Objective:
To compare the risk of readmission in those receiving no treatment, labetalol, nifedipine or both at hospital discharge following delivery complicated by presence of hypertension.
Study design:
Retrospective study at a single tertiary care center over a 4-year period (2017–2020). Those with peripartum hypertension (pHTN), defined as any SBP greater than 140 mmHg or DBP greater than 90 mmHg on two occasions 4 h apart during their admission for delivery were included. The primary outcome was postpartum readmission because of hypertensive complications. Analysis was stratified by medication prescribed at discharge (no treatment prescribed, labetalol, nifedipine, or both). The risks of readmission for the management of pHTN were estimated using logistic regression and adjusted for confounding variables.
Results:
Nineteen thousand, four hundred and twenty-five women gave birth during the study period and 4660 (24.0%) met the described definition of pHTN. Of those, 1232 (26.4%) were discharged on antihypertensive medication (s). There were 217 (4.7%) readmissions for hypertensive complications following discharge. Compared with patients who did not receive antihypertensive medication at discharge, any nifedipine prescription was found to significantly decrease the risk of readmission: monotherapy [aOR 0.27 (0.15–0.48)], nifedipine with labetalol [aOR 0.35 (0.16–0.77)]. Labetalol monotherapy was associated with increased risk of readmission [aOR 1.66 (1.06–2.61)].
Conclusion:
The risk of postpartum readmission for hypertensive complication was reduced by 65% when patients were discharged on nifedipine monotherapy and 56% with combined nifedipine and labetalol treatment when compared with no treatment. Patients discharged on labetalol monotherapy were nearly six times as likely to be readmitted for hypertensive complications when compared with patients on nifedipine monotherapy.
Keywords: antihypertensive, hypertension, labetalol, nifedipine, postpartum, preeclampsia, pregnancy, readmission
INTRODUCTION
In the United States, hypertension (HTN) during pregnancy accounts for approximately 7% of maternal mortality with approximately 70% of these deaths occurring in the postpartum period [1–3]. Although identification and management of HTN in the postpartum period remains a subject of research interest, there is a paucity of data comparing individual antihypertensive agents, blood pressure goals or data to affirm these medications provide benefit in the postpartum period. A recent publication from our group demonstrated the association between optimal blood pressure control in the immediate postpartum period and a reduced risk of postpartum readmission for hypertensive complications [4].
Labetalol, hydralazine and nifedipine are commonly used to manage acute HTN during pregnancy with a Cochrane review in 2013 finding no significant differences between the medications for management of acute HTN [5]. After acute HTN is controlled, current American College of Obstetrics and Gynecology guidelines allow permissive HTN (up to 150/100 mmHg) prior to treatment and recommend labetalol as the initial choice for ongoing therapy followed by nifedipine if adequate control is not achieved [6,7]. Although labetalol and nifedipine are well studied for acute treatment of HTN in the parturient, less is known on their effectiveness or safety in the postpartum period. Two small studies evaluated time to BP control and safety while initiating therapy but none have evaluated their efficacy beyond initiation of treatment in the postpartum period [6,8,9]. Nifedipine is a calcium channel blocker, which does not have a well defined mechanism of action but is thought to reduce blood pressure via a vasodilatory effect. In addition to direct vasodilation, it is theorized preferential vasodilation of the afferent renal vessels may increase renal blood flow, fluid clearance and reduce transient hypervolemia leading to faster recovery from pregnancy-related HTN [10,11]. In contrast, labetalol is a mixed alpha-adrenergic and beta-adrenergic receptor blocker. This mechanism is known to reduce BP through arterial vasodilation as well as directly affect cardiac inotropy as well as chronotropy [12].
Given the differences in pharmacologic mechanism between the two agents, we hypothesize nifedipine is superior to labetalol and will reduce the risk of complications requiring hospital readmission.
