Abstract
We aim to explore the link between maternal weekly temperature exposure and CHD in offspring and identify the relative contributions from heat and cold and from moderate and extreme atmospheric temperature. From January 2019 to December 2020, newborns who were diagnosed with CHD by echocardiography in the Network Platform for Congenital Heart Disease (NPCHD) from 11 cities in eastern China were enrolled in the present study. We appraised the exposure lag response relationship between temperature and CHDs in the distributed lag nonlinear model and further probed the pooled estimates by multivariate meta-analysis. We further performed the exposure–response curves in extreme temperature (5th percentile for cold and 95th for hot events). We also delve into the cumulative risk ratios (CRRs) of temperature on CHDs in general and subgroups. In this study, 5904 of 983, 523 infants were diagnosed with CHDs. The temperature-CHD combination performed positive significance in two exposure windows, gestational weeks 10–16 and 26–31, and reached the maximum effect in the 28th week. Compared with extreme cold (5th, 6.14℃), these effects were higher in extreme heat (95th, 29.26℃). The cumulative exposure–response curve showed a steep nonlinear rise in the hot tail but showed non-significance at low temperatures. In this range, the CRRs of temperature showed an increment to a ceiling of 3.781 (95% CI: 1.460–10.723). The temperature- CHD curves for both sex groups showed a general growth trend. No statistical significance was observed between these two groups (P = 0.106). The cumulative effect of the temperature related CHD was significant in regions with lower education levels (maximum CRR was 9.282 (3.019–28.535)). A degree centigrade increase in temperature exposure was associated with the increment of CHD risk in the first and second trimesters, especially in extreme heat. Neonates born in lower education regions were more vulnerable to temperature-related CHDs.
Supplementary Information
The online version contains supplementary material available at 10.1007/s11356-022-24396-5.
Keywords: Congenital heart disease, Temperature, Distributed lag nonlinear model, Multivariate meta-analysis, Neonate, Exposure–response effect
Introduction
Congenital heart disease refers to congenital malformations motivated by anormogenesis of the heart and large blood vessels in the embryonic stage. According to the Lancet’s 2019 Global Burden of Disease Report, more than 13 million people suffered from congenital heart disease worldwide, with a cumulative total of 210,000 deaths every year (Su et al. 2022). In China, congenital heart defects are ranked first in birth defect disease and are the leading cause of death among children under 5 years of age (China’s National Health Commission 2019).
Nearly all congenital heart abnormalities occur during the development process of the heart: cell migration, formation of cardiac hemodynamics, death of some cardiomyocytes, directed growth, and cell colonization (Sun et al. 2015). There are many predisposing factors that affect the development of the cardiovascular system and are generally divided into two main types of risk factors: genetic factors (chromosomal abnormalities and genetic mutations) and prenatal environment (including rubella virus and other infections, radiation, medication, and environmental pollution) (NIH 2021; Sun et al. 2015).
Some of the environmental factors influencing the onset of congenital heart disease and their pathogenesis have been elucidated, but there are still a considerable number of unspecified environmental teratogens. In particular, the link between prenatal extreme temperature exposure and congenital abnormalities warrants consideration with plausible mechanisms that support such linkages. First, maternal heat exposure is strongly associated with a series of adverse birth outcomes, indicating the sensitivity of pregnancy to heat exposure (Matthew Francis Chersich et al. 2020a, b). Second, numerous animal studies reposted a causal relationship between high temperatures and offspring abnormalities (Edwards 1969; Edwards et al. 1995; Wells 2002). Third, several epidemiologic and experimental studies have demonstrated that maternal fever, saunas, and antenatal exercise are associated with offspring birth defects (Acs et al. 2005; Davenport et al. 2019; Duong et al. 2011; Lipson et al. 1985; Moretti et al. 2005; Shi et al. 2014). Finally, exposure to certain teratogens may increase during periods of extreme temperatures, such as alcohol use in hot weather.
Meanwhile, the climate change raising concerns perform a negative impact on public health in recent years. In 2017, more than 9 million people were injured by new fire, heat, and hot substances, and 120,632 died from high atmospheric temperature (James et al. 2020). Several animal studies have reported that high temperature triggered teratogenic effects in early cardiac development (Botto et al. 2002; Martinez-Frias et al. 2001). However, there is a paucity of data on the teratogenicity of external temperature-generating sources that change maternal core temperature during pregnancy.
Several epidemiological studies have examined the effects of nonoptimal temperatures on adverse birth outcomes, but most of them mainly focus on the effects of extreme heat (Chersich et al. 2020a, b; Stingone et al. 2019; Wang et al. 2013). Limited studies have observed that congenital heart defects may be associated with maternal nonoptimal exposure during pregnancy. Most of the previous studies mainly focus on the early pregnancy period (3–8 weeks or first trimester) (Agay-Shay et al. 2013; Auger et al. 2017; Jiang et al. 2021; Lin et al. 2018), and little is known about the effect of maternal ambient temperature exposure during the total gestational week on CHD in offspring. Moreover, previous literature mainly illustrated heat exposure rather than full temperature spectrum (Agay-Shay et al. 2013; Auger et al. 2017; Jiang et al. 2021).
