Highlights
-
•
High temperature increases 8-OHdG and dsDNA expressions in tissues of American oyster.
-
•
High temperature induces cellular apoptosis and oxidative stress in oysters.
-
•
High temperature decreases extrapallial fluid protein concentrations in oysters.
Keywords: Heat stress, dsDNA breaks, HSP70, Caspase 3, Extrapallial fluid, Marine mollusks
Abbreviations: BAX, bcl-2-associate X; BSA, bovine serum albumin; CAS-3, caspase-3; dsDNA, double-stranded DNA; DSBs, double-stranded breaks; EP, extrapallial; γ-H2AX, γ-histone family member X; HSP70, heat shock protein 70; 8-OHdG, 8‑hydroxy-2′-deoxyguanosine; PBS, Phosphate buffer saline; qRT-PCR, quantitative real-time polymerase chain reaction; ssDNA, single-stranded DNA; SSBs, single-stranded breaks; TUNEL, terminal deoxynucleotidyl transferase (TdT) dUTP nick-end labeling
Abstract
Global temperature is increasing due to anthropogenic activities and the effects of elevated temperature on DNA lesions are not well documented in marine organisms. The American oyster (Crassostrea virginica, an edible and commercially important marine mollusk) is an ideal shellfish species to study oxidative DNA lesions during heat stress. In this study, we examined the effects of elevated temperatures (24, 28, and 32 °C for one-week exposure) on heat shock protein-70 (HSP70, a biomarker of heat stress), 8‑hydroxy-2′-deoxyguanosine (8-OHdG, a biomarker of pro-mutagenic DNA lesion), double-stranded DNA (dsDNA), γ-histone family member X (γH2AX, a molecular biomarker of DNA damage), caspase-3 (CAS-3, a key enzyme of apoptotic pathway) and Bcl-2-associated X (BAX, an apoptosis regulator) protein and/or mRNA expressions in the gills of American oysters. Immunohistochemical and qRT-PCR results showed that HSP70, 8-OHdG, dsDNA, and γH2AX expressions in gills were significantly increased at high temperatures (28 and 32 °C) compared with control (24°C). In situ TUNEL analysis showed that the apoptotic cells in gill tissues were increased in heat-exposed oysters. Interestingly, the enhanced apoptotic cells were associated with increased CAS-3 and BAX mRNA and/or protein expressions, along with 8-OHdG levels in gills after heat exposure. Moreover, the extrapallial (EP) fluid (i.e., extracellular body fluid) protein concentrations were lower; however, the EP glucose levels were higher in heat-exposed oysters. Taken together, these results suggest that heat shock-driven oxidative stress alters extracellular body fluid conditions and induces cellular apoptosis and DNA damage, which may lead to increased 8-OHdG levels in cells/tissues in oysters.
1. Introduction
Anthropogenic greenhouse gas emissions are causing a rise in global temperature, which has an impact on marine and coastal biota [1]. Rising temperatures in the coastal and oceanic environments may pose more harmful effects when compared with the terrestrial environment [2]. The increased sea surface water temperature has documented effects on growth, reproduction, and development in marine organisms (e.g., mussels, clams, corals, oysters, snails, fishes, etc.) [3]. For example, global warming is increasingly and profoundly threatening teleost fishes, resulting in an uncertain future for both fish diversity and global fisheries [4]. Notably, climate-driven warming reduces growth and impairs reproductive functions (i.e., gametogenesis to spawning) of teleost species over large spatial scales [4,5]. Similarly, high temperature leads to decreased gamete production in marine invertebrates such as the American oyster, Crassostrea virginica [6,7], and Atlantic Sea urchin, Arbacia punctulata [2], along with other species. In addition, high temperature increases the oxygen consumption rate in zebra mussel, Dreissena polymorpha [8], and crayfish (Orconectes immunis) [9]. Therefore, it is predicted that heat stress directly influences the many life stages (i.e., larvae, juvenile, young, and adult) and the physiological functions of marine organisms [10], [11], [12].
In addition to alteration in physiological functions, heat stress also causes up- and/or down-regulation of numerous genes and proteins related to various molecular and signaling pathways in terrestrial and aquatic organisms [13], [14], [15]. Recent genome-wide studies identified various heat shock proteins (HSPs) and transcription factors under heat stress in marine invertebrates including marine bivalve mollusks [13,16]. HSPs are chaperon proteins those help in stabilizing protein folding by binding with non-native proteins [17,18]. Remarkably, HSPs are a superfamily of genes and respond to a wide variety of environmental stressors such as cold shock, hypoxia (low dissolved oxygen, DO <2.0 mg/L), heat stress, etc. [19]. Chaperon proteins such as HSP20, HSP60, HSP70, HSP90, and HSP110 are the best studied HSPs in terrestrial and aquatic organisms [16,17,20]. Among them, HSP70 is the most prominent and highly characterized stress protein in animals [21], [22], [23]. In addition, heat shock cognate protein-70 (HSC70) is a constitutively expressed molecular chaperone that belongs to the HSP70 family [24]. Notably, HSC70 shares some structural similarities with HSP70, but it has different functional properties compared with HSP70 and other HSPs [25]. Especially, HSP70 is found to be environmentally inducible in cells and tissues of all organisms [23,25]. Moreover, HSP70 protects the animal body from various environmental stressors like high temperatures, toxicants, infections, etc. [26], [27], [28]. It can also induce wound healing in human tissues by upregulating protein [29]. Thus, HSP70 is considered a potential molecular chaperone biomarker in terrestrial and aquatic organisms exposed to environmental stress [12,30,31].
Environmental stressors like heat stress can lead to drastically increased reactive oxygen and reactive nitrogen species (ROS/RNS) that can attack proteins, lipids, RNA, and DNA [32,33]. The molecular and biochemical responses to heat stress have been studied extensively in terrestrial and aquatic organisms [34], [35], [36]; however, the effects of high temperatures on DNA strand breaks (i.e., single-stranded breaks [SSBs] and double-stranded breaks [DSBs]) are not well documented in oysters. It has been shown that heat stress increases single-stranded and double-stranded DNA breaks in hemocytes (i.e., free cells of mussel hemolymph that play fundamental roles in transport nutrients, detoxification of toxic compounds, as well as an immune defense; [37,38]) of invasive and native marine mussels (Mytilus galloprovincialis and Mytilus californianus) [39], thus DNA SSBs and DSBs can be used as specific molecular biomarkers in marine mollusks during heat stress.
8‑hydroxy-2′-deoxyguanosine (8-OHdG) is the modified base of DNA and is considered one of the best-characterized molecular biomarkers in vertebrates [40,41]. 8-OHdG is the result of oxidative DNA damage when deoxyguanosine, a component of DNA, is oxidized [41], [42], [43]. Notably, out of 20 different oxidative products that have been characterized, 8-OHdG is considered the major element of oxidative DNA damage [43]. Numerous studies in recent years have analyzed levels of 8-OHdG in fish and shellfish DNA in relation to environmental pollutants, hypoxia, transport stress, as well as thermal stress [43], [44], [45], [46], [47]. For example, Gleason et al. [45] reported that thermal stress increases 8-OHdG formation in rocky intertidal mussel (M. californianus) when collected from field sites. Therefore, it is very important to understand the potential molecular mechanisms and/or signaling pathways of 8-OHdG regulation and oxidative DNA damage in marine organisms during heat stress.
γH2AX is a subfamily of the H2A histone protein family which is central to nucleosome chromatin and DNA structure, and it represents a 2–25% component of the H2A complement in vertebrates [48]. High temperature causes the formation of nuclear foci and phosphorylation of histone γH2AX at serine 139 in human HeLa cells [49]. γH2AX formation is a quick and highly sensitive cellular response (e. g, double-stranded DNA breakage) to radiation [48]. Tumminello and Fuller-Espie [50] reported that heat stress decreases viability and increases ROS production as well as H2AX phosphorylation in coelomocytes of European nightcrawler (Eisenia hortensis, an earthworm). Therefore, γH2AX is considered a molecular biomarker for DNA damage of apoptotic cells and/or tissues in animals [51], [52], [53].
Apoptosis is a form of programmed cell death or controlled autodigestion cell death induced by acute cellular impairment [54]. Activation of apoptosis in cells and tissues involves mainly two mechanisms: (i) intrinsic or mitochondria-mediated, and (ii) extrinsic or death receptor-mediated pathways [55]. In response to various environmental stimuli, mitochondria releases cytochrome c and Smac/DIABLO (pro-apoptogenic factors) resulting in the activation of cellular apoptosis and caspase (CAS) activity in cells and tissues of organisms [55]. CASs are a large family (e.g., CAS-2, −3, −4, −5, −6, −7, −8, −9, −10, etc.) of cysteine proteases and are known as key mediators of apoptotic pathway [56]. Among them, CAS-3 plays a key role in apoptotic DNA fragmentation [[54], [57]]. CAS-3 is also able to regulate non-apoptotic function in many cells including keratinocytes and lens epithelium [58,59].
In addition to CAS-3, the Bcl-2 family proteins such as Bcl-2-associated X (BAX), also play a key role in regulating cellular apoptosis at the level of mitochondrial cytochrome c release [60]. BAX triggers multimerization and affects the permeabilization of the mitochondrial outer membrane [61]. Mitochondrial outer membrane permeabilization mediates the release of pro-apoptotic proteins (i.e., cytochrome c, Smac/DIABLO) during apoptosis [55]. Keep et al. [55] found cytochrome c is released even in the absence of BAX during Ngo- and cisplatin-induced apoptosis. In addition to this, cytochrome c release itself cannot drive the cell towards apoptosis and may require the formation of other pro-apoptotic factors from the mitochondria [55]. Therefore, BAX protein is considered as a pro-apoptotic protein member under the Bcl-2 family and performs a key role in cellular apoptosis during environmental stress [62,63].
High temperatures due to seasonal temperature fluctuations and/or long-term effects of global climate change challenge marine ectothermic organisms such as shellfish from different physiological aspects. Rising seawater temperatures may even pose additional threats to their survival due to their susceptibility to hypoxia, pollution, and pathogens under extreme temperature conditions [64]. For example, the effects of high temperature and a combination of cadmium-like pollutants were previously studied in mitochondrial functions and cellular apoptosis and necrosis in oysters [64], [65], [66], [67]. Moreover, few studies have also addressed the effects of high temperature on gene and/or protein expressions, and physiological functions in oysters [7,68]. Currently, there is no available information on the effects of elevated temperature on cellular oxidative DNA damage and the potential molecular mechanisms and/or signaling pathways of 8-OHdG, γH2AX, CAS-3, and BAX regulations by heat stress in marine oysters.
The American oyster (Bivalvia: Ostreidae) is an economically and commercially important marine shellfish species in the United States and is found along the Atlantic and Gulf coasts of North America [69]. The American oyster is an ectotherm (i.e., body temperature rises and falls along with the surrounding environmental temperature), inhabits shallow coastal waters and estuaries, and is frequently exposed to a variety of environmental stressors such as hypoxia, pollution, ocean acidification, temperature fluctuations, etc. [67,70,71]. Notably, extreme temperature conditions (i.e., heat waves) force them to use their stored energy to compensate for biochemical demands, ultimately decreasing their survival rate as well as increasing mortality during the post-spawning period [7,72].
Oysters are suspension feeders that filter suspended minute particles through their gills [73]. The oyster gills, being directly exposed to seawater, are extremely susceptible to environmental stress [74,75]; therefore, the aim of this study was to test the hypothesis that high temperature may induce the overproduction of reactive free radicals (i.e., ROS, RNS) that causes DNA strand breaks and induces apoptosis, consequently increasing the expression of γH2AX, BAX, and CAS-3, which may promote 8-OHdG levels in the gills of oysters. The main goals of this research were three-fold: (i) to determine the effects of high temperatures on morphological changes of gills in the American oyster, (ii) to determine the effects of high temperatures on 8-OHdG, γH2AX, HSP70, CAS-3, BAX expressions, DNA damage, cellular apoptosis and signaling pathways in gills, and (iii) to determine the effects of high temperatures on extrapallial fluid (also called extracellular fluid or body fluid that performs a variety of physiological functions including transport of oxygen and nutrients, as well as immune defense, shell repair, digestion, and excretion [76]) conditions of oysters.
2. Materials and methods
2.1. Collection of oysters
Young American oysters (Crassostrea virginica, average age: ∼2–3 years, average shell size: 8.48 ± 0.42 cm length, 4.29 ± 0.1 cm width, and meat weight: 7.87 ± 0.88 g) were randomly collected during low tide from oyster beds on the bay side of South Padre Island (geographical location: 26°04′30′'N, 97°09′59′'W) in Texas, USA. The collection site is adjacent to the recreational fishing area. The physio-chemical parameters such as salinity (32 ppt), pH (7.9) and water temperature (22 °C) were measured using a YSI probe (YSI Professional Plus 1020 Multiprobe System, Ywellow Springs, OH, USA) during oyster collection. After collection, oysters were kept in buckets (5–8 gallon capacity) and then quickly transported with aerated seawater to a laboratory at the University of Texas Rio Grande Valley (UTRGV) Brownsville campus.
2.2. Ethical approval
Oysters were collected under an approved scientific permit for research from Texas Parks & Wildlife Department (no. SPR-1018–274). The UTRGV Institutional Animal Care and Use Committee (IACUC) does not require any animal protocol for aquatic invertebrates including oysters. All oysters, however, were handled and cared according to the Guide for the Care and Use of Animals for research by the National Research Council Committee of the United States (https://grants.nih.gov/grants/olaw/guide-for-the-care-and-use-of-laboratory-animals.pdf).
2.3. Laboratory heat exposure experiment
For the laboratory heat exposure experiment, oysters were randomly selected and placed in six glass aquariums (12 oysters/aquarium; capacity: 20 gallons; Tetra, Blacksburg, VI, USA). Each aquarium was equipped with recirculating seawater flow-through system with a biological filter. Oysters were then acclimatized under controlled laboratory conditions (22 ± 0.5 °C; light/dark cycle 12:12 h) for 5 days. Two aquariums were set up for each of the three different treatment groups (control: 24 °C, medium temperature: 28 °C, and high temperature: 32 °C). Temperatures were gradually increased (∼1 °C/day) from 22 to 24 °C, 28 °C, or 32 °C within a 3- to 5-day period using a digital water heater (Top Fin, Franklin, WI, USA) and were maintained throughout the experiment. During the experimental period, temperature, pH, salinity, and dissolved oxygen were recorded with a YSI probe three times daily (morning, afternoon, and evening). There were no major changes in the physiochemical parameters. Oysters were fed frozen Marine Cuisine (San Francisco Bay Brand, San Francisco, CA, USA) once every other day during the experimental period. After a week of heat exposure, oysters were collected (12 oysters/aquarium) for analysis. Notably, the experimental temperatures (i.e., environmentally relevant temperatures: 24, 28, and 32 °C) and short-term (one-week) exposure period used in this study were based on previous publications in the American oysters [7,67,75,[77], [78], [79]]. No mortality occurred in control (24 °C) and medium- and high-temperature groups.
2.4. Sample collection and fixation
Oysters were cracked open using protective gloves and an oyster knife, and extrapallial (EP) fluid was carefully collected and pipetted in a 1.5 ml sterile microcentrifuge tube. For histological and immunohistochemical analyses, gill samples were collected and placed on an embedding plastic cassette (Fisher Scientific, Hampton, NH, USA), and kept in a polyethylene plastic container with 4% paraformaldehyde solution (Acros Organics, Morris, NJ, USA) for 6–7 days at 4 °C. For molecular analysis, gill samples were collected and placed in a 1.5 ml RNase-DNase free tube and stored at −80 °C for later analysis.