MATERIALS AND METHODS
We performed a retrospective cohort study of all pregnancies with peripartum hypertension (pHTN) during their admission for delivery at a single tertiary care center over a 4-year period (2017–2020). Peripartum hypertension was defined as SBP at least 140 mmHg or DBP at least 90 mmHg on at least two occasions, 4 h apart in the electronic medical record at any point during the parturient's admission for delivery. Inclusion criteria were any pregnancy and delivery during the study period in which HTN was identified based on the criteria described. This study was approved by the Institutional Review Board on 15 December 2020, IRB#1459.
The primary outcome of interest was hospital readmission for the treatment of hypertensive complications following delivery. To identify readmissions, all patients presenting to the emergency department or admitted to the hospital during the 4-year study period with a delivery at the study institution in the prior 12 months were identified. In order to identify, which visits to review, these encounters were electronically screened to identify if the patient had HTN during the emergency room (ER) visit or admission using patient-level BP data. Due to the abbreviated nature of some ER visits, any visit with a single elevated blood pressure based on the previously described criteria underwent thorough chart review. Each ER visit and readmission underwent chart review by study authors (T.L. and B.C.) to determine if the visit or readmission was related to complications of hypertension or preeclampsia. Readmissions because of other complications, gestational HTN or preeclampsia without severe features were excluded from analysis. Admissions determined to be related to HTN or HTN-related complications were included in the readmission cohort. In addition to typical complications of preeclampsia, hypertension-related complications were defined as eclampsia, pulmonary edema, cardiomyopathy, myocardial infarction, posterior reversible encephalopathy syndrome or cerebrovascular accident. Patients were also included in the readmission cohort if they were discharged from the emergency room visit but reviewers felt documentation was consistent with hemolysis, elevated liver enzymes, low-platelet syndrome (HELLP), severe gestational HTN, preeclampsia with severe features, and should have resulted in readmission.
The exposure of interest was the type of antihypertensive medication prescribed to the patient at the time of hospital discharge from delivery and the primary outcome was readmission to the hospital for hypertensive complications. The medication(s) used, dosing, and discharge timing were at the discretion of the primary provider as there were no current evidence-based guidelines for the management of pHTN during the period studied. Patient demographic information and clinical characteristics were compared between those who were not prescribed treatment, labetalol monotherapy, nifedipine monotherapy, or combined labetalol and nifedipine therapy (combination therapy) using appropriate univariate tests. The rates of readmission for each of the four groups were calculated and compared using chi squared test. A P value of less than 0.05 was considered statistically significant. Multivariate logistic regression analysis was performed to estimate the risk of readmission associated with each treatment regimen, using no treatment as the baseline comparison group. Based on cohort characteristics, the final regression model was adjusted for gestational age of delivery, BMI, chronic hypertension, blood pressure greater than 140/90 mmHg during the 12 h prior to hospital discharge, multiple pregnancy, mode of delivery and preeclampsia severity. These analyses were performed using Stata 12 (College Station, Texas, USA).
