Abstract
Granulomatous inflammation is characterized morphologically by a compact organized collection of macrophages and their derivatives. It is classified as either a hypersensitivity type or a foreign-body type. Lipid components of the Mycobacterium tuberculosis cell wall participate in the pathogenesis of infection. Strains of M. tuberculosis have cord factor (trehalose 6,6′-dimycolate [TDM]) on their surface. To clarify host responses to TDM, including immunogenicity and pathogenicity, we have analyzed the footpad reaction, histopathology, and cytokine profiles of experimental granulomatous lesions in immunized and unimmunized mice challenged with TDM. In the present study, we have demonstrated for the first time that TDM can induce both foreign-body-type (nonimmune) and hypersensitivity-type (immune) granulomas by acting as a nonspecific irritant and T-cell-dependent antigen. Immunized mice challenged with TDM developed more severe lesions than unimmunized mice. At the active lesion, we found monocyte chemotactic, proinflammatory, and immunoregulatory cytokines. The level was enhanced in immunized mice challenged with TDM. This result implies that both nonimmune and immune mechanisms participate in granulomatous inflammation induced by mycobacterial infection. Taken together with a previous report, this study shows that TDM is a pleiotropic molecule against the host and plays an important role in the pathogenesis of tuberculosis.
The pathogenesis of tuberculosis is a function of the pathogen, Mycobacterium tuberculosis, and of the immune response of the host to the pathogen (5, 14). Tuberculosis is a chronic infection with M. tuberculosis complex, including M. tuberculosis and Mycobacterium bovis, that is characterized morphologically by granulomatous inflammation, a compact organized collection of macrophages and their derivatives, such as epithelioid and giant cells, at the site of infection (19). The pathogenicity of M. tuberculosis is related to its ability to escape killing by macrophages and induce delayed-type hypersensitivity (DTH) (5, 14, 19).
Granulomatous inflammation can be broadly classified as either a hypersensitivity (immunologic, T-cell-dependent) type or a foreign-body (nonimmunologic, T-cell-independent) type (19, 20). There is much known, but we still have a long way to go to understand the mechanism of M. tuberculosis pathogenicity. Mycobacteria are rich in lipids. Lipid components of the M. tuberculosis cell wall participate in pathogenesis. Cord factor (trehalose 6,6′-dimycolate [TDM]), a surface glycolipid, causes M. tuberculosis to grow in serpentine cords in vitro. Virulent strains of M. tuberculosis have TDM on their surface (2), and injection of purified TDM into experimental animals induces lesions characterized by chronic granulomatous inflammation (6, 29).
To clarify host responses to mycobacterial TDM, including immunogenicity and pathogenicity, we have analyzed the footpad reactions, histopathology, and cytokine profiles of experimental granulomatous lesions in immunized and unimmunized mice challenged with TDM.
MATERIALS AND METHODS
Animals.
Specific-pathogen-free female euthymic and athymic nude nu/nu BALB/c mice at 8 weeks of age were purchased from SLC (Shizuoka, Japan). Experiments were conducted according to the standard guidelines for animal experiments of Osaka City University Graduate School of Medicine.
Preparation of TDM.
M. tuberculosis Aoyama B was grown in Sauton medium for 5 weeks at 37°C. Glycolipids were serially extracted with chloroform-methanol at 4:1, (vol/vol), 3:1, and 2:1. Each mycolate was purified by developing the lipids on a thin-layer plate of silica gel (Analtech, Inc., Newark, Del.) with chloroform-methanol-acetone-acetic acid (90:10:6:1) and subsequently with chloroform-methanol-water (90:10:1). This procedure was repeated until a single spot was obtained (25). The preparation contained less than 80 ng of protein/100 μg of TDM, as determined by a protein assay kit (Bio-Rad, Hercules, Calif.).
Preparation of w/o/w emulsion.
To prepare 100 μl of sample, 100 μg of purified TDM was dissolved in 3.2 μl of Freund's incomplete adjuvant (FIA) (Difco Laboratories, Detroit, Mich.) in a Teflon grinder. After addition of 3.2 μl of 0.1 M phosphate-buffered saline (PBS), a water-in-oil emulsion was made. Then, 93.6 μl of saline containing 0.2% Tween 80 was added at the final concentration (3.2%) of FIA, and a water-in-oil-in-water (w/o/w) emulsion was made by mixing (28). As controls, w/o/w micelles without TDM were used.
Immunization.
Mice were immunized by subcutaneous injections of 100 μg of methylated bovine serum albumin (MBSA) (Sigma Chemical Co., St. Louis, Mo.) emulsified with Freund's complete adjuvant (FCA) into the inguinal region, the front footpad, and the base of the tail. FCA was prepared by adding heat-killed M. tuberculosis Aoyama B in FIA at a concentration of 2 mg/ml (15).
Footpad assays for DTH.
