Abstract
Rationale
There is limited literature exploring the relationship between military exposures and idiopathic pulmonary fibrosis (IPF).
Objectives
To evaluate whether exposure to Agent Orange is associated with an increased risk of IPF among veterans.
Methods
We used Veterans Health Administration data to identify patients diagnosed with IPF between 2010 and 2019. We restricted the cohort to male Vietnam veterans and performed multivariate logistic regression to examine the association between presumptive Agent Orange exposure and IPF. We conducted sensitivity analyses restricting the cohort to army veterans (highest theoretical burden of exposure, surrogate for dose response) and a more specific case definition of IPF. Fine-Gray competing risk models were used to evaluate age to IPF diagnosis.
Measurements and Main Results
Among 3.6 million male Vietnam veterans, 948,103 (26%) had presumptive Agent Orange exposure. IPF occurred in 2.2% of veterans with Agent Orange exposure versus 1.9% without exposure (odds ratio, 1.14; 95% confidence interval [CI], 1.12–1.16; P < 0.001). The relationship persisted after adjusting for known IPF risk factors (odds ratio, 1.08; 95% CI, 1.06–1.10; P < 0.001). The attributable risk among exposed veterans was 7% (95% CI, 5.3–8.7%; P < 0.001). Numerically greater risk was observed when restricting the cohort to 1) Vietnam veterans who served in the army and 2) a more specific definition of IPF. After accounting for the competing risk of death, veterans with Agent Orange exposure were still more likely to develop IPF.
Conclusions
Presumptive Agent Orange exposure is associated with greater risk of IPF. Future research should validate this association and investigate the biological mechanisms involved.
Keywords: interstitial lung disease, epidemiology, Veterans health
At a Glance Commentary
Scientific Knowledge on the Subject
Observational studies of inhaled particulates and gases suggest that exposures such as smoking, chronic ambient air pollution, and vocational activities increase the risk of idiopathic pulmonary fibrosis (IPF). However, very little is known about the association between military exposures and IPF.
What This Study Adds to the Field
In this study, we leveraged the strength of the Veterans Health Administration electronic health record system to evaluate the association between exposure to Agent Orange, an herbicide and chemical defoliant used during the Vietnam War, and IPF. We found that presumptive Agent Orange exposure was associated with a 14% higher risk of developing IPF in an unadjusted analysis and an 8% higher risk of IPF after adjusting for known IPF risk factors. We noted numerically greater risk when limiting the cohort to veterans who served in the army (highest burden of exposure, surrogate for dose response) and when restricting the cohort to more specific case definitions of IPF.
Idiopathic pulmonary fibrosis (IPF) is a progressive fibrotic lung disease associated with high morbidity and mortality (1). In the current conceptual model of IPF, pulmonary parenchymal fibroproliferation develops with age as the lung is exposed to cumulative intrinsic and extrinsic stressors. Over time, this iterative cycle is hypothesized to lead to senescence of the alveolar epithelium and create a microenvironment of abnormal repair, manifesting in diffuse fibrotic lung disease (2).
Observational studies of inhaled particulates and gases suggest that exposures may increase the risk of IPF. Smoking is the most robustly defined of these risk factors (3–6). However, chronic ambient air pollution (7–9) and vocational activities associated with a high probability of dust, fume, or gas inhalation (10–16) have also been implicated. These airborne pollutants can trigger alterations in mucosal surfaces, induce oxidative stress, and modify epigenetics, all mechanisms that have been described in the pathogenesis of IPF (2).
The U.S. Veteran population is demographically primed to develop IPF. Recent literature has suggested that the prevalence of IPF among Veterans may be higher than what has been described in other epidemiologic studies (17, 18). In addition to traditional risk factors of older age and tobacco use, Veterans have unique military exposures that may accelerate the development of pulmonary fibrosis. Agent Orange, a chemical defoliant so named because it was stored in barrels identified by an orange band during the Vietnam War, is an exposure of particular interest to the Veteran Health Administration (VHA) and has been studied extensively by the National Academy of Sciences. Agent Orange refers to a 50:50 formulation of 2,4-dichlorphenoxyacetic acid (2,4-D) and 2,4,5-trichlorophenoxyacetic acid (2,4,5-T) that was sprayed from 1962 to 1971 to strip the jungle canopy. The component 2,4,5-T also contained the unintended contaminant 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD), commonly referred to as “dioxin.” The association between dioxin and malignancies such as soft tissue sarcoma, lymphoma, and chronic lymphocytic leukemia have previously been described (19). However, there is limited literature exploring the relationship between Vietnam service, Agent Orange exposure, and the subsequent development of IPF. In this study, we used the strength of the Veterans Affairs (VA) integrated electronic health record system to evaluate whether presumed exposure to Agent Orange is associated with an increased risk of IPF among a national cohort of male U.S. Veterans who served in the Vietnam War.
Methods
The University of California San Francisco and the San Francisco VA Healthcare System Institutional Review Boards approved this study.
