ABSTRACT
Semaglutide has little hepatic metabolism and is deemed low risk for causing drug-induced liver injury (DILI). We present a case of DILI from the US DILI Network. The case involved a 51-year-old man with type 2 diabetes who presented with jaundice and acute-on-chronic kidney disease 6 months after starting oral semaglutide. His liver injury progressed to biliary cirrhosis, accompanied by nephritis that led to end-stage renal disease. Extensive evaluations including liver and kidney biopsies revealed no alternative etiologies. Cholestatic gene sequencing revealed heterozygosity for ABCC2 and DHCR7. He eventually underwent combined liver and kidney transplantation.
INTRODUCTION
Semaglutide is a glucagon-like peptide-1 receptor agonist that was approved as an injectable for type 2 diabetes mellitus (T2DM) in December 2017.1,2 In September 2019, oral semaglutide was approved for T2DM.3,4 Oral semaglutide is coformulated with salcaprozate sodium to facilitate absorption.4,5 The estimated bioavailability of oral semaglutide is low at 0.4%–1%, and it is extensively bound to plasma albumin with an elimination half-life of approximately 1 week.4
Semaglutide is generally considered safe with common side effects being hypoglycemia and gastrointestinal intolerance. Serious adverse events include pancreatitis, diabetic retinopathy, and acute kidney injury.6 Isolated cases of acute kidney injury without recovery in patients with T2DM have been reported.7 Hepatobiliary events from semaglutide are rare and primarily attributed to gallstone-related complications.8 Because semaglutide is a recombinant polypeptide with little or no hepatic metabolism, it is deemed low risk for causing drug-induced liver injury (DILI). One meta-analysis of semaglutide showed a relative risk of 1.45 (0.29–7.19) for DILI compared with control.6 However, since its approval, a bona fide case of DILI from either form of semaglutide has never been reported.9,10 We describe the clinical phenotype of oral semaglutide DILI by review of the US DILI Network (DILIN) database.
CASE SUMMARY
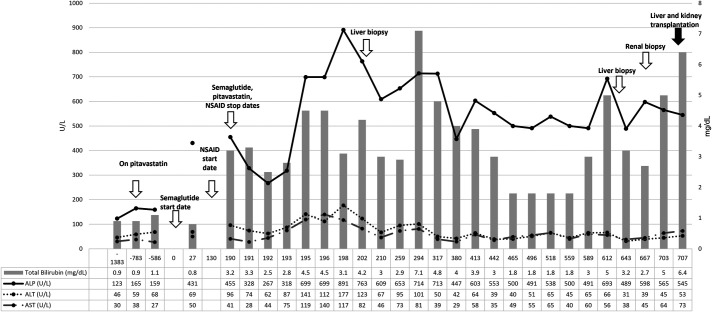
The patient is a 51-year-old Asian man with a medical history of hypertension, hyperlipidemia, T2DM, nonalcoholic fatty liver disease, and chronic kidney disease stage 3a. In December 2019, he started oral semaglutide 3 mg daily for T2DM. His other chronic medications included pitavastatin, losartan, and insulin. Semaglutide was increased to 7 mg once daily after 1 month. Liver tests before starting semaglutide were either normal or minimally elevated for several years. This was attributed to his nonalcoholic fatty liver disease, T2DM, and statin therapy. Liver tests 27 days after starting semaglutide showed an increase in alkaline phosphatase (ALP), alanine aminotransferase (ALT), and aspartate aminotransferase (AST) with normal total bilirubin (Figure 1). The patient was asymptomatic, and oral semaglutide was continued until he presented to the hospital in June 2020 with abdominal discomfort, new-onset jaundice, nausea, vomiting, diarrhea, and weakness. He had no change in medications, except for taking ibuprofen 400 mg twice daily for shoulder pain for the past 2 months. The patient was afebrile and tachycardic, with blood pressures of 90s/60s mm Hg. On physical examination, he had scleral icterus and dry oral mucosa. Laboratory test results showed a blood urea nitrogen of 58 mg/dL and creatinine of 2.89 mg/dL, with hepatic panel showing worsening liver tests (Figure 1). Coagulation tests and alpha-fetoprotein were normal. Imaging with magnetic resonance with cholangiogram did not show any abnormalities. Further workup for liver injury revealed negative serologies for autoimmune (antinuclear antibody, smooth muscle antibody, anti-mitochondrial antibody, liver-kidney microsome-1, and soluble liver antigen) and viral hepatitis (hepatitis A, B, C). COVID-19 was negative. The patient improved and was discharged home for outpatient follow-up of abnormal liver tests (Figure 1).
