Abstract
The antidiabetic medication metformin has been proposed to be the first drug tested to target aging and extend healthspan in humans. While there is extensive epidemiological support for the health benefits of metformin in patient populations, it is not clear if these protective effects apply to those free of age-related disease. Our previous data in older adults without diabetes suggest a dichotomous change in insulin sensitivity and skeletal muscle mitochondrial adaptations after metformin treatment when co-prescribed with exercise. Those who entered the study as insulin-sensitive had no change to detrimental effects while those who were insulin-resistant had positive changes. The objective of this clinical trial is to determine if (a) antecedent metabolic health and (b) skeletal muscle mitochondrial remodeling and function mediate the positive or detrimental effects of metformin monotherapy, independent of exercise, on the metabolism and biology of aging. In a randomized, double-blind clinical trial, adults free of chronic disease (n = 148, 40–75 years old) are stratified as either insulin-sensitive or resistant based on homeostatic model assessment of insulin resistance (≤2.2 or ≥2.5) and take 1 500 mg/day of metformin or placebo for 12 weeks. Hyperinsulinemic-euglycemic clamps and skeletal muscle biopsies are performed before and after 12 weeks to assess primary outcomes of peripheral insulin sensitivity and mitochondrial remodeling and function. Findings from this trial will identify clinical characteristics and cellular mechanisms involved in modulating the effectiveness of metformin treatment to target aging that could inform larger Phase 3 clinical trials aimed at testing aging as a treatment indication for metformin.
Clinical Trials Registration Number: NCT04264897.
Keywords: Geroscience, Healthspan, Insulin sensitivity, Mitochondria, Skeletal muscle
Rationale
The dramatic demographic shift due to worldwide population aging is one of the most critical societal challenges of our time (1). Within the next 30 years, the number of people older than the age of 65 is estimated to reach 2 billion (2). Increased longevity is accompanied by a parallel increase in the incidence of chronic diseases (2). Age is a primary risk factor for most chronic conditions, including type 2 diabetes (T2D), cardiovascular disease (CVD), arthritis, sarcopenia, dementia, and cancer (3,4). Additionally, ~63% of the population 65 years or older has 2 or more age-related chronic conditions which is nearly double that of people aged 45–64 years (5). A recent framework has divided life span into a time of life free of disease (healthspan) followed by the accumulation of one or more overt age-associated diseases and disabilities (4). There is an urgent need to develop strategies that can simultaneously decrease the risk of age-related comorbidities. Such strategies that delay the onset of age-related chronic conditions to improve healthspan must start before the onset of age-related disease (6). It is now critical to test new or repurposed existing drugs for healthspan extension in people who are currently free of chronic disease.
Metformin is a candidate to be the first pharmaceutical treatment to slow aging and extend healthspan (7). Metformin is the most widely prescribed oral antihyperglycemic medication and has 60 years of safety documentation, low cost, and is associated with decreased risk of mortality and diseases such as T2D, CVD, dementia, and cancer (8–12). While these findings provide rationale and fuel the excitement for metformin to treat aging, the completed studies were conducted in participants with T2D and none were in relatively healthy participants, absent of disease. Thus, the efficacy of metformin to improve outcomes associated with the aging process in human participants free of chronic disease remains incompletely understood.
While the pleiotropic effects of metformin treatment could potentially be advantageous for targeting the basic biology of aging, the health- and lifespan-extending effects of metformin are equivocal across multiple models, including Caenorhabditis elegans, mice, rats, and humans. Metformin increases mean life span when started in young C. elegans (13,14), yet the opposite is true in aged C. elegans where metformin decreases life span (15). However, insulin-resistant daf-2(e1370) mutant C. elegans were impervious to the decrease in life span by metformin (15). Dietary metformin (1 000 ppm) increased life span and indices of healthspan in male C57BL6 mice (16) but not in male and female UMHET3 mice (17). Metformin can even shorten life span in C57BL6 mice when used at higher dietary doses (10 000 ppm) (16). Collectively, these studies in model systems highlight that the positive effects of metformin are not universal.