Data Source and Patient Identification
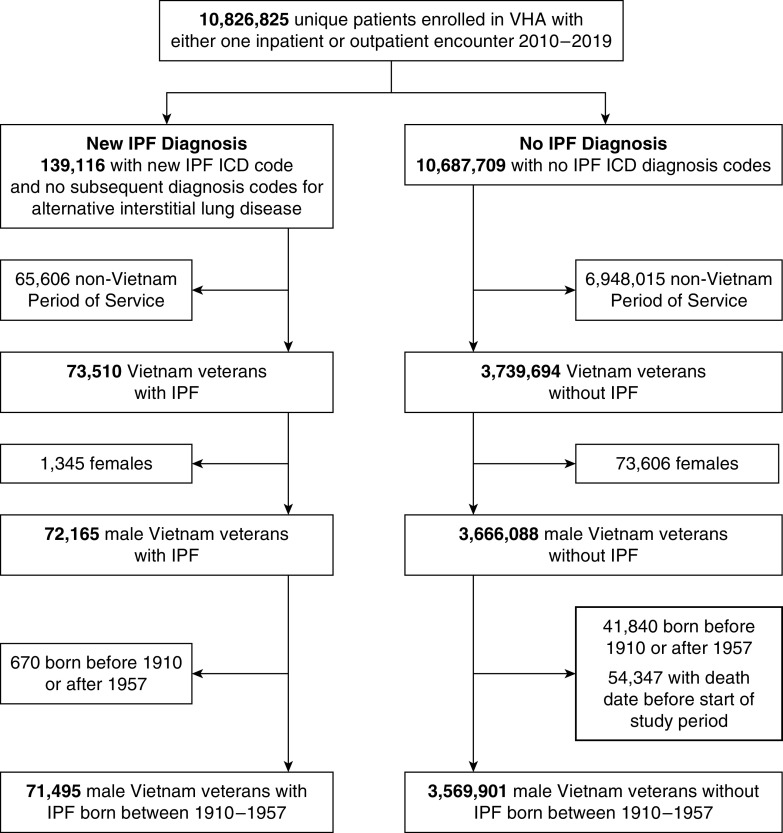
We extracted the electronic health record data of Veterans who were enrolled in the VHA and had at least one inpatient or outpatient encounter at a VHA facility or a non-VHA facility paid for by the VHA between 2010 and 2019 (Figure 1). We identified all patients who had an International Classification of Disease (ICD) diagnosis code for IPF (ICD-9-CM code 515, 516.3, 516.31 or ICD-10-CM code J84.111, J84.112, J84.89, J84.9, J84.10, J84.17) recorded between January 1, 2010, and December 31, 2019. Patients were considered to have IPF if they did not have any other diagnosis code for an alternative interstitial lung disease after the first diagnosis code for IPF as has been defined in other large electronic health record–based studies (17, 20–22). We restricted the analytic cohort to male Vietnam Veterans (there were very few females with Agent Orange exposure) who were born between 1910 and 1957 to ensure that patients would have been between the ages of 18 and 65 years old during the Vietnam War and excluded those who died before the study start date (January 1, 2010). More than 95% of patients were between the ages of 55 and 74 years old at the study start date, consistent with the majority of the cohort being in their 20s and 30s during Vietnam service. We chose to study the incidence of IPF between 2010 and 2019 rather than immediately after the Vietnam War to allow sufficient time lapse from exposure to disease as it is known that IPF develops as the lung ages, and to ensure consistency in the case definition of IPF per society guidelines.
Figure 1.
Cohort identification. ICD = International Classification of Disease; IPF = idiopathic pulmonary fibrosis; VHA = Veterans Health Administration.
Covariates including age, race, ethnicity, rural versus urban residence, military service branch, and smoking history were obtained from electronic heath records. The VA defines rurality using the U.S. Department of Agriculture’s Rural–Urban Commuting Area system (23), whose codes classify U.S. census tracts using measures of population density, urbanization, and daily commuting. All Veterans were categorized into rural versus urban residence based on home address at time of IPF diagnosis. A positive smoking history was defined as an ICD-9 or ICD-10 diagnosis code for tobacco use disorder during the study period but before IPF diagnosis. We also included a positive tobacco use assessment from the health factor database as indicative of smoking history. Patients were dichotomized into ever-smokers and never-smokers with unknown smoking history defaulting to the classification of never-smokers.
Presumptive Agent Orange exposure was identified by an Agent Orange Exposure Flag, which is determined by a Veteran’s military discharge paperwork. Veterans marked as having a Vietnam Campaign Medal, signifying military service with “boots on the ground” in Vietnam and thus at risk for Agent Orange exposure, received the Agent Orange Flag on their military discharge paperwork. Because of widespread spraying and unpredictability of wind dispersal patterns, it is not possible to quantify the exact extent of exposure for an individual veteran.
Statistical Analysis
We examined characteristics of Vietnam Veterans with and without presumptive Agent Orange exposure using t test for continuous variables and χ2 for categorical variables. We calculated adjusted odds ratios (ORs) using regression standardization via parametric g-computation (24) from the model that included potential confounders (age, race, ethnicity, smoking history, and rurality) that have been associated with IPF and were associated with Agent Orange in the bivariate analyses. We included rurality in our multivariable model on the basis of prior literature suggesting an association between rural residence and pulmonary fibrosis in the veteran population (17). Within the VA Healthcare System, rurality also correlates with socioeconomic status, as rural Veterans often have lower education and income than urban Veterans (25). In addition, rurality captures potential exposures such as agriculture and farming that have also been postulated to be associated with IPF.
We allowed for an interaction between age × Agent Orange and smoking × Agent Orange as data from other disease processes such as asbestosis and lung cancer have noted compounding risk among patients with both tobacco use and occupational exposures (26). Owing to interactions noted between Agent Orange × age and Agent Orange × smoking, we reported the odds of IPF among those with and without presumptive Agent Orange exposure by age and smoking in a subgroup analysis.
Attributable fraction of IPF among exposed (AFe) was calculated using the formula AFe = (Ie – Iu)/Ie, where Ie is the incidence in the exposed group and Iu is the incidence in the unexposed group to estimate the percentage of IPF cases among the exposed that could plausibly be attributed to Agent Orange.
