Abstract
In this study, we report the implementation of a comprehensive wastewater surveillance testing program at a university campus in Singapore to identify Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) infected individuals and the usage of pharmaceuticals and personal care products (PPCPs) as well as other emerging contaminants (ECs). This unique co-monitoring program simultaneously measured SARS-CoV-2 with chemical markers/contaminants as the COVID-19 situation evolved from pandemic to endemic stages, following a nationwide mass vaccination drive. SARS-CoV-2 RNA concentrations in wastewater from campus dormitories were measured using real-time reverse transcription-polymerase chain reaction (RT-qPCR) and corroborated with the number of symptomatic COVID-19 cases confirmed with the antigen rapid test (ART). Consistent results were observed where the concentrations of SARS-CoV-2 RNA detected in wastewater increased proportionately with the number of COVID-19 infected individuals residing on campus. Similarly, a wide range of ECs, including disinfectants and antibiotics, were detected through sensitive liquid chromatography with tandem mass spectrometry (LC-MS/MS) techniques to establish PPCPs consumption patterns during various stages of the COVID-19 pandemic in Singapore. Statistical correlation of SARS-CoV-2 RNA was observed with few ECs belonging to disinfectants, PCPs and antibiotics. A high concentration of disinfectants and subsequent positive correlation with the number of reported cases on the university campus indicates that disinfectants could serve as a chemical marker during such unprecedented times.
Keywords: SARS-CoV-2, Wastewater-based epidemiology, University campus, Emerging contaminants, Disinfectants
Graphical Abstract
1. Introduction
Wastewater testing for biological and chemical markers, i.e., wastewater based epidemiology (WBE), has been used to monitor health threats due to viral outbreaks, antimicrobial resistance and illicit drug use in populations [2], [1], [3], [4]. Since the beginning of the COVID-19 pandemic, scientific groups around the world, in collaboration with public health authorities, have used sewage testing for the monitoring of Severe Acute Respiratory Syndrome Coronavirus- 2 (SARS-CoV-2) as a tool to study infection and transmission dynamics in entire communities (“COVID-19 WBE Collaborative Dashboard,” 2020; [6], [5].
SARS-CoV-2 is an enveloped positive-sense single-stranded RNA virus (+ssRNA) belonging to the Betacoronavirus genus (family Coronaviridae), and its RNA has been detected in sewage samples through urinary and faecal matter excretion from symptomatic, asymptomatic and pre-symptomatic individuals [7], [8], [9], [10], [11], [12]. Although health professionals worldwide have tirelessly worked to diagnose infections, clinical testing is generally limited to symptomatic individuals and case ascertainment rate is influenced by surveillance system and health care seeking behavior or the community [13]. Based on different seroprevalence studies, the symptom-based PCR-testing strategy missed 62 % of COVID-19 diagnoses, and ∼36 % of individuals with SARS-CoV-2 infection were asymptomatic [14]. Therefore, monitoring SARS-CoV-2 in sewage provides an indication of SARS-CoV-2 transmission that is independent of prevailing clinical test protocols or clinical presentation [15].
During the COVID-19 pandemic, several attempts have been made where WBE was helpful for early case detection in passenger flights [16], cities [17], apartments [18] and schools [19]. Such surveillance can save manpower involved in door-to-door COVID-19 testing, costs in the purchase of reagents and, most importantly, time consumed and logistic costs in mass-scale testing regimes. WBE holds promise for the surveillance of university campuses, where a large numbers of students reside in close quarters and transmission could potentially be very rapid. Additionally, on-campus quarantine and stay-home practices on university campuses would increase the concentration of SARS-CoV-2 in campus sewage [20], [21], [22], [5]. Initial wastewater surveillance studies were focused mainly on reporting the presence of SARS-CoV-2 before diagnosis and reports of clinical cases, so that WBE could be used for early detection tool of any spread of infection [18]. With the help of WBE, asymptomatic individuals could be detected in a campus dorm in the University of North Carolina at Charlotte, USA, with a capacity of 150–200 residents [20]. Results obtained from WBE were also used to develop an automated wastewater notification system and corresponding alert for building residents at the University of California (UC) San Diego to take precautionary measures [23]. As the pandemic evolves to an endemic phase worldwide, the application of WBE has also changed. Bivins and Bibby recently studied the correlation of SARS-CoV-2 RNA with increased mass vaccination and observed a corresponding decrease in SARS-CoV-2 RNA in wastewater samples from a university campus in Notre Dame, USA [5]. However, studies have generally been limited to date.
Singapore reported its first confirmed case of COVID-19 on 23 January 2020, which quickly led to the implementation of risk mitigation measures. The country’s response to the pandemic was focused on rapid detection, active case finding, containment of infection and suppression of community transmission chains. Wastewater surveillance testing (WST) was also used early in the pandemic as a complementary tool to clinical testing. Singapore commenced WST in February 2020 and progressively rolled out a surveillance programme comprising a network of more than 500 surveillance sites. The country-wide testing points include water reclamation plants, regional residential hubs, high-density living premises types such as student dormitories, among others. WST supported the management of COVID-19 through several waves of transmission. In mid-April 2020 when COVID-19 cases were reported among migrant workers dormitories [24], WST was deployed to facilitate early case detection. Combined with good clinical surveillance and the timely implementation of infection control and prevention measures, the wave was effectively contained and did not spread further. Similarly, WST also supported the monitoring of student dormitories and residential blocks at risk of COVID-19 spread when the population was still being vaccinated and active case-finding was necessary. After majority of the population was vaccinated (Ministry of Health, Singapore), control measures were gradually eased with a shift towards living with COVID-19. In tandem with the overall management of COVID-19, WST also shifted from case detection to situational monitoring, providing an independent indicator of transmission rates, especially when new and more transmissible delta and omicron variants emerged in Oct/Nov 2021 and Jan/Feb 2022, respectively [25], [26].
The impact associated with unprecedented lockdowns from the virus outbreak may, from time to time, put a large sector of the population under stress, potentially leading to higher consumption of several antidepressants during this pandemic [27]. In some cases, the lack of knowledge has prompted people to take several antibiotics [28], antivirals [29] and nonsteroidal anti-inflammatory drugs (NSAIDs) [30] as a precautionary measure. After consumption, these drugs may undergo a series of biochemical transformations in the human body and are finally discharged both in conjugated and unconjugated forms with the potential to cause ecotoxicity effects [31], [32], [33]. Many of these pharmaceuticals and personal care products (PPCPs) are known to produce adverse secondary effects and thus, could pose chronic impacts to aquatic biota [34], [35], [36]. Previous studies have reported selected emerging contaminants (ECs) such as caffeine and N,N-diethyl-3-methylbenzamide (DEET) as chemical markers for anthropogenic pollution in surface water [37], [38]. WBE has also been used to track illicit drug consumption patterns in several countries to safeguard the public from drug abuse [39], [40], [41]. Recent studies reported increased consumption of recreational drugs during the pandemic [42], [43]. In addition to pharmaceuticals, there is a rapid surge in the use of disinfectants, with quaternary ammonium compounds (QACs) being the active ingredients [44]. Based on a recent list released by the U.S. EPA, out of 430 registered disinfectants, 216 contained QACs which may eventually enter wastewater treatment plants (WWTPs) through the sewerage network [44]. The National Environment Agency (NEA) in Singapore has also recommended using household disinfectants containing QACs such as benzalkonium chloride, and didecyldimethylammonium chloride as disinfectants against SARS-CoV-2 [45]. Another study reported increase in the QACs, surfactants and biocides load at WWTPs by 331 %, 196 % and 152 %, respectively, during the COVID-19 pandemic [46]. Thus, monitoring the presence of selected chemical markers and concomitant viral targets in wastewater could provide insights on community’s response to the pandemic as the situation evolves.
In this study, we applied WST across dormitories in a university campus that has a well-connected sewerage network. WST was used to track the spread of disease from Jan 2021 to Mar 2022, during which the nation went from strict movement control to the resumption of socioeconomic activities, as social and travel restrictions were progressively lifted following a successful vaccination program. This study was a part of a larger national effort to monitor SARS-CoV-2 in the community through WST. Here, we also sought to identify suitable chemical markers in wastewater that were associated with the virus during the pandemic and its transition to endemic phase. We report the combined occurrence of both SARS-CoV-2 with ECs in a university campus. By comparing the latter with pre-COVID measurements, we can establish health-related trends that reflect the evolving pandemic situation.
2. Methods
2.1. Sampling site, collection, and analysis approach
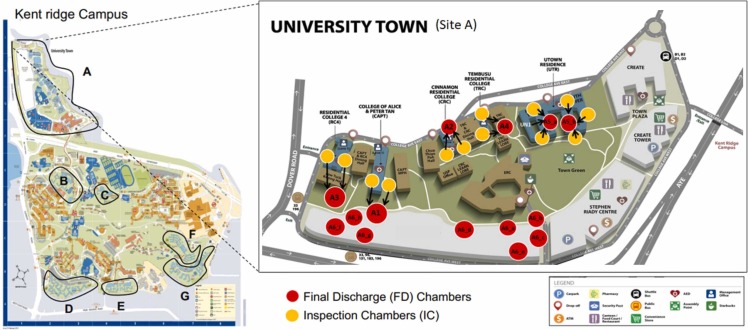
More than 30,000 students are currently enrolled at the National University of Singapore (NUS). The university offers 14 residential facilities, grouped into seven sites (A-G; Fig. 1) and divided into different blocks (Table S1). Although the main teaching mode was online during the pandemic, a considerable number of students were residing in various student dormitories, making the university campus a potential hot spot for disease transmission and consequent outbreaks. Thus, wastewater monitoring of SARS-CoV-2 was conducted in campus dormitories from January 2021 to March 2022, with the initial aim to trace the source of infection to prevent outbreaks, and subsequently, to track the prevalence of SARS-CoV-2 in the community as the situation evolved from pandemic to endemic stages. Usage patterns of PPCPs were also tracked to understand their correlation with SARS-CoV-2 viral loads.
Fig. 1.
Sampling sites for collection of wastewater samples. Left panel: Residential areas of Kent Ridge Campus of the National University of Singapore (NUS) monitored in this study. The hostel dormitories are divided in to 7 sampling sites, A-G. For details see Table S1. Right panel: Sampling distribution of Inspection Chambers (ICs) and Final Discharge (FD) Chambers are shown in detail for site A (University Town).
Systematic WST was implemented over 14 months, from January 2021 to March 2022. There were two main considerations for planning and executing the wastewater surveillance program in the campus dormitories – identification of appropriate (i) sampling points and (ii) sampling times. The sewage from each residential block/building drains into a manhole/inspection chamber (IC) and subsequently, sewage from these ICs drains into a final discharge (FD) chamber before entering the main sewer line (Fig. 1). 28 FD chambers were identified from 14 residential facilities and 110 auto-samplers were deployed at ICs and FD manholes throughout campus (Table S1). A two-tier “group testing strategy” was adopted to sample sewage from the university residential areas: Firstly, sewage samples collected from the FD chambers were tested for SARS-CoV-2. If these samples tested positive for SARS-CoV-2, the next step was to screen the corresponding upstream IC samples to help trace to the positive cases at the block level. A confirmed signal in the wastewater would result in PCR swab testing of the residents at the implicated block, followed by isolation and contact tracing of the residents of the affected dormitory.