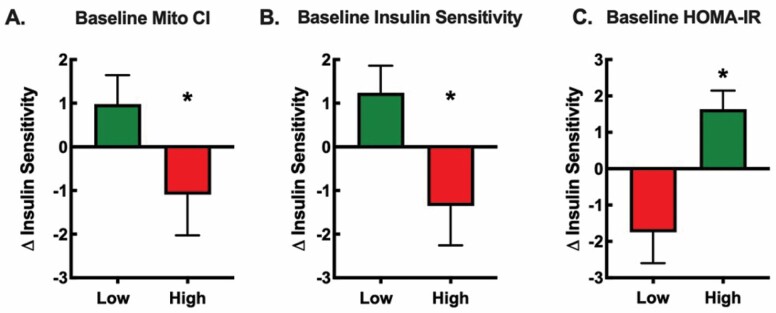
The landmark Diabetes Prevention Program trial examined the progression of participants with prediabetes to T2D (18). Over the 3 years of study, the metformin treatment group (1 700 mg/day; n = 1 073) had significantly less progression to T2D compared to controls. However, the effect of metformin was minimal in those with a lower body mass index and lower fasting glucose and tended to be less effective in older versus younger adults (18,19). Therefore, it remains unclear if metformin will be an effective treatment to improve aging processes in people free of chronic disease. These findings are in line with our recently completed randomized, double-blinded clinical trial investigating the impact of metformin on the healthspan-extending effects of exercise (20). Sedentary older participants (n = 53, 62 ± 2 years) completed 12 weeks of aerobic exercise training while taking either placebo or metformin (1 500–2 000 mg/day). On average, metformin prevented the expected exercise-induced increase in whole-body insulin sensitivity and mitochondrial complex I-linked respiration. However, there was a wide range of intersubject heterogeneity specific to the metformin group, but not the placebo group, where some participants demonstrated the expected exercise-induced improvement in insulin sensitivity while others had no improvement or a decrement in insulin sensitivity (20). Retrospectively, we used baseline subject characteristics of the metformin-treated group and rank sorted the participants to determine if any baseline characteristics were associated with the change in insulin sensitivity after exercise training and metformin treatment (Figure 1). There were significant differences in the change in insulin sensitivity associated with baseline mitochondrial complex I respiration, insulin sensitivity, and HOMA-IR, when participants were ranked and divided into 2 groups representing the upper 50% (high) and lower 50% (low). In other words, negative responders were participants who began the study with greater mitochondrial complex I respiration and insulin sensitivity and lower HOMA-IR. We interpreted these secondary analyses to suggest that participants who entered the study in a metabolically healthy state had detrimental changes in insulin sensitivity when taking metformin with exercise, while those that were relatively less metabolically healthy had positive changes. We do not yet know if these detrimental or positive responses happen with metformin monotherapy in the absence of exercise training. Therefore, if metformin is to be recommended to slow the onset of age-related disease, we need to further understand the characteristics of participants who do or do not benefit from metformin treatment.
Figure 1.
Retrospective analysis of baseline subject characteristics on the change in insulin sensitivity after metformin plus exercise. Participants (n = 25–27) from our previous study (21) were divided into 2 groups representing the upper 50% (high) and lower 50% (low) for baseline (A) skeletal muscle mitochondrial complex I (CI)-linked respiration, (B) insulin sensitivity, and (C) HOMA-IR. The negative responders, noted in the red bars, were the participants who began the study with greater mitochondrial CI-linked respiration, higher insulin sensitivity, and lower insulin resistance. The positive responders, noted in green bars, were the participants who began the study with lower mitochondrial CI-linked respiration, lower insulin sensitivity, and greater insulin resistance. These data suggest those who were metabolically healthy had a negative response while those who were relatively less healthy had a positive response to metformin adjuvant to exercise. As previously described (21), we performed skeletal muscle biopsies to assess skeletal muscle mitochondrial respiration in permeabilized fibers, a 75 g oral glucose tolerance test to determine insulin sensitivity via the Matsuda Index, and fasting insulin and glucose values to determine insulin resistance via HOMA-IR. *p <.05 vs. Low.
