Abstract
Background:
Major depressive disorder (MDD) is one of the most commonly diagnosed mental illnesses worldwide, with a higher prevalence in women than men. Although currently available pharmacological therapeutics help many individuals, they are not effective for most. Animal models have been important for the discovery of molecular alterations in stress and depression, but difficulties in adapting animal models of depression for females has impeded progress into developing novel therapeutic treatments that may be more efficacious for women.
Methods:
Using the California mouse social defeat model, we took a multidisciplinary approach to identify stress-sensitive molecular targets that have translational relevance for women. We determined the impact of stress on transcriptional profiles in male and female California mouse nucleus accumbens (NAc) and compared these results with data from post-mortem samples of the NAc from men and women diagnosed with MDD.
Results:
Our cross-species computational analyses identified regulator of G-protein signaling 2 (Rgs2) as a transcript downregulated by defeat stress in female California mice and in women with MDD. RGS2 plays a key role in signal regulation of neuropeptide and neurotransmitter receptors. Viral vector mediated overexpression of Rgs2 in the NAc restored social approach and sucrose preference in stressed female California mice.
Conclusions:
These studies show that Rgs2 acting in the NAc has functional properties that translate to changes in anxiety- and depression-related behavior. Future studies should investigate whether targeting Rgs2 represents a novel target for treatment-resistant depression in women.
Keywords: Peromyscus californicus, prefrontal cortex, ventral tegmental area, social defeat, nucleus accumbens, major depression, depression, reward
Introduction
Chronic stress is a risk factor for mental illnesses such as anxiety and major depressive disorder (MDD), which are leading causes of disability worldwide (1–3). These disorders place a burden on society by impacting performance in school or work settings, social relationships, and self-care. Although therapies are available, many individuals seeking treatment do not respond completely (4), and the remission rate is about 20% (5). Extensive research indicates that the nucleus accumbens (NAc), part of the ventral striatum, is altered in patients with MDD. In humans, reductions in brain volume and brain activity in the ventral striatum are associated with social anhedonia (6) and MDD (7,8). As stress is a risk factor for depression, rodent social stress models can be used to investigate the impact of stress on brain function.
Social stress reduces social approach behaviors, which are affected by anxiety and depression disorders. This phenotype is modulated in part by the NAc (9). The NAc is important for the processing of rewarding and aversive stimuli (10) and receives dopaminergic, serotonergic, and glutamatergic innervation from nearby regions (11,12). Through connections to motor regions the NAc aids in the selection and elicitation of directed behavior to salient stimuli (13–15), including both rewarding or aversive cues (16,17). In rodents, chronic stress alters transcription and neuronal morphology in the NAc (9). Ultimately, these changes can affect the functional activity and connectivity of these cells and contribute to depression-like phenotypes.
A limitation of previous rodent social stress studies is that most focus exclusively on males (18). This forms a gap in knowledge as women are more likely to develop MDD than men. Although there has been progress integrating females in preclinical models (19), females are still underrepresented in rodent models of MDD. Recent studies suggest that there are distinct molecular signatures in the NAc and other brain regions in men and women with MDD (20,21). Similar findings have been reported in rodents exposed to sub-chronic variable stress (20,22) or early life stress (23). These sex-specific effects highlight the need for further development of preclinical models using female rodents that can be used to identify novel molecular targets (24). A challenge for studying molecular mechanisms of social stress in females has been difficulty in establishing robust protocols in conventional rodents (25). In California mice (Peromyscus californicus), males and females exhibit vigorous aggressive behavior, which allows for both sexes to be exposed to similar levels of social stress in an ethologically valid approach (26).
We took a multidisciplinary approach using the California mouse social defeat model system to assess how defeat stress impacts the transcriptome of reward-related brain regions. We used RNAseq to examine transcriptional responses to social defeat in the NAc of male and female California mice and compared these data to transcriptional profiles from post-mortem samples of the NAc of patients with MDD. Our analyses identified the G-protein regulator Rgs2 as a transcript down-regulated in samples from stressed female mice and women with depression. This protein facilitates the process of GTP hydrolysis, which in turn terminates downstream G-protein coupled receptor signaling pathways (27). Replication experiments and viral overexpression of Rgs2 in the NAc suggest that stress-induced decreases in Rgs2 in female California mouse NAc contribute to depression- and anxiety-related behavior.
Methods and materials
Full details for all experimental procedures (28,29) are provided in supplementary materials.
Animals and housing conditions
All studies on California mice (Peromyscus californicus) were in accordance with the NIH Guide for the Care and Use of Laboratory Animals and approved by the Institutional Animal Care and Use Committee at the University of California, Davis.
Social Defeat Stress (SDS)
Mice were randomly assigned to control handling or three episodes of social defeat as previously described (30). Behavior tests and brain tissue collection were conducted two weeks after the last episode of social defeat.
Sucrose Anhedonia
Sucrose preference was assessed using a two-bottle choice test (30). Mice were habituated for two days with two bottles of tap water. The next day water in one bottle was replaced with a 1% sucrose solution for a 24 hr observation period. Percent sucrose preference was calculated as the amount of sucrose solution consumed (mL) over total amount of solution consumed (water and sucrose solution combined, mL).
Social Interaction Test
Social interaction testing was performed as previously described (30,31). We define time spent in the interaction zone with a target mouse as social approach. Social vigilance was scored during the acclimation and interaction phases by recording the amount of time the focal mouse spent with its head oriented towards the target mouse while outside the interaction zone.
RNA extraction and RNA-sequencing library preparation
Adult mice were euthanized 1 day after the final behavioral test. Brains were removed rapidly, and bilateral punches were made from VTA (16 gauge), NAc (14 gauge), and PFC (12 gauge) and flash-frozen in tubes on dry ice. Total RNA was isolated with TriZol reagent (Invitrogen) and purified with RNeasy Micro Kits (Qiagen). Purified RNA was used to prepare libraries using Truseq mRNA library prep kit (Illumina RS-122–2001/2). VTA, NAc, and PFC samples were prepared from individual animals and sequenced with 125-nt single-end reads at Beckman Coulter Genomics (currently Genewiz). Samples were multiplexed to produce >30M reads/sample. All reads and RNA-seq files have been deposited and are available through NCBI BioProject (ID: PRJNA700778). RNAseq data from human subjects used for analysis was published previously (20).
RNAseq Data Analysis
Raw reads were processed with expHTS (32) to trim low-quality reads and adapter contamination and to remove PCR duplicates. The processed reads were aligned to a California mouse brain transcriptome (PRJNA350325) using bwa mem (33). The average mapping rate was 90.2%. Read counts per transcript were combined to generate counts per gene. Genes with fewer than 2 counts per million reads in all samples were filtered prior to analysis, leaving 40,634 genes. Differential expression analyses were conducted using the limma-voom Bioconductor pipeline (34,35). Heatmaps were generated using Python and GO analyses on female differential expression analyses were performed using Kolmogorov-Smirnov tests, as implemented in the Bioconductor package topGO, to compare uncorrected differential expression p-values for genes annotated with a given term to those not annotated with a given term (36). Full threshold-free differential expression lists were performed using RRHO (37). Parameters for significant differential expression were set at an uncorrected p<0.05 and a logFC > |1.15| between stress comparisons (42, 44).
In-situ hybridization
In-situ hybridization was performed as previously described (38). Brains were coronally cryosectioned at 60μm, fixed, treated with proteinase K, acetylated, permeabilized, and equilibrated in hybridization solution. A riboprobe (0.3μg/mL) directed against Rgs2 corresponding to bases 74–550 of cDNA sequence XM_028884620.1 was hybridized overnight at 65°C. Slides were then washed and incubated with alkaline phosphatase-conjugated sheep anti-digoxigenin primary antibody (1:1000; Roche) overnight then developed in nitro blue tetrazolium and 5-bromo-4-chloro-3-indolyl-phosphate (Roche) at 37°C for 24 hrs.
Western blots
Protein was extracted from NAc punches and then separated with gel electrophoresis and transferred to polyvinylidine fluoride (PVDF) membranes (Bio-Rad, Hercules, CA), rinsed, and blocked. Membranes were incubated overnight in primary rabbit anti-RGS2 (Abcam, Boston, MA; ab155762) 1:1000 at 4°C that was validated using samples from Rgs2 knockout mice (Supplemental Fig. 1). Membranes were incubated in peroxidase-conjugated anti-rabbit secondary antibody (1:100, Vector, Burlingame, CA). Membranes were washed, developed, and imaged on a Bio-Rad ChemiDoc. Blots were probed for β-actin as a loading control (Cell Signaling, Danvers, MA, 1:1000), and RGS2 protein bands were normalized to their respective β-actin controls.