Figure 1.
Clinical course, trends in liver biochemistries over time as it relates to initiation of oral semaglutide, discontinuation, liver and kidney biopsy, and finally simultaneous liver and kidney transplantation.
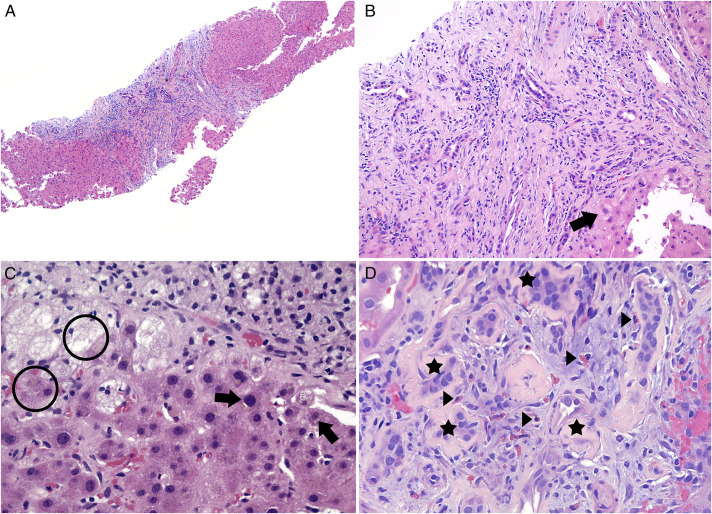
In clinic, worsening liver tests prompted repeat imaging and endoscopic retrograde cholangiopancreatogram (Figure 1). Both were unrevealing. The patient then underwent 2 liver biopsies several months apart with histology showing marked and diffuse bile duct injury with patchy ductular reaction and mild cholestasis evolving to portal tracts with expanded edema, marked ductular reaction, and chronic inflammatory infiltrate with damage to interlobular bile ducts but no duct loss (Figure 2). There was no evidence of steatohepatitis or interface hepatitis with plasma cell infiltrate (Figure 2). Cholestatic gene panel showed heterozygosity for ABCC2 (linked to Dubin-Johnson syndrome) NM_000392.5:c:656 G>A (p.R219H) and for DHCR7 (linked to Smith-Lemti-Optiz syndrome) NM_001360.2:2.1168C>T (p.H390Y).11 Owing to progressive renal disease, he also underwent renal biopsy that showed chronic active interstitial nephritis with eosinophils, patchy acute tubular damage, and moderate tubular atrophy with interstitial fibrosis (Figure 2). The patient eventually required hemodialysis.
Figure 2.
(A) Liver (×40, hematoxylin and eosin [H&E] stain): Largely preserved hepatic architecture with focal hepatocyte ballooning and marked and diffuse bile duct injury. (B) Liver (×200, H&E): Marked bile duct injury with mild to focally moderate inflammatory infiltrate consisting of lymphocytes and rare plasma cells. Hepatocyte ballooning (arrow). (C) Liver (×400, H&E): Mild cholestasis (arrow, circle). (D) Kidney (×400, H&E): Interstitial inflammatory infiltrate, including eosinophils (arrow), are present within a background of tubular atrophy (star). Extravasated red blood cells are seen on the right.
The patient was enrolled in DILIN's prospective study and underwent a protocol-specific workup for exclusion of competing etiologies. The protocols and study design of DILIN are previously reported.12–14 The committee adjudicated causality and severity scores of 3 (probable) and 5 (transplantation), respectively. The Roussel-Uclaf Causality Assessment Method score was 3, suggesting possible DILI.
The patient was treated with ursodiol, budesonide, mycophenolate mofetil, and rapamycin with no meaningful improvement in liver or kidney function. He ultimately received simultaneous liver and kidney transplantation with successful recovery. The liver explant revealed cirrhosis with marked ductular reaction, cholestasis, and bile infarcts, consistent with biliary cirrhosis (see Supplementary Figure 1, http://links.lww.com/ACGCR/A32).
