Abstract
Squamous cells are rarely found in biliary tract cytology specimens, and when present are typically scant in quantity. Over an 8‐year time period, two cases at our institution reporting abundant squamous cells were identified. Both patients underwent endoscopic retrograde cholangiopancreatography with bile duct brushings and removal of a migrated biliary stent. The migrated stents were retrieved using rat toothed forceps and required removal of the endoscope through the esophagus with the stent exposed to esophageal and oral mucosa outside of the endoscope. Cytologic examination of the accompanying biliary stent material accordingly revealed abundant benign squamous cells. However, bile duct brushings showed benign ductal epithelial cells without squamous cells. Prior and subsequent cytology and bile duct surgical pathology specimens did not show squamous metaplasia. Migrated biliary stents that require endoscopic withdrawal increase the risk of contaminating samples with squamous cells. Recognition of this unique scenario is important, as the differential diagnosis includes squamous metaplasia and squamous neoplasia.
Keywords: bile duct, contamination, cytology, endoscopy, metaplasia, squamous cells
1. INTRODUCTION
Biliary tract cytology is commonly used to evaluate the biliary system for a wide variety of pathologic lesions. Bile duct brushings obtained during endoscopic retrograde cholangiopancreatography (ERCP) are the preferred method for the initial evaluation of suspected pancreaticobiliary neoplasms, due to the high specificity for malignancy (98%–100%), low complication rate, and ability to sample extensively throughout the pancreaticobiliary tract. 1 , 2 , 3 , 4 , 5 , 6 , 7 However, there are some significant limitations of this technique, including the low sensitivity (6%–65%, mean 42%) and difficulty distinguishing reactive changes from a neoplastic process, even when evaluated by experienced cytopathologists. 1 , 2 , 3 , 4 , 5 , 6 , 7 , 8 , 9 , 10 , 11 Cytologic examination of cellular material from removed biliary stents, which are used to treat biliary strictures, is also frequently performed. 12 However, the role of biliary stent cytology in the diagnosis of malignancy has not been as well studied. Several early studies found sensitivities ranging from 36% 13 up to 78.6%, 14 while a more recent study showed a lower sensitivity of 11%–16%. 15
Bile ducts are lined by cuboidal epithelium in the smaller ducts and columnar epithelium in the larger ducts, 16 and thus ductal epithelial cells are the most common cell type seen in biliary tract cytology specimens. However, a variety of metaplastic changes in bile duct epithelium have been described in the literature. Hoang et al. reported that pyloric gland metaplasia was the most common type of metaplasia seen in extrahepatic bile ducts, while intestinal metaplasia and combined pyloric and intestinal metaplasia were less common. 17 Kurumaya et al. reported pseudopyloric gland metaplasia and intestinal metaplasia of the intrahepatic bile ducts in patients with hepatolithiasis. 18 To the best of our knowledge, these are the only reviews of bile duct metaplasia to date.
Squamous cells are only rarely found in cytology specimens from the biliary tract. Squamous metaplasia of the biliary tree is very rare and was reported in only one of the 20 cases with metaplastic changes in the extrahepatic bile ducts reviewed in the above series 17 ; it also occurred in association with both pyloric and intestinal metaplasia. In another series, Stewart et al. reviewed 406 pancreaticobiliary brush specimens to assess the diagnostic accuracy of brush cytology in patients with pancreaticobiliary strictures. 19 They reported that “cells reminiscent of squamous metaplasia” were variably present in 215 cases that had negative cytological diagnoses. An even more rare cause of squamous cells in biliary tract cytology is contamination by oral or esophageal squamous mucosa during removal of biliary stents. Given the paucity of information available on this subject, we share our experience with two such cases where biliary cytology samples contained abundant squamous cells, which to our knowledge are the first reported cases of this type.
2. MATERIALS AND METHODS
We searched the bile duct cytology archives in our laboratory information system. Only two cases with squamous cells were identified. We reviewed the patient demographics, clinical presentation, brushing procedure, stent removal technique, and cytology findings in these cases. The samples included bile duct brushings and biliary stents that were collected during ERCP and placed in CytoLyt fixative solution. After agitating the samples, ThinPrep slides were prepared and stained with a Papanicolaou stain. Formalin‐fixed cell blocks were prepared and stained with hematoxylin and eosin for the biliary stent specimens.