2.5. Dehydration and embedding of tissue samples
After a week of fixation in paraformaldehyde solution, gill samples were dehydrated with a series of ethanol dilutions (50%, 75%, 95%, and 100% 2x) for 30 min each. Tissue samples were immediately cleared by a common clearing agent, xylene (Fisher Scientific) for 30 min each (3 times). Samples were then incubated with a mixture of xylene and melted paraffin (1:1) overnight. Following this, samples were infiltrated with paraffin (1 h each for 3 times) and finally embedded in liquid paraffin (Paraplast Plus, Fisher Scientific) using an embedding cassette. The paraffin-embedded tissue blocks were sectioned at 7 µm using a rotary microtome machine (Leica, Wetzlar, Germany) and transferred on a positively charged glass slide (Superfrost Plus, Thermo Fisher, Waltham, MA, USA). Slides were then incubated on a slide warmer (35–40 °C for 24 h) to dry and remove extra water.
2.6. Histological staining of tissue samples
For histological analysis, gill tissue samples were stained according to routine histology procedures described by Haberkorn et al. [80] and Khondee et al. [81]. Briefly, paraffin-embedded tissue slides were deparaffinized in xylene (3 times, 5 min each). Slides were then rehydrated with a series of ethanol dilutions (100% 2x, 95%, 75%, and 50%) and incubated with hematoxylin stain (Millipore Sigma, Burlington, MA, USA) for 3–5 min. Following this, the slides were washed with deionized (DI) water until their deep blue color disappeared. Slides were stained with eosin solution (Millipore Sigma) for 30 min. Slides were then washed and dehydrated through an increasing concentration of ethanol dilutions (50%, 75%, 95%, and 100% 2x). Slides were cleared in xylene and mounted by using a mounting medium (Cytoseal XYL, Richard-Allan Scientific, MI, USA) and coverslip (Fisher Scientific). Slides were then kept dry at room temperature for 30 min prior to microscopic observation. Histological pictures were taken with a photometric Cool-SNAP camera (Nikon Eclipse E600, Nikon, Japan) using a light microscope (Photometrics, Tucson, AZ, USA).
2.7. Immunohistochemical analysis for protein expression
For immunohistochemical analysis, gill tissue sections from the same blocks were deparaffinized in xylene (3x, 5 min each). The slides were dehydrated with a series of ethanol dilutions and washed with 1x phosphate buffer saline (PBS, Fisher Scientific) solution for 3 times (10 min each). Slides were then incubated with 1% bovine serum albumin (BSA, Fisher Scientific) blocking solution for 1 h at room temperature to avoid non-specific binding. Following this, slides were washed again with 1x PBS solution and incubated with different primary antibodies (diluted 1:100 with 1x PBS solution); mouse anti-HSP70 (MilliporeSigma), mouse anti-8-OHdG (Japan Institute for the Control of Aging, Shizuoka, Japan), mouse anti-double-stranded DNA (anti-dsDNA; MilliporeSigma), rabbit anti-CAS-3 (Cell Signaling Technology, Danvers, MA, USA), rabbit anti-BAX (Cell Signaling), or rabbit anti-γH2AX (Novus Biologicals, Centennial, CO, USA) at 4 °C for 48 h. The negative tissue slides were incubated with 1x PBS instead of the primary antibody. The HSP70, 8-OHdG, and dsDNA antibodies have been validated in oyster tissues previously [7,75,79,82]. Slides (including negative control) were then washed with 1x PBS solution (3x, 10 min each). After washing, tissue slides were incubated with either anti-mouse (Cell Signaling Technology) or anti-rabbit secondary antibodies (Southern Biotech, Birmingham, AL, USA) (diluted 1:100 with 1x PBS solution) for 1 h at room temperature. Slides were washed again with 1x PBS solution for 3 times (10 min each). Then, 3,3,'-diaminobenzidine (DAB) peroxidase substrate (diluted 1 drop substrate with 1000 ml with DAB solution) (Vector Laboratories Inc., Burlingame, CA, USA) was added according to manufacturer's guidelines and incubated for an appropriate time until the color development to detect the immunoreactivity of HSP70, 8-OHdG, dsDNA, CAS-3, BAX, and γH2AX proteins in tissue sections. Afterward, slides were washed with DI water for 5 min to prevent the formation of deep background. Slides were then dehydrated in another series of ethanol dilutions, cleared in xylene, and mounted with Cytoseal. The immunoreactive (IR) signals of HSP70, 8-OHdG, dsDNA, γH2AX, BAX, and CAS-3 were captured by a Cool-SNAP camera (Photometrics). The IR intensities of protein expression were estimated by measuring the optical density (OD) of staining (N = 311–2634 measurements of OD per treatment) using ImageJ software described by Schneider et al. [83].
2.8. Quantitative real-time PCR analysis for mRNA level
Total RNA was extracted from gill tissues using TRI reagent (MilliporeSigma) and treated with DNase-I (Promega, Madison, WI, USA) according to the instructions provided. Quantitative real-time PCR (qRT-PCR) analysis was performed using a one-step 2x SYBR Green qRT-PCR master mix (Promega) and gene-specific primers by the CFX Connect Real-Time PCR System (Bio-Rad, Hercules, CA, USA) according to Rahman et al. [84]. The assay was performed in duplicate (N = 7–8 samples per treatment group), and the oyster elongation factor-1α (EF1) was used as an internal reference gene for the calculation of the relative expression level. Gene-specific primers of HSP70 and CAS-3 were designed using Primer3 software according to Untergasser et al. [85]. The primer sets of HSP70 and CAS-3 have been validated in oyster tissues previously [6,7,75,79] and were shown in Table 1. To determine the specificity of primers, the amplification and melting curves of the qRT-PCR cycle were analyzed (Supplementary Fig. 1). The qRT-PCR products of each gene were electrophoresed on agarose gel (1%) stained with ethidium bromide to ensure a single PCR amplicon of expected length (Supplementary Fig. 2). The relative mRNA levels of HSP70 and CAS-3 were calculated using the 2−ΔΔCt method [86].
Table 1.
Primer sequences used in real-time quantitative RT-PCR for mRNA levels analyses in the American oyster.
| Candidate gene | Length (bp) | Accession no | Sense primer (5′ - 3′) | Anti-sense primer (5′ - 3′) |
|---|---|---|---|---|
| HSP70 | 233 | AJ271444 | CACATCTGGGAGGTGAGGAT | CTCAAATCTGGCTCGTGTGA |
| CAS-3 | 245 | XM_022464608 | AATGACCCATCCAAACAGGA | GCGACATGCTTGAACAAAGA |
| EF-1α | 200 | JX117894 | AGGCTGACTGTGCTGTGTTG | TTCAGCCTTGATTTCGTTGA |
HSP70, heat shock protein 70; CAS-3, caspase-3; EF-1α, elongation factor-1α.
2.9. In situ TUNEL assay for cellular apoptosis
Apoptotic cells in gills were detected using terminal deoxynucleotidyl transferase (TdT) dUTP Nick-End labeling (TUNEL) detection kit (Dead End Colorimetric TUNEL system, Promega) according to Lacy et al. [84]. Briefly, paraffin-embedded gills were sectioned, deparaffinized with xylene, rehydrated with a series of ethanol dilutions, washed with 0.85% NaCl at room temperature for 5 min, and rinsed with PBS. Slides were then incubated with protease K (20 mg/ml) for 15 min at room temperature and rinsed with PBS. Endogenous peroxidase was quenched by treatment with 3% H2O2 in PBS for 5 min at room temperature. Sections were then incubated with TdT enzyme mix (equilibrium buffer: biotinylated nucleotide mix: rTdT enzyme, 98:1:1) in a humidified chamber at 37 °C for 1 h. Slides were then rinsed in PBS and incubated with SSC buffer for 15 min at room temperature, rinsed in PBS, incubated with streptavidin HRP solution (1:500 in PBS), and stained with DAB substrate for 5 min in dark conditions. Sections were then rinsed in DI water, incubated with 1% methylene green for 5 min, dehydrated with a series of ethanol dilutions, sequenced with xylene, and mounted with Cytoseal under a glass coverslip. The in situ TUNEL intensity of apoptotic nuclei was estimated by measuring the OD of staining (N = 484–1008 measurements of OD per treatment) using ImageJ software described by Lacy et al. [87].
2.10. DNA damage assay for 8-OHdG
DNA was extracted from gill tissues using nuclei lysis and protease K solutions, then treated with RNase A according to the manufacturer's protocol (Promega). To investigate whether DNA damage in the gill tissues was associated with oxidative stress, the 8-OHdG levels from DNA samples (duplicate samples: 100 ng DNA per sample) were measured by a competitive enzyme-linked immunosorbent assay (ELISA) according to the manufacturer's protocol (EpigenTek Group Inc., Farmingdale, NY, USA). To quantify the absolute amount of 8-OHdG, a standard curve was generated (5, 10, 20, 50, 100, and 200 pg/µl) and recorded the absorbance at 450 nm by a microplate reader (Multiskan SkyHigh, Thermo Fisher Scientific). The absorbances were then plotted (standard vs. samples), and the 8-OHdG level was determined (N = 8 samples per treatment group) and expressed as pg/µl.
2.11. Bradford assay for EP fluid protein concentration
EP fluid protein concentration was measured using the technique developed by Bradford [88]. Briefly, 5 µl of extrapallial fluid was pipetted into a 5 ml protein assay solution (Bio-Rad, Hercules, CA, USA) and incubated at room temperature for 5 min. A Nanodrop (Thermo Fisher Scientific) was used for protein measurement. A standard curve was made of BSA solution (0, 0.0625, 0.125, 0.25, 0.5, and 1 mg/ml) and absorbance was read at 595 nm. The absorbances (standard vs. samples) were plotted and the protein concentration was determined (N = 10–12 samples per treatment group) and expressed as mg/ml.
2.12. Microcuvette assay for EP fluid glucose level
EP fluid glucose level was measured using HemoCue Glucose 201 analyzer (Angel Holm, Sweden) and glucose microcuvette according to the manufacturer's protocol. Briefly, a drop of EP fluid from the experimental sample was taken and then loaded into a microcuvette. The microcuvette was placed in the HemoCue Glucose analyzer and the data was recorded (N = 10–12 samples per treatment group).
2.13. Statistical analysis
Prior to analysis, data values two times higher or lower (plus or minus) than the standard mean values were considered outliers and withdrawn from analysis [89]. All OD data were checked for normality using Shapiro-Wilk's test. One-way analysis of variance (ANOVA) followed by Tukey's multiple comparison tests was conducted on all experimental data. A Student's t-test was also performed to compare the unpaired means. The mean ± standard error of the mean (SEM) was reported for all data. A P value of <0.05 was considered statistically significant. All statistical analyses were performed using GraphPad Prism version 9.0 (GraphPad San Diego, CA, USA).
3. Result
3.1. Effects of short-term heat stress on morphology of gills
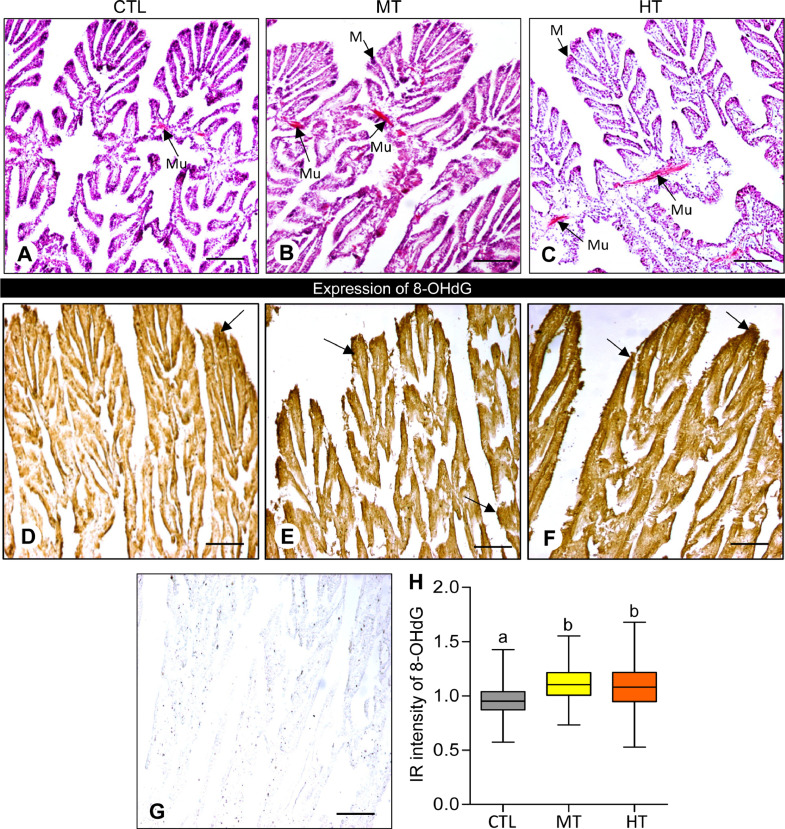
Histological observations showed that high temperature markedly changed the structure of gills (i.e., gill lamellae, hemocyte infiltration, gill epithelium, mucocyte, etc.) in oysters (Fig. 1A-C). The number of branchial lesions increased in medium and high temperatures (28 and 32 °C) compared with the control (24 °C). The number of mucocytes increased in gill filaments in 28 and 32 °C treatments compared with the control (Fig. 1B, C). Mucus was more robustly distributed at the base of demibranches in both medium- (28 °C) and high-temperature (32 °C) groups. The hemocyte infiltration within the intracellular space of the gill filaments was not prominent in all experimental samples (Fig. 1A-C).
Fig. 1.
Effects of one-week heat exposure on 8-hydroxy-2′-deoxyguanosine (8-OHdG) expression in gills of American oyster. (A-C) Histological appearance of representative photographs of gills collected from oysters exposed to control (CTL: 24 °C), medium temperature (MT: 28 °C), and high temperature (HT: 32 °C). Arrows indicate mucocyte (M) and mucus (Mu). (D-F) 8-OHdG expression in representative photographs of gills collected from oysters exposed to CTL, MT, and HT. Arrow indicates a higher 8-OHdG expression. (G) Negative control of 8-OHdG in gills of oyster. Scale bar = 100 µm. (H) Immunoreactive (IR) intensity of 8-OHdG in gills of oysters exposed to CTL, MT, and HT. Each value represents the mean ± SEM. Whiskers indicate the minimum and maximum values for each data set. The center line within the whisker box indicates the mean value. Data were analyzed with one-way ANOVA followed by Tukey's multiple comparison test. Different letters indicate significant differences (P<0.05) between experimental treatments (MT and HT) and the CTL.
3.2. Effects of short-term heat stress on 8-OHdG expression and level
Immunohistochemistry (IHC) results showed that there was an increased 8-OHdG expression in gills from different treatment groups (Fig. 1D-F). 8-OHdG expression appeared to be denser in both medium- (28 °C, Fig. 1E) and high-temperature (32 °C, Fig. 1F) groups compared with the control (24 °C, Fig. 1D). No IR signal of 8-OHdG expression was observed in the negative control (Fig. 1G).
The immunoreactive (IR) integrated optical density (OD) of 8-OHdG expression in gills was obtained through ImageJ software. There was a significant increase (P<0.05, one-way ANOVA, Tukey's test) of 8-OHdG expression in gills in both medium- (28 °C) and high- (32 °C) temperature groups compared with the control (Fig. 1H). The IR intensity of 8-OHdG increased ∼1.2-fold and ∼1.1-fold in medium- and high-temperature exposure groups, respectively, compared with the control, indicating more base modifications in response to heat stress.
