Abstract
The study of the astrocytic contribution to brain functions has been growing in popularity in the neuroscience field. In the last years, and especially since the demonstration of the involvement of astrocytes in synaptic functions, the astrocyte field has revealed multiple functions of these cells that seemed inconceivable not long ago. In parallel, cannabinoid investigation has also identified different ways by which cannabinoids are able to interact with these cells, modify their functions, alter their communication with neurons and impact behavior. In this review, we will describe the expression of different endocannabinoid system members in astrocytes. Moreover, we will relate the latest findings regarding cannabinoid modulation of some of the most relevant astroglial functions, namely calcium (Ca2+) dynamics, gliotransmission, metabolism, and inflammation.
Keywords: astrocytes, calcium, cannabinoids, gliotransmission, metabolism, neuroinflammation
Main Points
Astrocytes have a functional and diverse endocannabinoid system.
Cannabinoids are able to interact with astrocytes to modulate their functions and their communication with neurons.
1. ASTROCYTES AS INTEGRATORS OF INFORMATION
Astrocytes are a population of glial cells representing approximately 10%–20% of all the cells in the central nervous system (CNS) (Sun et al., 2017; Verkhratsky et al., 2017). Their unique morphological complexity, with intricate arborisation and specialized structures that contact other CNS elements such as other glial cells, blood vessels, and synapses, make astrocytes fundamental players in brain physiology, as well as in the development of several pathological processes (Valori et al., 2021). Many diverse functions have been attributed to these cells, from providing metabolic support for neurons to the exciting new concepts of astrocyte‐mediated de novo memory enhancement and active involvement in higher‐brain functions (Adamsky et al., 2018; Kastanenka et al., 2020; Mederos et al., 2021; Mederos & Perea, 2019; Navarrete et al., 2019; Zhou et al., 2021). Considering this, it has become clear that the studies of CNS physiology need to address the role of astrocytes (and probably other glial cells) to get a full picture of how the brain elements are interconnected and work together.
Due to their singular morphology, one single astrocyte is in contact with thousands of synapses through its thin distal processes (Bushong et al., 2002; Oberheim et al., 2009). Astrocytes are thus able to sense neuronal activity, adapting their physiology to neuronal demands and even participating in the synaptic function through the release of gliotransmitters (Allen & Eroglu, 2017; Araque et al., 2014; Durkee & Araque, 2019; Perea et al., 2009). On top of that, astrocytes express different neuromodulator receptors that are able to sense global environmental stimuli, integrating both synaptic and network information to finely tune neuronal connectivity (Ding et al., 2013; Paukert et al., 2014; Zhang et al., 2021). Thus, in response to both synaptic and extrasynaptic inputs, astrocytes are able to modify their physiology at different time points to orchestrate neuronal functions, being key for the proper function of brain physiology (Pacholko et al., 2020).
This review will address how the presence and the activity of one of these neuromodulator inputs, the endocannabinoid system (ECS), can impact astrocyte signaling and modulate key brain functions.
2. THE ENDOCANNABINOID SYSTEM IN ASTROCYTES
The ECS is a widely distributed, polyfunctional signaling system that is virtually involved in all brain functions (Fride, 2005). This signaling system was named after the discovery of the G‐protein coupled receptors (GPCRs) activated by the main active compound of Cannabis, (−)‐Δ 9‐tetrahydrocannabinol (THC), and the endogenous lipidic substances with cannabimimetic effects, so called endocannabinoids (eCBs) (Di Marzo, 1998). Thus, the ECS is classically considered to be composed by type 1 and type 2 cannabinoid receptors (CB1, CB2), anandamide (AEA), and 2‐arachidonoylglycerol (2‐AG), which are the best characterized eCBs derived from arachidonic acid (AA), and the enzymes responsible for their synthesis and degradation (for reviews on the ECS: Castillo et al., 2012; Hu & Mackie, 2015, 2015; Zou & Kumar, 2018; Cristino et al., 2020).
The boundaries of the ECS, however, are not well defined. Several reports have been pointing towards others cannabinoid‐like receptors and cation channels that are activated by eCBs or other biochemically related molecules (Di Marzo, 2018). Moreover, in addition to the “classical” AA‐derived AEA and 2‐AG, other endogenous ligands have been recently described as cannabinoid receptor modulators. For instance, the lipids Lipoxin A4 (Pamplona et al., 2012) and pregnenolone (Vallee et al., 2014) have been shown to act as positive and negative allosteric modulators of CB1 receptors, whereas a family of peptides (so‐called Pepcans) has been proposed to regulate cannabinoid signaling in the brain (Bauer et al., 2012). These additional elements introduce new layers of complexity that transform our perceptions on this signaling system, giving rise to the concept of an expanded ECS (Cristino et al., 2020; Di Marzo, 1998; Veilleux et al., 2019).
Functionally, a wide‐spread well‐characterized and powerful way through which the ECS regulates brain functions is the retrograde control of synaptic transmission and plasticity (Castillo et al., 2012; Kano et al., 2009). Briefly, this model indicates that the stimulation of postsynaptic neurons induces the mobilization of eCBs that travel retrogradely through the synaptic cleft, binds to presynaptic CB1 receptors and thereby reduces synaptic transmission and triggers synaptic plasticity (Piomelli, 2003). This can occur at many different types of synapses (excitatory, inhibitory, modulatory), thereby providing a very fine‐tuned control of interneuronal communication. However, besides this well‐described mechanism of action of the ECS, studies during the last decades revealed several additional ways through which the ECS impacts brain functions. One of the most unexpected and intriguing ones is the relatively recent discovery that astrocytes express several eCB‐related proteins, playing a key role in the control of their activity and functions (Busquets‐Garcia et al., 2018; Covelo et al., 2021; Oliveira da Cruz et al., 2016) (Figure 1).
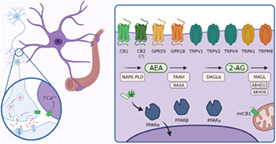
FIGURE 1.
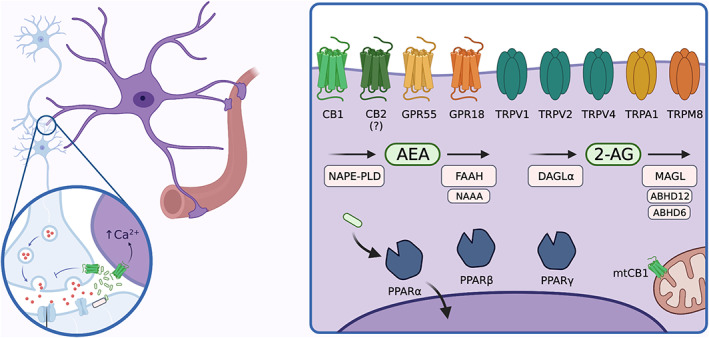
Endocannabinoid system elements in astrocytes. Upon depolarization, endocannabinoids (eCBs) are synthetized “on demand” and released from the postsynaptic terminal. eCBs will travel retrogradely to the presynaptic neuron to inhibit neurotransmitter release but also to the astrocytic thin processes, regulating astrocyte physiology. One of the most well‐known consequences of eCBs effects on astrocytes is the increase in cytosolic Ca2+ signals through cannabinoid (CB1) activation. However, astrocytes express other receptors associated to eCB signaling, which, upon activation may modulate several astroglial functions, such as release of gliotransmitters, metabolism or immune response via different signaling pathways. Of note, besides CB1 (and maybe CB2), other potential cannabinoid receptors have been described in astrocytes, such as GPR55 and GPR18 as well as different transient receptor potential (TRPs) such as TRPV1, TRPV2, TRPV4, TRPA1 or TRPM8. Moreover, the intracellular receptors peroxisome proliferator‐activated receptors (PPARs) (α, β and γ) have been described as potential cannabinoid receptors in astrocytes. Furthermore, functional CB1 receptors were found associated to astroglial mitochondria. Finally, astrocytes also express all the enzymes that take part in synthesis and catabolism of both anandamide (AEA) (N‐arachidonoylethanolamine phospholipase D [NAPE‐PLD] for synthesis and fatty acid amide hydrolase (FAAH) and N‐acylethanolamine acid amidase [NAAA] for degradation) and 2‐AG (diacylglycerol lipase α [DAGLα] for synthesis and monoacylglycerol lipase [MAGL], Alpha/beta‐hydrolase domain containing 12 [ABHD12], Alpha/beta‐hydrolase domain containing 6 [ABHD6] for catabolism), suggesting its implication in eCBs production and degradation.
The first evidence suggesting the expression of cannabinoid‐like receptors in astrocytes resulted from the observation that AEA treatment reduced the connection of cultured striatal astrocytes by gap junctions (Stella, 2010; Venance et al., 1995). Importantly, this effect seemed to be CB1‐independent, as it was not mimicked by CB1 synthetic agonists (CP55940 and WIN55) nor reverted by the CB1 antagonist rimonabant. Indeed, CB1 expression in astrocytes was initially controversial and early studies using cultured astrocytes addressing this question stated contradictory results. In the same way, the identification of astroglial CB1 by ultrastructural studies was ambiguous (Katona et al., 1999; Moldrich & Wenger, 2000; Rodríguez et al., 2001; Salio et al., 2002). However, CB1 expression on astrocytes was functionally demonstrated in the context of the hippocampal tripartite synapse, where local application of cannabinoids induced astroglial Ca2+ increases, an effect that was reverted by the CB1 selective antagonist AM251 (Navarrete & Araque, 2008, 2010). Complementarily, electron microscopic analyzes of double immunostaining of the astroglial marker glial fibrillary acid protein (GFAP) and CB1 in wild‐type and null CB1 mutant mice (CB1‐KO) provided definitive anatomical evidence for the existence of astroglial CB1, although in lower levels than neurons (Gutiérrez‐Rodríguez et al., 2018; Han et al., 2012).
The expression of CB1 receptors in astrocytes is rather complex. Besides the widely explored localization of CB1 in astrocytic plasma membranes close to synaptic terminals (Covelo et al., 2021), this receptor can be found in other subcellular localizations such as in mitochondria (Gutiérrez‐Rodríguez et al., 2018; Jimenez‐Blasco et al., 2020) and the plasmalemma of astrocytic perivascular end‐feet (Moldrich & Wenger, 2000; Rodríguez et al., 2001). Indeed, recent evidence has shown that activation of mitochondrial CB1 (mtCB1) in astrocytes via cannabinoids has a polyfunctional role that impacts glucose metabolism, Ca2+ signaling and behavior (Jimenez‐Blasco et al., 2020; Serrat et al., 2021).
Contrary to CB1, CB2 was initially considered to be absent from the brain (Atwood & Mackie, 2010). However, this receptor was later found to be present and functionally relevant in both microglia and possibly neurons (Jordan & Xi, 2019). Regarding astrocytes, CB2 presence seems to be questionable (Benyó et al., 2016; Dowie et al., 2014; Núñez et al., 2008; Stella, 2004). There are a few studies suggesting that CB2 is expressed in astrocytic cultures (Cassano et al., 2017; Köfalvi et al., 2016; Molina‐Holgado et al., 2002) but this can be a result of the impact that different culture protocols have on the astroglial transcriptome (Lange et al., 2012). Overall, there is a current lack of robust evidence for the presence of astroglial CB2 receptors in physiological conditions, although astrocytes may express this receptor under some pathological conditions. For instance, it is widely accepted that CB2 is upregulated in glial cells in response to some insults (Benito et al., 2008; Jordan & Xi, 2019). However, while some reports found this response to be specific to microglia (Núñez et al., 2008; Schmöle et al., 2015), others reported CB2 expression in astrocytes. For instance, CB2 was reported to be present on astrocytes from the spinal cord of a canine model for amyotrophic lateral sclerosis (Fernández‐Trapero et al., 2017). Moreover, CB2 is also present in human astrocytes derived from human fetal brain tissues (Sheng et al., 2005) and from postmortem studies of multiple sclerosis, spinocerebellar ataxia patients, and suicide victims (García‐Gutiérrez et al., 2018; Rodríguez‐Cueto et al., 2014; Zhang et al., 2011).
Besides CB1 and CB2, other GPCRs such as GPR55 and GPR18 have been proposed as putative cannabinoid receptors (Boczek & Zylinska, 2021; Cristino et al., 2020). These receptors have been identified in astrocytes, at both mRNA‐ and protein‐level (García‐Gutiérrez et al., 2018; Grabiec et al., 2019; Gutiérrez‐Rodríguez et al., 2018; Sawzdargo et al., 1999), however, their functional significance in astrocytes remains largely unexplored.
Additionally to GPCRs, receptors from other classes have also been associated to eCB signaling, namely a subset of transient receptor potential (TRP) channels and some isoforms of peroxisome proliferator‐activated receptors (PPARs) (Muller et al., 2019; O'Sullivan, 2016). TRPs are a superfamily of transmembrane cation channels that exhibit a remarkable diversity of activation mechanisms, including chemical and physical stimuli (Venkatachalam & Montell, 2007). In many cases, a single TRP channel can be modulated by distinct stimuli that can modify each other's responses, thereby acting as multiple signal integrators (Venkatachalam & Montell, 2007). Amongst TRP channels, there is evidence that cannabinoids can modulate the activity of TRP channels from the vanilloid (TRPV), ankyrin (TRPA), and melastatin (TRPM) subfamilies, namely TRPV1‐4, TRPA1 and TRPM8 (Boczek & Zylinska, 2021; Muller et al., 2019). In astrocytes, these so‐called “ionotropic cannabinoid receptors” are mainly associated to spatiotemporal coordination of Ca2+ and sodium (Na+) transients, that further transduce in various internal signaling cascades with multiple outcomes (Verkhratsky et al., 2014). The astrocytic expression and functional relevance of TRPV1, TRPV2, TRPV4, and TRPA1 is widely accepted (Benfenati et al., 2011; Luo et al., 2020; Shibasaki et al., 2013; Shigetomi et al., 2013; Tóth et al., 2005; Yang et al., 2019). Additionally, a recent study using primary cultures derived from piglet brains suggested the presence of TRPM8 in astrocytes (Fedinec et al., 2021). Finally, even though TRPV3 is known to be present in the brain, there is no evidence so far for its expression in astrocytes (Luo et al., 2020; Xu et al., 2002). However, the impact of (endo)cannabinoids on astroglial functions through this family of receptors is still to be established.
Regarding PPARs, these proteins constitute a family of nuclear hormone receptors that regulate gene expression by binding to DNA sequences called PPAR response elements upon ligand‐dependent activation (Bugge & Mandrup, 2010). All three subtypes of PPARs (α, β, and γ) have been associated to some of the CB1/CB2‐independent cannabinoid effects (O'Sullivan, 2016; Sun & Bennett, 2007). In mice, astrocytes have been reported to express PPARα and PPARγ, but no PPARβ (Warden et al., 2016). Interestingly, the expression of these receptors varies according to the brain region, with a lower expression in the ventral tegmental area compared to the prefrontal cortex, nucleus accumbens or amygdala, suggesting the idea of an area‐specific or circuit‐specific astrocytic cannabinoid signaling. Moreover, these receptors exhibit different subcellular distributions: while PPARα is localized in the astrocytic cell body and processes, astroglial PPARγ is mostly present in the cell body and scarce in processes (Warden et al., 2016). This suggests a potential for a PPAR‐mediated diversity of function of cannabinoids in astrocytes yet to be unveiled.