Eight days after immunization, hind footpads were challenged with 20 μl of TDM (1 mg/ml) in the form of w/o/w emulsion, w/o/w micelles alone, MBSA (1 mg/ml), or egg albumin (1 mg/ml) (grade V; Sigma Chemical Co.). Five mice were used for each group. Triplicate measurements of footpad thickness were performed with an engineer's micrometer (Mitsutoyo Co., Kanagawa, Japan) before and 24 h after the challenge (15). The difference between the measurements was calculated and expressed as the mean ± standard deviation (SD) in millimeters.
Induction of pulmonary granulomas.
Ten days after immunization, mice were injected intravenously with either 100 μg of TDM in 100 μl of w/o/w emulsion, 100 μl of w/o/w micelles alone, or 100 μg of MBSA in 100 μl of PBS. Unimmunized mice were similarly challenged. To exclude the possibility of boosting with repeated TDM exposures, we used two different sets of mice for DTH footpad assays and induction of lung granulomas throughout the study.
Determination of lung index.
To determine the activity of granulomatous inflammation, lung indices were used. Previous reports have indicated that a considerable proportion of the increase in lung weight as a consequence of granulomatous inflammation is due to an increase in cellularity in the organ (15, 30). The index was calculated as follows (6): lung index = lung weight (grams) × 100/body weight (grams).
Histology.
Lungs were fixed with 10% formalin for 5 days, dehydrated, and embedded in paraffin (15). Sections were stained with hematoxylin and eosin. For immunohistochemical analysis (13), lungs were fixed in a periodate–lysine–3% paraformaldehyde solution overnight at 4°C and then frozen in liquid nitrogen. Sections were made using a CM3000 cryostat (Leica Instruments GmbH, Nussloch, Germany) and immediately air dried. To block endogenous peroxidase activity, the sections were incubated with 0.3% hydrogen peroxide in methanol for 20 min at room temperature. After washing with PBS, the sections were incubated with normal rat serum for 20 min at room temperature. Subsequently, sections were incubated with diluted rat anti-mouse CD4 monoclonal antibody (1:300) (RM4-5; Pharmingen, San Diego, Calif.) overnight at 4°C. After being washed with PBS, they were treated with diluted biotinylated anti-rat immunoglobulin G antibody (1:400) (Dako, Copenhagen, Denmark) for 60 min at room temperature, followed by incubation with avidin-biotin-peroxidase complex (Vectastain kit; Vector Laboratories, Burlingame, Calif.). Reaction products were visualized after incubation with 0.025% diaminobenzidine and 0.003% hydrogen peroxide.
Enumeration of infiltrating cells.
The number of CD4+ cells in immunostained sections of the lung was counted 3 days after the challenge with TDM or w/o/w micelles in unimmunized and immunized mice. Three mice were used for each group, and 10 microscopic fields at a magnification of ×200 were randomly selected. The average number per microscopic field (0.34 mm2) was then calculated.
Protein expression of chemokines and cytokines in the lung.
Aqueous extracts of granulomatous lungs were prepared by a method described previously (15, 30). Briefly, lungs were inflated with 1 ml of PBS and homogenized in 1 ml of saline by using a Polytron (Brinkmann Instruments, Westbury, N.Y.) for 30 s. Homogenized tissues were then centrifuged in a refrigerated unit at 5,000 × g for 30 min, and the tissue pellet was discarded. Protein concentrations were determined with a protein assay kit (Bio-Rad). Aqueous lung extracts contained 2 to 4 mg of protein per ml of PBS (15, 30). The contents of cytokines and chemokines were measured by commercially available enzyme immunoassay (EIA) kits for murine interleukin-1β (IL-1β), IL-4, IL-12, tumor necrosis factor alpha (TNF-α), gamma interferon (IFN-γ), macrophage inflammatory protein-1α (MIP-1α), and monocyte chemotactic protein-1 (MCP-1) (Genzyme, Minneapolis, Minn.) and expressed as the amount per milligram of protein in the extract. The sensitivity was <3.0 pg/ml for IL-1β, <2.0 pg/ml for IL-4, <2.5 pg/ml for IL-12, <5.1 pg/ml for TNF-α, <2.0 pg/ml for IFN-γ, <1.5 pg/ml for MIP-1α, and <2.0 pg/ml for MCP-1, according to the manufacturer's instructions. The EIA was conducted in duplicate.
Statistical analyses.
Data were analyzed with a Power Macintosh G3 using StatView 5.0 (SAS Institute Inc., Cary, N.C.) and expressed as the mean ± SD. Data that appeared to be statistically significant were compared by an analysis of variance for comparing the means of multiple groups and were considered significant if P values were less than 0.05.
RESULTS
Induction of footpad DTH responses by TDM.
We found delayed-type footpad responses to specific antigens (TDM and MBSA) in immunized euthymic mice (Table 1). Although footpad responses induced by TDM, but not by MBSA, were seen in unimmunized euthymic mice and in athymic nude mice regardless of immunization, these responses were significantly lower than those in immunized euthymic mice. Regardless of immunization, w/o/w vehicles alone induced mild swelling of footpads. An irrelevant antigen, egg albumin, could not elicit the response. TDM could elicit both antigen-specific and nonspecific responses in euthymic mice, although athymic mice showed only nonspecific inflammatory responses.