Fine-Gray competing risk models examined time to IPF onset with age as the time scale and accounting for death as a competing risk. Time to event was calculated from age at the beginning of the study period (January 1, 2010) to the age of IPF diagnosis, death, or end of the study period (December 31, 2019) by assuming independent left truncation (27). We summarized age-specific risk using the subdistribution hazard ratio with associated 95% confidence interval (CI).
We conducted four additional sensitivity analyses. In the first, we required a computed tomography (CT) scan of the thorax or a lung biopsy before the last IPF diagnosis code. Of note, Veterans have several options for healthcare coverage, including dual enrollment through both VA and non-VA (Medicare, Medicaid, or employer-sponsored) health insurance plans. For dual users, if the CT scans were completed outside the VA, these patients may not have been captured in this narrower cohort. In the second sensitivity analysis, we restricted the cohort to Vietnam Veterans who served in the army as a surrogate measure of dose response. For this analysis, we excluded Veterans who had served in the navy, air force, marines, and coast guard during the Vietnam War. In the third sensitivity analysis, we restricted IPF diagnosis to patients with ICD codes 516.3, 516.31 or ICD-10-CM code J84.111, J84.112 and no subsequent codes for an alternative interstitial lung disease, because these codes have been shown to have improved specificity (albeit reduced sensitivity) for IPF. In the final sensitivity analysis, we restricted the cohort to both army Veterans and the most specific ICD codes. All analyses were conducted using StataCorp Analysis Software version 16.1.
Results
Among approximately 3.6 million male Vietnam Veterans who received care through the VHA between 2010 and 2019, 948,103 (26%) had an Agent Orange Exposure Flag, signifying presumptive dioxin exposure. The median age at the beginning of the study period among Veterans with Agent Orange exposure was 62.7 years old (Table 1). Veterans with Agent Orange exposure were more likely to be White (80% vs. 71%), non-Hispanic or -Latino (88% vs. 83%), live in rural areas (39% vs. 37%), and have served in the army (58% vs. 50%).
Table 1.
Characteristics of U.S. Vietnam Era Male Veterans by Agent Orange Exposure
| No Agent Orange Flag (N = 2,693,293) | Agent Orange Flag (N = 948,103) | |
|---|---|---|
| Idiopathic pulmonary fibrosis | 51,086 (1.9%) | 20,409 (2.2%) |
| Demographics | ||
| Median age (interquartile range) | 62.9 (59.5–66.9) | 62.7 (61.0–64.8) |
| Race (column %) | ||
| White | 1,922,947 (71%) | 762,049 (80%) |
| Black or African American | 333,894 (12%) | 85,772 (9%) |
| Asian/Native Hawaiian or Pacific Islander/American Indian | 49,599 (2%) | 17,707 (2%) |
| Unknown | 386,853 (14%) | 82,575 (9%) |
| Ethnicity (column %) | ||
| Hispanic or Latino | 101,550 (4%) | 36,761 (4%) |
| Not Hispanic or Latino | 2,226,246 (83%) | 836,539 (88%) |
| Unknown | 365,497 (14%) | 74,803 (8%) |
| Rurality | ||
| Rural | 989,166 (37%) | 371,684 (39%) |
| Unknown | 120,926 (4%) | 27,771 (3%) |
| Tobacco use | 1,656,838 (62%) | 588,190 (62%) |
| Military service | ||
| Army | 1,338,519 (50%) | 554,398 (58%) |
| Navy | 558,554 (21%) | 153,891 (16%) |
| Air Force | 487,227 (18%) | 105,045 (11%) |
| Marine | 265,104 (10%) | 131,193 (14%) |
| Coast Guard | 25,599 (1%) | 2,130 (0.2%) |
| Other | 18,290 (0.7%) | 1,446 (0.2%) |
Age calculated at the beginning of the study (January 1, 2010). P values were calculated with t test for continuous variables and chi-square test for categorical variables. All P values were less than 0.001.
A total of 71,495 cases of IPF among male Vietnam Veterans were identified over the 10-year study period. IPF occurred in 2.2% of the male Vietnam Veterans exposed to Agent Orange versus 1.9% without presumptive Agent Orange exposure (unadjusted OR, 1.14; 95% CI, 1.12–1.16; P < 0.001) (Table 2). The relationship held true after adjusting for known IPF risk factors including age, race, ethnicity, smoking, and rural residence and accounting for the interaction between Agent Orange × age (P = 0.008) and Agent Orange × smoking (P = 0.026). The odds of IPF among Vietnam Veterans with presumptive Agent Orange exposure was 8% higher than those with no exposure (adjusted OR, 1.08; 95% CI, 1.06–1.10; P < 0.001). The attributable fraction of IPF among Veterans with presumptive Agent Orange exposure was 7% (95% CI, 5.3–8.7%; P < 0.001). Similar results were observed when restricting the IPF cohort to Veterans who had a CT scan within the VA Healthcare System before their last IPF diagnosis (univariate OR, 1.12; 95% CI, 1.10–1.15; P < 0.001; multivariate OR, 1.06; 95% CI, 1.03–1.08).
Table 2.
Odds of Idiopathic Pulmonary Fibrosis among Male Vietnam Veterans with Agent Orange Exposure versus No Agent Orange Exposure
| Odds Ratio (95% Confidence Interval) | P Value | |
|---|---|---|
| Overall unadjusted | 1.14 (1.12–1.16) | <0.001 |
| Overall adjusted | 1.08 (1.06–1.10) | <0.001 |
Adjusted multivariable regression controlled for known idiopathic pulmonary fibrosis risk factors (age at the beginning of the study, race, ethnicity, smoking history, and rurality) and interactions between Agent Orange × age and Agent Orange × smoking.
