Abstract
OBJECTIVE
To investigate the relationship between long-term weight training and mortality in male health professionals with and without type 2 diabetes.
RESEARCH DESIGN AND METHODS
We analyzed 31,140 men without type 2 diabetes and 2,588 with type 2 diabetes from the Health Professionals Follow-up Study (1992–2018). Information on weight training was repeatedly assessed using a biennial questionnaire. Cox regression was used to estimate hazard ratios (HRs) and 95% CIs.
RESULTS
During up to 26 years of follow-up, we documented 12,607 deaths (988 deaths among men with type 2 diabetes). Among participants without type 2 diabetes, 1–59 and 60–149 min/week of long-term weight training were associated with 14% (HR 0.86; 95% CI 0.82–0.89) and 8% (HR 0.92; 95% CI 0.85–0.99) lower mortality versus no weight training, respectively, after adjustment for aerobic activity. However, ≥150 min/week of weight training was not significantly associated with mortality (HR 1.05; 95% CI 0.91–1.20; overall P trend = 0.94; P quadratic < 0.001). Meeting the recommended aerobic physical activity guideline (≥150 min/week) and performing any weight training were associated with 20–34% lower mortality. Among participants with type 2 diabetes, a moderate level of pre-diagnosis weight training was associated with lower mortality, whereas post-diagnosis weight training showed no association. Performing both weight training and aerobic activity before and after diagnosis was associated with lower mortality.
CONCLUSIONS
A moderate level of long-term weight training was associated with lower mortality, independently of aerobic activity, among male health professionals with and without type 2 diabetes. Addition of weight training to aerobic activities may provide further benefit in mortality risk reduction. Studies are required to confirm our findings in diverse populations.
Introduction
Current physical activity guidelines for U.S. adults recommend two or more times per week of muscle-strengthening activities in addition to ≥150 min/week of moderate to vigorous aerobic activity (1). Similarly, the American Diabetes Association recommends that adults with type 2 diabetes perform both aerobic physical activity and resistance training for optimal glycemic and health outcomes (2). Most adults with type 2 diabetes should engage in at least 150 min/week of aerobic physical activity, spread over at least three days, with no more than two consecutive days without activity, or 75 min/week of vigorous activity or interval training for younger and physically active individuals. In addition, adults with type 2 diabetes should engage in two to three sessions per week of resistance exercise on nonconsecutive days (2).
Numerous studies have consistently reported that regular aerobic physical activity is associated with decreased risks of major noncommunicable diseases (NCDs) (3,4). However, less is known about the association between weight training and subsequent health outcomes. Several prospective cohort studies have reported that moderate weight training (one to two sessions per week) is associated with lower risks of major NCDs, including cardiovascular disease (CVD), type 2 diabetes, and some cancers, as well as premature death (5–8). In adults with type 2 diabetes, weight training has been associated with improved glycemic control, strength, balance, and ability to engage in daily activities (2), but studies on mortality outcomes remain limited.
An inconsistent shape of the association between weight training and mortality, particularly in the higher range of weight training, has been reported (5). For example, a linear inverse association was reported for risk of type 2 diabetes, but no additional benefits or even increased risk was observed after a certain level of weight training for CVD or mortality outcomes (5). However, only a few cohort studies, including the Women’s Health Study, Aerobics Center Longitudinal Study, and Cancer Prevention Study II Nutrition Cohort had information on duration (min/week) of weight training (9–11). Also, most previous studies relied on a single baseline measurement, which is prone to measurement error (regression dilution bias) (12). Of note, measurement error and reverse causation may influence the dose-response shape of physical activity in relation to mortality; therefore, using repeated measures of weight training and applying at least a 2-year lag period likely comprise a more robust analytic approach to account for these biases (12).
A previous study of older women used repeated measures of weight training information to examine the association with mortality. This study found a J-shaped relationship between weight training and all-cause mortality, with an increased risk observed at >150 min/week, although a small number of deaths occurred in the high level of weight training (9). Moreover, studies including participants with type 2 diabetes have not used repeated measures of weight training. This information may be useful in assessing the association between weight training pre- and post-diagnosis of type 2 diabetes in relation to mortality. Given the limited evidence, more large prospective cohort studies with repeated measures of exposure over time are required to better inform the general public and patients with type 2 diabetes of the optimal amount of weight training, alone and in combination with aerobic activity, to improve health.
Therefore, we used a large prospective cohort study with up to 13 repeated measures of detailed physical activity to examine the dose-response relationship between long-term weight training and mortality in U.S. male health professionals with and without type 2 diabetes. We also examined the joint association of aerobic activity and weight training in relation to mortality. For participants with type 2 diabetes, we examined independent and joint associations of pre- and post-diagnosis weight training with all-cause mortality.
Research Design and Methods
Study Population
The Health Professionals Follow-up Study was initiated in 1986, when 51,529 male health professionals age 40–75 years were enrolled. Participants completed a questionnaire on demographic, lifestyle, and medical factors at enrollment and every 2 years. Response rate to the questionnaire exceeded 90%. The study protocol was approved by the institutional review boards of the Brigham and Women’s Hospital and Harvard T.H. Chan School of Public Health and those of participating registries as required. Participants were first asked about information on weight training in 1990. In the current study, we included participants with at least one measure of weight training at baseline. We excluded those previously diagnosed with cancer (except nonmelanoma skin cancer), CVD, or type 2 diabetes at baseline. In the final analysis, we included a total of 31,140 men without type 2 diabetes and 2,588 men with type 2 diabetes.
Weight Training Assessment
Starting from 1990 and every 2 years, participants were asked to report average weekly time spent weightlifting or using a nautilus or weight machine (i.e., 0, 1–4 min, 5–19 min, 20–59 min, 1 h, 1–1.5 h, 2–3 h, 4–6 h, 7–10 h, or ≥11 h). We collected up to 13 repeated measures of weight training information (median 11; 10th to 90th percentiles 4–13) over the follow-up. Aerobic activities, including walking, jogging, running, bicycling, swimming, tennis, squash or racquetball, calisthenics or rowing, and heavy outdoor work, were assessed using the same biennial questionnaire. Total moderate to vigorous aerobic activity was calculated by summing all activities, except for weight training, in minutes per week. The reproducibility and validity of physical activity questionnaires have been described elsewhere (13,14). The correlation between questionnaire-based physical activity and four single-week activity diaries administered across four different seasons was 0.62.
Covariate Assessment
Information on age, height, weight, smoking status, medical history, and sedentary behavior (watching television) was collected every 2 years. Dietary information, including alcohol intake and total calorie intake, was collected using a validated semiquantitative food frequency questionnaire every 4 years (15,16). Diet quality was assessed using the Alternative Health Eating Index (AHEI) score (17).
Type 2 Diabetes Assessment
Participants self-reported newly diagnosed diabetes through a biennial questionnaire, and a supplementary questionnaire was sent to confirm the cases. Consistent with the National Diabetes Data Group, we confirmed the diagnosis of type 2 diabetes if the participant met at least one of the following criteria: 1) one or more classic symptoms (e.g., excessive thirst, polyuria, weight loss, or hunger) plus fasting blood glucose ≥140 mg/dL or random blood glucose ≥200 mg/dL, 2) elevated blood glucose on two different occasions (e.g., fasting blood glucose ≥140 mg/dL or random blood glucose ≥200 mg/dL or blood glucose ≥200 mg/dL after 2-h oral glucose tolerance testing) with no symptoms, and 3) treatment with a hypoglycemic drug (e.g., insulin or oral hypoglycemic agent). Of note, the threshold for fasting blood glucose was changed to ≥126 mg/dL in 1998, and HbA1c ≥6.5% was added in 2010.