TABLE 1.
Footpad responses to antigens in euthymic and athymic micea
| Antigen | Difference in footpad thicknessb
|
|||
|---|---|---|---|---|
| Euthymic mice
|
Athymic mice
|
|||
| Unimmunized | Immunized | Unimmunized | Immunized | |
| TDM | 1.28 ± 0.32c | 1.88 ± 0.46cd | 1.10 ± 0.23c | 1.07 ± 0.15c |
| w/o/w | 0.46 ± 0.15 | 0.44 ± 0.07 | 0.29 ± 0.06 | 0.36 ± 0.06 |
| MBSA | 0.05 ± 0.02 | 0.43 ± 0.03d | 0.08 ± 0.10 | 0.16 ± 0.10 |
| Egg albumin | 0.19 ± 0.11 | 0.07 ± 0.07 | 0.11 ± 0.09 | 0.09 ± 0.05 |
BALB/c mice were immunized with MBSA emulsified in Freund's complete adjuvant. Eight days after immunization, mice were challenged with each antigen. The thickness of footpads was measured immediately before and 24 h after antigenic challenge. Differences were calculated.
Data represent the mean (millimeters) ± SD from three separate experiments for five mice each condition.
Statistically significant (P < 0.01) compared to mice challenged with w/o/w.
Statistically significant (P < 0.01) compared to athymic mice regardless of immunization and to unimmunized euthymic mice.
Granulomatous inflammation of the lung.
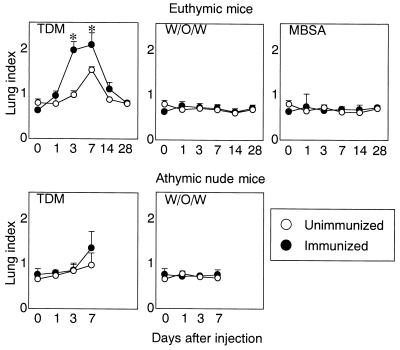
It has been demonstrated that intravenously injected TDM micelles are trapped in alveolar vessels and induce granulomatous inflammation of the lung (6, 8). A significant increase of lung indices was found in immunized euthymic mice 3 to 7 days after the challenge with TDM compared with unimmunized mice (Fig. 1). Regardless of immunization, athymic nude mice showed a moderate increase in the index. The kinetics and intensity were similar to those of unimmunized mice. The level showed a marked increase within 3 days, reached the maximum by day 7, and gradually declined thereafter. No significant increase was found in euthymic and athymic mice challenged with w/o/w alone or MBSA regardless of immunization.
FIG. 1.
Lung indices of unimmunized and immunized mice. The results are expressed as the mean ± SD obtained from three to six mice for each condition. The asterisks indicate a P value of <0.01, compared to unimmunized mice.
Histopathologic features of the lung.
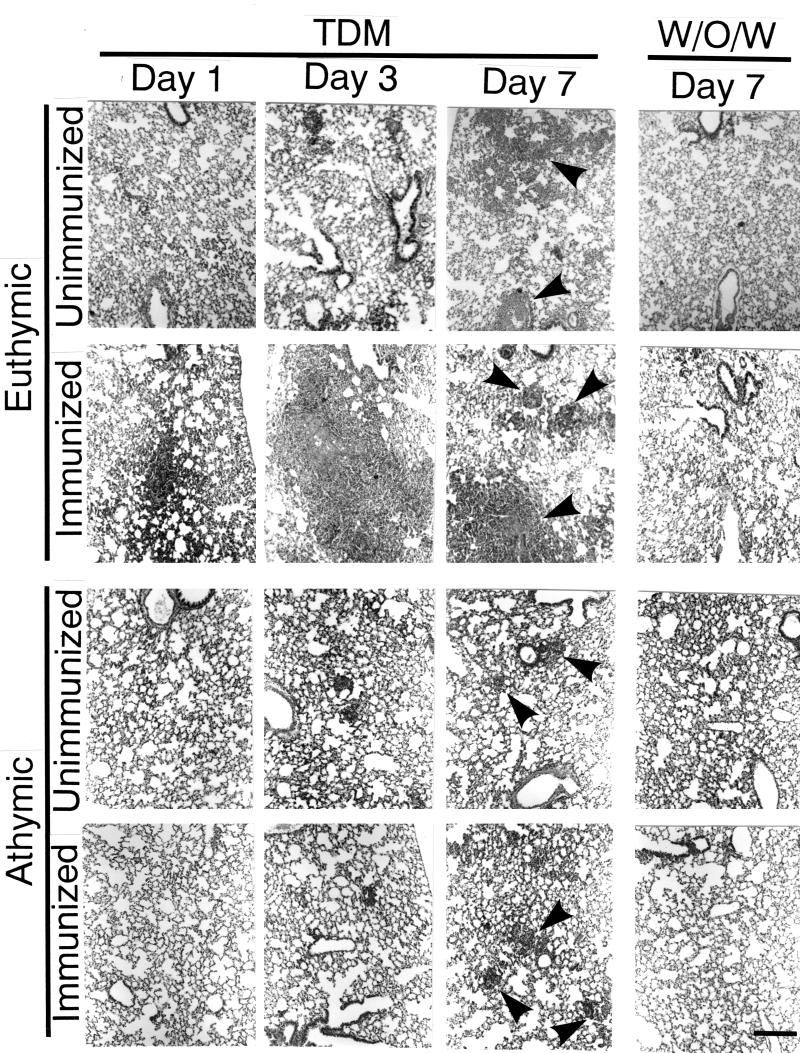
In unimmunized euthymic mice challenged with TDM, there was mild, diffuse and inflammatory cell infiltration in alveolar and perivascular areas at early stages (1 to 3 days) (Fig. 2). The infiltrate was composed primarily of macrophages, lymphocytes, and scattered neutrophils. At 1 and 2 weeks after the challenge, such mice showed randomly distributed, organized granulomas composed of macrophages and lymphocytes. The lesions subsided thereafter. Unimmunized mice challenged with w/o/w micelles exhibited no significant lesions. By contrast, accelerated and augmented lesions, including cell infiltration and granuloma formation in the alveolar and perivascular regions, were found in immunized euthymic mice challenged with TDM but not in those challenged with w/o/w micelles. The lesions in immunized mice were composed of macrophages, lymphocytes, and a few neutrophils. Athymic nude mice challenged with TDM, regardless of prior immunization, developed lesions that were similar to those in unimmunized mice.
FIG. 2.
