Abstract
Mind-body therapies (MBTs) use mental abilities to modify electrical neural activity across brain networks. Transcranial direct current stimulation (tDCS) is a non-invasive brain stimulation technique that modulates neuronal membrane potentials to enhance neuroplasticity. A combination of these treatment strategies may generate synergistic or additive effects, and thus has been more commonly tested in clinical trials, fostering a novel yet promising field of research. We conducted a literature search in four different databases including only randomized clinical trials (RCTs) that tested the combination of MBTs with tDCS. Ten studies (n=461) were included. Combined protocols included meditation/mindfulness (8/10), biofeedback (1/10), and hypnosis (1/10). The RCTs were heterogeneous with regards to population, design, and types of outcomes. Based on the findings of this search, we provide here a content description, methodological and practical insights, and future directions for the field. We hope this review will provide future authors with information to facilitate the development of trials with improved protocols.
Keywords: tDCS, mind body therapies, meditation, mindfulness, biofeedback, hypnosis
1. Introduction:
Mind-body therapies (MBTs) are a group of interventions strengthen on the premise that the relationship between the mind and the body can positively influence an individual’s overall health. Examples include meditation, yoga, tai chi, qigong, breathing exercises, biofeedback, hypnosis, and acupuncture, [1] and evidence shows their usefulness in different neuropsychiatry diseases.[2–10]
Transcranial direct current stimulation (tDCS) is a low-cost, safe, non-invasive neuromodulation technique. In its machinery, it modulates the neural membrane resting potentials. Clinically, it has shown to reduce symptomology and improve motor, sensory, and cognitive processing in many neuropsychiatric disorders.[11]
Since tDCS modulates and facilitates underlying neural activity, its optimal effect should be achieved when combined with another behavioral therapy.[12–14] On the other hand, there are data evidencing the direct effect of MBTs in the Central Nervous System and the recruitment of complex brain networks.[6, 7, 15] Therefore, the combination with tDCS could augment neuroplasticity in brain networks primed by the MBT and increase the effect of these interventions. A few randomized controlled trials have been performed in that matter, marking the emergence of a growing field.[16–26]
Our objective is to provide a discussion concerning the different perspectives, limitations, and challenges of combining tDCS with MBTs, backed on findings of a systematic literature search. We intend to aid future researchers interested in this topic by supplying a broad overview of the published literature and consistently suggesting future directions for the field.
2. Methods
In May 2021, we searched in online US-based databases (PubMed/Medline, Cochrane CENTRAL, and APA PsycNet), and in December 2021 in a non-US database (LILACS), using terms to identify tDCS studies with meditation/mindfulness therapies, hypnotherapy, biofeedback, yoga, acupuncture, qigong, and tai chi. These therapies were selected based on the most common MBTs used by patients and those studied in the neurology field.[27] We also checked the cited references in each one of the included articles for related studies.
2.1. Search Terms
Pubmed, Cochrane and LILACS:
(“transcranial direct current stimulation” OR tdcs) AND (“mind-body therapy” OR “mind-body therapies” OR meditation OR mindfulness OR mindful OR hypnosis OR biofeedback OR yoga OR tai chi OR qigong OR acupuncture OR “acupuncture therapy” OR “breathing exercises”)
APA PsycNet:
(‘transcranial direct current stimulation’ OR tdcs) AND (‘mind-body therapy’ OR ‘mind-body therapies’ OR meditation OR mindfulness OR mindful OR hypnosis OR biofeedback OR yoga OR tai chi OR qigong OR acupuncture OR ‘acupuncture therapy’ OR ‘breathing exercises’) Filtered by clinical trial
2.2. Inclusion Criteria
The inclusion criteria were: (i) being a randomized controlled trial; (ii) having at least one arm of combined MBT and tDCS; (iii) article available in English, Portuguese, Spanish, or French. We did not include case report, case series, reviews, and studies performed in animals.
2.3. Study Selection, data extraction, and critical appraisal
Abstract screening, full-text screening, and data extraction were performed by three independent authors (KV-A, JP, IR-S) and discrepancies were resolved by a third reviewer (KP-B). In the cases where data were reported in graphs and not in numerical values, the WebPlotDigitizer- Copyright- 2010–2020 Ankit Rohatgi was used to acquire the means and the upper limit values, that were later transformed into SE and SD using mathematical equations. If the results were reported in other forms, we excluded the study from the effect size calculation. To help illustrate the current findings, we calculated the within- and between- effect sizes of the included studies, using Hedges’ g statistic due to the small sample sizes.[28] For each outcome, we used the mean and the SD pre- and post-intervention, as well as the mean difference, with the MAVIS v1.1.3 effect size calculator. In the cases where only the change of the difference was reported and we had the values of pre-intervention, we calculated the post-intervention mean and SD using mathematical equations. The effect sizes of the main effects were calculated for factorial trials when possible. Moreover, to have an overall evaluation of the current methodological rigor of published articles, we used the Version 2 of the Cochrane risk-of-bias tool for randomized trials (RoB 2).[29]
3. Results
3.1. Included Studies
We identified 366 articles, 301 in the initial search in May and 59 from an additional search done in December 2021. Six titles were identified through manual and citation searching (Figure 1). After abstract and full-text review, ten studies were included and twelve reports analyzed [2 articles were secondary analyses (Pollinini L et al. 2020 [25] and Brown DR et al. 2019 [30])of two other trials (Ahn et al. 2019 [16] and Witkiewitz K et al. 2019 [24] respectively)]. We did not find published RCTs that combined tDCS with qigong, tai-chi, or acupuncture, although we did find three abstracts of yoga studies, but with no published results. We also found twelve ongoing trials, ten of which were of tDCS combined with meditation/mindfulness, one with acupuncture, and one with biofeedback (See Appendix 1 for complete list[31–42]).
