara M, Manabe O, Tomiyama Y, Magota K, et al. Impaired myocardial sympathetic innervation is associated with diastolic dysfunction in heart failure with preserved ejection fraction: 11C-hydroxyephedrine PET study. J Nucl Med 2017;58:784–790. [DOI] [PubMed] [Google Scholar]
- 126. Boitard SE, Marc Y, Keck M, Mougenot N, Agbulut O, Balavoine F, et al. Brain renin-angiotensin system blockade with orally active aminopeptidase A inhibitor prevents cardiac dysfunction after myocardial infarction in mice. J Mol Cell Cardiol 2019;127:215–222. [DOI] [PubMed] [Google Scholar]
- 127. Dewan P, Jackson A, Lam CSP, Pfeffer MA, Zannad F, Pitt B, et al. Interactions between left ventricular ejection fraction, sex and effect of neurohumoral modulators in heart failure. Eur J Heart Fail 2020;22:898–901. [DOI] [PubMed] [Google Scholar]
- 128. McMurray JJV, Jackson AM, Lam CSP, Redfield MM, Anand IS, Ge J, et al. Effects of sacubitril-valsartan versus valsartan in women compared with men with heart failure and preserved ejection fraction: insights from PARAGON-HF. Circulation 2020;141:338–351. [DOI] [PubMed] [Google Scholar]
- 129. Solomon SD, McMurray JJV, Anand IS, Ge J, Lam CSP, Maggioni AP, et al. Angiotensin-neprilysin inhibition in heart failure with preserved ejection fraction. N Engl J Med 2019;381:1609–1620. [DOI] [PubMed] [Google Scholar]
- 130. Suzuki H, Yasuda S, Shimokawa H. Brain-heart connection in Takotsubo syndrome before onset. Eur Heart J 2021;42:1909–1911. [DOI] [PubMed] [Google Scholar]
- 131. Lim GB. Brain–heart axis in Takotsubo syndrome. Nat Rev Cardiol 2019;16:258. [DOI] [PubMed] [Google Scholar]
- 132. Sancar F. For “broken heart” syndrome, brain may hold the key. JAMA 2019;321:2270–2271. [DOI] [PubMed] [Google Scholar]
- 133. Ghadri JR, Levinson RA, Lüscher TF, Jäncke L, Templin C. Neurocardiology: the brain–heart connection in Takotsubo syndrome. Eur Heart J 2019;40:3062–3063. [DOI] [PubMed] [Google Scholar]
- 134. Cammann VL, Szawan KA, Stähli BE, Kato K, Budnik M, Wischnewsky M, et al. Age-related variations in Takotsubo syndrome. J Am Coll Cardiol 2020;75:1869–1877. [DOI] [PubMed] [Google Scholar]
- 135. Wittstein IS. Why age matters in Takotsubo syndrome. J Am Coll Cardiol 2020;75:1878–1881. [DOI] [PubMed] [Google Scholar]
- 136. Mehta PK, Lima BB, Nelson MD, Bairey Merz CN. Adverse cardiovascular outcomes in women: blame the amygdala? Eur Heart J Cardiovasc Imaging 2019;20:633–635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Gebhard C, Bengs S, Haider A, Fiechter M. The neuro-inflammatory-vascular circuit: evidence for a sex-dependent interrelation? Front Neurosci 2020;14:614345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Pelliccia F, Kaski Juan C, Crea F, Camici Paolo G. Pathophysiology of Takotsubo syndrome. Circulation 2017;135:2426–2441. [DOI] [PubMed] [Google Scholar]
- 139. Lüscher TF, Templin C. Is takotsubo syndrome a microvascular acute coronary syndrome? Towards of a new definition. Eur Heart J 2016;37:2816–2820. [DOI] [PubMed] [Google Scholar]
- 140. Maredziak M, Bengs S, Portmann A, Haider A, Wijnen WJ, Warnock GI, et al. Microvascular dysfunction and sympathetic hyperactivity in women with supra-normal left ventricular ejection fraction (snLVEF). Eur J Nucl Med Mol Imaging 2020;47:3094–3106. [DOI] [PubMed] [Google Scholar]
- 141. Komesaroff PA, Esler MD, Sudhir K. Estrogen supplementation attenuates glucocorticoid and catecholamine responses to mental stress in perimenopausal women. J Clin Endocrinol Metab 1999;84:606–610. [DOI] [PubMed] [Google Scholar]
- 142. Sung BH, Ching M, Izzo JL, Dandona P, Wilson MF. Estrogen improves abnormal norepinephrine-induced vasoconstriction in postmenopausal women. J Hypertens 1999;17:523–528. [DOI] [PubMed] [Google Scholar]
- 143. Sader MA, Celermajer DS. Endothelial function, vascular reactivity and gender differences in the cardiovascular system. Cardiovasc Res 2002;53:597–604. [DOI] [PubMed] [Google Scholar]