Importantly, astrocytes are not only passive eCB “receivers” and some evidence has shown that these cells participate in eCB metabolism. First, astrocytes have a well‐recognized role on eCB degradation. In the context of retrograde signaling, astrocytes and neurons contribute synergistically to 2‐AG signaling termination through monoacylglycerol lipase (MAGL), its main degrading enzyme (Chen et al., 2016; Liu et al., 2016; Viader et al., 2015). Alpha/beta‐hydrolase domain containing 6 (ABHD6) and Alpha/beta‐hydrolase domain containing 12 (ABHD12) also contribute for astrocytic 2‐AG degradation, what can be particularly relevant in adaptative responses to certain pathological conditions (Marrs et al., 2010; Moreno‐García et al., 2020; Viader et al., 2016). Regarding AEA degradation, astrocytes dynamically express its main catabolic enzyme, fatty acid amide hydrolase (FAAH), which is upregulated in certain neuroinflammatory responses (Benito et al., 2007; Kallendrusch et al., 2012; Moreno‐García et al., 2020; Núñez et al., 2008). Moreover, astrocytes also express N‐acylethanolamine acid amidase (NAAA), an alternative AEA degradation enzyme, although in lower levels when compared to FAAH (Kallendrusch et al., 2012; Moreno‐García et al., 2020).
Finally, although it was early demonstrated that cultured astrocytes can produce both AEA and 2‐AG (Walter et al., 2002, 2004; Walter & Stella, 2003), there is an ongoing debate about the functional significance of astroglial‐derived eCBs (Covelo et al., 2021). Nonetheless, the expression of the main enzymes responsible for 2‐AG and AEA production, diacylglycerol lipase α (DAGLα) and N‐arachidonoylethanolamine phospholipase D (NAPE‐PLD) respectively, was characterized in cultured astrocytes (Kallendrusch et al., 2012; Viader et al., 2016) and their mRNA can also be detected ex vivo (Schüle et al., 2021). Interestingly, this machinery seems to be sensitive to changes in the environment, such as during maternal caloric restriction in rats (Tovar et al., 2021).
3. ROLE OF ENDOCANNABINOID SYSTEM IN ASTROGLIAL CALCIUM SIGNALING
3.1. Calcium signaling in astrocytes
Being electrically nonexcitable cells, astrocytes communicate with other cell types through different mechanisms that are largely regulated by intracellular Ca2+ levels. These Ca2+ fluctuations in astrocytes are then modulated by environmental conditions. Ca2+ increase is mediated either by Ca2+ entry from the extracellular space through the plasma membrane or by Ca2+ release from the intracellular Ca2+ stores such as the endoplasmic reticulum (ER) and mitochondria. The resulting Ca2+ events spread within the cell by passive diffusion and/or by regenerative mechanisms (Semyanov, 2019).
Plasma membrane entrance pathways consist in ligand‐activated cation permeable ionotropic receptors, such as N‐methyl‐d‐aspartate (NMDA), purinergic P2X(1/5) receptors (Lalo et al., 2006; Palygin et al., 2010), TRP channels that sense various changes in the environment (Verkhratsky et al., 2014), store operated Ca2+ entrance (Sakuragi et al., 2017), voltage‐gated Ca2+ channels (VGCCs) (Carmignoto et al., 1998), and the sodium/calcium exchanger (NCX) operating in reverse mode (Kirischuk et al., 2012). Note that these sources can work together with intracellular Ca2+ stores. For instance, it has been proposed that Ca2+ entry through VGCCs is very small in astrocytes, but it can trigger Ca2+ release from the ER (Carmignoto et al., 1998). The Ca2+ events are terminated by the inactivation of the Ca2+ source and by Ca2+ extrusion through the plasma membrane and/or uptake to the intracellular Ca2+ stores. The infinite possibilities of combinations result in a high diversity of Ca2+ events, whose decoding is still far to be achieved.
In astrocytes, Ca2+ release from ER is mediated by the Inositol trisphosphate Receptor (IP3R) (Kirischuk et al., 1999; Ullah et al., 2006). Ca2+ and IP3 are co‐agonists of IP3Rs (Bezprozvanny et al., 1991; Dupont & Goldbeter, 1993; Mak et al., 1998). Ca2+ elevations reaching the IP3R activation threshold can trigger a release of Ca2+ from the ER (Shinohara et al., 2011; Wu et al., 2019), a process known as Ca2+‐induced Ca2+ release. IP3Rs activation threshold depends on the level of IP3: when it increases, IP3Rs become more sensitive to Ca2+, which enhances their opening probability, and consequently IP3R‐mediated amplification and propagation of Ca2+ events (Mak et al., 1998; Shinohara et al., 2011). Astrocytes express several types of Gq‐protein coupled receptors which can trigger IP3 production via phospholipase C (PLC) activation: metabotropic glutamate receptor 5 (mGluR5) which can be activated following synaptic glutamate release (Bradley & Challiss, 2012; Nakahara et al., 1997; Umpierre et al., 2019), Gq‐protein coupled purinergic P2Y receptors (Fumagalli et al., 2003; Helen et al., 1992), serotoninergic 5HT2 A, adrenergic α1AR Gq‐protein coupled receptors (O'Donnell et al., 2015; Porter‐Stransky et al., 2019; Xu & Pandey, 2000) and CB1 (Navarrete & Araque, 2008). Of note, IP3R activity is regulated by cytosolic Ca2+ following a bell shape dependency: low concentrations of Ca2+ (50 nM–1 μM) increase the opening probability of IP3R, whereas higher concentrations (more than 1 μM) lower it. It has been proposed that high concentrations of Ca2+ at the mouth of the channel might prevent the propagation of the Ca2+ signal by inhibiting the channel activity (Foskett & Mak, 2010).
Other important players in astrocytic Ca2+ dynamics, and potentially astrocytes regulation of synaptic function, are mitochondria (Stephen et al., 2014). Mitochondria act globally as Ca2+ stores. Mitochondrial Ca2+ uptake is mostly mediated by the mitochondrial Ca2+ uniporter (MCU) and Ca2+ release by mitochondrial NCX, H+/Ca2+ exchanger, and the permeability transition pore (PTP) (O‐Uchi et al., 2012; Rizzuto et al., 2012). These transport mechanisms are located in the inner mitochondrial membrane, whereas the outer mitochondrial membrane is highly permeable to soluble molecules smaller than 5 kDa, including Ca2+. MCU drives the entry of large flux of Ca2+ into mitochondria, but it operates at micromolar cytosolic Ca2+ levels, which are usually only reached at the mouth of Ca2+ sources (Boyman & Lederer, 2020; Williams et al., 2013). When mitochondria are located in close proximity to the ER at the level of mitochondria/ER contacts (MERCs), MCU can contribute to the removal of Ca2+ released by IP3Rs (Boyman & Lederer, 2020; Marchi & Pinton, 2014; Rizzuto et al., 2012). MERCs have been observed in astrocytes processes and endfeet (Gӧbel et al., 2020), and the expression of some MERCs‐constitutive proteins induces Ca2+ transfer from the ER to mitochondria (Eraso‐Pichot et al., 2017). However, it is not clear how mitochondria Ca2+ uptake affects cytosolic Ca2+ transients in astrocytes. Some studies have reported that the impairment of the mitochondrial function increases the propagation and the duration of Ca2+ signals, which would suggest that mitochondria modulate negatively Ca2+ waves propagation (Boitier et al., 1999; Jackson & Robinson, 2015; Reyes & Parpura, 2008). On the other hand, other studies have observed that amplification sites for Ca2+ waves are characterized by accumulations of ER Ca2+ signaling proteins (calreticulin and IP3R), and by the presence of mitochondria (Simpson et al., 1997). Hence, ER‐mitochondria Ca2+ transfer could be needed for the propagation of cytosolic Ca2+ waves in astrocytes. Recently, we have shown that pharmacological inhibition or overexpression of a dominant negative mutant of MCU hamper the propagation of cytosolic Ca2+ events (Serrat et al., 2021). Altogether, these data suggest that mitochondria might prevent IP3R self‐inhibition by buffering the released Ca2+ at the mouth of the channel in astrocytes. Hence, depending on the studies, mitochondria appear as downregulators or enhancers of cytosolic Ca2+ signals. Discrepancies could originate from the different stimuli used (physiological synaptic stimulation vs pharmacological [ATP] or mechanical stimuli) and the type of mitochondrial drugs used (mitochondrial uncoupler or diverse MCU inhibitors).
3.2. Calcium patterns modulation following synaptic events
Astrocytes exhibit local and short Ca2+ events under resting conditions. The precise mechanisms by which those spontaneous Ca2+ transients are generated remain debated. Although it has been proposed that they could be linked to spontaneous synaptic vesicle release (Sun et al., 2014), astrocytes are still able to generate spontaneous Ca2+ events when neuronal and astrocytic vesicular release is blocked by bafilomycin A1 (Bowser & Khakh, 2007; Nett et al., 2002). Alternatively, stochastic opening of IP3Rs could underline spontaneous Ca2+ transients (Foskett & Mak, 2010). However, knockout of astrocytic IP3R type 2 still display spontaneous Ca2+ events (Sherwood et al., 2017; Srinivasan et al., 2015). Finally, a recent report has suggested that brief openings of PTP can be responsible for astrocytic spontaneous Ca2+ events in the absence of IP3R‐mediated Ca2+ release (Agarwal et al., 2017). Most likely, they are generated by stochastic Ca2+ fluxes through multiple pathways. Interestingly, changes in the properties of Ca2+ events (frequency, duration, and spread) occur in response to changes in environment, such as increased neuronal activity or high metabolic demand.
Extensive literature has shown that synaptic activity induces intracellular Ca2+ transients in astrocytes (Araque et al., 2001; Haydon & Carmignoto, 2006; Nedergaard et al., 2003; Panatier et al., 2011; Perea et al., 2009; Perea & Araque, 2005; Porter & McCarthy, 1996; Volterra & Meldolesi, 2005), through the activation of astroglial neurotransmitter receptors coupled to second messenger pathways. In turn, Ca2+ elevations cause the release of different signaling molecules, termed gliotransmitters, that modulate neuronal excitability and synaptic transmission (Araque et al., 1999; Beattie et al., 2002; Perea & Araque, 2007). Different patterns of neuronal activity are encoded in spatial and temporal properties of astroglial Ca2+ events. Thus, astrocytes display a highly complex repertoire of subcellular and intercellular Ca2+ signals that can be generated by different routes and involve different subregions of the cells.
For instance, spatial propagation of Ca2+ signals has been observed following application of mGluR agonists and low or high frequency stimulation of Schaffer collaterals (Navarrete & Araque, 2008; Sun et al., 2014; Wu et al., 2014). Propagation of Ca2+ transients is hypothesized to allow astrocytes to convey messages to distant synapses (Semyanov, 2019). This modulation of Ca2+ event properties was abolished by mGluR antagonists, by IP3 buffering via overexpression of an IP3 sponge (modified ligand‐binding domain of the IP3R) or by knocking out the astrocyte‐specific IP3R (Rungta et al., 2016; Sherwood et al., 2017; Srinivasan et al., 2015; Tanaka et al., 2013). The IP3R is the ideal support for the spatial propagation of Ca2+ transients upon neuronal stimulation. Indeed, glutamate, ATP, noradrenaline and eCBs can activate GPCRs and thereby increase the production of diacylglycerol and IP3 by PLC. The resulting high cytosolic IP3 levels increase the probability that spontaneous Ca2+ fluctuations are amplified by Ca2+‐induced Ca2+ release. This amplification can convert spatially restricted Ca2+ transient into propagating events. The central role of IP3R in the propagation of Ca2+ transient in astrocytes was illustrated in a couple of studies using KO mice for IP3R2 in astrocytes. Spontaneous Ca2+ fluctuations still occur in those mice, but they can no longer be amplified by Ca2+‐induced Ca2+ release (Rungta et al., 2016; Sherwood et al., 2017; Srinivasan et al., 2015; Tanaka et al., 2013). Recent work from our team also suggests that neuronal activity can modulate IP3R activity by tuning the Ca2+ uptake into mitochondria (Serrat et al., 2021). Interestingly, mtCB1 is involved in this mechanism, as will be discussed below.
3.3. Cannabinoid modulation of calcium signals in astrocytes
The most studied example of how cannabinoids may modulate neuronal communication through astrocytic Ca2+ signals so far is the CB1 receptor. In 2008, Alfonso Araque's group demonstrated that CB1 expressed in hippocampal astrocytes could be activated by exogenous cannabinoids as well as by eCBs released by neurons following neuronal stimulation. CB1 activation increased astrocyte Ca2+ levels through PLC‐dependent Ca2+ release from the ER, ultimately inducing cytosolic Ca2+ increases in astrocytes soma and processes. This CB1‐induced Ca2+ increase can spread along the cell and induce the release of gliotransmitters at distal regions (Navarrete & Araque, 2008). This propagation of astrocytic Ca2+ signals has been shown to induce short‐term (Covelo & Araque, 2018; Navarrete & Araque, 2010) or long‐term potentiation (Gómez‐Gonzalo et al., 2015) at distant synapses. In this aspect, astroglial CB1 act through specific signaling pathways that differ from the canonical neuronal ones that occur at short distances and result in a presynaptic inhibition of neurotransmitter release (Kano et al., 2009). Interestingly, other studies have shown that the physiological or the pharmacological activation of CB1 produces astrocytic Ca2+ increase (Hegyi et al., 2018; Kőszeghy et al., 2015; Robin et al., 2018). However, in these works, the mechanisms involved in the propagation of the Ca2+ signal were not explored.
At subcellular level, CB1 receptors in astrocytes have been observed not only at the plasma membrane but also associated with intracellular organelles like mitochondria (Gutiérrez‐Rodríguez et al., 2018; Jimenez‐Blasco et al., 2020). Contrary to other pools of CB1, astroglial mtCB1 activation has been associated with Gαi/o proteins, inhibition of mitochondrial soluble adenylyl cyclase (sAC) and reduction of mitochondrial respiration through the regulation of specific mitochondrial respiratory subunits (Hebert‐Chatelain et al., 2016; Jimenez‐Blasco et al., 2020). Our recent paper shows that the physiological spreading of astrocytic Ca2+ events in the context of lateral potentiation requires mtCB1 signaling (Serrat et al., 2021). Indeed, lateral potentiation is virtually abolished in mice expressing DN22 CB1, a mutant version of this receptor that is excluded from mitochondria (Hebert‐Chatelain et al., 2016; Soria‐Gomez et al., 2021). We hypothesize that the activation of mtCB1 enhances the transfer of Ca2+ between ER and mitochondria, removing the inhibition of IP3R by buffering the released Ca2+ at the mouth of the channel. Indeed, in cultured astrocytes, almost 100% of mitochondria are located less than 100 nm from ER, a distance that permit Ca2+ exchanges between the two organelles (Csordás et al., 2018). In addition, activation of mtCB1 receptors induces ER‐mitochondria Ca2+ transfer. mtCB1 has also been identified in astrocytic processes, in the vicinity of synapses (less than 1 μM away [Gutiérrez‐Rodríguez et al., 2018]). Hence, all the machinery needed for mtCB1‐regulated ER‐mitochondria Ca2+ transfer is present in astrocytic processes. In addition, in our hand, MCU inhibition affects the spatial and temporal spreading of subcellular Ca2+ events following strong stimulation of Schaffer collaterals in hippocampal slices, a protocol known to induce lateral potentiation (Serrat et al., 2021).