There were differential effects among Veterans with presumptive Agent Orange exposure by age and smoking with a higher point estimate of risk among the youngest age quartile (OR, 1.15; 95% CI, 1.11–1.20; P < 0.001) and among never-smokers exposed to Agent Orange (OR, 1.13; 95% CI, 1.08–1.18; P < 0.001) (Table 3).
Table 3.
Odds of Idiopathic Pulmonary Fibrosis among Male Vietnam Veterans with Agent Orange Exposure versus No Agent Orange Exposure by Subgroup
| Odds Ratio (95% Confidence Interval) | P Value | |
|---|---|---|
| Subgroups (adjusted) | ||
| Age at beginning of study period | ||
| Quartile 1: 53–60.1 yr old | 1.15 (1.11–1.20) | <0.001 |
| Quartile 2: 60.1–62.8 yr old | 1.08 (1.04–1.11) | <0.001 |
| Quartile 3: 62.8–66.3 yr old | 1.06 (1.03–1.10) | <0.001 |
| Quartile 4: 66.3–100 yr old | 1.02 (0.97–1.06) | 0.482 |
| Tobacco use | ||
| Smoker | 1.07 (1.05–1.09) | <0.001 |
| Non-Smoker | 1.13 (1.08–1.18) | <0.001 |
Owing to nonlinearity of variable age, age at the beginning of the study period (January 1, 2010) was subcategorized into equal quartiles. Adjusted multivariable regression controlled for idiopathic pulmonary fibrosis risk factors (age, race, ethnicity, smoking history, and rurality) and accounted for interactions between Agent Orange × age and Agent Orange × smoking in the model. The subgroup analysis compares risk of idiopathic pulmonary fibrosis among Veterans with Agent Orange exposure versus no Agent Orange exposure (reference group) for each interaction subgroup while holding other variables constant.
In a sensitivity analysis (Table 4) restricting the cohort to only Veterans who served in the army as a surrogate measure for dose response, the odds of IPF were 15% higher among army Veterans with presumptive Agent Orange exposure (unadjusted OR, 1.15; 95% CI, 1.13–1.18; P < 0.001) and 13% higher among army Veterans with presumptive Agent Orange exposure after controlling for other IPF risk factors (adjusted OR, 1.13; 95% CI, 1.09–1.17; P < 0.001). The odds of IPF were 10% higher among nonarmy Veterans (navy, air force, marines, or coast guard) with presumptive Agent Orange exposure (unadjusted OR, 1.10; 95% CI, 1.09–1.13; P < 0.001) and 5% higher among nonarmy Veterans with presumptive Agent Orange exposure after controlling for other IPF risk factors (adjusted OR, 1.05; 95% CI, 1.01–1.09).
Table 4.
Odds of Idiopathic Pulmonary Fibrosis among Male Vietnam Veterans with Agent Orange Exposure versus No Agent Orange Exposure (Sensitivity Analysis)
| N | Odds Ratio (95% Confidence Interval) | P Value | ||
|---|---|---|---|---|
| Restricted to army | IPF: 38,326 | No IPF: 1,854,591 | Unadjusted, 1.15 (1.13–1.18); | <0.001; |
| Adjusted, 1.13 (1.09–1.17) | <0.001 | |||
| Restricted to most specific IPF ICD codes | IPF: 8,818 | No IPF: 3,569,901 | Unadjusted, 1.17 (1.12–1.23); | <0.001; |
| Adjusted, 1.11 (1.05–1.17) | <0.001 | |||
| Restricted to army + most specific IPF ICD codes | IPF: 4,620 | No IPF: 1,854,591 | Unadjusted, 1.23 (1.16–1.31); | <0.001; |
| Adjusted, 1.17 (1.09–1.25) | <0.001 |
Definition of abbreviations: ICD = International Classification of Disease; IPF = idiopathic pulmonary fibrosis.
Adjusted multivariable regression controlled for age, race, ethnicity, smoking history, and rurality and accounted for interactions between Agent Orange × age and Agent Orange × smoking in the model.
In a second sensitivity analysis restricting IPF diagnosis to more specific ICD codes, the odds of IPF were 17% higher among Veterans with presumptive Agent Orange exposure (unadjusted OR, 1.17; 95% CI, 1.12–1.23; P < 0.001) and 11% higher among Veterans with presumptive Agent Orange exposure after controlling for other known IPF risk factors (adjusted OR, 1.11; 95% CI, 1.05–1.17; P < 0.001).
In a final sensitivity analysis restricting the cohort to army Veterans with more specific ICD diagnosis codes, the odds of IPF were 23% higher among army Veterans with presumptive Agent Orange exposure (unadjusted OR, 1.23; 95% CI, 1.16–1.31; P < 0.001) and 17% higher among army Veterans with Agent Orange exposure after adjusting for other IPF risk factors (adjusted OR, 1.17; 95% CI, 1.09–1.25; P < 0.001).