- 144. Rutten-Jacobs LC, Larsson SC, Malik R, Rannikmae K, MEGASTROKE consortium; International Stroke Genetics Consortium , et al. Genetic risk, incident stroke, and the benefits of adhering to a healthy lifestyle: cohort study of 306 473 UK Biobank participants. BMJ 2018;363:k4168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Virani SS, Alonso A, Aparicio HJ, Benjamin EJ, Bittencourt MS, Callaway CW, et al. Heart disease and stroke statistics-2021 update: a report from the American Heart Association. Circulation 2021;143:e254–e743. [DOI] [PubMed] [Google Scholar]
- 146. Reeves MJ, Bushnell CD, Howard G, Gargano JW, Duncan PW, Lynch G, et al. Sex differences in stroke: epidemiology, clinical presentation, medical care, and outcomes. Lancet Neurol 2008;7:915–926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Liberale L, Carbone F, Montecucco F, Gebhard C, Luscher TF, Wegener S, et al. Ischemic stroke across sexes: what is the status quo? Front Neuroendocrinol 2018;50:3–17. [DOI] [PubMed] [Google Scholar]
- 148. Poorthuis MH, Algra AM, Algra A, Kappelle LJ, Klijn CJ. Female- and male-specific risk factors for stroke: a systematic review and meta-analysis. JAMA Neurol 2017;74:75–81. [DOI] [PubMed] [Google Scholar]
- 149. Choi JW, Ryoo IW, Hong JY, Lee KY, Nam HS, Kim WC, et al. Clinical impact of estradiol/testosterone ratio in patients with acute ischemic stroke. BMC Neurol 2021;21:91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Battaglini D, Robba C, da Silva AL, Dos Santos Samary C, Leme Silva P, Dal Pizzol F, et al. Brain-heart interaction after acute ischemic stroke. Crit Care 2020;24:163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Emdin CA, Wong CX, Hsiao AJ, Altman DG, Peters SA, Woodward M, et al. Atrial fibrillation as risk factor for cardiovascular disease and death in women compared with men: systematic review and meta-analysis of cohort studies. BMJ 2016;532:h7013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Doehner W, Ural D, Haeusler KG, Celutkiene J, Bestetti R, Cavusoglu Y, et al. Heart and brain interaction in patients with heart failure: overview and proposal for a taxonomy. A position paper from the Study Group on Heart and Brain Interaction of the Heart Failure Association. Eur J Heart Fail 2018;20:199–215. [DOI] [PubMed] [Google Scholar]
- 153. Chen Z, Venkat P, Seyfried D, Chopp M, Yan T, Chen J. Brain-heart interaction: cardiac complications after stroke. Circ Res 2017;121:451–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Krause T, Werner K, Fiebach JB, Villringer K, Piper SK, Haeusler KG, et al. Stroke in right dorsal anterior insular cortex Is related to myocardial injury. Ann Neurol 2017;81:502–511. [DOI] [PubMed] [Google Scholar]
- 155. Min J, Farooq MU, Greenberg E, Aloka F, Bhatt A, Kassab M, et al. Cardiac dysfunction after left permanent cerebral focal ischemia: the brain and heart connection. Stroke 2009;40:2560–2563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156. Sposato LA, Lam M, Allen B, Shariff SZ, Saposnik G, Group PS. First-ever ischemic stroke and incident major adverse cardiovascular events in 93 627 older women and men. Stroke 2020;51:387–394. [DOI] [PubMed] [Google Scholar]
- 157. Akyea RK, Vinogradova Y, Qureshi N, Patel RS, Kontopantelis E, Ntaios G, et al. Sex, age, and socioeconomic differences in nonfatal stroke incidence and subsequent major adverse outcomes. Stroke 2021;52:396–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158. Barber M, Morton JJ, Macfarlane PW, Barlow N, Roditi G, Stott DJ. Elevated troponin levels are associated with sympathoadrenal activation in acute ischaemic stroke. Cerebrovasc Dis 2007;23:260–266. [DOI] [PubMed] [Google Scholar]
- 159. Hermanns N, Bascunana P, Langer BLN, Wolf B, Bankstahl J, Ross T, et al. Molecular imaging of inflammation in the brain-heart axis after ischemic stroke: comparison of two murine stroke models [abstract]. J Nucl Med 2020;61:88. [Google Scholar]
- 160. Yperzeele L, van Hooff RJ, Nagels G, De Smedt A, De Keyser J, Brouns R. Heart rate variability and baroreceptor sensitivity in acute stroke: a systematic review. Int J Stroke 2015;10:796–800. [DOI] [PubMed] [Google Scholar]