Interestingly, other members of the ECS are potential modulators of Ca2+ signals in astrocytes. Astrocytes express a huge variety of TRP channels that may control astrocytic Ca2+ signals (Verkhratsky et al., 2014) and are potentially modulated by both endogenous and exogenous cannabinoids (Muller et al., 2019). For instance, TRPA1, which has been implicated in the regulation of astrocyte resting Ca2+ (Shigetomi et al., 2013), has shown also cannabinoid‐mediated activity (Muller et al., 2019). Also, in Drosophila melanogaster, the TRP analog water witch (WTRW) has been seen to control Ca2+ dynamics in astrocytes, modulating neuronal activity and behavior (Ma et al., 2016). Interestingly, this same channel in astrocytes and neurons is modulated by AEA metabolites and protects against seizures (Jacobs & Sehgal, 2020), thus opening the door for further effects of cannabinoids on astrocyte Ca2+ regulation in a CB1‐independent manner.
3.4. Cannabinoid modulation of gliotransmission
The cannabinoid regulation of Ca2+ signals was linked to the astroglial release of a number of gliotransmitters. These active molecules, including ATP, glutamate, D‐Serine, and GABA, released in many different brain regions, such as the hippocampus, amygdala, striatum or spinal cord (Carlsen et al., 2021; Covelo & Araque, 2018; Martín et al., 2015; Martin‐Fernandez et al., 2017; Navarrete & Araque, 2008; Robin et al., 2018) were shown to modulate a number of short‐ and long‐term effects on synaptic transmission at both inhibitory and excitatory synapses (for recent review see [Covelo et al., 2021]). While the role of glial cells on cannabinoid‐mediated synaptic effects is well described, the molecular and cellular mechanisms of gliotransmitter release from astrocytes are yet to be fully understood.
One of the general mechanisms used by eukaryotic cells to export their contents to the extracellular space is through vesicular release dependent on SNARE proteins. These proteins, also expressed in astrocytes, were suggested to be major mediators of gliotransmission (Araque et al., 2000; Navarrete et al., 2019; Schwarz et al., 2017). Likewise, it was proposed that they are involved in a mechanism by which cannabinoid‐activated astrocytic Ca2+‐signaling affects synaptic function. Min and Nevian (2012) showed that eCB mediated spike timing‐dependent depression (t‐LTD) in the rat barrel cortex relies on SNARE‐dependent exocytosis of astroglial glutamate. Another study found that CB1 receptor activation triggers the release of D‐serine and ATP from neocortical astrocytes by SNARE‐dependent mechanism (Rasooli‐Nejad et al., 2014). Interestingly, one alternative, nonvesicular mechanism was recently proposed to rely on astroglial hemichannels (Labra et al., 2018). In agreement with this hypothesis, it was previously shown that AEA may facilitate the opening of astrocytic hemichannels in the healthy brain, suggesting that the eCB might participate in the mechanisms of ATP release (Vázquez et al., 2015). More in depth investigation of different mechanisms governing gliotransmission in the future might help to disentangle the complex eCB‐mediated effects on synaptic transmission.
In the classical model of cannabinoid signaling, eCBs are released from postsynaptic neurons upon neuronal activation and travel across synapses, activating CB1 on presynaptic axons and glia to modulate neurotransmission. Thus, so far, the majority of research on the cannabinoid effects on glia–neuron communication focused on CB1‐regulated gliotransmission. However, recently accumulating evidence suggests that glial cells are not only affected by cannabinoids, but, as we mentioned before, they also possess functional machinery to produce and release them. Early evidence showing that ATP stimulation increased 2‐AG production in astrocytes in vitro (Walter et al., 2004; Witting et al., 2004), was followed by recent work that demonstrated that cultured spinal astrocytes co‐express CB1 and the 2‐AG synthesizing enzyme, DAGLα, and that upon cannabinoid stimulation astrocytes produce 2‐AG in a Ca2+‐dependent manner (Hegyi et al., 2018). In line with this, (Schüle et al., 2021) showed that a subpopulation of astrocytes in the adult mouse brain expresses DAGLα and that genetic deletion of this enzyme in glutamate/aspartate transporter‐expressing astrocytes resulted in altered affective behaviors in female mice. Furthermore, one recent study suggested that astrocyte‐derived eCBs are involved in transient heterosynaptic depression in the hippocampus (Smith et al., 2020). Taken together, these results point out to a potential role of astrocyte‐derived eCBs in the regulation of brain functions, an interesting direction for future studies.
4. ENDOCANNABINOID SYSTEM REGULATION OF ASTROCYTE METABOLISM
4.1. Astrocyte metabolism
One of the key and best studied functions of astrocytes is their contribution to brain metabolism in general and to neuronal metabolism in particular. In that sense, metabolic compartmentalization between astrocytes and neurons has risen to a complex picture in which many metabolic pathways start in one cell type and end in the other (Bélanger et al., 2011). The prototypical example of this compartmentalization is the well‐known astrocyte‐neuron lactate shuttle (ANLS). The ANLS claims that astrocytes are capable to perform aerobic glycolysis and release lactate that will be in turn used by neighboring neurons as a metabolic or signaling molecule (Barros, 2013; Bélanger et al., 2011; Pellerin & Magistretti, 1994).
The discovery of the ANLS led to the idea that astrocytes are mainly glycolytic cells, while neurons rely on their mitochondrial metabolic activity to sustain their high energy demands. However, some recent studies propose that astrocytes show a more complex and versatile metabolism than previously thought. As an example, astrocytes are able to perform fatty acid oxidation (Ebert et al., 2003; Eraso‐Pichot et al., 2018; Fecher et al., 2019) although the contribution of this pathway to the general brain metabolism is still a matter of debate (Schönfeld & Reiser, 2013, 2021).
In addition, although to a lower level than some peripheral tissues such as the liver, astrocytes present glycogen reserves, which can be rapidly metabolized to obtain ATP and lactate (Bak et al., 2018). Finally, other metabolic pathways have been proposed to mediate this astrocyte‐neuron metabolic coupling and add layers to astrocyte metabolic complexity, such as the oxidation of glutamate and the glutamate‐glutamine cycle (Schousboe et al., 2014).
Interestingly, the metabolic complexity of astrocytes depends on environmental conditions. The best‐known example of astrocytic adaptation to neuronal demands is the aforementioned ANLS, where neuronal activity through glutamate release is able to inhibit astrocytic mitochondrial activity and increase aerobic glycolysis and lactate release (Pellerin et al., 2007). However, many other metabolic pathways in astrocytes have been demonstrated to be plastic upon neuronal activity such as the glycogenolysis (Magistretti et al., 1981), the oxidation of fatty acids (Eraso‐Pichot et al., 2018) or the glutamate‐glutamine cycle (Schousboe et al., 2014).
Cannabinoids are amongst the signaling molecules that may serve as environmental cues to induce changes in astrocytic metabolism, and could help explain how astroglial metabolic adaptations are necessary for brain physiology. Interestingly, exogenous and endogenous cannabinoids exert an effect on brain glucose levels that point to them as regulators of astrocytic metabolism (Brett et al., 2001; Margulies & Hammer, 1991; Nogueiras et al., 2009; Volkow et al., 1991).
4.2. Endocannabinoid impact on astrocyte metabolism
Evidence for the different roles of cannabinoids in astrocytic metabolism came mostly from the use of exogenous cannabinoids like THC to change astrocytic metabolic pathways. For instance, one of the earliest hints of the direct impact of cannabinoids in astrocyte glucose metabolism was provided by the observation that THC stimulates glucose utilization possibly through CB1 receptors or other alternative noncanonical pathways in cultured astrocytes (Sánchez et al., 1998). On the other hand, pharmacological activation of cannabinoid receptors induced suppression of glucose metabolism in the astrocytic tricarboxylic acid (TCA) cycle in hippocampal slices, blocked by a specific CB1 antagonist (AM251) (Duarte et al., 2012).
Recent studies revealed the link between astrocytic mtCB1 receptors, astrocyte glucose metabolism and THC‐induced defects in social behavior. In that study, 24 h THC treatment induced a decrease in complex I stability and function, eventually leading to a decrease of lactate production that resulted in impaired social behaviors. These metabolic changes were mediated by mtCB1 and were due to a decrease of phosphorylation of the Ser 173 residue of NADH: ubiquinone oxidoreductase subunit S4 (NDUFS4) by intra‐mitochondrial protein kinase A (PKA)/cAMP signaling (Jimenez‐Blasco et al., 2020). The changes in mitochondrial complex I affected not only mitochondrial respiration, but also reduced astrocytic mitochondrial reactive oxygen species (mROS). Astrocytes produce a higher amount of mROS than neurons, which plays a key role on the physiological regulation of glucose utilization and neuronal survival (Vicente‐Gutierrez et al., 2019). The transcription factor hypoxia‐inducible factor 1 (HIF‐1) senses mROS and regulates glycolysis, impacting the conversion of glucose to lactate (Kim et al., 2006). THC treatment decreased the expression of the alpha subunit of HIF‐1, which resulted in a decrease of lactate production, both in cultured astrocytes and in vivo, in an mtCB1 dependent manner, affecting social behaviors in mice (Jimenez‐Blasco et al., 2020). In summary, this study showed that exogenous cannabinoid‐induced bioenergetic stress in the astrocytes directly impacts neuronal functions in vivo.
In relation to eCBs, one study focusing on brain ischemia suggested a possible relation between 2‐AG and astrocytic glucose metabolism regulation: the authors showed that after short glucose and oxygen deprivation, treatment of primary astrocytes with 2‐AG increased their survival via STAT3 (Wang et al., 2016). STAT3 is a transcription factor that regulates aerobic glycolysis and ROS production in astrocytes (Sarafian et al., 2010) and while the mechanisms of the action of 2‐AG remain unexplored, this evidence could suggest a link between this endogenous messenger and astrocytic metabolism.
CB2 receptors have also been studied in the context of astrocytic metabolism, although scarcely. In vitro, activation of CB2 in cultured cortical astrocytes with the selective agonists GP1a and JWH133 led to an increase of glucose transport, which was blocked by AM630, a selective CB2 antagonist. Moreover, the inhibition of cyclooxygenase 2, an enzyme that, amongst other functions, have been shown to participate in the degradation of AEA, induced an increase in glucose uptake in ex vivo hippocampal slices, which was blocked by CB2 activation (Köfalvi et al., 2016). However, it is possible that this effect is not due to the direct stimulation of glucose metabolism by CB2 but more because of a change on transporter localization dynamics in the plasma membrane, since the CB1/CB2 agonist WIN55,212‐2 decreases mitochondrial oxidative glucose metabolism through CB1 receptors in astrocytes (Duarte et al., 2012).
As mentioned above, glutamate metabolism is another of the astrocytic functions that contribute to brain energy homeostasis and neuronal functions. Although glutamate is mostly metabolized into lactate in the TCA cycle, about 30% of the glutamate is transformed to glutamine to then be transported to neurons (Schousboe et al., 1993). When THC is injected in the brain of rats, the expression of the enzyme glutamine synthetase (GS) – that metabolizes synaptic glutamate into glutamine– is reduced (Suárez et al., 2002). Interestingly, endogenous 2‐AG protects against GS changes induced by lipopolysaccharide (LPS) activation of cultured primary astrocytes: during the early phase of the treatment, GS shows an upregulation linked to p38 phosphorylation that can be blocked by 2‐AG, an effect that is not present after treatment with AM630, a CB2 receptor antagonist. Moreover, during the late phase of activation via LPS, extracellular signal‐regulated kinases 1/2 (ERK1/2) phosphorylation mediates a downregulation of GS, which is reversed by 2‐AG. This effect is partially blocked by both AM281 and AM630 suggesting the role of both CB1 and CB2 receptors in this process (Wang et al., 2018). Although underexplored, this evidence suggests a role for eCB modulation of glutamate/glutamine metabolism in astrocytes.
Regarding cannabinoid‐modulation of other metabolic pathways in astrocytes, THC has been seen to activate carnitine palmitoyltransferase 1 (CPT1) and ketogenesis in cultured astrocytes in a CB1‐dependent manner (Blazquez et al., 1999). This effect, together with other observations in other organs showing a CB1‐depedent modulation of fatty acid oxidation (Jourdan et al., 2012), suggests a role for cannabinoids in the modulation of astrocytic fatty acid oxidation, which may be complementary to their effect in glucose usage. Finally, a work from our lab established an indirect regulation of glycogen content mediated by CB1 in astrocytes (Bosier et al., 2013), again opening new ways of cannabinoid regulation of astrocytic metabolism.
In summary, the impact of eCBs on astrocyte metabolism remains highly unexplored, but evidence of exogenous cannabinoids and receptor pharmacology points to a complex regulation of multiple pathways in these glial cells, which might link regulation of metabolism to brain functions and behavior.
5. ROLE OF THE ENDOCANNABINOID SYSTEM IN ASTROGLIAL INFLAMMATORY PROCESSES
5.1. Astrocyte implication in inflammation
Astrocytes are known to play key roles in the CNS inflammation in response to innate and adaptive immune responses. First, astrocytes are major players in the maintenance and permeability of the blood–brain barrier, thus controlling immune infiltration in the brain. Second, astrocytes are immune‐competent cells, meaning that they are able to respond to danger signals through the release of cytokines and chemokines, activating adaptive immune defense (Colombo & Farina, 2016).
Like many elements of these pathways, the role of astrocytes in the development and maintenance of inflammation in the CNS is multiple. For instance, depending on the nature of the stimulus as well as the phase of the inflammation process, astrocytic immune activation may be detrimental or protective (Sofroniew, 2020).
One of the most‐studied and best‐known indicators of astrocytic responses to danger, linked to the astrocyte contribution to CNS inflammation, is astrogliosis. Astrogliosis has been broadly studied for over a century as a hallmark of brain disease conditions, and it was considered to be a morphological change occurring in astrocytes through the increased expression of GFAP, a major protein constituent of astrocytic intermediate filaments. However, nowadays, and thanks to the contributions of years of research in different labs, its definition has changed to a complex process whereby astrocytes, in response to pathology, “engage in molecularly defined programs involving changes in transcriptional regulation, as well as biochemical, morphological, metabolic, and physiological remodeling, which ultimately result in gain of new function(s) or loss or upregulation of homeostatic ones” (Escartin et al., 2021). Interestingly, this process depends on the injury as well as the area affected and it can be protective or detrimental depending on the population, the nature of the danger or the state of the inflammation, so it seems meaningless to consider astrogliosis as a whole as a friend or a foe of the inflammatory processes (Escartin et al., 2021).