Quantitative Real-Time PCR
RNA was extracted from NAc tissue punches from SDS male and female mice (n = 5–8 per group) using an RNAeasy micro kit (Qiagen, 74004) and converted to cDNA using an iScript cDNA synthesis kit (Bio-Rad; 1708891). Real-time qPCR was performed using SybrGreen Fast master mix (Applied Biosystems) on a ViiA 7 Real-Time PCR system and analyzed by the 2−ΔΔCt method. For primer sequences see Supplemental Table 1.
Overexpression of Rgs2 within the NAc by HSV-mediated gene transfer.
To overexpress RGS2, we used a bicistronic p1005 herpes simplex virus (HSV) expressing GFP or GFP and Rgs2 (Origene). Expression of GFP is driven by a cytomegalovirus (CMV) promoter while the gene of interest is driven by the IE4/5 promoter (39,40). One week following defeat, mice received one bilateral 0.6uL injection of either the RGS2 vector or vector containing GFP alone into the NAc core (A/P: 0.84, M/L: ±1.5, D/V: 6.0). One week later mice were tested for sucrose anhedonia test and social interaction. To confirm that expression was limited to NAc, sections of the NAc were imaged to visualize GFP colocalization with Neurotrace (ThermoFisher). RGS2 overexpression was confirmed via western blot.
Statistical analyses
All statistical analyses were performed using R statistical software. Normality of data was assessed using Shapiro-tests. A Fligner-Killeen test was used to assess homogeneity of variance. Two-way ANOVA (sex and stress) was used to analyze qPCR data and behavior measures. An unpaired t-test was used for western blot data. One-way ANOVA was used to analyze behavior data for the RGS2 overexpression experiment. After ANOVA analyses that revealed significant interaction effects, we used a priori planned comparisons to test for effects of stress in males and females, or RGS2 versus GFP controls (41).
Results
Effects of social defeat on male and female transcriptional responses in the NAc of California mice
We performed behavioral and transcriptional analyses two weeks after social defeat or control manipulations (Fig. 1A). Similar to previous studies of California mice (42,43), stressed females but not males showed a decrease in social interaction ratio when the target was present (Fig. 1B, stress*sex interaction effect, F1,29=4.37, p<0.05) and planned comparisons showed an effect of stress in females but not males. There were no differences in the open field phase (Fig. 1C). We performed RNA-seq on samples of the NAc from these mice (Supplemental Table 2). We first set more liberal parameters for identifying alterations in transcription (uncorrected p<0.05, log2 fold-change (logFC) > |0.38|) to identify broad patterns of transcriptional changes (44,45). Heatmaps plotting normalized transcript expression (RPKMs) show contrasting transcriptional expression patterns in females, where low abundance transcripts in control females were more abundant in stressed females, and vice versa (Fig. 1D); this pattern is less distinct in males (Fig. 1D). Most of the top ten highly enriched terms following gene ontology analyses of differentially expressed transcripts in females are related to dopamine signaling pathways and G-alpha(q) second messenger signaling cascades (Fig. 1E). When comparing these datasets using RRHO, we found more overlap in overexpressed transcripts for males and females (Supplemental Fig. 2) and less overlap in transcripts that were reduced after defeat. When stricter parameters were used to identify differentially expressed genes (uncorrected p<0.05, logFC > |1.15) (44,46), we found a more robust effect of stress in females, where 320 transcripts were elevated in stressed females versus 36 transcripts in males (Fig. 1F). In contrast 140 transcripts were less abundant in females versus 31 transcripts in males. Volcano plots of these data also indicate stronger transcriptional responses in females compared to males (Supplemental Fig. 3). Together these results suggest that there may be sex-specific changes in transcriptomic responses in the NAc, with stronger responses occurring in females compared to males. For example, only 11 transcripts that were upregulated in stressed females were also upregulated in stressed males (Fig. 1F; Supplemental Table 3). For females, we also used RRHO analyses to assess the extent to which effects of stress on transcription in the NAC generalized to the PFC and VTA (9). Transcripts that were more abundant in the NAc of stressed females were also more abundant in the PFC and VTA (Supplemental Fig. 4) of stressed females. In contrast, distinct sets of transcripts were decreased by social defeat across the NAc, PFC, and VTA. A weakness of these analyses is that the vast majority of comparisons do not pass false discovery rate thresholds for significance, a common problem for bulk tissue RNAseq analyses (47). To determine the extent to which the patterns of gene expression in our study generalize across species, we used RRHO analyses to compare male and female California mouse NAc RNAseq results to data obtained from the NAc of men and women with MDD (GEO accession number: GSE102556).
Figure 1. Social defeat stress differentially expresses NAc transcriptional patterns in a sex-specific manner.
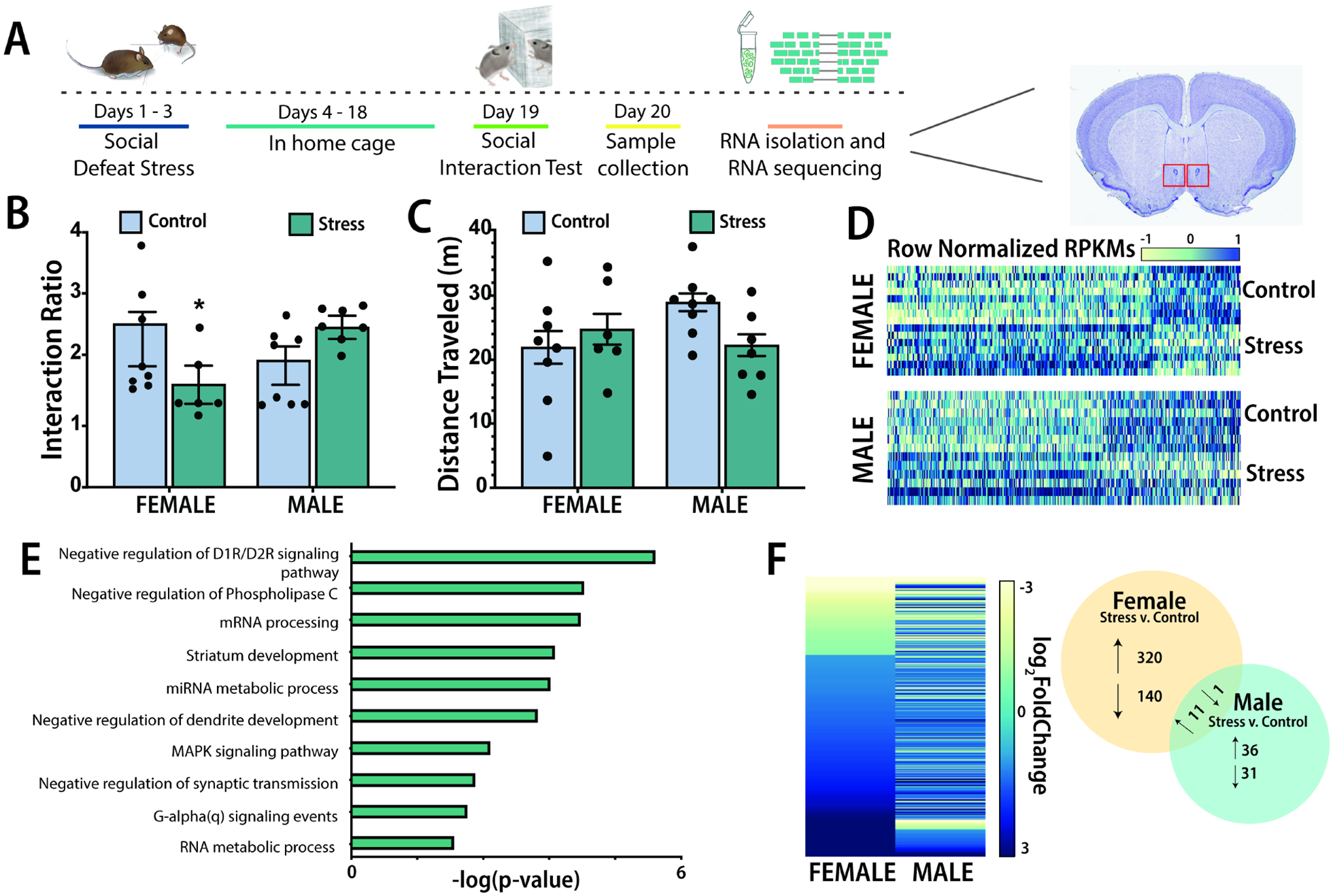
Timeline of experiment. Mice were run through social defeat and weeks later, they were run in a social interaction test. The next day, fresh punches of the NAc were collected for RNAseq (A). Social defeat reduces social approach in females but not males during the social interaction test (B). No differences were seen in distance traveled during the open field phase (C). Stressed female mice have different average transcript expression (RPKM) patterns compared to control females (D). Highly enriched differently expressed GO terms identified from overlapping male and female DEGs (E). Minimal overlap is present in DEGs between males and females (F). *planned comparison p<0.05 v. control. Group N’s: males/control: 8, male/stress: 7, female/control: 8, female/stress: 6.