Skeletal muscle is the largest organ in the body and is critical for maintaining physical function, energy balance, and metabolic health across the life span. With increasing age, there is an association between skeletal muscle mitochondrial respiration and walking speed (22), aerobic capacity (21,23), muscle strength (23), and insulin sensitivity (21). However, as previously reviewed (6,24), studies evaluating the effects of metformin on skeletal muscle mitochondria are limited with nearly an equal number of studies supporting or refuting the idea that metformin inhibits complex I activity. Studies that suggest an inhibitory effect of metformin on skeletal muscle mitochondria have used suprapharmacological doses (25–27). Our data were the first to show that a clinically relevant dose of metformin prevented the expected increase in complex I-linked mitochondrial respiration after aerobic exercise training (20). Whether metformin acts directly on the mitochondria or indirectly through mitochondrial protein remodeling remains unclear. We have previously shown that adding metformin to rapamycin in young UMHET3 mice decreases protein synthesis rates of complexes within the electron transport system, including proteins belonging to complex I (28). Therefore, it remains to be reconciled if complex I protein remodeling is a mechanism by which metformin monotherapy modulates skeletal muscle respiration and hydrogen peroxide (H2O2) emissions.
This clinical trial will address 2 unresolved questions: (a) Does antecedent metabolic health influence healthspan-related responses to metformin monotherapy? (b) Does metformin treatment lead to mitochondrial remodeling in skeletal muscle that changes mitochondrial function? We hypothesize that metformin treatment will improve insulin sensitivity and glucoregulation in insulin-resistant individuals, but will decrease or not improve insulin sensitivity and glucoregulation in insulin-sensitive individuals. We also hypothesize that metformin treatment will remodel skeletal muscle mitochondria in a way that improves mitochondrial function in participants who are insulin-resistant but decreases mitochondrial function in participants who are insulin-sensitive. We will explore if proposed biomarkers of aging (29) predict or correlate with changes in insulin sensitivity and mitochondrial metabolism in disease-free individuals. This is the first human clinical trial aimed at identifying the clinical and cellular characteristics that modulate the effectiveness of metformin on the metabolism and biology of aging in individuals free of disease.
Study Overview
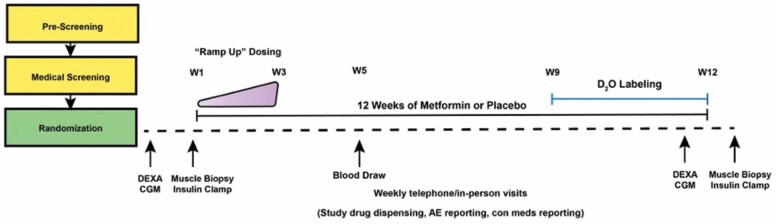
We have started a 12-week randomized, double-blind, placebo-controlled clinical trial at 2 sites: (a) Oklahoma Medical Research Foundation/University of Oklahoma Health Sciences Center (collectively referred to as OKC) led by B. F. Miller and (b) the University of Wisconsin–Madison (UWM) led by A. R. Konopka. The ongoing trial is registered at clinicaltrials.gov (NCT04264897). We are recruiting 148 participants, including men and women of all races and ethnic backgrounds between the ages of 40 and 75 who are not being treated with glucose-lowering drugs nor have overt chronic diseases (Table 1). Participants are eligible if they have impaired fasting glucose, obesity, hypertension, hyperlipidemia, or are physically inactive or on medications treating these CVD risk factors. Fifty percent of participants will be recruited at each site (OKC and UWM), where half of the participants are stratified as insulin-sensitive (n = 74) and half as insulin-resistant (n = 74) based on baseline HOMA-IR (≤2.2 or ≥2.5). As illustrated in Figure 2, eligible participants will complete a dual-energy x-ray absorptiometry scan to assess body composition, continuous glucose monitor wear period to evaluate ambulant glucose behavior, a skeletal muscle biopsy to determine mitochondrial respiration, hydrogen peroxide emissions, and protein remodeling, and a 3-hour hyperinsulinemic-euglycemic clamp to measure peripheral insulin sensitivity. Details of the study design, participants, and methodology are given in Supplementary Material.
Table 1.
