Abstract
Aims:
To evaluate whether the potent hypophagic and weight-suppressive effects of growth differentiation factor-15 (GDF15) and semaglutide combined would be a more efficacious antiobesity treatment than either treatment alone by examining whether the neural and behavioural mechanisms contributing to their anorectic effects were common or disparate.
Materials/Methods:
Three mechanisms were investigated to determine how GDF15 and semaglutide induce anorexia: the potentiation of the intake suppression by gastrointestinal satiation signals; the reduction in motivation to feed; and the induction of visceral malaise. We then compared the effects of short-term, combined GDF15 and semaglutide treatment on weight loss to the individual treatments. Rat pharmaco-behavioural experiments assessed whether GDF15 or semaglutide added to the satiating effects of orally gavaged food and exogenous cholecystokinin (CCK). A progressive ratio operant paradigm was used to examine whether GDF15 or semaglutide reduced feeding motivation. Pica behaviour (ie, kaolin intake) and conditioned affective food aversion testing were used to evaluate visceral malaise. Additionally, fibre photometry studies were conducted in agouti-related protein (AgRP)-Cre mice to examine whether GDF15 or semaglutide, alone or in combination with CCK, modulate calcium signalling in hypothalamic AgRP neurons.
Results:
Semaglutide reduced food intake by amplifying the feeding-inhibitory effect of CCK or ingested food, inhibited the activity of AgRP neurons when combined with CCK, reduced feeding motivation and induced malaise. GDF15 induced visceral malaise but, strikingly, did not affect feeding motivation, the satiating effect of ingested food or CCK signal processing. Combined GDF15 and semaglutide treatment produced greater food intake and body weight suppression than did either treatment alone, without enhancing malaise.
Conclusions:
GDF15 and semaglutide reduce food intake and body weight through largely distinct processes that produce greater weight loss and feeding suppression when combined.
Keywords: animal pharmacology, antiobesity drug, appetite control, GLP-1 analogue, neuropharmacology, weight control
1 |. INTRODUCTION
Obesity is a chronic disease which negatively impacts human health1 and is increasing in prevalence in the United States and worldwide.2,3 For many individuals, treating obesity with diet and exercise fails to facilitate weight loss or prevent weight regain.4,5 Although bariatric surgery is an effective treatment for weight management, it is invasive, irreversible, and can lead to undesirable side effects.6 Therefore, pharmacological therapies targeting biological pathways with similar efficacy to surgical treatments are needed.7
There is considerable interest in growth differentiation factor-15 (GDF15) as an antiobesity medication. Treatment with recombinant GDF15 reduces food intake and body weight in mice as well as nonhuman primates with obesity.8 Conversely, Gdf15 knockout increases food intake, body weight and adiposity in mice. These studies indicate a potential role for GDF15 in resistance to high-fat-diet-induced obesity9 and energy balance control.10 While these preclinical results highlight GDF15 as a potential antiobesity medication, there is a growing focus on developing combination obesity therapies to enhance efficacy. Recent evidence suggests cotreatment with glucagon-like peptide-1 (GLP-1) receptor agonists is an efficacious strategy.11–13 Semaglutide, a long-acting GLP-1 receptor agonist recently approved by the US Food and Drug Administration (FDA) for weight management, sets a new standard for monotherapies by achieving double-digit weight loss in patients with overweight/obesity.14,15 The combination of semaglutide with the long-acting amylin receptor agonist cagrilintide yields superior body weight loss to that achieved with semaglutide alone.11,14,15 These findings stress the value of GLP-1 receptor agonist-based dual therapies and highlight the potential for GDF15 and semaglutide combination therapy to produce greater food intake and weight suppression.
Achieving greater efficacy with drug combination would be more likely if the neuro-behavioural processes mediating the anorectic effect of each drug were distinct. We have previously shown that the food-intake-suppressive effect of GDF15 is mediated in part through induction of visceral malaise.16 GDF15-treated rats increased kaolin consumption,16,17 an accepted marker of nausea/malaise in nonvomiting species,18,19 and treated rats also displayed conditioned disgust/aversive reactions to foods associated with GDF15 treatment.16,20 Furthermore, there is conclusive evidence that GDF15 is an emetogenic agent in the shrew model.17 However, it is unknown whether additional neural and behavioural processes, such as reduced appetitive motivation or interactions with gastrointestinal (GI) satiation signals, such as cholecystokinin (CCK), contribute to the anorectic effect of GDF15. Clinical and preclinical data show that the GLP-1 receptor agonist exenatide engages multiple processes to reduce feeding and body weight, including enhancement of intake suppression by GI satiation signals and attenuation of appetitive processes such as food-seeking, motivation and reward.21–25 Additionally, although semaglutide induces nausea in human subjects, the mechanisms mediating its food-intake-suppressive effect have not been fully explored. Given semaglutide’s substantially different pharmacodynamic and pharmacokinetic profiles when compared with exenatide,26 it is important to investigate the mechanisms that mediate its food-intake- and body-weight-suppressive effects.
In this study, we used rat pharmaco-behavioural and mouse neural calcium signalling approaches to assess the processes mediating the anorectic effects of GDF15 and semaglutide. First, we examined whether GDF15 or semaglutide impact feeding motivation using a progressive ratio schedule of operant reinforcement. We next examined whether GDF15 or semaglutide interact with GI satiation signals to yield greater inhibition of food intake and hypothalamic agouti-related protein (AgRP) calcium signalling. Lastly, we tested whether combination treatment produces greater effects through actions on largely distinct neural substrates.27,28 To test this hypothesis, we examined the combinatorial effects of the long-acting GDF15 analogue Fc-GDF1516,29 and semaglutide on food intake, kaolin consumption and body weight.
2 |. MATERIALS AND METHODS
2.1 |. Experimental models and subject details
Adult male Sprague-Dawley rats (~250-265 g on arrival; Charles River, Wilmington, Massachusetts) and Agrp-IRES-Cre (Jackson Laboratories 012899, Agrptm1[cre]Lowl/J)30 mice were housed under a 12-hour light:12-hour dark cycle in a temperature- and humidity-controlled vivarium. Rats were individually housed in a hanging wire-bottomed cage with ad libitum access to a chow diet (Purina Laboratory Diet 5001; Nestlé-Purina, St Louis, Missouri) and tap water for all experiments except in Experiment 4, in which rats were food-deprived for 18 hours prior to experimentation. Rats in Experiments 1 and 6 also had ad libitum access to kaolin pellets (K50001, Research Diets, New Brunswick, USA). Rats were habituated to kaolin for at least 5 days prior to measuring kaolin consumption in pica testing. Mice were group-housed with ad libitum access to a standard rodent chow (Rodent 5001, LabDiet, St. Louis, USA) and water unless noted otherwise. Adult (at least 8 weeks old) male and female mice were used for fibre photometry experiments. All animals were naive to any experimental drug and test and were habituated to handling and experimental conditions prior to experimentation. All rat procedures conformed to the institutional standards of the University of Pennsylvania Institutional Animal Care and Use Committee and were consistent with the National Institutes of Health Guide for the Care and Use of Laboratory Animals. All mice procedures were approved by the Monell Chemical Senses Centre and University of Pennsylvania Institutional Animal Care and Use Committees.