3. CASE REPORTS
3.1. Case 1
A 65‐year‐old woman with a prior benign serous cystadenoma of the pancreas presented to the emergency department 6 years later with progressive obstructive jaundice. A CT scan showed a pancreatic head mass with obstruction of the common bile duct. An ERCP was performed, which revealed a bulging major papilla and biliary stricture with upstream dilation. A covered metal biliary stent was placed. Cells for cytology were obtained by brushing in the lower third and middle third of the common bile duct and revealed benign ductal epithelium with no malignant cells or squamous cells identified. The following day, a CT scan of the abdomen and pelvis was performed, which showed no significant change in the massive dilatation of the biliary tree. In addition, her stent was placed too distal to adequately decompress the biliary system. Hence, repeat ERCP was performed, and the stent was identified emerging from the major papilla. The stent appeared patent but had migrated intraductally since placement (Figure 1). The stent was removed from the biliary tree by grasping the stent with a rat toothed forceps and then withdrawing the endoscope from the patient. The stent was then sent for cytologic evaluation. A sphincterotomy was performed, and a new transpapillary stent was placed extending into the common hepatic duct. Microscopic examination of the cells from the removed stent revealed a highly cellular specimen consisting of benign ductal epithelium, inflammatory cells, debris, and scant bile. Numerous benign‐appearing squamous cells with bland round nuclei, finely granular chromatin, and abundant cytoplasm were also identified (Figure 2). The patient was subsequently discharged from the hospital, and a follow up ERCP was performed 8 weeks later for biliary stent exchange. Surgical intervention with a Whipple pancreaticoduodenectomy was attempted for definitive management of the bile duct obstruction. Due to high volume blood loss secondary to significant vascular collateralization of the large cystic mass in the pancreatic head, which was consistent with the patient's known serous cystadenoma, a palliative Roux‐en‐Y gastrojejunostomy and hepaticojejunostomy was performed instead.
FIGURE 1.

Bile duct stent is shown that migrated intraductally after placement because the metal flanges went into the duct, allowing the stent to freely migrate inward
FIGURE 2.

Microscopic examination of the cells from the removed stent in Case 1 revealed a highly cellular specimen consisting of benign ductal epithelium, inflammatory cells, debris, and scant bile. Numerous benign‐appearing squamous cells with bland round nuclei, finely granular chromatin, and abundant cytoplasm were also identified. Papanicolaou stain, 10× (A). Papanicolaou stain, 40× (B)
3.2. Case 2
A 48‐year‐old man with a history of a biliary stricture complicated by cholangitis had an ERCP with placement of a biliary stent. Bile duct brushings were reported as positive for adenocarcinoma. This outside specimen was not reviewed to confirm the diagnosis. He was referred to our institution for further evaluation and management. Repeat ERCP showed narrowing of the common bile duct with bilateral dilation of the intrahepatic ducts. The previously placed biliary stent was removed with a snare, replaced, and sent for cytologic analysis, which showed rare, atypical ductal epithelial cells and no squamous cells. Biopsy of the common bile duct showed reactive mucosal changes with no squamous metaplasia. Repeat ERCP was performed 7 weeks later for stent exchange. A partially occluded, plastic stent was identified. The stent was removed by grasping the stent with a rat toothed forceps and then withdrawing the endoscope from the patient. The biliary stent specimen was moderately cellular and consisted of abundant benign‐appearing squamous cells with round nuclei, finely granular chromatin, and abundant cytoplasm (Figure 3). There was background bile and abundant bacteria. No ductal epithelial cells or malignant cells were identified. Brushings from the remainder of the bile duct showed benign bile duct epithelium with no malignant cells or squamous cells. Biopsy of abnormal bile duct mucosa showed acutely inflamed mucosa. Four months later, the previously placed stent was removed with a snare and was not replaced as no obvious stricture was identified. The common bile duct was brushed and showed atypical ductal epithelial cells with no squamous cells. Fluorescence in situ hybridization (FISH) was performed on the bile duct brushings and showed no chromosomal gains associated with malignancy.
