Abstract
Objectives:
Coordinated Oral health Promotion (CO-OP) Chicago is a cluster randomized controlled trial testing the efficacy of a community health worker (CHW) intervention to improve tooth brushing in low-income children.
Methods:
420 children under three years old (mean 21.5 months) were recruited from 20 sites in Chicago, IL. Children were identified mainly as Black race (41.9%) or Hispanic ethnicity (53.8%) and most (85.2%) had Medicaid. Intervention families were offered four CHW home visits over one year. Brushing frequency was self-reported. Plaque score was determined from images collected in homes using disclosing solution. Analyses used GEE logistic models with variable selection at p<0.05.
Results:
At enrollment, 45.0% of families reported twice a day or more child brushing frequency, and child plaque scores were poor (mean of 1.9, SD 0.6). Data were obtained from 87.1% of children at 6-months and 86.2% at 12-months. In the CHW intervention arm (ten sites, N=211), 23.7% received 4 visits, 12.8% 3 visits, 21.3% 2 visits, 23.2% 1 visit, and 19% no visits from CHWs. No intervention effect was seen for brushing frequency or plaque score. Child brushing frequency improvement over time was associated with a range of child and caregiver factors. The only factor associated with a change in plaque score over time was parent involvement in brushing.
Conclusions:
Oral-health specific CHW services were not associated with improved brushing behaviors in these young children. However, caregiver involvement with brushing supported more quality brushing. More robust interventions are needed to support families during this critical developmental period.
Introduction
Despite expanded dental coverage for children in the United States, caries remains the most common chronic disease of childhood.1 Young children who develop severe disease often require treatment under sedation or general anesthesia.2 The cost of childhood caries includes the social cost of pain and adverse effects on cognitive development, school absenteeism, caregiver missed work, and lower oral health-related quality of life.3 Furthermore, these burdens continue over the life-course and are intergenerational.4,5
Caries disproportionately affects children from disadvantaged subgroups, with documented disparities in dental care utilization, access to healthy food choices, food security, oral health literacy, and exposure to high-risk behaviors.6–8 Therefore, addressing caries risk requires multi-level interventions that recognize threats to effective oral health behaviors on not only the individual/family level, but also the organization, community, and public health levels.9
Caries risk begins before birth, and early childhood is a time when formative patterns of behavior and beliefs are established.10 However, research to date on children under the age of three years old is limited. The Coordinated Oral Health Promotion (CO-OP) Chicago study was funded by the National Institute for Dental and Craniofacial Research (NIDCR) as part of a consortium to develop and test behavioral interventions to address oral health disparities in young children.11 The CO-OP Chicago intervention was based on Social Ecological Theory.12–13 The community health worker (CHW) intervention operated on the individual level with children (addressing knowledge and behaviors), the family level with their caregivers (addressing knowledge, beliefs, and behaviors through social modeling, social network engagement, and self-management skills support), and the organizational level with local social service agencies and medical clinics (addressing access and oral health messaging and social norms).11 The CHW intervention delivery was guided by Social Cognitive Theory.14 Behavior is shaped and maintained by consequences, particularly by immediate feedback from both objective sources (such as observation of brushing technique) and an individual’s social network (beliefs and traditions of family and friends). The oral health CHW intervention measured and supported these behaviors and social networks using self-management skills.11 Although a well-defined behavioral and social intervention in other disease areas, the ability of CHWs to improve oral health outcomes had not been rigorously tested in the United States.15–19
This study describes the primary outcomes of the CO-OP Chicago cluster-randomized trial. The trial tested if a multi-level oral health CHW intervention was associated with improved tooth brushing in low-income urban young children. The cluster design was chosen because a component of the intervention occurred at the site level, although outcomes were measured at the individual level. The primary oral health behavior for this age group was operationalized as tooth brushing frequency and quality because twice daily tooth brushing with fluoridated toothpaste is a cost-effective way to reduce caries.20 The hypothesis was that children offered oral heath CHW intervention in their homes and communities would brush more frequently and have less plaque than children not offered a CHW intervention.
Methods
See the Supplemental Protocol File for additional protocol details.
Design, Recruitment, and Randomization
The CO-OP Chicago Trial is a two-arm, cluster-randomized controlled trial with repeated measurements.11 Participants (420 child/caregiver dyads) were recruited from ten Special Supplemental Nutrition Program for Women, Infants, and Children (WIC) centers and ten pediatric medical clinics in the Chicago metro area (20 clusters total) from January 2018 to February 2019 (Figure 1). Active patients aged 6–36 months in one of these centers/clinics with at least two fully erupted primary maxillary incisors were included. The caregivers had to be 18 or older, speak English or Spanish, and be a primary caregiver living with the child at least five days out of the week. Children with medical conditions that would limit their ability to conduct trial activities were excluded. Matched randomization of clusters was conducted by the Coordinating Center prior to the start of recruitment. Once sites reached 90% of their recruitment goal, group allocation was revealed to specific unblinded CO-OP Chicago team members, who then informed the sites and participants. Cluster-level covariates included in randomization were race/ethnicity of the site population (Black, Hispanic, or White), site size (large, medium, small), and setting (WIC or clinic).11 All trial staff not working directly with CHWs were blind to study arm; participants and sites could not be blinded.