Study Inclusion and Exclusion Criteria
| Inclusion criteria |
|---|
| • Between the ages of 40 and 75 years |
| • No chronic illnesses |
| • Comprehension of the protocol as indicated by an ability to respond to questions about the study after reading the consent form |
| • Be able to use and be reached by phone • Self-mobility • HOMA-IR ≤2.2 or ≥2.5 |
| Exclusion criteria |
| • Pregnancy |
| • Heart disease (history, abnormal ECG, abnormal stress ECG) |
| • Cerebrovascular disease (history) |
| • Cancer (history) |
| • Chronic respiratory disease (history, FEV1/forced vital capacity <70, FEV1 <80% predicted) |
| • Chronic liver disease (history, abnormal blood liver panel defined as outside normal reference range for alanine aminotransferase: >52 units per liter (U/L)) |
| • Diabetes (history, HbA1C ≥6.5, fasting blood glucose ≥126 mg/dL) |
| • Alzheimer’s (history) |
| • Chronic kidney disease (history, abnormal blood kidney panel including serum creatinine >1.4 and glomerular filtration rate <45 mL/min/1.732) • Problems with bleeding, on medication that prolongs bleeding time |
| • Those on glucose-lowering drugs • Vitamin B12 deficiency (<193 pg/mL) |
| • Those planning to have imaging that requires intravenous contrast dye (within 6 weeks) or is on medications contraindicated with the use of metformin: Dofetilide, Lamotrigine, Pegvisomant, Somatropin, Trimethoprim, Trospium, Gatifloxacin, Cephalexin, Cimetidine, and Dalfampridine |
| • Tobacco use |
| • Allergies to lidocaine or metformin |
| • Alcohol use that is equal to or greater than the following guidelines of greater than 3 drinks on any single day or no more than 7 drinks a week for women, and no more than 4 drinks on a single day or greater than 14 drinks a week for men |
Note : ECG = electrocardiogram; FEV = forced expiratory volume.
Figure 2.
Study design schematic. To understand the translational potential of metformin to slow aging, this ongoing 12-week, double-blinded, randomized control trial aims to determine how antecedent metabolic health (insulin resistant vs insulin sensitive) and the relationship between skeletal muscle mitochondrial remodeling and function affect the potential healthspan-extending effects of metformin. After screening, eligible participants will be randomized to 12 weeks of placebo or metformin (1 500 mg/day) by the study statistician. Pre- and postintervention assessments include dual-energy x-ray absorptiometry (DEXA) to assess body composition, continuous glucose monitoring (CGM) to determine glucoregulation, proposed blood-based biomarkers of aging, hyperinsulinemic-euglycemic clamp to measure peripheral insulin sensitivity, and muscle biopsy samples to evaluate mitochondrial function by high-resolution fluorespirometry. We will also use deuterium oxide (D2O) stable isotope labeling during the last 4 weeks of the intervention to determine individual and complex-specific mitochondrial remodeling from postmuscle biopsy samples. W = Week; con meds = concomitant medications.
Conclusions
There is an urgent need to develop new therapeutic options to prevent or delay the deleterious changes of aging that contribute to morbidity among older adults. As we have illustrated, there are contradictory findings about chronic metformin treatment, and the effects of metformin treatment in those free of disease are largely unknown. Although there are studies using metformin to delay morbidity in rodent models, there is a noted lack of investigations examining metformin use in a middle-to-older-aged human population absent of metabolic disease. Importantly, within a population of disease-free individuals, there is a range of metabolic health and insulin sensitivity, which makes some more at risk for chronic age-related disease than others. With the geroscience goal of preventing or delaying morbidity and mortality, it is implied that treatment should begin before the accumulation of age-related morbidities. Therefore, our study will close the knowledge gap in understanding how metformin may affect aging-related processes in participants free of disease and identify the mechanisms through which metformin has potentially varying effects on aging-related outcomes. By the conclusion of this study, we will have provided insight into which participants could benefit from metformin treatment and thereby inform the design of a larger clinical trial aimed at using metformin to target aging.