Given the above concerns, we performed a time-series study to investigate the impact of ambient atmospheric temperature on CHDs among 11 cities in eastern China. We aim to reveal critical windows of prenatal exposures using distributed lag nonlinear models (DLNM) of temperature.
Materials and methods
Data collection
We collected time-series daily data, including CHD counts and weather variables, from 358 township medical institutions in 11 cities in eastern China. The geographical characteristics of the study area have been reported in our previous literature (Li et al. 2022). The weather indicators in this study were derived from Shanghai Qingyue (http: //data.epmap.org) which has been introduced in an earlier study (Li et al. 2022). Exposure assessments for all residents were represented by the mean daily temperature within a city. We also collected the city-specific daily average relative humidity to control the potential confusion.
The CHD case data were extracted from the NPCHD from Jan 1st, 2019, to Dec 31th, 2020, in 11 cities described in our previous article (Li et al. 2022). In brief, approximately 90.19% of neonates born in the study region accomplished the two-step screening method according to the Chinese National Comprehensive Program for the Prevention and Treatment of Birth Defects during the study period: First, POX (pulse oximetry) plus cardiac auscultation method was used within 3 days after birth. Then, the positive neonates were further confirmed using echocardiography. The adjustment variables of social economics in this study, including the urbanized population and the number of years of education per capita (AYE), were derived from the government work report of China’s seventh census in 2020. The coordinates of each city were derived from Baidu Maps API.
Statistical analysis
First stage analysis
This first-stage regression briefly included a natural cubic B-spline of time with 7 degrees of freedom per year to control seasonal and long-term trends. We used a distributed lag nonlinear model to describe the associations between ambient temperature and visits of neonatal CHDs. Because of the low incidence of CHD, we used the number of weekly CHD visits as the dependent variable. DLNM hypothesized that the exposure effect persists for a distinct time duration and evaluates the impact of various delays by fitting diverse parameter values. We established the DLNM in two steps: first, we modeled a two-dimensional matrix that combined temperature and gestational weeks; then, the two-dimensional matrix was joined to the generalized linear model (GLM) to evaluate the association between ambient temperature and CHDs. Gestational week and relative humidity were then adapted as continuous variables by natural cubic spline function. Our previous publication (Li et al. 2022) based on the same material found positive associations between ambient air pollution and CHD. Hence, we further adjusted ambient PM2.5 concentration level in GLM according to Mostofsky et al.’s literature (Mostofsky et al. 2012). We selected df for relative humidity in the variance analysis according to minimize Akaike information criterion (AIC) (Supplementary Table (SI)). We chose the optimal basic function to clarify the association between exposure and effect by the ANOVA test (Supplementary Table (SII)). Besides, we prolonged the lag weeks to 40 indicating different gestational weeks to scrutinize the critical windows of temperature-CHD combinations to estimate the effects (Zhang et al. 2021).
The structure of DLNM is as follows:
In this model, t is the gestational week of the exposure, E means the expected number of infants with CHDs on week t, α is the interception, represents for the cross-basis function of temperature, β stands for the regression coefficient, ns denotes a natural cubic spline function for nonlinear variables, humid is relative humidity, df stands for the degree of freedom, time is the week of gestation, and PM2.5 is a particulate matter with diameter less than 2.5 µm.
Second stage analysis
We further reduced the relevancy to the temperature-CHD correlation. Multivariate meta-analysis was applied to analyze the pooled estimates on restricted maximum likelihood (REML) introduced by Gasparrini et al.’s previous literature (Gasparrini and Armstrong 2013). The city-specific cumulative temperature-CHD combination was calculated, and multivariate meta-analysis was further applied. We presented the results of cumulative exposure–response curves. We further performed the effect estimates associated with a rise of 1 °C in the 5th (6.14 °C) and the 95th (29.26 °C) in consideration of nonlinear association between temperature and CHDs. To interpret the role of socioeconomic and geographic modifiers, we executed city-specific indicators as meta-predictors accounting for the potential and presented by Cochran Q test and I-squared (I2) statistics. The source of other modifiers, namely, latitude (geographic index), urbanized population, and AYE (socioeconomic indexes), is consistent with our previous article (Li et al. 2022).
Sensitivity analysis
Sex-specific associations between temperature and CHDs were evaluated in subgroup analysis. According to the average years of education in 11 cities, we hierarchized the overall CHD infants by education level (AYE < 10 years and AYE ≥ 10 years). Since the study period covered both pre-pandemic and the COVID-19 pandemic, participants’ exposure to ambient temperature may varied among different time periods. We split the CHDs into two groups by COVID-19 outbreak time (Jan 20th, 2020) to address the exposure bias. We calculated Z-test to assess the differences between subgroups following our previous article (Li et al. 2022).
All statistical analyses were performed using the R 4.0.4 software. A two-tailed P value of less than 0.05 was considered statistically significant.