Figure 1:
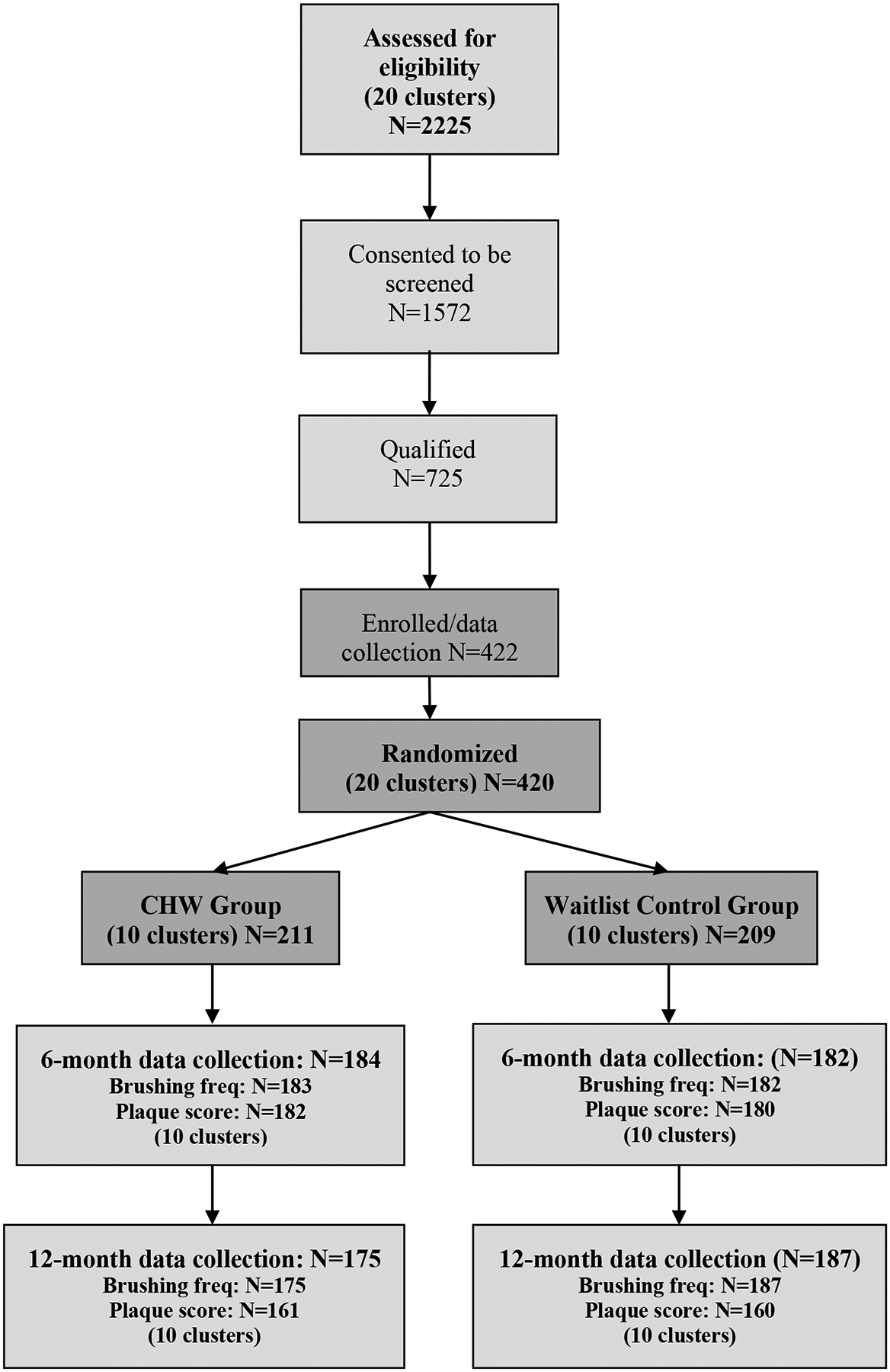
CO-OP Chicago Consort Diagram
Outcomes
The primary outcomes were tooth brushing frequency and plaque score; child dental visits were a secondary outcome. Participant-level data were collected by research assistants (RAs) at baseline, 6-, and 12-months post-randomization using a structured protocol that included caregiver self-report, clinical assessment, and observation. Data collection in homes was preferred, but families could choose other locations. RAs began by verbally asking caregivers about caregiver and child demographics, brushing frequency, previous dental visit frequency and purpose, insurance, and quality of life; child water source and sugary beverage and food consumption; and caregiver general oral health knowledge, social support, and psychosocial stressors.11 RAs then applied a plaque disclosing solution to the children’s primary maxillary incisors, photographing the teeth before and after application. Disclosed images were asynchronously scored by calibrated clinicians using the Oral Hygiene Index – Maxillary Incisor Simplified (OHI-MIS).21 The training and calibration associated with the plaque image process are described in the Supplemental Protocol File. Then RAs asked families to demonstrate how the children’s teeth are brushed; the observed process, technique, equipment used, and length of brushing activity were recorded. Caregivers received $40 after completion of each data collection visit.