Transcriptional patterns related to social defeat and major depressive disorder
Using RRHO we observed that the effects of social defeat stress on transcriptional responses in female California mice were broadly similar to differences observed in samples from women with MDD (Fig. 2A). This overlap was largely absent in samples from male California mice and samples from men with MDD (Fig. 2B). We then identified 17 transcripts present in both stressed female mice and women with MDD that had an uncorrected p<0.05 and log FC>|1.15| (Fig. 2A). One of these transcripts is Rgs2. We identified a sex-specific effect of stress on Rgs2 RPKMs in female California mice (Fig. 2C, sex*stress interaction effect, F1,29=4.762, p<0.05), with planned comparisons showing an effect of stress in females but not males. Importantly, Rgs2 was not identified as a DEG in the NAc of male mice or males with MDD. There was a positive correlation between Rgs2 expression and social approach in the social interaction test for females (Fig. 2D, Pearson r=0.6, p<0.05) but not males (Fig. 2E, r=0.098, p>0.05). No effects of stress were observed on Rgs2 RPKMs in the VTA or PFC (Supplemental Fig. 5). To determine the robustness of social defeat stress on Rgs2 expression, we measured gene expression in one set of biological replicates and RGS2 protein in a separate set of samples (Fig. 3A).
Figure 2. Social defeat stress induces similar gene expression patterns in the NAc in female California mice compared to women with major depression (MDD).
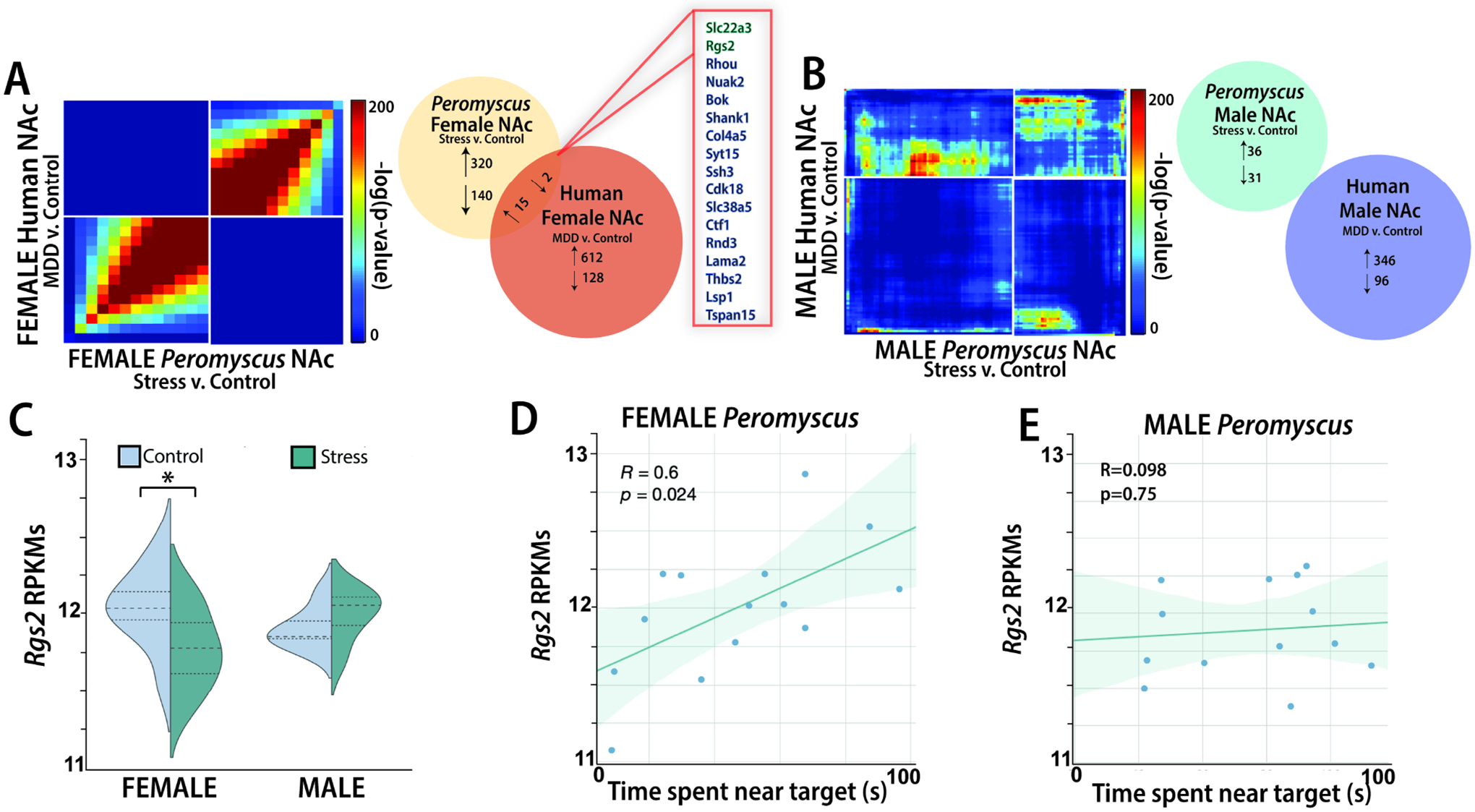
Social defeat stress induces similarities in transcriptional patterns in female California mice compared to transcriptional patterns observed in women with MDD (A); one transcript similarly affected in both data sets is Rgs2. No similarities in DEGs were observed in stressed male California mice and men with MDD (B). Stress reduces Rgs2 average expression in a sex-specific manner (C). Rgs2 expression is correlated to social avoidance behavior in female (D) but not male (E) California mice. *planned comparison p<0.05 v. control. Group N’s: males/control: 8, male/stress: 7, female/control: 8, female/stress: 8, women/MDD: 13, women/control: 9, men/MDD: 13, men/control: 13.
Figure 3. Social defeat stress reduces Rgs2 mRNA and protein expression in NAc of female California mice.