Supplementary Material
Acknowledgments
This study is ongoing and we would like to thank our participants for their time and dedication. We appreciate the efforts of Jaime Laurin and Katlyn Beecken at the initiation of the study and Dr. Julie Stoner (deceased) for assistance with creating the study protocol and statistical design. We also would like to thank Gina Herbert at OKC and Sara Decker, Tamara Kempken Mehring, Tammy Kiger, and Susan Johnston within the Clinical Research Unit and the Pharmaceutical Research Center at UWM for their detailed implementation and execution of the study protocol. We also express our gratitude to Samantha Lebsock at Belmar Pharmacy for leading study drug compounding and allocation. We apologize that we could not cite many studies using metformin due to reference limitations.
Contributor Information
Santosh Kumari, Division of Geriatrics and Gerontology, Department of Medicine, University of Wisconsin–Madison, Madison, Wisconsin, USA; Geriatric Research Education and Clinical Center, William S. Middleton Memorial Veterans Hospital, Madison, Wisconsin, USA.
Matthew T Bubak, Aging and Metabolism Research Program, Oklahoma Medical Research Foundation, Oklahoma City, Oklahoma, USA.
Hayden M Schoenberg, Division of Geriatrics and Gerontology, Department of Medicine, University of Wisconsin–Madison, Madison, Wisconsin, USA; Geriatric Research Education and Clinical Center, William S. Middleton Memorial Veterans Hospital, Madison, Wisconsin, USA.
Arik Davidyan, Aging and Metabolism Research Program, Oklahoma Medical Research Foundation, Oklahoma City, Oklahoma, USA.
Christian J Elliehausen, Division of Geriatrics and Gerontology, Department of Medicine, University of Wisconsin–Madison, Madison, Wisconsin, USA; Geriatric Research Education and Clinical Center, William S. Middleton Memorial Veterans Hospital, Madison, Wisconsin, USA.
Katrin G Kuhn, Hudson College of Public Health, University of Oklahoma Health Sciences Center, Oklahoma City, Oklahoma, USA.
Timothy M VanWagoner, Oklahoma Shared Clinical and Translational Resources, University of Oklahoma Health Sciences Center, Oklahoma City, Oklahoma, USA.
Rowan Karaman, Division of Endocrinology, Diabetes, and Metabolism, Department of Medicine, University of Wisconsin–Madison, Madison, Wisconsin, USA; Division of Endocrinology, William S. Middleton Memorial Veterans Hospital, Madison, Wisconsin, USA.
Robert Hal Scofield, Department of Veterans Affairs Medical Center, Oklahoma City, Oklahoma, USA; Arthritis & Clinical Immunology Program, Oklahoma Medical Research Foundation, Oklahoma City, Oklahoma, USA; Endocrinology, Diabetes and Metabolism Section, Department of Medicine, University of Oklahoma Health Sciences Center, Oklahoma City, Oklahoma, USA; Harold Hamm Diabetes Center, University of Oklahoma Health Sciences Center, Oklahoma City, Oklahoma, USA.
Benjamin F Miller, Aging and Metabolism Research Program, Oklahoma Medical Research Foundation, Oklahoma City, Oklahoma, USA; Harold Hamm Diabetes Center, University of Oklahoma Health Sciences Center, Oklahoma City, Oklahoma, USA; Oklahoma Center for Geroscience and Healthy Brain Aging, University of Oklahoma Health Sciences Center, Oklahoma City, Oklahoma, USA.
Adam R Konopka, Division of Geriatrics and Gerontology, Department of Medicine, University of Wisconsin–Madison, Madison, Wisconsin, USA; Geriatric Research Education and Clinical Center, William S. Middleton Memorial Veterans Hospital, Madison, Wisconsin, USA.
Funding
This work is supported by the National Institute on Aging, grant R01 AG064951 (to B.F.M.); the Clinical and Translational Science Award (CTSA) program through the NIH National Center for Advancing Translational Sciences (NCATS), grant UL1TR002373 (UWM), the Oklahoma Shared Clinical and Translational Resources, grant U54GM104938 (OUHSC), and Dexcom, Inc. (to A.R.K.). MTB and AD are supported by the National Institute on Aging T32AG052363. The work at UWM is supported using facilities and resources from the William S. Middleton Memorial Veterans Hospital. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH, the Department of Veterans Affairs, or the United States Government.