Mortality Assessment
Death was ascertained via the National Death Index, next of kin, or the postal system (18,19). Through these methods, >98% of deaths were confirmed. ICD-9 codes were used to classify deaths resulting from CVD (ICD-9 codes 390–459 and 795), cancer (ICD-9 codes 140–239), respiratory disease (ICD-9 codes 460–519), and other causes.
Statistical Analysis
Person-year was calculated from the time when weight training information was first available in 1990 until the time of death or the end of the study (January 2018), whichever occurred first. Cumulative average of repeated measures of weight training was used to reflect long-term behavior and mitigate measurement error. We also applied a 2-year lag between weight training assessment and time at risk of death to reduce reverse causation (12). For example, we used the baseline weight training information in 1990 for follow-up from 1992 to 1994 and cumulative average weight training of 1990 and 1992 for follow-up from 1994 to 1996 and so on. Weight training was categorized based on distribution, consistent with previous studies (0, 1–59, 60–149, and ≥150 min/week) (9,20). Cox proportional hazards regression with age as time scale and with stratification by calendar year was used after adjustment for potential confounders based on weight training and mortality literature. Covariates adjusted for included race (White or non-White), smoking status (never, past, or current [1–14, 15–24, and ≥25 cigarettes per day]), family history of myocardial infarction (yes or no), family history of cancer (yes or no), baseline hypertension (yes or no), alcohol consumption (none, 0.1–4.9, 5–14.9, 15–29.9, or ≥30 g/day), AHEI score 2010 (quintile), total calorie intake (quintile), time spent sitting to watch television (quintile), BMI (quintile), and aerobic activity (none, 1–59, 60–149, and ≥150 min/week).
We also used a restricted cubic spline with three knots at the 50th, 75th, and 90th percentiles to flexibly model the shape of the association between long-term weight training and all-cause and cause-specific mortality (9). To account for both types of exercise (i.e., aerobic and weight training), we conducted a joint analysis of weight training and aerobic activity in relation to mortality. Moreover, we conducted a stratified analysis by potential effect modifiers, such as age, BMI, and smoking status. Lastly, we conducted several sensitivity analyses using baseline or most recent weight training measure, applying longer lag times (4 years) and using different categorical cut points to improve power. We also excluded participants with high vigorous-intensity aerobic activity (>400 min/week) to exclude the possibility that any attenuation of association at higher levels of weight training was not due primarily to concurrently higher levels of vigorous-intensity aerobic activity, which may have attenuated benefit on mortality. We also conducted an analysis excluding each cause-specific death from all-cause mortality to see whether the shape of the dose-response relation was affected by cause-specific mortality.
For the analyses among participants with type 2 diabetes, we included men with incident type 2 diabetes diagnosed during follow-up (1990–2016). Person-year was calculated from the time of type 2 diabetes diagnosis until the time of death or the end of the study (January 2018), whichever occurred first. Pre- and post-diagnosis weight training were categorized using the same cut points (0, 1–59, 60–149, and ≥150 min/week). Cox proportional hazards regression with age as time scale and with stratification by calendar year was used after adjustment for potential confounders, including race (White or non-White), smoking status (never, past, or current [1–14 or ≥15 cigarettes per day]), family history of myocardial infarction (yes or no), family history of diabetes (yes or no), hypertension (yes or no), high cholesterol (yes or no), duration of diabetes (0–5, 6–10, or ≥10 years), multivitamin use (yes or no), aspirin use (yes or no), alcohol consumption (none, 0.1–4.9, 5–14.9, 15–29.9, or ≥30 g/day), AHEI score 2010 (quintile), BMI (quintile), and aerobic activity (none, 1–59, 60–149, or ≥150 min/week). For the post-diagnosis analysis, we performed an additional model further adjusting for potential confounders, including pre-diagnosis weight training and pre-diagnosis aerobic activity. In addition, we conducted two joint analyses of 1) pre- and post-diagnosis weight training and aerobic activity and 2) pre- and post-diagnosis weight training with mortality among participants with type 2 diabetes. All analyses were performed using SAS (version 9.4), and a P value of 0.05 was considered statistically significant.
Results
The mean age of participants at baseline was 59 years. Participants performing more weight training were younger, had a higher diet quality score, and engaged in more aerobic activity (Table 1). The mean age of participants with type 2 diabetes was 65 years. Participants performing more weight training had a higher diet quality score and engaged in more aerobic activity.
Table 1.
Age-standardized baseline characteristics of study participants with and without type 2 diabetes (Health Professionals Follow-up Study)
| Weight training, min/week | ||||
|---|---|---|---|---|
| 0 | 1–59 | 60–149 | ≥150 | |
| Participants without type 2 diabetes, n | 23,639 | 4,392 | 2,007 | 1,102 |
| Age, years* | 59.7 (9.4) | 55.6 (8.4) | 55 (8.1) | 54.4 (7.9) |
| BMI, kg/m2 | 25.7 (3.0) | 25.2 (2.6) | 25 (2.5) | 25.1 (2.7) |
| White race, % | 91.5 | 91.5 | 91.5 | 90.2 |
| Smoking status, % | ||||
| Never | 47.5 | 48.6 | 49.2 | 45.0 |
| Past | 44.9 | 47.3 | 46.5 | 49.6 |
| Current | 7.6 | 4.1 | 4.3 | 5.3 |
| Family history of myocardial infarction, % | 11.7 | 11.9 | 11.7 | 15.2 |
| Family history of cancer, % | 17.2 | 18.4 | 18.7 | 15.9 |
| High blood pressure, % | 23.2 | 20.5 | 19.8 | 21.2 |
| Calorie intake, kcal/day | 1,964 (559) | 1,973 (561) | 1,973 (556) | 1,982 (558) |
| Alcohol intake, g/day | 10.7 (14.1) | 10.9 (13.1) | 10.6 (12.3) | 10.4 (12.8) |
| AHEI score | 52.2 (10.7) | 55.3 (10.5) | 56.6 (10.4) | 57.2 (10.7) |
| Time spent sitting to watch television, h/week | 10.3 (7.2) | 9.5 (6.7) | 9.8 (6.9) | 9.7 (7) |
| Aerobic activity, min/week | 172 (203) | 252 (217) | 312 (238) | 348 (264) |
| Moderate intensity | 88 (158) | 96 (156) | 101 (165) | 115 (186) |
| Vigorous intensity | 84 (121) | 156 (152) | 211 (170) | 234 (185) |
| Participants with type 2 diabetes, n | 2,084 | 178 | 194 | 132 |
| Age, years* | 64.7 (8.7) | 64.9 (8.7) | 65.5 (7.9) | 65.2 (8.8) |
| BMI, kg/m2 | 29.2 (4.6) | 27.9 (4.3) | 28.5 (4.1) | 28.8 (4.1) |
| White race, % | 90.2 | 92.6 | 88.2 | 84.4 |
| Smoking status, % | ||||
| Never | 38.7 | 39.3 | 29.5 | 33.1 |
| Past | 54.2 | 58.5 | 67.9 | 62.6 |
| Current | 7.1 | 2.2 | 2.6 | 4.3 |
| Family history of myocardial infarction, % | 33.7 | 31.1 | 29.2 | 36.3 |
| Family history of cancer, % | 35.3 | 38.0 | 35.8 | 36.9 |
| Family history of diabetes, % | 37.6 | 39.1 | 38.2 | 38.2 |
| High blood pressure, % | 62.1 | 67.6 | 58.7 | 65.2 |
| Aspirin use, % | 49.4 | 46.0 | 44.4 | 53.0 |
| Multivitamin use, % | 50.9 | 43.7 | 41.4 | 46.6 |
| Duration of diabetes, years | 6.8 (5.5) | 7.7 (5.8) | 6.7 (5.6) | 6.5 (5.4) |
| Alcohol intake, g/day | 9.8 (14.5) | 9.9 (11.5) | 10.7 (13.4) | 9 (13.6) |
| AHEI score | 47.9 (11) | 51.9 (11.7) | 53.9 (11) | 54.3 (11.4) |
| Aerobic activity, min/week | 132 (288) | 216 (300) | 294 (348) | 402 (414) |
Values are given as mean (SD) for continuous variables and as percentage, number, or both for categorical variables and are standardized to the age distribution of the study population.