Results
The distribution of CHD cases and cumulative incidence in 11 cities from Jan 2019 to Dec 2020 was described elsewhere (Li et al. 2022). In brief, 5904 of 983, 523 infants were diagnosed with CHDs. In girls and warm seasons, their cumulative incidence rates of CHDs were higher than in other groups. A description of ambient temperature in the study area between 2018 and 2020 can be found in Table 1. Except for Zhoushan, where temperatures differed slightly, all cities experienced similar daily averages of ambient temperature. Figure 1 displays the distribution of atmospheric temperature and congenital heart disease cases in 11 cities in this study. It showed that a majority of the cities indicated that CHD cases increased along with the higher atmospheric temperature.
Table 1.
The distribution of atmospheric temperature and socioeconomic level in study area from 2018 to 2020 in 11 cities
| City | Latitude | Socioeconomic status | Temperature | PM2.5 | |||||
|---|---|---|---|---|---|---|---|---|---|
| Urbanized population (%) | AYE | Min | P25 | Median | P75 | Max | (μg/m3) | ||
| Huzhou | 30.90 | 83.29 | 9.95 | 1.79 | 11.45 | 18.13 | 24.37 | 32.10 | 26.99 (11.01–75.33) |
| Hangzhou | 30.28 | 65.64 | 11.65 | 3.79 | 11.93 | 19.12 | 25.08 | 31.93 | 30.10 (12.72–76.32) |
| Jiaxing | 31.25 | 71.34 | 10.97 | 3.01 | 11.68 | 18.20 | 24.44 | 32.43 | 29.05 (12.94–73.99) |
| Shaoxing | 30.00 | 71.02 | 10.31 | 4.25 | 11.94 | 19.06 | 25.11 | 32.28 | 31.37 (11.24–78.97) |
| Ningbo | 29.88 | 78.00 | 10.50 | 4.90 | 12.05 | 18.64 | 25.02 | 31.25 | 24.24 (8.23–68.46) |
| Taizhou | 28.68 | 61.98 | 9.72 | 4.93 | 13.28 | 18.72 | 25.28 | 31.72 | 24.40 (7.55–55.98) |
| Wenzhou | 29.08 | 72.16 | 9.88 | 7.30 | 13.33 | 19.17 | 25.38 | 30.88 | 25.94 (8.39–57.12) |
| Jinhua | 28.00 | 68.19 | 10.28 | 3.19 | 12.69 | 19.31 | 25.54 | 31.65 | 30.55 (10.55–112.80) |
| Quzhou | 28.93 | 57.57 | 9.75 | 1.89 | 12.32 | 19.31 | 25.20 | 31.96 | 27.81 (11.73–73.06) |
| Lishui | 28.45 | 61.82 | 9.98 | 3.86 | 13.48 | 19.60 | 25.47 | 31.64 | 22.66 (8.06–52.40) |
| Zhoushan | 30.00 | 71.89 | 11.40 | 5.65 | 11.51 | 18.00 | 23.72 | 28.67 | 16.64 (5.52–49.03) |
AYE, average years of education per capita
Fig. 1.
The distribution of atmospheric temperature and congenital heart disease cases in 11 cities in eastern China, 2019–2020
The overall lag-response curves in the different cities displayed in Fig. 2 showed a degree of centigrade increment in atmospheric temperature on CHDs amid all weeks of gestation. It demonstrated the region-specific aggregate linkage analyzed in the first step and the median of the multivariate meta-analysis anticipated by the main flexible model. More than half cities showed identical trajectory, but other cases exist variation. The temperature-CHD combinations demonstrated positive significance in two exposure windows, namely, during the gestational weeks 8–14 and 20–26, and arrived at the maximum impact in the 24th week (RR = 1.850, 95% CI: 1.335–2.840). The heterogeneity test showed highly significant among 11 cities (P value < 0.001).
Fig. 2.

Overall lag-response curves for a degree centigrade increment in temperature on CHDs during different stages of gestation
Figure 3 reveals the cumulative effects for temperature-related CHDs in the meta-analysis. The plot showed an increase in risk for CHDs for high temperatures, with a point of minimum CHD cases at about 18.5 °C. There was a steep nonlinear rise in the CRRs of temperature on CHDs in the hot tail but showed non-significance at low temperature. In this range, the CRRs of temperature showed a boost to a ceiling of 3.781 (95% CI: 1.460–10.723).
Fig. 3.

Overall lag-response curves for a degree centigrade increment in temperature exposure on CHDs among different atmospheric temperatures
The large differentiation in temperature-CHD curves between cities may be explained by different patterns of city-level factors—52.50% of the fluctuation was owing to authentic heterogeneousness among cities. Table 2 demonstrates the effects of meta-analysis after modifying city-specific socioeconomic and geographic factors (latitude, urbanized population, and years of education). The modifier for the urban population visibly reduced I2 to 51.8%, though the Cochran Q test remained significant. Meanwhile, the heterogeneity expanded after being adjusted for education. We also reported the city-level factors by the comparison of the AIC and BIC criteria to represent the importance in Table 2.
Table 2.