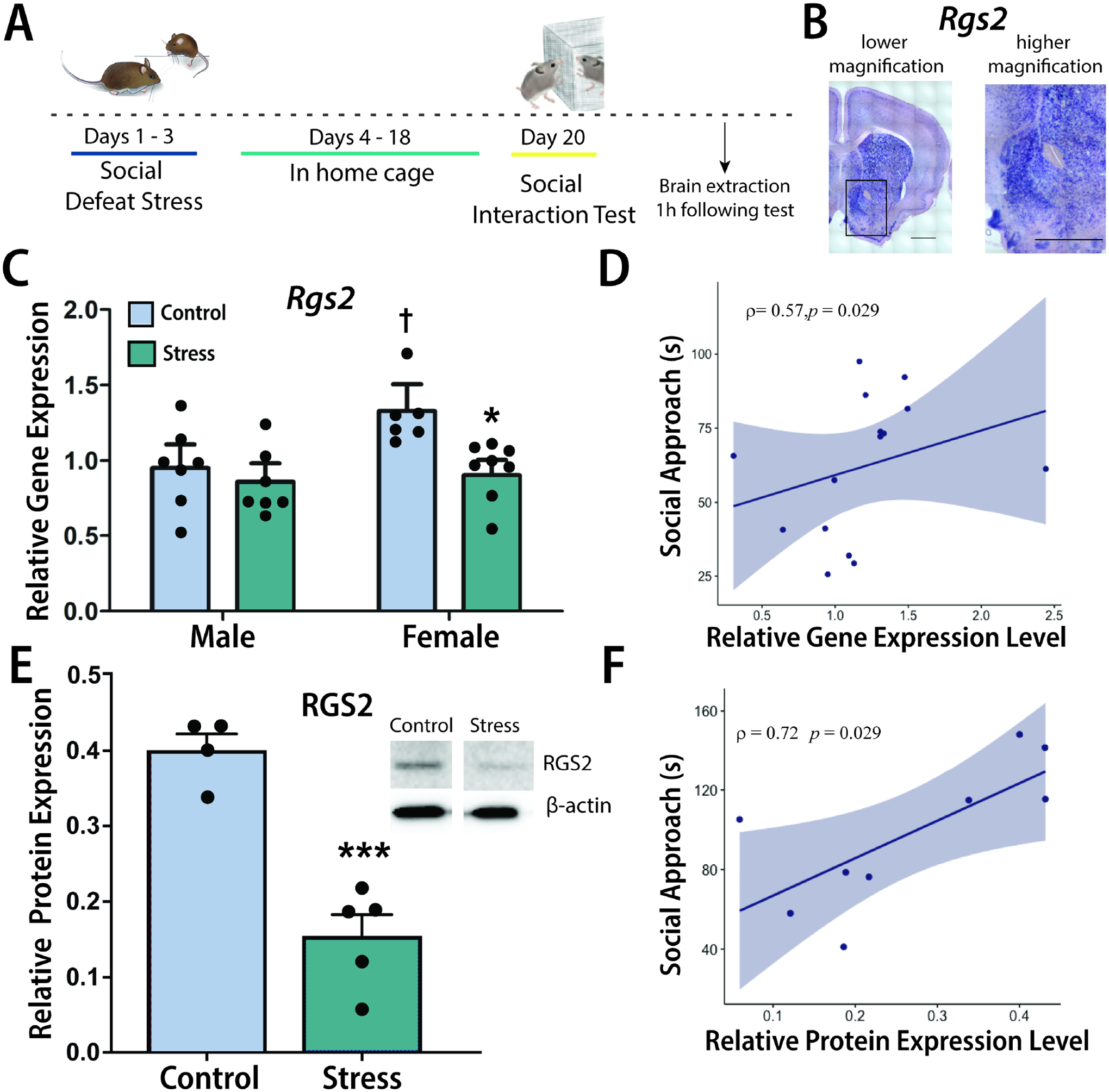
Timeline for experiment. Male and female California mice were run through control handling or social defeat stress. Two weeks later, mice were run through a social interaction test. Tissue samples were collected 1 hour following behavior testing for different cohorts of mice (A). Rgs2 expression in the NAc was confirmed using in-situ hybridization (B). Social defeat stress reduced Rgs2 mRNA in females but not males (C), and Rgs2 mRNA expression levels are positively correlated with social approach behavior (D). Stress reduced RGS2 protein levels in females (E), and these protein levels correlate with social approach (F). *p<0.05 planned comparison vs. female control p<0.05 v. control, ***p<0.001 independent t-test vs. control, † p<0.05 planned comparison v. male control. qPCR group N’s: male/control: 7, male/stress: 7, female/control: 6, female/stress: 8. Western blot group N’s control:4, stress:5.
Social defeat stress reduces Rgs2 mRNA and RGS2 protein expression in the female NAc
Using in-situ hybridization we confirmed Rgs2 expression in the NAc (Fig. 3B). Social defeat reduced social approach in females but not males when the target was present (Supplemental Fig. 6A, stress*sex, F1,26=5.995, p<0.05) but not when it was absent (Supplemental Fig. 6B). In these mice, real-time PCR analyses showed that social defeat reduced Rgs2 mRNA in the NAc in females but not males (Fig. 3C, stress*sex, F1,26=4.687, p<0.05). While RNAseq analyses showed no sex differences in Rgs2 mRNA in control mice, planned comparisons in the real-time PCR cohort showed that Rgs2 mRNA was higher in control females than control males (p<0.05). Similar to analyses of sequencing data, Rgs2 mRNA was positively correlated with social approach in females (Fig. 3D, Spearman ρ=0.57, p=0.03) but not in males (ρ=−0.27, p=0.39). In a separate group of biological replicates, social defeat significantly decreased RGS2 protein expression in females (Fig. 3E, t(7) = 6.9, p <0.001) and RGS2 protein was positively correlated with social approach (Fig. 3F, ρ=0.73, p=0.03).
Rgs2 overexpression in the NAc blocks depression-like behavior in stressed females
Overexpression of Rgs2 in the NAc via viral gene transfer was used to assess the effect of increasing Rgs2 on depression- and anxiety-like phenotypes in stressed females (Fig. 4A, B). Viral expression occurred in neurons and the Rgs2 virus increased RGS2 protein in the NAc (Fig. 4C). There were no differences in sucrose preference prior to defeat stress (Fig. 4D). After defeat, there were significant differences in sucrose preference (one-way ANOVA F2,14=36.4, p<0.001), with females receiving the Rgs2 virus in the NAc consuming more sucrose than GFP (planned comparison p<0.001) whereas misplaced Rgs2 viral injections did not differ from GFP. In the social interaction test, there were significant differences in social approach (Fig. 4E, F2,14=11.66, p<0.01) and social vigilance (Fig. 4F, F2,14=36.39, p<0.01). Mice that received the Rgs2 virus in the NAc had higher social approach (planned comparison, p<0.01) and lower social vigilance (planned comparison, p<0.05) compared to females receiving GFP. Mice with misplaced Rgs2 injections were not different from GFP controls. Rgs2 overexpression had no effects on behavior during the acclimation phase when the target was absent (Fig. 4G, 4H, both p’s >0.05). During the open field phase of the social interaction test, Rgs2 overexpression had no effects on distance traveled (Fig. 4I, p>0.05) or on time spent in the center of the arena (Fig. 4J, p>0.05).
Figure 4. Overexpression of Rgs2 in the NAc reverses stress-induced depression-like behavior in female California mice.