- 161. Spychala MS, Honarpisheh P, McCullough LD. Sex differences in neuroinflammation and neuroprotection in ischemic stroke. J Neurosci Res 2017;95:462–471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162. Salk RH, Hyde JS, Abramson LY. Gender differences in depression in representative national samples: meta-analyses of diagnoses and symptoms. Psychol Bull 2017;143:783–822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163. Vaccarino V, Badimon L, Bremner JD, Cenko E, Cubedo J, Dorobantu M, et al. Depression and coronary heart disease: 2018 position paper of the ESC working group on coronary pathophysiology and microcirculation. Eur Heart J 2020;41:1687–1696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164. Goldstein BI, Carnethon MR, Matthews KA, McIntyre RS, Miller GE, Raghuveer G, et al. Major depressive disorder and bipolar disorder predispose youth to accelerated atherosclerosis and early cardiovascular disease: a scientific statement from the American Heart Association. Circulation 2015;132:965–986. [DOI] [PubMed] [Google Scholar]
- 165. Hare DL, Toukhsati SR, Johansson P, Jaarsma T. Depression and cardiovascular disease: a clinical review. Eur Heart J 2014;35:1365–1372. [DOI] [PubMed] [Google Scholar]
- 166. Parashar S, Rumsfeld JS, Reid KJ, Buchanan D, Dawood N, Khizer S, et al. Impact of depression on sex differences in outcome after myocardial infarction. Circ Cardiovasc Qual Outcomes 2009;2:33–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167. Kim JM, Stewart R, Lee YS, Lee HJ, Kim MC, Kim JW, et al. Effect of escitalopram vs placebo treatment for depression on long-term cardiac outcomes in patients with acute coronary syndrome: a randomized clinical trial. JAMA 2018;320:350–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168. Doyle F, McGee H, Conroy R, Conradi HJ, Meijer A, Steeds R, et al. Systematic review and individual patient data meta-analysis of sex differences in depression and prognosis in persons with myocardial infarction: a MINDMAPS study. Psychosom Med 2015;77:419–428. [DOI] [PubMed] [Google Scholar]
- 169. Hamilton JP, Etkin A, Furman DJ, Lemus MG, Johnson RF, Gotlib IH. Functional neuroimaging of major depressive disorder: a meta-analysis and new integration of base line activation and neural response data. Am J Psychiatry 2012;169:693–703. [DOI] [PubMed] [Google Scholar]
- 170. Kaczkurkin AN, Moore TM, Ruparel K, Ciric R, Calkins ME, Shinohara RT, et al. Elevated amygdala perfusion mediates developmental sex differences in trait anxiety. Biol Psychiatry 2016;80:775–785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171. Berntson GG, Bigger JT, Eckberg DL, Grossman P, Kaufmann PG, Malik M, et al. Heart rate variability: origins, methods, and interpretive caveats. Psychophysiology 1997;34:623–648. [DOI] [PubMed] [Google Scholar]
- 172. Kemp AH, Quintana DS, Gray MA, Felmingham KL, Brown K, Gatt JM. Impact of depression and antidepressant treatment on heart rate variability: a review and meta-analysis. Biol Psychiatry 2010;67:1067–1074. [DOI] [PubMed] [Google Scholar]
- 173. Voss A, Boettger MK, Schulz S, Gross K, Bar KJ. Gender-dependent impact of major depression on autonomic cardiovascular modulation. Prog Neuropsychopharmacol Biol Psychiatry 2011;35:1131–1138. [DOI] [PubMed] [Google Scholar]
- 174. Koch C, Wilhelm M, Salzmann S, Rief W, Euteneuer F. A meta-analysis of heart rate variability in major depression. Psychol Med 2019;49:1948–1957. [DOI] [PubMed] [Google Scholar]
- 175. Halaris A. Inflammation-associated co-morbidity between depression and cardiovascular disease. Curr Top Behav Neurosci 2017;31:45–70. [DOI] [PubMed] [Google Scholar]
- 176. Khandaker GM, Zuber V, Rees JMB, Carvalho L, Mason AM, Foley CN, et al. Shared mechanisms between coronary heart disease and depression: findings from a large UK general population-based cohort. Mol Psychiatry 2020;25:1477–1486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177. Valkanova V, Ebmeier KP, Allan CL. CRP, IL-6 and depression: a systematic review and meta-analysis of longitudinal studies. J Affect Disord 2013;150:736–744. [DOI] [PubMed] [Google Scholar]