Statistics of intercity heterogeneity in random-effect models
| Cochran Q test | I2 | Information criteria | ||||
|---|---|---|---|---|---|---|
| Q | P | % | AIC | BIC | ||
| Temperature | ||||||
| Intercept only | 210.38 | 0.000 | 52.50 | − 234.40 | − 65.07 | |
| Latitude | 182.66 | 0.000 | 50.70 | − 139.65 | 47.83 | |
| Urban population | 179.56 | 0.000 | 49.90 | − 111.16 | 76.32 | |
| AYE | 197.18 | 0.000 | 54.40 | − 150.00 | 37.48 | |
AIC, Akaike information criterion; BIC, Bayesian information criterion; AYE, average years of education per capita
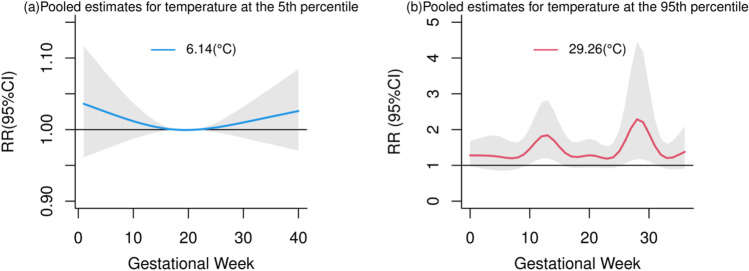
Figure 4 shows the RRs for CHDs associated with a centigrade increment in temperature exposures across the 40 lags gestational weeks. We failed to observe statistical significance with CHDs among different gestational weeks in the 5th percentile of temperature (6.14℃) (Fig. 4a). Positive associations were observed with more CHD acceleration at the first and second trimesters of gestation in the 95th percentile of temperature (29.26℃) (Fig. 4b). We found the increase of CHDs is related to temperature exposure in the second trimester of gestation.
Fig. 4.
The risk ratios for temperature-related CHDs for a centigrade increment in temperature (℃) when the reference is set at the 5th percentile (6.14℃) (a) and at the 95th percentile (29.26℃) (b) across the different gestational weeks
Figure 5a and b illustrate that the temperature-CHD effect varied in different sexes. In both the boys’ and girls’ groups, positive associations between CHD and atmospheric temperature were found, and no statistical significance was observed between the two groups (P = 0.106, see Table 3). The temperature-CHD curves for both groups showed a general growth trend, with the peaked CRRs of 2.041 (95% CI: 1.075–3.874) in boys and 4.210 (95% CI: 1.518–11.675) in girls. The cumulative impact of the temperature-CHD combination was significant in lower education level regions (see Fig. 5c and d), and the maximum CRR was 9.282 (3.019–28.535). No statistical significance was observed in the higher education level group.
Fig. 5.
The cumulative effects on the association between temperature and CHDs stratified by sex and education
Table 3.
Cumulative risk ratios for overall, sex-specific subgroups on temperature-related CHDs in eastern China, from 2019 to 2020
| Temperature | Z test | |||
|---|---|---|---|---|
| Z | P | |||
| Subgroup | ||||
| Sex | Boy | 2.041 (1.075, 3.874)* | 1.178 | 0.106 |
| Girl | 4.210 (1.518, 11.675)* | |||
| AYE | High | 1.911 (0.588, 6.210) | 1.903 | 0.026 |
| Low | 9.282 (3.019, 28.535)* | |||
High, AYE > 10 years; low, AYE ≤ 10 years. * stands for statistical significance
Compared to COVID-19 pandemic time period, the effect estimates for ambient temperature-related CHD were higher than those in the pre-pandemic time (see Fig. 6). The temperature-CHD curves for the pre-pandemic group presented fluctuation within a narrow range in the first second trimester and sharply reached the peak with CRR of 5.198 (95% CI: 2.501–11.038) in Fig. 6a. The highest estimate of CHD-related ambient temperature during COVID-19 pandemic was in the 39th gestational week, with CRR of 2.036 (95% CI: 1.477–2.890).
Fig. 6.
The cumulative effects on the association between ambient temperature and CHD stratified by COVID-19 outbreak in China
Discussion
Using population-based data for a significant number of neonates in all trimesters in a multicity study, we illustrate that exposure to heat was associated with an increased risk of neonatal congenital heart diseases with nonlinear temperature effects. The suspicious exposure window was in the first and second gestational trimesters. We further pinpointed the potential adjustments by socioeconomic and geographical characteristics.
This study confirmed evidence from previous literature that the growth in multiple CHD visits was associated with maternal exposure to heat during pregnancy. Several studies have assessed the impact of temperature exposure on congenital heart anomalies (Agay-Shay et al. 2013; Auger et al. 2017; Jiang et al. 2021; Lin et al. 2018; Stingone et al. 2019). A case–control study conducted in the USA found that daily maximum temperature (> 90%) was associated with ventricular septal defects (VSDs) (Lin et al. 2018). A retrospective cohort study in Israel illustrated that risk of multiple CHDs increased 1.13-fold (95% CI = 1.06, 1.21) and isolated ASDs 1.10-fold (95% CI = 1.02, 1.19) when exposed to the extreme heat (Agay-Shay et al. 2013). A self-reported study showed an increased risk of CHD when exposed to heat (~ 38 °C) in early pregnancy (Judge et al. 2004). Some studies showed the opposite. A case–control study focused on monthly air pollutants/temperature interaction on CHDs in the first trimester in China showed no statistical significance (Jiang et al. 2021). No association was found in Tikkanen et al.’s study aimed at assessing the effect between self-reported exposure to temperatures during the first trimester of pregnancy high-temperature environment and the risk of cardiac malformation (Tikkanen and Heinonen 1991).