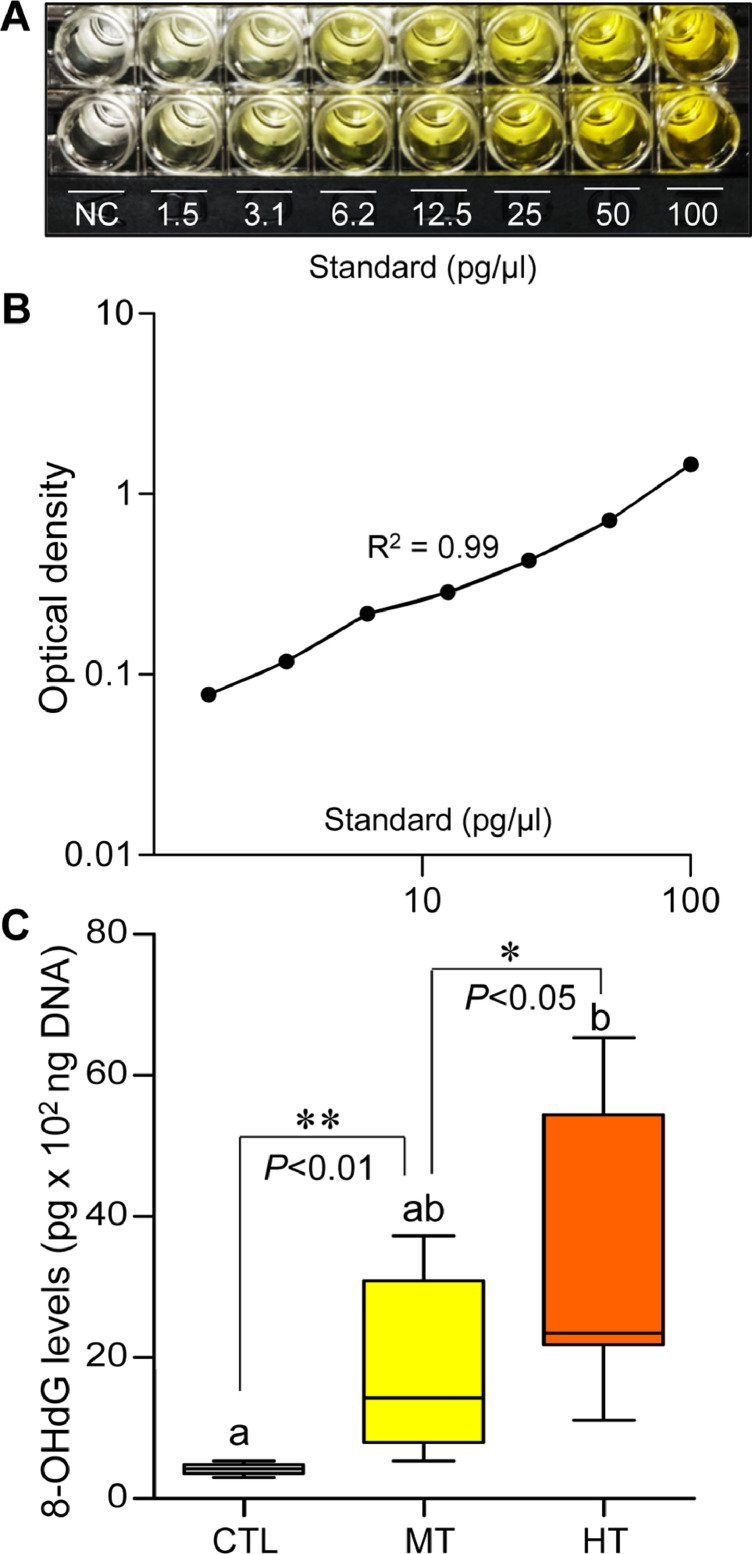
Colorimetric analysis showed there was a marked increase in 8-OHdG levels in both medium- and high-temperature groups compared with the control (Fig. 2). The 8-OHdG levels increased significantly around 4.2-fold (P<0.05, Student t-test).) in medium temperature, and ∼7.8-fold (P<0.05, Tukey's test) in high-temperature groups compared with the control.
Fig. 2.
Effects of one-week heat exposure on 8-hydroxy-2′-deoxyguanosine (8-OHdG) levels in gills of American oyster. (A) A representative photograph of 8-well assay strips containing serial dilutions of 8-OHdG standards and negative control (NC). (B) Standard curve of 8-OHdG. Each value represents the mean of duplicate determinations. (C) 8-OHdG levels. Each value represents the mean ± SEM. Whiskers indicate the minimum and maximum values for each data set. Center line within the whisker box indicates mean value. Asterisks indicate significant difference (Student t-test). Data were analyzed with one-way ANOVA followed by Tukey's multiple comparion test. Different letters indicate significant differences (P<0.05). CTL, control (24 °C); MT, medium temperature (28 °C), HT, high temperature (32 °C).
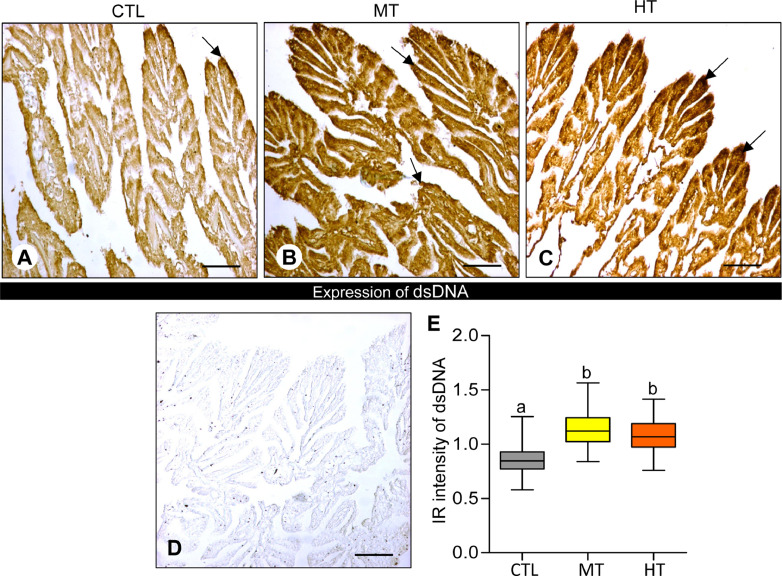
3.3. Effects of short-term heat stress on dsDNA expression
Oysters exposed to high temperatures (28 and 32 °C for 1 week) showed an increase in dsDNA expression in the gills of different experimental groups (Fig. 3A-C). A dense IR signal of dsDNA was found in both medium- (28 °C, Fig. 3B) and high-temperature (32 °C, Fig. 3C) groups compared with the control (24 °C, Fig. 3A). However, negative control tissues demonstrated no IR signal of dsDNA (Fig. 3D).
Fig. 3.
Effects of one-week heat exposure on double-stranded DNA (dsDNA) expression in gills of American oyster. (A-C) dsDNA expression in representative photographs of gills collected from oysters exposed to control (CTL: 24 °C), medium temperature (MT: 28 °C), and high temperature (HT: 32 °C). Arrow indicates higher dsDNA expression. (D) Negative control of dsDNA expression in gills of oysters. Scale bar = 100 µm. (E) Immunoreactive (IR) intensity of dsDNA in gills of oysters exposed to CTL, MT, and HT. Each value represents the mean ± SEM. Whiskers indicate the minimum and maximum values for each data set. The center line within the whisker box indicates the mean value. Data were analyzed with one-way ANOVA followed by Tukey's multiple comparion test. Different letters indicate significant differences (P<0.05).
The measured integrated OD of dsDNA from ImageJ analysis showed there was a marked increase in dsDNA IR intensity in both medium- and high-temperature groups compared with the control (Fig. 3E). The IR intensity of dsDNA increased significantly (P<0.05, Tukey's test) around 1.3-fold in medium temperature, and ∼1.3-fold in high-temperature groups compared with the control.
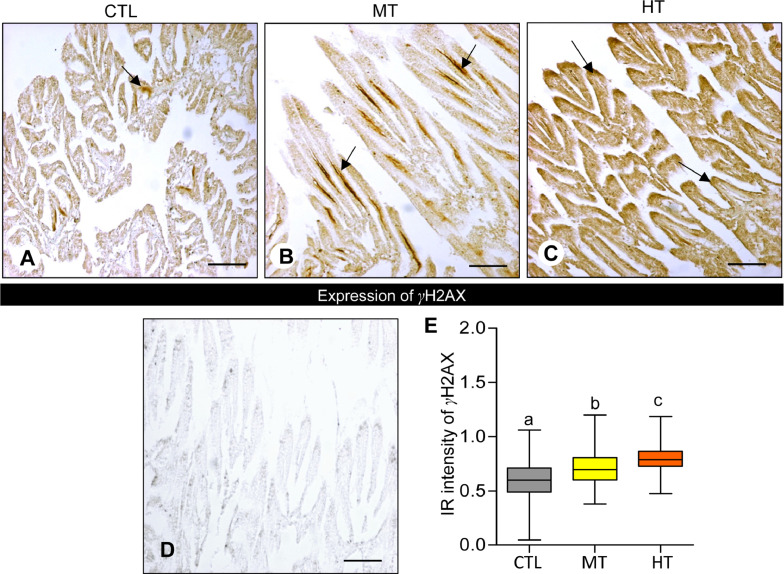
3.4. Effects of short-term heat stress on γH2AX expression
Oysters exposed to high temperatures (28 and 32 °C for 1 week) showed an increase in γH2AX expression in their gills (Fig. 4A-C). γH2AX expression appeared to be stronger in both medium- (28 °C) and high-temperature (32 °C) groups (Fig. 4B, C) compared with the control (Fig. 4A). No IR of γH2AX was found in negative control slides (Fig. 4D).
Fig. 4.
Effects of one-week heat exposure on γ-histone family member X (γ-H2AX) protein expression in gills of American oyster. (A-C) γ-H2AX expression in representative photographs of gills collected from oysters exposed to control (CTL: 24 °C), medium temperature (MT: 28 °C), and high temperature (HT: 32 °C). Darker brown color (arrow) indicates higher γ-H2AX expression. (D) Negative control of γ-H2AX expression in gills of oysters. Scale bar = 100 µm. (E) Immunoreactive (IR) intensity of γ-H2AX in gills of oysters exposed to CTL, MT, and HT. Each value represents the mean ± SEM. Whiskers indicate the minimum and maximum values for each data set. The center line within the whisker box indicates the mean value. Data were analyzed with one-way ANOVA followed by Tukey's multiple comparion test. Different letters indicate significant differences (P<0.05).
The integrated IR signal of γH2AX was significantly (P<0.05, Tukey's test) increased around 1.2-fold in medium temperature groups compared with control groups (Fig. 4E). Similarly, the IR intensity of γH2AX was also significantly (P<0.05) escalated ∼1.3-fold in high-temperature groups compared with the control (Fig. 4E).
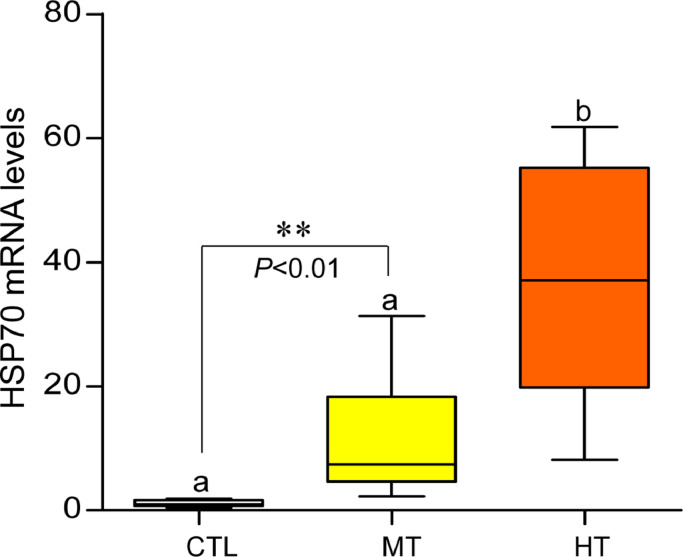
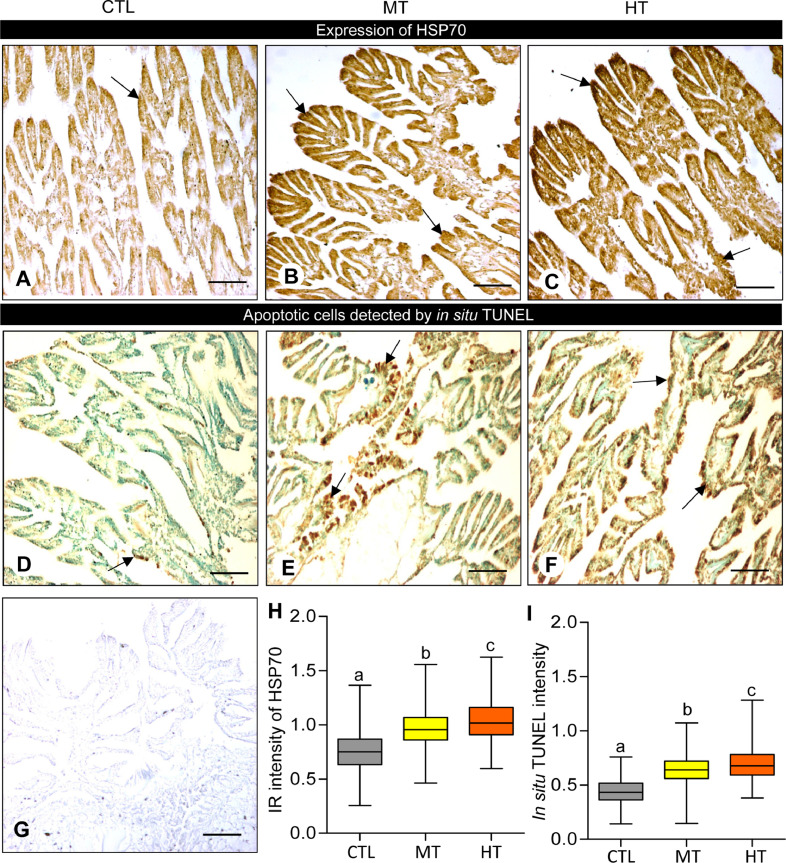
3.5. Effects of short-term heat stress on HSP70 expression and cellular apoptosis
The monitoring of HSP70 expression is a key tool for detecting stress tolerance. Therefore, we examined the effects of high temperatures on HSP70 mRNA levels and protein expression in the gills of oysters by qRT-PCR and IHC techniques, respectively. Oysters exposed to high temperatures showed significantly increased HSP70 mRNA levels (∼11.8-fold at 28 °C, and ∼38.8-fold at 32 °C) in gills compared with the control (24 °C, Fig. 5). Similarly, HSP70 protein expression appeared to be high in medium- (28 °C) and high-temperature (32 °C) groups compared with the control (Fig. 6A-C). No IR signal of HSP70 was detected in gill tissues of negative control (Fig. 6G). The measured integrated OD values obtained by ImageJ software demonstrated that the integrated IR signal of HSP70 increased significantly (P<0.05, Tukey's test) around 1.3-fold in the medium temperature group and ∼1.4-fold in a high-temperature group compared with the control (Fig. 6H), which indicates the synthesis of a higher amount of HSP70 expression in response to rising seawater temperature.