2.2 |. Methods details
For detailed experimental designs and methods see supplementary information.
2.3 |. Statistical analysis
All rat experiments were analysed using Prism 8 GraphPad Software (San Diego, California). In all acute studies, food intake, kaolin intake, lever presses and body weight change data were analysed using ordinary or repeated-measures one-way or two-way ANOVA; when indicated Tukey’s or Holm-Sidak’s were used for tests of multiple comparisons. Semaglutide and rh-GDF15’s effect on pellets earned were analysed via paired Students t-tests. For the analysis of conditioned taste aversion and the effects of semaglutide and Fc-GDF15 cotreatment, a longitudinal mixed-effects model using a compound symmetry covariance matrix was used, followed by Tukey’s post hoc test. All fibre photometry data were analysed using two-way repeated-measures ANOVA, followed by a Holm-Sidak, or paired two-tailed t-test as appropriate. All body weight changes were calculated by subtracting body weight on any given post-treatment day from the rat’s weight on the day of injection. All data are expressed as mean ± SEM. Information on replicates and significance is reported in the figure legends.
3 |. RESULTS
3.1 |. Experiment 1: Systemic semaglutide administration dose-dependently induced anorexia; higher doses also produced pica behaviour
Using a rat model, we examined the effects of semaglutide treatment on food intake, kaolin intake and body weight. Semaglutide reduced food intake in a dose-dependent manner (Figure 1A). While 1 nmol/kg had no effects on chow intake, administration of 3, 10 and 30 nmol/kg semaglutide significantly reduced chow intake from 24 to 96 hours relative to vehicle (all P < 0.0001). At 24 hours, the average percent food intake reduction was as follows: 3 nmol/kg: −20 ± 3%; 10 nmol/kg: −41 ± 5%; and 30 nmol/kg: −69 ± 2%, compared to vehicle (all P < 0.0001). The two highest doses, 10 and 30 nmol/kg, also triggered a pica response (ie, kaolin intake), while 3 nmol/kg did not (Figure 1B). Beginning at 4 hours and up to 96 hours post-drug administration, 30 nmol/kg semaglutide significantly increased kaolin intake, while 10 nmol/kg only significantly increased kaolin intake at 24 hours post-drug administration relative to vehicle (both P < 0.05). Semaglutide also dose-dependently reduced body weight at 24 hours (all P < 0.0001; Figure 1C), with 10 and 30 nmol/kg significantly reducing body weight for the duration of the experiment compared to vehicle (all P < 0.0001).
FIGURE 1.
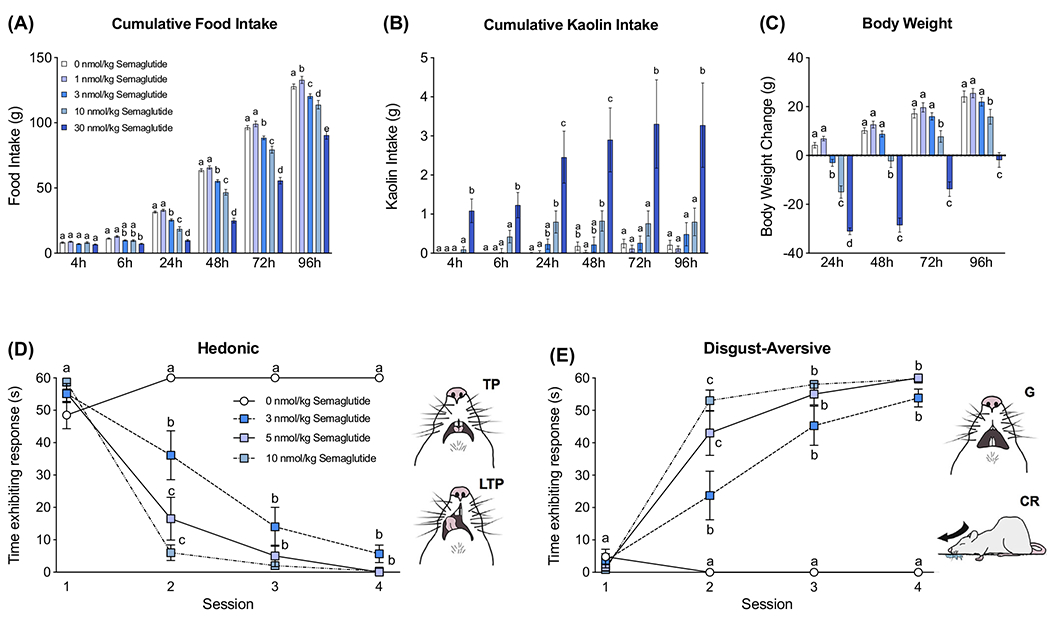
Systemic delivery of semaglutide produces anorexia, weight loss, pica behaviour and a conditioned taste aversion. A, Systemically delivered semaglutide dose-dependently reduced food intake (1, 3, 10 and 30 nmol/kg, n = 16/group). B, Semaglutide administered systemically induced kaolin intake (ie, pica behaviour, a proxy for visceral malaise in rodents; 1, 3, 10 and 30 nmol/kg, n = 16/group). C, The anorectic effect of semaglutide was paralleled by body weight loss (1, 3, 10 and 30 nmol/kg, n = 16/group). D, E, Successive associations of semaglutide (s.c. doses of 3, 5 and 10 nmol/kg) with a food stimulus decreased time spent expressing hedonic (D) and increased disgust/aversive (E) responses (n = 6-7/group). In (D) and (E), representative sketches taken from single frames of a videotape depicting hedonic responses (ie, tongue protrusions [TP] and lateral tongue protrusions [LTP]) and disgust/aversive affective responses (ie, gapes [G] and chin rubs [CR]) are shown next to the respective graphs. All data are expressed as mean ± SEM. A, B, and C were analysed with a repeated-measures two-way ANOVA, followed by the Tukey’s post hoc test. D and E were analysed using a longitudinal mixed-effects model using a compound symmetry covariance matrix, followed by Tukey’s post hoc test. Means with different letters are significantly different from each other (P < 0.05)
3.2 |. Experiment 2: Systemic semaglutide dose-dependently conditioned affective food aversion
To examine whether semaglutide conditions an affective food aversion, we used the taste reactivity paradigm to analyse orofacial and other affective responses elicited by food stimuli associated with drug treatment. Before treatment (ie, Session 1) all rats showed primarily hedonic responses to a novel, palatable food taste. After one, two and three drug-food associations (Sessions 2, 3 and 4; Figures 1D,E, respectively), all anorectic doses of semaglutide conditioned a significant decrease in the time spent displaying hedonic responses (Figure 1D) and a significant increase in time spent displaying disgust-aversive responses to the same taste across sessions (Figure 1E).