Intervention
Details of the CHW intervention have been published previously.11,22 CHWs were hired to match the target population on educational background, race, ethnicity, and language skills. They were trained in oral health education, motivational interviewing, care coordination, mental health first aid, resource navigation, and self-management skills development. The family-level CHW intervention consisted of four in-person visits that were followed 1–2 weeks later by phone follow-ups. Families were offered two visits/calls during months 0–6, and two visits/calls during months 7–12. CHWs administered the non-clinical portion of the Caries-risk Assessment Tool23 first to explore family needs. CHWs then covered oral health topics that included oral health basics (what are caries, causes, prevention), tooth brushing (when to start, frequency, technique, equipment, toothpaste), fluoride (water, other sources), weaning (when, sippy cups, nighttime feeds), nutrition (sugar-sweetened beverages, juice, healthy foods), and seeing the dentist (when, what to expect, role of pediatricians).26 CHWs used motivational interviewing and employed formal self-management skills, including problem-solving, self-discovery, and action planning. The timing and amount of focus on topics varied by family needs and interest. The CHWs also discussed non-oral health topics that arose, such as insurance, immigration, and childcare. CHWs ended visits with an action planning exercise. The site-level CHW intervention consisted of CHWs providing brief general oral health education for several hours weekly to anyone interested in the waiting areas of the clinics and WIC centers randomized to CHW intervention. CHW interventions included no monetary incentives.
Wait-list Control
The control group received usual care during the trial. After final data collection, control group families and sites were offered a CHW intervention.
Participants
Institutional Review Boards at the University of Illinois Chicago [2017–1090], University of California San Francisco [16–19920], and Chicago Department of Public Health [16–06] approved the trial. Caregivers provided written informed consent prior to randomization. Sites did not require consent. Trial oversight was also provided by a Data Safety Monitoring Board, an external monitor reporting to the funder, and a Community Advisory Board.
Analyses
Power was intended to detect an effect size of an odds ratio (OR) of 2.0 for frequency of brushing and 0.40 for plaque score.11 Allowing for repeated observations and 15% attrition at 12-months, 21 participants from each of the 20 sites (clusters) equaled a sample size of 420 with 80% power. This calculation controlled for the multiplicity of having two outcome measures by Bonferroni adjustment (Type 1 error = 0.025).
Missing data was limited, with missingness for the outcome variables between 9% - 13%. In order to perform intention-to-treat (ITT) analysis, multiple imputation was performed to allow all participants and observations to be included in the final models. For categorical outcomes, chained equations were used to allow flexibility.24 For numeric values, linear regression was used while ordered logit latent variable models were used for categorical variables. The variables used for stratifying clusters before randomization (type of site, site size, and site majority race/ethnicity) were included in all models. To account for within-site clustering, variables that differed by site11 were included in all models: child age, length of time since child’s last dental visit, caregiver highest degree, caregiver race/ethnicity, and caregiver health insurance presence/source. Finally, group, time and group-by-time interaction were included in all models. To avoid multicollinearity, variables of interest that were highly intercorrelated (Spearman’s rho > 0.4) were excluded. Backward elimination (significance at p<0.05) was then used to reduce the number of additional covariates from the remaining 23 to those included in the final models.
Brushing frequency was collapsed into a dichotomous variable (less than 2x/day versus 2x/day or more) and was analyzed using logistic regression with generalized estimating equations (GEE) to account for the repeated measures. Brushing frequency intra-class correlation (ICC) was estimated using Laplace (approximate maximum likelihood) using variance components for the type of the variance-covariance G matrix. ICC on the child level was 0.53, on the site level was 0.00.
Plaque score was treated as a continuous variable and modeled using a linear mixed model (LMM) to account for the repeated measures. Using restricted maximum likelihood, the ICC for plaque score on the child level was 0.26 and the site level was 0.03.
As a secondary analysis, child dental utilization was collapsed into a dichotomous variable (in the last six months versus not in the last six months) and analyzed in the same way as brushing frequency.
All data analyses were performed using SAS/STAT Version 9.4 (Cary, NC, USA).
Results
Recruitment ran from January 20, 2018, to February 23, 2019; data collection continued for one year after the date of enrollment. (Data collection officially ended August 20, 2020, after an extension granted due to the COVID-19 pandemic to collect data from the final 34 participants.) At enrollment (Table 1),11 the mean child age was 21.5 (SD 6.9) months, with a range of 9 to 35 months. Forty-five percent of caregivers reported brushing their children’s teeth the recommended twice a day or more. The mean OHI-MIS plaque score was 1.9 (SD 0.6), indicating a “high” level of plaque.25 Over half of children (59.7%) had never been to a dentist.