A survey was initially conducted to identify the peak usage of toilets for campus residents and to determine the appropriate sampling time intervals. Two autosamplers namely Aquacell (W2 Industrial Services Pte Ltd, Singapore) and Maxx (Horiba Instruments Singapore Pte Ltd, Singapore) were used in this study. The samplers were programmed and 4 bottles were placed to collect 6-hour composite samples (i.e., 4 times a day) at 2 or 15 mins intervals from 05:00–11:00, 11:00–17:00, 17:00–23:00 and 23:00–05:00 on a daily basis, including weekends and public holidays. Later, as surveillance objectives shifted from case detection to situational monitoring (i.e. from November, 2021 onwards), the 6 h composite samples were changed to 12 h composites from 05:00–17:00 and 17:00–05:00. Of note, although the composite timing was changed, the sensitivity of detection was not compromised.
2.2. Sample preparation, virus concentration and RNA extraction
As described above, only wastewater samples from the FD manholes were tested daily, following the two-tier group sampling strategy. All wastewater samples were first heat-inactivated in a water bath (60 oC for 30 min) prior to laboratory analysis to ensure the safety of laboratory personnel [47]. The effectiveness of thermal treatment for virus inactivation was reported for SARS-CoV-2 without affecting RNA integrity [49], [48]. After heat inactivation, samples were then subjected to viral concentration. Briefly, an aliquot of 20 mL of heat-inactivated raw sewage was centrifuged at 4000 g for 30 min at 4 oC to remove larger solid particles/debris and bacteria. 15 mL of this supernatant was then concentrated using centrifugal ultrafiltration at 4000 g for 20 min at 4 oC (Amicon® Ultra-15, Merck) to produce virus concentrates. Viral RNA was extracted from 200 µl of the concentrated sample using the KingFisher Flex System and MagMAX Viral Pathogen II Kit (ThermoFisher, USA), according to manufacturer's instructions.
2.3. Molecular detection of SARS-CoV-2
One-step quantitative real-time reverse transcription-polymerase chain reaction (RT-qPCR) assay was used to quantify SARS-CoV-2 and Pepper mild mottle virus (PMMoV) gene markers in wastewater samples. All the primers and probes used in this study are listed in Table S2. The faecal indicator, PMMoV, was used as a process control to act as an indirect indicator of PCR inhibition. Each amplification reaction was carried out in a 20 µl final reaction volume containing 2.5 µl of template RNA, one step reaction mix (2x) and RT enzyme mix (2x) were used from Luna Universal Probe One-Step RT-qPCR Kit (NEB, USA) with either 0.5 μM (N1) or 0.9 μM (PMMoV) of each primer and either 0.25 μM (N1) or 0.2 μM (PMMoV) of the probe. The thermal cycling protocol consisted of reverse transcription for 10 min at 55 oC, initial denaturation at 95 oC for 1 min, and 45 amplification cycles of denaturation at 95 oC for 10 s and extension at 55 oC for 30 s. The SARS-CoV-2 RNA synthetic control (complete genome based, Twist Synthetic SARS-CoV-2 RNA Control 2, MN908947.3; Twist Bioscience, USA) and PMMoV RNA synthetic control (110 bp; Integrated DNA Technologies, Singapore) were used to generate their respective standard curves and used to convert cycle threshold (Ct) values into copies per well and further, into Copies/L of sewage. The PMMoV quantification was subsequently used to derive a normalised SARS-CoV-2 RNA concentration by accounting for differences in building occupancy and human waste input [50]. Limits of detections (LOD) were determined based on the lowest copy number of the synthetic RNA templates with detectable Ct values. The LOD for both gene targets was five gene copies per reaction.
2.4. Epidemiological data source and individual level testing
In early August 2021, NUS conducted a pilot-scale study to introduce self-testing Antigen Rapid Test (ART) kits for individual COVID-19 testing. These tests were subsequently made mandatory for monthly self-swab testing from 10 August 2021 onwards. However, when SARS-CoV-2 transmission rates increased during Delta variant wave, NUS implemented a mandatory COVID-19 testing policy once and twice a week for vaccinated and unvaccinated individuals, respectively. This lasted from about 4 October 2021–3 April 2022. Test results from ART kits were individually uploaded into the university’s COVID-19 health declaration database, and anonymized data from NUS regulatory authorities were made available for subsequent correlation analysis with the WBE results on SARS-CoV-2 and emerging contaminants.
2.5. Sample preparation and target analysis of emerging contaminants
A portion of the wastewater samples from the FDs collected for SARS-CoV-2 analysis was used for chemical analysis. Sampling sites were grouped into three major final discharges corresponding to A2, A4 and A5 (Fig. 1). The chemical samples were analyzed biweekly from 28 June 2021–11 December 2021 and subsequently, weekly from 11 December 2021–28 March 2022 for ECs. Sampling frequency was increased from biweekly to weekly as the number of COVID positive cases increased on campus as discussed in Section 3.1.
2.6. Chemicals and reagent preparation
LC-MS grade pharmaceuticals standards and labelled isotopes were procured from Sigma Aldrich (US) at their best available purity. LC-MS grade solvents such as acetonitrile, methanol and acetone were also procured for solid-phase extraction and LC-MS analysis.
2.7. Sample preparation
200 mL of the wastewater samples obtained after centrifugation was subjected to filtration through 0.45 µm membrane (PALL, corporation, US) filtration followed by pH adjustment to 3 by 37 % HCl solution. The acidified samples were spiked with ethylenediaminetetraacetic acid (EDTA) and surrogate isotope labelled standards which accounted for analyte losses during sample preparation and matrix effects due to organic matter during LC-MS analysis [51], [32], [52], [53]. The final concentration of these standards in the 1 mL of the concentrated sample would be 100 ppb at 100 % recovery. After several hours, samples were subjected to solid pahse extraction (SPE) as previously described [37], [38]. Briefly, HRX cartridges were conditioned with methanol (MeOH 5 mL) and acidified water (5 mL) and the wastewater was allowed to pass through preconditioned cartridges at 5 mL/minute flow rate. The HRX cartridges were then cleaned with acidified water, vacuum dried and finally eluted with MeOH (5 mL) MeOH:acetone (50:50) (5 mL). The eluent was evaporated under nitrogen, reconstituted with MeOH:water (50:50) to 1 mL, and stored at − 20 °C before analysis with liquid chromatography with tandem mass spectrometry (LC-MS/MS) instrument.
2.8. LC-MS/MS analysis of ECs
Isotope dilution technique was used to quantify the targeted ECs in wastewater samples where the contaminants were separated using an Agilent Poroshell 120 EC-C18 reverse-phase column (100 × 4.6 mm i.d.; 2.7 µm particle size) and water (0.1 % formic acid (FA)) and acetonitrile:MeOH (50:50 % and 0.1 % FA) as mobile phases within 25 min runtime, as previously described [37], [38]. The ECs were analysed using HPLC–MS/MS, i.e., Agilent based 1290 Infinity LC coupled to an Agilent-based 6490 Triple Quadrupole MS/MS system at their optimised collision voltage and cell accelerator voltage. The list of ECs and corresponding surrogate isotope labelled standards used to analyse these compounds are presented in Table S3. Table S3 also lists the MRM transition for ECs. Detailed optimised parameters for HPLC-MS/MS operation are provided in our previously published manuscripts [38]. The analytes were quantified via the calibration curve constructed from the peak area response ratio of each analyte to the corresponding surrogate isotope labelled standards.
2.9. Quality control
To curtail contamination during (i) nucleic acid extraction, (ii) preparation for RT-qPCR reagents, and (iii) RT-qPCR, these procedures were carried out in separate laboratories. An extraction negative control was included during nucleic acid extraction. The RT-qPCR procedure included the extraction negative control and the reagent negative control. All negative controls were negative for the analyzed targets. Similarly, appropriate QA/QC criteria were adopted for ECs’ analysis as per our previously published protocols [54]. Briefly, the linearity of the method was evaluated by least-squares regression analysis for 15 concentrations ranging from 0.01 ng/mL to 240 ng/mL and the regression coefficients were all greater than 99.8 %. MeOH-solvent blank and calibration verification standards were injected after every 10 samples to monitor the instrument background and the validity of the calibration. The method detection limit (MDL) and method quantification limit (MQL) were defined to be the lowest observable concentration of analytes in spiked extracts at a signal-to-noise (S/N) of 3 and 10, respectively. The method validation data, including MDLs, MQLs and relative recoveries (RR) are shown in Table S4. The repeated injection (five times) (100 ng/mL) during the same day (repeatability) and on different days yielded method precision reported as relative standard deviation (reproducibility). Both were found to be less than 20 %. In addition, field and procedural water blanks were prepared and analyzed to check for possible contamination during the sampling and sample preparation. All analytes in these blanks were below MDLs.
2.10. Statistical data analysis
The correlation between SARS-CoV-2 (Copy/L) detected in wastewater samples and the number of reported COVID-19 cases on campus were analysed at both the dormitory’s cluster and campus levels. Accordingly, total SARS-CoV-2 (Copy/L) concentrations for each cluster were derived from the mean concentrations detected in the wastewater samples from FD network belonging to the same cluster. The sum of new COVID-19 cases recorded in a week was used to estimate the total number of cases for that particular week, with weekly SARS-CoV-2 (Copy/L) average values used to account for variations in weekly sample frequency. Furthermore, the correlations between weekly average SARS-COV-2 (Copy/L) normalised by PMMoV (Copy/L) was also calculated.
To examine the correlation of ECs with pandemic markers, ECs were grouped into six groups, namely, antibiotics, antivirals, antimalarials, acetaminophen, caffeine and disinfectants. The COVID-19 pandemic markers chosen for this correlation analysis were SARS-CoV-2 viral load (Copy/L), normalised SARS (Copy/L per PMMoV Copy/L) detected in wastewater, and the number of COVID-19 cases and COVID-19 cases normalised by the student population in the dormitories. The analysis was performed on average EC concentrations from 3 hostels from Site A (i.e., A2, A4 and A5). As ECs were analysed once weekly, the weekly average of the pandemic markers was used for the correlation analysis.
R version 4.1.1 was used for the correlation analysis and the strength and direction of the correlations were quantified using Spearman’s Rank Correlation Coefficient (ρ). The smoothing method was used for the trend analysis of SARS-CoV-2 viral load (Copy/L) and normalised SARS-CoV-2 (Copy/L per PMMoV Copy/L) in sewage samples [20].
3. Results and discussion
3.1. Prevalence of SARS-CoV-2 in wastewater
3.1.1. Temporal trends in the university campus
The wastewater samples from seven sites encompassing 28 sampling FD chambers were tested daily using the protocol described in the Methods (Fig. 1, Table S1) from January 2021 to March 2022. The first positive signal was detected in wastewater samples collected on 20th March 2021 with low levels of SARS-CoV-2 RNA in wastewater collected from the FD chamber of the North tower hostel block with an estimated population of approximately 450 residents (A5, Table S1). Based on the positive FD result, stored samples which had earlier been concurrently collected from three ICs upstream of the chamber were subsequently tested for tracing. Of these, only one of the IC samples tested positive for SARS-CoV-2. As this IC served 192 residents, the smaller population, instead of 450 of the whole apartment block, underwent PCR swab tests the next morning (21st March 2021). However, none of them was found to be positive, despite persistent positive signals from the samples collected from the same locations the following day, i.e., 21st March 2021. A thorough examination of the health records later discovered a student who had just been recently discharged from an external quarantine facility where he was treated for COVID-19. When the student was shifted to another facility outside the campus monitoring network, the positive signal from the A5 FD/ICs ceased. This data suggests that the positive signal detected in the wastewater was likely due to the prolonged shedding of SARS-CoV-2 in the faeces of a recovered patient. Wong et al. [18] also reported the detection of positive signals from wastewater samples due to viral shedding from recovered cases and suggested continued monitoring and trend analysis to distinguish signals from a new or recovered case. Nevertheless, this detection provides evidence of the sensitivity of the adopted approach and highlights the need for careful interpretation of results from wastewater testing [55], [18].