Conflict of Interest
None declared.
Author Contributions
A.R.K. is the Co-I for the project and PI for the UWM site. B.F.M. is the PI for the project and OKC site. A.R.K. and B.F.M. are responsible for study design, study execution, data collection, data analysis, and data interpretation. S.K., M.B., A.D., C.J.E., and T.M.V. are responsible for data collection and analysis. K.G.K. is a biostatistician and is responsible for randomization and data analysis. R.K. and R.H.S. are study physicians and provide medical oversight.
References
- 1. Burch JB, Augustine AD, Frieden LA, et al. Advances in geroscience: impact on healthspan and chronic disease. J Gerontol A Biol Sci Med Sci. 2014;69(suppl 1):S1–S3. doi: 10.1093/gerona/glu041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. UNFPA and HelpAge International. Ageing in the twenty-first century. UNFPA and HelpAge International. Accessed May 10, 2021. https://www.unfpa.org/publications/ageing-twenty-first-century
- 3. Niccoli T, Partridge L. Ageing as a risk factor for disease. Curr Biol. 2012;22(17):R741–R752. doi: 10.1016/j.cub.2012.07.024 [DOI] [PubMed] [Google Scholar]
- 4. Seals DR, Justice JN, LaRocca TJ. Physiological geroscience: targeting function to increase healthspan and achieve optimal longevity. J Physiol. 2016;594(8):2001–2024. doi: 10.1113/jphysiol.2014.282665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Boersma P. Prevalence of multiple chronic conditions among US adults, 2018. Prev Chronic Dis. 2020;17. doi: 10.5888/pcd17.200130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Konopka AR, Miller BF. Taming expectations of metformin as a treatment to extend healthspan. Geroscience. 2019;41(2):101–108. doi: 10.1007/s11357-019-00057-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Barzilai N, Crandall JP, Kritchevsky SB, Espeland MA. Metformin as a tool to target aging. Cell Metab. 2016;23(6):1060–1065. doi: 10.1016/j.cmet.2016.05.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bannister CA, Holden SE, Jenkins-Jones S, et al. Can people with type 2 diabetes live longer than those without? A comparison of mortality in people initiated with metformin or sulphonylurea monotherapy and matched, non-diabetic controls. Diabetes Obes Metab. 2014;16(11):1165–1173. doi: 10.1111/dom.12354 [DOI] [PubMed] [Google Scholar]
- 9. Effect of intensive blood-glucose control with metformin on complications in overweight patients with type 2 diabetes (UKPDS 34). UK Prospective Diabetes Study (UKPDS) Group. Lancet. 1998;352(9131):854–865. [PubMed] [Google Scholar]
- 10. Jw W, Dm B, Y P, Ni S, An F. Commonly used diabetes and cardiovascular medications and cancer recurrence and cancer-specific mortality: a review of the literature. Expert Opin Drug Saf. 2014;13(8):1071–1099. doi: 10.1517/14740338.2014.926887 [DOI] [PubMed] [Google Scholar]
- 11. Ng TP, Feng L, Yap KB, Lee TS, Tan CH, Winblad B. Long-term metformin usage and cognitive function among older adults with diabetes. J Alzheimers Dis. 2014;41(1):61–68. doi: 10.3233/JAD-131901 [DOI] [PubMed] [Google Scholar]
- 12. Campbell JM, Bellman SM, Stephenson MD, Lisy K. Metformin reduces all-cause mortality and diseases of ageing independent of its effect on diabetes control: a systematic review and meta-analysis. Ageing Res Rev. 2017;40:31–44. doi: 10.1016/j.arr.2017.08.003 [DOI] [PubMed] [Google Scholar]
- 13. Onken B, Driscoll M. Metformin induces a dietary restriction-like state and the oxidative stress response to extend C. elegans healthspan via AMPK, LKB1, and SKN-1. PLoS One. 2010;5(1):e8758. doi: 10.1371/journal.pone.0008758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Cabreiro F, Au C, Leung KY, et al. Metformin retards aging in C. elegans by altering microbial folate and methionine metabolism. Cell. 2013;153(1):228–239. doi: 10.1016/j.cell.2013.02.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Espada L, Dakhovnik A, Chaudhari P, et al. Loss of metabolic plasticity underlies metformin toxicity in aged Caenorhabditis elegans. Nat Metab. 2020;2(11):1316–1331. doi: 10.1038/s42255-020-00307-1 [DOI] [PubMed] [Google Scholar]