Interestingly, both endogenous and exogenous cannabinoids have been shown to be key players in the inflammatory responses of the brain, even being considered as potential targets in the treatment of some diseases that involve inflammatory processes (Kasatkina et al., 2021). The cellular mediators of these effects, however, are not clear yet. Given their role in the control of inflammatory responses, astrocytes are potential players in the modulation of inflammation by cannabinoids; however, the direct link is still missing. Here we will present some findings that point to astrocytes as possible mediators of the cannabinoid‐mediated effects on CNS inflammation.
5.2. Cannabinoid impact on astrocyte‐mediated inflammation
Evidence on the role of cannabinoids in astrocyte‐mediated inflammation came from studies in astrocytic cultures. In these early works, AEA was shown to modulate astrocytic immune responses to different stimuli, such as infection with Theiler's murine encephalomyelitis virus (TMEV) or treatment with LPS. In these works, AEA treatment decreases nitric oxide and tumor necrosis factor α (TNFα) release in response to these insults (Molina‐Holgado et al., 1997), as well as to increase the release of interleukin‐6, considered to be anti‐inflammatory and immunosuppressive, in astrocytic cultures infected with TMEV (Molina‐Holgado et al., 1998). Moreover, AEA and the synthetic cannabinoid analog CP‐55940 reduced nitric oxide production induced by LPS stimulation in a CB1 and CB2 dependent mechanism (Molina‐Holgado et al., 2002). In the same line, application of the synthetic cannabinoid WIN55,212‐2 was shown to inhibit the generation of inflammatory mediators in interleukin‐1β‐stimulated human astrocytes (Sheng et al., 2005). Finally, UCM707, a potent and selective AEA uptake inhibitor, was able to reduce the production of proinflammatory molecules in LPS‐treated astrocytes also in a CB1 and CB2 dependent manner, thus emphasizing a role of astroglial eCBs in this process (Ortega‐Gutiérrez et al., 2005). These early works suggested a role of cannabinoids in the modulation of astrocytic inflammatory responses via different signaling mechanisms.
Interestingly, contrary to the other astrocytic functions explained in this review, the regulation of the astrocytic inflammatory processes by cannabinoids has been described to be modulated through different mechanisms. In all these early works, many of the cannabinoid effects were found to be modulated, but not completely blocked by CB1 and CB2 antagonism, suggesting an involvement of other ECS members. As an example, recent evidence point to a protective role of palmitoylethanolamide (PEA), which can be considered as an “extended endocannabinoid” (Cristino et al., 2020), in different models of Alzheimer's disease (Beggiato et al., 2018; Bronzuoli et al., 2018; Scuderi et al., 2011). PEA has been demonstrated to exert some of its effects in astrocytes, reducing the release of inflammatory mediators and thus promoting neuronal survival (Beggiato et al., 2020). Interestingly, PEA seems to act through PPARα receptors, which are expressed in astrocytes but are not the classic cannabinoid receptors (Scuderi et al., 2012).
Another mechanism by which astrocytes contribute to inflammatory processes seems to be the degradation of the eCB signals. In fact, it has been demonstrated that astrocytes play key roles in terminating the cannabinoid signals from neurons via degradation enzymes such as MAGL (Viader et al., 2015). In fact, astrocytic‐specific deletion of MAGL attenuates LPS‐induced neuroinflammation. Given that MAGL is the enzyme responsible of degradation of 2‐AG to AA, a precursor of prostaglandin synthesis, the authors suggest that the reduction in the inflammation is due to the decrease in the synthesis of prostaglandins in the brain, rather than by an increased eCB tone (Grabner et al., 2016). Anyhow, the link between the eCBs and inflammatory molecules and their shared pathways in astrocytes needs to be addressed in more detail and seems also a promising candidate for the cannabinoid regulation of astrocytic‐mediated inflammatory processes.
Nowadays, different cannabis‐derived cannabinoids have gained interest due to their anti‐inflammatory effects in different pathological models (Graczyk et al., 2021). Given the relevant role of astrocytes in brain inflammation, it seems logic that some of these effects of cannabinoids are mediated by astrocytes. As reviewed here, some evidences have found this link between cannabinoids, astrocytes, and inflammation. Interestingly, many of these effects seem to be mediated by other receptors than CB1 such as CB2 or nonclassical cannabinoid receptors, opening new possibilities by which cannabinoids may regulate the other astrocytic functions. However, the exact mechanism of the different cannabinoids in regulating CNS inflammation through astrocytes ‐especially in vivo‐ are still lacking and represent a great target for future studies and application of cannabinoids as brain anti‐inflammatory agents.
6. CONCLUDING REMARKS
Astrocytes emerged as active players in multiple CNS functions, from memory encoding to the immune defense of the brain. Interestingly, some of these functions have been discovered only recently, suggesting that we may have uncovered only the tip of the iceberg of astrocytic implication in brain processes. At the same time, although the study of cannabinoids has traveled a longer way, astrocytic implication in some of the effects of cannabinoids is just starting to emerge. As seen in this review, most of the studies so far regarding the cannabinoid modulation of astroglial functions are focused on the CB1 receptor. However, astrocyte express other ECS members which are gaining more interest since they may be targets of the newly‐discovered promising therapeutic components of the cannabis sativa plant, such as cannabidiol or cannabigerol. Thus, the field should address the potential modulation of astroglial functions by these new components through other ECS members, to have a complete picture of the role of cannabinoids in astrocytes in physiology and pathology. Also, cannabinoids might be implicated in other astrocytic functions with therapeutic interest not addressed in this review, such as in neurotransmitter or ionic uptake (Egaña‐Huguet et al., 2021). To summarize, the field is just starting to address the role of astrocytes in some cannabinoid‐mediated effects in the CNS, as seen in some recent discoveries discussed in this review. These studies, together with the possible implications of other ECS members in astrocyte physiology, suggest new research paths to understand how (endo)cannabinoid effects and functions can be exerted through astrocytes and, conversely, how astrocytes can contribute to higher‐brain functions via cannabinoid signaling.”
AUTHOR CONTRIBUTIONS
All authors conceived, wrote and edited this review.
CONFLICT OF INTEREST
The authors declare no conflicts of interest.
ACKNOWLEDGMENTS
We thank all the members of Marsicano's lab for useful discussions and for their invaluable support. This work was funded by: INSERM, European Research Council (Endofood, ERC‐2010‐StG‐260515 and CannaPreg, ERC‐2014‐PoC‐640923, MiCaBra, ERC‐2017‐AdG‐786467), Fondation pour la Recherche Medicale (FRM, DRM20101220445, SPF201809006908, SPF201909009268), Université de Bordeaux (IdEx Bordeaux), Region Nouvelle Aquitaine, Agence Nationale de la Recherche (ANR, NeuroNutriSens ANR‐13‐BSV4‐0006; ORUPS ANR‐16‐CE37‐0010‐01; CaCoVi ANR 18‐CE16‐0001‐02; MitObesity ANR 18‐CE14‐0029‐01; BRAIN ANR‐10‐LABX‐0043). Graphical abstract created with biorender.com.
Eraso‐Pichot, A. , Pouvreau, S. , Olivera‐Pinto, A. , Gomez‐Sotres, P. , Skupio, U. , & Marsicano, G. (2023). Endocannabinoid signaling in astrocytes. Glia, 71(1), 44–59. 10.1002/glia.24246
Funding information Agence Nationale de la Recherche, Grant/Award Numbers: ANR‐10‐LABX‐0043, ANR 18‐CE14‐0029‐01, ANR 18‐CE16‐0001‐02, ANR‐16‐CE37‐0010‐01, ANR‐13‐BSV4‐0006; Université de Bordeaux (IdEx Bordeaux); Fondation pour la Recherche Medicale, Grant/Award Numbers: SPF201909009268, SPF201809006908, DRM20101220445; European Research Council, Grant/Award Numbers: ERC‐2017‐AdG‐786467, ERC‐2014‐PoC‐640923, ERC‐2010‐StG‐260515; INSERM
DATA AVAILABILITY STATEMENT
Data sharing is not applicable to this article as no new data were created or analyzed in this study.
REFERENCES
- Adamsky, A. , Kol, A. , Kreisel, T. , Doron, A. , Ozeri‐Engelhard, N. , Melcer, T. , Refaeli, R. , Horn, H. , Regev, L. , Groysman, M. , London, M. , & Goshen, I. (2018). Astrocytic activation generates De novo neuronal potentiation and memory enhancement. Cell, 174, 59–71.e14. [DOI] [PubMed] [Google Scholar]
- Agarwal, A. , Wu, P.‐H. , Hughes, E. G. , Fukaya, M. , Tischfield, M. A. , Langseth, A. J. , Wirtz, D. , & Bergles, D. E. (2017). Transient opening of the mitochondrial permeability transition pore induces microdomain calcium transients in astrocyte processes. Neuron, 93, 587–605.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen, N. J. , & Eroglu, C. (2017). Cell biology of astrocyte‐synapse interactions. Neuron, 96, 697–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Araque, A. , Carmignoto, G. , & Haydon, P. G. (2001). Dynamic signaling between astrocytes and neurons. Annual Review of Physiology, 63, 795–813. [DOI] [PubMed] [Google Scholar]
- Araque, A. , Carmignoto, G. , Haydon, P. G. , Oliet, S. H. R. , Robitaille, R. , & Volterra, A. (2014). Gliotransmitters travel in time and space. Neuron, 81, 728–739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Araque, A. , Li, N. , Doyle, R. T. , & Haydon, P. G. (2000). SNARE protein‐dependent glutamate release from astrocytes. Journal of Neuroscience: The Official Journal of the Society for Neuroscience, 20, 666–673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Araque, A. , Parpura, V. , Sanzgiri, R. P. , & Haydon, P. G. (1999). Tripartite synapses: Glia, the unacknowledged partner. Trends in Neurosciences, 22, 208–215. [DOI] [PubMed] [Google Scholar]
- Atwood, B. K. , & Mackie, K. (2010). CB2: A cannabinoid receptor with an identity crisis. British Journal of Pharmacology, 160, 467–479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bak, L. K. , Walls, A. B. , Schousboe, A. , & Waagepetersen, H. S. (2018). Astrocytic glycogen metabolism in the healthy and diseased brain. The Journal of Biological Chemistry, 293, 7108–7116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barros, L. F. (2013). Metabolic signaling by lactate in the brain. Trends in Neurosciences, 36, 396–404. [DOI] [PubMed] [Google Scholar]
- Bauer, M. , Chicca, A. , Tamborrini, M. , Eisen, D. , Lerner, R. , Lutz, B. , Poetz, O. , Pluschke, G. , & Gertsch, J. (2012). Identification and quantification of a new family of peptide endocannabinoids (Pepcans) showing negative allosteric modulation at CB1 receptors. The Journal of Biological Chemistry, 287, 36944–36967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beattie, E. C. , Stellwagen, D. , Morishita, W. , Bresnahan, J. C. , Ha, B. K. , Zastrow, M. V. , Beattie, M. S. , & Malenka, R. C. (2002). Control of synaptic strength by glial TNFα. Science, 295, 2282–2285. [DOI] [PubMed] [Google Scholar]
- Beggiato, S. , Borelli, A. C. , Ferraro, L. , Tanganelli, S. , Antonelli, T. , & Tomasini, M. C. (2018). Palmitoylethanolamide blunts amyloid‐β42‐induced astrocyte activation and improves neuronal survival in primary mouse cortical astrocyte‐neuron co‐cultures. Journal of Alzheimer's Disease, 61, 389–399. [DOI] [PubMed] [Google Scholar]
- Beggiato, S. , Cassano, T. , Ferraro, L. , & Tomasini, M. C. (2020). Astrocytic palmitoylethanolamide pre‐exposure exerts neuroprotective effects in astrocyte‐neuron co‐cultures from a triple transgenic mouse model of Alzheimer's disease. Life Sciences, 257, 118037. [DOI] [PubMed] [Google Scholar]
- Bélanger, M. , Allaman, I. , & Magistretti, P. J. (2011). Brain energy metabolism: Focus on astrocyte‐neuron metabolic cooperation. Cell Metabolism, 14, 724–738. [DOI] [PubMed] [Google Scholar]
- Benfenati, V. , Caprini, M. , Dovizio, M. , Mylonakou, M. N. , Ferroni, S. , Ottersen, O. P. , & Amiry‐Moghaddam, M. (2011). An aquaporin‐4/transient receptor potential vanilloid 4 (AQP4/TRPV4) complex is essential for cell‐volume control in astrocytes. Proceedings of the National Academy of Sciences, 108, 2563–2568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benito, C. , Romero, J. P. , Tolón, R. M. , Clemente, D. , Docagne, F. , Hillard, C. J. , Guaza, C. , & Romero, J. (2007). Cannabinoid CB1 and CB2 receptors and fatty acid amide hydrolase are specific markers of plaque cell subtypes in human multiple sclerosis. The Journal of Neuroscience, 27, 2396–2402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benito, C. , Tolón, R. M. , Pazos, M. R. , Núñez, E. , Castillo, A. I. , & Romero, J. (2008). Cannabinoid CB2 receptors in human brain inflammation. British Journal of Pharmacology, 153, 277–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benyó, Z. , Ruisanchez, É. , Leszl‐Ishiguro, M. , Sándor, P. , & Pacher, P. (2016). Endocannabinoids in cerebrovascular regulation. American Journal of Physiology‐Heart and Circulatory Physiology, 310, H785–H801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bezprozvanny, I. , Watras, J. , & Ehrlich, B. E. (1991). Bell‐shaped calcium‐response curves of ins(1,4,5)P3‐ and calcium‐gated channels from endoplasmic reticulum of cerebellum. Nature, 351, 751–754. [DOI] [PubMed] [Google Scholar]