Fig. 5.
Effects of one-week heat exposure on heat shock protein 70 (HSP70) mRNA levels in gills of American oyster. Each value represents the mean ± SEM. Whiskers indicate the minimum and maximum values for each data set. Center line within the whisker box indicates mean value. Data were analyzed with one-way ANOVA followed by Tukey's multiple comparion test. Different letters indicate significant differences (P<0.05). Asterisks indicate significant difference (Student t-test). CTL, control (24 °C); MT, medium temperature (28 °C), HT, high temperature (32 °C).
Fig. 6.
Effects of one-week heat exposure on heat shock protein-70 (HSP70) expression and apoptotic cells in gills of American oyster stained with immunohistochemistry and in situ TUNEL assay, respectively. (A-C) HSP70 expression in representative photographs of gills collected from oysters exposed to (A) control temperature (CTL: 24 °C), (B) medium temperature (MT: 28 °C), and (C) high temperature (HT: 32 °C). Arrow indicates higher HSP70 expression. (D-F) The presence of apoptotic nuclei shown as dark brown staining (arrow). (G) Negative control of HSP70 expression in gills of oysters. Scale bar = 100 µm. (H, I) Immunoreactive (IR) intensity of HSP70 and apoptotic nuclei in gills of oysters exposed to CTL, MT, and HT. Each value represents the mean ± SEM. Whiskers indicate the minimum and maximum values for each data set. The center line within the whisker box indicates the mean value. Data were analyzed with one-way ANOVA followed by Tukey's multiple comparion test. Different letters indicate significant differences (P<0.05).
In addition to HSP70 regulation during heat stress, an increase in cellular temperature incurs protein denaturation and interrupts critical cellular functions, resulting in apoptosis and/or cell death in terrestrials and aquatic organisms including shellfish species [7]. Thus, after heat stress, apoptotic cells in oyster gills were measured by in situ TUNEL assay using colorimetric staining. Oysters experienced high temperatures (28 and 32 °C) and showed an increase in the number of apoptotic cells in the gills in medium- and high-temperature groups compared with the control (Fig. 6D-F). ImageJ analysis showed that there was a significant increase (P<0.05, Tukey's test) in the integrated OD of apoptotic cells in both medium- (∼1.5-fold) and high-temperature (∼1.6-fold) groups compared with the control (Fig. 6I).
3.6. Effects of short-term heat stress on BAX expression
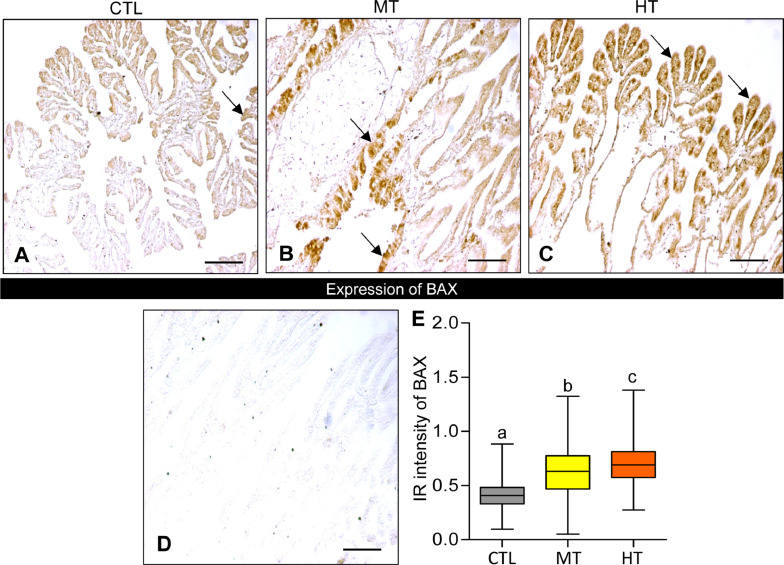
Oysters exposed to high temperatures (28 and 32 °C for 1 week) showed an increase in BAX expression in their gills (Fig. 7A-C). BAX expression appeared to be higher in both medium- (28 °C) and high-temperature (32 °C) groups compared with the control (24 °C). No IR signal of BAX was observed in the negative control (Fig. 7D).
Fig. 7.
Effects of one-week heat exposure on Bcl-2-associated-X (BAX) protein expression in gills of American oyster. (A-C) BAX protein expression in representative photographs of gills collected from oysters exposed to control (CTL: 24 °C), medium temperature (MT: 28 °C), and high temperature (HT: 32 °C). Arrow indicates higher BAX expression. (D) Negative control of BAX expression in gills of oyster. Scale bar = 100 µm. (E) Immunoreactive (IR) intensity of BAX in gills of oysters exposed to CTL, MT, and HT. Each value represents the mean ± SEM. Whiskers indicate the minimum and maximum values for each data set. The center line within the whisker box indicates the mean value. Data were analyzed with one-way ANOVA followed by Tukey's multiple comparion test. Different letters indicate significant differences (P<0.05).
The measured integrated OD of BAX significantly (P<0.05, Tukey's test) increased in medium- and high-temperature groups (Fig. 7E). The IR intensity of BAX increased ∼1.5-fold in medium temperature, and ∼1.7-fold in high-temperature groups compared with the control, indicating an increased rate of apoptosis with high-temperature exposure.
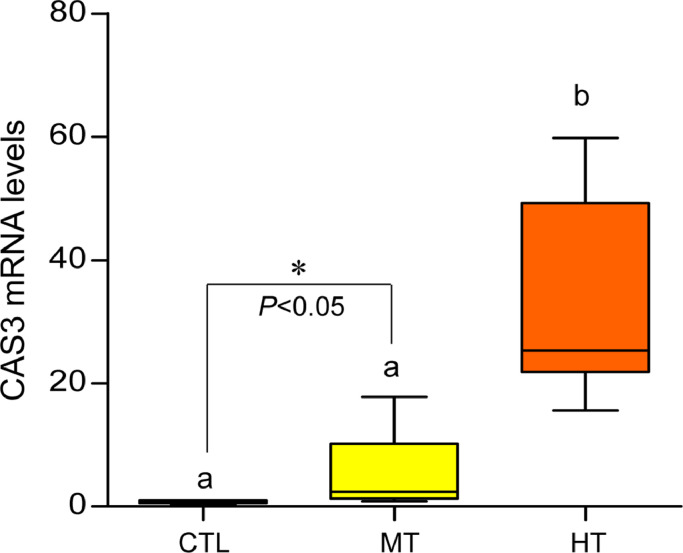
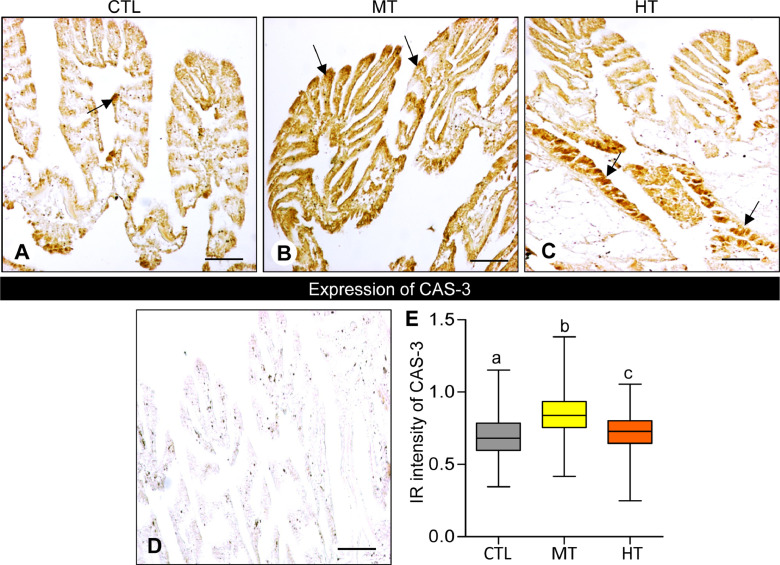
3.7. Effects of short-term heat stress on CAS-3 expression
Oysters exposed to high temperatures (28 and 32 °C for 1 week) showed an increase in CAS-3 mRNA levels in gills (∼6.7- and ∼42.1-fold at medium- and high-temperature, respectively, Fig. 8). Similarly, the expression of CAS-3 protein showed to be high in medium- (28 °C) and high-temperature (32 °C) groups compared with the control (Fig. 9A-C). No IR signal of CAS-3 was detected in gill tissues of negative control (Fig. 9D).
Fig. 8.
Effects of one-week heat exposure on caspase-3 (CAS-3) mRNA levels in gills of American oyster. Each value represents the mean ± SEM. Whiskers indicate the minimum and maximum values for each data set. Center line within the whisker box indicates mean value. Data were analyzed with one-way ANOVA followed by Tukey's multiple comparion test. Different letters indicate significant differences (P<0.05). Asterisks indicate significant difference (Student t-test). CTL, control (24 °C); MT, medium temperature (28 °C), HT, high temperature (32 °C).
Fig. 9.
Effects of one-week heat exposure on caspase-3 (CAS-3) protein expression in gills of American oyster. (A-C) CAS-3 protein expression in representative photographs of gills collected from oysters exposed to control (CTL: 24 °C), medium temperature (MT: 28 °C), and high temperature (HT: 32 °C). Arrow indicates higher HSP70 expression. (D) Negative control of CAS-3 expression in gills of oyster. Scale bar = 100 µm. (E) Immunoreactive (IR) intensity of CAS-3 in gills of oysters exposed to CTL, MT, and HT. Each value represents the mean ± SEM. Whiskers indicate the minimum and maximum values for each data set. The center line within the whisker box indicates the mean value. Data were analyzed with one-way ANOVA followed by Tukey's multiple comparion test. Different letters indicate significant differences (P<0.05).
The integrated OD of CAS-3 after ImageJ analysis showed a marked increase (P<0.05, Tukey's test) of IR intensity in both medium- (∼1.2-fold) and high-temperature (∼1.04-fold) groups (Fig. 8E), indicating a higher rate of apoptosis activity in gills exposed to high temperatures.
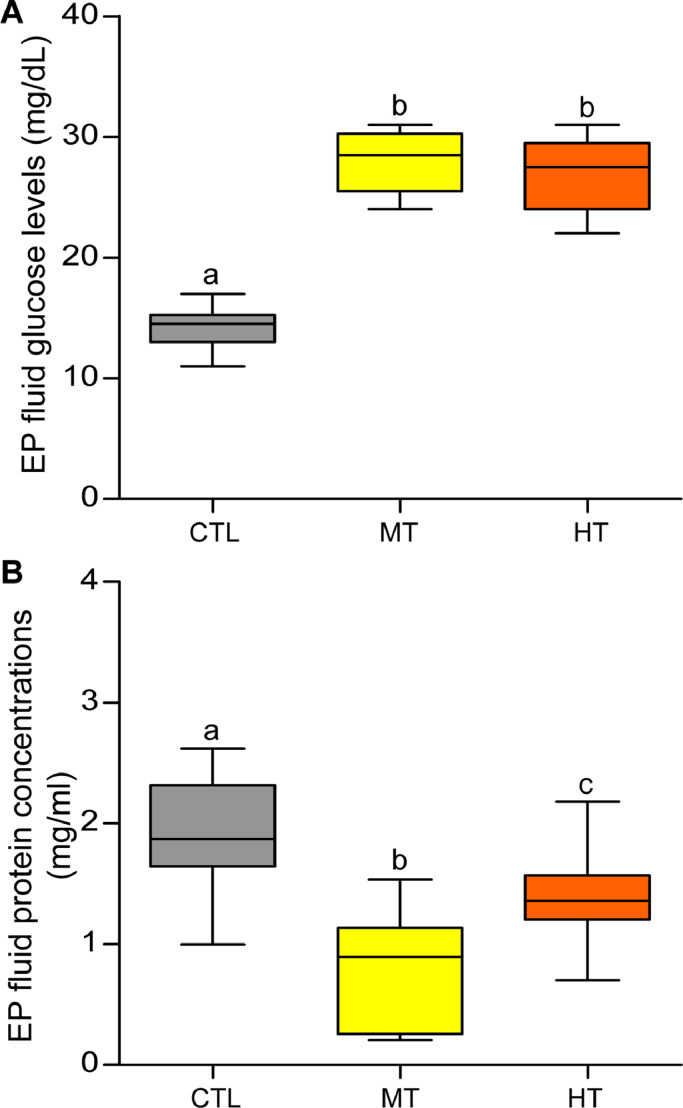
3.8. Effects of short-term heat stress on EP fluid conditions
Oysters exposed to high temperatures (28 and 32 °C for 1 week) significantly increased (∼1.5-fold, P<0.05, Tukey's test) in EP fluid glucose levels compared with the control (Fig. 10A). Contrarily, EP fluid protein concentrations tended to decrease (∼0.5–0.7-fold, P<0.05) in both medium- and high-temperature groups of oysters compared with the control (Fig. 10B).
Fig. 10.
Effects of one-week heat exposure on extrapallial (EP) fluid glucose levels and protein concentrations of American oyster. Each value represents the mean ± SEM. Whiskers indicate the minimum and maximum values for each data set. The center line within the whisker box indicates mean value. Data were analyzed with one-way ANOVA followed by Tukey's multiple comparion test. Different letters indicate significant differences (P<0.05). CTL, control (24 °C); MT, medium temperature (28 °C), HT, high temperature (32 °C).
4. Discussion
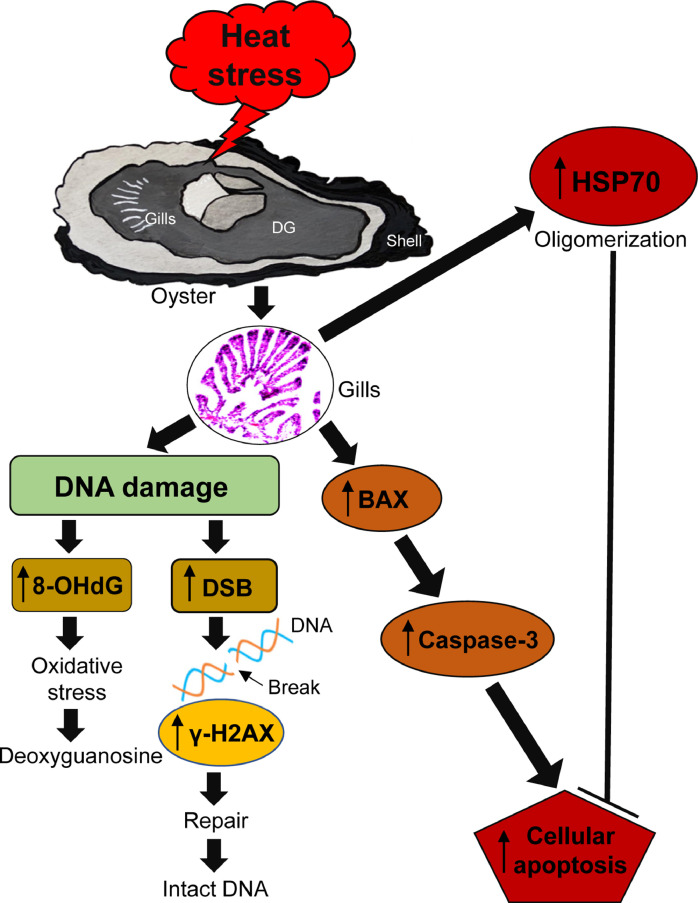
Molecular and biochemical responses to heat stress have previously been investigated in oysters, particularly in their gonads [6,7] and gills [75,79]. A few studies have reported heat stress and DNA damage in mussels, with very little information about its effects on DNA strand breaks [39,45,90]. However, none of the above studies have clearly described the oxidative DNA lesions and signaling pathways in marine oysters. In this study, we demonstrated that rising seawater temperatures (28 and 32 °C) increase the mRNA and protein expressions of HSP70, 8-OHdG, γH2AX, and dsDNA in the gills of oysters. Interestingly, BAX and CAS-3 protein expressions also increased in relation to increased apoptotic cells in gills, which distinctly indicate DNA damage in the tissues of oysters. We also demonstrated that elevated temperature changes the composition of EP fluid (i.e., EF glucose and protein concentrations) in oysters. Together, these results imply that rising seawater temperature drastically induces oxidative DNA damage and cellular apoptosis in oyster tissues (Fig. 11).