Table 1:
Baseline Demographics and Oral Health Behaviors11
| Usual Care Group, N=209 | CHW Group N=211 |
Total N=420 |
|
|---|---|---|---|
| Caregiver female (%) | 197 (94.3) | 208 (98.6) | 405 (96.4) |
| Caregiver age (years), mean (SD) | 29.7 (6.3) | 29.5 (6.9) | 29.6 (6.6) |
| Caregiver highest degree earned (%) | |||
| Less than high school | 30 (14.4) | 38 (18.0) | 68 (16.2) |
| High school/GED | 68 (32.5) | 64 (30.3) | 132 (31.3) |
| More than high school | 111 (53.1) | 109 (51.7) | 220 (52.4) |
| Caregiver relationship status (%) | |||
| Single | 72 (34.5) | 70 (33.2) | 142 (33.8) |
| Living with partner/spouse | 130 (62.2) | 127 (60.2) | 257 (61.2) |
| Separated/Divorced/Widowed | 7 (3.4) | 14 (6.6) | 21 (5.0) |
| Child female (%) | 109 (52.2) | 104 (49.3) | 213 (50.7) |
| Child’s age (months), mean (SD | 20.8 (7.0) | 22.4 (6.8) | 21.5 (6.9) |
| Child Hispanic (%) | 111 (53.1) | 115 (54.5) | 226 (53.8) |
| Child Hispanic ethnicity (%)a | |||
| Mexican | 78 (69.6) | 88 (76.5) | 166 (73.1) |
| Other | 34 (30.4) | 27 (23.5) | 61 (26.9) |
| Child race (%) | |||
| White | 33 (15.8) | 21 (10.0) | 54 (12.9) |
| Black | 85 (40.7) | 91 (43.1) | 176 (41.9) |
| Other | 91 (43.5) | 99 (46.9) | 190 (45.2) |
| Household size, mean (SD) | 5.0 (1.8) | 4.7 (1.5) | 4.8 (1.7) |
| Children in household, mean (SD) | 2.6 (1.3) | 2.4 (1.3) | 2.5 (1.3) |
| Child has health insurance (%) | 202 (96.7) | 199 (94.3) | 401 (95.5) |
| Child health insurance source (%)b | |||
| Medicaid | 175 (86.6) | 183 (92.0) | 358 (89.3) |
| Other | 27 (13.4) | 16 (8.0) | 43 (10.7) |
| Child has dental insurance (%) | 172 (82.3) | 172 (81.5) | 344 (81.9) |
| Child dental insurance source (%)c | |||
| Medicaid | 151 (87.8) | 160 (93.0) | 311 (90.4) |
| Other | 21 (12.2) | 12 (7.0) | 33 (9.6) |
| Length since child’s last dental visit (%)d | |||
| Never has been | 123 (59.1) | 127 (60.2) | 250 (59.7) |
| ≤ 6 months | 69 (33.2) | 70 (33.2) | 139 (33.2) |
| > 6 months | 16 (7.7) | 14 (6.6) | 30 (7.2) |
| Child’s brushing/wiping frequency (%) | |||
| Never | 12 (5.7) | 13 (6.2) | 25 (6.0) |
| Sometimes, but not everyday | 25 (12.0) | 39 (18.5) | 64 (15.2) |
| Once a day | 73 (34.9) | 69 (32.7) | 142 (33.8) |
| Twice a day | 89 (42.6) | 79 (37.4) | 168 (40.0) |
| More than twice a day | 10 (4.8) | 11 (5.2) | 21 (5.0) |
| Plaque score, mean (SD)d | 1.9 (0.6) | 2.0 (0.6) | 1.9 (0.6) |
N=227 for Total; N=112 for Usual Care; N=115 for CHW;
N=401 for Total; N=202 for Usual Care; N=199 for CHW;
N=344 for Total; N=172 for Usual Care; N=172 for CHW;
N=419 for Total; N=208 for Usual Care; N=211 for CHW
Missing data fell within the anticipated limits for sample size calculations, with 86.2% complete at 12-months (Figure 1). All sites completed the trial and this did not differ by group.
CHW visits and calls occurred between April 2018 and February of 2020. Among individual participants in the CHW group (N=211, 420 total visits), 19% received no CHW visits; 23.2% received one, 21.3% received two, 12.8% received three, and 23.7% received four CHW visits. Completed CHW visits varied by site (Table 2). Follow-up calls followed a similar pattern. Most visits (93.1%) occurred in homes. The mean visit duration was 63.7 (SD 21.8) minutes, with a range of 9–195 minutes. The mean follow-up call duration was 4.7 (SD 5.5) minutes. When the child was present (N=347), the child actively participated by playing a game or practicing a behavior with the caregiver and CHW a lot of the time (65.1%) and some of the time (31.1%). CHWs also provided approximately 1,255 hours of general oral health education at the 10 sites, with sites receiving 9–11 months of CHW intervention. CHWs documented conversations with 867 individuals, although this is an underestimate as some individuals listened peripherally and were not counted. No adverse events were reported.