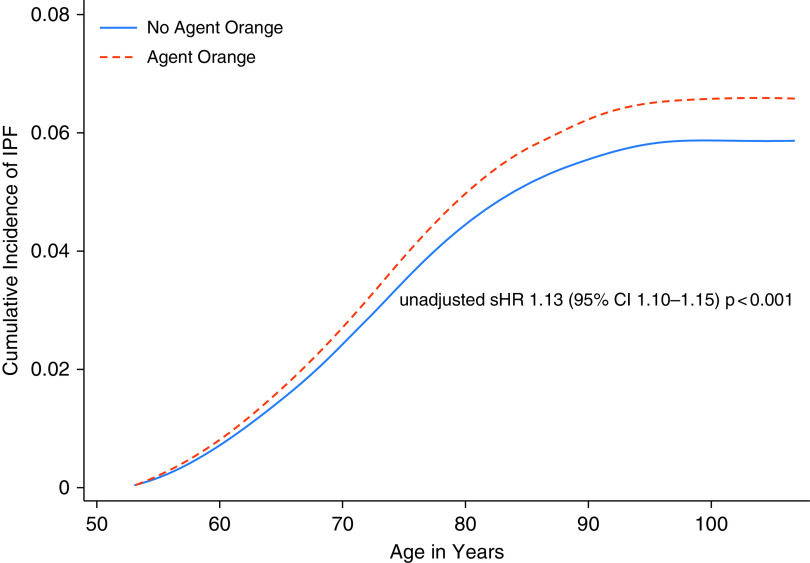
There were 810,808 (22%) deaths without IPF diagnosis during the study period. After accounting for competing risk of death, Veterans with presumptive Agent Orange exposure were still more likely to develop IPF (unadjusted subdivision hazard ratio, 1.13; 95% CI, 1.10–1.15; P < 0.001). Difference in percentage cumulative incidence of IPF diagnosis over the 10-year study period in those with and without Agent Orange exposure using age as the time scaler is shown in Figure 2.
Figure 2.
Cumulative incidence of idiopathic pulmonary fibrosis (IPF) in veterans with and without Agent Orange exposure. Time to event was calculated using age as the time scaler from the beginning of the study period (January 1, 2010) to the date of IPF diagnosis, death, or the end of the study period (December 31, 2019), assuming independent left truncation. CI = confidence interval; sHR = subdistribution hazard ratio.
Discussion
Among a national cohort of 3.6 million male Vietnam Veterans, presumptive exposure to Agent Orange was associated with a 14% higher risk of developing IPF in an unadjusted analysis and an 8% higher risk of IPF after adjusting for age, race, ethnicity, smoking history, and rural residence. Numerically higher odds were estimated when limiting the cohort to Veterans who served in the army (highest theoretical burden of Agent Orange exposure and surrogate measure for dose response) and when restricting the cohort to a more specific case definition of IPF. This study is the first to examine the impact of presumptive Agent Orange exposure on the subsequent development of IPF and contributes to a growing body of literature implicating the interplay between genetics and exposures in IPF pathogenesis.
Smoking is among the most robustly defined risk factors associated with IPF (3–6), with epidemiological studies demonstrating a more than twofold increase in risk of IPF among ever-smokers (10, 17, 28–30). Recent literature has suggested that other environmental and occupational exposures such as ambient air pollution (7–9) and activities associated with exposure to vapors, gases, dusts, and fumes (10–15) may increase the risk of IPF as well. Recognizing the need to review this emerging evidence, the American Thoracic Society and European Respiratory Society published a joint statement examining the impact of occupational exposures on the burden of nonmalignant respiratory diseases (16). The pooled population attributable fraction of workplace exposure on IPF was 26% for vapors, gases, dusts, and fumes, with variability across exposure subtype. However, this literature has largely excluded the systematic evaluation of military exposures, an important vocational risk factor particularly relevant to Veterans.
The impact of military exposures on health is an area of concern for the VA. Prior literature examining the epidemiology of IPF among the veteran population has noted higher incidence and prevalence rates than registry-based studies. This may in part be because of underlying demographics of the source population, such as older age and tobacco use, as well as vocational exposures unique to the veteran population (17, 18). Agent Orange in particular has gained attention as an exposure of interest owing to a combination of toxicology data documenting the harmful effects of dioxin and epidemiologic studies of disease among patients with occupational or environmental dioxin exposures. Although no longer used commercially in the United States, dioxin is a highly persistent chemical pollutant that accumulates in fatty tissues. Once absorbed, the elimination half-life is estimated to be 7–11 years (31), which suggests lingering health implications even after an initial inhalational insult (32). U.S. Public Law 102–3, the Agent Orange Act of 1991, has directed the National Academy of Sciences to regularly review new scientific evidence describing the association between dioxin and health outcomes of Veterans (29). The committee categorizes health outcomes related to Agent Orange exposure into four groups based on the strength of epidemiologic literature and biological plausibility of the association: 1) sufficient evidence of an association; 2) limited or suggestive evidence of an association; 3) inadequate or insufficient evidence to determine an association; or 4) limited or suggestive evidence of no association. To date, soft tissue sarcomas, lymphomas, and chronic lymphocytic leukemia have the strongest tier-one-level associations, whereas most nonmalignant respiratory conditions have had inadequate evidence to determine association (19). Our study adds to the literature and may support a future change in the categorization of IPF.
There are limited data examining the direct causal relationship between dioxin exposure in animal models and subsequent development of pulmonary fibrosis. However, several potential mechanisms through which dioxin may cause fibrosis have been proposed. Current literature examining the impact of dioxin exposure in animal models suggests that dioxin is immunotoxic (33) and has broad effects on gene expression (34, 35). Dioxin binds to and activates the Ahr (aryl hydrocarbon receptor), an important modulator of adaptative immune response and thus may contribute to pulmonary fibrosis through its chronic effects on lung inflammation (36). Toxicology studies have demonstrated that dioxin induces migration of pulmonary fibroblast through the AhR-axis (37) and that dioxin exposure is associated with portal fibrosis and cholangiofibrosis in rats (38). Ahr activation has also been shown to act on lung epithelial cells to alter mucin expression. Given the known association between a mucin gene polymorphism associated with mucous oversecretion and pulmonary fibrosis, direct effects on the airway epithelium could also be important. Further exploration of these mechanisms of actions and studies that evaluate whether dioxin exposure in animals causes pulmonary fibrosis are needed to support the biological plausibility of the epidemiologic association found in this study.