Histopathologic features of the lung. In unimmunized euthymic mice challenged with TDM, mild, diffuse and inflammatory cell infiltration in alveolar and perivascular areas at early stages (1 to 3 days). The infiltrate was composed primarily of macrophages, lymphocytes, and scattered neutrophils. One week after the challenge, such mice showed organized granulomas (arrowheads) composed of macrophages and lymphocytes. The lesions subsided thereafter. Unimmunized mice challenged with w/o/w micelles exhibited no significant lesions. By contrast, accelerated and augmented granulomatous lesions were found in immunized euthymic mice challenged with TDM but not in those challenged with w/o/w micelles. The lesions in immunized mice were composed of macrophages, lymphocytes, and a few neutrophils. In athymic nude mice, slight granuloma formation was seen at day 7 regardless of preimmunization. Hematoxylin and eosin staining was used. Bar, 200 μm.
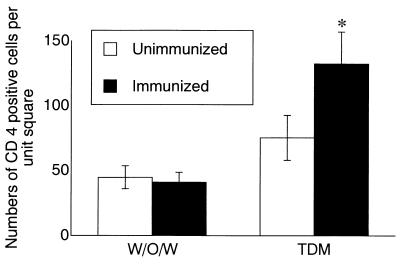
Immunohistochemical studies demonstrated that CD4+ cells (Th cells) were abundant in granulomas and perivascular cell infiltration of unimmunized and immunized mice 3 days after TDM challenge, although the number of CD4+ cells was significantly increased in immunized mice compared to unimmunized mice (Fig. 3). Regardless of preimmunization, the number did not differ in mice challenged with w/o/w alone.
FIG. 3.
Immunocytochemical analyses of CD4-positive cells in the lesion. The number of CD4+ cells per unit square (0.34 mm2) in the lungs of unimmunized and immunized mice was calculated 3 days after the challenge with either TDM or w/o/w micelles alone. Ten microscopic fields were examined at a magnification of ×200. Data represent the mean ± SD from three mice. The asterisk indicates a P value of <0.05 compared to unimmunized mice.
Protein expression of chemokines and cytokines.
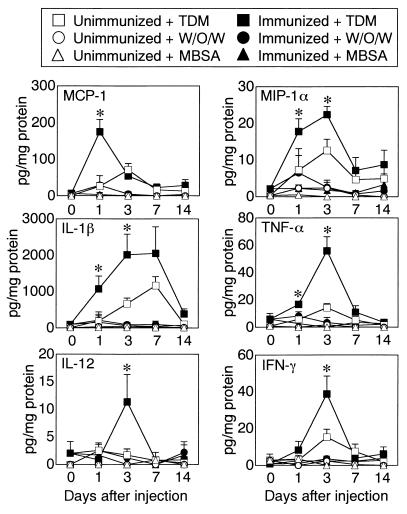
We next studied the profile of cytokines and chemokines in the lung (Fig. 4), because a prominent accumulation of mononuclear cells, such as granulomatous inflammation, was seen. A significant amount of CC chemokines, including MCP-1 and MIP-1α, was detected in lung extracts from immunized mice challenged with TDM compared to unimmunized mice. In immunized and unimmunized mice, the peak activity was present 1 to 3 days after the challenge and declined thereafter. Lung extracts from mice challenged with w/o/w micelles alone contained a smaller amount of CC chemokines regardless of preimmunization. Proinflammatory cytokines such as IL-1β and TNF-α were prominent in immunized mice challenged with TDM. In contrast to the case for immunized mice, the level of proinflammatory cytokines in the extracts from unimmunized mice challenged with w/o/w micelles alone was not only considerably less but also was maintained for a shorter time. A considerable amount of Th1- and IFN-γ-inducing immunoregulatory cytokines, IL-12 and IFN-γ, was detected in the extracts prepared from immunized mice challenged with TDM, but there was much less from immunized mice challenged with w/o/w micelles alone and unimmunized mice challenged with TDM. The peak was reached by day 3, and the level declined thereafter. Regardless of prior immunization, IL-4 was not found in the extracts from mice challenged with TDM or w/o/w micelles alone (data not shown). To examine the effects of the procedure on enzymatic degradation and half-lives of cytokines, we measured cytokine levels in the extracts prepared from either immunized or unimmunized mice in the presence of standard recombinant cytokines, including chemokines. The results showed that the levels of all cytokines tested in our study were not affected by procedures of immunization and extract preparation (data not shown).