Table 2:
Completed Community Health Worker (CHW) Visits by Site in Intervention Arm
| # | %* | # | %* | # | %* | # | %* | # | %* | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| All Sites | N=211 | 40 | 19.0 | 49 | 23.2 | 45 | 21.3 | 27 | 12.8 | 50 | 23.7 |
| Clinic Sites | N=99 | 21 | 22.2 | 26 | 26.3 | 23 | 23.2 | 11 | 11.1 | 18 | 18.2 |
| 1 | N=21 | 4 | 19.0 | 6 | 28.6 | 7 | 33.3 | 0 | 0.0 | 4 | 19.0 |
| 2 | N=18 | 4 | 22.2 | 2 | 11.1 | 6 | 33.3 | 3 | 16.7 | 3 | 16.7 |
| 3 | N=19 | 1 | 5.3 | 6 | 31.6 | 5 | 26.3 | 6 | 31.6 | 1 | 5.3 |
| 4 | N=22 | 3 | 13.6 | 6 | 27.3 | 4 | 18.2 | 0 | 0.0 | 9 | 40.9 |
| 5 | N=19 | 9 | 47.4 | 6 | 31.6 | 1 | 5.3 | 2 | 10.5 | 1 | 5.3 |
| WIC Sites | N=112 | 19 | 17.0 | 23 | 20.5 | 22 | 19.6 | 16 | 14.3 | 32 | 28.6 |
| 6 | N=23 | 2 | 8.7 | 3 | 13.0 | 4 | 17.4 | 6 | 26.1 | 8 | 34.8 |
| 7 | N=23 | 3 | 13.0 | 4 | 17.4 | 3 | 13.0 | 6 | 26.1 | 7 | 30.4 |
| 8 | N=23 | 5 | 21.7 | 4 | 17.4 | 6 | 26.1 | 0 | 0.0 | 8 | 34.8 |
| 9 | N=21 | 4 | 19.0 | 0 | 0.0 | 7 | 33.3 | 3 | 14.3 | 7 | 33.3 |
| 10 | N=22 | 5 | 22.7 | 12 | 54.5 | 2 | 9.1 | 1 | 4.5 | 2 | 9.1 |
Denominator is row (site) specific
Child brushing frequency did not differ by intervention group at 12-months (Table 3). Child brushing frequency improved for all over time and for children who were older at the start, children who had seen the dentist in the last six months compared to those who had never seen the dentist, and children who “always” had adults to help brush their teeth or “sometimes/most of the time” compared to “never”. Child brushing frequency was worse for children whose caregivers brushed less than twice a day, whose caregivers had “fair” or “good/very good” mouth conditions compared to those with “poor” mouth conditions, and those whose family activities of daily living interfered with brushing routines “always/most of the time” and “sometimes” compared to “never”.
Table 3:
Multiple logistic regression analysis to compare the odds ratio (OR) of child twice daily or more brushing frequency over 12-months by group (CHW intervention compared to wait-list control), controlling for co-variates*
| Parameter | OR | 95% CL | P value | |
|---|---|---|---|---|
| Intercept | 0.82 | 0.25 | 2.74 | 0.75 |
| CHW group [vs wait-list control group] | 0.82 | 0.55 | 1.23 | 0.34 |
| Time | 1.06 | 1.02 | 1.10 | <0.01 |
| Group by time interaction | 1.00 | 0.95 | 1.06 | 0.89 |
| Child age | 1.05 | 1.03 | 1.08 | <0.01 |
| More than 6 months ago | 0.83 | 0.54 | 1.30 | 0.42 |
| Less than high school | 0.96 | 0.64 | 1.45 | 0.85 |
| Non-Hispanic Black | 0.97 | 0.55 | 1.71 | 0.91 |
| Private insurance | 1.02 | 0.70 | 1.48 | 0.92 |
| Caregiver brushes once/day or less [vs twice or more] | 0.17 | 0.12 | 0.24 | <0.01 |
| Good/very good | 0.49 | 0.30 | 0.78 | <0.01 |
| Sometimes/most of the time | 1.99 | 1.20 | 3.27 | 0.01 |
| Sometimes | 0.49 | 0.34 | 0.71 | <0.01 |
| WIC site [vs healthcare clinic] | 0.85 | 0.61 | 1.18 | 0.33 |
| Small | 0.94 | 0.66 | 1.32 | 0.71 |
| Mixed non-Hispanic Black/Hispanic | 0.72 | 0.49 | 1.05 | 0.09 |
Variables in the final model were selected through backward elimination or they were included because they had been previously shown to differ by site (child age, child’s last dental visit, and caregiver degree and health insurance)11 or were used for the cluster randomization (type of site, site size, and site race/ethnicity).
Plaque score was not different by intervention group at 12-months (Table 4). When controlling for all other variables, children whose caregivers reported that adults “always” helped their children brush their teeth had lower plaque scores than children whose caregivers reported adults never helped. No other factors were associated with plaque score.