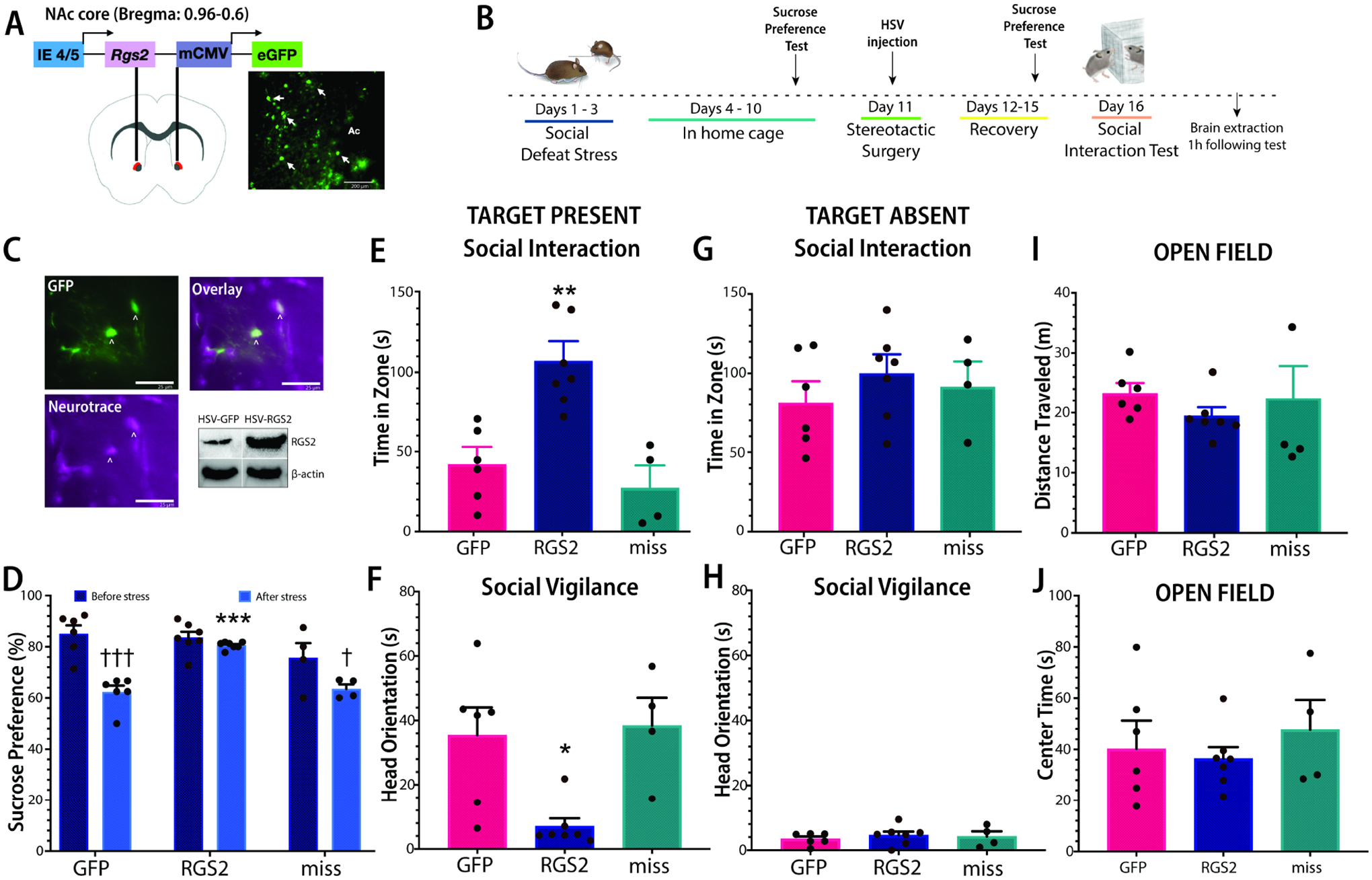
Schematic of placement site and viral construct and image of viral transfection (GFP in NAc) (A). Timeline of experiment. We took baseline sucrose preference levels from all mice before stress exposure. All females were exposed to social defeat and then one week later, viral vectors (GFP or Rgs2) were microinjected into the NAc core. Four days later, stressed mice received a sucrose preference test followed by a social interaction test (B). Neurotrace staining shows that GFP expression occurs in neurons and western blot analysis demonstrates that HSV-RGS2 vectors increase RGS2 protein in the NAc (C). Rgs2 overexpression reversed anhedonia-like phenotypes in the sucrose preference test (D). Rgs2 overexpression increased social approach (E) and decreased social vigilance (F) when the target was present but not while the target was absent (G, H). Rgs2 overexpression did not alter distance traveled (I) or center time (J) during the open field phase. * p<0.05 planned comparison v. GFP. **p<0.01 planned comparison v. GFP. ***p<0.001 planned comparison v. GFP. †p<0.05 paired t-test with baseline (before stress). †††p<0.001 paired t-test with baseline (before stress). Group N’s: RGS2: 7, GFP: 6, miss: 4. Scale bar in 4A=200μM. Scale bar in 4C=25μM.
Discussion
An important question in psychiatry is why rates of depression and anxiety are elevated in women versus men. There is growing evidence that distinct neurobiological responses can be evoked by stress in women and men. Here we demonstrate that social defeat stress in female California mice induces broad patterns of transcriptional changes in the NAc that are correlated with transcriptional patterns reported in postmortem NAc samples collected from women diagnosed with depression. This finding suggests a strong translational potential for female California mice in studying biological mechanisms related to depression and anxiety that are relevant for women. These analyses identified Rgs2 as a stress-sensitive transcript in the NAc of females. RGS2 protein regulates the activity of neuropeptide and neurotransmitter receptors, and its overexpression blocked stress-induced sucrose anhedonia and social avoidance. Overexpression of Rgs2 also reduced social vigilance, which is modulated by the bed nucleus of the stria terminalis (48), suggesting that Rgs2 modulates direct or indirect connections within the extended amygdala. Consistent with prior studies, stressed males did not exhibit social avoidance and had fewer transcriptional changes in the NAc. However, stressed male California mice exhibit alternative phenotypes such as reduced cognitive flexibility (49), suggesting that in males stress could have stronger effects on transcription outside of the NAc. Thus, when utilizing rodent models to study social stress in both males and females it is important to consider a broad range of behavioral and neurobiological phenotypes.
Genetic variants of the Rgs2 gene that have less stable Rgs2 mRNA (50) are correlated with increased risk for depression (51), anxiety (52–56), and risk for suicide (57–59). Disruptions in Rgs2 gene expression are also linked to patients with treatment-resistant depression (54), suggesting that Rgs2 may play some role in a lack of efficacy to currently available treatments. These studies included both men and women and adjusted genetic analyses for sex. However, none of these studies tested whether Rgs2 gene variations had stronger associations with health outcomes in women versus men. Preclinical studies in male Mus musculus showed that Rgs2 deletion increased anxiety-like responses and passive coping responses (60,61). These studies had important limitations. Global knockout approaches cannot distinguish whether behavioral changes are due to developmental effects of Rgs2 or altered gene function in the adult brain. Our results show that stress-induced decreases in Rgs2 expression in the adult brain can contribute to depression-like behaviors. Furthermore, Rgs2 is widely distributed throughout the brain, so global knockout approaches have little precision for identifying the brain circuits mediating Rgs2 action on social behavior. In addition, behavioral studies focused primarily on males. In our qPCR experiment Rgs2 expression was higher in control females versus control males, although this difference was not replicated in the RNAseq dataset. There are few data on RGS2 expression in male and female brains, although higher Rgs2 expression was reported in female rat brainstem versus males (62). Further study is needed to determine whether baseline differences in Rgs2 expression are consistent across species or brain regions. Previous work showed that Rgs2 expression in the brain can be stress sensitive (63), but to our knowledge no study has tested whether these changes contribute to behavioral outcomes via RGS2 manipulation. Our experiments show that in females, Rgs2 mRNA and protein in the NAc are decreased by social defeat, and that viral overexpression of RGS2 in NAc is sufficient to reduce stress-induced social avoidance, social vigilance, and sucrose anhedonia. These findings agree with clinical findings, suggesting that Rgs2 is an important modulator for behaviors with translational relevance.
A main function of RGS proteins is to potentiate the process of GTP hydrolysis, which effectively switches off downstream G-protein coupled receptor signaling pathways (27,64,65). Reduced production of RGS2 protein disrupts this process (66), which consequently interferes with the function of neuropeptide and neurotransmitter receptors (67,68). In addition to the GTPase activating action, RGS proteins may modulate GPCR responses by several other mechanisms. For example, they may act as effector antagonists for G alpha subunits or as regulators of epigenetic and transcriptional processes (69). RGS2 specifically regulates G-alpha(q) signaling events (70). Given this distinction, an important question to consider is which receptor signaling pathways within the NAc are being impacted by stress-induced reductions in RGS2. Prior findings indicate that RGS2 may regulate dopamine receptor 1 (D1R)-expressing neurons in the NAc (71–73). Single-cell RNAseq analyses of striatum showed that Rgs2 gene in the striatum clusters significantly with D1R-expressing neurons but not D2R-expressing neurons (74). Although these data lack anatomical specificity, it suggests that RGS2 could be impacting social behavior through a D1R-driven mechanism within the striatum, and potentially specifically within the NAc. Consistent with this hypothesis, D1R agonist infusions in the NAc are sufficient to reduce social approach in unstressed female California mice (75). Confirming whether Rgs2 modulates signaling pathways in the NAc through a D1R driven mechanism, or through other receptor signaling systems, will lead to novel insights on a cell-type-specific mechanism through which Rgs2 modulates social behavior and deficits in social behavior that are relevant to stress disorders. Other members of the RGS family have been shown to modulate stress, but they have distinct functions. Prevention of Rgs7 action reduces anxiety-like behaviors in response to environmental stimuli in male mice (76), whereas deletion of the Rgs4 gene decreases the efficacy of monoamine-targeting antidepressants and promotes the actions of ketamine (77).