DISCUSSION
We report a novel case of cholestatic liver injury attributed to oral semaglutide that progressed to biliary cirrhosis. The case is compelling because of its comprehensive evaluation and structured causality assessment with long-term follow-up until simultaneous liver and kidney transplantation. Determining causality was challenging because of a lack of prior published reports. Although this patient used ibuprofen for 2 months (day 130–190) before hospitalization and DILI cases have been reported with its use, this patient's ALP was significantly elevated just 27 days after initiating oral semaglutide and before ibuprofen use.15 It is plausible that the patient may have been genetically predisposed, and prolonged exposure of oral semaglutide after the initial increase in ALP may have resulted in progressive biliary duct injury resulting in biliary cirrhosis.
One aspect of oral semaglutide is the excipient, salcaprozate sodium. It is FDA-approved as medical food and has been generally regarded as safe.16 Finally, we are currently unable to link his double heterozygosity for ABCC2 and DHCR7 with semaglutide DILI. ABCC2 encodes multidrug resistance-associated protein 2, which is involved in biliary transport and renal excretion.17,18 DHCR7 encodes the last enzyme in the cholesterol synthesis pathway and does seem to be a recessive gene, and therefore, we do not anticipate that heterozygotes would have sufficiently reduced function to have an effect.19,20
In summary, we present a case of DILI from oral semaglutide. The clinical presentation appears to be a cholestatic injury leading to biliary cirrhosis. Although previous cases of kidney injury requiring hemodialysis have been reported with semaglutide, this is the first case of simultaneous liver and kidney injury resulting in end-stage liver and renal disease requiring combined liver kidney transplantation.
DISCLOSURES
Author contributions: All authors contributed substantially to the design, analysis, and interpretation of the data for this manuscript and actively participated in drafting the manuscript for intellectual content and approval of the final version to be published. Raj Vuppalanchi is the article guarantor.
Acknowledgments: We acknowledge Professor Richard Thompson, Professor of Molecular Hepatology at King's College, London, and Prof Laura Bull, Professor at University of California, San Francisco, for their input regarding the significance of ABCC2 and DHCR7 variants.
Financial disclosure: R. Vuppalanchi has no financial conflicts relevant for this paper. R. Vuppalanchi has paid consulting agreements with Labcorp, Medpace, Worldwide trials, and Enyo. He also receives institutional funding for clinical trial research from Eli Lilly, Zydus Therapeutics, Intercept, Astra Zeneca, Galectin Therapeutics, and Pliant. N. Chalasani has no financial conflicts relevant for this paper. N. Chalasani has paid consulting agreements with AbbVie, Altimmune, Lilly, Madrigal, Zydus, Galectin, Boehringer-Ingelheim, and Foresite. He receives research funding from Exact Sciences and DSM. The remaining authors have no financial conflicts relevant for this paper.
The DILIN (https://dilin.dcri.duke.edu/) is supported by the National Institute of Diabetes and Digestive and Kidney Diseases of the National Institutes of Health (NIH) as a Cooperative Agreement (U01 s) under the following grants: U01-DK065176 and U24-DK065176 (Duke), U01-DK065211 (Indiana), U01-DK065201 (UNC), U01-DK065184 (Michigan), U01-DK065193 (UConn), U01-DK065238 (UCSF/CPMC), U01-DK083023 (UTSW), U01-DK083020 (USC), U01-DK082992 (Mayo), U01-DK083027 (TJH/UPenn), and U01-DK100928 (Icahn).
Informed consent was obtained for this case report.
Supplementary Material
Footnotes
SUPPLEMENTARY MATERIAL accompanies this paper at http://links.lww.com/ACGCR/A32
Contributor Information
Jiayi Ma, Email: joema@iu.edu.
Karan Mathur, Email: kdmathur@iu.edu.
Jessica L. Muldoon, Email: jlmuldoo@iupui.edu.
Marwan Ghabril, Email: mghabril@iu.edu.
Naga Chalasani, Email: nchalasa@iu.edu.