Value is not age adjusted.
Association Between Long-term Weight Training and All-Cause and Cause-Specific Mortality in Participants Without Type 2 Diabetes at Baseline
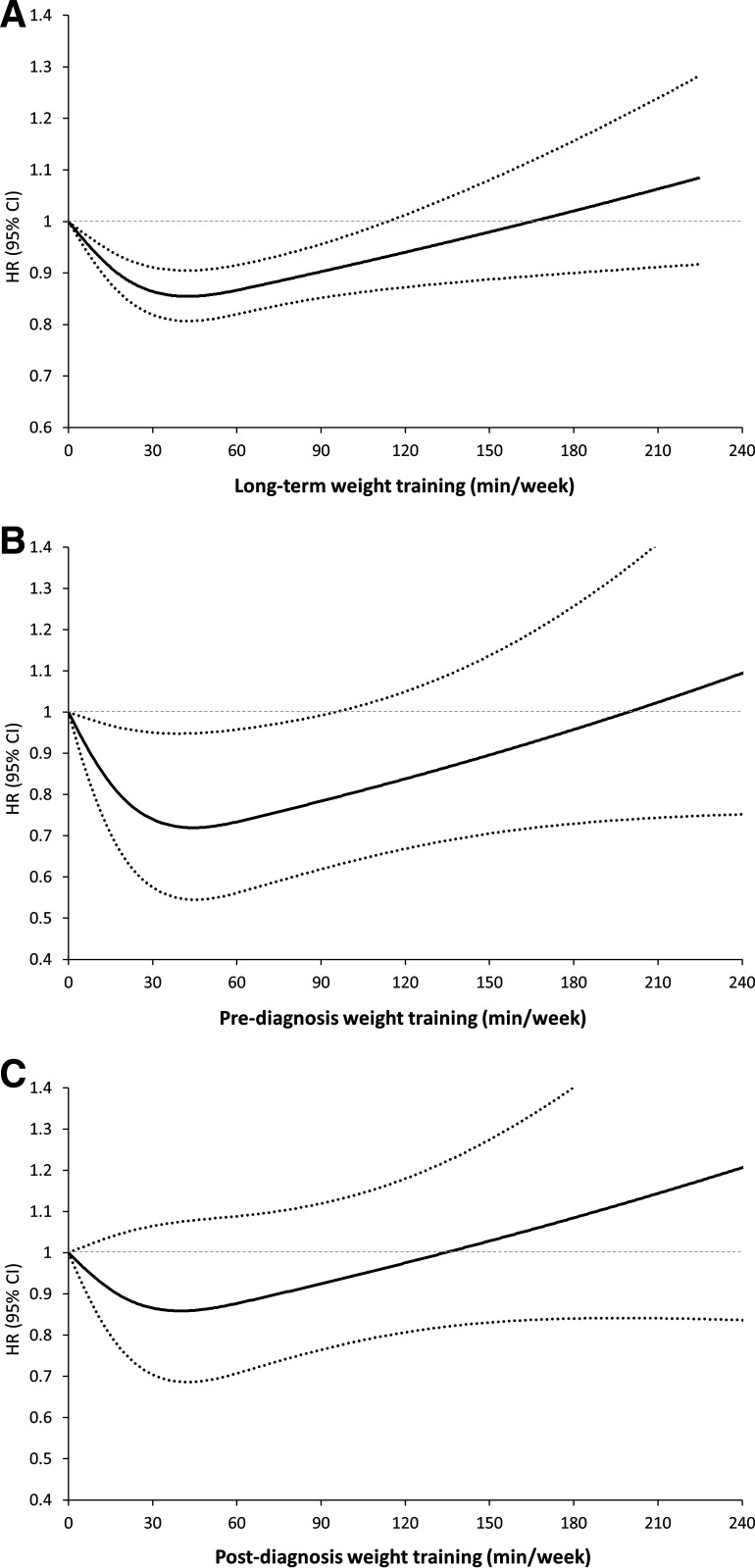
During up to 26 years of follow-up, we documented 12,607 deaths. More weight training was associated with a lower risk of all-cause mortality in participants without type 2 diabetes at baseline (Table 2). In the multivariable-adjusted models including potential confounders and aerobic activity, compared with participants with no weight training, participants with 1–59 and 60–149 min/week of long-term weight training had 14% (hazard ratio [HR] 0.86; 95% CI 0.82–0.89) and 8% (HR 0.92; 95% CI 0.85–0.99) lower risks of all-cause mortality, respectively. However, participants with ≥150 min/week of weight training did not have a decreased risk of all-cause mortality (HR 1.05; 95% CI 0.91–1.20) compared with those with no weight training. A significant quadratic association was observed for all-cause mortality (P trend = 0.94; P quadratic < 0.001). In the dose-response analysis, we found a J-shaped association between long-term weight training and all-cause mortality, with the lowest risk of mortality observed at ∼30–60 min/week and then the inverse association attenuated afterward (P nonlinearity < 0.001) (Fig. 1A and Supplementary Table 1).
Table 2.