Although Rgs2 was not differentially expressed in the VTA or PFC, at a broad level RRHO analyses comparing the NAc with VTA and PFC detected more overlap in transcripts upregulated by stress than transcripts downregulated by stress. In C57Bl6/J, unpredictable chronic mild stress induced similar transcriptional changes in the NAc and PFC in male but not female mice, while in females similar gene expression profiles were observed in the NAc and BLA (78). In analyses of human post-mortem samples, RRHO analyses detected little overlap in gene expression across NAc and cortical regions in either males or females. Numerous studies have reported sex-specific neural transcriptional responses to stress using bulk RNAseq methods (79), in which different cells are combined during the RNA extraction process. A weakness of these studies, including ours, is that this approach generally does not provide sufficient power to detect differential expression that passes false discovery correction (but see (80)). Thus, although RRHO analyses identified broad similarities in gene expression signatures in female rodent and human NAc samples, few transcripts met criteria for differential expression (Fig. 2A). Despite this weakness, when combined with follow-up analyses of different biological replicates, these approaches have led to the successful identification of numerous transcripts with sex-specific transcriptional responses to stress such as Dusp4, Dnmt3a, and Emx1. Here we showed that bulk sequencing approaches are effective for hypothesis generation when paired with replication and manipulation of candidate gene function. Our analyses also identified Slc22A3 as a transcript down-regulated by stress in California mice and decreased in samples from women diagnosed with depression. One protein encoded by this transcript is organic cation transporter 3 (OCT3), a low-affinity high-capacity transporter for monoamines (81). Although less is known about OCT3, it enhances place preference responses to cocaine (82) and is sensitive to glucocorticoids (83). Our sequencing data suggests that it is an intriguing target for further study. Moving forward, greater use of strategies that have more statistical power is needed. This could be achieved using larger sample sizes or analyses of more defined cell populations via single-cell analyses of transcription (84). This will increase the utility of comparisons across stress models and enhance our ability to assess the extent to which depression and anxiety disorders are linked to sex-specific molecular signatures.
These studies demonstrate that Rgs2 is a stress-sensitive transcript in the NAc that modulates depression- and anxiety-like behavior. Our results suggest that facilitating Rgs2 activity could have important therapeutic properties, especially in females. This is important because women are twice as likely to develop MDD and some underlying mechanisms may be distinct from men. Identifying distinct mechanisms could facilitate sex-specific targets for therapeutic intervention. These studies in California mice highlight the utility of model systems in which social stress can be studied in males and females.
Supplementary Material
KEY RESOURCES TABLE
| Resource Type | Specific Reagent or Resource | Source or Reference | Identifiers | Additional Information |
|---|---|---|---|---|
| Add additional rows as needed for each resource type | Include species and sex when applicable. | Include name of manufacturer, company, repository, individual, or research lab. Include PMID or DOI for references; use “this paper” if new. | Include catalog numbers, stock numbers, database IDs or accession numbers, and/or RRIDs. RRIDs are highly encouraged; search for RRIDs at https://scicrunch.org/resources. | Include any additional information or notes if necessary. |
| Antibody | rabbit anti-RGS2 | Abcam | ab155762, RRID:AB_2916033 | |
| Antibody | Goat Anti-Rabbit IgG Antibody (H+L), Peroxidase | Vector Labs | PI-1000-1, RRID:AB_2916034 | |
| Antibody | rabbit anti-b-actin 13E5 | Cell Signaling | 4970, RRID:AB_2223172 | |
| Deposited Data; Public Database | California mouse RNAseq data | NCBI Bioproject | PRJNA700778 | |
| Deposited Data; Public Database | Human post-mortem brain RNAseq data | NCBI GEO DataSets | GSE102556 | |
| Commercial Assay Or Kit | Neurotrace 530/615 | ThermoFisher | N21482 | |
| Primers for RT-qPCR, see Table S1 | RGS2 and Hprt primers | This paper, Invitrogen | ||
| Viral vector | HSV-Rgs2 | Mass General Brigham Gene Delivery Technology Core | CMV promoter for GFP and IE4/5 promoter for Rgs2 |
Acknowledgements
We thank Cindy Clayton, Marcus Petagara, and vivarium staff for coordinating animal care, M. Wu and J. Tollkuhn for technical assistance, and Andrew Fox and Jill Silverman for helpful discussions.
Funding and disclosure
Supported by K99/R00 MH115096 to CJP, R01MH051399 to EJN, NS086444 to VZ, NS086444S1 (RAS), R01MH121829S1 to AVM, and R01MH121829 and R01MH103322 to BCT. The authors report no biomedical financial interests or potential conflicts of interest.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Goel N, Bale TL (2009): Examining the intersection of sex and stress in modelling neuropsychiatric disorders. J Neuroendocr 21: 415–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bale TN, Epperson CN (2015): Sex differences and stress across the lifespan. Nat Neurosci 18: 1413–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Laman-Maharg A, Trainor BC (2017): Stress, sex, and motivated behaviors. J Neurosci Res 95: 83–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Culpepper L (2010): Why do you need to move beyond first-line therapy for major depression? J Clin Psychiatry 71: 4–9. [DOI] [PubMed] [Google Scholar]
- 5.Depression: How Effective Are Antidepressants? (2017): Institute for Quality and Efficiency in Health Care (IQWiG). Retrieved March 1, 2019, from https://www.ncbi.nlm.nih.gov/books/NBK361016/ [Google Scholar]
- 6.Enneking V, Krüssel P, Zaremba D, Dohm K, Grotegerd D, Förster K, et al. (2018): Social anhedonia in major depressive disorder: a symptom-specific neuroimaging approach. Neuropsychopharmacology. 10.1038/s41386-018-0283-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nauczyciel C, Robic S, Dondaine T, Verin M, Robert G, Drapier D, et al. (2013): The nucleus accumbens: a target for deep brain stimulation in resistant major depressive disorder. J Mol Psychiatry 1: 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Delaloye S, Holtzheimer PE (2014): Deep brain stimulation in the treatment of depression. Clin Res 16: 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Russo SJ, Nestler EJ (2013): The brain reward circuitry in mood disorders. Nat Rev Neurosci 14: 609–625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Carlezon WA Jr, Thomas MJ (2009): Biological substrates of reward and aversion: A nucleus accumbens activity hypothesis. Neuropharmacology 56: 122–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Britt JP, Benaliouad F, McDevitt RA, Stuber GD, Wise RA, Bonci A (2012): Synaptic and Behavioral Profile of Multiple Glutamatergic Inputs to the Nucleus Accumbens. Neuron 76: 790–803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sesack SR, Grace AA (2010): Cortico-Basal Ganglia Reward Network: Microcircuitry. Neuropsychopharmacology 35: 27–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mogenson G, Jones D, Yim C (1980): From motivation to action: Functional interface between the limbic system and the motor system. Prog Neurobiol 14: 69–97. [DOI] [PubMed] [Google Scholar]
- 14.Roitman MF, Wheeler RA, Carelli RM (2005): Nucleus Accumbens Neurons Are Innately Tuned for Rewarding and Aversive Taste Stimuli, Encode Their Predictors, and Are Linked to Motor Output. Neuron 45: 587–597. [DOI] [PubMed] [Google Scholar]
- 15.Humphries MD, Prescott TJ (2010): The ventral basal ganglia, a selection mechanism at the crossroads of space, strategy, and reward. Prog Neurobiol 90: 385–417. [DOI] [PubMed] [Google Scholar]
- 16.Parkinson JA, Willoughby PJ, Robbins TW, Everitt BJ (2000): Disconnection of the anterior cingulate cortex and nucleus accumbens core impairs Pavlovian approach behavior: Further evidence for limbic cortical-ventral striatopallidal systems. Behav Neurosci 114: 42–63. [PubMed] [Google Scholar]