- 178. Bucciarelli V, Caterino AL, Bianco F, Caputi CG, Salerni S, Sciomer S, et al. Depression and cardiovascular disease: the deep blue sea of women’s heart. Trends Cardiovasc Med 2020;30:170–176. [DOI] [PubMed] [Google Scholar]
- 179. Klein SL. The effects of hormones on sex differences in infection: from genes to behavior. Neurosci Biobehav Rev 2000;24:627–638. [DOI] [PubMed] [Google Scholar]
- 180. Freeman EW, Sammel MD, Lin H, Nelson DB. Associations of hormones and menopausal status with depressed mood in women with no history of depression. Arch Gen Psychiatry 2006;63:375–382. [DOI] [PubMed] [Google Scholar]
- 181. Frokjaer VG, Pinborg A, Holst KK, Overgaard A, Henningsson S, Heede M, et al. Role of serotonin transporter changes in depressive responses to sex-steroid hormone manipulation: a positron emission tomography study. Biol Psychiatry 2015;78:534–543. [DOI] [PubMed] [Google Scholar]
- 182. Slavich GM, Sacher J. Stress, sex hormones, inflammation, and major depressive disorder: extending social signal transduction theory of depression to account for sex differences in mood disorders. Psychopharmacology (Berl) 2019;236:3063–3079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183. Walther A, Breidenstein J, Miller R. Association of testosterone treatment with alleviation of depressive symptoms in men: a systematic review and meta-analysis. JAMA Psychiatry 2019;76:31–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184. Livingston G, Huntley J, Sommerlad A, Ames D, Ballard C, Banerjee S, et al. Dementia prevention, intervention, and care: 2020 report of the Lancet Commission. Lancet 2020;396:413–446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185. Neu SC, Pa J, Kukull W, Beekly D, Kuzma A, Gangadharan P, et al. Apolipoprotein E genotype and sex risk factors for Alzheimer disease: a meta-analysis. JAMA Neurol 2017;74:1178–1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186. Breteler MM, Claus JJ, Grobbee DE, Hofman A. Cardiovascular disease and distribution of cognitive function in elderly people: the Rotterdam Study. BMJ 1994;308:1604–1608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187. Qiu C, Winblad B, Marengoni A, Klarin I, Fastbom J, Fratiglioni L. Heart failure and risk of dementia and Alzheimer disease: a population-based cohort study. Arch Intern Med 2006;166:1003–1008. [DOI] [PubMed] [Google Scholar]
- 188. Austin SA, Santhanam AV, Katusic ZS. Endothelial nitric oxide modulates expression and processing of amyloid precursor protein. Circ Res 2010;107:1498–1502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189. de Montgolfier O, Pincon A, Pouliot P, Gillis MA, Bishop J, Sled JG, et al. High systolic blood pressure induces cerebral microvascular endothelial dysfunction, neurovascular unit damage, and cognitive decline in mice. Hypertension 2019;73:217–228. [DOI] [PubMed] [Google Scholar]
- 190. Yin C, Ackermann S, Ma Z, Mohanta SK, Zhang C, Li Y, et al. ApoE attenuates unresolvable inflammation by complex formation with activated C1q. Nat Med 2019;25:496–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191. Volgman AS, Bairey Merz CN, Aggarwal NT, Bittner V, Bunch TJ, Gorelick PB, et al. Sex differences in cardiovascular disease and cognitive impairment: another health disparity for women? J Am Heart Assoc 2019;8:e013154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192. Iadecola C, Yaffe K, Biller J, Bratzke LC, Faraci FM, Gorelick PB, et al. Impact of hypertension on cognitive function: a scientific statement from the American Heart Association. Hypertension 2016;68:e67–e94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193. Ernst ME, Ryan J, Chowdhury EK, Margolis KL, Beilin LJ, Reid CM, et al. Long-term blood pressure variability and risk of cognitive decline and dementia among older adults. J Am Heart Assoc 2021;10:e019613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194. Sible IJ, Bangen KJ, Blanken