Figure 1:
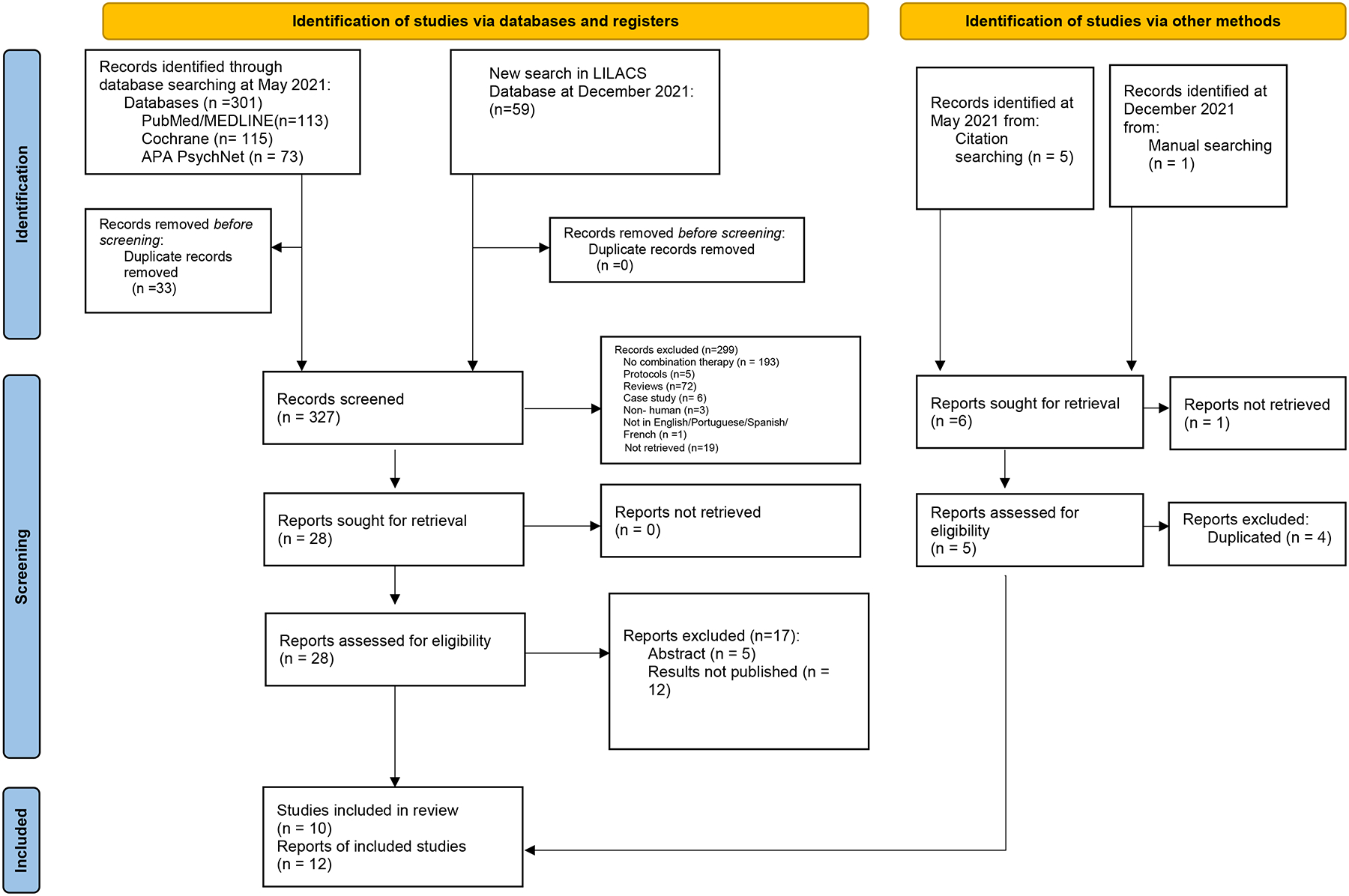
Studies selection flow diagram
3.2. Studies’ characteristics
Table 1 summarizes the information regarding the study design, population characteristics, and interventions of each one of the studies. The studies’ populations were healthy subjects, patients with major depressive disorder, alcohol use disorder, or pain due to knee osteoarthritis. The articles were composed mostly of small to moderate sample sizes (95 subjects being the largest) or over-stratified samples, as is the case of Robinson et al. that conducted a 2×2×2 factorial design with 87 participants. Most of the studies were outpatient, aside from Ahn et al. that used home-based tDCS concurrently with home-guided mindfulness-based meditation. They evaluated subjects’ satisfaction with the devices used and the way the procedure occurred and found great responses in terms of confidence, easiness, and helpfulness, with no important differences between intervention groups. Table 2 summarizes the tDCS and MBT protocols of each study.
Table 1:
Design and description of the included studies
| Author, year | Country | Study design | Sample size | Population | Age, yearsMean ± SD | Sex, n (%) | Intervention |
|---|---|---|---|---|---|---|---|
| Mindfulness/Meditation | |||||||
| Ahn et al. 2019 [16] | USA | Randomized, double-blinded, 2-arm parallel trial | 30 | Knee OA | 59.47 ± 6.91 |
M: 12 (40)
F: 18 (60) |
Arm 1: active tDCS + meditation. Arm2: sham TDCS + meditation Concomitant interventions |
| Badran et al. 2017 [17] | USA | Randomized, double-blinded, cross over, washout period of 1 week. | 15 | Healthy | 28.2 ± 6.8 |
M: 7 (46.66)
F: 8 (53.34) |
Arm 1: 2 mA- active-tDCS + meditation
Arm 2: 1 mA- active-tDCS + meditation Arm 3: sham-tDCS + meditation Concomitant interventions |
| Clarke et al. 2020 [18] | Australia | Randomized, single-blinded, 2×2 factorial design | 95 | Healthy | 22.13 ± 6.23 |
M: 22 (23.16)
F: 73 (76.84) |
Arm 1: active tDCS+ Mindful-focus
Arm 2: active tDCS+ Mindful-wandering Arm 3: sham tDCS+ Mindful-focus Arm 4: sham tDCS+ Mind-wandering Concomitant interventions |
| Chin-Lu Hung et al. 2019 [19] | Taiwan | Randomized, 3-arm parallel trial | 26 | Treatment resistant unipolar depression | 47.85 ± 10.5 |
M: 8 (30.8)
F: 18 (69.2) |
Arm 1: active tDCS + mindfulness
Arm 2: active tDCS Arm 3: sham tDCS Not concomitant to or immediately after stimulation |
| Hunter et al. 2018 [21] | USA | Randomized, double-blinded, 2-arm parallel trial | 29 | Healthy | 27.59 ± 5.7 |
M: 18 (62.06)
F: 11 (37.94) |
Arm1: active tDCS+ Mindfulness base meditation
Arm2: sham-tDCS + sham mindfulness-based meditation Concomitant interventions |
| Monnart et al. 2019 [22] | Belgium | Randomized, open-label, 2-arm parallel design | 31 | Treatment resistant Major depressive disorder | 50.16 ± 6.79 |
M: 11 (35.48)
F: 20 (64,52) |
Arm 1: active tDCS + mindfulness-based cognitive therapy (MBCT)
Arm 2: active tDCS + relaxation MBT applied immediately after stimulation |
| Robinson et al. 2017 [23] | USA | Randomized, double-blinded, 2×2×2 factorial design | 87 | Healthy | 20.16 ± 4.34 |
M: 24 (27.58)
F: 63 (72.42) |
Arm 1: active tDCS + left dlPFC + loving kindness meditation
Arm 2: active tDCS + left dlPFC + sham meditation Arm 3: active tDCS + right TPJ + loving kindness meditation Arm 4: active tDCS + right TPJ + sham meditation Arm 5: sham-tDCS + left dlPFC + loving kindness meditation Arm 6: sham-tDCS + left dlPFC + sham meditation Arm7: sham-tDCS + right TPJ + loving-kindness meditation Arm 8: sham-tDCS + right TPJ + sham meditation Concomitant interventions |
| Witkiewitz et al. 2019 [24] | USA | Randomized, double-blinded, 2-arm parallel trial | 84 | Alcohol use disorder | 52.27 ± 13 |
M: 50 (59.5)
F: 34 (40.5) |
Arm 1: active tDCS+ guided meditation + Mindfulness-based relapse prevention (MBRP)
Arm 2: sham tDCS+ guided meditation + MBRP Concomitant interventions |
| Hypnotherapy | |||||||
| Beltran Serrano et al. (2020) [26] | Brazil | Randomized, double-blinded, washout period of 7 days | 48 | Healthy | 26.03 ± 7.251 |
M: 0 (0%)
F: 48 (100%) |
Arm 1: Active tDCS
Arm 2: Hypnotic Analgesia Suggestion Arm 3: Active tDCS + Hypnotic Analgesia Suggestion Arm 4: Sham tDCS + Hypnotic Analgesia Suggestion |
| Biofeedback | |||||||
| Guleken et al. 2020 [20] | Turkey | Randomized, single-blinded, 2-arm parallel trial | 16 | Healthy | 21.21 ± 5.76 |
M:12 (75)
F: 4 (25) |
Arm 1: active tDCS+ neurofeedback (NFB)
Arm 2: Neurofeedback MBT applied immediately after stimulation |
OA= osteoarthritis; M= male; F= Female, dlPFC= dorsolateral prefrontal cortex; TPJ= temporoparietal junction;
This mean and SD were calculated based on the information provided in the article regarding the arm 1 and arm 3 in the first trial. No information regarding the age of Arm 2 nor 4.