There is no consensus regarding the critical windows of exposure to heat during pregnancy. Most of the previous studies mainly used exposure assessment based on early pregnancy in gestational weeks 3–8 according to the general agreement about heart development (Auger et al. 2017; Lin et al. 2018; Miao et al. 2021; Tan and Lewandowski 2020). Agay-Shay et al.’s study in Israel reported that extreme heat during weeks 3–8 of pregnancy increased the incidence of CHDs using the nearest monitoring station approach (Agay-Shay et al. 2013). Auger found that several heat days (> 30℃) were associated with a higher risk of noncritical CHDs in early pregnancy (Auger et al. 2017). An epidemiological study in southeast China investigating maternal temperature viability in gestation and birth defects among 4.78 million newborns showed that the susceptive exposure window for cardiac anomalies was in weeks 3–8 of gestation and highest in the 5th week (Miao et al. 2021). Lin et al.’s case–control study found that 3–11 days of extreme heat events (90th percentile of daily maximum temperature) during summer and spring were significantly associated with ventricular septal defects (Lin et al. 2018).
However, Wen et al.’s study also failed to spot significant effects of maternal temperature-related CHDs during the early pregnancy (Jiang et al. 2021). Except for early pregnancy, our study also found that heat exposure in later gestation was associated with overall congenital heart defects. Another study also considered that the susceptivity window of atmospheric temperature may not be along with the critical stage of cardiac development (Stingone et al. 2019). The mechanism of later pregnancy exposure and cardiac developmental defects is unclear. Precise procedures of cardiac physical development that are more vulnerable to heat may partly account for the result (Jiang et al. 2021). Most notably, whether the results of different studies can be summarized remains to be discussed. The difference in effects between studies may, in part, be explained by variants of climate zones among studies. It is noteworthy that several studies reported no statistical significance because their observations were performed in countries with moderate climates where is seldom to reach the threshold level to trigger the defect (Van Zutphen et al. 2012).
In subgroup analysis, no statistical sex difference in temperature-related CHDs was observed, and we did not find any studies that perform a similar hierarchical analysis. However, previous studies indicated fetal-maternal interactions and sex differences in growth trajectories (Deegan et al. 2021; Intapad et al. 2014). A previous study pointed to the opposite view that male embryos are more susceptible to dysplasia than females (Sanghavi and Rutherford 2014). Our study confirmed the results in previous literature that neonates in lower education regions tended to be more susceptible to CHD-temperature combinations. The education level is positively related to economic development in our study area. People in underdeveloped regions often have fewer ways to avoid extreme weather, which may lead to exposure bias.
The mechanism of temperature with heart defects was not clear yet, but some of the literature illustrated potential mediators in maternal heat exposure and cardiac malformation in offspring. Animal studies found that maternal exposure to high temperature probably induces fetal cell death, eventually resulting in congenital malformations (Bennett 2010). In addition, it is reported that hyperthermia induced by external heat sources or fever interferes with protein synthesis through heat shock proteins, resulting in embryo death, growth retardation, and developmental defects (Edwards et al. 2003). Previous studies reported that the increment of body temperature caused by fever was linked to congenital heart malformation (Shi et al. 2014). During extreme temperatures, exposure to certain teratogens may increase, such as alcoholic beverage in summer. Hence, the elevated atmospheric temperature can further conduct a reasonable impact on congenital cardiac defects. The variation for CHD-temperature exposure–response curves between pre- and post-COVID-19 outbreak confirmed the robustness of maternal external temperature exposure and CHD onset in offspring CHDs. Pregnant women might have more opportunity to heat exposure in pre-pandemic time period which lead to higher estimates for these effects.
To the best of our knowledge, this is the first study to scrutinize the weekly effect of maternal ambient temperature exposure on CHDs in offspring in multicities in China. We evaluated weekly effects rather than the commonly considered organogenetic period to quantify the effects during the whole pregnancy period. We are also the first to use the daily mean temperature rather than extreme heat to comprehensively appraise full-spectrum temperature-CHD effects. Using NPCHD data with good sample representation and less biased estimates is an exclusive advantage of this study.
Our study is subject to several limitations. First, the temperature exposure estimates in this study applied the mean value of the city-specific monitoring station approach, which may introduce bias in individual exposure assessment. Second, maternal and family history factors, maternal BMI, maternal smoking, and alcohol consumption during pregnancy that are highly associated with the occurrence of CHDs were not considered in this study, which may affect the size of the estimates. Then, the approach of the random-effect model dealing with no small heterogeneity may result in potential bias in this study. In addition, due to the limitations of time-series studies, we simply set the gestational week of all mothers at 40 weeks, which may lead to potential bias in the selection of peak/valley week on temperature-related CHDs. Lastly, indoor temperature was not estimated in this study, which is a potential confounding to evaluate temperature exposure for pregnant woman.