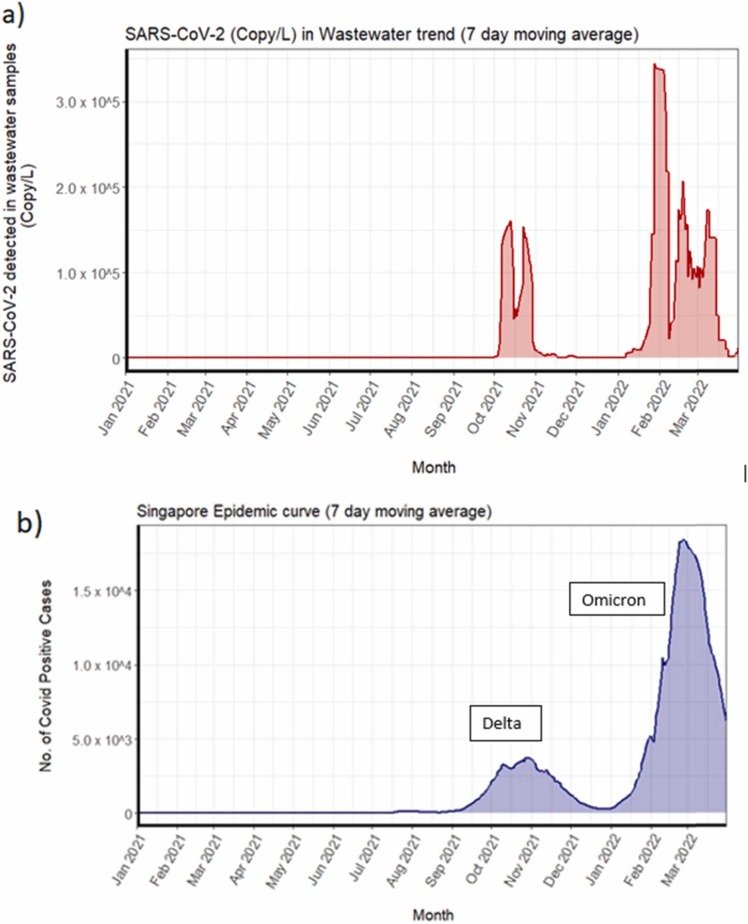
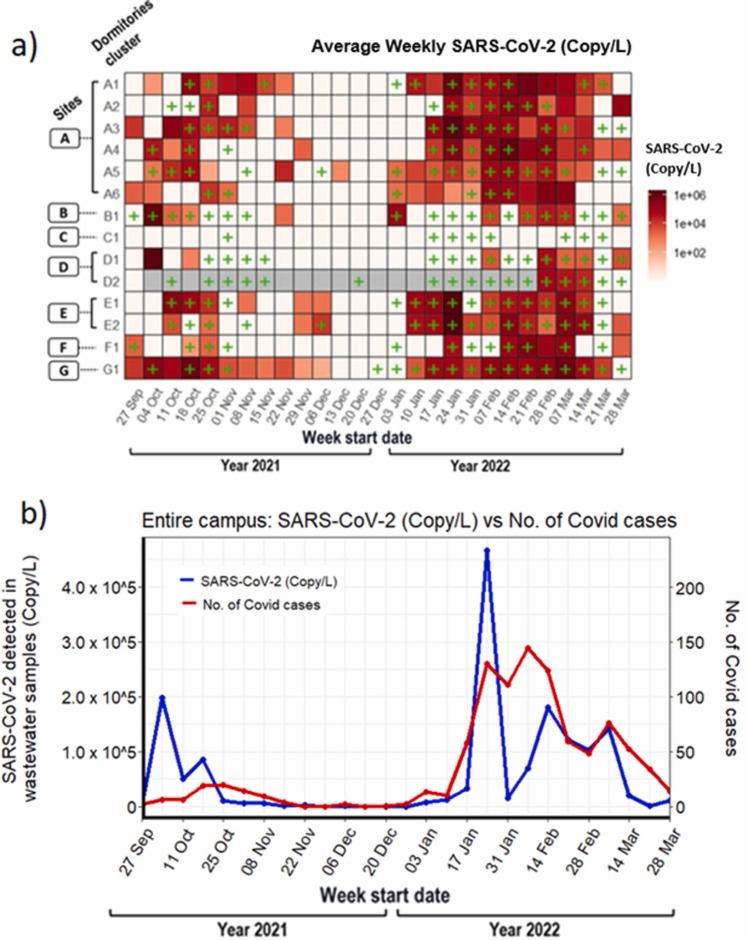
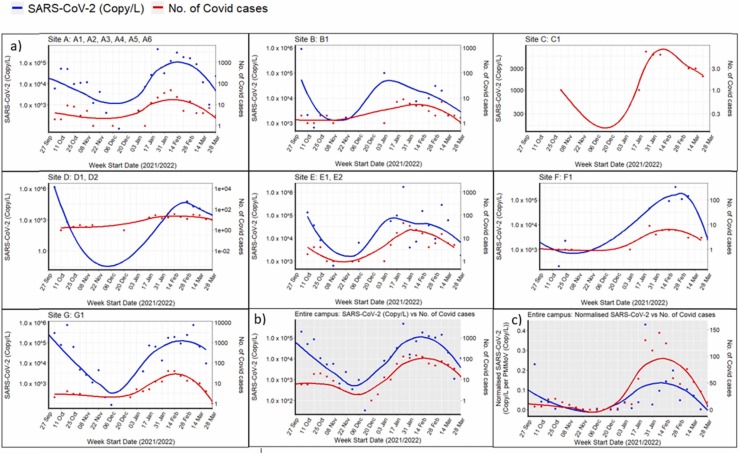
No further positive signals in wastewater were detected on campus until 30th September 2021. It was followed by two waves of signals which corresponded with two waves of reported COVID-19 infection: the first wave was detected in the week of 27th September and lasted till mid-December 2021 while the second wave was observed from January 2022 to March 2022 ( Fig. 2a). In the initial week of first wave, we detected SARS-CoV-2 RNA signals from four dormitory locations, i.e., A3, A6, F1, G1 ( Fig. 3a and Fig. 4a). Almost immediately, in the ensuing week, the number of locations that showed positive signals rose to 7 and rapidly reached its peak (Fig. 3 and Fig. 4a). Thereafter, both virus signals and the number of positive cases subsided, reaching a minimum by mid-December 2021 (Fig. 3). This first transmission wave from October to November 2021 was reportedly due to widespread dissemination of the Delta variant of SARS-CoV-2 in Singapore at that time (Fig. 2b) [25], which was subsequently confirmed by the detection of mutation P681R and L452R in spike protein (using Promega variant panel Cat No: CS3174B02) and absence of E484Q in positive wastewater samples from the campus. The second transmission wave started in the second week of January 2022, resulting in rapidly rising positive signals in wastewater samples (Fig. 3), culminating in a peak around mid-February 2022 with 9 locations affected (Fig. 3a), and subsequent decline by late March 2022 (Fig. 3b). This second peak corroborated the widespread transmission of the Omicron variant throughout the country (Fig. 2b; MOH, Singapore). The representative positive wastewater samples of the second wave showed the detection of mutation N510Y, K417N, and H69-/V70- in the spike protein, that further indicates the presence of the Omicron variant in the campus during that time [56].
Fig. 2.
Wastewater surveillance results from Jan 2021 – March 2022: (a) Cumulative viral load (SARS-CoV-2 copy/L) in wastewater samples from the university campus dormitories and (b) national epidemic curve (positive COVID-19 cases) in Singapore showing predominant variant
(Source: MOH Singapore).
Fig. 3.
Weekly details of WST results from Sep 2021 – March 2022:(a) Overview of sample collection and detection from 7 sites and their residential hostel dormitories, showing the weekly viral load (SARS-CoV-2 Copy/L) detected by RT-qPCR for SARS-CoV-2 N1 gene. COVID-19 cases detected using ART kits is shown by “+ ”, (b) Spearman co-relation between viral load in wastewater samples and reported positive cases on the campus. Each data point represents the aggregate viral load of SARS-CoV-2 (Copy/L, blue line) and FET cases (red line) from each of the hostels on campus.
Fig. 4.
Correlation analysis between viral loads in wastewater samples and reported COVID positive cases A) Site level B) Campus level C) Normalization at campus level. Each data point represents the aggregate viral load of SARS-CoV-2 (Copy/L, blue line) and ART cases (red line) from each of the hostel dormitories.
3.1.2. Correlation between wastewater surveillance and antigen rapid test (ART) detection of SARS-CoV-2
Viral load in WWT was analysed with the number of COVID-19 cases detected with the antigen rapid test (ART), according to the data from the health declaration database. For this study, campus dormitories were divided into 7 clusters based on their proximity with each other. The goal of this analysis was to investigate the effect of different population size on the utilisation of WST as a tool to study infection and transmission dynamics in communities with low disease incidence and prevalence.
3.1.3. Site level correlation analysis
Fig. 4 shows an overview of the weekly SARS-CoV-2 virus detection across different sites with corresponding COVID-19 cases. Site A had 6 hostel dormitories (A1, A2, A3, A4, A5 and A6) and is linked to a population size of around 3060 residents (Table S1). A reasonably strong correlation with COVID-19 cases was observed for the site A (Fig. 4a; Spearman’s correlation, ρ = 0.75, p < 0.05). Similar to site A, site E that had two hostels, E1 and E2, with a population size of 1030 residents also showed strong correlation (ρ = 0.76, p < 0.05). Sites B, C and D showed no statistically significant correlation. This is likely due to frequently missing samples from sites B-D due to low or negligible wastewater flows. Site F has around 400 residents, whereas site G has a population size of ∼ 2500 residents but both showed moderately positive correlation and association with COVID-19 cases [site F (ρ = 0.50, p < 0.05); site G (ρ = 0.62, p < 0.05)].
3.1.4. Campus level correlation analysis
Overall, the weekly average SARS-CoV-2 concentrations in wastewater samples correlated well with the weekly average of COVID-19 cases reported on campus hostels (Fig. 4b, ρ = 0.76, p < 0.05), suggesting a strong positive association between the SARS-CoV-2 concentrations in wastewater samples and the number of COVID-19 cases. Our results showed that the viral load present in wastewater correlated with the reported COVID-19 cases at both site level (Fig. 4a) and campus level (Fig. 4b). This is consistent with the earlier study where authors showed association of SARS-CoV-2 RNA with COVID-19 cases at individual, multiple-building level and campus level [57]. Other studies also showed that wastewater signals largely correlated with COVID-19 cases at different scale from individual college dormitories to the larger sewer shed area at the municipality or county level [59], [58]. Although many studies report success in the detection and quantitation of SARS-CoV-2 RNA, challenges do exist with assay sensitivity and systematic variation in establishing correlation with COVID-19 cases, particularly in regions with low COVID-19 prevalence [60].
During the Delta wave (October – December 2021), SARS-CoV-2 concentrations in wastewater peaked in the week of 4th October 2021 whereas the peak in the number of COVID-19 cases was observed about a week later, i.e., week of 18th October 2021 (Fig. 3b). This lag in clinical detection (by 2–9 days) relative to wastewater detection has been reported in other cohorts [61], [62], [63], [64]. However, during the Omicron wave (January – March 2022), the wastewater SARS-CoV-2 concentrations mirrored the fluctuations in COVID-19 cases (Fig. 3b), similar to correlations studies by Duvallet and colleagues [59]. They studied temporal trends from 55 locations distributed across 39 counties in USA and found that wastewater measurements mirrored reported COVID-19 cases in most of the locations with variations in the correlation factor. The difference in correlation for the Omicron wave could also be explained by the commencement of the school term in January 2022.