RESULTS
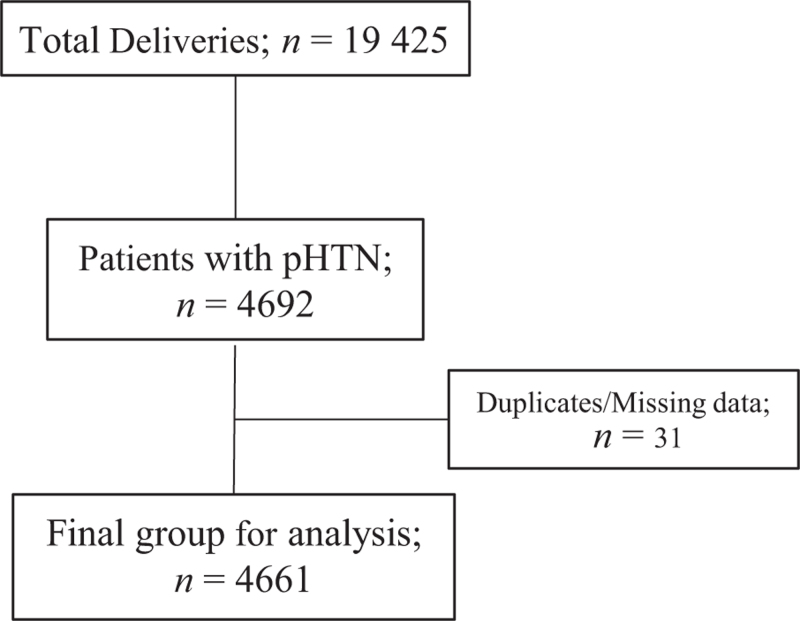
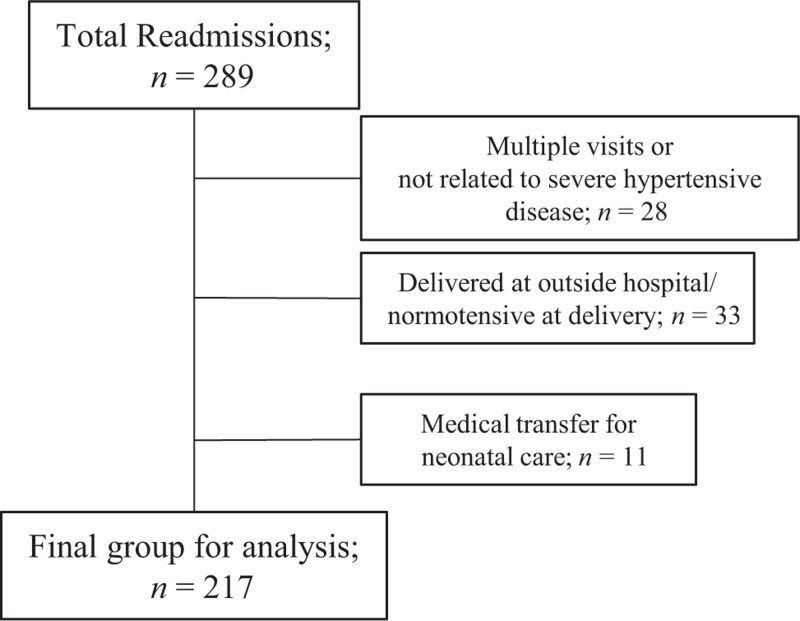
Between January 2017 and December 2020 19 425 deliveries occurred of which 4660 (24%) met inclusion criteria (Fig. 1). During this period, 217 women were readmitted because of postpartum hypertensive complications for an overall readmission rate of 4.7% (Fig. 2). Compared with patients who did not require treatment at hospital discharge, those discharged with labetalol monotherapy, nifedipine monotherapy or both were statistically different in the median gestational age at delivery, BMI, length of stay, rate of hypertension in the 12 h prior to hospital discharge, chronic hypertension, cesarean delivery and severity of pHTN. Approximately two-thirds of patients with preeclampsia with severe features or superimposed preeclampsia were discharged with a prescription for antihypertensive medication. Patients with severe pHTN (preeclampsia with severe features, HELLP, superimposed preeclampsia and eclampsia) discharged on a single medication were more likely to be discharged on nifedipine monotherapy than labetalol monotherapy (77 vs. 23%). (Table 1).
FIGURE 1.
Flow diagram for hypertensive cohort.
FIGURE 2.
Flow diagram for readmissions.
TABLE 1.