FIGURE 3.
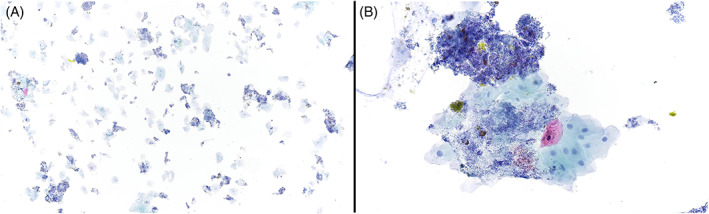
The biliary stent specimen from Case 2 was moderately cellular and consisted of abundant benign‐appearing squamous cells with round nuclei, finely granular chromatin, and abundant cytoplasm. There was background bile and abundant bacteria. No ductal epithelial cells or malignant cells were identified. Papanicolaou stain, 10× (A). Papanicolaou stain, 40× (B)
4. DISCUSSION/CONCLUSION
Review of the literature indicates that the presence of squamous cells in biliary tract cytology is a rare finding, and we were able to find only two such cases at our institution over an 8‐year period. The differential diagnosis includes squamous metaplasia, usually secondary to infection or inflammation; contamination from the upper gastrointestinal tract; a squamous‐lined cyst; or, rarely, squamous cell carcinoma or adenosquamous carcinoma of the pancreatobiliary tree.
Contamination from the gastrointestinal tract can occur when a stent is removed by grasping the stent and then withdrawing the endoscope. Upon investigation, there was endoscopic evidence of proximal stent migration in both of our cases with abundant squamous cells. When a stent has migrated, especially proximally, the endoscopist may be unable to grasp the stent in the proper position to allow for removal through the endoscope channel. As a result, the endoscope must be removed through the esophagus while the stent is exposed to the esophageal and oral mucosa outside of the endoscope. This method of stent removal thus increases the risk of contamination with esophageal and oral squamous cells. The fact that prior and subsequent pathology specimens in the above cases did not show squamous metaplasia is further evidence that the squamous cells were due to contamination from the upper gastrointestinal tract, rather than true squamous metaplasia.
As noted previously, squamous metaplasia in the bile ducts is also very rare. In the Hoang et al. series referenced above, 17 there was only a single case of squamous metaplasia, which occurred in a patient with an inflammatory stricture of the common bile duct. Histopathologic evaluation of the bile duct revealed replacement of the surface columnar biliary epithelium by stratified mature squamous epithelium, and there was concurrent pyloric and intestinal metaplasia. Kline et al. reported a case of a patient with a history of acquired immunodeficiency syndrome (AIDS), Cryptosporidium infection, and cholangitis who was found to have extensive squamous metaplasia and mild inflammation in a fluoroscopically guided biopsy of the bile duct mucosa. 20 Cryptosporidia could be visualized at the mucosal surface adjacent to the area of metaplastic change. Squamous metaplasia has also been reported rarely in the gallbladder due to chronic cholecystitis. 21
Squamous cells in biliary tract cytology may also represent the presence of a lesion such as a squamous‐lined cyst or squamous cell carcinoma. Kwon reported a case of a choledochal cyst entirely lined with stratified squamous epithelium instead of the usual bile duct epithelial lining. 22 There were adjacent foci of squamous metaplasia in the nearby bile duct branches. This lesion was considered most consistent with an epidermoid cyst arising from a background of squamous metaplasia of the bile duct. Squamous cell carcinoma of the bile duct is rare, with approximately 35 cases described in the literature. One such case, reported by Bacha et al., demonstrated the presence of atypical squamous cells with irregular nuclei, keratinization, and mitoses on bile duct brushing cytology. 23
Squamous cells may also infrequently be seen in pancreatic aspirates, which are sometimes obtained simultaneously with biliary brushings. Olson et al. reported that squamous cells were found in 2.5% of pancreatic fine needle aspiration specimens in a review of 4094 specimens. 24 Nearly half of these cases were ultimately diagnosed as primary adenosquamous carcinoma of the pancreas. Recognition of adenosquamous carcinoma is particularly important given its poor prognosis compared to conventional ductal adenocarcinoma. In addition to primary or metastatic adenosquamous or squamous cell carcinoma, the differential diagnosis includes lymphoepithelial cysts and squamous metaplasia associated with chronic pancreatitis or pancreatic as well as biliary stents. 24 , 25
In summary, we present two cases in which numerous squamous cells were present due to contamination from esophageal mucosa during stent removal. To our knowledge, this is the first report of cases of this kind. Recognition of this scenario is important to avoid misdiagnosis, as the differential diagnosis includes squamous metaplasia and other squamous lesions, including squamous neoplasms.