Association between long-term weight training and all-cause mortality in participants without type 2 diabetes at baseline (Health Professionals Follow-up Study, 1992–2018)
| Weight training, min/week | P | |||||
|---|---|---|---|---|---|---|
| 0 | 1–59 | 60–149 | ≥150 | Linear trend | Quadratic | |
| Median (Q1–Q3) | 0 | 17 (7–31) | 85 (70–103) | 188 (150–263) | ||
| N of deaths | 8,344 | 3,266 | 775 | 222 | ||
| Person-years | 400,202 | 197,513 | 58,448 | 20,797 | ||
| Age-adjusted model | 1 (ref) | 0.76 (0.72–0.79) | 0.77 (0.71–0.83) | 0.89 (0.78–1.02) | <0.001 | <0.001 |
| MV1 | 1 (ref) | 0.82 (0.78–0.86) | 0.86 (0.79–0.93) | 0.98 (0.85–1.12) | 0.04 | <0.001 |
| MV2 | 1 (ref) | 0.86 (0.82–0.89) | 0.92 (0.85–0.99) | 1.05 (0.91–1.20) | 0.94 | <0.001 |
Age-adjusted model: adjusted for age (months) and calendar year. Multivariable model 1 (MV1): age-adjusted model plus race (White or non-White), smoking status (never, past, or current [1–14, 15–24, or ≥25 cigarettes per day]), family history of myocardial infarction (yes or no), family history of cancer (yes or no), baseline hypertension (yes or no), alcohol consumption (none, 0.1–4.9, 5–14.9. 15–29.9, or ≥30 g/day), AHEI score 2010 (quintile), total calorie intake (quintile), time spent sitting to watch television (quintile), and BMI (quintile). MV2: MV1 plus aerobic activity (none, 1–59, 60–149, or ≥150 min/week). We applied a 2-year lag time between weight training assessment and the time at risk of death for all models.
Figure 1.
Dose-response relationship between long-term weight training and all-cause mortality in participants with and without type 2 diabetes (Health Professionals Follow-up Study, 1992–2018). Restricted cubic spline model with three knots specified at 50th, 75th, and 90th percentiles was performed after adjustment for the same covariates included in multivariable model shown in Table 2 (participants without type 2 diabetes) and Table 4 (participants with type 2 diabetes). The solid line indicates HRs, and the dashed lines indicate 95% CIs. The reference is at 0 min/week of weight training. We applied a 2-year lag time between weight training assessment and the time at risk of death. P nonlinearity < 0.001 for participants without type 2 diabetes. P nonlinearity = 0.02 for pre-diagnosis weight training and 0.13 for post-diagnosis for participants with type 2 diabetes. A: Participants without type 2 diabetes. B and C: Participants with type 2 diabetes pre- (B) and post-diagnosis (C).
For cause-specific mortality, participants with 1–59 or 60–149 min/week of long-term weight training had 16–19% lower CVD mortality, 7% lower cancer mortality, 13–18% lower respiratory disease mortality, and 13–18% lower other causes of death (Table 3). Engaging in ≥150 min/week of long-term weight training showed a positive association for CVD and cancer mortality and other causes of death and an inverse association for respiratory disease mortality, but none were statistically significant. A significant quadratic association was observed for CVD mortality and other causes of death but not for cancer or respiratory disease mortality. In the dose-response analysis, we found a J-shaped association of long-term weight training with CVD and cancer mortality and other causes of death and inverse association for respiratory disease mortality (Supplementary Fig. 1 and Supplementary Table 1).
Table 3.
Association between long-term weight training and cause-specific mortality in participants without type 2 diabetes at baseline (Health Professionals Follow-up Study, 1992–2018)
| Weight training, min/week | P | |||||
|---|---|---|---|---|---|---|
| 0 | 1–59 | 60–149 | ≥150 | Trend | Quadratic | |
| CVD mortality | ||||||
| N of deaths | 2,448 | 876 | 180 | 64 | ||
| Person-years | 406,723 | 200,376 | 59,108 | 20,961 | ||
| Age-adjusted model | 1 (ref) | 0.74 (0.68–0.80) | 0.67 (0.57–0.78) | 0.99 (0.77–1.27) | <0.001 | <0.001 |
| MV1 | 1 (ref) | 0.81 (0.74–0.88) | 0.76 (0.65–0.90) | 1.09 (0.84–1.41) | 0.12 | 0.02 |
| MV2 | 1 (ref) | 0.84 (0.77–0.91) | 0.81 (0.69–0.95) | 1.14 (0.88–1.48) | 0.48 | 0.10 |
| Cancer mortality | ||||||
| N of deaths | 2,315 | 919 | 251 | 69 | ||
| Person-years | 406,944 | 200,360 | 59,060 | 20,976 | ||
| Age-adjusted model | 1 (ref) | 0.82 (0.75–0.89) | 0.91 (0.80–1.04) | 0.89 (0.70–1.14) | 0.11 | 0.02 |
| MV1 | 1 (ref) | 0.88 (0.81–0.95) | 1.01 (0.88–1.16) | 0.98 (0.77–1.25) | 0.91 | 0.23 |
| MV2 | 1 (ref) | 0.93 (0.85–1.01) | 1.10 (0.96–1.27) | 1.08 (0.84–1.38) | 0.23 | 0.73 |
| Respiratory disease mortality | ||||||
| N of deaths | 660 | 245 | 48 | 10 | ||
| Person-years | 408,647 | 201,157 | 59,280 | 21,031 | ||
| Age-adjusted model | 1 (ref) | 0.71 (0.61–0.83) | 0.63 (0.47–0.85) | 0.57 (0.30–1.07) | 0.001 | 0.001 |
| MV1 | 1 (ref) | 0.79 (0.68–0.93) | 0.70 (0.52–0.96) | 0.65 (0.34–1.22) | 0.02 | 0.02 |
| MV2 | 1 (ref) | 0.87 (0.74–1.02) | 0.82 (0.60–1.12) | 0.76 (0.40–1.44) | 0.20 | 0.12 |
| Other causes of death | ||||||
| N of deaths | 2,921 | 1,226 | 296 | 79 | ||
| Person-years | 406,053 | 199,914 | 58,982 | 20,959 | ||
| Age-adjusted model | 1 (ref) | 0.73 (0.68–0.79) | 0.76 (0.67–0.86) | 0.88 (0.70–1.11) | 0.006 | <0.001 |
| MV1 | 1 (ref) | 0.79 (0.74–0.85) | 0.84 (0.74–0.95) | 0.97 (0.77–1.22) | 0.32 | <0.001 |
| MV2 | 1 (ref) | 0.82 (0.76–0.88) | 0.87 (0.76–0.99) | 1.01 (0.80–1.27) | 0.90 | <0.001 |
Age-adjusted model: adjusted for age (month) and calendar year. Multivariable model 1 (MV1): age-adjusted model plus race (White or non-White), smoking status (never, past, or current [1–14, 15–24, and ≥25 cigarettes per day]), family history of myocardial infarction (yes or no), family history of cancer (yes or no), baseline hypertension (yes or no), alcohol consumption (none, 0.1–4.9, 5–14.9. 15–29.9, or ≥30 g/day), AHEI score 2010 (quintile), total calorie intake (quintile), time spent sitting to watch television (quintile), and BMI (quintile). MV2: MV1 plus aerobic activity (none, 1–59, 60–149, or ≥150 min/week). We applied a 2-year lag time between weight training assessment and the time at risk of death for all models.