Conclusion
In this study, we pinpointed that a degree centigrade increase in temperature exposure was related to the increase in CHD risk in the first and second trimesters. Statistical significance was observed in cumulative effects in temperature-CHD combinations in extreme heat. We then clarified education act as a potential modifier in these effects. Further research is needed to authenticate the current findings in present work.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
The authors wish to thank all the staff members at the Network Platform of Congenital Heart Disease, National Clinical Research Center for Child Health, and Shanghai Qingyue for their strong support of this study.
Author contribution
All authors contributed to the study conception and design. Material preparation and data collection were performed by Ting Wu, Wei Cheng, Lulu Pan, Jing An, Jing Li, Yueqin Jin, Hongliang Lou, Chengyin Huang, Junqiu Xu, and Yongliang Lei. Data analysis was performed by Die Li, Weize Xu, and Feixia Pan. The first draft of the manuscript was written by Weize Xu and Die Li. Zehua Shao revised this draft critically for important intellectual content. All authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Funding
This research was supported by the Zhejiang Provincial Natural Science Foundation of China under Grant (No. LQ22H260004).
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Declarations
Ethics approval
The study was approved by the Ethical Committee of Children’s Hospital, Zhejiang University School of Medicine.
Consent to participate
Not applicable.
Consent for publication
Not applicable in this paper.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Weize Xu, Die Li, Zehua Shao and Yanqin You contributed equally to this article.
References
- Acs N, Banhidy F, Puho E, Czeizel AE. Maternal influenza during pregnancy and risk of congenital abnormalities in offspring. Birth Defects Res A-Clin Mole Teratol. 2005;73(12):989–996. doi: 10.1002/bdra.20195. [DOI] [PubMed] [Google Scholar]
- Agay-Shay K, Friger M, Linn S, Peled A, Amitai Y, Peretz C. Ambient temperature and congenital heart defects. Hum Reprod. 2013;28(8):2289–2297. doi: 10.1093/humrep/det244. [DOI] [PubMed] [Google Scholar]
- Auger N, Fraser WD, Sauve R, Bilodeau-Bertrand M, Kosatsky T. Risk of congenital heart defects after ambient heat exposure early in pregnancy. Environ Health Perspect. 2017;125(1):8–14. doi: 10.1289/ehp171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett GD. Hyperthermia: malformations to chaperones. Birth Defects Res B-Dev Reprod Toxicol. 2010;89(4):279–288. doi: 10.1002/bdrb.20254. [DOI] [PubMed] [Google Scholar]
- Botto LD, Erickson JD, Mulinare J, Lynberg MC, Liu YC. Maternal fever, multivitamin use, and selected birth defects: evidence of interaction? Epidemiology. 2002;13(4):485–488. doi: 10.1097/00001648-200207000-00019. [DOI] [PubMed] [Google Scholar]
- Chersich MF, Minh Duc P, Areal A, Haghighi MM, Manyuchi A, Swift CP, Wernecke B, Robinson M, Hetem R, Boeckmann M, Hajat S, Climate Change Heat-Hlth Study, G Associations between high temperatures in pregnancy and risk of preterm birth, low birth weight, and stillbirths: systematic review and meta-analysis. Bmj-British Med J. 2020;371:m3811. doi: 10.1136/bmj.m3811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chersich MF, Pham MD, Areal A, Haghighi MM, Manyuchi A, Swift CP, Wernecke B, Robinson M, Hetem R, Boeckmann M, Hajat S, Climate Change Heat-Hlth Study, G Associations between high temperatures in pregnancy and risk of preterm birth, low birth weight, and stillbirths: systematic review and meta-analysis. Bmj-British Med J. 2020;371:m3811. doi: 10.1136/bmj.m3811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- China’s National Health Commission (2019) Report on the development of maternal and child health in China (2019). http://www.nhc.gov.cn/fys/s7901/201905/bbd8e2134a7e47958c5c9ef032e1dfa2.shtml. Accessed 28 Dec 2022
- Davenport MH, Yoo C, Mottola MF, Poitras VJ, Garcia AJ, Gray CE, Barrowman N, Davies GA, Kathol A, Skow RJ, Meah VL, Riske L, Sobierajski F, James M, Nagpal TS, Marchand A-A, Slater LG, Adamo KB, Barakat R, Ruchat S-M (2019) Effects of prenatal exercise on incidence of congenital anomalies and hyperthermia: a systematic review and meta-analysis. Br J Sports Med 53(2):116-+. 10.1136/bjsports-2018-099653 [DOI] [PubMed]