Cohort characteristics
| No meds | Labetalol | Nifedipine | Both | P value | |
| N | 3428 | 259 | 704 | 269 | |
| Maternal age | 32 [28–36] | 33 [29–36] | 32 [28–36] | 32 [28–36] | 0.56 |
| Gestational age at delivery | 39 [37–39] | 37 [36–38] | 37 [35–38] | 35 [32–37 | <0.0001 |
| Parity | 1 [1–2] | 1 [1–2] | 1 [1–2] | 1 [1–2] | 0.12 |
| Race | |||||
| Black/African American | 171 | 11 | 40 | 18 | 0.87 |
| Non-Hispanic white | 2798 | 213 | 590 | 223 | |
| Multiple | 133 | 10 | 23 | 8 | |
| Native American | 24 | 1 | 3 | 3 | |
| Asian | 64 | 6 | 7 | 1 | |
| Native Hawaiian | 5 | 0 | 0 | 0 | |
| Hispanic or Latinx | 143 | 11 | 23 | 9 | |
| Other/declined | 90 | 7 | 18 | 7 | |
| Private insurance | 766 (22.3) | 60 (23.1) | 160 (22.7) | 67 (24.9) | 0.8 |
| Multiple pregnancy | 154 (4.5) | 22 (8.5) | 77 (10.9) | 33 (12.3) | <0.0001 |
| CHTN | 159 (4.6) | 33 (12.7) | 58 (8.2) | 25 (9.3) | <0.0001 |
| BMI | 34.3 [30.0–39.5] | 36.9 [30.5–43.4] | 34.5 [29.5–40.1] | 35.7 [32–41.5] | 0.0003 |
| Cesarean delivery | 1511 (44.1) | 140 (54.1) | 365 (51.9) | 191 (71) | <0.0001 |
| LOS | 2.6 [2–3.2] | 2.8 [2–3.5] | 2.5 [2–3.2] | 2.4 [2.0–3.1] | 0.92 |
| Preeclampsia severity (by ICD10 coding) | |||||
| No code for hypertension | 2204 (64.3) | 77 (29.7) | 110 (15.6) | 20 (7.4) | <0.001 |
| Without severe features | 932 (27.2) | 76 (29.3) | 243 (34.5) | 43 (16.0) | |
| With severe features | 212 (6.2) | 68 (26.3) | 273 (38.8) | 144 (53.5) | |
| Superimposed | 81 (2.4) | 37 (14.3) | 77 (10.9) | 60 (22.3) | |
| Eclampsia | 0 | 1 (0.1) | 1 (0.1) | 2 (0.7) | |
| Highest SBP (12 h before discharge) | 135.5{10.6} | 143.5{10.7} | 141.4{9.0} | 142.8{10.3} | <0.0001 |
| Highest DBP (12 h before discharge) | 81.2{9.2} | 85.9{7.8} | 86.1{7.6} | 85.7{8.5} | <0.0001 |
| Highest SBP (During admission) | 150.7{9.8} | 160.9{13.0} | 160.2{13.4} | 166.2{13.1} | <0.0001 |
| Highest DBP (during admission) | 89.3{7.5} | 93.5{5.9} | 94.1{5.3} | 95.6{3.9} | <0.0001 |
| Magnesium Sulfate infusion | 243 (7.1) | 85 (32.8) | 262 (37.2) | 134 (49.8) | <0.001 |
Normality assessed with Shapiro–Wilks test. Data presented as n (%), median [IQR] or median {SD}. CHTN, chronic hypertension; LOS, length of stay; pHTN, peripartum hypertension.
The rate of readmission in the untreated group was 4.8%, compared with a rate of 10.8% in patients treated with labetalol monotherapy, 2.4% in patients treated with nifedipine monotherapy, and 3% in patients treated with both agents. Nifedipine monotherapy was associated with a significantly lower risk of readmission when compared with the referent even after adjusting for potential confounders [adjusted odds ratio (aOR): 0.27, 95% confidence interval (CI) 0.15–0.48). In contrast, labetalol monotherapy was associated with an increased risk of readmission (aOR: 1.66, 95% CI 1.06–2.61). A lower risk of readmission was also seen in patients discharged on combination therapy with nifedipine and labetalol (aOR: 0.35, 95% CI 0.16–0.77) (Table 2).
TABLE 2.