- Blazquez, C. , Sanchez, C. , Daza, A. , Galve‐Roperh, I. , & Guzman, M. (1999). The stimulation of ketogenesis by cannabinoids in cultured astrocytes defines carnitine palmitoyltransferase I as a new ceramide‐activated enzyme. Journal of Neurochemistry, 72, 1759–1768. [DOI] [PubMed] [Google Scholar]
- Boczek, T. , & Zylinska, L. (2021). Receptor‐dependent and independent regulation of voltage‐gated Ca2+ channels and Ca2+‐permeable channels by endocannabinoids in the brain. International Journal of Molecular Sciences, 22, 8168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boitier, E. , Rea, R. , & Duchen, M. R. (1999). Mitochondria exert a negative feedback on the propagation of intracellular Ca2+ waves in rat cortical astrocytes. The Journal of Cell Biology, 145, 795–808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosier, B. , Bellocchio, L. , Metna‐Laurent, M. , Soria‐Gomez, E. , Matias, I. , Hebert‐Chatelain, E. , Cannich, A. , Maitre, M. , Leste‐Lasserre, T. , Cardinal, P. , Mendizabal‐Zubiaga, J. , Canduela, M. J. , Reguero, L. , Hermans, E. , Grandes, P. , Cota, D. , & Marsicano, G. (2013). Astroglial CB1 cannabinoid receptors regulate leptin signaling in mouse brain astrocytes. Molecular metabolism, 2, 393–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowser, D. N. , & Khakh, B. S. (2007). Vesicular ATP is the predominant cause of intercellular calcium waves in astrocytes. The Journal of General Physiology, 129, 485–491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyman, L. , & Lederer, W. J. (2020). How the mitochondrial calcium uniporter complex (MCUcx) works. Proceedings of the National Academy of Sciences, 117, 22634–22636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley, S. J. , & Challiss, R. A. J. (2012). G protein‐coupled receptor signalling in astrocytes in health and disease: A focus on metabotropic glutamate receptors. Biochemical Pharmacology, 84, 249–259. [DOI] [PubMed] [Google Scholar]
- Brett, R. , MacKenzie, F. , & Pratt, J. (2001). Delta 9‐tetrahydrocannabinol‐induced alterations in limbic system glucose use in the rat. Neuroreport, 12, 3573–3577. [DOI] [PubMed] [Google Scholar]
- Bronzuoli, M. R. , Facchinetti, R. , Steardo, L. , Romano, A. , Stecca, C. , Passarella, S. , Steardo, L. , Cassano, T. , & Scuderi, C. (2018). Palmitoylethanolamide dampens reactive Astrogliosis and improves neuronal trophic support in a triple transgenic model of Alzheimer's disease: In vitro and in vivo evidence. Oxidative Medicine and Cellular Longevity, 2018, 4720532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bugge, A. , & Mandrup, S. (2010). Molecular mechanisms and genome‐wide aspects of PPAR subtype specific transactivation. PPAR Research, 2010, 169506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bushong, E. A. , Martone, M. E. , Jones, Y. Z. , & Ellisman, M. H. (2002). Protoplasmic astrocytes in CA1 stratum Radiatum occupy separate anatomical domains. The Journal of Neuroscience, 22, 183–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busquets‐Garcia, A. , Bains, J. , & Marsicano, G. (2018). CB1 receptor signaling in the brain: Extracting specificity from ubiquity. Neuropsychopharmacology, The Official Publication of the American College of Neuropsychopharmacology, 43, 4–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlsen, E. M. M. , Falk, S. , Skupio, U. , Robin, L. , Pagano Zottola, A. C. , Marsicano, G. , & Perrier, J.‐F. (2021). Spinal astroglial cannabinoid receptors control pathological tremor. Nature Neuroscience, 24, 658–666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmignoto, G. , Pasti, L. , & Pozzan, T. (1998). On the role of voltage‐dependent calcium channels in calcium signaling of astrocytes in situ. The Journal of Neuroscience, 18, 4637–4645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassano, T. , Calcagnini, S. , Pace, L. , De Marco, F. , Romano, A. , & Gaetani, S. (2017). Cannabinoid receptor 2 signaling in neurodegenerative disorders: From pathogenesis to a promising therapeutic target. Frontiers in Neuroscience, 11, 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castillo, P. E. , Younts, T. J. , Chávez, A. E. , & Hashimotodani, Y. (2012). Endocannabinoid signaling and synaptic function. Neuron, 76, 70–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Liu X, Vickstrom CR, Liu MJ, Zhao L, Viader A, Cravatt BF, Liu Q. 2016. Neuronal and astrocytic Monoacylglycerol lipase limit the spread of endocannabinoid signaling in the cerebellum. eNeuro 3:ENEURO.0048–16.2016. [DOI] [PMC free article] [PubMed]
- Colombo, E. , & Farina, C. (2016). Astrocytes: Key regulators of Neuroinflammation. Trends in Immunology, 37, 608–620. [DOI] [PubMed] [Google Scholar]
- Covelo, A. , & Araque, A. (2018). Neuronal activity determines distinct gliotransmitter release from a single astrocyte. eLife, 7, e32237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Covelo, A. , Eraso‐Pichot, A. , Fernández‐Moncada, I. , Serrat, R. , & Marsicano, G. (2021). CB1R‐dependent regulation of astrocyte physiology and astrocyte‐neuron interactions. Neuropharmacology, 195, 108678. [DOI] [PubMed] [Google Scholar]
- Cristino, L. , Bisogno, T. , & Di Marzo, V. (2020). Cannabinoids and the expanded endocannabinoid system in neurological disorders. Nature Reviews. Neurology, 16, 9–29. [DOI] [PubMed] [Google Scholar]
- Csordás, G. , Weaver, D. , & Hajnóczky, G. (2018). Endoplasmic reticulum‐mitochondrial Contactology: Structure and signaling functions. Trends in Cell Biology, 28, 523–540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Marzo, V. (1998). “Endocannabinoids” and other fatty acid derivatives with cannabimimetic properties: Biochemistry and possible physiopathological relevance. Biochimica et Biophysica Acta (BBA)‐Lipids and Lipid Metabolism, 1392, 153–175. [DOI] [PubMed] [Google Scholar]
- Di Marzo, V. (2018). New approaches and challenges to targeting the endocannabinoid system. Nature Reviews. Drug Discovery, 17, 623–639. [DOI] [PubMed] [Google Scholar]
- Ding, F. , O'Donnell, J. , Thrane, A. S. , Zeppenfeld, D. , Kang, H. , Xie, L. , Wang, F. , & Nedergaard, M. (2013). α1‐adrenergic receptors mediate coordinated Ca2+ signaling of cortical astrocytes in awake, behaving mice. Cell Calcium, 54, 387–394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dowie, M. J. , Grimsey, N. L. , Hoffman, T. , Faull, R. L. M. , & Glass, M. (2014). Cannabinoid receptor CB2 is expressed on vascular cells, but not astroglial cells in the post‐mortem human Huntington's disease brain. Journal of Chemical Neuroanatomy, 59–60, 62–71. [DOI] [PubMed] [Google Scholar]
- Duarte, J. M. N. , Ferreira, S. G. , Carvalho, R. A. , Cunha, R. A. , & Köfalvi, A. (2012). CB₁ receptor activation inhibits neuronal and astrocytic intermediary metabolism in the rat hippocampus. Neurochemistry International, 60, 1–8. [DOI] [PubMed] [Google Scholar]
- Dupont, G. , & Goldbeter, A. (1993). One‐pool model for Ca2+ oscillations involving Ca2+ and inositol 1,4,5‐trisphosphate as co‐agonists for Ca2+ release. Cell Calcium, 14, 311–322. [DOI] [PubMed] [Google Scholar]
- Durkee, C. A. , & Araque, A. (2019). Diversity and specificity of astrocyte‐neuron communication. Neuroscience, 396, 73–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebert, D. , Haller, R. G. , & Walton, M. E. (2003). Energy contribution of octanoate to intact rat brain metabolism measured by 13C nuclear magnetic resonance spectroscopy. Journal of Neuroscience: The Official Journal of the Society for Neuroscience, 23, 5928–5935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egaña‐Huguet, J. , Soria‐Gómez, E. , & Grandes, P. (2021). The endocannabinoid system in glial cells and their profitable interactions to treat epilepsy: Evidence from animal models. International Journal of Molecular Sciences, 22, 13231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eraso‐Pichot, A. , Brasó‐Vives, M. , Golbano, A. , Menacho, C. , Claro, E. , Galea, E. , & Masgrau, R. (2018). GSEA of mouse and human mitochondriomes reveals fatty acid oxidation in astrocytes. Glia, 66, 1724–1735. [DOI] [PubMed] [Google Scholar]
- Eraso‐Pichot, A. , Larramona‐Arcas, R. , Vicario‐Orri, E. , Villalonga, R. , Pardo, L. , Galea, E. , & Masgrau, R. (2017). CREB decreases astrocytic excitability by modifying subcellular calcium fluxes via the sigma‐1 receptor. Cellular and Molecular Life Sciences, 74, 937–950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Escartin, C. , Galea, E. , Lakatos, A. , O'Callaghan, J. P. , Petzold, G. C. , Serrano‐Pozo, A. , Steinhäuser, C. , Volterra, A. , Carmignoto, G. , Agarwal, A. , Allen, N. J. , Araque, A. , Barbeito, L. , Barzilai, A. , Bergles, D. E. , Bonvento, G. , Butt, A. M. , Chen, W.‐T. , Cohen‐Salmon, M. , … Verkhratsky, A. (2021). Reactive astrocyte nomenclature, definitions, and future directions. Nature Neuroscience, 24, 312–325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fecher, C. , Trovò, L. , Müller, S. A. , Snaidero, N. , Wettmarshausen, J. , Heink, S. , Ortiz, O. , Wagner, I. , Kühn, R. , Hartmann, J. , Karl, R. M. , Konnerth, A. , Korn, T. , Wurst, W. , Merkler, D. , Lichtenthaler, S. F. , Perocchi, F. , & Misgeld, T. (2019). Cell‐type‐specific profiling of brain mitochondria reveals functional and molecular diversity. Nature Neuroscience, 22, 1731–1742. [DOI] [PubMed] [Google Scholar]
- Fedinec, A. L. , Liu, J. , Zhang, R. , Harsono, M. , Pourcyrous, M. , & Parfenova, H. (2021). The cold receptor TRPM8 activation leads to attenuation of endothelium‐dependent cerebral vascular functions during head cooling. Journal of Cerebral Blood Flow & Metabolism. official Journal of International Society for Cerebral Blood Flow & Metabolism, 41, 2897–2906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernández‐Trapero, M. , Espejo‐Porras, F. , Rodríguez‐Cueto, C. , Coates, J. R. , Pérez‐Díaz, C. , de Lago, E. , & Fernández‐Ruiz, J. (2017). Upregulation of CB2 receptors in reactive astrocytes in canine degenerative myelopathy, a disease model of amyotrophic lateral sclerosis. Disease Models & Mechanisms, 10, 551–558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foskett, J. K. , & Mak, D.‐O. D. (2010). Regulation of IP3R channel gating by Ca2+ and Ca2+ binding proteins. Current Topics in Membranes, 66, 235–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fride, E. (2005). Endocannabinoids in the central nervous system: From neuronal networks to behavior. Current Drug Targets. CNS and Neurological Disorders, 4, 633–642. [DOI] [PubMed] [Google Scholar]
- Fumagalli, M. , Brambilla, R. , D'Ambrosi, N. , Volonté, C. , Matteoli, M. , Verderio, C. , & Abbracchio, M. P. (2003). Nucleotide‐mediated calcium signaling in rat cortical astrocytes: Role of P2X and P2Y receptors. Glia, 43, 218–230. [DOI] [PubMed] [Google Scholar]
- García‐Gutiérrez, M. S. , Navarrete, F. , Navarro, G. , Reyes‐Resina, I. , Franco, R. , Lanciego, J. L. , Giner, S. , & Manzanares, J. (2018). Alterations in gene and Protein expression of cannabinoid CB2 and GPR55 receptors in the dorsolateral prefrontal cortex of suicide victims. Neurotherapeutics, Journal of the American Society for Experimental Neurotherapeutics, 15, 796–806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gӧbel, J. , Engelhardt, E. , Pelzer, P. , Sakthivelu, V. , Jahn, H. M. , Jevtic, M. , Folz‐Donahue, K. , Kukat, C. , Schauss, A. , Frese, C. K. , Giavalisco, P. , Ghanem, A. , Conzelmann, K.‐K. , Motori, E. , & Bergami, M. (2020). Mitochondria‐endoplasmic reticulum contacts in reactive astrocytes promote vascular remodeling. Cell Metabolism, 31, 791–808.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gómez‐Gonzalo, M. , Navarrete, M. , Perea, G. , Covelo, A. , Martín‐Fernández, M. , Shigemoto, R. , Luján, R. , & Araque, A. (2015). Endocannabinoids induce lateral Long‐term potentiation of transmitter release by stimulation of Gliotransmission. Cerebral Cortex, 1991(25), 3699–3712. [DOI] [PubMed] [Google Scholar]
- Grabiec, U. , Hohmann, T. , Ghadban, C. , Rothgänger, C. , Wong, D. , Antonietti, A. , Groth, T. , Mackie, K. , & Dehghani, F. (2019). Protective effect of N‐Arachidonoyl glycine‐GPR18 signaling after Excitotoxical lesion in murine Organotypic hippocampal slice cultures. International Journal of Molecular Sciences, 20, 1266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grabner, G. F. , Eichmann, T. O. , Wagner, B. , Gao, Y. , Farzi, A. , Taschler, U. , Radner, F. P. W. , Schweiger, M. , Lass, A. , Holzer, P. , Zinser, E. , Tschöp, M. H. , Yi, C.‐X. , & Zimmermann, R. (2016). Deletion of Monoglyceride lipase in astrocytes attenuates lipopolysaccharide‐induced Neuroinflammation *. The Journal of Biological Chemistry, 291, 913–923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graczyk, M. , Lewandowska, A. A. , & Dzierżanowski, T. (2021). The therapeutic potential of cannabis in counteracting oxidative stress and inflammation. Molecules, 26, 4551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutiérrez‐Rodríguez, A. , Bonilla‐Del Río, I. , Puente, N. , Gómez‐Urquijo, S. M. , Fontaine, C. J. , Egaña‐Huguet, J. , Elezgarai, I. , Ruehle, S. , Lutz, B. , Robin, L. M. , Soria‐Gómez, E. , Bellocchio, L. , Padwal, J. D. , van der Stelt, M. , Mendizabal‐Zubiaga, J. , Reguero, L. , Ramos, A. , Gerrikagoitia, I. , Marsicano, G. , & Grandes, P. (2018). Localization of the cannabinoid type‐1 receptor in subcellular astrocyte compartments of mutant mouse hippocampus. Glia, 66, 1417–1431. [DOI] [PubMed] [Google Scholar]
