Abstract
Background and Aims
The association between birth weight (BW) and metabolic outcomes has been described since the 1980s but NAFLD has been rarely studied. This study aimed to investigate the association between BW and NAFLD occurrence in adult subjects.
Approach and Results
The study population consisted of participants from the French nationwide Constances cohort from 2012 to 2019. Participants with a history of chronic viral hepatitis or excessive alcohol consumption were excluded. Noninvasive diagnosis of NAFLD and fibrosis was performed using a combination of the Fatty Liver Index (FLI) and the Forns Index. The relationship between BW and NAFLD was analyzed with a sex‐stratified logistic regression model adjusted for sociodemographic parameters, lifestyle, and birth term, whereas liver fibrosis was analyzed with a sex‐stratified linear regression model. In total, 55,034 individuals with reliable BW were included (43% men, mean age: 38 years). NAFLD (FLI ≥ 60) was present in 5530 individuals (10%). Multivariate logistic regression showed a significant U‐shaped relationship between BW and NAFLD, with no significant interaction with sex. A significant and slightly decreasing association was found between BW and Forns Index (β = −0.05; p = 0.04). Premature birth (OR, 1.23; 95% CI, 1.03–1.48 for birth between 33 and 37 weeks versus ≥ 37 weeks) was associated with NAFLD, with a significant direct effect of premature birth, and without an indirect effect of low BW in mediation analysis. Forns Index was not significantly higher in participants with preterm birth compared to full‐term birth.
Conclusions
This large prospective adult‐based cohort confirms the relationship between BW and NAFLD occurrence.

Abbreviations
- BMI
body mass index
- BW
birth weight
- FLI
Fatty Liver Index
- GGT
gamma‐glutamyl transpeptidase
- HBW
high birth weight
- HDL
high‐density lipoprotein
- ISCED
International Standard Classification of Education
- LBW
low birth weight
INTRODUCTION
The association between birth weight (BW) and the development of metabolic and cardiovascular diseases has been described since the 1980s.[ 1 , 2 , 3 , 4 , 5 ] This observation led to the Developmental Origins of Health and Disease concept. According to this concept, environmental exposures during the periconceptional period, pregnancy, and early childhood may influence later health and, particularly, the development of metabolic and cardiovascular diseases in children and adults.
NAFLD is a common chronic liver disease whose frequency has progressively increased in populations exposed to overweight and obesity.[ 6 ] Pathogenesis is based on the accumulation of hepatic triglycerides (steatosis), resulting from free fatty acid metabolism changes associated with insulin resistance. NAFLD is strongly associated with metabolic syndrome, and screening for liver fibrosis is recommended in people presenting one or more factors, such as type 2 diabetes.[ 7 ] NAFLD can lead to less common but more severe liver diseases, such as NASH, fibrosis, and irreversible cirrhosis. Currently, NAFLD is the leading cause of cirrhosis in Western countries.[ 8 ] Because NAFLD is associated with metabolic disorders, it is legitimate to hypothesize a shared developmental origin. However, the early origins of NAFLD remain unclear. A systematic review by Querter et al.[ 9 ] identified maternal pre‐pregnancy overweight and obesity as a risk factor for pediatric NAFLD, whereas breastfeeding was associated with a reduced risk for NAFLD, and being born preterm or small for gestational age had an unclear impact on the development of NAFLD. Altogether, few studies have investigated this relationship, and existing studies were mostly performed in children and adolescents.[ 10 , 11 ] Moreover, analyses did not attempt to distinguish the role of prematurity from that of intrauterine growth retardation. In adults, two Finnish studies[ 12 , 13 ] showed an association between being small for gestational age and NAFLD and showed a higher prevalence of NAFLD in those born preterm.
Our study aimed to assess the relationship among BW, preterm birth, and NAFLD in adults from a large French population‐based cohort.
PARTICIPANTS AND METHODS
Study population
The study included individuals from the “general purpose” population‐based epidemiological French cohort “Constances,” designed to be representative of the French general population.[ 14 ] Constances is a French national sample of more than 200,000 volunteers aged 18–69 at baseline living in 21 selected French “Départements” (administrative divisions) throughout metropolitan France.[ 14 , 15 ] Participants were drawn from individuals covered by the national general health insurance scheme or partner health insurance companies (85% of the French population) using a random sampling scheme stratified by place of residence, age, sex, occupation and socioeconomic status. Eligible individuals were invited by mail to participate in the study.
Data collection
Individuals were included in the cohort from February 2012 to December 2019.
Sociodemographic and lifestyle data were obtained with a standardized self‐administered questionnaire at the time of inclusion in the cohort. This self‐administered questionnaire included information on lifestyle (food, frequency of alcohol consumption, smoking, physical activities), level of education, geographical origin, and parents’ occupation. Information on BW (from each individual’s health booklet, which is systematically given out at birth in France since 1945) and medical history (diabetes, high blood pressure, and chronic viral hepatitis) was recorded by a physician in a medical center. Blood tests were also performed in a medical center.
Additional data related to early childhood (birth term) were also collected from the health booklet through a self‐administered follow‐up questionnaire in 2019.
Birth term was considered as reliable if it was consistent with BW, that is, between −5SD and +5SD in French reference tables that give the distribution of BW by birth term. For graphical representations, BW was sex‐specifically categorized in 200 g intervals starting from the 2.5th percentile: from 2360 to 4360 g in men and from 2245 to 4245 g in women.
Education level was categorized according to the 2011 International Standard Classification of Education (ISCED).
Overweight was defined as a body mass index (BMI) between 25 and 30 kg/m2 and obesity was defined as BMI > 30 kg/m2. Abdominal obesity was defined as waist circumference > 102 cm for men and >88 cm for women. Hypertension was defined as a history of high blood pressure (physician‐administrated questionnaire) or a mean systolic blood pressure > 140 mm Hg or a mean diastolic blood pressure > 90 mm Hg after two measures on the same arm during examination in the medical center.
Impaired fasting glucose was defined by a history of diabetes (physician‐administrated questionnaire) or fasting glycaemia > 110 mg/dl after the blood test in the medical center. Hypertriglyceridemia was defined as triglycerides > 1.5 g/L or treatment for hypertriglyceridemia, and low high‐density lipoprotein (HDL)‐cholesterol was defined as <40 mg/dl for men and <50 mg/dl in women. A “metabolic impairment” variable was defined as having at least one anomaly from among abdominal obesity, hypertension, impaired fasting glucose, hypertriglyceridemia, and low HDL‐cholesterol, as defined above.
Leisure physical activity was defined as “never” in the absence of sports activity, “mild” for <2 h of exercise per week, and “high” for ≥2 h per week.