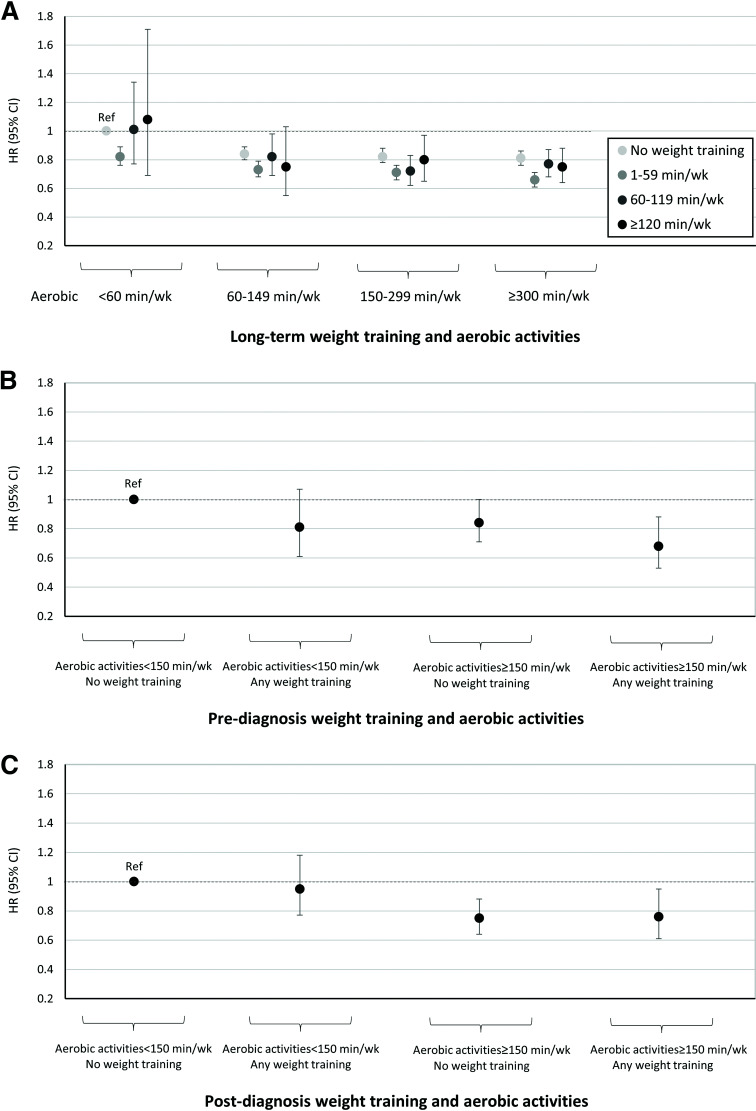
In the joint analysis of long-term aerobic activity and weight training, we observed a 20–34% lower risk of all-cause mortality among participants meeting the recommended aerobic physical activity guideline and performing any weight training versus those performing insufficient aerobic activity with no weight training (Fig. 2A). Stratified analyses showed that the association between long-term weight training and all-cause mortality did not differ by age, BMI, or smoking status (Supplementary Table 2). In sensitivity analyses, using different categories/intensities of aerobic activity and weight training or excluding those with a high level of vigorous-intensity aerobic activity did not substantially change the overall results (Supplementary Tables 3–5). Moreover, excluding each cause-specific death from all-cause mortality did not change the association of weight training and aerobic activity with mortality (Supplementary Table 6). Application of 4-year lag times between cumulative average weight training assessment and the time at risk of death did not materially change the results (Supplementary Table 7). Compared with repeated measures, the use of a single baseline measure of weight training showed a weaker inverse association. Also, when, instead of using the cumulative measure described above, we used the most recent single measure (simple updated) of weight training, we found a modestly stronger inverse association (Supplementary Table 7).
Figure 2.
Joint association of long-term weight training and aerobic activity with all-cause mortality in participants with and without type 2 diabetes (Health Professionals Follow-up Study, 1992–2018). All models were adjusted for the same covariates included in multivariable model shown in Table 2 (participants without type 2 diabetes) and Table 4 (participants with type 2 diabetes). A: Participants without type 2 diabetes. B and C: Participants with type 2 diabetes pre- (B) and post-diagnosis (C).
Association Between Long-term (Pre- and Post-Diagnosis of Type 2 Diabetes) Weight Training and All-Cause Mortality in Participants With Type 2 Diabetes
Among 2,588 participants with type 2 diabetes, we documented 988 deaths. In multivariable-adjusted models including potential confounders and aerobic activities, compared with participants with type 2 diabetes with no pre-diagnosis weight training, participants with type 2 diabetes with 1–59 and 60–149 min/week of pre-diagnosis weight training had 32% (HR 0.68; 95% CI 0.48–0.96) and 27% (HR 0.73; 95% CI 0.54–0.99) lower risks of all-cause mortality, respectively (Table 4). However, participants with ≥150 min/week of pre-diagnosis weight training did not have a decreased risk of all-cause mortality (HR 1.11; 95% CI 0.80–1.55). No significant quadratic association was observed for all-cause mortality (P trend = 0.38; P quadratic = 0.55). Moreover, a moderate level of post-diagnosis weight training was weakly inversely associated with all-cause mortality in participants with type 2 diabetes after adjustment for potential confounders, but the association became statistically nonsignificant after additional adjustment for post-diagnosis aerobic activity. In the dose-response analysis, we observed a similar J-shaped association with all-cause mortality for pre- and post-diagnosis weight training (P nonlinearity = 0.02 for pre-diagnosis and 0.13 for post-diagnosis) (Fig. 1B and C).
Table 4.
Association between pre- and post-diagnosis weight training and all-cause mortality in participants with type 2 diabetes (Health Professionals Follow-up Study)
| Weight training, min/week | P | |||||
|---|---|---|---|---|---|---|
| 0 | 1–59 | 60–149 | ≥150 | Trend | Quadratic | |
| Pre-diagnosis weight training | ||||||
| N of deaths | 854 | 36 | 55 | 43 | ||
| Person-years | 31,581 | 2,104 | 2,864 | 1,632 | ||
| Age-adjusted model | 1 (ref) | 0.62 (0.44–0.88) | 0.63 (0.48–0.84) | 0.87 (0.64–1.19) | 0.06 | 0.05 |
| MV1 | 1 (ref) | 0.67 (0.48–0.96) | 0.69 (0.52–0.93) | 1.00 (0.73–1.38) | 0.20 | 0.53 |
| MV2 | 1 (ref) | 0.68 (0.48–0.96) | 0.73 (0.54–0.99) | 1.11 (0.80–1.55) | 0.38 | 0.55 |
| Post-diagnosis weight training | ||||||
| N of deaths | 994 | 81 | 70 | 51 | ||
| Person-years | 35,246 | 3,140 | 3,698 | 2,278 | ||
| Age-adjusted model | 1 (ref) | 0.96 (0.76–1.21) | 0.65 (0.51–0.83) | 0.82 (0.61–1.09) | 0.28 | 0.03 |
| MV1 | 1 (ref) | 1.05 (0.83–1.32) | 0.77 (0.59–0.99) | 0.98 (0.73–1.31) | 0.64 | 0.12 |
| MV2 | 1 (ref) | 1.06 (0.84–1.35) | 0.83 (0.64–1.08) | 1.08 (0.80–1.45) | 0.31 | 0.13 |
| MV3 | 1 (ref) | 1.26 (0.96–1.66) | 0.91 (0.67–1.24) | 1.04 (0.73–1.49) | 0.11 | 0.40 |
Age-adjusted model: adjusted for age and calendar year. Multivariable model 1 (MV1): age-adjusted model plus race (White or non-White), smoking status (never, past, or current [1–14 or ≥15 cigarettes per day]), family history of myocardial infarction (yes or no), family history of diabetes (yes or no), hypertension (yes or no), high cholesterol (yes or no), duration of diabetes (0–5, 6–10, or ≥10 years), multivitamin use (yes or no), aspirin use (yes or no), alcohol consumption (none, 0.1–4.9, 5–14.9, 15–29.9, or ≥30 g/day), AHEI score 2010 (quintile), and BMI (quintile). MV2: MV1 plus aerobic activity (none, 1–59, 60–149, or ≥150 min/week). MV3: MV2 plus pre-diagnosis weight training and pre-diagnosis aerobic activity.