Table 2:
TDCS and Mind-Body therapies parameters
| Author, Year | tDCS (1) Timing with respect to mind-body intervention, (2) Polarization of the active electrode, (3) Active electrode location, (4) Reference electrode Location, (5) Current intensity, (6) Duration, (7) Number of sessions, (8) Frequency of sessions per week, (9) Surface area, (10) Use of Sham (11) Setting, (12) Current density. |
Mind-body interventions Order: (1) Duration, (2) Number of sessions (3) Brief description, (4) use of sham, (5) Extra assignments. |
|---|---|---|
| Ahn et al. 2019 [16] | Concurrent, anodal, M1 contralateral to affected knee, Supraorbital ipsilateral to affected knee, 2mA, 20min, 10 sessions, 5 times per week, 35cm2, yes, home-based, 0.571A/m2 | 20 min, 10 sessions, participants listened to a meditation instructional CD with mindfulness-based instructions, yes (the sham meditation consisted in relaxation and breathing techniques without mindfulness-based instructions), no |
| Badran et al. 2017 [17] | Concurrent, anodal, EEG F8*, left supraorbital, 1 or 2mA, 20min, 3 sessions, 1 time per week, 25.8cm2, yes, outpatient, 0.775A/m2 | 20 min, 3 sessions, participants listened to a meditation instructional CD, no sham, no |
| Beltran Serrano et al. 2020 [26] | Concurrent, anodal, EEG F3*, EEG F4*, 2mA, 20min, 1 session, -, 25 cm2, yes, outpatient, 0.8A/m2 | 20 min; not reported; Guided by a licensed psychologist the standard hypnotic protocol begins with an induction where the subjects focus their attention on a single stimulus and associate this with breathing and relaxation. Through the 8 final minutes of the induction, suggestions are used to reduce the pain of the participants and increase control over their own sensations, no. |
| Clarke et al. 2020 [18] | Concurrent, anodal, left dlPFC, left superior trapezius muscle, 2mA, 20min, 1 session, -, 30cm2, yes, outpatient, 0.667A/m2 | 14 min, 1 session, Participants listened to a guided body-scan meditation recording, yes (the sham was mind wandering), no |
| Chin-Lu Hung et al. 2019 [19] | Different day from the mindfulness training session, anodal, EEG F3, EEG F4, 2mA, 30min, 12 sessions, 2 per week, -, -, yes, outpatient, - | 60 min, 6 sessions, one-to-one mindfulness training, no, after each session participants had homework assignments containing mindfulness practices for 20 minutes and mindful daily activities such as eating and walking. |
| Guleken et al. 2020 [20] | Immediately before the NFB, anodal, Right Primary Motor cortex (EEG C4), Left Mastoid region, 2mA, 10min, 10 sessions, 2 times per week, 35cm2, no, outpatient, 0.571A/m2 | 30 min, 10 sessions, Participants played a game that was controlled by biofeedback parameters, no, no |
| Hunter et al. 2018 [21] | Concurrent, anodal, Right Inferior Gyrus (EEG F10), Contralateral Biceps, 2mA, 30min, 8 sessions, 2 times per week, 11 cm2, yes, outpatient, 1.818A/m2 | 30 min, 20 session/8 session concurrently with tDCS, Participants listened to a guided mindfulness meditation (they could choose between focus -attention or open monitoring practices) no, there was a weekly voluntary 50-minute mindfulness webinar which allowed participants to ask questions and provide feedback about their practice |
| Monnart et al. 2019 [22] | Immediately before the Mind-body intervention, anodal, left DLPFC (F3), right PFDLC (F4), 2mA, 20min, 9 sessions, -, -, no, outpatient, - | 120 min, 9 sessions, MBCT session, yes (30 min Jacobson relaxation session), no |
| Robinson et al. 2017 [23] | Concurrent, anodal, Left dlPFC (F3) or Right TPJ (CP6), Contralateral tricep, 2mA, 30min, 1 session, -, 25cm2, yes, outpatient, 0.8A/m2 | 30 min, 1 session, Participants listened to a recording of guided loving-kindness meditation (Guided Loving Kindness (Metta) Meditation with Sharon Salzberg), yes (recording Reflection for Resilience and Stress Busters), no |
| Witkiewitz et al. 2019 [24] | Concurrent, anodal, right IFG (EEG F10), left upper arm, 2mA, 30min, 8 sessions, 1 time per week, 15cm2, yes, outpatient, 1.333A/m2. | 120 min, 8 sessions, 30 minutes of guided meditation followed by 90 minutes of Mindfulness-Based relapse prevention (discussions of mindfulness and session-specific mindfulness practices), no, Participants were given audio format files (mp3s or CDs) of guided meditation practices developed specifically for MBRP for practice outside of sessions. |
According to the EEG 10–20 international systems; MBCT= mindfulness-based cognitive therapy; dlPFC= dorsolateral prefrontal cortex; TPJ= temporoparietal junction
Tables summarizing the calculated within- and between- effect sizes are available on Appendix 2, according to different categories of outcome: pain (Table S1), cognitive (Table S2), addiction (Table S3), mindfulness state & emotional intelligence outcomes (Table S4), psychological (Table S5). Regarding safety, there was no report of important adverse events, although Witkiewitz et al. described having patients that dropped out of the study due to not tolerating sensations of tDCS. Only three out of ten trials collected mechanistic data, i.e., electroencephalogram (EEG) patterns (Hunter et al. and Guleken et al.) and quantitative sensory testing (QST) measures (Ahn et al.). The overall risk of bias ranged from moderate to high (See Appendix 3).