Consumption of high‐sugar and high‐fat foods was assessed with an ad hoc nutrition score designed from the self‐administrated food frequency questionnaire. Nine categories of foods or beverages were selected: sweet drinks, fast food, fried food, aperitifs, cookies or chocolate bars, sweet desserts, cakes and pastries, cheese, and cured meats. For each, individuals could choose six levels of consumption, each of which was associated with a coefficient averaging intake in times/per week: never (coefficient = 0), less than once a week (coefficient = 0.5), once a week (coefficient = 1), 2–3 times a week (coefficient = 2.5), 4–6 times a week (coefficient = 5), and every day (coefficient = 7). Then, we created a score ranging from 0 (very low high‐sugar and high‐fat foods consumption) to 63 (very high level of consumption) by summing the coefficients for each individual.
Exclusion criteria
The study only included individuals with reliable BW (collected from individuals’ health booklets). Therefore, individuals without a personal health booklet (lost or not given at birth) were not included. Individuals aged over 60 were excluded from the study because the proportion of birth data available in this population was too small. Finally, people with BW below 500 g or above 6000 g were excluded because the data were considered to be potentially erroneous.
To avoid misclassification of NAFLD diagnosis, individuals with excessive alcohol consumption (over 20 g per day for women and 30 g per day for men) were excluded. Likewise, individuals with a history of chronic liver disease (chronic viral or autoimmune hepatitis or other hepatitis) were also excluded.
Outcomes
NAFLD and liver fibrosis diagnosis methodology in the Constances cohort have previously been described by Nabi et al.[ 15 ] NAFLD was defined as Fatty Liver Index (FLI) ≥ 60.[ 16 ]
FLI = (exp(0.953 × log(triglycerides) + 0.139 × BMI + 0.718 × log(GGT) + 0.053 × waist circumference −15.745)) / (1 + exp(0.953 × log(triglycerides) + 0.139 × BMI + 0.718 × log(GGT) + 0.053 × waist circumference – 15.745)) × 100.
For individuals with FLI ≥ 60, the Forns Index[ 17 ] was also calculated to assess liver fibrosis level.
Forns index = 7.811 – 3.131 × ln(platelet count) + 0.781 × ln(GGT) + 3.467 × ln(age) – 0.014 × (total cholesterol). A score <4.2 is associated with a low likelihood of significant fibrosis, whereas a score >6.9 is associated with a high probability of significant fibrosis.
Statistical analysis
Descriptive statistics
Individuals’ characteristics are presented as means and standard deviation for continuous variables and number (percentage) for categorical variables. The characteristics of individuals without reliable BW (and therefore not included) are described to account for possible selection bias.
The relation between prediction of NAFLD and BW is graphically presented with BW as a categorical variable (in 200 g intervals). Crude and adjusted predicted NAFLD probabilities by BW categories were calculated using a logistic procedure with a contrast statement.
Association between BW and liver outcomes
The association between BW and each parameter used to calculate FLI and Forns Index (BMI, waist circumference, triglycerides, gamma‐glutamyl transpeptidase [GGT], platelets, age, total cholesterol) was tested with linear regression models by considering each parameter as a continuous variable. For biological variables whose distribution was not normal (triglycerides and GGT), logarithmic transformation was performed.
The association between NAFLD (FLI ≥ 60) and BW was assessed with univariate and multivariate logistic regression models, including BW as a continuous variable and using a quadratic model to test a U‐shaped relation. Models were adjusted for factors associated with both BW and NAFLD: age, level of education (ISCED‐2011), level of leisure physical activity, and diet (high‐sugar and high‐fat foods score). A mediation analysis was performed to identify the pathways of the association between both low and high BW (defined as BW ≤10th and ≥90th sex‐specific percentiles, compared to the middle category). The mediation model is summarized in the directed acyclic graph presented in Figure S1. The mediator was defined as the “metabolic impairment” variable and BMI as a mediator‐outcome confounder affected by “BW category” exposure. The mediation analysis was performed in men and women separately.
The association between birth term in three categories (≥37 weeks, between 33 and 37 weeks, <33 weeks of gestation) and NAFLD was tested by logistic regression adjusted for age, level of education (ISCED‐2011), level of leisure physical activity, and the high‐sugar and high‐fat foods score. A mediation analysis was performed to assess whether low birth weight (LBW) mediated the association. The mediation analyses were performed using the g‐formula method[ 18 ] with the CMAverse R‐package.[ 19 ]
The association between BW and liver fibrosis (Forns Index) was tested in individuals with FLI > 60. Linear regression models were performed to assess the relationship between liver fibrosis and BW, considering the Forns Index as a continuous variable due to the small number of individuals with a Forns Index higher than 6.9. Models were adjusted for age.
Sex stratification
Because BW distribution and the prevalence of metabolic outcomes are different between men and women, analyses were a priori stratified by sex. Differences between men and women were subsequently tested with an interaction term.
Imputation
Because of the large amount of missing data related to the consumption of high‐sugar and high‐fat foods (25%), multiple imputations were performed for the nutrition score. Five independent datasets were imputed using the “fully conditional specification” method (SAS software: Multiple imputation [MI] procedure), and pooled effect estimates were calculated (MI analysis procedure). In addition to the nutrition score, data were also imputed for the following adjustment variables: level of education, level of leisure physical activity, and metabolic impairment items. For each of these variables, the percentage of missing data was less than 5%. In addition to these variables, imputation also took into account BW, all the clinical and biological parameters included in the scores, FLI and Forns Index, tobacco consumption, and parents’ geographical origins and occupation.
A p value < 0.05 was considered to be statistically significant. Analyses were performed using SAS version 9.4. and R studio version 4.0.3.
The authors ensure that a) all participants included in the Constances cohort have given their informed and written consent for the use of their data b) the study protocol is conformed to the ethical guidelines of the 1975 Declaration of Helsinki as reflected in a priori approval by the appropriate review committee. The data collection within the Constances cohort obtained authorization from the French National Commission for Information Technology and Liberties and the institutional review board of the National Institute for Medical Research (Inserm). All studies using the Constances cohort data have received approval from a specific ethics committee.
RESULTS
Population selection and analysis
At the time of analysis, data were available for 199,711 participants in the Constances cohort (Figure 1).
FIGURE 1.