- Deegan DF, Nigam P, Engel N. Sexual dimorphism of the heart: genetics, epigenetics, and development. Front Cardiovasc Med. 2021;8:668252. doi: 10.3389/fcvm.2021.668252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duong HT, Hashmi SS, Ramadhani T, Canfield MA, Scheuerle A, Waller DK, Natl Birth Defects Prevention, S Maternal use of hot tub and major structural birth defects. Birth Defects Res A-Clin Mole Teratol. 2011;91(9):836–841. doi: 10.1002/bdra.20831. [DOI] [PubMed] [Google Scholar]
- Edwards MJ. Congenital defects in guinea pigs - fetal resorptions, abortions, and malformations following induced hyperthermia during early gestation. Teratology. 1969;2(4):313–0. doi: 10.1002/tera.1420020406. [DOI] [PubMed] [Google Scholar]
- Edwards MJ, Saunders RD, Shiota K. Effects of heat on embryos and foetuses. Int J Hyperth. 2003;19(3):295–324. doi: 10.1080/0265673021000039628. [DOI] [PubMed] [Google Scholar]
- Edwards MJ, Shiota K, Smith MSR, Walsh DA. Hyperthermia and birth-defects. Reprod Toxicol. 1995;9(5):411–425. doi: 10.1016/0890-6238(95)00043-a. [DOI] [PubMed] [Google Scholar]
- Gasparrini A, Armstrong B. Reducing and meta-analysing estimates from distributed lag non-linear models. Bmc Med Res Methodol. 2013;13:1. doi: 10.1186/1471-2288-13-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Intapad S, Ojeda NB, Dasinger JH, Alexander BT. Sex differences in the developmental origins of cardiovascular disease. Physiology. 2014;29(2):122–132. doi: 10.1152/physiol.00045.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James SL, Lucchesi LR, Bisignano C, Castle CD, Dingels ZV, Fox JT, Hamilton EB, Henry NJ, McCracken D, Roberts NLS, Sylte DO, Ahmadi A, Ahmed MB, Alahdab F, Alipour V, Andualem Z, Antonio CAT, Arabloo J, Badiye AD, Bagherzadeh M, Banstola A, Barnighausen TW, Barzegar A, Bayati M, Bhaumik S, Bijani A, Bukhman G, Carvalho F, Crowe CS, Dalal K, Daryani A, Nasab MD, Do HT, Do HP, Endries AY, Fernandes E, Filip I, Fischer F, Fukumoto T, Gebremedhin KBB, Gebremeskel GG, Gilani SA, Haagsma JA, Hamidi S, Hostiuc S, Househ M, Igumbor EU, Ilesanmi OS, Irvani SSN, Jayatilleke AU, Kahsay A, Kapoor N, Kasaeian A, Khader YS, Khalil IA, Khan EA, Khazaee-Pool M, Kokubo Y, Lopez AD, Madadin M, Majdan M, Maled V, Malekzadeh R, Manafi N, Manafi A, Mangalam S, Massenburg BB, Meles HG, Menezes RG, Meretoja TJ, Miazgowski B, Miller TR, Mohammadian-Hafshejani A, Mohammadpourhodki R, Morrison SD, Negoi I, Nguyen TH, Nguyen SH, Nguyen CT, Nixon MR, Olagunju AT, Olagunju TO, Padubidri JR, Polinder S, Rabiee N, Rabiee M, Radfar A, Rahimi-Movaghar V, Rawaf S, Rawaf DL, Rezapour A, Rickard J, Roro EM, Roy N, Safari-Faramani R, Salamati P, Samy AM, Satpathy M, Sawhney M, Schwebel DC, Senthilkumaran S, Sepanlou SG, Shigematsu M, Soheili A, Stokes MA, Tohidinik HR, Tran BX, Valdez PR, Wijeratne T, Yisma E, Zaidi Z, Zamani M, Zhang ZJ, Hay SI, Mokdad AH. Epidemiology of injuries from fire, heat and hot substances: global, regional and national morbidity and mortality estimates from the Global Burden of Disease 2017 study. Inj Prev. 2020;26(SUPP_1):36–45. doi: 10.1136/injuryprev-2019-043299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang W, Liu Z, Ni B, Xie W, Zhou H, Li X. Independent and interactive effects of air pollutants and ambient heat exposure on congenital heart defects. Reprod Toxicol. 2021;104:106–113. doi: 10.1016/j.reprotox.2021.07.007. [DOI] [PubMed] [Google Scholar]
- Judge CM, Chasan-Taber L, Gensburg L, Nasca PC, Marshall EG. Physical exposures during pregnancy and congenital cardiovascular malformations. Paediatr Perinat Epidemiol. 2004;18(5):352–360. doi: 10.1111/j.1365-3016.2004.00586.x. [DOI] [PubMed] [Google Scholar]
- Li D, Xu W, Qiu Y, Pan F, Lou H, Li J, Jin Y, Wu T, Pan L, An J, Xu J, Cheng W, Tao L, Lei Y, Huang C, Yin F, Shu Q. Maternal air pollution exposure and neonatal congenital heart disease: a multi-city cross-sectional study in eastern China. Int J Hyg Environ Health. 2022;240:113898–113898. doi: 10.1016/j.ijheh.2021.113898. [DOI] [PubMed] [Google Scholar]
- Lin S, Lin ZQ, Ou YQ, Soim A, Shrestha S, Lu Y, Sheridan S, Luben TJ, Fitzgerald E, Bell E, Shaw GM, Reefhuis J, Langlois PH, Romitti P, Feldkamp ML, Malik S, Pantea C, Nayak S, Hwang SA, Browne M, Natl Birth Defects Prevention, S Maternal ambient heat exposure during early pregnancy in summer and spring and congenital heart defects - a large US population-based, case-control study. Environ Int. 2018;118:211–221. doi: 10.1016/j.envint.2018.04.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipson A, Webster W, Edwards M. Sauna and birth-defects. Teratology. 1985;32(1):147–148. doi: 10.1002/tera.1420320120. [DOI] [PubMed] [Google Scholar]