AE, Ho JK, Nation DA. Antemortem visit-to-visit blood pressure variability predicts cerebrovascular lesion burden in autopsy-confirmed Alzheimer’s disease. J Alzheimers Dis 2021;83:65–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195. Stegmann T, Chu ML, Witte VA, Villringer A, Kumral D, Riedel-Heller SG, et al. Heart failure is independently associated with white matter lesions: insights from the population-based LIFE-Adult Study. ESC Heart Fail 2021;8:697–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196. Roy B, Woo MA, Wang DJJ, Fonarow GC, Harper RM, Kumar R. Reduced regional cerebral blood flow in patients with heart failure. Eur J Heart Fail 2017;19:1294–1302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197. Zilberman JM, Cerezo GH, Del Sueldo M, Fernandez-Perez C, Martell-Claros N, Vicario A. Association between hypertension, menopause, and cognition in women. J Clin Hypertens (Greenwich) 2015;17:970–976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198. Heart rate variability: standards of measurement, physiological interpretation and clinical use. Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. Circulation 1996;93:1043–1065. [PubMed] [Google Scholar]
- 199. Kubota Y, Chen LY, Whitsel EA, Folsom AR. Heart rate variability and lifetime risk of cardiovascular disease: the Atherosclerosis Risk in Communities Study. Ann Epidemiol 2017;27:619–625 e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200. Soares-Miranda L, Sattelmair J, Chaves P, Duncan GE, Siscovick DS, Stein PK, et al. Physical activity and heart rate variability in older adults: the Cardiovascular Health Study. Circulation 2014;129:2100–2110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201. Schroeder EB, Liao D, Chambless LE, Prineas RJ, Evans GW, Heiss G. Hypertension, blood pressure, and heart rate variability: the Atherosclerosis Risk in Communities (ARIC) study. Hypertension 2003;42:1106–1111. [DOI] [PubMed] [Google Scholar]
- 202. Schroeder EB, Chambless LE, Liao D, Prineas RJ, Evans GW, Rosamond WD, et al. Atherosclerosis risk in communities. Diabetes, glucose, insulin, and heart rate variability: the Atherosclerosis Risk in Communities (ARIC) study. Diabetes Care 2005;28:668–674. [DOI] [PubMed] [Google Scholar]
- 203. Nolan J, Batin PD, Andrews R, Lindsay SJ, Brooksby P, Mullen M, et al. Prospective study of heart rate variability and mortality in chronic heart failure: results of the United Kingdom heart failure evaluation and assessment of risk trial (UK-heart). Circulation 1998;98:1510–1516. [DOI] [PubMed] [Google Scholar]
- 204. Goldenberg I, Goldkorn R, Shlomo N, Einhorn M, Levitan J, Kuperstein R, et al. Heart rate variability for risk assessment of myocardial ischemia in patients without known coronary artery disease: the HRV-DETECT (Heart Rate Variability for the Detection of Myocardial Ischemia) Study. J Am Heart Assoc 2019;8:e014540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205. Tsuji H, Larson MG, Venditti FJ Jr, Manders ES, Evans JC, Feldman CL, et al. Impact of reduced heart rate variability on risk for cardiac events. The Framingham Heart Study. Circulation 1996;94:2850–2855. [DOI] [PubMed] [Google Scholar]
- 206. Gebhard C, Messerli M, Lohmann C, Treyer V, Bengs S, Benz DC, et al. Sex and age differences in the association of heart rate responses to adenosine and myocardial ischemia in patients undergoing myocardial perfusion imaging. J Nucl Cardiol 2020;27:159–170. [DOI] [PubMed] [Google Scholar]
- 207. Bhatheja R, Francis GS, Pothier CE, Lauer MS. Heart rate response during dipyridamole stress as a predictor of mortality in patients with normal myocardial perfusion and normal electrocardiograms. Am J Cardiol 2005;95:1159–1164. [DOI] [PubMed] [Google Scholar]
- 208. Abidov A, Hachamovitch R, Hayes SW, Ng CK, Cohen I, Friedman JD, et al. Prognostic impact of hemodynamic response to adenosine in patients older than age 55 years undergoing vasodilator stress myocardial perfusion study. Circulation 2003;107:2894–2899. [DOI] [PubMed] [Google Scholar]