Our study has a number of limitations. First, we used an ICD code–based algorithm to identify cases of IPF, which have not been individually case validated. Accurate identification of IPF cases using billing code–based algorithms depend on the characteristics of the underlying source population. Prior case validation studies of electronic health record cohorts have reported positive predictive values ranging from 44% to 83% depending on the demographics of the population and the specificity of the algorithm used (22, 39). We hypothesize that given the underlying demographics of the veteran population (older age, male sex, and high prevalence of tobacco use), the pretest probability of IPF is higher than in cohorts with younger, more heterogeneous populations. We thus started with a broader, more sensitive case definition of IPF and subsequently conducted sensitivity analysis with more specific IPF case definitions to ensure consistency of results. Reassuringly, the conclusions remained internally consistent and were in fact numerically greater when using more specific ICD codes for IPF. Second, we used an Agent Orange Exposure Flag, which is derived from military discharge paperwork, as indicative of dioxin exposure. Patients receive an Agent Orange Exposure Flag if they served with boots on the ground in Vietnam. Although it would be informative to allow differentiation among Veterans with Agent Orange Exposure Flags over a gradient of exposure likelihood, for example, by deployment to areas of highest defoliant use, these data are not currently available in the electronic health record. We thus conducted a sensitivity analysis restricting the cohort to army Veterans as a surrogate for dose response, as they were most likely to have greater time with boots on the ground. Although the above limitations likely introduce some level of false positives into our case definition and exposure determination, we would expect such systematic classification error (i.e., including minimally exposed Veterans in the exposure category) to bias results toward the null. That an association was still observed, therefore, is notable. Third, we accounted for the competing risk of death via Fine-Gray modeling. This model accounts for survival bias during the study period (2010–2019) but not before study entry. Analysis of cases before 2010 was beyond the scope of this study. Fourth, although limited animal studies demonstrate that dioxin induces migration of pulmonary fibroblasts and is associated with fibrogenesis in the liver, studies examining whether dioxin exposure in animals causes pulmonary fibrosis are needed. Lastly, although we adjusted for known IPF risk factors, observational data can never completely rule out the possibility of confounding. It is possible that the association noted in this study is due to an upstream risk factor or a coexposure. For example, although Agent Orange was the primary herbicide used during the Vietnam War, an arsenic-based herbicide, Agent Blue, was also used in South Vietnam. Although chronic arsenic exposure has not been linked epidemiologically with IPF, it has been associated with respiratory conditions in humans and fibrosis in animal models (40, 41). For the above reasons, further exploration of the relationship among military service during Vietnam, Agent Orange, and IPF is warranted before definitive conclusions can be drawn. Further studies that investigate the association between Agent Orange exposure and IPF among the resident civilian and military populations of Vietnam could also lend important insight on the impact of dioxin exposure in IPF pathogenesis.
Conclusions
Our findings suggest that Vietnam Veterans with presumptive Agent Orange exposure are at higher risk for IPF. Additional work is needed to evaluate whether this association between Agent Orange and fibrosis is also seen in other forms of interstitial lung disease. Future studies that further refine the methodology for case and exposure identification and examine the potential biological mechanisms involved are needed and will help facilitate better understanding of pulmonary fibrosis among the U.S. veteran population.
Acknowledgments
Acknowledgment
The authors thank Dr. Dean Sheppard for his comments on potential biological mechanisms involved in Agent Orange and IPF pathogenesis.
Footnotes
Supported by the NHLBI of the NIH under Awards K12HL138046 and KL2TR001870. The funder had no role in the design or conduct of this study. The views expressed do not necessarily reflect the position or policy of the United States Government.
Author Contributions: B.K., J.S.L., D.V.G., P.D.B., H.R.C., and M.A.W. contributed to study conception, study design, and data interpretation. M.A.W. and N.Z. contributed to data acquisition. B.K., D.V.G., and M.A.W. contributed to analysis. B.K. drafted the report, and all authors revised it critically. All authors approved the final version.