REFERENCES
- 1.Warren M, Chaykin L, Trachtenbarg D, Nayak G, Wijayasinghe N, Cariou B. Semaglutide as a therapeutic option for elderly patients with type 2 diabetes: Pooled analysis of the SUSTAIN 1-5 trials. Diabetes Obes Metab. 2018;20(9):2291–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dhillon S. Semaglutide: First global approval. Drugs. 2018;78(2):275–84. [DOI] [PubMed] [Google Scholar]
- 3.Powell J, Piszczatoski C, Taylor JR. Oral semaglutide: The first-available noninjectable glucagon-like peptide 1 receptor agonist. Clin Ther. 2020;42(10):2100–16. [DOI] [PubMed] [Google Scholar]
- 4.https://www.novo-pi.com/rybelsus.pdf.
- 5.Oral semaglutide (Rybelsus) for type 2 diabetes. Med Lett Drugs Ther. 2019;61(1583):166–8. [PubMed] [Google Scholar]
- 6.Yin DG, Ding LL, Zhou HR, Qiu M, Duan XY. Comprehensive analysis of the safety of semaglutide in type 2 diabetes: A meta-analysis of the SUSTAIN and PIONEER trials. Endocr J. 2021;68(6):739–42. [DOI] [PubMed] [Google Scholar]
- 7.Leehey DJ, Rahman MA, Borys E, Picken MM, Clise CE. Acute kidney injury associated with semaglutide. Kidney Med. 2021;3(2):282–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhong P, Zeng H, Huang M, Fu W, Chen Z. Efficacy and safety of once-weekly semaglutide in adults with overweight or obesity: A meta-analysis. Endocrine. 2022;75(3):718–24. [DOI] [PubMed] [Google Scholar]
- 9.https://www.ncbi.nlm.nih.gov/books/NBK547852/. Accessed May 30, 2022.
- 10.Yabe D, Deenadayalan S, Horio H, et al. Efficacy and safety of oral semaglutide in Japanese patients with type 2 diabetes: A subgroup analysis by baseline variables in the PIONEER 9 and PIONEER 10 trials. J Diabetes Investig. 2022;13(6):975–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.https://www.egl-eurofins.com/about/news-and-events/news/2014/01/next-generation-sequencing-panels/index.html. Accessed May 30, 2022.
- 12.Chalasani N, Bonkovsky HL, Fontana R, et al. Features and outcomes of 899 patients with drug-induced liver injury: The DILIN prospective study. Gastroenterology. 2015;148(7):1340–52.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chalasani N, Fontana RJ, Bonkovsky HL, et al. Causes, clinical features, and outcomes from a prospective study of drug-induced liver injury in the United States. Gastroenterology. 2008;135(6):1924–34. 1934.e1-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fontana RJ, Watkins PB, Bonkovsky HL, et al. Drug-induced liver injury Network (DILIN) prospective study: Rationale, design and conduct. Drug Saf. 2009;32(1):55–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.https://www.ncbi.nlm.nih.gov/books/NBK547845/. Accessed May 30, 2022.
- 16.Twarog C, Fattah S, Heade J, Maher S, Fattal E, Brayden DJ. Intestinal permeation enhancers for oral delivery of macromolecules: A comparison between salcaprozate sodium (SNAC) and sodium caprate (C10). Pharmaceutics. 2019;11(2):78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ieiri I, Higuchi S, Sugiyama Y. Genetic polymorphisms of uptake (OATP1B1, 1B3) and efflux (MRP2, BCRP) transporters: Implications for inter-individual differences in the pharmacokinetics and pharmacodynamics of statins and other clinically relevant drugs. Expert Opin Drug Metab Toxicol. 2009;5(7):703–29. [DOI] [PubMed] [Google Scholar]
- 18.Keppler D. Progress in the molecular characterization of hepatobiliary transporters. Dig Dis. 2017;35(3):197–202. [DOI] [PubMed] [Google Scholar]
- 19.Witsch-Baumgartner M, Loffler J, Utermann G. Mutations in the human DHCR7 gene. Hum Mutat. 2001;17(3):172–82. [DOI] [PubMed] [Google Scholar]
- 20.Fitzky BU, Witsch-Baumgartner M, Erdel M, et al. Mutations in the Δ7-sterol reductase gene in patients with the Smith–Lemli–Opitz syndrome. Proc Natl Acad Sci U S A. 1998;95(14):8181–6. [DOI] [PMC free article] [PubMed] [Google Scholar]