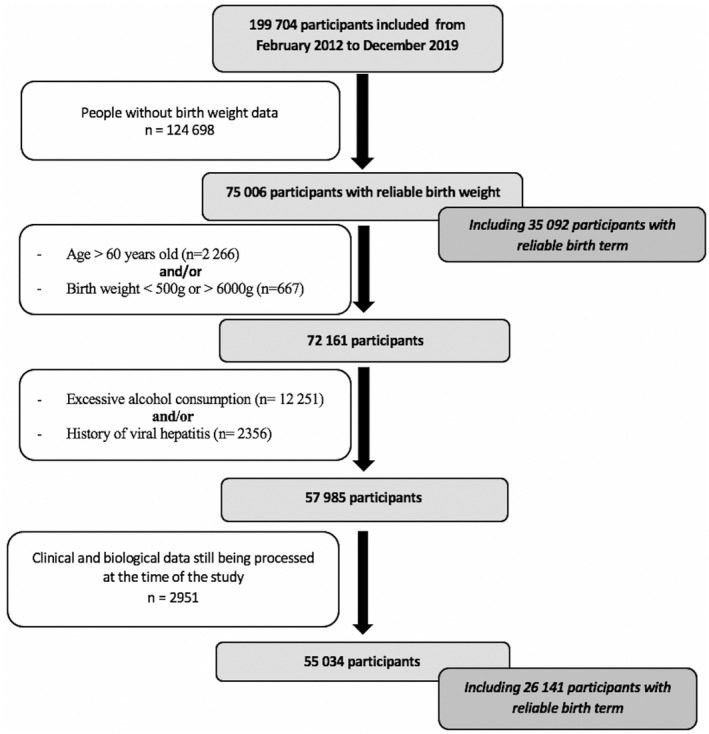
Flow chart
After exclusion of participants aged over 60 years, BW was available (from individuals’ health booklets) and reliable (500–6000 g) for 72,159 participants.
When comparing individuals under 60 years old with and without reliable and available BW data, those with reliable BW were younger (38 vs. 45 years), were thinner (BMI: 24 vs. 25 kg/m2), and had higher education level and parental socioprofessional category. They were more often women (58% vs. 52%), and the prevalence of diabetes/Impaired fasting glucose (4.3% vs. 9.8%), hypertension (12% vs. 22%), and metabolic impairment (at least one of the metabolic syndrome anomalies: 34% vs. 47%) was lower. After exclusion of all participants with excessive alcohol consumption and/or history of chronic hepatitis, when comparing individuals under 60 years old, NAFLD prevalence was significantly higher in the population without available/reliable BW (17%) than in the population with BW data (10%), even after adjustment for age and sex.
Finally, 55,034 participants were selected for the study. The mean (± SD) age was 38 (± 9) years, 43% were men, and, regarding BMI, 25% were overweight and 8% were obese. The average BW was 3307 ± 487 g (3386 and 3247 g for men and women, respectively). BW distribution is presented in Figure S2. General characteristics by sex are displayed in Table 1.
TABLE 1.
Population characteristics (n = 55,034)
| Men (n = 23,780) | Women (n = 31,254) | |||||
|---|---|---|---|---|---|---|
| Age (years) | 37.8 | (9.1) | 38 | (9.5) | ||
| Education level: ISCED‐2011 n (%) | ||||||
| ≤4 | 6856 | (29) | 8129 | (26) | ||
| 5–6 | 6447 | (27) | 10,755 | (35) | ||
| ≥7 | 10,270 | (44) | 12,096 | (39) | ||
| Missing data on education level (n) | 207 | 274 | ||||
| Birth weight (g) | ||||||
| Mean (SD) | 3386 | (491) | 3247 | (475) | ||
| Median (Q1–Q3) | 3400 | (3100–3700) | 3250 | (2980–3550) | ||
| Birth weight distribution by 200 g classes n (%) | ||||||
| <2360 g | 593 | (2.5) | <2245 g | 781 | (2.5) | |
| 2360–2560 g | 487 | (2.1) | 2245–2445 g | 610 | (2) | |
| 2560–2760 g | 966 | (4.1) | 2445–2645 g | 1348 | (4.3) | |
| 2760–2960 | 1825 | (7.7) | 2645–2845 g | 2602 | (8.3) | |
| 2960–3160 g | 3168 | (13.3) | 2845–3045 g | 4344 | (13.9) | |
| 3160–3360 g | 4115 | (17.3) | 3045–3245 g | 5568 | (17.8) | |
| 3360–3560 g | 4132 | (17.4) | 3245–3445 g | 5565 | (17.8) | |
| 3560–3760 g | 3583 | (15.1) | 3445–3645 g | 4583 | (14.7) | |
| 3760–3960 g | 2373 | (10) | 3645–3845 g | 2967 | (9.5) | |
| 3960–4160 g | 1345 | (5.7) | 3845–4045 g | 1601 | (5.1) | |
| 4160–4360 g | 661 | (2.8) | 4045–4245 g | 746 | (2.4) | |
| ≥4360 g | 532 | (2.2) | ≥4245 g | 539 | (1.7) | |
| Impaired fasting glycaemia n (%) | 1305 | (5.5) | 989 | (3.2) | ||
| High blood pressure a n (%) | 4109 | (17.3) | 2554 | (8.2) | ||
| Lifestyle n (%) | ||||||
| Leisure physical activity n (%) | ||||||
| Never | 7252 | (31) | 9932 | (32) | ||
| <2 h a week | 6914 | (29) | 11646 | (38) | ||
| ≥2 h a week | 9446 | (40) | 9421 | (30) | ||
| Missing data on physical exercise (n) | 168 | 255 | ||||
| Nutrition score (mean (SD)) | 13.7 | (7) | 11.5 | (6) | ||
| Missing data on nutrition score (n) | 6441 | 9221 | ||||
| Clinical parameters (mean (SD)) | ||||||
| Body mass index (kg/m2) | 24.5 | (3.6) | 23.7 | (4.5) | ||
| Waist circumference (cm) | 86 | (10) | 77 | (11) | ||
| Biological parameters (mean (SD)) | ||||||
| Fasting glucose (mmol/L) | 5.3 | (0.6) | 5.0 | (0.6) | ||
| Total cholesterol (mmol/L) | 5.2 | (1) | 5.1 | (0.9) | ||
| Fasting triglycerides (mmol/L) | 1.2 | (0.7) | 0.9 | (0.4) | ||
| HDL‐cholesterol (mmol/L) | 1.36 | (0.33) | 1.59 | (0.38) | ||
| Platelets (G/L) | 234 | (49) | 255 | (56) | ||
| ALT (IU/L) | 31 | (19) | 20 | (12) | ||
| GGT (IU/L) | 30 | (28) | 20 | (17) | ||
| Metabolic impairement b , n (%) | 9203 | (39) | 8787 | (28) | ||
| Liver disease scores (mean (SD)) | ||||||
| Fatty liver index | 29 | (25) | 16 | (20) | ||
| Forns index (calculated if FLI ≥ 60, n = 5530) | 3.4 | (1.2) | 2.7 | (1.3) | ||
Abbreviations: ALAT, alanine aminotransferases; GGT, gamma glutamyl‐transferase; HDL, high‐density lipoprotein.