- Han, J. , Kesner, P. , Metna‐Laurent, M. , Duan, T. , Xu, L. , Georges, F. , Koehl, M. , Abrous, D. N. , Mendizabal‐Zubiaga, J. , Grandes, P. , Liu, Q. , Bai, G. , Wang, W. , Xiong, L. , Ren, W. , Marsicano, G. , & Zhang, X. (2012). Acute cannabinoids impair working memory through Astroglial CB1 receptor modulation of hippocampal LTD. Cell, 148, 1039–1050. [DOI] [PubMed] [Google Scholar]
- Haydon, P. G. , & Carmignoto, G. (2006). Astrocyte control of synaptic transmission and neurovascular coupling. Physiological Reviews, 86, 1009–1031. [DOI] [PubMed] [Google Scholar]
- Hebert‐Chatelain, E. , Desprez, T. , Serrat, R. , Bellocchio, L. , Soria‐Gomez, E. , Busquets‐Garcia, A. , Pagano Zottola, A. C. , Delamarre, A. , Cannich, A. , Vincent, P. , Varilh, M. , Robin, L. M. , Terral, G. , García‐Fernández, M. D. , Colavita, M. , Mazier, W. , Drago, F. , Puente, N. , Reguero, L. , … Marsicano, G. (2016). A cannabinoid link between mitochondria and memory. Nature, 539, 555–559. [DOI] [PubMed] [Google Scholar]
- Hegyi, Z. , Oláh, T. , Kőszeghy, Á. , Piscitelli, F. , Holló, K. , Pál, B. , Csernoch, L. , Di Marzo, V. , & Antal, M. (2018). CB1 receptor activation induces intracellular Ca2+ mobilization and 2‐arachidonoylglycerol release in rodent spinal cord astrocytes. Scientific Reports, 8, 10562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helen, C. , Kastritsis, C. , Salm, A. K. , & McCarthy, K. (1992). Stimulation of the P2Y purinergic receptor on type 1 Astroglia results in inositol phosphate formation and calcium mobilization. Journal of Neurochemistry, 58, 1277–1284. [DOI] [PubMed] [Google Scholar]
- Hu, S. S.‐J. , & Mackie, K. (2015). Distribution of the endocannabinoid system in the central nervous system. In Pertwee R. G. (Ed.), Endocannabinoids. Handbook of experimental pharmacology (pp. 59–93). Springer International Publishing. 10.1007/978-3-319-20825-1_3 [DOI] [PubMed] [Google Scholar]
- Jackson, J. G. , & Robinson, M. B. (2015). Reciprocal regulation of mitochondrial dynamics and calcium signaling in astrocyte processes. The Journal of Neuroscience, 35, 15199–15213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs, J. A. , & Sehgal, A. (2020). Anandamide metabolites protect against seizures through the TRP Channel water witch in Drosophila melanogaster. Cell Reports, 31, 107710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jimenez‐Blasco, D. , Busquets‐Garcia, A. , Hebert‐Chatelain, E. , Serrat, R. , Vicente‐Gutierrez, C. , Ioannidou, C. , Gómez‐Sotres, P. , Lopez‐Fabuel, I. , Resch‐Beusher, M. , Resel, E. , Arnouil, D. , Saraswat, D. , Varilh, M. , Cannich, A. , Julio‐Kalajzic, F. , Bonilla‐Del Río, I. , Almeida, A. , Puente, N. , Achicallende, S. , … Marsicano, G. (2020). Glucose metabolism links astroglial mitochondria to cannabinoid effects. Nature, 583, 603–608. [DOI] [PubMed] [Google Scholar]
- Jordan, C. J. , & Xi, Z.‐X. (2019). Progress in brain cannabinoid CB2 receptor research: From genes to behavior. Neuroscience and Biobehavioral Reviews, 98, 208–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jourdan, T. , Demizieux, L. , Gresti, J. , Djaouti, L. , Gaba, L. , Vergès, B. , & Degrace, P. (2012). Antagonism of peripheral hepatic cannabinoid receptor‐1 improves liver lipid metabolism in mice: Evidence from cultured explants. Hepatology, 55, 790–799. [DOI] [PubMed] [Google Scholar]
- Kallendrusch, S. , Hobusch, C. , Ehrlich, A. , Ziebell, S. , Ueda, N. , Geisslinger, G. , Koch, M. , & Dehghani, F. (2012). Site‐specific and time‐dependent activation of the endocannabinoid system after transection of Long‐range projections. PLoS One, 7, e33537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kano, M. , Ohno‐Shosaku, T. , Hashimotodani, Y. , Uchigashima, M. , & Watanabe, M. (2009). Endocannabinoid‐mediated control of synaptic transmission. Physiological Reviews, 89, 309–380. [DOI] [PubMed] [Google Scholar]
- Kasatkina, L. A. , Rittchen, S. , & Sturm, E. M. (2021). Neuroprotective and immunomodulatory action of the endocannabinoid system under Neuroinflammation. International Journal of Molecular Sciences, 22, 5431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kastanenka, K. V. , Moreno‐Bote, R. , De Pittà, M. , Perea, G. , Eraso‐Pichot, A. , Masgrau, R. , Poskanzer, K. E. , & Galea, E. (2020). A roadmap to integrate astrocytes into systems neuroscience. Glia, 68, 5–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katona, I. , Sperlágh, B. , Sík, A. , Käfalvi, A. , Vizi, E. S. , Mackie, K. , & Freund, T. F. (1999). Presynaptically located CB1 cannabinoid receptors regulate GABA release from axon terminals of specific hippocampal interneurons. Journal of Neuroscience: The Official Journal of the Society for Neuroscience, 19, 4544–4558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim, J. , Tchernyshyov, I. , Semenza, G. L. , & Dang, C. V. (2006). HIF‐1‐mediated expression of pyruvate dehydrogenase kinase: A metabolic switch required for cellular adaptation to hypoxia. Cell Metabolism, 3, 177–185. [DOI] [PubMed] [Google Scholar]
- Kirischuk, S. , Kirchhoff, F. , Matyash, V. , Kettenmann, H. , & Verkhratsky, A. (1999). Glutamate‐triggered calcium signalling in mouse Bergmann glial cells in situ: Role of inositol‐1,4,5‐trisphosphate‐mediated intracellular calcium release. Neuroscience, 92, 1051–1059. [DOI] [PubMed] [Google Scholar]
- Kirischuk, S. , Parpura, V. , & Verkhratsky, A. (2012). Sodium dynamics: Another key to astroglial excitability? Trends in Neurosciences, 35, 497–506. [DOI] [PubMed] [Google Scholar]
- Köfalvi, A. , Lemos, C. , Martín‐Moreno, A. M. , Pinheiro, B. S. , García‐García, L. , Pozo, M. A. , Valério‐Fernandes, Â. , Beleza, R. O. , Agostinho, P. , Rodrigues, R. J. , Pasquaré, S. J. , Cunha, R. A. , & de Ceballos, M. L. (2016). Stimulation of brain glucose uptake by cannabinoid CB2 receptors and its therapeutic potential in Alzheimer's disease. Neuropharmacology, 110, 519–529. [DOI] [PubMed] [Google Scholar]
- Kőszeghy, Á. , Kovács, A. , Bíró, T. , Szücs, P. , Vincze, J. , Hegyi, Z. , Antal, M. , & Pál, B. (2015). Endocannabinoid signaling modulates neurons of the pedunculopontine nucleus (PPN) via astrocytes. Brain Structure & Function, 220, 3023–3041. [DOI] [PubMed] [Google Scholar]
- Labra, V. C. , Santibáñez, C. A. , Gajardo‐Gómez, R. , Díaz, E. F. , Gómez, G. I. , & Orellana, J. A. (2018). The neuroglial dialog between cannabinoids and Hemichannels. Frontiers in Molecular Neuroscience, 11, 79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lange, S. C. , Bak, L. K., Waagepetersen, H. S., Schousboe, A., & Norenberg, M. D. (2012). Primary cultures of astrocytes: their value in understanding astrocytes in health and disease. Neurochemical research, 37(11), 2569–2588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lalo, U. , Pankratov, Y. , Kirchhoff, F. , North, R. A. , & Verkhratsky, A. (2006). NMDA receptors mediate neuron‐to‐glia signaling in mouse cortical astrocytes. The Journal of Neuroscience, 26, 2673–2683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, X. , Chen, Y. , Vickstrom, C. R. , Li, Y. , Viader, A. , Cravatt, B. F. , & Liu, Q. (2016). Coordinated regulation of endocannabinoid‐mediated retrograde synaptic suppression in the cerebellum by neuronal and astrocytic monoacylglycerol lipase. Scientific Reports, 6, 35829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo, H. , Saubamea, B. , Chasseigneaux, S. , Cochois, V. , Smirnova, M. , Glacial, F. , Perrière, N. , Chaves, C. , Cisternino, S. , & Declèves, X. (2020). Molecular and Functional Study of Transient Receptor Potential Vanilloid 1–4 at the Rat and Human Blood–Brain Barrier Reveals Interspecies Differences. Frontiers in Cell and Development Biology, 8, 1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma, Z. , Stork, T. , Bergles, D. E. , & Freeman, M. R. (2016). Neuromodulators signal through astrocytes to alter neural circuit activity and behaviour. Nature, 539, 428–432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magistretti, P. J. , Morrison, J. H. , Shoemaker, W. J. , Sapin, V. , & Bloom, F. E. (1981). Vasoactive intestinal polypeptide induces glycogenolysis in mouse cortical slices: A possible regulatory mechanism for the local control of energy metabolism. Proceedings of the National Academy of Sciences of the United States of America, 78, 6535–6539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mak, D.‐O. D. , McBride, S. , & Foskett, J. K. (1998). Inositol 1,4,5‐tris‐phosphate activation of inositol tris‐phosphate receptor Ca2+ channel by ligand tuning of Ca2+ inhibition. Proceedings of the National Academy of Sciences, 95, 15821–15825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchi, S. , & Pinton, P. (2014). The mitochondrial calcium uniporter complex: Molecular components, structure and physiopathological implications. The Journal of Physiology, 592, 829–839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margulies, J. E. , & Hammer, R. P. (1991). Delta 9‐tetrahydrocannabinol alters cerebral metabolism in a biphasic, dose‐dependent manner in rat brain. European Journal of Pharmacology, 202, 373–378. [DOI] [PubMed] [Google Scholar]
- Marrs, W. R. , Blankman, J. L. , Horne, E. A. , Thomazeau, A. , Lin, Y. H. , Coy, J. , Bodor, A. L. , Muccioli, G. G. , Hu, S. S.‐J. , Woodruff, G. , Fung, S. , Lafourcade, M. , Alexander, J. P. , Long, J. Z. , Li, W. , Xu, C. , Möller, T. , Mackie, K. , Manzoni, O. J. , … Stella, N. (2010). The serine hydrolase ABHD6 controls the accumulation and efficacy of 2‐AG at cannabinoid receptors. Nature Neuroscience, 13, 951–957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martín, R. , Bajo‐Grañeras, R. , Moratalla, R. , Perea, G. , & Araque, A. (2015). Circuit‐specific signaling in astrocyte‐neuron networks in basal ganglia pathways. Science, 349, 730–734. [DOI] [PubMed] [Google Scholar]
- Martin‐Fernandez, M. , Jamison, S. , Robin, L. M. , Zhao, Z. , Martin, E. D. , Aguilar, J. , Benneyworth, M. A. , Marsicano, G. , & Araque, A. (2017). Synapse‐specific astrocyte gating of amygdala‐related behavior. Nature Neuroscience, 20, 1540–1548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mederos, S. , & Perea, G. (2019). GABAergic‐astrocyte signaling: A refinement of inhibitory brain networks. Glia, 67, 1842–1851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mederos, S. , Sánchez‐Puelles, C. , Esparza, J. , Valero, M. , Ponomarenko, A. , & Perea, G. (2021). GABAergic signaling to astrocytes in the prefrontal cortex sustains goal‐directed behaviors. Nature Neuroscience, 24, 82–92. [DOI] [PubMed] [Google Scholar]
- Min, R. , & Nevian, T. (2012). Astrocyte signaling controls spike timing‐dependent depression at neocortical synapses. Nature Neuroscience, 15, 746–753. [DOI] [PubMed] [Google Scholar]
- Moldrich, G. , & Wenger, T. (2000). Localization of the CB1 cannabinoid receptor in the rat brain. An immunohistochemical study. Peptides, 21, 1735–1742. [DOI] [PubMed] [Google Scholar]
- Molina‐Holgado, F. , Lledó, A. , & Guaza, C. (1997). Anandamide suppresses nitric oxide and TNF‐alpha responses to Theiler's virus or endotoxin in astrocytes. Neuroreport, 8, 1929–1933. [DOI] [PubMed] [Google Scholar]
- Molina‐Holgado, F. , Molina‐Holgado, E. , & Guaza, C. (1998). The endogenous cannabinoid anandamide potentiates interleukin‐6 production by astrocytes infected with Theiler's murine encephalomyelitis virus by a receptor‐mediated pathway. FEBS Letters, 433, 139–142. [DOI] [PubMed] [Google Scholar]
- Molina‐Holgado, F. , Molina‐Holgado, E. , Guaza, C. , & Rothwell, N. J. (2002). Role of CB1 and CB2 receptors in the inhibitory effects of cannabinoids on lipopolysaccharide‐induced nitric oxide release in astrocyte cultures. Journal of Neuroscience Research, 67, 829–836. [DOI] [PubMed] [Google Scholar]
- Moreno‐García, Á. , Bernal‐Chico, A. , Colomer, T. , Rodríguez‐Antigüedad, A. , Matute, C. , & Mato, S. (2020). Gene expression analysis of astrocyte and microglia endocannabinoid signaling during autoimmune demyelination. Biomolecules, 10, 1228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller, C. , Morales, P. , & Reggio, P. H. (2019). Cannabinoid ligands targeting TRP channels. Frontiers in Molecular Neuroscience, 11, 487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakahara, K. , Okada, M. , & Nakanishi, S. (1997). The metabotropic glutamate receptor mGluR5 induces calcium oscillations in cultured astrocytes via protein kinase C phosphorylation. Journal of Neurochemistry, 69, 1467–1475. [DOI] [PubMed] [Google Scholar]
- Navarrete, M. , & Araque, A. (2008). Endocannabinoids mediate neuron‐astrocyte communication. Neuron, 57, 883–893. [DOI] [PubMed] [Google Scholar]
- Navarrete, M. , & Araque, A. (2010). Endocannabinoids potentiate synaptic transmission through stimulation of astrocytes. Neuron, 68, 113–126. [DOI] [PubMed] [Google Scholar]
- Navarrete, M. , Cuartero, M. I. , Palenzuela, R. , Draffin, J. E. , Konomi, A. , Serra, I. , Colié, S. , Castaño‐Castaño, S. , Hasan, M. T. , Nebreda, Á. R. , & Esteban, J. A. (2019). Astrocytic p38α MAPK drives NMDA receptor‐dependent long‐term depression and modulates long‐term memory. Nature Communications, 10, 2968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nedergaard, M. , Ransom, B. , & Goldman, S. A. (2003). New roles for astrocytes: Redefining the functional architecture of the brain. Trends in Neurosciences, 26, 523–530. [DOI] [PubMed] [Google Scholar]