3.3 |. Experiment 3: Semaglutide but not rh-GDF15 reduced motivation to work for sucrose pellets
To investigate whether semaglutide or rh-GDF15 treatment altered motivation to work for a palatable food, we measured operant responding for sucrose pellets under a progressive ratio schedule of reinforcement. Semaglutide reduced operant responding for sucrose pellets at 6 hours post semaglutide injection (Figure 2A), such that 10 nmol/kg semaglutide reduced active lever presses compared with vehicle (P < 0.01), without affecting inactive lever presses. Rats treated with 10 nmol/kg semaglutide also earned significantly fewer pellets than those treated with vehicle (P < 0.01; Figure 2B).
FIGURE 2.
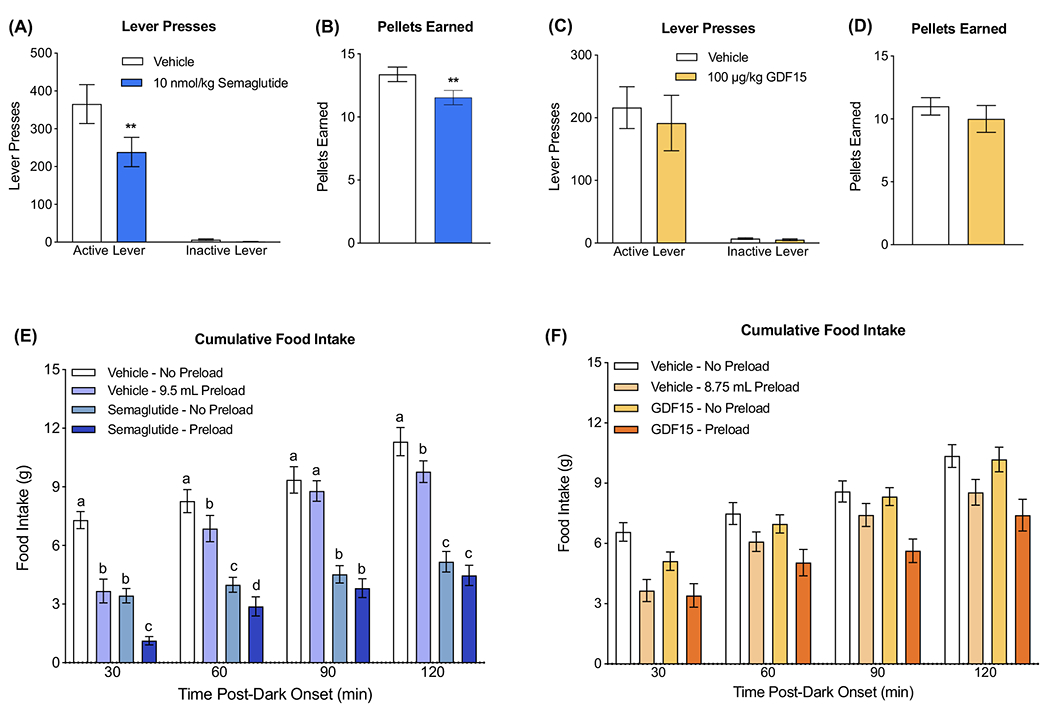
Semaglutide but not growth differentiation factor 15 (GDF15) reduces motivation to work for palatable food and adds to the food-intake-inhibitory effects of a mixed-meal preload. A, Semaglutide (10 nmol/kg) significantly reduced lever presses for sucrose pellets on a progressive ratio schedule of reinforcement (n = 13/group). B, Semaglutide (10 nmol/kg) reduced the number of sucrose pellets earned (n = 13/group). C, GDF15 (100 μg/kg) did not affect lever presses for sucrose pellets on a progressive ratio schedule of reinforcement (n = 10/group). D, GDF15 (100 μg/kg) did not affect pellets earned (n = 10/group). E, Semaglutide (10 nmol/kg) amplified the food-intake-inhibitory effects of a mixed-meal preload (9.5 mL Ensure) relative to either individual treatment at 30 and 60 minutes post-mixed-meal delivery (n = 14/group). Semaglutide suppressed feeding at all tested time points, while preload suppressed intake at 30, 90 and 120 minutes. F, GDF15 (100 μg/kg) did not impact the food-intake-inhibitory effects of a mixed-meal preload at any time point tested (8.75 mL Ensure; n = 11/group). All data are expressed as mean ± SEM. A, C, E and F were analysed with repeated-measures two-way ANOVA, followed by the Tukey’s or Holm-Sidak’s post hoc test. Means with different letters are significantly different from each other (P < 0.05). (B) and (D) were analysed with paired Students t-tests. **P < 0.01
In contrast to semaglutide, 100 μg/kg rh-GDF15 did not impact operant responding or pellets earned 8 hours post-injection (Figure 2C,D). Similarly, there were no effects on operant responding or pellets earned at 4 or 24 hours post-injection (S1A-D). However, at all time points tested (4, 8 and 24 hours), animals pressed significantly more on the active versus the inactive lever under both vehicle and rh-GDF15 treatment (all P < 0.001). This indicates that rats could discriminate between the active and inactive lever.
3.4 |. Experiment 4: Semaglutide but not rh-GDF15 potentiated the intake-inhibitory effect of a mixed-meal preload
To examine whether semaglutide or GDF15 interact with the intake-inhibitory effects of GI satiation signals released by a meal, the drugs were separately administered in combination with a mixed-meal preload. This preload is known to stimulate the release of GI satiation signals, for example, CCK, GLP-1 and peptide YY.31–34 When combined with the meal preload, semaglutide significantly added to the food intake suppression (Figure 2E). Rats treated with semaglutide in combination with a mixed-meal preload reduced chow intake by 84 ± 3% compared to those treated with vehicle, while individually semaglutide or preload reduced intake by 52 ± 5% and 50 ± 8%, respectively, at 30 minutes post-preload delivery (all P values <0.0001). There was a similar trend 60 minutes post-preload delivery, where rats treated with the combination of semaglutide and preload significantly reduced food intake compared to either treatment alone (all P < 0.05). Semaglutide significantly reduced food intake at all time points tested, while preload significantly reduced food intake at 30, 60 and 120 minutes (all P < 0.01).
By contrast, when rh-GDF15 was combined with a mixed-meal preload, there was no additive effect on food intake suppression compared to either treatment alone (main effect of preload, but no main effect of GDF15; Figure 2F). In follow-up experiments that examined other doses (rh-GDF15; 2, 10 and 20 μg/kg) and other preload meal volumes (3 and 6.5 mL) combinations, there was also no effect of combining GDF15 and the satiating effects of a preload (Figure S1E–S1G).