Fig. 11.
A representative summary of the results of current study. Schematic diagram of heat stress and oxidative DNA damage and cellular apoptosis in gills of American oysters. Synthesis of 8-hydroxy-2′-deoxyguanosine (8-OHdG) and γ-histone family member X (γ-H2AX) proteins, and DNA double-stranded break (DSBs). Oligomerization of heat shock protein-70 (HSP70) and synthesis of apoptotic proteins (e.g., Bcl-2-associated-X (BAX), caspase-3, etc.) in gills of oysters.
4.1. Effects of heat stress in morphology of gills in oysters
An anticipated finding of this study is that elevated temperatures from 28 to 32 °C cause a change in gill structure and increase branchial lesions and mucus secretion. One of the roles of mucus is associated with host (oyster) defense against physical, chemical, and biological invasions from surrounding water [79,91]. This characteristic is gained by the organism due to the presence of bioactive molecules (i.e., HSPs, CASs, etc.) embedded in their matrix that counteracts diverse stressors the host tissue faces. Increasing mucus secretions in gills thus pose a threat to the host body's immune system by counteracting the defense system against rising temperature. Histological observation in the gills of oysters also revealed an increase in branchial lesions found at the periphery of the gills possibly due to the exposure to seawater proximity, although apoptotic cells were not clearly visible under the microscope within the lesions in all treatment groups. Da Silva et al. [92] observed extreme branchial lesions with abundant apoptotic cells in the gills of flat oyster, Ostrea edulis, a close relative species to the American oyster, after they were exposed to increased temperature (25 °C). However, we were able to show apoptotic cells in different treatment groups of higher temperatures after in situ TUNEL analysis. Since the gills, an important organ of bivalve respiration, acts as a vital organ in gaseous exchange [93], the high temperature could limit its functional capacity from different aspects. Also, Kennedy and Mihursky [93] showed that high temperature (30 °C) decreases the respiratory metabolism in soft-shell clam, Mya arenaria, and dwarf-surf clam, Mulinaia lateralis. Overall, these results indicate that elevated temperatures cause changes in the morphology of gills and reduce their functions to a certain level.
4.2. Effects of heat stress on 8-OHdG expression in oysters
An important finding of this study is that elevated temperatures (28 and 32 °C for 1-week exposure) increase the expression of 8-OHdG in the gills of oysters. 8-OHdG is one of the most prevalent types of oxidative DNA damage that occur in a wide range of organisms [94]. Notably, the hydroxyl radical (HO•), is one of the most important oxygen-free radicals that attacks and damages DNA strands [41]. The interaction of this hydroxyl radical with the nucleobase guanine results in the formation of C8-hydroxyguanine or 8-OHdG [41]. Recently, Gleason et al. [45] demonstrated that exposure to high temperatures (35.7 °C for 23 days) caused an increase in 8-OHdG expression in the gills of California mussels. Remarkably, increased expression of 8-OHdG is not only associated with heat stress and linked to other environmental stressors. For example, Canova et al. [95] observed a significant increase of 8-OHdG levels in the gills and digestive glands of Mediterranean blue mussel, Mytilus galloprovincialis, after a 2 to 3-day exposure to benzo[a]pyrene (BaP). In addition, Torres et al. [96] detected higher levels of 8-OHdG in the digestive glands of the mangrove mussel, Mytella guyanensis, collected from a polluted mangrove area contaminated with trace metals (i.e., Hg, Pb, Cr, and Cd). However, Akcha et al. [97] observed BaP contamination (3–28 days exposure) does not make any difference in the levels of 8-OHdG in the gills of Mediterranean blue mussels. In addition to shellfish, a significant increase in 8-OHdG levels was found in the livers of English sole (Parophrys vetulus, a marine flatfish) collected from polluted areas [98]. Collectively, these results in conjunction with the present study suggest that elevated temperature, like other environmental stressors (i.e., pollutants), induces oxidative DNA damage in both fish and shellfish species. It is also envisaged that DNA damage in an organism may not occur solely from a single stressor and could be linked to other environmental factors.
4.3. Effects of heat stress on DNA damage in oysters
Heat shock acts as a noxious agent causing DNA damage and inhibiting the DNA excision repair system that consists of base excision repair and nucleotide excision repair [87]. DNA strand breaks (i.e., single-strand breaks [SSBs]; and double-strand breaks [DSBs]) are the most frequent type of DNA damage and are often repaired without any mistakes [99]. Importantly, DNA DSBs form through the inhibition of DNA topoisomerase II (top2), introducing temporary DSB fragments into DNA [100]. DNA DSBs are also correlated with DNA inhibition of both replication and transcription processes [101]. The results of the present study showed that the expression of DSBs increased around 1.32-fold at 28 °C and ∼1.26-fold at 32 °C in the gills of oysters after 1-week exposure compared to control. Similarly, short-term heat stress (32 °C for 8-h) drastically increases (∼44-fold) DSBs in the hemocytes of California mussels [39]. Moreover, it has been reported that high temperature (30 °C) induces p38 MAPK phosphorylation, a biomarker of DNA damage, which increases around 2.18-fold within 15 min in the mantle tissues of Mediterranean blue mussels [102]. Together, our findings imply that DNA DSBs are more common during heat stress and that if they occur, they are unrepairable, causing cellular apoptosis and so posing a higher threat to cellular integrity in marine mollusks.
4.4. Effects of heat stress on γ-H2AX expression in oysters
γ-H2AX is a novel biomarker of DNA DSBs which is induced by various environmental stressors such as heat, UV radiation, chemical exposure, etc. [51,52,103,104]. Notably, DSBs lead to phosphorylation of histone H2AX, a variant of the H2A protein family (i.e., a component of the histone octamer in nucleosomes) [51]. The newly phosphorylated proteins, γ-H2AX foci, subsequently recruit and localize DNA repair proteins, some of which are directly attached to γ-H2AX, while others are associated with the binding of proteins [51]. In the present study, IHC analysis of gill tissues showed a significant increase in γ-H2AX protein expression, concomitant with high temperature (28 and 32 °C) exposure, indicating DSBs in the gills of oysters. It has been demonstrated that short-term heat stress (45.5 °C for 20-min exposure) increases γ-H2AX foci formation in human H1299 cells in vitro [105]. In addition to heat stress, γ-H2AX was found to be upregulated from other environmental stressors. Gonzalez-Romero et al. [106] demonstrated that γ-H2AX mRNA levels were increased in the gills of oysters (C. virginica) exposed to red tides on the Florida coast. Recently, Vernon et al. [107] investigated the interactive effects of 32P and Cu on marine (M. galloprovincialis) and freshwater (Dreissena polymorpha) mussels using a multi-biomarker approach. They have shown that γ-H2AX foci were significantly higher in the gill cells following 10-day exposure to 32P and Cu in mussels. Sayed et al. [108] reported increasing degrees of γ-H2AX expression in the blood cells of Japanese medaka (Oryzias latipes, a freshwater fish) exposed to γ-irradiation for 24 h. Taken together, our results support the concept that heat stress induces oxidative stress, which leads to increased DNA DSBs and γ-H2AX expressions in oyster tissues that could negatively affect genetic characteristics in marine mollusks, particularly when they are chronically exposed to heat stress and/or heat waves.
4.5. Effects of heat stress on HSP70 expression and cellular apoptosis in oysters
HSP70 is one of the key chaperon proteins because it plays a central role in folding and unfolding peptides and protecting cells and tissues during environmental stress [109]. In the present study, we observed that short-term exposure to high temperatures (28 and 32°C) increased HSP70 mRNA levels (∼25-fold) and protein expression (∼1.3-fold) in the gills of oysters. A similar trend of HSP70 gene and protein expressions was also observed in the gills, digestive glands, and gonads of oysters exposed to short-term high temperatures (28 and 32°C for 1 week), which support our findings [7,75]. Similarly, Kefaloyianni et al. [102] demonstrated that short-term exposure to high temperature (30 °C for 120 min) induced HSP70 protein expression around 4.2-fold in the gills of blue mussels. Ivanina et al. [110] reported that long-term exposure to high temperatures (20, 24, 28, and 32 °C for 45–50 days) resulted in significantly elevated (∼9–18 fold) HSP69, an inducible isoform of the HSP70 family, expression in the gills of American oyster. Additionally, Piano et al. [111] found that high temperature (35 °C for 1 h) induced HSP69 mRNA transcript in the gills of flat oysters (O. edulis). Similarly, acute heat stress (37 °C for 6-h exposure) increased HSP70 mRNA levels in the gills of Hong Kong oyster, C. hongkongensis [112]. Moreover, Tomanek and Zuzow [112] investigated multiple HSP70 isoforms and have shown that HSP70 (11) isoform expression was higher at 28 °C, but HSP70 (14), HSP70 (15), and HSP70 (16) isoforms expressions were higher at 32 °C in the gills of Mediterranean blue mussels after 1-h heat stress. It is worth noting that HSP70 expression varies among isoforms in oysters after exposure to sublethal and lethal heat stress [[113], [114]]. However, the temperature at which the responses occur varies from species to species compared to their natural environment [115]. Since HSP70 is a bioactive molecule that is housed in the tissues of mollusks [109], it may protect cells against environmental stress; however, excessive heat stress and/or heat waves can overwhelm and cause severe tissue damage including apoptosis induction in cells and tissues of oysters [7,79].
The apoptotic cell death process is a defense mechanism that is adopted by organisms against any environmental stressors as they eliminate dead or infected cells [116]. An important finding of this study is that short-term exposure to high temperatures (28 and 32°C for 1 week) increased the apoptotic cells in the gills of oysters. Yang et al. [117] found acute heat stress (25 °C) for 36 d and 60 h significantly increased the apoptosis rate of hemocytes in the Pacific oysters. Cherkasov et al. [118] demonstrated that high temperature (28 °C) significantly increases the apoptosis in hemocytes of American oysters. High temperature also induces the increased apoptotic cell in other marine invertebrates. Johnstone et al. [2] found that high temperature (32 °C) induced the apoptotic cells around 3-fold in the testicular tissues and ∼1.4-fold in the ova of Atlantic Sea urchin, and increased protein carbonyl, a biomarker of ROS, contents in gonads. Several studies have also shown high-temperature induced cellular apoptosis in tissues of teleost species [119], [120], [121]. For example, Sleadd et al. [122] found that sub-lethal heat stress induces apoptosis in hepatocytes of common Antarctic fish (Trematomus bernacchii). Since apoptosis is considered an integral aspect of the development and homeostasis process in aquatic organisms [116], increasing apoptosis in cells and tissues may jeopardize their lives and limits their survival capacity in the warming coastal and/or marine ecosystems.
4.6. Effects of heat stress on BAX expression in oysters
BAX is considered a novel pro-apoptotic regulator protein [10]. Arguably, there is little information regarding how heat stress affects BAX regulation in terrestrial and aquatic organisms. A few in vitro and in vivo studies have been done in mice and rats. Bleicken et al. [123] found that elevated temperatures (from 20 to 37 °C) increased the activation of BAX in BL21 (DE3) RIPL cells in vitro. Luo et al. [124] observed that high temperatures (40 and 43 °C) increased BAX mRNA levels in granulosa cells in mice in vitro. It has also been shown that high temperature (43 °C) induced cytochrome c release (by BAX) in mouse liver from the mitochondrial outer membrane, thus indicating the role of BAX in apoptosis [125]. Thus, cytochrome c released from the mitochondria through ion channels combines with apoptotic protease-activating factor, leading to active CAS-3, a key effector enzyme in inducing cellular apoptosis [124]. Likewise, Topal et al. [126] recently showed that short-term exposure to high temperatures (20 and 25 °C for 8 h) increased BAX mRNA levels around 1.5-fold in the brain of rainbow trout (Oncorhynchus mykiss). Moreover, Wang et al. [127] demonstrated that short-term heat stress (37 °C for 24 h) induced BAX mRNA levels (∼4-fold) and apoptosis in the gills of razor clam (Sinonovacula constricta). Similarly, we found that high temperatures (28 and 32 °C) increased BAX expression (∼1.7-fold) in the gills of oysters. These findings complying with our results suggest that elevated temperature acts as an environmental stressor that stimulates BAX to induce cellular apoptosis in terrestrial and aquatic organisms.
4.7. Effects of heat stress on CAS-3 expression in oysters
Similar to BAX, CAS-3, a family of cysteine protease, is a key biomarker in signaling pathways of DNA damage and programmed cell death (apoptosis) in bivalve mollusks [128]. Steinert [42] and Sokolova et al. [65] showed that DNA damage was induced due to the induction of apoptosis and the level of necrosis in somatic and germ cells in mussels, and hemocytes in oysters exposed to high temperatures and pollutants. In this study, we have shown that the number of apoptotic cells, and BAX and CAS-3 proteins, were increased in a similar fashion (∼1.5–1.9-fold), indicating their similar roles during heat stress in the gills of oysters. Recently, our research groups have shown that short-term exposure to high temperatures (28 and 32 °C for 1 week) induced CAS-3/7 activity (around 4-fold) and cellular apoptosis in the testes and ovaries of oysters [6,7]. Similarly, Chang et al. [129] showed that CAS-3 mRNA expression, CAS-3 activity, and apoptosis rate were significantly increased in hemocytes of white shrimp, Litopenaeus vannamei, exposed to 22 °C for 1 week. Kefaloyianni et al. [102] found that acute thermal stress (30 °C) increased CAS-3 activity in the gills of Mediterranean blue mussels. These findings, in conjunction with our results, suggest that high-temperature triggers and activates CAS-3 in the cells and tissues which causes cellular apoptosis and DNA damage, leading to physiological function deviation in marine mollusks.
4.8. Effects of heat stress on EP fluid conditions in oysters
EP fluid is an important body fluid as it maintains osmoregulatory processes, body temperature, and various physiological functions in oysters [7,75]. EP fluid shows functional similarity to the hemolymphs of lower invertebrates, and blood of vertebrates [7]. Importantly, EP fluid contains proteins, carbohydrates, lipids, amino acids, glucose, etc., in mollusks [130]. In aquatic organisms, the water temperature greatly influences the blood/EP fluid glucose and protein levels, which can be used as bioindicators in fish and shellfish species. In the present study, we have shown that high temperatures (28 and 32 °C for 1 week) increased EP fluid glucose levels (∼1.9-fold) in oysters. Similarly, Billah and Rahman [64] demonstrated that EP fluid glucose levels in oysters were upregulated in elevated seawater temperatures (∼1.02-fold at 28 °C and ∼1.18-fold at 32 °C) suggesting increased metabolism during heat stress. Caldari-Torres et al. [130] recorded significantly higher hemolymph glucose levels (∼1.5-fold; 45 ± 14.3 mg/dL) in stressed crayfish (genus Orconestes). In addition, Nash et al. [7] showed that short-term exposure to elevated temperatures (28 and 32 °C for 1 week) causes decreased EP fluid protein concentrations in oysters. In the present study, we also demonstrated that high temperatures markedly decreased EP fluid protein concentrations in oysters. Since the EP fluid protein binds to Ca2+ and may act as a precursor of the soluble organic matrix in the shell [131], a reduction of EP fluid protein concentrations may inhibit the growth and development of oysters. Moreover, the biomolecular components of EP fluid are involved in shell formation [132], defense, and regulatory processes [75]; therefore, an increase or decrease in any of its components may negatively affect the physiological functions and cellular integrity (i.e., DNA lesions) in oysters during heat stress.