Risk of readmission by management at time of discharge
| Rate of readmission | P value | OR | 95% CI | aOR | 95% CI | |||
| Antihypertensive Treatment(s) | ||||||||
| No treatment | 165 (4.8) | <0.001 | Reference | |||||
| Labetalol | 28 (10.8) | 2.40 | 1.57 | 3.66 | 1.66 | 1.06 | 2.61 | |
| Nifedipine | 16 (2.3) | 0.46 | 0.27 | 0.77 | 0.27 | 0.15 | 0.48 | |
| Labetalol+nifedipine | 8 (3.0) | 0.61 | 0.29 | 1.25 | 0.35 | 0.16 | 0.77 | |
Adjusted for: gestational age of delivery, BMI, chronic hypertension, hypertension prior to discharge, multiple pregnancy, mode of delivery, type of pHTN. Bold indicates clinically significant final result. aOR, adjusted odds ratio; CI, confidence interval; OR, odds ratio.
DISCUSSION
Principle findings
This study demonstrated a significant risk reduction for postpartum readmission in patients who required antihypertensive treatment and were discharged on Nifedipine either as a single agent or in combination with labetalol. Comparatively, patients treated with labetalol saw a 66% increase in risk of readmission for postpartum hypertension complications despite this group having less severe hypertensive disease.
The finding labetalol may increase risk of hypertensive complications does not have a previously described biologic plausibility in the obstetric literature. One possible explanation for how nifedipine may mitigate this effect is via the improvement in renal blood flow, while labetalol may impair cardiac adaptation. Studies on physiology of preeclampsia show similarities to acute heart failure; increased systemic vascular resistance, decreased stroke volume, hypervolemia, decreased cardiac output and derangements of the renin–angiotensin–aldosterone system. Beta blockers have been shown to increase mortality in acute heart failure, and it is possible the increased risk of hypertensive complications and readmission in patients on labetalol is related to impairment in adaptation to postpartum preeclampsia. Prospective RCTs and physiologic studies would lend further data to confirm these findings and further delineate the underlying cause [8,12–18]. Our findings support the use of nifedipine for the management of pregnancy-related hypertension, and our findings suggest that it may be superior in the ongoing management of HTN following delivery [19–22]. Given prior recommendations are based on expert opinion, this data coupled with our previously published results would suggest treating patients to normotension for at least 12 h prior to discharge with nifedipine as the first-line agent is the regimen associated with lowest risk of readmission.
The major strengths of our study were the methods for selecting both the overall cohort and the readmission cohort. By including all patients who met the definition of peripartum hypertension, we chose a method that would result in the most inclusive hypertension cohort and a method to result in the most accurate readmission group. Individual patient-level data collection by specialists in the field of Maternal–Fetal Medicine ensured high-quality data and accurate identification of patients with hypertensive disorders and complications requiring admission and treatment. The principle findings of nifedipine monotherapy were associated with a lower risk of readmission, which was bolstered by consistent parallel findings: patients treated with nifedipine monotherapy were more likely to have the most severe forms of pHTN; patients with nifedipine monotherapy were more likely to receive magnesium during delivery; patients who required combination therapy (nifedipine and labetalol) had a lower risk of readmission compared with those receiving no medication or labetalol MT; and there was no clinically significant differences in blood pressures during admission or in the 12 h prior to discharge in those receiving labetalol or nifedipine monotherapy.
We acknowledge limitations to this study include the biases implicit in retrospective study design. The initial cohort (24%) is large compared with rates of pHTN previously reported in the literature using ICD10 coding, likely because of our use of individual patient-level data [23–26]. Because of our method to identify the study cohort, there may be a risk of some patients being included that did not have pHTN and met criteria because of spurious blood pressure measurements. Despite this potential limitation or bias, our data did demonstrate this cohort was at significantly increased risk of readmission compared with previously published studies on all cause postpartum readmission. Additionally, because of our sample size, it was necessary to use readmission as a surrogate for the true outcomes of interest since they occur with such rarity: maternal death, pulmonary edema, acute heart failure, eclampsia, hypertensive encephalopathy and stroke. We also acknowledge attrition bias, as we are unable to determine which or how many patients were readmitted outside our hospital system, affecting the overall risk of readmission and potentially impacting the demographic characteristics of the readmission cohort. Last, of those who were prescribed an antihypertensive medication, it is unknown if they were filled or taken as directed potentially resulting in further selection bias.