In summary, our analysis showed that the concentration of SARS-CoV-2 detected in wastewater increased proportionately with the number of COVID-19 cases and thus, wastewater surveillance could be used as a tool for monitoring trends of COVID-19 burden in the community. However, the effect of the SARS-CoV-2 variant on the temporal variations in wastewater concentrations and COVID-19 cases cannot be ruled out. Since, WST relies on the viral RNA shedding from infected individuals the differences between RNA concentration per COVID-19 case observed could also be due to the different dominant variant in the two waves. Of note, Delta variant has been associated with higher viral load and longer duration of shedding [65]. Overall, though WST is able to capture the evolving spread of COVID-19, the interpretation of disease burden of a community must take the varying shedding of different variants into consideration.
We next normalized the raw SARS-CoV-2 RNA concentrations across different sites with the PMMoV concentration to account for variations in population over time (Fig. 4c). The normalization by PMMoV did not substantially improve the correlation of COVID-19 cases with viral load in waste water (Fig. 4c, ρ = 0.78, p < 0.05); that was observed with raw virus concentrations (Fig. 4b, ρ = 0.76, p < 0.05). Similar to ours, others have reported no improvement when normalized SARS-CoV-2 RNA concentrations were used [66], [57]. In contrast, normalization with PMMoV has been shown to improve the correlation between viral loads present in wastewater and COVID-19 incidence data [67], [22], [68]. Normalization accounts for changes in wastewater dilution and differences in relative human waste input over time and also account for viral losses that occur between faecal inputs into the manhole and quantification losses in the laboratory.
3.1.5. Variation in the concentrations of emerging contaminants and correlation analysis
Parallel to SARS-CoV-2 monitoring, several ECs belonging to various classes were also monitored on the university campus to understand the patterns of pharmaceutical consumption, personal care products and disinfectants usage, and to establish correlations between ECs with SARS-CoV-2 viral loads detected in wastewater and the number of COVID-19 cases (raw and normalised). Although PMMoV was found to be the promising population biomarker as discussed before, population chemical markers were also investigated, as discussed below.
3.1.6. Variation in the total concentration
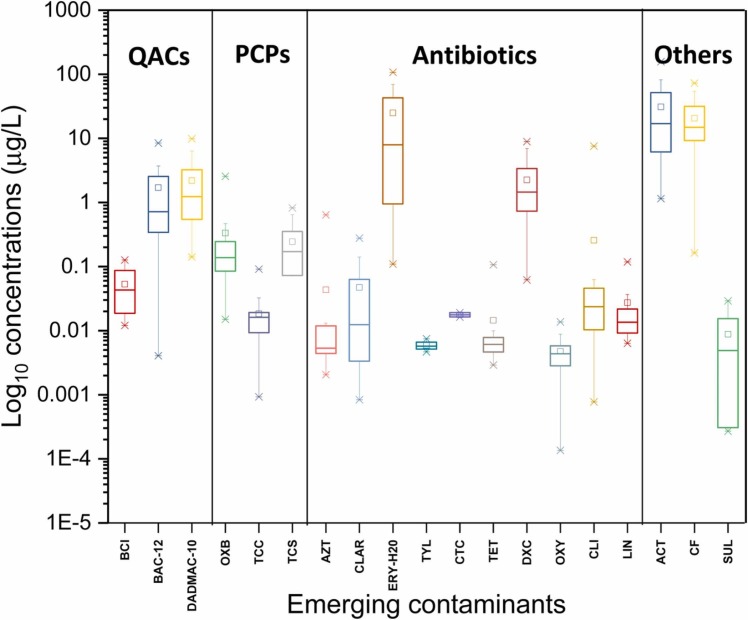
The overall variation in the concentrations of ECs in wastewater across all the sampling points is shown in Table 1. Among the contaminants analyzed, acetaminophen (ACT), erythromycin (ERY-H2O), caffeine (CF), doxycycline (DXC), and benzyldimethyldodecylammonium chloride (BAC-12) were detected at high concentrations in campus wastewater compared to the other analytes. Among 19 analyzed compounds, a maximum concentration of 157.2 µg/L was detected for the over-the-counter medication, ACT (paracetamol/panadol), commonly used as a NSAID. This was followed by ERY-H2O and CF, with concentrations detected up to 130.7 and 72.9 µg/L, respectively (Table 1). Overall, the total concentrations of ECs ranged from 1.7 to 468 µg/L. A considerable variation in the concentrations of individual ECs was seen ( Fig. 5), reflecting PPCPs consumption patterns during the pandemic, as discussed in the next section. Due to the non-availability of EC concentrations in university wastewater samples prior to the COVID-19 pandemic, the EC results obtained from this study were compared with raw wastewater samples taken as influent into a local WWTP in pre-COVID-19 times, where available.
Table 1.
Minimum, maximum, mean, stand deviation and frequency of detection of the emerging contaminants.
| Target ECs | Abbreviations | Min (µg/L) | Max (µg/L) | Mean (µg/L) | Stand deviation | Frequency (%) |
|---|---|---|---|---|---|---|
| Acetaminophen | ACT | 1.144 | 157.207 | 35.519 | 35.372 | 86 |
| Azithromycin | AZT | 0.002 | 0.640 | 0.044 | 0.106 | 90 |
| Benzethonium Cl | BCl | 0.007 | 0.192 | 0.057 | 0.048 | 37 |
| Benzyldimethyldodecylammonium chloride | BAC-12 | 0.004 | 24.767 | 4.481 | 5.441 | 99 |
| Caffeine | CF | 0.163 | 72.857 | 14.941 | 13.164 | 99 |
| Clarithromycin | CLAR | 0.001 | 0.486 | 0.062 | 0.101 | 47 |
| Clindamycin | CLI | 0.001 | 7.566 | 0.161 | 0.903 | 96 |
| Chlortetracycline | CTC | 0.014 | 0.027 | 0.018 | 0.003 | 18 |
| Didecyldimethylammonium chloride | DADMAC-10 | 0.141 | 9.884 | 2.073 | 1.782 | 99 |
| Doxycycline | DXC | 0.062 | 59.107 | 7.204 | 11.604 | 79 |
| Erythromycin-H2O | ERY-H2O | 0.109 | 130.738 | 16.329 | 30.100 | 79 |
| Lincomycin | LIN | 0.004 | 0.151 | 0.030 | 0.030 | 82 |
| Oxybenzone | OXB | 0.015 | 2.565 | 0.317 | 0.410 | 99 |
| Oxytetracycline | OXY | 0.000 | 0.014 | 0.005 | 0.003 | 25 |
| Sulpiride | SUL | 0.000 | 0.031 | 0.013 | 0.008 | 41 |
| Triclocarban | TCC | 0.001 | 0.167 | 0.021 | 0.024 | 89 |
| Triclosan | TCS | 0.017 | 0.871 | 0.243 | 0.194 | 90 |
| Tetracycline | TET | 0.003 | 0.758 | 0.071 | 0.142 | 55 |
| Tylosin | TYL | 0.005 | 0.010 | 0.007 | 0.002 | 15 |
Fig. 5.
The log10 concentrations of emerging contaminants in the university campus over 9 months of sampling.
3.1.7. Variation in the concentrations of disinfectants
The overall variation in the concentrations of disinfectants (BCl, DADMAC-10, and BAC-12) is shown in Fig. S1 . Among the disinfectants, benzethonium chloride (BCl) was detected in only 37 % of the samples analysed, with an average concentration of 0.05 ± 0.04 µg/L, possibly due to their limited application as a topical antimicrobial agent in first aid antiseptics. BAC-12 and DADMAC-10 were detected at concentrations ranging from 0.004 to 24.8 and 0.1–9.9 μg/L, respectively (Table 1). Both these compounds were detected at a detection frequency of 99 %, with the maximum detected concentration of BAC-12 being 24.8 μg/L. Both BAC-12 and DADMAC-10 are the most common cationic surfactants used as the active components in disinfectants, biocides, detergents and personal care products. As an active ingredient of many disinfectants, it is expected that high concentrations of these compounds should be detected in wastewater during the pandemic and their total concentrations specifically peaked in the second wave i.e., from January 2022 to March 2022 ( Fig. 6, Fig S1). Unfortunately, there is no data on these compounds in wastewaters in Singapore for comparison. Nevertheless, the concentrations for BAC-12 detected in our campus wastewater (0.004–24.8 μg/L) were considerably higher than concentrations reported in raw wastewater from China (0.3–06 μg/L) [69] and Sweden (0.4–1.4 μg/L) [70] measured prior to the pandemic. However, the concentrations of DADMAC-10 (0.141–9.884 μg/L) were lower than the concentration reported for wastewater in Sweden (1.6–208 μg/L) before the pandemic [70].
Fig. 6.
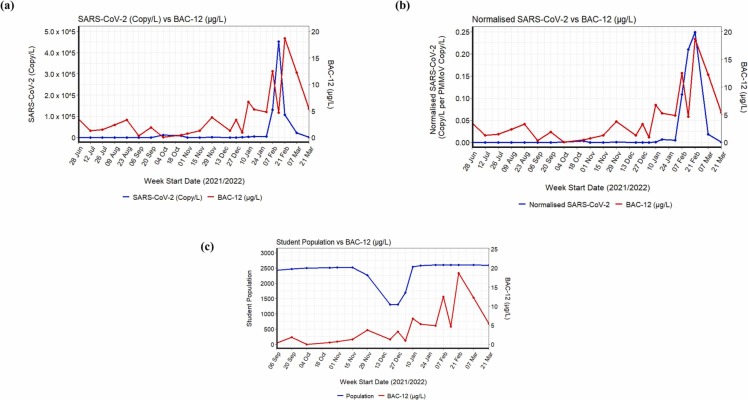
Correlation analysis between BAC-12 and (a) SARS-CoV-2 (copy/L) (p < 0.05), (b) normalized SARS-CoV-2 (Copy/L per PMMoV Copy/L) (p < 0.05), (c) student population (p < 0.05).
Among several ECs considered for the correlation analysis, the total concentrations of disinfectants showed a relatively strong positive association with the SARS-CoV-2 viral load in wastewater (Spearman’s Coefficient, ρ = 0.64, p < 0.05) (Fig. S2a) and with the normalised SARS-CoV-2 data (ρ = 0. 69, p < 0.05) (Fig. S2b). The concentration of disinfectants also showed a moderate positive association with the number of COVID-19 cases (ρ = 0.45, p < 0.05) (Fig. S2c) and the number of student population (ρ = 0.7, p < 0.05) (Fig. S2d). Thus, when individual disinfectants were tested, except BAC-12, other disinfectants (BCl and DADMAC-10) did not show any statistical correlation with the pandemic markers, i.e., SARS-CoV-2 viral load, normalised SARS-CoV-2, and the number of student population (Fig. 6). The spatial-temporal variation and subsequent correlation of BAC-12 with the SARS-CoV-2 viral load in wastewater (ρ = 0.5, p < 0.05) (Fig. 6a) and with the normalised SARS-CoV-2 data (ρ = 0. 6, p < 0.05) (Fig. 6b) highlights the increased use of disinfectants with BAC-12 as an active ingredient among hostel residents during the second wave of COVID-19 (January 2022 to March 2022). In addition, a moderate positive correlation was observed between disinfectant use and the number of the student population (ρ = 0.7, p < 0.05) (Fig. 6c), indicating BAC-12 is a reliable population chemical marker during the pandemic. The moderate association could possibly also be attributed to consistent disinfectant use irrespective of the first COVID-19 peak seen between 30 September and mid-December 2021 attributed to the delta variant discussed before (Fig. 2).