High blood pressure was defined as a history of high blood pressure (physician‐administrated questionnaire) or a mean systolic blood pressure > 140 mmHg or a mean diastolic blood pressure > 90 mmHg after 2 measures on the same arm during examination in the medical center.
Defined as having at least one anomaly from among: abdominal obesity, hypertension, impaired fasting glucose, hypertriglyceridemia, low HDL‐cholesterol.
According to FLI ≥ 60, a total of 5530 (10%) were considered to have NAFLD (15% in men and 6% in women). Among these individuals, average age was 41 ± 9 years, 66% were men, and mean BW was 3346 ± 516 g. Among these subjects with NAFLD (5530), 1153 had a Forns Index between 4.2 and 6.9, and only 12 individuals had a Forns Index ≥ 6.9. FLI and Forns Index distribution are given in Figures S3 and S4, respectively.
The comparison of participants according to FLI status is displayed in Table S1.
Association between BW and liver score parameters
Univariate analysis showed a significant positive linear association between BW and BMI, and between BW and waist circumference at adult age in both sexes (Figure S5, Supporting Information). Conversely, the relationships with triglycerides level and GGT were decreasing (Figure S6, Supporting Information).
For individuals with FLI ≥ 60, there was a significant linear and increasing relationship between age and BW in univariate analysis only in men. A significant linear and decreasing relationship was found between BW and GGT in both men and women, and to a lesser degree with total cholesterol in men. No significant association was shown between platelet level and BW.
Association between BW and NAFLD
The relationship between BW and prediction of risk for NAFLD is presented by sex in Figure 2 before (model 0) and after adjustment for age and education level (model 1) and additionally for nutrition score and leisure physical activity (model 2).
FIGURE 2.
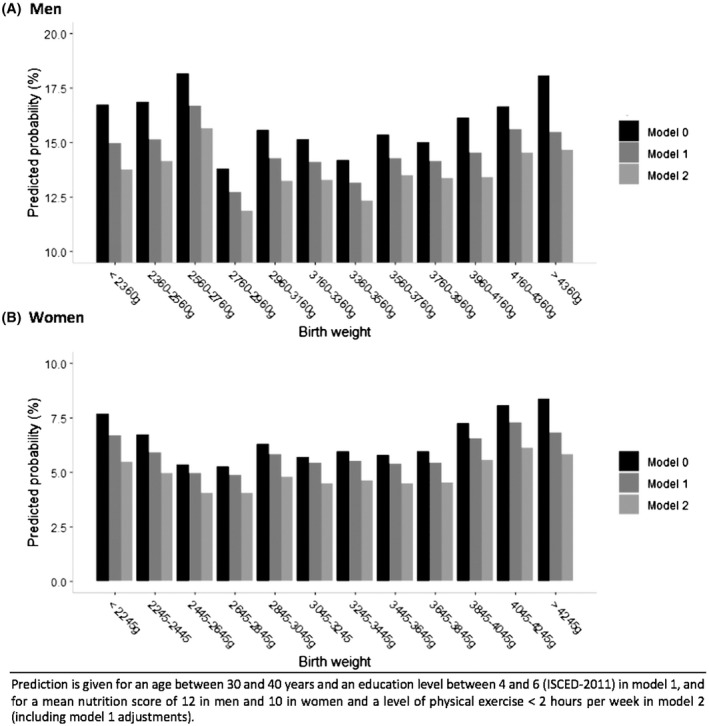
Relationship between BW and prediction of NAFLD by sex: (A) men, (B) women
Figure 2 shows a U‐shaped association between BW and estimated crude and adjusted prevalence of NAFLD by logistic regression. For men in the age class 30–40 years and education level from 5 to 6 (ISCED 2011), the predicted prevalence of NAFLD was lower (between 12.7% and 14.2%) in subjects with BW between 2760 and 3960 g and higher (between 14.5% and 16.7 %) in subjects with BW <2760 g or >3960 g. In women of the same age and educational level class, the predicted prevalence ranged from 5% to 5.4% for BW between 2445 and 3845 g and from 5.9% to 7.3% for the lowest and highest BW categories. This U‐shaped association was statistically confirmed with BW analyzed as a continuous quadratic variable in logistic regression models (Table 2) and remained significant after adjustment for age and education level. After adjustment for nutrition score and leisure physical activity, the association also remained significant for women and borderline for men. When men and women were combined in a single analysis, the interaction term between BW and sex was not significant. The linear and quadratic terms for BW remained significant in all models. The likelihood ratio test confirmed a better fit of the model with a quadratic term for BW than with only a linear term (p = 0.005).
TABLE 2.
Association between BW and NAFLD in men and women, using multiple logistic regression
| Men (n = 23,780) | β coefficient | Model 0 | β coefficient | Model 1 | β coefficient | Model 2 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| OR | 95% CI | p value | OR | 95% CI | p value | OR | 95% CI | p value | ||||
| BW (kg) | −0.84 | 0.0008 | − 0.53 | 0.0444 | −0.51 | 0.0506 | ||||||
| BW2 (kg2) | 0.13 | 0.0005 | 0.08 | 0.042 | 0.08 | 0.0446 | ||||||
| Age (years) | 0.05 | 1.05 | (1.05–1.06) | <0.0001 | 0.05 | 1.05 | (1.05–1.06) | <0.0001 | ||||
| Education level (ISCED‐2011) | <0.0001 | <0.0001 | ||||||||||
| ≤4 | 0 | 1 | (Ref) | 0 | 1 | (Ref) | ||||||
| Between 5 and 6 | − 0.31 | 0.73 | (0.66–0.80) | −0.27 | 0.76 | (0.69–0.83) | ||||||
| ≥7 | −0.82 | 0.44 | (0.41–0.48) | −0.73 | 0.48 | (0.44–0.52) | ||||||
| Leisure physical activity | ||||||||||||
| Never | 0 | 1 | (Ref) | <0.0001 | ||||||||
| <2 h a week | −0.54 | 0.58 | (0.53–0.64) | |||||||||
| ≥2 h a week | −0.94 | 0.39 | (0.36–0.43) | |||||||||
| Nutrition score (unit = 1) | −0.01 | 0.99 | (0.99–1.00) | 0.06 | ||||||||
| Women (n = 31,254) | β coefficient | Model 0 | β coefficient | Model 1 | β coefficient | Model 2 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| OR | 95% CI | p value | OR | 95% CI | p value | OR | 95% CI | p value | ||||
| BW (kg) | −1.27 | <0.0001 | −0.97 | 0.0031 | −0.99 | 0.003 | ||||||
| BW2 (kg2) | 0.22 | <0.0001 | 0.16 | 0.0017 | 0.17 | 0.001 | ||||||
| Age (years) | 0.03 | 1.03 | (1.03–1.04) | <0.0001 | 0.04 | 1.04 | (1.03–1.04) | <0.0001 | ||||
| Education level (ISCED‐2011) | <0.0001 | <0.0001 | ||||||||||
| ≤4 | 0 | 1 | (Ref) | 0 | 1 | (Ref) | ||||||
| Between 5 and 6 | −0.48 | 0.62 | (0.56–0.69) | −0.42 | 0.66 | (0.59–0.74) | ||||||
| ≥7 | −1.08 | 0.34 | (0.30–0.38) | −0.99 | 0.37 | (0.33–0.42) | ||||||
| Leisure physical activity | <0.0001 | |||||||||||
| Never | 0 | 1 | (Ref) | |||||||||
| <2 h a week | −0.63 | 0.53 | (0.48–0.60) | |||||||||
| ≥2 h a week | −1.02 | 0.36 | (0.32–0.42) | |||||||||
| Nutrition score (unit = 1) | 0 | 1 | (0.99–1.01) | 0.46 | ||||||||
Model 0: crude association. Model 1: Model 0 + adjustment for age and education level (ISCED). Model 2: Model 1 + adjustment for nutrition score and leisure physical activity. Only beta coefficients are given for BW, as there are two terms for this variable in the model.