In the joint analysis of weight training and aerobic activity, we observed 32% (HR 0.68; 95% CI 0.53–0.88 for pre-diagnosis) and 24% (HR 0.76; 95% CI 0.61–0.95 for post-diagnosis) lower risks of all-cause mortality among participants with type 2 diabetes meeting the recommended aerobic physical activity guideline and performing any weight training before and after diagnosis, respectively, compared with those performing insufficient aerobic activity with no weight training before and after diagnosis (Fig. 2B and C and Supplementary Table 8). Moreover, compared with participants with neither pre- nor post-diagnosis weight training, participants with both any pre- and post-diagnosis weight training had a 24% (HR 0.76; 95% CI 0.59–0.97) lower risk of all-cause mortality (Supplementary Table 9).
Conclusions
In this large prospective cohort study of 31,440 U.S. men using up to 13 repeated measures of weight training during 26 years of follow-up, a moderate level of long-term weight training (30–60 min/week) was associated with a lower risk of mortality, independently of aerobic activity. However, no mortality benefit was observed at >150 min/week of weight training, suggesting a J-shaped relationship. Moreover, participants performing the recommended aerobic physical activity (≥150 min/week) and any level of weight training had a 20–34% decreased risk of all-cause mortality, compared with those with insufficient aerobic activity and no weight training. In a subgroup of 2,588 men with type 2 diabetes, we found a similar benefit of mortality risk reduction for pre-diagnosis weight training, but no association was observed for post-diagnosis weight training, independent of aerobic activities. Our study supports the current physical activity guidelines and provides additional evidence for the optimal amount of long-term weight training independently and jointly with aerobic activity to maximize longevity benefits in adults with and without type 2 diabetes.
It is well established that moderate to vigorous aerobic activity is associated with lower risks of major NCDs and mortality (3,4). In adults with type 2 diabetes, moderate to vigorous aerobic physical activity has also been recommended for several health benefits (2). However, whether weight training is independently associated with mortality remains unclear. Using the National Health and Nutrition Examination (21–23) and the National Health Insurance Survey data (24,25), several studies examined the association between frequency of muscle-strengthening activities and mortality. These studies suggested that meeting the physical activity guideline of muscle-strengthening activities (two or more times per week) was associated with a lower risk of mortality. However, some studies showed no significant inverse association after adjustment for aerobic activity or for participants engaging in a higher frequency of muscle-strengthening activities (21,23). Moreover, none of these studies evaluated the dose-response relationship.
A few prospective cohort studies have examined the dose-response relationship of weight training with mortality (8–11). A study of middle-aged U.S. adults from the Aerobics Center Longitudinal Study found that weekly weight training of 1–59 min/week was significantly associated with a 36% lower risk of all-cause mortality, after adjustment for aerobic activity, but the inverse association was attenuated at >60 min/week of weight training: HRs of 0.84 (95% CI 0.53–1.34) for 60–119 min/week and 1.03 (95% CI 0.59–1.80) for ≥120 min/week (10). Another study of older adults from the Cancer Prevention Study 2 Nutrition Cohort showed similar results, suggesting a quadratic relationship between weight training and all-cause mortality (11). Multivariable-adjusted HRs were 0.88 (95% CI 0.82–0.94) for 1–59 min/week, 0.90 (95% CI 0.84–0.97) for 60–119 min/week, and 1.01 (95% CI 0.93–1.09) for ≥120 min/week, compared with no weight training. In the Women’s Health Study, a J-shaped association was also observed in older women, with the lowest mortality shown at 82 min/week of strength training (HR 0.87) (9). The benefits of strength training attenuated until 146 min/week, and mortality increased afterward (HR >1), although the estimates were nonsignificant, with wide CIs. Although the outcome was not mortality, a similar J-shaped association was observed between weight training and risks of major chronic diseases in men from the Health Professionals Follow-up Study (26). Our study including middle-aged and older U.S. men supports these previous findings that a moderate level of weight training (<150 min/week) is associated with an approximately 10–20% lower risk of mortality, independently of aerobic activities.
Consistent with previous studies (8–11), we observed attenuated mortality benefits for participants performing >150 min/week of weight training. The reason for the lack of association or even harmful association with a high level of weight training is unclear. Some biologic evidence suggests that a high level of weight training may cause arterial stiffness and thickening and consequently increase the risk of CVD (27,28). Previous studies reported that the attenuation of mortality benefit with a high level of weight training was more pronounced for CVD outcomes than for others, such as type 2 diabetes and cancer (5). For cause-specific mortality, we also found a stronger J shape with a more steeply increased slope for CVD mortality compared with other causes of death. However, it is unlikely that CVD mortality primarily explains the observed J-shaped association for all-cause mortality because we continued to see a similar pattern after excluding deaths resulting from CVD from total deaths.
Second, methodologic issues in weight training assessment may partially explain the lack of association between a high level of weight training and mortality. Currently, there is no standard objective device to assess weight training. All existing studies (including ours) have relied on self-reported questionnaires, which are prone to measurement error. Duration and intensity of weight training can be variable, and measurement error is likely to be larger at higher levels. If the duration and intensity of weight training were overreported at the higher levels, the magnitude of the association at the higher levels of weight training may have been underestimated, resulting in a nonlinear inverse association between weight training and mortality. Two studies, including ours (9), used repeated measures of weight training to reduce measurement error and better capture long-term activity behavior, but a J-shaped association between weight training and mortality remained. Additionally, the limited distribution of weight training in most study populations compromised the ability to accurately estimate the association at higher levels. Fewer than 5% of study participants performed 150 min/week, and low statistical power hampers precise estimation. Moreover, it is possible that participants with a high level of weight training may include the use of anabolic agents and/or protein-enriched dietary modifications, which may be associated with higher mortality. Residual confounding by these factors may also partially explain the J-shape association. More studies are required to improve more accurate measurement of weight training and investigate the effect of higher amount and intensity of weight training on overall health. Exploring the detailed characteristics of participants with a high level of weight training may provide additional insights to understand the observed J-shaped association with mortality.