3.5 |. Experiment 5: Semaglutide, but not rh-GDF15, amplified CCK-induced AgRP neuron inhibition
Hypothalamic AgRP-expressing neurons are highly active during hunger35,36 and are inhibited by the GI satiation signal CCK at high doses.37 To determine whether treatment with 100 μg/kg rh-GDF15 or 10 nmol/kg semaglutide synergized with the effects of a low and ineffective CCK dose (3μg/kg) on AgRP neuron activity, we used fibre photometry to monitor calcium signalling (GCaMP6s fluorescence) as a proxy for the neural activity of these neurons in AgRP-Cre mice (Figure 3A,B). For a positive control, mice were treated with 30 μg/kg CCK, which resulted in robust inhibition of AgRP neuron activity (Figure 3C), consistent with previous findings.37,38 When administered alone, neither rh-GDF15 nor semaglutide directly affected AgRP neuron activity (no main effect of treatment; Figures 3D–G).
FIGURE 3.
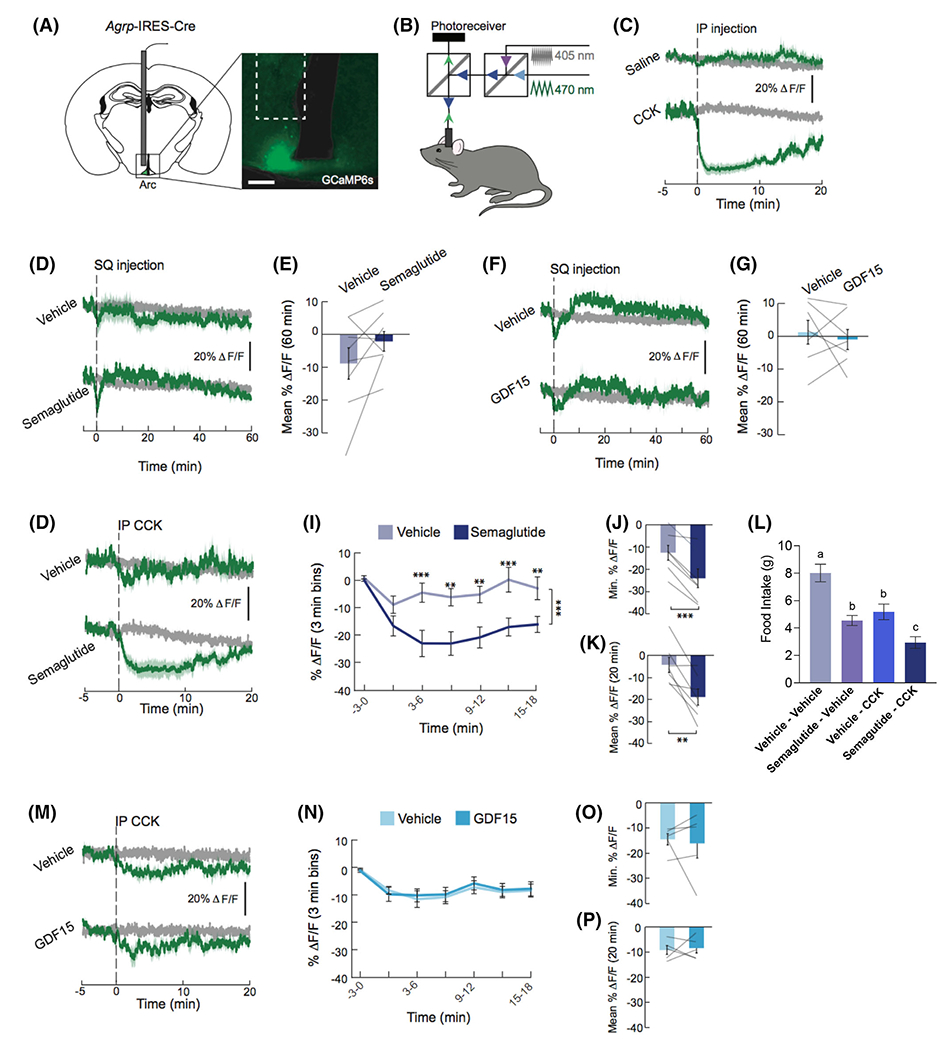
Semaglutide but not growth differentiation factor 15 (GDF15) potentiates cholecystokinin (CCK)-induced inhibition of agouti-related protein (AgRP) neuron activity. A, Representative image showing GCaMP6s expression in AgRP neurons of Agrp-IRES-Cre mice. Scale bar, 200 μm. B, Schematic of the dual-wavelength fibre photometry set-up used to monitor AgRP neuron activity. C, CCK (30 μg/kg) robustly inhibits AgRP neuron activity (n = 6 /group). Traces represent ΔF/F of GCaMP6s signals in AgRP neurons. Signals are aligned to intraperitoneal (IP) injection. Green: 470-nm calcium signal; grey: 405-nm control signal. Dark lines represent means and lighter shaded areas represent SEM. D, Semaglutide (10 nmol/kg) did not alter AgRP neuron calcium activity (n = 8/group). Traces represent average ΔF/F of GCaMP6s signals in AgRP neurons. Signals are aligned to injection of vehicle or semaglutide. E, Mean ΔF/F of the 470-nm signal from 0 to 60 minutes. F, GDF15 (100 μg/kg) did not alter AgRP neuron calcium activity (n = 7/group). Traces represent average ΔF/F of GCaMP6s signals in AgRP neurons. Signals are aligned to injection of vehicle or GDF15. G, Mean ΔF/F of the 470-nm signal from 0 to 60 min. H, Semaglutide (10 nmol/kg) augments CCK (3 μg/kg)-induced reductions in calcium signalling in AgRP neurons (n = 7/group). Traces represent ΔF/F of GCaMP6s signals in AgRP neurons. I, Mean ΔF/F of the 470-nm signal (3-minute bins). J, Minimum ΔF/F of the 470-nm signal. K, Mean ΔF/F of the 470-nm signal from 0 to 20 minutes. L, Semaglutide (10 nmol/kg) and CCK (3 μg/kg) treatments each suppressed feeding. Dual treatment produced greater food intake suppression relative to either treatment alone at 30 minutes post-CCK injection (n = 10/group). M, GDF15 (100 μg/kg) did not alter CCK (3 μg/kg)-induced reductions in calcium signals in AgRP neurons (n = 5/group). Traces represent ΔF/F of GCaMP6s signals in AgRP neurons. Signals are aligned to CCK injection. Green: 470-nm calcium signal; grey: 405-nm control signal. Dark lines represent means and lighter shaded areas represent SEM. N, Mean ΔF/F of the 470-nm signal (3-minute bins). O, Minimum ΔF/F of the 470-nm signal. P, Mean ΔF/F of the 470-nm signal from 0 to 20 minutes. All data are expressed as mean ± SEM. E, G, J, K, N and O were analysed using a paired Student’s t-tests. I and M were analysed with a repeated-measures two-way ANOVA, followed by a Holm-Sidak post hoc test. **P < 0.01 and ***P < 0.001
We next explored whether combining semaglutide or GDF15 with CCK inhibits AgRP neuron activity. Mice were treated with 3 μg/kg CCK, which alone does not inhibits AgRP neuron activity37. Pretreatment with semaglutide (Figure 3H–K) but not rh-GDF15 (Figure 3M–P) significantly augmented AgRP neuron inhibition induced by a low dose of CCK (3 μg/kg).