- Nett, W. J. , Oloff, S. H. , & McCarthy, K. D. (2002). Hippocampal astrocytes in situ exhibit calcium oscillations that occur independent of neuronal activity. Journal of Neurophysiology, 87, 528–537. [DOI] [PubMed] [Google Scholar]
- Nogueiras, R. , Diaz‐Arteaga, A. , Lockie, S. H. , Velásquez, D. A. , Tschop, J. , López, M. , Cadwell, C. C. , Diéguez, C. , & Tschöp, M. H. (2009). The endocannabinoid system: Role in glucose and energy metabolism. Pharmacological Research, 60, 93–98. [DOI] [PubMed] [Google Scholar]
- Núñez, E. , Benito, C. , Tolón, R. M. , Hillard, C. J. , Griffin, W. S. T. , & Romero, J. (2008). Glial expression of cannabinoid CB2 receptors and fatty acid amide hydrolase are beta amyloid–linked events in Down's syndrome. Neuroscience, 151, 104–110. [DOI] [PubMed] [Google Scholar]
- Oberheim, N. A. , Takano, T. , Han, X. , He, W. , Lin, J. H. C. , Wang, F. , Xu, Q. , Wyatt, J. D. , Pilcher, W. , Ojemann, J. G. , Ransom, B. R. , Goldman, S. A. , & Nedergaard, M. (2009). Uniquely hominid features of adult human astrocytes. Journal of Neuroscience: The Official Journal of the Society for Neuroscience, 29, 3276–3287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Donnell, J. , Ding, F. , & Nedergaard, M. (2015). Distinct functional states of astrocytes during sleep and wakefulness: Is norepinephrine the master regulator? Current Sleep Medicine Reports, 1, 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliveira da Cruz, J. F. , Robin, L. M. , Drago, F. , Marsicano, G. , & Metna‐Laurent, M. (2016). Astroglial type‐1 cannabinoid receptor (CB1): A new player in the tripartite synapse. Neuroscience, 323, 35–42. [DOI] [PubMed] [Google Scholar]
- Ortega‐Gutiérrez, S. , Molina‐Holgado, E. , & Guaza, C. (2005). Effect of anandamide uptake inhibition in the production of nitric oxide and in the release of cytokines in astrocyte cultures. Glia, 52, 163–168. [DOI] [PubMed] [Google Scholar]
- O'Sullivan, S. E. (2016). An update on PPAR activation by cannabinoids. British Journal of Pharmacology, 173, 1899–1910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O‐Uchi, J. , Pan, S. , & Sheu, S.‐S. (2012). Molecular identities of mitochondrial Ca2+ influx mechanism: Updated passwords for accessing mitochondrial Ca2+−linked health and disease. The Journal of General Physiology, 139, 435–443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pacholko, A. G. , Wotton, C. A. , & Bekar, L. K. (2020). Astrocytes‐the ultimate effectors of Long‐range Neuromodulatory networks? Frontiers in Cellular Neuroscience, 14, 581075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palygin, O. , Lalo, U. , Verkhratsky, A. , & Pankratov, Y. (2010). Ionotropic NMDA and P2X1/5 receptors mediate synaptically induced Ca2+ signalling in cortical astrocytes. Cell Calcium, 48, 225–231. [DOI] [PubMed] [Google Scholar]
- Pamplona, F. A. , Ferreira, J. , Menezes de Lima, O. , Duarte, F. S. , Bento, A. F. , Forner, S. , Villarinho, J. G. , Bellocchio, L. , Bellochio, L. , Wotjak, C. T. , Lerner, R. , Monory, K. , Lutz, B. , Canetti, C. , Matias, I. , Calixto, J. B. , Marsicano, G. , Guimarães, M. Z. P. , & Takahashi, R. N. (2012). Anti‐inflammatory lipoxin A4 is an endogenous allosteric enhancer of CB1 cannabinoid receptor. Proceedings of the National Academy of Sciences of the United States of America, 109, 21134–21139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panatier, A. , Vallée, J. , Haber, M. , Murai, K. K. , Lacaille, J.‐C. , & Robitaille, R. (2011). Astrocytes are endogenous regulators of basal transmission at central synapses. Cell, 146, 785–798. [DOI] [PubMed] [Google Scholar]
- Paukert, M. , Agarwal, A. , Cha, J. , Doze, V. A. , Kang, J. U. , & Bergles, D. E. (2014). Norepinephrine controls astroglial responsiveness to local circuit activity. Neuron, 82, 1263–1270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pellerin, L. , Bouzier‐Sore, A.‐K. , Aubert, A. , Serres, S. , Merle, M. , Costalat, R. , & Magistretti, P. J. (2007). Activity‐dependent regulation of energy metabolism by astrocytes: An update. Glia, 55, 1251–1262. [DOI] [PubMed] [Google Scholar]
- Pellerin, L. , & Magistretti, P. J. (1994). Glutamate uptake into astrocytes stimulates aerobic glycolysis: A mechanism coupling neuronal activity to glucose utilization. Proceedings of the National Academy of Sciences of the United States of America, 91, 10625–10629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perea, G. , & Araque, A. (2005). Glial calcium signaling and neuron–glia communication. Cell Calcium, 38, 375–382. [DOI] [PubMed] [Google Scholar]
- Perea, G. , & Araque, A. (2007). Astrocytes potentiate transmitter release at single hippocampal synapses. Science, 317, 1083–1086. [DOI] [PubMed] [Google Scholar]
- Perea, G. , Navarrete, M. , & Araque, A. (2009). Tripartite synapses: Astrocytes process and control synaptic information. Trends in Neurosciences, 32, 421–431. [DOI] [PubMed] [Google Scholar]
- Piomelli, D. (2003). The molecular logic of endocannabinoid signalling. Nature Reviews. Neuroscience, 4, 873–884. [DOI] [PubMed] [Google Scholar]
- Porter, J. T. , & McCarthy, K. D. (1996). Hippocampal astrocytes in situ respond to glutamate released from synaptic terminals. The Journal of Neuroscience, 16, 5073–5081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter‐Stransky, K. A. , Centanni, S. W. , Karne, S. L. , Odil, L. M. , Fekir, S. , Wong, J. C. , Jerome, C. , Mitchell, H. A. , Escayg, A. , Pedersen, N. P. , Winder, D. G. , Mitrano, D. A. , & Weinshenker, D. (2019). Noradrenergic transmission at Alpha1‐adrenergic receptors in the ventral periaqueductal gray modulates arousal. Biological Psychiatry, 85, 237–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasooli‐Nejad, S. , Palygin, O. , Lalo, U. , & Pankratov, Y. (2014). Cannabinoid receptors contribute to astroglial Ca2+‐signalling and control of synaptic plasticity in the neocortex. Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences, 369, 20140077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reyes, R. C. , & Parpura, V. (2008). Mitochondria modulate Ca2+−dependent glutamate release from rat cortical astrocytes. Journal of Neuroscience: The Official Journal of the Society for Neuroscience, 28, 9682–9691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizzuto, R. , Stefani, D. D. , Raffaello, A. , & Mammucari, C. (2012). Mitochondria as sensors and regulators of calcium signalling. Nature Reviews. Molecular Cell Biology, 13, 566–578. [DOI] [PubMed] [Google Scholar]
- Robin, L. M. , Oliveira da Cruz, J. F. , Langlais, V. C. , Martin‐Fernandez, M. , Metna‐Laurent, M. , Busquets‐Garcia, A. , Bellocchio, L. , Soria‐Gomez, E. , Papouin, T. , Varilh, M. , Sherwood, M. W. , Belluomo, I. , Balcells, G. , Matias, I. , Bosier, B. , Drago, F. , Van Eeckhaut, A. , Smolders, I. , Georges, F. , … Marsicano, G. (2018). Astroglial CB1 receptors determine synaptic D‐serine availability to enable recognition memory. Neuron, 98, 935–944.e5. [DOI] [PubMed] [Google Scholar]
- Rodríguez, J. J. , Mackie, K. , & Pickel, V. M. (2001). Ultrastructural localization of the CB1 cannabinoid receptor in μ‐opioid receptor patches of the rat caudate putamen nucleus. The Journal of Neuroscience, 21, 823–833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodríguez‐Cueto, C. , Benito, C. , Fernández‐Ruiz, J. , Romero, J. , Hernández‐Gálvez, M. , & Gómez‐Ruiz, M. (2014). Changes in CB1 and CB2 receptors in the post‐mortem cerebellum of humans affected by spinocerebellar ataxias. British Journal of Pharmacology, 171, 1472–1489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rungta, R. L. , Bernier, L.‐P. , Dissing‐Olesen, L. , Groten, C. J. , LeDue, J. M. , Ko, R. , Drissler, S. , & MacVicar, B. A. (2016). Ca2+ transients in astrocyte fine processes occur via Ca2+ influx in the adult mouse hippocampus. Glia, 64, 2093–2103. [DOI] [PubMed] [Google Scholar]
- Sakuragi, S. , Niwa, F. , Oda, Y. , Mikoshiba, K. , & Bannai, H. (2017). Astroglial Ca2+ signaling is generated by the coordination of IP3R and store‐operated Ca2+ channels. Biochemical and Biophysical Research Communications, 486, 879–885. [DOI] [PubMed] [Google Scholar]
- Salio, C. , Doly, S. , Fischer, J. , Franzoni, M. F. , & Conrath, M. (2002). Neuronal and astrocytic localization of the cannabinoid receptor‐1 in the dorsal horn of the rat spinal cord. Neuroscience Letters, 329, 13–16. [DOI] [PubMed] [Google Scholar]
- Sánchez, C. , Galve‐Roperh, I. , Rueda, D. , & Guzmán, M. (1998). Involvement of sphingomyelin hydrolysis and the mitogen‐activated protein kinase Cascade in the Δ9‐tetrahydrocannabinol‐induced stimulation of glucose metabolism in primary astrocytes. Molecular Pharmacology, 54, 834–843. [DOI] [PubMed] [Google Scholar]
- Sarafian, T. A. , Montes, C. , Imura, T. , Qi, J. , Coppola, G. , Geschwind, D. H. , & Sofroniew, M. V. (2010). Disruption of astrocyte STAT3 signaling decreases mitochondrial function and increases oxidative stress in vitro. PLoS One, 5, e9532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawzdargo, M. , Nguyen, T. , Lee, D. K. , Lynch, K. R. , Cheng, R. , Heng, H. H. , George, S. R. , & O'Dowd, B. F. (1999). Identification and cloning of three novel human G protein‐coupled receptor genes GPR52, PsiGPR53 and GPR55: GPR55 is extensively expressed in human brain. Brain Research. Molecular Brain Research, 64, 193–198. [DOI] [PubMed] [Google Scholar]
- Schmöle, A.‐C. , Lundt, R. , Gennequin, B. , Schrage, H. , Beins, E. , Krämer, A. , Zimmer, T. , Limmer, A. , Zimmer, A. , & Otte, D.‐M. (2015). Expression analysis of CB2‐GFP BAC transgenic mice. PLoS One, 10, e0138986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schönfeld, P. , & Reiser, G. (2013). Why does brain metabolism not favor burning of fatty acids to provide energy? Reflections on disadvantages of the use of free fatty acids as fuel for brain. Journal of Cerebral Blood Flow & Metabolism. official Journal of International Society for Cerebral Blood Flow & Metabolism, 33, 1493–1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schönfeld, P. , & Reiser, G. (2021). How the brain fights fatty acids' toxicity. Neurochemistry International, 148, 105050. [DOI] [PubMed] [Google Scholar]
- Schousboe, A. , Scafidi, S. , Bak, L. K. , Waagepetersen, H. S. , & McKenna, M. C. (2014). Glutamate metabolism in the brain focusing on astrocytes. Advances in neurobiology, 11, 13–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schousboe, A. , Westergaard, N. , Sonnewald, U. , Petersen, S. B. , Huang, R. , Peng, L. , & Hertz, L. (1993). Glutamate and glutamine metabolism and compartmentation in astrocytes. Developmental Neuroscience, 15, 359–366. [DOI] [PubMed] [Google Scholar]
- Schüle, L.‐L. , Glasmacher, S. , Gertsch, J. , Roggan, M. D. , Transfeld, J.‐L. , Bindila, L. , Lutz, B. , Kolbe, C.‐C. , Bilkei‐Gorzo, A. , Zimmer, A. , & Leidmaa, E. (2021). Diacylglycerol lipase alpha in astrocytes is involved in maternal care and affective behaviors. Glia, 69, 377–391. [DOI] [PubMed] [Google Scholar]
- Schwarz, Y. , Zhao, N. , Kirchhoff, F. , & Bruns, D. (2017). Astrocytes control synaptic strength by two distinct v‐SNARE‐dependent release pathways. Nature Neuroscience, 20, 1529–1539. [DOI] [PubMed] [Google Scholar]
- Scuderi, C. , Esposito, G. , Blasio, A. , Valenza, M. , Arietti, P. , Steardo, L., Jr. , Carnuccio, R. , De Filippis, D. , Petrosino, S. , Iuvone, T. , Marzo, V. D. , & Steardo, L. (2011). Palmitoylethanolamide counteracts reactive astrogliosis induced by β‐amyloid peptide. Journal of Cellular and Molecular Medicine, 15, 2664–2674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scuderi, C. , Valenza, M. , Stecca, C. , Esposito, G. , Carratù, M. R. , & Steardo, L. (2012). Palmitoylethanolamide exerts neuroprotective effects in mixed neuroglial cultures and organotypic hippocampal slices via peroxisome proliferator‐activated receptor‐α. Journal of Neuroinflammation, 9, 49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semyanov, A. (2019). Spatiotemporal pattern of calcium activity in astrocytic network. Cell Calcium, 78, 15–25. [DOI] [PubMed] [Google Scholar]
- Serrat, R. , Covelo, A. , Kouskoff, V. , Delcasso, S. , Ruiz‐Calvo, A. , Chenouard, N. , Stella, C. , Blancard, C. , Salin, B. , Julio‐Kalajzić, F. , Cannich, A. , Massa, F. , Varilh, M. , Deforges, S. , Robin, L. M. , De Stefani, D. , Busquets‐Garcia, A. , Gambino, F. , Beyeler, A. , … Marsicano, G. (2021). Astroglial ER‐mitochondria calcium transfer mediates endocannabinoid‐dependent synaptic integration. Cell Reports, 37, 110133. [DOI] [PubMed] [Google Scholar]
- Sheng, W. S. , Hu, S. , Min, X. , Cabral, G. A. , Lokensgard, J. R. , & Peterson, P. K. (2005). Synthetic cannabinoid WIN55,212‐2 inhibits generation of inflammatory mediators by IL‐1β‐stimulated human astrocytes. Glia, 49, 211–219. [DOI] [PubMed] [Google Scholar]
- Sherwood, M. W. , Arizono, M. , Hisatsune, C. , Bannai, H. , Ebisui, E. , Sherwood, J. L. , Panatier, A. , Oliet, S. H. R. , & Mikoshiba, K. (2017). Astrocytic IP3 Rs: Contribution to Ca2+ signalling and hippocampal LTP. Glia, 65, 502–513. [DOI] [PubMed] [Google Scholar]
- Shibasaki, K. , Ishizaki, Y. , & Mandadi, S. (2013). Astrocytes express functional TRPV2 ion channels. Biochemical and Biophysical Research Communications, 441, 327–332. [DOI] [PubMed] [Google Scholar]