3.3. Combination with mindfulness
Eight out of ten trials combined tDCS with meditation/mindfulness. Due to the lack of consensus regarding its definition, we accepted the definitions stablished by the authors themselves. Mindfulness and meditation have been used interchangeably on several occasions, although they are subtle different terms. Mindfulness means a non-judgmental awareness to the present moment, while meditation is a mindfulness technique that consists in formal practice of self-regulated attention to enhance awareness of ourselves and the environment. [43–45] However, the consensual definition of mindfulness/meditation practice is not defined, and there are no conventional treatment guidelines available,[46] allowing multiple technique variants to be implemented. In this review, none of the eight articles that studied meditation therapies used the same protocol. They varied in terminology, number and duration of sessions, and administration mode (self or group guided). The number of tDCS sessions ranged from one to twelve, with an average of 6.5 sessions. Most of the sample populations were healthy individuals (4/8), but the combination was also tested on treatment-resistant depressive disorder (2/8) and chronic knee OA (1/10). Significant between-group differences were found in mindfulness associated with tDCS in pain related outcomes, such as pain scales [Numeric Rating Scale for pain (NRS), Western Ontario and McMaster Universities Osteoarthritis index (WOMAC)], and quantitative sensory testing (pain thresholds and conditioned pain modulation) in knee OA population; l the Digit Span Forward measure in patients with treatment-resistant major depressive disorder; and specifically in the inhibitory control measured by the Stop Signal Test in alcohol users.
3.3.1. Methodological insights
Most studies did not specify how the training was performed or accounted for the therapy training or previous experience effects. While most of the trials were performed in healthy individuals, only Hunter et al.[21] screened subjects considering their previous experience with meditation. Not only it might be harder to detect improvements in healthy individuals, but there seems to be structural and functional connectivity differences between the brains of experienced meditators and the average population.[8, 47–49] Selecting non-naïve individuals might increase even more the likelihood of a ceiling effect, especially if studying outcomes such as mindfulness state, given that we would expect these subjects to reach the ceil of improvement more easily.
On top of that, considering that experienced meditators don’t need as much instruction to reach a mindfulness state as would a naïve meditator, it might also be that they were benefited from sham meditation in control groups, jeopardizing the detection of between-group differences. It is crucial that trials applying MBT interventions have at least a reasonable level of uniformed practice between groups. What adds concern is that most of the trials did not report the MBT training strategy or its timeline, when we expect these skills to be laborious to develop (e.g., reaching a mindfulness state).
3.4. Combination with hypnotherapy
Hypnotherapy is the psychotherapeutic use of the hypnotic induction to achieve better clinical outcomes.[50] Hypnosis is defined by the American Psychological Association (APA) as a state of consciousness with a focused attention, reduction of peripheral awareness, and an increased response to suggestion.[51] The only study on this review assessing the combination of hypnotherapy and tDCS was Beltran Serrano et al.[26] The study compared four different healthy groups: 1) active tDCS; 2) hypnotic suggestion (HS); 3) active tDCS and HS; and 4) sham tDCS and HS. The authors identified within group differences some pain outcomes for the hypnotherapy and sham tDCS group on the heat pain threshold and on the cold pressor thresholds, and in the hypnotherapy and active tDCS, only differences on the heat pain threshold.
3.4.1. Methodological insights
Hypnotherapy effects, on the contrary of meditation’s, don’t seem to be related to the amount of practice done by the subject, but rather to his/her inner susceptibility. Interestingly, research in the field has far identified that there is variability in the competence to respond to hypnotic induction and/or suggestion between different individuals. Currently, there are scales to identify different hypnotizability levels, which have been associated to different EEG and neuroimaging metrics,[52–54] and clinical response.[55] The study of Beltran Serrano et al. did apply a screening hypnotizability scale and chose a cut-off point to select individuals according to their capability to respond to hypnotic suggestion. Although much still is discussed over the applicability of these scales, in this matter there seems to be an advantage for hypnotherapy research.
3.5. Combination with biofeedback
Biofeedback was defined as a loop intervention that involves the measurement of a physiological parameter and its transformation into an auditory and/or visual signal – monitored with the purpose of teaching the patient how to modify it.[56] In accordance, motor imagery, visual mirror feedback, and brain-computer interface were not considered biofeedback interventions in our review, and only one study of biofeedback plus tDCS was found. Guleken et al. conducted a single-blinded, randomized trial with 16 healthy young individuals to analyze the supportive effects of tDCS when added to neurofeedback (NFB) in cognitive outcomes of selective attention, response time and suppression. The participants were assigned to either neurofeedback (i.e., electroencephalography feedback through visual representations on a screen) or neurofeedback plus tDCS, with no full control group. The calculated averaged effect sizes are reported in Table S2 and show significant within-group differences but no between-group differences on Continuous Performance Task (CPT) sub-items. However, the authors reported that the EEG frequency bands between the NFB plus tDCS group when compared to only NFB, had a statistically significant increase in alpha(F(3.21)= 3.807, p=0.025) beta2(F(3.21)= 3.570, P=0.031) and theta/beta(F(3.21)=4.270,P0.017).
3.5.1. Methodological insights
Guleken et al. [20] did not report addressing for the effects of training practice. Again, techniques such as interpreting physiological surrogates in biofeedback can be laborious to develop. It is important to consider that using biofeedback techniques in a trial usually requires the use of complex devices that can be unintuitive to the participant. Therefore, it is necessary to consider the exposure of each participant to similar devices and software, since the familiarity to the technique can predict a better performance. For this to be addressed, it is necessary a training phase, where every individual must undergo a few practice sessions with a brief test in the end to assure that everyone starts the intervention in the same level of expertise. These test scores could be used to adjust the analysis to level of expertise at baseline.
3.6. MBT combined with tDCS: a growing field.
The prevalence of chronic pain and psychiatry conditions (i.e., major depressive disorder, anxiety disorders) are escalating globally, as well as the incidence of opioid and antianxiety drugs dependence.[57, 58] As supported by the findings of this search, we consider the combination of MBT and tDCS promising as a safe, non-addicting, and unexpensive alternative for the treatment of neuropsychiatry conditions. Also, based on our search, the first articles combining tDCS with MBTs were published in 2017, and since then, we have observed an exponential increase of publications (Figure 2), with 12 on-going trials. This points to a novel yet growing and expandable field, especially due to the need for more remote treatments to reach rural and underrepresented populations, and to fit the new requirements of post-pandemic world. The combination of MBTs with tDCS in home-based protocols seems feasible, as supported by the feasibility reports of the included study of Ahn et al. and the numerous trials applying home-based biofeedback, meditation, hypnotherapy or tDCS. [59–62] This is largely facilitated by the evolution of portable user-friendly tDCS devices, and health software compatible to smartphones.
Figure 2: Scientific production of mind-body therapies and tDCS combination.