3.1.8. Variation in the concentrations of Personal care products
Out of three personal care products (TCC, TCS, OXB) shown in Fig. S3, the UV sunscreen, OXB, was detected at a high frequency (99 %) with a maximum concentration of 2.6 μg/L (Table 1). It is reasonable to have OXB in wastewater at a high frequency due to its frequent use in tropical Singapore. However, its mean concentration was one order of magnitude less than the mean concentration (∼2.6 μg/L) reported for raw wastewater samples in Singapore in our previous study [38]. OXB is also an active ingredient in several hand sanitisers, which might have contributed to its concentrations in wastewater. As a result of which OXB was moderately correlated with SARS-CoV-2 viral load in wastewater (ρ = 0.6, p < 0.05) (Fig. S4a), normalised SARS-CoV-2 data (ρ = 0. 6, p < 0. 05) (Fig. S4b), and the number of COVID-19 cases (ρ = 0.5, p < 0.05) (Fig. S4c). Compared with the concentrations reported previously [38], the detected maximum concentrations of TCS (0.2 µg/L) and TCC (0.9 µg/L) in the campus wastewater (Table 1) were much lower probably due to their restricted use in soap and other personal care products to avoid the development of antimicrobial resistance [71]. Since 2016, TCS along with 8 other antimicrobial chemicals from household soap products have been banned by the United States Food and Drug Administration (FDA), which was later adopted by other countries. Even when they are present at a low concentration, TCC showed statistically significant correlation with SARS-CoV-2 viral load in wastewater (ρ = 0.6, p < 0.05) (Fig. S5a), normalised SARS-CoV-2 data (ρ = 0. 6, p < 0. 05) (Fig. S5b), and the number of COVID-19 cases (ρ = 0.5, p < 0.05) (Fig. S5c). In the contrary, there was no significant correlation between TCC and the total number of campus residents, indicating increased use of disinfectants containing TCC among the COVID-positive students. Although the quantum of sanitisers sold in Singapore during the pandemic is not available, the available data indicates that the mean revenue generated from hand sanitisers between 2020 and 2022 was 2.2 million USD higher than in 2019 [72]. Thus, such disinfectants could also serve as a reliable population chemical marker during such an unprecedented time.
3.1.9. Variation in the concentrations of antibiotics
Several antibiotics were detected in the wastewater (Table 1, Fig. 5); however, antibiotics belonging to tetracyclines, lincosamide, and macrolides showed a significant correlation with various pandemic markers.
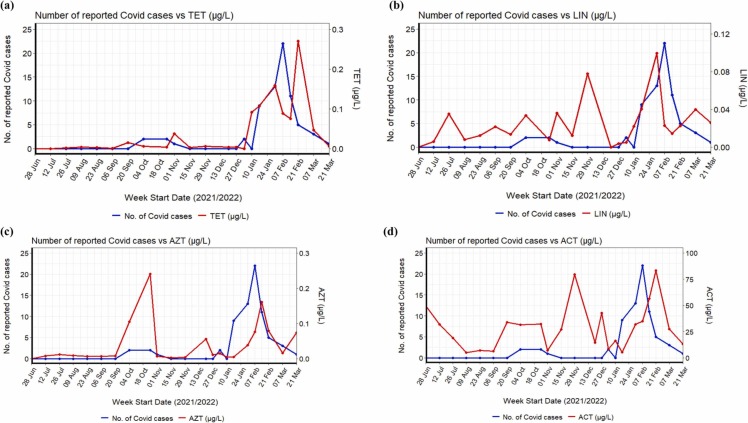
The total concentrations of tetracyclines (Fig. S6, Fig. S7) peaked from January 2022 to March 2022, i.e., second wave of the pandemic ( Fig. 7b). Among the tetracyclines, DXC was detected at a maximum concentration of 59.1 µg/L with a 79 % detection frequency (Table 1). Its detected concentrations during the pandemic were higher than the pre-pandemic concentrations in wastewater samples (2.4 µg/L) collected in Germany [73]. It is also one of the most commonly used antibiotics to treat COVID-19 respiratory symptoms in UK [74]. CLI was detected at a high frequency among the lincosamides (Fig. S8). The concentrations of CLI were in the range of 0.001–7.6 µg/L, which is higher than the concentrations reported for wastewater from Singapore (0.024–0.03 µg/L) [37], [75]. Literature evidence suggests that among the lincosamides, CLI was used to treat bacteria-related infections more frequently among COVID-19 patients in the neighbouring country of Singapore, i.e., Malaysia [28]. Even though the concentration DXC and CLI were present at high concentrations, when each subgroup of tetracyclines and lincosamides were considered individually, only TET (Fig. 7a) and LIN (Fig. 7b) showed a moderate positive correlation with the number of COVID-19 cases (ρ = 0.7 and 0.5, respectively, p < 0.05). Compared to LIN (Fig. S9), a strong correlation was observed for TET with SARS-CoV-2 viral load in wastewater (ρ = 0.7, p < 0.001) (Fig. S9a), normalised SARS-CoV-2 data (ρ = 0. 75, p < 0. 001) (Fig. S9b) indicating TET as a reliable population chemical marker.
Fig. 7.
Time series and correlation analysis between the number of reported COVID cases and (a) tetracycline (p < 0.05), (b) lincomycin (p < 0.05), (c) azithromycin (p < 0.05), and (d) acetaminophen (p > 0.05).
Macrolides such as AZT [76], [77], [78] and ERY-H2O [79] were suggested for COVID-19 treatment. Earlier studies also suggested combining AZT and hydroxychloroquine for COVID-19 treatment [78]. Thus, in this sewage surveillance study, macrolides were also targeted. Among the macrolides, ERY-H2O was detected at high levels with a frequency of 79 % (Fig. S10, Table 1). On the other hand, AZT was detected at a higher frequency (∼90 %), but with concentrations far below ERY-H2O, ranging from 0.002 to 0.6 µg/L (Table 1). While the maximum concentration for AZT was several orders lower than its reported concentration in wastewater (1.5–2.9 µg/L) before the pandemic [37], [75], detected concentrations for ERY-H2O were higher than previously reported concentrations in wastewater (07–2.9 µg/L) from Singapore [37], [75]. However, only AZT showed statistical correlation with pandemic markers such as SARS-CoV-2 viral load in wastewater (ρ = 0.6, p < 0.05) (Fig. S11a), normalised SARS-CoV-2 data (ρ = 0.5, p < 0. 05) (Fig. S11b), and the number of reported COVID-19 cases (ρ = 0.62, p < 0.05) (Fig. S7c) monitored in the university campus. Such correlation indicates that AZT might be prescribed on the university campus during the pandemic, which can also be used as a chemical marker. Even in Singapore, the health authority found an increase in antibiotic use among patients suspected or confirmed COVID-19, raising concerns about misuse [80].
3.1.10. Variation in the concentrations of other pharmaceuticals
Among other pharmaceuticals presented in Fig. S12, acetaminophen followed by caffeine was detected at high levels with concentrations ranging from 1.1 to 157.2 µg/L and 0.2–72.9 µg/L, respectively (Table 1). While the detected concentrations of caffeine were only slightly higher than previously reported concentrations (0.8–60.5 µg/L) in wastewaters in Asia [38], acetaminophen was detected at a higher concentration than previously reported values (0.07–147.7 µg/L). With a high excretion rate (∼90 %), a large amount of acetaminophen can be expected in wastewater due to its frequent use as an NSAID during the pandemic and it is readily available over the counter. It was also recommended as the main medication to relieve symptoms arising from mass-vaccination which was carried out on a nationwide scale during the sampling period and around 81 % of the population in Singapore were already vaccinated by early September 2021. A recent study conducted in the USA also indicated that the proportion of acetaminophen ingestion increased from 0.13 % to 0.28 % between 2016 and 2018, decreased to 0.24 % in 2019, then increased to 0.44 % in 2020 during the pandemic [30]. From the time series analysis of acetaminophen (Fig. 7d), it can be clearly seen that acetaminophen concentration peaked during late June, September, November 2021 and January/February 2022, possibly attributed to its consumption during mass vaccination and various COVID waves. Even a surge in Panadol (acetaminophen-based products in Singapore) use was noticed during the second half of 2021 and in the first quarter of 2022 amid the Omicron wave [81]. Thus, the concentrations detected in the campus wastewater were possibly attributed to its use for vaccination and recovery from COVID-19; hence the concentrations of acetaminophen were not statistically correlated with the pandemic markers considered in this study (Fig. S13, Table S5).
No clear trend in coffee consumption during the pandemic was reported [82]. Even though caffeine was suggested as a population chemical marker [37], [38], both caffeine and acetaminophen did not show any statistically significant correlation with the pandemic markers even though students were quarantined in the university dorms from the beginning of the first wave, i.e., September 2021 (Fig. S14, Table S6).
4. Conclusions
Due to the probability of evolution of different variants of SARS-CoV-2 and their immune escape mechanism, a cost-effective, nonintrusive, mass surveillance tool is required for the long-term monitoring of SARS-CoV-2 and ECs in the endemic phase of COVID-19. This study demonstrates that WST can be used as a core epidemiological indicator of infection prevalence for supporting policy-making as it allows for passive community-wide screening without interfering in the normal activities of the general population. This study also presented the chemical consumption pattern of various ECs during the same period, reflecting the effect of lockdowns, mass vaccination and other measures, including health and lifestyle changes on the campus residents. A strong positive correlation was established between WBE results and the campus ART results. Similarly, except for a few ECs, disinfectants (BAC-12), PCPs (TCS and OXB), and antibiotics (TET, LIN and AZT) showed a statistically significant correlation with the number of COVID-19 cases, suggesting that changes in PPCP use patterns were likely attributed to COVID-19. Our study suggests these selected ECs could potentially be used as a chemical marker during the COVID-19 pandemic and possibly, other similar viral epidemics in the future.
Environmental implication
The continuous evolution of different variants of SARS-CoV-2 and the use of pharmaceuticals and QACs based disinfectants during the COVID-19 pandemic has raised the necessity for a cost-effective, nonintrusive, and mass surveillance tool. Besides the SARS-CoV-2 monitoring, there is a change in the consumption pattern of emerging contaminants reflecting the effect of lockdowns, mass vaccination and other measures, including health and lifestyle changes on the campus residents. In general, this paper provides a comprehensive discussion on the occurrence and correlation analysis between several pandemic markers, including SARS-CoV-2, the number of active cases and emerging contaminants.
CRediT authorship contribution statement
Sanjeeb Mohapatra: Experimental analysis, Methodology, Data processing, Investigation, Writing – original draft, Writing – review & editing. Sumedha Bhatia: Experimental analysis, Methodology, Data processing, Investigation, Writing – original draft, Writing – review & editing. Kavindra Yohan Kuhatheva Senaratna: Experimental analysis, Data processing, Investigation, Writing – review & editing. Mui-Choo Jong: Experimental analysis, Data processing, Investigation, Writing – review & editing. Chun Min Benjamin Lim: Experimental analysis, Methodology. G Reuben Gangesh: Experimental analysis, Methodology. Jia Xiong Lee: Experimental analysis, Methodology. Goh Shin Giek: Experimental analysis, Methodology. Callie Cheung: Experimental analysis, Methodology. Lin Yutao: Experimental analysis, Methodology. You Luhua: Experimental analysis, Methodology. Ng How Yong: Field sampling, Methodology. Lim Cheh Peng: Review of clinical COVID cases. Judith Chui Ching Wong: Writing – review & editing. Ng Lee Ching: Writing – review & editing, Funding acquisition. Karina Yew-Hoong Gin: Supervision, Project lead, Writing – review & editing.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgement
This research is co-funded by the National University of Singapore (NUS), National Environment Agency (NEA) and the National Research Foundation (NRF), Prime Minister’s Office, Singapore under its Campus for Research Excellence and Technological Enterprise (CREATE) program (Intra-CREATE Thematic Project NRF2019-THE001-0003), administered by the NUS Environmental Research Institute (NERI), Singapore. We are grateful for the contributions to the sampling and lab analysis from You Fang, Umamaheswari Muniasamy, Lam Yuan Chang, Jolen Nivetha, and Felix Gaffu Tandadjaja. Thanks also to the NUS hostel administration for providing ART data. We appreciate the support from NEA’s Wastewater Based Epidemiology Branch for their technical advice in wastewater sampling.