Finally, mediation analysis was performed and represented in Figure 3. Compared to normal bBW (i.e., BWt between the 10th and 90th sex‐specific percentiles), a high BW (HBW) was associated with NAFLD with a significant direct and indirect (accounting for BMI and metabolic impairment) effect only in women, whereas an indirect effect was protective in men. Total effect was significant only in women (OR, 1.17; CI, 1.08–1.36). Conversely, the total effect of LBW on NAFLD was significant only in men (OR, 1.12; CI, 1.05–1.19) and almost significant in women. The direct effect was not significant in either men or women. The indirect effect was significant only in men and almost significant in women.
FIGURE 3.
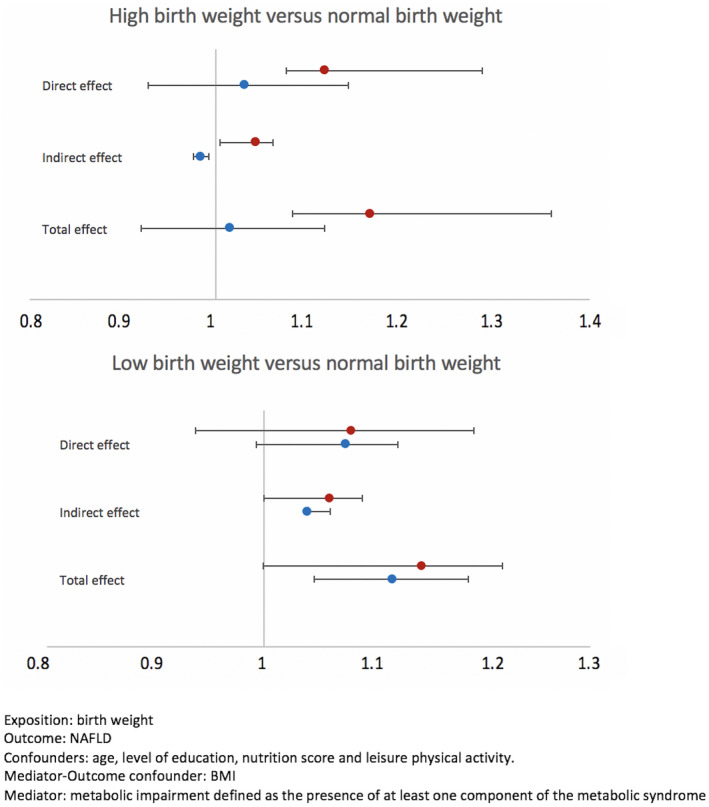
Mediation analysis of the association between BW and NAFLD
Association between BW and liver fibrosis
In total, 3638 men and 1892 women presented FLI ≥ 60. The analysis did not find any significant quadratic or linear association between BW and Forns Index in either men or women (Table 3).
TABLE 3.
Association between birth weight and liver fibrosis as assessed by the Forns index in men and women, using multiple linear regression
| Model 0 | Model 1 | |||||
|---|---|---|---|---|---|---|
| ß | 95%CI | p value | ß | 95%CI | p value | |
| Men (n = 3638) | ||||||
| Birth weight (kg) | 0.01 | (−0.07 to 0.09) | 0.82 | −0.05 | (−0.12 to 0.01) | 0.08 |
| Age (years) | 0.09 | (0.08 to 0.09) | <0.0001 | |||
| Women (n = 1892) | ||||||
| Birth weight (kg) | −0.01 | (−0.13 to 0.11) | 0.85 | −0.05 | (−0.14 to 0.04) | 0.30 |
| Age (years) | 0.09 | (0.09 to 0.10) | <0.0001 | |||
Model 0: crude association. Model 1: Model 0 + adjustment for age.
When men and women were combined in a single analysis, there was no interaction between BW and sex, and a significant trend toward an increase in Forns Index with decreasing BW (β = −0.05; p = 0.04) was observed.
Association between birth term and liver outcomes
Among the 55,034 participants, 26,141 reported reliable birth term. Among these, 24,366 (93.2%) reported a full‐term birth (≥37 weeks); 1392 (5.3%) reported a birth between 33 and 37 weeks; 383 (1.5%) reported a birth before 33 weeks. The mean age was 39 years, mean BW was 3316 g, 61% were women, and 2409 (9%) had FLI ≥ 60.
Due to the lack of significant interaction between sex and BW in the main analysis and the smaller sample size, the entire population was analyzed without sex stratification.
Premature birth was associated with NAFLD after adjustment for age, educational level, nutrition score, and leisure physical activity (Table 4).
TABLE 4.