The effect of semaglutide on CCK-driven inhibition of AgRP neuron calcium signalling led us to investigate whether semaglutide would also amplify CCK-induced food intake inhibition in a rat model. Semaglutide combined with CCK produced a significantly greater magnitude of intake inhibition than CCK or semaglutide alone (Figure 3L). Semaglutide/CCK cotreatment produced a 61 ± 7% suppression in food intake relative to vehicle, while semaglutide and CCK individually decreased intake by 40 ± 5% and 34 ± 6% compared to vehicle (all P < 0.05).
3.6 |. Experiment 6: Combined semaglutide and GDF15 treatment induced additive effects on anorexia and weight loss but not on pica
In this study, we examined whether the combination treatment of long-acting Fc-GDF15 and GLP-1 receptor agonist, semaglutide, would produce greater effects on anorexia, weight loss, and pica behaviour compared to either drug administered alone. The magnitude of food intake inhibition in rats treated with the combination of 10 nmol/kg semaglutide and 50 μg/kg Fc-GDF15 was significantly greater than either treatment across time (Figure 4A). On Days 2, 3 and 5 to 8, the combined treatment significantly reduced intake compared with Fc-GDF15 or semaglutide alone, indicating greater anorexia with combination therapy (all P values <0.05). Maximal food intake suppression after cotreatment occurred on Day 2, with a 73 ± 3% food intake reduction compared with vehicle, while each treatment alone only resulted in 36 ± 3% and 38 ± 3% reductions in intake (all P values <0.01). Importantly, the combination treatment also produced significantly greater weight loss than either treatment alone throughout the experiment. Weight loss on Days 2, 3 and 5 to 7 of rats receiving the combined treatment was significantly greater than those treated with either treatment alone (all P < 0.05; Figure 4B). On Day 5, the largest body weight suppression after cotreatment occurred, with a 14 ± 1% reduction compared to baseline weight, while Fc-GDF15 led to a 5 ± 1% and semaglutide led to a 1 ± 1% reduction (all P values <0.05).
FIGURE 4.

Combined semaglutide and growth differentiation factor 15 (GDF15) treatment induced additive effects on anorexia and weight loss but not on pica. A, Semaglutide (10 nmol/kg) and Fc-GDF15 (50 μg/kg) treatments each suppressed feeding. Semaglutide/Fc-GDF15 cotreatment produced a greater magnitude of food intake suppression than either treatment alone over the 8-day period (n = 7-9/group). B, Body weight loss following dual treatment was significantly greater compared to each treatment alone (n = 7-9/group). C, Co-treating semaglutide with Fc-GDF15 did not amplify kaolin intake compared to Fc-GDF15 treatment alone over an 8-day period (n = 7-9/group). All data are expressed as mean ± SEM and analysed using a longitudinal mixed-effects model using a compound symmetry covariance matrix, followed by Tukey’s post hoc test. Means with different letters are significantly different from each other (P < 0.05)
In contrast to the greater effects of combination treatment on body weight loss and food intake inhibition, kaolin intake was not greater after combined treatment, indicating no additive effect on pica behaviour with semaglutide cotreatment (Figure 4C). Fc-GDF15 significantly elevated pica beginning on Day 4 and up to the last day, Day 8, relative to vehicle (P < 0.05) and beginning on Day 5 relative to semaglutide (P < 0.01). For the duration of the experiment, kaolin intake after Fc-GDF15 treatment did not significantly differ from than the combination treatment.
4 |. DISCUSSION
To evaluate the potential of semaglutide and GDF15 combined treatment as a novel antiobesity medication, we first examined whether the food- intake- inhibitory actions of semaglutide and GDF15 monotreatments were mediated by distinct processes. We then assessed their combined effects on food intake, body weight loss and visceral malaise in a short-term treatment paradigm. Results showed that the anorectic effects of the two treatments were indeed mediated by largely different processes, and the combined treatment produced greater intake and weight suppression than either treatment alone.
The anorectic response following semaglutide was mediated, in part, by its additive effects on the feeding-suppressive effect of gavaged food or CCK as well as by suppressing appetitive feeding motivation. Three types of complementary data support semaglutide’s additive effect on food or CCK signalling responses. First, combining semaglutide with a satiating, mixed-meal preload (ie, the Ensure diet) produced a larger magnitude of feeding inhibition. Second, in contrast to the effect of either treatment alone, semaglutide/CCK dual adminstration inhibited AgRP-neuron calcium signalling. Third, semaglutide and CCK dual treatment produced greater anorexia than either treatment alone. These data are consistent with clinical and preclinical work showing that other GLP-1 receptor agonists enhance fullness in human subjects and rodents.39,40,41 The combination of current and prior evidence strongly supports the notion that GLP-1 receptor signalling amplifies the neural processing of GI signals to reduce food intake.
In addition to potentiating the neural and behavioural effects of GI signals, semaglutide treatment also reduced rats’ willingness to work for palatable food on a progressive ratio operant responding task. These results are consistent with the appetitive behaviour-reducing effects of other long-acting GLP-1 receptor agonists that decrease affective food liking40 and food cue-driven neuronal activation of appetitive feeding centres in the brain25 in human subjects with obesity or type 2 diabetes. Consistent with these human data, results from animal studies show pharmacological GLP-1 receptor activation of distinct brain nuclei reduces food seeking, motivation, and reward.22–24,42 Taken together, results show that the intake-inhibitory effect of semaglutide is mediated by several neural and behavioural processes.
In contrast to our findings for semaglutide, GDF15 treatment failed to interact with the feeding-inhibitory effects of GI signals. These findings are supported by follow-up studies where the effects of three different combinations of GDF15 doses and Ensure volumes (Figure S1E–G) also failed to amplify anorexia (Figure 2H). Furthermore, GDF15, alone or in combination with CCK, did not affect hypothalamic AgRP calcium signaling. GDF15 treatment also did not impact operant responding to obtain sucrose pellets in a progressive ratio paradigm, suggesting that GDF15 also does not affect feeding motivation. Additional analyses examining whether GDF15 altered feeding motivation at two other time points (Figure S1A–D) showed comparable results (ie, no impact on motivation to work for sucrose pellets at all time points). Collectively, the pattern of results was consistent across all design variants, highlighting visceral malaise as the primary mechanism mediating the anorectic effects of GDF15.