Table 4:
Multiple linear regression analysis to compare child plaque scores (reported as beta estimates [Est] in the table) over 12-months by group (CHW intervention compared to wait-list control), controlling for co-variates*
| Parameter | Est | 95% CL | P value | |
|---|---|---|---|---|
| Intercept | 2.06 | 1.69 | 2.43 | <0.01 |
| CHW group [vs wait-list control group] | 0.08 | −0.04 | 0.19 | 0.19 |
| Time | −0.00 | −0.01 | 0.01 | 0.49 |
| Group by time interaction | −0.01 | −0.02 | 0.01 | 0.46 |
| Child age | 0.00 | −0.00 | 0.01 | 0.40 |
| More than 6 months ago | −0.01 | −0.14 | 0.11 | 0.86 |
| Less than high school | 0.04 | −0.07 | 0.14 | 0.49 |
| Non-Hispanic Black | 0.03 | −0.15 | 0.21 | 0.75 |
| Private insurance | 0.01 | −0.10 | 0.12 | 0.82 |
| Sometimes/most of the time | −0.11 | −0.25 | 0.03 | 0.12 |
| WIC site [vs healthcare clinic] | −0.03 | −0.14 | 0.08 | 0.58 |
| Small | −0.04 | −0.16 | 0.07 | 0.48 |
| Mixed non-Hispanic Black/Hispanic | −0.06 | −0.18 | 0.07 | 0.40 |
Variables in the final model were selected through backward elimination or they were included because they had been previously shown to differ by site (child age, child’s last dental visit, and caregiver degree and health insurance,)11 or were used for the cluster randomization (type of site, site size, and site race/ethnicity).
Child brushing frequency and plaque score did not vary by amount of CHW intervention received (Supplemental Figure)
As a secondary analysis, changes in children seeing the dentist in the past six months were assessed. There was no difference by intervention group (Supplemental Table). Children were more likely to see the dentist over time if they were older at baseline, if the child brushed twice a day or more, if the child brushed for 1–2 minutes compared to more than 2 minutes, if the child used toothpaste with fluoride, and if the caregiver had been to the dentist in the past year. Children from households with more chaos and from sites that were mixed race/ethnicity were less likely to have been to the dentist.
Discussion
The primary aim of this research was to determine whether a multi-level oral health CHW intervention was associated with improved tooth brushing behaviors after 12-months in low-income urban children under three years old. The families that participated met these criteria, providing new information on a population underrepresented in prior oral health research. Intervention delivery went as expected, with 81% receiving some CHW intervention and 24% completing the entire intervention protocol. This degree of CHW intervention uptake is consistent with other CHW studies and better than many clinical interventions.26–28 Despite the strong intervention evidence base and careful fidelity monitoring, oral health CHW services were not associated with changes in brushing frequency or plaque score.
Although CO-OP Chicago measured behaviors, not caries, the findings appear to conflict with those obtained by similar interventions in Brazil, England, and Australia, where home visits for oral health education and support were associated with reduced caries incidence for very young children.15–17 The interventions in those studies were comparable to CO-OP Chicago but the countries have different health services structures that may have played a role in intervention responsiveness. Despite CO-OP CHWs employing evidenced-based methods to support behavior change,14,22,29,30 perhaps the oral health messaging and family and organizational support were not delivered frequently enough or in sufficient depth to enact behavior change. It may also be that the intervention did not address change at a time of developmental readiness or did not address behavior changes of highest priority for these families who struggled with many social and financial issues. The CHWs may have needed more advanced training in motivational interviewing. Another theory is that families of these young children were not sufficiently motivated to make oral health behavior changes owing to a lack of visible consequences of poor oral health behaviors. Oral health CHW intervention may be more effective when embedded as part of overall health messaging and coordination.31 Another strategy may be to incorporate CHWs in secondary or tertiary prevention when caries is present. Most CHW interventions that have shown success in other disease areas were for individuals struggling with chronic disease management and acute illness.26–28,32
Despite a lack of intervention effect, this trial offers an opportunity to better understand brushing behaviors in young children over time. Brushing frequency is a common way to measure oral health behaviors and is known to prevent caries.20 Not surprisingly, child brushing frequency was higher for older children, those with established dental care, and children who received more adult help. Caregiver behaviors also aligned with brushing frequency, with less child brushing when caregivers brushed their own teeth less and in households with chaotic brushing routines. Others have reported similar patterns.33 Of interest, caregivers who reported better oral health for themselves also reported lower child brushing frequency. In CO-OP’s pilot work that included focus groups with caregivers, some caregivers described less concern for their children’s oral health if their own oral health was good.34 This type of attitude is concerning. Child nutrition is different than it was several decades ago with much higher consumption of sugar and processed foods among some populations.35 Access to fluoride is also changing as more people consume nonfluoridated bottled water.36 Sugar plays a major causal role in caries development and fluoridated water is protective.35,36
Plaque score was used as a clinician-rated verification of brushing behavior and obtained very different findings than with self-reported brushing. Plaque scores started out poor and did not improve. In fact, the only factor associated with a change in plaque score was if caregivers always brush their children’s teeth. This suggests that only effective brushing by an adult is sufficient to reduce plaque. Parents consider it a positive milestone when they teach their children to brush their own teeth, but young children have limited manual dexterity and developmental capacity;37 they may simply just not be up to the task.
This trial has several limitations. Self-reported brushing frequency is a standard measure in the field, but it remains prone to respondent bias. Although plaque is one known risk factor for future caries, its predictive power has limitations. While careful attention was paid to the design and monitoring of the plaque protocol, this is a new adaptation and therefore has no comparison. Due to the age of the children, the trial focused only on their four maxillary incisors and did not measure caries. The trial did not capture all the factors that influence brushing and plaque. One year may be a short duration for follow-up when measuring a behavior change that is complicated by a range of family and social issues. And finally, the trial was conducted with only low-income urban families, which limits the ability to apply these results to all children.