Editor: Lingxin Chen
Footnotes
Supplementary data associated with this article can be found in the online version at doi:10.1016/j.jhazmat.2022.130690.
Appendix A. Supplementary material
Supplementary material.
.
Data Availability
The data that has been used is confidential.
References
- 1.Asghar H., Diop O.M., Weldegebriel G., Malik F., Shetty S., El Bassioni L., Akande A.O., Al Maamoun E., Zaidi S., Adeniji A.J., Burns C.C., Deshpande J., Oberste M.S., Lowther S.A. Environmental surveillance for polioviruses in the global polio eradication initiative. J Infect Dis. 2014;210(Suppl 1):S294–S303. doi: 10.1093/infdis/jiu384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Choi P.M., Bowes D.A., O'Brien J.W., Li J., Halden R.U., Jiang G., Thomas K.V., Mueller J.F. Do food and stress biomarkers work for wastewater-based epidemiology? a critical evaluation. Sci Total Environ. 2020;736 doi: 10.1016/j.scitotenv.2020.139654. [DOI] [PubMed] [Google Scholar]
- 3.Petrie B., Barden R., Kasprzyk-Hordern B. A review on emerging contaminants in wastewaters and the environment: current knowledge, understudied areas and recommendations for future monitoring. Water Res. 2015;72:3–27. doi: 10.1016/j.watres.2014.08.053. [DOI] [PubMed] [Google Scholar]
- 4.Zuccato E., Chiabrando C., Castiglioni S., Calamari D., Bagnati R., Schiarea S., Fanelli R. Cocaine in surface waters: a new evidence-based tool to monitor community drug abuse. Environ Health. 2005;4:14. doi: 10.1186/1476-069x-4-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bivins A., Bibby K. Wastewater surveillance during Mass COVID-19 vaccination on a college campus. Environ Sci Technol Lett. 2021;8(9):792–798. doi: 10.1021/acs.estlett.1c00519. [DOI] [PubMed] [Google Scholar]
- 6.Mohapatra S., Menon N.G., Mohapatra G., Pisharody L., Pattnaik A., Menon N.G., Bhukya P.L., Srivastava M., Singh M., Barman M.K., Gin K.Y., Mukherji S. The novel SARS-CoV-2 pandemic: Possible environmental transmission, detection, persistence and fate during wastewater and water treatment. Sci Total Environ. 2021;765 doi: 10.1016/j.scitotenv.2020.142746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kumar M., Jiang G., Kumar Thakur A., Chatterjee S., Bhattacharya T., Mohapatra S., Chaminda T., Kumar Tyagi V., Vithanage M., Bhattacharya P., Nghiem L.D., Sarkar D., Sonne C., Mahlknecht J. Lead time of early warning by wastewater surveillance for COVID-19: Geographical variations and impacting factors. Chem Eng J. 2022;441 doi: 10.1016/j.cej.2022.135936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Menon N.G., Mohapatra S. The COVID-19 pandemic: Virus transmission and risk assessment. Curr Opin Environ Sci Health. 2022;28 doi: 10.1016/j.coesh.2022.100373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nishiura H., Linton N.M., Akhmetzhanov A.R. Serial interval of novel coronavirus (COVID-19) infections. Int J Infect Dis. 2020;93:284–286. doi: 10.1016/j.ijid.2020.02.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Petrillo M., Brogna C., Cristoni S., Querci M., Piazza O., Van den Eede G. Increase of SARS-CoV-2 RNA load in faecal samples prompts for rethinking of SARS-CoV-2 biology and COVID-19 epidemiology. F1000Res. 2021;10:370. doi: 10.12688/f1000research.52540.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Treibel T.A., Manisty C., Burton M., McKnight Á., Lambourne J., Augusto J.B., Couto-Parada X., Cutino-Moguel T., Noursadeghi M., Moon J.C. COVID-19: PCR screening of asymptomatic health-care workers at London hospital. Lancet. 2020;395(10237):1608–1610. doi: 10.1016/s0140-6736(20)31100-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang Y., Cen M., Hu M., Du L., Hu W., Kim J.J., Dai N. Prevalence and persistent shedding of fecal SARS-CoV-2 RNA in patients with COVID-19 infection: a systematic review and meta-analysis. Clin Transl Gastroenterol. 2021;12(4) doi: 10.14309/ctg.0000000000000343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tran B.X., Vu G.T., Le H.T., Pham H.Q., Phan H.T., Latkin C.A., Ho R.C. Understanding health seeking behaviors to inform COVID-19 surveillance and detection in resource-scarce settings. J Glob Health. 2020;10(2):0203106. doi: 10.7189/jogh.10.0203106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ng O.T., Koh V., Chiew C.J., Marimuthu K., Thevasagayam N.M., Mak T.M., Chua J.K., Ong S.S.H., Lim Y.K., Ferdous Z., Johari A.K.B., Chen M.I., Maurer-Stroh S., Cui L., Lin R.T.P., Tan K.B., Cook A.R., Leo P.Y., Lee P.V.J. Impact of delta variant and vaccination on SARS-CoV-2 secondary attack rate among household close contacts. Lancet Reg Health West Pac. 2021;17 doi: 10.1016/j.lanwpc.2021.100299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wu F., Zhang J., Xiao A., Gu X., Lee W.L., Armas F., Kauffman K., Hanage W., Matus M., Ghaeli N., Endo N., Duvallet C., Poyet M., Moniz K., Washburne A.D., Erickson T.B., Chai P.R., Thompson J., Alm E.J. SARS-CoV-2 titers in wastewater are higher than expected from clinically confirmed cases. mSystems. 2020;5(4) doi: 10.1128/mSystems.00614-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ahmed W., Bertsch P.M., Angel N., Bibby K., Bivins A., Dierens L., Edson J., Ehret J., Gyawali P., Hamilton K.A., Hosegood I., Hugenholtz P., Jiang G., Kitajima M., Sichani H.T., Shi J., Shimko K.M., Simpson S.L., Smith W.J.M., Symonds E.M., Thomas K.V., Verhagen R., Zaugg J., Mueller J.F. Detection of SARS-CoV-2 RNA in commercial passenger aircraft and cruise ship wastewater: a surveillance tool for assessing the presence of COVID-19 infected travellers. J Travel Med. 2020;27(5) doi: 10.1093/jtm/taaa116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Srivastava V., Gupta S., Patel A.K., Joshi M., Kumar M. Reflections of COVID-19 cases in the wastewater loading of SARS-CoV-2 RNA: a case of three major cities of Gujarat, India. Case Stud Chem Environ Eng. 2021;4 doi: 10.1016/j.cscee.2021.100115. 100115-100115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wong J.C.C., Tan J., Lim Y.X., Arivalan S., Hapuarachchi H.C., Mailepessov D., Griffiths J., Jayarajah P., Setoh Y.X., Tien W.P., Low S.L., Koo C., Yenamandra S.P., Kong M., Lee V.J.M., Ng L.C. Non-intrusive wastewater surveillance for monitoring of a residential building for COVID-19 cases. Sci Total Environ. 2021;786 doi: 10.1016/j.scitotenv.2021.147419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hassard F., Lundy L., Singer A.C., Grimsley J., Di Cesare M. Innovation in wastewater near-source tracking for rapid identification of COVID-19 in schools. Lancet Microbe. 2021;2(1):e4–e5. doi: 10.1016/s2666-5247(20)30193-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gibas C., Lambirth K., Mittal N., Juel M.A.I., Barua V.B., Roppolo Brazell L., Hinton K., Lontai J., Stark N., Young I., Quach C., Russ M., Kauer J., Nicolosi B., Chen D., Akella S., Tang W., Schlueter J., Munir M. Implementing building-level SARS-CoV-2 wastewater surveillance on a university campus. Sci Total Environ. 2021;782 doi: 10.1016/j.scitotenv.2021.146749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rennert L., McMahan C., Kalbaugh C.A., Yang Y., Lumsden B., Dean D., Pekarek L., Colenda C.C. Surveillance-based informative testing for detection and containment of SARS-CoV-2 outbreaks on a public university campus: an observational and modelling study. Lancet Child Adolesc Health. 2021;5(6):428–436. doi: 10.1016/S2352-4642(21)00060-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Scott L.C., Aubee A., Babahaji L., Vigil K., Tims S., Aw T.G. Targeted wastewater surveillance of SARS-CoV-2 on a university campus for COVID-19 outbreak detection and mitigation. Environ Res. 2021;200 doi: 10.1016/j.envres.2021.111374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Karthikeyan S., Nguyen A., McDonald D., Zong Y., Ronquillo N., Ren J., Zou J., Farmer S., Humphrey G., Henderson D., Javidi T., Messer K., Anderson C., Schooley R., Martin N.K., Knight R. Rapid, large-scale wastewater surveillance and automated reporting system enable early detection of nearly 85% of covid-19 cases on a University Campus. mSystems. 2021;6(4) doi: 10.1128/mSystems.00793-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gorny A.W., Bagdasarian N., Koh A.H.K., Lim Y.C., Ong J.S.M., Ng B.S.W., Hooi B., Tam W.J., Kagda F.H., Chua G.S.W., Yong M., Teoh H.L., Cook A.R., Sethi S., Young D.Y., Loh T., Lim A.Y.T., Aw A.K., Mak K.S.W., Fisher D. SARS-CoV-2 in migrant worker dormitories: geospatial epidemiology supporting outbreak management. Int J Infect Dis. 2021;103:389–394. doi: 10.1016/j.ijid.2020.11.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Song H., Fan G., Liu Y., Wang X., He D. The second wave of COVID-19 in South and Southeast Asia and the effects of vaccination. Front Med (Lausanne) 2021;8 doi: 10.3389/fmed.2021.773110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang B., Goh Y.S., Fong S.W., Young B.E., Ngoh E.Z.X., Chavatte J.M., Salleh S.N.M., Yeo N.K., Amrun S.N., Hor P.X., Loh C.Y., Lee C.Y., Chan Y.H., Chang Z.W., Tay M.Z., Rouers A., Torres-Ruesta A., Carissimo G., Soh M.K., Lee R.T.C., Xu Y., Pada S., Lin R.T.P., Leo Y.S., Lye D.C., Maurer-Stroh S., Ng L.F.P., Renia L., Wang C.I. Resistance of SARS-CoV-2 Delta variant to neutralization by BNT162b2-elicited antibodies in Asians. Lancet Reg Health West Pac. 2021;15 doi: 10.1016/j.lanwpc.2021.100276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Varma P., Junge M., Meaklim H., Jackson M.L. Younger people are more vulnerable to stress, anxiety and depression during COVID-19 pandemic: a global cross-sectional survey. Prog Neuropsychopharmacol Biol Psychiatry. 2021;109 doi: 10.1016/j.pnpbp.2020.110236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mohamad I.-N., Wong C.K.-W., Chew C.-C., Leong E.L., Lee B.-H., Moh C.-K., Chenasammy K., Lim S.C.-L., Ker H.-B. The landscape of antibiotic usage among COVID-19 patients in the early phase of pandemic: a Malaysian national perspective. J Pharm Policy Pract. 2022;15(1):4. doi: 10.1186/s40545-022-00404-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kumar M., Mazumder P., Mohapatra S., Kumar Thakur A., Dhangar K., Taki K., Mukherjee S., Kumar Patel A., Bhattacharya P., Mohapatra P., Rinklebe J., Kitajima M., Hai F.I., Khursheed A., Furumai H., Sonne C., Kuroda K. A chronicle of SARS-CoV-2: Seasonality, environmental fate, transport, inactivation, and antiviral drug resistance. J Hazard Mater. 2021;405 doi: 10.1016/j.jhazmat.2020.124043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Moss R., Hertzberg E., Person H., Zackai S., Bucuvalas J., Gillen J. 617: Increasing rates of acetaminophen ingestion for self-harm in the pediatric population. Crit Care Med. 2022;50(1):301. doi: 10.1097/01.ccm.0000808792.12260.0a. [DOI] [Google Scholar]