5. Conclusion
The present study provides, to the best of our knowledge, the first conclusive evidence and potential pathways of oxidative DNA damage, cellular apoptosis, DNA DSBs, and 8-OHdG regulation in the gills of American oysters during heat stress. Notably, the American oyster habitat includes the coastal environment of a wide fluctuation of temperature (from 4 to 5 °C during winter to 30–31 °C in summer months) [109]. Importantly, this species may represent an ideal model species to monitor the thermal impacts on marine invertebrates as well as the overall health of the environment in the Gulf of Mexico and along the Atlantic coast. Also, the American oyster is an important marine bivalve species, both ecologically and economically, and it is vital to conserve this species in its proper habitat. The study of heat stress on oysters contributes to the body of knowledge on the effects of global warming and /or heat waves on multiple scales, ranging from molecular to cellular, and biochemical to physiological levels in oysters. Our results showed that high water temperature leads to morphological changes in the oyster gills by causing an increase in expression levels of BAX, CAS-3, 8-OHdG, dsDNA, γ-H2AX, and apoptosis, and a decrease in EP fluid protein concentrations. Future studies may explore the prospects of DNA repairs in the cells and/or tissues to identify potential genes involved in the repair mechanisms in the tissues of oysters.
Funding information
This study was funded in part by the UTRGV College of Sciences Dean Graduate Research Assistant (DGRA) to Md Faizur Rahman, and the start-up fund and University of Texas Rio Grandy Valley (UTRGV) College of Science SEED grant (grant no. 210000371) to Dr. Md Saydur Rahman.
CRediT authorship contribution statement
Md Faizur Rahman: Conceptualization, Methodology, Formal analysis, Funding acquisition, Investigation, Methodology, Writing – original draft, Writing – review & editing. Mohammad Maruf Billah: Conceptualization, Methodology, Writing – review & editing. Richard J. Kline: Conceptualization, Writing – review & editing. Md Saydur Rahman: Conceptualization, Methodology, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Supervision, Writing – original draft, Writing – review & editing.
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
We would like to thank Britney Lacy and Md Sadequr Rahman, School of Earth, Environmental and Marine Sciences (SEEMS), University of Texas Rio Grande Valley (UTRGV) for their help in oyster dissection. The authors are grateful to Dr. Drew Davis, SEEMS, UTRGV, and Kristen Kline, Department of History, UTRGV, for critically reviewing and editing the manuscript. We also thank Esmirna Cantu, SEEMS, UTRGV, for drawing the oyster anatomy. We would like to thank and truly appreciate the two anonymous reviewers for their valuable time, suggestions, and constructive comments on the manuscript.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.fsirep.2022.100079.
Appendix. Supplementary materials
Data Availability
Data will be made available on request.
References
- 1.Root T.L., Price J.T., Hall K.R., Schneider S.H., Rosenzweig C., Pounds J.A. Fingerprints of global warming on wild animals and plants. Nature. 2003;421(6918):57–60. doi: 10.1038/nature01333. [DOI] [PubMed] [Google Scholar]
- 2.Johnstone J., Nash S., Hernandez E., Rahman M.S. Effects of elevated temperature on gonadal functions, cellular apoptosis and oxidative stress in Atlantic sea urchin Arabacia punculata. Mar. Environ. Res. 2019;149:40–49. doi: 10.1016/j.marenvres.2019.05.017. [DOI] [PubMed] [Google Scholar]
- 3.Chua C.M., Leggat W., Moya A., Baird A.H. Temperature affects the early life history stages of corals more than near future ocean acidification. Mar. Ecol. Prog. Series. 2013;475:85–92. doi: 10.3354/MEPS10077. [DOI] [Google Scholar]
- 4.Huang M., Ding L., Wang J., Ding C., Tao J. The impacts of climate change on fish growth: a summary of conducted studies and current knowledge. Ecol Ind. 2021;121 doi: 10.1016/j.ecolind.2020.106976. [DOI] [Google Scholar]
- 5.Alix M., Kjesbu O.S., Anderson K.C. From gametogenesis to spawning: how climate-driven warming affects teleost reproductive biology. J. Fish Biol. 2020;97(3):607–632. doi: 10.1111/jfb.14439. [DOI] [PubMed] [Google Scholar]
- 6.Nash S., Rahman M.S. Short-term heat stress impairs testicular functions in the American oyster, Crassostrea virginica: molecular mechanisms and induction of oxidative stress and apoptosis in spermatogenic cells. Mol. Reprod. Develop. 2019;86(10):1444–1458. doi: 10.1002/mrd.23268. [DOI] [PubMed] [Google Scholar]
- 7.Nash S., Johnstone J., Rahman M.S. Elevated temperature attenuates ovarian functions and induces apoptosis and oxidative stress in the American oyster, Crassostrea virginica: potential mechanisms and signaling pathways. Cell Stress Chaper. 2019;24:957–967. doi: 10.1007/s12192-019-01023-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rao D.G.V.P., Khan M.A.Q. Zebra mussels: enhancement of copper toxicity by high temperature and its relationship with respiration and metabolism. Water Environ. Res. 2000;72(2):175–178. doi: 10.2175/106143000X137257. [DOI] [Google Scholar]
- 9.Khan M.A.Q., Ahmed S.A., Catalin B., Khodadoust A., Ajayi O., Vaughn M. Effects of Temperature on heavy metal toxicity to juvenile crayfish, Orconectes immunis (Hagen) Environ. Toxicol. 2006;21:513–520. doi: 10.1002/tox.20213. [DOI] [PubMed] [Google Scholar]
- 10.Chan M.W.H., Hasan K.A., Balthazar-Silva D., Asghar M., Mirani Z.A. Surviving under pollution stress: antibacterial and antifungal activities of the oyster species (Magallana bilineata and Magallana cuttackensis) Fish Shellfish Immunol. 2021;108:142–146. doi: 10.1016/j.fsi.2020.11.021. [DOI] [PubMed] [Google Scholar]
- 11.Chan M.W.H., Ali A., Ullah A., Mirani Z.A., Balthazar-Silva D. A size-dependent bioaccumulation of metal pollutants, antibacterial and antifungal activities of Telescopium telescopium, Nerita albicilla and Lunella coronata. Environ. Toxicol. Pharmacol. 2021;87 doi: 10.1016/j.etap.2021.103722. [DOI] [PubMed] [Google Scholar]
- 12.Aslam S., Chan M.W.H., Siddiqui G., Boczkaj G., Kazmi S.J.H., Kazmi M.R. A comprehensive assessment of environmental pollution by means of heavy metal analysis for oysters' reefs at Hab River Delta, Balochistan, Pakistan. Mar. Poll. Bull. 2020;153 doi: 10.1016/j.marpolbul.2020.110970. [DOI] [PubMed] [Google Scholar]
- 13.Zhao C.H., Liu H.Q., Cao R., Ji A.L., Zhang L., Wang F., Yang R.H. Effects of dietary fish oil on learning functions and apoptosis of hippocampal neurons in streptozotocin-diabetic rats. Brain Res. 2012;1457:33–43. doi: 10.1016/j.brainres.2012.03.067. [DOI] [PubMed] [Google Scholar]
- 14.Slimen B.I., Najar T., Ghram A., Abdrrabba M. Heat stress effects on livestock: molecular, cellular and metabolic aspects, a review. J. Ani. Physiol. Ani. Nutr. 2016;100(3):401–412. doi: 10.1111/jpn.12379. [DOI] [PubMed] [Google Scholar]
- 15.Bernal M.A., Schunter C., Lehmann R., Lightfoot D.J., Allan B.J., Veilleux H.D.…Ravasi T. Species-specific molecular responses of wild coral reef fishes during a marine heatwave. Sci. Adv. 2020;6(12):eaay3423. doi: 10.1126/sciadv.aay3423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Huang X., Li S., Gao Y., Zhan A. Genome-wide identification, characterization and expression analyses of heat shock protein-related genes in a highly invasive ascidian Ciona savignyi. Front. Physioi. 2018;9:1043. doi: 10.3389/fphys.2018.01043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Doberentz E., Genneper L., Wagner R., Madea B. Expression times for hsp27 and hsp70 as an indicator of thermal stress during death due to fire. Int. J. Legal Med. 2017;131(6):1707–1718. doi: 10.1007/s00414-017-1566-x. [DOI] [PubMed] [Google Scholar]
- 18.Aghdassi A., Phillips P., Dudeja V., Dhaulakhandi D., Sharif R., Dawra R., Lerch M.M., Saluja A. Heat shock protein 70 increases tumorigenicity and inhibits apoptosis in pancreatic adenocarcinoma. Cancer Res. 2007;67(2):616–625. doi: 10.1158/0008-5472.CAN-06-1567. [DOI] [PubMed] [Google Scholar]
- 19.Mikami T., Sumida S., Ishibashi Y., Ohta S. Endurance exercise training inhibits activity of plasm GOT and liver casepase-3 of rats exposed to stress by induction of heat shock protein 70. J. Appl. Physiol. 2004;96:1776–1781. doi: 10.1152/japplphysiol.00795.2002. [DOI] [PubMed] [Google Scholar]
- 20.Barnett A.F., Gledhill J.H., Griffitt R.J., Slattery M., Gochfeld D.J., Willett K.L. Combined and independent effects of hypoxia and tributyltin on mRNA expression and physiology of the Eastern oyster (Crassostrea virginica) Sci. Rep. 2020;10(1):1–13. doi: 10.1038/s41598-020-67650-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bierkens J.A. Applications and pitfalls of stress proteins in biomonitoring. Toxicology. 2000;153(1–3):61–72. doi: 10.1016/s0300-483x(00)00304-8. [DOI] [PubMed] [Google Scholar]
- 22.Ahamed M., Posgai R., Gorey T.J., Nielsen M., Hussain S.M., Rowe J.J. Silver nanoparticles induced heat shock protein 70, oxidative stress and apoptosis in Drosophila melanogaster. Toxicol. Appl. Pharmacol. 2009;242:263–269. doi: 10.1016/j.taap.2009.10.016. [DOI] [PubMed] [Google Scholar]
- 23.Zhang G., Fang X., Guo X., Li L.I., Luo R., Xu F.…Wang J. The oyster genome reveals stress adaptation and complexity of shell formation. Nature. 2012;490(7418):49–54. doi: 10.1038/nature11413. [DOI] [PubMed] [Google Scholar]
- 24.Liu D., Chen Z. The expression and induction of heat shock proteins in molluscs. Protein Peptide Lett. 2013;20(5):602–606. doi: 10.2174/0929866511320050014. [DOI] [PubMed] [Google Scholar]
- 25.Evans C.G., Chang L., Gestwicki J.E. Heat Shock Protein 70 (Hsp70) as an emerging Drug Target. J. Med. Chem. Persp. 2010;53(12):4585–4602. doi: 10.1021/jm100054f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Clark M.S., Fraser K.P., Peck L.S. Antarctic marine molluscs do have an HSP70 heat shock response. Cell Stress Chaper. 2008;13(1):39–49. doi: 10.1007/s12192-008-0014-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Clark M.S., Peck L.S. HSP70 heat shock proteins and environmental stress in Antarctic marine organisms: a mini-review. Mar. Genom. 2009;1(2):11–18. doi: 10.1016/j.margen.2009.03.003. [DOI] [PubMed] [Google Scholar]
- 28.Hassan F.U., Nawaz A., Rehman M.S., Ali M.A., Dilshad S.M., Yang C. Prospects of HSP70 as a genetic marker for mmune-tolerance and mmune-modulation in animals under climate change scenario. Anim. Nutr. 2019;5(4):340–350. doi: 10.1016/j.aninu.2019.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Oberringer M., Baum H.P., Jung V., Welter C., Frank J., Kuhlmann M., Mutschler W., Hanselmann R.G. Differential expression of heat shock protein 70 in well healing and chronic human wound tissue. Biochem. Biophy. Res. Comm. 1995;214(3):1009–1014. doi: 10.1006/bbrc.1995.2386. [DOI] [PubMed] [Google Scholar]
- 30.Ait-Aissa S., Porcher J.M., Arrigo A.P., Lambre C. Activation of the hsp70 promoter by environmental inorganic and organic chemicals: relationships with cytotoxicity and lipophilicity. Toxicology. 2000;145(2–3):147–157. doi: 10.1016/s0300-483x(00)00145-1. [DOI] [PubMed] [Google Scholar]
- 31.Siddique H.R., Gupta S.C., Mitra K., Bajpai V.K., Mathur N., Murthy R.C., Saxena D.K., Chowdhuri D.K. Adverse effect of tannery waste leachates in transgenic Drosophila melanogaster: role of ROS in modulation of Hsp70, oxidative stress and apoptosis. J. Appl. Toxicol. 2008;28(6):734–748. doi: 10.1002/jat.1332. [DOI] [PubMed] [Google Scholar]
- 32.Belhadj Slimen I., Najar T., Ghram A., Dabbebi H., Ben Mrad M., Abdrabbah M. Reactive oxygen species, heat stress and oxidative-induced mitochondrial damage. a review. Int. J. Hypertherm. 2014;30(7):513–523. doi: 10.3109/02656736.2014.971446. [DOI] [PubMed] [Google Scholar]
- 33.Nikitaki Z., Hellweg C.E., Georgakilas A.G., Ravant J.L. Stress-induced DNA damage biomarkers: applications and limitations. Front. Chem. 2015;3:1–15. doi: 10.3389/fchem.2015.00035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sanders B.M. Stress proteins in aquatic organisms: an environmental perspective. Critical Rev. Toxicol. 1993;23(1):49–75. doi: 10.3109/10408449309104074. [DOI] [PubMed] [Google Scholar]
- 35.Brierley A.S., Kingsford M.J. Impacts of climate change on marine organisms and ecosystems. Curr. Biol. 2009;19(14):R602–R614. doi: 10.1016/j.cub.2009.05.046. [DOI] [PubMed] [Google Scholar]
- 36.Binnaser S.Y. Global warming, marine invertebrates, and Saudi Arabia coast on the red sea: an updated review. Egyptian J. Aquat. Biol. Fish. 2021;25(4):221–240. doi: 10.21608/ejabf.2021.187702. [DOI] [Google Scholar]
- 37.Parrino V., Costa G., Cannavà C., Fazio E., Bonsignore M., Concetta S., Piccione G., Fazio F. Flow cytometry and micro-Raman spectroscopy: identification of hemocyte populations in the mussel Mytilus galloprovincialis (Bivalvia: mytilidae) from Faro Lake and Tyrrhenian Sea (Sicily, Italy) Fish Shellfish Immunol. 2019;87:1–8. doi: 10.1016/j.fsi.2018.12.067. [DOI] [PubMed] [Google Scholar]