Originally published in Press as DOI: 10.1164/rccm.202112-2724OC on May 13, 2022
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1. Raghu G, Remy-Jardin M, Myers JL, Richeldi L, Ryerson CJ, Lederer DJ, et al. American Thoracic Society, European Respiratory Society, Japanese Respiratory Society, and Latin American Thoracic Society Diagnosis of idiopathic pulmonary fibrosis. An official ATS/ERS/JRS/ALAT clinical practice guideline. Am J Respir Crit Care Med . 2018;198:e44–e68. doi: 10.1164/rccm.201807-1255ST. [DOI] [PubMed] [Google Scholar]
- 2. Wolters PJ, Collard HR, Jones KD. Pathogenesis of idiopathic pulmonary fibrosis. Annu Rev Pathol . 2014;9:157–179. doi: 10.1146/annurev-pathol-012513-104706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Baumgartner KB, Samet JM, Stidley CA, Colby TV, Waldron JA. Cigarette smoking: a risk factor for idiopathic pulmonary fibrosis. Am J Respir Crit Care Med . 1997;155:242–248. doi: 10.1164/ajrccm.155.1.9001319. [DOI] [PubMed] [Google Scholar]
- 4. Steele MP, Speer MC, Loyd JE, Brown KK, Herron A, Slifer SH, et al. Clinical and pathologic features of familial interstitial pneumonia. Am J Respir Crit Care Med . 2005;172:1146–1152. doi: 10.1164/rccm.200408-1104OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ekström M, Gustafson T, Boman K, Nilsson K, Tornling G, Murgia N, et al. Effects of smoking, gender and occupational exposure on the risk of severe pulmonary fibrosis: a population-based case-control study. BMJ Open . 2014;4:e004018. doi: 10.1136/bmjopen-2013-004018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. García-Sancho Figueroa MC, Carrillo G, Pérez-Padilla R, Fernández-Plata MR, Buendía-Roldán I, Vargas MH, et al. Risk factors for idiopathic pulmonary fibrosis in a Mexican population. A case-control study. Respir Med . 2010;104:305–309. doi: 10.1016/j.rmed.2009.08.013. [DOI] [PubMed] [Google Scholar]
- 7. Johannson KA, Balmes JR, Collard HR. Air pollution exposure: a novel environmental risk factor for interstitial lung disease? Chest . 2015;147:1161–1167. doi: 10.1378/chest.14-1299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Johannson KA, Vittinghoff E, Lee K, Balmes JR, Ji W, Kaplan GG, et al. Acute exacerbation of idiopathic pulmonary fibrosis associated with air pollution exposure. Eur Respir J . 2014;43:1124–1131. doi: 10.1183/09031936.00122213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Winterbottom CJ, Shah RJ, Patterson KC, Kreider ME, Panettieri RA, Jr, Rivera-Lebron B, et al. Exposure to ambient particulate matter is associated with accelerated functional decline in idiopathic pulmonary fibrosis. Chest . 2018;153:1221–1228. doi: 10.1016/j.chest.2017.07.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Andersson M, Blanc PD, Torén K, Järvholm B. Smoking, occupational exposures, and idiopathic pulmonary fibrosis among Swedish construction workers. Am J Ind Med . 2021;64:251–257. doi: 10.1002/ajim.23231. [DOI] [PubMed] [Google Scholar]
- 11. Gustafson T, Dahlman-Höglund A, Nilsson K, Ström K, Tornling G, Torén K. Occupational exposure and severe pulmonary fibrosis. Respir Med . 2007;101:2207–2212. doi: 10.1016/j.rmed.2007.02.027. [DOI] [PubMed] [Google Scholar]
- 12. Hubbard R, Cooper M, Antoniak M, Venn A, Khan S, Johnston I, et al. Risk of cryptogenic fibrosing alveolitis in metal workers. Lancet . 2000;355:466–467. doi: 10.1016/S0140-6736(00)82017-6. [DOI] [PubMed] [Google Scholar]
- 13. Iwai K, Mori T, Yamada N, Yamaguchi M, Hosoda Y. Idiopathic pulmonary fibrosis. Epidemiologic approaches to occupational exposure. Am J Respir Crit Care Med . 1994;150:670–675. doi: 10.1164/ajrccm.150.3.8087336. [DOI] [PubMed] [Google Scholar]
- 14. Lee SH, Kim DS, Kim YW, Chung MP, Uh ST, Park CS, et al. Association between occupational dust exposure and prognosis of idiopathic pulmonary fibrosis: a Korean national survey. Chest . 2015;147:465–474. doi: 10.1378/chest.14-0994. [DOI] [PubMed] [Google Scholar]
- 15. Park Y, Ahn C, Kim TH. Occupational and environmental risk factors of idiopathic pulmonary fibrosis: a systematic review and meta-analyses. Sci Rep . 2021;11:4318. doi: 10.1038/s41598-021-81591-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Blanc PD, Annesi-Maesano I, Balmes JR, Cummings KJ, Fishwick D, Miedinger D, et al. The occupational burden of nonmalignant respiratory diseases. An Official American Thoracic Society and European Respiratory Society Statement. Am J Respir Crit Care Med . 2019;199:1312–1334. doi: 10.1164/rccm.201904-0717ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kaul B, Lee JS, Zhang N, Vittinghoff E, Sarmiento K, Collard HR, et al. Epidemiology of idiopathic pulmonary fibrosis among U.S. Veterans, 2010–2019. Ann Am Thorac Soc . 2022;19:196–203. doi: 10.1513/AnnalsATS.202103-295OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Tighe RM, Chaudhary S. Uncovering the epidemiology of idiopathic pulmonary fibrosis in the Veterans Affairs Health System. Ann Am Thorac Soc . 2022;19:161–162. doi: 10.1513/AnnalsATS.202108-972ED. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.National Academies of Sciences, Engineering, and Medicine. Veterans and Agent Orange: update 2014. Washington, DC: The National Academies Press; 2016. [PubMed] [Google Scholar]