- 31.Menon N.G., Mohapatra S., Padhye L.P., Tatiparti S.S.V., Mukherji S. In: Emerging Issues in the Water Environment during Anthropocene: A South East Asian Perspective. Kumar M., Snow D.D., Honda R., editors. Springer Singapore; Singapore: 2020. Review on occurrence and toxicity of pharmaceutical contamination in Southeast Asia; pp. 63–91. [DOI] [Google Scholar]
- 32.Mohapatra S., Sharma N., Mohapatra G., Padhye L.P., Mukherji S. Seasonal variation in fluorescence characteristics of dissolved organic matter in wastewater and identification of proteins through HRLC-MS/MS. J Hazard Mater. 2021;413 doi: 10.1016/j.jhazmat.2021.125453. [DOI] [PubMed] [Google Scholar]
- 33.Mohapatra S., Yutao L., Goh S.G., Ng C., Luhua Y., Tran N.H., Gin K.Y. Quaternary ammonium compounds of emerging concern: Classification, occurrence, fate, toxicity and antimicrobial resistance. J Hazard Mater. 2022;445 doi: 10.1016/j.jhazmat.2022.130393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dubey M., Mohapatra S., Tyagi V.K., Suthar S., Kazmi A.A. Occurrence, fate, and persistence of emerging micropollutants in sewage sludge treatment. Environ Pollut. 2021;273 doi: 10.1016/j.envpol.2021.116515. [DOI] [PubMed] [Google Scholar]
- 35.Mohapatra S., Mrozik W., Acharya K., Menon N.G. In: Legacy and Emerging Contaminants in Water and Wastewater: Monitoring, Risk Assessment and Remediation Techniques. Chakraborty P., Snow D., editors. Springer International Publishing; Cham: 2022. Suspect and nontarget screening of pharmaceuticals in water and wastewater matrices; pp. 77–92. [DOI] [Google Scholar]
- 36.Tong X., Mohapatra S., Zhang J., Tran N.H., You L., He Y., Gin K.Y. Source, fate, transport and modelling of selected emerging contaminants in the aquatic environment: Current status and future perspectives. Water Res. 2022;217 doi: 10.1016/j.watres.2022.118418. [DOI] [PubMed] [Google Scholar]
- 37.Tran N.H., Chen H., Reinhard M., Mao F., Gin K.Y. Occurrence and removal of multiple classes of antibiotics and antimicrobial agents in biological wastewater treatment processes. Water Res. 2016;104:461–472. doi: 10.1016/j.watres.2016.08.040. [DOI] [PubMed] [Google Scholar]
- 38.Tran N.H., Reinhard M., Khan E., Chen H., Nguyen V.T., Li Y., Goh S.G., Nguyen Q.B., Saeidi N., Gin K.Y. Emerging contaminants in wastewater, stormwater runoff, and surface water: application as chemical markers for diffuse sources. Sci Total Environ. 2019;676:252–267. doi: 10.1016/j.scitotenv.2019.04.160. [DOI] [PubMed] [Google Scholar]
- 39.Cruz-Cruz C., Yargeau V., Vidaña-Perez D., Schilmann A., Pineda M.A., Lobato M., Hernández-Avila M., Villatoro J.A., Barrientos-Gutierrez T. Opioids, stimulants, and depressant drugs in fifteen Mexican Cities: a wastewater-based epidemiological study. Int J Drug Policy. 2021;88 doi: 10.1016/j.drugpo.2020.103027. [DOI] [PubMed] [Google Scholar]
- 40.Gonzalez-Marino I., Baz-Lomba J.A., Alygizakis N.A., Andres-Costa M.J., Bade R., Bannwarth A., Barron L.P., Been F., Benaglia L., Berset J.D., Bijlsma L., Bodik I., Brenner A., Brock A.L., Burgard D.A., Castrignano E., Celma A., Christophoridis C.E., Covaci A., Delemont O., de Voogt P., Devault D.A., Dias M.J., Emke E., Esseiva P., Fatta-Kassinos D., Fedorova G., Fytianos K., Gerber C., Grabic R., Gracia-Lor E., Gruner S., Gunnar T., Hapeshi E., Heath E., Helm B., Hernandez F., Kankaanpaa A., Karolak S., Kasprzyk-Hordern B., Krizman-Matasic I., Lai F.Y., Lechowicz W., Lopes A., Lopez de Alda M., Lopez-Garcia E., Love A.S.C., Mastroianni N., McEneff G.L., Montes R., Munro K., Nefau T., Oberacher H., O'Brien J.W., Oertel R., Olafsdottir K., Pico Y., Plosz B.G., Polesel F., Postigo C., Quintana J.B., Ramin P., Reid M.J., Rice J., Rodil R., Salgueiro-Gonzalez N., Schubert S., Senta I., Simoes S.M., Sremacki M.M., Styszko K., Terzic S., Thomaidis N.S., Thomas K.V., Tscharke B.J., Udrisard R., van Nuijs A.L.N., Yargeau V., Zuccato E., Castiglioni S., Ort C. Spatio-temporal assessment of illicit drug use at large scale: evidence from 7 years of international wastewater monitoring. Addiction. 2020;115(1):109–120. doi: 10.1111/add.14767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mao K., Yang Z., Zhang H., Li X., Cooper J.M. Paper-based nanosensors to evaluate community-wide illicit drug use for wastewater-based epidemiology. Water Res. 2021;189 doi: 10.1016/j.watres.2020.116559. [DOI] [PubMed] [Google Scholar]
- 42.Galani A., Alygizakis N., Aalizadeh R., Kastritis E., Dimopoulos M.A., Thomaidis N.S. Patterns of pharmaceuticals use during the first wave of COVID-19 pandemic in Athens, Greece as revealed by wastewater-based epidemiology. Sci Total Environ. 2021;798 doi: 10.1016/j.scitotenv.2021.149014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zaami S., Marinelli E., Varì M.R. New trends of substance abuse during COVID-19 pandemic: an international perspective. Front Psychiatry. 2020:11. doi: 10.3389/fpsyt.2020.00700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hora P.I., Pati S.G., McNamara P.J., Arnold W.A. Increased use of quaternary ammonium compounds during the SARS-CoV-2 pandemic and beyond: consideration of environmental implications. Environ Sci Technol Lett. 2020;7(9):622–631. doi: 10.1021/acs.estlett.0c00437. [DOI] [PubMed] [Google Scholar]
- 45.NEA, 2022. National Environment Agency | List of household disinfectants and self-disinfecting surface coating products against COVID-19 virus. https://www.nea.gov.sg/our-services/public-cleanliness/environmental-cleaning-guidelines/guidelines/list-of-household-products-and-active-ingredients-for-disinfection-of-covid-19.
- 46.Alygizakis N., Galani A., Rousis N.I., Aalizadeh R., Dimopoulos M.A., Thomaidis N.S. Change in the chemical content of untreated wastewater of Athens, Greece under COVID-19 pandemic. Sci Total Environ. 2021;799 doi: 10.1016/j.scitotenv.2021.149230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.La Rosa G., Iaconelli M., Mancini P., Bonanno Ferraro G., Veneri C., Bonadonna L., Lucentini L., Suffredini E. First detection of SARS-CoV-2 in untreated wastewaters in Italy. Sci Total Environ. 2020;736 doi: 10.1016/j.scitotenv.2020.139652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Batéjat C., Grassin Q., Manuguerra J.-C., Leclercq I. Heat inactivation of the severe acute respiratory syndrome coronavirus 2. bioRxiv. 2020 doi: 10.1101/2020.05.01.067769. 2020.2005.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pastorino B., Touret F., Gilles M., de Lamballerie X., Charrel R.N. Heat inactivation of different types of SARS-CoV-2 samples: what protocols for biosafety, molecular detection and serological diagnostics? Viruses. 2020;12(7):735. doi: 10.3390/v12070735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Haramoto E., Kitajima M., Kishida N., Konno Y., Katayama H., Asami M., Akiba M. Occurrence of pepper mild mottle virus in drinking water sources in Japan. Appl Environ Microbiol. 2013;79(23):7413–7418. doi: 10.1128/aem.02354-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mohapatra S., Padhye L.P., Mukherji S. In: Environmental Contaminants. Energy, Environment, and Sustainability. Gupta T., Agarwal A., Agarwal R., Labhsetwar N., editors. Springer; Singapore: 2018. Challenges in Detection of Antibiotics in Wastewater Matrix. [DOI] [Google Scholar]
- 52.Mohapatra S., Snow D., Shea P., Gálvez-Rodríguez A., Kumar M., Padhye L.P., Mukherji S. Photodegradation of a mixture of five pharmaceuticals commonly found in wastewater: Experimental and computational analysis. Environmental Research. 2023;216:114659. doi: 10.1016/j.envres.2022.114659. [DOI] [PubMed] [Google Scholar]
- 53.Priyanka M.S., Mohapatra S. Transactions in Civil and Environmental Engineering. Springer; Singapore: 2020. Shifts and Trends in Analysis of Contaminants of Emerging Concern: Sulfonamides. [DOI] [Google Scholar]
- 54.You L., Nguyen V.T., Pal A., Chen H., He Y., Reinhard M., Gin K.Y. Investigation of pharmaceuticals, personal care products and endocrine disrupting chemicals in a tropical urban catchment and the influence of environmental factors. Sci Total Environ. 2015;536:955–963. doi: 10.1016/j.scitotenv.2015.06.041. [DOI] [PubMed] [Google Scholar]