Association between birth term and NAFLD, using multiple logistic regression
| Model 0 | Model 0 | Model 1 | Model 2 | Model 3 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| OR | 95% CI | p value | OR | 95% CI | p value | OR | p value | OR | 95% CI | p value | ||
| Birth term | 0.02 | 0.025 | 0.03 | 0.04 | ||||||||
| Full‐term birth (≥37 weeks) | 1 | (Ref) | 1 | (Ref) | 1 | (Ref) | 1 | (Ref) | ||||
| Preterm birth 33–37 weeks | 1.18 | (0.99–1.41) | 1.18 | (0.99–1.41) | 1.24 | (1.04–1.49) | 1.23 | (1.03–1.48) | ||||
| Preterm birth <33 weeks | 1.40 | (1.03–1.91) | 1.39 | (1.02–1.90) | 1.24 | (0.90–1.71) | 1.23 | (0.89–1.70) | ||||
| Sex | <0.0001 | <0.0001 | <0.0001 | |||||||||
| Women | 1 | (Ref) | 1 | (Ref) | 1 | (Ref) | ||||||
| Men | 2.93 | (2.69–3.20) | 3.09 | (2.83–3.38) | 3.22 | (2.94–3.53) | ||||||
Model 0: crude association. Model 1: Model 0 + adjustment for sex. Model 2: Model 1 + adjustment for age and education level (ISCED). Model 3: Model 2 + adjustment for nutrition score and leisure physical activity level.
In mediation analysis adjusted for the same variables, adding LBW as a mediator variable (<10th percentile), the total effect of premature birth (<37 weeks) was significant (OR, 1.21; CI, 1.16–1.31), driven by a significant direct effect, whereas the indirect effect (LBW) was not significantly associated with NAFLD (Figure 4).
FIGURE 4.
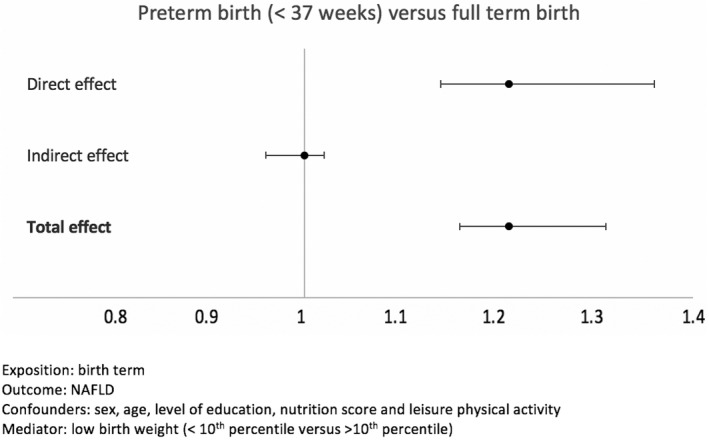
Mediation analysis of the association between birth term and NAFLD
Finally, linear regression was performed to study the relationship between birth term and liver fibrosis (Forns Index) in the 2409/26,141 participants (9%) with FLI ≥ 60 and reliable birth term. In the whole sample of men and women, the Forns Index was not significantly different between birth term classes (p = 0.11): 3.28 for full‐term participants, 3.17 for those born between 33 and 37 weeks, and 3.65 for birth under 33 weeks.
DISCUSSION
This study assesses the relationship between BW, gestational age, and NAFLD in a large sample of the general population. It confirms a U‐shaped relation, with a higher risk of NAFLD in people with low or high BW. This relationship persisted after adjustment for confounding factors related to socioeconomic background and adult lifestyle, whereas the association seems to be mediated by a high overall fat mass for HBW and by metabolic impairment for LBW. Our study further suggests that premature birth explains the association between LBW and NAFLD, as the indirect effect of LBW was not significant in the association between preterm birth and NAFLD. Concerning liver fibrosis, our data failed to show a strong association but favor a higher Forns Index in people with LBW.
NAFLD prevalence
The prevalence of NAFLD was 10 % in the sample selected for this analysis. In a previous study based on the Constances cohort, using the FLI to define NAFLD, Nabi et al.[ 15 ] reported a prevalence of 18.2%. This result is in line with a meta‐analysis[ 6 ] that found an NAFLD prevalence of 23% in the European population, considering multiple diagnosis methods (imaging, liver biopsy, and blood tests). The selection of the population for the present study was mostly based on the availability of the health booklet, which is associated with younger age and higher education level. This can explain the difference observed here compared to the data reported by Nabi et al., where the mean age of participants was 47 years versus 38 years in our sample. Accordingly, the prevalence of overweight (30% vs. 25%) and obesity (12% vs. 8%) were also higher in the study by Nabi et al.
Association between BW and liver outcomes
In our cohort of young adults, NAFLD prevalence varies from 13.8% to 18.1% according to BW categories in men and from 5.3% to 8.4 % in women. To the best of our knowledge, no comparable study has been conducted in a general population. The association between BW and NAFLD was mostly studied in small samples of children and young adults heretofore, with a case‐control design.[ 20 ] Nobili et al.[ 21 ] found a fourfold higher prevalence of LBW (BW below <10th percentile for gestational age) in children with NAFLD (n = 90) compared to the general population of their pediatric department. Newton et al.[ 10 ] studied the distribution of BW in 538 children with NAFLD and found an overrepresentation of HBW (>4000 g; OR, 1.82; 95% CI, 1.15–2.88) compared to the general population. In contrast, liver fibrosis was associated with LBW (OR, 2.23; CI, 1.08–4.62), but the study did not consider gestational age. Finally, in adults, Suomela et al.[ 12 ] found a significant association between small BW (for gestational age) and NAFLD in a Finnish cohort. Other studies failed to demonstrate any significant association.[ 11 , 22 ]
Pathophysiological assumptions
HBW
Children born with macrosomia have an increased body fat mass.[ 23 ] Leading causes of fetal macrosomia are gestational diabetes and maternal obesity. Maternal medical history was not considered in the present study. Nevertheless, animal models have validated the hypothesis of fetal programming of these diseases even in macrosomia without identified maternal pathology.[ 24 ]
Our results show that HBW is associated with NAFLD and that the association is mediated by fat mass and metabolic impairment, especially in women. An increased body fat mass in adulthood likely explains part of this association, as FLI includes BMI and waist circumference in its calculation. Because BMI is a better proxy for fat mass in women than in men,[ 25 ] it may explain our finding of a stronger association in women than in men.
LBW and prematurity
On the other hand, the mediation analysis showed an indirect effect (metabolic impairment) in the association between LBW and NAFLD. LBW does not appear to confer an increased risk of NAFLD by itself, as its indirect effect was not significant in the association between birth term and NAFLD. There is a broad amount of literature on the association between “small for gestational age” and NAFLD based on the “thrifty phenotype” theory.[ 21 , 23 , 24 , 26 ] This theory assumes a metabolic sparing phenotype in the fetus in response to a nutrient‐restricted uterine environment. Indeed, multiple data confirmed an association between intrauterine growth retardation and insulin resistance.[ 27 ] This theory could make sense in the case of NAFLD, as its physiopathology is strongly associated with insulin resistance. However, our results suggest a role of prematurity rather than growth retardation.