- 17.Stefanik MT, Kupchik YM, Brown RM, Kalivas PW (2013): Optogenetic Evidence That Pallidal Projections, Not Nigral Projections, from the Nucleus Accumbens Core Are Necessary for Reinstating Cocaine Seeking. J Neurosci 33: 13654–13662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Williams AV, Trainor BC (2018): The impact of sex as a biological variable in the search for novel antidepressants. Front Neuroendocrinol 50: 107–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Will TR, Proaño SB, Thomas AM, Kunz LM, Thompson KC, Ginnari LA, et al. (2017): Problems and Progress regarding Sex Bias and Omission in Neuroscience Research. eNeuro 4. 10.1523/ENEURO.0278-17.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Labonté B, Engmann O, Purushothaman I, Menard C, Wang J, Tan C, et al. (2017): Sex-specific transcriptional signatures in human depression. Nat Med 23: 1102–1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Seney ML, Huo Z, Cahill K, French L, Puralewski R, Zhang J, et al. (2018): Opposite Molecular Signatures of Depression in Men and Women. Biol Psychiatry 84: 18–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hodes GE, Pfau ML, Purushothaman I, Ahn HF, Golden SA, Christoffel DJ, et al. (2015): Sex Differences in Nucleus Accumbens Transcriptome Profiles Associated with Susceptibility versus Resilience to Subchronic Variable Stress. J Neurosci 35: 16362–16376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ordoñes Sanchez E, Bavley CC, Deutschmann AU, Carpenter R, Peterson DR, Karbalaei R, et al. (2021): Early life adversity promotes resilience to opioid addiction-related phenotypes in male rats and sex-specific transcriptional changes. Proc Natl Acad Sci U S A 118. 10.1073/pnas.2020173118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bangasser DA, Cuarenta A (2021): Sex differences in anxiety and depression: circuits and mechanisms [no. 11]. Nat Rev Neurosci 22: 674–684. [DOI] [PubMed] [Google Scholar]
- 25.Kuske JX, Trainor BC (2021): Mean Girls: Social Stress Models for Female Rodents. Curr Top Behav Neurosci. 10.1007/7854_2021_247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Trainor BC, Pride MC, Villalon Landeros R, Knoblauch NW, Takahashi EY, Silva AL, Crean KK (2011): Sex differences in social interaction behavior following social defeat stress in the monogamous California mouse (Peromyscus californicus). PLOS One 6: e17405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Watson N, Linder ME, Drueyt KM, Kehrlt JH, Blumer KJ (1996): RGS family members: GTPase-activating proteins for heterotrimeric G-protein a-subunits. 383: 4. [DOI] [PubMed] [Google Scholar]
- 28.Butler-Struben HM, Kentner AC, Trainor BC (2022): What’s wrong with my experiment?: The impact of hidden variables on neuropsychopharmacology research. Neuropsychopharmacology 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.du Sert NP, Hurst V, Ahluwalia A, Alam S, Avey MT, Baker M, et al. (2020): The ARRIVE guidelines 2.0: Updated guidelines for reporting animal research. PLOS Biol 18: e3000410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Williams AV, Laman-Maharg A, Armstrong CV, Ramos-Maciel S, Minie VA, Trainor BC (2018): Acute inhibition of kappa opioid receptors before stress blocks depression-like behaviors in California mice. Prog Neuropsychopharmacol Biol Psychiatry 86: 166–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Williams AV, Duque-Wilckens N, Ramos-Maciel S, Campi KL, Bhela SK, Xu CK, et al. (2020): Social approach and social vigilance are differentially regulated by oxytocin receptors in the nucleus accumbens. Neuropsychopharmacology 45: 1423–1430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Streett DA, Petersen KR, Gerritsen AT, Hunter SS, Settles ML (2015): expHTS: analysis of high throughput sequence data in an experimental framework. Proceedings of the 6th ACM Conference on Bioinformatics, Computational Biology and Health Informatics 523–524. [Google Scholar]
- 33.Li H (2013): Aligning sequence reads, clone sequences and assembly contigs with BWA-MEM. ArXiv13033997 Q-Bio. Retrieved June 2, 2021, from http://arxiv.org/abs/1303.3997 [Google Scholar]
- 34.Robinson MD, McCarthy DJ, Smyth GK (2010): edgeR: a Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics 26: 139–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ritchie ME, Phipson B, Wu D, Hu Y, Law CW, Shi W, Smyth GK (2015): limma powers differential expression analyses for RNA-sequencing and microarray studies. Nucleic Acids Res 43: e47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Alexa A, Rahnenfuhrer J (2020): topGO: Enrichment Analysis for Gene Ontology. R package version 2.41.0.
- 37.Cahill KM, Huo Z, Tseng GC, Logan RW, Seney ML (2018): Improved identification of concordant and discordant gene expression signatures using an updated rank-rank hypergeometric overlap approach. Sci Rep 8: 9588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wu MV, Tollkuhn J (2017): Estrogen receptor alpha is required in GABAergic, but not glutamatergic, neurons to masculinize behavior. Horm Behav 95: 3–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Neve RL, Neve KA, Nestler EJ, Carlezon WA (2005): Use of herpes virus amplicon vectors to study brain disorders. BioTechniques 39: 381–391. [DOI] [PubMed] [Google Scholar]
- 40.Peña CJ, Kronman HG, Walker DM, Cates HM, Bagot RC, Purushothaman I, et al. (2017): Early life stress confers lifelong stress susceptibility in mice via ventral tegmental area OTX2. Science 356: 1185–1188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Keppel G (1991): Design and Analysis: A Researcher’s Handbook, 3rd ed. Upper Saddle River, NJ: Prentice Hall. [Google Scholar]
- 42.Greenberg GD, Laman-Maharg A, Campi KL, Voigt H, Orr VN, Schaal L, Trainor BC (2014): Sex differences in stress-induced social withdrawal: role of brain derived neurotrophic factor in the bed nucleus of the stria terminalis. Front Behav Neurosci 7: 223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Trainor BC, Takahashi EY, Campi KL, Florez SA, Greenberg GD, Laman-Maharg A, et al. (2013): Sex differences in stress-induced social withdrawal: independence from adult gonadal hormones and inhibition of female phenotype by corncob bedding. Horm Behav 63: 543–550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Peña CJ, Smith M, Ramakrishnan A, Cates HM, Bagot RC, Kronman HG, et al. (2019): Early life stress alters transcriptomic patterning across reward circuitry in male and female mice [no. 1]. Nat Commun 10: 5098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Walker DM, Zhou X, Ramakrishnan A, Cates HM, Cunningham AM, Peña CJ, et al. (2020): Adolescent Social Isolation Reprograms the Medial Amygdala: Transcriptome and Sex Differences in Reward. Neuroscience. 10.1101/2020.02.18.955187 [DOI] [Google Scholar]
- 46.Walker DM, Cates HM, Loh Y-HE, Purushothaman I, Ramakrishnan A, Cahill KM, et al. (2018): Cocaine self-administration alters transcriptome-wide responses in the brain’s reward circuitry. Biol Psychiatry 84: 867–880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ruiz-Ortiz J, Tollkuhn J (2021): Specificity in sociogenomics: Identifying causal relationships between genes and behavior. Horm Behav 127: 104882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wright EC, Hostinar CE, Trainor BC (2020): Anxious to see you: Neuroendocrine mechanisms of social vigilance and anxiety during adolescence. Eur J Neurosci 52: 2516–2529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Laredo SA, Steinman MQ, Robles CF, Ferrer E, Ragen BJ, Trainor BC (2015): Effects of defeat stress on behavioral flexibility in males and females: Modulation by the mu-opioid receptor. Eur J Neurosci 41. 10.1111/ejn.12824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Semplicini A, Lenzini L, Sartori M, Papparella I, Calò L, Pagnin E, et al. (2006): Reduced expression of regulator of G-protein signaling 2 (RGS2) in hypertensive patients increases calcium mobilization and ERK1/2 phosphorylation induced by angiotensin II. J Hypertens 24: 1115–1124. [DOI] [PubMed] [Google Scholar]
- 51.Asselmann E, Hertel J, Schmidt C-O, Homuth G, Nauck M, Beesdo‐Baum K, et al. (2018): Interplay between RGS2 and childhood adversities in predicting anxiety and depressive disorders: Findings from a general population sample. Depress Anxiety 35: 1104–1113. [DOI] [PubMed] [Google Scholar]