Despite the J-shaped association between weight training and mortality, those performing both a high amount of weight training (≥2 h weekly) and sufficient aerobic activity still had a 15–20% lower mortality than those with low levels of both. Higher levels of both aerobic activity and weight training were not necessarily linearly inversely associated with lower mortality, with the lowest mortality observed for those with a high level of aerobic activity and moderate level (up to 1 h weekly) of weight training. The results were consistent after excluding each cause-specific death from all-cause mortality or participants with high-intensity vigorous aerobic activity. This finding alleviates the potential concern about performing a high level of weight training and further suggests that performing both aerobic activity and weight training could provide substantial benefits to improve survival for the general population. A recent large study from the National Health Interview Survey reported that meeting both aerobic (≥150 min/week of moderate or ≥75 min/week of vigorous activity) and muscle-strengthening activities (two or more times per week) was associated with reduction of all-cause and cause-specific mortality, compared with participants meeting the aerobic activity guideline alone or those who did not meet the physical activity guideline for aerobic or muscle-strengthening activities (25). Our study adds new evidence on the optimal amount, beyond the frequency, with which nearly maximal mortality benefits can be achieved by performing ≥300 min/week of aerobic activity with 30–60 min/week of weight training over the life course.
In adults with type 2 diabetes, weight training has been shown to be effective in improving glycemic control and metabolic health in a number of randomized controlled trials (29,30). However, little is known about whether pre- or post-diagnosis weight training is associated with long-term health outcomes in adults with type 2 diabetes. In the current study, we leveraged repeated measures of weight training to examine the association of pre- and post-diagnosis weight training with mortality among 2,588 men with type 2 diabetes. Independently of pre-diagnosis aerobic activity, a moderate level of pre-diagnosis weight training (1–149 min/week vs. no weight training) was associated with lower mortality, whereas no association was shown for post-diagnosis weight training after adjustment for post-diagnosis aerobic activity. However, participants who performed both pre- and post-diagnosis weight training had the lowest mortality compared with those who did not perform pre- or post-diagnosis weight training or neither. Moreover, we clearly observed lower mortality in participants with type 2 diabetes who performed both the recommended level of aerobic activity and any weight training before and after type 2 diabetes diagnosis. Our findings support the current physical activity guideline for type 2 diabetes, which encourages 2–3 days/week of resistance training (2,31) and provide new evidence for the benefits of long-term weight training (pre- and post-diagnosis) for long-term survival in adults with type 2 diabetes.
Several biologic mechanisms support the observed benefits of long-term weight training, independently of and jointly with aerobic activity, on mortality reduction. First, weight training increases muscle mass and strength, which improve glycemic control and insulin sensitivity. Muscle weakness and insulin resistance have been associated with increased risks of major chronic diseases and premature death (32–34). Second, studies have shown that weight training reduces mediating risk factors (e.g., abdominal adiposity, high blood pressure, and diabetes) (20,35,36) and improves metabolic profiles (e.g., lipids and inflammation) (37,38). Moreover, aerobic activity that involves muscle-strengthening aspects (e.g., high-intensity interval training) may have the additional benefit of building muscle mass, which could partially explain the largely reduced mortality in participants performing both aerobic activity and weight training.
Our study has some limitations. First, weight training was assessed using a self-reported questionnaire, and therefore, measurement error is inevitable. However, there is no currently available objective method to validate weight training. Previous studies using the same questionnaire in our cohort reported plausible findings showing a linear inverse association of weight training with long-term waist circumference change (36) and risk of type 2 diabetes (20). Moreover, we used repeated measures of weight training, which allowed us to better capture long-term weight training behavior and reduce within-person measurement error. Second, because of the nature of observational studies, residual confounding was possible. However, we had detailed information on several potential confounders. Third, our study primarily consisted of 91.5% White male health professionals (i.e., dentists, pharmacists, optometrists, osteopath physicians, podiatrists, and veterinarians), which allowed enhanced internal validity as a result of similar demographic characteristics, including social economic status, but it may have reduced the generalizability of our findings (39). Future studies are required to examine the association of long-term weight training with health outcomes in diverse populations, particularly women and/or non-White populations because participation in muscle-strengthening activities and body composition (i.e., muscle and adiposity) may vary by sex and race/ethnicity. In conclusion, engaging in 30–60 min/week of long-term weight training was associated with lower risks of all-cause and cause-specific mortality, independently of aerobic activities. Moreover, the optimal benefit of mortality risk reduction was found in individuals performing above the recommended aerobic activity and any weight training. Similar findings were observed in adults with type 2 diabetes, suggesting the benefit of engaging in moderate levels of pre- and post-diagnosis weight training, with aerobic activity, to reduce mortality.
Article Information
Acknowledgments. The authors thank the participants and staff of the Health Professionals Follow-up Study for their valuable contributions as well as the following state cancer registries for their help: Alabama, Arizona, Arkansas, California, Colorado, Connecticut, Delaware, Florida, Georgia, Idaho, Illinois, Indiana, Iowa, Kentucky, Louisiana, Maine, Maryland, Massachusetts, Michigan, Nebraska, New Hampshire, New Jersey, New York, North Carolina, North Dakota, Ohio, Oklahoma, Oregon, Pennsylvania, Rhode Island, South Carolina, Tennessee, Texas, Virginia, Washington, and Wyoming.
Funding. This work was supported by the National Institutes of Health (U01 CA167552 and R01 HL35464).
The funders had no role in the design or conduct of the study; collection, management, analysis, or interpretation of the data; preparation, review, or approval of the manuscript; or the decision to submit the manuscript for publication.
Duality of Interest. No potential conflicts of interest relevant to this article were reported.
Author Contributions. D.H.L. wrote the first draft of the manuscript. D.H.L. and X.L. conducted analyses. D.H.L., X.L., X.Z., and E.L.G. designed the research. L.F.M.R., H.-K.J., N.K., E.B.R., and F.K.T. provided statistical expertise. All authors contributed to the interpretation of the results and critical revision of the manuscript for important intellectual content and approved the final version of the manuscript. D.H.L. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Footnotes
This article contains supplementary material online at https://doi.org/10.2337/figshare.21397860.
D.H.L. and X.L. contributed equally as first authors. X.Z. and E.L.G. contributed equally as last authors.