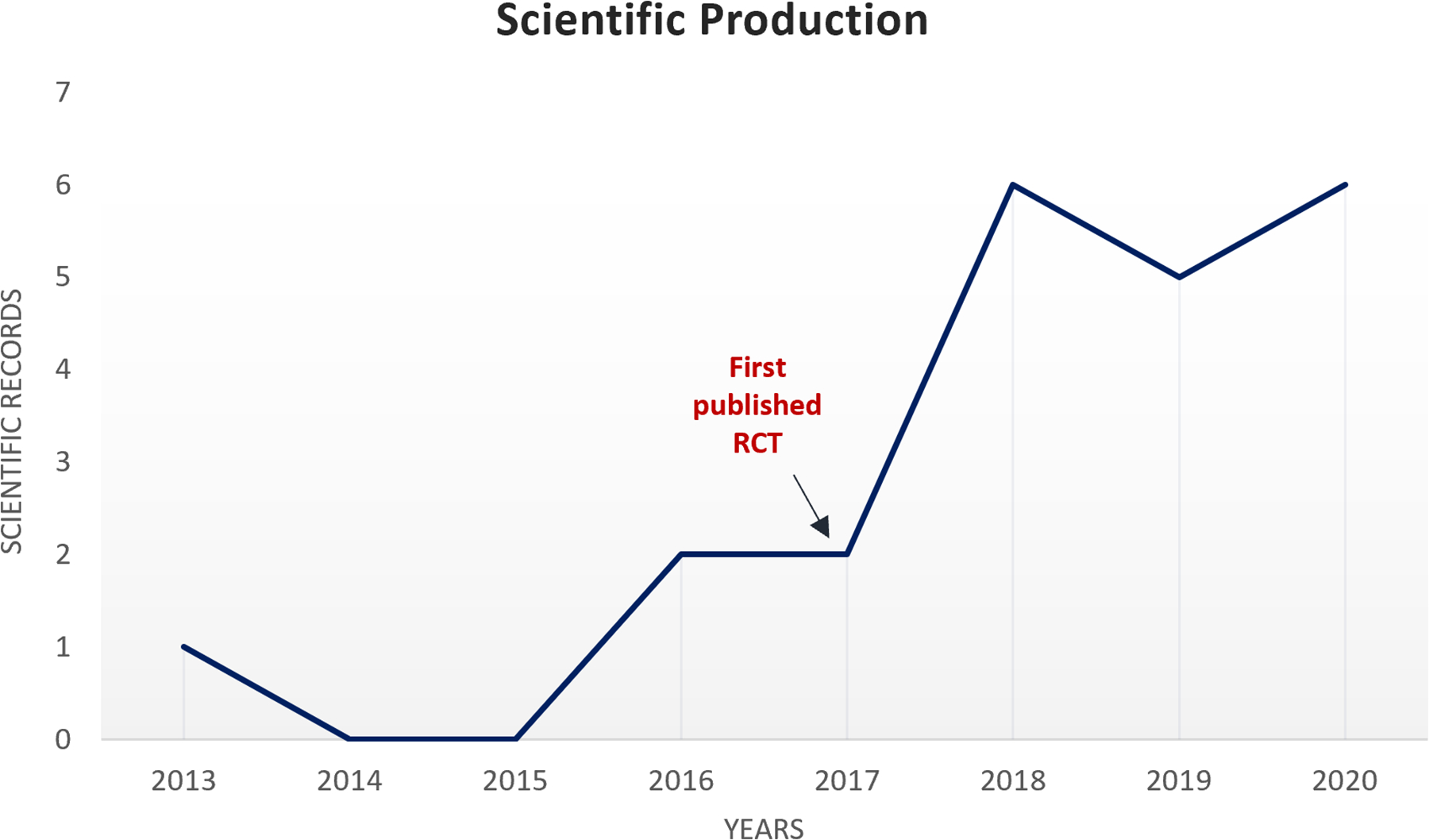
This graph represents the scientific records to date related to the combination of tDCS and mind-body therapies, including ongoing clinical trials and published articles.
4. Discussion
The combination of tDCS with MBTs is feasible and safe, with mindfulness apparently being the most feasible adjunct therapy. Most likely that is due to its low cost, easy implementation (guided recordings, no need for an in-person therapist), and increased evidence in the literature compared to other MBTs.[46] Nonetheless, the lack of studies on therapies such as tai-chi, qigong and acupuncture may be partially due to high costs related to the need of personalized guidance and moderate infrastructure (i.e. materials and equipped spaces). The concomitant association of tDCS with MBTs that demand physical movement (e.g., tai-chi, qigong) requires higher complexity.
However, the data for combined tDCS and MBTs – even for mindfulness/meditation – come mostly from pilot and proof-of-concept studies testing clinical outcomes in healthy subjects, still far from impacting real clinical practice. Assessments of neurophysiological correlates (including multimodal neuroimaging [EEG and fMRI], autonomic response [heart rate variability], and cortical excitability studies with TMS) are still scarce and certainly missed. These surrogates are essential to better explore MBTs’ mechanisms of actions and neural signatures. Therefore, there is a need for more mechanistic trials in the field. Data from mechanistic trials could help the development of biomarkers or monitoring tools for the subjects’ state of engagement to the MBT and thus allow for it be controlled for in the analysis. Furthermore, they could serve to the purpose of identifying different patterns of susceptibility of response across different subjects, and help foster more individually-tailored approaches. In fact, researchers should build prediction models of response, including both clinical and physiological covariates, to identify the subset of individuals that benefit the most from the combined therapy and the factors associated to it. In that matter, the rationale for sample selection should also be revisited. If not added as sample selection criteria, the previous experience with the MBT needs to be accounted for in the design or in the analysis, especially for studies combining tDCS with meditation/mindfulness.
Also, mechanistic data will serve to the development of optimized targeted protocols with tDCS, since a fully comprehension of the physiological framework that underlie the MBT is essential to propose any mechanism of synergism with tDCS. This is a brain stimulation technique that does not induce action potentials, rather it changes the neuronal membranes’ excitability and modulates circuits’ response to different stimuli. Thus, a spatial correlation between the tDCS target area (electrodes placement) and brain circuits primed by the MBT in question is needed.[63, 64] For this reason, we suggest categorizing the MBTs in cognitive/emotional oriented (e.g., meditation/mindfulness, biofeedback, hypnosis) and sensory-motor oriented MBTs (e.g., yoga, qigong, tai-chi), based on the hypothesis that certain MBTs may activate similar neural networks according to the predominant tasks associated with their practice. Cognitive/emotional-oriented MBTs, such as meditation[65] and hypnosis,[66] might use neural networks associated with emotional and executive processing such as prefrontal networks. Therefore, the association of cognitive/emotional oriented MBTs with tDCS may strengthen the modulation of its respective networks. Sensory-motor oriented MBTs would involve body movement (such as yoga, qigong, tai chi) and movement representation techniques (such as motor imagery), and we could infer that they would activate predominantly areas that are also activated during the specific exercises, as the primary motor cortex (M1) and supplementary motor areas.[67] Therefore, the previously mentioned areas could be used to explore the best location for the tDCS stimulation when combined with MBTs.