AUTHOR CONTRIBUTION
All authors contributed equally to the manuscript preparation and review.
CONFLICT OF INTEREST
No conflict of interest declared for all authors.
Tomm NK, Lamps LW, Ko C, Kwon RS, Cantley R, Pantanowitz L. Pronounced squamous cell contamination in biliary tract cytology: A diagnostic pitfall. Diagnostic Cytopathology. 2022;50(11):E320‐E324. doi: 10.1002/dc.25008
DATA AVAILABILITY STATEMENT
Data sharing is not applicable to this article as no new data were created or analyzed in this study.
REFERENCES
- 1. Avadhani V, Hacihasanoglu E, Memis B, et al. Cytologic predictors of malignancy in bile duct brushings: a multi‐reviewer analysis of 60 cases. Mod Pathol. 2017;30:1273‐1286. [DOI] [PubMed] [Google Scholar]
- 2. Layfield LJ, Wax TD, Lee JG, Cotton PB. Accuracy and morphologic aspects of pancreatic and biliary duct brushings. Acta Cytol. 1995;39(1):11‐18. [PubMed] [Google Scholar]
- 3. Cohen MB, Wittchow RJ, Johlin FC, Bottles K, Raab SS. Brush cytology of the extrahepatic biliary tract: comparison of cytologic features of adenocarcinoma and benign biliary strictures. Mod Pathol. 1995;8(5):498‐502. [PubMed] [Google Scholar]
- 4. Barr Fritcher EG, Caudill JL, Blue JE, et al. Identification of malignant cytologic criteria in pancreatobiliary brushings with corresponding positive fluorescence in situ hybridization results. Am J Clin Pathol. 2011;136(3):442‐449. [DOI] [PubMed] [Google Scholar]
- 5. Govil H, Reddy V, Kluskens L, et al. Brush cytology of the biliary tract: retrospective study of 278 cases with histopathologic correlation. Diagn Cytopathol. 2002;26(5):273‐277. [DOI] [PubMed] [Google Scholar]
- 6. Alizadeh MAH, Mousavi M, Salehi B, et al. Biliary brush cytology in the assessment of biliary strictures at a tertiary center in Iran. Asian Pac J Cancer Prev. 2011;12:2793‐2796. [PubMed] [Google Scholar]
- 7. Stoos‐Veić T, Bilić B, Kaić G, Ostović KT, Babić Z, Kujundzić M. Biliary brush cytology for the diagnosis of malignancy: a single center experience. Coll Antropol. 2010;34(1):139‐143. [PubMed] [Google Scholar]
- 8. Burnett AS, Calvert TJ, Chokshi RJ. Sensitivity of endoscopic retrograde cholangiopancreatography standard cytology: 10‐y review of the literature. J Surg Res. 2013;184(1):304‐311. [DOI] [PubMed] [Google Scholar]
- 9. Draganov PV, Chauhan S, Wagh MS, et al. Diagnostic accuracy of conventional and cholangioscopy‐guided sampling of indeterminate biliary lesions at the time of ERCP: a prospective, long‐term follow‐up study. Gastrointest Endosc. 2012;75(2):347‐353. [DOI] [PubMed] [Google Scholar]
- 10. Duggan MA, Brasher P, Medlicott SA. ERCP‐directed brush cytology prepared by the Thinprep method: test performance and morphology of 149 cases. Cytopathology. 2004;15(2):80‐86. [DOI] [PubMed] [Google Scholar]
- 11. Hart J, Parab M, Mandich D, Cartun RW, Ligato S. IMP3 immunocytochemical staining increases sensitivity in the routine cytologic evaluation of biliary brush specimens. Diagn Cytopathol. 2012;40(4):321‐326. [DOI] [PubMed] [Google Scholar]