In conclusion, our findings suggest the risk of postpartum readmission for hypertensive complications was reduced by 65% when patients with pHTN were discharged on nifedipine monotherapy compared with no treatment and by 56% with combined nifedipine and labetalol treatment. Patients discharged on labetalol monotherapy were six times more likely to be readmitted for hypertensive complications than patients discharged on nifedipine monotherapy. Future study considerations include corroboration and validation of our findings with prospective analysis in addition to evaluation of other antihypertensive agents.
ACKNOWLEDGEMENTS
Each author has confirmed compliance with the journal's requirements for authorship.
Prior publication: this article is a secondary analysis of data previously reported. Reference: Lovgren T, Connealy B, Yao R, Dahlke JD. Postpartum management of hypertension and effect on readmission rates. Am J Obstet Gynecol MFM. 2022 Jan;4(1):100517. doi: 10.1016/j.ajogmf.2021.100517. Epub 30 October 2021. PMID: 34757235.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Petersen EE, Davis NL, Goodman D, Cox S, Mayes N, Johnston E, et al. Vital signs: pregnancy-related deaths, United States, 2011–2015, and strategies for prevention, 13 states, 2013–2017. MMWR Morb Mortal Wkly Rep 2019; 68:423–429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Say L, Chou D, Gemmill A, Tunçalp Ö, Moller AB, Daniels J, et al. Global causes of maternal death: a WHO systematic analysis. Lancet Glob Health 2014; 2:e323–e333. [DOI] [PubMed] [Google Scholar]
- 3.Davis NL, Smoots AN, Goodman DA. Pregnancy-related deaths: data from 14 U.S. Maternal Mortality Review Committees, 2008–2017. Atlanta, GA: Centers for Disease Control and Prevention, U.S. Department of Health and Human Services; 2019. [Google Scholar]
- 4.Lovgren T, Connealy B, Yao R, Dahlke JD. Postpartum management of hypertension and effect on readmission rates. Am J Obstet Gynecol MFM 2022; 4:100517. [DOI] [PubMed] [Google Scholar]
- 5.Duley L, Meher S, Jones L. Drugs for treatment of very high blood pressure during pregnancy. Cochrane Database Syst Rev 2013; 2013:CD001449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gestational hypertension and preeclampsia. ACOG Practice Bulletin No. 222. American College of Obstetricians and Gynecologists. Obstet Gynecol 2020; 135:e237–e260. [DOI] [PubMed] [Google Scholar]
- 7.Hypertension in pregnancy. Report of the American College of Obstetricians and Gynecologists’ Task Force on Hypertension in Pregnancy. Obstet Gynecol 2013; 122:1122–1131. [DOI] [PubMed] [Google Scholar]
- 8.Sharma KJ, Greene N, Kilpatrick SJ. Oral labetalol compared to oral nifedipine for postpartum hypertension: a randomized controlled trial. Hypertens Pregnancy 2017; 36:44–47. [DOI] [PubMed] [Google Scholar]
- 9.Ainuddin J, Javed F, Kazi S. Oral labetalol versus oral nifedipine for the management of postpartum hypertension a randomized control trial. Pak J Med Sci 2019; 35:1428–1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Barton JR, Hiett AK, Conover WB. The use of Nifedipine during the postpartum period in patients with severe preeclampsia. Am J Obstet Gynecol 1990; 162:788–792. [DOI] [PubMed] [Google Scholar]
- 11. Khan KM, Patel J, Schaefer TJ. Nifedipine. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2021 January. Available at: https://www.ncbi.nlm.nih.gov/books/NBK537052/ [Accessed November 2021] [Google Scholar]