It is also essential that the trials optimize the number of tDCS sessions and follow current evidence on the montage and current density for each health condition. For example, we found an average of 6.3 tDCS sessions overall, lower than those used in previous positive trials, ranging from ten to even 60.[68–70] It has been suggested that an extended number of tDCS sessions is fundamental to induce long-lasting neuroplasticity.[64, 71] In fact, findings from a dose-response RCT suggest that a minimum of 15 daily sessions is needed to achieve a clinically meaningful result (50% decrease in reported pain) in patients with fibromyalgia using high definition tDCS.[72] To compare, only three out of ten trials performed ≥10 tDCS sessions (Ahn et al. 2019, Guleken et al. 2020, Galen et al. 2019). However, many studies were feasibility pilot trials, as the state of evidence at this point requires.
A final suggestion for future researchers is that the study design should consider a further exploration of main and combined effects. Therefore, to (i) explore the potential placebo effect; (ii) detangle main effects; and (iii) investigate synergistic or additive effects of the interventions, the ideal is to use a full-factorial design, which should be considered parallel to feasibility issues of conducting such trials.
5. Conclusion
The combination of tDCS and MBTs is a growing field that needs further research with larger sample sizes, standardized, evidence-based tDCS and MBTs protocols, and better study reporting. We hope this review will provide future authors with information to facilitate the development of improved trials’ protocols.
Supplementary Material
Key Messages:
The MBT most commonly tested in combination with tDCS is mindfulness/meditation, followed by hypnosis and biofeedback. This is likely due to feasibility matters in comparison to MBTs that require physical movements and moderate infrastructure.
This field of research is promising as it complies with the world’s trend towards the development of home-based, non-pharmacological, non-additive treatments for neuropsychiatry disorders, and this is evidenced by the increased number of both published and on-going clinical trials testing this combination since 2017.
There is a need for more mechanistic data to fully comprehend the mechanisms of action and patterns of response susceptibility to the MBT as well as to the combined synergic/additive effects with tDCS. A full-factorial design is thus ideal to detangle the effects.
Source(s) of support:
This work was supported by National Center for Complementary and Alternative Medicine (R01 AT009491–01A1)
Footnotes
Presentation at a meeting: No previous presentation.
Conflicting Interest: The authors report no conflicts of interest.
References:
- 1.Wahbeh H, Elsas S-M, Oken BS. Mind-body interventions: applications in neurology. Neurology. 2008;70(24):2321–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barrós-Loscertales A, Hernández SE, Xiao Y, González-Mora JL, Rubia K. Resting State Functional Connectivity Associated With Sahaja Yoga Meditation. Frontiers in Human Neuroscience. 2021;15(65). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Doll A, Hölzel BK, Boucard CC, Wohlschläger AM, Sorg C. Mindfulness is associated with intrinsic functional connectivity between default mode and salience networks. Frontiers in Human Neuroscience. 2015;9(461). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jiang H, White MP, Greicius MD, Waelde LC, Spiegel D. Brain Activity and Functional Connectivity Associated with Hypnosis. Cerebral Cortex. 2016;27(8):4083–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kilpatrick LA, Suyenobu BY, Smith SR, Bueller JA, Goodman T, Creswell JD, et al. Impact of mindfulness-based stress reduction training on intrinsic brain connectivity. NeuroImage. 2011;56(1):290–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kral TRA, Imhoff-Smith T, Dean DC III, Grupe D, Adluru N, Patsenko E, et al. Mindfulness-Based Stress Reduction-related changes in posterior cingulate resting brain connectivity. Social Cognitive and Affective Neuroscience. 2019;14(7):777–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tao J, Chen X, Egorova N, Liu J, Xue X, Wang Q, et al. Tai Chi Chuan and Baduanjin practice modulates functional connectivity of the cognitive control network in older adults. Scientific reports. 2017;7(1):41581-. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.van Lutterveld R, van Dellen E, Pal P, Yang H, Stam CJ, Brewer J. Meditation is associated with increased brain network integration. Neuroimage. 2017;158:18–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tang YY, Hölzel BK, Posner MI. The neuroscience of mindfulness meditation. Nat Rev Neurosci. 2015;16(4):213–25. [DOI] [PubMed] [Google Scholar]
- 10.Demertzi A, Soddu A, Faymonville ME, Bahri MA, Gosseries O, Vanhaudenhuyse A, et al. Hypnotic modulation of resting state fMRI default mode and extrinsic network connectivity. Prog Brain Res. 2011;193:309–22. [DOI] [PubMed] [Google Scholar]
- 11.Bikson M, Grossman P, Thomas C, Zannou AL, Jiang J, Adnan T, et al. Safety of Transcranial Direct Current Stimulation: Evidence Based Update 2016. Brain Stimul. 2016;9(5):641–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li LM, Violante IR, Leech R, Ross E, Hampshire A, Opitz A, et al. Brain state and polarity dependent modulation of brain networks by transcranial direct current stimulation. Hum Brain Mapp. 2019;40(3):904–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cardenas-Rojas A, Pacheco-Barrios K, Giannoni-Luza S, Rivera-Torrejon O, Fregni F. Noninvasive brain stimulation combined with exercise in chronic pain: a systematic review and meta-analysis. Expert review of neurotherapeutics. 2020;20(4):401–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stagg CJ, Antal A, Nitsche MA. Physiology of transcranial direct current stimulation. The journal of ECT. 2018;34(3):144–52. [DOI] [PubMed] [Google Scholar]
- 15.Demertzi A, Soddu A, Faymonville ME, Bahri MA, Gosseries O, Vanhaudenhuyse A, et al. Hypnotic modulation of resting state fMRI default mode and extrinsic network connectivity. Progress in Brain Research. 2011;193:309–22. [DOI] [PubMed] [Google Scholar]
- 16.Ahn H, Zhong C, Miao H, Chaoul A, Park L, Yen IH, et al. Efficacy of combining home-based transcranial direct current stimulation with mindfulness-based meditation for pain in older adults with knee osteoarthritis: A randomized controlled pilot study. Journal of clinical neuroscience : official journal of the Neurosurgical Society of Australasia. 2019;70:140–5. [DOI] [PubMed] [Google Scholar]