FIG. 4.
Protein expression of chemokines and cytokines in the lung. Results represent the mean ± SD from three (unimmunized) or six (immunized) mice challenged with TDM, w/o/w micelles, or MBSA. The asterisks indicate a P value of <0.05 compared to unimmunized mice. The EIA was conducted in duplicate.
DISCUSSION
In the present study, we have demonstrated for the first time that TDM derived from virulent M. tuberculosis can induce both foreign-body-type (nonimmune) and hypersensitivity-type (immune) granulomas by acting as a nonspecific irritant and a T-cell-dependent antigen. This result implies that both nonimmune and immune mechanisms participate in granulomatous inflammation induced by mycobacterial infection.
Granulomatous inflammation is manifested in chronic inflammatory diseases that often result in tissue destruction and end-stage fibrosis. The common histologic feature of granulomatous inflammation, i.e., infiltrating mononuclear leukocytes and their derivatives (epithelioid cells and multinucleated giant cells), is observed in a variety of granulomatous diseases caused by infectious agents (tuberculosis, leprosy, schistosomiasis, leishmaniasis, and histoplasmosis), non-infectious agents (silicosis and berylliosis), or unknown agents (sarcoidosis, Crohn's disease, and Wegener's granulomatosis) (20). The lesion is usually surrounded by a collar of lymphocytes and occasionally eosinophils. Granulomatous inflammation can be broadly classified as either a hypersensitivity (immunologic, T-cell-dependent) type or a foreign-body (nonimmunologic, T-cell-independent) type (19, 20). The classification is based on the participation of antigen-specific T lymphocytes in the lesions. The granulomas induced by M. bovis bacillus Calmette-Guérin (BCG) in mice were developed regardless of preimmunization with M. tuberculosis or BCG, although augmented lesions were observed in preimmunized mice (15, 30).
Disease progression to active tuberculosis is dependent on an interplay between bacterial and host factors. Mycobacteria are rich in lipids. These include mycolic acids, which are long-chain fatty acids (C78 to C90) (2). The pathogenicity of M. tuberculosis is related to its ability to induce cell-mediated immunity and DTH. (5). Strains of M. tuberculosis have cord factor (TDM), a surface glycolipid. A variety of foreign lipids and glycolipids, including several found in the cell walls and cell membranes of pathogenic mycobacteria, are recognized by CD1-restricted T cells in humans, and the CD1 system provides a novel mechanism for host responses to mycobacterial infection, such as the development of cell-mediated immunity (21, 27). To confirm the contribution of T cells in TDM-induced hypersensitivity, it is necessary to demonstrate TDM-specific T cells, although in the present study this was not done. This approach will also explore the possibility that the putative T-cell contribution might arise from contamination with minute amounts of protein in the TDM preparation. The immune response to microbial pathogens relies on both innate and adaptive components. The role of CD1, CD14, and Toll-like receptors (1, 26) in host responses to TDM is intriguing, although this is not addressed in our present study. Future studies are needed to clarify the issue.
In the present study we have demonstrated that TDM can induce hypersensitivity granulomas in euthymic mice immunized with FCA and also can elicit foreign-body lesions in unimmunized euthymic and athymic nude mice. This contention is supported by the result that footpad DTH responses to TDM were augmented in immunized mice compared to unimmunized euthymic and athymic nude mice. In addition, TDM itself acts as a nonspecific inflammatory stimulus, because similar and moderate footpad swelling was observed in euthymic and athymic mice. The precise mechanism of the delayed granulomatous response in athymic nude mice remains unknown, however, mature T lymphocytes may be involved in the response, because athymic mice lack them. Collectively, our results imply that mechanisms of granulomatous inflammation in tuberculosis are composed of both foreign-body and hypersensitivity types.