- 38.Parrino V., Costa G., Giannetto A., De Marco G., Cammilleri G., Acar Ü., Piccione G., Fazio F. Trace elements (Al, Cd, Cr, Cu, Fe, Mn, Ni, Pb and Zn) in Mytilus galloprovincialis and Tapes decussatus from Faro and Ganzirri Lakes (Sicily, Italy): flow cytometry applied for hemocytes analysis. J. Trace Elem. Med. Biol. 2021;68 doi: 10.1016/j.jtemb.2021.126870. [DOI] [PubMed] [Google Scholar]
- 39.Yao C.L., Somero G.N. The impact of acute temperature stress on hemocytes of invasive and native mussels (Mytilus galloprovincialis and Mytilus californianus): DNA damage, membrane integrity, apoptosis and signaling pathways. J. Exp. Biol. 2012;215(24):4267–4277. doi: 10.1242/jeb.073577. [DOI] [PubMed] [Google Scholar]
- 40.Min K.S., Horie T., Tetsutchikawahara N., Onosaka S. Metallothionein suppresses the formation of 8-hydroxy-2’-deoxyguanosine in DNA induced by ferric nitrilotriacetate in vitro. J. Health Sc. 2005;51(4):497–503. doi: 10.1248/jhs.51.497. [DOI] [Google Scholar]
- 41.Valavanidis A., Vlachogianni T., Fiotakis C. 8-hydroxy-2’-deoxyguanosine (8-OHdG): a critical biomarker of oxidative stress and carcinogenesis. J. Environ. Sci. Health. Part C. 2009;27(2):120–139. doi: 10.1080/10590500902885684. [DOI] [PubMed] [Google Scholar]
- 42.Steinert S.A. DNA damage as a bivalve biomarker. Biomarkers. 1999;4(6):492–496. doi: 10.1080/135475099230651. [DOI] [PubMed] [Google Scholar]
- 43.Domijan A.M., Peraica M. Determination of 8-hydroxy-2’deoxyguanosine in urine using HPLC with electrochemical detection. Arch. Ind. Hygiene Toxicol. 2008;59(4):277–282. doi: 10.2478/10004-1254-59-2008-1879. [DOI] [PubMed] [Google Scholar]
- 44.Michel C., Vincent-Hubert F. DNA oxidation and DNA repair in gills of zebra mussels exposed to cadmium and benzo(a)pyrene. Ecotoxicology. 2015;24(9):2009–2016. doi: 10.1007/s10646-015-1536-3. [DOI] [PubMed] [Google Scholar]
- 45.Gleason L.U., Miller L.P., Winnikoff J.R., Somero G.N., Yancey P.H., Bratz D., Dowd W.W. Thermal history and gape of individual Mytilus californianus correlate with oxidative damage and thermoprotective osmolytes. J. Exp. Biol. 2017;220:4292–4304. doi: 10.1242/jeb.168450. [DOI] [PubMed] [Google Scholar]
- 46.Bortoletti M., Maccatrozzo L., Radaelli G., Caberlotto S., Bertotto D. Muscle cortisol levels, expression of glucocorticoid receptor and oxidative stress markers in the teleost fish Argyrosomus regius exposed to transport stress. Animals. 2021;11(4):1160. doi: 10.3390/ani11041160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Luo S.Y., Liu C., Ding J., Gao X.M., Wang J.Q., Zhang Y.B., Du C., Hou C.C., Zhu J.Q., Lou B., Wu X.F. Scavenging reactive oxygen species is a potential strategy to protect Larimichthys crocea against environmental hypoxia by mitigating oxidative stress. Zool. Res. 2021;42(5):592. doi: 10.24272/j.issn.2095-8137.2021.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rogakou E.P., Pilch D.R., Orr A.H., Ivanova V.S., Bonner W.M. DNA double-stranded breaks induce histone H2AX phosphorylation on serine 139*. J. Biol. Chem. 1998;273(10):5858–5868. doi: 10.1074/jbc.273.10.5858. [DOI] [PubMed] [Google Scholar]
- 49.Tuul M., Kitao H., Limori M., Matsuoka K., Kiyonari S., Saeki H., Oki E., Morita M., Maehara Y. Rad9, Rad17, TopBP1 and claspin play essential roles in heat-induced activation of ATP kinase and heat tolerance. PLoS One. 2013;8(2):1–12. doi: 10.1371/journal.pone.0055361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tumminello R.A., Fuller-Espie S.L. Heat stress induces ROS production and histone phosphorylation in celomocytes of Eisenia hortensis. Invert. Sur. J. 2013;10(1):50–57. [Google Scholar]
- 51.Kuo L.J., Yang L.X. γH2AX- a novel biomarker for DNA double-strand breaks. In Vivo. 2008;22(3):305–309. doi: 10.1016/j.ecoenv.2014.03.035. [DOI] [PubMed] [Google Scholar]
- 52.Mah L.J., El-Osta A., Karagiannis T.C. γh2AX: a sensitive molecular marker of DNA damage and repair. Leukemia. 2010;24:679–686. doi: 10.1038/leu.2010.6. [DOI] [PubMed] [Google Scholar]
- 53.Whittemore K., Martinez-Nevado E., Blasco M.A. Slower rates of accumulation of DNA damage in leukocytes correlet with longer lifespans across several species of birds and mammals. Aging. 2019;11(21):9829–9845. doi: 10.18632/aging.102430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Thompson C.B. Apoptosis in the pathogenesis and treatment of disease. Science. 1995;267(5403):1456–1462. doi: 10.1126/science.7878464. [DOI] [PubMed] [Google Scholar]
- 55.Kepp O., Rajalingam K., Kimmig S., Rubel T. Bak and Bax are non-redundant during infection-and DNA damage-induced apoptosis. EMBO J. 2007;26:825–834. doi: 10.1038/sj.emboj.7601533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Nicholas D.W., Thornberry N.A. Caspases: killer proteases. Trends Biomed. Sci. 1997;22(8):299–306. doi: 10.1016/s0968-0004(97)01085-2. [DOI] [PubMed] [Google Scholar]
- 57.Wolf B.B., Schuler M., Echeverri F., Green D.R. Casepase-3 is the primary activator of cpoptotic DNA fragmentation via DNA fragmentation factor-45/inhibitor of caspase-activated DNase inactivation. J. Biol. Chem. 1999;274(43):30651–30656. doi: 10.1074/jbc.274.43.30651. [DOI] [PubMed] [Google Scholar]
- 58.Ishizaki Y., Jacobson M.D., Raff M.C. A role for caspases in lens fiber differentiation. J. Cell Biol. 1998;140(1):153–158. doi: 10.1083/jcb.140.1.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Weil M., Raff M.C., Braga V.M. Caspase activation in the terminal differentiation of human epidermal keratinocytes. Curr. Biol. 1999;9(7):361–364. doi: 10.1016/s0960-9822(99)80162-6. [DOI] [PubMed] [Google Scholar]
- 60.Danial N.K., Korsmeyer S.J. Cell death: critical control points. Cell. 2004;116(2):205–219. doi: 10.1016/s0092-867(04)00046-7. [DOI] [PubMed] [Google Scholar]
- 61.Korsmeyer S.J., Wei M.C., Saito M., Weiler S., Oh K.J., Schlesinger P.H. Pro-apoptotic cascade activates BID, which oligomerizes BAK or BAX into pores that result in the release of cytochrome c. Cell Death Diff. 2000;7:1166–1173. doi: 10.1038/sj.cdd.4400783. [DOI] [PubMed] [Google Scholar]
- 62.MacGibbon G.A., Lawlor P.A., Sirimanne E.S., Walton M.R., Connor B., Young D., Williams C., Gluckman P., Faull R.L.M., Hughes P., Dragunow M. Bax expression in mammalian neurons undergoing apoptosis, and in Alzheimer's disease hippocampus. Brain Res. 1997;750(1–2):223–234. doi: 10.1016/s0006-8993(96)01351-0. [DOI] [PubMed] [Google Scholar]
- 63.Wei M.C., Zong W.-.X., Cheng E.H.Y., Lindsten T., Panoutsakopoulou V., Ross A.J., Roth K.A., MacGregor G.R., Thompson C.B., Korsmeyer S.J. Pro-apoptotic BAX and BAK: a requisite gateway to mitochondrial dysfunction and death. Science. 2001;292(5517):727–730. doi: 10.1126/science.1059108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Billah M.M., Rahman M.S. Impacts of anthropogenic contaminants and elevated temperature on prevalence and proliferation of Escherichia coli in the wild-caught American oyster, Crassostrea virginica in the southern Gulf of Mexico coast. Mar. Biol. Res. 2021;17(9–10):775–793. doi: 10.1080/17451000.2022.2053161. [DOI] [Google Scholar]
- 65.Sokolova I.M. Cadmium effects on mitochondrial function are enhanced by elevated temperatures in a marine poikilotherm, Crassostrea virginica Gmelin (Bivalvia: ostreidae) J. Exp. Biol. 2004;207(15):2639–2648. doi: 10.1242/jeb.01054. [DOI] [PubMed] [Google Scholar]
- 66.Sokolova I.M., Evans S., Hughes F.M. Cadmium-induced apoptosis in oyster hemocytes involves disturbance of cellular energy balance but not mitochondrial permeability transition. J. Exp. Biol. 2004;207(19):3369–3380. doi: 10.1242/jeb.01152. [DOI] [PubMed] [Google Scholar]
- 67.Lannig G., Flores J.F., Sokolova I.M. Temperature-dependent stress response in oysters, Crassostrea virginica: pollution reduces temperature tolerance in oysters. Aquat. Toxicol. 2006;79:278–287. doi: 10.1016/j.aquatox.2006.06.017. [DOI] [PubMed] [Google Scholar]
- 68.Jones H.R., Johnson K.M., Kelly M.W. Synergistic effects of temperature and salinity on the gene expression and physiology of Crassostrea virginica. Integ. Comp. Biol. 2019;59:306–319. doi: 10.1093/icb/icz035. [DOI] [PubMed] [Google Scholar]
- 69.MacKenzie C.L., Jr, Burrell V.G., Jr, Rosenfield A., Hobart W.L. 1997. The History, Present Condition, and Future of the Molluscan Fisheries of North and Central America and Europe; p. 234.https://spo.nmfs.noaa.gov/sites/default/files/tr127opt.pdf A Technical Report of the Fishery Bulletin, Vol 1, Atlantic and Gulf Coasts. U.S. Dept. Commer., NOAA Tech. Rep. 127. [Google Scholar]
- 70.Shumway S.E. In: The Eastern Oyster Crassostrea virginica. Kennedy V.S., Newell R.I.E., Eble A.F., editors. Maryland Sea Grant College; College Park: 1996. Natural environmental factors; pp. 467–513. [Google Scholar]
- 71.Marshall D.A., Coxe N.C., La Peyre M.K., Walton W.C., Rikard F.S., Pollack J.B., Kelly M.W., La Peyre J.F. Tolerance of northern Gulf of Mexico eastern oysters to chronic warming at extreme salinities. J. Thermal. Biol. 2021;100 doi: 10.1016/j.jtherbio.2021.103072. [DOI] [PubMed] [Google Scholar]
- 72.Li Y., Qin J.G., Abbott C.A., Li X., Benkendorff K. Synergistic impacts of heat shock and spawning on the physiology and immune health of Crassostrea gigas: an explanation for summer mortality in Pacific oysters. Amer. J. Physiol. Reg. Integr. Comp. Physiol. 2007;293(6):R2353–R2362. doi: 10.1152/ajpregu.00463.2007. [DOI] [PubMed] [Google Scholar]
- 73.Haven D.S., Morales-Alamo R. Filtration of particles from suspension by the American oyster Crassostrea virginica. Biol. Bull. 1970;139(2):248–264. doi: 10.2307/1540081. [DOI] [PubMed] [Google Scholar]
- 74.Jørgensen C.B., Larsen P.S., Riisgård H.U. Effects of temperature on the mussel pump. Mar. Ecol. Prog. Series. 1990:89–97. [Google Scholar]
- 75.Rahman M.S., Rahman M.S. Elevated seasonal temperature disrupts prooxidant-antioxidant homeostasis and promotes cellular apoptosis in the American oyster, Crassostrea virginica, in the Gulf of Mexico: a field study. Cell Stress Chapr. 2021;26:917–936. doi: 10.1007/s12192-021-01232-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Allam B., Paillard C. Defense factors in clam extrapallial fluids. Dis. Aquat. Org. 1998;33:123–128. doi: 10.3354/dao033123. [DOI] [Google Scholar]
- 77.Lowe M.R., Sehlinger T., Soniat T.M., La Peyre M.K. Interactive effects of water temperature and salinity on growth and mortality of eastern oysters, Crassostrea virginica: a meta-analysis using 40 years of monitoring data. J. Shellfish Res. 2017;36:683–697. doi: 10.2983/035.036.0318. [DOI] [Google Scholar]
- 78.Casas S.M., Filgueira R., Lavaud R., Comeau L.A., La Peyre M.K., La Peyere J.F. Combined effects of temperature and salinity on the physiology of two geographically distant Eastern oyster populations. J. Exper. Mar. Biol. Ecol. 2018;506:82–90. doi: 10.1016/j.jembe.2018.06.001. [DOI] [Google Scholar]
- 79.Rahman M.S., Rahman M.S. Effects of elevated temperature on prooxidant-antioxidant homeostasis and redox status in the American oyster: signaling pathways of celllular apoptosis during heat stress. Environ. Res. 2021;196 doi: 10.1016/j.envres.2020.110428. [DOI] [PubMed] [Google Scholar]
- 80.Haberkorn H., Lambert C., Le Goïc N., Moal J., Suquet M., Guéguen M., Sunila I., Soudant P. Effects of Alexandrium minutum exposure on nutrition-related processes and reproductive output in oysters Crassostrea gigas. Harmful Algae. 2010;9(5):427–439. doi: 10.1016/j.hal.2010.01.003. [DOI] [Google Scholar]
- 81.Khondee P., Srisomsap C., Chokchaichamnankit D., Svasti J., Simpson R.J., Kingtong S. Histopathological effect and stress response of mantle proteome following TBT exposure in the Hooded oyster Saccostrea cucullata. Environ. Pollut. 2016;218:855–862. doi: 10.1016/j.envpol.2016.08.011. [DOI] [PubMed] [Google Scholar]
- 82.Mohan K.M. School of Earth, Environmental, and Marine Sciences, University of Texas Rio Grande Valley; 2022. Effects of Tributyltin Exposure On 8-OHdG and dsDNA expressions, Oxidative and Nitrative Stress in the American oyster, Crassostrea virginica; p. 61. M.Sc. Thesis. [Google Scholar]
- 83.Schneider C.A., Rasband W.S., Eliceiri K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods. 2012;9(7):671–675. doi: 10.1038/nmeth.2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Rahman M.S., Kline R.J., Vázquez O.A., Khan I.A., Thomas P. Molecular characterization and expression of arginine vasotocin V1a2 receptor in Atlantic croaker brain: potential mechanisms of its downregulation by PCB77. J. Biochem. Mol. Toxicol. 2020 doi: 10.1002/jbt.22500. e22500, 1–12. [DOI] [PubMed] [Google Scholar]