- 12. Miller M, Kerndt CC, Maani CV. Labetalol. [Updated 16 July 2021]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2021 January. Available at: https://www.ncbi.nlm.nih.gov/books/NBK534787/ [Accessed November 2021] [Google Scholar]
- 13.Benedetti TJ, Kates R, Williams V. Hemodynamic observations in severe preeclampsia complicated by pulmonary edema. Am J Obstet Gynecol 1985; 152:330–334. [DOI] [PubMed] [Google Scholar]
- 14.Timokhina E, Kuzmina T, Strizhakov A, Pitskhelauri E, Ignatko I, Belousova V. Maternal cardiac function after normal delivery, preeclampsia, and eclampsia: a prospective study. J Pregnancy 2019; 2019:9795765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ghi T, Degli Esposti D, Montaguti E, Rosticci M, De Musso F, Youssef A, et al. Postpartum evaluation of maternal cardiac function after severe preeclampsia. J Matern-Fet Neonat Med 2014; 27:696–701. [DOI] [PubMed] [Google Scholar]
- 16.Ghossein-Doha C, van Neer J, Wissink B, Breetveld NM, de Windt LJ, van Dijk AP, et al. Preeclampsia: an important risk factor for asymptomatic heart failure. Ultrasound Obstet Gynecol 2017; 49:143–149. [DOI] [PubMed] [Google Scholar]
- 17.Schwinger RHG. Pathophysiology of heart failure. Cardiovasc Diagn Ther 2021; 11:263–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nanayakkara S, Haykowsky M, Mariani J, Van Empel V, Maeder MT, Vizi D, et al. Hemodynamic profile of patients with heart failure and preserved ejection fraction vary by age. J Am Heart Assoc 2017; 6:e005434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Easterling T, Mundle S, Bracken H, Parvekar S, Mool S, Magee LA, et al. Oral antihypertensive regimens (Nifedipine retard, Labetalol, and methyldopa) for management of severe hypertension in pregnancy: an open-label, randomised controlled trial. Lancet 2019; 394:1011–1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zulfeen M, Tatapudi R, Sowjanya R. IV Labetalol and oral Nifedipine in acute control of severe hypertension in pregnancy-a randomized controlled trial. Eur J Obstet Gynecol Reprod Biol 2019; 236:46–52. [DOI] [PubMed] [Google Scholar]
- 21.Vermillion ST, Scardo JA, Newman RB, Chauhan SP. A randomized, double-blind trial of oral Nifedipine and intravenous Labetalol in hypertensive emergencies of pregnancy. Am J Obstet Gynecol 1999; 181:858–861. [DOI] [PubMed] [Google Scholar]
- 22.Shekhar S, Sharma C, Thakur S, Verma S. Oral Nifedipine or intravenous Labetalol for hypertensive emergency in pregnancy: a randomized controlled trial. Obstet Gynecol 2013; 122:1057–1063. [DOI] [PubMed] [Google Scholar]
- 23.Matas JL, Mitchell LE, Sharma SV, Louis JM, Salemi JL. Severe maternal morbidity at delivery and postpartum readmission in the United States. Paediatr Perinat Epidemiol 2021; 35:627–634. [DOI] [PubMed] [Google Scholar]
- 24.Nam JY, Park EC. The relationship between severe maternal morbidity and a risk of postpartum readmission among Korean women: a nationwide population-based cohort study. BMC Pregnancy Childbirth 2020; 20:148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Corrigan L, O’Farrell A, Moran P, Daly D. Hypertension in pregnancy: prevalence, risk factors and outcomes for women birthing in Ireland. Pregnancy Hypertens 2021; 24:1–6. [DOI] [PubMed] [Google Scholar]
- 26.Lo JO, Mission JF, Caughey AB. Hypertensive disease of pregnancy and maternal mortality. Curr Opin Obstet Gynecol 2013; 25:124–132. [DOI] [PubMed] [Google Scholar]