- 55.Badu K., Oyebola K., Zahouli J.Z.B., Fagbamigbe A.F., de Souza D.K., Dukhi N., Amankwaa E.F., Tolba M.F., Sylverken A.A., Mosi L., Mante P.K., Matoke-Muhia D., Goonoo N. SARS-CoV-2 viral shedding and transmission dynamics: implications of WHO COVID-19 discharge guidelines. Front Med. 2021:8. doi: 10.3389/fmed.2021.648660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Karthikeyan S., Levy J.I., De Hoff P., Humphrey G., Birmingham A., Jepsen K., Farmer S., Tubb H.M., Valles T., Tribelhorn C.E., Tsai R., Aigner S., Sathe S., Moshiri N., Henson B., Mark A.M., Hakim A., Baer N.A., Barber T., Belda-Ferre P., Chacón M., Cheung W., Cresini E.S., Eisner E.R., Lastrella A.L., Lawrence E.S., Marotz C.A., Ngo T.T., Ostrander T., Plascencia A., Salido R.A., Seaver P., Smoot E.W., McDonald D., Neuhard R.M., Scioscia A.L., Satterlund A.M., Simmons E.H., Abelman D.B., Brenner D., Bruner J.C., Buckley A., Ellison M., Gattas J., Gonias S.L., Hale M., Hawkins F., Ikeda L., Jhaveri H., Johnson T., Kellen V., Kremer B., Matthews G., McLawhon R.W., Ouillet P., Park D., Pradenas A., Reed S., Riggs L., Sanders A., Sollenberger B., Song A., White B., Winbush T., Aceves C.M., Anderson C., Gangavarapu K., Hufbauer E., Kurzban E., Lee J., Matteson N.L., Parker E., Perkins S.A., Ramesh K.S., Robles-Sikisaka R., Schwab M.A., Spencer E., Wohl S., Nicholson L., McHardy I.H., Dimmock D.P., Hobbs C.A., Bakhtar O., Harding A., Mendoza A., Bolze A., Becker D., Cirulli E.T., Isaksson M., Schiabor Barrett K.M., Washington N.L., Malone J.D., Schafer A.M., Gurfield N., Stous S., Fielding-Miller R., Garfein R.S., Gaines T., Anderson C., Martin N.K., Schooley R., Austin B., MacCannell D.R., Kingsmore S.F., Lee W., Shah S., McDonald E., Yu A.T., Zeller M., Fisch K.M., Longhurst C., Maysent P., Pride D., Khosla P.K., Laurent L.C., Yeo G.W., Andersen K.G., Knight R. Wastewater sequencing reveals early cryptic SARS-CoV-2 variant transmission. Nature. 2022 doi: 10.1038/s41586-022-05049-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zambrana W., Catoe D., Coffman M.M., Kim S., Anand A., Solis D., Sahoo M.K., Pinsky B.A., Bhatt A.S., Boehm A.B., Wolfe M.K. SARS-CoV-2 RNA and N antigen quantification via wastewater at the campus level, building cluster level, and individual-building level. ACS EST Water. 2022 doi: 10.1021/acsestwater.2c00050. [DOI] [PubMed] [Google Scholar]
- 58.Betancourt W.Q., Schmitz B.W., Innes G.K., Prasek S.M., Pogreba Brown K.M., Stark E.R., Foster A.R., Sprissler R.S., Harris D.T., Sherchan S.P., Gerba C.P., Pepper I.L. COVID-19 containment on a college campus via wastewater-based epidemiology, targeted clinical testing and an intervention. Sci Total Environ. 2021;779 doi: 10.1016/j.scitotenv.2021.146408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Duvallet C., Wu F., McElroy K.A., Imakaev M., Endo N., Xiao A., Zhang J., Floyd-O’Sullivan R., Powell M.M., Mendola S., Wilson S.T., Cruz F., Melman T., Sathyanarayana C.L., Olesen S.W., Erickson T.B., Ghaeli N., Chai P., Alm E.J., Matus M. Nationwide Trends in COVID-19 Cases and SARS-CoV-2 RNA Wastewater Concentrations in the United States. ACS EST Water. 2022 doi: 10.1021/acsestwater.1c00434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Randazzo W., Truchado P., Cuevas-Ferrando E., Simón P., Allende A., Sánchez G. SARS-CoV-2 RNA in wastewater anticipated COVID-19 occurrence in a low prevalence area. Water Res. 2020;181 doi: 10.1016/j.watres.2020.115942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Huisman J.S., Scire J., Caduff L., Fernandez-Cassi X., Ganesanandamoorthy P., Kull A., Scheidegger A., Stachler E., Boehm A.B., Hughes B., Knudson A., Topol A., Wigginton K.R., Wolfe M.K., Kohn T., Ort C., Stadler T., Julian T.R. Wastewater-based estimation of the effective reproductive number of SARS-CoV-2. Environ Health Perspect. 2022;130(5) doi: 10.1289/EHP10050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kaplan E.H., Zulli A., Sanchez M., Peccia J. Scaling SARS-CoV-2 wastewater concentrations to population estimates of infection. Sci Rep. 2022;12(1):3487. doi: 10.1038/s41598-022-07523-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Medema G., Heijnen L., Elsinga G., Italiaander R., Brouwer A. Presence of SARS-coronavirus-2 RNA in sewage and correlation with reported COVID-19 prevalence in the early stage of the epidemic in The Netherlands. Environ Sci Technol Lett. 2020;7(7):511–516. doi: 10.1021/acs.estlett.0c00357. [DOI] [PubMed] [Google Scholar]
- 64.Peccia J., Zulli A., Brackney D.E., Grubaugh N.D., Kaplan E.H., Casanovas-Massana A., Ko A.I., Malik A.A., Wang D., Wang M., Warren J.L., Weinberger D.M., Arnold W., Omer S.B. Measurement of SARS-CoV-2 RNA in wastewater tracks community infection dynamics. Nat Biotechnol. 2020;38(10):1164–1167. doi: 10.1038/s41587-020-0684-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Liu Y., Rocklöv J. The reproductive number of the Delta variant of SARS-CoV-2 is far higher compared to the ancestral SARS-CoV-2 virus. J Travel Med. 2021;28(7) doi: 10.1093/jtm/taab124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Feng S., Roguet A., McClary-Gutierrez J.S., Newton R.J., Kloczko N., Meiman J.G., McLellan S.L. Evaluation of sampling, analysis, and normalization methods for SARS-CoV-2 concentrations in wastewater to assess COVID-19 burdens in wisconsin communities. ACS EST Water. 2021;1(8):1955–1965. doi: 10.1021/acsestwater.1c00160. [DOI] [Google Scholar]
- 67.D'Aoust P.M., Mercier E., Montpetit D., Jia J.-J., Alexandrov I., Neault N., Baig A.T., Mayne J., Zhang X., Alain T., Langlois M.-A., Servos M.R., MacKenzie M., Figeys D., MacKenzie A.E., Graber T.E., Delatolla R. Quantitative analysis of SARS-CoV-2 RNA from wastewater solids in communities with low COVID-19 incidence and prevalence. Water Res. 2021;188 doi: 10.1016/j.watres.2020.116560. 116560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wolfe M.K., Archana A., Catoe D., Coffman M.M., Dorevitch S., Graham K.E., Kim S., Grijalva L.M., Roldan-Hernandez L., Silverman A.I., Sinnott-Armstrong N., Vugia D.J., Yu A.T., Zambrana W., Wigginton K.R., Boehm A.B. Correction for “Scaling of SARS-CoV-2 RNA in settled solids from multiple wastewater treatment plants to compare incidence rates of laboratory-confirmed COVID-19 in Their Sewersheds”. Environ Sci Technol Lett. 2021;8(9) doi: 10.1021/acs.estlett.1c00626. [DOI] [PubMed] [Google Scholar]
- 69.Zhu F.J., Ma W.L., Xu T.F., Ding Y., Zhao X., Li W.L., Liu L.Y., Song W.W., Li Y.F., Zhang Z.F. Removal characteristic of surfactants in typical industrial and domestic wastewater treatment plants in Northeast China. Ecotoxicol Environ Saf. 2018;153:84–90. doi: 10.1016/j.ecoenv.2018.02.001. [DOI] [PubMed] [Google Scholar]
- 70.Alexandre B., Barbara G., Laure W., Bruno D., Adriana G.O., Emmanuelle V. Development of a multiple-class analytical method based on the use of synthetic matrices for the simultaneous determination of commonly used commercial surfactants in wastewater by liquid chromatography-tandem mass spectrometry. J Chromatogr A. 2016;1450:64–75. doi: 10.1016/j.chroma.2016.04.078. [DOI] [PubMed] [Google Scholar]
- 71.Carey D.E., McNamara P.J. The impact of triclosan on the spread of antibiotic resistance in the environment. Front Microbiol. 2015:5. doi: 10.3389/fmicb.2014.00780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.STATISTA, 2022. Revenue hand sanitizers Singapore 2025 | Statista. https://www.statista.com/forecasts/1196375/revenue-hand-sanitizers-singapore.
- 73.Rossmann J., Schubert S., Gurke R., Oertel R., Kirch W. Simultaneous determination of most prescribed antibiotics in multiple urban wastewater by SPE-LC–MS/MS. J Chromatogr B. 2014;969:162–170. doi: 10.1016/j.jchromb.2014.08.008. [DOI] [PubMed] [Google Scholar]
- 74.Butler C.C., Yu L.M., Dorward J., Gbinigie O., Hayward G., Saville B.R., Van Hecke O., Berry N., Detry M.A., Saunders C., Fitzgerald M., Harris V., Djukanovic R., Gadola S., Kirkpatrick J., de Lusignan S., Ogburn E., Evans P.H., Thomas N.P.B., Patel M.G., Hobbs F.D.R. Doxycycline for community treatment of suspected COVID-19 in people at high risk of adverse outcomes in the UK (PRINCIPLE): a randomised, controlled, open-label, adaptive platform trial. Lancet Respir Med. 2021;9(9):1010–1020. doi: 10.1016/s2213-2600(21)00310-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Tran N.H., Reinhard M., Gin K.Y. Occurrence and fate of emerging contaminants in municipal wastewater treatment plants from different geographical regions-a review. Water Res. 2018;133:182–207. doi: 10.1016/j.watres.2017.12.029. [DOI] [PubMed] [Google Scholar]
- 76.Bleyzac N., Goutelle S., Bourguignon L., Tod M. Azithromycin for COVID-19: More Than Just an Antimicrobial. Clin Drug Investig. 2020;40(8):683–686. doi: 10.1007/s40261-020-00933-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kamara I.F., Kumar A.M.V., Maruta A., Fofanah B.D., Njuguna C.K., Shongwe S., Moses F., Tengbe S.M., Kanu J.S., Lakoh S., Mansaray A.H.D., Selvaraj K., Khogali M., Zachariah R. Antibiotic Use In Suspected And Confirmed COVID-19 patients admitted to health facilities in sierra leone in 2020-2021: practice does not follow policy. Int J Environ Res Public Health. 2022;19(7) doi: 10.3390/ijerph19074005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Lauriola M., Pani A., Ippoliti G., Mortara A., Milighetti S., Mazen M., Perseghin G., Pastori D., Grosso P., Scaglione F. Effect of combination therapy of hydroxychloroquine and azithromycin on mortality in patients with COVID-19. Clin Transl Sci. 2020;13(6):1071–1076. doi: 10.1111/cts.12860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Batiha G.E.-S., Zayed M.A., Awad A.A., Shaheen H.M., Mustapha S., Herrera-Calderon O., Pagnossa J.P., Algammal A.M., Zahoor M., Adhikari A., Pandey I., Elazab S.T., Rengasamy K.R.R., Cruz-Martins N., Hetta H.F. Management of SARS-CoV-2 infection: key focus in macrolides efficacy for COVID-19. Front Med. 2021:8. doi: 10.3389/fmed.2021.642313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Lim S.Y.C., Zhou Y.P., Yii D., Chin Z., Hung K.C., Lee L.W., Lim J.L., Loo L.W., Koomanan N., Chua N.G., Liew Y., Cherng B.P.Z., Thien S.Y., Lee W.H.L., Kwa A.L.H., Chung S.J. Stemming the rise of antibiotic use for community-acquired acute respiratory infections during COVID-19 pandemic. Antibiot (Basel) 2022;11(7) doi: 10.3390/antibiotics11070846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.CNA, 2022. Manufacturer GSK increases supply of Panadol in Singapore after recent surge in demand .https://www.channelnewsasia.com/singapore/panadol-supply-surge-demand-gsk-covid-19-2619586.
- 82.Castellana F., De Nucci S., De Pergola G., Di Chito M., Lisco G., Triggiani V., Sardone R., Zupo R. Trends in coffee and tea consumption during the COVID-19 Pandemic. Foods. 2021;10(10):2458. doi: 10.3390/foods10102458. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material.
Data Availability Statement
The data that has been used is confidential.