- 85.Untergasser A., Cutcutache I., Koressaar T., Ye J., Faircloth B.C., Remm M., Rozen S.G. Primer3—New capabilities and interfaces. Nucleic. Acids. Res. 2012;40(15):e115. doi: 10.1093/nar/gks596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Livak K.J., Schmittgen T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−∆∆Ct method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 87.Lacy B., Rahman M.S., Rahman M.S. Potential mechanisms of Na+/K+-ATPase attenuation by heat and pesticides co-exposure in goldfish: role of cellular apoptosis, oxidative/nitrative stress and antioxidants in gills. Environ. Sci. Pollut. Res. 2022;29:57376–57394. doi: 10.1007/s11356-022-19779-7. [DOI] [PubMed] [Google Scholar]
- 88.Bradford M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976;72:248–254. doi: 10.1016/0003/-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 89.Rousseeuw P.J., Croux C. Alternatives to the median absolute deviation. J Amer. Stat Assoc. 1993;88(424):1273–1283. doi: 10.1080/01621459.1993.10476408. [DOI] [Google Scholar]
- 90.Kantidze O.L., Velichko A.K., Luzhin A.V., Razin S.V. Heat stress-induced DNA damage. Acta Nat. 2016;8(2):75–78. [PMC free article] [PubMed] [Google Scholar]
- 91.Espionosa E.P., Koller A., Allam B. Proteomic characterization of mucosal secretions in the eastern oyster, Crassostrea virginica. J. Proteom. 2016;132:63–76. doi: 10.1016/j.jprot.2015.11.018. [DOI] [PubMed] [Google Scholar]
- 92.Da Silva P.M., Villalba A., Sunila I. Branchial lesions associated with abundant apoptotic cells in oysters Ostrea edulis of Galicia (NW Spain) Dis. Aquat. Org. 2006;70:129–137. doi: 10.3354/dao070129. [DOI] [PubMed] [Google Scholar]
- 93.Khondee P., Srisomsap C., Chokchaichamnankit D., Svasti J., Simpson R.J., Kingtong S. Histopathological effect and stress response of mantle proteome following TBT exposure in the Hooded oyster Saccostrea cucullata. Environ. Pollut. 2016;218:855–862. doi: 10.1016/j.envpol.2016.08.011. [DOI] [PubMed] [Google Scholar]
- 94.Li C.-.S., Wu K.-.Y., Chang-Chien G.-.P., Chou C.-.C. Analysis of oxidative DNA damage 8-hydroxy-2’-deoxyguanosine as a biomarker of exposures to persistent pollutants for marine mammals. Environ. Sci. Technol. 2005;39(8):2455–2460. doi: 10.1021/es0487123. [DOI] [PubMed] [Google Scholar]
- 95.Canova S., Degan P., Peters L.D., Livingstone D.R., Voltan R., Venier P. Tissue dose, DNA adducts, oxidative DNA damage and CYP1A-immunopositive proteins in mussels exposed to waterborne benzo[a]pyrene. Mut. Res. 1998;399:17–30. doi: 10.1016/s0027-5107(97)00263-7. [DOI] [PubMed] [Google Scholar]
- 96.Torres M.A., Testa C.P., Gaspari C., Masutti M.B., Panitz C.M.N., Curi-Pedrosa R., de Almeida E.A., Di Mascio P., Filho D.W. Oxidative stress in the mussel Mytella guyanensis from polluted mangroves on Santa Catarina Island, Brazil. Marine Pollut. Bull. 2002;44:923–932. doi: 10.1016/s0025-326x(02)00142-x. [DOI] [PubMed] [Google Scholar]
- 97.Akcha F., Ruiz S., Zampieron C., Venier P., Burgeot T., Cadet J., Narbonne J.F. Benzo[a]pyrene-induced DNA damage in Mytilus galloprovincialis: measurement of bulky DNA adducts and DNA oxidative damage in terms of 8-oxo-7,8-dihydro-2’-deoxoyguanosine formation. Biomarkers. 2000;5(5):355–367. doi: 10.1080/135475000424366. [DOI] [PubMed] [Google Scholar]
- 98.Malins D.C., Haimanot R. The etiology of cancer: hydroxyl radical-induced DNA lesions in histologically normal livers of fish from a population with liver tumors. Aquat. Toxicol. 1991;20:123–130. doi: 10.1016/0166-445X(91)90011-W. [DOI] [Google Scholar]
- 99.Wallace S.S. DNA damages processed by base excision repair: biological consequences. Int. J. Rad. Biol. 1994;66:579–589. doi: 10.1080/09553009414551661. [DOI] [PubMed] [Google Scholar]
- 100.Nitiss, J.L., 2009. Targeting DNA topoisomerase II in cancer chemotherapy. Nat. Rev. Cancer. 9(5), 338–350. https://doi.org/10.1038%2Fnrc2607. [DOI] [PMC free article] [PubMed]
- 101.Velichko A.K., Petrova N.V., Razin S.V., Kantidze O.L. Mechanism of heat stress-induced cellular senescence elucidates the exclusive vulnerability of early S-phase cells to mild genotoxic stress. Nucl. Acids Res. 2015;43(13):6309–6320. doi: 10.1093/nar/gkv573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Kefaloyianni E., Gourgou E., Ferle V., Kotsakis E., Gaitanaki C., Beis I. Acute thermal stress and various heavy metals induce tissue-specific pro-or anti-apoptotic events via the p38-MAPK signal transduction pathway in Mytilus galloprovincialis (Lam.) J. Exp. Biol. 2005;208:4427–4436. doi: 10.1242/jeb.01924. [DOI] [PubMed] [Google Scholar]
- 103.Sarkar A., Ray D., Shrivastava A.N., Sarker S. Molecular biomarkers: their significance and application in marine pollution monitoring. Ecotoxicology. 2006;15(4):333–340. doi: 10.1007/s10646-006-0069-1. [DOI] [PubMed] [Google Scholar]
- 104.Gerić M., Gajski G., Garaj-Vrhovac V. γ-H2AX as a biomarker for DNA double-strand breaks in ecotoxicology. Ecotoxicol. Environ. Saf. 2014;105:13–21. doi: 10.1016/j.ecoenv.2014.03.035. [DOI] [PubMed] [Google Scholar]
- 105.Takahashi A., Matsumoto H., Nagayama K., Kitano M., Hirose S., Tanaka H., Mori E., Yamakawa N., Yasumoto J., Yuki K., Ohnishi K., Ohnishi T. Evidence for the involvement of double-strand breaks in heat-induced cell killing. Cancer Res. 2004;64(24):8839–8845. doi: 10.1158/0008-5472.can-04-1876. [DOI] [PubMed] [Google Scholar]
- 106.Gonzalez-Romero R., Suarez-Ulloa V., Rodriguez-Casariego J., Garcia-Souto D., Diaz G., Smith A., Pasantes J.J., Rand G. Effects of Florida red tides on histone variant expression and DNA methylation in the Eastern oyster Crassostrea virginica. Aquat. Toxicol. 2017;186:196–204. doi: 10.1016/j.aquatox.2017.03.006. [DOI] [PubMed] [Google Scholar]
- 107.Vernon E.L., Bean T.P., Jha A.N. Assessing relative biomarker responses in marine and freshwater bivalve molluscs following exposure to phosphorus 32 (32P): application of genotoxicological and molecular biomarkers. J. Environ. Rad. 2020;213 doi: 10.1016/j.jenvrad.2019.106120. [DOI] [PubMed] [Google Scholar]
- 108.Sayed A.E.D.H., Igarashi K., Watanabe-Asaka T., Mitani H. Double strand break repair and γ-H2AX formation in erythrocytes of medaka (Oryzias latipes) after γ-irradiation. Environ. Pollut. 2017;224:35–43. doi: 10.1016/j.envpol.2016.11.050. [DOI] [PubMed] [Google Scholar]
- 109.Liu T., Daniels C.K., Cao S. Comprehensive review on the HSC70 functions, interactions with related molecules and involvement in clinical diseases and therapeutic potential. Pharmacol. Therap. 2012;136(3):354–374. doi: 10.1016/j.pharmthera.2012.08.014. [DOI] [PubMed] [Google Scholar]
- 110.Ivanina A.V., Taylor C., Sokolova I.M. Effects of elevated temperature and cadmium exposure on stress protein response in eastern oysters, Crassostrea virginica (Gmelin) Aquat. Toxicol. 2009;91:245–254. doi: 10.1016/j.aquatox.2008.11.016. [DOI] [PubMed] [Google Scholar]
- 111.Piano, A., Valbonesi, P., Fabbri, E., 2004. Expression of cytoprotective proteins, heat shock protein 70and metallothioneins, in tissues of Ostrea edulis exposed to heat and heavy metals. Cell Stress Chaper. 9 (2), 134. https://doi.org/10.1379%2F483.1. [DOI] [PMC free article] [PubMed]
- 112.Zhan Z., Zhang Q. Molecular cloning, characterization and expression of heat shock protein 70 gene from the oyster Crassostrea hongkongensis responding to thermal stress and exposure of Cu2+ and malachite green. Gene. 2012;497:172–180. doi: 10.1016/j.gene.2012.01.058. [DOI] [PubMed] [Google Scholar]
- 113.Tomanek L., Zuzow M.J. The proteomic response of the mussel congeners Mytilus galloprovincialis and M. trossulus to acute heat stress: implications for thermal tolerance limits and metabolic costs of thermal stress. J. Exp. Biol. 2010;213(20):3559–3574. doi: 10.1242/jeb.041228. [DOI] [PubMed] [Google Scholar]
- 114.Encomio V.G., Chu F.L. Heat shock protein (hsp70) expression and thermal tolerance in sublethally heat-shocked eastern oysters Crassostrea virginica infected with the parasite Perkinsus marinus. Dis. Aquat. Organ. 2007;76(3):251–260. doi: 10.3354/dao076251. [DOI] [PubMed] [Google Scholar]
- 115.Clark M.S., Geissler P., Waller C., Fraser K.P.P., Barnes D.K.A., Peck L.S. Low heat shock thresholds in wild Antarctic inter-tidal limpets (Nacella concinna) Cell Stress Chaper. 2008;13:51–58. doi: 10.1007/s12192-008-0015-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Ophelie G., Tristan R., Isabelle A. Induction of apoptosis by UV in the flat oyster, Ostrea edulis. Fish Shellfish Immunol. 2015;46(2):232–242. doi: 10.1016/j.fsi.2015.05.046. [DOI] [PubMed] [Google Scholar]
- 117.Yang C., Gao Q., Liu C., Wang L., Zhou Z., Gong C., Zhang H., Qiu L., Song L. The transcriptional response of the Pacific oyster Crassostrea gigas against acute heat stress. Fish Shellfish Immunol. 2017;68:132–143. doi: 10.1016/j.fsi.2017.07.016. [DOI] [PubMed] [Google Scholar]
- 118.Cherkasov A.S., Grewal S., Sokolova I.M. Combined effects of temperature and cadmium exposure on haemocyte apoptosis and cadmium accumulation in the eastern oyster Crassostrea virginica (Gmelin) J. Thermal. Biol. 2007;32:162–170. doi: 10.1016/j.jtherbio.2007.01.005. [DOI] [Google Scholar]
- 119.Yabu T., Ishibashi Y., Yamashita M. Stress-induced apoptosis by heat shock, UV and γ ray irradiation in zebrafish embryos detected by increased caspase activity and whole-mount TUNEL staining. Fish. Sci. 2001;67:333–340. doi: 10.1046/j.1444-2906.2001.00233.x. [DOI] [Google Scholar]
- 120.Yabu T., Ishibashi Y., Yamashita M. Stress-induced apoptosis in larval embryos of Japanese flounder. Fish. Sci. 2003;69:1218–1223. doi: 10.1111/j.0919-9268.2003.00748.x. [DOI] [Google Scholar]
- 121.Takle H., McLeord A., Andersen O. Cloning and characterization of th executioner caspases 3, 6, 7 and Hsp70 in hyperthermic Atlantic salmon (Salmo salar) embryos. Comp. Biochem. Physiol. B. 2006;144:188–198. doi: 10.1016/j.cbpb.2006.02.006. [DOI] [PubMed] [Google Scholar]
- 122.Sleadd I.M., Lee M., Hassumani D.O., Stecyk T.M.A., Zeitz O.K., Buckley B.A. Sub-lethal heat stress causes apoptosis in an Antarctic fish that lacks an inducible heat shock response. J. Thermal. Biol. 2014;44:119–125. doi: 10.1016/j.jtherbio.2014.06.007. [DOI] [PubMed] [Google Scholar]
- 123.Bleicken S., Classen M., Padmavathi P.V.L., Ishikawa T., Zeth K., Steinhoff H.J., Bordignon E. Molecular details of Bax activation, oligomerization, and membrane insertion. J. Biol. Chem. 2010;285(9):6636–6647. doi: 10.1074/jbc.M109.081539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Luo M., Li L., Xiao C., Sun Y., Wang G. Heat stress impairs mice granulosa cell function by diminishing steroids production and inducing apoptosis. Mol. Cell. Biochem. 2016;412:81–90. doi: 10.1007/s11010-015-2610-0. [DOI] [PubMed] [Google Scholar]
- 125.Pagliari L.J., Kuwana T., Bonzon C., Newmeyer D.D., Tu S., Beere H.M., Green D.R. The multidomain proapoptotic molecules Bax and Bak are directly activated by heat. Proc. Nat. Acad. Sci. 2005;102(50):17975–17980. doi: 10.1073/pnas.0506712102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Topal A., Özdemir S., Arslan H., Çomaklı S. How does elevated water temperature affect fish brain? A neurophysiological and experimental study: assessment of brain derived neurotrophic factor, cFOS, apoptotic genes, heat shock genes, ER-stress genes and oxidative stress genes) Fish Shellfish Immunol. 2021;115:198–204. doi: 10.1016/j.fsi.2021.05.002. [DOI] [PubMed] [Google Scholar]
- 127.Wang Y., Han Y., Wang Y., Lv M., Li Y., Niu D. Expression of p38MAPK and its regulation of apoptosis under high temperature stress in the razor clam Sinonovacula constricta. Fish Shellfish Immunol. 2022;122:288–297. doi: 10.1016/j.fsi.2022.02.020. [DOI] [PubMed] [Google Scholar]
- 128.Vogeler S., Carboni S., Li X., Joyce A. Phylogenetic analysis of the caspase family in bivalves: implications for programmed cell death, immune response and development. BMC Genom. 2021;22(1):1–17. doi: 10.1186/s12864-021-07380-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Chang C.C., Yeh M.S., Cheng W. Cold shock-induced norepinephrine triggers apoptosis of haemocytes via caspase-3 in the white shrimp, Litopenaeus vannamei. Fish Shellfish Immunol. 2009;27(6):695–700. doi: 10.1016/j.fsi.2009.08.010. [DOI] [PubMed] [Google Scholar]
- 130.Yin Y., Huang J., Paine M.L., Reinhold V.N., Chasteen N.D. Structural characterization of the major extrapallial fluid protein of the mollusc Mytilus edulis: implications for function. Biochemistry. 2005;44(31):10720–10731. doi: 10.1021/bi0505565. [DOI] [PubMed] [Google Scholar]
- 131.Caldari-Torres C., Banta-Long W., Bruss A., Choi E., Fiegel H., Jollis M.S., Ly S., Viswanathan S. Hemolymph glucose levels as a measure of crayfish stress: a methodology using a human glucometer. FASEB J. 2018;32(S1):lb224. doi: 10.1096/fasebj.2018.32.1_supplement.lb224. [DOI] [Google Scholar]
- 132.Misogianes M.J., Chasteen N.D. A chemical and spectral characterization of the extrapallial fluid of Mytilus edulis. Anal. Biochem. 1979;100(2):324. doi: 10.1016/0003-2697(79)90236-7. 2697(79)90236-7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be made available on request.