- 52.Smoller JW, Paulus MP, Fagerness JA, Purcell S, Yamaki LH, Hirshfeld-Becker D, et al. (2008): Influence of RGS2 on Anxiety-Related Temperament, Personality, and Brain Function. Arch Gen Psychiatry 65: 298–308. [DOI] [PubMed] [Google Scholar]
- 53.Koenen KC, Amstadter AB, Ruggiero KJ, Acierno R, Galea S, Kilpatrick DG, Gelernter J (2009): RGS2 and generalized anxiety disorder in an epidemiologic sample of hurricane-exposed adults. Depress Anxiety 26: 309–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Stein MB, Keshaviah A, Haddad SA, Van Ameringen M, Simon NM, Pollack MH, Smoller JW (2014): Influence of RGS2 on Sertraline Treatment for Social Anxiety Disorder. Neuropsychopharmacology 39: 1340–1346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hohoff C, Weber H, Richter J, Domschke K, Zwanzger PM, Ohrmann P, et al. (2015): RGS2 genetic variation: Association analysis with panic disorder and dimensional as well as intermediate phenotypes of anxiety. Am J Med Genet B Neuropsychiatr Genet 168: 211–222. [DOI] [PubMed] [Google Scholar]
- 56.Otowa T, Shimada T, Kawamura Y, Sugaya N, Yoshida E, Inoue K, et al. (2011): Association of RGS2 variants with panic disorder in a Japanese population. Am J Med Genet B Neuropsychiatr Genet 156: 430–434. [DOI] [PubMed] [Google Scholar]
- 57.Amstadter AB, Koenen KC, Ruggiero KJ, Acierno R, Galea S, Kilpatrick DG, Gelernter J (2009): Variant in RGS2 moderates posttraumatic stress symptoms following potentially traumatic event exposure. J Anxiety Disord 23: 369–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Amstadter AB, Koenen KC, Ruggiero KJ, Acierno R, Galea S, Kilpatrick DG, Gelernter J (2009): Variation in RGS2 is Associated with Suicidal Ideation in an Epidemiological Study of Adults Exposed to the 2004 Florida Hurricanes. Arch Suicide Res 13: 349–357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Cui H, Nishiguchi N, Ivleva E, Yanagi M, Fukutake M, Nushida H, et al. (2008): Association of RGS2 Gene Polymorphisms with Suicide and Increased RGS2 Immunoreactivity in the Postmortem Brain of Suicide Victims. Neuropsychopharmacology 33: 1537–1544. [DOI] [PubMed] [Google Scholar]
- 60.Lifschytz T, Broner EC, Zozulinsky P, Slonimsky A, Eitan R, Greenbaum L, Lerer B (2012): Relationship between Rgs2 gene expression level and anxiety and depression-like behaviour in a mutant mouse model: serotonergic involvement. Int J Neuropsychopharmacol 15: 1307–1318. [DOI] [PubMed] [Google Scholar]
- 61.Oliveira-dos-Santos AJ, Matsumoto G, Snow BE, Bai D, Houston FP, Whishaw IQ, et al. (2000): Regulation of T cell activation, anxiety, and male aggression by RGS2. Proc Natl Acad Sci 97: 12272–12277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Nakahara T, Hashimoto K, Hirano M, Rajendram R, Martin CR, Ar, Preedy VR (2010): Gender differences in the relative abundance of RGS2 mRNA in brain-stem, cortex, cerebellum and midbrain and the effects of chronic alcohol feeding. Proc Nutr Soc 69. 10.1017/S0029665110001473 [DOI] [Google Scholar]
- 63.Okimoto N, Bosch OJ, Slattery DA, Pflaum K, Matsushita H, Wei F-Y, et al. (2012): RGS2 mediates the anxiolytic effect of oxytocin. Brain Res 1453: 26–33. [DOI] [PubMed] [Google Scholar]
- 64.Hepler JR (1999): Emerging roles for RGS proteins in cell signalling. Trends Pharmacol Sci 20: 376–382. [DOI] [PubMed] [Google Scholar]
- 65.Heximer SP, Watson N, Linder ME, Blumer KJ, Hepler JR (1997): RGS2/G0S8 is a selective inhibitor of Gqalpha function. Proc Natl Acad Sci USA 94: 14389–14393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Phan HTN, Sjögren B, Neubig RR (2017): Human Missense Mutations in Regulator of G Protein Signaling 2 Affect the Protein Function Through Multiple Mechanisms. Mol Pharmacol 92: 451–458. [DOI] [PubMed] [Google Scholar]
- 67.Siderovski DP, Hessel A, Chung S, Mak TW, Tyers M (1996): A new family of regulators of G-protein-coupled receptors? Curr Biol 6: 211–212. [DOI] [PubMed] [Google Scholar]
- 68.Koelle MR, Horvitz HR (1996): EGL-10 Regulates G Protein Signaling in the C. elegans Nervous System and Shares a Conserved Domain with Many Mammalian Proteins. Cell 84: 115–125. [DOI] [PubMed] [Google Scholar]
- 69.Sakloth F, Polizu C, Bertherat F, Zachariou V (2020): Regulators of G Protein Signaling in Analgesia and Addiction. Mol Pharmacol 98: 739–750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Differential effects of RGS proteins on Gαq and Gα11 activity - ScienceDirect (n.d.): Retrieved March 10, 2021, from https://www.sciencedirect.com/science/article/abs/pii/S0898656806001410?via%3Dihub
- 71.Taymans J-M, Leysen JE, Langlois X (2003): Striatal gene expression of RGS2 and RGS4 is specifically mediated by dopamine D1 and D2 receptors: clues for RGS2 and RGS4 functions. J Neurochem 84: 1118–1127. [DOI] [PubMed] [Google Scholar]
- 72.Taymans J-M, Kia HK, Claes R, Cruz C, Leysen J (2004): Dopamine receptor-mediated regulation of RGS2 and RGS4 mRNA differentially depends on ascending dopamine projections and time. Eur J Neurosci 19: 2249–2260. [DOI] [PubMed] [Google Scholar]
- 73.Stanwood GD, Parlaman JP, Levitt P (2006): Genetic or pharmacological inactivation of the dopamine D1 receptor differentially alters the expression of regulator of G-protein signalling (Rgs) transcripts. Eur J Neurosci 24: 806–818. [DOI] [PubMed] [Google Scholar]
- 74.Saunders A, Macosko EZ, Wysoker A, Goldman M, Krienen FM, de Rivera H, et al. (2018): Molecular Diversity and Specializations among the Cells of the Adult Mouse Brain. Cell 174: 1015–1030.e16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Campi KL, Greenberg GD, Kapoor A, Ziegler TE, Trainor BC (2014): Sex differences in effects of dopamine D1 receptors on social withdrawal. Neuropharmacology 77: 208–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Sutton LP, Khalatyan N, Savas JN, Martemyanov KA (2021): Striatal RGS7 Regulates Depression-Related Behaviors and Stress-Induced Reinstatement of Cocaine Conditioned Place Preference. eNeuro 8. 10.1523/ENEURO.0365-20.2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Stratinaki M, Varidaki A, Mitsi V, Ghose S, Magida J, Dias C, et al. (2013): Regulator of G protein signaling 4 is a crucial modulator of antidepressant drug action in depression and neuropathic pain models. Proc Natl Acad Sci U S A 110: 8254–8259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Paden W, Barko K, Puralewski R, Cahill KM, Huo Z, Shelton MA, et al. (2020): Sex differences in adult mood and in stress-induced transcriptional coherence across mesocorticolimbic circuitry [no. 1]. Transl Psychiatry 10: 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Scarpa JR, Fatma M, Loh Y-HE, Traore SR, Stefan T, Chen TH, et al. (2020): Shared Transcriptional Signatures in Major Depressive Disorder and Mouse Chronic Stress Models. Biol Psychiatry 88: 159–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.McCann KE, Sinkiewicz DM, Rosenhauer AM, Beach LQ, Huhman KL (2019): Transcriptomic Analysis Reveals Sex-Dependent Expression Patterns in the Basolateral Amygdala of Dominant and Subordinate Animals After Acute Social Conflict. Mol Neurobiol 56: 3768–3779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Gasser PJ (2019): Roles for the uptake2 transporter OCT3 in regulation of dopaminergic neurotransmission and behavior. Neurochem Int 123: 46–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.McReynolds JR, Taylor A, Vranjkovic O, Ambrosius T, Derricks O, Nino B, et al. (2017): Corticosterone Potentiation of Cocaine-Induced Reinstatement of Conditioned Place Preference in Mice is Mediated by Blockade of the Organic Cation Transporter 3 [no. 3]. Neuropsychopharmacology 42: 757–765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Amphoux A, Vialou V, Drescher E, Brüss M, La Cour CM, Rochat C, et al. (2006): Differential pharmacological in vitro properties of organic cation transporters and regional distribution in rat brain. Neuropharmacology 50: 941–952. [DOI] [PubMed] [Google Scholar]
- 84.Gegenhuber B, Wu MV, Bronstein R, Tollkuhn J (2022): Gene regulation by gonadal hormone receptors underlies brain sex differences [no. 7912]. Nature 606: 153–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