- 17.Badran BW, Austelle CW, Smith NR, Glusman CE, Froeliger B, Garland EL, et al. A Double-Blind Study Exploring the Use of Transcranial Direct Current Stimulation (tDCS) to Potentially Enhance Mindfulness Meditation (E-Meditation). Brain stimulation. 2017;10(1):152–4. [DOI] [PubMed] [Google Scholar]
- 18.Clarke PJF, Sprlyan BF, Hirsch CR, Meeten F, Notebaert L. tDCS increases anxiety reactivity to intentional worry. Journal of psychiatric research. 2020;120:34–9. [DOI] [PubMed] [Google Scholar]
- 19.Chin-Lun Hung G Proceedings #3: Effects of Combining Transcranial Direct Current Stimulation with Mindfulness Training in Patients with Treatment-Resistant Depression: A Pilot Study. Brain stimulation. 2019;12(2):e59–e60. [Google Scholar]
- 20.Guleken Z, Eskikurt G, Karamürsel S. Investigation of the effects of transcranial direct current stimulation and neurofeedback by continuous performance test. Neuroscience letters. 2020;716:134648-. [DOI] [PubMed] [Google Scholar]
- 21.Hunter MA, Lieberman G, Coffman BA, Trumbo MC, Armenta ML, Robinson CSH, et al. Mindfulness-based training with transcranial direct current stimulation modulates neuronal resource allocation in working memory: A randomized pilot study with a nonequivalent control group. Heliyon. 2018;4(7):e00685–e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Monnart A, Vanderhasselt M-A, Schroder E, Campanella S, Fontaine P, Kornreich C. Treatment of Resistant Depression: A Pilot Study Assessing the Efficacy of a tDCS-Mindfulness Program Compared With a tDCS-Relaxation Program. Frontiers in psychiatry. 2019;10:730-. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Robinson C, Armenta M, Combs A, Lamphere ML, Garza GJ, Neary J, et al. Modulating affective experience and emotional intelligence with loving kindness meditation and transcranial direct current stimulation: A pilot study. Social neuroscience. 2019;14(1):10–25. [DOI] [PubMed] [Google Scholar]
- 24.Witkiewitz K, Stein ER, Votaw VR, Wilson AD, Roos CR, Gallegos SJ, et al. Mindfulness-Based Relapse Prevention and Transcranial Direct Current Stimulation to Reduce Heavy Drinking: A Double-Blind Sham-Controlled Randomized Trial. Alcoholism, clinical and experimental research. 2019;43(6):1296–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pollonini L, Montero-Hernandez S, Park L, Miao H, Mathis K, Ahn H. Functional Near-Infrared Spectroscopy to Assess Central Pain Responses in a Nonpharmacologic Treatment Trial of Osteoarthritis. Journal of neuroimaging. 2020;30(6):808–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Beltran Serrano G, Pooch Rodrigues L, Schein B, Zortea M, Torres ILS, Fregni F, et al. The Hypnotic Analgesia Suggestion Mitigated the Effect of the Transcranial Direct Current Stimulation on the Descending Pain Modulatory System: A Proof of Concept Study. Journal of pain research. 2020;13:2297–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wolsko PM, Eisenberg DM, Davis RB, Phillips RS. Use of mind-body medical therapies. J Gen Intern Med. 2004;19(1):43–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hedges LV. Distribution Theory for Glass’s Estimator of Effect Size and Related Estimators. Journal of Educational Statistics. 1981;6(2):107–28. [Google Scholar]
- 29.Sterne JAC, Savović J, Page MJ, Elbers RG, Blencowe NS, Boutron I, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. Bmj. 2019;366:l4898. [DOI] [PubMed] [Google Scholar]
- 30.Brown DR, Jackson TCJ, Claus ED, Votaw VR, Stein ER, Robinson CSH, et al. Decreases in the Late Positive Potential to Alcohol Images Among Alcohol Treatment Seekers Following Mindfulness-Based Relapse Prevention. Alcohol and Alcoholism. 2019;55(1):78–85. [DOI] [PubMed] [Google Scholar]
- 31.Ahn H. Self Transcranial Direct Current Stimulation and Mindfulness-based Meditation for Pain in Older Adults With Knee Osteoarthritis. clinicaltrials.gov, US National Library of Medicine; 2018. [Google Scholar]
- 32.Ahn H. Combination Therapy of Home-based Transcranial Direct Current Stimulation and Mindfulness-based Meditation for Self-management of Clinical Pain and Symptoms in Older Adults With Knee Osteoarthritis. clinicaltrials.gov, US National Library of Medicine; 2020. [Google Scholar]
- 33.Centre for Addiction and Mental Health. Mindfulness-Based Stress Reduction (MBSR) and Transcranial Direct Current Stimulation (tDCS). clinicaltrials.gov, US National Library of Medicine; 2018. [Google Scholar]
- 34.Conklin Cynthia. Mindfulness + tDCS to Reduce Urgency Incontinence in Women. clinicaltrials.gov, US National Library of Medicine; 2020. [Google Scholar]
- 35.Blumberger Daniel. Maintenance of Response After rTMS for Depression Using tDCS. clinicaltrials.gov, US National Library of Medicine; 2018. [Google Scholar]
- 36.Lenze Eric. Brain Stimulation and Enhancing Cognition in Older Adults. clinicaltrials.gov, US National Library of Medicine; 2018. [Google Scholar]
- 37.Kong Jian. Enhancing Acupuncture Treatment Effect Through Non-invasive Neuromodulation. clinicaltrials.gov, US National Library of Medicine; 2018. [Google Scholar]
- 38.Medical University of South Carolina. A Pilot Study Investigating Transcranial Direct Current Stimulation (tDCS) to Enhance Mindfulness Meditation. clinicaltrials.gov, US National Library of Medicine; 2016. [Google Scholar]
- 39.Freedman Steven. tDCS for the Management of Chronic Visceral Pain in Patients With Chronic Pancreatitis. clinicaltrials.gov, US National Library of Medicine; 2013. [Google Scholar]
- 40.Andrade Suellen Marinho. Transcranial Direct Current Stimulation Associated With Mindfulness in Chronic Migraine. clinicaltrials.gov, US National Library of Medicine; 2020. [Google Scholar]
- 41.Besse-Hammer Tatiana. Study of the Synergistic Effects of Biofeedback and Transcranial Electrical Stimulation in Anxio-depressive Disorders. clinicaltrials.gov, US National Library of Medicine; 2019. [Google Scholar]
- 42.University of New Mexico. Mindfulness-Based Intervention and Transcranial Direct Current Brain Stimulation to Reduce Heavy Drinking. clinicaltrials.gov, US National Library of Medicine; 2016. [Google Scholar]
- 43.Zhang Q, Wang Z, Wang X, Liu L, Zhang J, Zhou R. The Effects of Different Stages of Mindfulness Meditation Training on Emotion Regulation. 2019;13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Behan C The benefits of meditation and mindfulness practices during times of crisis such as COVID-19. Ir J Psychol Med. 2020;37(4):256–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Perez-de-Albeniz A, Holmes J. Meditation: Concepts, effects and uses in therapy. International Journal of Psychotherapy. 2000;5(1):49–58. [Google Scholar]