In conclusion, this trial demonstrated suboptimal brushing behaviors in a cohort of low-income children under the age of three years old. Oral health CHW intervention did not improve these behaviors. These findings conflict with a large body of evidence showing the utility of the CHW model in marginalized and disadvantaged populations around the world in a range of disease areas. Therefore, CHWs remain of interest in oral health. The American Dental Association has been funding a type of CHW called Community Dental Health Coordinators since 2006 to support oral health services and outreach. The specific role of CHWs in oral health requires additional investigation, especially for young children who are establishing their first oral health behaviors. Recognizing CHW influences at multiple levels, not just the individual, and using a community-based participatory research approach in this future research will enhance understanding of the practical utility of CHWs in oral health.9,38
Supplementary Material
Funding:
Research reported in this publication was supported by the National Institutes of Dental & Craniofacial Research of the National Institutes of Health under Award Number UH3DE025483, Principal Investigator: Molly A. Martin, and Coordinating Center Award Number U01DE025507, Principal Investigator: Stuart A. Gansky, University of California, San Francisco. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Conflict of interest disclosure: The authors have no conflicts of interest to disclose.
Ethics approval statement: Institutional Review Boards at the University of Illinois at Chicago [2017–1090], the University of California San Francisco [16–19920], and the Chicago Department of Public Health [16–06] approved the trial. Caregivers provided written informed consent.
Clinical trials registration: NCT03397589
Data availability statement:
The data that support the findings of this trial are available from the corresponding author upon reasonable request.
References
- 1.Fleming E, Afful J. Prevalence of Total and Untreated Dental Caries Among Youth: United States, 2015–2016. 2018. NCHS Data Brief, no 307. Hyattsville, MD. [PubMed] [Google Scholar]
- 2.Policy on Hospitalization and Operating Room Access for Oral Care of Infants, Children, Adolescents, and Individuals with Special Health Care Needs. Pediatr Dent. 2017;39(6):104–105. [PubMed] [Google Scholar]
- 3.Casamassimo PS, Thikkurissy S, Edelstein BL, Maiorini E. Beyond the dmft: The Human and Economic Cost of Early Childhood Caries. J Am Dent Assoc. 2009;140(6):650–657. [DOI] [PubMed] [Google Scholar]
- 4.Shearer DM, Thomson WM, Broadbent JM, Poulton R. Maternal oral health predicts their children’s caries experience in adulthood. J Dent Res. 2011;90(5):672–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Goettems ML, Nascimento GG, Peres MA, et al. Influence of maternal characteristics and caregiving behaviours on children’s caries experience: An intergenerational approach. Community Dent Oral Epidemiol. 2018;46(5):435–441. [DOI] [PubMed] [Google Scholar]
- 6.Gupta N, Vujicic M, Yarbrough C, Harrison B. Disparities in untreated caries among children and adults in the U.S., 2011–2014. BMC Oral Health. 2018;18(1):30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schwendicke F, Dörfer CE, Schlattmann P, et al. Socioeconomic Inequality and Caries: A Systematic Review and Meta-Analysis. J Den Res. 2015;94:10–18. [DOI] [PubMed] [Google Scholar]
- 8.Horowitz AM, Kleinman DV. Oral health literacy: a pathway to reducing health disparities in Maryland. J Public Health Dent. 2012;72(Suppl 1):S26–30. [DOI] [PubMed] [Google Scholar]
- 9.Fisher-Owens SA, Gansky SA, Platt LJ, et al. Influences on children’s oral health: a conceptual model. Pediatrics. 2007;120(3):e510–20. [DOI] [PubMed] [Google Scholar]
- 10.Broadbent JM, Zeng J, Foster Page LA, et al. Oral Health-related Beliefs, Behaviors, and Outcomes through the Life Course. J Dent Res. 2016;95(7):808–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Martin MA, Zimmerman LJ, Rosales GF, et al. Design and sample characteristics of Coordinated Oral health Promotion (CO-OP) Chicago: A cluster-randomized controlled trial. Contemp Clin Trials. 2020;92:105919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Glanz K, Bishop DB. The role of behavioral science theory in development and implementation of public health interventions. An Rev Pub Health. 2010;31:399–418. [DOI] [PubMed] [Google Scholar]
- 13.Newton JT, Bower EJ. The social determinants of oral health: new approaches to conceptualizing and researching complex causal networks. Community Dent Oral Epidemiol. 2005; 33(1):25–34. [DOI] [PubMed] [Google Scholar]
- 14.Bandura A. Self-efficacy: toward a unifying theory of behavioral change. Psychological Review. 1977;84:191–215. [DOI] [PubMed] [Google Scholar]
- 15.Feldens CA, Giugliani ERJ, Duncan BB, et al. Long-term effectiveness of a nutritional program in reducing early childhood caries: A randomized trial. Community Dent Oral Epidemiol. 2010;38(4):324–332. [DOI] [PubMed] [Google Scholar]