Granuloma formation is the expression of a series of complex inflammatory events. Evidence suggests that proinflammatory cytokines play important roles in the initiation and maintenance of granuloma formation (9, 10, 31). Histopathologically, the bulk of both hypersensitive and foreign-body granulomas are composed of macrophages and their derivatives. In most tissues the presence of inflammatory macrophages results from the recruitment of peripheral blood monocytes. Besides granulomas, our studies showed prominent perivascular infiltration of mononuclear cells around the lesion. This may be attributed to recruitment of blood and tissue mononuclear cells through local expression of CC chemokines for monocytes and lymphocytes, such as MCP-1 and MIP-1α (4, 7). MIP-1α is known to be an efficient chemoattractant for Th1 cells, although MCP-1 exerts chemotactic activity for both Th1 and Th2 cells (32). This may lead to expression of cell-mediated immunity in immunized mice challenged with TDM, because the expression of MIP-1α (days 1 to 3) persisted longer than that of MCP-1 (day 1) in the lesion.
The very early expression of CC chemokines was detected within 1 to 3 days after the challenge with TDM. Human blood monocytes preferentially produce CC chemokines in response to M. tuberculosis (11, 12). Both MCP-1 and MIP-1α may be pivotal in the initial recruitment of monocytes to sites of subsequent granuloma formation. It has been demonstrated that proinflammatory cytokines such as IL-1 and TNF-α participate in granulomatous inflammation (9, 10, 19, 20, 31), although they lack direct chemotactic activity for monocytes (22). However, chemokines are inducible by stimulating macrophages/monocytes with IL-1 and TNF-α (23). The cytokine network may form a powerful amplification circuit of granulomatous inflammation.
IL-12, a cytokine produced mainly by macrophages in response to mycobacteria, augments cytotoxicity and cytokine production by T cells and NK cells and initiates development of CD4+ Th1 cells (33). CD4+ Th1 and NK cells stimulated with IL-12 produce and secrete IFN-γ, which activates macrophages to inhibit or kill intracellular mycobacteria via reactive nitrogen intermediates (3). Thus, macrophages accumulate at the site of microbial growth and become activated through the CD4+ Th1 cell-cytokine-macrophage axis (14). IL-12 induces the differentiation of Th1 cells from uncommitted T cells and, consequently, initiates cell-mediated immunity, which plays a protective role in infections with mycobacteria. This cytokine represents an important regulatory bridge between innate resistance and adaptive immunity.
TDM can stimulate production of IL-12 from mouse macrophages (24), and IL-12 is found in the active lesion elicited by experimental mycobacterial infection in mice, including that with Mycobacterium avium (17, 18) and Mycobacterium leprae (16). In immunized mice challenged with TDM, lesional IL-12 and IFN-γ reached peak levels concomitantly 3 days after challenge, whereas unimmunized mice express less of them. The temporal profiles of IL-12 and IFN-γ may indicate their close functional relationship. Our data that mice bearing granulomas showed significant expression of IL-12 and IFN-γ but not IL-4 suggest that TDM challenge may favor dominance of the Th1 response via through the local cytokine profile. Indeed, such mice did not produce anti-TDM antibodies that might reflect a Th2 response (data not shown).
Infection with mycobacteria results in either host defense or disease expression such as granulomatous inflammation (5, 14, 19). Our present study suggests that TDM, a surface glycolipid derived from the cell walls of virulent strains of M. tuberculosis, plays a critical role in the process. Taken together with the previous reports that mycobacterial TDM can induce apoptosis (6) and angiogenesis (29), this study indicates that TDM is a pleiotropic molecule against the host and participates in the pathogenesis of tuberculosis.
ACKNOWLEDGMENTS
This work was supported by grants from the Ministry of Health and Welfare (Research on Emerging and Re-emerging Infectious Diseases, Health Sciences Research Grants) and the U.S.-Japan Cooperative Medical Science Program against Tuberculosis and Leprosy.