- 46.Zhang D, Lee EKP, Mak ECW, Ho CY, Wong SYS. Mindfulness-based interventions: an overall review. Br Med Bull. 2021;138(1):41–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Li C, Kee YH, Lam LS. Effect of brief mindfulness induction on university athletes’ sleep quality following night training. Frontiers in psychology. 2018;9:508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Luders E, Kurth F. The neuroanatomy of long-term meditators. Current opinion in psychology. 2019;28:172–8. [DOI] [PubMed] [Google Scholar]
- 49.Fox KCR, Nijeboer S, Dixon ML, Floman JL, Ellamil M, Rumak SP, et al. Is meditation associated with altered brain structure? A systematic review and meta-analysis of morphometric neuroimaging in meditation practitioners. Neuroscience & Biobehavioral Reviews. 2014;43:48–73. [DOI] [PubMed] [Google Scholar]
- 50.Mamoune S, Mener E, Chapron A, Poimboeuf J. Hypnotherapy and insomnia: A narrative review of the literature. Complementary Therapies in Medicine. 2022;65:102805. [DOI] [PubMed] [Google Scholar]
- 51.Elkins GR, Barabasz AF, Council JR, Spiegel D. Advancing research and practice: the revised APA Division 30 definition of hypnosis. The International journal of clinical and experimental hypnosis. 2015;63(1):1–9. [DOI] [PubMed] [Google Scholar]
- 52.McGeown WJ, Mazzoni G, Venneri A, Kirsch I. Hypnotic induction decreases anterior default mode activity. Consciousness and cognition. 2009;18(4):848–55. [DOI] [PubMed] [Google Scholar]
- 53.McGeown WJ, Mazzoni G, Vannucci M, Venneri A. Structural and functional correlates of hypnotic depth and suggestibility. Psychiatry Research: Neuroimaging. 2015;231(2):151–9. [DOI] [PubMed] [Google Scholar]
- 54.Jamieson GA, Burgess AP. Hypnotic induction is followed by state-like changes in the organization of EEG functional connectivity in the theta and beta frequency bands in high-hypnotically susceptible individuals. Frontiers in human neuroscience. 2014;8:528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Milling LS, Coursen EL, Shores JS, Waszkiewicz JA. The predictive utility of hypnotizability: The change in suggestibility produced by hypnosis. Journal of consulting and clinical psychology. 2010;78(1):126. [DOI] [PubMed] [Google Scholar]
- 56.McKee MG. Biofeedback: an overview in the context of heart-brain medicine. Cleve Clin J Med. 2008;75 Suppl 2:S31–4. [DOI] [PubMed] [Google Scholar]
- 57.Vos T, Lim SS, Abbafati C, Abbas KM, Abbasi M, Abbasifard M, et al. Global burden of 369 diseases and injuries in 204 countries and territories, 1990–2019: a systematic analysis for the Global Burden of Disease Study 2019. The Lancet. 2020;396(10258):1204–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Macfarlane GJ. The epidemiology of chronic pain. Pain. 2016;157(10):2158–9. [DOI] [PubMed] [Google Scholar]
- 59.Rutten J, Vlieger AM, Frankenhuis C, George EK, Groeneweg M, Norbruis OF, et al. Home-Based Hypnotherapy Self-exercises vs Individual Hypnotherapy With a Therapist for Treatment of Pediatric Irritable Bowel Syndrome, Functional Abdominal Pain, or Functional Abdominal Pain Syndrome: A Randomized Clinical Trial. JAMA pediatrics. 2017;171(5):470–7. [DOI] [PubMed] [Google Scholar]
- 60.Sakuma Y, Sasaki-Otomaru A, Ishida S, Kanoya Y, Arakawa C, Mochizuki Y, et al. Effect of a home-based simple yoga program in child-care workers: a randomized controlled trial. Journal of alternative and complementary medicine (New York, NY). 2012;18(8):769–76. [DOI] [PubMed] [Google Scholar]
- 61.Rao SSC, Valestin JA, Xiang X, Hamdy S, Bradley CS, Zimmerman MB. Home-based versus office-based biofeedback therapy for constipation with dyssynergic defecation: a randomised controlled trial. The lancet Gastroenterology & hepatology. 2018;3(11):768–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Cappon D, den Boer T, Jordan C, Yu W, Lo A, LaGanke N, et al. Safety and Feasibility of Tele-Supervised Home-Based Transcranial Direct Current Stimulation for Major Depressive Disorder. Frontiers in aging neuroscience. 2021;13:765370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Woods AJ, Antal A, Bikson M, Boggio PS, Brunoni AR, Celnik P, et al. A technical guide to tDCS, and related non-invasive brain stimulation tools. Clinical Neurophysiology. 2016;127(2):1031–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Pacheco-Barrios K, Cardenas-Rojas A, Thibaut A, Costa B, Ferreira I, Caumo W, et al. Methods and strategies of tDCS for the treatment of pain: current status and future directions. Expert Review of Medical Devices. 2020;17(9):879–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Marchand WR. Neural mechanisms of mindfulness and meditation: Evidence from neuroimaging studies. World J Radiol. 2014;6(7):471–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Huber A, Lui F, Porro CA. Hypnotic susceptibility modulates brain activity related to experimental placebo analgesia. Pain. 2013;154(9):1509–18. [DOI] [PubMed] [Google Scholar]
- 67.Wang B, Xiao S, Yu C, Zhou J, Fu W. Effects of Transcranial Direct Current Stimulation Combined With Physical Training on the Excitability of the Motor Cortex, Physical Performance, and Motor Learning: A Systematic Review. Frontiers in Neuroscience. 2021;15(336). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Brietzke AP, Zortea M, Carvalho F, Sanches PRS, Silva DPJ, Torres I, et al. Large Treatment Effect With Extended Home-Based Transcranial Direct Current Stimulation Over Dorsolateral Prefrontal Cortex in Fibromyalgia: A Proof of Concept Sham-Randomized Clinical Study. J Pain. 2020;21(1–2):212–24. [DOI] [PubMed] [Google Scholar]
- 69.Brunoni AR, Valiengo L, Baccaro A, Zanão TA, de Oliveira JF, Goulart A, et al. The Sertraline vs Electrical Current Therapy for Treating Depression Clinical Study: Results From a Factorial, Randomized, Controlled Trial. JAMA Psychiatry. 2013;70(4):383–91. [DOI] [PubMed] [Google Scholar]
- 70.Simis M, Uygur-Kucukseymen E, Pacheco-Barrios K, Battistella LR, Fregni F. Beta-band oscillations as a biomarker of gait recovery in spinal cord injury patients: A quantitative electroencephalography analysis. Clinical Neurophysiology. 2020;131(8):1806–14. [DOI] [PubMed] [Google Scholar]
- 71.Berryhill M Longitudinal tDCS: Consistency across Working Memory Training Studies. AIMS Neuroscience. 2017;4:71–86. [Google Scholar]
- 72.Castillo-Saavedra L, Gebodh N, Bikson M, Diaz-Cruz C, Brandao R, Coutinho L, et al. Clinically effective treatment of fibromyalgia pain with high-definition transcranial direct current stimulation: phase II open-label dose optimization. The Journal of Pain. 2016;17(1):14–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