The absence of an effect of GDF15 on GI signals and feeding motivation is notable. The area postrema and adjacent nucleus tractus solitarius (AP/NTS) of the dorsal medulla are well known for their role in integrating and processing GI satiation signals.43 Given the exclusive hindbrain expression of the GDF15 receptor GFRAL,8,28 it is surprising that GDF15 does not reduce food intake by modulating the effects of GI satiation signals. It is also interesting that GDF15 does not reduce feeding motivation, as there is an accumulating body of evidence that other NTS-acting signals impact food intake through alterations in feeding motivation.22,44–46 In fact, many AP/NTS-acting signals affect food intake via one or both mechanisms. Although the intake-suppressive effects of GDF15 contrast with those of other hindbrain-acting signals, the current data are consistent with neuro-anatomical evidence showing that GFRAL neurons are not activated by gastric lipid load or CCK,47 and with emerging evidence defining the neuronal phenotype of GFRAL neurons and neural circuitry mediating GDF15 anorexia. Importantly, these data suggest that GDF15 acts on a population of neurons that do not modulate the processing of satiation signals or feeding motivation.47–49 Our results show that GDF15 systemic administration, alone or in combination with CCK, fails to produce an effect on hypothalamic AgRP neuron signalling, consistent with the overwhelming consensus that GDF15-induced anorexia is driven primarily by causing malaise.16
In contrast to the known malaise-inducing effects of all anorectic GDF15 doses,16,17 in the current investigation of semaglutide, two of the three semaglutide doses that significantly decreased food intake also increased kaolin consumption, while the lower dose did not. By contrast, all anorectic doses of semaglutide conditioned disgust/aversive affective responses when associated with a palatable food. These novel preclinical pica and affective conditioned food aversion data are consistent with reports that GLP-1-receptor-based treatment in humans is often accompanied by GI adverse effects. Countless medical reports highlight nausea and emesis as the principal reported side effect and major reason for treatment discontinuation with existing GLP-1-receptor-based agents, including semaglutide.50,51 Importantly, these adverse effects are caused by direct central activation of various GLP-1-receptor-expressing nuclei, including those located in the hindbrain. The use of uptitration designs in semaglutide and in the prescribing protocols for other GLP-1 receptor agonists14,15 are partially effective in limiting the percentage of subjects experiencing GI adverse effects, reducing patient attrition.52 Whether similar titration and dose escalation paradigms using just GDF15 or semaglutide and GDF15 combination treatment can mitigate visceral malaise in chronic, preclinical models remains an avenue for future research. More generally, the efficacy and feasibility of GDF15-based therapeutics, alone or in combination, as an obesity treatment in humans remains to be clarified. Similarly, whether the overall beneficial effects of GDF15-based treatments outweigh their side effects (ie, visceral malaise), as in the case of various FDA-approved GLP-1 analogues, is an empirical question that awaits clinical assessment.
Considering that GDF15 and semaglutide reduce food intake through mostly distinct mechanisms, it is not surprising that the individual ligands activate largely distinct neural elements. While GFRAL-RET is expressed solely in the AP/NTS,8,28 GLP-1 receptor is expressed in numerous and anatomically distributed sites across the CNS.53 From pharmacological and genetic manipulation studies, it is clear that food intake suppression can be triggered by increasing GLP-1-receptor signalling in multiple, individual sites. While there is a small subgroup of AP/NTS neurons that express both GFRAL-RET and GLP-1 receptor54–56 the clear majority of GLP-1-receptor-expressing neurons within AP/NTS do not co-express GFRAL. As a result, even within the same nuclei, GDF15 and semaglutide do not activate the same neuronal populations.47 These largely distinct receptor systems lead to independent effects on energy balance, supported by data showing Glp1r knockout does not impact GDF15-induced anorexia and body weight loss, and Gfral knockout does not prevent liraglutide’s effects on energy balance.27,28
Given our findings highlighting the largely independent effects of GDF15 and semaglutide on energy balance, we examined the combined, short-term effects of semaglutide and a long-acting Fc-GDF15 administration. In combination, semaglutide and Fc-GDF15 produced significantly greater food intake suppression and body weight loss than either drug alone. While it has previously been shown that acute combination of human GDF15 with liraglutide, a GLP-1 receptor agonist of lesser efficacy, led to greater food intake and weight loss in mice,27 our results showing short-term administration of semaglutide and Fc-GDF15 combined produce greater anorexia and weight loss without increasing pica provide useful additional information. Despite evidence that each drug independently produces visceral malaise, when the two drugs were combined there was not a greater magnitude of kaolin intake. This suggests that both drugs in combination have little if any additive or additional negative impact on tolerability from a malaise perspective. Although further work using different treatment regimens and doses is warranted, the greater feeding and body weight effects without an increase in adverse events suggest this drug combination merits clinical evaluation as an antiobesity medication.
We conclude that the food-intake-inhibitory effect underlying the impressive weight-reducing effect of semaglutide is mediated by three processes: (1) potentiating the neural and behavioural effects of CCK or GI food; (2) reducing motivation to work for preferred foods; and (3) inducing visceral malaise. By contrast, the feeding-suppressive effect of GDF15 resulted primarily from inducing visceral malaise16 as it does not alter the neural and behavioural effects of CCK or feeding motivation. Importantly, we showed that short-term combination of semaglutide and GDF15 greatly suppressed food intake and body weight, probably due to their separate actions on energy balance. Therefore, semaglutide and GDF15 combination therapy may represent a novel and efficacious strategy for weight reduction that merits clinical investigation.
Supplementary Material
ACKNOWLEDGMENTS
The authors would like to thank Nitsan Goldstein and Juliana Vollmer for technical assistance and Bei Zhang, Zhidan Wu and Don Bennett for their valuable suggestions. This work was supported by NIH-DK21397 (to H.J.G.), NIH-DK119574 (to A.L.A.), NIH-DK-112812 (to B.C.D.), the Swiss National Science Foundation (P400PB-186728 to T.B.), and by research funds from Pfizer, Inc (to H.J.G.).
Funding information
National Institute of Diabetes and Digestive and Kidney Diseases, Grant/Award Numbers: 112812, 119574, 21397; Pfizer, Inc; Schweizerischer Nationalfonds zur Förderung der Wissenschaftlichen Forschung, Grant/Award Number: P400PB-186728
CONFLICT OF INTEREST
Research funding from Pfizer to H.J.G. was used to partially support these studies. H.J.G. is a consultant and advisory board member for Novo Nordisk. B.C.D. also received funding from Eli Lilly that was not used in support of these studies. T.B. and B.C.D. are co-inventors and owners of a patent for a proprietary compound related to the GDF15/GFRAL system (serial no. 62/801391). D.B. is an employee of Pfizer. All other authors declare no competing interests.