REFERENCES
- 1.Aderem A, Ulevitch R J. Toll-like receptors in the induction of the innate immune response. Nature. 2000;406:782–787. doi: 10.1038/35021228. [DOI] [PubMed] [Google Scholar]
- 2.Besra G S, Chatterjee D. Lipids and carbohydrates of Mycobacterium tuberculosis. In: Bloom B R, editor. Tuberculosis: pathogenesis, protection, and control. Washington, D.C.: ASM Press; 1994. pp. 285–306. [Google Scholar]
- 3.Chan J, Xing Y, Magliozzo R S, Bloom B R. Killing of virulent Mycobacterium tuberculosis by reactive nitrogen intermediates produced by activated murine macrophages. J Exp Med. 1992;175:1111–1122. doi: 10.1084/jem.175.4.1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chensue S W, Warmington K S, Ruth J H, Sanghi P S, Lincoln P, L. K S. Role of monocyte chemoattractant protein-1 (MCP-1) in Th1 (mycobacterial) and Th2 (schistosomal) antigen-induced granuloma formation: relationship to local inflammation, Th cell expression, and IL-12 production. J Immunol. 1996;157:4602–4607. [PubMed] [Google Scholar]
- 5.Dannenberg A M., Jr Delayed-type hypersensitivity and cell-mediated immunity in the pathogenesis of tuberculosis. Immunol Today. 1991;12:228–233. doi: 10.1016/0167-5699(91)90035-R. [DOI] [PubMed] [Google Scholar]
- 6.Hamasaki N, Isowa K I, Kamada K, Terano Y, Matsumoto T, Arakawa T, Kobayashi K, Yano I. In vivo administration of mycobacterial cord factor (trehalose 6,6′-dimycolate) can induce lung and liver granulomas and thymic atrophy in rabbits. Infect Immun. 2000;68:3704–3709. doi: 10.1128/iai.68.6.3704-3709.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hogaboam C M, Bone-Larson C L, Lipinski S, Lukacs N W, Chensue S W, Strieter R M, Kunkel S L. Differential monocyte chemoattractant protein-1 and chemokine receptor 2 expression by murine lung fibroblasts derived from Th1- and Th2-type pulmonary granuloma models. J Immunol. 1999;163:2193–2201. [PubMed] [Google Scholar]
- 8.Kaneda K, Sumi Y, Kurano F, Kato Y, Yano I. Granuloma formation and hemopoiesis induced by C36–48-mycolic acid-containing glycolipids from Nocardia rubra. Infect Immun. 1986;54:869–875. doi: 10.1128/iai.54.3.869-875.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kasahara K, Kobayashi K, Shikama Y, Yoneya I, Kaga S, Hashimoto M, Odagiri T, Soejima K, Ide H, Takahashi T, et al. The role of monokines in granuloma formation in mice: the ability of interleukin 1 and tumor necrosis factor-α to induce lung granulomas. Clin Immunol Immunopathol. 1989;51:419–425. doi: 10.1016/0090-1229(89)90040-8. [DOI] [PubMed] [Google Scholar]
- 10.Kasahara K, Kobayashi K, Shikama Y, Yoneya I, Soezima K, Ide H, Takahashi T. Direct evidence for granuloma-inducing activity of interleukin-1. Induction of experimental pulmonary granuloma formation in mice by interleukin-1-coupled beads. Am J Pathol. 1988;130:629–638. [PMC free article] [PubMed] [Google Scholar]
- 11.Kasahara K, Sato I, Ogura K, Takeuchi H, Kobayashi K, Adachi M. Expression of chemokines and induction of rapid cell death in human blood neutrophils by Mycobacterium tuberculosis. J Infect Dis. 1998;178:127–137. doi: 10.1086/515585. [DOI] [PubMed] [Google Scholar]
- 12.Kasahara K, Tobe T, Tomita M, Mukaida N, Shao-Bu S, Matsushima K, Yoshida T, Sugihara S, Kobayashi K. Selective expression of monocyte chemotactic and activating factor/monocyte chemoattractant protein 1 in human blood monocytes by Mycobacterium tuberculosis. J Infect Dis. 1994;170:1238–1247. doi: 10.1093/infdis/170.5.1238. [DOI] [PubMed] [Google Scholar]
- 13.Kasama T, Yamazaki J, Hanaoka R, Miwa Y, Hatano Y, Kobayashi K, Negishi M, Ide H, Adachi M. Biphasic regulation of the development of murine type II collagen-induced arthritis by interleukin-12: possible involvement of endogenous interleukin-10 and tumor necrosis factor α. Arthritis Rheum. 1999;42:100–109. doi: 10.1002/1529-0131(199901)42:1<100::AID-ANR13>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 14.Kaufmann S H E. Immunity to intracellular microbial pathogens. Immunol Today. 1995;16:338–343. doi: 10.1016/0167-5699(95)80151-0. [DOI] [PubMed] [Google Scholar]
- 15.Kobayashi K, Allred C, Castriotta R, Yoshida T. Strain variation of bacillus Calmette-Guerin-induced pulmonary granuloma formation is correlated with anergy and the local production of migration inhibition factor and interleukin 1. Am J Pathol. 1985;119:223–235. [PMC free article] [PubMed] [Google Scholar]
- 16.Kobayashi K, Kai M, Gidoh M, Nakata N, Endoh M, Singh R P, Kasama T, Saito H. The possible role of interleukin (IL)-12 and interferon-γ-inducing factor/IL-18 in protection against experimental Mycobacterium leprae infection in mice. Clin Immunol Immunopathol. 1998;88:226–231. doi: 10.1006/clin.1998.4533. [DOI] [PubMed] [Google Scholar]