- Martinez-Frias ML, Mazario MJG, Caldas CF, Gallego MPC, Bermejo E, Rodriguez-Pinilla E. High maternal fever during gestation and severe congenital limb disruptions. Am J Med Genet. 2001;98(2):201–203. doi: 10.1002/1096-8628(20010115)98:2<201::Aid-ajmg1031>3.0.Co;2-p. [DOI] [PubMed] [Google Scholar]
- Miao H, Wu H, Zhu Y, Kong L, Yu X, Zeng Q, Chen Y, Zhang Q, Guo P, Wang D. Congenital anomalies associated with ambient temperature variability during fetal organogenesis period of pregnancy: evidence from 4.78 million births. Sci Total Environ. 2021;798:149305. doi: 10.1016/j.scitotenv.2021.149305. [DOI] [PubMed] [Google Scholar]
- Moretti ME, Bar-Oz B, Fried S, Koren G. Maternal hyperthermia and the risk for neural tube defects in offspring systematic review and meta-analysis. Epidemiology. 2005;16(2):216–219. doi: 10.1097/01.ede.0000152903.55579.15. [DOI] [PubMed] [Google Scholar]
- Mostofsky E, Schwartz J, Coull BA, Koutrakis P, Wellenius GA, Suh HH, Gold DR, Mittleman MA. Modeling the association between particle constituents of air pollution and health outcomes. Am J Epidemiol. 2012;176(4):317–326. doi: 10.1093/aje/kws018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NIH (2021) Congenital heart disease. https://www.nhs.uk/conditions/congenital-heart-disease/. Accessed 28 Dec 2022
- Sanghavi M, Rutherford JD. Cardiovascular physiology. Circulation. 2014;130(12):1003–1008. doi: 10.1161/circulationaha.114.009029. [DOI] [PubMed] [Google Scholar]
- Shi QY, Zhang JB, Mi YQ, Song Y, Ma J, Zhang YL. Congenital heart defects and maternal fever: systematic review and meta-analysis. J Perinatol. 2014;34(9):677–682. doi: 10.1038/jp.2014.76. [DOI] [PubMed] [Google Scholar]
- Stingone JA, Luben TJ, Sheridan SC, Langlois PH, Shaw GM, Reefhuis J, Romitti PA, Feldkamp ML, Nembhard WN, Browne ML, Lin S, National Birth Defects Prevention, S Associations between fine particulate matter, extreme heat events, and congenital heart defects. Environ Epidemiol (Philadelphia, Pa) 2019;3(6):e071. doi: 10.1097/ee9.0000000000000071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su Z, Zou Z, Hay SI, Liu Y, Li S, Chen H, Naghavi M, Zimmerman MS, Martin GR, Wilner LB, Sable CA, Murray CJL, Kassebaum NJ, Patton GC, Zhang H. Global, regional, and national time trends in mortality for congenital heart disease, 1990–2019: an age-period-cohort analysis for the Global Burden of Disease 2019 study. Eclinicalmedicine. 2022;43:101249. doi: 10.1016/j.eclinm.2021.101249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun R, Liu M, Lu L, Zheng Y, Zhang P. Congenital heart disease: causes, diagnosis, symptoms, and treatments. Cell Biochem Biophys. 2015;72(3):857–860. doi: 10.1007/s12013-015-0551-6. [DOI] [PubMed] [Google Scholar]
- Tan CMJ, Lewandowski AJ. The transitional heart: from early embryonic and fetal development to neonatal life. Fetal Diagn Ther. 2020;47(5):373–386. doi: 10.1159/000501906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tikkanen J, Heinonen OP (1991) Maternal hyperthermia during pregnancy and cardiovascular malformations in the offspring. Eur J Epidemiol 7(6):628–635. <Go to ISI>://WOS:A1991GR78300007 [DOI] [PubMed]
- Van Zutphen AR, Lin S, Fletcher BA, Hwang SA. A population-based case-control study of extreme summer temperature and birth defects. Environ Health Perspect. 2012;120(10):1443–1449. doi: 10.1289/ehp.1104671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Williams G, Guo Y, Pan X, Tong S. Maternal exposure to heatwave and preterm birth in Brisbane, Australia. Bjog-an Int J Obstetrics Gynaecol. 2013;120(13):1631–1641. doi: 10.1111/1471-0528.12397. [DOI] [PubMed] [Google Scholar]
- Wells JCK. Thermal environment and human birth weight. J Theor Biol. 2002;214(3):413–425. doi: 10.1006/jtbi.2001.2465. [DOI] [PubMed] [Google Scholar]
- Zhang Q, Sun S, Sui XM, Ding L, Yang M, Li CL, Zhang C, Zhang XJ, Hao JH, Xu YC, Lin SL, Ding R, Cao JY. Associations between weekly air pollution exposure and congenital heart disease. Sci Total Environ. 2021;757:143821. doi: 10.1016/j.scitotenv.2020.143821. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.