Footnotes
SUPPORTING INFORMATION
Additional supporting information may be found in the online version of the article at the publisher’s website.
DATA AVAILABILITY STATEMENT
The raw data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1.Bray GA, Kim KK, JPH W, World Obesity F. Obesity: a chronic relapsing progressive disease process. A position statement of the World Obesity federation. Obes Rev. 2017;18(7):715–723. [DOI] [PubMed] [Google Scholar]
- 2.Ng M, Fleming T, Robinson M, et al. Global, regional, and national prevalence of overweight and obesity in children and adults during 1980-2013: a systematic analysis for the global burden of disease study 2013. Lancet. 2014;384(9945):766–781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hales CM, Carroll MD, Fryar CD, Ogden CL. Prevalence of obesity and severe obesity among adults: United States, 2017-2018. In: 2020. [PubMed]
- 4.Unick JL, Hogan PE, Neiberg RH, et al. Evaluation of early weight loss thresholds for identifying nonresponders to an intensive lifestyle intervention. Obesity (Silver Spring). 2014;22(7):1608–1616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Christian JG, Tsai AG, Bessesen DH. Interpreting weight losses from lifestyle modification trials: using categorical data. Int J Obes (Lond). 2010;34(1):207–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Azagury DE, Morton JM. Bariatric surgery: overview of procedures and outcomes. Endocrinol Metab Clin North Am. 2016;45(3):647–656. [DOI] [PubMed] [Google Scholar]
- 7.Ryan DH. Semaglutide for obesity: four STEPs forward, but more to come. Lancet Diabetes Endocrinol. 2021;9(5):252–254. [DOI] [PubMed] [Google Scholar]
- 8.Mullican SE, Lin-Schmidt X, Chin CN, et al. GFRAL is the receptor for GDF15 and the ligand promotes weight loss in mice and nonhuman primates. Nat Med. 2017;23(10):1150–1157. [DOI] [PubMed] [Google Scholar]
- 9.Tsai VW, Zhang HP, Manandhar R, et al. GDF15 mediates adiposity resistance through actions on GFRAL neurons in the hindbrain AP/NTS. Int J Obes (Lond). 2019;43(12):2370–2380. [DOI] [PubMed] [Google Scholar]
- 10.Tsai VW, Macia L, Johnen H, et al. TGF-b superfamily cytokine MIC-1/GDF15 is a physiological appetite and body weight regulator. PLoS One. 2013;8(2):e55174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Enebo LB, Berthelsen KK, Kankam M, et al. Safety, tolerability, pharmacokinetics, and pharmacodynamics of concomitant administration of multiple doses of cagrilintide with semaglutide 2.4 mg for weight management: a randomised, controlled, phase 1b trial. Lancet. 2021;397(10286):1736–1748. [DOI] [PubMed] [Google Scholar]
- 12.Gimeno RE, Briere DA, Seeley RJ. Leveraging the gut to treat metabolic disease. Cell Metab. 2020;31(4):679–698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Frias JP, Nauck MA, Van J, et al. Efficacy and safety of LY3298176, a novel dual GIP and GLP-1 receptor agonist, in patients with type 2 diabetes: a randomised, placebo-controlled and active comparator- controlled phase 2 trial. Lancet. 2018;392(10160):2180–2193. [DOI] [PubMed] [Google Scholar]
- 14.Wilding JPH, Batterham RL, Calanna S, et al. Once-weekly Semaglutide in adults with overweight or Obesity. N Engl J Med. 2021;384(11):989–1002. [DOI] [PubMed] [Google Scholar]
- 15.Wadden TA, Bailey TS, Billings LK, et al. Effect of subcutaneous Semaglutide vs placebo as an adjunct to intensive behavioral therapy on body weight in adults with overweight or Obesity: the STEP 3 randomized clinical trial. JAMA. 2021;325(14):1403–1413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Borner T, Wald HS, Ghidewon MY, et al. GDF15 induces an aversive visceral malaise state that drives anorexia and weight loss. Cell Rep. 2020;31(3):107543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Borner T, Shaulson ED, Ghidewon MY, et al. GDF15 induces anorexia through nausea and emesis. Cell Metab. 2020;31(2):351–362e355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mitchell D, Wells C, Hoch N, Lind K, Woods SC, Mitchell LK. Poison induced pica in rats. Physiol Behav. 1976;17(4):691–697. [DOI] [PubMed] [Google Scholar]
- 19.Takeda N, Hasegawa S, Morita M, Matsunaga T. Pica in rats is analogous to emesis: an animal model in emesis research. Pharmacol Biochem Behav. 1993;45(4):817–821. [DOI] [PubMed] [Google Scholar]
- 20.Patel S, Alvarez-Guaita A, Melvin A, et al. GDF15 provides an endocrine signal of nutritional stress in mice and humans. Cell Metab. 2019;29(3):707–718 e708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Konanur VR, Hsu TM, Kanoski SE, Hayes MR, Roitman MF. Phasic dopamine responses to a food-predictive cue are suppressed by the glucagon-like peptide-1 receptor agonist Exendin-4. Physiol Behav. 2020;215:112771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Alhadeff AL, Grill HJ. Hindbrain nucleus tractus solitarius glucagon-like peptide-1 receptor signaling reduces appetitive and motivational aspects of feeding. Am J Physiol Regul Integr Comp Physiol. 2014;307(4):R465–R470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Alhadeff AL, Baird JP, Swick JC, Hayes MR, Grill HJ. Glucagon-like Peptide-1 receptor signaling in the lateral parabrachial nucleus contributes to the control of food intake and motivation to feed. Neuropsychopharmacology. 2014;39(9):2233–2243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ong ZY, Liu JJ, Pang ZP, Grill HJ. Paraventricular thalamic control of food intake and reward: role of glucagon-like Peptide-1 receptor signaling. Neuropsychopharmacology. 2017;42(12):2387–2397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.van Bloemendaal L, IJzerman RG, Ten Kulve JS, et al. GLP-1 receptor activation modulates appetite- and reward-related brain areas in humans. Diabetes. 2014;63(12):4186–4196. [DOI] [PubMed] [Google Scholar]
- 26.Knudsen LB, Lau J. The discovery and development of Liraglutide and Semaglutide. Front Endocrinol (Lausanne). 2019;10:155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Frikke-Schmidt H, Hultman K, Galaske JW, Jorgensen SB, Myers MG Jr, Seeley RJ. GDF15 acts synergistically with liraglutide but is not necessary for the weight loss induced by bariatric surgery in mice. Mol Metab. 2019;21:13–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hsu JY, Crawley S, Chen M, et al. Non-homeostatic body weight regulation through a brainstem-restricted receptor for GDF15. Nature. 2017;550(7675):255–259. [DOI] [PubMed] [Google Scholar]