- Shigetomi, E. , Jackson‐Weaver, O. , Huckstepp, R. T. , O'Dell, T. J. , & Khakh, B. S. (2013). TRPA1 channels are regulators of astrocyte basal calcium levels and Long‐term potentiation via constitutive d‐serine release. The Journal of Neuroscience, 33, 10143–10153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinohara, T. , Michikawa, T. , Enomoto, M. , Goto, J.‐I. , Iwai, M. , Matsu‐ura, T. , Yamazaki, H. , Miyamoto, A. , Suzuki, A. , & Mikoshiba, K. (2011). Mechanistic basis of bell‐shaped dependence of inositol 1,4,5‐trisphosphate receptor gating on cytosolic calcium. Proceedings of the National Academy of Sciences, 108, 15486–15491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson, P. B. , Mehotra, S. , Lange, G. D. , & Russell, J. T. (1997). High density distribution of endoplasmic reticulum proteins and mitochondria at specialized Ca2+ release sites in oligodendrocyte processes. The Journal of Biological Chemistry, 272, 22654–22661. [DOI] [PubMed] [Google Scholar]
- Smith, N. A. , Bekar, L. K. , & Nedergaard, M. (2020). Astrocytic endocannabinoids mediate hippocampal transient Heterosynaptic depression. Neurochemical Research, 45, 100–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sofroniew, M. V. (2020). Astrocyte reactivity: Subtypes, states, and functions in CNS innate immunity. Trends in Immunology, 41, 758–770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soria‐Gomez, E. , Pagano Zottola, A. C. , Mariani, Y. , Desprez, T. , Barresi, M. , Bonilla‐Del Río, I. , Muguruza, C. , Le Bon‐Jego, M. , Julio‐Kalajzić, F. , Flynn, R. , Terral, G. , Fernández‐Moncada, I. , Robin, L. M. , Oliveira da Cruz, J. F. , Corinti, S. , Amer, Y. O. , Goncalves, J. , Varilh, M. , Cannich, A. , … Bellocchio, L. (2021). Subcellular specificity of cannabinoid effects in striatonigral circuits. Neuron, 109, 1513–1526.e11. [DOI] [PubMed] [Google Scholar]
- Srinivasan, R. , Huang, B. S. , Venugopal, S. , Johnston, A. D. , Chai, H. , Zeng, H. , Golshani, P. , & Khakh, B. S. (2015). Ca2+ signaling in astrocytes from Ip3r2−/− mice in brain slices and during startle responses in vivo. Nature Neuroscience, 18, 708–717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stella, N. (2004). Cannabinoid signaling in glial cells. Glia, 48, 267–277. [DOI] [PubMed] [Google Scholar]
- Stella, N. (2010). Cannabinoid and cannabinoid‐like receptors in microglia, astrocytes, and astrocytomas. Glia, 58, 1017–1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephen, T.‐L. , Gupta‐Agarwal, S. , & Kittler, J. T. (2014). Mitochondrial dynamics in astrocytes. Biochemical Society Transactions, 42, 1302–1310. [DOI] [PubMed] [Google Scholar]
- Suárez, I. , Bodega, G. , Fernández‐Ruiz, J. J. , Ramos, J. A. , Rubio, M. , & Fernández, B. (2002). Reduced glial fibrillary acidic protein and glutamine Synthetase expression in astrocytes and Bergmann glial cells in the rat cerebellum caused by Δ9‐tetrahydrocannabinol administration during development. Developmental Neuroscience, 24, 300–312. [DOI] [PubMed] [Google Scholar]
- Sun, M.‐Y. , Devaraju, P. , Xie, A. X. , Holman, I. , Samones, E. , Murphy, T. R. , & Fiacco, T. A. (2014). Astrocyte calcium microdomains are inhibited by Bafilomycin A1 and cannot be replicated by low‐level Schaffer collateral stimulation in situ. Cell Calcium, 55, 1–16. [DOI] [PubMed] [Google Scholar]
- Sun, W. , Cornwell, A. , Li, J. , Peng, S. , Osorio, M. J. , Aalling, N. , Wang, S. , Benraiss, A. , Lou, N. , Goldman, S. A. , & Nedergaard, M. (2017). SOX9 is an astrocyte‐specific nuclear marker in the adult brain outside the neurogenic regions. The Journal of Neuroscience, 37, 4493–4507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun, Y. , & Bennett, A. (2007). Cannabinoids: A new Group of Agonists of PPARs. PPAR Research, 2007, 23513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka, M. , Shih, P.‐Y. , Gomi, H. , Yoshida, T. , Nakai, J. , Ando, R. , Furuichi, T. , Mikoshiba, K. , Semyanov, A. , & Itohara, S. (2013). Astrocytic Ca2+ signals are required for the functional integrity of tripartite synapses. Molecular Brain, 6, 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tóth, A. , Boczán, J. , Kedei, N. , Lizanecz, E. , Bagi, Z. , Papp, Z. , Édes, I. , Csiba, L. , & Blumberg, P. M. (2005). Expression and distribution of vanilloid receptor 1 (TRPV1) in the adult rat brain. Molecular Brain Research, 135, 162–168. [DOI] [PubMed] [Google Scholar]
- Tovar, R. , Vargas, A. , Aranda, J. , Sánchez‐Salido, L. , González‐González, L. , Chowen, J. A. , Rodríguez de Fonseca, F. , Suárez, J. , & Rivera, P. (2021). Analysis of both lipid metabolism and endocannabinoid signaling reveals a new role for hypothalamic astrocytes in maternal caloric restriction‐induced perinatal programming. International Journal of Molecular Sciences, 22, 6292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ullah, G. , Jung, P. , & Cornell‐Bell, A. H. (2006). Anti‐phase calcium oscillations in astrocytes via inositol (1, 4, 5)‐trisphosphate regeneration. Cell Calcium, 39, 197–208. [DOI] [PubMed] [Google Scholar]
- Umpierre, A. D. , West, P. J. , White, J. A. , & Wilcox, K. S. (2019). Conditional Knock‐out of mGluR5 from astrocytes during epilepsy development impairs high‐frequency glutamate uptake. The Journal of Neuroscience, 39, 727–742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallee, M. , Vitiello, S. , Bellocchio, L. , Hebert‐Chatelain, E. , Monlezun, S. , Martin‐Garcia, E. , Kasanetz, F. , Baillie, G. L. , Panin, F. , Cathala, A. , Roullot‐Lacarriere, V. , Fabre, S. , Hurst, D. P. , Lynch, D. L. , Shore, D. M. , Deroche‐Gamonet, V. , Spampinato, U. , Revest, J.‐M. , Maldonado, R. , … Piazza, P. V. (2014). Pregnenolone can protect the brain from cannabis intoxication. Science, 343, 94–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valori, C. F. , Possenti, A. , Brambilla, L. , & Rossi, D. (2021). Challenges and opportunities of targeting astrocytes to halt neurodegenerative disorders. Cell, 10, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vázquez, C. , Tolón, R. M. , Pazos, M. R. , Moreno, M. , Koester, E. C. , Cravatt, B. F. , Hillard, C. J. , & Romero, J. (2015). Endocannabinoids regulate the activity of astrocytic hemichannels and the microglial response against an injury: In vivo studies. Neurobiology of Disease, 79, 41–50. [DOI] [PubMed] [Google Scholar]
- Veilleux, A. , Di Marzo, V. , & Silvestri, C. (2019). The expanded endocannabinoid system/Endocannabinoidome as a potential target for treating diabetes mellitus. Current Diabetes Reports, 19, 117. [DOI] [PubMed] [Google Scholar]
- Venance, L. , Piomelli, D. , Glowinski, J. , & Giaume, C. (1995). Inhibition by anandamide of gap junctions and intercellular calcium signalling in striatal astrocytes. Nature, 376, 590–594. [DOI] [PubMed] [Google Scholar]
- Venkatachalam, K. , & Montell, C. (2007). TRP Channels. Annual Review of Biochemistry, 76, 387–417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verkhratsky, A. , Reyes, R. C. , & Parpura, V. (2014). TRP channels coordinate ion Signalling in Astroglia. Reviews of Physiology, Biochemistry and Pharmacology, 166, 1–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verkhratsky, A. , Zorec, R. , & Parpura, V. (2017). Stratification of astrocytes in healthy and diseased brain. Brain Pathology, 27, 629–644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viader, A. , Blankman, J. L. , Zhong, P. , Liu, X. , Schlosburg, J. E. , Joslyn, C. M. , Liu, Q.‐S. , Tomarchio, A. J. , Lichtman, A. H. , Selley, D. E. , Sim‐Selley, L. J. , & Cravatt, B. F. (2015). Metabolic interplay between astrocytes and neurons regulates endocannabinoid action. Cell Reports, 12, 798–808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viader, A. , Ogasawara, D. , Joslyn, C. M. , Sanchez‐Alavez, M. , Mori, S. , Nguyen, W. , Conti, B. , & Cravatt, B. F. (2016). A chemical proteomic atlas of brain serine hydrolases identifies cell type‐specific pathways regulating neuroinflammation. eLife, 5, e12345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vicente‐Gutierrez, C. , Bonora, N. , Bobo‐Jimenez, V. , Jimenez‐Blasco, D. , Lopez‐Fabuel, I. , Fernandez, E. , Josephine, C. , Bonvento, G. , Enriquez, J. A. , Almeida, A. , & Bolaños, J. P. (2019). Astrocytic mitochondrial ROS modulate brain metabolism and mouse behaviour. Nature Metabolism, 1, 201–211. [DOI] [PubMed] [Google Scholar]
- Volkow, N. D. , Gillespie, H. , Mullani, N. , Tancredi, L. , Grant, C. , Ivanovic, M. , & Hollister, L. (1991). Cerebellar metabolic activation by delta‐9‐tetrahydro‐cannabinol in human brain: A study with positron emission tomography and 18F‐2‐fluoro‐2‐deoxyglucose. Psychiatry Research, 40, 69–78. [DOI] [PubMed] [Google Scholar]
- Volterra, A. , & Meldolesi, J. (2005). Astrocytes, from brain glue to communication elements: The revolution continues. Nature Reviews. Neuroscience, 6, 626–640. [DOI] [PubMed] [Google Scholar]
- Walter, L. , Dinh, T. , & Stella, N. (2004). ATP induces a rapid and pronounced increase in 2‐arachidonoylglycerol production by astrocytes, a response limited by monoacylglycerol lipase. Journal of Neuroscience: The Official Journal of the Society for Neuroscience, 24, 8068–8074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walter, L. , Franklin, A. , Witting, A. , Möller, T. , & Stella, N. (2002). Astrocytes in culture produce anandamide and other Acylethanolamides *. The Journal of Biological Chemistry, 277, 20869–20876. [DOI] [PubMed] [Google Scholar]
- Walter, L. , & Stella, N. (2003). Endothelin‐1 increases 2‐arachidonoyl glycerol (2‐AG) production in astrocytes. Glia, 44, 85–90. [DOI] [PubMed] [Google Scholar]
- Wang, F. , Li, M. , Li, X. , Kinden, R. , Zhou, H. , Guo, F. , Wang, Q. , & Xiong, L. (2016). 2‐Arachidonylglycerol protects primary astrocytes exposed to oxygen‐glucose deprivation through a blockade of NDRG2 signaling and STAT3 phosphorylation. Rejuvenation Research, 19, 215–222. [DOI] [PubMed] [Google Scholar]
- Wang, S. , Zhang, H. , Geng, B. , Xie, Q. , Li, W. , Deng, Y. , Shi, W. , Pan, Y. , Kang, X. , & Wang, J. (2018). 2‐arachidonyl glycerol modulates astrocytic glutamine synthetase via p38 and ERK1/2 pathways. Journal of Neuroinflammation, 15, 220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warden, A. , Truitt, J. , Merriman, M. , Ponomareva, O. , Jameson, K. , Ferguson, L. B. , Mayfield, R. D. , & Harris, R. A. (2016). Localization of PPAR isotypes in the adult mouse and human brain. Scientific Reports, 6, 27618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams, G. S. B. , Boyman, L. , Chikando, A. C. , Khairallah, R. J. , & Lederer, W. J. (2013). Mitochondrial calcium uptake. Proceedings of the National Academy of Sciences of the United States of America, 110, 10479–10486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witting, A. , Walter, L. , Wacker, J. , Möller, T. , & Stella, N. (2004). P2X7 receptors control 2‐arachidonoylglycerol production by microglial cells. Proceedings of the National Academy of Sciences of the United States of America, 101, 3214–3219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu, Y.‐W. , Gordleeva, S. , Tang, X. , Shih, P.‐Y. , Dembitskaya, Y. , & Semyanov, A. (2019). Morphological profile determines the frequency of spontaneous calcium events in astrocytic processes. Glia, 67, 246–262. [DOI] [PubMed] [Google Scholar]
- Wu, Y.‐W. , Tang, X. , Arizono, M. , Bannai, H. , Shih, P.‐Y. , Dembitskaya, Y. , Kazantsev, V. , Tanaka, M. , Itohara, S. , Mikoshiba, K. , & Semyanov, A. (2014). Spatiotemporal calcium dynamics in single astrocytes and its modulation by neuronal activity. Cell Calcium, 55, 119–129. [DOI] [PubMed] [Google Scholar]
- Xu, H. , Ramsey, I. S. , Kotecha, S. A. , Moran, M. M. , Chong, J. A. , Lawson, D. , Ge, P. , Lilly, J. , Silos‐Santiago, I. , Xie, Y. , DiStefano, P. S. , Curtis, R. , & Clapham, D. E. (2002). TRPV3 is a calcium‐permeable temperature‐sensitive cation channel. Nature, 418, 181–186. [DOI] [PubMed] [Google Scholar]
- Xu, T. , & Pandey, S. C. (2000). Cellular localization of serotonin2A (5HT2A) receptors in the rat brain. Brain Research Bulletin, 51, 499–505. [DOI] [PubMed] [Google Scholar]
- Yang, X.‐L. , Wang, X. , Shao, L. , Jiang, G.‐T. , Min, J.‐W. , Mei, X.‐Y. , He, X.‐H. , Liu, W.‐H. , Huang, W.‐X. , & Peng, B.‐W. (2019). TRPV1 mediates astrocyte activation and interleukin‐1β release induced by hypoxic ischemia (HI). Journal of Neuroinflammation, 16, 114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, H. , Hilton, D. A. , Hanemann, C. O. , & Zajicek, J. (2011). Cannabinoid receptor and N‐acyl phosphatidylethanolamine phospholipase D—Evidence for altered expression in multiple sclerosis. Brain Pathology, 21, 544–557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, K. , Förster, R. , He, W. , Liao, X. , Li, J. , Yang, C. , Qin, H. , Wang, M. , Ding, R. , Li, R. , Jian, T. , Wang, Y. , Zhang, J. , Yang, Z. , Jin, W. , Zhang, Y. , Qin, S. , Lu, Y. , Chen, T. , … Chen, X. (2021). Fear learning induces α7‐nicotinic acetylcholine receptor‐mediated astrocytic responsiveness that is required for memory persistence. Nature Neuroscience, 24, 1686–1698. [DOI] [PubMed] [Google Scholar]
- Zhou, Z. , Okamoto, K. , Onodera, J. , Hiragi, T. , Andoh, M. , Ikawa, M. , Tanaka, K. F. , Ikegaya, Y. , & Koyama, R. (2021). Astrocytic cAMP modulates memory via synaptic plasticity. Proceedings of the National Academy of Sciences, 118, e2016584118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou, S. , & Kumar, U. (2018). Cannabinoid receptors and the endocannabinoid system: Signaling and function in the central nervous system. International Journal of Molecular Sciences, 19, 833. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article as no new data were created or analyzed in this study.