Sipola‐Leppänen et al.[ 13 ] also found a higher prevalence of intermediate and high FLI in adults born early preterm or late preterm compared with adults born at term in Northern Finland.
The main hypothesis is the postnatal weight gain induced by dense nutrition given to premature babies, suggesting fat storage that would be more deleterious from a qualitative than quantitative point of view, favoring insulin resistance. Liver immaturity and/or disturbances in adipogenesis, which occurs in the third trimester of pregnancy, could also be hypothesized,[ 28 ] as Morrison et al. found an increased risk of glucose and blood pressure disorders in individuals born prematurely, unrelated to excessive weight gain in early childhood.[ 29 ]
Limitations
Selection bias
Although there is a random selection system adjusted for the probability of individual nonresponse for inclusion in the cohort, people selected in Constances are volunteers whose characteristics are different from the general population, particularly in terms of education level and lifestyle. These characteristics lead to a lower prevalence of metabolic diseases and, therefore, to a loss of power in statistical association measures. Furthermore, our study’s main selection criterion was the exclusion of individuals who could not provide their child health booklet. The comparison between included and excluded subjects in our analysis illustrates that it further selected subjects born in France with higher parental socioprofessional category and personal education level. However, we see no reason why these two limitations would distort the relationship between birth conditions and liver disease.
NAFLD diagnosis
The second point concerns the potential diagnosis misclassification related to the FLI and Forns Index. The FLI was developed in 2006 by Italian researchers.[ 16 ] With a threshold of 60, the sensitivity and specificity are respectively 87% and 64%.
The same questions may arise concerning the Forns Index. Nevertheless, the results of a recent study using data from a Swedish cohort[ 30 ] showed that the performance of this index was equivalent to that of the Fibrosis‐4 (FIB‐4) and NAFLD fibrosis scores, which are recommended by the European Association for the Study of the Liver for noninvasive liver fibrosis screening.[ 7 ] The accuracy (sensitivity × prevalence + specificity × [1 – prevalence]) for the Forns Index was 97.3% versus 96.8% for the FIB‐4 and 98.8% for the NAFLD fibrosis score.
Gestational age assessment and maternal data
Due to a lack of precision in gestational age data, recorded in months rather than in gestational weeks for a large number of participants, we had to categorize birth term into three classes to avoid misclassification. This led to a loss of precision in the assessment of the relationship with NAFLD risk.
Finally, maternal medical history (including obesity) was not considered in the adjustment strategy, even though this information would probably help to further understand the mechanisms of the association between BW and NAFLD. This will be considered for future studies on the cohort.
This study in the general population confirms an increased risk of NAFLD in individuals with low or high BW. Excess body fat mass in adulthood likely contributes to the increased risk in subjects born with HBW. At the other end of BW distribution, premature birth, rather than intrauterine growth retardation, is associated with an increased risk of NAFLD. Whether the increased risk in people with LBW is mediated by fat mass accumulated during the first months of life warrants investigation in future research in the same cohort. NAFLD should be considered as one of the adult chronic diseases with roots in early life, and BW should be considered as a significant element when it comes to the prevention of metabolic diseases in young people.
CONFLICTs OF INTEREST
Dr. Amadou consults for and received grants from Lilly, Sanofi, Diabeloop, and Medtrum. Dr. Lacombe consults for and received grants from Gilead.
AUTHOR CONTRIBUTIONS
Coralie Amadou (methodology, statistical analysis, original draft, review, and editing); Oumarou Nabi (review and editing); Lawrence Serfaty (review and editing); Karine Lacombe (review and editing); Jérôme Boursier (review and editing), Philippe Mathurin (review and editing); Céline Ribet (review and editing); Victor de Ledinghen (review and editing); Marie Zins (conceptualization, methodology, review, and editing); and Marie‐Aline Charles (conceptualization, methodology, original draft, review, and editing).
DATA AVILABILITY STATEMENT
Data from this study are protected by health data regulations from the French National Commission on Informatics and Liberty (Commission Nationale de l'Informatique et des Libertés, CNIL). Data can be made available upon reasonable request to the steering committee of the CONSTANCES cohort study, after legal verification of the use of the data.
Supporting information
Fig S1
Fig S2
Fig S3
Fig S4
Fig S5
Fig S6
Table S1
Amadou C, Nabi O, Serfaty L, Lacombe K, Boursier J, Mathurin P, et al. Association between birth weight, preterm birth, and nonalcoholic fatty liver disease in a community‐based cohort. Hepatology. 2022;76:1438–1451. 10.1002/hep.32540
Funding information
The Constances Cohort Study is supported and funded by the Caisse nationale d’assurance maladie. The Constances Cohort Study is an “Infrastructure nationale en Biologie et Santé” and received a grant from the National Research Agency, ANR (ANR‐11‐INBS‐0002), and from the French Ministry of Research. Constances is also partly funded by Merck Sharp and Dohme, AstraZeneca, and Lundbeck. The findings and conclusions in this study are those of the authors and do not necessarily represent the views of the sponsors.