References
- 1. Piercy KL, Troiano RP, Ballard RM, et al. The physical activity guidelines for Americans. JAMA 2018;320:2020–2028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Colberg SR, Sigal RJ, Yardley JE, et al. Physical activity/exercise and diabetes: a position statement of the American Diabetes Association. Diabetes Care 2016;39:2065–2079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Arem H, Moore SC, Patel A, et al. Leisure time physical activity and mortality: a detailed pooled analysis of the dose-response relationship. JAMA Intern Med 2015;175:959–967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ekelund U, Tarp J, Steene-Johannessen J, et al. Dose-response associations between accelerometry measured physical activity and sedentary time and all cause mortality: systematic review and harmonized meta-analysis. BMJ 2019;366:l4570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Giovannucci EL, Rezende LFM, Lee DH. Muscle-strengthening activities and risk of cardiovascular disease, type 2 diabetes, cancer and mortality: a review of prospective cohort studies. J Intern Med 2021;290:789–805 [DOI] [PubMed] [Google Scholar]
- 6. Nascimento W, Ferrari G, Martins CB, et al. Muscle-strengthening activities and cancer incidence and mortality: a systematic review and meta-analysis of observational studies. Int J Behav Nutr Phys Act 2021;18:69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Saeidifard F, Medina-Inojosa JR, West CP, et al. The association of resistance training with mortality: a systematic review and meta-analysis. Eur J Prev Cardiol 2019;26:1647–1665 [DOI] [PubMed] [Google Scholar]
- 8. Momma H, Kawakami R, Honda T, Sawada SS. Muscle-strengthening activities are associated with lower risk and mortality in major non-communicable diseases: a systematic review and meta-analysis of cohort studies. Br J Sports Med 2022;56:755–763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kamada M, Shiroma EJ, Buring JE, Miyachi M, Lee IM. Strength training and all-cause, cardiovascular disease, and cancer mortality in older women: a cohort study. J Am Heart Assoc 2017;6:e007677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Liu Y, Lee DC, Li Y, et al. Associations of resistance exercise with cardiovascular disease morbidity and mortality. Med Sci Sports Exerc 2019;51:499–508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Patel AV, Hodge JM, Rees-Punia E, Teras LR, Campbell PT, Gapstur SM. Relationship between muscle-strengthening activity and cause-specific mortality in a large US cohort. Prev Chronic Dis 2020;17:E78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lee DH, Rezende LFM, Ferrari G, et al. Physical activity and all-cause and cause-specific mortality: assessing the impact of reverse causation and measurement error in two large prospective cohorts. Eur J Epidemiol 2021;36:275–285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Wolf AM, Hunter DJ, Colditz GA, et al. Reproducibility and validity of a self-administered physical activity questionnaire. Int J Epidemiol 1994;23:991–999 [DOI] [PubMed] [Google Scholar]
- 14. Chasan-Taber S, Rimm EB, Stampfer MJ, et al. Reproducibility and validity of a self-administered physical activity questionnaire for male health professionals. Epidemiology 1996;7:81–86 [DOI] [PubMed] [Google Scholar]
- 15. Al-Shaar L, Yuan C, Rosner B, et al. Repro-ducibility and validity of a semiquantitative food frequency questionnaire in men assessed by multiple methods. Am J Epidemiol 2021;190:1122–1132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Rimm EB, Giovannucci EL, Stampfer MJ, Colditz GA, Litin LB, Willett WC. Reproducibility and validity of an expanded self-administered semiquantitative food frequency questionnaire among male health professionals. Am J Epidemiol 1992;135:1114–1126; discussion 1127–1136 [DOI] [PubMed] [Google Scholar]
- 17. Chiuve SE, Fung TT, Rimm EB, et al. Alternative dietary indices both strongly predict risk of chronic disease. J Nutr 2012;142:1009–1018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Rich-Edwards JW, Corsano KA, Stampfer MJ. Test of the national death index and Equifax nationwide death search. Am J Epidemiol 1994;140:1016–1019 [DOI] [PubMed] [Google Scholar]
- 19. Stampfer MJ, Willett WC, Speizer FE, et al. Test of the National Death Index. Am J Epidemiol 1984;119:837–839 [DOI] [PubMed] [Google Scholar]
- 20. Grøntved A, Rimm EB, Willett WC, Andersen LB, Hu FB. A prospective study of weight training and risk of type 2 diabetes mellitus in men. Arch Intern Med 2012;172:1306–1312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Dankel SJ, Loenneke JP, Loprinzi PD. Dose-dependent association between muscle-strengthening activities and all-cause mortality: prospective cohort study among a national sample of adults in the USA. Arch Cardiovasc Dis 2016;109:626–633 [DOI] [PubMed] [Google Scholar]
- 22. Evenson KR, Wen F, Herring AH. Associations of accelerometry-assessed and self-reported physical activity and sedentary behavior with all-cause and cardiovascular mortality among US adults. Am J Epidemiol 2016;184:621–632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Zhao G, Li C, Ford ES, et al. Leisure-time aerobic physical activity, muscle-strengthening activity and mortality risks among US adults: the NHANES linked mortality study. Br J Sports Med 2014;48:244–249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kraschnewski JL, Sciamanna CN, Poger JM, et al. Is strength training associated with mortality benefits? A 15year cohort study of US older adults. Prev Med 2016;87:121–127 [DOI] [PubMed] [Google Scholar]
- 25. Zhao M, Veeranki SP, Magnussen CG, Xi B. Recommended physical activity and all cause and cause specific mortality in US adults: prospective cohort study. BMJ 2020;370:m2031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Chomistek AK, Cook NR, Flint AJ, Rimm EB. Vigorous-intensity leisure-time physical activity and risk of major chronic disease in men. Med Sci Sports Exerc 2012;44:1898–1905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Barauna VG, Rosa KT, Irigoyen MC, de Oliveira EM. Effects of resistance training on ventricular function and hypertrophy in a rat model. Clin Med Res 2007;5:114–120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Miyachi M. Effects of resistance training on arterial stiffness: a meta-analysis. Br J Sports Med 2013;47:393–396 [DOI] [PubMed] [Google Scholar]
- 29. Gordon BA, Benson AC, Bird SR, Fraser SF. Resistance training improves metabolic health in type 2 diabetes: a systematic review. Diabetes Res Clin Pract 2009;83:157–175 [DOI] [PubMed] [Google Scholar]
- 30. Lee J, Kim D, Kim C. Resistance training for glycemic control, muscular strength, and lean body mass in old type 2 diabetic patients: a meta-analysis. Diabetes Ther 2017;8:459–473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. O’Hagan C, De Vito G, Boreham CA. Exercise prescription in the treatment of type 2 diabetes mellitus: current practices, existing guidelines and future directions. Sports Med 2013;43:39–49 [DOI] [PubMed] [Google Scholar]
- 32. Someya Y, Tamura Y, Kaga H, et al. Insulin resistance and muscle weakness are synergistic risk factors for silent lacunar infarcts: the Bunkyo Health Study. Sci Rep 2021;11:21093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Someya Y, Tamura Y, Takeno K, et al. Decreased muscle strength of knee flexors is associated with impaired muscle insulin sensitivity in non-diabetic middle-aged Japanese male subjects. Diabetes Ther 2020;11:2401–2410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Hurley BF, Hanson ED, Sheaff AK. Strength training as a countermeasure to aging muscle and chronic disease. Sports Med 2011;41:289–306 [DOI] [PubMed] [Google Scholar]
- 35. Cornelissen VA, Fagard RH. Effect of resistance training on resting blood pressure: a meta-analysis of randomized controlled trials. J Hypertens 2005;23:251–259 [DOI] [PubMed] [Google Scholar]
- 36. Mekary RA, Grøntved A, Despres JP, et al. Weight training, aerobic physical activities, and long-term waist circumference change in men. Obesity (Silver Spring) 2015;23:461–467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Kelley GA, Kelley KS. Impact of progressive resistance training on lipids and lipoproteins in adults: a meta-analysis of randomized controlled trials. Prev Med 2009;48:9–19 [DOI] [PubMed] [Google Scholar]
- 38. Lee DH, de Rezende LFM, Eluf-Neto J, Wu K, Tabung FK, Giovannucci EL. Association of type and intensity of physical activity with plasma biomarkers of inflammation and insulin response. Int J Cancer 2019;145:360–369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Wang P, Giovannucci EL. Are exposure-disease relationships assessed in cohorts of health professionals generalizable?: a comparative analysis based on WCRF/AICR systematic literature reviews. Cancer Causes Control. 5 October 2022 [Epub ahead of print]. DOI: 10.1007/s10552-022-01633-3 [DOI] [PubMed] [Google Scholar]