- 12. Alagappan M, Darras N, Yang L, et al. Yield of biliary stent cytology: is it time to think lean? Endosc Int Open. 2019;7(4):E545‐E550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Foutch PG, Kerr DM, Harlan JR, Kummet TD. A prospective, controlled analysis of endoscopic cytotechniques for diagnosis of malignant biliary strictures. Am J Gastroenterol. 1991;86(5):577‐580. [PubMed] [Google Scholar]
- 14. Leung JW, Sung JY, Chung SC, Chan KM. Endoscopic scraping biopsy of malignant biliary strictures. Gastrointest Endosc. 1989;35(1):65‐66. [DOI] [PubMed] [Google Scholar]
- 15. Devereaux BM, Fogel EL, Bucksot L, Shelly LA, Lehman GA, Sherman S. Clinical utility of stent cytology for the diagnosis of pancreaticobiliary neoplasms. Am J Gastroenterol. 2003;98(5):1028‐1031. [DOI] [PubMed] [Google Scholar]
- 16. Strazzabosco M, Fabris L. Functional anatomy of Normal bile ducts. Anat Rec. 2008;291(6):653‐660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hoang MP, Murakata LA, Padilla‐Rodriguez AL, Albores‐Saavedra J. Metaplastic lesions of the extrahepatic bile ducts: a morphologic and immunohistochemical study. Mod Pathol. 2001;14:1119‐1125. [DOI] [PubMed] [Google Scholar]
- 18. Kurumaya H, Terada T, Nakanuma Y. ‘Metaplastic lesions’ in intrahepatic bile ducts in hepatolithiasis: a histochemical and immunohistochemical study. J Gastroenterol Hepatol. 1990;5:530‐536. [DOI] [PubMed] [Google Scholar]
- 19. Stewart CJR, Mills PR, Carter R, et al. Brush cytology in the assessment of pancreatico‐biliary strictures: a review of 406 cases. J Clin Pathol. 2001;54:449‐455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kline TJ, De Las MT, O'Brien M, Smith BF, Afdhal NH. Squamous metaplasia of extrahepatic biliary system in an AIDS patient with cryptosporidia and cholangitis. Dig Dis Sci. 1993;38(5):960‐962. [DOI] [PubMed] [Google Scholar]
- 21. Teo CHY, Leow CK, Chang SA. A pseudoepidermoid cyst arising from exuberant squamous metaplasia of the gallbladder. Arch Pathol Lab Med. 2005;129(6):e138‐e140. [DOI] [PubMed] [Google Scholar]
- 22. Kwon D. Primary epidermoid cyst of biliary duct presenting as choledochal cyst. Am J Clin Pathol. 2016;146:S104‐S142. [DOI] [PubMed] [Google Scholar]
- 23. Bacha D, Hajri M, Ferjaoui W, et al. Primary squamous cell carcinoma of the common bile duct with liver metastases. Arq Bras Cir Dig. 2021;34(1):e1564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Olson MT, Siddiqui MT, Ali SZ. The differential diagnosis of squamous cells in pancreatic aspirates: from contamination to adenosquamous carcinoma. Acta Cytol. 2013;57(2):139‐146. [DOI] [PubMed] [Google Scholar]
- 25. Layfield LJ, Cramer H, Madden J, Gopez EV, Liu K. Atypical squamous epithelium in cytologic specimens from the pancreas: cytological differential diagnosis and clinical implications. Diagn Cytopathol. 2001;25(1):38‐42. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article as no new data were created or analyzed in this study.