REFERENCES
- 1. Barker DJ, Winter PD, Osmond C, Margetts B, Simmonds SJ. Weight in infancy and death from ischaemic heart disease. Lancet. 1989;2(8663):577–80. [DOI] [PubMed] [Google Scholar]
- 2. Hales CN, Barker DJ, Clark PM, Cox LJ, Fall C, Osmond C, Winter PD, et al. Fetal and infant growth and impaired glucose tolerance at age 64. BMJ. 1991;303(6809):1019–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Phipps K, Barker DJ, Hales CN, Fall CH, Osmond C, Clark PM. Fetal growth and impaired glucose tolerance in men and women. Diabetologia. 1993;36(3):225–8. [DOI] [PubMed] [Google Scholar]
- 4. Barker DJP, Osmond C, Forsén TJ, Kajantie E, Eriksson JG. Trajectories of growth among children who have coronary events as adults. N Engl J Med. 2005;353(17):1802–9. [DOI] [PubMed] [Google Scholar]
- 5. Barker DJP, Eriksson JG, Forsén T, Osmond C. Fetal origins of adult disease: strength of effects and biological basis. Int J Epidemiol. 2002;31(6):1235–9. [DOI] [PubMed] [Google Scholar]
- 6. Younossi ZM, Koenig AB, Abdelatif D, Fazel Y, Henry L, Wymer M. Global epidemiology of nonalcoholic fatty liver disease—Meta‐analytic assessment of prevalence, incidence, and outcomes. Hepatology. 2016;64(1):73–84. [DOI] [PubMed] [Google Scholar]
- 7. European Association for Study of Liver, Asociacion Latinoamericana para el Estudio del Higado . EASL‐ALEH Clinical Practice Guidelines: non‐invasive tests for evaluation of liver disease severity and prognosis. J Hepatol. 2015;63(1):237–64. [DOI] [PubMed] [Google Scholar]
- 8. Younossi ZM, Stepanova M, Afendy M, Fang Y, Younossi Y, Mir H, Srishord M, et al. Changes in the prevalence of the most common causes of chronic liver diseases in the United States from 1988 to 2008. Clin Gastroenterol Hepatol. 2011;9(6):524–530.e1; quiz e60. [DOI] [PubMed] [Google Scholar]
- 9. Querter I, Pauwels NS, De Bruyne R, Dupont E, Verhelst X, Devisscher L, et al. Maternal and perinatal risk factors for pediatric non‐alcoholic fatty liver disease: a systematic review. Clin Gastroenterol Hepatol. 2022;20(4):740–55. [DOI] [PubMed] [Google Scholar]
- 10. Newton KP, Feldman HS, Chambers CD, Wilson L, Behling C, Clark JM, et al. Low and high birth weights are risk factors for nonalcoholic fatty liver disease in children. J Pediatr. 2017;187:141–6.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Breij LM, Kerkhof GF, Hokken‐Koelega ACS. Accelerated Infant weight gain and risk for nonalcoholic fatty liver disease in early adulthood. J Clin Endocrinol Metab. 2014;99(4):1189–95. [DOI] [PubMed] [Google Scholar]
- 12. Suomela E, Oikonen M, Pitkänen N, Ahola‐Olli A, Virtanen J, Parkkola R, et al. Childhood predictors of adult fatty liver. The Cardiovascular Risk in Young Finns Study. J Hepatol. 2016;65(4):784–90. [DOI] [PubMed] [Google Scholar]
- 13. Sipola‐Leppänen M, Vääräsmäki M, Tikanmaki M, Matinolli HM, Miettola S, Hovi P, et al. Cardiometabolic risk factors in young adults who were born preterm. Am J Epidemiol. 2015;181(11):861–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Zins M, Bonenfant S, Carton M, Coeuret‐Pellicer M, Guéguen A, Gourmelen J, et al. The CONSTANCES cohort: an open epidemiological laboratory. BMC Public Health. 2010;10(1):479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Nabi O, Lacombe K, Boursier J, Mathurin P, Zins M, Serfaty L. Prevalence and risk factors of nonalcoholic fatty liver disease and advanced fibrosis in general population: The French Nationwide NASH‐CO Study. Gastroenterology. 2020;159(2):791–3.e2. [DOI] [PubMed] [Google Scholar]
- 16. Bedogni G, Bellentani S, Miglioli L, Masutti F, Passalacqua M, Castiglione A, et al. The Fatty Liver Index: a simple and accurate predictor of hepatic steatosis in the general population. BMC Gastroenterol. 2006;6(1):33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Forns X, Ampurdanès S, Llovet JM, Aponte J, Quintó L, Martínez‐Bauer E, et al. Identification of chronic hepatitis C patients without hepatic fibrosis by a simple predictive model. Hepatology. 2002;36(4 Pt 1):986–92. [DOI] [PubMed] [Google Scholar]
- 18. Robins J. A new approach to causal inference in mortality studies with a sustained exposure period—Application to control of the healthy worker survivor effect. Mathematical Modelling. 1986;7(9–12):1393–512. [Google Scholar]
- 19. Shi B, Choirat C, Coull BA, VanderWeele TJ, Valeri L. CMAverse: a suite of functions for reproducible causal mediation analyses. Epidemiology. 2021;32(5):e20–e22. [DOI] [PubMed] [Google Scholar]
- 20. Mouzaki M, Ling SC. The highs and lows of fetal programming for fatty liver disease. J Pediatr. 2017;187:13–5. [DOI] [PubMed] [Google Scholar]
- 21. Nobili V, Marcellini M, Marchesini G, Vanni E, Manco M, Villani A, Bugianesi E, et al. Intrauterine growth retardation, insulin resistance, and nonalcoholic fatty liver disease in children. Diabetes Care. 2007;30(10):2638–40. [DOI] [PubMed] [Google Scholar]
- 22. Ayonrinde OT, Olynyk JK, Marsh JA, Beilin LJ, Mori TA, Oddy WH, et al. Childhood adiposity trajectories and risk of nonalcoholic fatty liver disease in adolescents. J Gastroenterol Hepatol. 2015;30(1):163–71. [DOI] [PubMed] [Google Scholar]
- 23. Heerwagen MJR, Miller MR, Barbour LA, Friedman JE. Maternal obesity and fetal metabolic programming: a fertile epigenetic soil. Am J Physiol Regul Integr Comp Physiol. 2010;299(3):R711–R722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hermann GM, Dallas LM, Haskell SE, Roghair RD. Neonatal macrosomia is an independent risk factor for adult metabolic syndrome. Neonatology. 2010;98(3):238–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Gallagher D, Visser M, Sepúlveda D, Pierson RN, Harris T, Heymsfield SB. How useful is body mass index for comparison of body fatness across age, sex, and ethnic groups? Am J Epidemiol. 1996;143(3):228–39. [DOI] [PubMed] [Google Scholar]
- 26. McCance DR, Pettitt DJ, Hanson RL, Jacobsson LT, Knowler WC, Bennett PH. Birth weight and non‐insulin dependent diabetes: thrifty genotype, thrifty phenotype, or surviving small baby genotype? BMJ. 1994;308(6934):942–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Priante E, Verlato G, Giordano G, Stocchero M, Visentin S, Mardegan V, et al. Intrauterine growth restriction: new insight from the metabolomic approach. Metabolites. 2019;9(11):267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Desai M, Beall M, Ross MG. Developmental origins of obesity: programmed adipogenesis. Curr Diab Rep. 2013;13(1):27–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Morrison KM, Ramsingh L, Gunn E, Streiner D, Van Lieshout R, Boyle M, et al. Cardiometabolic health in adults born premature with extremely low birth weight. Pediatrics. 2016;138(4):e20160515. [DOI] [PubMed] [Google Scholar]
- 30. Hagström H, Talbäck M, Andreasson A, Walldius G, Hammar N. Ability of noninvasive scoring systems to identify individuals in the population at risk for severe liver disease. Gastroenterology. 2020;158(1):200–14. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig S1
Fig S2
Fig S3
Fig S4
Fig S5
Fig S6
Table S1