- 20. Raghu G, Chen SY, Hou Q, Yeh WS, Collard HR. Incidence and prevalence of idiopathic pulmonary fibrosis in US adults 18-64 years old. Eur Respir J . 2016;48:179–186. doi: 10.1183/13993003.01653-2015. [DOI] [PubMed] [Google Scholar]
- 21. Raghu G, Chen SY, Yeh WS, Maroni B, Li Q, Lee YC, et al. Idiopathic pulmonary fibrosis in US Medicare beneficiaries aged 65 years and older: incidence, prevalence, and survival, 2001–11. Lancet Respir Med . 2014;2:566–572. doi: 10.1016/S2213-2600(14)70101-8. [DOI] [PubMed] [Google Scholar]
- 22. Ley B, Urbania T, Husson G, Vittinghoff E, Brush DR, Eisner MD, et al. Code-based diagnostic algorithms for idiopathic pulmonary fibrosis. Case validation and improvement. Ann Am Thorac Soc . 2017;14:880–887. doi: 10.1513/AnnalsATS.201610-764OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Economic Research Service. 2019. https://www.ers.usda.gov/data-products/rural-urban-commuting-area-codes/
- 24. Snowden JM, Rose S, Mortimer KM. Implementation of G-computation on a simulated data set: demonstration of a causal inference technique. Am J Epidemiol . 2011;173:731–738. doi: 10.1093/aje/kwq472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rural veterans. 2021. https://www.ruralhealth.va.gov/aboutus/ruralvets.asp
- 26. Ngamwong Y, Tangamornsuksan W, Lohitnavy O, Chaiyakunapruk N, Scholfield CN, Reisfeld B, et al. Additive synergism between asbestos and smoking in lung cancer risk: a systematic review and meta-analysis. PLoS One . 2015;10:e0135798. doi: 10.1371/journal.pone.0135798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Cologne J, Hsu WL, Abbott RD, Ohishi W, Grant EJ, Fujiwara S, et al. Proportional hazards regression in epidemiologic follow-up studies: an intuitive consideration of primary time scale. Epidemiology . 2012;23:565–573. doi: 10.1097/EDE.0b013e318253e418. [DOI] [PubMed] [Google Scholar]
- 28. Abramson MJ, Murambadoro T, Alif SM, Benke GP, Dharmage SC, Glaspole I, et al. Australian IPF Registry Occupational and environmental risk factors for idiopathic pulmonary fibrosis in Australia: case-control study. Thorax . 2020;75:864–869. doi: 10.1136/thoraxjnl-2019-214478. [DOI] [PubMed] [Google Scholar]
- 29. Anderson C. Agent Orange: veterans sue to force study. Nature . 1990;346:498. doi: 10.1038/346498a0. [DOI] [PubMed] [Google Scholar]
- 30. Bellou V, Belbasis L, Evangelou E. Tobacco smoking and risk for pulmonary fibrosis: a prospective cohort study from the UK Biobank. Chest . 2021;160:983–993. doi: 10.1016/j.chest.2021.04.035. [DOI] [PubMed] [Google Scholar]
- 31.Dioxin and their effects on human health fact sheet. 2016. https://www.who.int/news-room/fact-sheets/detail/dioxins-and-their-effects-on-human-health
- 32.Learn about dioxin. https://www.epa.gov/dioxin/learn-about-dioxin
- 33. Kerkvliet NI. AHR-mediated immunomodulation: the role of altered gene transcription. Biochem Pharmacol . 2009;77:746–760. doi: 10.1016/j.bcp.2008.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Papoutsis AJ, Selmin OI, Borg JL, Romagnolo DF. Gestational exposure to the AhR agonist 2,3,7,8-tetrachlorodibenzo-p-dioxin induces BRCA-1 promoter hypermethylation and reduces BRCA-1 expression in mammary tissue of rat offspring: preventive effects of resveratrol. Mol Carcinog . 2015;54:261–269. doi: 10.1002/mc.22095. [DOI] [PubMed] [Google Scholar]
- 35. Somm E, Stouder C, Paoloni-Giacobino A. Effect of developmental dioxin exposure on methylation and expression of specific imprinted genes in mice. Reprod Toxicol . 2013;35:150–155. doi: 10.1016/j.reprotox.2012.10.011. [DOI] [PubMed] [Google Scholar]
- 36. Chiba T, Uchi H, Tsuji G, Gondo H, Moroi Y, Furue M. Arylhydrocarbon receptor (AhR) activation in airway epithelial cells induces MUC5AC via reactive oxygen species (ROS) production. Pulm Pharmacol Ther . 2011;24:133–140. doi: 10.1016/j.pupt.2010.08.002. [DOI] [PubMed] [Google Scholar]
- 37. Su HH, Lin HT, Suen JL, Sheu CC, Yokoyama KK, Huang SK, et al. Aryl hydrocarbon receptor-ligand axis mediates pulmonary fibroblast migration and differentiation through increased arachidonic acid metabolism. Toxicology . 2016;370:116–126. doi: 10.1016/j.tox.2016.09.019. [DOI] [PubMed] [Google Scholar]
- 38. National Toxicology Program. NTP technical report on the toxicology and carcinogenesis studies of 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) (CAS No. 1746-01-6) in female Harlan Sprague-Dawley rats (Gavage Studies) Natl Toxicol Program Tech Rep Ser . 2006;521:4–232. [PubMed] [Google Scholar]
- 39. Esposito DB, Lanes S, Donneyong M, Holick CN, Lasky JA, Lederer D, et al. Idiopathic pulmonary fibrosis in United States automated claims. Incidence, prevalence, and algorithm validation. Am J Respir Crit Care Med . 2015;192:1200–1207. doi: 10.1164/rccm.201504-0818OC. [DOI] [PubMed] [Google Scholar]
- 40. Bencko V, Yan Li Foong F. The history of arsenical pesticides and health risks related to the use of Agent Blue. Ann Agric Environ Med . 2017;24:312–316. doi: 10.26444/aaem/74715. [DOI] [PubMed] [Google Scholar]
- 41. Wang W, Zheng F, Zhang A. Arsenic-induced lung inflammation and fibrosis in a rat model: contribution of the HMGB1/RAGE, PI3K/AKT, and TGF-β1/SMAD pathways. Toxicol Appl Pharmacol . 2021;432:115757. doi: 10.1016/j.taap.2021.115757. [DOI] [PubMed] [Google Scholar]