- 16. Martin-Montalvo A, Mercken EM, Mitchell SJ, et al. Metformin improves healthspan and lifespan in mice. Nat Commun. 2013;4:2192. doi: 10.1038/ncomms3192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Strong R, Miller RA, Antebi A, et al. Longer lifespan in male mice treated with a weakly estrogenic agonist, an antioxidant, an α-glucosidase inhibitor or a Nrf2-inducer. Aging Cell. 2016;15(5):872–884. doi: 10.1111/acel.12496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Knowler WC, Barrett-Connor E, Fowler SE, et al. ; Diabetes Prevention Program Research Group . Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346(6):393–403. doi: 10.1056/NEJMoa012512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Diabetes Prevention Program Research Group, Crandall J, Schade D, et al. The influence of age on the effects of lifestyle modification and metformin in prevention of diabetes. J Gerontol A Biol Sci Med Sci. 2006;61(10):1075–1081. doi: 10.1093/gerona/61.10.1075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Konopka AR, Laurin JL, Schoenberg HM, et al. Metformin inhibits mitochondrial adaptations to aerobic exercise training in older adults. Aging Cell. 2019;18(1):e12880. doi: 10.1111/acel.12880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Short KR, Bigelow ML, Kahl J, et al. Decline in skeletal muscle mitochondrial function with aging in humans. Proc Natl Acad Sci U S A. 2005;102(15):5618–5623. doi: 10.1073/pnas.0501559102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Coen PM, Jubrias SA, Distefano G, et al. Skeletal muscle mitochondrial energetics are associated with maximal aerobic capacity and walking speed in older adults. J Gerontol A Biol Sci Med Sci. 2013;68(4):447–455. doi: 10.1093/gerona/gls196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Gonzalez-Freire M, Scalzo P, D’Agostino J, et al. Skeletal muscle ex vivo mitochondrial respiration parallels decline in vivo oxidative capacity, cardiorespiratory fitness, and muscle strength: the Baltimore Longitudinal Study of Aging. Aging Cell. 2018;17(2):e12725. doi: 10.1111/acel.12725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Miller BF, Thyfault JP. Exercise-pharmacology interactions: metformin, statins, and healthspan. Physiology (Bethesda). 2020;35(5):338–347. doi: 10.1152/physiol.00013.2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Wessels B, Ciapaite J, van den Broek NM, Nicolay K, Prompers JJ. Metformin impairs mitochondrial function in skeletal muscle of both lean and diabetic rats in a dose-dependent manner. PLoS One. 2014;9(6):e100525. doi: 10.1371/journal.pone.0100525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kane DA, Anderson EJ, Price JW 3rd, et al. Metformin selectively attenuates mitochondrial H2O2 emission without affecting respiratory capacity in skeletal muscle of obese rats. Free Radic Biol Med. 2010;49(6):1082–1087. doi: 10.1016/j.freeradbiomed.2010.06.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Brunmair B, Staniek K, Gras F, et al. Thiazolidinediones, like metformin, inhibit respiratory complex I: a common mechanism contributing to their antidiabetic actions? Diabetes. 2004;53(4):1052–1059. doi: 10.2337/diabetes.53.4.1052 [DOI] [PubMed] [Google Scholar]
- 28. Wolff CA, Lawrence MM, Porter H, et al. Sex differences in changes of protein synthesis with rapamycin treatment are minimized when metformin is added to rapamycin. Geroscience. 2021;43(2):809–828. doi: 10.1007/s11357-020-00243-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Justice JN, Ferrucci L, Newman AB, et al. A framework for selection of blood-based biomarkers for geroscience-guided clinical trials: report from the TAME Biomarkers Workgroup. Geroscience. 2018;40(5–6):419–436. doi: 10.1007/s11357-018-0042-y [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.