- 16.Kowash MB. Effectiveness on oral health of a long-term health education programme for mothers with young children. Br Dent J. 2000;188(4):201–205. [DOI] [PubMed] [Google Scholar]
- 17.Plonka KA, Pukallus ML, Barnett A, et al. A controlled, longitudinal study of home visits compared to telephone contacts to prevent early childhood caries. Int J Paediatr Dent. 2013;23(1):23–31. [DOI] [PubMed] [Google Scholar]
- 18.Grover J. The community dental health coordinator. A valued new member of the dental team. Todays FDA. 2014;26(2):56–7. [PubMed] [Google Scholar]
- 19.Martino S. Oral health behavioral and social intervention research concepts and methods. J Public Health Dent. 2011;71(Suppl 1):S2–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Walsh T, Worthington HV, Glenny A, et al. Fluoride toothpastes of different concentrations for preventing dental caries. Cochrane Database Syst Rev. 2019;April:3(3): CD007868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Greene JG, Vermillion JR. The Simplified Oral Hygiene Index. J Am Dent Assoc. 1964;68(1):7–13. [DOI] [PubMed] [Google Scholar]
- 22.Martin M, Frese W, Lumsden C, Sandoval A. Building a pediatric oral health training curriculum for community health workers. J Public Health Manag Pract. 2018;24(3):e9–e18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Guideline on Caries-risk Assessment and Management for Infants, Children, and Adolescents. Ped Dent. 2016;38(6):142–149. [PubMed] [Google Scholar]
- 24.Azur MJ, Stuart EA, Frangakis C, Leaf PJ. Multiple imputation by chained equations: what is it and how does it work? Int J Methods Psychiatr Res. 2011;20(1):40–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wei SH, Lang NP. Periodontal epidemiological indices for children and adolescents: II. Evaluation of oral hygiene; III. Clinical applications. Pediatr Dent. 1982;4(1):64–73. [PubMed] [Google Scholar]
- 26.Campbell JD, Brooks M, Hosokawa P, et al. Community Health Worker Home Visits for Medicaid-Enrolled Children With Asthma: Effects on Asthma Outcomes and Costs. Am J Public Health. 2015;105(11):2366–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rothschild SK, Martin MA, Swider SM, et al. Mexican American trial of community health workers: a randomized controlled trial of a community health worker intervention for Mexican Americans with type 2 diabetes mellitus. Am J Public Health. 2014;104(8):1540–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Martin MA, Pugach O, Mosnaim G, et al. Community Health Worker Asthma Interventions for Children: Results From a Clinically Integrated Randomized Comparative Effectiveness Trial (2016‒2019). Am J Public Health. 2021:111(7):1328–1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Colvara BC, Faustino-Silva DD, Meyer E, et al. Motivational interviewing for preventing early childhood caries: A systematic review and meta-analysis. Community Dent Oral Epidemiol. 2021;49(1):10–16. [DOI] [PubMed] [Google Scholar]
- 30.Tsai C, Raphael S, Agnew C, et al. Health promotion interventions to improve oral health of adolescents: A systematic review and meta-analysis. Community Dent Oral Epidemiol. 2020;48(6):549–560. [DOI] [PubMed] [Google Scholar]
- 31.Balcazar H, Rosenthal EL, Brownstein JN, Rush CH, Matos S, Hernandez L. Community health workers can be a public health force for change in the United States: three actions for a new paradigm. Am J Public Health. 2011;101(12):2199–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pinto D, Carroll-Scott A, Christmas T, Heidig M, Turchi R. Community health workers: improving population health through integration into healthcare systems. Curr Opin Pediatr. 2020;32(5):674–682. [DOI] [PubMed] [Google Scholar]
- 33.Aliakbari E, Gray-Burrows KA, Vinall-Collier KA, et al. Facilitators and barriers to home-based toothbrushing practices by parents of young children to reduce tooth decay: a systematic review. Clin Oral Investig. 2021;25(6):3383–3393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Martin MA, Lee HH, Landa J, et al. Formative Research Implications on Design of a Randomized Controlled Trial for Oral Health Promotion in Children. Pilot Feasibility Stud. 2018;4:155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bleich SN, Vercammen KA, Koma JW, Li Z. Trends in Beverage Consumption Among Children and Adults, 2003–2014. Obesity. 2018;26(2):432–441. [DOI] [PubMed] [Google Scholar]
- 36.Vieux F, Maillot M, Rehm CD, et al. Trends in tap and bottled water consumption among children and adults in the United States: analyses of NHANES 2011–16 data. Nutr J. 2020;19(1):10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mentes A, Atukeren J. A study of manual toothbrushing skills in children aged 3 to 11 years. J Clin Pediatr Dent. 2002;27(1)91–94. [DOI] [PubMed] [Google Scholar]
- 38.Rosenthal EL, Balcazar HG, De Heer HD, Wise S, Flores L, Aguirre M. Critical reflections on the role of CBPR within an RCT community health worker prevention intervention. J Ambul Care Manage. 2014;37(3):241–9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this trial are available from the corresponding author upon reasonable request.