- 29.Fung E, Kang L, Sapashnik D, et al. Fc-GDF15 glyco-engineering and receptor binding affinity optimization for body weight regulation. Sci Rep. 2021;11(1):8921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tong Q, Ye CP, Jones JE, Elmquist JK, Lowell BB. Synaptic release of GABA by AgRP neurons is required for normal regulation of energy balance. Nat Neurosci. 2008;11(9):998–1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dailey MJ, Tamashiro KL, Terrillion CE, Moran TH. Nutrient specific feeding and endocrine effects of jejunal infusions. Obesity (Silver Spring). 2010;18(5):904–910. [DOI] [PubMed] [Google Scholar]
- 32.Duca FA, Swartz TD, Sakar Y, Covasa M. Decreased intestinal nutrient response in diet-induced obese rats: role of gut peptides and nutrient receptors. Int J Obes (Lond). 2013;37(3):375–381. [DOI] [PubMed] [Google Scholar]
- 33.Nolan LJ, Guss JL, Liddle RA, Pi-Sunyer FX, Kissileff HR. Elevated plasma cholecystokinin and appetitive ratings after consumption of a liquid meal in humans. Nutrition. 2003;19(6):553–557. [DOI] [PubMed] [Google Scholar]
- 34.Liddle RA, Goldfine ID, Rosen MS, Taplitz RA, Williams JA. Cholecystokinin bioactivity in human plasma. Molecular forms, responses to feeding, and relationship to gallbladder contraction. J Clin Invest. 1985;75(4):1144–1152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Betley JN, Xu S, Cao ZFH, et al. Neurons for hunger and thirst transmit a negative-valence teaching signal. Nature. 2015;521(7551):180–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Takahashi KA, Cone RD. Fasting induces a large, leptin-dependent increase in the intrinsic action potential frequency of orexigenic arcuate nucleus neuropeptide Y/agouti-related protein neurons. Endocrinology. 2005;146(3):1043–1047. [DOI] [PubMed] [Google Scholar]
- 37.Su Z, Alhadeff AL, Betley JN. Nutritive, post-ingestive signals are the primary regulators of AgRP neuron activity. Cell Rep. 2017;21(10):2724–2736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Alhadeff AL, Goldstein N, Park O, Klima ML, Vargas A, Betley JN. Natural and drug rewards engage distinct pathways that converge on coordinated hypothalamic and reward circuits. Neuron. 2019;103(5):891–908 e896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.van Can J, Sloth B, Jensen CB, Flint A, Blaak EE, Saris WH. Effects of the once-daily GLP-1 analog liraglutide on gastric emptying, glycemic parameters, appetite and energy metabolism in obese, non-diabetic adults. Int J Obes (Lond). 2014;38(6):784–793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tronieri JS, Wadden TA, Walsh O, et al. Effects of liraglutide on appetite, food preoccupation, and food liking: results of a randomized controlled trial. Int J Obes (Lond). 2020;44(2):353–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hayes MR, Bradley L, Grill HJ. Endogenous hindbrain glucagon-like peptide-1 receptor activation contributes to the control of food intake by mediating gastric satiation signaling. Endocrinology. 2009;150(6):2654–2659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Alhadeff AL, Mergler BD, Zimmer DJ, et al. Endogenous glucagon-like Peptide-1 receptor signaling in the nucleus Tractus Solitarius is required for food intake control. Neuropsychopharmacology. 2017;42(7):1471–1479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Grill HJ, Hayes MR. Hindbrain neurons as an essential hub in the neuroanatomically distributed control of energy balance. Cell Metab. 2012;16(3):296–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kanoski SE, Alhadeff AL, Fortin SM, Gilbert JR, Grill HJ. Leptin signaling in the medial nucleus tractus solitarius reduces food seeking and willingness to work for food. Neuropsychopharmacology. 2014;39(3):605–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wald HS, Chandra A, Kalluri A, Ong ZY, Hayes MR, Grill HJ. NTS and VTA oxytocin reduces food motivation and food seeking. Am J Physiol Regul Integr Comp Physiol. 2020;319(6):R673–R683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kay K, Parise EM, Lilly N, Williams DL. Hindbrain orexin 1 receptors influence palatable food intake, operant responding for food, and food-conditioned place preference in rats. Psychopharmacology (Berl). 2014;231(2):419–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Worth AA, Shoop R, Tye K, et al. The cytokine GDF15 signals through a population of brainstem cholecystokinin neurons to mediate anorectic signalling. Elife. 2020;9:1–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Roman CW, Sloat SR, Palmiter RD. A tale of two circuits: CCK(NTS) neuron stimulation controls appetite and induces opposing motivational states by projections to distinct brain regions. Neuroscience. 2017;358:316–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.D’Agostino G, Lyons DJ, Cristiano C, et al. Appetite controlled by a cholecystokinin nucleus of the solitary tract to hypothalamus neurocircuit. Elife. 2016;5:1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bettge K, Kahle M, Abd El Aziz MS, Meier JJ, Nauck MA. Occurrence of nausea, vomiting and diarrhoea reported as adverse events in clinical trials studying glucagon-like peptide-1 receptor agonists: a systematic analysis of published clinical trials. Diabetes Obes Metab. 2017;19(3):336–347. [DOI] [PubMed] [Google Scholar]
- 51.Sikirica MV, Martin AA, Wood R, Leith A, Piercy J, Higgins V. Reasons for discontinuation of GLP1 receptor agonists: data from a real-world cross-sectional survey of physicians and their patients with type 2 diabetes. Diabetes Metab Syndr Obes. 2017;10:403–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lean ME, Carraro R, Finer N, et al. Tolerability of nausea and vomiting and associations with weight loss in a randomized trial of liraglutide in obese, non-diabetic adults. Int J Obes (Lond). 2014;38(5):689–697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gabery S, Salinas CG, Paulsen SJ, et al. Semaglutide lowers body weight in rodents via distributed neural pathways. JCI Insight. 2020;5(6):e133429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Borner T, Geisler CE, Fortin SM, et al. GIP receptor Agonism attenuates GLP-1 receptor agonist induced nausea and emesis in preclinical models. Diabetes. 2021;70:2545–2553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhang C, Kaye JA, Cai Z, Wang Y, Prescott SL, Liberles SD. Area Postrema cell types that mediate nausea-associated behaviors. Neuron. 2021;109(3):461–472 e465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ludwig MQ, Cheng W, Gordian D, et al. A genetic map of the mouse dorsal vagal complex and its role in obesity. Nat Metab. 2021;3(4):530–545. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The raw data that support the findings of this study are available from the corresponding author upon reasonable request.