- 17.Kobayashi K, Nakata N, Kai M, Kasama T, Hanyuda Y, Hatano Y. Decreased expression of cytokines that induce type 1 helper T cell/interferon-γ responses in genetically susceptible mice infected with Mycobacterium avium. Clin Immunol Immunopathol. 1997;85:112–116. doi: 10.1006/clin.1997.4421. [DOI] [PubMed] [Google Scholar]
- 18.Kobayashi K, Yamazaki J, Kasama T, Katsura T, Kasahara K, Wolf S F, Shimamura T. Interleukin (IL)-12 deficiency in susceptible mice infected with Mycobacterium avium and amelioration of established infection by IL-12 replacement therapy. J Infect Dis. 1996;174:564–573. doi: 10.1093/infdis/174.3.564. [DOI] [PubMed] [Google Scholar]
- 19.Kobayashi K, Yoshida T. The immunopathogenesis of granulomatous inflammation induced by Mycobacterium tuberculosis. Methods. 1996;9:204–214. doi: 10.1006/meth.1996.0027. [DOI] [PubMed] [Google Scholar]
- 20.Kunkel S L, Chensue S W, Strieter R M, Lynch J P, Remick D G. Cellular and molecular aspects of granulomatous inflammation. Am J Respir Cell Mol Biol. 1989;1:439–448. doi: 10.1165/ajrcmb/1.6.439. [DOI] [PubMed] [Google Scholar]
- 21.Moody D B, Reinhold B B, Guy M R, Beckman E M, Frederique D E, Furlong S T, Ye S, Reinhold V N, Sieling P A, Modlin R L, Besra G S, Porcelli S A. Structural requirements for glycolipid antigen recognition by CD1b-restricted T cells. Science. 1997;278:283–286. doi: 10.1126/science.278.5336.283. [DOI] [PubMed] [Google Scholar]
- 22.Oppenheim J J, Neta R. Pathophysiological roles of cytokines in development, immunity, and inflammation. FASEB J. 1994;8:158–162. doi: 10.1096/fasebj.8.2.8119486. [DOI] [PubMed] [Google Scholar]
- 23.Oppenheim J J, Zachariae C O C, Mukaida N, Matsushima K. Properties of the novel proinflammatory supergene “intercrine” cytokine family. Annu Rev Immunol. 1991;9:617–648. doi: 10.1146/annurev.iy.09.040191.003153. [DOI] [PubMed] [Google Scholar]
- 24.Oswald I P, Dozois C M, Petit J F, Lemaire G. Interleukin-12 synthesis is a required step in trehalose dimycolate-induced activation of mouse peritoneal macrophages. Infect Immun. 1997;65:1364–1369. doi: 10.1128/iai.65.4.1364-1369.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ozeki Y, Kaneda K, Fujiwara N, Morimoto M, Oka S, Yano I. In vivo induction of apoptosis in the thymus by administration of mycobacterial cord factor (trehalose 6,6′-dimycolate) Infect Immun. 1997;65:1793–1799. doi: 10.1128/iai.65.5.1793-1799.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Park S H, Bendelac A. CD1-restricted T-cell responses and microbial infection. Nature. 2000;406:788–792. doi: 10.1038/35021233. [DOI] [PubMed] [Google Scholar]
- 27.Porcelli S A, Modlin R L. The CD1 system: antigen-presenting molecules for T cell recognition of lipids and glycolipids. Annu Rev Immunol. 1999;17:297–329. doi: 10.1146/annurev.immunol.17.1.297. [DOI] [PubMed] [Google Scholar]
- 28.Saita N, Fujiwara N, Yano I, Soejima K, Kobayashi K. Trehalose 6,6′-dimycolate (cord factor) of Mycobacterium tuberculosis induces corneal angiogenesis in rats. Infect Immun. 2000;68:5991–5997. doi: 10.1128/iai.68.10.5991-5997.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sakaguchi I, Ikeda N, Nakayama M, Kato Y, Yano I, Kaneda K. Trehalose 6,6′-dimycolate (cord factor) enhances neovascularization through vascular endothelial growth factor production by neutrophils and macrophages. Infect Immun. 2000;68:2043–2052. doi: 10.1128/iai.68.4.2043-2052.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sato I Y, Kobayashi K, Kasama T, Kaga S, Kasahara K, Kanemitsu H, Nakatani K, Takahashi T, Nakamura R M, Skamene E, Yoshida T. Regulation of Mycobacterium bovis BCG and foreign body granulomas in mice by the Bcg gene. Infect Immun. 1990;58:1210–1216. doi: 10.1128/iai.58.5.1210-1216.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shikama Y, Kobayashi K, Kasahara K, Kaga S, Hashimoto M, Yoneya I, Hosoda S, Soejima K, Ide H, Takahashi T. Granuloma formation by artificial microparticles in vitro. Macrophages and monokines play a critical role in granuloma formation. Am J Pathol. 1989;134:1189–1199. [PMC free article] [PubMed] [Google Scholar]
- 32.Siveke J T, Hamann A. T helper 1 and T helper 2 cells respond differentially to chemokines. J Immunol. 1998;160:550–554. [PubMed] [Google Scholar]
- 33.Trinchieri G. Interleukin-12: a proinflammatory cytokine with immunoregulatory functions that bridge innate resistance and antigen-specific adaptive immunity. Annu Rev Immunol. 1995;13:251–276. doi: 10.1146/annurev.iy.13.040195.001343. [DOI] [PubMed] [Google Scholar]